Submitted:
11 January 2024
Posted:
12 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
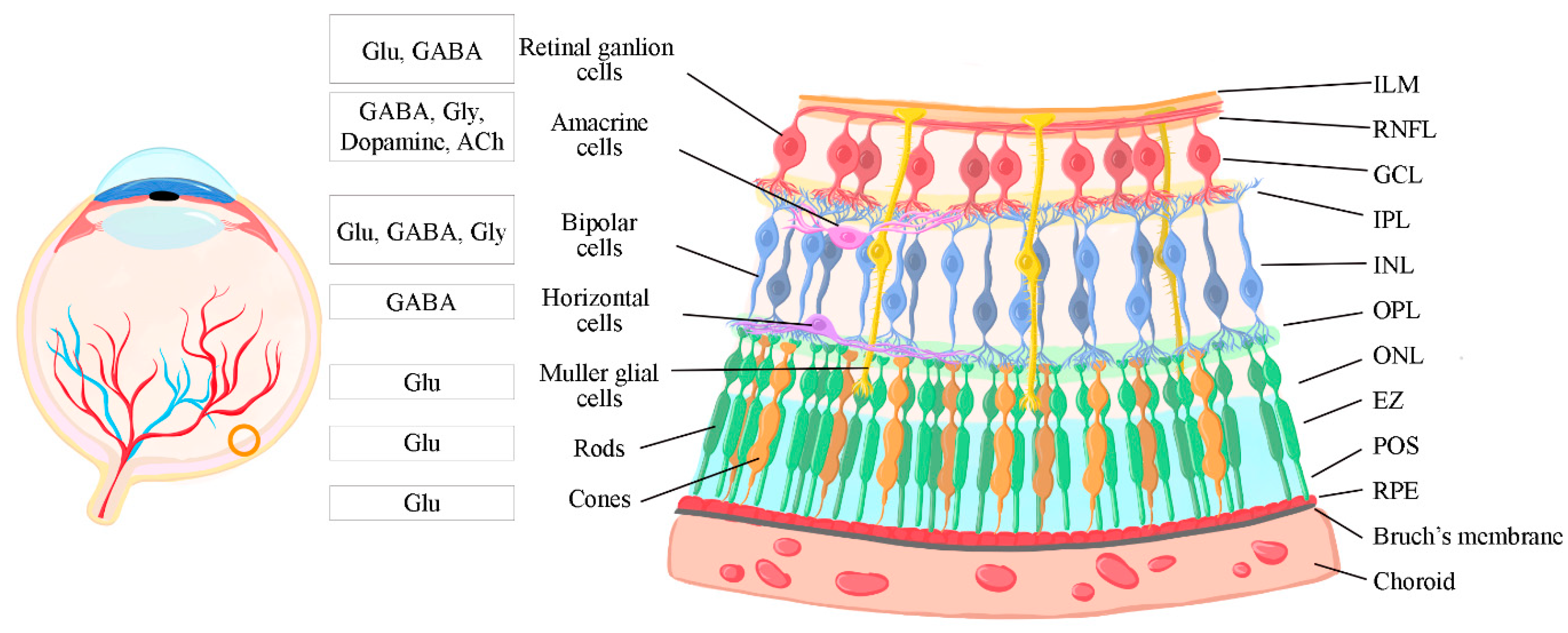
1.1. The retina
1.1. Retinal structure and information processing
1.2. Retinal Synapses
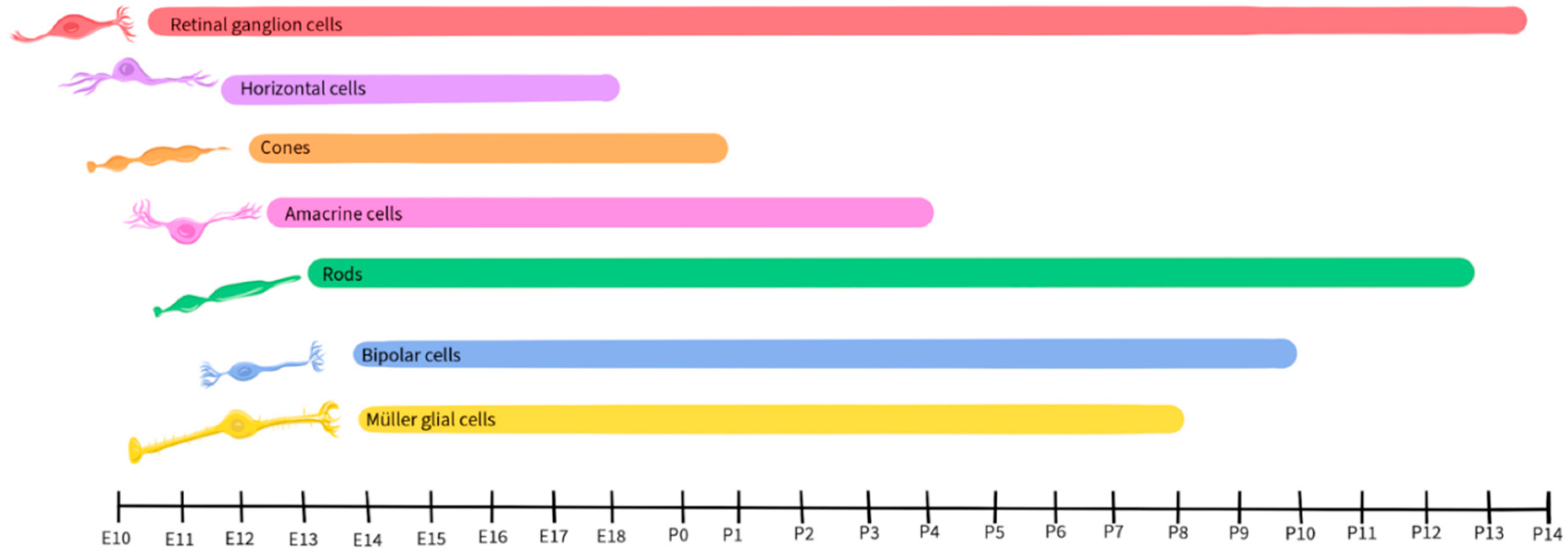
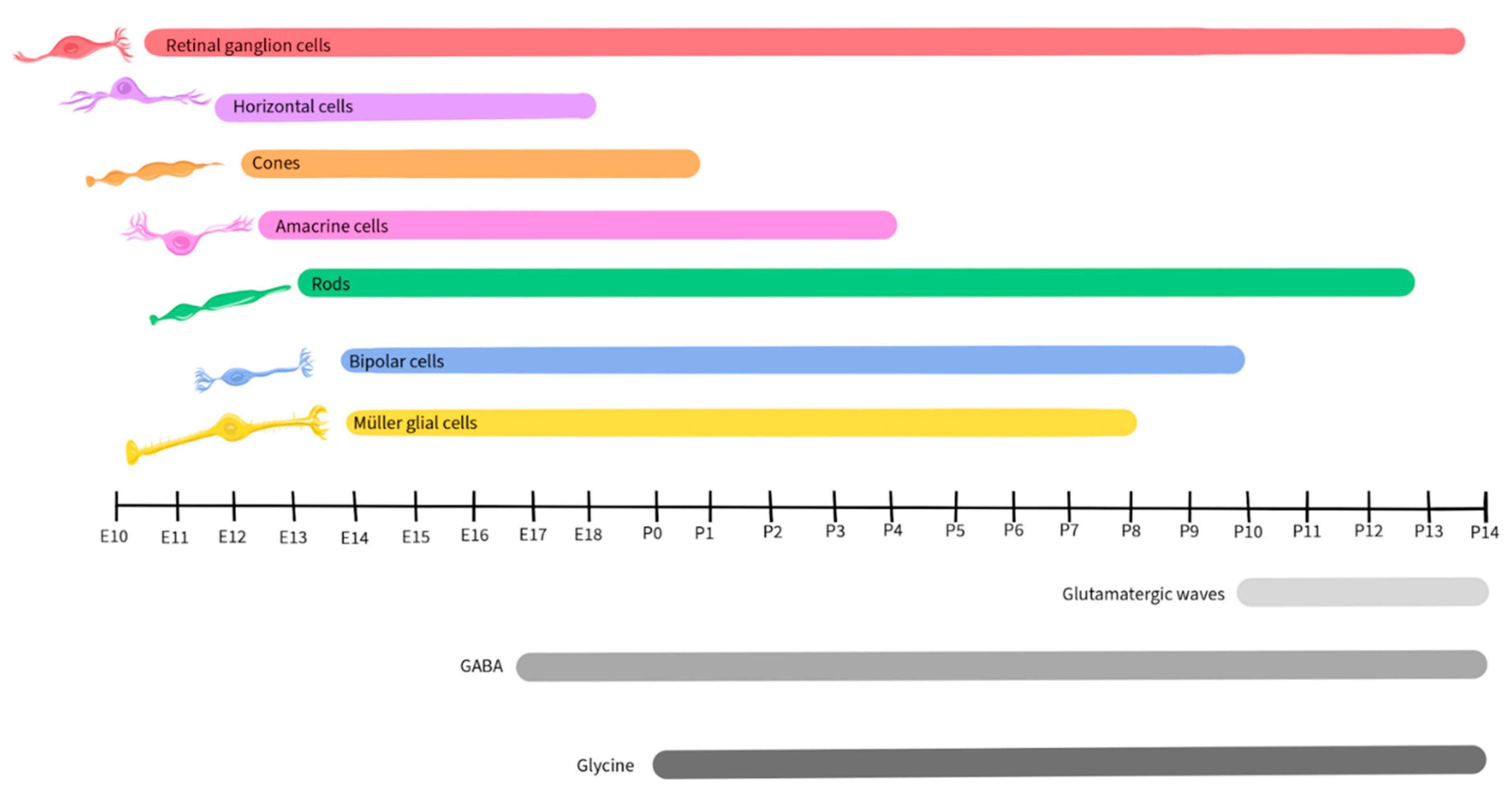
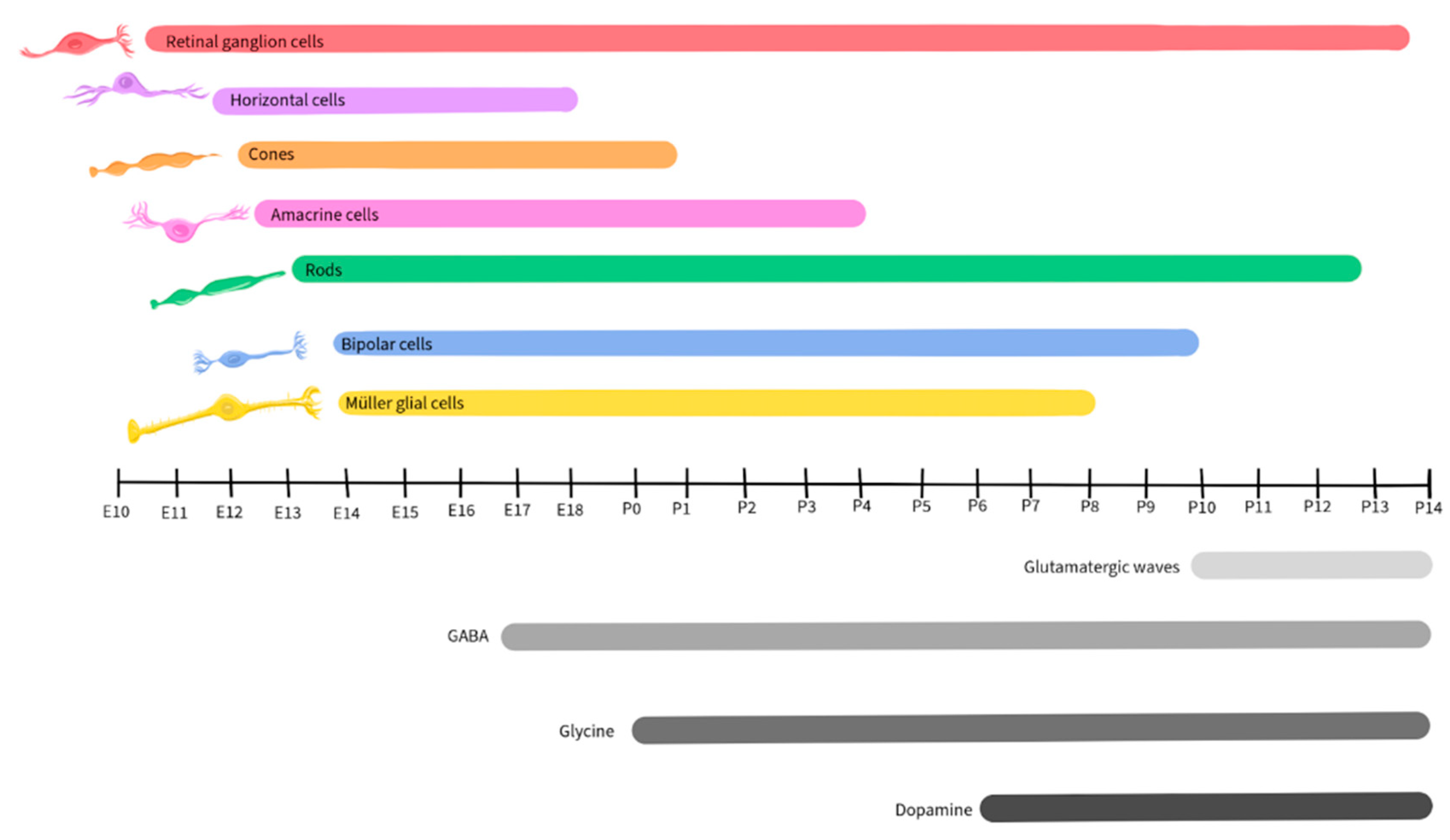
1.3. Retinal Development
Neurotransmission
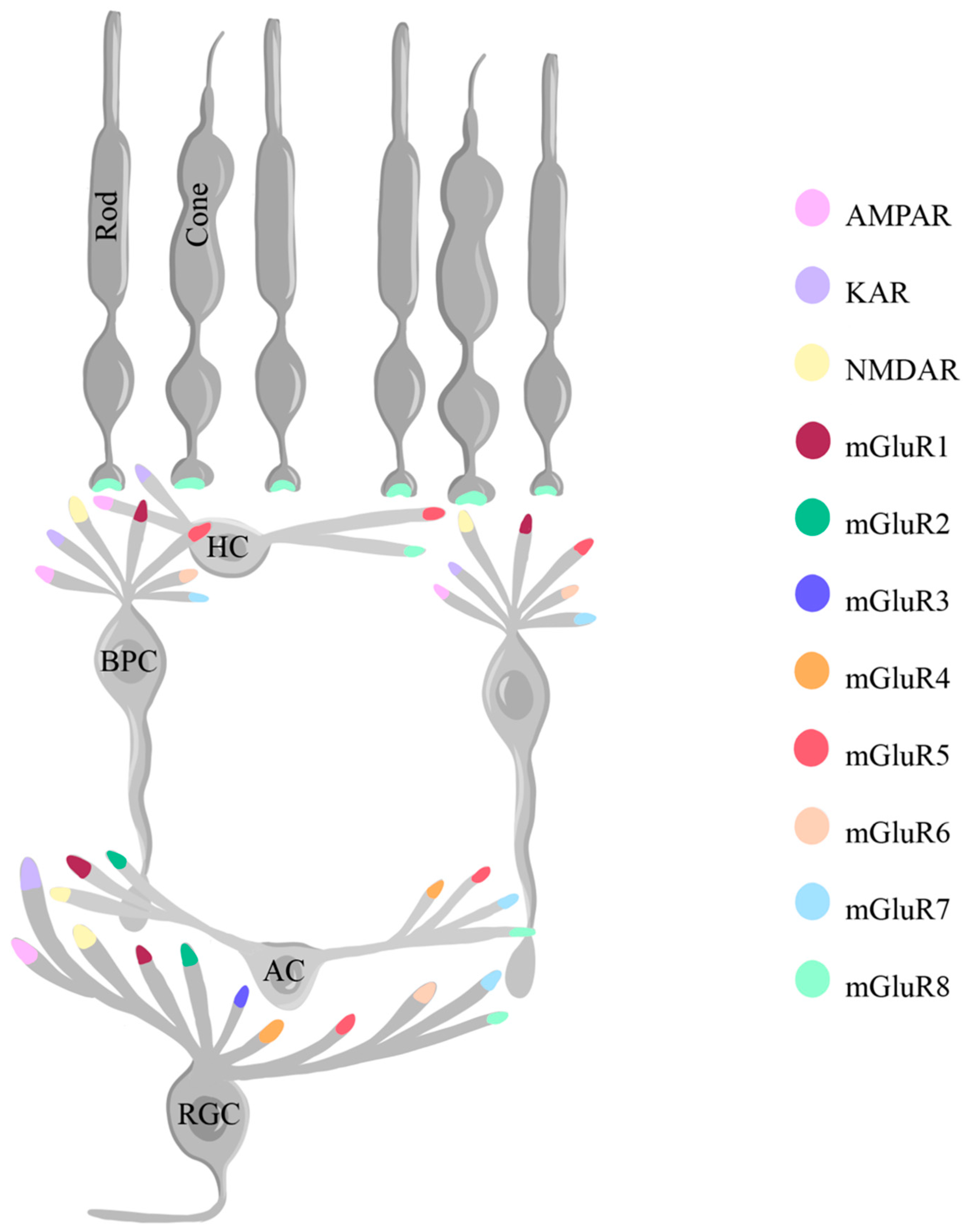
1.4. Glutamate
2.1.1. The Role of Glutamate in Retinal Development
2.1.2. The Role of Glutamate in Normal Retinal Physiology
2.1.3. The Role of Glutamate in Retinal Diseases
2.1.4. The Interplay of Glutamate and Calcium
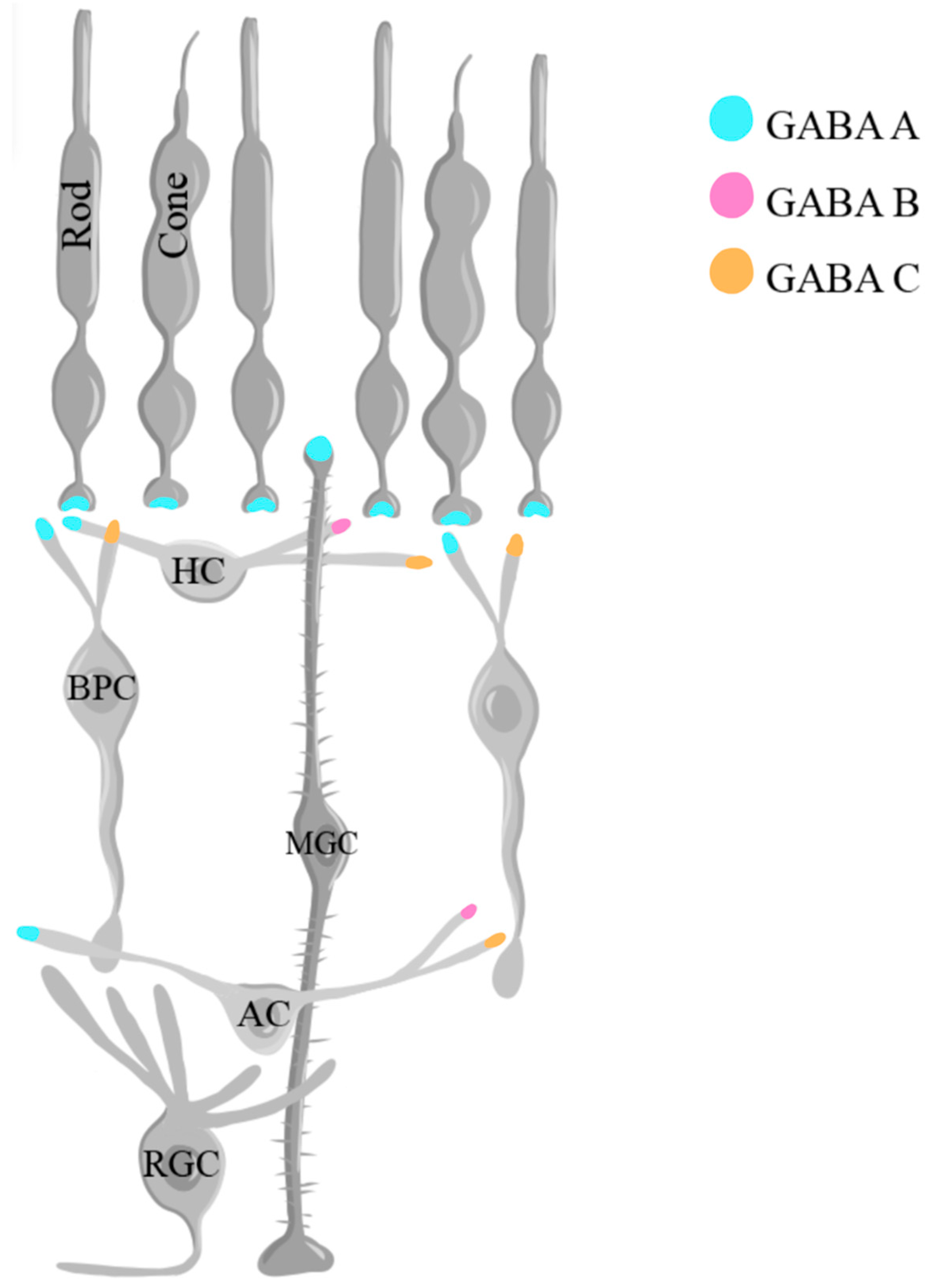
1.5. Gamma-aminobutyric acid (GABA)
2.2.1. The Role of GABA in Retinal Development
2.2.2. The Role of GABA in Normal Retinal Physiology
2.2.3. The Role of GABA in Retinal Diseases
2.2.4. The Interplay of GABA and Calcium
1.6. Glycine
2.3.1. The Role of Glycine in Retinal Development
2.3.2. The Role of Glycine in Normal Retinal Physiology
2.3.3. The Role of Glycine in Retinal Diseases
2.3.4. The Interplay of Glycine and Calcium
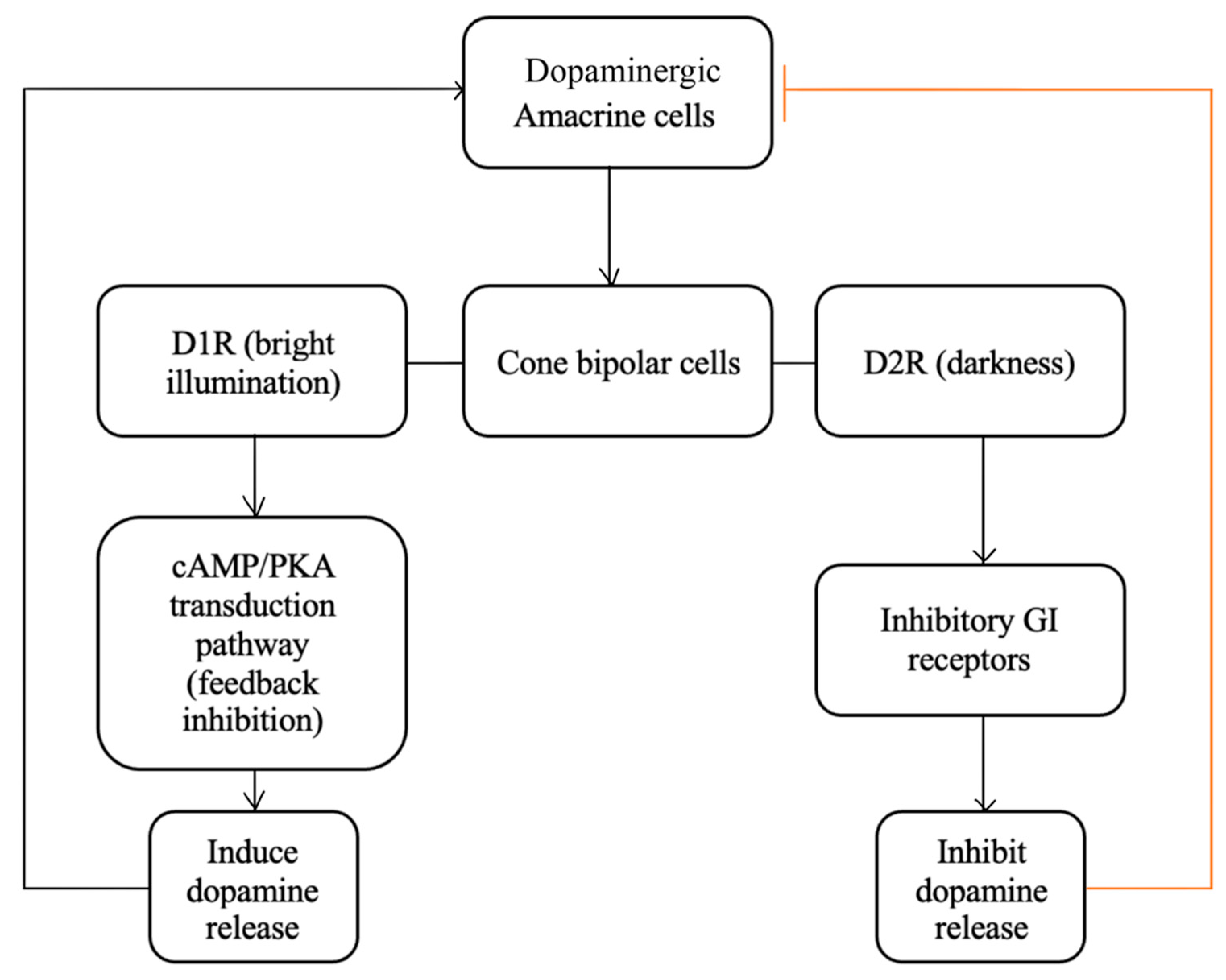
1.7. Dopamine
2.4.1. The Role of Dopamine in Retinal Development
2.4.2. The Role of Dopamine in Normal Retinal Physiology
2.4.3. The Role of Dopamine in Retinal Diseases
2.4.4. The Interplay of Dopamine and Calcium
1.8. Acetylcholine
2.5.1. The Role of Acetylcholine in Retinal Development
2.5.2. The Role of Acetylcholine in Normal Retinal Physiology
2.5.3. The Role of Acetylcholine in Retinal Diseases
2.5.4. The Interplay of Acetylcholine and Calcium
5. Conclusion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masland Richard, H. The Neuronal Organization of the Retina. Neuron. 2012, 76, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Myers CE, Klein BE, Meuer SM. Retinal thickness measured by spectral-domain optical coherence tomography in eyes without retinal abnormalities: the Beaver Dam Eye Study. Am J Ophthalmol. 2015, 159, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Muraoka Y, Ikeda HO, Nakano N, Hangai M, Toda Y, Okamoto-Furuta K, Kohda H, Kondo M, Terasaki H, Kakizuka A, et al. Real-Time Imaging of Rabbit Retina with Retinal Degeneration by Using Spectral-Domain Optical Coherence Tomography. PLOS ONE. 2012, 7, e36135. [Google Scholar] [CrossRef]
- Quint WH, Tadema KCD, Crins JHC, Kokke N, Meester-Smoor MA, Willemsen R, Klaver CCW, Iglesias AI. Zebrafish: An In Vivo Screening Model to Study Ocular Phenotypes. Transl Vis Sci Technol. 2022, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Guo L, Normando E, Nizari S, Lara D, Cordeiro MF. Tracking Longitudinal Retinal Changes in Experimental Ocular Hypertension Using the cSLO and Spectral Domain-OCT. Investigative ophthalmology & visual science. 2010, 51, 6504–6513. [Google Scholar] [CrossRef]
- Pycock, CJ. Retinal neurotransmission. Survey of Ophthalmology. 1985, 29, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Wu SM, Maple BR. Amino acid neurotransmitters in the retina: a functional overview. Vision Research. 1998, 38, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Brockie PJ, Madsen DM, Zheng Y, Mellem J, Maricq AV. Differential expression of glutamate receptor subunits in the nervous system of Caenorhabditis elegans and their regulation by the homeodomain protein UNC-42. J Neurosci. 2001, 21, 1510–1522. [Google Scholar] [CrossRef]
- Fedorovich SV, Waseem TV, Lavrukevich TV, Konev SV. Role of Calcium in Exocytosis Induced by Hypotonic Swelling. Annals of the New York Academy of Sciences. 2005, 1048, 337–340. [Google Scholar] [CrossRef]
- Cerella C, Diederich M, Ghibelli L. The dual role of calcium as messenger and stressor in cell damage, death, and survival. Int J Cell Biol. 2010, 2010, 546163. [Google Scholar] [CrossRef]
- Vaithianathan T, Henry D, Akmentin W, Matthews G. Nanoscale dynamics of synaptic vesicle trafficking and fusion at the presynaptic active zone. eLife. 2016, 5, e13245. [Google Scholar] [CrossRef]
- Mennerick S, Matthews G. Ultrafast exocytosis elicited by calcium current in synaptic terminals of retinal bipolar neurons. Neuron. 1996, 17, 1241–1249. [Google Scholar] [CrossRef]
- Dong F, Zhi Z, Pan M, Xie R, Qin X, Lu R, Mao X, Chen JF, Willcox MD, Qu J, et al. Inhibition of experimental myopia by a dopamine agonist: different effectiveness between form deprivation and hyperopic defocus in guinea pigs. Mol Vis. 2011, 17, 2824–2834. [Google Scholar]
- Esteve-Rudd J, Fernández-Sánchez L, Lax P, De Juan E, Martín-Nieto J, Cuenca N. Rotenone induces degeneration of photoreceptors and impairs the dopaminergic system in the rat retina. Neurobiol Dis. 2011, 44, 102–115. [Google Scholar] [CrossRef]
- Ishikawa A, Ishiguro S, Tamai M. Changes in GABA metabolism in streptozotocin-induced diabetic rat retinas. Curr Eye Res. 1996, 15, 63–71. [Google Scholar] [CrossRef]
- Napper GA, Pianta MJ, Kalloniatis M. Localization of amino acid neurotransmitters following in vitro ischemia and anoxia in the rat retina. Vis Neurosci. 2001, 18, 413–427. [Google Scholar] [CrossRef]
- Ortuño-Lizarán I, Sánchez-Sáez X, Lax P, Serrano GE, Beach TG, Adler CH, Cuenca N. Dopaminergic Retinal Cell Loss and Visual Dysfunction in Parkinson Disease. Ann Neurol. 2020, 88, 893–906. [Google Scholar] [CrossRef]
- Pisani F, Costa C, Caccamo D, Mazzon E, Gorgone G, Oteri G, Calabresi P. Tiagabine and vigabatrin reduce the severity of NMDA-induced excitotoxicity in chick retina. Experimental brain research. 2006, 171, 511–515. [Google Scholar] [CrossRef]
- Rosenlund, BL. Effects of insulin on free amino acids in plasma and the role of the amino acid metabolism in the etiology of diabetic microangiopathy. Biochem Med Metab Biol. 1993, 49, 375–391. [Google Scholar] [CrossRef]
- Stone RA, Lin T, Laties AM, Iuvone PM. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci U S A. 1989, 86, 704–706. [Google Scholar] [CrossRef]
- van Dijk HW, Verbraak FD, Kok PH, Stehouwer M, Garvin MK, Sonka M, DeVries JH, Schlingemann RO, Abràmoff MD. Early neurodegeneration in the retina of type 2 diabetic patients. Invest Ophthalmol Vis Sci. 2012, 53, 2715–2719. [Google Scholar] [CrossRef]
- Amini E, Moghaddasi M, Habibi SAH, Azad Z, Miri S, Nilforushan N, Mirshahi R, Cubo E, Mohammadzadeh N, Rohani M. Huntington's disease and neurovascular structure of retina. Neurol Sci. 2022, 43, 5933–5941. [Google Scholar] [CrossRef]
- Zhang Y, Zhang X, Yue Y, Tian T. Retinal Degeneration: A Window to Understand the Origin and Progression of Parkinson's Disease? Front Neurosci. 2021, 15, 799526. [Google Scholar] [CrossRef]
- Liao C, Xu J, Chen Y. Retinal Dysfunction in Alzheimer's Disease and Implications for Biomarkers. Biomolecules 2021, 11. [Google Scholar] [CrossRef]
- Marchesi N, Fahmideh F, Boschi F. Ocular Neurodegenerative Diseases: Interconnection between Retina and Cortical Areas. Cells 2021, 10. [Google Scholar] [CrossRef]
- Wang YX, Pan Z, Xue CC, Xie H, Wu X, Jonas JB. Macular outer nuclear layer, ellipsoid zone and outer photoreceptor segment band thickness, axial length and other determinants. Scientific Reports. 2023, 13, 5386. [Google Scholar] [CrossRef]
- Kawamura S, Tachibanaki S. Rod and cone photoreceptors: Molecular basis of the difference in their physiology. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2008, 150, 369–377. [Google Scholar] [CrossRef]
- Thoreson WB, Mangel SC. Lateral interactions in the outer retina. Progress in Retinal and Eye Research. 2012, 31, 407–441. [Google Scholar] [CrossRef]
- Martemyanov KA, Sampath AP. The Transduction Cascade in Retinal ON-Bipolar Cells: Signal Processing and Disease. Annu Rev Vis Sci. 2017, 3, 25–51. [Google Scholar] [CrossRef]
- Euler T, Haverkamp S, Schubert T, Baden T. Retinal bipolar cells: elementary building blocks of vision. Nature Reviews Neuroscience. 2014, 15, 507–519. [Google Scholar] [CrossRef]
- Grimes WN, Aytürk DG, Hoon M, Yoshimatsu T, Gamlin C, Carrera D, Nath A, Nadal-Nicolás FM, Ahlquist RM, Sabnis A, et al. A high-density narrow-field inhibitory retinal interneuron with direct coupling to Müller glia. J Neurosci. 2021, 41, 6018–6037. [Google Scholar] [CrossRef]
- Yu DY, Cringle SJ, Balaratnasingam C, Morgan WH, Yu PK, Su EN. Retinal ganglion cells: Energetics, compartmentation, axonal transport, cytoskeletons and vulnerability. Prog Retin Eye Res. 2013, 36, 217–246. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach A, Bringmann A. New functions of Müller cells. Glia. 2013, 61, 651–678. [Google Scholar] [CrossRef] [PubMed]
- Christensen I, Lu B, Yang N, Huang K, Wang P, Tian N. The Susceptibility of Retinal Ganglion Cells to Glutamatergic Excitotoxicity Is Type-Specific. Front Neurosci. 2019, 13, 219. [Google Scholar] [CrossRef] [PubMed]
- Zhang F, Kurokawa K, Lassoued A, Crowell JA, Miller DT. Cone photoreceptor classification in the living human eye from photostimulation-induced phase dynamics. Proceedings of the National Academy of Sciences. 2019, 116, 7951–7956. [Google Scholar] [CrossRef] [PubMed]
- Park, PS. Constitutively active rhodopsin and retinal disease. Adv Pharmacol. 2014, 70, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Connaughton VP, Graham D, Nelson R. Identification and morphological classification of horizontal, bipolar, and amacrine cells within the zebrafish retina. J Comp Neurol. 2004, 477, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Song PI, Matsui JI, Dowling JE. Morphological types and connectivity of horizontal cells found in the adult zebrafish (Danio rerio) retina. J Comp Neurol. 2008, 506, 328–338. [Google Scholar] [CrossRef]
- Bertalmío, M. Chapter 2 - The biological basis of vision: the retina. In Vision Models for High Dynamic Range and Wide Colour Gamut Imaging; Bertalmío M, ed, Ed.; Academic Press, 2020; pp. 11–46. [Google Scholar]
- Matthews, GG. Neurobiology: Molecules, Cells and Systems; Wiley, 2000. [Google Scholar]
- Balasubramanian R, Gan L. Development of Retinal Amacrine Cells and Their Dendritic Stratification. Curr Ophthalmol Rep. 2014, 2, 100–106. [Google Scholar] [CrossRef]
- Werblin, FS. Regenerative hyperpolarization in rods. The Journal of Physiology. 1975, 244, 53–81. [Google Scholar] [CrossRef]
- Oakley B, 2nd, Flaming DG, Brown KT. Effects of the rod receptor potential upon retinal extracellular potassium concentration. Journal of General Physiology. 1979, 74, 713–737. [Google Scholar] [CrossRef]
- Nakatani K, Yau KW. Calcium and magnesium fluxes across the plasma membrane of the toad rod outer segment. The Journal of Physiology. 1988, 395, 695–729. [Google Scholar] [CrossRef] [PubMed]
- Euler T, Masland RH. Light-Evoked Responses of Bipolar Cells in a Mammalian Retina. Journal of Neurophysiology. 2000, 83, 1817–1829. [Google Scholar] [CrossRef]
- Baden T, Berens P, Franke K, Román Rosón M, Bethge M, Euler T. The functional diversity of retinal ganglion cells in the mouse. Nature. 2016, 529, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Field GD, Gauthier JL, Sher A, Greschner M, Machado TA, Jepson LH, Shlens J, Gunning DE, Mathieson K, Dabrowski W, et al. Functional connectivity in the retina at the resolution of photoreceptors. Nature. 2010, 467, 673–677. [Google Scholar] [CrossRef]
- La Vail MM, Rapaport DH, Rakic P. Cytogenesis in the monkey retina. J Comp Neurol. 1991, 309, 86–114. [Google Scholar] [CrossRef] [PubMed]
- Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J Comp Neurol. 1979, 188, 245–262. [Google Scholar] [CrossRef]
- Haverkamp S, Wässle H, Duebel J, Kuner T, Augustine GJ, Feng G, Euler T. The primordial, blue-cone color system of the mouse retina. J Neurosci. 2005, 25, 5438–5445. [Google Scholar] [CrossRef]
- Nikonov SS, Kholodenko R, Lem J, Pugh EN, Jr. Physiological features of the S- and M-cone photoreceptors of wild-type mice from single-cell recordings. J Gen Physiol. 2006, 127, 359–374. [Google Scholar] [CrossRef]
- Wikler KC, Williams RW, Rakic P. Photoreceptor mosaic: number and distribution of rods and cones in the rhesus monkey retina. J Comp Neurol. 1990, 297, 499–508. [Google Scholar] [CrossRef]
- Wässle H, Boycott BB. Functional architecture of the mammalian retina. Physiol Rev. 1991, 71, 447–480. [Google Scholar] [CrossRef] [PubMed]
- Wässle, H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004, 5, 747–757. [Google Scholar] [CrossRef]
- Roorda A, Metha AB, Lennie P, Williams DR. Packing arrangement of the three cone classes in primate retina. Vision Res. 2001, 41, 1291–1306. [Google Scholar] [CrossRef] [PubMed]
- Dowling JE, Wells GP. Synaptic organization of the frog retina: an electron microscopic analysis comparing the retinas of frogs and primates. Proceedings of the Royal Society of London Series B Biological Sciences. 1997, 170, 205–228. [Google Scholar] [CrossRef]
- Sterling P, Matthews G. Structure and function of ribbon synapses. Trends Neurosci. 2005, 28, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Zenisek D, Horst NK, Merrifield C, Sterling P, Matthews G. Visualizing synaptic ribbons in the living cell. J Neurosci. 2004, 24, 9752–9759. [Google Scholar] [CrossRef]
- Vaithianathan T, Matthews G. Visualizing synaptic vesicle turnover and pool refilling driven by calcium nanodomains at presynaptic active zones of ribbon synapses. Proceedings of the National Academy of Sciences. 2014, 111, 8655–8660. [Google Scholar] [CrossRef]
- Heidelberger R, Wang MM, Sherry DM. Differential distribution of synaptotagmin immunoreactivity among synapses in the goldfish, salamander, and mouse retina. Vis Neurosci. 2003, 20, 37–49. [Google Scholar] [CrossRef]
- Henrique von G, Gary M. Depletion and Replenishment of Vesicle Pools at a Ribbon-Type Synaptic Terminal. The Journal of Neuroscience. 1997, 17, 1919. [Google Scholar] [CrossRef]
- von Gersdorff H, Vardi E, Matthews G, Sterling P. Evidence That Vesicles on the Synaptic Ribbon of Retinal Bipolar Neurons Can Be Rapidly Released. Neuron. 1996, 16, 1221–1227. [Google Scholar] [CrossRef]
- Gary M, Peter S. Evidence That Vesicles Undergo Compound Fusion on the Synaptic Ribbon. The Journal of Neuroscience. 2008, 28, 5403. [Google Scholar] [CrossRef]
- Ling-Gang W, Timothy AR, Leon L. Modes of Vesicle Retrieval at Ribbon Synapses, Calyx-Type Synapses, and Small Central Synapses. The Journal of Neuroscience. 2007, 27, 11793. [Google Scholar] [CrossRef]
- Cho S, von Gersdorff H. Ca(2+) influx and neurotransmitter release at ribbon synapses. Cell Calcium. 2012, 52, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Coggins M, Zenisek D. Evidence that exocytosis is driven by calcium entry through multiple calcium channels in goldfish retinal bipolar cells. J Neurophysiol. 2009, 101, 2601–2619. [Google Scholar] [CrossRef] [PubMed]
- Zenisek D, Davila V, Wan L, Almers W. Imaging calcium entry sites and ribbon structures in two presynaptic cells. J Neurosci. 2003, 23, 2538–2548. [Google Scholar] [CrossRef] [PubMed]
- Pangrsic T, Singer JH, Koschak A. Voltage-Gated Calcium Channels: Key Players in Sensory Coding in the Retina and the Inner Ear. Physiological Reviews. 2018, 98, 2063–2096. [Google Scholar] [CrossRef] [PubMed]
- Kushner J, Ferrer X, Marx SO. Roles and Regulation of Voltage-gated Calcium Channels in Arrhythmias. J Innov Card Rhythm Manag. 2019, 10, 3874–3880. [Google Scholar] [CrossRef]
- Fox AP, Nowycky MC, Tsien RW. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. The Journal of Physiology. 1987, 394, 149–172. [Google Scholar] [CrossRef] [PubMed]
- Neelands TR, King APJ, Macdonald RL. Functional Expression of L-, N-, P/Q-, and R-Type Calcium Channels in the Human NT2-N Cell Line. Journal of Neurophysiology. 2000, 84, 2933–2944. [Google Scholar] [CrossRef] [PubMed]
- Griguoli M, Sgritta M, Cherubini E. Presynaptic BK channels control transmitter release: physiological relevance and potential therapeutic implications. J Physiol. 2016, 594, 3489–3500. [Google Scholar] [CrossRef]
- Wang, ZW. Regulation of synaptic transmission by presynaptic CaMKII and BK channels. Mol Neurobiol. 2008, 38, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Robitaille R, Charlton MP. Presynaptic calcium signals and transmitter release are modulated by calcium-activated potassium channels. J Neurosci. 1992, 12, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Morita K, Barrett EF. Evidence for two calcium-dependent potassium conductances in lizard motor nerve terminals. J Neurosci. 1990, 10, 2614–2625. [Google Scholar] [CrossRef] [PubMed]
- Grimes WN, Li W, Chávez AE, Diamond JS. BK channels modulate pre- and postsynaptic signaling at reciprocal synapses in retina. Nat Neurosci. 2009, 12, 585–592. [Google Scholar] [CrossRef]
- Jian Wei X, Malcolm MS. Large-Conductance Calcium-Activated Potassium Channels Facilitate Transmitter Release in Salamander Rod Synapse. The Journal of Neuroscience. 2005, 25, 7660. [Google Scholar] [CrossRef]
- Naoyuki T, Vithiyanjali S, Thomas E, Peter R, Mathias WS, Timm S. BK Channels Mediate Pathway-Specific Modulation of Visual Signals in the <em>In Vivo</em> Mouse Retina. The Journal of Neuroscience. 2012, 32, 4861. [Google Scholar] [CrossRef]
- Van Cruchten S, Vrolyk V, Perron Lepage M-F, Baudon M, Voute H, Schoofs S, Haruna J, Benoit-Biancamano M-O, Ruot B, Allegaert K. Pre- and Postnatal Development of the Eye: A Species Comparison. Birth Defects Research. 2017, 109, 1540–1567. [Google Scholar] [CrossRef]
- Graw, J. Eye development. Curr Top Dev Biol. 2010, 90, 343–386. [Google Scholar] [CrossRef]
- Harada T, Harada C, Parada LF. Molecular regulation of visual system development: more than meets the eye. Genes Dev. 2007, 21, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Marquardt T, Gruss P. Generating neuronal diversity in the retina: one for nearly all. Trends Neurosci. 2002, 25, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Cantrup R, Kaushik G, Schuurmans C. Control of Retinal Development by Tumor Suppressor Genes. In Tumor Suppressor Genes; Cheng Y, ed, Ed.; IntechOpen: London, U.K., 2012. [Google Scholar]
- Turner DL, Snyder EY, Cepko CL. Lineage-independent determination of cell type in the embryonic mouse retina. Neuron. 1990, 4, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Wetts R, Fraser SE. Multipotent Precursors Can Give Rise to All Major Cell Types of the Frog Retina. Science. 1988, 239, 1142–1145. [Google Scholar] [CrossRef]
- Holt CE, Bertsch TW, Ellis HM, Harris WA. Cellular determination in the Xenopus retina is independent of lineage and birth date. Neuron. 1988, 1, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987, 328, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Hernan G, de Mera-Rodríguez JA, Gañán Y, Solana-Fajardo J, Martín-Partido G, Rodríguez-León J, Francisco-Morcillo J. Development and postnatal neurogenesis in the retina: a comparison between altricial and precocial bird species. Neural Regen Res. 2021, 16, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Finlayson PG, Iezzi R. Glutamate stimulation of retinal ganglion cells in normal and s334ter-4 rat retinas: a candidate for a neurotransmitter-based retinal prosthesis. Invest Ophthalmol Vis Sci. 2010, 51, 3619–3628. [Google Scholar] [CrossRef] [PubMed]
- Lee S, Chen L, Chen M, Ye M, Seal RP, Zhou ZJ. An unconventional glutamatergic circuit in the retina formed by vGluT3 amacrine cells. Neuron. 2014, 84, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Ruan G-X, Allen GC, Yamazaki S, McMahon DG. An Autonomous Circadian Clock in the Inner Mouse Retina Regulated by Dopamine and GABA. PLOS Biology. 2008, 6, e249. [Google Scholar] [CrossRef]
- Hartwick AT, Hamilton CM, Baldridge WH. Glutamatergic calcium dynamics and deregulation of rat retinal ganglion cells. J Physiol. 2008, 586, 3425–3446. [Google Scholar] [CrossRef]
- Roth, BL. Molecular pharmacology of metabotropic receptors targeted by neuropsychiatric drugs. Nat Struct Mol Biol. 2019, 26, 535–544. [Google Scholar] [CrossRef]
- Wong, RO. Retinal waves and visual system development. Annu Rev Neurosci. 1999, 22, 29–47. [Google Scholar] [CrossRef]
- Bodnarenko SR, Chalupa LM. Stratification of ON and OFF ganglion cell dendrites depends on glutamate-mediated afferent activity in the developing retina. Nature. 1993, 364, 144–146. [Google Scholar] [CrossRef]
- Bodnarenko SR, Jeyarasasingam G, Chalupa LM. Development and regulation of dendritic stratification in retinal ganglion cells by glutamate-mediated afferent activity. J Neurosci. 1995, 15, 7037–7045. [Google Scholar] [CrossRef] [PubMed]
- Galli L, Maffei L. Spontaneous impulse activity of rat retinal ganglion cells in prenatal life. Science. 1988, 242, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang RW, Du WJ, Prober DA, Du JL. Müller Glial Cells Participate in Retinal Waves via Glutamate Transporters and AMPA Receptors. Cell Rep. 2019, 27, 2871–2880. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki T, Yamasaki M, Hashimoto K, Kohda K, Yuzaki M, Shimamoto K, Tanaka K, Kano M, Watanabe M. Glutamate transporter GLAST controls synaptic wrapping by Bergmann glia and ensures proper wiring of Purkinje cells. Proc Natl Acad Sci U S A. 2017, 114, 7438–7443. [Google Scholar] [CrossRef]
- Blankenship AG, Ford KJ, Johnson J, Seal RP, Edwards RH, Copenhagen DR, Feller MB. Synaptic and extrasynaptic factors governing glutamatergic retinal waves. Neuron. 2009, 62, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Xu H-P, Burbridge TJ, Ye M, Chen M, Ge X, Zhou ZJ, Crair MC. Retinal Wave Patterns Are Governed by Mutual Excitation among Starburst Amacrine Cells and Drive the Refinement and Maintenance of Visual Circuits. The Journal of Neuroscience. 2016, 36, 3871–3886. [Google Scholar] [CrossRef] [PubMed]
- Liets LC, Chalupa LM. Glutamate-mediated responses in developing retinal ganglion cells. Prog Brain Res. 2001, 134, 1–16. [Google Scholar] [CrossRef]
- Chang Y-C, Chen C-Y, Chiao C-C. Visual Experience–Independent Functional Expression of NMDA Receptors in the Developing Rabbit Retina. Investigative Ophthalmology & Visual Science. 2010, 51, 2744–2754. [Google Scholar] [CrossRef]
- Gründer T, Kohler K, Kaletta A, Guenther E. The distribution and developmental regulation of NMDA receptor subunit proteins in the outer and inner retina of the rat. J Neurobiol. 2000, 44, 333–342. [Google Scholar] [CrossRef]
- Hartveit E, Brandstätter JH, Sassoè-Pognetto M, Laurie DJ, Seeburg PH, Wässle H. Localization and developmental expression of the NMDA receptor subunit NR2A in the mammalian retina. J Comp Neurol. 1994, 348, 570–582. [Google Scholar] [CrossRef]
- Pourcho RG, Qin P, Goebel DJ. Cellular and subcellular distribution of NMDA receptor subunit NR2B in the retina. J Comp Neurol. 2001, 433, 75–85. [Google Scholar] [CrossRef] [PubMed]
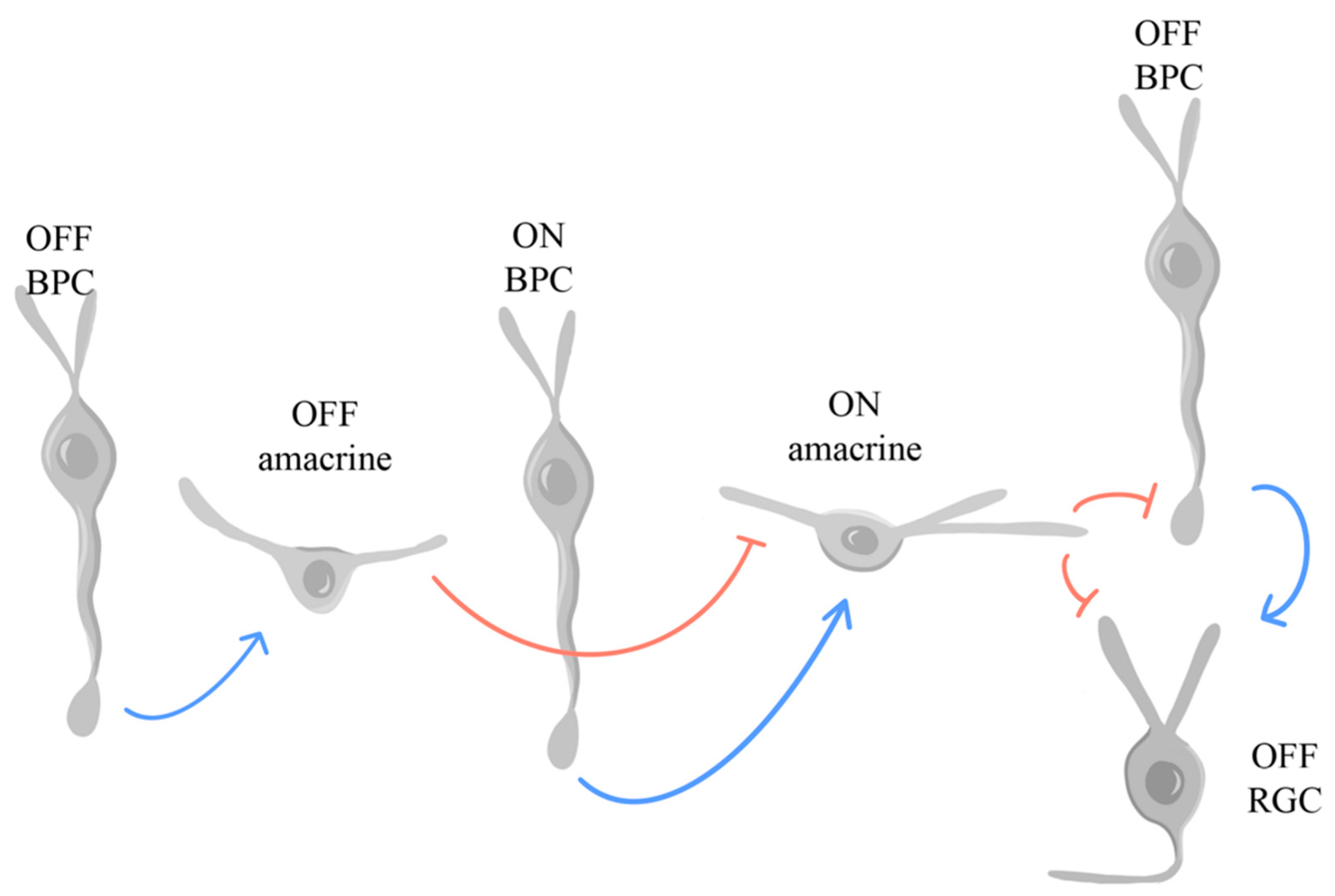
- Popova, E. ON-OFF Interactions in the Retina: Role of Glycine and GABA. Curr Neuropharmacol. 2014, 12, 509–526. [Google Scholar] [CrossRef]
- Mehta B, Ke J-B, Zhang L, Baden AD, Markowitz AL, Nayak S, Briggman KL, Zenisek D, Singer JH. Global Ca2+ Signaling Drives Ribbon-Independent Synaptic Transmission at Rod Bipolar Cell Synapses. The Journal of Neuroscience. 2014, 34, 6233–6244. [Google Scholar] [CrossRef] [PubMed]
- Mehta B, Snellman J, Chen S, Li W, Zenisek D. Synaptic ribbons influence the size and frequency of miniature-like evoked postsynaptic currents. Neuron. 2013, 77, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Thoreson, WB. Transmission at rod and cone ribbon synapses in the retina. Pflugers Arch. 2021, 473, 1469–1491. [Google Scholar] [CrossRef]
- Werblin FS, Dowling JE. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969, 32, 339–355. [Google Scholar] [CrossRef]
- Kaneko, A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970, 207, 623–633. [Google Scholar] [CrossRef]
- DeVries SH, Schwartz EA. Kainate receptors mediate synaptic transmission between cones and 'Off' bipolar cells in a mammalian retina. Nature. 1999, 397, 157–160. [Google Scholar] [CrossRef]
- Zhang J, Diamond JS. Distinct perisynaptic and synaptic localization of NMDA and AMPA receptors on ganglion cells in rat retina. J Comp Neurol. 2006, 498, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Gustafson EC, Morgans CW, Tekmen M, Sullivan SJ, Esguerra M, Konno R, Miller RF. Retinal NMDA receptor function and expression are altered in a mouse lacking D-amino acid oxidase. J Neurophysiol. 2013, 110, 2718–2726. [Google Scholar] [CrossRef] [PubMed]
- Dhingra A, Vardi N. "mGlu Receptors in the Retina" - WIREs Membrane Transport and Signaling. Wiley Interdiscip Rev Membr Transp Signal. 2012, 1, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Nawy, S. The metabotropic receptor mGluR6 may signal through G(o), but not phosphodiesterase, in retinal bipolar cells. J Neurosci. 1999, 19, 2938–2944. [Google Scholar] [CrossRef]
- Shen Y, Heimel JA, Kamermans M, Peachey NS, Gregg RG, Nawy S. A transient receptor potential-like channel mediates synaptic transmission in rod bipolar cells. J Neurosci. 2009, 29, 6088–6093. [Google Scholar] [CrossRef]
- Morgans CW, Brown RL, Duvoisin RM. TRPM1, the endpoint of the mGluR6 signal transduction cascade in retinal ON-bipolar cells. Bioessays. 2010, 32, 609–614. [Google Scholar] [CrossRef]
- Lie-Venema H, Hakvoort TB, van Hemert FJ, Moorman AF, Lamers WH. Regulation of the spatiotemporal pattern of expression of the glutamine synthetase gene. Prog Nucleic Acid Res Mol Biol. 1998, 61, 243–308. [Google Scholar] [CrossRef]
- Novelli A, Reilly JA, Lysko PG, Henneberry RC. Glutamate becomes neurotoxic via the N-methyl-D-aspartate receptor when intracellular energy levels are reduced. Brain Res. 1988, 451, 205–212. [Google Scholar] [CrossRef]
- Nguyen D, Alavi MV, Kim KY, Kang T, Scott RT, Noh YH, Lindsey JD, Wissinger B, Ellisman MH, Weinreb RN, et al. A new vicious cycle involving glutamate excitotoxicity, oxidative stress and mitochondrial dynamics. Cell Death Dis. 2011, 2, e240. [Google Scholar] [CrossRef]
- Peichl, L. Alpha ganglion cells in mammalian retinae: common properties, species differences, and some comments on other ganglion cells. Vis Neurosci. 1991, 7, 155–169. [Google Scholar] [CrossRef]
- Sanes JR, Masland RH. The Types of Retinal Ganglion Cells: Current Status and Implications for Neuronal Classification. Annual Review of Neuroscience. 2015, 38, 221–246. [Google Scholar] [CrossRef]
- Lucas DR, Newhouse JP. The toxic effect of sodium L-glutamate on the inner layers of the retina. AMA Arch Ophthalmol. 1957, 58, 193–201. [Google Scholar] [CrossRef]
- Izumi Y, Shimamoto K, Benz AM, Hammerman SB, Olney JW, Zorumski CF. Glutamate transporters and retinal excitotoxicity. Glia. 2002, 39, 58–68. [Google Scholar] [CrossRef]
- Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol. 1998, 53, 195–201. [Google Scholar] [CrossRef]
- Wasilewa P, Hockwin O, Korte I. Glycogen concentration changes in retina, vitreous body and other eye tissues caused by disturbances of blood circulation. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1976, 199, 115–120. [Google Scholar] [CrossRef]
- Kosenko E, Llansola M, Montoliu C, Monfort P, Rodrigo R, Hernandez-Viadel M, Erceg S, Sánchez-Perez AM, Felipo V. Glutamine synthetase activity and glutamine content in brain: modulation by NMDA receptors and nitric oxide. Neurochem Int. 2003, 43, 493–499. [Google Scholar] [CrossRef]
- Fernandez DC, Chianelli MS, Rosenstein RE. Involvement of glutamate in retinal protection against ischemia/reperfusion damage induced by post-conditioning. Journal of Neurochemistry. 2009, 111, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004, 23, 91–147. [Google Scholar] [CrossRef] [PubMed]
- Barnett NL, Pow DV, Bull ND. Differential perturbation of neuronal and glial glutamate transport systems in retinal ischaemia. Neurochem Int. 2001, 39, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Liu Y, Wang C, Su G. Cellular Signaling in Müller Glia: Progenitor Cells for Regenerative and Neuroprotective Responses in Pharmacological Models of Retinal Degeneration. J Ophthalmol. 2019, 2019, 5743109. [Google Scholar] [CrossRef]
- Bringmann A, Uckermann O, Pannicke T, Iandiev I, Reichenbach A, Wiedemann P. Neuronal versus glial cell swelling in the ischaemic retina. Acta Ophthalmol Scand. 2005, 83, 528–538. [Google Scholar] [CrossRef]
- Barnett NL, Grozdanic SD. Glutamate transporter localization does not correspond to the temporary functional recovery and late degeneration after acute ocular ischemia in rats. Exp Eye Res. 2004, 79, 513–524. [Google Scholar] [CrossRef]
- Casson, RJ. Possible role of excitotoxicity in the pathogenesis of glaucoma. Clin Exp Ophthalmol. 2006, 34, 54–63. [Google Scholar] [CrossRef]
- Naskar R, Vorwerk CK, Dreyer EB. Concurrent downregulation of a glutamate transporter and receptor in glaucoma. Invest Ophthalmol Vis Sci. 2000, 41, 1940–1944. [Google Scholar]
- Harada T, Harada C, Nakamura K, Quah HM, Okumura A, Namekata K, Saeki T, Aihara M, Yoshida H, Mitani A, et al. The potential role of glutamate transporters in the pathogenesis of normal tension glaucoma. J Clin Invest. 2007, 117, 1763–1770. [Google Scholar] [CrossRef]
- Harada C, Nakamura K, Namekata K, Okumura A, Mitamura Y, Iizuka Y, Kashiwagi K, Yoshida K, Ohno S, Matsuzawa A, et al. Role of apoptosis signal-regulating kinase 1 in stress-induced neural cell apoptosis in vivo. Am J Pathol. 2006, 168, 261–269. [Google Scholar] [CrossRef]
- Osaka N, Takahashi T, Murakami S, Matsuzawa A, Noguchi T, Fujiwara T, Aburatani H, Moriyama K, Takeda K, Ichijo H. ASK1-dependent recruitment and activation of macrophages induce hair growth in skin wounds. J Cell Biol. 2007, 176, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Ambati J, Chalam KV, Chawla DK, D'Angio CT, Guillet EG, Rose SJ, Vanderlinde RE, Ambati BK. Elevated gamma-aminobutyric acid, glutamate, and vascular endothelial growth factor levels in the vitreous of patients with proliferative diabetic retinopathy. Arch Ophthalmol. 1997, 115, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Hernández C, Simó R. Neuroprotection in diabetic retinopathy. Curr Diab Rep. 2012, 12, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Villagrana AR, Garcia-Román J, González-Espinosa C, Lamas M. Pharmacological inhibition of N-methyl d-aspartate receptor promotes secretion of vascular endothelial growth factor in müller cells: effects of hyperglycemia and hypoxia. Curr Eye Res. 2010, 35, 733–741. [Google Scholar] [CrossRef]
- Kusari J, Zhou SX, Padillo E, Clarke KG, Gil DW. Inhibition of Vitreoretinal VEGF Elevation and Blood–Retinal Barrier Breakdown in Streptozotocin-Induced Diabetic Rats by Brimonidine. Investigative Ophthalmology & Visual Science. 2010, 51, 1044–1051. [Google Scholar] [CrossRef]
- Li Q, Puro DG. Diabetes-induced dysfunction of the glutamate transporter in retinal Müller cells. Invest Ophthalmol Vis Sci. 2002, 43, 3109–3116. [Google Scholar]
- Zeng K, Xu H, Mi M, Zhang Q, Zhang Y, Chen K, Chen F, Zhu J, Yu X. Dietary taurine supplementation prevents glial alterations in retina of diabetic rats. Neurochem Res. 2009, 34, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Paradies G, Petrosillo G, Paradies V, Ruggiero FM. Role of cardiolipin peroxidation and Ca2+ in mitochondrial dysfunction and disease. Cell Calcium. 2009, 45, 643–650. [Google Scholar] [CrossRef]
- Aydın A, Orhan H, Sayal A, Özata M, Şahin G, Işımer A. Oxidative stress and nitric oxide related parameters in type II diabetes mellitus: effects of glycemic control. Clinical Biochemistry. 2001, 34, 65–70. [Google Scholar] [CrossRef]
- Dong LY, Jin J, Lu G, Kang XL. Astaxanthin attenuates the apoptosis of retinal ganglion cells in db/db mice by inhibition of oxidative stress. Mar Drugs. 2013, 11, 960–974. [Google Scholar] [CrossRef]
- Mittal R, Kumar A, Singh DP, Bishnoi M, Nag TC. Ameliorative potential of rutin in combination with nimesulide in STZ model of diabetic neuropathy: targeting Nrf2/HO-1/NF-kB and COX signalling pathway. Inflammopharmacology. 2018, 26, 755–768. [Google Scholar] [CrossRef]
- Xu Z, Wei Y, Gong J, Cho H, Park JK, Sung ER, Huang H, Wu L, Eberhart C, Handa JT, et al. NRF2 plays a protective role in diabetic retinopathy in mice. Diabetologia. 2014, 57, 204–213. [Google Scholar] [CrossRef]
- Firl A, Sack GS, Newman ZL, Tani H, Feller MB. Extrasynaptic glutamate and inhibitory neurotransmission modulate ganglion cell participation during glutamatergic retinal waves. Journal of Neurophysiology. 2013, 109, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Wong, RO. Effects of glutamate and its analogs on intracellular calcium levels in the developing retina. Vis Neurosci. 1995, 12, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Mesnard CS, Barta CL, Sladek AL, Zenisek D, Thoreson WB. Eliminating Synaptic Ribbons from Rods and Cones Halves the Releasable Vesicle Pool and Slows Down Replenishment. Eliminating Synaptic Ribbons from Rods and Cones Halves the Releasable Vesicle Pool and Slows Down Replenishment. Int J Mol Sci. 2022, 23. [Google Scholar] [CrossRef]
- Frank T, Rutherford MA, Strenzke N, Neef A, Pangršič T, Khimich D, Fejtova A, Gundelfinger ED, Liberman MC, Harke B, et al. Bassoon and the synaptic ribbon organize Ca²+ channels and vesicles to add release sites and promote refilling. Neuron. 2010, 68, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Chen M, Van Hook MJ, Zenisek D, Thoreson WB. Properties of ribbon and non-ribbon release from rod photoreceptors revealed by visualizing individual synaptic vesicles. J Neurosci. 2013, 33, 2071–2086. [Google Scholar] [CrossRef] [PubMed]
- Krizaj D, Lai FA, Copenhagen DR. Ryanodine stores and calcium regulation in the inner segments of salamander rods and cones. J Physiol. 2003, 547, 761–774. [Google Scholar] [CrossRef]
- Sattler R, Tymianski M. Molecular mechanisms of calcium-dependent excitotoxicity. Journal of Molecular Medicine. 2000, 78, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Smith, SB. Diabetic Retinopathy and the NMDA Receptor. Drug News Perspect. 2002, 15, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Wu X-S, Wu L-G. The Yin and Yang of Calcium Effects on Synaptic Vesicle Endocytosis. The Journal of Neuroscience. 2014, 34, 2652–2659. [Google Scholar] [CrossRef] [PubMed]
- Haverkamp S, Wässle H. Immunocytochemical analysis of the mouse retina. J Comp Neurol. 2000, 424, 1–23. [Google Scholar] [CrossRef]
- Vaughn JE, Famiglietti EV, Barber Jr. RP, Saito K, Roberts E, Ribak CE. GABAergic amacrine cells in rat retina: Immunocytochemical identification and synaptic connectivity. Journal of Comparative Neurology. 1981, 197, 113–127. [Google Scholar] [CrossRef]
- Mosinger JL, Yazulla S, Studholme KM. GABA-like immunoreactivity in the vertebrate retina: a species comparison. Exp Eye Res. 1986, 42, 631–644. [Google Scholar] [CrossRef]
- Davanger S, Ottersen OP, Storm-Mathisen J. Glutamate, GABA, and glycine in the human retina: an immunocytochemical investigation. J Comp Neurol. 1991, 311, 483–494. [Google Scholar] [CrossRef]
- Agardh E, Ehinger B, Wu JY. GABA and GAD-like immunoreactivity in the primate retina. Histochemistry. 1987, 86, 485–490. [Google Scholar] [CrossRef]
- Yu BCY, Watt CB, Lam DMK, Fry KR. GABAergic ganglion cells in the rabbit retina. Brain Research. 1988, 439, 376–382. [Google Scholar] [CrossRef]
- Sandell, JH. GABA as a developmental signal in the inner retina and optic nerve. Perspect Dev Neurobiol. 1998, 5, 269–278. [Google Scholar] [PubMed]
- Shaye H, Stauch B, Gati C. Molecular mechanisms of metabotropic GABA(B) receptor function. Sci Adv 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Catsicas M, Mobbs P. GABAb receptors regulate chick retinal calcium waves. J Neurosci. 2001, 21, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer J, Rusoff AC. Horizontal cells of the mouse retina contain glutamic acid decarboxylase-like immunoreactivity during early developmental stages. J Neurosci. 1984, 4, 2948–2955. [Google Scholar] [CrossRef] [PubMed]
- Fischer KF, Lukasiewicz PD, Wong RO. Age-dependent and cell class-specific modulation of retinal ganglion cell bursting activity by GABA. J Neurosci. 1998, 18, 3767–3778. [Google Scholar] [CrossRef]
- Buddhala C, Hsu CC, Wu JY. A novel mechanism for GABA synthesis and packaging into synaptic vesicles. Neurochem Int. 2009, 55, 9–12. [Google Scholar] [CrossRef]
- Mikkelsen M, Harris AD, Edden RAE, Puts NAJ. Macromolecule-suppressed GABA measurements correlate more strongly with behavior than macromolecule-contaminated GABA+ measurements. Brain Res. 2018, 1701, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Hyland N, Cryan J. A Gut Feeling about GABA: Focus on GABAB Receptors. Frontiers in Pharmacology 2010, 1. [Google Scholar] [CrossRef] [PubMed]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994, 17, 569–602. [Google Scholar] [CrossRef] [PubMed]
- Bormann, J. Electrophysiology of GABAA and GABAB receptor subtypes. Trends in Neurosciences. 1988, 11, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Wang S, Du L, Peng G, Li W. GABA inhibits proliferation and self-renewal of mouse retinal progenitor cell. Cell Death Discovery. 2019, 5, 80. [Google Scholar] [CrossRef] [PubMed]
- Koulen P, Malitschek B, Kuhn R, Bettler B, Wässle H, Brandstätter JH. Presynaptic and postsynaptic localization of GABAB receptors in neurons of the rat retina. European Journal of Neuroscience. 1998, 10, 1446–1456. [Google Scholar] [CrossRef]
- Kamermans M, Werblin F. GABA-mediated positive autofeedback loop controls horizontal cell kinetics in tiger salamander retina. The Journal of Neuroscience. 1992, 12, 2451–2463. [Google Scholar] [CrossRef]
- Matthews G, Ayoub GS, Heidelberger R. Presynaptic inhibition by GABA is mediated via two distinct GABA receptors with novel pharmacology. J Neurosci. 1994, 14, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, EA. Depolarization without calcium can release gamma-aminobutyric acid from a retinal neuron. Science. 1987, 238, 350–355. [Google Scholar] [CrossRef]
- Warrier A, Borges S, Dalcino D, Walters C, Wilson M. Calcium from internal stores triggers GABA release from retinal amacrine cells. J Neurophysiol. 2005, 94, 4196–4208. [Google Scholar] [CrossRef]
- Brecha NC, Weigmann C. Expression of GAT-1, a high-affinity gamma-aminobutyric acid plasma membrane transporter in the rat retina. J Comp Neurol. 1994, 345, 602–611. [Google Scholar] [CrossRef]
- Honda S, Yamamoto M, Saito N. Immunocytochemical localization of three subtypes of GABA transporter in rat retina. Molecular Brain Research. 1995, 33, 319–325. [Google Scholar] [CrossRef]
- Schousboe, A. Role of astrocytes in the maintenance and modulation of glutamatergic and GABAergic neurotransmission. Neurochem Res. 2003, 28, 347–352. [Google Scholar] [CrossRef]
- Moran J, Pasantes-Morales H, Redburn DA. Glutamate receptor agonists release [3H]GABA preferentially from horizontal cells. Brain Research. 1986, 398, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Marc, RE. Structural organization of GABAergic circuitry in ectotherm retinas. Prog Brain Res. 1992, 90, 61–92. [Google Scholar] [CrossRef] [PubMed]
- Barnett NL, Osborne NN. Redistribution of GABA immunoreactivity following central retinal artery occlusion. Brain Research. 1995, 677, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Malmgren K, Ben-Menachem E, Frisén L. Vigabatrin Visual Toxicity: Evolution and Dose Dependence. Epilepsia. 2001, 42, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Biermann J, Grieshaber P, Goebel U, Martin G, Thanos S, Di Giovanni S, Lagrèze WA. Valproic acid-mediated neuroprotection and regeneration in injured retinal ganglion cells. Invest Ophthalmol Vis Sci. 2010, 51, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Schur RM, Gao S, Yu G, Chen Y, Maeda A, Palczewski K, Lu ZR. New GABA modulators protect photoreceptor cells from light-induced degeneration in mouse models. FASEB J. 2018, 32, 3289–3300. [Google Scholar] [CrossRef] [PubMed]
- Sernagor E, Young C, Eglen SJ. Developmental modulation of retinal wave dynamics: shedding light on the GABA saga. J Neurosci. 2003, 23, 7621–7629. [Google Scholar] [CrossRef]
- Eggers ED, Klein JS, Moore-Dotson JM. Slow changes in Ca2(+) cause prolonged release from GABAergic retinal amacrine cells. J Neurophysiol. 2013, 110, 709–719. [Google Scholar] [CrossRef]
- Hirano AA, Vuong HE, Kornmann HL, Schietroma C, Stella SL, Barnes S, Brecha NC. Vesicular Release of GABA by Mammalian Horizontal Cells Mediates Inhibitory Output to Photoreceptors. Vesicular Release of GABA by Mammalian Horizontal Cells Mediates Inhibitory Output to Photoreceptors. Frontiers in Cellular Neuroscience. 2020, 14. [Google Scholar] [CrossRef]
- Verweij J, Kamermans M, Spekreijse H. Horizontal cells feed back to cones by shifting the cone calcium-current activation range. Vision Res. 1996, 36, 3943–3953. [Google Scholar] [CrossRef]
- Moore-Dotson JM, Eggers ED. Reductions in Calcium Signaling Limit Inhibition to Diabetic Retinal Rod Bipolar Cells. Invest Ophthalmol Vis Sci. 2019, 60, 4063–4073. [Google Scholar] [CrossRef]
- Czapiński P, Blaszczyk B, Czuczwar SJ. Mechanisms of action of antiepileptic drugs. Curr Top Med Chem. 2005, 5, 3–14. [Google Scholar] [CrossRef]
- Chintalapudi SR, Maria D, Di Wang X, Bailey JNC, Allingham R, Brilliant M, Budenz D, Fingert J, Gaasterland D, Gaasterland T, et al. Systems genetics identifies a role for Cacna2d1 regulation in elevated intraocular pressure and glaucoma susceptibility. Nature Communications. 2017, 8, 1755. [Google Scholar] [CrossRef] [PubMed]
- Pow DV, Barnett NL. Changing patterns of spatial buffering of glutamate in developing rat retinae are mediated by the Müller cell glutamate transporter GLAST. Cell Tissue Res. 1999, 297, 57–66. [Google Scholar] [CrossRef]
- Sassoe-Pognetto M, Wassle H, Grunert U. Glycinergic synapses in the rod pathway of the rat retina: cone bipolar cells express the alpha 1 subunit of the glycine receptor. The Journal of Neuroscience. 1994, 14, 5131–5146. [Google Scholar] [CrossRef]
- Lynch, JW. Native glycine receptor subtypes and their physiological roles. Neuropharmacology. 2009, 56, 303–309. [Google Scholar] [CrossRef]
- Kirsch J, Betz H. Glycine-receptor activation is required for receptor clustering in spinal neurons. Nature. 1998, 392, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Chávez G, Velázquez-Flores MÁ, Ruiz Esparza-Garrido R, Salceda R. Glycine receptor subunits expression in the developing rat retina. Neurochemistry International. 2017, 108, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Young TL, Cepko CL. A Role for Ligand-Gated Ion Channels in Rod Photoreceptor Development. Neuron. 2004, 41, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Haverkamp, S. Glycine Receptor Diversity in the Mammalian Retina. In Webvision: The Organization of the Retina and Visual System; Kolb H, Nelson R, Fernandez E, Ed.; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 1995. [Google Scholar]
- Famiglietti EV, Jr. , Kolb H. A bistratified amacrine cell and synaptic cirucitry in the inner plexiform layer of the retina. Brain Res. 1975, 84, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Hsueh HA, Molnar A, Werblin FS. Amacrine-to-amacrine cell inhibition in the rabbit retina. J Neurophysiol. 2008, 100, 2077–2088. [Google Scholar] [CrossRef]
- Bormann J, Rundström N, Betz H, Langosch D. Residues within transmembrane segment M2 determine chloride conductance of glycine receptor homo- and hetero-oligomers. Embo j. 1993, 12, 3729–3737. [Google Scholar] [CrossRef]
- Betz, H. Structure and function of inhibitory glycine receptors. Q Rev Biophys. 1992, 25, 381–394. [Google Scholar] [CrossRef]
- Guidry, C. The role of Müller cells in fibrocontractive retinal disorders. Prog Retin Eye Res. 2005, 24, 75–86. [Google Scholar] [CrossRef]
- Gholami S, Kamali Y, Reza Rostamzad M. Glycine Supplementation Ameliorates Retinal Neuronal Damage in an Experimental Model of Diabetes in Rats: A Light and Electron Microscopic Study. J Ophthalmic Vis Res. 2019, 14, 448–456. [Google Scholar] [CrossRef]
- Hou M, Duan L, Slaughter MM. Synaptic inhibition by glycine acting at a metabotropic receptor in tiger salamander retina. J Physiol. 2008, 586, 2913–2926. [Google Scholar] [CrossRef] [PubMed]
- Zhang P-P, Zhang G, Zhou W, Weng S-J, Yang X-L, Zhong Y-M. Signaling mechanism for modulation by ATP of glycine receptors on rat retinal ganglion cells. Scientific Reports. 2016, 6, 28938. [Google Scholar] [CrossRef]
- Marc AM, Veeramuthu B, Xiaohan W, Henrique von G. Glycine Release Is Potentiated by cAMP via EPAC2 and Ca<sup>2+</sup> Stores in a Retinal Interneuron. The Journal of Neuroscience. 2021, 41, 9503. [Google Scholar] [CrossRef]
- Freed MA, Liang Z. Synaptic noise is an information bottleneck in the inner retina during dynamic visual stimulation. The Journal of Physiology. 2014, 592, 635–651. [Google Scholar] [CrossRef]
- Witkovsky, P. Dopamine and retinal function. Documenta Ophthalmologica. 2004, 108, 17–39. [Google Scholar] [CrossRef]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-Containing Retinal Ganglion Cells: Architecture, Projections, and Intrinsic Photosensitivity. Science. 2002, 295, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Versaux-Botteri C, Verney C, Zecevic N, Nguyen-Legros J. Early appearance of tyrosine hydroxylase immunoreactivity in the retina of human embryos. Developmental Brain Research. 1992, 69, 283–287. [Google Scholar] [CrossRef]
- Wulle I, Schnitzer J. Distribution and morphology of tyrosine hydroxylase-immunoreactive neurons in the developing mouse retina. Developmental Brain Research. 1989, 48, 59–72. [Google Scholar] [CrossRef]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine Receptors: From Structure to Function. Physiological Reviews. 1998, 78, 189–225. [Google Scholar] [CrossRef] [PubMed]
- Iuvone PM, Tosini G, Pozdeyev N, Haque R, Klein DC, Chaurasia SS. Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog Retin Eye Res. 2005, 24, 433–456. [Google Scholar] [CrossRef]
- Goel M, Mangel SC. Dopamine-Mediated Circadian and Light/Dark-Adaptive Modulation of Chemical and Electrical Synapses in the Outer Retina. Frontiers in Cellular Neuroscience 2021, 15. [Google Scholar] [CrossRef]
- Troy JB, Shou T. The receptive fields of cat retinal ganglion cells in physiological and pathological states: where we are after half a century of research. Prog Retin Eye Res. 2002, 21, 263–302. [Google Scholar] [CrossRef] [PubMed]
- Hammond, P. Receptive field mechanisms of sustained and transient retinal ganglion cells in the cat. Exp Brain Res. 1975, 23, 113–128. [Google Scholar] [CrossRef]
- Harnois C, Di Paolo T. Decreased dopamine in the retinas of patients with Parkinson's disease. Invest Ophthalmol Vis Sci. 1990, 31, 2473–2475. [Google Scholar]
- Moschos MM, Tagaris G, Markopoulos L, Margetis L, Tsapakis S, Kanakis M, Koutsandrea C. Morphologic Changes and Functional Retinal Impairment in Patients with Parkinson Disease without Visual Loss. European Journal of Ophthalmology. 2011, 21, 24–29. [Google Scholar] [CrossRef]
- Mao J, Liu S, Qin W, Li F, Wu X, Tan Q. Levodopa inhibits the development of form-deprivation myopia in guinea pigs. Optom Vis Sci. 2010, 87, 53–60. [Google Scholar] [CrossRef]
- Gao Q, Liu Q, Ma P, Zhong X, Wu J, Ge J. Effects of direct intravitreal dopamine injections on the development of lid-suture induced myopia in rabbits. Graefes Arch Clin Exp Ophthalmol. 2006, 244, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Ashby RS, Schaeffel F. The effect of bright light on lens compensation in chicks. Invest Ophthalmol Vis Sci. 2010, 51, 5247–5253. [Google Scholar] [CrossRef]
- Backhouse S, Collins AV, Phillips JR. Influence of periodic vs continuous daily bright light exposure on development of experimental myopia in the chick. Ophthalmic Physiol Opt. 2013, 33, 563–572. [Google Scholar] [CrossRef]
- Lan W, Feldkaemper M, Schaeffel F. Intermittent episodes of bright light suppress myopia in the chicken more than continuous bright light. PLoS One. 2014, 9, e110906. [Google Scholar] [CrossRef]
- Ivanova TN, Alonso-Gomez AL, Iuvone PM. Dopamine D4 receptors regulate intracellular calcium concentration in cultured chicken cone photoreceptor cells: Relationship to dopamine receptor-mediated inhibition of cAMP formation. Brain Research. 2008, 1207, 111–119. [Google Scholar] [CrossRef]
- Jackson CR, Chaurasia SS, Zhou H, Haque R, Storm DR, Iuvone PM. Essential roles of dopamine D4 receptors and the type 1 adenylyl cyclase in photic control of cyclic AMP in photoreceptor cells. J Neurochem. 2009, 109, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, ML. Melatonin is a potent modulator of dopamine release in the retina. Nature. 1983, 306, 782–784. [Google Scholar] [CrossRef]
- Nowak JZ, Z̵urawska E, Zawilska J. Melatonin and its generating system in vertebrate retina: circadian rhythm, effect of environmental lighting and interaction with dopamine. Neurochemistry International. 1989, 14, 397–406. [Google Scholar] [CrossRef]
- Ford KJ, Feller MB. Assembly and disassembly of a retinal cholinergic network. Vis Neurosci. 2012, 29, 61–71. [Google Scholar] [CrossRef]
- Feller MB, Wellis DP, Stellwagen D, Werblin FS, Shatz CJ. Requirement for Cholinergic Synaptic Transmission in the Propagation of Spontaneous Retinal Waves. Science. 1996, 272, 1182–1187. [Google Scholar] [CrossRef]
- Burbridge Timothy J, Xu H-P, Ackman James B, Ge X, Zhang Y, Ye M-J, Zhou ZJ, Xu J, Contractor A, Crair Michael C. Visual Circuit Development Requires Patterned Activity Mediated by Retinal Acetylcholine Receptors. Neuron. 2014, 84, 1049–1064. [Google Scholar] [CrossRef]
- Zhou ZJ, Fain GL. Starburst amacrine cells change from spiking to nonspiking neurons during retinal development. Proc Natl Acad Sci U S A. 1996, 93, 8057–8062. [Google Scholar] [CrossRef]
- Sernagor E, Grzywacz NM. Influence of spontaneous activity and visual experience on developing retinal receptive fields. Curr Biol. 1996, 6, 1503–1508. [Google Scholar] [CrossRef]
- Wong WT, Wong RO. Changing specificity of neurotransmitter regulation of rapid dendritic remodeling during synaptogenesis. Nat Neurosci. 2001, 4, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Mehta V, Sernagor E. Early neural activity and dendritic growth in turtle retinal ganglion cells. Eur J Neurosci. 2006, 24, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Arroyo DA, Kirkby LA, Feller MB. Retinal Waves Modulate an Intraretinal Circuit of Intrinsically Photosensitive Retinal Ganglion Cells. J Neurosci. 2016, 36, 6892–6905. [Google Scholar] [CrossRef] [PubMed]
- Zeisel SH, Da Costa K-A, Franklin PD, Alexander EA, Lamont JT, Sheard NF, Beiser A. Choline, an essential nutrient for humans. The FASEB Journal. 1991, 5, 2093–2098. [Google Scholar] [CrossRef]
- Voigt, T. Cholinergic amacrine cells in the rat retina. Journal of Comparative Neurology. 1986, 248, 19–35. [Google Scholar] [CrossRef]
- Townes-Anderson E, Vogt BA. Distribution of muscarinic acetylcholine receptors on processes of isolated retinal cells. J Comp Neurol. 1989, 290, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Strang CE, Long Y, Gavrikov KE, Amthor FR, Keyser KT. Nicotinic and muscarinic acetylcholine receptors shape ganglion cell response properties. Journal of Neurophysiology. 2014, 113, 203–217. [Google Scholar] [CrossRef]
- Hall LM, Hellmer CB, Koehler CC, Ichinose T. Bipolar Cell Type-Specific Expression and Conductance of Alpha-7 Nicotinic Acetylcholine Receptors in the Mouse Retina. Invest Ophthalmol Vis Sci. 2019, 60, 1353–1361. [Google Scholar] [CrossRef]
- Strang CE, Renna JM, Amthor FR, Keyser KT. Nicotinic acetylcholine receptor expression by directionally selective ganglion cells. Vis Neurosci. 2007, 24, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Keyser KT, MacNeil MA, Dmitrieva N, Wang F, Masland RH, Lindstrom JM. Amacrine, ganglion, and displaced amacrine cells in the rabbit retina express nicotinic acetylcholine receptors. Vis Neurosci. 2000, 17, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva NA, Strang CE, Keyser KT. Expression of alpha 7 nicotinic acetylcholine receptors by bipolar, amacrine, and ganglion cells of the rabbit retina. J Histochem Cytochem. 2007, 55, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Fischer AJ, McKinnon LA, Nathanson NM, Stell WK. Identification and localization of muscarinic acetylcholine receptors in the ocular tissues of the chick. J Comp Neurol. 1998, 392, 273–284. [Google Scholar] [CrossRef]
- Ruan Y, Patzak A, Pfeiffer N. Muscarinic Acetylcholine Receptors in the Retina-Therapeutic Implications. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Bernard V, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing muscarinic receptor genes. The Journal of Neuroscience. 1992, 12, 3591–3600. [Google Scholar] [CrossRef]
- Laspas P, Zhutdieva MB, Brochhausen C, Musayeva A, Zadeh JK, Pfeiffer N, Xia N, Li H, Wess J, Gericke A. The M1 muscarinic acetylcholine receptor subtype is important for retinal neuron survival in aging mice. Scientific Reports. 2019, 9, 5222. [Google Scholar] [CrossRef]
- Elstrott J, Anishchenko A, Greschner M, Sher A, Litke AM, Chichilnisky EJ, Feller MB. Direction selectivity in the retina is established independent of visual experience and cholinergic retinal waves. Neuron. 2008, 58, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Lee S, Kim K, Zhou ZJ. Role of ACh-GABA Cotransmission in Detecting Image Motion and Motion Direction. Neuron. 2010, 68, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Vaney DI, Sivyer B, Taylor WR. Direction selectivity in the retina: symmetry and asymmetry in structure and function. Nature Reviews Neuroscience. 2012, 13, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Tiriac A, Bistrong K, Pitcher MN, Tworig JM, Feller MB. The influence of spontaneous and visual activity on the development of direction selectivity maps in mouse retina. Cell Rep. 2022, 38, 110225. [Google Scholar] [CrossRef] [PubMed]
- Hellmer CB, Hall LM, Bohl JM, Sharpe ZJ, Smith RG, Ichinose T. Cholinergic feedback to bipolar cells contributes to motion detection in the mouse retina. Cell Rep. 2021, 37, 110106. [Google Scholar] [CrossRef] [PubMed]
- Lusthaus J, Goldberg I. Current management of glaucoma. Med J Aust. 2019, 210, 180–187. [Google Scholar] [CrossRef]
- Shen J, Wang Y, Yao K. Protection of retinal ganglion cells in glaucoma: Current status and future. Exp Eye Res. 2021, 205, 108506. [Google Scholar] [CrossRef]


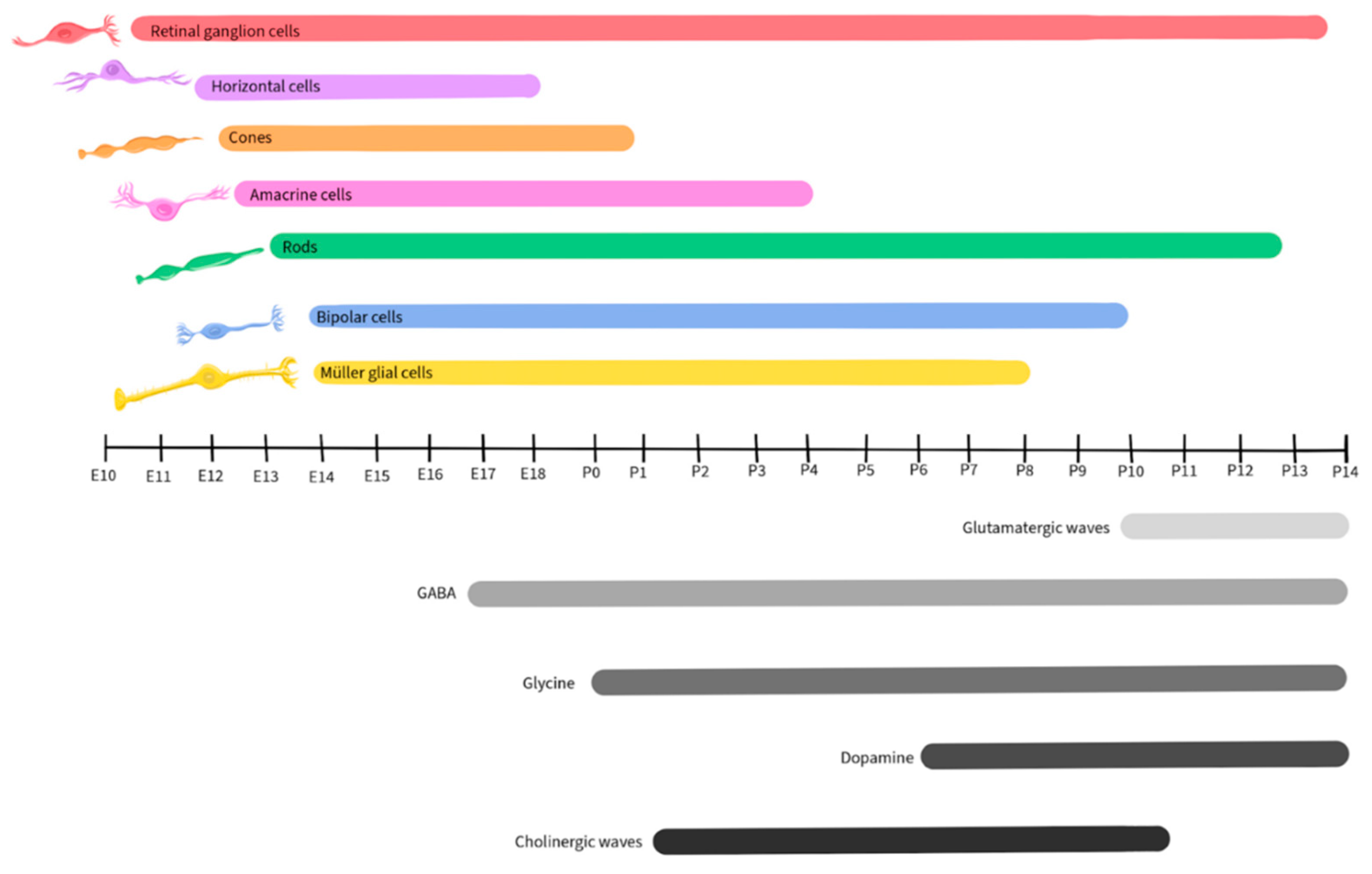
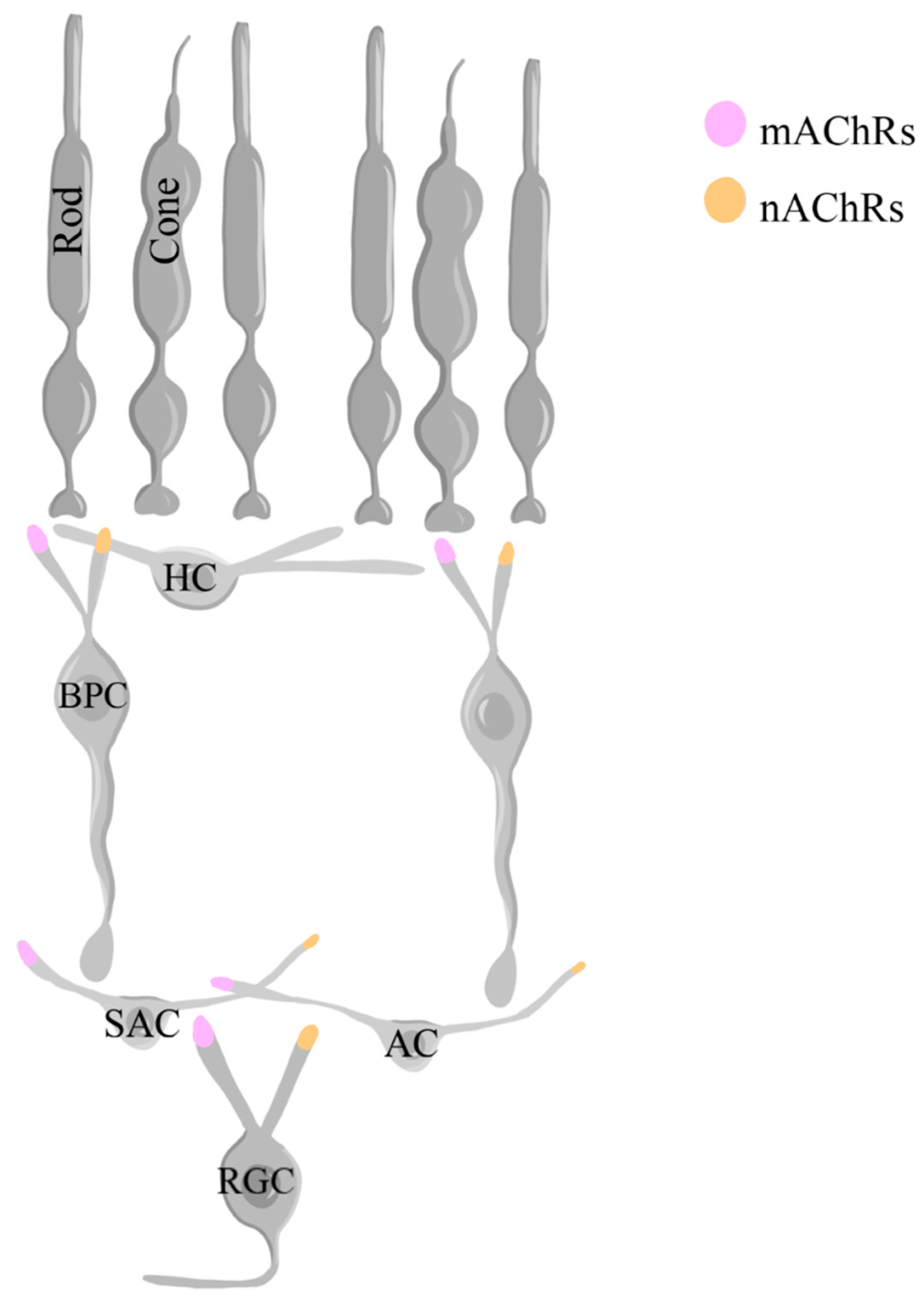
| Neurotransmitter | Retinal Development | Normal Retinal Physiology |
|---|---|---|
| Glutamate |
|
|
| GABA |
|
|
| Glycine |
|
|
| Dopamine |
|
|
| Acetylcholine (ACh) |
|
|
| Glaucoma | Retinal Ischemia | Diabetic Retinopathy | Parkinson’s Disease Visual Impairment | Form-Depravation Myopia | |
|---|---|---|---|---|---|
| Glutamate |
|
|
|
Unknown. | Unknown. |
| GABA | Unknown. |
|
|
Unknown. | Unknown. |
| Glycine | Unknown. | Unknown. |
|
Unknown. | Unknown. |
| Dopamine | Unknown. | Unknown. | Unknown. |
|
|
| Acetylcholine (ACh) |
|
Unknown. | Unknown. | Unknown. | Unknown. |
| Neurotransmitter | Role of Calcium Interplay |
|---|---|
| Glutamate |
|
| GABA |
|
| Glycine |
|
| Dopamine |
|
| Acetylcholine (ACh) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





