1. Introduction
Arteries constitute a critical part of the cardiovascular system, being the blood vessels aimed at supplying the whole body with oxygen and nutrients. Structural and functional features characterize the vascular beds of different anatomical locations within the arterial network. This arterial heterogeneity exerts a significant influence on cardiovascular diseases (CVDs), prompting the search for therapies and surgical options specific for the injured vascular bed.
In the present review, we will discuss the arterial tree heterogeneity on a structural and cellular basis, focusing on the differences in atherosclerotic plaques among different arteries. Secondly, the role of microRNA (miRNAs) as potential epigenetic regulators of arterial heterogeneity and differentiation will be proposed, with particular emphasis on the abdominal aorta and femoral artery.
2. The heterogeneity of the arterial tree: structural and cellular basis
The cardiovascular system consists of heart and blood vessels, which are arteries, veins and capillaries. Arteries nourish tissue and organs by delivering blood with oxygen and nutrients from heart to the whole body and are classified into elastic, muscular and arterioles, according to size and structure. The complexity and heterogeneity of the vascular beds within the arterial tree depend on the different local demand of oxygen and nutrients. Aorta and carotid artery belong to the elastic arteries, collect blood from the heart, characterized by a large content of elastic fibres in the tunica media that allow the vessel to stretch during systole and contract during diastole to allow blood distribution within the cardiovascular system [
1]. Muscular arteries include brachial, radial and femoral arteries, are medium-sized, collect and deliver blood from elastic arteries to organs, and are equipped with strong connective tissue and less elastic fibres than elastic arteries [
1]. Arterioles lead blood into capillaries, are smaller in size and wall thickness, own a prominent elastic internal membrane and perform pressure and blood flow regulation [
2]. The vascular heterogeneity also stem from vascular cell features and transcriptome. Endothelial cells (ECs) represent the inner cell layer within the blood vessels and regulate a broad range of processes including vascular homeostasis, blood flow regulation, blood cells luminal adherence and vascular permeability. Despite a shared structure and gene signature, a phenotypic and functional heterogeneity emerge in the endothelium from different vascular beds, mirroring differential antigen and transcriptional patterns. Endothelium heterogeneity can be related to embryological origin [
3,
4,
5], microenvironment [
5,
6], epigenetic [
5,
7], site-specific demands and artery size. In vivo [
8,
9,
10] and in vitro [
11] studies support anatomical differences among ECs. Site-specific transcriptional signatures were observed in bovine endothelial cells derived from glomerular and aortic endothelium [
8]. Microarray analysis also demonstrated a different transcriptome existing between porcine endothelial cells from coronary and iliac arteries [
9]. Differences in terms of growth rate and biochemical properties were also found in ECs isolated from human cerebral and peripheral muscular arteries [
11].
Smooth muscle cells (SMCs) are located within the media layer of the arterial wall, regulate the luminal diameter by contraction and relaxation for adapting to the blood flow and keeping blood pressure, and synthesize many components of the extracellular matrix (ECM). SMCs display a significant plasticity, through the switching from a quiescent contractile phenotype to a synthetic proliferative one [
12,
13]. Beyond the phenotypic plasticity, SMC populations arising from different progenitors can be found within the arterial tree, contributing to vascular heterogeneity [
13,
14]. SMC heterogeneity was also observed in different areas of the same vessel, as documented by the different expression of desmin and connexin 43 in SMCs of the internal thoracic artery [
15,
16].
Vascular stem cells critically affect arterial differentiation with respect to structural/functional heterogeneity and disease pathogenic features. A broad range of evidences supports the existence of the vasculogenic niche, which is the adventitial reservoir of stem cells arising from bone marrow, circulation, large and small blood vessels [
17]. A noteworthy contribution is exerted by the adventitial Mesenchymal Stromal Cells (MSCs), multipotent stem cells engaged with differentiation potential into the adipogenic, osteogenic, chondrogenic, leyomiogenic lineages [
18,
19]. Functional peculiarities regarding the differentiation potential among MSCs located in different anatomical sites of the arterial tree also exist as elucidated by comparative studies study of our group. Thoracic aorta hMSCs were shown to be more osteogenic than femoral artery ones, whereas the latter displayed higher propensity to differentiate into the adipogenic lineage in according with higher lipid droplet production and peroxisome proliferator activated receptor (PPAR)-γ expression [
20]. The chondrogenic potential resulted more pronounced in hMSCs from brachiocephalic artery and thoracic aorta than in hMSCs of femoral artery [
21].
This functional heterogeneity mirrors the different pathological patterns and progressions of CVDs, supporting the higher prevalence of obstructive diseases in femoral vascular beds and the common occurrence of calcified plaques within carotid and thoracic segments, where the degree of osteo-chondrogenic differentiation is higher [
21].
The identification of an artery-specific genetic signature would represent the key point in the cardiovascular research, in the light of more effective therapies and surgical options for CVDs.
3. The arterial heterogeneity in atherosclerosis
Atherosclerosis is the main cause of CVDs including myocardial infarction, stroke and heart failure. This chronic and inflammatory affection determines the accumulation of lipids, inflammatory cells and SMCs leading to the intimal and medial thickening of medium-sized arteries, progressively reducing the vascular lumen [
22] . Smoke, lipid-rich diet, hypertension, obesity and high glucose levels are the most common risk factors in atherosclerosis, however they do not affect all the arterial segments in the same manner. Differences in atherosclerotic plaque pathogenesis, morphology and progression exist along the arterial tree. Further, not all arterial segments are vulnerable atherosclerotic sites, like the internal mammary artery. The study of atherosclerosis and CVD heterogeneity within the arterial tree would be pivotal for the improvement of the clinical and therapeutic options.
In atherosclerosis, the endothelial dysfunction is considered the initiating event, characterized by the loss of the EC barrier, the recruitment of monocytes and the infiltration of low-density lipoproteins (LDLs) within the intima. LDL oxidation is followed by foam cell formation, upstream process in the inflammatory cascade; SMCs migrate and proliferate into the intima, giving rise to the main component of the atherosclerotic plaque. All these conditions culminate in the sharpening of inflammation, activation of matrix-degrading enzymes and occurrence of secondary mechanisms like ectopic calcification, that is the calcium deposition in the vascular wall associated with plaque instability, rupture and increase of mortality risk [
23]. A broad range of evidence supports an anatomical specificity for atherosclerosis and heterogeneity in the morphological and pathogenic mechanisms, due to differences in crucial factors like hemodynamic, wall architecture and vascular cell characteristics (i.e. SMC developmental origin) among different arteries [
24,
25]. Morphological and haemodynamic differences can be seen in carotid and coronary plaques; indeed, carotid plaques exhibit thicker fibrous cap, higher prevalence of intraplaque haemorrhage and calcification compared to coronary plaques [
26].
A morphological study based on the American Heart Association (AHA) grading, calcification and lipid content highlighted differences between plaques of carotid and femoral arteries. Carotid plaques were mainly characterized by a fibrous cap atheroma, whereas fibrocalcific plaques were mainly observed in femoral arteries; further, femoral plaques displayed higher calcium and lower cholesterol contents than carotid plaques [
24]. The relevance of vascular cell heterogeneity has been elucidated in a histological and transcriptomic study assessed in healthy and atherosclerotic plaques from different vascular beds. Here, femoral plaques resulted the most calcified arteries and the transcriptomic profile of SMCs was consistent with a prominent mineralization activity, whereas SMCs from other arterial segments like abdominal and thoracic aortas were less prone to calcification [
27]. As introduced above, the developmental origin and epigenetic are some of the proposed mechanisms responsible for vascular cell heterogeneity. Epigenetic refers to the modulation of gene expression through histone modifications, DNA methylation, and noncoding RNAs (ncRNAs), without involving DNA structure [
28]. ncRNAs include microRNA (miRNA), a class of small non-coding endogenous RNAs (10-22 nucleotides) that regulate the expression of different genes involved in many biological processes. Their discovery is quite recent, the first miRNA was discovered in 1993 by Victor Ambros, Rosalind Lee e Rhonda Feinbaum while studying the gene lin-4, that controls the timing of
C. elegans larval development [
29]. Considering the key regulatory role of miRNAs in vascular cell phenotype and function, it could be reasonable hypothesizing a miRNA-based molecular signature of arterials beds useful for deepening atherosclerosis and CVD pathogenesis differentiation and for developing more targeted and effective preventive/therapeutic interventions. In the management of CVDs, the identification of frail patients that are at higher mortality risk should be included. Frailty refers to an age-related clinical syndrome with multiple organ affections including CVDs, and higher vulnerability to negative health outcomes [
30,
31]. Age is a well-known risk factor, but also genetic, epigenetic and environmental stressors are potent stressors in frail condition. However, frailty has not been fully defined yet and the identification of biomarkers able to screen frail patients would be promising in CVD management.
4. microRNAs (miRNAs)
4.1. miRNAs as endogenous regulators of vascular biology and vascular heterogeneity
miRNAs regulate specific cellular processes through inhibition of the expression of their gene targets by inducing messenger RNA (mRNA) degradation or inhibiting mRNA translation, resulting in inhibition of protein synthesis [
32].
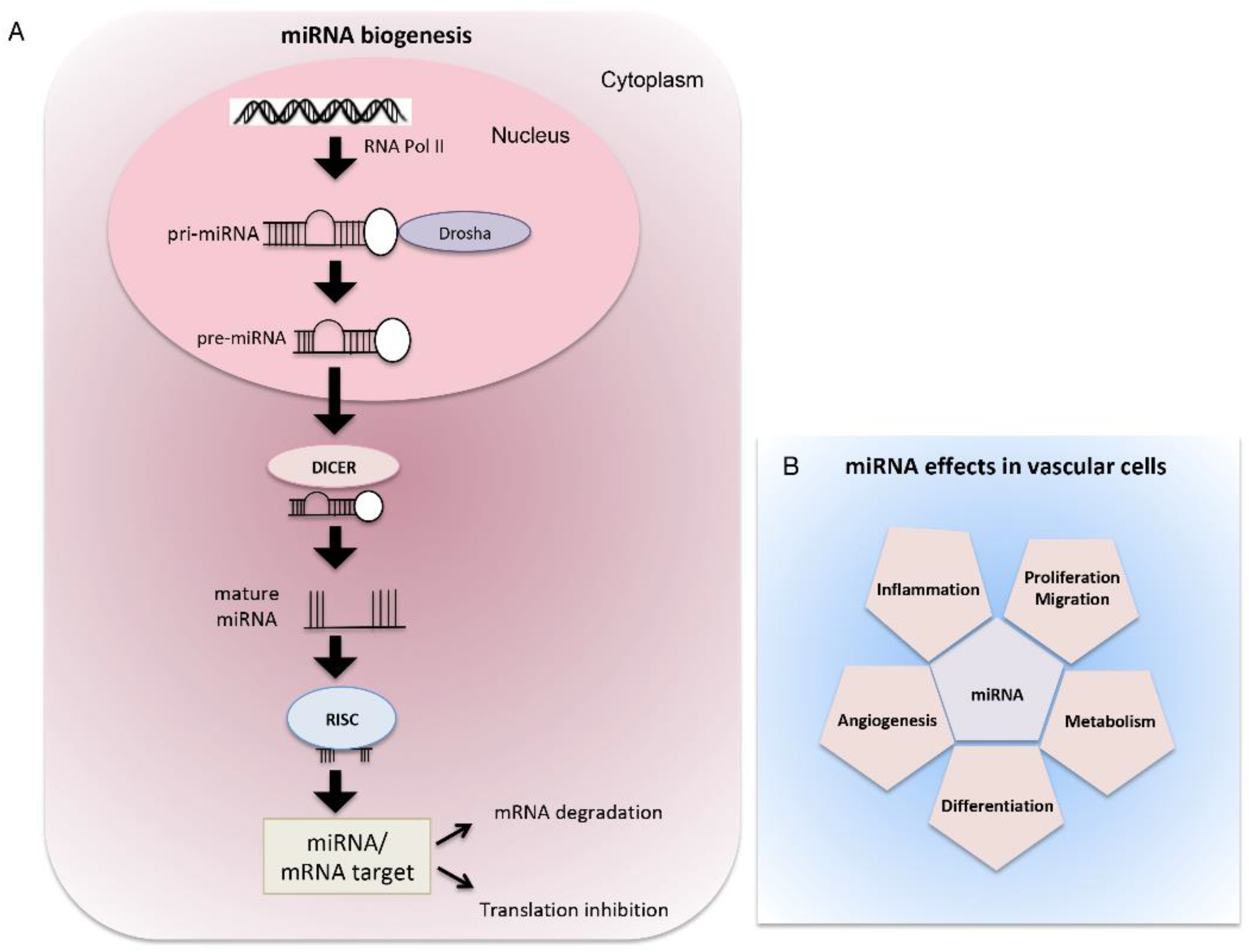
The canonical biogenesis of miRNAs begins with the transcription of their coding sequences by the RNA Polymerase II (POL II), which leads to the formation of their precursors called primary miRNA transcripts (pri-miRNAs) that are folded into characteristic hairpin structures. In the nucleus, pri-miRNAs are cleaved by the ribonuclease DROSHA to form shorter temporary miRNAs (pre-miRNAs) that are then transported from the nucleus to the cytoplasm through the action of a protein called Exportin-5 (XPO-5). In the cytoplasm, pre-miRNAs are recognized by another ribonuclease, DICER, and further processed into short double-stranded immature miRNAs. Subsequently, one strand is degraded by the component 3 promoter of RNA-induced silencing complex (C3PO) while the guide strand is loaded into the RNA-induced silencing complex (RISC). Here, miRNAs can bind to their mRNA targets and inhibit their expression through the endonuclease cleavage activity of the protein Argonaute 2 (AGO2) [
33] (
Figure 1).
miRNAs contribute to the regulation of both coding and non-coding RNA transcriptome in the nucleus, by blocking or promoting pri-miRNA maturation and regulating long non-coding RNAs levels. Moreover, in the nucleus, miRNAs induce remodeling of chromatin structure, regulate alternative splicing, and in turn, regulate itself. miRNAs can also mediate transcriptional gene activation or transcriptional gene silencing. Other nuclear miRNAs co-localize with ribosomal RNAs (rRNAs) in the nucleolus, where they are stored, or in the cytoplasm where they influence the abundance of rRNAs and/or regulate ribosomes interaction with accessory proteins. All these miRNA activities have recently been reviewed [
34].
A large number of miRNAs are involved in different biological and pathological processes such as cell proliferation, differentiation and migration [
35,
36], inflammation [
37,
38], nervous system diseases [
39,
40,
41], cancer [
42] and diabetes [
43].
In the cardiovascular system, miRNAs regulate the development process as well as the pathogenesis of many diseases (
Figure 1).
Fish et al. observed that miR-126 is highly expressed in endothelial cells and it is important for angiogenesis and for maintenance of vascular integrity
in vivo as it stimulates vascular endothelial growth factor (VEGF) signaling by directly inhibiting SPRED1 and PIK3R2, which are negative regulators of VEGF signalling [
44]. Moreover, miR-23 and miR-27 are positive regulators of angiogenesis in vivo, inhibiting Sprouty2 and semaphoring 6A (SEMA6A), which negatively regulate MAPK and VEGF2 signalling [
45,
46]. In endothelial cells, miR-210 overexpression led to the up-regulation of the Notch1 pathway, which is responsible for the enhanced blood vessel formation of the endothelium [
47]. In vascular SMCs one of the most expressed miRNA is miR-15b/16, whose overexpression promotes the contractility of VSMCs while mitigating their proliferation through the inhibition of the oncoprotein Yes-associated protein (YAP) [
48]. Also, it has been found that the levels of miR-146 and miR-31 expression are higher in proliferative VSMCs and silencing these miRNAs resulted in the inhibition of VMSCs proliferative and migratory abilities, thus demonstrating that they have a pro-proliferative and anti-apoptotic function [
49,
50].
Alteration of miRNAs expression and function can lead to vascular disease. Indeed, miRNAs are endogenous regulators of several pathological processes, including atherosclerosis and arterial calcification. In recent years, the contribution of miRNAs in vascular calcification has been studied. Zhou et al. quantified serum expression levels of miR-30-5p family in patients with atherosclerosis compared to a normal group, observing that the expression levels of miR-30-5p were higher in the atherosclerosis group and supporting the miR-30a-5p association with cardiovascular disease [
51]. Moreover, a study on atherosclerotic plaques showed that miR-30a-5p and miR-30d were downregulated in calcified carotid plaques, identifying them as potential contributors to vascular calcification [
52]. Indeed, in an
in vitro study in human umbilical vein endothelial cells (HUVEC), miR-30a-5p and miR-30d resulted downregulated following osteogenic differentiation, and their over-expression during the osteogenic induction assay revealed a decrease of the mineralization activity, implicating that these miRNAs potentially exert a regulatory role in atherosclerotic calcification [
53]. In addition, Han et al. demonstrated that miR-223-3p expression increases in medial and atherosclerotic calcified aortas while suppressing vascular calcification by targeting IL-6/STAT3 signaling [
54]. The involvement of miRNAs in vascular disease dynamics is very complex and versatile, deepening their role and mechanisms would pave the way to miRNAs use as markers for diagnostic purposes and as a potential foundation for therapeutic strategies.
Table 1 summarizes the main findings reported in this section.
4.2. miRNAs and vascular heterogeneity: focus on femoral artery and abdominal aorta
miRNAs are fine tuners of cell biology and phenotype, suggesting their contribution to the vascular heterogeneity. So far, there are few studies addressing the diversity of miRNAs expression in normal tissues. However, it has been observed the presence of many organ-specific miRNAs in mice [
53], but the possible differences existing among cells belonging to the same tissue have not been elucidated yet. A recent comparative study identified three miRNAs (miR-20b, miR-99b, let-7b) that were differently expressed among ECs cultured from aorta, coronary artery, umbilical vein, pulmonary, dermal and brain microvasculature [
56]. The literature is poor also regarding comparative studies based on miRNA expression profiles in different arterial districts under pathological conditions. A systematic review found both a common and a site-specific profile of circulating miRNAs in individuals with and without atherosclerosis of large or medium size [
57]. Some miRNAs displayed opposite expression trends, like miR-126 that was upregulated in renal artery stenosis and downregulated in carotid and lower limbs plaques [
57].
The recent knowledge acquired on miRNA genome regulation even more endorses hypothesis on their central contribution to arteries structure differences and atherosclerosis development. In this regard, Collura et al. [
58] showed that miR profiling in non-pathological femoral, abdominal and carotid arteries presents high similarity between abdominal aorta and carotid arteries, while major difference is observed between femoral artery and the others two arteries. Authors identified three miRNAs significantly altered when comparing normal arteries i.e., miR-27a-5p, -139-5p, and -155-5p. In particular, miR-155-5p and miR-27a-5p turned out to be more highly expressed in normal aorta/carotid than femoral arteries, while miR-139-5p showed the opposite direction suggesting a different epigenetic pattern in the normal arteries. These three miRNAs also have relevance under pathological conditions. In fact, the same authors showed that miR-155-5p and miR-27a-5p expression increases in femoral atheroma when compared with the normal counterpart, thus becoming more like normal abdominal/carotid aorta arteries. Furthermore, some targets of the identified miRNAs such as CD44, E-cadherin and vimentin, were shown to be differently expressed under physiological and disease conditions between femoral and abdominal/carotid arteries, also suggesting that in these arteries there is a different activation of the main molecular drivers of pathological condition. This current topic is almost neglected and deserves additional research that should also be focused on blood/vesicles circulating miRNAs, in order to investigate their effects on atheroma development in femoral and abdominal aorta arteries. Interestingly, in obese animal model extracellular vesicles with miR-221-3p, derived from perivascular adipose tissue, mediates vascular remodelling and dysfunction in femoral artery [
59]. In this regard, further studies should be crucial to identify both common and different epigenetic molecular patterns to pave the way for future artery-specific therapeutic applications [
60].
5. Conclusions
The complex nature of the arterial network is reflected on physiological functionality of the vascular tissues as well as on the pathogenic mechanisms affecting the vascular beds according to the anatomical localization. The investigation of this heterogeneity, including the different responsiveness to the main pathology risk factors and the several pathways activated during disease occurrence, would be the key for more effective and targeted therapies for the management of CVDs. Among the pathogenic mechanisms, the vascular stem cell differentiation performs a pivotal role for CVD progression, contributing to the main morphological features of CVDs from different arterial segments. The identification of a miRNA signature that can be associated with the differentiation process and specific for each vascular district would open novel research directions for the future of artery-specific approaches. In this future perspective, blood circulating miRNAs can be employed as diagnostic tool for risk stratification also in the elderly population [
61] in order to identify vascular frail patients more subjected to cardiovascular mortality.
Author Contributions
Conceptualization, C.C. and G.P., writing—original draft preparation, C.C and I.M.; writing—review and editing, C.C., I.M., M.C., M.G., and G.P.; supervision, G.P.
Funding
The authors acknowledge co-funding from Next Generation EU, in the context of the National Recovery and Resilience Plan, Investment PE8 – Project Age-It: “Ageing Well in an Ageing Society”. This resource was co-financed by the Next Generation EU [DM 1557 11.10.2022]. The views and opinions expressed are only those of the authors and do not necessarily reflect those of the European Union or the European Commission. Neither the European Union nor the European Commission can be held responsible for them.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tucker, W.D.; Arora, Y.; Mahajan, K. Anatomy, Blood Vessels. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2022.
- Fleischer, J.R.; Jodszuweit, C.A.; Ghadimi, M.; De Oliveira, T.; Conradi, L.-C. Vascular Heterogeneity With a Special Focus on the Hepatic Microenvironment. Frontiers in Physiology 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Endothelial Cell Heterogeneity and Atherosclerosis. Curr Atheroscler Rep 2006, 8, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Phenotypic Heterogeneity of the Endothelium. Circulation Research 2007, 100, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Aird, W.C. Endothelial Cell Heterogeneity. Cold Spring Harb Perspect Med 2012, 2, a006429. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Mäkinen, T. Vascular Heterogeneity and Specialization in Development and Disease. Nat Rev Mol Cell Biol 2017, 18, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.-T.; Chang, H.Y.; Haraldsen, G.; Jahnsen, F.L.; Troyanskaya, O.G.; Chang, D.S.; Wang, Z.; Rockson, S.G.; van de Rijn, M.; Botstein, D.; et al. Endothelial Cell Diversity Revealed by Global Expression Profiling. Proc Natl Acad Sci U S A 2003, 100, 10623–10628. [Google Scholar] [CrossRef] [PubMed]
- Sengoelge, G.; Luo, W.; Fine, D.; Perschl, A.M.; Fierlbeck, W.; Haririan, A.; Sorensson, J.; Rehman, T.-U.; Hauser, P.; Trevick, J.S.; et al. A SAGE-Based Comparison between Glomerular and Aortic Endothelial Cells. American Journal of Physiology-Renal Physiology 2005, 288, F1290–F1300. [Google Scholar] [CrossRef]
- Zhang, J.; Burridge, K.A.; Friedman, M.H. In Vivo Differences between Endothelial Transcriptional Profiles of Coronary and Iliac Arteries Revealed by Microarray Analysis. Am J Physiol Heart Circ Physiol 2008, 295, H1556–H1561. [Google Scholar] [CrossRef] [PubMed]
- Simmons, G.H.; Padilla, J.; Laughlin, M.H. Heterogeneity of Endothelial Cell Phenotype within and amongst Conduit Vessels of the Swine Vasculature. Experimental Physiology 2012, 97, 1074–1082. [Google Scholar] [CrossRef]
- Thorin, E.; Shatos, M.A.; Shreeve, S.M.; Walters, C.L.; Bevan, J.A. Human Vascular Endothelium Heterogeneity. Stroke 1997, 28, 375–381. [Google Scholar] [CrossRef]
- Rensen, S.S.M.; Doevendans, P.A.F.M.; van Eys, G.J.J.M. Regulation and Characteristics of Vascular Smooth Muscle Cell Phenotypic Diversity. Neth Heart J 2007, 15, 100–108. [Google Scholar] [CrossRef]
- Wall, V.Z.; Bornfeldt, K.E. Arterial Smooth Muscle. Arteriosclerosis, Thrombosis, and Vascular Biology 2014, 34, 2175–2179. [Google Scholar] [CrossRef]
- Majesky, M.W. Developmental Basis of Vascular Smooth Muscle Diversity. Arteriosclerosis, Thrombosis, and Vascular Biology 2007, 27, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.-S.; Yeh, H.-I.; Haw, M.; Dupont, E.; Kaba, R.; Plenz, G.; Robenek, H.; Severs, N.J. Differential Expression of Connexin43 and Desmin Defines Two Subpopulations of Medial Smooth Muscle Cells in the Human Internal Mammary Artery. Arteriosclerosis, Thrombosis, and Vascular Biology 1999, 19, 1669–1680. [Google Scholar] [CrossRef]
- Li, S.; Fan, Y.-S.; Chow, L.H.; Van Den Diepstraten, C.; van der Veer, E.; Sims, S.M.; Pickering, J.G. Innate Diversity of Adult Human Arterial Smooth Muscle Cells. Circulation Research 2001, 89, 517–525. [Google Scholar] [CrossRef]
- Ciavarella, C.; Valente, S.; Pasquinelli, G. The Characteristics and Survival Potential Under Sub-Lethal Stress of Mesenchymal Stromal/Stem Cells Isolated from the Human Vascular Wall. Stem Cells 2022, 40, 1071–1077. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, A.; Yuan, F.; Yan, Z.; Liu, B.; Chu, J.S.; Helms, J.A.; Li, S. Differentiation of Multipotent Vascular Stem Cells Contributes to Vascular Diseases. Nat Commun 2012, 3, 875. [Google Scholar] [CrossRef] [PubMed]
- Pasquinelli, G.; Pacilli, A.; Alviano, F.; Foroni, L.; Ricci, F.; Valente, S.; Orrico, C.; Lanzoni, G.; Buzzi, M.; Luigi Tazzari, P.; et al. Multidistrict Human Mesenchymal Vascular Cells: Pluripotency and Stemness Characteristics. Cytotherapy 2010, 12, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Pasanisi, E.; Ciavarella, C.; Valente, S.; Ricci, F.; Pasquinelli, G. Differentiation and Plasticity of Human Vascular Wall Mesenchymal Stem Cells, Dermal Fibroblasts and Myofibroblasts: A Critical Comparison Including Ultrastructural Evaluation of Osteogenic Potential. Ultrastructural Pathology 2019, 43, 261–272. [Google Scholar] [CrossRef]
- Sabrina, V.; Gianandrea, P. Phenotypic and Functional Mapping of Mesenchymal Stem Cells Harvested from Different Portions of the Human Arterial Tree. In Mesenchymal Stem Cells - Isolation, Characterization and Applications; IntechOpen, 2017 ISBN 978-953-51-3616-3.
- Lahoz, C.; Mostaza, J.M. Atherosclerosis As a Systemic Disease. Rev Esp Cardiol 2007, 60, 184–195. [Google Scholar] [CrossRef]
- Stary Herbert C.; Chandler A. Bleakley; Dinsmore Robert E.; Fuster Valentin; Glagov Seymour; Insull William; Rosenfeld Michael E.; Schwartz Colin J.; Wagner William D.; Wissler Robert W. A Definition of Advanced Types of Atherosclerotic Lesions and a Histological Classification of Atherosclerosis. Circulation 1995, 92, 1355–1374. [CrossRef]
- Herisson, F.; Heymann, M.-F.; Chétiveaux, M.; Charrier, C.; Battaglia, S.; Pilet, P.; Rouillon, T.; Krempf, M.; Lemarchand, P.; Heymann, D.; et al. Carotid and Femoral Atherosclerotic Plaques Show Different Morphology. Atherosclerosis 2011, 216, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Artery-Related Differences in Atherosclerosis Expression | Stroke. Available online: https://www.ahajournals.org/doi/10.1161/STROKEAHA.107.486480 (accessed on 5 January 2023).
- Sigala, F.; Oikonomou, E.; Antonopoulos, A.S.; Galyfos, G.; Tousoulis, D. Coronary versus Carotid Artery Plaques. Similarities and Differences Regarding Biomarkers Morphology and Prognosis. Current Opinion in Pharmacology 2018, 39, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Espitia, O.; Chatelais, M.; Steenman, M.; Charrier, C.; Maurel, B.; Georges, S.; Houlgatte, R.; Verrecchia, F.; Ory, B.; Lamoureux, F.; et al. Implication of Molecular Vascular Smooth Muscle Cell Heterogeneity among Arterial Beds in Arterial Calcification. PLOS ONE 2018, 13, e0191976. [Google Scholar] [CrossRef] [PubMed]
- Aavik, E.; Babu, M.; Ylä-Herttuala, S. DNA Methylation Processes in Atherosclerotic Plaque. Atherosclerosis 2019, 281, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. Elegans Heterochronic Gene Lin-4 Encodes Small RNAs with Antisense Complementarity to Lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Stewart, R.; White, H. Importance of Frailty in Patients with Cardiovascular Disease. European Heart Journal 2014, 35, 1726–1731. [Google Scholar] [CrossRef] [PubMed]
- Dato, S.; Crocco, P.; Iannone, F.; Passarino, G.; Rose, G. Biomarkers of Frailty: miRNAs as Common Signatures of Impairment in Cognitive and Physical Domains. Biology (Basel) 2022, 11, 1151. [Google Scholar] [CrossRef]
- Small, E.M.; Olson, E.N. Pervasive Roles of microRNAs in Cardiovascular Biology. Nature 2011, 469, 336–342. [Google Scholar] [CrossRef]
- Stavast, C.J.; Erkeland, S.J. The Non-Canonical Aspects of MicroRNAs: Many Roads to Gene Regulation. Cells 2019, 8, 1465. [Google Scholar] [CrossRef]
- Annese, T.; Tamma, R.; De Giorgis, M.; Ribatti, D. microRNAs Biogenesis, Functions and Role in Tumor Angiogenesis. Frontiers in Oncology 2020, 10. [Google Scholar] [CrossRef]
- Wang, D.; Atanasov, A.G. The microRNAs Regulating Vascular Smooth Muscle Cell Proliferation: A Minireview. Int J Mol Sci 2019, 20, 324. [Google Scholar] [CrossRef] [PubMed]
- Regulatory Role of microRNAs in the Proliferation and Differentiation of Adipose-Derived Stem Cells. Histology and Histopathology 2016, 1–10. [CrossRef]
- miR-21 and miR-146a: The microRNAs of Inflammaging and Age-Related Diseases - ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S1568163721001215?via%3Dihub (accessed on 6 September 2023).
- Mahesh, G.; Biswas, R. MicroRNA-155: A Master Regulator of Inflammation. J Interferon Cytokine Res 2019, 39, 321–330. [Google Scholar] [CrossRef]
- microRNAs at the Synapse | Nature Reviews Neuroscience. Available online: https://www.nature.com/articles/nrn2763 (accessed on 6 September 2023).
- Zheng, K.; Li, H.; Huang, H.; Qiu, M. microRNAs and Glial Cell Development. Neuroscientist 2012, 18, 114–118. [Google Scholar] [CrossRef]
- Zingale, V.D.; Gugliandolo, A.; Mazzon, E. MiR-155: An Important Regulator of Neuroinflammation. Int J Mol Sci 2021, 23, 90. [Google Scholar] [CrossRef]
- Lee, Y.S.; Dutta, A. MicroRNAs in Cancer. Annual Review of Pathology: Mechanisms of Disease 2009, 4, 199–227. [Google Scholar] [CrossRef]
- Tang, X.; Tang, G.; Özcan, S. Role of MicroRNAs in Diabetes. Biochim Biophys Acta 2008, 1779, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Fish, J.E.; Santoro, M.M.; Morton, S.U.; Yu, S.; Yeh, R.-F.; Wythe, J.D.; Bruneau, B.G.; Stainier, D.Y.R.; Srivastava, D. miR-126 Regulates Angiogenic Signaling and Vascular Integrity. Dev Cell 2008, 15, 272–284. [Google Scholar] [CrossRef]
- Zhou, Q.; Gallagher, R.; Ufret-Vincenty, R.; Li, X.; Olson, E.N.; Wang, S. Regulation of Angiogenesis and Choroidal Neovascularization by Members of microRNA-23∼27∼24 Clusters. Proc Natl Acad Sci U S A 2011, 108, 8287–8292. [Google Scholar] [CrossRef]
- Urbich, C.; Kaluza, D.; Frömel, T.; Knau, A.; Bennewitz, K.; Boon, R.A.; Bonauer, A.; Doebele, C.; Boeckel, J.-N.; Hergenreider, E.; et al. MicroRNA-27a/b Controls Endothelial Cell Repulsion and Angiogenesis by Targeting Semaphorin 6A. Blood 2012, 119, 1607–1616. [Google Scholar] [CrossRef]
- Lou, Y.-L.; Guo, F.; Liu, F.; Gao, F.-L.; Zhang, P.-Q.; Niu, X.; Guo, S.-C.; Yin, J.-H.; Wang, Y.; Deng, Z.-F. miR-210 Activates Notch Signaling Pathway in Angiogenesis Induced by Cerebral Ischemia. Mol Cell Biochem 2012, 370, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Ahmed, A.S.I.; Kang, X.; Hu, G.; Liu, F.; Zhang, W.; Zhou, J. MicroRNA-15b/16 Attenuates Vascular Neointima Formation by Promoting the Contractile Phenotype of Vascular Smooth Muscle through Targeting YAP. Arterioscler Thromb Vasc Biol 2015, 35, 2145–2152. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, Y.; Chen, X.; Yang, J.; Xu, L.; Zhang, C. MicroRNA-31 Regulated by the Extracellular Regulated Kinase Is Involved in Vascular Smooth Muscle Cell Growth via Large Tumor Suppressor Homolog 2. Journal of Biological Chemistry 2011, 286, 42371–42380. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Xiong, W.; Yuan, J.; Li, J.; Liu, J.; Xu, X. MiRNA-146a Regulates the Maturation and Differentiation of Vascular Smooth Muscle Cells by Targeting NF-κB Expression. Molecular Medicine Reports 2013, 8, 407–412. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, Y.; Zhang, D.; Wu, S.; Liu, T.; Cai, G.; Qin, S. MicroRNA-30-3p Suppresses Inflammatory Factor-Induced Endothelial Cell Injury by Targeting TCF21. Mediators Inflamm 2019, 2019. [Google Scholar] [CrossRef]
- Vasuri, F.; Ciavarella, C.; Fittipaldi, S.; Pini, R.; Vacirca, A.; Gargiulo, M.; Faggioli, G.; Pasquinelli, G. Different Histological Types of Active Intraplaque Calcification Underlie Alternative miRNA-mRNA Axes in Carotid Atherosclerotic Disease. Virchows Arch. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ciavarella, C.; Motta, I.; Vasuri, F.; Fittipaldi, S.; Valente, S.; Pollutri, D.; Ricci, F.; Gargiulo, M.; Pasquinelli, G. Involvement of miR-30a-5p and miR-30d in Endothelial to Mesenchymal Transition and Early Osteogenic Commitment under Inflammatory Stress in HUVEC. Biomolecules 2021, 11, 226. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, J.; Huang, S.; Cheng, N.; Zhang, C.; Li, Y.; Wang, X.; Liu, J.; You, B.; Du, J. MicroRNA-223-3p Inhibits Vascular Calcification and the Osteogenic Switch of Vascular Smooth Muscle Cells. J Biol Chem 2021, 296, 100483. [Google Scholar] [CrossRef]
- Lagos-Quintana, M.; Rauhut, R.; Yalcin, A.; Meyer, J.; Lendeckel, W.; Tuschl, T. Identification of Tissue-Specific MicroRNAs from Mouse. Current Biology 2002, 12, 735–739. [Google Scholar] [CrossRef]
- McCall, M.N.; Kent, O.A.; Yu, J.; Fox-Talbot, K.; Zaiman, A.L.; Halushka, M.K. MicroRNA Profiling of Diverse Endothelial Cell Types. BMC Medical Genomics 2011, 4, 78. [Google Scholar] [CrossRef]
- Pereira-da-Silva, T.; Coutinho Cruz, M.; Carrusca, C.; Cruz Ferreira, R.; Napoleão, P.; Mota Carmo, M. Circulating microRNA Profiles in Different Arterial Territories of Stable Atherosclerotic Disease: A Systematic Review. Am J Cardiovasc Dis 2018, 8, 1–13. [Google Scholar] [PubMed]
- Collura, S.; Ciavarella, C.; Morsiani, C.; Motta, I.; Valente, S.; Gallitto, E.; Abualhin, M.; Pini, R.; Vasuri, F.; Franceschi, C.; et al. MicroRNA Profiles of Human Peripheral Arteries and Abdominal Aorta in Normal Conditions: MicroRNAs-27a-5p, -139-5p and -155-5p Emerge and in Atheroma Too. Mechanisms of Ageing and Development 2021, 198, 111547. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ballantyne, L.L.; Yu, Y.; Funk, C.D. Perivascular Adipose Tissue–Derived Extracellular Vesicle miR-221-3p Mediates Vascular Remodeling. FASEB J 2019, 33, 12704–12722. [Google Scholar] [CrossRef] [PubMed]
- Collura, S.; Morsiani, C.; Vacirca, A.; Fronterrè, S.; Ciavarella, C.; Vasuri, F.; D’Errico, A.; Franceschi, C.; Pasquinelli, G.; Gargiulo, M.; et al. The Carotid Plaque as Paradigmatic Case of Site-Specific Acceleration of Aging Process: The microRNAs and the Inflammaging Contribution. Ageing Research Reviews 2020, 61, 101090. [Google Scholar] [CrossRef]
- Salvioli, S.; Basile, M.S.; Bencivenga, L.; Carrino, S.; Conte, M.; Damanti, S.; De Lorenzo, R.; Fiorenzato, E.; Gialluisi, A.; Ingannato, A.; et al. Biomarkers of aging in frailty and age-associated disorders: State of the art and future perspective. Ageing Research Reviews 2023, 91, 102044. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).