Submitted:
12 January 2024
Posted:
12 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Bitter Taste Receptors and Polyphenols
2.1. Bitter Taste Receptors Expressed Extra-Oral Cavity and GI Hormones.
2.2. Interactions between Bitter Taste Receptors and Polyphenols
3. Astringent Taste Receptors and Polyphenols
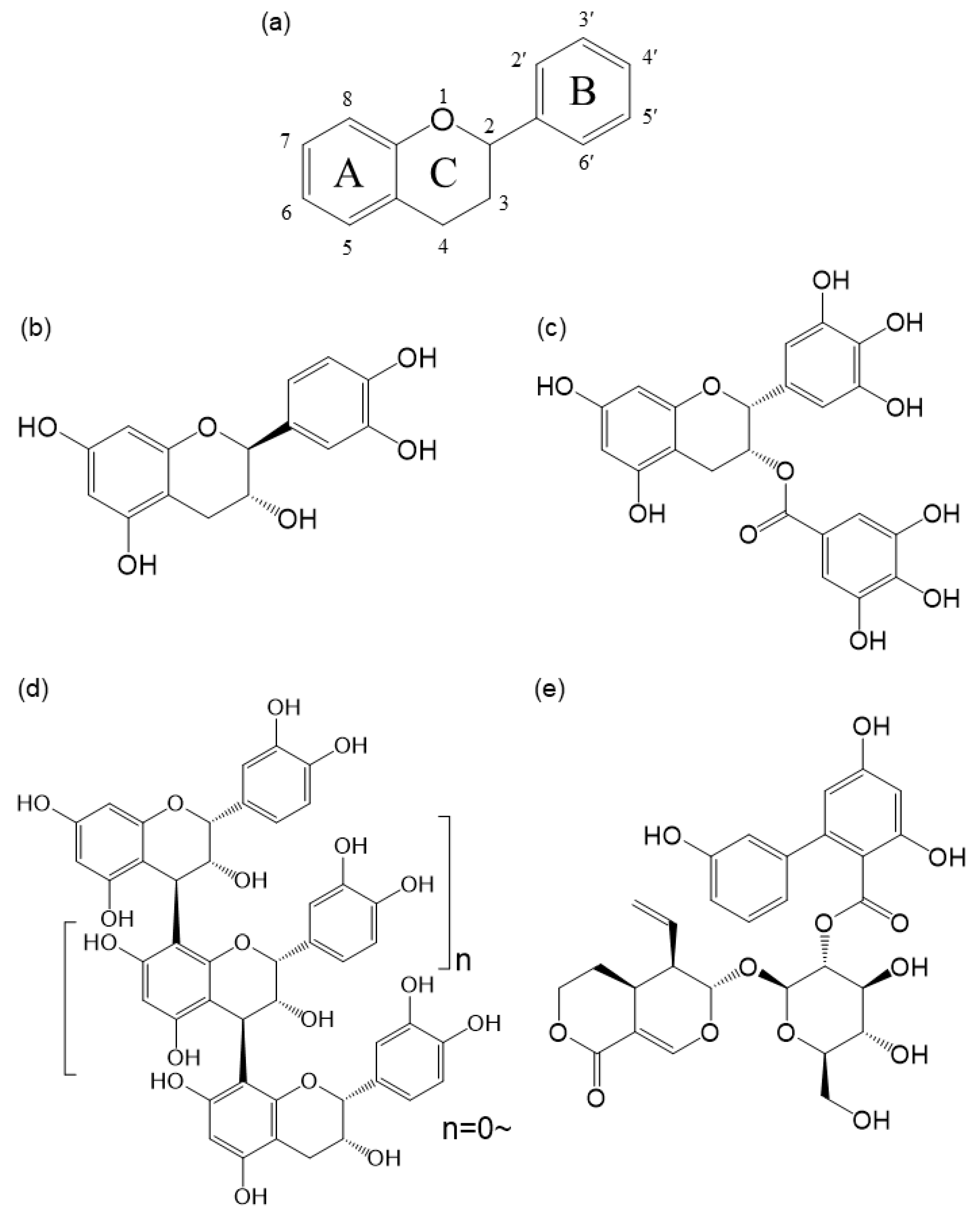
3.1. Mechanisms of Astringent Taste Perception
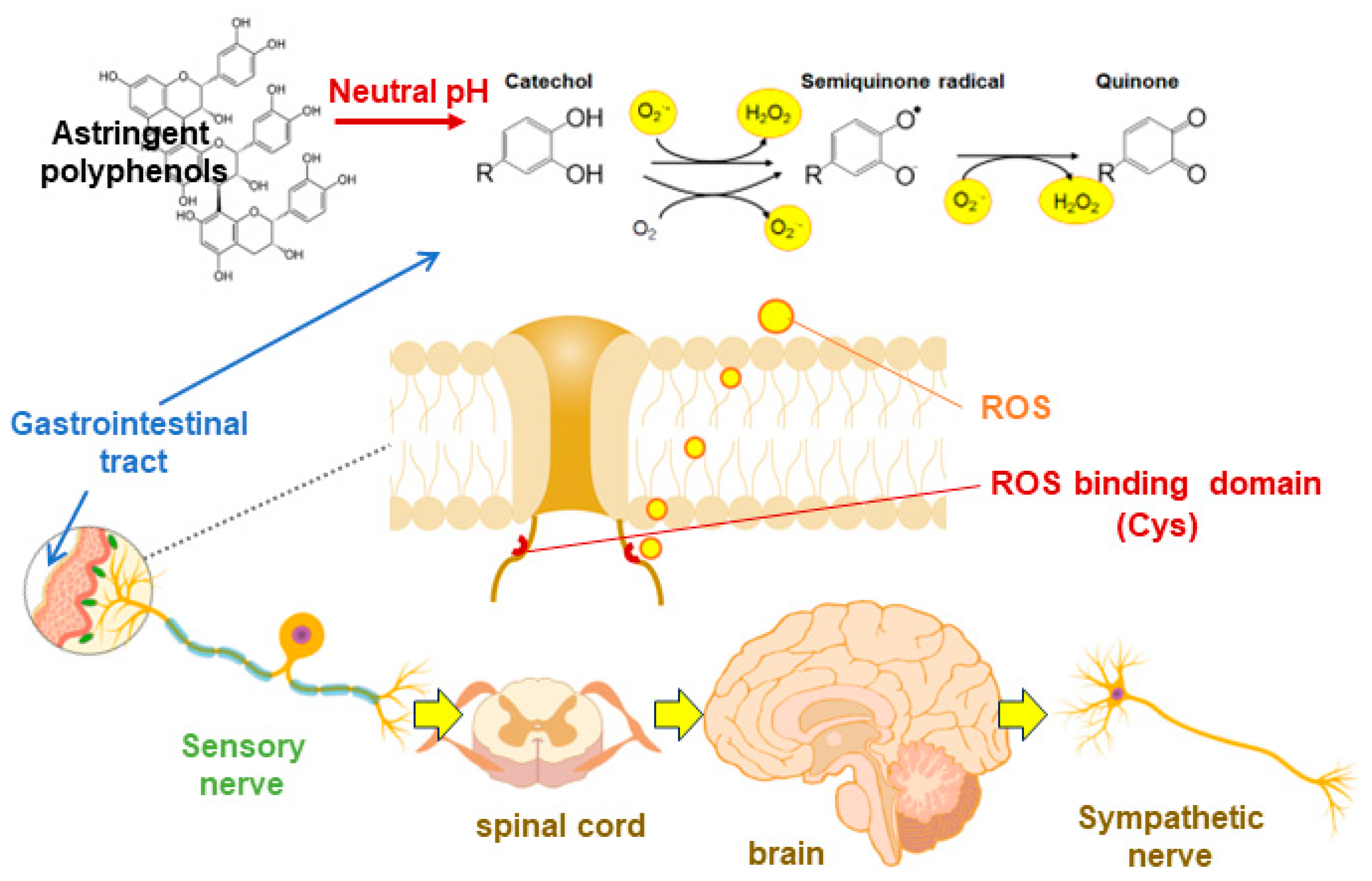
3.2. Astringent Polyphenols and Stress Response
3.3. Astringent Polyphenols and TRP Channels
4. Bioavailability of Polyphenols
5. Biological Regulation through Bitter and Astringency of Polyphenols
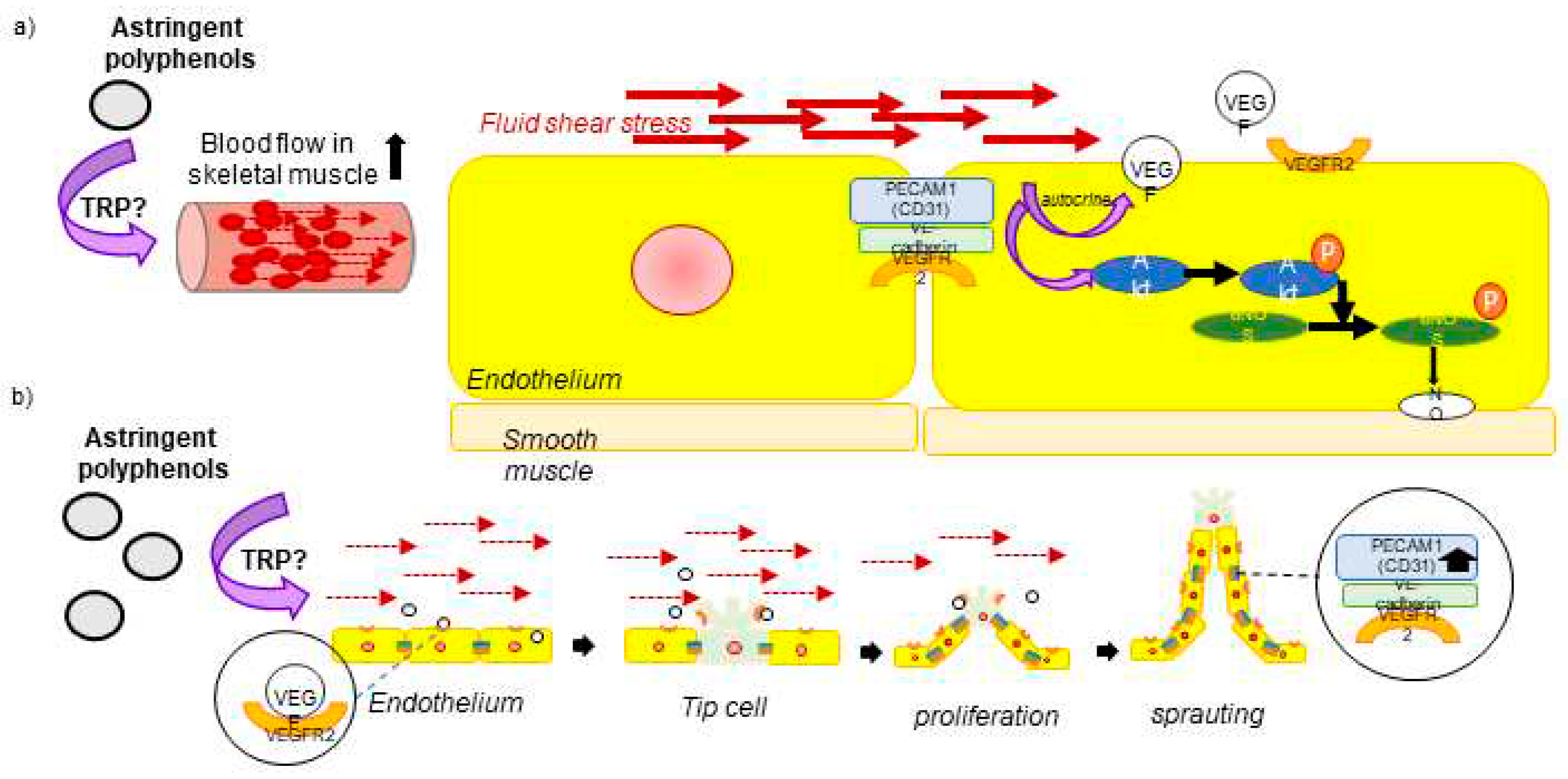
5.1. Circulation and Polyphenol
5.2. Blood Glucose and Polyphenols
5.3. Obesity and Polyphenols
5.4. Brain Function and Polyphenol
6. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Reed, D.R.; Mainland, J.D.; Arayata, C.J. Sensory nutrition: The role of taste in the reviews of commercial food products. Physiology & behavior 2019, 209, 112579. [Google Scholar]
- Reed, D.R.; Alhadeff, A.L.; Beauchamp, G.K.; Chaudhari, N.; Duffy, V.B.; Dus, M.; Fontanini, A.; Glendinning, J.I.; Green, B.G.; Joseph, P.V.; et al. NIH Workshop Report: sensory nutrition and disease. The American journal of clinical nutrition 2021, 113, 232–245. [Google Scholar] [CrossRef]
- Lindemann, B. Receptors and transduction in taste. Nature 2001, 413, 219–225. [Google Scholar] [CrossRef]
- Lee, S.J.; Depoortere, I.; Hatt, H. Therapeutic potential of ectopic olfactory and taste receptors. Nat Rev Drug Discov 2019, 18, 116–138. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, R.; Aier, I.; Semwal, R.; Tyagi, P.; Varadwaj, P. Sense of Smell: Structural, Functional, Mechanistic Advancements and Challenges in Human Olfactory Research. Current neuropharmacology 2019, 17, 891–911. [Google Scholar] [CrossRef]
- Kato, A.; Touhara, K. Mammalian olfactory receptors: pharmacology, G protein coupling and desensitization. Cell Mol Life Sci 2009, 66, 3743–3753. [Google Scholar] [CrossRef]
- Spehr, M.; Munger, S.D. Olfactory receptors: G protein-coupled receptors and beyond. J Neurochem 2009, 109, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Dubovski, N.; Fierro, F.; Margulis, E.; Ben Shoshan-Galeczki, Y.; Peri, L.; Niv, M.Y. Taste GPCRs and their ligands. Prog Mol Biol Transl Sci 2022, 193, 177–193. [Google Scholar]
- Chaudhari, N.; Roper, S.D. The cell biology of taste. J Cell Biol 2010, 190, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Roper, S.D. TRPs in taste and chemesthesis. Handbook of experimental pharmacology 2014, 223, 827–871. [Google Scholar]
- Zufall, F. TRPs in olfaction. Handbook of experimental pharmacology 2014, 223, 917–933. [Google Scholar] [PubMed]
- Green, B.G. Chemesthesis and the chemical senses as components of a "chemofensor complex". Chem Senses 2012, 37, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Guichard, E.; Barba, C.; Thomas-Danguin, T.; Tromelin, A. Multivariate Statistical Analysis and Odor-Taste Network To Reveal Odor-Taste Associations. Journal of agricultural and food chemistry 2020, 68, 10318–10328. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M.; Prescott, J. Odor/taste integration and the perception of flavor. Exp Brain Res 2005, 166, 345–357. [Google Scholar] [CrossRef]
- Tong, T.; Wang, Y.; Kang, S.G.; Huang, K. Ectopic Odorant Receptor Responding to Flavor Compounds: Versatile Roles in Health and Disease. Pharmaceutics 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, O.; Drago, F. Pharmacological significance of extra-oral taste receptors. Eur J Pharmacol 2021, 910, 174480. [Google Scholar] [CrossRef] [PubMed]
- Parmentier, M.; Libert, F.; Schurmans, S.; Schiffmann, S.; Lefort, A.; Eggerickx, D.; Ledent, C.; Mollereau, C.; Gérard, C.; Perret, J.; et al. Expression of members of the putative olfactory receptor gene family in mammalian germ cells. Nature 1992, 355, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Griffin, C.A.; Kafadar, K.A.; Pavlath, G.K. MOR23 promotes muscle regeneration and regulates cell adhesion and migration. Dev Cell 2009, 17, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Park, J.; Moon, C.; Park, T. Regulation of Adipogenesis and Thermogenesis through Mouse Olfactory Receptor 23 Stimulated by α-Cedrene in 3T3-L1 Cells. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Giusepponi, M.E.; Kern, M.; Chakaroun, R.; Wohland, T.; Kovacs, P.; Dietrich, A.; Schön, M.R.; Krohn, K.; Pucci, M.; Polidori, C.; et al. Gene expression profiling in adipose tissue of Sprague Dawley rats identifies olfactory receptor 984 as a potential obesity treatment target. Biochem Biophys Res Commun 2018, 505, 801–806. [Google Scholar] [CrossRef]
- Bellono, N.W.; Bayrer, J.R.; Leitch, D.B.; Castro, J.; Zhang, C.; O’Donnell, T.A.; Brierley, S.M.; Ingraham, H.A.; Julius, D. Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell 2017, 170, 185–198.e16. [Google Scholar] [CrossRef] [PubMed]
- Laffitte, A.; Neiers, F.; Briand, L. Functional roles of the sweet taste receptor in oral and extraoral tissues. Current opinion in clinical nutrition and metabolic care 2014, 17, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Zhai, K.; Yang, Z.; Zhu, X.; Nyirimigabo, E.; Mi, Y.; Wang, Y.; Liu, Q.; Man, L.; Wu, S.; Jin, J.; et al. Activation of bitter taste receptors (tas2rs) relaxes detrusor smooth muscle and suppresses overactive bladder symptoms. Oncotarget 2016, 7, 21156–21167. [Google Scholar] [CrossRef] [PubMed]
- Xie, C. Role of Intestinal Bitter Sensing in Enteroendocrine Hormone Secretion and Metabolic Control. Frontiers in endocrinology (Lausanne) 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Ansoleaga, B.; Garcia-Esparcia, P.; Pinacho, R.; Haro, J.M.; Ramos, B.; Ferrer, I. Decrease in olfactory and taste receptor expression in the dorsolateral prefrontal cortex in chronic schizophrenia. J Psychiatr Res 2015, 60, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.R.; Blank, K.; See Hoe, L.E.; Behrens, M.; Meyerhof, W.; Peart, J.N.; Thomas, W.G. Bitter taste receptor agonists elicit G-protein-dependent negative inotropy in the murine heart. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 2014, 28, 4497–4508. [Google Scholar] [CrossRef] [PubMed]
- Tizzano, M.; Finger, T.E. Chemosensors in the nose: guardians of the airways. Physiology (Bethesda) 2013, 28, 51–60. [Google Scholar] [CrossRef]
- Xu, J.; Cao, J.; Iguchi, N.; Riethmacher, D.; Huang, L. Functional characterization of bitter-taste receptors expressed in mammalian testis. Mol Hum Reprod 2013, 19, 17–28. [Google Scholar] [CrossRef]
- Lund, T.C.; Kobs, A.J.; Kramer, A.; Nyquist, M.; Kuroki, M.T.; Osborn, J.; Lidke, D.S.; Low-Nam, S.T.; Blazar, B.R.; Tolar, J. Bone Marrow Stromal and Vascular Smooth Muscle Cells Have Chemosensory Capacity via Bitter Taste Receptor Expression. PloS one 2013, 8, e58945. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed Engl 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Vancleef, L.; Van Den Broeck, T.; Thijs, T.; Steensels, S.; Briand, L.; Tack, J.; Depoortere, I. Chemosensory signalling pathways involved in sensing of amino acids by the ghrelin cell. Sci Rep 2015, 5, 15725. [Google Scholar] [CrossRef]
- Li, F. Taste perception: from the tongue to the testis. Mol Hum Reprod 2013, 19, 349–360. [Google Scholar] [CrossRef]
- Hill, M.A.; Sowers, J.R. Mineralocorticoid antagonists and ENaC inhibitors in hyperaldosteronism. Journal of clinical hypertension (Greenwich, Conn.) 2019, 21, 929–931. [Google Scholar] [CrossRef]
- Fallah, H.P.; Ahuja, E.; Lin, H.; Qi, J.; He, Q.; Gao, S.; An, H.; Zhang, J.; Xie, Y.; Liang, D. A Review on the Role of TRP Channels and Their Potential as Drug Targets_An Insight Into the TRP Channel Drug Discovery Methodologies. Frontiers in pharmacology 2022, 13, 914499. [Google Scholar] [CrossRef]
- Emery, E.C.; Diakogiannaki, E.; Gentry, C.; Psichas, A.; Habib, A.M.; Bevan, S.; Fischer, M.J.; Reimann, F.; Gribble, F.M. Stimulation of GLP-1 secretion downstream of the ligand-gated ion channel TRPA1. Diabetes 2015, 64, 1202–1210. [Google Scholar] [CrossRef]
- Mayer, F.; Gunawan, A.L.; Tso, P.; Aponte, G.W. Glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide stimulate release of substance P from TRPV1- and TRPA1-expressing sensory nerves. Am J Physiol Gastrointest Liver Physiol 2020, 319, G23–g35. [Google Scholar] [CrossRef]
- Arts, I.C.; Hollman, P.C. Polyphenols and disease risk in epidemiologic studies. The American journal of clinical nutrition 2005, 81, 317s–325s. [Google Scholar] [CrossRef]
- Dabas, D. Polyphenols as Colorants. Advances in Food Technology and Nutritional Sciences - Open Journal 2018, SE, S1–S6. [Google Scholar]
- Soares, S.; Kohl, S.; Thalmann, S.; Mateus, N.; Meyerhof, W.; De Freitas, V. Different phenolic compounds activate distinct human bitter taste receptors. Journal of agricultural and food chemistry 2013, 61, 1525–1533. [Google Scholar] [CrossRef]
- Soares, S.; Silva, M.S.; García-Estevez, I.; Groβmann, P.; Brás, N.; Brandão, E.; Mateus, N.; de Freitas, V.; Behrens, M.; Meyerhof, W. Human Bitter Taste Receptors Are Activated by Different Classes of Polyphenols. Journal of agricultural and food chemistry 2018, 66, 8814–8823. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; de Freitas, V. Tannins in Food: Insights into the Molecular Perception of Astringency and Bitter Taste. Molecules (Basel, Switzerland) 2020, 25. [Google Scholar] [CrossRef]
- Ferrer-Gallego, R.; Quijada-Morín, N.; Brás, N.F.; Gomes, P.; de Freitas, V.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Characterization of Sensory Properties of Flavanols - A Molecular Dynamic Approach. Chem Senses 2015, 40, 381–390. [Google Scholar] [CrossRef]
- Labbe, D.; Damevin, L.; Vaccher, C.; Morgenegg, C.; Martin, N. Modulation of perceived taste by olfaction in familiar and unfamiliar beverages. Food Quality and Preference 2006, 17, 582–589. [Google Scholar] [CrossRef]
- Mattes, R.D. Influences on acceptance of bitter foods and beverages. Physiology & behavior 1994, 56, 1229–1236. [Google Scholar]
- Stein, L.J.; Nagai, H.; Nakagawa, M.; Beauchamp, G.K. Effects of repeated exposure and health-related information on hedonic evaluation and acceptance of a bitter beverage. Appetite 2003, 40, 119–129. [Google Scholar] [CrossRef]
- Dugo, L.; Tripodo, G.; Santi, L.; Fanali, C. Cocoa Polyphenols: Chemistry, Bioavailability and Effects on Cardiovascular Performance. Curr Med Chem 2018, 25, 4903–4917. [Google Scholar] [CrossRef]
- Zhang, X.; Molsberry, S.A.; Yeh, T.S.; Cassidy, A.; Schwarzschild, M.A.; Ascherio, A.; Gao, X. Intake of Flavonoids and Flavonoid-Rich Foods and Mortality Risk Among Individuals With Parkinson Disease: A Prospective Cohort Study. Neurology 2022, 98, e1064–e1076. [Google Scholar] [CrossRef]
- Holland, T.M.; Agarwal, P.; Wang, Y.; Leurgans, S.E.; Bennett, D.A.; Booth, S.L.; Morris, M.C. Dietary flavonols and risk of Alzheimer dementia. Neurology 2020, 94, e1749–e1756. [Google Scholar] [CrossRef]
- Tang, D.; Tran, Y.; Shekhawat, G.S.; Gopinath, B. Dietary Flavonoid Intake and Chronic Sensory Conditions: A Scoping Review. Antioxidants (Basel, Switzerland) 2022, 11. [Google Scholar] [CrossRef]
- Imamura, F.; Schulze, M.B.; Sharp, S.J.; Guevara, M.; Romaguera, D.; Bendinelli, B.; Salamanca-Fernández, E.; Ardanaz, E.; Arriola, L.; Aune, D.; et al. Estimated Substitution of Tea or Coffee for Sugar-Sweetened Beverages Was Associated with Lower Type 2 Diabetes Incidence in Case-Cohort Analysis across 8 European Countries in the EPIC-InterAct Study. The Journal of nutrition 2019, 149, 1985–1993. [Google Scholar] [CrossRef]
- Ma, L.; Hu, Y.; Alperet, D.J.; Liu, G.; Malik, V.; Manson, J.E.; Rimm, E.B.; Hu, F.B.; Sun, Q. Beverage consumption and mortality among adults with type 2 diabetes: prospective cohort study. BMJ (Clinical research ed.) 2023, 381, e073406. [Google Scholar] [CrossRef]
- Sesso, H.D.; Manson, J.E.; Aragaki, A.K.; Rist, P.M.; Johnson, L.G.; Friedenberg, G.; Copeland, T.; Clar, A.; Mora, S.; Moorthy, M.V.; et al. Effect of cocoa flavanol supplementation for prevention of cardiovascular disease events: The COSMOS randomized clinical trial. The American journal of clinical nutrition 2022. [Google Scholar]
- Brickman, A.M.; Yeung, L.K.; Alschuler, D.M.; Ottaviani, J.I.; Kuhnle, G.G.C.; Sloan, R.P.; Luttmann-Gibson, H.; Copeland, T.; Schroeter, H.; Sesso, H.D.; et al. Dietary flavanols restore hippocampal-dependent memory in older adults with lower diet quality and lower habitual flavanol consumption. Proceedings of the National Academy of Sciences of the United States of America 2023, 120, e2216932120. [Google Scholar] [CrossRef]
- Osakabe, N.; Terao, J. Possible mechanisms of postprandial physiological alterations following flavan 3-ol ingestion. Nutr Rev 2018, 76, 174–186. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants (Basel, Switzerland) 2022, 11. [Google Scholar] [CrossRef]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Frontiers in nutrition 2019, 6, 188. [Google Scholar] [CrossRef]
- Yan, J.; Tong, H. An overview of bitter compounds in foodstuffs: Classifications, evaluation methods for sensory contribution, separation and identification techniques, and mechanism of bitter taste transduction. Compr Rev Food Sci Food Saf 2023, 22, 187–232. [Google Scholar] [CrossRef]
- Brockhoff, A.; Behrens, M.; Niv, M.Y.; Meyerhof, W. Structural requirements of bitter taste receptor activation. Proceedings of the National Academy of Sciences of the United States of America 2010, 107, 11110–11115. [Google Scholar] [CrossRef]
- Meyerhof, W. Elucidation of mammalian bitter taste. Reviews of physiology, biochemistry and pharmacology 2005, 154, 37–72. [Google Scholar]
- Ahmad, R.; Dalziel, J.E. G Protein-Coupled Receptors in Taste Physiology and Pharmacology. Frontiers in pharmacology 2020, 11, 587664. [Google Scholar] [CrossRef]
- Lossow, K.; Hübner, S.; Roudnitzky, N.; Slack, J.P.; Pollastro, F.; Behrens, M.; Meyerhof, W. Comprehensive Analysis of Mouse Bitter Taste Receptors Reveals Different Molecular Receptive Ranges for Orthologous Receptors in Mice and Humans. J Biol Chem 2016, 291, 15358–15377. [Google Scholar] [CrossRef]
- Wooding, S.P.; Ramirez, V.A.; Behrens, M. Bitter taste receptors: Genes, evolution and health. Evol Med Public Health 2021, 9, 431–447. [Google Scholar] [CrossRef]
- Ye, L.; Liddle, R.A. Gastrointestinal hormones and the gut connectome. Curr Opin Endocrinol Diabetes Obes 2017, 24, 9–14. [Google Scholar] [CrossRef]
- Clark, A.A.; Liggett, S.B.; Munger, S.D. Extraoral bitter taste receptors as mediators of off-target drug effects. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 2012, 26, 4827–4831. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes Metab 2018, 20 Suppl 1, 5–21. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Pfeiffer, A.F.H. The evolving story of incretins (GIP and GLP-1) in metabolic and cardiovascular disease: A pathophysiological update. Diabetes Obes Metab 2021, 23 Suppl 3, 5–29. [Google Scholar] [CrossRef]
- Xie, C.; Wang, X.; Young, R.L.; Horowitz, M.; Rayner, C.K.; Wu, T. Role of Intestinal Bitter Sensing in Enteroendocrine Hormone Secretion and Metabolic Control. Frontiers in endocrinology 2018, 9, 576. [Google Scholar] [CrossRef]
- Miller, L.J.; Harikumar, K.G.; Wootten, D.; Sexton, P.M. Roles of Cholecystokinin in the Nutritional Continuum. Physiology and Potential Therapeutics. Frontiers in endocrinology 2021, 12, 684656. [Google Scholar] [CrossRef]
- Tack, J.; Verbeure, W.; Mori, H.; Schol, J.; Van den Houte, K.; Huang, I.H.; Balsiger, L.; Broeders, B.; Colomier, E.; Scarpellini, E.; et al. The gastrointestinal tract in hunger and satiety signalling. United European Gastroenterol J 2021, 9, 727–734. [Google Scholar] [CrossRef]
- Kim, W.; Egan, J.M. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacological reviews 2008, 60, 470–512. [Google Scholar] [CrossRef]
- Holst, J.J.; Gasbjerg, L.S.; Rosenkilde, M.M. The Role of Incretins on Insulin Function and Glucose Homeostasis. Endocrinology 2021, 162. [Google Scholar] [CrossRef]
- Zhao, T.C. Glucagon-like peptide-1 (GLP-1) and protective effects in cardiovascular disease: a new therapeutic approach for myocardial protection. Cardiovasc Diabetol 2013, 12, 90. [Google Scholar] [CrossRef]
- Christensen, M.; Bagger, J.I.; Vilsbøll, T.; Knop, F.K. The alpha-cell as target for type 2 diabetes therapy. Rev Diabet Stud 2011, 8, 369–381. [Google Scholar] [CrossRef]
- Deacon, C.F.; Ahrén, B. Physiology of incretins in health and disease. Rev Diabet Stud 2011, 8, 293–306. [Google Scholar] [CrossRef]
- Rezaie, P.; Bitarafan, V.; Rose, B.D.; Lange, K.; Mohammadpour, Z.; Rehfeld, J.F.; Horowitz, M.; Feinle-Bisset, C. Effects of Quinine on the Glycaemic Response to, and Gastric Emptying of, a Mixed-Nutrient Drink in Females and Males. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Rose, B.D.; Bitarafan, V.; Rezaie, P.; Fitzgerald, P.C.E.; Horowitz, M.; Feinle-Bisset, C. Comparative Effects of Intragastric and Intraduodenal Administration of Quinine on the Plasma Glucose Response to a Mixed-Nutrient Drink in Healthy Men: Relations with Glucoregulatory Hormones and Gastric Emptying. The Journal of nutrition 2021, 151, 1453–1461. [Google Scholar] [CrossRef]
- Verbeure, W.; Deloose, E.; Tóth, J.; Rehfeld, J.F.; Van Oudenhove, L.; Depoortere, I.; Tack, J. The endocrine effects of bitter tastant administration in the gastrointestinal system: intragastric versus intraduodenal administration. American journal of physiology. Endocrinology and metabolism 2021, 321, E1–e10. [Google Scholar] [CrossRef]
- Koh, G.Y.; Rowling, M.J.; Pritchard, S.K. Possible role of type 1 and type 2 taste receptors on obesity-induced inflammation. Nutr Rev 2022, 80, 1919–1926. [Google Scholar] [CrossRef]
- Medapati, M.R.; Bhagirath, A.Y.; Singh, N.; Chelikani, P. Pharmacology of T2R Mediated Host-Microbe Interactions. Handbook of experimental pharmacology 2022, 275, 177–202. [Google Scholar]
- Li, W.; Chen, H.; Xu, B.; Wang, Y.; Zhang, C.; Cao, Y.; Xing, X. Research progress on classification, sources and functions of dietary polyphenols for prevention and treatment of chronic diseases. Journal of Future Foods 2023, 3, 289–305. [Google Scholar] [CrossRef]
- Tarragon, E.; Moreno, J.J. Polyphenols and taste 2 receptors. Physiological, pathophysiological and pharmacological implications. Biochemical pharmacology 2020, 178, 114086. [Google Scholar] [CrossRef]
- Behrens, M.; Brockhoff, A.; Batram, C.; Kuhn, C.; Appendino, G.; Meyerhof, W. The human bitter taste receptor hTAS2R50 is activated by the two natural bitter terpenoids andrographolide and amarogentin. Journal of agricultural and food chemistry 2009, 57, 9860–9866. [Google Scholar] [CrossRef] [PubMed]
- Roland, W.S.; Vincken, J.P.; Gouka, R.J.; van Buren, L.; Gruppen, H.; Smit, G. Soy isoflavones and other isoflavonoids activate the human bitter taste receptors hTAS2R14 and hTAS2R39. Journal of agricultural and food chemistry 2011, 59, 11764–11771. [Google Scholar] [CrossRef]
- Narukawa, M.; Noga, C.; Ueno, Y.; Sato, T.; Misaka, T.; Watanabe, T. Evaluation of the bitterness of green tea catechins by a cell-based assay with the human bitter taste receptor hTAS2R39. Biochem Biophys Res Commun 2011, 405, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Narukawa, M.; Mochizuki, M.; Misaka, T.; Watanabe, T. Activation of the hTAS2R14 human bitter-taste receptor by (-)-epigallocatechin gallate and (-)-epicatechin gallate. Bioscience, biotechnology, and biochemistry 2013, 77, 1981–1983. [Google Scholar] [CrossRef] [PubMed]
- Intelmann, D.; Batram, C.; Kuhn, C.; Haseleu, G.; Meyerhof, W.; Hofmann, T. Three TAS2R Bitter Taste Receptors Mediate the Psychophysical Responses to Bitter Compounds of Hops (Humulus lupulus L.) and Beer. Chemosensory Perception 2009, 2, 118–132. [Google Scholar] [CrossRef]
- Roland, W.S.; van Buren, L.; Gruppen, H.; Driesse, M.; Gouka, R.J.; Smit, G.; Vincken, J.P. Bitter taste receptor activation by flavonoids and isoflavonoids: modeled structural requirements for activation of hTAS2R14 and hTAS2R39. Journal of agricultural and food chemistry 2013, 61, 10454–10466. [Google Scholar] [CrossRef] [PubMed]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses 2010, 35, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Misaka, T.; Ishiguro, M.; Masuda, K.; Sugawara, T.; Ito, K.; Kobayashi, T.; Matsuo, S.; Ishimaru, Y.; Asakura, T.; et al. Characterization of the beta-D-glucopyranoside binding site of the human bitter taste receptor hTAS2R16. J Biol Chem 2010, 285, 28373–28378. [Google Scholar] [CrossRef]
- Xu, W.; Wu, L.; Liu, S.; Liu, X.; Cao, X.; Zhou, C.; Zhang, J.; Fu, Y.; Guo, Y.; Wu, Y.; et al. Structural basis for strychnine activation of human bitter taste receptor TAS2R46. Science 2022, 377, 1298–1304. [Google Scholar] [CrossRef]
- Cheynier, V. Polyphenols in foods are more complex than often thought. The American journal of clinical nutrition 2005, 81, 223s–229s. [Google Scholar] [CrossRef]
- Gibbons, J.R.; Sadiq, N.M. Neuroanatomy, Neural Taste Pathway. In StatPearls, StatPearls Publishing Copyright © 2023; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Huang, R.; Xu, C. An overview of the perception and mitigation of astringency associated with phenolic compounds. Compr Rev Food Sci Food Saf 2021, 20, 1036–1074. [Google Scholar] [CrossRef] [PubMed]
- Schöbel, N.; Radtke, D.; Kyereme, J.; Wollmann, N.; Cichy, A.; Obst, K.; Kallweit, K.; Kletke, O.; Minovi, A.; Dazert, S.; et al. Astringency is a trigeminal sensation that involves the activation of G protein-coupled signaling by phenolic compounds. Chem Senses 2014, 39, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Mouritsen, O.G. Gastrophysics of the Oral Cavity. Curr Pharm Des 2016, 22, 2195–2203. [Google Scholar] [CrossRef] [PubMed]
- Kishi, M.; Sadachi, H.; Nakamura, J.; Tonoike, M. Functional magnetic resonance imaging investigation of brain regions associated with astringency. Neurosci Res 2017, 122, 9–16. [Google Scholar] [CrossRef] [PubMed]
- García-Estévez, I.; Ramos-Pineda, A.M.; Escribano-Bailón, M.T. Interactions between wine phenolic compounds and human saliva in astringency perception. Food Funct 2018, 9, 1294–1309. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rochera, B.; Manjón, E.; Escribano-Bailón, M.T.; García-Estévez, I. Role of Anthocyanins in the Interaction between Salivary Mucins and Wine Astringent Compounds. Foods 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Kurogi, M.; Saitoh, O. The diversity in sensitivity of TRPA1 and TRPV1 of various animals to polyphenols. Biomed Res 2021, 42, 43–51. [Google Scholar] [CrossRef]
- Kurogi, M.; Miyashita, M.; Emoto, Y.; Kubo, Y.; Saitoh, O. Green tea polyphenol epigallocatechin gallate activates TRPA1 in an intestinal enteroendocrine cell line, STC-1. Chem Senses 2012, 37, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Kurogi, M.; Kawai, Y.; Nagatomo, K.; Tateyama, M.; Kubo, Y.; Saitoh, O. Auto-oxidation products of epigallocatechin gallate activate TRPA1 and TRPV1 in sensory neurons. Chem Senses 2015, 40, 27–46. [Google Scholar] [CrossRef]
- Amoah, I.; Lim, J.J.; Osei, E.O.; Arthur, M.; Tawiah, P.; Oduro, I.N.; Aduama-Larbi, M.S.; Lowor, S.T.; Rush, E. Effect of Cocoa Beverage and Dark Chocolate Consumption on Blood Pressure in Those with Normal and Elevated Blood Pressure: A Systematic Review and Meta-Analysis. Foods 2022, 11. [Google Scholar] [CrossRef]
- Ried, K.; Sullivan, T.R.; Fakler, P.; Frank, O.R.; Stocks, N.P. Effect of cocoa on blood pressure. The Cochrane database of systematic reviews 2012, 8, Cd008893. [Google Scholar]
- Hooper, L.; Kay, C.; Abdelhamid, A.; Kroon, P.A.; Cohn, J.S.; Rimm, E.B.; Cassidy, A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials. The American journal of clinical nutrition 2012, 95, 740–751. [Google Scholar] [CrossRef]
- Ebaditabar, M.; Djafarian, K.; Saeidifard, N.; Shab-Bidar, S. Effect of dark chocolate on flow-mediated dilatation: Systematic review, meta-analysis, and dose-response analysis of randomized controlled trials. Clin Nutr ESPEN 2020, 36, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, N.; Fushimi, T.; Fujii, Y. Hormetic response to B-type procyanidin ingestion involves stress-related neuromodulation via the gut-brain axis: Preclinical and clinical observations. Frontiers in nutrition 2022, 9, 969823. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zimmermann, D.; De Castro, C.A.; Actis-Goretta, L. Dose-response relationship between cocoa flavanols and human endothelial function: a systematic review and meta-analysis of randomized trials. Food Funct 2019, 10, 6322–6330. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Osakabe, N.; Di Paola, R.; Siracusa, R.; Fusco, R.; D’Amico, R.; Impellizzeri, D.; Cuzzocrea, S.; Fritsch, T.; Abdelhameed, A.S.; et al. Hormesis defines the limits of lifespan. Ageing research reviews 2023, 91, 102074. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Cornelius, C.; Cuzzocrea, S.; Iavicoli, I.; Rizzarelli, E.; Calabrese, E.J. Hormesis, cellular stress response and vitagenes as critical determinants in aging and longevity. Mol Aspects Med 2011, 32, 279–304. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, C.; Graziano, A.; Calabrese, E.J.; Calabrese, V. Hormesis and vitagenes in aging and longevity: mitochondrial control and hormonal regulation. Horm Mol Biol Clin Investig 2013, 16, 73–89. [Google Scholar] [CrossRef]
- Fushimi, T.; Fujii, Y.; Koshino, H.; Inagawa, K.; Saito, A.; Koizumi, R.; Shibata, M.; Osakabe, N. Method for detecting hemodynamic alterations following a single gavage in rats. Exp Anim 2021, 70, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Ingawa, K.; Aruga, N.; Matsumura, Y.; Shibata, M.; Osakabe, N. Alteration of the systemic and microcirculation by a single oral dose of flavan-3-ols. PloS one 2014, 9, e94853. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Inagawa, K.; Ebe, R.; Fukase, S.; Horikoshi, Y.; Shibata, M.; Osakabe, N. Onset of a hypotensive effect following ingestion of flavan 3-ols involved in the activation of adrenergic receptors. Free radical biology & medicine 2016, 99, 584–592. [Google Scholar]
- Charkoudian, N.; Rabbitts, J.A. Sympathetic neural mechanisms in human cardiovascular health and disease. Mayo Clinic proceedings 2009, 84, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Charkoudian, N.; Wallin, B.G. Sympathetic neural activity to the cardiovascular system: integrator of systemic physiology and interindividual characteristics. Comprehensive Physiology 2014, 4, 825–850. [Google Scholar] [PubMed]
- Koizumi, R.; Fushimi, T.; Sato, Y.; Fujii, Y.; Sato, H.; Osakabe, N. Relationship between hemodynamic alteration and sympathetic nerve activation following a single oral dose of cinnamtannin A2. Free radical research 2020, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, A.; Horikoshi, Y.; Fushimi, T.; Saito, A.; Koizumi, R.; Fujii, Y.; Hu, Q.Q.; Hirota, Y.; Aizawa, K.; Osakabe, N. Acylated anthocyanins derived from purple carrot (Daucus carota L.) induce elevation of blood flow in rat cremaster arteriole. Food Funct 2019, 10, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Tentolouris, N.; Liatis, S.; Katsilambros, N. Sympathetic system activity in obesity and metabolic syndrome. Annals of the New York Academy of Sciences 2006, 1083, 129–152. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Muta, O.; Teshima, T.; Hirasima, N.; Odaka, M.; Fushimi, T.; Fujii, Y.; Osakabe, N. Repeated Oral Administration of Flavan-3-ols Induces Browning in Mice Adipose Tissues through Sympathetic Nerve Activation. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Muta, O.; Oyama, S.; Odaka, M.; Shimizu, K.; Katsuragawa, S.; Suzuki, K.; Fushimi, T.; Fujii, Y.; Akagi, R.; Osakabe, N. Cinnamtannin A2, (-)-epicatechin tetramer, attenuates skeletal muscle wasting in disuse atrophy model mice induced by hindlimb suspension. J Clin Biochem Nutr 2023, 73, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Ishimura, K.; Oya, S.; Kamino, M.; Fujii, Y.; Nanba, F.; Toda, T.; Ishii, T.; Adachi, T.; Suhara, Y.; et al. Comparison of the sympathetic stimulatory abilities of B-type procyanidins based on induction of uncoupling protein-1 in brown adipose tissue (BAT) and increased plasma catecholamine (CA) in mice. PloS one 2018, 13, e0201203. [Google Scholar] [CrossRef]
- Ogawa, N.; Kurokawa, T.; Mori, Y. Sensing of redox status by TRP channels. Cell Calcium 2016, 60, 115–122. [Google Scholar] [CrossRef]
- Machado, S.A.; Pasquarelli-do-Nascimento, G.; da Silva, D.S.; Farias, G.R.; de Oliveira Santos, I.; Baptista, L.B.; Magalhães, K.G. Browning of the white adipose tissue regulation: new insights into nutritional and metabolic relevance in health and diseases. Nutrition & metabolism 2022, 19, 61. [Google Scholar]
- Molina-Hidalgo, C.; Stillman, C.M.; Collins, A.M.; Velazquez-Diaz, D.; Ripperger, H.S.; Drake, J.A.; Gianaros, P.J.; Marsland, A.L.; Erickson, K.I. Changes in stress pathways as a possible mechanism of aerobic exercise training on brain health: a scoping review of existing studies. Frontiers in physiology 2023, 14, 1273981. [Google Scholar] [CrossRef] [PubMed]
- Wadsworth, M.E.; Broderick, A.V.; Loughlin-Presnal, J.E.; Bendezu, J.J.; Joos, C.M.; Ahlkvist, J.A.; Perzow, S.E.D.; McDonald, A. Co-activation of SAM and HPA responses to acute stress: A review of the literature and test of differential associations with preadolescents’ internalizing and externalizing. Dev Psychobiol 2019, 61, 1079–1093. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Suzuki, K.; Adachi, T.; Taira, S.; Osakabe, N. Corticotropin-releasing hormone is significantly upregulated in the mouse paraventricular nucleus following a single oral dose of cinnamtannin A2 as an (-)-epicatechin tetramer. J Clin Biochem Nutr 2019, 65, 29–33. [Google Scholar] [CrossRef]
- Fujii, Y.; Suzuki, K.; Hasegawa, Y.; Nanba, F.; Toda, T.; Adachi, T.; Taira, S.; Osakabe, N. Single oral administration of flavan 3-ols induces stress responses monitored with stress hormone elevations in the plasma and paraventricular nucleus. Neuroscience letters 2018, 682, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Szallasi, A. Transient receptor potential (TRP) channels: a clinical perspective. British journal of pharmacology 2014, 171, 2474–2507. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yu, M.; Liu, Y.; Yu, S. TRP channel functions in the gastrointestinal tract. Seminars in immunopathology 2016, 38, 385–396. [Google Scholar] [CrossRef]
- Uchida, K.; Dezaki, K.; Yoneshiro, T.; Watanabe, T.; Yamazaki, J.; Saito, M.; Yada, T.; Tominaga, M.; Iwasaki, Y. Involvement of thermosensitive TRP channels in energy metabolism. J Physiol Sci 2017, 67, 549–560. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.H. Neural control of blood pressure: focusing on capsaicin-sensitive sensory nerves. Cardiovascular & hematological disorders drug targets 2007, 7, 37–46. [Google Scholar]
- Fushimi, T.; Hirahata, C.; Hiroki, K.; Fujii, Y.; Calabrese, V.; Suhara, Y.; Osakabe, N. Activation of transient receptor potential channels is involved in reactive oxygen species (ROS)-dependent regulation of blood flow by (-)-epicatechin tetramer cinnamtannin A2. Biochemical pharmacology 2023, 214, 115682. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, K.C.; Duke, S.O. Function of polyphenol oxidase in higher plants. Physiologia Plantarum 1984, 60, 106–112. [Google Scholar] [CrossRef]
- Tan, J.; de Bruijn, W.J.C.; van Zadelhoff, A.; Lin, Z.; Vincken, J.-P. Browning of Epicatechin (EC) and Epigallocatechin (EGC) by Auto-Oxidation. Journal of agricultural and food chemistry 2020, 68, 13879–13887. [Google Scholar] [CrossRef]
- Fang, J. Bioavailability of anthocyanins. Drug metabolism reviews 2014, 46, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Kozai, D.; Ogawa, N.; Mori, Y. Redox regulation of transient receptor potential channels. Antioxidants & redox signaling 2014, 21, 971–986. [Google Scholar]
- Ogawa, N.; Kurokawa, T.; Fujiwara, K.; Polat, O.K.; Badr, H.; Takahashi, N.; Mori, Y. Functional and Structural Divergence in Human TRPV1 Channel Subunits by Oxidative Cysteine Modification*. Journal of Biological Chemistry 2016, 291, 4197–4210. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; Chen, W.; Kawaguchi, K.; Nyasha, M.R.; Sasaki, S.; Hatakeyama, H.; Kaneko, T.; Kanzaki, M. TRPA1 and TRPV1 channels participate in atmospheric-pressure plasma-induced [Ca(2+)](i) response. Sci Rep 2020, 10, 9687. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Chen, L. Polyphenols and bioavailability: an update. Crit Rev Food Sci Nutr 2019, 59, 2040–2051. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Xing, X.; Wang, S. Health benefits of dietary polyphenols: insight into interindividual variability in absorption and metabolism. Current Opinion in Food Science 2022, 48, 100941. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. The American journal of clinical nutrition 2005, 81, 230s–242s. [Google Scholar] [CrossRef]
- Felgines, C.; Talavéra, S.; Gonthier, M.P.; Texier, O.; Scalbert, A.; Lamaison, J.L.; Rémésy, C. Strawberry anthocyanins are recovered in urine as glucuro- and sulfoconjugates in humans. The Journal of nutrition 2003, 133, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Bitsch, R.; Netzel, M.; Sonntag, S.; Strass, G.; Frank, T.; Bitsch, I. Urinary Excretion of Cyanidin Glucosides and Glucuronides in Healthy Humans After Elderberry Juice Ingestion. J Biomed Biotechnol 2004, 2004, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Koirala, N.; Thuan, N.H.; Ghimire, G.P.; Thang, D.V.; Sohng, J.K. Methylation of flavonoids: Chemical structures, bioactivities, progress and perspectives for biotechnological production. Enzyme and Microbial Technology 2016, 86, 103–116. [Google Scholar] [CrossRef] [PubMed]
- de Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. British journal of pharmacology 2014, 171, 3268–3282. [Google Scholar] [CrossRef]
- Luo, C.; Wei, X.; Song, J.; Xu, X.; Huang, H.; Fan, S.; Zhang, D.; Han, L.; Lin, J. Interactions between Gut Microbiota and Polyphenols: New Insights into the Treatment of Fatigue. Molecules (Basel, Switzerland) 2022, 27. [Google Scholar] [CrossRef]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Frontiers in nutrition 2019, 6. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J.; Zhang, X. Dietary Polyphenols as Prospective Natural-Compound Depression Treatment from the Perspective of Intestinal Microbiota Regulation. Molecules (Basel, Switzerland) 2022, 27. [Google Scholar] [CrossRef]
- Rojas, M.; Chávez-Castillo, M.; Pirela, D.; Parra, H.; Nava, M.; Chacín, M.; Angarita, L.; Añez, R.; Salazar, J.; Ortiz, R.; et al. Metabolic Syndrome: Is It Time to Add the Central Nervous System? Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Kiyimba, T.; Yiga, P.; Bamuwamye, M.; Ogwok, P.; Van der Schueren, B.; Matthys, C. Efficacy of Dietary Polyphenols from Whole Foods and Purified Food Polyphenol Extracts in Optimizing Cardiometabolic Health: A Meta-Analysis of Randomized Controlled Trials. Advances in Nutrition 2023, 14, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Fairlie-Jones, L.; Davison, K.; Fromentin, E.; Hill, A.M. The Effect of Anthocyanin-Rich Foods or Extracts on Vascular Function in Adults: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2017, 9, 908. [Google Scholar] [CrossRef] [PubMed]
- Mohammadipoor, N.; Shafiee, F.; Rostami, A.; Kahrizi, M.S.; Soleimanpour, H.; Ghodsi, M.; Ansari, M.J.; Bokov, D.O.; Jannat, B.; Mosharkesh, E.; et al. Resveratrol supplementation efficiently improves endothelial health: A systematic review and meta-analysis of randomized controlled trials. Phytother Res 2022, 36, 3529–3539. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Liu, X.X.; Bai, Y.Y.; Wang, X.J.; Sun, K.; Chen, J.Z.; Hui, R.T. Effect of oral isoflavone supplementation on vascular endothelial function in postmenopausal women: a meta-analysis of randomized placebo-controlled trials. The American journal of clinical nutrition 2010, 91, 480–486. [Google Scholar] [CrossRef]
- Fairlie-Jones, L.; Davison, K.; Fromentin, E.; Hill, A.M. The Effect of Anthocyanin-Rich Foods or Extracts on Vascular Function in Adults: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Currenti, W.; Micek, A.; Falzone, L.; Libra, M.; Giampieri, F.; Forbes-Hernández, T.Y.; Quiles, J.L.; Battino, M.; et al. The Effect of Dietary Polyphenols on Vascular Health and Hypertension: Current Evidence and Mechanisms of Action. Nutrients 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.D.; Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cassidy, A. Relative impact of flavonoid composition, dose and structure on vascular function: a systematic review of randomised controlled trials of flavonoid-rich food products. Mol Nutr Food Res 2012, 56, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Inagawa, K.; Shibata, M.; Osakabe, N. Flavan-3-ol fraction from cocoa powder promotes mitochondrial biogenesis in skeletal muscle in mice. Lipids in health and disease 2014, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Fushimi, T.; Oyama, S.; Koizumi, R.; Fujii, Y.; Osakabe, N. Impact of cyanidin 3-O-glucoside on rat micro-and systemic circulation, possibly thorough angiogenesis. J Clin Biochem Nutr 2023, 72, 132–138. [Google Scholar] [CrossRef]
- Resnick, N.; Yahav, H.; Shay-Salit, A.; Shushy, M.; Schubert, S.; Zilberman, L.C.; Wofovitz, E. Fluid shear stress and the vascular endothelium: for better and for worse. Progress in biophysics and molecular biology 2003, 81, 177–199. [Google Scholar] [CrossRef]
- Li, Y.S.; Haga, J.H.; Chien, S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech 2005, 38, 1949–1971. [Google Scholar] [CrossRef] [PubMed]
- Gorski, T.; De Bock, K. Metabolic regulation of exercise-induced angiogenesis. Vasc Biol 2019, 1, H1–h8. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, H.; Golding, M.; Fruttiger, M.; Ruhrberg, C.; Lundkvist, A.; Abramsson, A.; Jeltsch, M.; Mitchell, C.; Alitalo, K.; Shima, D.; et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 2003, 161, 1163–1177. [Google Scholar] [CrossRef]
- Galie, P.A.; Nguyen, D.H.; Choi, C.K.; Cohen, D.M.; Janmey, P.A.; Chen, C.S. Fluid shear stress threshold regulates angiogenic sprouting. Proceedings of the National Academy of Sciences of the United States of America 2014, 111, 7968–7973. [Google Scholar] [CrossRef]
- Koizumi, R.; Fushimi, T.; Sato, Y.; Fujii, Y.; Sato, H.; Osakabe, N. Relationship between hemodynamic alteration and sympathetic nerve activation following a single oral dose of cinnamtannin A2. Free radical research 2021, 55, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Yanagimoto, A.; Matsui, Y.; Yamaguchi, T.; Saito, S.; Hanada, R.; Hibi, M. Acute Dose-Response Effectiveness of Combined Catechins and Chlorogenic Acids on Postprandial Glycemic Responses in Healthy Men: Results from Two Randomized Studies. Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.E.G.; Paiva, C.; Amato, A.A.; Lofrano-Porto, A.; Wassell, S.; Bluck, L.J.C.; Dórea, J.G.; da Costa, T.H.M. Decaffeinated coffee improves insulin sensitivity in healthy men. The British journal of nutrition 2018, 119, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Jokura, H.; Watanabe, I.; Umeda, M.; Hase, T.; Shimotoyodome, A. Coffee polyphenol consumption improves postprandial hyperglycemia associated with impaired vascular endothelial function in healthy male adults. Nutrition research (New York, N.Y.) 2015, 35, 873–881. [Google Scholar] [CrossRef]
- Carnevale, R.; Silvestri, R.; Loffredo, L.; Novo, M.; Cammisotto, V.; Castellani, V.; Bartimoccia, S.; Nocella, C.; Violi, F. Oleuropein, a component of extra virgin olive oil, lowers postprandial glycaemia in healthy subjects. British journal of clinical pharmacology 2018, 84, 1566–1574. [Google Scholar] [CrossRef]
- Castro-Acosta, M.L.; Smith, L.; Miller, R.J.; McCarthy, D.I.; Farrimond, J.A.; Hall, W.L. Drinks containing anthocyanin-rich blackcurrant extract decrease postprandial blood glucose, insulin and incretin concentrations. J Nutr Biochem 2016, 38, 154–161. [Google Scholar] [CrossRef]
- Liu, C.Y.; Huang, C.J.; Huang, L.H.; Chen, I.J.; Chiu, J.P.; Hsu, C.H. Effects of green tea extract on insulin resistance and glucagon-like peptide 1 in patients with type 2 diabetes and lipid abnormalities: a randomized, double-blinded, and placebo-controlled trial. PloS one 2014, 9, e91163. [Google Scholar] [CrossRef] [PubMed]
- Yanagimoto, A.; Matsui, Y.; Yamaguchi, T.; Hibi, M.; Kobayashi, S.; Osaki, N. Effects of Ingesting Both Catechins and Chlorogenic Acids on Glucose, Incretin, and Insulin Sensitivity in Healthy Men: A Randomized, Double-Blinded, Placebo-Controlled Crossover Trial. Nutrients 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Zibadi, S.; Rohdewald, P.J.; Park, D.; Watson, R.R. Reduction of cardiovascular risk factors in subjects with type 2 diabetes by Pycnogenol supplementation. Nutrition research (New York, N.Y.) 2008, 28, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Matsumae, T.; Kataoka, T.; Yazaki, Y.; Yamaguchi, H. Effect of acacia polyphenol on glucose homeostasis in subjects with impaired glucose tolerance: A randomized multicenter feeding trial. Exp Ther Med 2013, 5, 1566–1572. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Urpi-Sarda, M.; Ros, E.; Valderas-Martinez, P.; Casas, R.; Arranz, S.; Guillén, M.; Lamuela-Raventós, R.M.; Llorach, R.; Andres-Lacueva, C.; et al. Effects of red wine polyphenols and alcohol on glucose metabolism and the lipid profile: a randomized clinical trial. Clin Nutr 2013, 32, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Cesar, T.B.; Ramos, F.M.M.; Ribeiro, C.B. Nutraceutical Eriocitrin (Eriomin) Reduces Hyperglycemia by Increasing Glucagon-Like Peptide 1 and Downregulates Systemic Inflammation: A Crossover-Randomized Clinical Trial. Journal of medicinal food 2022, 25, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin extract for prevention of type 2 diabetes. Diabetes care 2012, 35, 2121–2127. [Google Scholar] [CrossRef] [PubMed]
- Brasnyó, P.; Molnár, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, A.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. The British journal of nutrition 2011, 106, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Thazhath, S.S.; Wu, T.; Bound, M.J.; Checklin, H.L.; Standfield, S.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Administration of resveratrol for 5 wk has no effect on glucagon-like peptide 1 secretion, gastric emptying, or glycemic control in type 2 diabetes: a randomized controlled trial. The American journal of clinical nutrition 2016, 103, 66–70. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Sarian, M.N.; Khattak, M.; Khatib, A.; Sabere, A.S.M.; Yusoff, Y.M.; Latip, J. Flavonoids as Antidiabetic and Anti-Inflammatory Agents: A Review on Structural Activity Relationship-Based Studies and Meta-Analysis. International journal of molecular sciences 2022, 23. [Google Scholar] [CrossRef]
- Aloo, S.O.; Ofosu, F.K.; Kim, N.H.; Kilonzi, S.M.; Oh, D.H. Insights on Dietary Polyphenols as Agents against Metabolic Disorders: Obesity as a Target Disease. Antioxidants (Basel, Switzerland) 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. International journal of molecular sciences 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Balasooriya, H.; Sirisena, S.; Ng, K. The effectiveness of dietary polyphenols in obesity management: A systematic review and meta-analysis of human clinical trials. Food Chem 2023, 404, 134668. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules (Basel, Switzerland) 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Wang, H.N.; Xiang, J.Z.; Qi, Z.; Du, M. Plant extracts in prevention of obesity. Crit Rev Food Sci Nutr 2022, 62, 2221–2234. [Google Scholar] [CrossRef] [PubMed]
- Kawser Hossain, M.; Abdal Dayem, A.; Han, J.; Yin, Y.; Kim, K.; Kumar Saha, S.; Yang, G.M.; Choi, H.Y.; Cho, S.G. Molecular Mechanisms of the Anti-Obesity and Anti-Diabetic Properties of Flavonoids. International journal of molecular sciences 2016, 17, 569. [Google Scholar] [CrossRef] [PubMed]
- Raven, L.M.; Stoita, A.; Feller, R.B.; Brown, C.; Greenfield, J.R. Delayed Gastric Emptying with Perioperative Use of Glucagon-like Peptide-1 Receptor Agonists. Am J Med 2023. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab 2021, 46, 101102. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Natsume, M.; Yasuda, A.; Nakamura, Y.; Tamura, T.; Osakabe, N.; Kanegae, M.; Kondo, K. Plasma LDL and HDL cholesterol and oxidized LDL concentrations are altered in normo- and hypercholesterolemic humans after intake of different levels of cocoa powder. The Journal of nutrition 2007, 137, 1436–1441. [Google Scholar] [CrossRef]
- Baba, S.; Osakabe, N.; Kato, Y.; Natsume, M.; Yasuda, A.; Kido, T.; Fukuda, K.; Muto, Y.; Kondo, K. Continuous intake of polyphenolic compounds containing cocoa powder reduces LDL oxidative susceptibility and has beneficial effects on plasma HDL-cholesterol concentrations in humans. The American journal of clinical nutrition 2007, 85, 709–717. [Google Scholar] [CrossRef]
- Kaelberer, M.M.; Buchanan, K.L.; Klein, M.E.; Barth, B.B.; Montoya, M.M.; Shen, X.; Bohórquez, D.V. A gut-brain neural circuit for nutrient sensory transduction. Science 2018, 361. [Google Scholar] [CrossRef] [PubMed]
- Kaelberer, M.M.; Rupprecht, L.E.; Liu, W.W.; Weng, P.; Bohórquez, D.V. Neuropod Cells: The Emerging Biology of Gut-Brain Sensory Transduction. Annu Rev Neurosci 2020, 43, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Fraser, K.A.; Davison, J.S. Cholecystokinin-induced c-fos expression in the rat brain stem is influenced by vagal nerve integrity. Experimental physiology 1992, 77, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Rinaman, L.; Baker, E.A.; Hoffman, G.E.; Stricker, E.M.; Verbalis, J.G. Medullary c-Fos activation in rats after ingestion of a satiating meal. Am J Physiol 1998, 275, R262–R268. [Google Scholar] [CrossRef] [PubMed]
- Suarez, A.N.; Hsu, T.M.; Liu, C.M.; Noble, E.E.; Cortella, A.M.; Nakamoto, E.M.; Hahn, J.D.; de Lartigue, G.; Kanoski, S.E. Gut vagal sensory signaling regulates hippocampus function through multi-order pathways. Nature communications 2018, 9, 2181. [Google Scholar] [CrossRef] [PubMed]
- Klarer, M.; Arnold, M.; Günther, L.; Winter, C.; Langhans, W.; Meyer, U. Gut vagal afferents differentially modulate innate anxiety and learned fear. J Neurosci 2014, 34, 7067–7076. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Yan, Q.; Ma, Y.; Fang, J.; Yang, Y. Recognizing the role of the vagus nerve in depression from microbiota-gut brain axis. Front Neurol 2022, 13, 1015175. [Google Scholar] [CrossRef]
- Lightman, S.L.; Birnie, M.T.; Conway-Campbell, B.L. Dynamics of ACTH and Cortisol Secretion and Implications for Disease. Endocr Rev 2020, 41. [Google Scholar] [CrossRef]
- Timmermans, S.; Souffriau, J.; Libert, C. A General Introduction to Glucocorticoid Biology. Frontiers in immunology 2019, 10, 1545. [Google Scholar] [CrossRef]
- Bermejo, J.L.; Valldecabres, R.; Villarrasa-Sapiña, I.; Monfort-Torres, G.; Marco-Ahulló, A.; Ribeiro Do Couto, B. Increased cortisol levels caused by acute resistance physical exercise impair memory and learning ability. PeerJ 2022, 10, e13000. [Google Scholar] [CrossRef]
- Knezevic, E.; Nenic, K.; Milanovic, V.; Knezevic, N.N. The Role of Cortisol in Chronic Stress, Neurodegenerative Diseases, and Psychological Disorders. Cells 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; McEwen, B.S.; Friston, K. Uncertainty and stress: Why it causes diseases and how it is mastered by the brain. Prog Neurobiol 2017, 156, 164–188. [Google Scholar] [CrossRef] [PubMed]
- Kanaley, J.A.; Weltman, J.Y.; Pieper, K.S.; Weltman, A.; Hartman, M.L. Cortisol and growth hormone responses to exercise at different times of day. J Clin Endocrinol Metab 2001, 86, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Stranahan, A.M.; Lee, K.; Mattson, M.P. Central mechanisms of HPA axis regulation by voluntary exercise. Neuromolecular Med 2008, 10, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Adlard, P.A.; Cotman, C.W. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience 2004, 124, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, T.J.; Gould, E. Stress, stress hormones, and adult neurogenesis. Exp Neurol 2012, 233, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Nakagawa, S.; Kitaichi, Y.; An, Y.; Omiya, Y.; Song, N.; Koga, M.; Kato, A.; Inoue, T.; Kusumi, I. The role of medial prefrontal corticosterone and dopamine in the antidepressant-like effect of exercise. Psychoneuroendocrinology 2016, 69, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Nakagawa, S.; An, Y.; Ito, K.; Kitaichi, Y.; Kusumi, I. The exercise-glucocorticoid paradox: How exercise is beneficial to cognition, mood, and the brain while increasing glucocorticoid levels. Frontiers in neuroendocrinology 2017, 44, 83–102. [Google Scholar] [CrossRef]
- Fujii, Y.; Sakata, J.; Sato, F.; Onishi, K.; Yamato, Y.; Sakata, K.; Taira, S.; Sato, H.; Osakabe, N. Impact of short-term oral dose of cinnamtannin A2, an (-)-epicatechin tetramer, on spatial memory and adult hippocampal neurogenesis in mouse. Biochem Biophys Res Commun 2021, 585, 1–7. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




