0. Introduction
In the last three years, many scientists dedicated their efforts to the analysis of pandemic data. The severity of COVID-19 infections and the intensive use of hospital resources have been driving factors behind these studies. One of the goals of these research efforts is to develop updated pandemic plans, that provide alert indicators. These indicators would enable the early detection of critical situations [
1,
2,
3]. A critical aspect of COVID 19 pandemic is the presence of a large number of asymptomatic cases, which has contributed to the rapid worldwide spread [
4,
5], making the early detection of aberrations a crucial point. At the end of February 2020, the first case was detected in Italy, and the virus began to spread rapidly, especially in the northern regions of Italy. Despite, a national lockdown being enforced throughout Italy on the 9th of March 2020, just 15 days after the first case was discovered, three years later in March 2023, the Italian former prime minister, the former health minister and 17 others including the current president of Lombardy region were placed under investigation on suspicion of aggravated culpable epidemic. This investigation was in connection with an alleged delayed government response and the high number of deaths in the Bergamo area, which registered a significant excess of deaths during the initial outbreak of the virus [
6,
7,
8,
9,
10].
Considering the incidence rate, more precisely the 7-day incidence rate per 100,000 inhabitants, an indicator based on known cases that was used in successive waves for surveillance and establishing thresholds to identify critical areas, nothing conclusive can be drawn at the beginning of March 2020. Indeed, analysing the trend of the incidence rate, reveals that the values found on 9 March 2020 for Italy (11.88) and the Lombardy region (42.07) were both well below the "red zone" alert threshold (250), and even lower than the "white zone" alert threshold (50). The latter was used as a first warning threshold, not associated with drastic measures..
However, there are other indicators that could have been used at the first stages. For example the Test Positivity Rate (TPR), which ia the percentage of positive tests over total tests [
11], proved to be more suitable for modelling under-ascertainment due to asymptomatic carriers [
12,
13,
14,
15,
16]. This indicator was demonstrated to be correlated with excess deaths in the first wave of the pandemic [
17], and, most importantly, it anticipates incidence [
11].
The aim of this paper is to analyse pandemic data for Italy and Lombardy region in the first 10 days of the pandemic, spanning from the 24th of February 2020 to the 4th of March 2020. The goal is to determine whether the use of early warning indicators, such as TPR, would have made it possible to identify critical increases in infections, thus enabling the timely formulation of strategies for pandemic containment, reducing the number of deaths.
Finally, to translate these observations into practical guidelines, we propose a low cost early warning method for infectious respiratory diseases with asymptomatic carriers.
1. Methods
The goal of early warning methods is to identify abnormal distribution of infectious diseases that may have the potential to develop into outbreaks with significant fatalities. We analyse the first 10 days of the pandemic in Italy and Lombardy to identify a set of conditions in public health records that could be used to define an early warning method suitable for infectious diseases with asymptomatic carriers.
Making valuable predictions based on such a short time interval and limited data is a challenging task. For example, useful predictions based on positive cases and ICU admissions, concerning the potential strain on ICU beds in Italy, were developed approximately 10 days after [
18]. The presence of asymptomatic carries makes the task even more challenging due to under-ascertainment issues in indicators based on known cases, such as the incidence rate.
In our analysis, we consider test positivity and community-level indicators to model the impact of COVID-19 on hospitalization, aiming to capture significant variations within a 7-day interval to generate a warning signal. Our objective is to answer the question: is it possible to define an early warning method within a period of one weak at the beginning of a pandemic that correlates with excess mortality?
1.1. Modelling Test Positivity
Following the approach of [
11] we use a definition of TPR based on two levels, one level deals with data collection issues and the other with epidemiological issues. Let
,
,⋯
be the daily TPR time series, TPR at time
i, when
and
can be modelled by computing the trend as follows:
Using this approach, TPR in a given day is modelled by computing the average value of the days preceding and following it. Then we introduce a second level to compute the final TPR value at day
d considering epidemiological issues. The TPR value is defined as the average of the last
days of the previous time series, where
is the incubation period.
Where
on the onset [
19], and
in the Omicron outbreak [
20,
21]. The details of the calculation are presented in [
11].
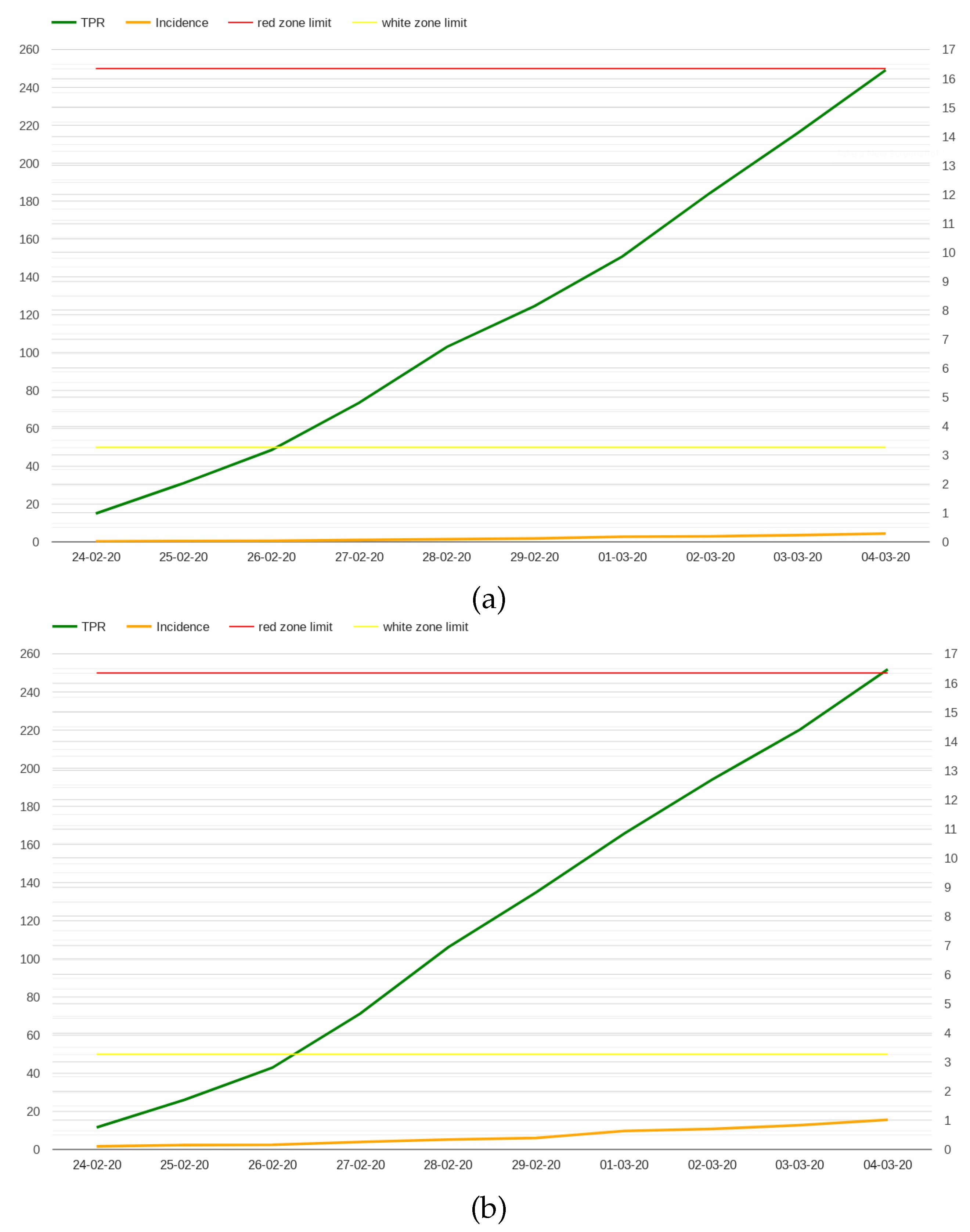
A comparison between the incidence rate and TPR in the first 10 days of the pandemic is presented in
Figure 1 considering whole Italy (a) and Lombardy (b). While the values of the incidence rate are relatively low in the first 10 days of the pandemic, the TPR rises to 15% for both Italy (16.29%) and Lombardy (16.47%), not far from the 20% threshold that World Health Organization sets for the highest Level of community transmission [
22].
The low values for the incidence rate are, of course, related to the limited volume of tests conducted during those days; however this is a typical condition at the beginning of an outbreak.
Would a significant increase of test positivity be a sufficient signal to justify the early enactment of drastic measures, such as the lockdown of the 9th of March 2020? Although a relationship between test positivity and mortality was discovered in the first COVID-19 outbreak in Italy [
17], the analysis of pandemic data in the successive waves shows that an increase of test positivity alone is not a sufficient condition to identify emergency situations [
11]. For example, a similar increase was observed in the Omicron outbreak at the end of December 2021, without the need to make equally drastic decisions. The point is that community-level indicators[
23], which measure the impact of COVID-19 in terms of hospitalizations and strain on the healthcare system, should be also considered when making a decision.
1.2. Modelling Admission in Hospital and Intensive care Units
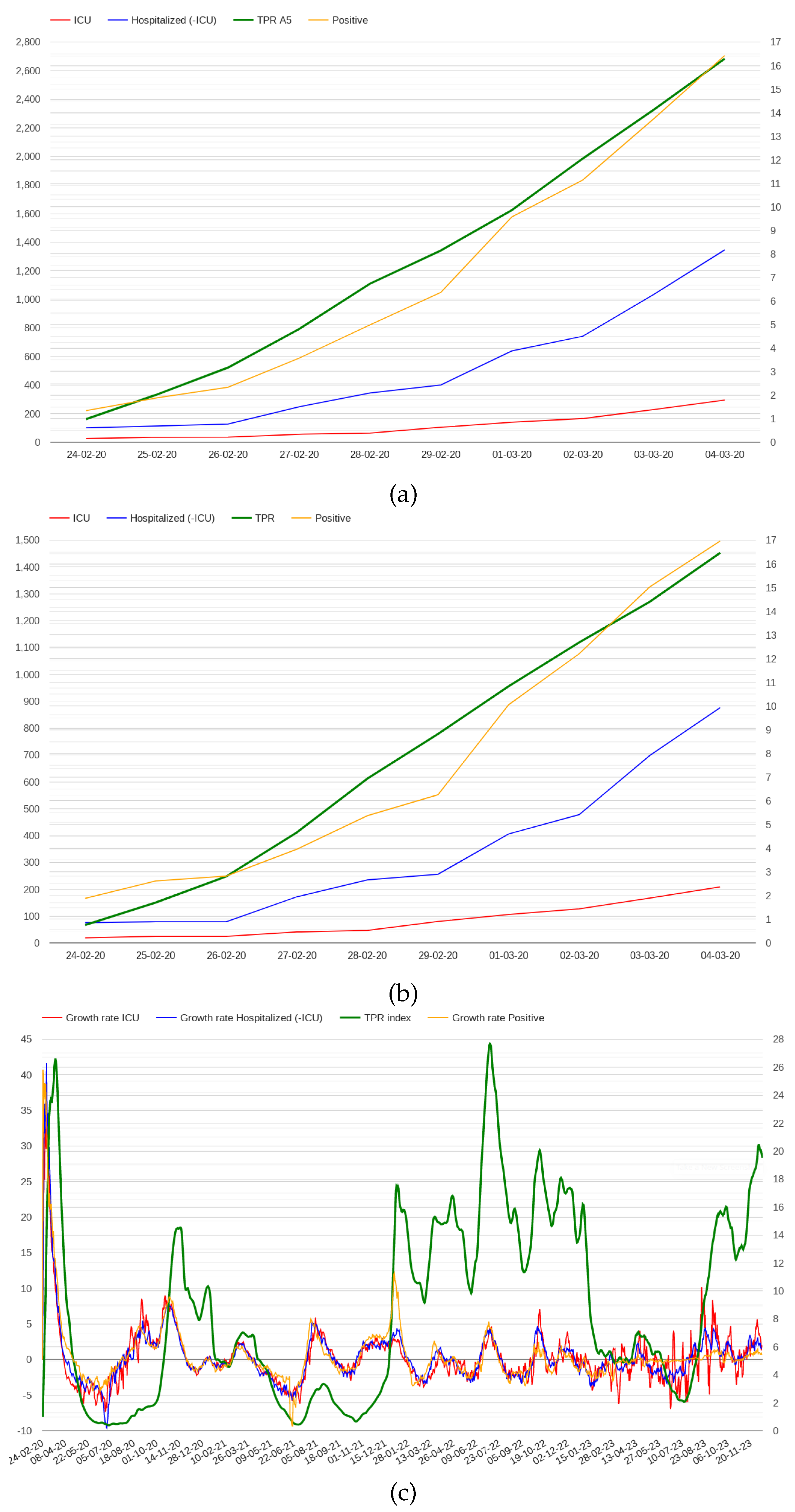
A possible alarm signal could be associated with an estimate of the number of potential deaths, which were only 28 in the first 10 days. Such an estimate can be obtained by analysing the trend positive cases and hospitalization during those days.
Figure 2 presents the number of positive cases, the number of patients admitted in hospitals and ICUs in the first 10 days in Italy and Lombardy. Although all the indicators show growth, the number of cases was still limited to infer a critical situation in those days.
Analysing COVID-19 data from February 2020 to December 2023, we observed that the growth rates of positive and hospitalized patients were higher in the first few days than in the rest of the pandemic, as shown in
Figure 2 (c). This figure presents the growth rate trends of positive cases and hospitalized patients in Italy throughout the pandemic; a similar trend can be observed in all Italian regions. To delve into this point, we investigated whether high growth rates of positive cases and admissions to hospitals correlate with excess mortality. We considered average growth rates in 7-day intervals, observing that the regions where growth rates are higher than 40% include Lazio, Puglia and Toscana where the mortality rate was not particularly high. We also found that restricting the analysis to hospitals and ICUs only, does not improve the results. For example, considering a growth rate threshold of 45% for admitted in hospitals and ICUs, Lazio and Puglia are still included while Lombardy, the first region of Italy considering mortality rate, is not.
In the light of these considerations, we argue that growth rates of positive and hospitalized cases are not precise indicators for the purpose of defining an early warning method capable of detecting the risk of high mortality rates. On the contrary, we discovered two community-level indicators that appear to be more precise and suitable for our aims:
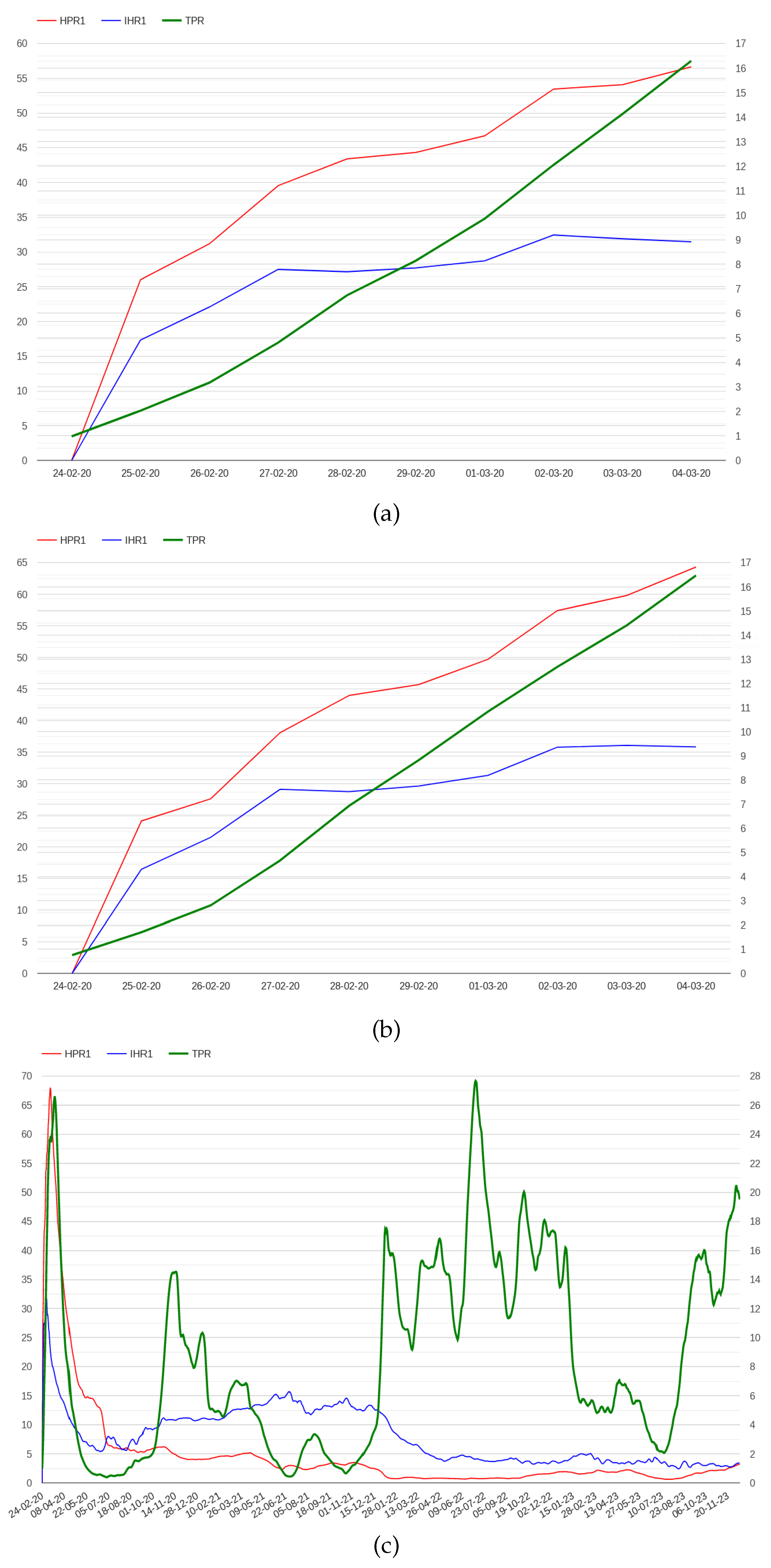
The N hospital/positive ratio (HPRN), which is defined as the ratio between hospitalized patients and the number of positive cases N days before.
The N ICU/hospital ratio (IHRN), which is defined as the ratio between patients admitted in ICU and hospitalized patients N days before.
Where N varies depending on the disease, for example 4 could be a reasonable number for COVID-19 [
24]. However, posing N equal to 4 generates a delay of 4 days in the computation of these indicators which is not compatible with the objective of providing an aberration signal in a week. Moreover, the value of 4 which is specific of COVID-19 may be not adequate for other diseases. However, we have found that posing N equal to 1, still preserves both indicators properties, which instead are lost posing N equal to 0. The trend of HPR1 and IHR1 in Italy and Lombardy in the first days of the pandemic are presented in
Figure 3.
1.3. Defining an Early Warning Method
Combining these two indicators with test positivity, an early warning method can be developed using the following rules of thumb which trigger warning signals in a 7-day interval:
IF TPR grows more than 5 points
AND the TPR is monotone
AND the growth rate of the HPR1 is greather than 5
AND the growth rate of the 1 IHR1 is greather than 5
AND The average value of IHR1 is greather than 15
THEN an aberration signal is detected
IF the number of patients admitted in ICU is 0 for one day in the week.
AND TPR grows more than 8 points
AND the TPR is monotone
AND The average value of HPR1 is greather than 20
AND The average value of IHR1 is greather than 10
THEN an aberration signal is detected
The monotonicity condition on TPR has been added to consider periods in which the TPR effectively grows, excluding weeks that could contain possible peaks. The minimum threshold on the average value of the IHR1 allows us to discriminate critical situations. Indeed, after the spread of the Omicron variant at the end of December 2021 the IHR1 dropped by half [
11] reducing healthcare system strain.
The second rule addresses situations in which data on ICU are missing in the first days. This was the case of three Italian regions: Valle d’Aosta, Piemonte and Alto Adige. For these regions the first rule does not trigger because the data on ICU are missing, namely, the number of accesses in ICU is 0, and thus the two conditions that use growth rates do not hold simultaneously. To resolve this issue we relaxed the condition on the average value of the IHR1, making other conditions more selective. However, the reported problem could be also related to delays in reporting data, a crucial issue for early warning systems and surveillance in general [
25].
2. Results
Using the above early warning method, an abnormal signal can be detected the 1st of March for both Italy and Lombardy, more than one week before the 9th of March. The alarm conditions also hold on the 2nd and the 3rd of March for both Lombardy and Italy. After the 4th of March the IHR1 drops to 2.274 for Italy and to 3.524 for Lombardy.
To assess the proposed method, we have tested it using data from all the Italian regions to check if the alarm signals are associated with high mortality rates. The alarm signals fire only at the beginning of the first wave of the pandemic, in the other waves, the conditions on HPR1 and IHR1 do not hold simultaneously. Thus, to asses the method, we considered the COVID-19 mortality rate in the first wave only until the 30th of June 2020 [
26].
Table 1 summarises the results, which appear to be promising because the alarm condition only fires for the 9 regions with higher mortality rate and for whole Italy.
However, recent studies indicates that the deaths cases reported in official data are underestimated [
27]. Considering excess mortality computed using data provided by the National Statistical Institute (ISTAT) by subtracting the average deaths counted in February-April over the prior 5 years (2015-2019) from the deaths counted in the same period in 2020 [
17], the results are similar. Indeed the 8 regions with higher mortality rates are individuated by our method, with the exception that data of Trentino and Alto Adige are aggregated.
Instead, if we look at the estimations of the maxima curves obtained taking the largest between DPC [
26] and ISTAT datum in each day [
17], Friuli Venezia Giulia, which is not selected by our method, goes beyond Veneto. We hypothesize that this issue is due to lack of precision in the functional estimation method used in [
17], because the DPC ranking they present for the period February-April 2020 differs from the COVID-19 mortality rates computed at the end of the first wave, and at the end of April 2020 too [
26].
3. Discussion
Beyond the seemingly good results, a thorough analysis is required. As a first point we observe that what likely happens is that the data of Italy are influenced by Lombardy and other regions where the alarm signal also fires at the start. Thus, containment strategies that could have been implemented for whole Italy, would not necessarily be those suggested for Lombardy.
Concerning the accusation of culpable epidemic, no new conclusions can be drawn, because the indicators that compose our method were almost unknown (unused) at the beginning of March 2020. For example, the predictive capacity of test positivity with respect to the trend of admissions in hospital and ICUs [
15], and its correlation with excess mortality [
17], have been theorized only afterward.
The question is, would the proposed method be able to improve the COVID-19 response strategy in Italy if these alarms were made available? Certainly, the generated alarm signal alone is not enough to support decision-making; it is just another piece of information that could be considered together with others, such as the three methods used in CIDARS [
25,
28,
29,
30]. However, adding to these known methods a specific component for infectious diseases with asymptomatic carriers, which can be used along with other signals, can undoubtedly improve surveillance tools for making the right decision in time.
The rationale of this method comes from a surprise effect, given that the earliest cases in Italy were detected with a considerable delay (up to 3 weeks) [
31], when the first case was detected in Lombardy, the virus had already spread to some area, and consequently some indicators had out of range values in the initial phase.
Associating these anomalous values to a possible excess of mortality in the near future could have been valuable information at that time, perhaps enough for suddenly implement a lockdown limited to Lombardia, Emilia-Romagna, Veneto and Marche in the first days of March 2020, probably avoiding a large number of deaths. With regards to the other regions, Piemonte could have been added a few day later, while for the remaining regions additional consideration have to be done. Indeed, the Liguria, Trentino and Valle d’Aosta data would probably be affected by an anticipated lockdown in the neighbouring regions, due to mobility issues[
17].
Considering the state of the art of early warning systems in the detection of infectious diseases outbreaks [
3], the proposed method suffers from the limitations of all the approaches based on public health records which rely on cases reported by clinicians. Thus they are not working in general for diseases that are still unknown [
25]. Indeed, for using test positivity the disease should be known, as the diagnostic test available..
4. Conclusion
Analysing pandemic data in Italy on the first day of the outbreak, we identified anomalous conditions in TPR and in two novel community-level indicators. These conditions allowed us to define an early warning method, capable of signaling a risk for excess mortality in a few days. This method specific for infectious diseases with asymptomatic carries, can be considered in the implementation of next generation early warning systems. Using this method, a lockdown limited to Lombardia, Emilia-Romagna, Veneto and Marche could have been implemented in the first days of March 2020, However, it is clear that these knowledge was not available at the beginning of March 2020; thus, nothing new can be concluded concerning the reported accusation of culpable epidemic.
Beyond these seemingly positive results, the generality of the approach requires further investigations. Presumably it could apply to other respiratory diseases with asymptomatic carriers under a similar criticality COVID-19 condition. Future work will concern a more extensive validation, considering data from other countries and diseases. We also would like to test if values which exceed lower thresholds for TPR, HPR1 and IHR1 indicators are associated to other critical points in the pandemic, and correlates with high mortality rates.
Funding
This research received no external funding.
Data Availability Statement
Acknowledgments
The author would like to thank the Italian Civil Protection Department, and all the staff involved for providing the data of the outbreak used in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Russell, T.W.; Golding, N.; Hellewell, J.; Abbott, S.; Wright, L.; Pearson, C.A.; van Zandvoort, K.; Jarvis, C.I.; Gibbs, H.; Liu, Y.; others. Reconstructing the early global dynamics of under-ascertained COVID-19 cases and infections. BMC medicine 2020, 18, 1–9. [Google Scholar] [CrossRef]
- Beatrice, F.; Calleja, N. Early warning indicators of COVID-19 burden for a prosilient European pandemic response. European Journal of Public Health 2021, 31, iv21–iv26. [Google Scholar] [CrossRef]
- Meckawy, R.; Stuckler, D.; Mehta, A.; Al-Ahdal, T.; Doebbeling, B.N. Effectiveness of early warning systems in the detection of infectious diseases outbreaks: a systematic review. BMC public health 2022, 22, 1–62. [Google Scholar] [CrossRef]
- Yu, X.; Yang, R. COVID-19 transmission through asymptomatic carriers is a challenge to containment. Influenza and other respiratory viruses 2020, 14, 474. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, X.; Deng, Y.; Tang, Y.; Lu, J. COVID-19: asymptomatic carrier transmission is an underestimated problem. Epidemiology & Infection 2020, 148. [Google Scholar] [CrossRef]
- Usuelli, M. The Lombardy region of Italy launches the first investigative COVID-19 commission. The Lancet 2020, 396, e86–e87. [Google Scholar] [CrossRef]
- Goumenou, M.; Sarigiannis, D.; Tsatsakis, A.; Anesti, O.; Docea, A.O.; Petrakis, D.; Tsoukalas, D.; Kostoff, R.; Rakitskii, V.; Spandidos, D.A.; others. COVID-19 in Northern Italy: An integrative overview of factors possibly influencing the sharp increase of the outbreak. Molecular medicine reports 2020, 22, 20–32. [Google Scholar] [CrossRef]
- Bernucci, C.; Brembilla, C.; Veiceschi, P. Effects of the COVID-19 outbreak in Northern Italy: perspectives from the Bergamo Neurosurgery Department. World neurosurgery 2020, 137, 465. [Google Scholar] [CrossRef]
- Palladino, R.; Bollon, J.; Ragazzoni, L.; Barone-Adesi, F. Excess Deaths and Hospital Admissions for COVID-19 Due to a Late Implementation of the Lockdown in Italy. International Journal of Environmental Research and Public Health 2020, 17. [Google Scholar] [CrossRef]
- Perico, N.; Fagiuoli, S.; Di Marco, F.; Laghi, A.; Cosentini, R.; Rizzi, M.; Gianatti, A.; Rambaldi, A.; Ruggenenti, P.; La Vecchia, C.; others. Bergamo and COVID-19: how the dark can turn to light. Frontiers in Medicine 2021, 8, 609440. [Google Scholar] [CrossRef]
- Gaspari, M. The impact of test positivity on surveillance with asymptomatic carriers. Epidemiologic Methods 2023, 11, 20220125. [Google Scholar] [CrossRef]
- Fasina, C.; Salami, M.; Fasina, M.; Otekunrin, O, A. ; Hoogesteijn, A.; Hittner, J.B. Test positivity–evaluation of a new metric to assess epidemic dispersal mediated by non-symptomatic cases. Methods 2021, 195, 15–22. [Google Scholar] [CrossRef]
- Furuse, Y.; Ko, Y.K. and Ninomiya, K.; Suzuki, M.and Oshitani, H. Relationship of test positivity rates with COVID-19 epidemic dynamics. International journal of environmental research and public health 2021, 18, 4655. [Google Scholar] [CrossRef] [PubMed]
- Al Dallal, A.; AlDallal, U.; Al Dallal, J. Positivity rate: an indicator for the spread of COVID-19. Current Medical Research and Opinion 2021, 37, 2067–2076. [Google Scholar] [CrossRef]
- Fenga, L.; Gaspari, M. Predictive capacity of covid-19 test positivity rate. Sensors 2021, 21, 2435. [Google Scholar] [CrossRef]
- Vong, S.; Kakkar, M. Monitoring COVID-19 where capacity for testing is limited: use of a three-step analysis based on test positivity ratio. WHO South-East Asia journal of public health 2020, 9, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Boschi, T.; Di Iorio, J.; Testa, L.; Cremona, M.A.; Chiaromonte, F. Functional data analysis characterizes the shapes of the first COVID-19 epidemic wave in Italy. Scientific reports 2021, 11, 17054. [Google Scholar] [CrossRef] [PubMed]
- Remuzzi, A.; Remuzzi, G. COVID-19 and Italy: what next? The lancet 2020, 395, 1225–1228. [Google Scholar] [CrossRef]
- Lauer, S.A.; Grantz, K.H.; Bi, Q.; Jones, F.K.; Zheng, Q.; Meredith, H.R.; Azman, A.S.; Reich, N.G.; Lessler, J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Annals of internal medicine 2020, 172, 577–582. [Google Scholar] [CrossRef]
- Jansen, L.; Tegomoh, B.; Lange, K.; Showalter, K.; Figliomeni, J.; Abdalhamid, B.; Iwen, P.C.; Fauver, J.; Buss, B.; Donahue, M. Investigation of a SARS-CoV-2 B. 1.1. 529 (omicron) variant cluster—Nebraska, November–December 2021. Morbidity and Mortality Weekly Report 2021, 70, 1782. [Google Scholar] [CrossRef]
- Brandal, L.T.; MacDonald, E.; Veneti, L.; Ravlo, T.; Lange, H.; Naseer, U.; Feruglio, S.; Bragstad, K.; Hungnes, O.; Ødeskaug, L.E.; others. Outbreak caused by the SARS-CoV-2 Omicron variant in Norway, November to December 2021. Eurosurveillance 2021, 26, 2101147. [Google Scholar] [CrossRef] [PubMed]
- Organization, W.H.; others. Considerations for implementing and adjusting public health and social measures in the context of COVID-19: interim guidance, 4 November 2020. Technical report, World Health Organization, 2020.
- for Immunization, N.C.; others. Science Brief: Indicators for Monitoring COVID-19 Community Levels and Making Public Health Recommendations. In CDC COVID-19 Science Briefs [Internet]; Centers for Disease Control and Prevention (US), 2022.
- Faes, C.; Abrams, S.; Van Beckhoven, D.; Meyfroidt, G.; Vlieghe, E.; Hens, N.; on COVID-19 Hospital Surveillance, B.C.G. Time between symptom onset, hospitalisation and recovery or death: statistical analysis of Belgian COVID-19 patients. International journal of environmental research and public health 2020, 17, 7560. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, Z.; Lan, Y.; Wang, J.; Ma, J.; Jin, L.; Sun, Q.; Lv, W.; Lai, S.; Liao, Y.; others. A nationwide web-based automated system for outbreak early detection and rapid response in China. Western Pacific surveillance and response journal: WPSAR 2011, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Presidenza del Consiglio dei Ministri.; Dipartimento di Protezione Civile. Dati COVID-19 Italia, 2020.
- Roccetti, M. Excess mortality and COVID-19 deaths in Italy: A peak comparison study. Mathematical Biosciences and Engineering 2023, 20, 7042–7055. [Google Scholar] [CrossRef] [PubMed]
- Kulldorff, M.; Nagarwalla, N. Spatial disease clusters: detection and inference. Statistics in medicine 1995, 14, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Kulldorff, M. A spatial scan statistic. Communications in Statistics-Theory and methods 1997, 26, 1481–1496. [Google Scholar] [CrossRef]
- Widdowson, M.A.; Bosman, A.; van Straten, E.; Tinga, M.; Chaves, S.; van Eerden, L.; van Pelt, W. Automated, laboratory-based system using the Internet for disease outbreak detection, the Netherlands. Emerging Infectious Diseases 2003, 9, 1046. [Google Scholar]
- Riccardo, F.; Ajelli, M.; Andrianou, X.D.; Bella, A.; Del Manso, M.; Fabiani, M.; Bellino, S.; Boros, S.; Urdiales, A.M.; Marziano, V.; others. Epidemiological characteristics of COVID-19 cases and estimates of the reproductive numbers 1 month into the epidemic, Italy, 28 January to 31 March 2020. Eurosurveillance 2020, 25, 2000790. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).