Submitted:
13 January 2024
Posted:
15 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Dietary Sources and Determinants the Body Burden of Cadmium
2.1. Estimation of Exposure to Cadmium in the Human Diet
2.2. The Intestinal Absorption Rate of Cadmium
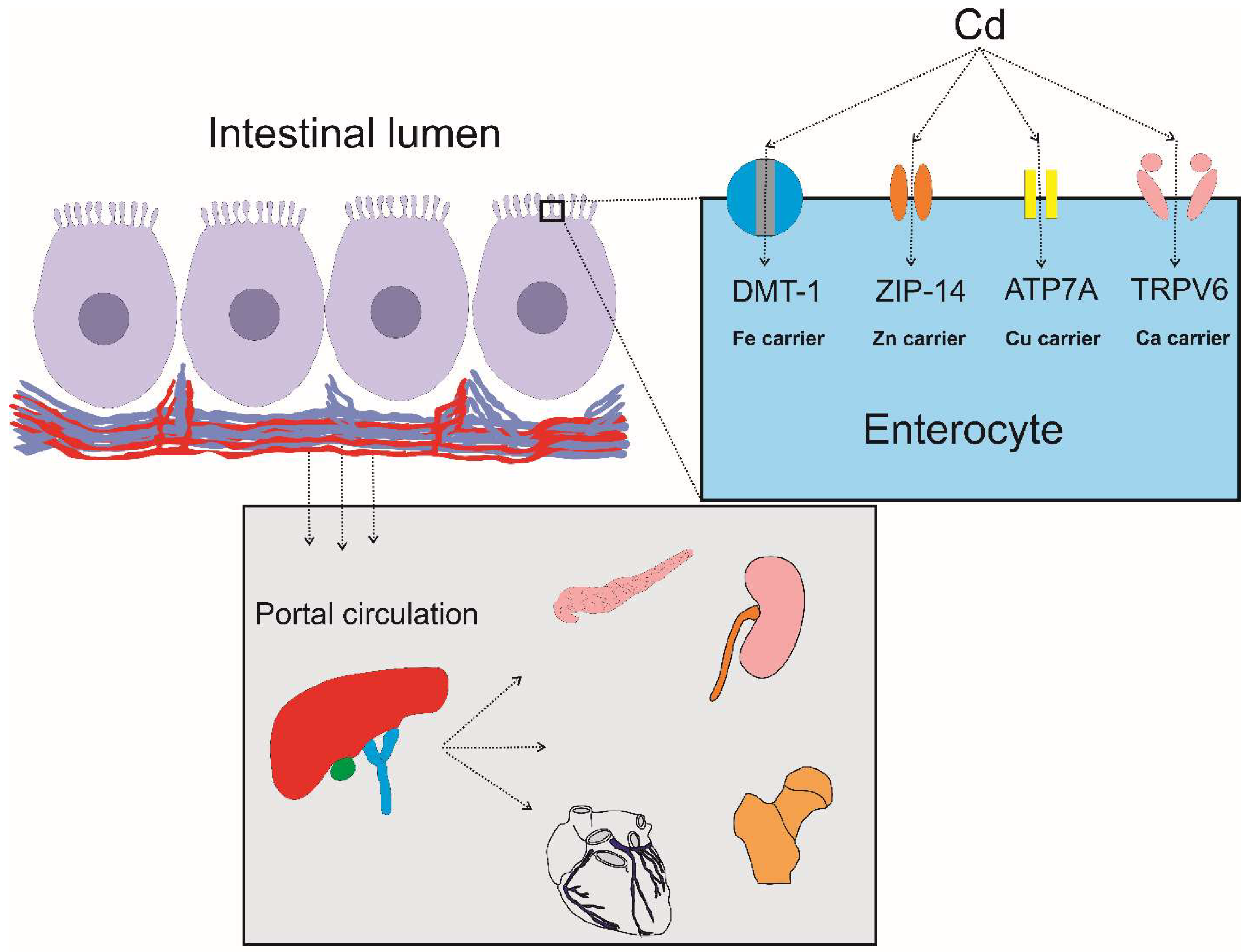
2.3. Metal Transporters Involved in Cadmium Absorption
2.3. Determinants of Cadmium Absorption Rate
2.3.1. Body Iron Stores and Iron Deficiency
2.3.2. Dietary Factors: Diet Quality
2.3.3. Genetic Factors: The ZIP8, ZIP14, TFR and H63D variants
2.4. Summary: Determinants of Cadmium Absorption Rate
3. Cadmium as a Multi-Tissue Carcinogen: Epidemiologic Studies
3.1. Cadmium and Human Cancer: Evidence from Meta-Analysis
3.1. Impact of Cadmium on Cancer Risk
3.2. Cadmium and Hepatocellular Carcinoma
3.3. Cadmium and Other Types of Cancer
3.4. Implications for Heath Risk Calculation
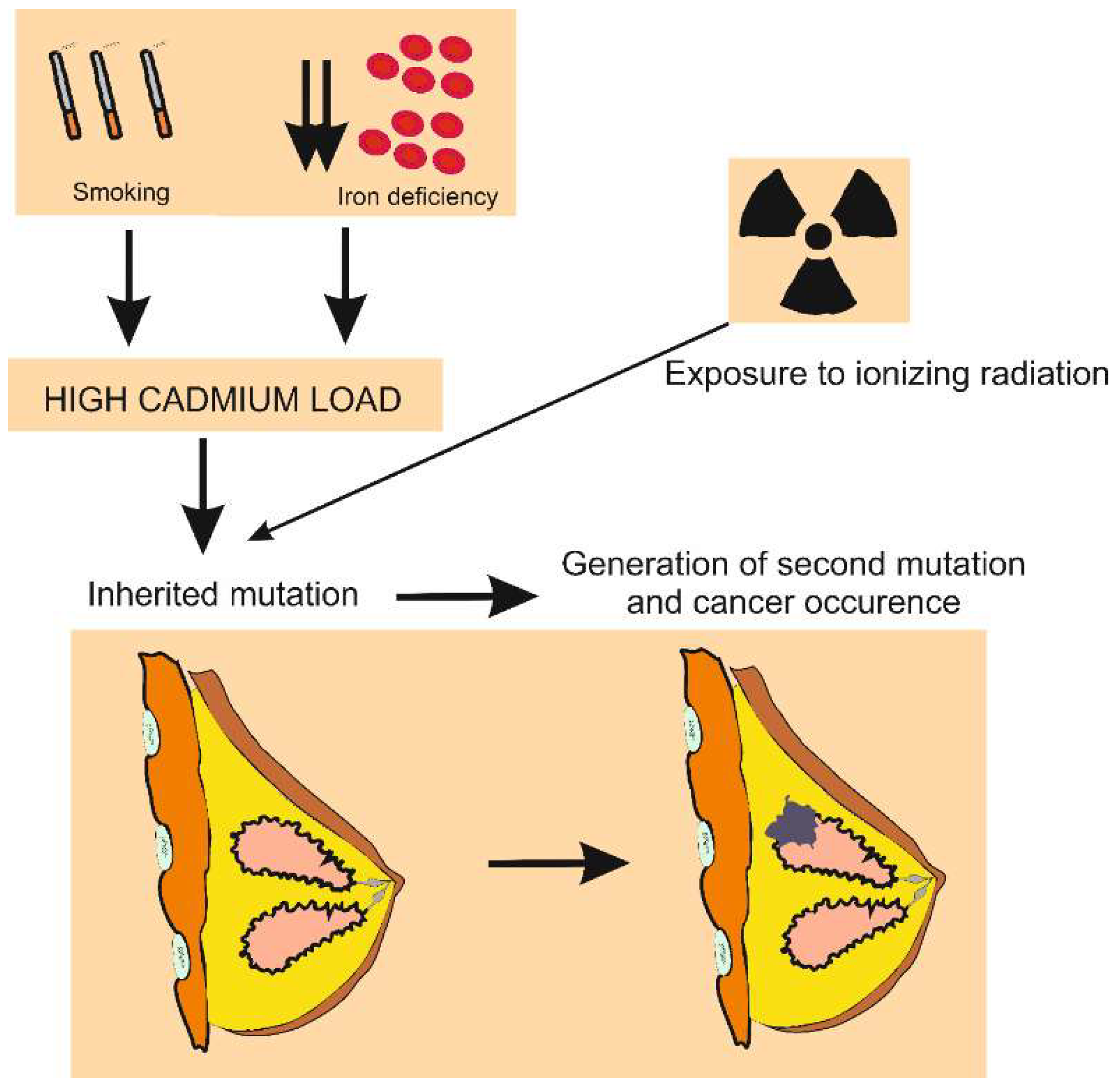
3.5. Cadmium, Breast Cancer, and Iron Supplement
3.6. Role of Cadmium in Genesis of Breast Cancer
4. Cadmium as a Multi-Tissue Carcinogen: Experimental Studies
4.1. Cadmium and Tumor Formation in Mice
4.2. The Genesis of Lung and Liver Carcinomas after Cadmium Exposure
4.3. Induced Formation of Cancer Cells
4.4. Accquired Resistance to the Cytoxicity of Cadmium: Role of Metal Transporters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Satarug, S.; Vesey, D.A.; Gobe, G.C.; Phelps, K.R. Estimation of health risks associated with dietary cadmium exposure. Arch. Toxicol. 2023, 97, 329–358. [Google Scholar] [CrossRef]
- Callan, A.; Hinwood, A.; Devine, A. (2014) Metals in commonly eaten groceries in Western Australia: a market basket survey and dietary assessment. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2014, 31, 1968–1981. [Google Scholar] [CrossRef]
- Fechner, C.; Hackethal, C.; Höpfner, T.; Dietrich, J.; Bloch, D.; Lindtner, O.; Sarvan, I. Results of the BfR MEAL Study: In Germany, mercury is mostly contained in fish and seafood while cadmium, lead, and nickel are present in a broad spectrum of foods. Food Chem X. 2022, 14, 100326. [Google Scholar] [CrossRef]
- Mhungu, F.; Chen, K.; Wang, Y.; Liu, Y.; Zhang, Y.; Pan, X.; Cheng, Y.; Liu, Y.; Zhang, W. Probabilistic risk assessment of dietary exposure to cadmium in residents of Guangzhou, China-young children potentially at a health risk. Int. J. Environ. Res. Public Health 2022, 19, 9572. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kataoka, Y.; Hayashi, K.; Matsuda, R.; Uneyama, C. Dietary exposure of the Japanese general population to elements: Total diet study 2013-2018. Food Saf. (Tokyo) 2022, 10, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Pappas, R.S.; Fresquez, M.R.; Watson, C.H. Cigarette smoke cadmium breakthrough from traditional filters: Implications for exposure. J. Anal. Toxicol. 2015, 39, 45–51. [Google Scholar] [CrossRef]
- Świetlik, R.; Trojanowska, M. Chemical fractionation in environmental studies of potentially toxic particulate-bound elements in urban air: A critical review. Toxics 2022, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Sunderman, F.W. Jr. (2001) Nasal toxicity, carcinogenicity, and olfactory uptake of metals. Ann. Clin. Lab. Sci. 2001, 31, 3–24. [Google Scholar] [PubMed]
- Cory-Slechta, D.A.; Allen, J.L.; Conrad, K.; Marvin, E.; Sobolewski, M. Developmental exposure to low level ambient ultrafine particle air pollution and cognitive dysfunction. Neurotoxicology 2018, 69, 217–231. [Google Scholar] [CrossRef]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuča, K.; Musílek, K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef]
- Moulis, J.M.; Bourguinon, J.; Catty, P. Chapter 23 Cadmium. In RSC Metallobiology Series No. 2, Binding, Transport. and Storage of Metal. Ions in Biological Cells; Wolfgang, M., Anthony, W., Eds.; The Royal Society of Chemistry: London, UK, 2014; pp. 695–746. [Google Scholar]
- Petering, D.H. Reactions of the Zn proteome with Cd2+ and other xenobiotics: Trafficking and toxicity. Chem. Res. Toxicol. 2016, 30, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Earley, B.J.; Cubillas, C.; Warnhoff, K.; Ahmad, R.; Alcantar, A.; Lyon, M.D.; Schneider, D.L.; Kornfeld, K. Cadmium hijacks the high zinc response by binding and activating the HIZR-1 nuclear receptor. Proc. Natl. Acad. Sci. USA 2021, 118, e2022649118. [Google Scholar] [CrossRef]
- Cannino, G.; Ferruggia, E.; Luparello, C.; Rinaldi, A.M. Cadmium and mitochondria. Mitochondrion 2009, 9, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Luparello, C.; Sirchia, R.; Longo, A. Cadmium as a transcriptional modulator in human cells. Crit. Rev. Toxicol. 2011, 41, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, A. Metal interaction with redox regulation: an integrating concept in metal carcinogenesis? Free Radic. Biol. Med. 2013, 55, 63–72. [Google Scholar] [CrossRef]
- Patel, C.J.; Rehkopf, D.H.; Leppert, J.T.; Bortz, W.M.; Cullen, M.R.; Chertow, G.M.; Ioannidis, J.P. Systematic evaluation of environmental and behavioural factors associated with all-cause mortality in the United States national health and nutrition examination survey. Int. J. Epidemiol. 2013, 42, 1795–1810. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Y.; Tao, C.; Yan, W.; Huang, Y.; Qian, H.; Xu, Q.; Wan, T.; Chen, Y.; Qin, Y.; Lu, C. Association between exposure to cadmium and risk of all-cause and cause-specific mortality in the general US adults: A prospective cohort study. Chemosphere 2022, 307, 136060. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Lee, J.; Yu, J.M.; Choi, H.; Choi, S.; Park, J.; Choi, K.; Kim, E.; Kim, H.; Kim, M.J.; Park, Y.J. Association between environmental cadmium exposure and increased mortality in the U.S. National Health and Nutrition Examination Survey (1999-2018). J. Expo. Sci. Environ. Epidemiol. 2023, 33, 874–882. [Google Scholar] [CrossRef]
- Nogawa, K.; Watanabe, Y.; Sakuma, S.; Sakurai, M.; Nishijo, M.; Ishizaki, M.; Morikawa, Y.; Kido, T.; Nakagawa, H.; Suwazono, Y. Renal tubular dysfunction and cancer mortality in the Japanese general population living in cadmium-non-contaminated areas. J. Appl. Toxicol. 2022, 42, 1458–1466. [Google Scholar] [CrossRef]
- Xing, X.; Xu, M.; Yang, L.; Shao, C.; Wang, Y.; Qi, M.; Niu, X.; Gao, D. Association of selenium and cadmium with heart failure and mortality based on the National Health and Nutrition Examination Survey. J. Hum. Nutr. Diet 2023, 36, 1496–1506. [Google Scholar] [CrossRef]
- Sears, C.G.; Eliot, M.; Raaschou-Nielsen, O.; Poulsen, A.H.; Harrington, J.M.; Howe, C.J.; James, K.A.; Roswall, N.; Overvad, K.; Tjønneland, A.; et al. Urinary cadmium and incident heart failure: A case-cohort analysis among never-smokers in Denmark. Epidemiology 2022, 33, 185–192. [Google Scholar] [CrossRef]
- Shi, J.-W.; Fan, D.-X.; Li, M.-Q. The Relationship between cadmium exposure and mortality in postmenopausal females: A cohort study of 2001–2018 NHANES. Nutrients 2023, 15, 4604. [Google Scholar] [CrossRef] [PubMed]
- Suwazono, Y.; Nogawa, K.; Morikawa, Y.; Nishijo, M.; Kobayashi, E.; Kido, T.; Nakagawa, H.; Nogawa, K. Renal tubular dysfunction increases mortality in the Japanese general population living in cadmium non-polluted areas. J. Expo. Sci/ Environ. Epidemiol. 2015, 25, 399–404. [Google Scholar] [CrossRef]
- Kim, J.; Song, H.; Lee, J.; Kim, Y.J.; Chung, H.S.; Yu, J.M.; Jang, G.; Park, R.; Chung, W.; Oh, C.M.; Moon, S. Smoking and passive smoking increases mortality through mediation effect of cadmium exposure in the United States. Sci. Rep. 2023, 13, 3878. [Google Scholar] [CrossRef] [PubMed]
- Elinder, C.G.; Lind, B.; Kjellstorm, T.; Linnman, L.; Friberg, L. Cadmium in kidney cortex, liver and pancreas from Swedish autopsies: Estimation of biological half time in kidney cortex, considering calorie intake and smoking habits. Arch. Environ. Health 1976, 31, 292–301. [Google Scholar] [CrossRef]
- Elinder, C.G.; Kjellstöm, T.; Lind, B.; Molander, M.L.; Silander, T. Cadmium concentrations in human liver, blood, and bile: comparison with a metabolic model. Environ Res. 1978, 17, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Suwazono, Y.; Kido, T.; Nakagawa, H.; Nishijo, M.; Honda, R.; Kobayashi, E.; Dochi, M.; Nogawa, K. Biological half-life of cadmium in the urine of inhabitants after cessation of cadmium exposure. Biomarkers 2009, 14, 77–81. [Google Scholar] [CrossRef]
- Ishizaki, M.; Suwazono, Y.; Kido, T.; Nishijo, M.; Honda, R.; Kobayashi, E.; Nogawa, K.; Nakagawa, H. Estimation of biological half-life of urinary cadmium in inhabitants after cessation of environmental cadmium pollution using a mixed linear model. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2015, 32, 1273–1276. [Google Scholar] [CrossRef]
- Satarug, S.; Nishijo, M.; Ujjin, P.; Vanavanitkun, Y.; Baker, J.R.; Moore, M.R. Evidence for concurrent effects of exposure to environmental cadmium and lead on hepatic CYP2A6 phenotype and renal function biomarkers in nonsmokers. Environ Health Perspect. 2004, 112, 1512–1518. [Google Scholar] [CrossRef]
- Satarug, S.; Tassaneeyakul, W.; Na-Bangchang, K.; Cashman, J.R.; Moore, M.R. Genetic and environmental influences on therapeutic and toxicity outcomes: studies with CYP2A6. Curr. Clin. Pharmacol. 2006, 1, 291–309. [Google Scholar] [CrossRef]
- Apinan, R.; Tassaneeyakul, W.; Mahavorasirikul, W.; Satarug, S.; Kajanawart, S.; Vannaprasaht, S.; Ruenweerayut, R.; Na-Bangchang, K. The influence of CYP2A6 polymorphisms and cadmium on nicotine metabolism in Thai population. Environ. Toxicol. Pharmacol. 2009, 28, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Haswell-Elkins, M.R.; Moore, M.R. Safe levels of cadmium intake to prevent renal toxicity in human subjects. Br. J. Nutr. 2000, 84, 791–802. [Google Scholar] [CrossRef]
- Guo, S.; Frazer, D.M.; Anderson, G.J. Iron homeostasis: transport, metabolism, and regulation. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Knez, M.; Graham, R.D.; Welch, R.M.; Stangoulis, J.C. New perspectives on the regulation of iron absorption via cellular zinc concentrations in humans. Crit. Rev. Food Sci. Nutr. 2017, 57, 2128–2143. [Google Scholar] [CrossRef] [PubMed]
- Nishito, Y.; Kambe, T. (2018) Absorption mechanisms of iron, copper, and zinc: An overview. J. Nutr. Sci. Vitaminol. (Tokyo) 2018, 64, 1–7. [Google Scholar] [CrossRef]
- Kondaiah, P.; Yaduvanshi, P.S.; Sharp, P.A.; Pullakhandam, R. Iron and zinc homeostasis and interactions: Does enteric zinc excretion cross-talk with intestinal iron absorption? Nutrients 2019, 11, 1885. [Google Scholar] [CrossRef] [PubMed]
- Charlebois, E.; Pantopoulos, K. Nutritional aspects of iron in health and disease. Nutrients 2023, 15, 2441. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Nomiyama, T.; Kumagai, N.; Dekio, F.; Uemura, T.; Takebayashi, T.; Nishiwaki, Y.; Matsumoto, Y.; Sano, Y.; Hosoda, K.; et al. Uptake of cadmium in meals from the digestive tract of young non-smoking Japanese female volunteers. J. Occup. Health 2003, 45, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, H.; Oguma, E.; Sasaki, S.; Miyamoto, K.; Ikeda, Y.; Machida, M.; Kayama, F. Comprehensive study of the effects of age, iron deficiency, diabetes mellitus, and cadmium burden on dietary cadmium absorption in cadmium-exposed female Japanese farmers. Toxicol. Appl. Pharmacol. 2004, 196, 114–123. [Google Scholar] [CrossRef]
- JECFA. JECFA. In Summary and Conclusions. In Proceedings of the Joint FAO/WHO Expert Committee on Food Additives and Contaminants, Seventy-Third Meeting, Geneva, Switzerland, 8–17 June 2010; JECFA/73/SC; Food and Agriculture Organization of the United Nations/World Health Organization: Geneva, Switzerland, In Summary and Conclusions. In Proceedings of the Joint FAO/WHO Expert Committee on Food Additives and Contaminants, Seventy-Third Meeting, Geneva, Switzerland, 8–17 June 2010; JECFA/73/SC; Food and Agriculture Organization of the United Nations/World Health Organization: Geneva, Switzerland, 2011. Available online: https://apps.who.int/iris/handle/10665/44521 (accessed on 3 January 2024).
- Fujita, Y.; el Belbasi, H.I.; Min, K.S.; Onosaka, S.; Okada, Y.; Matsumoto, Y.; Mutoh, N.; Tanaka, K. Fate of cadmium bound to phytochelatin in rats. Res. Commun. Chem. Pathol. Pharmacol. 1993, 82, 357–365. [Google Scholar]
- Langelueddecke, C.; Roussa, E.; Fenton, R.A.; Thévenod, F. Expression and function of the lipocalin-2 (24p3/NGAL) receptor in rodent and human intestinal epithelia. PLoS ONE 2013, 8, e71586. [Google Scholar] [CrossRef]
- Langelueddecke, C.; Lee, W.K.; Thévenod, F. Differential transcytosis and toxicity of the hNGAL receptor ligands cadmium-metallothionein and cadmium-phytochelatin in colon-like Caco-2 cells: Implications for in vivo cadmium toxicity. Toxicol. Lett. 2014, 226, 228–235. [Google Scholar] [CrossRef]
- Schneider, S.N.; Liu, Z.; Wang, B.; Miller, M.L.; Afton, S.E.; Soleimani, M.; Nebert, D.W. Oral cadmium in mice carrying 5 versus 2 copies of the Slc39a8 gene: Comparison of uptake, distribution, metal content, and toxicity. Int. J. Toxicol. 2014, 33, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Jorge-Nebert, L.F.; Gálvez-Peralta, M.; Landero Figueroa, J.; Somarathna, M.; Hojyo, S.; Fukada, T.; Nebert, D.W. Comparing gene expression during cadmium uptake and distribution: Untreated versus oral Cd-treated wild-type and ZIP14 knockout mice. Toxicol. Sci. 2015, 143, 26–35. [Google Scholar] [CrossRef]
- Fujishiro, H.; Hamao, S.; Tanaka, R.; Kambe, T.; Himeno, S. Concentration-dependent roles of DMT1 and ZIP14 in cadmium absorption in Caco-2 cells. J. Toxicol. Sci. 2017, 42, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, T.B.; Cousins, R.J. The multiple faces of the metal transporter ZIP14 (SLC39A14). J. Nutr. 2018, 148, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, G.J.; Aydemir, T.B.; Troche, C.; Martin, A.B.; Chang, S.M.; Cousins, R.J. Influence of ZIP14 (slc39A14) on intestinal zinc processing and barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G171–G178. [Google Scholar] [CrossRef]
- Kippler, M.; Goessler, W.; Nermell, B.; Ekström, E.C.; Lönnerdal, B.; El Arifeen, S.; Vahter, M. Factors influencing intestinal cadmium uptake in pregnant Bangladeshi women--a prospective cohort study. Environ. Res. 2009, 109, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Kippler, M.; Ekström, E.C.; Lönnerdal, B.; Goessler, W.; Akesson, A.; El Arifeen, S.; Persson, L.A.; Vahter, M. Influence of iron and zinc status on cadmium accumulation in Bangladeshi women. Toxicol. Appl. Pharmacol. 2007, 222, 221–226. [Google Scholar] [CrossRef]
- Rentschler, G.; Kippler, M.; Axmon, A.; Raqib, R.; Skerfving, S.; Vahter, M.; Broberg, K. Cadmium concentrations in human blood and urine are associated with polymorphisms in zinc transporter genes. Metallomics 2014, 6, 885–891. [Google Scholar] [CrossRef]
- Rentschler, G.; Kippler, M.; Axmon, A.; Raqib, R.; Ekström, E.C.; Skerfving, S.; Vahter, M.; Broberg, K. Polymorphisms in iron homeostasis genes and urinary cadmium concentrations among nonsmoking women in Argentina and Bangladesh. Environ. Health Perspect. 2013, 121, 467–472. [Google Scholar] [CrossRef]
- Ng, E.; Lind, P.M.; Lindgren, C.; Ingelsson, E.; Mahajan, A.; Morris, A.; Lind, L. Genome-wide association study of toxic metals and trace elements reveals novel associations. Hum. Mol. Genet. 2015, 24, 4739–4745. [Google Scholar] [CrossRef]
- Leazer, T.M.; Liu, Y.; Klaassen, C.D. Cadmium absorption and its relationship to divalent metal transporter-1 in the pregnant rat. Toxicol. Appl. Pharmacol. 2002, 185, 18–24. [Google Scholar] [CrossRef]
- Flanagan, P.R.; McLellan, J.S.; Haist, J.; Cherian, M.G.; Chamberlain, M.J.; Valberg, L.S. Increased dietary cadmium absorption in mice and human subjects with iron deficiency. Gastroenterol. 1978, 46, 609–623. [Google Scholar] [CrossRef]
- Silver, M.K.; Lozoff, B.; Meeker, J.D. Blood cadmium is elevated in iron deficient U.S. children: A cross-sectional study. Environ. Health 2013, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Schildroth, S.; Friedman, A.; Bauer, J.A.; Claus, Henn, B. Associations of a metal mixture with iron status in U.S. adolescents: Evidence from the National Health and Nutrition Examination Survey. New Dir. Child Adolesc. Dev. 2022, 2022, 67–89. [Google Scholar] [CrossRef]
- Ahmed, F.; Coyne, T.; Dobson, A.; McClintock, C. Iron status among Australian adults: Findings of a population- based study in Queensland, Australia. Asia Pac. J. Clin. Nutr. 2008, 17, 40–47. [Google Scholar]
- Suh, Y.J.; Lee, J.E.; Lee, D.H.; Yi, H.G.; Lee, M.H.; Kim, C.S.; Nah, J.W.; Kim, S.K. Prevalence and relationships of iron deficiency anemia with blood cadmium and vitamin D levels in Korean women. J. Korean Med. Sci. 2016, 31, 25–32. [Google Scholar] [CrossRef]
- Meltzer, H.M.; Brantsaeter, A.L.; Borch-Iohnsen, B.; Ellingsen, D.G.; Alexander, J.; Thomassen, Y.; Stigum, H.; Ydersbond, T.A. Low iron stores are related to higher blood concentrations of manganese, cobalt and cadmium in non-smoking, Norwegian women in the HUNT 2 study. Environ. Res. 2010, 110, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Finley, J.W. Manganese absorption and retention by young women is associated with serum ferritin concentration. Am. J. Clin. Nutr. 1999, 70, 37–43. [Google Scholar] [CrossRef]
- Satarug, S.; Ujjin, P.; Vanavanitkun, Y.; Baker, J.R.; Moore, M.R. Influence of body iron store status and cigarette smoking on cadmium body burden of healthy Thai women and men. Toxicol. Lett. 2004, 148, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Vahter, M.; Berglund, M.; Nermell, B.; Akesson, A. Bioavailability of cadmium from shellfish and mixed diet in women. Toxicol. Appl. Pharmacol. 1996, 136, 332–341. [Google Scholar] [CrossRef]
- Haswell-Elkins, M.; Imray, P.; Satarug, S.; Moore, M.R.; O'dea, K. Urinary excretion of cadmium among Torres Strait Islanders (Australia) at risk of elevated dietary exposure through traditional foods. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 372–377. [Google Scholar] [CrossRef]
- Copes, R.; Clark, N.A.; Rideout, K.; Palaty, J.; Teschke, K. Uptake of cadmium from Pacific oysters (Crassostrea gigas) in British Columbia oyster growers. Environ. Res. 2008, 107, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Garner, R.; Levallois, P. Cadmium levels and sources of exposure among Canadian adults. Health Rep. 2016, 27, 10–18. [Google Scholar]
- Rimbach, G.; Pallauf, J. Cadmium accumulation, zinc status, and mineral bioavailability of growing rats fed diets high in zinc with increasing amounts of phytic acid. Biol. Trace Elem. Res. 1997, 57, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Lind, Y.; Engman, J.; Jorhem, L.; Glynm, A.W. Accumulation of cadmium from wheat bran, sugar-beet fibre, carrots and cadmium chloride in the liver and kidneys of mice. Br. J. Nutr. 1998, 80, 205–211. [Google Scholar] [CrossRef]
- Groten, J.P.; Sinkeldam, E.J.; Muys, T.; Luten, J.B.; van Bladeren, P.J. Interaction of dietary Ca, P, Mn, Cu, Fe, Zn and Se with accumulation and oral toxicity of cadmium in rats. Food Chem. Toxicol. 1991, 29, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Min, K.S.; Ueda, H.; Kihara, T.; Tanaka, K. Increased hepatic accumulation of ingested Cd is associated with upregulation of several intestinal transporters in mice fed diets deficient in essential metals. Toxicol Sci. 2008, 106, 284–289. [Google Scholar] [CrossRef]
- Min, K.S.; Sano, E.; Ueda, H.; Sakazaki, F.; Yamada, K.; Takano, M.; Tanaka, K. Dietary deficiency of calcium and/or iron, an age-related risk factor for renal accumulation of cadmium in mice. Biol. Pharm. Bull. 2015, 38, 1557–1563. [Google Scholar] [CrossRef]
- Walter, A.; Rimbach, G.; Most, E.; Pallauf, J. Effect of citric acid supplements to a maize-soya diet on the in vitro availability of minerals, trace elements and heavy metals. J. Vet. Med. 1998, 45, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Kukongviriyapan, U.; Pannangpetch, P.; Kukongviriyapan, V.; Donpunha, W.; Sompamit, K.; Surawattanawan, P. 2014. Curcumin protects against cadmium-induced vascular dysfunction, hypertension and tissue cadmium accumulation in mice. Nutrients 2014, 6, 1194–1208. [Google Scholar] [CrossRef]
- Li, X.; Jiang, X.; Sun, J.; Zhu, C.; Li, X.; Tian, L.; Liu, L.; Bai, W. Cytoprotective effects of dietary flavonoids against cadmium-induced toxicity. Ann. NY Acad. Sci. 2017, 1398, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Oh, C.; Shim, S.Y.; Jeong, S.; Kim, H.S.; Kim, M.S. Reduction in prevalence of hypertension and blood heavy metals among curry-consumed Korean. Tohoku J. Exp. Med. 2018, 244, 219–229. [Google Scholar] [CrossRef]
- Illing, A.C.; Shawki, A.; Cunningham, C.L.; Mackenzie, B. Substrate profile and metal-ion selectivity of human divalent metal-ion transporter-1. J. Biol. Chem. 2012, 287, 30485–30496. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, T.H.; Schwartz, J.; Bellinger, D.C.; Hauser, R.; Amarasiriwardena, C.; Sparrow, D.; Wright, R.O. Iron-processing genotypes, nutrient intakes, and cadmium levels in the Normative Aging Study: Evidence of sensitive subpopulations in cadmium risk assessment. Environ. Int. 2018, 119, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Li, C.; Zhao, D.; Huang, L. Associations of micronutrients exposure with cadmium body burden among population: A systematic review. Ecotoxicol. Environ. Saf. 2023, 256, 114878. [Google Scholar] [CrossRef]
- IARC (International Agency for Research on Cancer). Cadmium. IARC Monogr. Eval. Carcinog. Risk Hum. 1993, 58, 119–238. [Google Scholar]
- Nawrot, T.S.; Martens, D.S.; Hara, A.; Plusquin, M.; Vangronsveld, J.; Roels, H.A.; Staessen, J.A. Association of total cancer and lung cancer with environmental exposure to cadmium: the meta-analytical evidence. Cancer Causes Control. 2015, 26, 1281–1288. [Google Scholar] [CrossRef]
- Chen, C.; Xun, P.; Nishijo, M.; He, K. Cadmium exposure and risk of lung cancer: a meta-analysis of cohort and case-control studies among general and occupational populations. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 437–444. [Google Scholar] [CrossRef]
- Song, Jk.; Luo, H.; Yin, Xh.; Huang, G.l.; Luo, Sy.; Lin, du R. ; Yuan, D.B.; Zhang, W.; Zhu, Jg. Association between cadmium exposure and renal cancer risk: a meta-analysis of observational studies. Sci. Rep. 2015, 5, 17976. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xun, P.; Nishijo, M.; Sekikawa, A.; He, K. Cadmium exposure and risk of pancreatic cancer: a meta-analysis of prospective cohort studies and case-control studies among individuals without occupational exposure history. Environ. Sci. Pollut. Res. Int. 2015, 22, 17465–17474. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Orsini, N.; Wolk, A. Urinary cadmium concentration and risk of breast cancer: A systematic review and dose-response meta-analysis. Am. J. Epidemiol. 2015, 182, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhang, F.; Lei, Y. Dietary intake and urinary level of cadmium and breast cancer risk: A meta-analysis. Cancer Epi-demiol. 2016, 42, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Jouybari, L.; Saei Ghare Naz, M.; Sanagoo, A.; Kiani, F.; Sayehmiri, F.; Sayehmiri, K.; Hasanpour Dehkordi, A. Toxic elements as biomarkers for breast cancer: A meta-analysis study. Cancer Manag. Res. 2018, 10, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Van Maele-Fabry, G.; Lombaert, N.; Lison, D. Dietary exposure to cadmium and risk of breast cancer in postmenopausal women: A systematic review and meta-analysis. Environ. Int. 2016, 86, 1–13. [Google Scholar] [CrossRef]
- Florez-Garciam, V.A.; Guevara-Romero, E.C.; Hawkins, M.M.; Bautista, L.E.; Jenson, T.E.; Yu, J.; Kalkbrenner, A.E. Cadmium exposure and risk of breast cancer: A meta-analysis. Environ. Res. 2023, 219, 115109. [Google Scholar] [CrossRef]
- Filippini, T.; Torres, D.; Lopes, C.; Carvalho, C.; Moreira, P.; Naska, A.; Kasdagli, M.I.; Malavolti, M.; Orsini, N.; Vinceti, M. Cadmium exposure and risk of breast cancer: A dose-response meta-analysis of cohort studies. Environ. Int. 2020, 142, 105879. [Google Scholar] [CrossRef]
- Anđelković, M.; Djordjevic, A.B.; Miljaković, E.A.; Javorac, D.; Čolaković, N.; Oprić, S.; Petričević, S.; Granić, M.; Kotur-Stevuljević, J.; Antonijević, B.; et al. Cadmium tissue level in women diagnosed with breast cancer - A case control study. Environ. Res. 2021, 199, 111300. [Google Scholar] [CrossRef]
- Kim, J.U.; Shariff, M.I.; Crossey, M.M.; Gomez-Romero, M.; Holmes, E.; Cox, I.J.; Fye, H.K.; Njie, R.; Taylor-Robinson, S.D. Hepatocellular carcinoma: Review of disease and tumor biomarkers. World J. Hepatol. 2016, 8, 471–484. [Google Scholar] [CrossRef]
- Kew, M.C. Epidemiology of chronic hepatitis B virus infection, hepatocellular carcinoma, and hepatitis B virus-induced hepatocellular carcinoma. Pathologie Biologie 2010, 58, 273–277. [Google Scholar] [CrossRef]
- Bartosch B, Thimme R, Blum HE, Zoulim F. Hepatitis C virus-induced hepatocarcinogenesis. J. Hepatology 2009, 51, 810–820.
- Besaratinia, A.; Kim, S.I.; Hainaut, P.; Pfeifer, G.P. In vitro recapitulating of TP53 mutagenesis in hepatocellular carcinoma associated with dietary aflatoxin B1 exposure. Gastroenterol., 2009, 137, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Millonig, G.; Seitz, H.K. Alcoholic liver disease and hepatitis C: A frequently underestimated combination. World J. Gastroenterol. 2009, 15, 3462–3471. [Google Scholar]
- Savas, N; Canan, O. ; Ozcay, F.; Bilezikci, B.; Karakayali, H.; Yilmaz, U.; Haberal, M. Hepatocellular carcinoma in Wilson's disease: a rare association in childhood. Pediatric Transplantation 2006, 10, 639–643.
- Giannitrapani, L.; Soresi, M.; La Spada, E.; Cervello, M.; D'Alessandro, N.; Montalto, G. Sex hormones and risk of liver tumor. Ann. N.Y. Acad. Sci. 2006, 1089, 228–236. [Google Scholar] [CrossRef]
- Bartley, J.; Loddenkemper, C.; Lange, J.; Mechsner, S.; Radke, C.; Neuhaus, P.; Ebert, A.D. Hepatocellular adenoma and focal nodular hyperplasia after long-term use of danazol for endometriosis: a case report. Arch. Gynecol. Obstet. 2004, 269, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Cattan, S.; Wendum, D.; Chazouilleres, O.; Schmitz, J.; Gendre, J.P. Hepatocellular carcinoma and focal hepatic glycogenosis after prolonged azathioprine therapy. Human Pathol. 2000, 31, 874–876. [Google Scholar] [CrossRef]
- Ishida, M. ; Naka S; Shiomi, H. ; Tsujikawa, T.; Andoh, A.; Nakahara, T.; Saito, Y.; Kurumi, Y.; Takikita-Suzuki, M.; Kojima, F.; Hotta, M.; Tani, T.; Fujiyama, Y.; Okabe, H. Hepatocellular carcinoma occurring in a Crohn's disease patient. World J. Gastroenterol. 2010, 16, 3215–3218. [Google Scholar]
- Bannasch, P. Hepatocellular glycogenosis and hepatic neoplasms. Toxicol. Pathol. 010, 38, 1000-1002.
- Campbell, T.C.; Chen, J.S.; Liu, C.B.; Li, J.Y.; Parpia, B. Non-association of aflatoxin with primary liver cancer in a cross-sectional ecological survey in the People's Republic of China. Cancer Res. 1990, 50, 6882–6893. [Google Scholar]
- Jiang, A.; Gong, L.; Ding, H.; Wang, M. Cancer mortality and long-term environmental exposure of cadmium in contaminated community based on a third retrospective cause of death investigation of residents living in the Guangdong Province from 2004 to 2005. Biol. Trace. Elem. Res. 2021, 199, 4504–4515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Li, T.; Zhuo, W.; Zhu, Y. Elevated serum and hair levels of cadmium as a risk factor for liver carcinoma: A meta-analysis. Nutr. Cancer 2023, 75, 1438–1447. [Google Scholar] [CrossRef]
- Satarug, S.; Baker, J.R.; Reilly, P.E.; Moore, M.R.; Williams, D.J. Cadmium levels in the lung, liver, kidney cortex, and urine samples from Australians without occupational exposure to metals. Arch. Environ. Health 2002, 57, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Baker, J.R.; Reilly, P.E.; Moore, M.R.; Williams, D.J. Changes in zinc and copper homeostasis in human livers and kidneys associated with exposure to environmental cadmium. Hum. Exp. Toxicol. 2001, 20, 205–213. [Google Scholar] [CrossRef]
- Satarug, S. Long-term exposure to cadmium in food and cigarette smoke, liver effects and hepatocellular carcinoma. Curr. Drug Metab. 2012, 13, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Hyder, O.; Chung, M.; Cosgrove, D.; Herman, J.M.; Li, Z.; Firoozmand, A.; Gurakar, A.; Koteish, A.; Pawlik, T.M. Cadmium exposure and liver disease among US adults. J. Gastrointest. Surg. 2013, 17, 1265–1273. [Google Scholar] [CrossRef]
- Hong, D.; Min, J.Y.; Min, K.B. Association between cadmium exposure and liver function in adults in the United States: A cross-sectional study. J. Prev. Med. Public Health 2021, 54, 471–480. [Google Scholar] [CrossRef]
- Xu, Z.; Weng, Z.; Liang, J.; Liu, Q.; Zhang, X.; Xu, J.; Xu, C.; Gu, A. Association between urinary cadmium concentrations and liver function in adolescents. Environ. Sci. Pollut. Res. Int. 2022, 29, 39768–39776. [Google Scholar] [CrossRef]
- Kang, M.Y.; Cho, S.H.; Lim, Y.H.; Seo, J.C.; Hong, Y.C. Effects of environmental cadmium exposure on liver function in adults. Occup. Environ. Med. 2013, 70, 268–273. [Google Scholar] [CrossRef]
- Kim, D.W.; Ock, J.; Moon, K.W.; Park, C.H. Association between Pb, Cd, and Hg Exposure and Liver Injury among Korean Adults. Int. J. Environ. Res. Public Health 2021, 18, 6783. [Google Scholar] [CrossRef]
- Park, E.; Kim, J.; Kim, B.; Park, E.Y. Association between environmental exposure to cadmium and risk of suspected non-alcoholic fatty liver disease. Chemosphere 2021, 266, 128947. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Sung, G.H.; Lee, S.; Han, K.J.; Han, H.J. Serum cadmium is associated with hepatic steatosis and fibrosis: Korean national health and nutrition examination survey data IV-VII. Medicine (Baltimore) 2022, 101, e28559. [Google Scholar] [CrossRef]
- Seo, M.N.; Eom, S.Y.; Lim, J.A.; Lee, J.E.; Choi, B.S.; Kwon, H.J.; Hong, Y.S.; Kim, H.; Park, J.D. Effects of environmental cadmium exposure on the liver in Korean adults: Cross-sectional and longitudinal studies. Arch. Environ. Contam. Toxicol. 2023, 84, 237–247. [Google Scholar] [CrossRef] [PubMed]
- McElroy, J.A.; Hunter, M.I. Cadmium: a new risk factor for endometrial cancer? Expert Rev. Anticancer Ther. 2019, 19, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Cirovic, A.; Cirovic, A. Iron deficiency as promoter of heavy metals-induced acute myeloid leukemia. Leuk. Res. 2021, 112, 106755. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. The evolving role for zinc and zinc transporters in cadmium tolerance and urothelial cancer. Stresses 2021, 1, 105–118. [Google Scholar] [CrossRef]
- Satir, S. The relationship between oral cancer and cadmium: a review. Mol. Biol. Rep. 2022, 49, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli Pirzaman, A.; Mansoori, R.; Hosseini, S.M.; Abolhosseini, A.; Khosravi, S.; Moghadamnia, A.A.; Kazemi, S. Toxic mechanisms of cadmium and exposure as a risk factor for oral and gastrointestinal carcinomas. Hum. Exp. Toxicol. 2024, 43, 9603271231223506. [Google Scholar] [CrossRef]
- Rezapour, M.; Rezapour, H.A.; Chegeni, M.; Khanjani, N. Exposure to cadmium and head and neck cancers: a meta-analysis of observational studies. Rev. Environ. Health 2021, 36, 577–584. [Google Scholar] [CrossRef]
- Chen, C.; Xun, P.; Nishijo, M.; Carter, S.; He, K. Cadmium exposure and risk of prostate cancer: a meta-analysis of cohort and case-control studies among the general and occupational populations. Sci. Rep. 2016, 6, 25814. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Y.; Hao, R.; Shao, M.; Luo, Y. Cadmium levels in tissue and plasma as a risk factor for prostate carcinoma: A meta-analysis. Biol. Trace Elem. Res. 2016, 172, 86–92. [Google Scholar] [CrossRef]
- McElroy, J.A.; Shafer, M.M.; Trentham-Dietz, A.; Hampton, J.M.; Newcomb, P.A. Cadmium exposure and breast cancer risk. J. Natl. Cancer Inst. 2006, 98, 869–873. [Google Scholar] [CrossRef]
- Gallagher, C.M.; Chen, J.J.; Kovach, J.S. Environmental cadmium and breast cancer risk. Aging (Albany NY) 2010, 2, 804–814. [Google Scholar] [CrossRef]
- Moffett, D.B.; Mumtaz, M.M.; Sullivan, D.W., Jr.; Whittaker, M.H. Chapter 13, General Considerations of Dose-Effect and Dose-Response Relationships. In Handbook on the Toxicology of Metals, 5th ed.; Volume I: General, Considerations, Nordberg, G., Costa, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 299–317. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Oh, T.K.; Song, I.A. Anemia may increase the overall risk of cancer: Findings from a Cohort Study with a 12-year follow-up period in South Korea. Cancer Epidemiol. Biomarkers Prev. 2021, 30, 1440–1448. [Google Scholar] [CrossRef]
- Lee, P.-J.; Jhuang, J.-R.; Chen, Y.-C.; Su, S.-Y.; Chiang, C.-J.; Yang, Y.-W.; Hsieh, P.-C.; Chen, M.-J.; Lee, W.-C. Urban–rural disparity in birth cohort effects on breast cancer incidence. J. Urban Health 2023, 100, 341–354. [Google Scholar] [CrossRef]
- Ronchetti, S.A.; Miler, E.A.; Duvilanski, B.H.; Cabilla, J.P. Cadmium mimics estrogen-driven cell proliferation and prolactin secretion from anterior pituitary cells. PLoS One 2013, 8, e81101. [Google Scholar] [CrossRef] [PubMed]
- Siewit, C.L.; Gengler, B.; Vegas, E.; Puckett, R.; Louie, M.C. Cadmium promotes breast cancer cell proliferation by potentiating the interaction between ERalpha and c-Jun. Mol Endocrinol. 2010, 24, 981–992. [Google Scholar] [CrossRef]
- Nalwoga, H.; Ahmed, L.; Arnes, J.B.; Wabinga, H.; Akslen, L.A. Strong expression of hypoxia-inducible factor-1α (HIF-1α) is associated with Axl expression and features of aggressive tumors in African breast cancer. PLoS One 2016, 11, e0146823. [Google Scholar] [CrossRef] [PubMed]
- Habashy, H.O.; Powe, D.G.; Staka, C.M.; Rakha, E.A.; Ball, G.; Green, A.R.; Aleskandarany, M.; Paish, E.C.; Douglas Macmillan, R.; Nicholson, R.I.; et al. Transferrin receptor (CD71) is a marker of poor prognosis in breast cancer and can predict response to tamoxifen. Breast Cancer Res. Treat. 2010, 119, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, X.; Dong, D.; Zhang, B.; Xue, Y.; Shang, P. Transferrin receptor 1 in cancer: a new sight for cancer therapy. Am. J. Cancer Res. 2018, 8, 916–931. [Google Scholar]
- Abul-Husn, N.S.; Soper, E.R.; Odgis, J.A.; Cullina, S.; Bobo, D.; Moscati, A.; Rodriguez, J.E.; CBIPM Genomics Team; Regeneron Genetics Center; et al. Exome sequencing reveals a high prevalence of BRCA1 and BRCA2 founder variants in a diverse population-based biobank. Genome Med. 2019, 31, 12. [Google Scholar] [CrossRef] [PubMed]
- Kachamakova-Trojanowska, N.; Podkalicka, P.; Bogacz, T.; Barwacz, S.; Józkowicz, A.; Dulak, J.; Łoboda, A. HIF-1 stabilization exerts anticancer effects in breast cancer cells in vitro and in vivo. Biochem. Pharmacol. 2020, 175, 113922. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Pi, H.; Liao, L.; Tan, M.; Deng, P.; Yue, Y.; Xi, Y.; Tian, L.; Xie, J.; Chen, M.; et al. Cadmium promotes breast cancer cell proliferation, migration and invasion by inhibiting ACSS2/ATG5-mediated autophagy. Environ. Pollut. 2021, 273, 116504. [Google Scholar] [CrossRef]
- Joseph, P. Mechanisms of cadmium carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 272–279. [Google Scholar] [CrossRef]
- Candéias, S.; Pons, B.; Viau, M.; Caillat, S.; Sauvaigo, S. Direct inhibition of excision/synthesis DNA repair activities by cadmium: analysis on dedicated biochips. Mutat. Res. 2010, 694, 53–59. [Google Scholar] [CrossRef]
- Waalkes, M.P.; Rehm, S. Chronic toxic and carcinogenic effects of cadmium chloride in male DBA/2NCr and NFS/NCr mice: strain-dependent association with tumors of the hematopoietic system, injection site, liver, and lung. Fundam. Appl. Toxicol. 1994, 23, 21–31. [Google Scholar] [CrossRef]
- Huff, J.; Lunn, R.M.; Waalkes, M.P.; Tomatis, L.; Infante, P.F. Cadmium-induced cancers in animals and in humans. Int. J. Occup. Environ. Health 2007, 13, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Tokar, E.J.; Benbrahim-Tallaa, L.; Waalkes, M.P. Metal ions in human cancer development. Met. Ions Life Sci. 2011, 8, 375–401. [Google Scholar]
- Zhu, Y.; Costa, M. Metals and molecular carcinogenesis. Carcinogenesis 2020, 41, 1161–1172. [Google Scholar] [CrossRef]
- Alexandrov, K.; Rojas, M.; Satarug, S. The critical DNA damage by benzo(a)pyrene in lung tissues of smokers and approaches to preventing its formation. Toxicol. Lett. 2010, 198, 63–68. [Google Scholar] [CrossRef]
- Sens, D.A.; Park, S.; Gurel, V.; Sens, M.A.; Garrett, S.H.; Somji, S. Inorganic cadmium- and arsenite-induced malignant transformation of human bladder urothelial cells. Toxicol. Sci. 2004, 79, 56–63. [Google Scholar] [CrossRef]
- Hoggarth, Z.E.; Osowski, D.B.; Freeberg, B.A.; Garrett, S.H.; Sens, D.A.; Sens, M.A.; Zhou, X.D.; Zhang, K.K.; Somji, S. The urothelial cell line UROtsa transformed by arsenite and cadmium display basal characteristics associated with muscle invasive urothelial cancers. PLoS ONE 2018, 13, e0207877. [Google Scholar] [CrossRef] [PubMed]
- Benbrahim-Tallaa, L.; Tokar, E.J.; Diwan, B.A.; Dill, A.L.; Coppin, J.-F.; Waalkes, M.P. Cadmium malignantly transforms normal human breast epithelial cells into a basal-like phenotype. Environ. Health Perspect. 2009, 117, 1847–1852. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Liu, L.Z.; Jiang, Y.; Zhu, Y.; Guo, N.L.; Barnett, J.; Rojanasakul, Y.; Agani, F.; Jiang, B.H. Cadmium increases HIF-1 and VEGF expression through ROS, ERK, and AKT signaling pathways and induces malignant transformation of human bronchial epithelial cells. Toxicol. Sci. 2012, 125, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Person, R.J.; Tokar, E.J.; Xu, Y.; Orihuela, R.; Ngalame, N.N.; Waalkes, M.P. Chronic cadmium exposure in vitro induces cancer cell characteristics in human lung cells. Toxicol. Appl. Pharmacol. 2013, 273, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Tokar, E.J.; Kim, A.J.; Bell, M.W.; Waalkes, M.P. Chronic cadmium exposure in vitro causes acquisition of multiple tumor cell characteristics in human pancreatic epithelial cells. Environ. Health Perspect. 2012, 120, 1265–1271. [Google Scholar] [CrossRef]
- Qu, W.; Diwan, B.A.; Reece, J.M.; Bortner, C.D.; Pi, J.; Liu, J.; Waalkes, M.P. Cadmium-induced malignant transformation in rat liver cells: Role of aberrant oncogene expression and minimal role of oxidative stress. Int. J. Cancer 2005, 114, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Takiguchi, M.; Achanzar, W.E.; Qu, W.; Li, G.; Waalkes, M.P. Effects of cadmium on DNA-(Cytosine-5) methyltransferase activity and DNA methylation status during cadmium-induced cellular transformation. Exp. Cell Res. 2003, 286, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Schidberger, H.; Mayer, A. The Warburg effect: essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg effect: historical dogma versus current understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Drochioiu, G. Multifactorial distress, the Warburg effect, and respiratory and pH imbalance in cancer development. Stresses 2023, 3, 500–528. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Zhu, Y.; Yu, Z.; Shao, M.; Luo, Y. Identification and characterization of cadmium-related genes in liver carcinoma. Biol. Trace Elem. Res. 2018, 182, 238–247. [Google Scholar] [CrossRef]
- Forcella, M.; Lau, P.; Fabbri, M.; Fusi, P.; Oldani, M.; Melchioretto, P.; Gribaldo, L.; Urani, C. Is cadmium toxicity tissue-specific? Toxicogenomics studies reveal common and specific pathways in pulmonary, hepatic, and neuronal cell models. Int. J. Mol. Sci. 2022, 23, 1768. [Google Scholar] [CrossRef]
- Luparello, C. Cadmium-associated molecular signatures in cancer cell models. Cancers (Basel) 2021, 13, 2823. [Google Scholar] [CrossRef]
- Rossi, M.R.; Masters, J.R.W.; Park, S.; Todd, J.H.; Garrett, S.H.; Sens, M.A.; Somji, S.; Nath, J.; Sens, D.A. The immortalized UROtsa cell line as a potential cell culture model of human urothelium. Environ. Health Perspect. 2001, 109, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Petzoldt, L.; Leigh, I.M.; Duffy, P.G.; Sexton, C.; Masters, R.W. Immortalisation of human urothelial cells. Urol. Res. 1995, 23, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Takiguchi, M.; Cherrington, N.J.; Hartley, D.P.; Klaassen, C.D.; Waalkes, M.P. Cyproterone acetate induces a cellular tolerance to cadmium in rat liver epithelial cells involving reduced cadmium accumulation. Toxicology 2001, 165, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Ohana, E.; Sekler, I.; Kaisman, T.; Kahn, N.; Cove, J.; Silverman, W.F.; Amsterdam, A.; Hershfinkel, M. Silencing of ZnT-1 expression enhances heavy metal influx and toxicity. J. Mol. Med. 2006, 84, 753–763. [Google Scholar] [CrossRef]
- Nishito, Y.; Kambe, T. Zinc transporter 1 (ZNT1) expression on the cell surface is elaborately controlled by cellular zinc levels. J. Biol. Chem. 2019, 294, 15686–15697. [Google Scholar] [CrossRef]
- Satarug, S.; Garrett, S.H.; Somji, S.; Sens, M.A.; Sens, D.A. Zinc, zinc transporters, and cadmium cytotoxicity in a cell culture model of human urothelium. Toxics 2021, 9, 94. [Google Scholar] [CrossRef] [PubMed]
| Foods | Cd, mg/kg | Intake rate, g/day | Exposure, µg/day | ||
|---|---|---|---|---|---|
| Maximum | Typical | High | Average | ||
| Vegetables; potatoes, included | 0.1 | 0.05 | 250 | 25 | 12.5 |
| Cereals, pulses, legume; rice, wheat grain included | 0.2 | 0.05 | 200 | 40 | 10 |
| Fruit | 0.05 | 0.01 | 150 | 7.5 | 1.5 |
| Oilseeds and cocoa beans | 1.0 | 0.5 | 1 | 1 | 0.5 |
| Meat; cattle, poultry, pig, sheep | 0.1 | 0.02 | 150 | 15 | 3.0 |
| Liver; cattle, poultry, pig, sheep | 0.5 | 0.1 | 5 | 2.5 | 0.5 |
| Kidney; cattle, poultry, pig, sheep | 2.0 | 0.5 | 1 | 2 | 0.5 |
| Fish | 0.05 | 0.02 | 30 | 1.5 | 0.6 |
| Crustaceans, mollusks | 2 | 0.25 | 3 | 6 | 0.75 |
| TOTAL | − | − | − | 93.5 | 30 |
| Cancer | Exposure and Risk Estimates | References |
|---|---|---|
| Lung | Comparing the highest vs. lowest category of urinary Cd, respective OR (95% CI) values for lung cancer risk and cancer mortality were 1.68 (1.47–1.92) and 1.22 (1.13–1.31). The average urinary Cd excretions across three cohorts ranged from 0.25 to 0.93 µg/g creatinine. |
Nawrot et al. 2015 [81] |
| Lung | Comparing the highest vs. lowest Cd dose category, respective OR (95% CI) values for lung cancer were 1.42 (0.91 − 2.23), 0.68 (0.33 − 1.41) and 1.61 (0.94 − 2.75) in the general population, occupationally-exposed population, and case–control studies, respectively. | Chen et al. 2016 [82] |
| Kidney | Comparing the highest vs. lowest category of Cd dose metrics, OR (95% CI) values for renal cancer risk was 1.47 (1.27−1.71) | Song et al. 2015 [83] |
| Pancreas | Comparing the highest vs. lowest category of Cd dose metrics, respective RR (95% CI) values for pancreatic cancer risk in all subjects, men and women were 2.05 (1.58−2.66), 1.78 (1.04–3.05) and 1.02, (0.63–1.65). | Chen et al. 2015 [84] |
| Breast | Comparing the highest vs. lowest category of urinary Cd, OR value (95% CI) for breast cancer risk was 2.24 (1.50–3.34). For each 0.5-µg/g creatinine increase of urinary Cd, the OR for breast cancer was increased 1.66-fold (95% CI: 1.23–2.25) |
Larsson et al. 2015 [85] |
| Breast | Comparing the highest vs. lowest quartile of urinary Cd, OR value (95% CI) for breast cancer was 2.24 (1.49–3.35) Dietary Cd exposure level was not associated with breast cancer risk (RR 1.01, 95% CI: 0.89–1.15). |
Lin et al. 2016 [86] |
| Breast | Respective mean (95% CI) values for Cd and Ni in scalp hair and toenail samples from women with breast cancer were 2.65 (1.57–3.73) and 2.06 (1.20–3.32), higher than controls. | Jouybari et al. 2018 [87] |
| Breast | Among postmenopausal women, dietary Cd exposure level was not associated with breast cancer risk (RR, 1.03, 95% CI: 0.89–1.19) |
Van Maele-Fabry et al. 2016 [88] |
| Study Population | Exposure Levels and Effects Observed | Reference |
|---|---|---|
| United States NHANES 1988 − 1994 n 12,732, ≥ 20 years |
Urinary Cd levels ≥ 0.83 μg/g creatinine were associated with 1.26-fold increase in risk of liver inflammation in women. Urinary Cd ≥ 0.65 μg/g creatinine were associated with liver inflammation (OR 2.21), NAFLD (OR 1.30), and NASH (OR 1.95) in men |
Hyder et al. 2013 [109] |
| United States NHANES 1999 − 2015 n 11, 838, ≥ 20 years |
A tenfold increment of urinary Cd was associated with elevated plasma levels of ALT (OR 1.36), and AST (OR 1.31) | Hong et al. 2021 [110] |
| United States NHANES 1999 − 2016 n 4411 adolescents |
Urinary Cd quartile 4 was associated with elevated plasma ALT (OR 1.40) and AST (OR 1.64). Liver effect of Cd was larger in boys than girls |
Xu et al. 2022 [111] |
| South Korea, n 3914, ≥ 19 years |
Blood Cd levels ≥ 1.98 μg/L were associated with elevated plamsa levels of AST (OR 3.61) and ALT (OR 2.40) | Kang et al. 2013 [112] |
| Korean National Environmental Health Survey ((2015-2017) n 2953, ≥ 19 year |
Geometric mean urinary Cd was 0.42 µg/L. Top urinary Cd quartile (> 0.88 μg/L) was associated with higher plasma AST and GGT, compared with quartile 1. Urinary Cd quartiles 4, 3, and 2 were associated with higher plasma ALT, compared with bottom. quartile |
Kim et al. 2021 [113] |
| South Korea, n 12,099, ≥ 19 years |
Blood Cd levels higher than 0.96 and 1.41 μg/L were associated with NAFLD (OR 1.91) and NASH (OR 1.41) | Park et al. 2021 [114] |
| Korean NHANES 2008–2017 n 15,783, ≥ 20 years, mean age 46 |
Serum Cd levels >1.41 µg/dL were respectively associated with 1.90, 1.26-, 1.73- and 2.53-fold increases in risk of ALT elevation, hepatic steatosis, fibrosis, and AST elevation, compared to serum Cd levels < 0.651 µg/dL | Han et al. 2022 [115] |
| South Korea Baseline (2011), n 2,086 adults Follow-up (2015), n 503 adult |
The geometric mean blood Cd was higher in females than males (1.16 vs. 0.96 μg/L). No change in blood Cd levels both genders over 5-year duration. ALT and GGT rose only in females. |
Seo et al. 2023 [116] |
| Immortal Cell Line, Reference |
[Cd], Exposure Duration | Transformant and Tumor Characteristics |
|---|---|---|
| Human urothelium, UROtsa [146,147] | 1 μM Cd2+ 8-10 weeks. |
Soft agar colony formation. Tumorigenic in nude mice with the transitional cell carcinoma phenotypes; gene expression profiles like the basal subtype of muscle invasive bladder carcinomas. Acquired Cd tolerance as an adaptive survival mechanism. |
| Human breast epithelium, MCF-10A [148] |
2.5 μM Cd2+ 40 weeks. |
Basal-like breast cancer cells; ER-α negative, HER2 negative, diminished BRCA1 expression, persistent cell proliferation, elevated expression of cytokeratin 5 and p63, overexpressed c-myc and Kras, and displayed a global DNA hypomethylation state. Tumors were ER-α negative. |
| Human bronchial epithelium, BEAS-2B [149] |
1 µM Cd2+ 6 months. Passage-matched cells as controls. |
Activated ERK and AKT signaling, elevated HIF-1 and VEGF expression. Inhibition of the ROS generation attenuated the activation of ERK, AKT, p70S6K1 and expression of HIF-1α. Tumorigenic in nude mice. Formation of tubes in an in vitro test for angiogenesis. |
| Human peripheral lung epithelium, HPL-1D [150] |
5 μM Cd2+ 20 weeks. |
Soft agar colony formation. Decreased expression of the tumor suppressor genes p16 and SLC38A3, increased expression of the oncoproteins KRAS and NRAS and vimentin, the epithelial-to-mesenchymal transition marker, overexpressed MT-1A, MT-2A, HO-1, HIF-1A, ZnT1, ZnT5 and ZIP8. Acquired Cd tolerance as an adaptive survival mechanism. |
| Human pancreatic ductal epithelium, HPDE [151] |
1 μM Cd2+ 29 weeks. |
Increased secretion of matrix metalloproteinase-9 (MMP-9) and over-expressed the pancreatic cancer marker S100P. Formation of poorly differentiated glandular-like structures on soft agar. |
| Rat liver epithelium, TRL1215 [152,153]. |
2.5 μM Cd2+ for 10 weeks 1 μM Cd2+ for 28 weeks |
Hyperproliferation, increased invasiveness, and reduced dependency on serum, increased DNA methylation, enhanced DNA methyltransferase activity. Over expression of c-myc and c-jun oncogenes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




