Submitted:
12 January 2024
Posted:
14 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
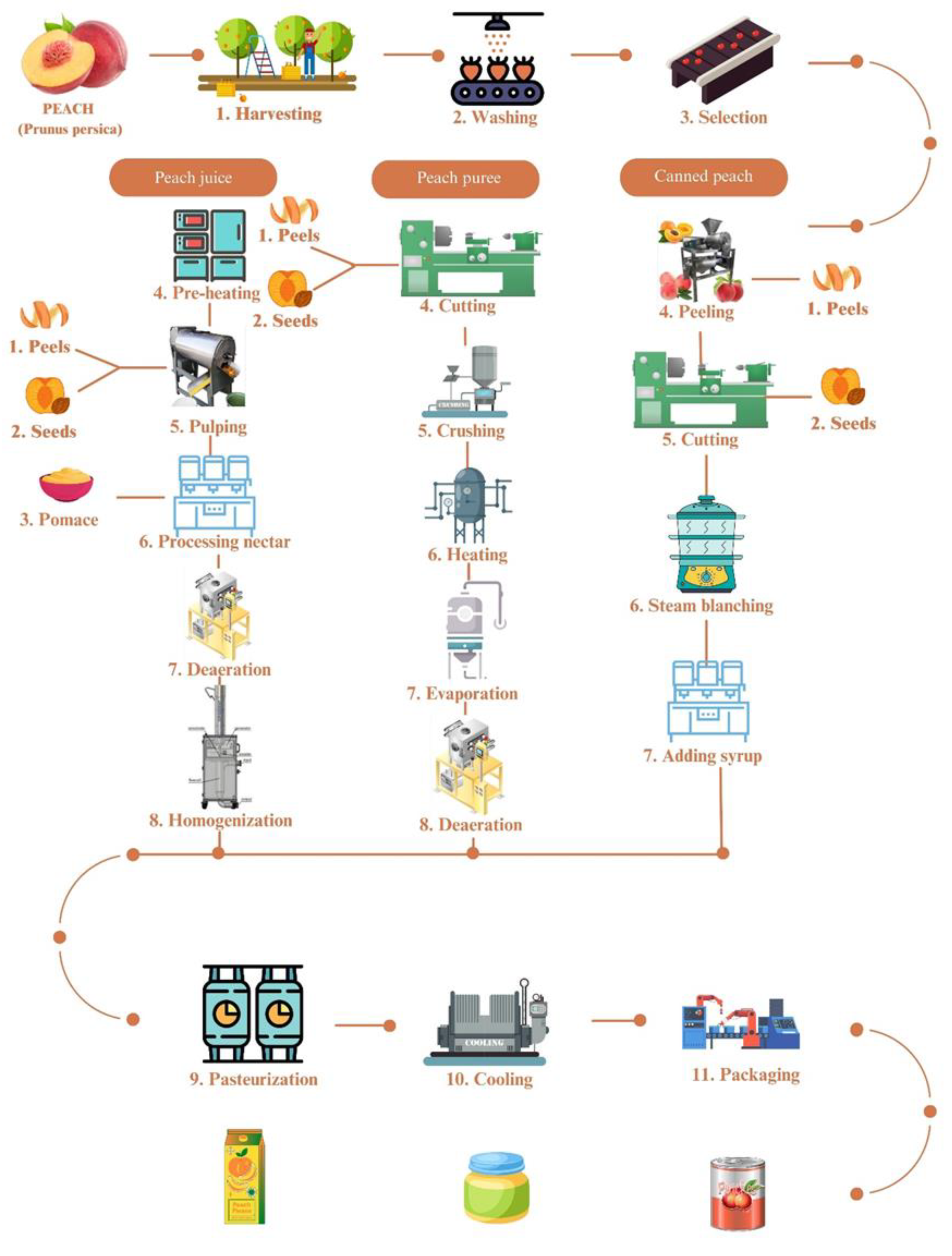
2. Peach and its products and by-products
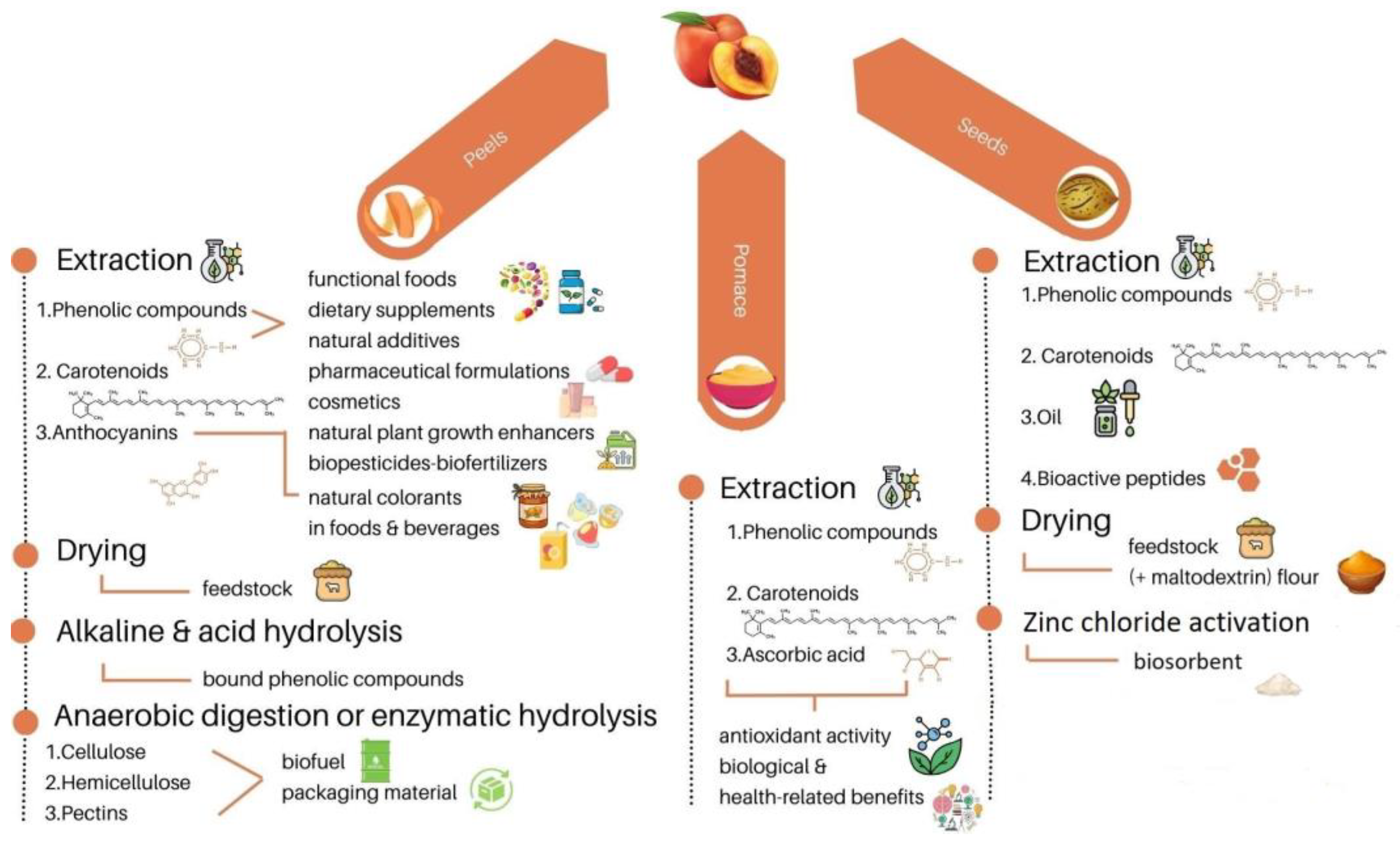
3. Peach peels
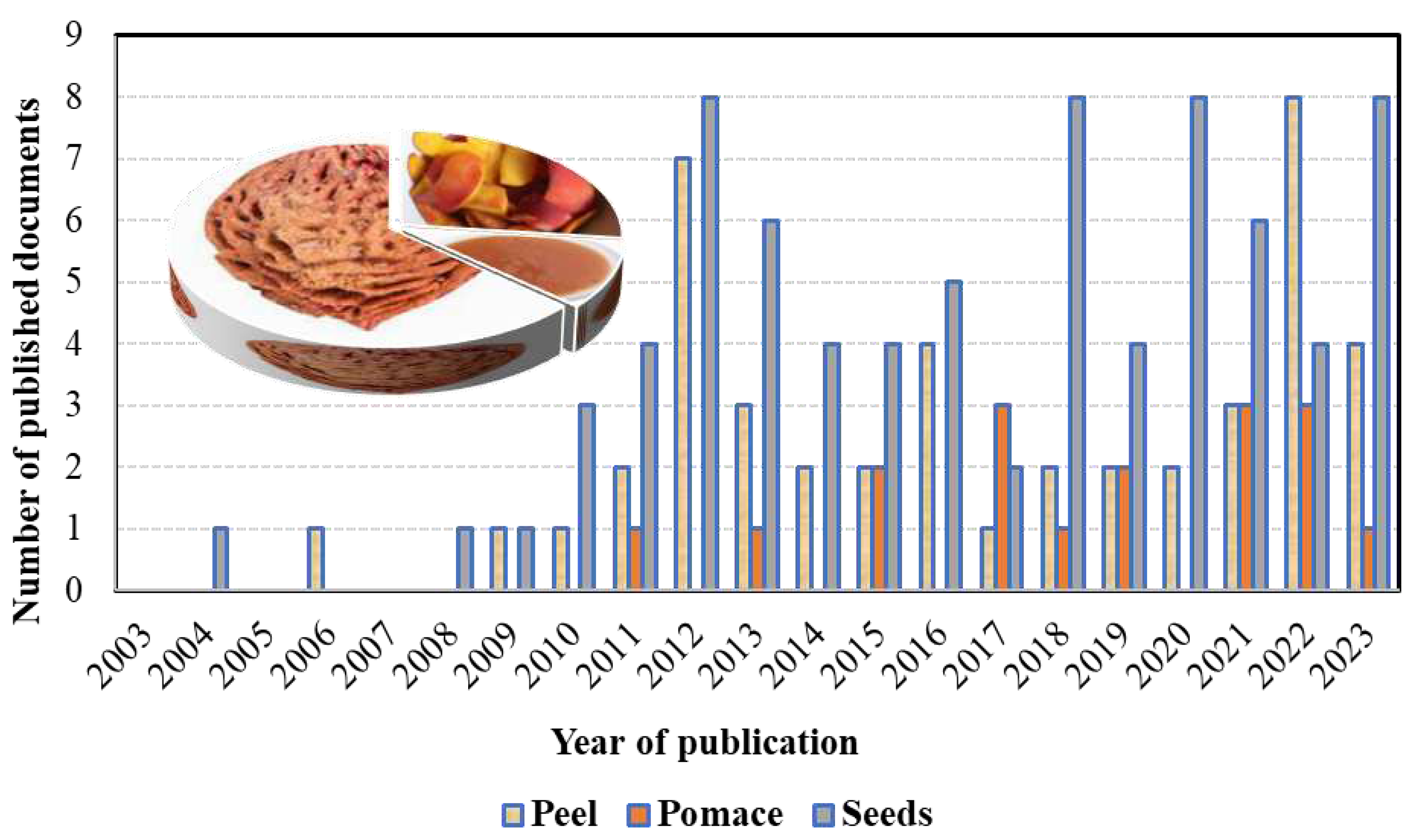
3.1. Peach peels identification
3.2. Valorization of peach peels
3.2.1. Maceration extraction of phenolic compounds
3.2.2. Ultrasound-assisted extraction of phenolic compounds
3.2.4. Exploitation of peach peels extract
3.2.5. Other uses of peach peels
4. Peach seeds
4.1. Peach seeds identification
4.2. Valorization of peach seeds
4.2.1. Extraction of oil
4.2.2. Exploitation of peach seeds oil
4.2.3. Recovery of bioactive components
4.2.4. Other uses of peach seeds
5. Peach pomace
5.1. Peach pomace identification
5.2. Valorization of peach pomace
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agricultural Organization of the United Nations (FAOSTAT) Website. 2020. https://www.fao.org/faostat/en/#data/QCL.
- Manzoor, M.; Anwar, F.; Mahmood, Z.; Rashid, U.; Ashraf, M. Variation in minerals, phenolics and antioxidant activity of peel and pulp of different varieties of peach (Prunus persica L.) fruit from Pakistan. Molecules 2012, 17(6), 6491–6506. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, S. A Complete Course in Canning and Related Processes: Volume 1 Fundemental Information on Canning; Woodhead Publishing, Cambridge, UK, 2015.
- Nowicka, P.; Wojdyło, A. Content of bioactive compounds in the peach kernels and their antioxidant, anti-hyperglycemic, anti-aging properties. Eur Food Res Technol 2019, 245(5), 1123–1136. [Google Scholar] [CrossRef]
- Uysal, T.; Duman, G.; Onal, Y.; Yasa, I.; Yanik, J. Production of activated carbon and fungicidal oil from peach stone by two-stage process. J Anal Appl Pyrolysis 2014, 108, 47–55. [Google Scholar] [CrossRef]
- Kamenidou, I.; Tzimitra-Kalogianni, I.; Zotos, Y.; Mattas, K. Household purchasing and consumption behaviour towards processed peach products. New Medit 2002, 1(1), 45–49. [Google Scholar]
- Plazzotta, S.; Ibarz, R.; Manzocco, L.; Martín-Belloso, O. Optimizing the antioxidant biocompound recovery from peach waste extraction assisted by ultrasounds or microwaves. Ultrason Sonochem 2020, 63, 104954. [Google Scholar] [CrossRef]
- Vásquez-Villanueva, R.; Marina, M.L.; García, M.C. Revalorization of a peach (Prunus persica (L.) Batsch) byproduct: Extraction and characterization of ACE-inhibitory peptides from peach stones. J Funct Foods 2015, 18, 137–146. [Google Scholar] [CrossRef]
- Wu, H.; Shi, J.; Xue, S.; Kakuda, Y.; Wang, D.; Jiang, Y.; Ye, X.; Li, Y.; Subramanian, J. Essential oil extracted from peach (Prunus persica) kernel and its physicochemical and antioxidant properties. LWT-Food Sci Technol 2011, 44(10), 2032–2039. [Google Scholar] [CrossRef]
- Rudke, C.R.M.; Zielinski, A.A.F.; Ferreira, S.R.S. From biorefinery to food product design: Peach (Prunus persica) by-products deserve attention. Food Bioproc Technol 2023, 16(6), 1197–1215. [Google Scholar] [CrossRef] [PubMed]
- Zhivkova, V. Evaluation of Nutritional and Mineral Composition of Apricot, Peach and Nectarine Wasted Peels. Calitatea 2020, 21(179), 144–146. [Google Scholar]
- Saidani, F.; Giménez, R.; Aubert, C.; Chalot, G.; Betrán, J. A.; Gogorcena, Y. Phenolic, sugar and acid profiles and the antioxidant composition in the peel and pulp of peach fruits. J Food Compos Anal 2017, 62, 126–133. [Google Scholar] [CrossRef]
- Mihaylova, D.; Popova, A.; Desseva, I.; Dincheva, I.; Tumbarski, Y. Valorization of Peels of Eight Peach Varieties: GC–MS Profile, Free and Bound Phenolics and Corresponding Biological Activities. Antioxidants 2023, 12(1), 205. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, M.; Karagiannis, E.; Nasiopoulou, E.; Skodra, C.; Molassiotis, A.; Tanou, G. Peach, apple, and pear fruit quality: To peel or not to peel? Horticulturae 2021, 7(4), 85. [Google Scholar] [CrossRef]
- Bassi, D.; Mignani, I.; Spinardi, A.; Tura, D. Peach (Prunus persica (L.) Batsch). In Nutritional composition of fruit cultivars, Simmonds, M.S.J.; Preedy, V.R., Eds.; Academic Press, USA, 2016; pp. 535-571. [CrossRef]
- Brown, A.F.; Yousef, G.G.; Guzman, I.; Chebrolu, K.K.; Werner, D.J.; Parker, M.; Gasic, K.; Perkins-Veazie, P. Variation of carotenoids and polyphenolics in peach and implications on breeding for modified phytochemical profiles. J Am Soc Hortic Sci 2014, 139(6), 676–686. [Google Scholar] [CrossRef]
- Mannino, G.; Ricciardi, M.; Gatti, N.; Serio, G.; Vigliante, I.; Contartese, V.; Gentile, C.; Bertea, C.M. Changes in the Phytochemical Profile and Antioxidant Properties of Prunus persica Fruits after the Application of a Commercial Biostimulant Based on Seaweed and Yeast Extract. Int J Mol Sci 2022, 23(24), 15911. [Google Scholar] [CrossRef] [PubMed]
- Dabbou, S.; Maatallah, S.; Castagna, A.; Guizani, M.; Sghaeir, W.; Hajlaoui, H.; Ranieri, A. Carotenoids, phenolic profile, mineral content and antioxidant properties in flesh and peel of Prunus persica fruits during two maturation stages. Plant Foods Hum Nutr 2017, 72, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Bento, C.; Goncalves, A.C.; Silva, B.; Silva, L.R. Peach (Prunus persica): Phytochemicals and health benefits. Food Rev Int 2022, 38(8), 1703–1734. [Google Scholar] [CrossRef]
- Redondo, D.; Gimeno, D.; Calvo, H.; Venturini, M.E.; Oria, R.; Arias, E. Antioxidant activity and phenol content in different tissues of stone fruits at thinning and at commercial maturity stages. Waste Biomass Valor 2021, 12, 1861–1875. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.H. Comparative study of proteasome inhibitory, synergistic antibacterial, synergistic anticandidal, and antioxidant activities of gold nanoparticles biosynthesized using fruit waste materials. Int J Nanomed 2016, 4691–4705. [Google Scholar] [CrossRef]
- Petruccelli, R.; Bonetti, A.; Ciaccheri, L.; Ieri, F.; Ganino, T.; Faraloni, C. Evaluation of the fruit quality and phytochemical compounds in peach and nectarine cultivars. Plants 2023, 12(8), 1618. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Z.; Ma, Q.; Mu, J.; Li, X.; Liu, H. Preparation and characterization of yellow peach peel/sodium alginate/glycerol antioxidant film applicable for oil package. Polymers 2022, 14(9), 1693. [Google Scholar] [CrossRef]
- de Escalada Pla, M.F.; González, P.; Sette, P.; Portillo, F.; Rojas, A.M.; Gerschenson, L.N. Effect of processing on physico-chemical characteristics of dietary fibre concentrates obtained from peach (Prunus persica L.) peel and pulp. Food Res Int, 2012; 49(1), 184–192. [Google Scholar] [CrossRef]
- Colaric, M.; Veberic, R.; Stampar, F.; Hudina, M. Evaluation of peach and nectarine fruit quality and correlations between sensory and chemical attributes. J Sci Food Agric 2005, 85(15), 2611–2616. [Google Scholar] [CrossRef]
- Liu, T.; Song, S.; Yuan, Y.; Wu, D.; Chen, M.; Sun, Q.; Zhang, B.; Xu, C.; Chen, K. Improved peach peel color development by fruit bagging. Enhanced expression of anthocyanin biosynthetic and regulatory genes using white non-woven polypropylene as replacement for yellow paper. Sci Hortic, 2015b; 184, 142–148. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Yin, X.; Su, M.; Sun, C. , Li; X., Chen, K. Phenolic composition and antioxidant properties of different peach [Prunus persica (L.) Batsch] cultivars in China. Int J Mol Sci 2015, 16(3), 5762–5778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Antioxidant effect of peach skin extracts from 13 varieties of South Carolina grown peaches. Doctoral dissertation, Clemson University, USA, August 2014.
- Chang, S.; Tan, C.; Frankel, E.N.; Barrett, D.M. Low-density lipoprotein antioxidant activity of phenolic compounds and polyphenol oxidase activity in selected clingstone peach cultivars. J Agric Food Chem 2000, 48(2), 147–151. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.; Cao, J.; Ma, L. Evaluation of antioxidant properties of extractable and nonextractable polyphenols in peel and flesh tissue of different peach varieties. J Food Proc Preserv 2018, 42(6), e13624. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, Z.; Ye, Z. Key proteins associated to coloured compounds of peach peel using iTRAQ proteomic techniques during development and postharvest. Sci Hortic 2018, 239, 123–132. [Google Scholar] [CrossRef]
- Yusoff, I.M.; Taher, Z.M.; Rahmat, Z.; Chua, L.S. A review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins. Food Res Int 2022, 157, 111268. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem 2014, 152, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Verma, B.; Hucl, P.; Chibbar, R.N. Phenolic acid composition and antioxidant capacity of acid and alkali hydrolysed wheat bran fractions. Food Chem 2009, 116(4), 947–954. [Google Scholar] [CrossRef]
- Chen, G.L.; Zhang, X.; Chen, S.G.; Han, M.D.; Gao, Y.Q. Antioxidant activities and contents of free, esterified and insoluble-bound phenolics in 14 subtropical fruit leaves collected from the south of China. J Funct Foods 2017, 30, 290–302. [Google Scholar] [CrossRef]
- Layne, D.; Bassi, D. The peach: botany, production and uses, Cabi., 2008.
- Pokorny, J.; Yanishlieva, N.; Gordon, M. Antioxidants in Food: Practical Application. In The development of oxidative rancidity in foods, Gordon, M., Ed.; Woodhead Publishing Limited, Florida, USA, 2001; pp 11.
- Rossato, S.B.; Haas, C.; Raseira, M.D.C.B.; Moreira, J.C.F.; Zuanazzi, J.A.S. Antioxidant potential of peels and fleshes of peaches from different cultivars. J Med Food 2009, 12(5), 1119–1126. [Google Scholar] [CrossRef]
- Kan, J.; Chen, C.; Huo, T.; Xie, W.; Hui, Y.; Liu, J.; Jin, C. Polyphenolic-enriched peach peels extract regulates lipid metabolism and improves the gut microbiota composition in high fat diet-fed mice. J Funct Foods 2020, 72, 104082. [Google Scholar] [CrossRef]
- Kultys, E.; Kurek, M.A. Green extraction of carotenoids from fruit and vegetable byproducts: A review. Molecules 2022, 27(2), 518. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Sun, M.; Li, J.; Su, Z.; Cai, Z.; Shen, Z.; Ma, R.; Yan, J.; Yu, M. Carotenoid profiling of yellow-flesh peach fruit. Foods 2022, 11(12), 1669. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, M.; Bakshi, M.P.S.; Makkar, H.P.S. Wastes to worth: value added products from fruit and vegetable wastes. CABI Reviews 2016, 2015, 1–25. [Google Scholar] [CrossRef]
- Schilderman, P.A.E.L.; Ten Vaarwerk, F.J.; Lutgerink, J.T.; Van der Wurff, A.; Ten Hoor, F.; Kleinjans, J.C.S. Induction of oxidative DNA damage and early lesions in rat gastro-intestinal epithelium in relation to prostaglandin H synthase-mediated metabolism of butylated hydroxyanisole. Food Chem Toxicol 1995, 33(2), 99–109. [Google Scholar] [CrossRef] [PubMed]
- Raturi, R.; Singh, H.; Bahuguna, P.; Sati, S.C.; Badoni, P.P. Antibacterial and antioxidant activity of methanolic extract of bark of Prunus persica. J Appl Nat Sci 2011, 3(2), 312–314. [Google Scholar] [CrossRef]
- Yao, X.C.; Cao, Y.; Wu, S.J. Antioxidant activity and antibacterial activity of peach gum derived oligosaccharides. Int J Biol Macromol 2013, 62, 1–3. [Google Scholar] [CrossRef]
- Nanni, V. Antimicrobial Activity of Peach and Grapevine Defensins, Doctoral dissertation, Università di Bologna, Bologna, Italy, 2012.
- Kumar, D.R.; Kumar, M.A.; Naidu, P.B. Evaluation of Anthelmintic Activity of Prunus Persica (L.). Asian J Pharm Clin Res 2015, 4(5), 716–721. [Google Scholar]
- Angulo-López, J.E.; Flores-Gallegos, A.C.; Ascacio-Valdes, J.A.; Contreras Esquivel, J.C.; Torres-León, C.; Rúelas-Chácon, X.; Aguilar, C.N. Antioxidant dietary fiber sourced from agro-industrial byproducts and its applications. Foods 2022, 12(1), 159. [Google Scholar] [CrossRef]
- Sousa, S.D.F.; da Silva, F.B.; de Araújo, A.C.; Gomes, J.P. Determinação das propriedades físicas e físico-químicas de pêssegos cultivar Rubimel. Rev Bras Tecnol Agroind 2018, 12(2). [Google Scholar] [CrossRef]
- Shahid, I.; Dildar, A. Nutritional and physicochemical studies on fruit pulp, seed and shell of indigenous Prunus persica. J Med Plants Res 2011, 5(16), 3917–3921. [Google Scholar]
- Zerva, E.; Abatis, D.; Skaltsounis, A.L.; Fokialakis, N. Development and application of a methodology for the recovery of high added value products from peach industry waste. Planta Medica 2012, 78(11), PJ98. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Gil, M.I.; Cremin, P.; Waterhouse, A.L.; Hess-Pierce, B.; Kader, A.A. HPLC− DAD− ESIMS analysis of phenolic compounds in nectarines, peaches, and plums. J Agric Food Chem 2001, 49(10), 4748–4760. [Google Scholar] [CrossRef] [PubMed]
- Mokrani, A.; Krisa, S.; Cluzet, S.; Da Costa, G.; Temsamani, H.; Renouf, E.; Mérillon, J.M.; Madani, K.; Mesnil, M.; Monvoisin, A.; Richard, T. Phenolic contents and bioactive potential of peach fruit extracts. Food Chem 2016a, 202, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Wang, Z.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. High-throughput screening and characterization of phenolic compounds in stone fruits waste by LC-ESI-QTOF-MS/MS and their potential antioxidant activities. Antioxidants 2021, 10(2), 234. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, C.; Ravaglia, D.; Ragaini, A.; Costa, G. Phenolic compounds in peach (Prunus persica) cultivars at harvest and during fruit maturation. Ann Appl Biol 2008, 153(1), 11–23. [Google Scholar] [CrossRef]
- de Ancos, B.; González, E.M.; Cano, M.P. Ellagic acid, vitamin C, and total phenolic contents and radical scavenging capacity affected by freezing and frozen storage in raspberry fruit. J Agric Food Chem 2000, 48(10), 4565–4570. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, P.; Wojdyło, A.; Samoticha, J. Evaluation of phytochemicals, antioxidant capacity, and antidiabetic activity of novel smoothies from selected Prunus fruits. J Funct Foods 2016, 25, 397–407. [Google Scholar] [CrossRef]
- Nüβlein, B.; Kreis, W. Purification and characterization of a cynaroside 7-O-β-D-glucosidase from Cynarae scolymi folium. In IV International Congress on Artichoke, Bari, Italy, 2000, October; pp. 413-420. [CrossRef]
- Campbell, O.E.; Padilla-Zakour, O.I. Phenolic and carotenoid composition of canned peaches (Prunus persica) and apricots (Prunus armeniaca) as affected by variety and peeling. Food Res Int 2013, 54(1), 448–455. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. J Agric Food Chem 2002, 50(17), 4976–4982. [Google Scholar] [CrossRef]
- Giuffrida, D.; Torre, G.; Dugo, P.; Dugo, G. Determination of the carotenoid profile in peach fruits, juice and jam. Fruits 2013, 68(1), 39–44. [Google Scholar] [CrossRef]
- Buratti, C.; Foschini, D.; Barbanera, M.; Fantozzi, F. Fermentable sugars production from peach tree prunings: Response surface model optimization of NaOH alkaline pretreatment. Biomass Bioenerg 2018, 112, 128–137. [Google Scholar] [CrossRef]
- Conde-Mejia, C.; Jimenez-Gutierrez, A.; El-Halwagi, M. A comparison of pretreatment methods for bioethanol production from lignocellulosic materials. Proc Saf Environ Prot 2012, 90(3), 189–202. [Google Scholar] [CrossRef]
- Shukla, R.K.; Kant, R. Assessment of phytochemical screening by Fourier Transform Infrared spectroscopic analysis of peach (Prunus persica) seed biomass from Uttarakhand region of India. J Appl Nat Sci 2020, 12(4), 519–524. [Google Scholar] [CrossRef]
- Rahma, E.H.; Abd El-Aal, M.H. Chemical characterization of peach kernel oil and protein: Functional properties, in vitro digestibility and amino acids profile of the flour. Food Chem 1988, 28(1), 31–43. [Google Scholar] [CrossRef]
- Hao, E.; Pang, G.; Du, Z.; Lai, Y.H.; Chen, J.R.; Xie, J.; Zhou, K.; Hou, X.; Hsia, C.D.; Deng, J. Peach kernel oil downregulates expression of tissue factor and reduces atherosclerosis in ApoE knockout mice. Int J Mol Sci 2019, 20(2), 405. [Google Scholar] [CrossRef] [PubMed]
- Sodeifian, G.; Sajadian, S.A. Antioxidant capacity, physicochemical properties, thermal behavior, and oxidative stability of nectarine (Prunus persica var. nucipersica) kernel oil. J Food Proc Preserv 2021, 45(2), e15198. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, E.; Marina, M.L.; García, M.C.; Righetti, P.G.; Fasoli, E. Identification of plum and peach seed proteins by nLC-MS/MS via combinatorial peptide ligand libraries. J Proteom 2016, 148, 105–112. [Google Scholar] [CrossRef]
- Gettens, C.S. Propriedades funcionais, nutricionais e atividade antimicrobiana de subprodutos agroindustriais de pêssego e sua aplicação em cookies. Doctoral dissertation, Dissertação (mestrado)–Programa de Pós-Graduação em Nutrição e Alimentos. Universidade Federal de Pelotas, 2016.
- Lazos, E.S. Composition and oil characteristics of apricot, peach and cherry kernel. Grasas y Aceites 1991, 42(2), 127–131. [Google Scholar] [CrossRef]
- Mezzomo, N.; Mileo, B.R.; Friedrich, M.T.; Martínez, J.; Ferreira, S.R. Supercritical fluid extraction of peach (Prunus persica) almond oil: process yield and extract composition. Biores Technol 2010, 101(14), 5622–5632. [Google Scholar] [CrossRef]
- Martínez, M.L.; Mattea, M.A.; Maestri, D.M. Pressing and supercritical carbon dioxide extraction of walnut oil. J Food Eng 2008, 88(3), 399–404. [Google Scholar] [CrossRef]
- Oliveira, R.; Fátima Rodrigues, M.; Gabriela Bernardo-Gil, M. Characterization and supercritical carbon dioxide extraction of walnut oil. J Am Oil Chem Soc 2002, 79(3), 225–230. [Google Scholar] [CrossRef]
- Özkal, S.G.; Salgın, U.; Yener, M.E. Supercritical carbon dioxide extraction of hazelnut oil. J Food Eng 2005, 69(2), 217–223. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical fluid extraction and fractionation of natural matter. J Supercrit Fluids 2006, 38(2), 146–166. [Google Scholar] [CrossRef]
- Kahla, N.E.; SafeKordi, A. Evaluation of temperature & solvent effect on peach kernel oil extraction & determination & quantification of its fatty. Evaluation 2012, 2(2). [Google Scholar]
- Mezzomo, N.; Martínez, J.; Ferreira, S.R. Supercritical fluid extraction of peach (Prunus persica) almond oil: kinetics, mathematical modeling and scale-up. J Supercrit Fluids 2009, 51(1), 10–16. [Google Scholar] [CrossRef]
- Barwick, V.J. Strategies for solvent selection—a literature review. Trends Anal Chem 1997, 16(6), 293–309. [Google Scholar] [CrossRef]
- Markom, M.; Hasan, M.; Daud, W.R.W.; Singh, H.; Jahim, J.M. Extraction of hydrolysable tannins from Phyllanthus niruri Linn.: Effects of solvents and extraction methods. Sep Purif Technol 2007, 52(3), 487–496. [Google Scholar] [CrossRef]
- Jouyban, A.; Chan, H.K.; Foster, N.R. Mathematical representation of solute solubility in supercritical carbon dioxide using empirical expressions. J Supercrit Fluids 2002, 24(1), 19–35. [Google Scholar] [CrossRef]
- Michielin, E.M.; Bresciani, L.F.; Danielski, L.; Yunes, R.A.; Ferreira, S.R. Composition profile of horsetail (Equisetum giganteum L.) oleoresin: comparing SFE and organic solvents extraction. J Supercrit Fluids 2005, 33(2), 131–138. [Google Scholar] [CrossRef]
- Vági, E.; Simándi, B.; Suhajda, A.; Hethelyi, E. Essential oil composition and antimicrobial activity of Origanum majorana L. extracts obtained with ethyl alcohol and supercritical carbon dioxide. Food Res Int 2005, 38(1), 51–57. [Google Scholar] [CrossRef]
- Danielski, L.; Michielin, E.M.; Ferreira, S.R. Horsetail (Equisetum giganteum L.) oleoresin and supercritical CO2: Experimental solubility and empirical data correlation. J Food Eng 2007, 78(3), 1054–1059. [Google Scholar] [CrossRef]
- Kitzberger, C.S.; Lomonaco, R.H.; Michielin, E.M.; Danielski, L.; Correia, J.; Ferreira, S.R. Supercritical fluid extraction of shiitake oil: curve modeling and extract composition. J Food Eng 2009, 90(1), 35–43. [Google Scholar] [CrossRef]
- Karadimou, C.C.; Koletti, A.E.; Moschona, A.; Gika, H.G.; Vlachos, D.; Assimopoulou, A.N. Assimopoulou, A.N. Peach Kernel: A Potential Source for Cosmeceuticals. In Metrology Promoting Harmonization & Standardization in Food & Nutrition, Thessaloniki, Greece, 1st–4th October 2017.
- Li, W.; Xing, L.; Cai, Y.; Qu, H. Classification and quantification analysis of Radix scutellariae from different origins with near infrared diffuse reflection spectroscopy. Vib Spectrosc 2011, 55(1), 58–64. [Google Scholar] [CrossRef]
- Ordoudi, S.A.; Bakirtzi, C.; Tsimidou, M.Z. The potential of tree fruit stone and seed wastes in Greece as sources of bioactive ingredients. Recycling 2018, 3(1), 9. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Olšteine, A.; Krasnova, I.; Pugajeva, I.; Lācis, G.; Siger, A.; Michalak, M.; Soliven, A.; Segliņa, D. Phenolic compounds in different fruit parts of crab apple: Dihydrochalcones as promising quality markers of industrial apple pomace by-products. Ind Crops Prod 2015, 74, 607–612. [Google Scholar] [CrossRef]
- Rodrigues, F.; Pimentel, F.B.; Oliveira, M.B.P. Olive by-products: Challenge application in cosmetic industry. Ind Crops Prod 2015, 70, 116–124. [Google Scholar] [CrossRef]
- de Jesus Raposo, M.F.; de Morais, R.M.S.C.; de Morais, A.M.M.B. Health applications of bioactive compounds from marine microalgae. Life Sci 2013, 93(15), 479–486. [Google Scholar] [CrossRef]
- Matthaeus, B.; Oezcan, M.M. Fatty acids and tocopherol contents of some Prunus spp. kernel oils. J Food Lipids 2009, 16(2), 187–199. [Google Scholar] [CrossRef]
- Qi, Z.X.; Lei, C.H.E.N. Effect of Chinese drugs for promoting blood circulation and eliminating blood stasis on vascular endothelial growth factor expression in rabbits with glucocorticoid-induced ischemic necrosis of femoral head. J Tradit Chin Med 2009, 29(2), 137–140. [Google Scholar] [CrossRef]
- Kutlu, T.; Durmaz, G.; Ates, B.; Erdogan, A. Protective effect of dietary apricot kernel oil supplementation on cholesterol levels and antioxidant status of liver in hypercholesteremic rats. J Food Agric Environ 2009, 7, 61–65. [Google Scholar]
- Li, Y.; Wang, L. Genetic resources, breeding programs in China, and gene mining of peach: a review. Hortic Plant J 2020, 6(4), 205–215. [Google Scholar] [CrossRef]
- Anagnostopoulou, M.A.; Kefalas, P.; Papageorgiou, V.P.; Assimopoulou, A.N.; Boskou, D. Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chem 2006, 94(1), 19–25. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, Y.P.; Seo, E.M.; Choi, Y.K.; Kim, H.S. Antioxidant effect of natural plant extracts on the microencapsulated high oleic sunflower oil. J Food Eng 2008, 84(2), 327–334. [Google Scholar] [CrossRef]
- Redondo, D.; Venturini, M.E.; Oria, R.; Arias, E. Inhibitory effect of microwaved thinned nectarine extracts on polyphenol oxidase activity. Food Chem 2016, 197, 603–610. [Google Scholar] [CrossRef]
- Zheng, H.Z.; Kim, Y.I.; Chung, S.K. A profile of physicochemical and antioxidant changes during fruit growth for the utilization of unripe apples. Food Chem 2012, 131(1), 106–110. [Google Scholar] [CrossRef]
- Martins, C.M.; Ferro, D.M.; de Brito, E.S.; Ferreira, S.R.S. Industrial relevance of Tamarindus indica L. by-products as source of valuable active metabolites. Innov Food Sci Emerg Technol 2020, 66, 102518. [Google Scholar] [CrossRef]
- Lara, M.V.; Bonghi, C.; Famiani, F.; Vizzotto, G.; Walker, R.P.; Drincovich, M.F. Stone fruit as biofactories of phytochemicals with potential roles in human nutrition and health. Front Plant Sci 2020, 11, 562252. [Google Scholar] [CrossRef]
- Vásquez-Villanueva, R.; Orellana, J.M.; Marina, M.L.; García, M.C. Isolation and characterization of angiotensin converting enzyme inhibitory peptides from peach seed hydrolysates: In vivo assessment of antihypertensive activity. J Agric Food Chem 2019, 67(37), 10313–10320. [Google Scholar] [CrossRef]
- Kaltschmitt, M. Renewable energy from biomass: introduction; Springer, New York, USA, 2019; pp. 1-14.
- Paraskevopoulou, C.; Vlachos, D.; Bechtsis, D.; Tsolakis, N. An assessment of circular economy interventions in the peach canning industry. Int J Prod Econ 2022, 249, 108533. [Google Scholar] [CrossRef]
- Skoulou, V.; Zabaniotou, A. Investigation of agricultural and animal wastes in Greece and their allocation to potential application for energy production. Renew Sust Energ Rev 2007, 11(8), 1698–1719. [Google Scholar] [CrossRef]
- Qiu, P.L.; Liu, S.Y.; Bradshaw, M.; Rooney-Latham, S.; Takamatsu, S.; Bulgakov, T.S.; Tang, S.R.; Feng, J.; Jin, D.N.; Aroge, T.; Li, Y.; Wang, L.L.; Braun, U. Multi-locus phylogeny and taxonomy of an unresolved, heterogeneous species complex within the genus Golovinomyces (Ascomycota, Erysiphales), including G. ambrosiae, G. circumfusus and G. spadiceus. BMC Microbiol 2020, 20, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kul, A.R.; Aldemir, A.; Hasan, E.L.İ.K. Adsorption of Acid Blue 25 on peach seed powder: Isotherm, kinetic and thermodynamic studies. Environ Res Technol 2019, 2(4), 233–242. [Google Scholar] [CrossRef]
- Lima, B.NB.; Lima, F.F.; Tavares, M.I.B.; Costa, A.M.M.; Pierucci, A.P.T.R. Determination of the centesimal composition and characterization of flours from fruit seeds. Food Chem 2014, 151, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Pelentir, N.; Block, J.M.; Monteiro Fritz, A.R.; Reginatto, V.; Amante, E.R. Production and chemical characterization of peach (Prunus persica) kernel flour. J Food Proc Eng 2011, 34(4), 1253–1265. [Google Scholar] [CrossRef]
- Liu, H.; Cao, J.; Jiang, W. Evaluation and comparison of vitamin C, phenolic compounds, antioxidant properties and metal chelating activity of pulp and peel from selected peach cultivars. LWT - Food Sci Technol 2015a, 63, 1042-1048. [CrossRef]
- Loizzo, M.R.; Pacetti, D.; Lucci, P.; Núñez, O.; Menichini, F.; Frega, N.G.; Tundis, R. Prunus persica var. platycarpa (Tabacchiera Peach): Bioactive Compounds and Antioxidant Activity of Pulp, Peel and Seed Ethanolic Extracts. Plant Foods Human Nutr 2015, 70, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Argun, H.; Dao, S. Bio-hydrogen production from waste peach pulp by dark fermentation: Effect of inoculum addition. Int J Hydrog Energy 2017, 42(4), 2569–2574. [Google Scholar] [CrossRef]
- Geduk, A.S.; Atsız, S. LC-MS/MS phenolic composition of peach (Prunus persica (L.) Batsch) extracts and an evaluation of their antidiabetic, antioxidant, and antibacterial activities. S Afr J Bot 2022, 147, 636–645. [Google Scholar] [CrossRef]
- Vizzotto, M.; Cisneros-Zevallos, L.; Byrne, D.H. Large Variation Found in the Phytochemical and Antioxidant Activity of Peach and Plum Germplasm. J Am Soc Hortic Sci 2007, 132(3), 334–340. [Google Scholar] [CrossRef]
- Montevecchi, G.; Simone, G.V.; Masino, F.; Bignami, C.; Antonelli, A. Physical and chemical characterization of Pescabivona, a Sicilian white flesh peach cultivar [Prunus persica (L.) Batsch]. Food Res Int 2012, 45, 123–131. [Google Scholar] [CrossRef]
- Wu, J.; Fan, J.; Li, Y.; Cao, K.; Chen, C.; Wang, X.; Fang, W.; Zhu, G.; Wang, L. Characterizing of carotenoid diversity in peach fruits affected by the maturation and varieties. J Food Compos Anal 2022, 113, 104711. [Google Scholar] [CrossRef]
- Zuo, J.; Tang, W.; Xu, Y. Chapter 68-Anti-hepatitis b virus activity of chlorogenic acid and its related compounds. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 607–613. [Google Scholar]
- Gutiérrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-López, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, J.B. Flavonoids and Phenolic Acids from Oregano: Occurrence, Biological Activity and Health Benefits. Plants 2017, 7(1), 2. [Google Scholar] [CrossRef] [PubMed]
- Goula, A.M.; Ververi, M.; Adamopoulou, A.; Kaderides, K. Green ultrasound-assisted extraction of carotenoids from pomegranate wastes using vegetable oils. Ultrason Sonochem 2017, 34, 821–830. [Google Scholar] [CrossRef]
- Cao, S.; Liang, M.; Shi, L.; Shao, J.; Song, C.; Bian, K.; Chen, W.; Yang, Z. Accumulation of carotenoids and expression of carotenogenic genes in peach fruit. Food Chem 2017, 214, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Falchi, R.; Vendramin, E.; Zanon, L.; Scalabrin, S.; Cipriani, G.; Verde, I.; Vizzotto, G.; Morgante, M. Three distinct mutational mechanisms acting on a single gene underpin the origin of yellow flesh in peach. Plant J 2013, 76(2), 175–187. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhao, B.; Cai, Z.; Yan, J.; Ma, R.; Yu, M. Amino Acid Profiles in Peach (Prunus persica L.) Fruit. Foods 2022, 11, 1718. [Google Scholar] [CrossRef]
- Cirilli, M.; Bassi, D.; Ciacciulli, A. Sugars in peach fruit: a breeding perspective. Hortic Res 2016, 3, 12. [Google Scholar] [CrossRef]
- Zhang, X.; Su, M.; Du, J.; Zhou, H.; Li, X.; Zhang, M.; Hu, Y.; Ye, Z. Analysis of the free amino acid content and profile of 129 peach (Prunus persica (L.) Batsch) germplasms using LC-MS/MS without derivatization. J Food Compos Anal 2022, 114, 104811. [Google Scholar] [CrossRef]
- Planchet, E.; Rannou, O.; Ricoult, C.; Boutet-Mercey, S.; Maia-Grondard, A.; Limami, A.M. Nitrogen metabolism responses to water deficit act through both abscisic acid (ABA)-dependent and independent pathways in Medicago truncatula during post-germination. J Exp Bot 2011, 2, 605–615. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, Z. Understanding different regulatory mechanisms of proteinaceous and non-proteinaceous amino acid formation in tea (Camellia sinensis) provides new insights into the safe and effective alteration of tea flavor and function. Critical Rev Food Sci Nutr 2020, 60(5), 844–858. [Google Scholar] [CrossRef]
- Urano, K.; Maruyama, K.; Ogata, Y.; Morishita, Y.; Takeda, M.; Sakurai, N.; Suzuki, H.; Saito, K.; Shibata, D.; Kobayashi, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Characterization of the ABA-regulated global responses to dehydration in Arabidopsis by metabolomics. Plant J 2009, 57, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savoure, A. Proline: a multifunctional amino acid. Trends Plant Sci 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.; Sodek, L.; Licausi, F.; Hameed, M.W.; Dornelas, M.C.; van Dongen, J.T. Analysis of alanine aminotransferase in various organs of soybean (Glycine max) and independence of different nitrogen fertilisers during hypoxic stress. Amino Acids 2010, 39, 1043–1053. [Google Scholar] [CrossRef]
- Bae, J.; Shin, D.H.; Chun, B.Y.; Choi, B.Y.; Kim, M.K.; Shin, M.H.; Lee, Y.H.; Park, P.S.; Kim, S.K. The effect of vitamin C intake on the risk of hyperuricemia and serum uric acid level in Korean Multi-Rural Communities Cohort. Joint Bone Spine 2014, 81, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Cao, K.; Fang, W.; Zhu, G.; Chen, C.; Wang, X.; Wang, L. Evaluation of phenolic components (anthocyanins, flavanols, phenolic acids, and flavonols) and their antioxidant properties of peach fruits. Sci Hortic 2020, 268, 109365. [Google Scholar] [CrossRef]
- Mokrani, A.; Madani, K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep Purif Technol 2016b, 162, 68–76. [Google Scholar] [CrossRef]
- Tsiaka, T.; Lantzouraki, D.Z.; Polychronaki, G.; Sotiroudis, G.; Kritsi, E.; Sinanoglou, V.J.; Kalogianni, D.P.; Zoumpoulakis, P. Optimization of ultrasound-and microwave-assisted extraction for the determination of phenolic compounds in peach byproducts using experimental design and liquid chromatography–tandem mass spectrometry. Molecules 2023, 28(2), 518. [Google Scholar] [CrossRef]
- de Vargas, E.F.; Jablonski, A.; Flôres, S.H.; Rios, A.O. Waste from peach (Prunus persica) processing used for optimisation of carotenoids ethanolic extraction. Int J Food Sc Technol 2017, 52(3), 757–762. [Google Scholar] [CrossRef]
| Component | Content | Reference |
|---|---|---|
| Moisture | 88.04 ± 0.30% | [11] |
| Sugars | 7.58 ± 0.25% 9.29 – 18.96 mg/g dw 5.42 – 10.2 g/100 g fw |
[11,12,13] |
| Total dietary fibers | 1.31 ± 0.20% | [11] |
| Protein | 1.14 ± 0.15% | [11] |
| Fat | 0.08% | [11] |
| Total ash | 0.49 ± 0.01% | [11] |
| Phenolic compound | Content | Reference |
|---|---|---|
| Flavonols | ||
| 3’-Methylquercetin | 6.98 – 12.58 mg/100 g fw | [17] |
| Dihydrokaempferol | 0.28 – 0.45 mg/100 g fw | [17] |
| Dihydromyricetin | 0.31 – 1.45 mg/100 g fw | [17] |
| Dihydromyricetin-3-glucoside | 3.17 – 12.68 mg/100 g fw | [17] |
| Dihydroquercetin-3-galactoside | 23.09 – 160.55 mg/100 g fw | [17] |
| Dihydroquercetin-3-glucoside | 38.96 – 130.8 mg/100 g fw | [17] |
| Isorhamnetin-3-rutinoside | 4.61 – 22.66 mg/100 g fw | [17] |
| Kaempferol | 0.18 – 2.98 mg/100 g fw | [17] |
| Kaempferol-3-galactoside | 14.73 – 211.08 mg/100 g fw | [17] |
| Kaempferol-3-glucuronide | 3.76 – 21.32 mg/100 g fw | [17] |
| Kaempferol-3-glucoside | 0.08 – 78.89 mg/100 g fw | [12,17] |
| Kaempferol-3-rhamnoside | 65.64 – 129.32 mg/100 g fw | [17] |
| Quercetin | 5.34 – 12.81 mg/100 g fw | [17] |
| Quercetin-3-galactoside | 0.16 – 79.11 mg/100 g fw | [12,15,17,18] |
| Quercetin-3-glucoside | 0.52 – 9.69 mg/100 g fw | [15] |
| Quercetin-3-rutinoside | 0.53 – 50.61 mg/100 g fw | [15,17,18,19] |
| Anthocyanins | ||
| Cyanidin-3-glucoside | 0.07 – 32.51 mg/100 g fw | [12,15,18,19] |
| Cyanidin-3-rutinoside | 0.18 – 6.35 mg/100 g fw | [15,18,19] |
| Hydroxycinnamic acids | ||
| 4-caffeoylquinic acid | 0.08 – 0.70 mg/100 g fw | [12] |
| Caffeoylquinic acid derivative | 0.25 – 0.98 mg/100 g fw | [12] |
| Chlorogenic acid | 0.1 – 47.05 mg/100 g fw 84.2 – 355.9 mg/100 g dw |
[12,13,15,18,19,20] |
| Coumaric acid | 2.9 – 3.2 mg/100 g fw | [20] |
| Ferulic acid | 1.2 mg/100 g fw | [20] |
| Neochlorogenic acid | 0.03 – 34.6 mg/100 g fw | [12,15,18,19,20] |
| p-coumaroylquinic acid | 0.03 – 0.12 mg/100 g fw | [12] |
| trans-caffeic acid | 2.6 – 12.8 mg/100g dw | [13] |
| trans-ferulic acid | 2.6 – 13.7 mg/100 g dw | [13] |
| trans-p-coumaric acid | 8.3 – 18.5 mg/100 g dw | [13] |
| trans-sinapic acid | 2.2 – 7.2 mg/100 g dw | [13] |
| Hydroxybenzoic acids | ||
| Gallic acid | 4.47 – 8.48 mg/100 g fw | [18] |
| Protocatechuic acid | 3.3 – 27.3 mg/100 g dw | [13] |
| Flavan-3-ols | ||
| Catechin | 0.12 – 31.89 mg/100 g fw | [12,15,17,18,19,20] |
| Catechin 3’,5-diglucoside | 2.25 – 4.32 mg/100 g fw | [17] |
| Epicatechin | 0.64 – 20.6 mg/100 g fw | [15,17,18,19,20] |
| Flavanones | ||
| Eriodictyol | 0.36 – 0.51 mg/100 g fw | [17] |
| Eriodictyol-7-glucoside | 0.45 – 1.97 mg/100 g fw | [17] |
| Eriodictyol-7-neohesperidoside | 0.33 – 16.95 mg/100 g fw | [17] |
| Eriodictyol-7-rutinoside | 5.2 – 29.83 mg/100 g fw | [17] |
| Hesperetin | 3.43 – 13.75 mg/100 g fw | [17] |
| Hesperetin-7-rutinoside | 2.63 – 55.79 mg/100 g fw | [17] |
| Naringenin | 0.56 – 2.13 mg/100 g fw | [17] |
| Naringenin-7-glucoside | 3.2 – 32.46 mg/100 g fw | [17] |
| Naringenin-7-glucuronide | 3.79 – 13.01 mg/100 g fw | [17] |
| Naringenin-7-rutinoside | 1.32 – 9.43 mg/100 g fw | [17] |
| Flavones | ||
| Luteolin | 0.05 – 2.97 mg/100g fw | [17] |
| Luteolin-7-glucuronide | 8.45 – 156.89 mg/100g fw | [17] |
| Luteolin-7-rutinoside | 0.64 – 20.3 mg/100g fw | [17] |
| Proanthocyanidins | ||
| PAC-A type dimer | 2.87 – 7.24 mg/100 g fw | [17] |
| PAC-A type trimer | 0.07 – 0.29 mg/100 g fw | [17] |
| PAC-B type dimer | 119.13 – 1762.13 mg/100 g fw | [17] |
| PAC-B type tetramer | 0.44 – 3.53 mg/100 g fw | [17] |
| Procyanidin B1 | 0.04 – 49.23 mg/100 g fw | [12,15,19] |
| Procyanidin B2 | 0.02 – 0.10 mg/100 g fw | [12] |
| Extraction method | Conditions | Target compound | Yield | Reference |
|---|---|---|---|---|
| Maceration | 0.05% HCl in methanol, dark | Anthocyanins | Total anthocyanin content: 1-8 mg cyanidin-3-glucoside equivalents/100 g fw | [26] |
| Ultrasound-assisted extraction | 80% MeOH, 60 kHz, 30 W, 30 min | Phenolic compounds | Neochlorogenic acid: 5.77 – 342.75 mg/kg dw Chlorogenic acid: 52.2 – 1631.25 mg/kg dw Procyanidin B1: 54.76 – 539.22 mg/kg dw Catechin: 60.14 – 1030.06 mg/kg dw Cyanidin-3-glucoside: 9.33 – 670.59 mg/kg dw Quercetin-3-galactoside: 8.45 – 396.49 mg/kg dw Quercetin-3-glucoside: 2.45 – 581.21 mg/kg dw Quercetin-3-rutinoside: 59.15 – 193.25 mg/kg dw Kaempferol-3-rutinoside: 16.91 – 110.86 mg/kg dw Total phenolics: 4.58 – 12.68 mg gallic acid equivalents/g dw |
[27] |
| Ultrasound-assisted extraction | 50% EtOH, 42 kHz, 30 min, room temperature | Phenolic compounds | Total phenolic content: 8.38 – 18.81 mg gallic acid equivalents/g dw | [28] |
| Maceration | 80% MeOH, 1 min blending | Phenolic compounds | Total phenolic content: 877 – 1896 mg gallic acid equivalents/kg Neochlorogenic acid: 22.5 – 80.9 mg chlorogenic acid equivalents/kg Chlorogenic acid: 164.4 – 470.5 mg/kg Malvin: 0.0 – 75.6 mg/kg Rutin: 7.1 – 81.3 mg/kg Isoquercetin: 38.0 – 96.9 mg rutin equivalents/kg Procyanidin B1: 69.6 – 279 mg catechin equivalents/kg Catechin: 53.1 – 434 mg/kg |
[29] |
| Ultrasound-assisted extraction (free phenolics) Alkaline hydrolysis (bound phenolics) Acid hydrolysis (bound phenolics) |
80% EtOH, 50oC, 30 min (free) 2M NaOH, 18h digestion, 30 oC, 6M HCl to pH 1.5-2.0, extraction with ethyl acetate (bound-alkaline) MeOH/H2SO4 (90:10), 70oC, 24h, sonication, 10M NaOH to pH 12.0, extraction with ethyl acetate (bound-acid) |
Phenolic compounds | Total phenolic content: 6.82 – 13.12 mg gallic acid equivalents/g dw (free) 7 – 31% of total phenolics (bound) Total flavonoids: 164.14 – 515.83 μg quercetin equivalents/g dw (free) 0 – 76.93% of total flavonoids (bound) Total monomeric anthocyanins: 327.84 – 1246.77 μg cyanidin-3-glucoside/g dw (free) 0 – 49% of total anthocyanins (bound) |
[13] |
| Maceration | 80% MeOH, 8h, room temperature, shaking | Phenolic compounds | Total phenolic content: 1209.3 – 1354.5 mg gallic acid equivalents/100 g dw Total flavonoids: 599.7 – 785.5 mg catechin equivalents/100 g dw |
[2] |
| Maceration (free phenolics) Acid hydrolysis (bound phenolics) |
50% EtOH, pH 2.0, 1h, shaking, 70% acetone, shaking (free) MeOH/H2SO4 (90:10), 85 oC, 20h (bound) |
Phenolic compounds | Total phenolic content: 79.14 – 167.10 mg gallic acid equivalents/100 g fw (free) 52.93 – 84.02 mg gallic acid equivalents/100 g fw (bound) |
[30] |
| Maceration | MeOH/H2O/formic acid (60:38:2) | Phenolic compounds | Total phenolic content: 88.9 – 277.0 mg gallic acid equivalents/100 g fw Total flavonoids: 39.3 – 245 mg catechin equivalents/100 g fw Total anthocyanin content: 0.55 – 17.6 mg cyanidin-3-glucoside equivalents/100 g fw Quercetin-3-galactoside: 0.16 – 0.78 mg/100 g fw Quercetin-3-O-glucoside plus quercetin-3-O-rutinoside: 0.30 – 1.84 mg/100 g fw Kaempferol-3-O-glucoside: 0.08 – 0.40 mg/100 g fw Neochlorogenic acid: 1.02 – 7.98 mg/100 g fw p-coumaroylquinic acid: 0.03 – 0.12 mg/100 g fw Chlorogenic acid: 6.74 – 31.2 mg/100g fw 4-caffeoylquinic acid: 0.09 – 0.70 mg/100g fw Caffeoylquinic acid derivative: 0.25 – 0.98 mg/100g fw Procyanidin dimer B1: 0.04 – 0.77 mg/100g fw (+)-catechin: 0.12 – 0.80 mg/100g fw Procyanidin dimer B2: 0.02 – 0.10 mg/100g fw Cyanidin-3-glucoside: 0.07 – 4.89 mg/100g fw |
[12] |
| Maceration | 1% HCl/EtOH, pH 3.0, 60oC, 1h (anthocyanins) Acetone + BHT, 24h, 4oC (carotenoids, zeaxanthin, lycopene) |
Chlorophyll a content: 2.34 – 81.36 g/kg fw Chlorophyll b content: 2.94 – 31.13 g/kg fw Carotenoid content: 1.78 – 19.83 g/kg fw Lycopene content: 0.73 – 1.49 mg/kg fw Zeaxanthin content: 0 – 0.30 mg/kg fw B-carotenoid content: 0.31 – 10.63 mg/kg fw Anthocyanins content: 0 – 3.58 g/kg fw |
[31] | |
| Maceration | 1M NaOH, vacuum, 25oC, 18h, HCl to pH < 2.0 | Phenolic compounds | Total phenolic content: 0.61 – 0.91 g/100 g dw | [24] |
| Maceration | Hexane, 20 min, shaking 180 rpm, saponification with 0.1% methanolic KOH, 6 oC, 45 min (carotenoids) MeOH/H2O/formic acid (47.5:47.5:5), 20 min (phenolic compounds) |
Carotenoids and phenolic compounds | Cyanidin-3-glucoside: 74 – 178 mg/100g dw Cyanidin-3-rutinoside: 1 – 6 mg/100g dw Neochlorogenic acid: 23 – 27 mg/100g dw Chlorogenic acid: 52 – 136 mg/100g dw Caffeic acid: 1 – 3 mg/100g dw Procyanidin B1: 84 – 148 mg/100g dw Procyanidin B2: 12 – 41 mg/100g dw Procyanidin B3: 80 – 128 mg/100g dw Procyanidin B4: 2 – 9 mg/100g dw Catechin: 69 – 106 mg/100g dw Epicatechin: 4 – 10 mg/100g dw Quercetin-3-glucoside: 8 – 19 mg/100g dw Quercetin-3-rutinoside: 8 – 13 mg/100g dw Neoxanthin: 10.3 – 13.6 μg/g dw Violaxanthin: 5.6 – 36.5 μg/g dw Lutein epoxide: 8.2 – 20.6 μg/g dw Lutein: 9.6 – 15.1 μg/g dw Zeaxanthin: 10.1 – 18.7 μg/g dw β-cryptoxanthin: 6.3 – 13.2 μg/g dw β-carotene: 7.5 – 16.4 μg/g dw |
[16] |
| Component | Content | Reference |
|---|---|---|
| Moisture | 6.9 ± 0.06% | [50] |
| Sugars | 47.44 ± 0.02% | [50] |
| Total dietary fibers | 1.8 ± 0.05% | [50] |
| Protein | 2.67 ± 0.10% | [50] |
| Fat | 37.69% | [50] |
| Total ash | 3.36 ± 0.02% | [50] |
| Phenolic compound | Content | Reference |
|---|---|---|
| Flavonols | ||
| Isorhamnetin-3-O-glucoside | 0.53 – 66.67 mg/100 g dw | [4] |
| Keampferol-3-O-glucoside | 1.98 – 63.14 mg/100 g dw | [4] |
| Quercetin-3-O-glucoside | 2.87 mg/100 g dw | [4] |
| Luteolin-7-glucoside | 1.61 mg/100 g dw | [4] |
| Keampferol-7-neohesperidoside | 0.62 mg/100 g dw | [4] |
| Hesperidin-7-rutinoside | 0.55 mg/100 g dw | [4] |
| Hydroxycinnamic acids | ||
| Chlorogenic acid | 72.92– 1727.05 mg/100 g dw | [4] |
| Neochlorogenic acid | 130.07 mg/100 g dw | [4] |
| Gallic acid | 2.98 mg/100 g dw | [54] |
| Caffeic acid | 0.98 mg/100 g dw | [54] |
| 2-O-caffeoyl-L-malate | 17 – 130.52 mg/100g dw | [4] |
| 3-O-p-coumaroyloquinic acid | 9.6 – 70.22 mg/100 g dw | [4] |
| cis-5-p-coumaroyloquinic acid | 21.93 – 190.8 mg/100 g dw | [4] |
| Hydroxybenzoic acids | ||
| Ellagic acid | 0.77 – 9.42 mg/100 g dw | [4] |
| p-hydroxybenzoic acid | 18.64 mg/100 g dw | [54] |
| Flavan-3-ols | ||
| Epicatechin | 18.62 – 33.74 mg/100 g dw | [4] |
| Procyanidin B1 | 150.65 mg/100 g dw | [4] |
| Procyanidin B2 | 28.12 mg/100 g dw | [4] |
| Fatty acids | Content | Reference |
|---|---|---|
| Unsaturated fatty acids | ||
| Oleic acid | 55 – 74% | [67] |
| Linoleic acid | 12 – 31% | [67] |
| Saturated fatty acids | ||
| Palmitic acid | 7.97% | [65] |
| Stearic acid | 23.7% | [65] |
| α-linolenic acid | 0.11% | [65] |
| Extraction method | Conditions | Yield* | Fatty acids composition | Reference |
|---|---|---|---|---|
| Soxhlet | Hexane, 70/80/90oC | 38% | Oleic acid: 74%Linoleic acid: 15% | [76] |
| Maceration | Hexane/ethanol | 22%/17% | Oleic acid: 74%Linoleic acid: 15% | [76] |
| Supercritical fluid extraction | 5% ethanol at 50°C/300 bar | 24% | Oleic acid: 60 – 65%Linoleic acid: 15 – 20% | [10,77] |
| Maceration | 130 mL petroleum ether at 65°C for 2.5 h | 30 – 50% | Oleic acid: 55.2 %Linoleic acid: 30.8%Palmitic acid: 7.97%Stearic acid: 2.37%α- linoleic acid: 0.11% | [65] |
| Soxhlet | n-hexane | 46.4 ± 1.3% | Oleic acid: 74.55%Linoleic acid: 16.85% | [67] |
| Component | Content | Reference |
|---|---|---|
| Moisture | 65.84 – 84.76% | [110,111] |
| Sugars | 12.14 – 26.38% 10.8 – 15.7 g/100 g fw |
[12,110,111] |
| Total dietary fibers | 1.78% | [110] |
| Protein | 0.68% | [110] |
| Fat | 0.21% | [110] |
| Total ash | 0.43 – 0.56% | [110,111] |
| Component | Content | Reference |
|---|---|---|
| Total phenolics | 105.1 ± 1.21 mg GAE/g extract | [112] |
| 3.62 – 19.4 mg GAE/100 g fw | [12] | |
| 24.83 – 86.33 mg of GAE/100 g fw | [109] | |
| 3.5 - 4.5 mg/g dw | [55] | |
| 711.7 – 881.3 mg GAE/100 g dw | [2] | |
| Flavonoids | 726.5 ± 8.2 mg QCT/100 g fw | [110] |
| 17.76 ± 130.17 mg RE/100 g fw | [109] | |
| 301.3 – 499.7 mg CE/100 g | [2] | |
| Phenols | 921.8 ± 2.5 mg CGA/100 g fw 461 ± 308 mg CGA/100 g fw |
[110,113] |
| Anthocyanins | 148.7 ± 83 mg C3G/100 g ft | [113] |
| Hydroxycinnamic acids | 103 – 303 mg/kg | [114] |
| 3.45 – 18.1 mg/100 g fw | [12] | |
| Chlorogenic acid | 15.029 ± 1.3 mg/kg extract | [109] |
| 0.12 – 1.82 mg/g dw | [55] | |
| 3.58 – 14.22 mg/100 g fw | [109] | |
| Neo-chlorogenic acid | 2.13 – 12.14 mg/100 g fw | [109] |
| Flavan-3-ols | 1160 – 2147 mg/kg | [114] |
| 0.05 – 1.89 mg/g dw | [55] | |
| Total carotenoids | 13.79 ± 2.45 μg/g fw | [115] |
| 61.9 ± 1.8 mg β-catotene/100 g fw | [110] | |
| 2.8 ± 0.9 mg β-catotene/100 g fw | [113] | |
| β-cryptoxanthin | 2.19 – 88.05 µg/g dw | [115] |
| β-carotene | 5.07 – 28.9 µg/g dw | [115] |
| lutein | 0.83 – 10.8 µg/g dw | [115] |
| zeaxanthin | 1.33 – 19.08 µg/g dw | [115] |
| (E/Z)-phytoene | 0.41 – 8.8 µg/g dw | [115] |
| Ascorbic acid | 4.15 – 14.2 mg/100 g fw | [12] |
| 2.48 – 5.54 mg/100 g fw | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





