Submitted:
11 January 2024
Posted:
12 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
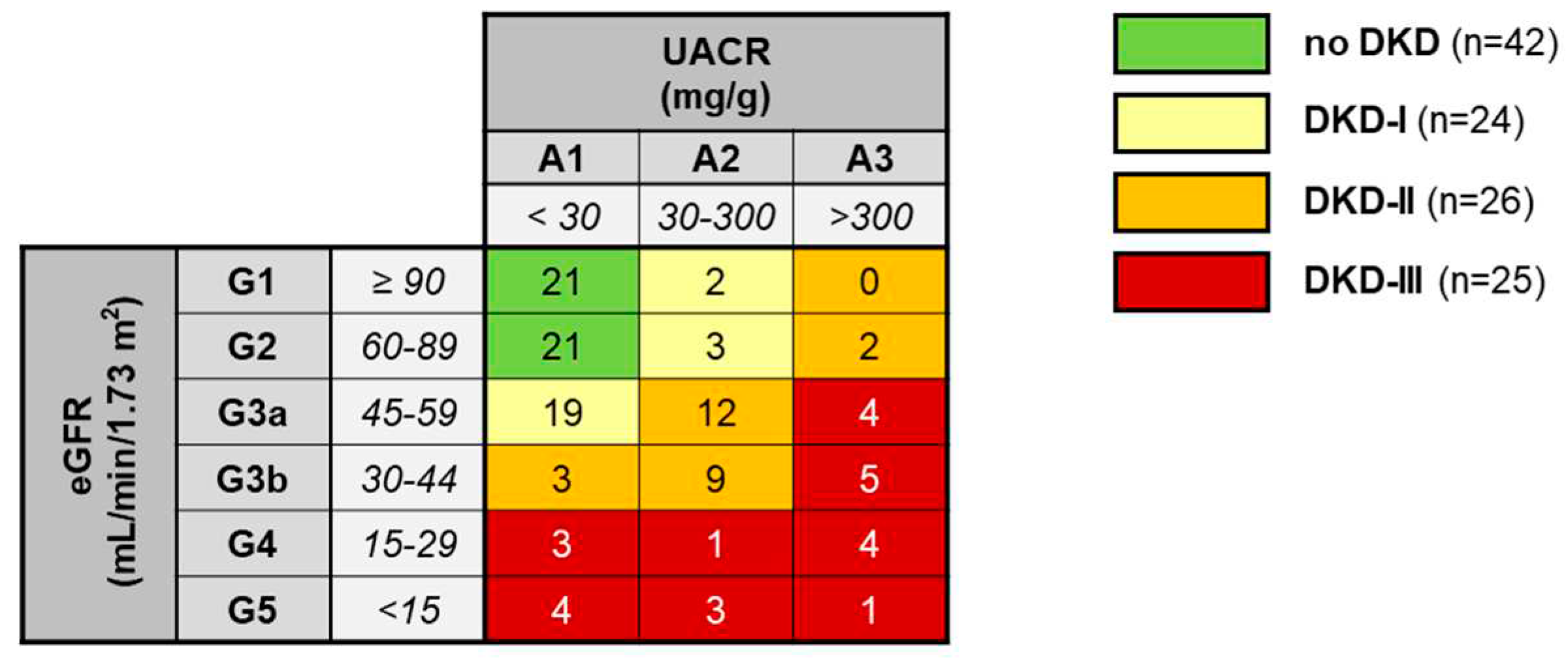
2.1. Subject population
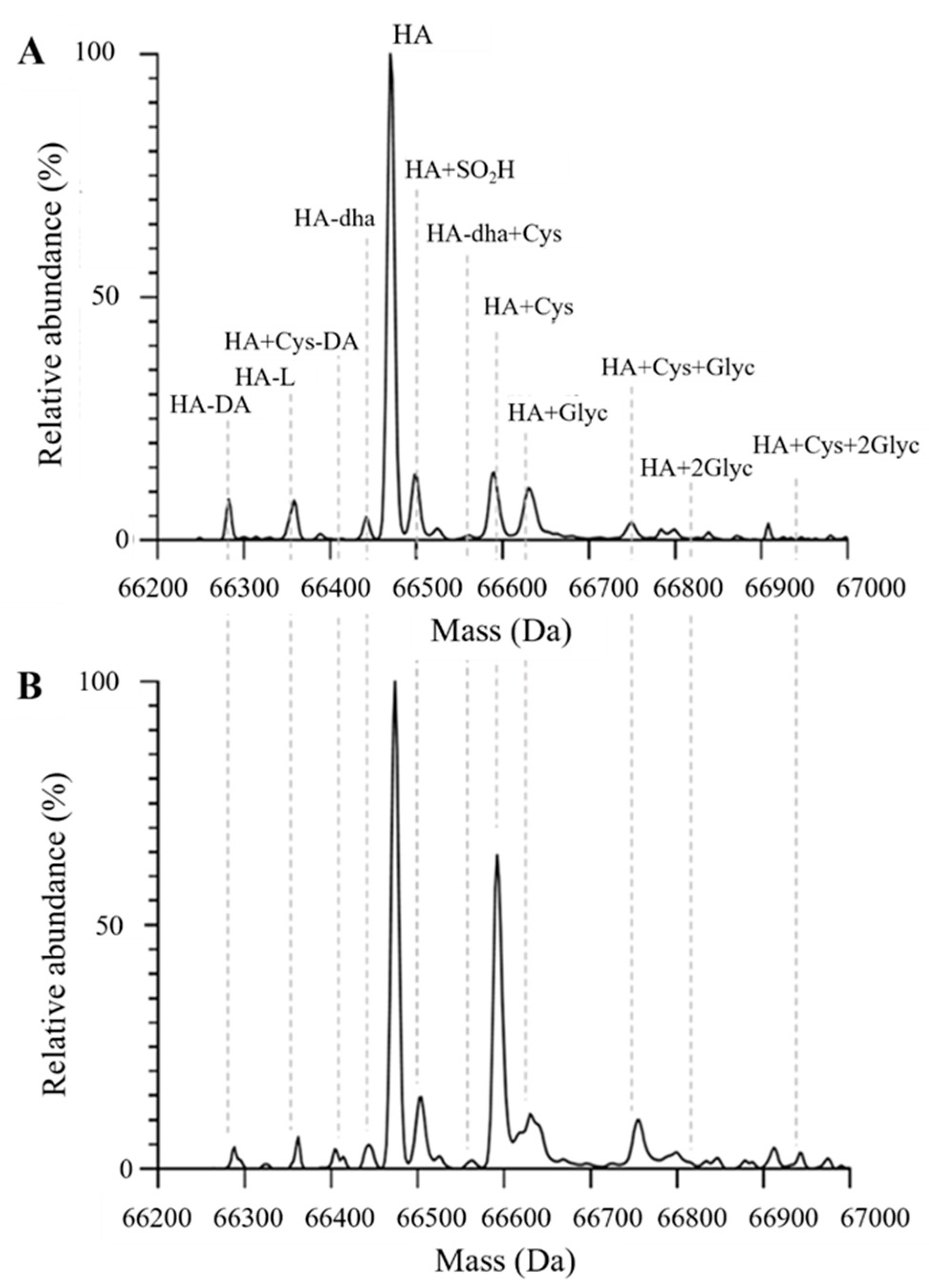
2.2. Evaluation of albumin structure
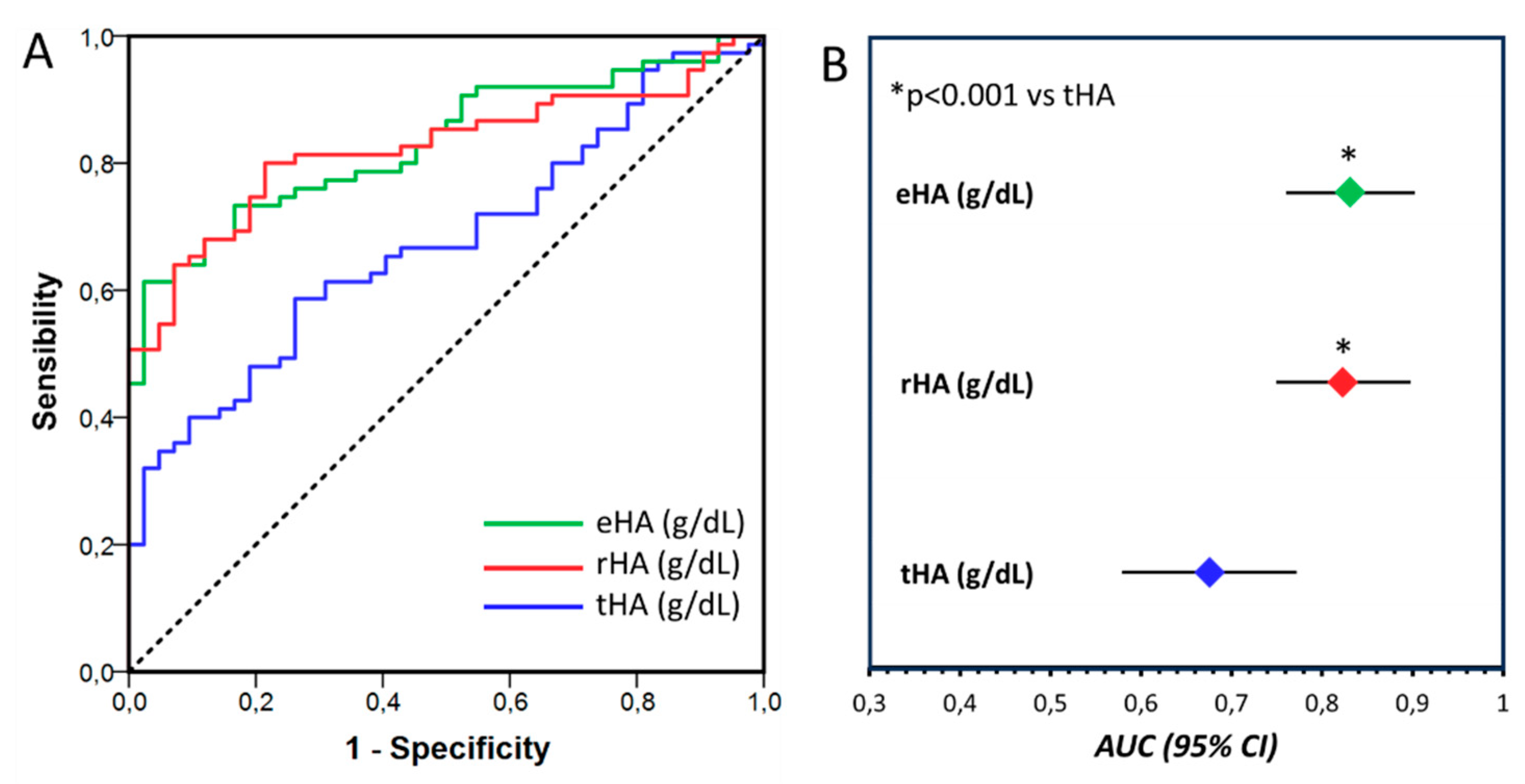
2.3. Total, effective, and reduced albumin concentration
2.4. Correlation of tHA, rHA and eHA with biochemical parameters
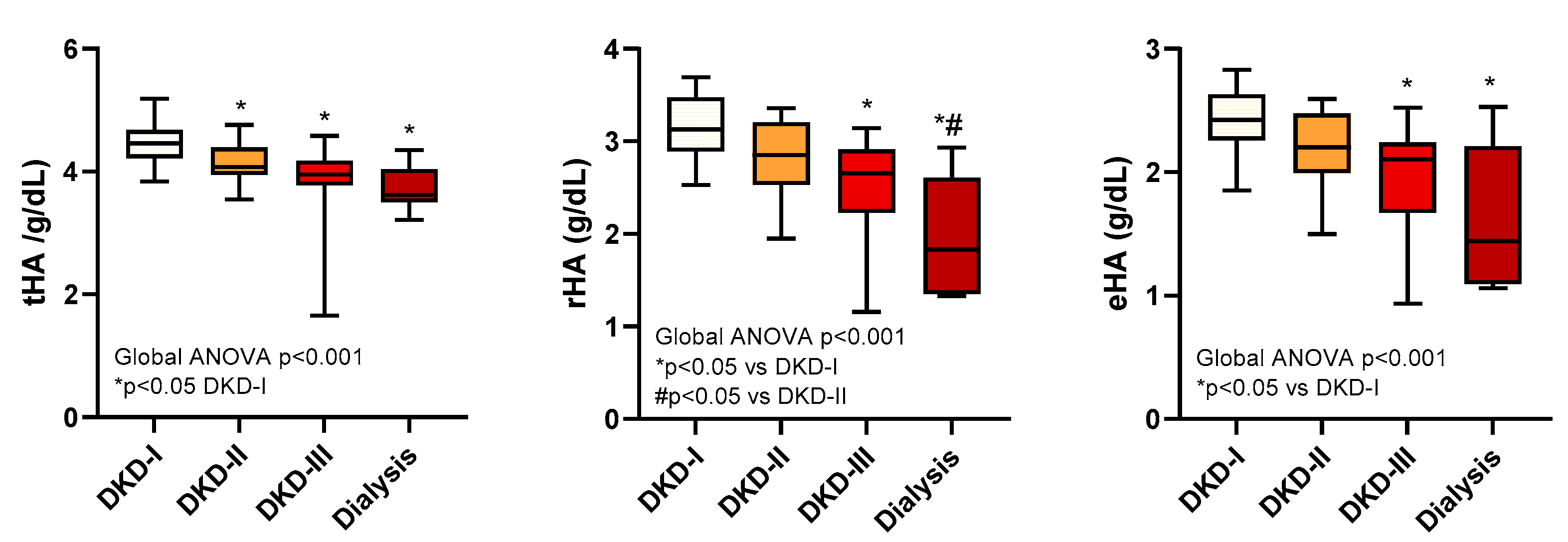
2.5. Albumin and severity of diabetic kidney disease
3. Discussion
4. Materials and Methods
4.1. Patients and study design
4.2. Bromo cresol green (BCG) colorimetric assay
4.3. Liquid chromatography-mass spectrometry (LC-MS) analysis
4.4. Assessment of eHA and rHA
4.5. Statistical analysis
Supplementary Materials
CRediT authorship contribution statement
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
References
- Persson, F.; Rossing, P. Diagnosis of Diabetic Kidney Disease: State of the Art and Future Perspective. Kidney Int. Suppl. 2018, 8, 2–7.
- Sugahara, M.; Pak, W.L.W.; Tanaka, T.; Tang, S.C.W.; Nangaku, M. Update on Diagnosis, Pathophysiology, and Management of Diabetic Kidney Disease. Nephrology 2021, 26, 491–500. [CrossRef]
- Yamanouchi, M.; Furuichi, K.; Hoshino, · Junichi; Ubara, Y.; Wada, T. Nonproteinuric Diabetic Kidney Disease. Clin. Exp. Nephrol. 2020, 24, 573–581. [CrossRef]
- Hoogeveen, E.K. The Epidemiology of Diabetic Kidney Disease. Kidney Dial. 2022, 2, 433–442. [CrossRef]
- Solini, A.; Penno, G.; Bonora, E.; Fondelli, C.; Orsi, E.; Arosio, M.; Trevisan, R.; Vedovato, M.; Cignarelli, M.; Andreozzi, F.; et al. Diverging Association of Reduced Glomerular Filtration Rate and Albuminuria with Coronary and Noncoronary Events in Patients with Type 2 Diabetes: The Renal Insufficiency and Cardiovascular Events (RIACE) Italian Multicenter Study. Diabetes Care 2012, 35, 143–149. [CrossRef]
- Oshima, M.; Shimizu, M.; Yamanouchi, M.; Toyama, T.; Hara, A.; Furuichi, K.; Wada, T. Trajectories of Kidney Function in Diabetes: A Clinicopathological Update. Nat. Rev. Nephrol. 2021, 17, 740–750. [CrossRef]
- Sauriasari, R.; Safitri, D.D.; Azmi, N.U. Current Updates on Protein as Biomarkers for Diabetic Kidney Disease: A Systematic Review. Ther. Adv. Endocrinol. Metab. 2021, 12. [CrossRef]
- Thomas, M.C.; Brownlee, M.; Susztak, K.; Sharma, K.; Jandeleit-Dahm, K.A.M.; Zoungas, S.; Rossing, P.; Groop, P.H.; Cooper, M.E. Diabetic Kidney Disease. Nat. Rev. Dis. Prim. 2015 11 2015, 1, 1–20. [CrossRef]
- Pena, M.J.; Mischak, H.; Heerspink, H.J.L. Proteomics for Prediction of Disease Progression and Response to Therapy in Diabetic Kidney Disease. Diabetologia. [CrossRef]
- Yan, Z.; Wang, G.; Shi, X. Advances in the Progression and Prognosis Biomarkers of Chronic Kidney Disease. Front. Pharmacol. 2021, 12, 3731. [CrossRef]
- Piwkowska, A.; Zdrojewski, Ł.; Heleniak, Z.; Dębska-ślizień, A. Novel Markers in Diabetic Kidney Disease—Current State and Perspectives. Diagnostics 2022, Vol. 12, Page 1205 2022, 12, 1205. [CrossRef]
- Baldassarre, M.; Naldi, M.; Zacchierini, G.; Bartoletti, M.; Agnese, A.; Laggetta, M.; Gagliardi, M.; Tufoni, M.; Domenicali, M.; Waterstradt, K.; et al. Determination of Effective Albumin in Patients With Decompensated Cirrhosis: Clinical and Prognostic Implications. Hepatology 2021, 74. [CrossRef]
- Marjolaine Roche, Philippe Rondeau, N.S.; Evalyne Tarnus, E.B. The Antioxidant Properties of Serum Albumin.Pdf. FEBS Lett. 2008, 582, 1783–1787.
- Jagdish, R.K.; Maras, J.S.; Sarin, S.K. Albumin in Advanced Liver Diseases: The Good and Bad of a Drug!; John Wiley and Sons Inc, 2021; Vol. 74; ISBN 0000000175.
- Michelis, R.; Kristal, B.; Zeitun, T.; Shapiro, G.; Fridman, Y.; Geron, R.; Sela, S. Albumin Oxidation Leads to Neutrophil Activation in Vitro and Inaccurate Measurement of Serum Albumin in Patients with Diabetic Nephropathy. Free Radic. Biol. Med. 2013, 60, 49–55. [CrossRef]
- Friedman, A.N.; Fadem, S.Z. Reassessment of Albumin as a Nutritional Marker in Kidney Disease. J. Am. Soc. Nephrol. 2010, 21, 223–230. [CrossRef]
- Zhang, J.; Zhang, R.; Wang, Y.; Li, H.; Han, Q.; Wu, Y.; Wang, T.; Liu, F. The Level of Serum Albumin Is Associated with Renal Prognosis in Patients with Diabetic Nephropathy. J. Diabetes Res. 2019, 2019. [CrossRef]
- Cheng, T.; Wang, X.; Han, Y.; Hao, J.; Hu, H.; Hao, L. The Level of Serum Albumin Is Associated with Renal Prognosis and Renal Function Decline in Patients with Chronic Kidney Disease. BMC Nephrol. 2023, 24, 1–17. [CrossRef]
- Paramasivan, S.; Adav, S.S.; Ngan, S.F.C.; Dalan, R.; Leow, M.K.S.; Ho, H.H.; Sze, S.K. Serum Albumin Cysteine Trioxidation Is a Potential Oxidative Stress Biomarker of Type 2 Diabetes Mellitus. Sci. Rep. 2020, 10, 1–12. [CrossRef]
- Watanabe, H. Oxidized Albumin: Evaluation of Oxidative Stress as a Marker for the Progression of Kidney Disease. Biol. Pharm. Bull. 2022, 45, 1728–1732. [CrossRef]
- International, K. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020, 98, S1–S115. [CrossRef]
- Berg, A.H.; Drechsler, C.; Wenger, J.; Buccafusca, R.; Hod, T.; Kalim, S.; Ramma, W.; Parikh, S.M.; Steen, H.; Friedman, D.J.; et al. Carbamylation of Serum Albumin as a Risk Factor for Mortality in Patients with Kidney Failure. Sci. Transl. Med. 2013. [CrossRef]
- Akchurin, O.M.; Kaskel, F. Update on Inflammation in Chronic Kidney Disease. Blood Purif. 2015, 39, 84–92. [CrossRef]
- Andrade-Oliveira, V.; Foresto-Neto, O.; Watanabe, I.K.M.; Zatz, R.; Câmara, N.O.S. Inflammation in Renal Diseases: New and Old Players. Front. Pharmacol. 2019, 10, 1–19. [CrossRef]
- Naldi, M.; Baldassarre, M.; Domenicali, M.; Bartolini, M.; Caraceni, P. Structural and Functional Integrity of Human Serum Albumin: Analytical Approaches and Clinical Relevance in Patients with Liver Cirrhosis. J. Pharm. Biomed. Anal. 2017, 144, 138–153. [CrossRef]
- Terawaki, H.; Yoshimura, K.; Hasegawa, T.; Matsuyama, Y.; Negawa, T.; Yamada, K.; Matsushima, M.; Nakayama, M.; Hosoya, T.; Era, S. Oxidative Stress Is Enhanced in Correlation with Renal Dysfunction: Examination with the Redox State of Albumin. Kidney Int. 2004, 66, 1988–1993. [CrossRef]
- Baldassarre, M.; Domenicali, M.; Naldi, M.; Laggetta, M.; Giannone, F.A.; Biselli, M.; Patrono, D.; Bertucci, C.; Bernardi, M.; Caraceni, P. Albumin Homodimers in Patients with Cirrhosis: Clinical and Prognostic Relevance of a Novel Identified Structural Alteration of the Molecule. Sci. Rep. 2016, 6. [CrossRef]
- Domenicali, M.; Baldassarre, M.; Giannone, F.A.; Naldi, M.; Mastroroberto, M.; Biselli, M.; Laggetta, M.; Patrono, D.; Bertucci, C.; Bernardi, M.; et al. Posttranscriptional Changes of Serum Albumin: Clinical and Prognostic Significance in Hospitalized Patients with Cirrhosis. Hepatology 2014. [CrossRef]
- Jha, J.C.; Banal, C.; Chow, B.S.M.; Cooper, M.E.; Jandeleit-Dahm, K. Diabetes and Kidney Disease: Role of Oxidative Stress. Antioxidants Redox Signal. 2016, 25, 657–684. [CrossRef]
- Jung, S.W.; Moon, J.Y. The Role of Inflammation in Diabetic Kidney Disease. Korean J. Intern. Med. 2021, 36, 753–766. [CrossRef]
- Rico-Fontalvo, J.; Aroca, G.; Cabrales, J.; Daza-Arnedo, R.; Yánez-Rodríguez, T.; Martínez-Ávila, M.C.; Uparella-Gulfo, I.; Raad-Sarabia, M. Molecular Mechanisms of Diabetic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 1–16. [CrossRef]
- Gremese, E.; Bruno, D.; Varriano, V.; Perniola, S.; Petricca, L.; Ferraccioli, G. Serum Albumin Levels: A Biomarker to Be Repurposed in Different Disease Settings in Clinical Practice. J. Clin. Med. 2023, 12. [CrossRef]
- Prakash, S. Role of Human Serum Albumin and Oxidative Stress in Diabetes. J. Appl. Biotechnol. Bioeng. 2017, 3, 281–285. [CrossRef]
- Clària, J.; Stauber, R.E.; Coenraad, M.J.; Moreau, R.; Jalan, R.; Pavesi, M.; Amorós, À.; Titos, E.; Alcaraz-Quiles, J.; Oettl, K.; et al. Systemic Inflammation in Decompensated Cirrhosis: Characterization and Role in Acute-on-Chronic Liver Failure. Hepatology 2016, 64, 1249–1264. [CrossRef]
- Imafuku, T.; Watanabe, H.; Oniki, K.; Yoshida, A.; Kato, H.; Nakano, T.; Tokumaru, K.; Fujita, I.; Arimura, N.; Maeda, H.; et al. Cysteinylated Albumin as a Potential Biomarker for the Progression of Kidney Disease in Patients With Type 2 Diabetes. Diabetes Care 2021, 44, e115–e117. [CrossRef]
- Beng, C.G.; Rasanayagam, L.J.; Lim, K.L.; Lau, K.S. Solubility and Absorption Spectra of Complexes Resulting from Interaction among Human Albumin, Bromcresol Green and Detergents. Clin. Chim. Acta 1974, 52, 257–269. [CrossRef]
- Naldi, M.; Baldassarre, M.; Domenicali, M.; Giannone, F.A.; Bossi, M.; Montomoli, J.; Sandahl, T.D.; Glavind, E.; Vilstrup, H.; Caraceni, P.; et al. Mass Spectrometry Characterization of Circulating Human Serum Albumin Microheterogeneity in Patients with Alcoholic Hepatitis. J. Pharm. Biomed. Anal. 2016, 122. [CrossRef]
| No DKD n = 42 |
DKD n = 75 |
p value | |
|---|---|---|---|
| Anthropometric data | |||
| Age (years) | 63 (56-67) | 70 (64-74) | <0.001 |
| Male sex (n, %) | 29 (69) | 52 (69) | 1.000 |
| BMI | 28.7 (25.8-34.4) | 32.3 (27.4-36.5) | 0.114 |
| Drug therapy | |||
| Anti-hypertensives (n, %) | 31 (74) | 62 (83) | 0.340 |
| ACE inhibitors (n, %) | 15 (48) | 29 (47) | 0.883 |
| Angiotensin receptor blockers (n, %) | 13 (42) | 27 (44) | 0.882 |
| Diuretics (n, %) | 6 (14) | 32 (43) | 0.002 |
| Metformin (n, %) | 29 (70) | 42 (56) | 0.175 |
| Insulin (n, %) | 15 (36) | 40 (53) | 0.083 |
| Other glucose-lowering therapies (n, %) | 22 (52) | 43 (57) | 0.699 |
| Sulfonylureas (n, %) | 4 (18) | 10 (23) | 0.222 |
| DPP-4 inhibitors (n, %) | 7 (32) | 13 (30) | 0.896 |
| GLP-1 Receptor agonists (n, %) | 8 (36) | 21 (49) | 0.298 |
| SGLT-2 Inhibitors (n, %) | 5 (12) | 3 (4) | 0.067 |
| Statin/fibrates (n, %) | 26 (62) | 50 (67) | 0.687 |
| Biochemical parameters | |||
| HbA1c (%) | 7.0 (6.4-7.6) | 7.1 (6.2-7.6) | 0.952 |
| Glucose (mg/dL) | 133 (110-155) | 123 (110-151) | 0.496 |
| Total cholesterol (mg/dL) | 172 ± 35 | 149 ± 31 | 0.001 |
| HDL (mg/dL) | 47 ± 9 | 41 ± 9 | 0.005 |
| LDL (mg/dL) | 101 ± 31 | 79 ± 27 | 0.002 |
| Triglycerides (mg/dL) | 122 (87-177) | 166 (116-204) | 0.032 |
| Creatinine (mg/dL) | 0.8 (0.8-0.9) | 1.4 (1.2-1.7) | <0.001 |
| UACR (mg/g) | 7 (5-10) | 66 (17-229) | <0.001 |
| eGFR (mL/min/1.73m2) | 89 (77-97) | 48 (34-56) | <0.001 |
| Relative Abundance (%) | p value* | ||
|---|---|---|---|
| HA forms | no DKD n = 42 |
DKD n = 75 |
|
| nHA | 59.0 (57.1 - 60.6) | 52.5 (48.3 - 56.7) | < 0.0001 |
| HMA | 75.7 (73.2 - 78.4) | 67.1 (62.5 - 72.7) | < 0.0001 |
| HNA1 | 16.4 (13.7 - 19) | 25.7 (19.8 - 30.9) | < 0.0001 |
| HNA2 | 8.9 (8.1 - 9.6) | 7.9 (7 - 8.6) | < 0.0001 |
| Truncated | 6.5 (5.1 - 7.9) | 5.2 (4.4 - 7) | 0.0242 |
| Glycated | 10.8 (9.2 - 12.2) | 12.5 (10.5 - 14.7) | 0.0028 |
| Relative Abundance (%) | p value* | ||
|---|---|---|---|
| HA forms | No DKD n = 42 |
DKD (I-III) n = 75 |
|
| tHA (g/dL) | 4.3 (4.1 - 4.5) | 4.1 (3.9 - 4.4) | 0.001 |
| rHA (g/dL) | 3.3 (3.2 - 3.4) | 2.8 (2.5 - 3.1) | < 0.001 |
| eHA (g/dL) | 2.5 (2.4 - 2.7) | 2.2 (1.9 - 2.4) | < 0.001 |
| tHA | rHA | eHA | ||
| Rho (p value) | Rho (p value) | Rho (p value) | ||
| Baseline | Creatinine (mg/dL) | -0.260 (0.006) | -0.567 (<0.001) | -0.548 (<0.001) |
| eGFR | 0.337 (<0.001) | 0.659 (<0.001) | 0.649 (<0.001) | |
| Albuminuria | -0.497 (<0.001) | -0.546 (<0.001) | -0.554 (<0.001) | |
| Follow-up | Creatinine (mg/dL) | -0.172 (0.077) | -0.508 (<0.001) | -0.509 (<0.001) |
| eGFR | 0.172 (0.076) | 0.562 (<0.001) | 0.584 (<0.001) | |
| Albuminuria | -0.363 (<0.001) | -0.461 (<0.001) | -0.407 (<0.001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





