1. Introduction
Insomnia, a widespread challenge in contemporary society, is defined by insufficient sleep quantity or quality, marked by difficulties in falling asleep, frequent awakenings, and post-wake fatigue [
1,
2]. Contributing factors such as stress, anxiety, and various mental or physical health conditions have been associated with heightened arousal in individuals contending with insomnia [
3,
4].
The pathophysiology of insomnia can be broadly classified into two models: the cognitive model and the physiological or neurophysiological model [
3]. The cognitive model posits that life stress and worries disrupt sleep, leading to acute episodes of insomnia. Conversely, the physiological model emphasizes factors such as metabolic rate, heart rate, and neuroendocrine processes. Insomnia, seen as a dysregulation of the sleep system, involves mutual inhibition between sleep-promoting areas (e.g., ventrolateral preoptic nucleus and median preoptic nucleus) and wake-promoting areas (such as tuberomammillary nucleus, dorsal raphe, and locus coeruleus) in the sleep switch model [
2,
4].
The sleep switch model highlights the crucial role of the ventrolateral preoptic nucleus (VLPO) in the regulation of sleep [
4,
5,
6,
7]. Situated in the anterior hypothalamus, VLPO consists of a small group of neurons located above and to the side of the human brain’s optic chiasm. During sleep, VLPO demonstrates notably high activity, sending inhibitory signals to wake-promoting brain areas and suppressing their effects [
5]. Insomnia onset has been linked to damage in the VLPO, while its activation directly promotes sleep [
8,
9], rendering it a focal point for numerous methods in the treatment of insomnia.
Treatment options for insomnia encompass both non-pharmacological interventions rooted in cognitive-behavioral approaches and pharmacological therapy, with widespread use of benzodiazepine receptor agonists (BzRAs) [
2,
10]. BzRAs, which include both benzodiazepines (BZD) and non-benzodiazepine medications, bind to gamma-aminobutyric acid (GABAA) receptors, inducing a sedative and hypnotic effect [
10,
11]. However, concerns about side effects, drug abuse, dependence, alterations in sleep architecture, and rebound insomnia impede the broad utilization of these drugs [
11]. The quest for treatments with fewer side effects and increased effectiveness has renewed interest in an age-old therapy: transcranial direct current stimulation (tDCS) [
12].
TDCS, a non-invasive brain stimulation technique, involves placing positive (anode) and negative (cathode) electrodes on the scalp and applying a weak electrical current to modulate neural activity [
13]. Unlike inducing action potentials, tDCS modulates resting membrane potential, facilitating depolarization or hyperpolarization and altering neuronal excitability [
14]. Research suggests that tDCS has the capacity to modulate neural plasticity, generating effects akin to long-term potentiation (LTP) and long-term depression (LTD) [
15,
16,
17]. Anodal stimulation induces excitation, while cathodal stimulation produces inhibitory effects [
14,
18].
In numerous studies on tDCS, consistent emphasis is placed on the importance of appropriately configuring the conditional parameters of an effective tDCS device. These parameters include, but are not limited to:
(a) Current Intensity and Duration: Many studies suggest that the impact of current duration and intensity on outcomes is not necessarily linear [
19]. An example study indicated that excessively long stimulation times may affect the expected effects of polarity, such as anodal stimulation causing inhibition of cortical excitability or vice versa [
20].
(b) Electrode Placement: For tDCS to form a complete circuit, two electrodes are required. The target electrode (anode or cathode) is placed on the specific brain region to be modulated, while the reference or counter electrode (the one with the opposite polarity to the target electrode) is placed in the corresponding area based on the hypothesis or target configuration [
21,
22,
23]. When placing the reference electrode, it is necessary to consider the distance between the two electrodes so that the current can pass through the target brain region. If the distance between the two electrodes is too close, it may lead to a shunting effect, causing the current to dissipate through the scalp [
22]. In clinical applications, especially in the field of human psychiatric disorders, selected brain stimulation areas often include the motor cortex and dorsolateral prefrontal cortex. Some research results have shown that after stimulating these regions, patients experience significant improvements in sleep quality [
24] and related indicators such as sleep efficiency or number of awakenings [
25,
26].
(c) Current Density and Charge Density: Current density (A/m²) is the amount of electric charge passing through a unit area during a given period, while charge density (C/m²) is the multiplication of current density and the duration. In a study investigating brain tissue damage in rats following tDCS stimulation, the findings suggested that brain tissue can be harmed when both current density and charge density surpass a certain threshold [
27]. This implies that even with high current density, if the duration is very short, the impact on the tissue may be weak. Conversely, when applying extremely low current density and prolonging the duration, even with a high final charge density, it does not result in tissue damage. Factors influencing current density also include the electrode’s contact area with the skull, essentially the size of the electrode. Therefore, when setting the current intensity, adjustments should be made based on the electrode’s contact area used, rather than using the same current intensity directly. Furthermore, reducing the impedance between the electrode and the contact surface is also of significant importance [
22]. In most experiments, the primary method for ensuring conductivity involves the use of saline, while some studies employ conductive gel [
21]. However, in our experiment, the extremely low current we applied led to significant resistance when using saline as the conducting solution. This resistance prevented the formation of a complete circuit. As a result, we opted for chloride-free conductive gel. This not only ensured the flow of current but also prevented any potential chemical reactions between the electrodes and the conducting solution.
TDCS has found application in diverse psychiatric and neurological disorders, demonstrating efficacy in conditions such as major depressive disorder, drug addiction, Alzheimer’s disease, and attention deficit hyperactivity disorder (ADHD) [
28]. The dorsolateral prefrontal cortex (DLPFC), crucial for executive functions, is often the targeted region in tDCS applications [
29,
30,
31]. Despite the promise shown in psychiatric disorders, the effectiveness of tDCS in treating insomnia varies. In a study involving 19 insomnia patients, tDCS administered before sleep demonstrated no changes in sleep continuity or structure [
32]. Another trial with seven chronic insomnia patients, conducted as a one-month, double-blind, randomized, sham-controlled experiment, revealed improvements in total sleep time (TST) and sleep efficiency (SE) in the anodal stimulation group [
33], indicating potential efficacy but underscoring the necessity for further research and exploration in the realm of insomnia treatment.
Various models have been developed to simulate insomnia symptoms in animals. One approach involves inducing insomnia through drug administration, commonly using caffeine [
34]. The effects and duration of this method vary based on dosage [
35] and the animal strain [
36]. Another prevalent model is stress-induced, with stress considered the most typical cause among various factors contributing to insomnia [
37]. In humans, the “first night effect” (FNE) is a widely recognized phenomenon associated with sleeping in an unfamiliar environment for the first time. It is characterized by a reduction in total sleep duration and decreased sleep efficiency [
38]. A similar phenomenon is observed in rodents. When placed in a new environment or when their cages are changed, there is an immediate delay in both non-rapid eye movement (NREM) and rapid eye movement (REM) sleep, accompanied by a decrease in the overall duration of NREM and REM sleep [
36,
39]. The insomnia induced by cage-exchanging has been confirmed in our research, revealing an immediate acute stress response after the bedding change, followed by acute insomnia within the subsequent hours [
40].
Our study endeavors to establish a mouse tDCS model and investigate its influence on sleep and neural mechanisms. Objectives encompass the setup of the tDCS device, assessing its effects on sleep in both normal and insomnia mice, and exploring potential neural mechanisms through chemogenetic techniques. Despite the absence of a direct equivalent to the human dorsolateral prefrontal cortex (DLPFC) in mice, our hypothesis posits that tDCS can activate the infralimbic area (IL), prompting excitatory neuron conduction and subsequent activation of the ventrolateral preoptic nucleus (VLPO), thereby enhancing sleep [
41,
42,
43].
2. Materials and Methods
For our investigation, we utilized male C57BL/6 mice (20-25g; BioLASCO Taiwan Co., Ltd). The mice were housed in a temperature-controlled environment (23-24 °C) following a 12:12 light-dark cycle. Adequate food and water were available ad libitum. Post-surgery, a recovery period of at least 7 days was observed, and handling was carried out as required for experimental purposes to mitigate stress-related effects resulting from the procedures. Approval for all experimental procedures was obtained from the National Taiwan University Institutional Animal Care and Use Committee (IACUC).
2.1. Electroencephalography (EEG) and tDCS Electrodes Implantation
Anesthesia was induced through an intraperitoneal injection of a Zoletil (10 mg/kg, Carros, France) and xylazine (12 mg/kg, Sigma-Aldrich, USA) mixture. Once reflexes and muscle tension loss were confirmed in the mice, the surgical area, spanning approximately from the midpoint between the eyes to the back of the ears, was shaved. Ensuring a clear airway, the mouse’s tongue was secured, and it was affixed to the stereotaxic apparatus. Following the application of eye ointment, the surgical area underwent alternating disinfection using cotton swabs soaked in povidone-iodine and alcohol (70%), with the process repeated until the cotton swab no longer retained fine hairs.
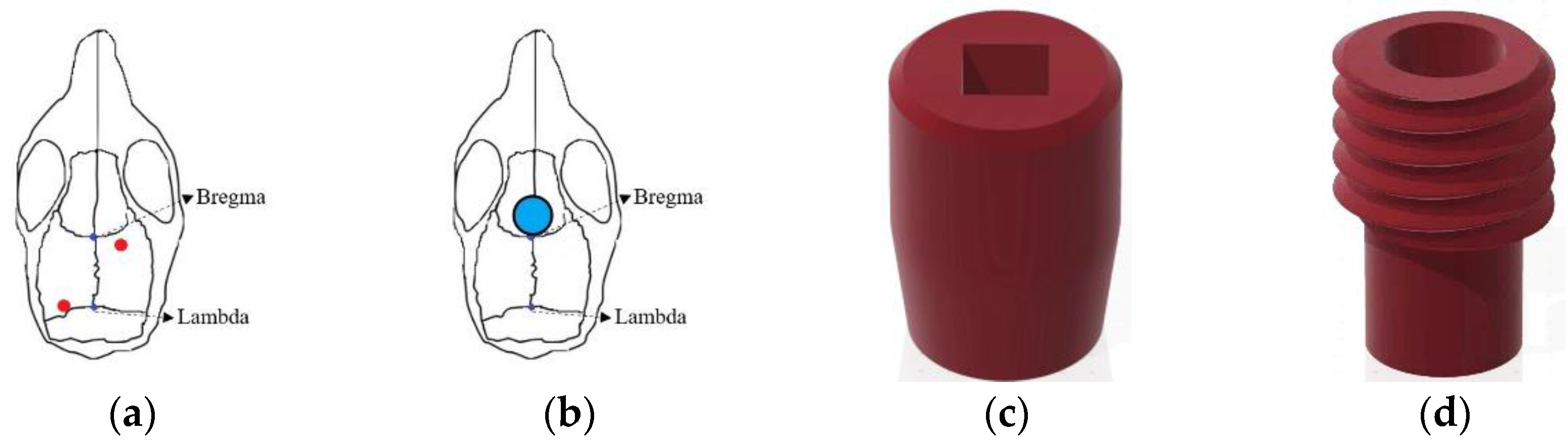
The positions of the two EEG electrodes were as follows: Anteroposterior (AP): −0.5 mm, Mediolateral (ML): +1.5 mm relative to bregma, and ML: −1.7 mm relative to lambda (
Figure 1a). After electrode implantation, bone cement (Tempron, GC Co., Tokyo, Japan) was meticulously applied drop by drop to cover the skull’s surface and the lower portion of the electrode. A hill-like structure was constructed layer by layer to ensure implant stability. Neomycin ointment was applied around the surgical site with a cotton swab to prevent infection. Following surgery, mice were individually housed, and ibuprofen (0.4 g/250 mL, Yung Shin Pharm. Ind. Co. Ltd) was added to their drinking water for pain relief. Mice rested in their cages for at least one week before subsequent experimental manipulation and recording procedures.
During the surgery for implanting EEG electrodes, the tDCS electrode was concurrently placed. The stimulating electrodes were divided into two parts: the cap (
Figure 1c) for installing the needle electrode and the implant placed on the mouse’s skull (
Figure 1d) to connect to the cap. In the subsequent text, the term “tDCS electrode” specifically refers to the implant on the mouse’s skull. Simultaneously with EEG electrode implantation, the tDCS electrode was positioned following the aforementioned steps. However, during implant fixation, an additional step involved delicately applying super glue to its base to ensure proper positioning when covered with bone cement at the surgery’s conclusion. As illustrated in
Figure 1b, the implant’s center was located at AP: +1.7 mm, relative to bregma, directly targeting the infralimbic region. The contact area between the electrode and the skull was 9.61 mm², with the capacity to hold a volume of conductive solution around 80 μL.
2.2. Microinjection of Fluorogold
While the neural circuit from the infralimbic to the VLPO has been identified in rats [
44], there is limited research on the relevant pathways in mice. To verify the existence of the presumed neural pathway, we injected fluorogold into the VLPO of mice. Fluorogold, a retrograde synaptic transport tracer, is widely employed for tracing neural system pathways [
45,
46]. After surgically exposing the mouse skull by cutting the skin, we used a drill to create an opening on the skull at coordinates AP: -0.1 mm, ML: ±0.75 mm, relative to bregma. Subsequently, we performed microinjection at coordinates AP: −0.1mm, ML: ±0.75 mm, DV: −5.6 mm, relative to bregma, injecting 200 nl of fluorogold at a rate of 1 nl/sec and allowing an 8-min retention time. Following the injection, the opening was sealed with bone cement, and the scalp was sutured using 3/0 stitches. Subsequently, the mice were returned to individual cages to rest.
2.3. Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) and Cre/DIO System
Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) are artificially modified G-protein coupled receptors (GPCRs) primarily engineered from human muscarinic receptors. These receptors respond exclusively to specific small-molecule drugs, including clozapine-N-oxide (CNO), clozapine (CLZ), and Compound 21 (Cmpd-21) [
47,
48,
49]. Commonly used DREADDs can be categorized into four main pathways of activation: Gq, Gi, Gs, and β-arrestin. Our research specifically focuses on inhibitory Gi-DREADDs, particularly hm4Di, derived from the muscarinic M4 receptor. Activation by CNO or the mentioned drugs initiates inhibitory G protein (Gi) signaling cascades, thereby suppressing neural signal transmission and excitability [
50,
51].
To achieve cell-type-specific regulation, we combined DREADDs with the Cre/DIO system. Cre (Cyclization Recombination Enzyme) is a recombinase originally discovered in the P1 bacteriophage, recognizing LoxP sites in DNA sequences and facilitating genetic recombination between two LoxP sites [
52]. DIO (Double-Floxed Inverted Open reading frame), also known as a gene switch FLEX based on LoxP sites, activates gene expression through Cre-mediated recombination. For exploring the excitatory neural pathway from the IL to VLPO, we injected pENN.AAV.CamKII.HI.GFP-Cre.WPRE.SV40 into the IL. This specific viral vector, a gift from James M. Wilson, was selected for its CamKII promoter properties, anticipating the infection and expression of GFP-Cre in excitatory neurons projecting from the IL. Paired with it is pAAV-hSyn-DIO-hM4D(Gi)-mCherry (AAVrg), a gift from Bryan Roth. This virus, injected into the VLPO, carries a Cre-dependent hM4D(Gi) receptor, allowing infection only in cells expressing Cre. Upon activation through clozapine (CLZ), it facilitates the blockade of the neural pathway projecting from the IL to VLPO.
The surgical procedure for virus injection, akin to EEG implantation, involved creating openings in the mouse’s skull. In addition to the two EEG electrode locations, four holes were made on both sides of the VLPO and both sides of the IL region specifically for virus injection. A glass capillary was employed for microinjection due to the relatively small size of the targeted brain regions. AAV-hSyn-DIO-hM4D(Gi)-mCherry (AAVrg) was injected into the ventrolateral preoptic area, and pENN.AAV.CamKII.HI.GFP-Cre.WPRE.SV40 was injected into the IL region. Injection coordinates and volumes were precisely controlled, and after completion, injection holes were filled with bone cement. Subsequently, the installation surgery for tDCS electrodes and EEG electrodes was performed, and mice were individually housed. To allow the virus to fully infect the IL-to-VLPO pathway, mice were given an eight-week rest period before commencing subsequent experimental procedures and EEG recordings.
2.4. EEG Recording and Analysis
The day before commencing EEG recording, we affixed the EEG electrodes to the mouse’s head and connected them to recording wires, allowing for acclimation to the wired condition. Subsequently, the collected EEG signals underwent a 10,000-fold amplification using a Colbourn Instruments amplifier (Lehigh Valley, PA, USA; model V75-01) and were then filtered to preserve analog signals within the 0.1 to 40 Hz range. Following this, the filtered signals were converted into digital signals at a rate of 128 Hz using a signal converter (NI PCI-6033E, National Instrument, Austin, TX, USA). The obtained signals were then displayed on a computer and manually analyzed using ICELUS® software (M. OPP, University of Colorado Boulder).
Mouse sleep stages, categorized into wakefulness, non-rapid eye movement (NREM), and rapid eye movement (REM) sleep, were delineated during the EEG analysis [
53]. Each state exhibited distinct features in peak frequency and corresponding amplitudes. Our analysis was performed in 12-s intervals, referred to as epochs, utilizing Fourier frequency transformation to extract frequency-domain features within each epoch. This methodology facilitated the capture of primary frequencies during wakefulness, NREM sleep, and REM sleep.
During wakefulness, mice exhibited a combination of delta (0.5-4.0 Hz) and theta (6.0-9.0 Hz) waves in the EEG, indicating diverse activation across brain regions. The NREM stage displayed synchronized brain activity predominantly characterized by delta waves, with this stage showing the highest amplitude among the three stages. REM sleep featured brain activity resembling wakefulness but with predominant theta waves, distinguishing it from the mixed frequencies observed during wakefulness. Using these criteria, we conducted an analysis of the percentage of each sleep stage and sleep architecture within the recording period. Furthermore, we assessed the sleep quality of mice based on delta power [
54].
2.5. Brain Slice Preparation
After concluding experimental procedures, mice were administered intraperitoneal anesthesia to induce a deep anesthetic state. Upon confirming the absence of all reflexes, a thoracotomy was performed to initiate perfusion. Initially, blood in the mouse’s body was replaced with a phosphate-buffered saline (PBS) solution. Using a butterfly needle inserted into the left ventricle, the right atrium was incised, and perfusion continued until the liquid flowing from the right atrium became transparent. Subsequently, the PBS was replaced with 4 % formalin (P6148, Sigma-Aldrich, USA), perfusing until the entire mouse body became rigid, completing the perfusion step.
After perfusion, the mouse’s brain was extracted and submerged in 4 % formalin at room temperature for one day. Subsequently, the brain was moved to a 20 % sucrose solution dissolved in 0.1 % phosphate buffer (PB) and kept at 4 °C for one day before being transferred to a 30 % sucrose solution for an additional day. This sequential process ensures thorough dehydration of the brain, preventing the formation of ice crystals and tissue damage during subsequent frozen sectioning.
After completing the dehydration process, the brain was embedded in an optimal cutting temperature (OCT) compound (Sakura Finetek USA, Inc) and preserved at -80 °C for subsequent frozen sectioning. The prepared brain tissue will be sliced into sections with a thickness of 40 micrometers. These sections will then be mounted on glass slides and examined under a fluorescent microscope. Since the viruses and neural tracers we have selected inherently fluoresce, no additional staining procedures will be conducted.
2.6. Experimental Protocols
2.6.1. Establishing a Suitable tDCS Parameters
To enhance the mouse tDCS model parameters for impacting sleep, our primary goal in the initial experiment is to ascertain a current intensity that is both safe and effective. According to Liebetanz et al., a rat lesion threshold is reported at 52400 C/m
2 [
31]. In reverse calculation, taking into account the contact area of our tDCS electrode with the skull (9.61 mm
2), we determine a maximum safe current intensity of 839.24 μA. Notably, this aligns with the customary values employed in conventional mouse studies [
23,
55].
In the preliminary assessments using a 0.2 mA current, mice displayed apparent signs of itching and discomfort. This discomfort was likely linked to the placement of the EEG electrodes, acting as the reference (cathode) electrode, situated farther from the tDCS electrode. As a response, we methodically decreased the current intensity. Eventually, at 0.06 mA or lower, mice exhibited no disruption in ongoing activities, such as foraging or sleeping, although occasional itching persisted. Consequently, we designated 0.06 mA as the configuration for testing the tDCS effect.
In anticipation of evaluating the potential effects of this current intensity on mice, one day prior to the commencement of baseline recording and stimulation, we pre-loaded the conductive gel into the tDCS implant on the mouse’s head and affixed the cover with connected wires. This procedure enabled the mice to acclimate to the weight of the cover and potential discomfort resulting from contact with the conductive gel. The tDCS settings were consistently set at a current intensity of 0.06 mA, administered for a duration of 8 min.
Our experimental procedures are illustrated in the diagram provided (
Figure 2). EEG recordings on the first and third days commence during the dark cycle and extend for a duration of 24 h, while tDCS stimulation concludes prior to the onset of the dark cycle on the third day. Initiating recording at the beginning of the dark cycle is intentional, as our hypothesis posits that mice typically experience reduced sleep during this period compared to the light cycle. This strategic choice improves our capacity to identify potential variations in sleep patterns if tDCS exerts any influence.
2.6.2. tDCS Effects on Stress-Induced Insomnia Model
In our investigation, we chose the stress-induced insomnia model for its operational simplicity and its capacity to mimic conditions akin to acute insomnia in humans. Expanding upon the insights gained from the initial experiment, where we examined the influence of anodal stimulation at a current intensity of 0.06 mA for 8 min on sleep, our objective was to explore whether these parameters could potentially have a therapeutic impact on insomnia in mice.
For this experiment, EEG recordings commenced at the beginning of the light cycle. After recording baseline EEG values, mice were divided into two groups. The first group constituted the insomnia group, where a mouse was transferred to a cage already occupied by another mouse a week prior to the initiation of EEG recording. The introduction of the other mouse’s odor and the unavoidable nature of the new environment triggered an immediate acute stress response phase in the relocated mouse, resulting in acute insomnia over the subsequent hours. In contrast, the second group, the treatment group, underwent tDCS therapy in the modified environment following the cage change. The comprehensive process is illustrated in the diagram below (
Figure 3).
2.6.3. Validate the Projection from IL to VLPO
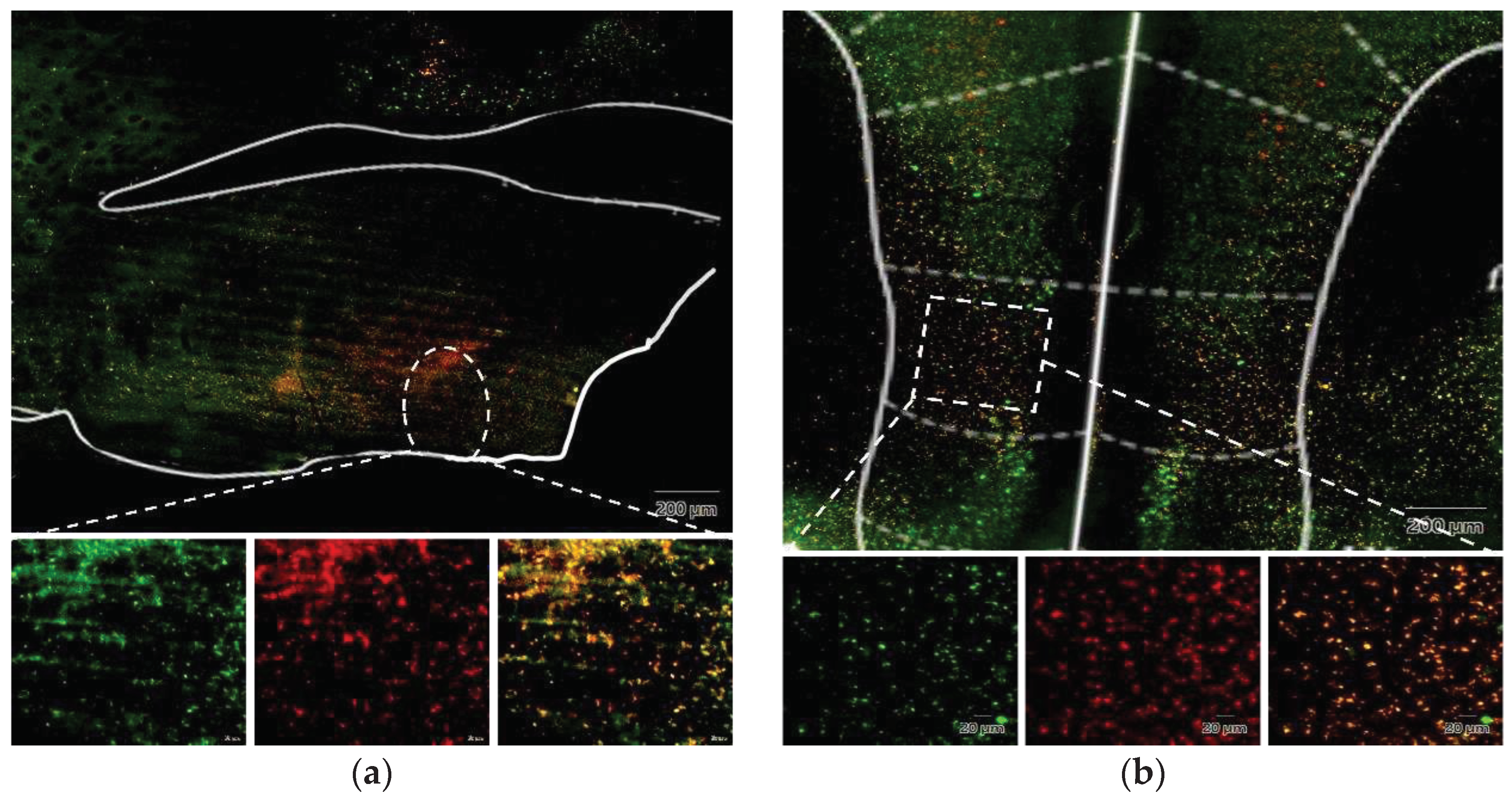
As mentioned earlier, the pathway from the IL to the VLPO has not been thoroughly investigated. Thus, the aim of this experiment is to validate the existence of this pathway in the mouse brain. The methodology included injecting the retrograde tracer Fluorogold into the VLPO. After an 8-week post-surgery period, mice were euthanized, and their brains were meticulously dissected, sectioned, and mounted on slides. Subsequently, using a fluorescent microscope, we scrutinized whether the Fluorogold tracer retrogradely travels from the VLPO to the IL brain region.
2.6.4. Determine the Effect of tDCS on Stress-Induced Insomnia Is Mediated by the IL-VLPO Pathway
To delve deeper into the potential contribution of the IL-to-VLPO pathway to tDCS-induced improvements in sleep, we utilized chemogenetic modulation of this circuit. After surgery, an 8-week period was allotted to ensure comprehensive viral infection along this pathway. The activation of hM4D(Gi) was initiated by injecting Clozapine, leading to the inhibition of excitatory neurons from the IL to VLPO. If the mechanism by which tDCS enhances sleep aligns with our hypothesis, the impact of tDCS should be hindered when this pathway is suppressed.
Between the surgery and the actual recording sessions, we implemented daily handling sessions to habituate the mice to the forthcoming Clozapine injection procedure. The day preceding the formal recording, we connected the EEG to the amplifier, filled the tDCS implant with conductive gel, and affixed the cap to acquaint them with the upcoming procedures and recording. Baseline sleep states were then recorded on the first day.
On the third day, mice underwent three distinct procedures in a randomized order:
1. The first group received an intraperitoneal injection of saline. After a 15-min interval, the mouse was relocated to a cage already occupied by another mouse to induce insomnia. EEG recording commenced after tDCS stimulation.
2. The second group received an intraperitoneal injection of clozapine (clz, 0.4mg/kg) to inhibit the activation of the IL to VLPO pathway [
49]. Fifteen minutes after the intraperitoneal injection, we conducted cage exchanging and initiated tDCS stimulation with parameters consistent with the previous settings. EEG recording began after completing the stimulation.
3. The third group received an intraperitoneal injection of saline. After a 15-min interval, they underwent cage exchanging, followed by EEG recording.
In this experiment, each mouse experienced a minimum one-week interval between different procedures to safeguard against potential influences from preceding cage-exchanging operations and tDCS effects on subsequent experiments. The initiation of all EEG recordings occurred during the light cycle, and all specified procedures were concluded before transitioning from the dark cycle to the light cycle. The detailed procedure is depicted in the figure below (
Figure 4).
2.7. Statistical Analysis
The statistical analysis was carried out using SPSS version 25.0 (IBM, SPSS, Armonk, NY, USA). In the first experiment, paired t-tests were employed to assess differences in sleep proportions and sleep structure before and after administering tDCS to mice. For the second and third experiments, a mixed-model repeated measures ANOVA was utilized, complemented by Bonferroni post-hoc comparisons, to evaluate the interaction between different experimental groups and the time factor. Sleep proportion differences between groups at specific time points, sleep percentages during particular periods, and sleep structure were subjected to statistical analysis using one-way ANOVA with Bonferroni post-hoc tests.
4. Discussion
This study investigates the impact of tDCS on stress-induced insomnia through three experimental phases. Additionally, it delves into the role of the neural pathway from the IL to the VLPO. The selected current intensity (0.06 mA) for tDCS was deliberately lower than commonly employed in animal studies [
21]. This decision stemmed from two primary considerations. Firstly, the impact of tDCS does not demonstrate a linear correlation with an escalation in current intensity. Secondly, elevated currents have the potential to cause discomfort in animals, manifesting as itching or disruption of ongoing activities. Notably, the choice of current intensity was also influenced by the placement of the reference electrode; in this study, we opted for EEG placement above the cerebellum as opposed to methods like chest patching.
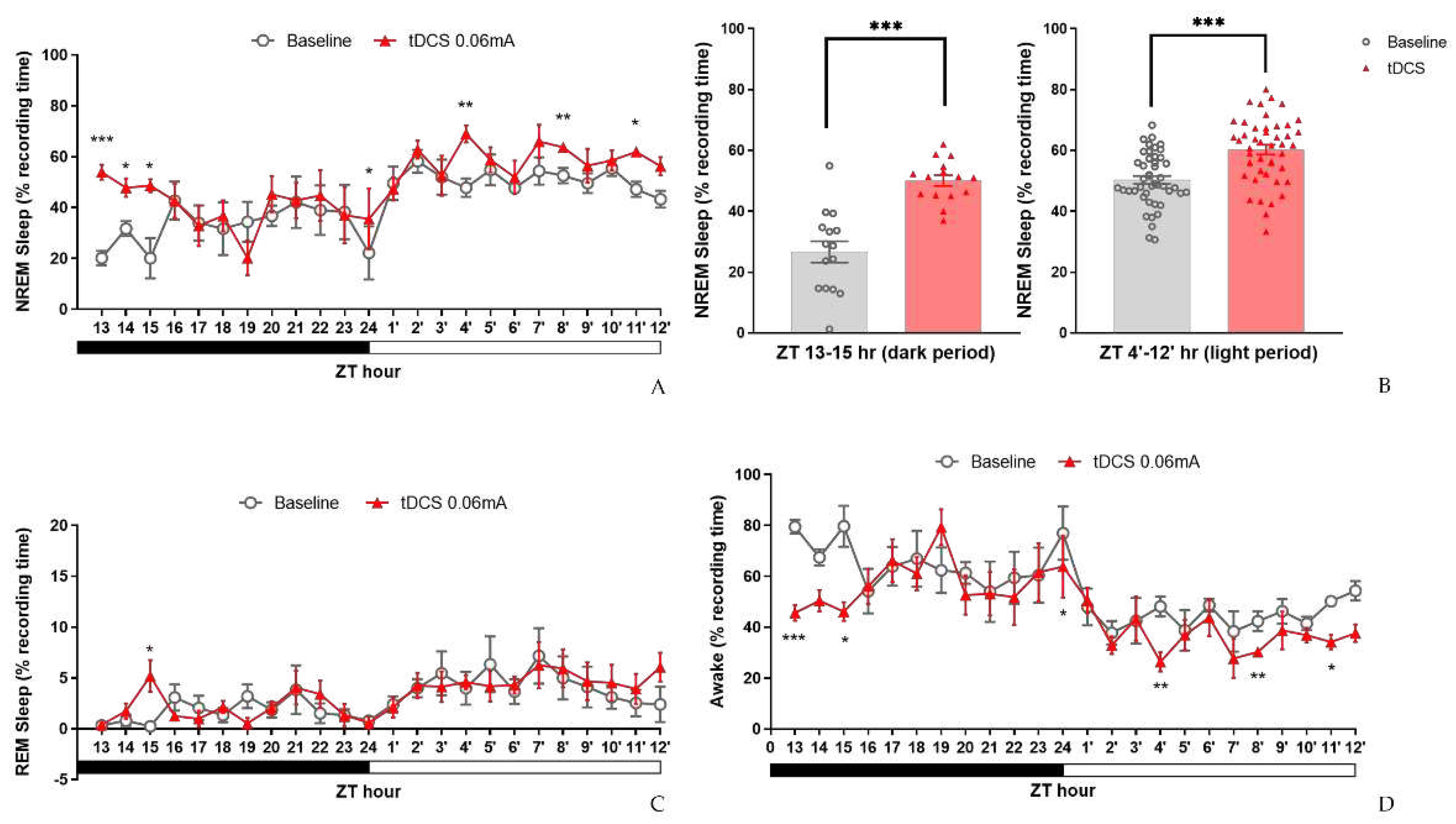
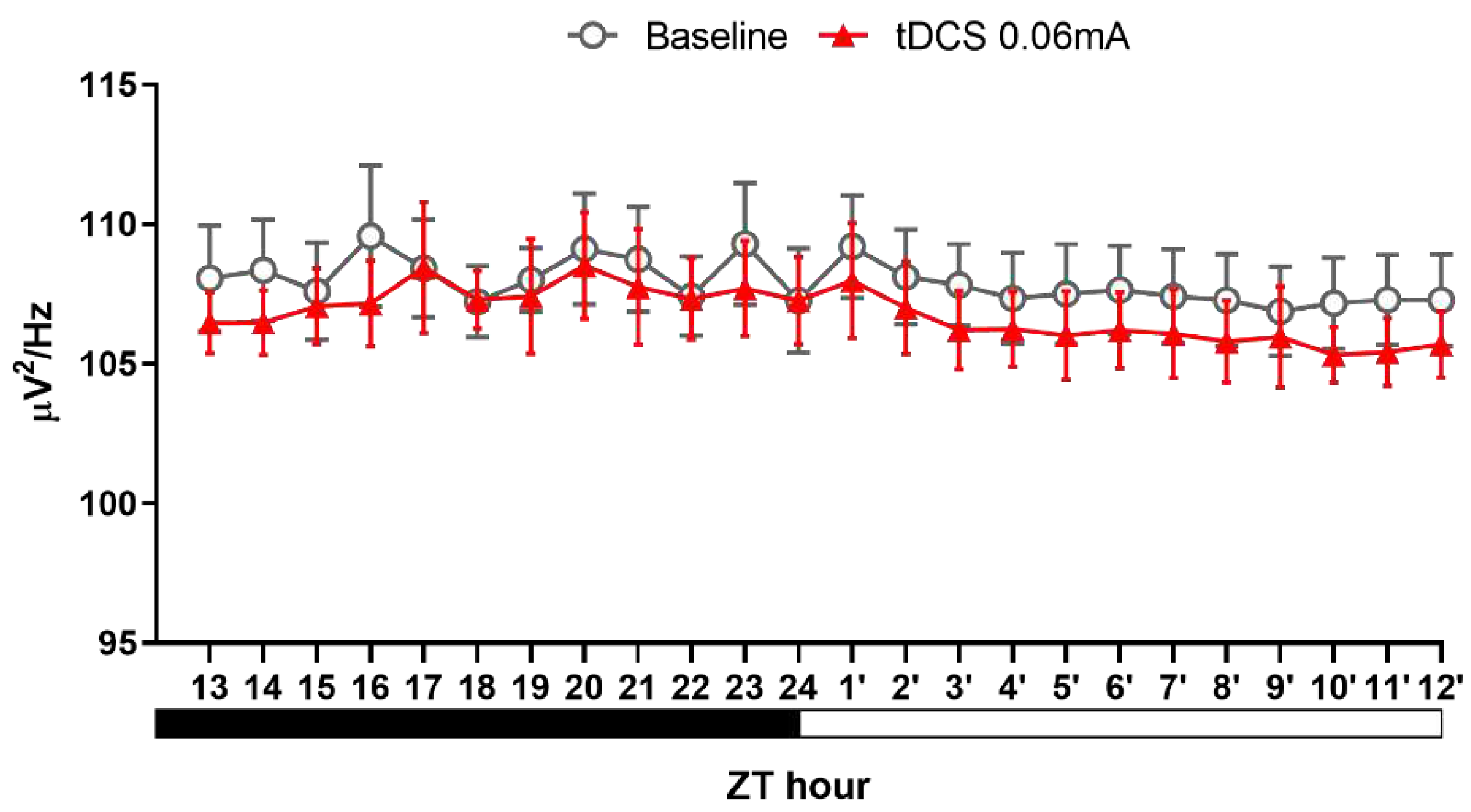
The outcomes of our experiment revealed a noteworthy augmentation in the percentage of NREM sleep for both naïve and stress-induced insomnia mice. This consistent trend across both experiments provides preliminary confirmation of the potential efficacy of tDCS in promoting sleep. Notably, tDCS exhibited an influence on REM sleep, particularly in the post-stimulation hours, where there was a significant upsurge in the percentage of REM sleep. Crucially, the durability of these effects for over 12 h post-stimulation suggests that the mechanism of tDCS in promoting sleep may encompass not only immediate impacts but also the modulation of neural plasticity, leading to more enduring effects [
56,
57].
In the stress-induced insomnia mice, changes in NREM sleep were delineated into two stages: the acute stress response stage and the acute insomnia stage. In both stages, the ameliorative effect of tDCS on NREM sleep was corroborated, primarily characterized by an increase in sleep duration rather than alterations in depth. Conversely, REM sleep exhibited a gradual rise in its proportion within the initial 3-4 h post-electrical stimulation, followed by a lack of significant effects. In summary, these findings underscore the potential effectiveness of tDCS as a non-pharmacological intervention for sleep, presenting promising prospects for clinical applications.
We substantiated the existence of the IL to VLPO projection pathway in the mouse brain using Fluorogold labeling. This validation revealed not only the anticipated upper edge region but also additional areas, laying a robust foundation for future investigations into modulating tDCS effects through chemical interventions within this pathway. While IL is typically associated with behavioral regulation, such as fear conditioning [
58], reward processing [
59], and habit learning [
60], we selected this pathway due to the widespread use of the DLPFC in human tDCS studies, despite the lack of a direct equivalent in mice [
42,
43]. Some studies suggest that the prelimbic and infralimbic areas in rodents may serve as a model for the human DLPFC [
41].
Experimental results inhibiting the IL to VLPO projection pathway indicated that tDCS could enhance NREM sleep even when the pathway was suppressed during the ZT8-10 period. However, a certain degree of attenuation was observed at the initial time points (ZT1-2). The limited inhibitory effects may be associated with the duration of clozapine, as studies propose that clozapine reaches peak blood concentration 15 min after intraperitoneal injection, rapidly decreasing thereafter [
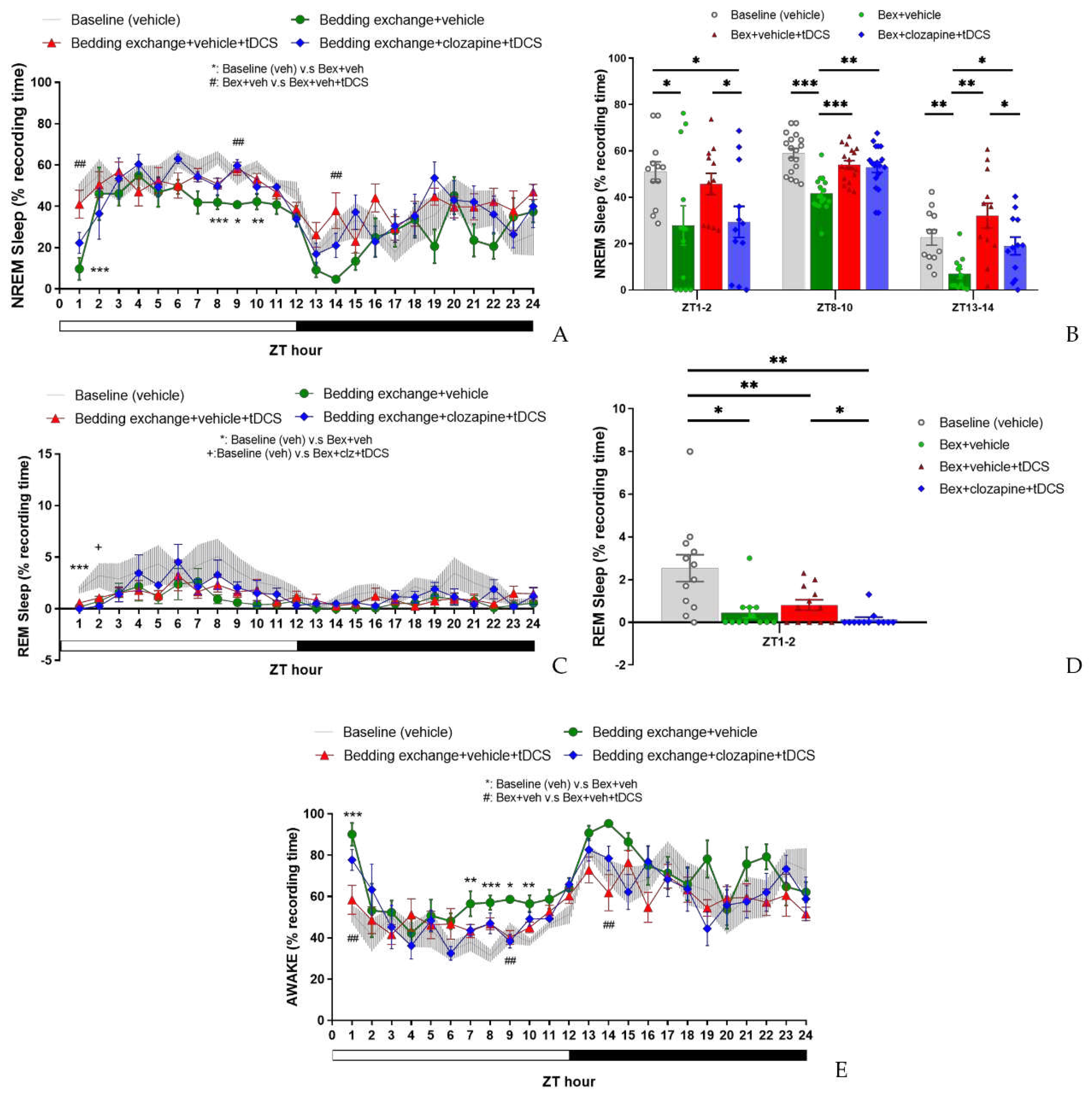
49]. Consequently, tDCS initiation 15 min after clozapine administration aimed to synchronize the interventions. Nonetheless, the rapid metabolism of clozapine might hinder sustained inhibition of the after-effects post-tDCS. This could elucidate the significantly lower NREM sleep in the inhibited pathway group compared to the non-inhibited group during the initial acute stress response period (ZT1-2), with no statistical difference during the subsequent acute insomnia period (ZT8-10). In summary, these experimental results suggest that the IL to VLPO projection pathway plays a modulating role in the sleep improvement induced by tDCS, particularly concerning NREM sleep.
A distinctive aspect in the third experiment was the absence of changes in REM sleep in the initial hours, irrespective of pathway inhibition. Across all experimental groups, no statistical differences in REM proportions were observed after ZT3. This variability could be attributed to individual differences among mouse groups, akin to the inconsistency in the sleep decline period exhibited by the Bex group in experiments two and three.
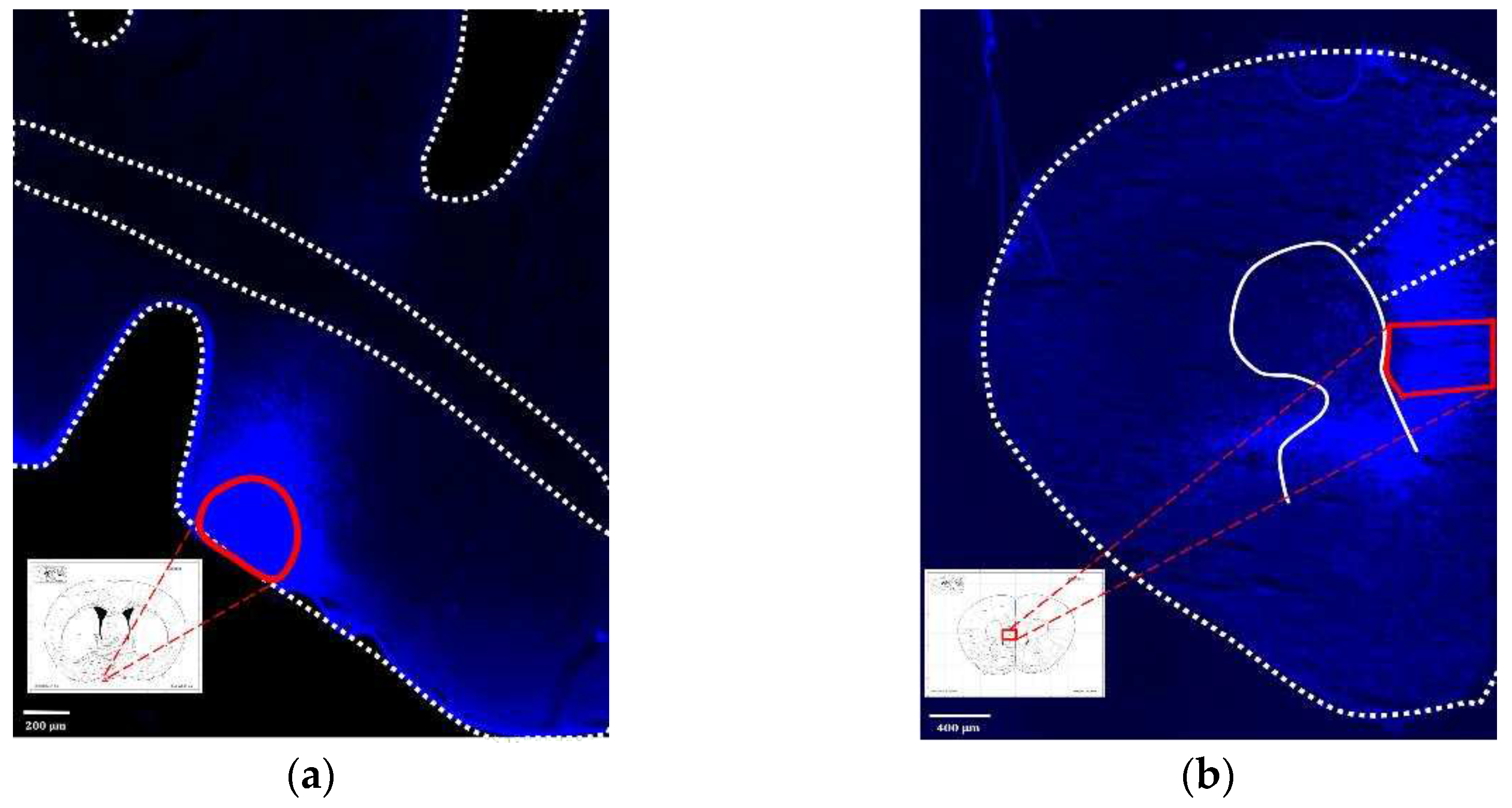
Figure 1.
Schematic diagram of (a) EEG electrodes position; (b) tDCS implant location; (c) tDCS electrode—cap part; (d) tDCS electrode—implant part.
Figure 1.
Schematic diagram of (a) EEG electrodes position; (b) tDCS implant location; (c) tDCS electrode—cap part; (d) tDCS electrode—implant part.
Figure 2.
Experimental protocol for testing the effect of tDCS.
Figure 2.
Experimental protocol for testing the effect of tDCS.
Figure 3.
Experimental protocols for determining tDCS effects on stress-induced insomnia mice.
Figure 3.
Experimental protocols for determining tDCS effects on stress-induced insomnia mice.
Figure 4.
Experimental protocols for modulating the IL-VLPO pathway to assess the impact of tDCS on stress-induced insomnia.
Figure 4.
Experimental protocols for modulating the IL-VLPO pathway to assess the impact of tDCS on stress-induced insomnia.
Figure 5.
Brain tissue sections positioned directly beneath the stimulation electrode.
Figure 5.
Brain tissue sections positioned directly beneath the stimulation electrode.
Figure 7.
NREM delta power after tDCS treatment in naïve mice.
Figure 7.
NREM delta power after tDCS treatment in naïve mice.
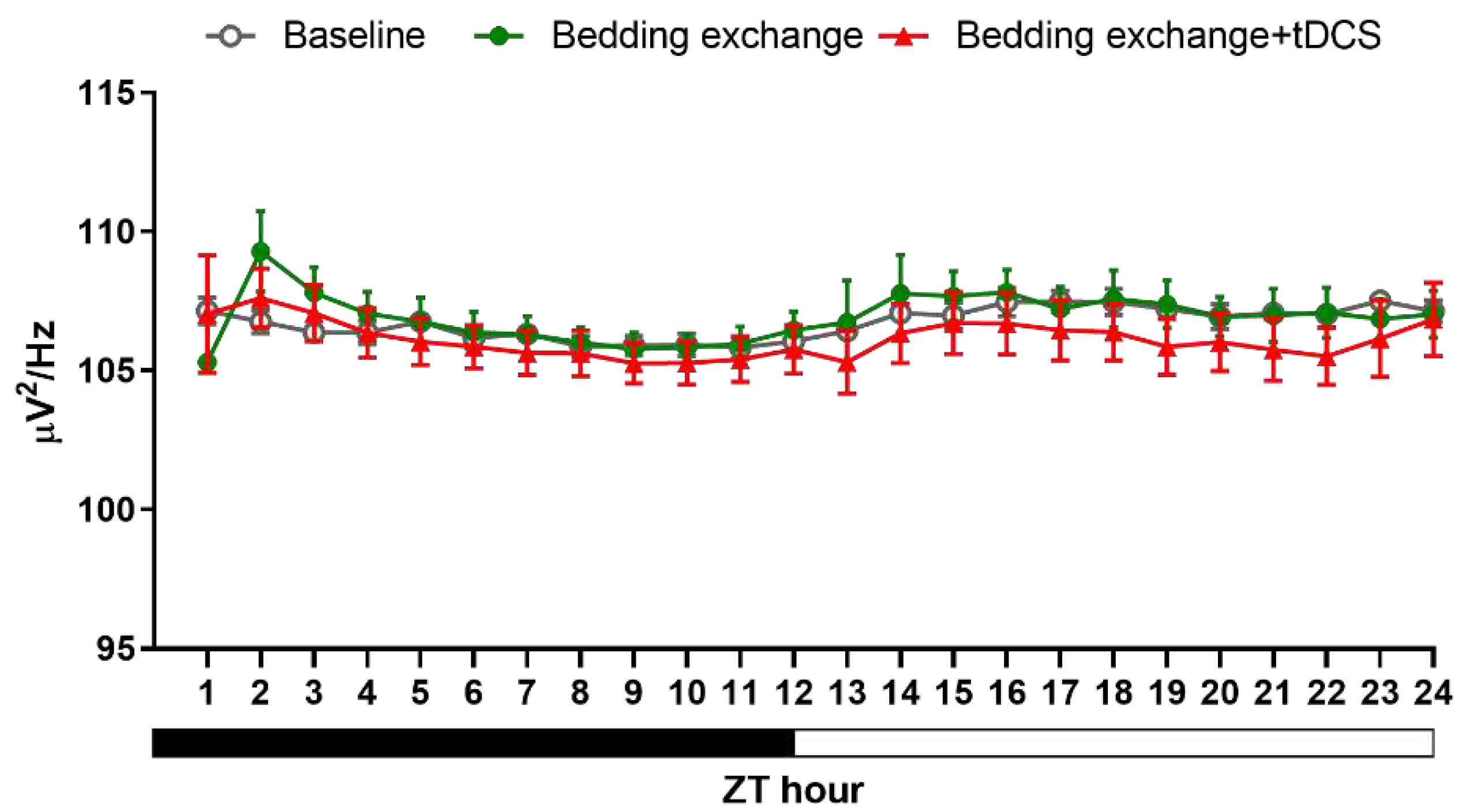
Figure 8.
Effects of tDCS on sleep in stress-induced insomnia mice. (A) The decrease of NREM sleep in mice caused by bedding exchange primarily occurs in the first 12 h, distinguishing between the acute stress response phase (ZT1-2) and the subsequent acute insomnia phase (ZT10-12). When comparing with Bex group, the group treated with tDCS significantly enhances NREM sleep in both time periods; (B) During the ZT1-2, ZT10-12, and ZT14-16 periods, the Bex group consistently exhibited significantly lower NREM sleep compared to the baseline group. Moreover, the Bex+tDCS group demonstrated a reversal of insomnia effects across all time intervals.; (C) After bedding exchange, mice exhibited a significant decrease of REM sleep during the first two hours, with a trend of overall lower percentages compared to baseline in subsequent time points. In the tDCS treated group, no statistical difference from the Bex group was observed during the first two hours, but a significant increase occurred at ZT4; (D) During ZT1-2, the Bex group showed a lower percentage of REM sleep, and there was no reversal effect after the tDCS administration. At ZT4, the tDCS group exhibited a higher REM sleep percentage compared to both the Bex and baseline groups; (E) We noticed a notable rise in wakefulness percentage for the Bex group during the ZT1-2 and ZT10-12 periods compared to the baseline, indicative of acute stress response and acute insomnia phases. Conversely, in the Bex+tDCS group, a significant reduction in wakefulness percentage was evident at ZT2, lasting until the fourth hour. Additionally, at ZT12, the Bex+tDCS group demonstrated a significant decrease in wakefulness percentage compared to the Bex group. (*P < 0.05, **P < 0.01, ***P < 0.001 vs Baseline, #P < 0.05, ##P < 0.01 vs Bex; Repeated-measures ANOVA followed by the Bonferroni test). Data are presented as the mean ± SEM. The sample sizes for the baseline, Bex, and Bex+tDCS groups are 11, 6, and 6, respectively; ZT: zeitgeber time.
Figure 8.
Effects of tDCS on sleep in stress-induced insomnia mice. (A) The decrease of NREM sleep in mice caused by bedding exchange primarily occurs in the first 12 h, distinguishing between the acute stress response phase (ZT1-2) and the subsequent acute insomnia phase (ZT10-12). When comparing with Bex group, the group treated with tDCS significantly enhances NREM sleep in both time periods; (B) During the ZT1-2, ZT10-12, and ZT14-16 periods, the Bex group consistently exhibited significantly lower NREM sleep compared to the baseline group. Moreover, the Bex+tDCS group demonstrated a reversal of insomnia effects across all time intervals.; (C) After bedding exchange, mice exhibited a significant decrease of REM sleep during the first two hours, with a trend of overall lower percentages compared to baseline in subsequent time points. In the tDCS treated group, no statistical difference from the Bex group was observed during the first two hours, but a significant increase occurred at ZT4; (D) During ZT1-2, the Bex group showed a lower percentage of REM sleep, and there was no reversal effect after the tDCS administration. At ZT4, the tDCS group exhibited a higher REM sleep percentage compared to both the Bex and baseline groups; (E) We noticed a notable rise in wakefulness percentage for the Bex group during the ZT1-2 and ZT10-12 periods compared to the baseline, indicative of acute stress response and acute insomnia phases. Conversely, in the Bex+tDCS group, a significant reduction in wakefulness percentage was evident at ZT2, lasting until the fourth hour. Additionally, at ZT12, the Bex+tDCS group demonstrated a significant decrease in wakefulness percentage compared to the Bex group. (*P < 0.05, **P < 0.01, ***P < 0.001 vs Baseline, #P < 0.05, ##P < 0.01 vs Bex; Repeated-measures ANOVA followed by the Bonferroni test). Data are presented as the mean ± SEM. The sample sizes for the baseline, Bex, and Bex+tDCS groups are 11, 6, and 6, respectively; ZT: zeitgeber time.
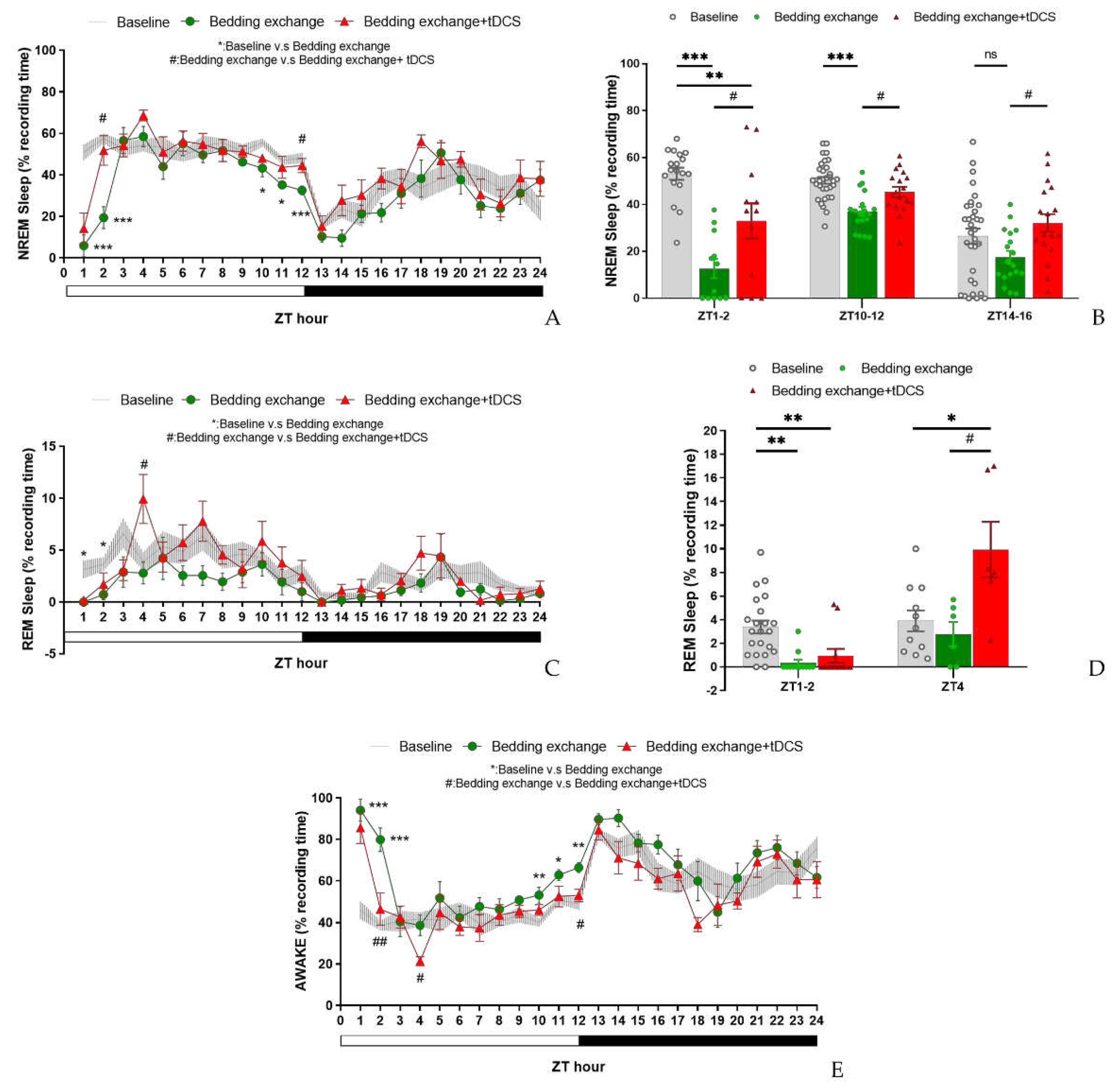
Figure 9.
NREM delta power between different groups in second experiment.
Figure 9.
NREM delta power between different groups in second experiment.
Figure 10.
(a) Injection site of fluorogold, VLPO; (b) The neural tracer retrogradely traveled to the IL and its surrounding brain regions.
Figure 10.
(a) Injection site of fluorogold, VLPO; (b) The neural tracer retrogradely traveled to the IL and its surrounding brain regions.
Figure 12.
NREM delta power obtained from the experiment 3.
Figure 12.
NREM delta power obtained from the experiment 3.
Table 1.
Sleep architecture obtained from the experiment 1.
Table 1.
Sleep architecture obtained from the experiment 1.
| Group |
ZT
Hour
|
L:D cycle |
bout number |
bout duration |
| NREM |
WAKE |
REM |
NREM |
WAKE |
REM |
| Control |
13-24 |
D |
9.5±2.0 |
6.7±1.5 |
1.1±0.2 |
2.1±0.4 |
10.2±3.7 |
0.5±0.1 |
| tDCS |
13-24 |
D |
8.4±1.4 |
5.9±0.7 |
1.1±0.1 |
3.0±0.2*
|
8.0±2.2 |
0.6±0.1 |
| Control |
1′-12′ |
L |
14.3±2.0 |
9.7±1.3 |
1.9±0.5 |
2.4±0.4 |
2.9±0.5 |
1.0±0.2 |
| tDCS |
1′-12′ |
L |
12.3±1.3 |
8.2±0.9 |
2.1±0.2 |
3.2±0.4 |
2.7±0.3 |
1.2±0.2 |
| Control |
13-15 |
D |
8.3±1.7 |
5.9±1.3 |
0.3±0.1 |
1.6±0.1 |
12.0±3.8 |
0.3±0.1 |
| tDCS |
13-15 |
D |
9.6±1.6 |
6.1±0.8 |
1.3±0.2*
|
3.8±0.7*
|
4.5±0.6 |
0.7±0.1 |
| Control |
4′-12′ |
L |
15.3±2.0 |
10.4±1.4 |
1.8±0.6 |
2.1±0.3 |
2.6±0.4 |
1.0±0.2 |
| tDCS |
4′-12′ |
L |
13.2±1.2 |
8.5±0.9 |
2.2±0.1 |
3.0±0.3*
|
2.3±0.2 |
1.3±0.2 |
Table 2.
Sleep architecture obtained from the experiment 2.
Table 2.
Sleep architecture obtained from the experiment 2.
| Group |
ZT
Hour
|
L:D
cycle
|
bout number |
bout duration |
| NREM |
WAKE |
REM |
NREM |
WAKE |
REM |
| Control |
1-12 |
L |
13.8±1.4 |
9.5±0.9 |
1.9±0.2 |
2.6±0.2 |
3.1±0.4 |
1.0±0.1 |
| Bex |
1-12 |
L |
11.2±0.9 |
7.9±0.5 |
1.2±0.3 |
2.2±0.2 |
8.7±1.3**
|
0.6±0.2 |
| Bex+tDCS |
1-12 |
L |
13.4±1.3 |
8.5±0.9 |
1.9±0.4 |
2.3±0.2 |
5.6±1.4 |
1.0±0.2 |
| Control |
13-24 |
D |
8.6±1.2 |
6.3±0.9 |
1.0±0.1 |
2.2±0.2 |
12.2±3.1 |
0.6±0.1 |
| Bex |
13-24 |
D |
7.8±1.0 |
5.5±0.8 |
0.6±0.1*
|
2.2±0.3 |
12.3±2.1 |
0.4±0.1 |
| Bex+tDCS |
13-24 |
D |
9.4±1.3 |
6.6±0.9 |
0.9±0.1 |
2.3±0.2 |
10.1±3.1 |
0.6±0.1 |
| Control |
1-2 |
L |
12.9±1.9 |
7.9±0.9 |
2.0±0.2 |
2.9±0.3 |
3.3±0.5 |
1.0±0.1 |
| Bex |
1-2 |
L |
2.2±0.7*
|
1.8±0.5**
|
0.3±0.2***
|
1.9±0.6 |
34.5±7.0***
|
0.1±0.1**
|
| Bex+tDCS |
1-2 |
L |
7.5±1.8 |
5.6±1.6 |
0.4±0.3***
|
2.0±0.2 |
19.2±7.0 |
0.3±0.2**
|
| Control |
4 |
L |
14.7±1.4 |
11.2±1.0 |
2.2±0.4 |
2.3±0.3 |
2.2±0.3 |
0.9±0.1 |
| Bex |
4 |
L |
11.3±1.5 |
7.8±0.9 |
1.8±0.7 |
3.2±0.4 |
2.7±0.6 |
0.6±0.2 |
| Bex+tDCS |
4 |
L |
14.8±1.9 |
7.7±0.7 |
4.5±0.9+
|
2.9±0.4 |
1.1±0.2+
|
1.4±0.2+
|
| Control |
10-12 |
L |
15.0±1.3 |
10.8±0.8 |
1.3±0.3 |
2.1±0.2 |
2.6±0.3 |
0.7±0.2 |
| Bex |
10-12 |
L |
13.2±0.9 |
9.7±0.6 |
1.1±0.4 |
1.6±0.1 |
3.9±0.3*
|
0.6±0.2 |
| Bex+tDCS |
10-12 |
L |
15.9±1.7 |
10.5±1.0 |
1.6±0.5 |
1.8±0.1 |
2.9±0.4 |
1.0±0.3 |
| Control |
14-16 |
D |
7.6±1.4 |
5.3±0.9 |
0.8±0.2 |
1.8±0.2 |
15.3±4.7 |
0.5±0.1 |
| Bex |
14-16 |
D |
4.7±1.2 |
3.2±0.8 |
0.4±0.1 |
2.4±0.4 |
18.2±2.9 |
0.2±0.1 |
| Bex+tDCS |
14-16 |
D |
8.5±1.8 |
5.6±1.0 |
0.6±0.3 |
2.5±0.2 |
9.3±2.5 |
0.5±0.3 |
Table 3.
Sleep architecture obtained from the experiment 3.
Table 3.
Sleep architecture obtained from the experiment 3.
| Group |
ZT
Hour
|
L:D
cycle
|
bout number |
Bout duration |
| NREM |
WAKE |
REM |
NREM |
WAKE |
REM |
| Control |
1-12 |
L |
11.8±0.6 |
7.6±0.3 |
1.9±0.6 |
2.9±0.2 |
3.4±0.3**
|
0.7±0.1 |
| Bex+veh |
1-12 |
L |
11.5±0.4 |
8.5±0.3 |
0.9±0.4 |
2.3±0.2 |
8.3±1.2 |
0.4±0.2 |
| Bex+veh+tDCS |
1-12 |
L |
14.3±1.3 |
10.3±1.1 |
1.3±0.3 |
2.2±0.2 |
3.2±0.4**
|
0.4±0.1 |
| Bex+clz+tDCS |
1-12 |
L |
12.0±0.8 |
8.4±0.7 |
1.1±0.2 |
2.6±0.3 |
4.8±1.0*
|
0.6±0.1 |
| Control |
13-24 |
D |
6.8±0.6 |
5.2±0.4 |
1.0±0.2 |
2.6±0.2 |
11.2±1.7 |
0.4±0.1 |
| Bex+veh |
13-24 |
D |
5.7±1.1 |
4.4±0.7 |
0.3±0.1 |
2.4±0.3 |
16.5±2.9 |
0.1±0.1 |
| Bex+veh+tDCS |
13-24 |
D |
10.4±0.6*
|
7.6±0.6*
|
0.7±0.2 |
2.1±0.2 |
5.6±0.6*
|
0.3±0.1 |
| Bex+clz+tDCS |
13-24 |
D |
7.2±1.4 |
5.5±0.7 |
0.7±0.2 |
2.7±0.2 |
10.9±2.2 |
0.3±0.1 |
| Control |
1-2 |
L |
9.3±1.1*
|
5.9±0.7 |
1.9±0.5**
|
3.4±0.3 |
4.6±0.7**
|
0.7±0.1 |
| Bex+veh |
1-2 |
L |
3.5±1.0 |
2.7±0.7 |
0.3±0.2 |
3.0±0.9 |
30.5±8.1 |
0.2±0.1 |
| Bex+veh+tDCS |
1-2 |
L |
12.0±1.7**
|
9.2±1.1***
|
0.8±0.3 |
2.2±0.2 |
4.2±0.8**
|
0.4±0.1 |
| Bex+clz+tDCS |
1-2 |
L |
5.8±1.0#
|
5.4±0.9#
|
0.2±0.2++
|
2.9±0.8 |
13.1±5.7 |
0.0±0.0++
|
| Control |
8-10 |
L |
13.6±1.1 |
8.5±0.5##
|
2.3±0.7 |
2.8±0.3**
|
2.4±0.2 |
0.7±0.2 |
| Bex+veh |
8-10 |
L |
15.2±1.0 |
11.5±0.3 |
0.7±0.2 |
1.6±0.2 |
3.1±0.2 |
0.4±0.1 |
| Bex+veh+tDCS |
8-10 |
L |
17.7±1.4 |
13.2±1.3 |
1.4±0.2 |
1.9±0.2+
|
2.0±0.2**
|
0.6±0.2 |
| Bex+clz+tDCS |
8-10 |
L |
14.9±0.7 |
9.6±0.3##
|
1.2±0.4 |
2.2±0.1 |
2.6±0.2 |
0.8±0.3 |
| Control |
13-14 |
D |
6.6±0.9 |
4.4±0.9 |
0.3±0.2 |
2.0±0.3 |
12.4±2.3 |
0.2±0.1 |
| Bex+veh |
13-14 |
D |
1.8±0.4 |
1.8±0.3 |
0.0±0.0 |
1.8±0.5 |
28.6±5.1 |
0.0±0.0 |
| Bex+veh+tDCS |
13-14 |
D |
8.9±1.6**
|
6.3±0.8**
|
0.5±0.3 |
1.9±0.3 |
7.7±2.6*
|
0.2±0.1 |
| Bex+clz+tDCS |
13-14 |
D |
6.0±1.7 |
4.4±1.0 |
0.4±0.2 |
1.7±0.4 |
16.2±5.3 |
0.3±0.1 |