Submitted:
12 January 2024
Posted:
15 January 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Materials and Methods
Study Design
Population
Primary Exposure
Data Source
Measure Outcomes
Immunosuppression
Drug Exposure Assay
Special Considerations
Pre-Transplant Management of HCC
Cut-Offs and Definitions
Statistical Analyses
Results
Demographics and Clinical Characteristics of the Original Cohort
Stabilized IPTW Effect
Results in the Balanced Groups
Re-transplantation
HCC Recurrence
immunosuppression
Risk Factors for Recurrence-Free and Overall Survival
Discussion
Funding
Conflicts of interest
Abbreviations:
References
- Di Maira, T.; Little, E.C.; Berenguer, M. Immunosuppression in liver transplant. Best Pract Res Clin Gastroenterol 2020, 46-47, 101681. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.; Terrec, F.; Malvezzi, P.; Rostaing, L. Adverse effects of immunosuppression after liver transplantation. Best Pract Res Clin Gastroenterol, 2021; 54-55, 101762. [Google Scholar]
- De Simone, P.; Nevens, F.; De Carlis, L.; Metselaar, H.J.; Beckebaum, S.; Saliba, F.; et al. Everolimus with Reduced Tacrolimus Improves Renal Function in De Novo Liver Transplant Recipients: A Randomized Controlled Trial. Am J Transplant 2012, 12, 3008–3020. [Google Scholar] [CrossRef] [PubMed]
- Saliba, F.; De Simone, P.; Nevens, F.; De Carlis, L.; Metselaar, H.J.; Beckebaum, S.; et al. Renal function at two years in liver transplant patients receiving everolimus: Results of a randomized, multicenter study. Am J Transplant 2013, 13, 1734–1745. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.; Saliba, F.; Kaiser, G.M.; De Carlis, L.; Metselaar, H.J.; De Simone, P.; et al. Three-year outcomes in de novo liver transplant patients receiving everolimus with reduced tacrolimus: Follow-up results from a randomized, multicenter study. . Transplantation. 2015, 99, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Levy, G.; Schmidili, H.; Punch, J.; Tuttle-Newhall, E.; Mayer, D.; Neuhaus, P.; et al. Safety, tolerability, and efficacy of everolimus in de novo liver transplant recipients: 12- and 36-month results. Liver Transpl 2006, 12, 1640–1648. [Google Scholar] [CrossRef]
- Saliba, F.; Dharancy, S.; Salamé, E.; Conti, F.; Eyraud, D.; Radenne, S.; et al. Time to conversion to an everolimus-based regimen: Renal outcomes in liver transplant recipients from the EVEROLIVER registry. Liver Transpl 2020, 26, 1465–1476. [Google Scholar] [CrossRef] [PubMed]
- De Simone, P.; Fagiuoli, S.; Cescon, M.; De Carlis, L.; Tisone, G.; Volpes, R.; Cillo, U. Use of everolimus in liver transplantation: Recommendations from a working group. Transplantation 2017, 101, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Jeng, L.B.; Saliba, F.; Singh Soin, A.; Lee, W.C.; De Simone, P.; et al. Efficacy and safety of everolimus with reduced tacrolimus in liver transplant recipients: 24-month results from the pooled analysis of 2 randomized controlled trials. Transplantation 2021, 105, 1564–1575. [Google Scholar] [CrossRef]
- Lin, M.; Mittal, S.; Sahebjam, F.; Rana, A.; Sood, G.K. Everolimus with early withdrawal or reduced dose calcineurin inhibitors improves renal function in liver transplant recipients: A systematic review and meta-analysis. Clin Transplant 2017, 31. [Google Scholar] [CrossRef]
- Guan, T.W.; Lin, Y.J.; Ou, M.Y.; Chen, K.B. Efficacy and safety of everolimus treatment on liver transplant recipients: A meta-analysis. Eur J Clin Invest 2019, 49, e13179. [Google Scholar] [CrossRef]
- Yan, X.; Huang, S.; Yang, Y.; Lu, Z.; Li, F.; Jiang, L.; et al. Sirolimus or everolimus improves survival after liver transplantation for hepatocellular carcinoma: A systematic review and meta-analysis. Liver Transpl 2022, 28, 1063–1077. [Google Scholar] [CrossRef]
- [No authors listed] Banff schema for grading liver allograft rejection: An international consensus document. Hepatology 1997, 25, 658–663. [CrossRef]
- EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol 2012, 56, 908–943. [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, A.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Olthoff, K.M.; Kulik, L.; Samstein, B.; Kaminski, M.; Abecassis, M.; Emond, J.; Shaked, A.; Christie, J.D. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl 2010, 16, 943–949. [Google Scholar] [CrossRef] [PubMed]
- KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney International Supplements 2012, 2, 1–141.
- Tonon, M.; Rosi, S.; Gambino, C.G.; Piano, S.; Calvino, V.; Romano, A.; Martini, A.; Pontisso, P.; Angeli, P. Natural history of acute kidney disease in patients with cirrhosis. J Hepatol 2021, 74, 578–583. [Google Scholar] [CrossRef]
- Diabetes Standards of Care: ADA guidelines (2018). Available at: http://diabetesed.net/wp-content/uploads/2017/12/2018-ADA-Standards-of-Care.pdf. Accessed 1st September 2023.
- Tejada, S.; Martinez-Revejo, R.; Nogueira, T.A.; Gòmez, A.; Pont, T.; Liao, X.; et al. The effect of sex inequality on solid organ transplantation: A systematic review and meta-analysis. Eur J Int Med 2023, 109, 58–67. [Google Scholar] [CrossRef]
- Gil, E.; Kim, J.M.; Jeon, K.; Park, H.; Kang, D.; Cho, J.; Suh, G.Y.; Park, H. Recipient age and mortality after liver transplantation: A population-based cohort study. Transplantation 2018, 102, 2025–2032. [Google Scholar] [CrossRef]
- Bhamidimarri, K.R.; Satapathy, S.K.; Martin, P. Hepatitis C virus and liver transplantation. Gastroenterol Hepatol (N Y) 2017, 13, 214–220. [Google Scholar]
- Brodosi, L.; Petta, S.; Petroni, M.L.; Marchesini, G.; Morelli, M.C. Management of diabetes in candidates for liver transplantation and in transplant recipients. Transplantation 2022, 106, 462–478. [Google Scholar] [CrossRef]
- Cullaro, G.; Verna, E.C.; Lee, B.P.; Lai, J.C. Chronic Kidney Disease in Liver Transplant Candidates: A rising burden impacting post-liver transplant outcomes. Liver Transpl 2020, 26, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Avolio, A.W.; Franco, A.; Schlegel, A.; Lai, Q.; Meli, S.; Burra, P.; et al. Development and validation of a comprehensive model to estimate early allograft failure among patients requiring early liver retransplant. JAMA Surg 2020, 155, e204095. [Google Scholar] [CrossRef]
- Sarkar, M.; Watt, K.D.; Terrault, N.; Berenguer, M. Outcomes in liver transplantation: Does sex matter? J Hepatol 2015, 62, 946–955. [Google Scholar] [CrossRef]
- Kanneganti, M.; Olthoff, K.M.; Bittermann, T. Impact of older donor age on recipient and graft survival after LDLT: The US Experience. Transplantation 2023, 107, 162–171. [Google Scholar] [CrossRef]
- Singhal, A.K.; Sheng, X.; Drakos, S.G.; Stehlik, J. Impact of donor cause of death on transplant outcomes: UNOS registry analysis. Transplant Proc 2009, 41, 3539–3544. [Google Scholar] [CrossRef]
- Tingle, S.J.; Dobbins, J.J.; Thompson, E.R.; Figueiredo, R.S.; Mahendran, B.; Pandanaboyana, S.; Wilson, C. Machine perfusion in liver transplantation. Cochrane Database of Systematic Reviews 2023, Issue 9. Art. No.: CD014685. [CrossRef]
- Figiel, W.; Smoter, P.; Krasnodębski, M.; Rykowski, P.; Morawski, M.; Grąt, M.; Patkowski, W.; Zieniewicz, K. Predictors of long-term outcomes after liver transplantation depending on the length of cold ischemia time. Transpl Proc 2022, 54, 1025–1028. [Google Scholar] [CrossRef]
- Lingiah, V.A.; Niazi, M.; Olivo, R.; Paterno, F.; Guarrera, J.V.; Pyrsopoulos, N.T. Liver transplantation beyond Milan criteria. J Clin Transl Hepatol 2020, 8, 69–75. [Google Scholar] [CrossRef]
- Duvoux, C.; Roudot-Thoraval, F.; Decaens, T.; Pessione, F.; Badran, H.; Piardi, P.; et al. Liver transplantation for hepatocellular carcinoma: A model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012, 143, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Guy, J.; Frenette, C.T.; Dodge, J.L.; Osorio, R.W.; Minteer, W.B.; Roberts, J.P.; Yao, F.Y. Excellent outcomes of liver transplantation following down-staging of hepatocellular carcinoma to within Milan criteria: A multicenter study. Clin Gastroenterol Hepatol 2018, 16, 955–964. [Google Scholar] [CrossRef]
- Jonas, S.; Bechstein, W.O.; Steinmueller, T.; Hermann, M.; Radke, C.; Berg, T.; Settmacher, U.; Neuhaus, P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology 2001, 33, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Perálvarez, M.; Tsochatzis, E.; Naveas, M.C.; Pieri, G.; García-Caparrós, C.; O'Beirne, J.; et al. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J Hepatol 2013, 59, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Aloun, A.; Abu-Zeid, E.E.D.; Garzali, I.U. Does mTORi based immunosuppression offer survival advantage after liver transplantation for hepatocellular carcinoma? Systematic review and meta-analysis of randomized controlled trials. Hepatol Forum 2023, 4, 82–88. [Google Scholar] [PubMed]
- Zhang, G.; Duan, B.; Li, G. mTORi-based immunosuppression reduces HCC recurrence at the expenses of increased adverse side effects: A systematic review and meta-analysis. Clin Transplant 2022, 36, e14823. [Google Scholar] [CrossRef] [PubMed]
- Grigg SE, Sarri GL, Gow PJ; Yeomans ND. Systematic review with meta-analysis: Sirolimus- or everolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther 2019, 49, 1260–1273.
- Tarantino, G.; Magistri, P.; Ballarin, R.; Di Francia, R.; Berretta, M.; Di Benedetto, F. Oncological impact of mTOR inhibitor immunosuppressive therapy after liver transplantation for hepatocellular carcinoma: Review of the literature. Front Pharmacol 2016, 7, 387. [Google Scholar] [CrossRef] [PubMed]
- Holdaas, H.; De Simone, P.; Zuckermann, A. Everolimus and malignancy after solid organ transplantation: A clinical update. J Transplant 2016, 2016, 4369574. [Google Scholar] [CrossRef] [PubMed]
- Geissler, E.K.; Schnitzbauer, A.A.; Zülke, C.; Lamby, P.E.; Proneth, A.; Duvoux, C.; et al. Sirolimus use in liver transplant recipients with hepatocellular carcinoma: A randomized, multicenter, open-label Phase 3 trial. Transplantation 2016, 100, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Sapisochin, G.; Lee, W.C.; Joo Dj Joh, J.W.; Hata, K.; Soin, A.S.; et al. Long-term effects of everolimus-facilitated tacrolimus reduction in living donor liver transplant recipients with hepatocellular carcinoma. Ann Transplant 2022, 27, e937988. [Google Scholar] [CrossRef]
- Rodriguez-Peralvarez, M.; Guerrero, M.; Barrera, L.; Ferrin, G.; Alamo, J.M.; et al. Impact of early initiated everolimus on the recurrence of hepatocellular carcinoma after liver transplantation. Transplantation 2018, 102, 2056–2064. [Google Scholar] [CrossRef]
- Kang, I.; Lee, J.G.; Choi, S.H.; Kim, H.J.; Han, D.H.; Choi, G.H.; et al. Impact of everolimus on survival after liver transplantation for hepatocellular carcinoma. Clin Mol Hepatol 2021, 27, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Wasilewicz, M.P.; Moczydlowska, D.; Janik, M.; Grat, M.; Zienewicz, K.; Raszeja-Wyszomirska, J. Immunosuppressive treatment with everolimus in patients after liver transplant: 4 years of single-center experience. Pol Arch Intern Med 2019, 129, 686–691. [Google Scholar] [PubMed]
- Cholongitas, E.; Antoniadis, N.; Goulis, I.; Theocharidou, E.; Imvrios, G.; Giouleme, O.; et al. Trough levels of everolimus are associated with recurrence rates of hepatocellular carcinoma after liver transplantation. Transplant Proc 2019, 51, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Engl, T.; Rutz, J.; Maxeiner, S.; Juengel, E.; Roos, F.; Khoder, W.; et al. mTOR inhibition reduces growth and adhesion of hepatocellular carcinoma in vitro. Mol Med Rep 2017, 16, 7064–7071. [Google Scholar] [CrossRef] [PubMed]
- Ande, A.; Chaar, M.; Ait-Oudhia, S. Multiscale systems pharmacological analysis of everolimus action in hepatocellular carcinoma. J Pharmacokinet Pharmacodyn 2018, 45, 607–620. [Google Scholar] [CrossRef]
- Cillo, U.; De Carlis, L.; Del Gaudio, M.; De Simone, P.; Fagiuoli, S.; Lupo, F.; Tisone, G.; Volpes, R. Immunosuppressive regimens for adult liver transplant recipients in real-life practice: Consensus recommendations from an Italian working group. Liver Int 2020, 146, 930–943. [Google Scholar] [CrossRef]
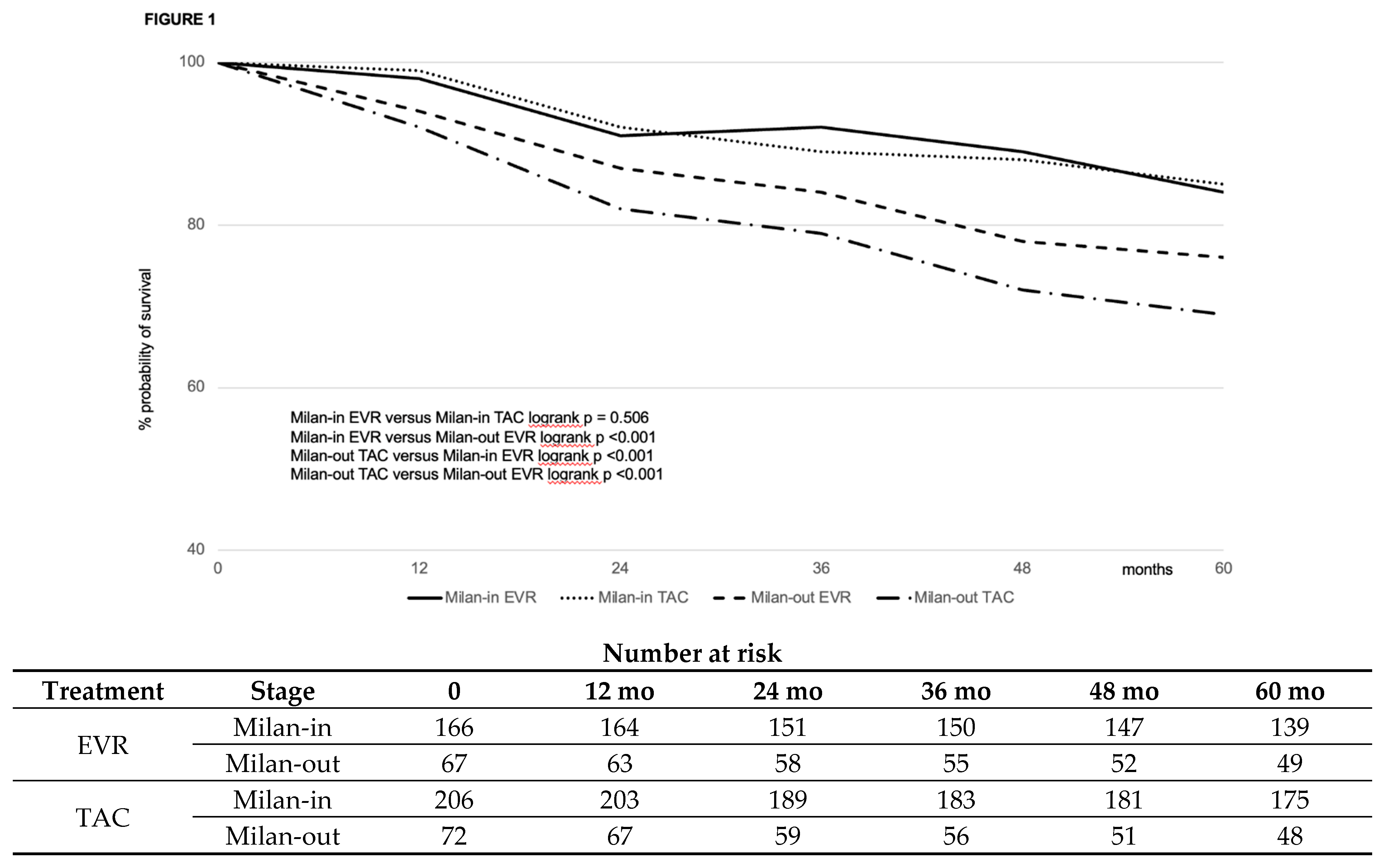
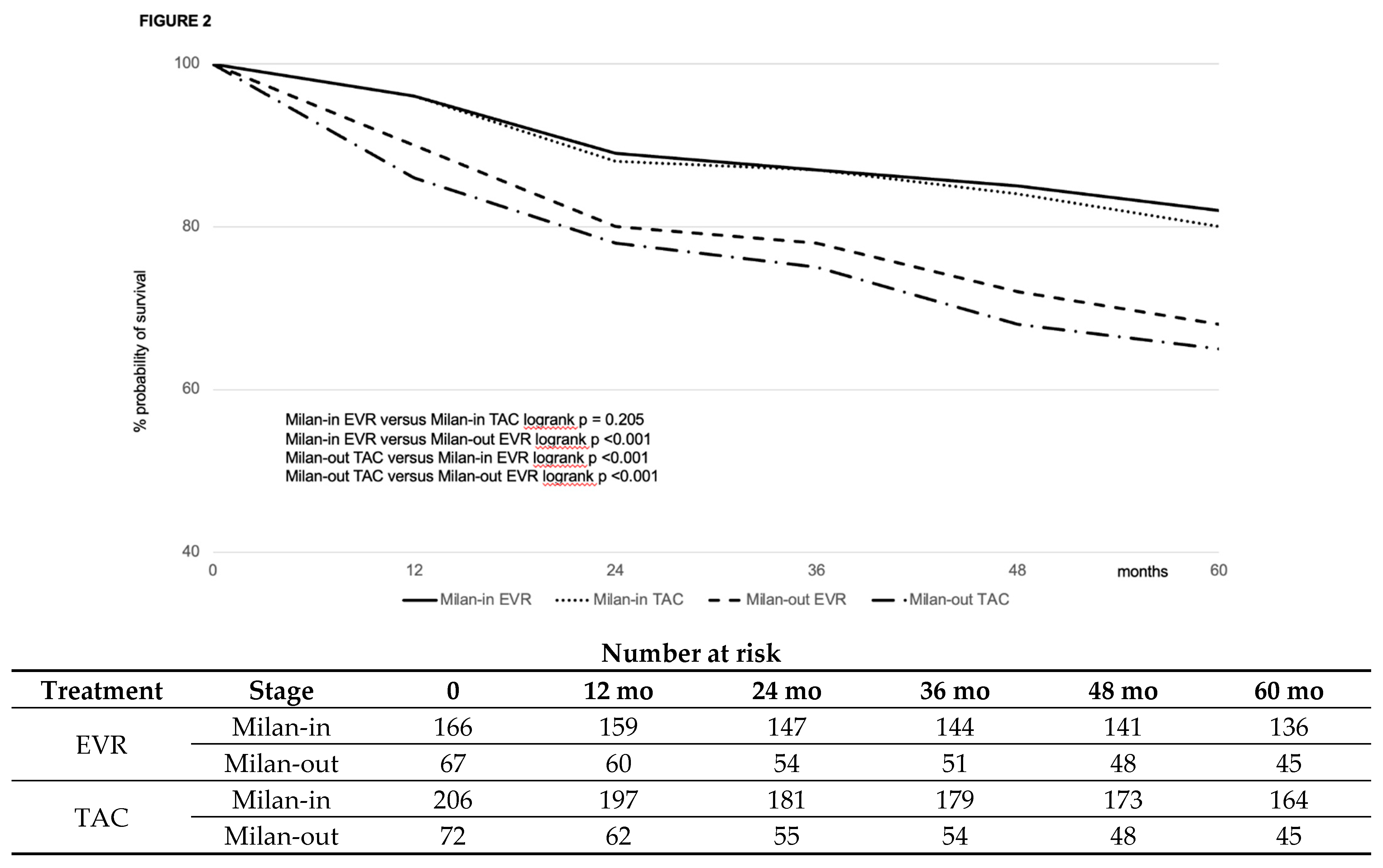
| Variable | EVR (#463) | CNI (#556) | P |
|---|---|---|---|
| RECIPIENT | |||
| Male sex, n (%) | 386 (83.4) | 487 (87.6) | 0.55 |
| Age at transplant (median, IQR), years | 56 (10) | 56 (10) | 0.28 |
| Indication to transplant, n (%) HCV HBV (±HDV) HCV-HBV(±HDV) Alcohol NAFLD Autoimmune/PSC |
237 (55.5) 123 (26.5) 17 (3.7) 54 (11.6) 20 (4.3) 12 (5.2) |
294 (52.8) 153 (27.5) 20 (3.5) 59 (9.5) 24 (4.3) 6 (1.1) |
0.59 0.73 0.94 0.59 0.99 0.67 |
| Lab-MELD at transplant (median, IQR) | 7 (6) | 8 (7) | 0.45 |
| DM at transplant, n (%) | 113 (24.4) | 144 (25.8) | 0.58 |
| CKD at transplant, n (%) | 27 (5.8) | 39 (7.0) | 0.44 |
| Hypertension at transplant, n (%) | 69 (14.9) | 76 (13.6) | 0.57 |
| <2013, n (%) | 147 (31.7) | 387 (66.0) | <0.0001 |
| TAC, n (%) | 403 (86.8) | 313 (56.3) | <0.0001 |
| Mean TAC exposure >10 ng/mL within the first month post-transplantation | 127 (27.4) | 172 (31.0) | 0.22 |
| DONOR | |||
| Male sex, n (%) | 241 (52.0) | 281 (50.5) | 0.63 |
| Age, median (IQR) | 69 (25) | 67 (26) | 0.78 |
| ICU stay, median (IQR) days | 3 (4) | 3 (4) | 0.67 |
| CVA as cause of death, n (%) | 333 (71.9) | 411 (73.9) | 0.47 |
| Anti-HCV-positive, n (%) | 4 (0.86) | 0 (0) | 0.58 |
| Anti-HBc-positive, n (%) | 60 (12.9) | 77 (13.8) | 0.67 |
| Cardiac arrest episodes, n (%) | 43 (9.3) | 42 (7.5) | 0.31 |
| Use of inotropes, n (%) | 407 (87.9) | 483 (86.8) | 0.62 |
| HCC | |||
| Tumor nodules*, median (IQR) | 2 (1) | 2 (1) | 0.78 |
| Largest nodule size*, median (IQR) (mm) | 28 (18) |
25 (15) |
0.04 |
| Total tumor size*, median (IQR) (mm) | 39.5 (25) |
36.5 (36) |
0.003 |
| Exceeding Milan criteria at transplant *, n (%) | 152 (32.8) | 101 (18.1) | <0.0001 |
| Pre-transplant treatment, n (%) None, n (%) TACE, n (%) RFA/MW, n (%) PEI, n (%) Resection, n (%) TACE + RFA/MW, n (%) TARE, n (%) Successful downstage**, n (%) |
141 (30.4) 229 (49.4) 33 (7.1) 6 (1.3) 6 (1.3) 42 (9.1) 6 (1.3) 75 (16.2) |
209 (37.6) 307 (55.2) 22 (3.9) 12 (2.1) 4 (0.7) 2 (0.5) 0 (0) 45 (8.1) |
0.01 0.06 0.02 0.29 0.35 <0.0001 0.008 0.0006 |
| AFP at transplant, median (IQR) (ng/mL) | 46.3 (28) |
4.7 (19) |
0.002 |
| Milan-out at explant histology, n (%) | 120 (25.9) |
167 (30.0) | 0.98 |
| G3-4, n (%) | 148 (31.9) | 140 (25.1) | 0.01 |
| Microvascular infiltration, n (%) | 88 (39.5) | 182 (32.7) | 0.02 |
| TRANSPLANTATION | |||
| CIT, median (IQR) (min) | 424 (89) | 420 (101) | 0.09 |
| MP, n (%) | 9 (1.9) | 7 (1.2) | 0.89 |
| Re-transplantation, n (%) | 18 (3.8) | 23 (4.1) | 0.45 |
| Variables | Pre-IPTW | Post-IPTW | ||||
|---|---|---|---|---|---|---|
| EVR (n=463) | CNI (n=556) | Cohen’s D-value | EVR (n=233) | TAC (n=278) | Cohen’s D-value | |
| Mean (±SD) | Mean (±SD) | |||||
| Patient male sex | 0.83±0.15 | 0.87±0.14 | 0.05 | 0.81±0.17 | 0.82±0.15 | 0.05 |
| Patient age, years | 55.9±3.92 | 56.4±3.46 | -0.20 | 55.1±0.55 | 55.3±0.53 | -0.03 |
| HCV | 55.5±0.70 | 55.8±0.58 | -0.42 | 24.3±0.56 | 24.1±0.52 | 0.01 |
| Patient diabetes | 0.24±0.50 | 0.26±0.45 | 0.12 | 0.23±0.50 | 0.24±0.50 | 0.00 |
| Patient CKD | 0.05±0.02 | 0.07±0.42 | 0.42 | 0.05±0.01 | 0.05±0.01 | 0.01 |
| MELD | 0.07±0.26 | 0.11±0.33 | -0.15 | 0.08±0.38 | 0.07±0.37 | 0.01 |
| Donor male sex | 0.52±0.38 | 0.50±0.41 | -0.08 | 0.51±0.28 | 0.50±0.28 | 0.01 |
| Donor age, years | 0.69±0.65 | 0.67±0.64 | 0.01 | 0.68±0.38 | 0.67±0.37 | 0.03 |
| Donor cause of death (CVA) | 0.71±0.50 | 0.73±0.40 | 0.13 | 0.71±0.46 | 0.72±0.45 | -0.02 |
| MP | 0.09±0.02 | 0.08±0.02 | 0.01 | 0.08±0.02 | 0.08±0.02 | 0.00 |
| CIT, minutes | 431.77±79.02 | 423.50±85.79 | 0.10 | 0.53±0.50 | 0.53±0.50 | 0.01 |
| Milan-out stage, radiologic | 32.8±2.33 | 19±1.65 | 0.42 | 29.2±1.2 | 26.3±0.9 | 0.02 |
| Tumor downstaging | 0.17±0.05 | 0.08±0.04 | 0.43 | 0.10±0.02 | 0.08±0.08 | 0.04 |
| AFP at transplant | 0.45±0.27 | 0.04±0.02 | 0.38 | 0.23±0.04 | 0.19±0.06 | 0.14 |
| Milan-out stage, histology | 26.1±1.5 | 29.1±2.3 | 0.16 | 24.0±2.3 | 22.0±2.8 | 0.12 |
| G3-G4 | 32.0±2.4 | 25±1.8 | 0.23 | 29.0±2.3 | 28.2±2.0 | 0.21 |
| Microinfiltration | 40.2±1.2 | 33.7±1.7 | 0.26 | 38.1±2.3 | 32.5±1.9 | 0.20 |
| Mean TAC exposure >10 ng/mL within the first month | 0.28±0.04 | 0.32±0.45 | 0.13 | 0.15±0.02 | 0.13±0.03 | 0.00 |
| NOTE: AFP, alpha-fetoprotein; CIT, cold ischemia time; CKD, chronic kidney disease; CVA, cerebro-vascular accident; G, grading; HCV, hepatitis C virus; IPTW, inverse probability therapy weighting; MP, machine perfusion; n, number; SD, standard deviation; TAC, tacrolimus. | ||||||
| Variable | EVR (#233) | TAC (#278) | P |
|---|---|---|---|
| RECIPIENT | |||
| Male sex, n (%) | 192 (82.4) | 228 (82.0) | 0.90 |
| Age at transplant (median, IQR), years | 55.5 (9) | 55.3 (10) | 0.89 |
| HCV, n (%) | 58 (24.3) | 69 (24.8) | 1 |
| Lab-MELD at transplant (median, IQR) * | 8 (6) | 7 (7) | 0.78 |
| DM at transplant, n (%) | 53 (22.7) | 66 (23.7) | 0.83 |
| CKD at transplant, n (%) | 12 (5.1) | 14 (5.0) | 1 |
| Mean TAC exposure >10 ng/mL within the first month post-transplantation | 35 (15.0) | 36 (12.9) | 0.52 |
| DONOR | |||
| Male sex, n (%) | 118 (50.6) | 140 (50.3) | 1 |
| Age, median (IQR) | 68.0 (23) | 67 (26) | 0.89 |
| CVA as cause of death, n (%) | 181 (77.7) | 200 (71.9) | 0.15 |
| HCC | |||
| Exceeding Milan criteria at transplant *, n (%) | 67 (28.7) | 72 (25.8) | 0.48 |
| Successful downstaging**, n (%) | 24 (10.3) | 23 (8.2) | 0.44 |
| AFP at transplant, median (IQR) (ng/mL) | 23.3 (18) | 19 (11) | 0.56 |
| Milan-out at explant histology, n (%) | 55 (23.6) |
62 (22.3) | 0.75 |
| G3-4, n (%) | 67 (28.7) | 78 (28.1) | 0.92 |
| Microvascular infiltration, n (%) | 88 (37.8) | 91 (32.7) | 0.26 |
| TRANSPLANTATION | |||
| CIT, median (IQR) (min) | 432 (89) | 489 (101) | 0.06 |
| MP, n (%) | 9 (1.9) | 7 (1.2) | 0.89 |
| Variable | EVR (#233) | TAC (#278) | P |
|---|---|---|---|
| Death, n (%) HCC recurrence, n (%) HCV recurrence, n (%) Incomplete/delayed graft function, n (%) MACE, n (%) Intra/peri-operative, n (%) Ischemic cholangiopathy, n (%) Infection/sepsis, n (%) De novo malignancy, n (%) Stroke, n (%) |
62 (26.6) 16 (6.8) 16 (6.9) 1 (0.4) 2 (0.8) 2 (0.8) 4 (1.7) 12 (5.1) 5 (2.1) 4 (1.7) |
105 (37.8) 42 (15.1) 15 (5.4) 2 (0.7) 6 (2.1) 3 (1.1) 7 (2.5) 16 (5.7) 11 (3.9) 3 (1.1) |
0.007 0.003 0.48 0.22 0.30 1 0.76 0.84 0.31 0.70 |
| Re-transplantation, n (%) Ischemic cholangiopathy, n (%) PNF, n (%) HAT, n % Chronic rejection, n (%) HCV recurrence, n (%) |
9 (3.9) 3 (1.3) 3 (1.3) 2 (0.8) 1 (0.4) 0 (0) |
11 (3.9) 3 (1.1) 5 (1.8) 1 (0.3) 1 (0.3) 1 (0.3) |
1 1 0.73 0.59 1 0.99 |
| HCC recurrence, n (%) Liver only, n (%) Liver and lung, n (%) Liver and bone, n (%) Lung only, n (%) Bone only, n (%) Lung and bone, n (%) Nodes, n (%) >2 organs, n (%) |
18 (7.7) 7 (3.0) 1 (0.4) 0 (0) 4 (1.7) 4 (1.7) 1 (0.4) 1 (0.4) 2 (0.8) |
47 (16.9) 15 (5.4) 8 (2.8) 4 (1.4) 9 (1.8) 1 (0.2) 4 (1.4) 6 (2.1) 16 (5.7) |
0.002 0.19 0.04 0.12 0.39 0.18 0.38 0.13 0.002 |
| Variable | EVR (#233) |
|---|---|
| Reason for EVR use, n (%) HCC recurrence prophylaxis, n (%) Deteriorating renal function *, n (%) Neurologic complication *, n (%) MACE *, n (%) |
212 (91.0) 14 (6.0) 4 (1.7) 3 (1.2) |
| Timing of EVR introduction, median (IQR) (days) * |
30 (16) |
| Duration of EVR treatment, median (IQR) (months) * |
46.6 (36.1) |
| EVR whole-blood exposure, median (IQR) (ng/mL) * |
5.8 (1.7) |
| Variable | Recurring HCC (#18) | Non-recurring HCC (#215) | P |
|---|---|---|---|
| Timing of EVR introduction, median (IQR) (days) * | 52 (26.4) | 30 (12) | <0.001 |
| Duration of EVR treatment, median (IQR) (months) * | 46.5 (57.0) | 69.9 (24.8) | <0.001 |
| EVR whole-blood exposure, median (IQR) (ng/mL) * | 3.65 (0.55) | 5.9 (1.4) | <0.001 |
| Variable | Coefficients (95%CI) |
SE | z | HR | p |
|---|---|---|---|---|---|
| OS | |||||
| Successful pre-transplant downstaging | 0.6 (0.15; 1.06) | 0.23 | 2.6 | 0.79 | 0.006 |
| Within Milan criteria at transplant | -1.15 (-1.61;-0.7) | 0.23 | 5.02 | 0.67 | <0.01 |
| Within Milan criteria at histology | 0.01 (0; 0.01) | 0 | 2.41 | 0.78 | 0.02 |
| Micro-infiltration | 0.39 (-0.01; 0.78) | 0.2 | 1.91 | 1.13 | 0.056 |
| G3-G4 | 0.25 (0.01; 0.5) | 0.12 | 2.02 | 1.18 | 0.077 |
| EVR | -0.59 (-1.02; -0.16) | 0.22 | 2.7 | 0.69 | 0.009 |
| RFS | |||||
| Successful pre-transplant downstaging | 0.57 (0.12; 1.02) | 0.23 | 2.47 | 0.65 | 0.01 |
| Within Milan criteria at transplant | -1.18 (-1.63;-0.72) | 0.23 | 5.11 | 0.56 | 0.01 |
| Within Milan criteria at histology | 0.01 (0; 0.01) | 0 | 2.52 | 0.68 | 0.012 |
| Micro-infiltration | 0.42 (0.02; 0.81) | 0.2 | 2.06 | 1.22 | 0.04 |
| G3-G4 | 0.22 (-0.02; 0.47) | 0.13 | 1.77 | 1.27 | 0.04 |
| EVR | -0.78 (1.2; -0.36) | 0.21 | 3.66 | 0.46 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





