Submitted:
12 January 2024
Posted:
15 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Study population and collecting blood samples
2.2. Cell culture
2.3. Chemicals and antibodies
2.4. Enzyme-linked immunosorbent assay (ELISA)
2.5. Quantitative real time-polymerase chain reaction (qRT-PCR)
2.6. Transfection of siRNA
2.7. Western blotting
2.8. Statistical analysis
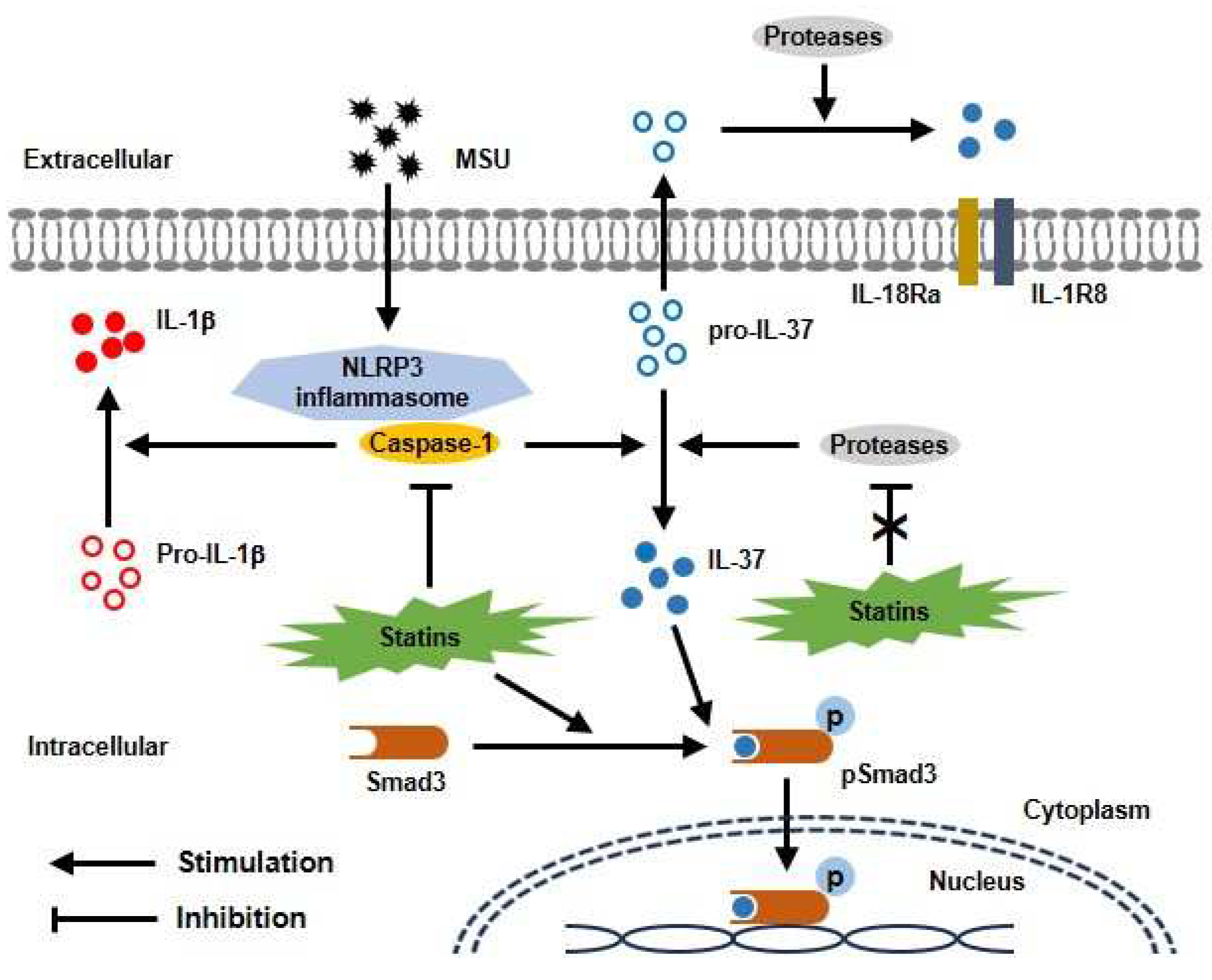
3. Results
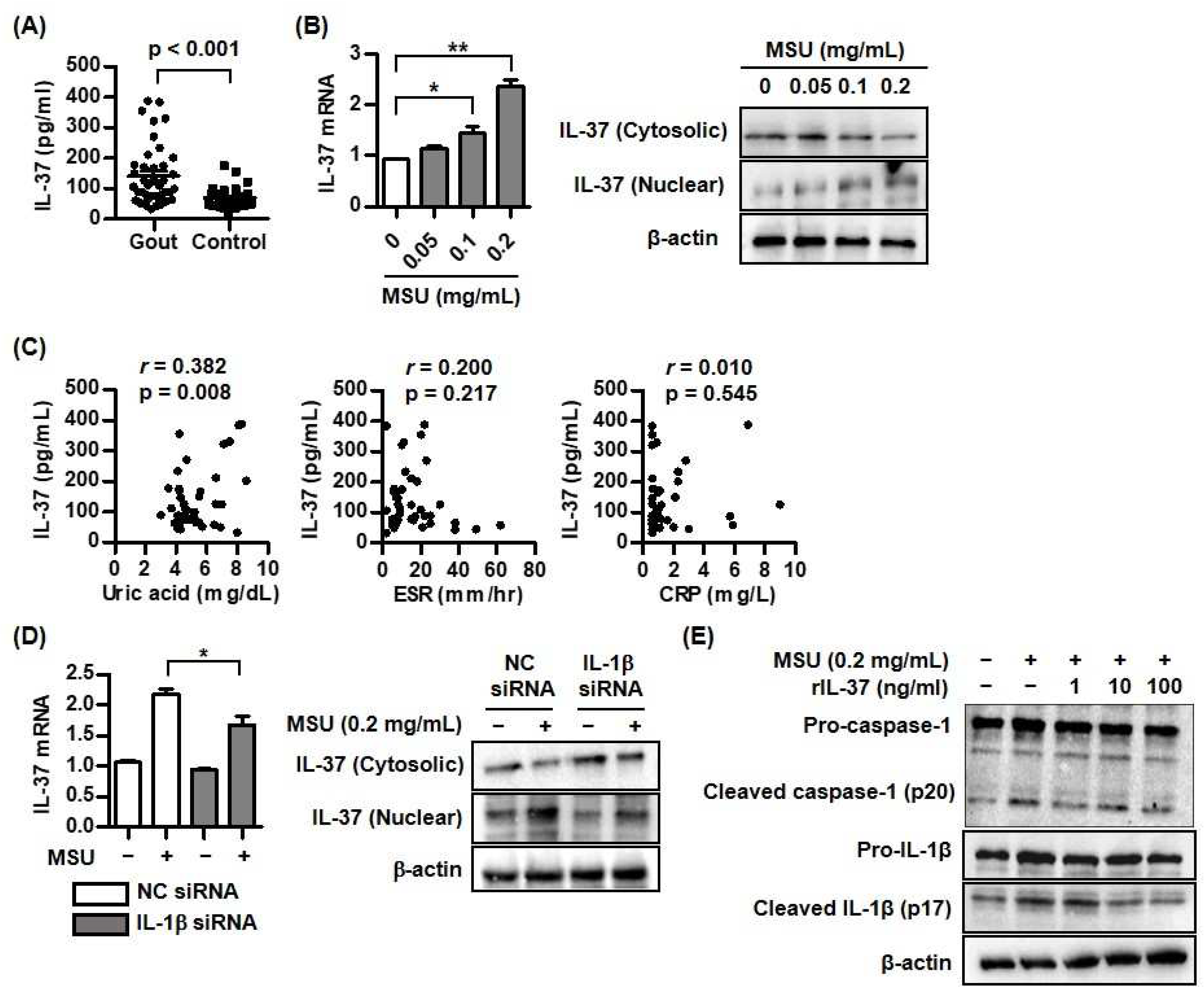
3.1. IL-37 expression in MSU-induced inflammation
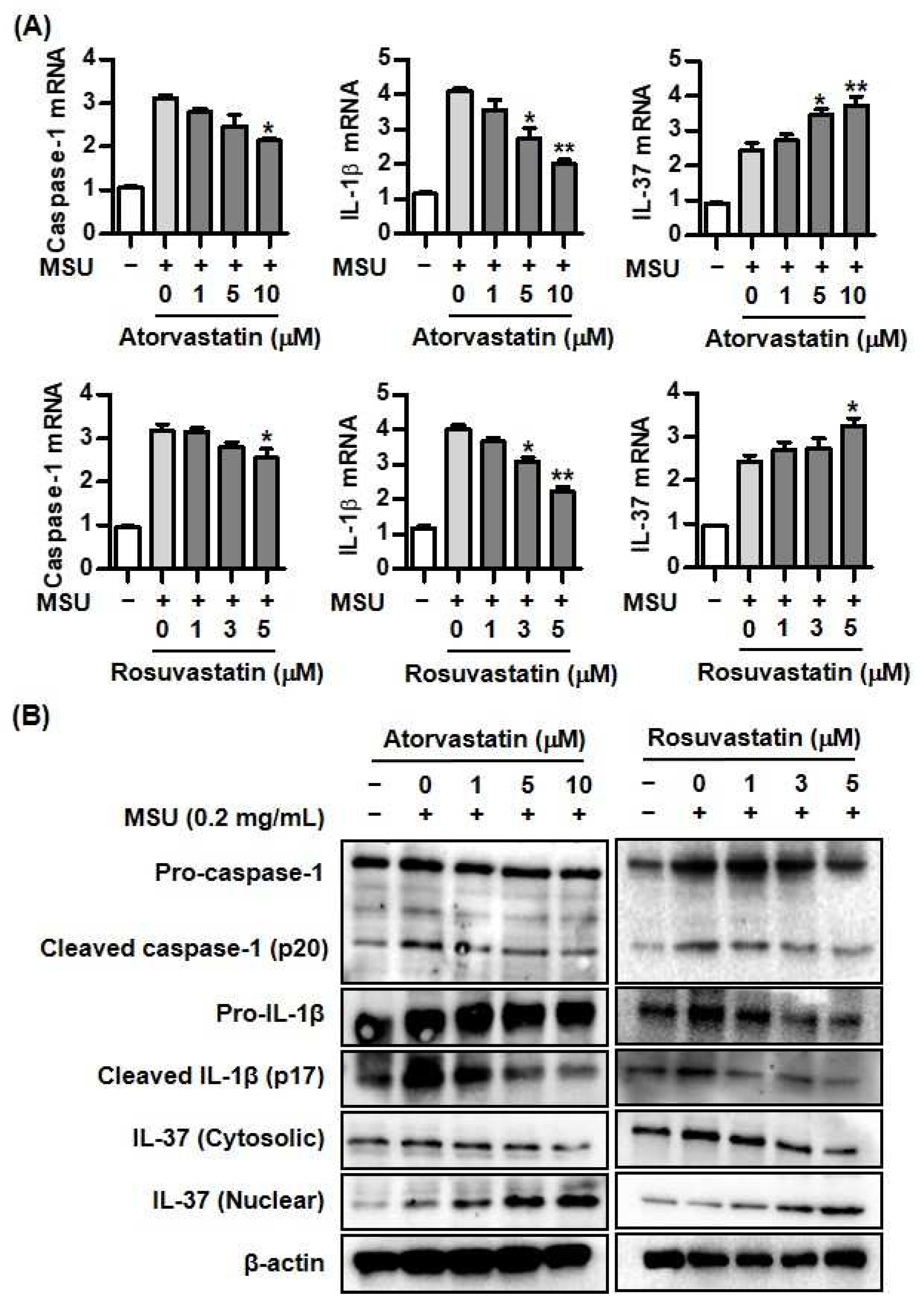
3.2. Effect of statins on caspase-1, IL-1, and IL-37 expression in MSU-induced inflammatory response
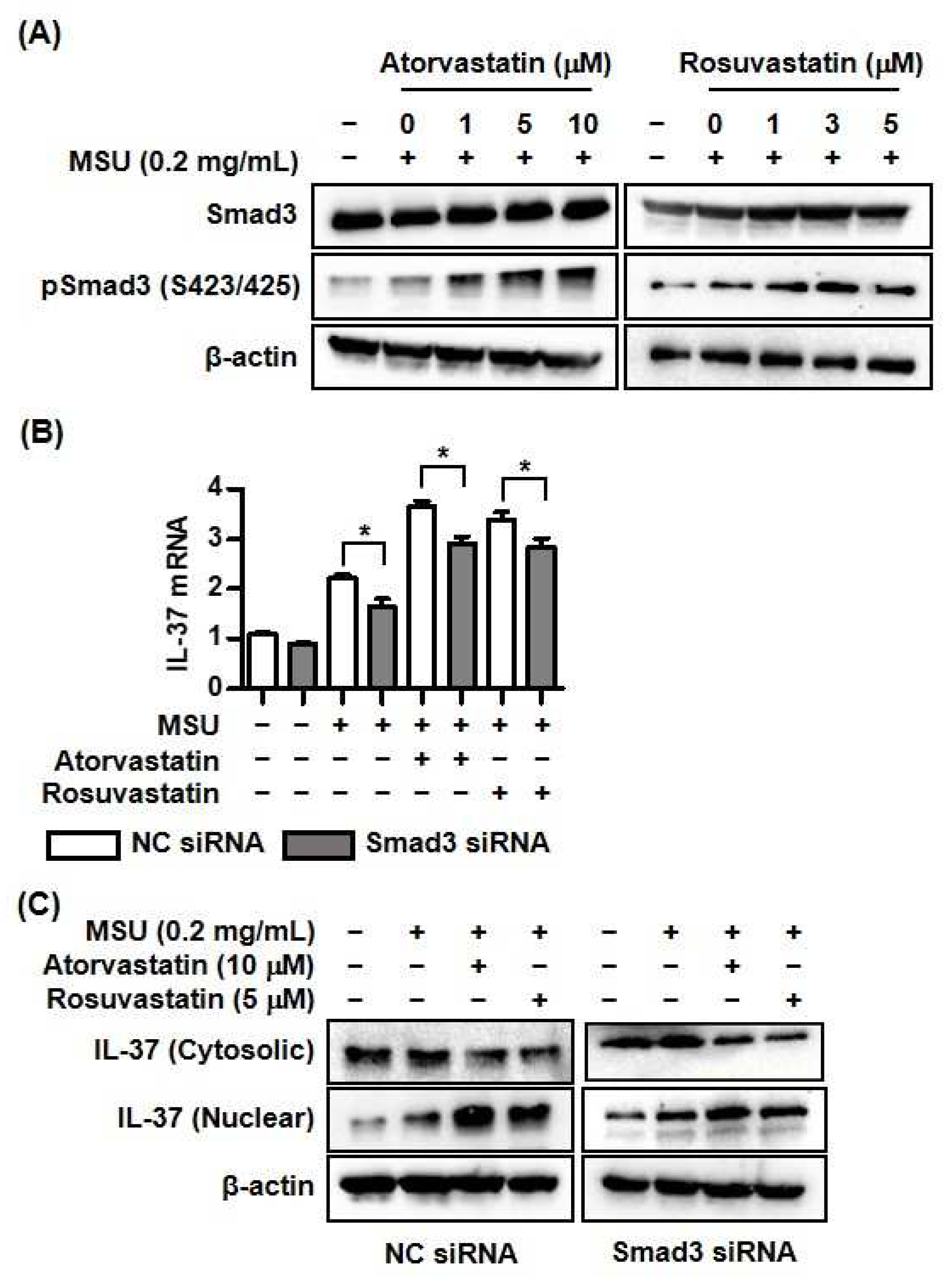
3.3. Effects of statins on interactions with Smad3 and IL-37 in MSU-induced inflammation
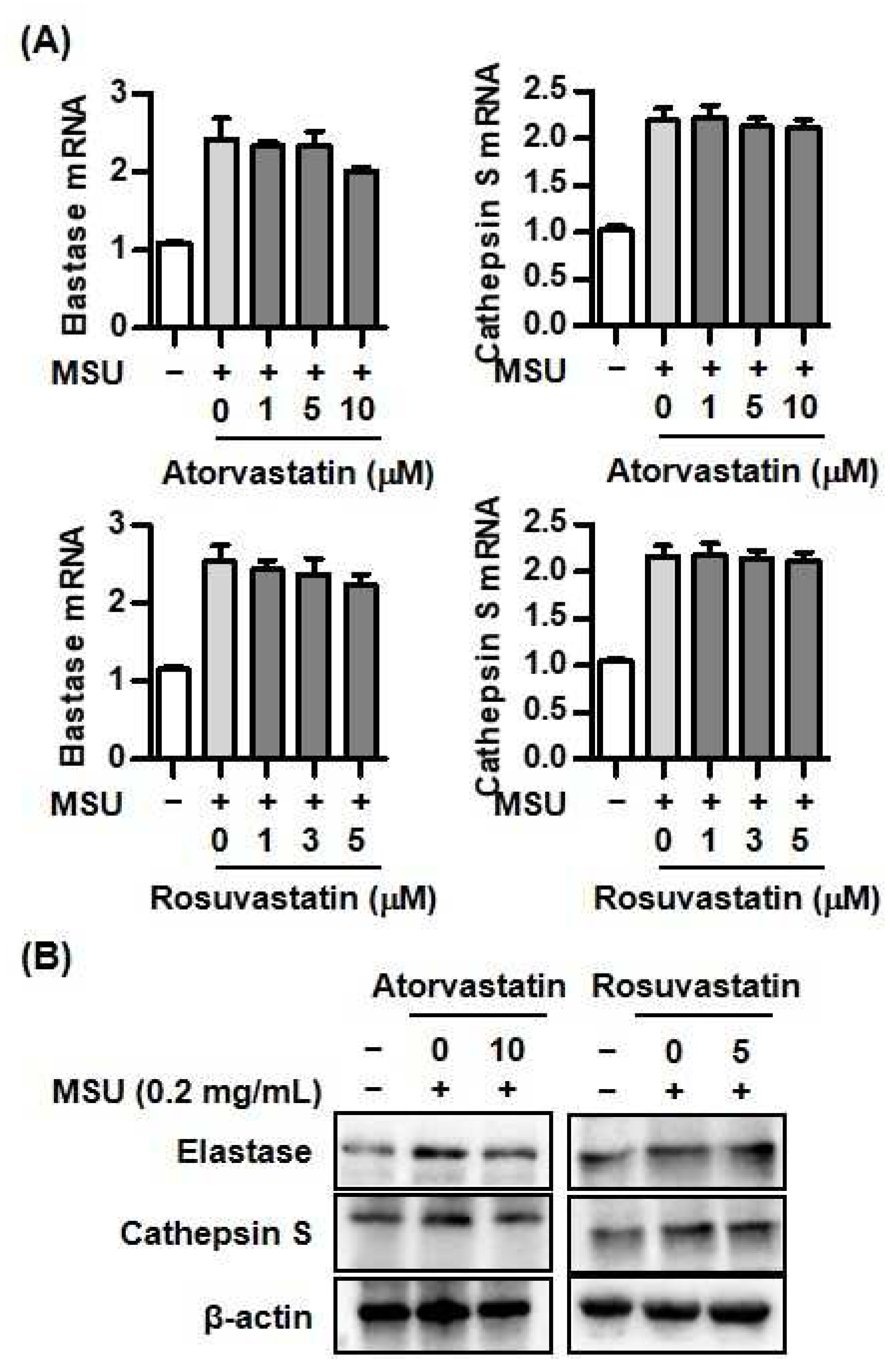
3.4. Effect of statins on proteolytic activity in the process of IL-37 activation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, M.; Tian, Y.; Wang, Q.; Guo, C. Gout: a disease involved with complicated immunoinflammatory responses: a narrative review. Clin. Rheumatol. 2020, 39, 2849–2859. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K. The Mechanism of the NLRP3 Inflammasome Activation and Pathogenic Implication in the Pathogenesis of Gout. J. Rheum. Dis. 2022, 29, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Pétrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006, 440, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-. Mol. Cell. 2002, 10, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.K.; Kim, J.K.; Shin, D.M.; Sasakawa, C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol. 2016, 13, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Akbal, A.; Dernst, A.; Lovotti, M.; Mangan, M.S.J.; McManus, R.M.; Latz, E. How location and cellular signaling combine to activate the NLRP3 inflammasome. Cell. Mol Immunol. 2022, 19, 1201–1214. [Google Scholar] [CrossRef]
- Nold, M.F.; Nold-Petry, C.A.; Zepp, J.A.; Palmer, B.E.; Bufler, P.; Dinarello, C.A. IL-37 is a fundamental inhibitor of innate immunity. Nat. Immunol. 2010, 11, 1014–1022. [Google Scholar] [CrossRef]
- Jia, H.; Liu, J.; Han, B. Reviews of Interleukin-37: Functions, Receptors, and Roles in Diseases. Biomed. Res. Int. 2018, 2018, 3058640. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Quan, Y.; Yue, Y.; Heng, X.; Che, F. Interleukin-37: A crucial cytokine with multiple roles in disease and potentially clinical therapy. Oncol. Lett. 2018, 15, 4711–4719. [Google Scholar] [CrossRef]
- Su, Z.; Tao, X. Current Understanding of IL-37 in Human Health and Disease. Front. Immunol. 2021, 12, 696605. [Google Scholar] [CrossRef]
- Zhao, P.W.; Jiang, W.G.; Wang, L.; Jiang, Z.Y.; Shan, Y.X.; Jiang, Y.F. Plasma levels of IL-37 and correlation with TNF-α, IL-17A, and disease activity during DMARD treatment of rheumatoid arthritis. PLoS. One. 2014, 9, e95346. [Google Scholar] [CrossRef]
- Xia, T.; Zheng, X.F.; Qian, B.H.; Fang, H.; Wang, J.J.; Zhang, L.L.; Pang, Y.F.; Zhang, J.; Wei, X.Q.; Xia, Z.F.; et al. Plasma Interleukin-37 Is Elevated in Patients with Rheumatoid Arthritis: Its Correlation with Disease Activity and Th1/Th2/Th17-Related Cytokines. Dis. Markers. 2015, 2015, 795043. [Google Scholar] [CrossRef]
- Song, L.; Qiu, F.; Fan, Y.; Ding, F.; Liu, H.; Shu, Q.; Liu, W.; Li, X. Glucocorticoid regulates interleukin-37 in systemic lupus erythematosus. J. Clin. Immunol. 2013, 33, 111–117. [Google Scholar] [CrossRef]
- Ding, L.; Hong, X.; Sun, B.; Huang, Q.; Wang, X.; Liu, X.; Li, L.; Huang, Z.; Liu, D. IL-37 is associated with osteoarthritis disease activity and suppresses proinflammatory cytokines production in synovial cells. Sci. Rep. 2017, 7, 11601. [Google Scholar] [CrossRef]
- Chen, B.; Huang, K.; Ye, L.; Li, Y.; Zhang, J.; Zhang, J.; Fan, X.; Liu, X.; Li, L.; Sun, J.; et al. Interleukin-37 is increased in ankylosing spondylitis patients and associated with disease activity. J. Transl. Med. 2015, 13, 36. [Google Scholar] [CrossRef]
- Zeng, M.; Dang, W.; Chen, B.; Qing, Y.; Xie, W.; Zhao, M.; Zhou, J. IL-37 inhibits the production of pro-inflammatory cytokines in MSU crystal-induced inflammatory response. Clin. Rheumatol. 2016, 35, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Li, H.; Sun, B.; Wang, T.; Meng, S.; Huang, Q.; Hong, X.; Liu, D. Elevated interleukin-37 associated with tophus and pro-inflammatory mediators in Chinese gout patients. Cytokine. 2021, 141, 155468. [Google Scholar] [CrossRef]
- Liu, L.; Xue, Y.; Zhu, Y.; Xuan, D.; Yang, X.; Liang, M.; Wang, J.; Zhu, X.; Zhang, J.; Zou, H. Interleukin 37 limits monosodium urate crystal-induced innate immune responses in human and murine models of gout. Arthritis. Res. Ther. 2016, 18, 268. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, T.; Yang, X.; Cao, L.; Xu, R.; Liu, J.; Lin, C.; Yu, Y.; Xuan, D.; Zhu, X.; et al. IL-37 blocks gouty inflammation by shaping macrophages into a non-inflammatory phagocytic phenotype. Rheumatology. (Oxford). 2022, 61, 3841–3853. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, S.I.; Muniyappa, R.; Francisco, R.; Sowers, J.R. Clinical review 145: Pleiotropic effects of statins: lipid reduction and beyond. J. Clin. Endocrinol. Metab. 2002, 87, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.K.; Laufs, U. Pleiotropic effects of statins. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 89–118. [Google Scholar] [CrossRef]
- Kim, S.K.; Choe, J.Y.; Kim, J.W.; Park, K.Y. HMG-CoA Reductase Inhibitors Suppress Monosodium Urate-Induced NLRP3 Inflammasome Activation through Peroxisome Proliferator-Activated Receptor- Activation in THP-1 Cells. Pharmaceuticals. (Basel). 2023, 16, 522. [Google Scholar] [CrossRef]
- Cui, H.; Soga, K.; Tamehiro, N.; Adachi, R.; Hachisuka, A.; Hirose, A.; Kondo, K.; Nishimaki-Mogami, T. Statins repress needle-like carbon nanotube- or cholesterol crystal-stimulated IL-1β production by inhibiting the uptake of crystals by macrophages. Biochem. Pharmacol. 2021, 188, 114580. [Google Scholar] [CrossRef]
- Neogi, T.; Jansen, T.L.; Dalbeth, N.; Fransen, J.; Schumacher, H.R.; Berendsen, D.; Brown, M.; Choi, H.; Edwards, N.L.; Janssens, H.J.; et al. 2015 Gout Classification Criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis. Rheumatol. 2015, 67, 2557–2568. [Google Scholar] [CrossRef]
- Choe, J.Y.; Jung, H.Y.; Park, K.Y.; Kim, S.K. Enhanced p62 expression through impaired proteasomal degradation is involved in caspase-1 activation in monosodium urate crystal-induced interleukin-1b expression. Rheumatology. (Oxford). 2014, 53, 1043–1053. [Google Scholar] [CrossRef]
- Bulau, A.M.; Nold, M.F.; Li, S.; Nold-Petry, C.A.; Fink, M.; Mansell, A.; Schwerd, T.; Hong, J.; Rubartelli, A.; Dinarello, C.A.; et al. Role of caspase-1 in nuclear translocation of IL-37, release of the cytokine, and IL-37 inhibition of innate immune responses. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 2650–2655. [Google Scholar] [CrossRef]
- Milionis, H.J.; Kakafika, A.I.; Tsouli, S.G.; Athyros, V.G.; Bairaktari, E.T.; Seferiadis, K.I.; Elisaf, M.S. Effects of statin treatment on uric acid homeostasis in patients with primary hyperlipidemia. Am. Heart. J. 2004, 148, 635–640. [Google Scholar] [CrossRef]
- Lin, G.L.; Lin, H.C.; Lin, H.L.; Keller, J.J.; Wang, L.H. Association between statin use and the risk of gout in patients with hyperlipidemia: A population-based cohort study. Front. Pharmacol. 2023, 14, 1096999. [Google Scholar] [CrossRef]
- Klück, V.; van Deuren, R.C.; Cavalli, G.; Shaukat, A.; Arts, P.; Cleophas, M.C.; Crișan, T.O.; Tausche, A.K.; Riches, P.; Dalbeth, N.; et al. Rare genetic variants in interleukin-37 link this anti-inflammatory cytokine to the pathogenesis and treatment of gout. Ann. Rheum. Dis. 2020, 79, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Shaoyuan, C.; Ming, D.; Yulang, H.; Hongcheng, F. Increased IL-37 in Atherosclerotic Disease could be Suppressed by Atorvastatin Therapy. Scand. J. Immunol. 2015, 82, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, R.; He, S.; He, L.; Zhao, H.; Deng, X.; Chen, Z. Tripterygium wilfordii Glycosides Upregulate the New Anti-Inflammatory Cytokine IL-37 through ERK1/2 and p38 MAPK Signal Pathways. Evid. Based. Complement. Alternat. Med. 2017, 2017, 9148523. [Google Scholar] [CrossRef]
- Shi, X.; Lai, C.; Zhao, L.; Zhang, M.; Liu, X.; Peng, S.; Guo, W.; Xu, Q.; Chen, S.; Chen, G.X. Chloroquine and Rapamycin Augment Interleukin-37 Expression via the LC3, ERK, and AP-1 Axis in the Presence of Lipopolysaccharides. J. Immunol. Res. 2020, 2020, 6457879. [Google Scholar] [CrossRef]
- Afonina, I.S.; Müller, C.; Martin, S.J.; Beyaert, R. Proteolytic Processing of Interleukin-1 Family Cytokines: Variations on a Common Theme. Immunity. 2015, 42, 991–1004. [Google Scholar] [CrossRef]
- Sullivan, G.P.; Davidovich, P.; Muñoz-Wolf, N.; Ward, R.W.; Hernandez Santana, Y.E.; Clancy, D.M.; Gorman, A.; Najda, Z.; Turk, B.; Walsh, P.T.; et al. Myeloid cell-derived proteases produce a proinflammatory form of IL-37 that signals via IL-36 receptor engagement. Sci. Immunol. 2022, 7, eade5728. [Google Scholar] [CrossRef]
- Climent, E.; Benaiges, D.; Pedro-Botet, J. Hydrophilic or Lipophilic Statins. Front. Cardiovasc. Med. 2021, 8, 687585. [Google Scholar] [CrossRef]
- Chang, C.H.; Hsu, Y.M.; Chen, Y.C.; Lin, F.H.; Sadhasivam, S.; Loo, S.T.; Savitha, S. Anti-inflammatory effects of hydrophilic and lipophilic statins with hyaluronic acid against LPS-induced inflammation in porcine articular chondrocytes. J. Orthop. Res. 2014, 32, 557–565. [Google Scholar] [CrossRef]
- Kwon, M.J.; Kim, J.H.; Kim, J.H.; Park, H.R.; Kim, N.Y.; Hong, S.; Choi, H.G. Incident Rheumatoid Arthritis Following Statin Use: From the View of a National Cohort Study in Korea. J. Pers. Med. 2022, 12, 559. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




