Submitted:
15 January 2024
Posted:
15 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
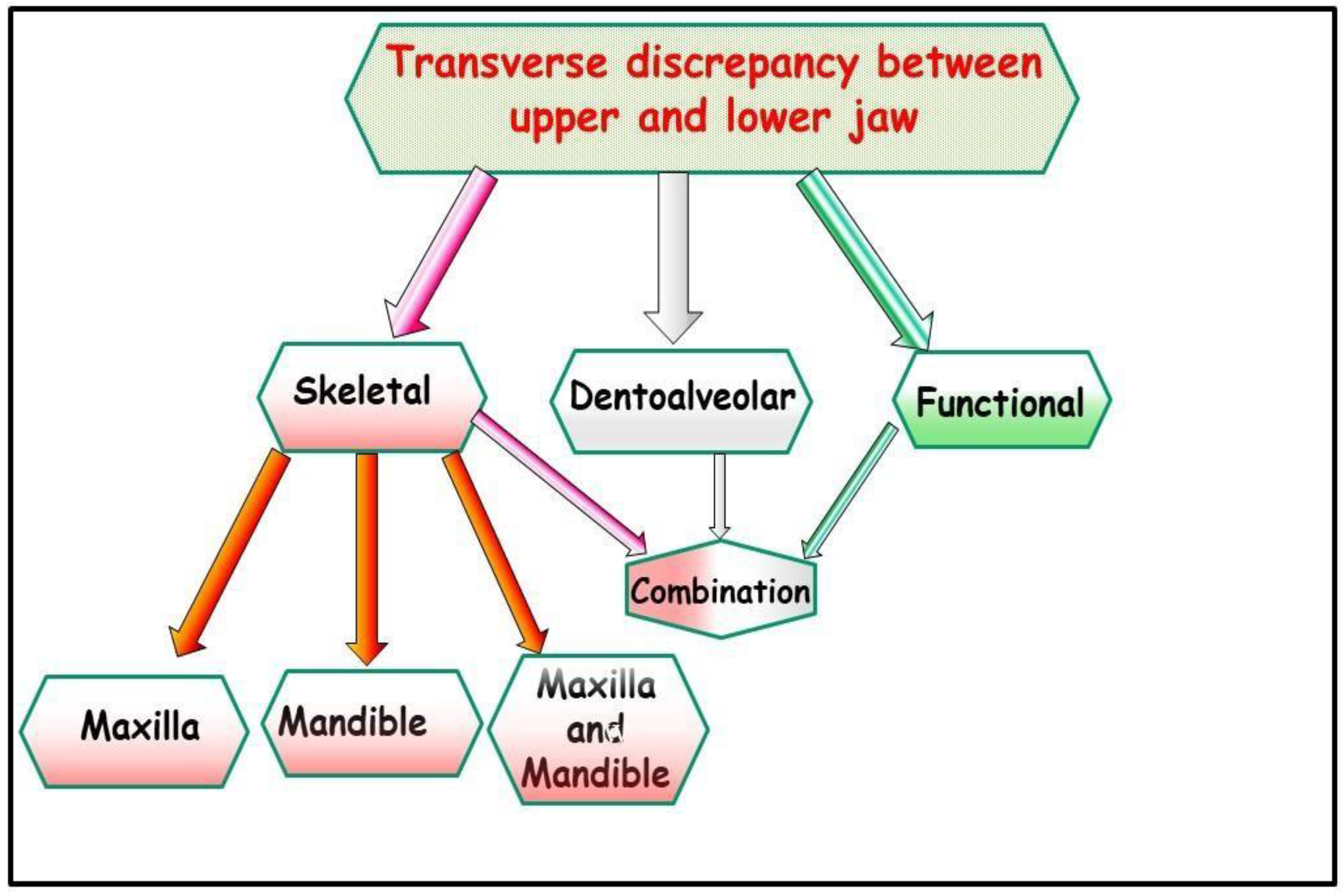
2. Transverse Deficiency and CR/CO Discrepancy
3. Transverse Deficiency and Working/Nonworking Interferences
4. Transverse Deficiency and the Periodontium
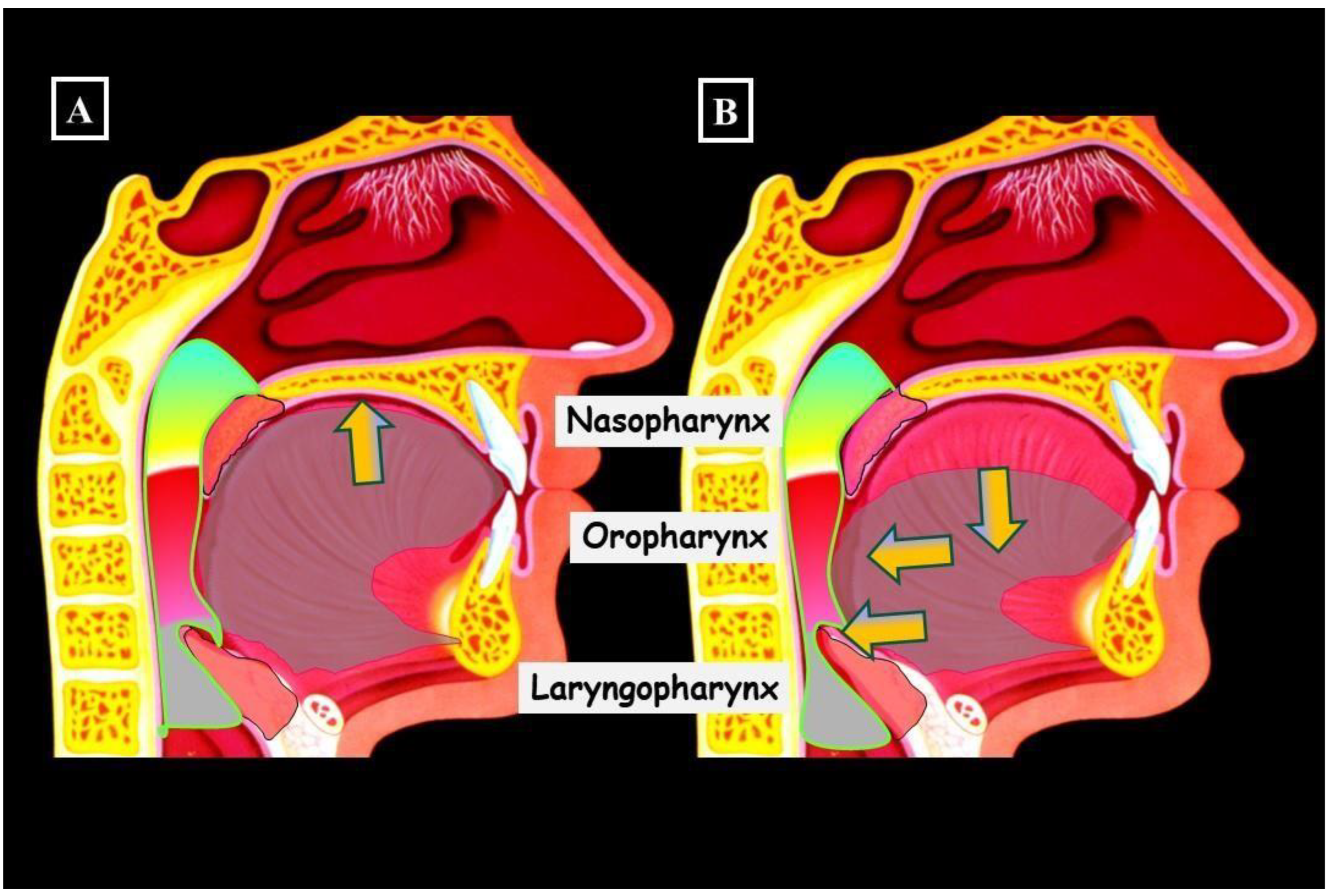
5. Transverse Deficiency and the Airway
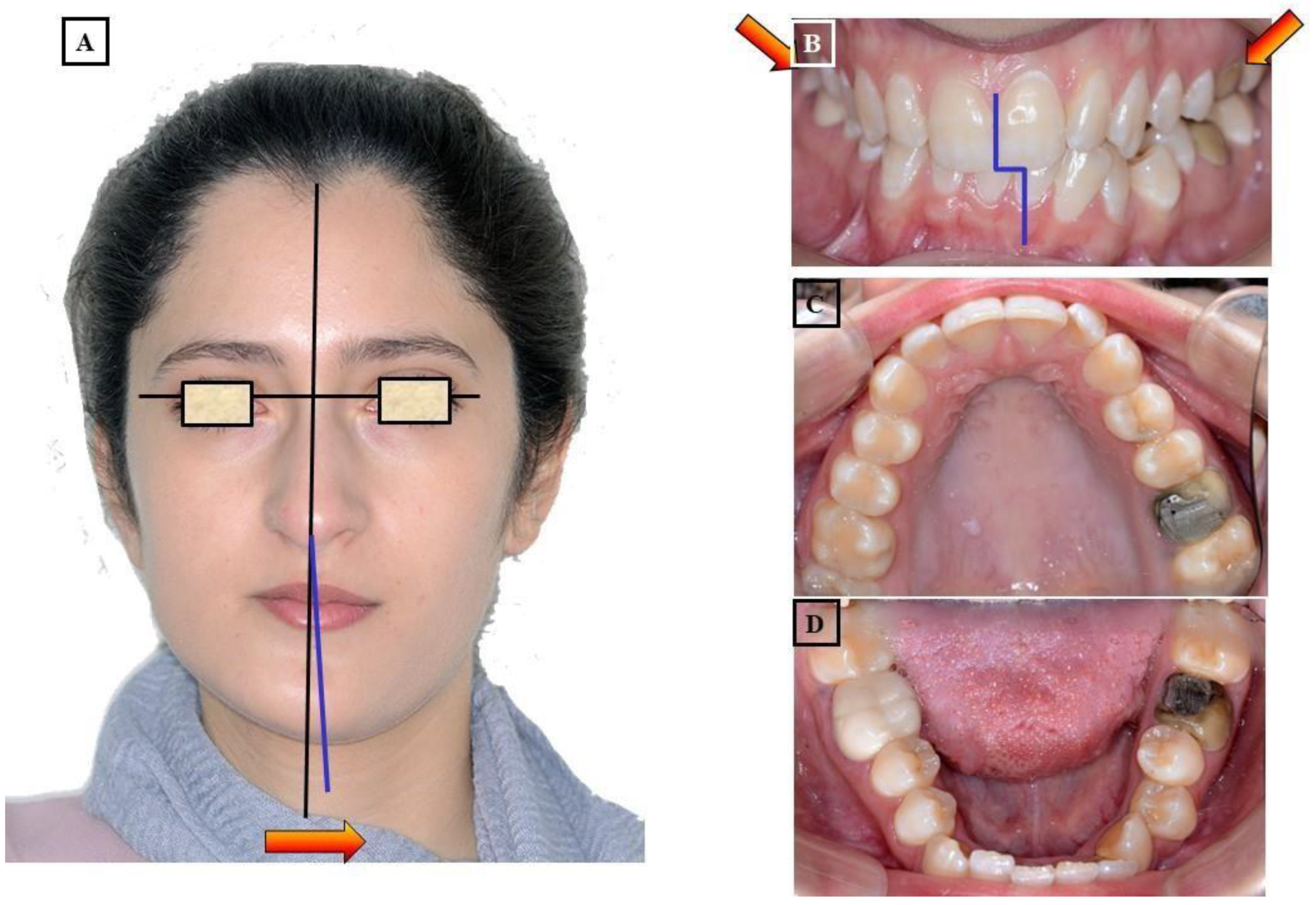
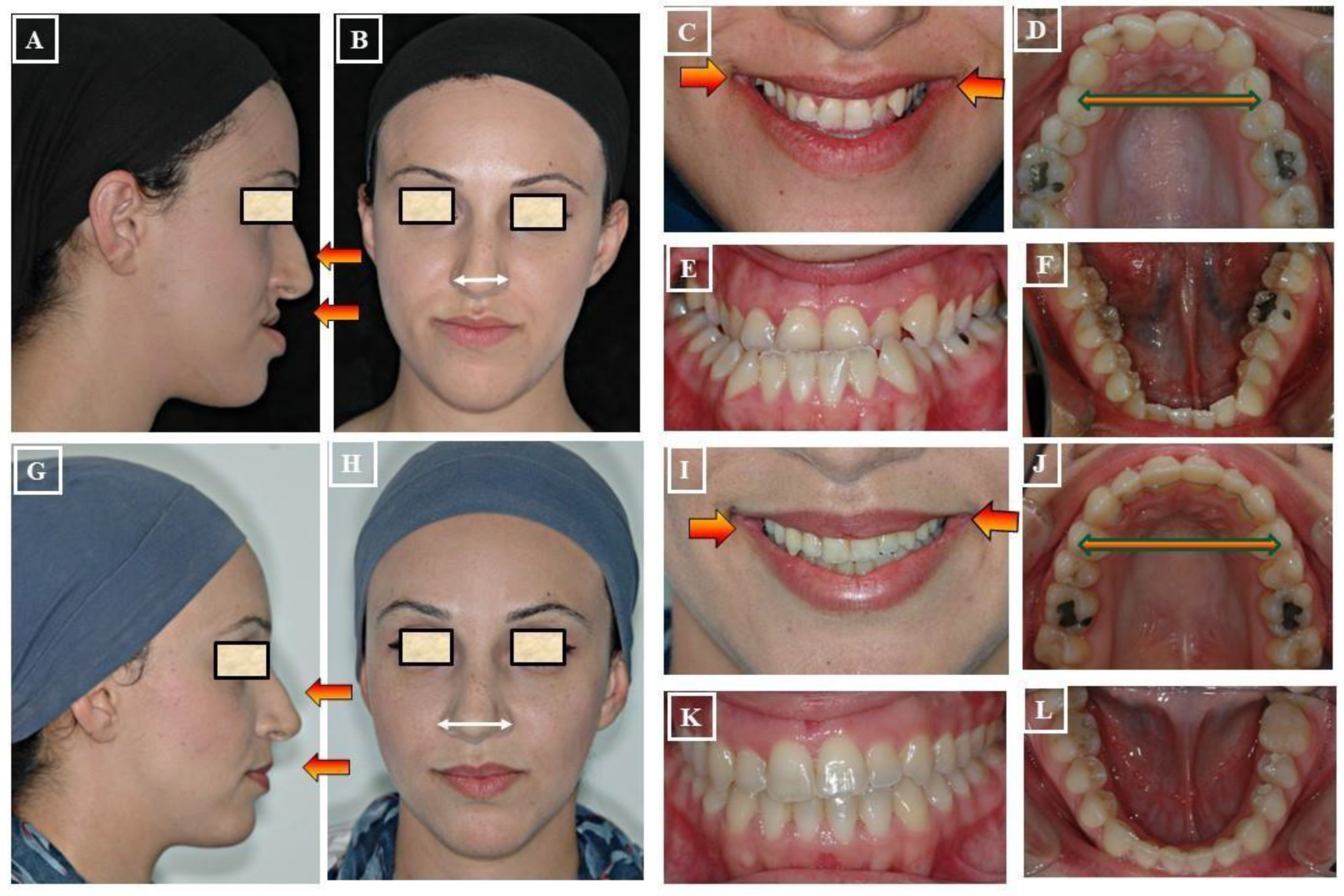
6. Methods of Transverse Diagnosis
7. Diagnosis
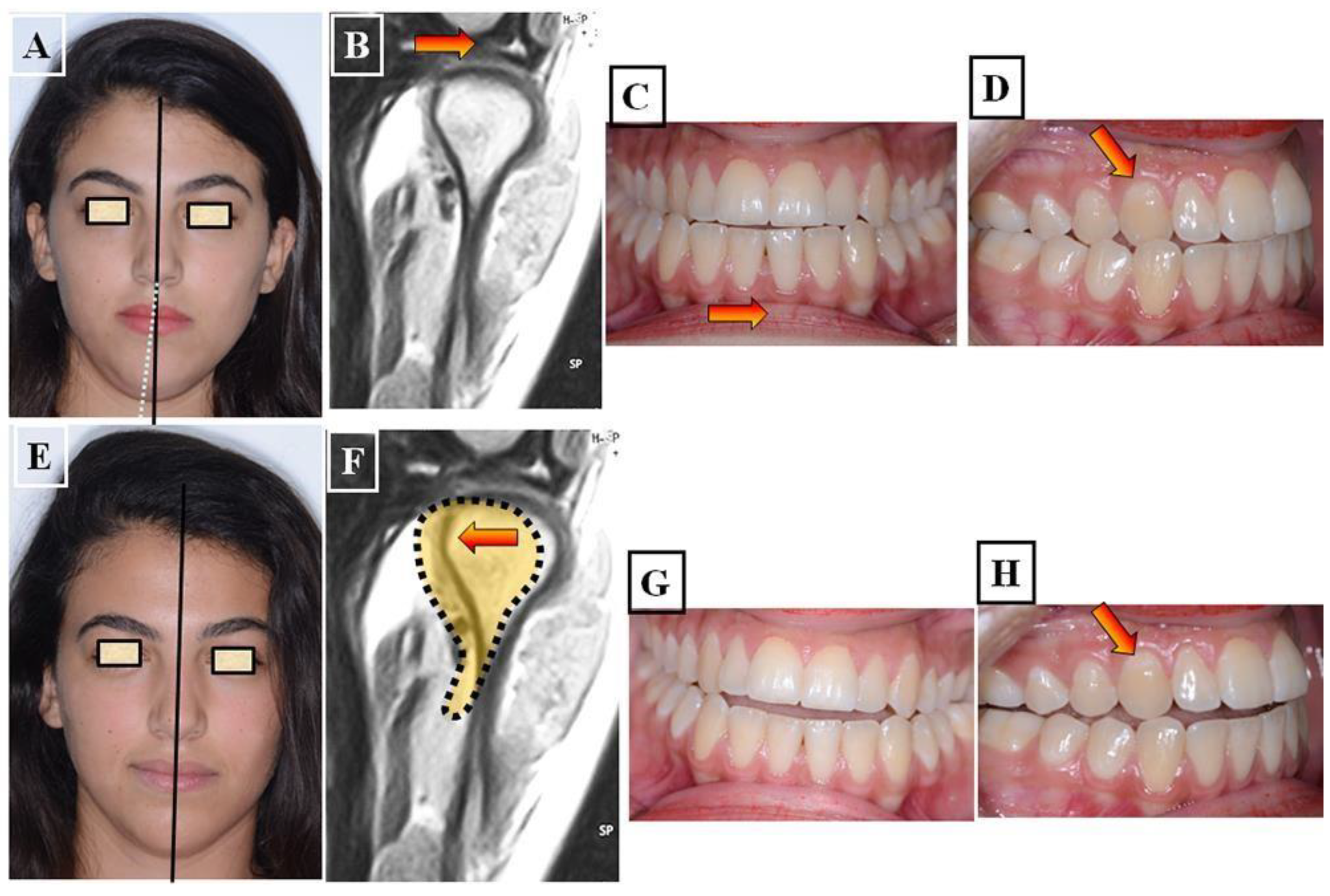
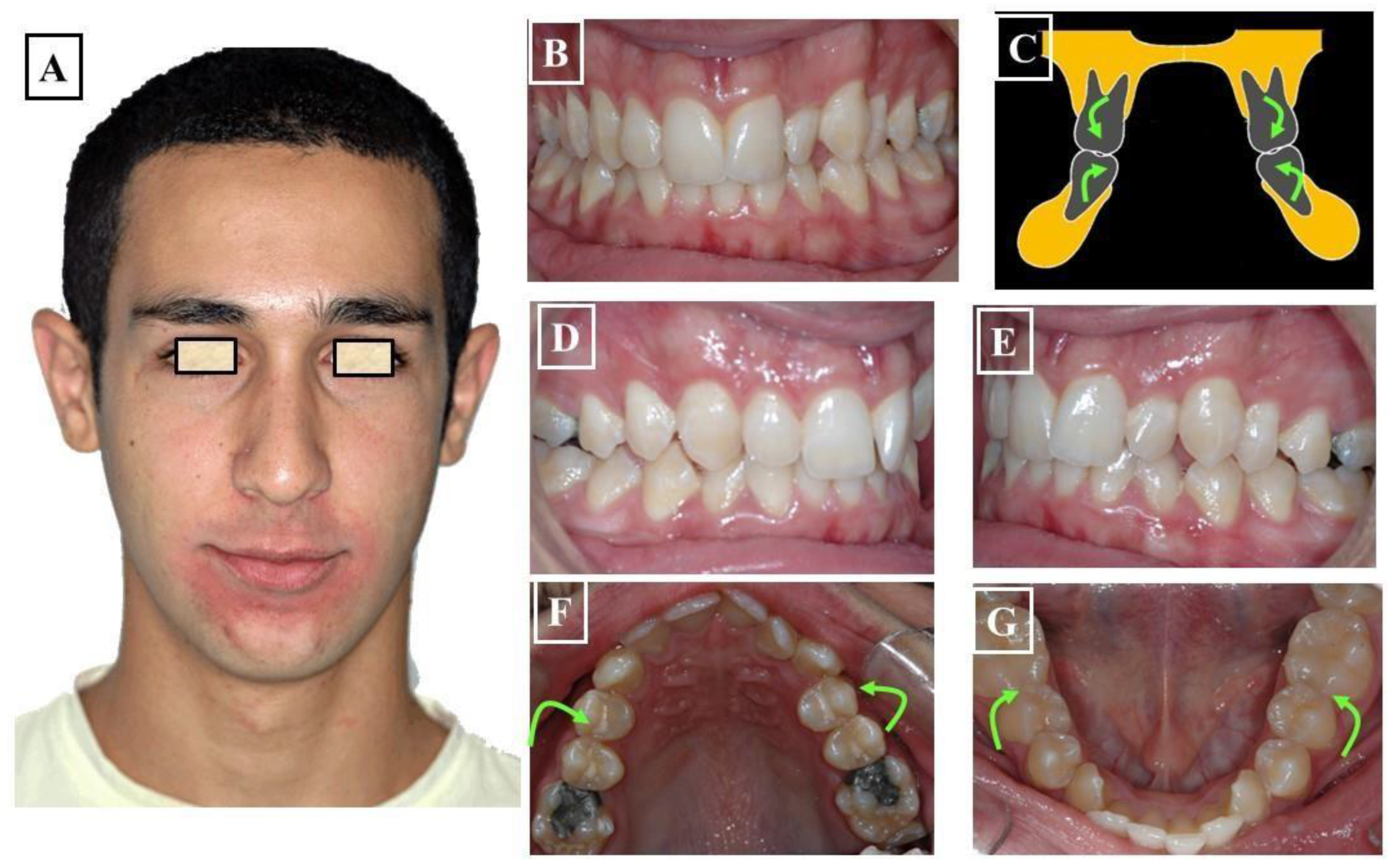
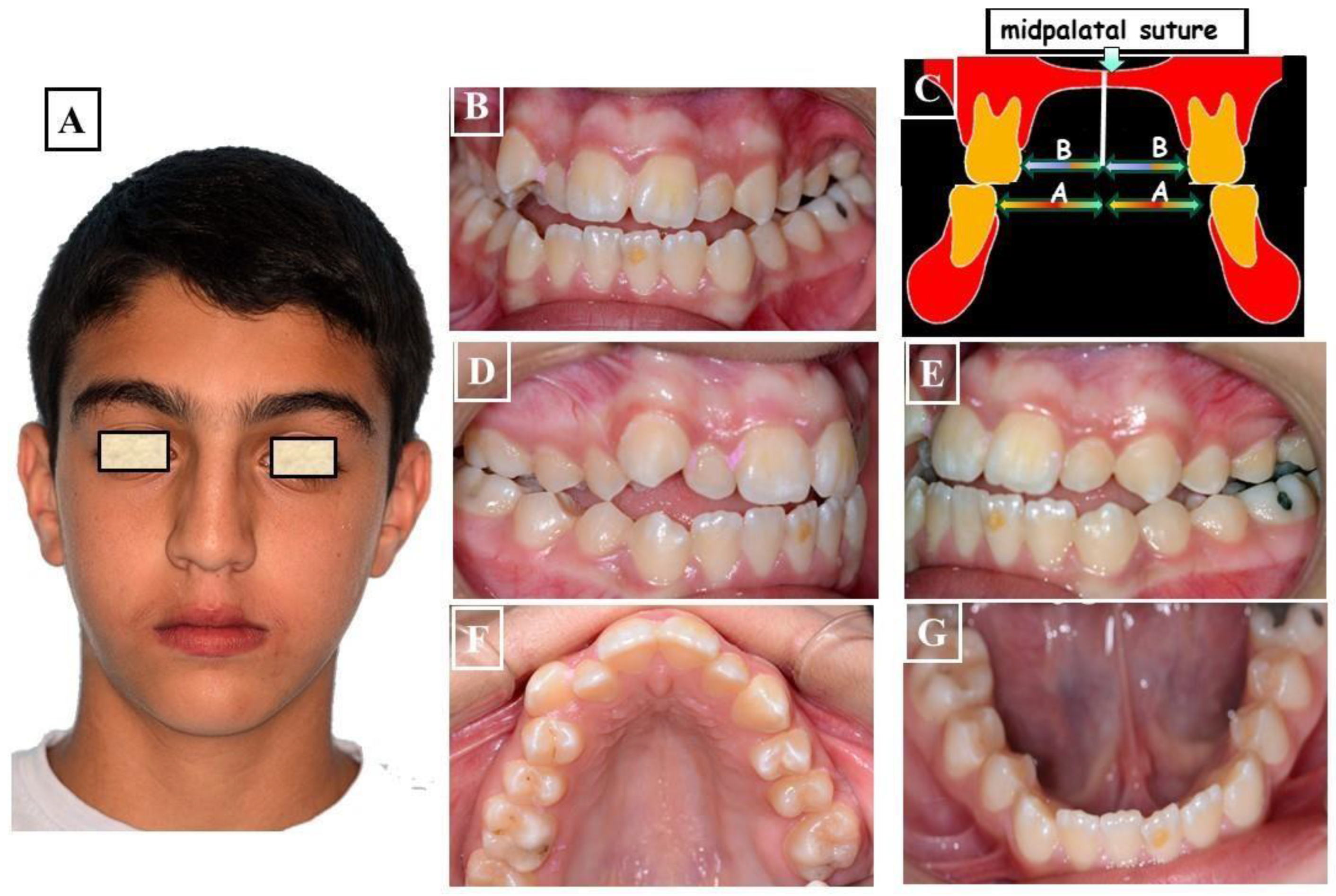
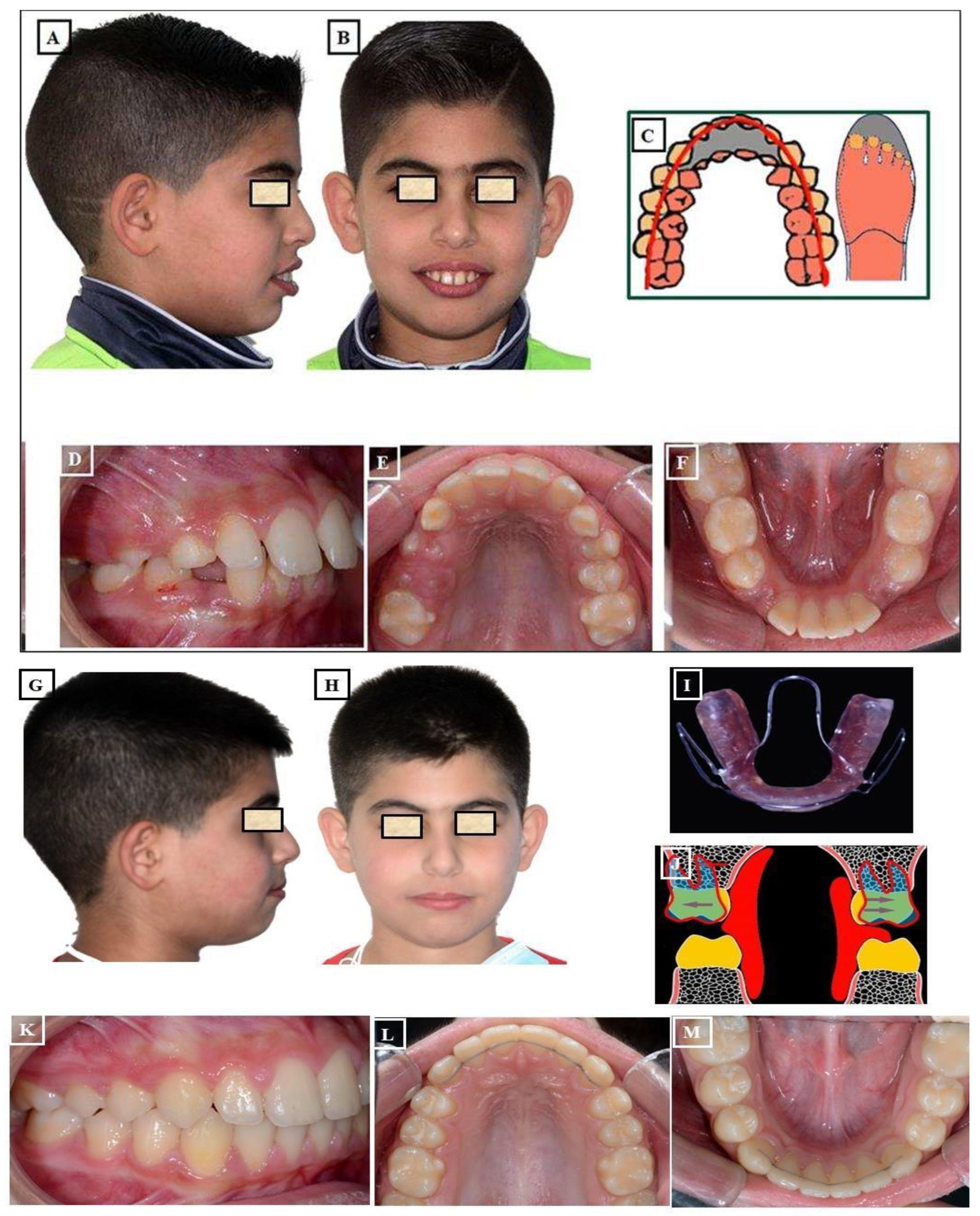
- Functional: In this situation, two different occlusions with two different condyle positions can be observed - a habitual occlusion with a habitual condyle position and a centric occlusion with a centric condyle position. Typically, facial asymmetry is observed in habitual occlusion or habitual condyle position. Manual functional analysis can achieve the centric condyle position with the corresponding change in occlusion. This helps reduce or completely eliminate facial asymmetry. A dentoalveolar malalignment is often involved in most cases (Figure 7A-H).
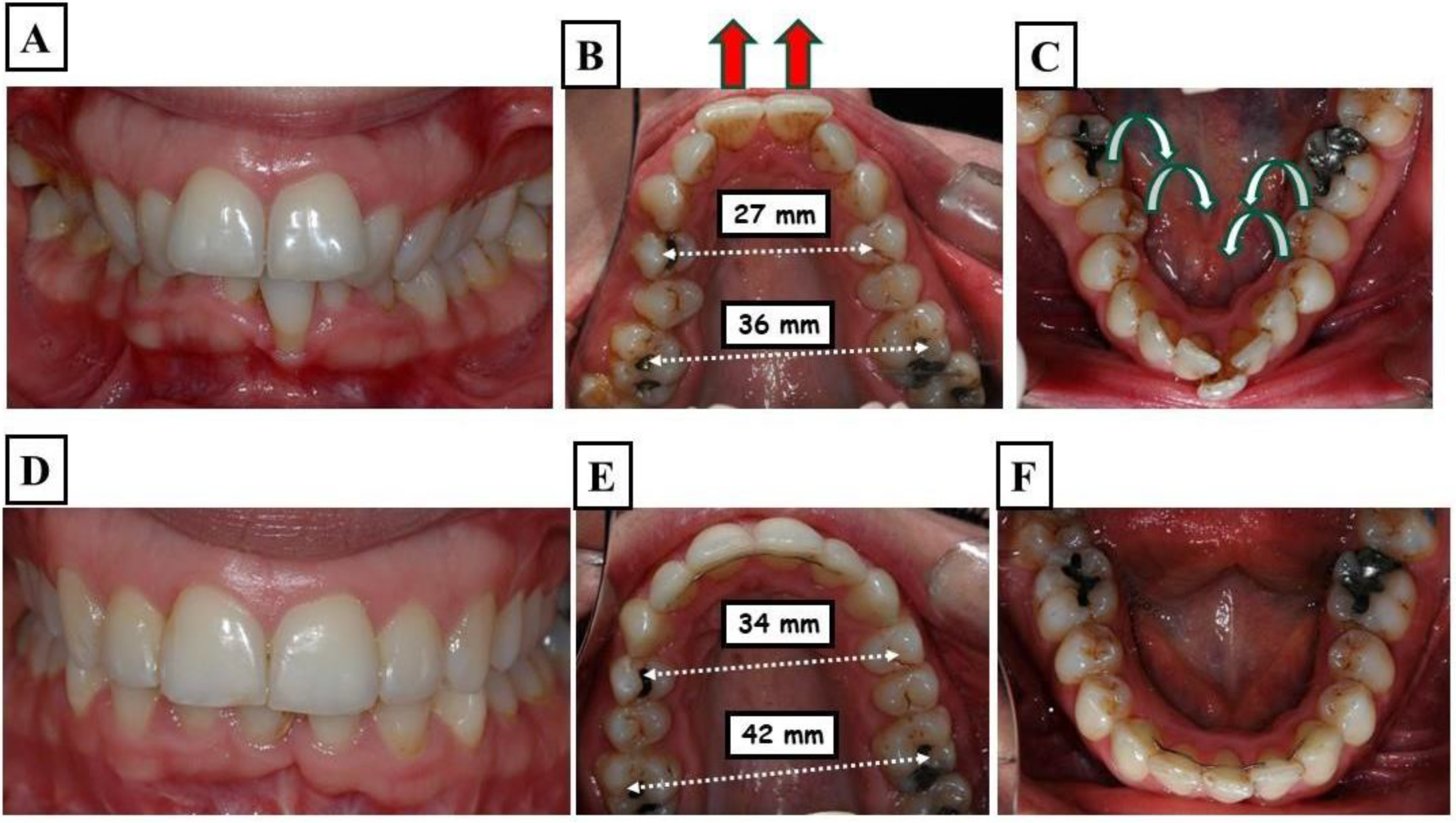
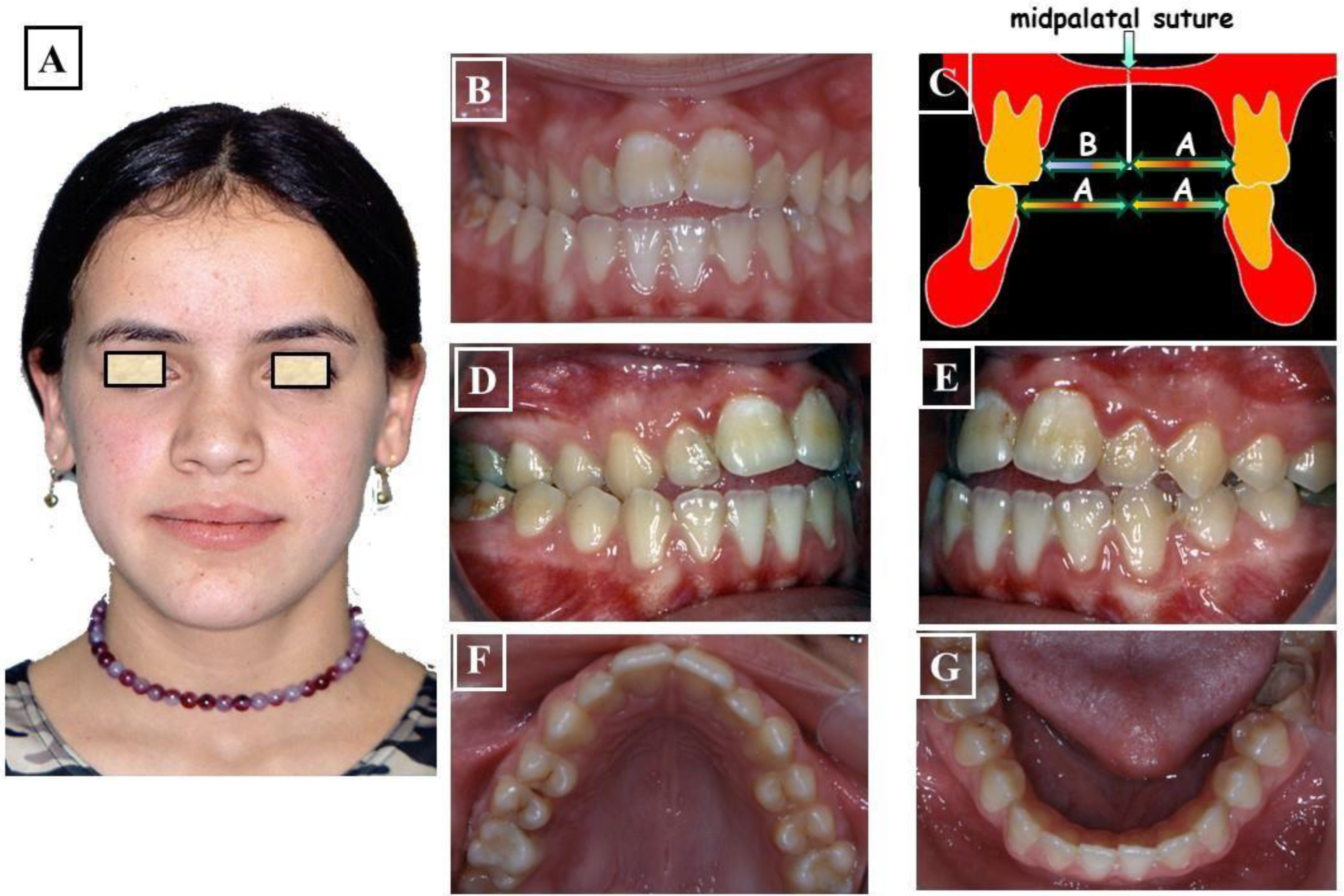
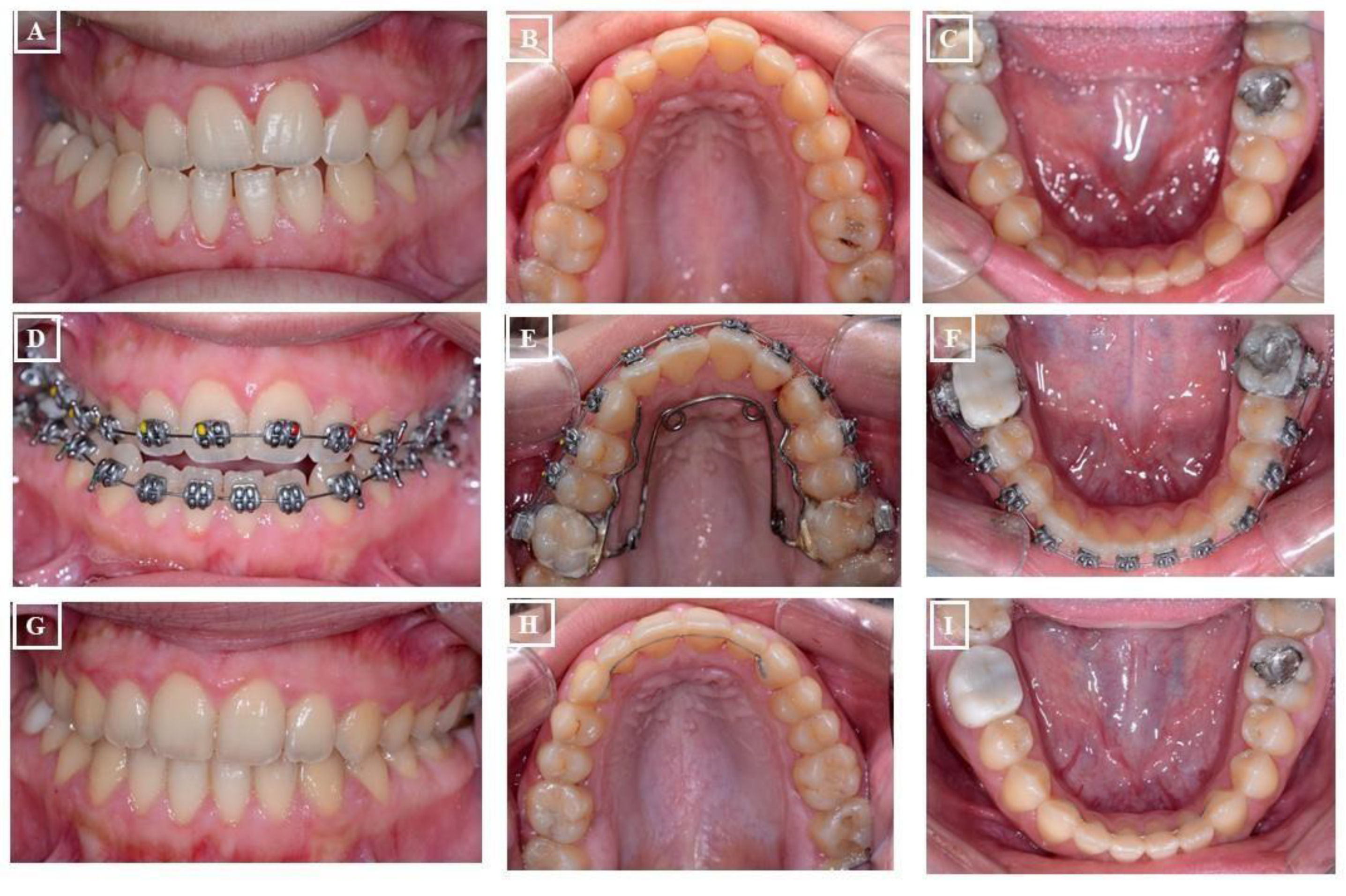
- Dentoalveolar: In this situation, it involves a dental misalignment in the transverse dimension without affecting the centric condyle position. There is a discrepancy in the transverse dimension, and the cause lies in the upper jaw. In these cases, no facial asymmetry is observed (Figure 8A-G).
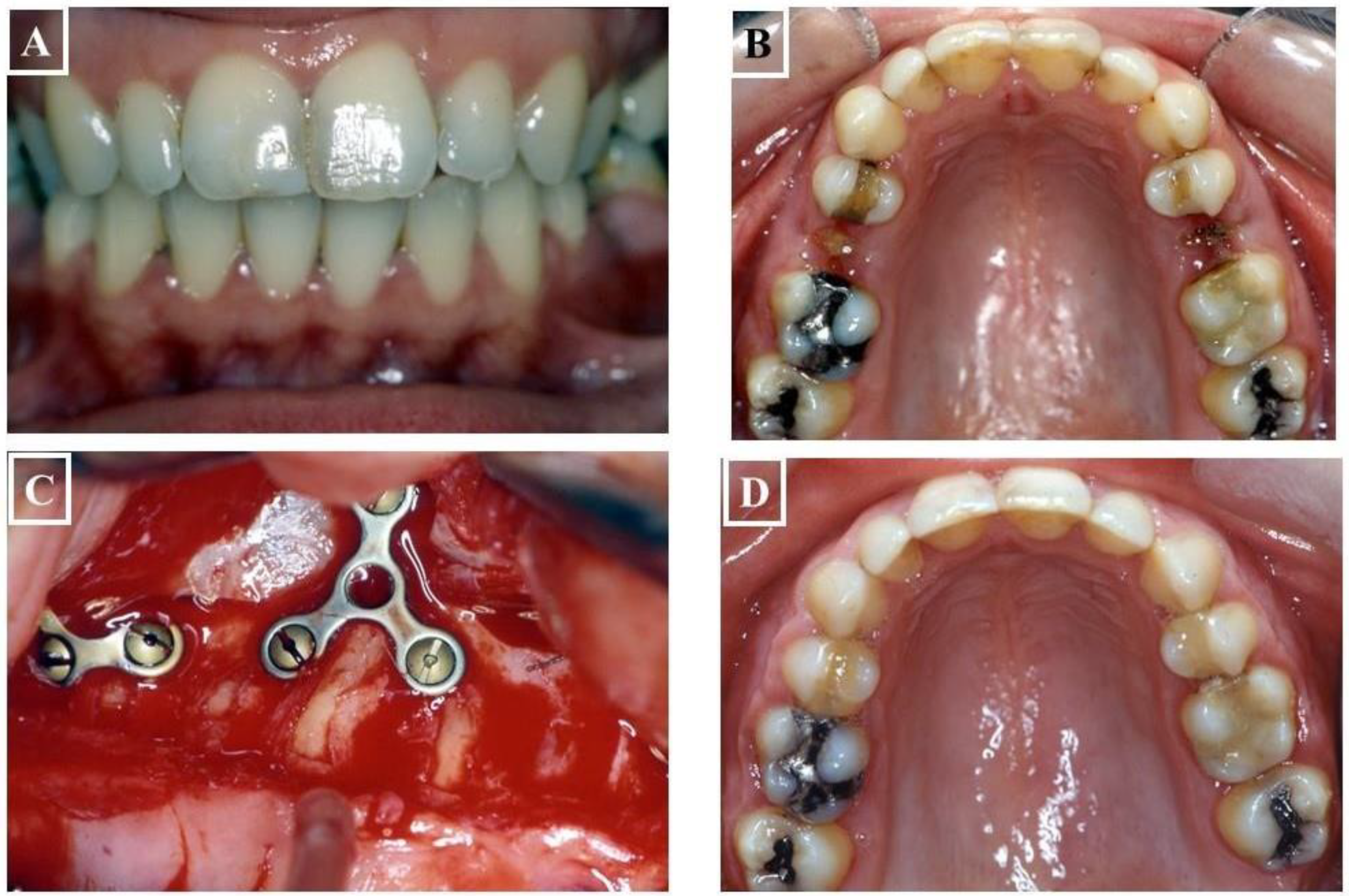
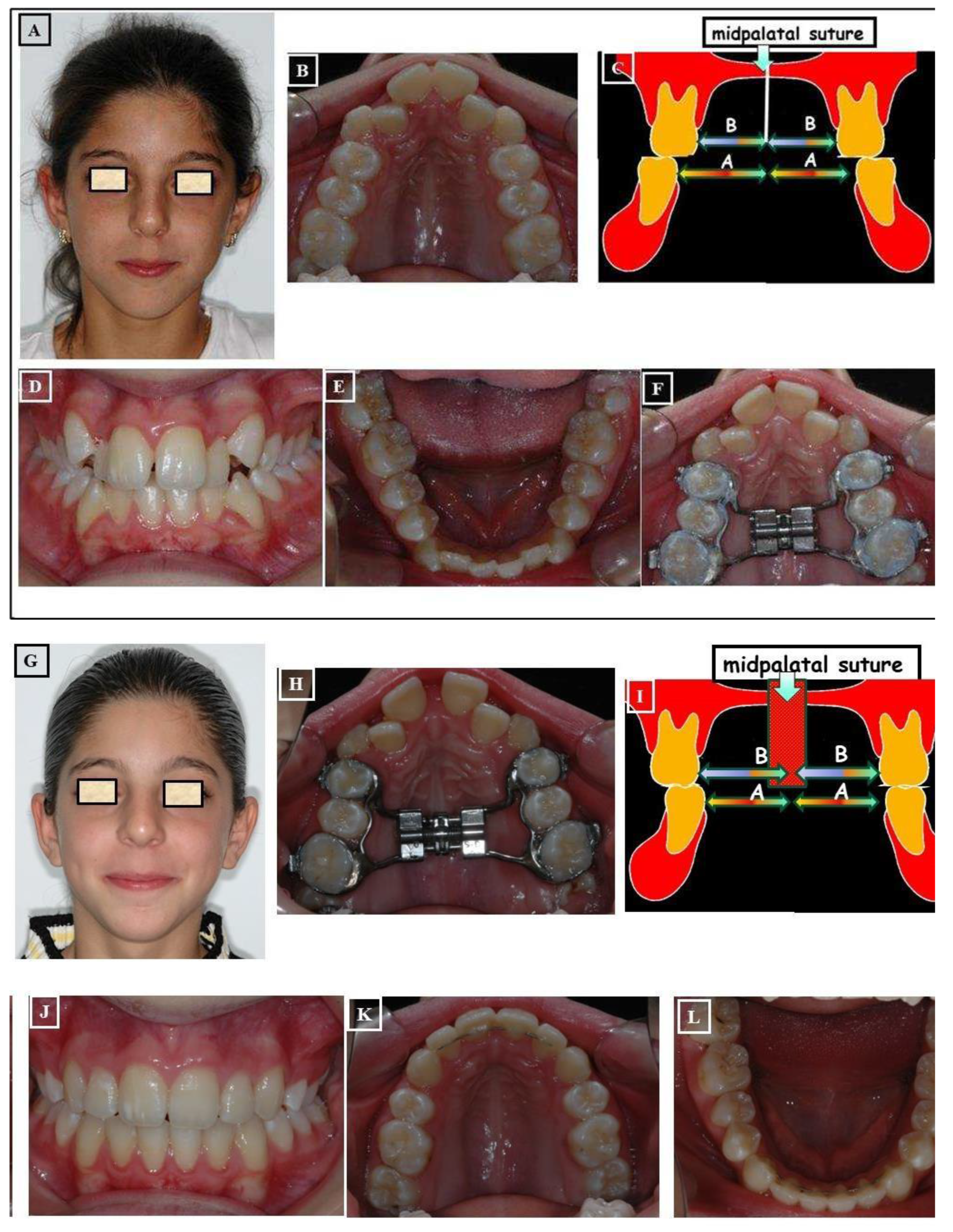
- Skeletal: This skeletal dysplasia can occur in the upper jaw (narrow upper jaw base). In this situation, the upper jaw can be narrow on one side of the jaw, while the other half is normal (unilateral crossbite) (Figure 9A-G), or the entire upper jaw base is narrow. In these situations, no facial asymmetries are observed (Figure 10A-G).
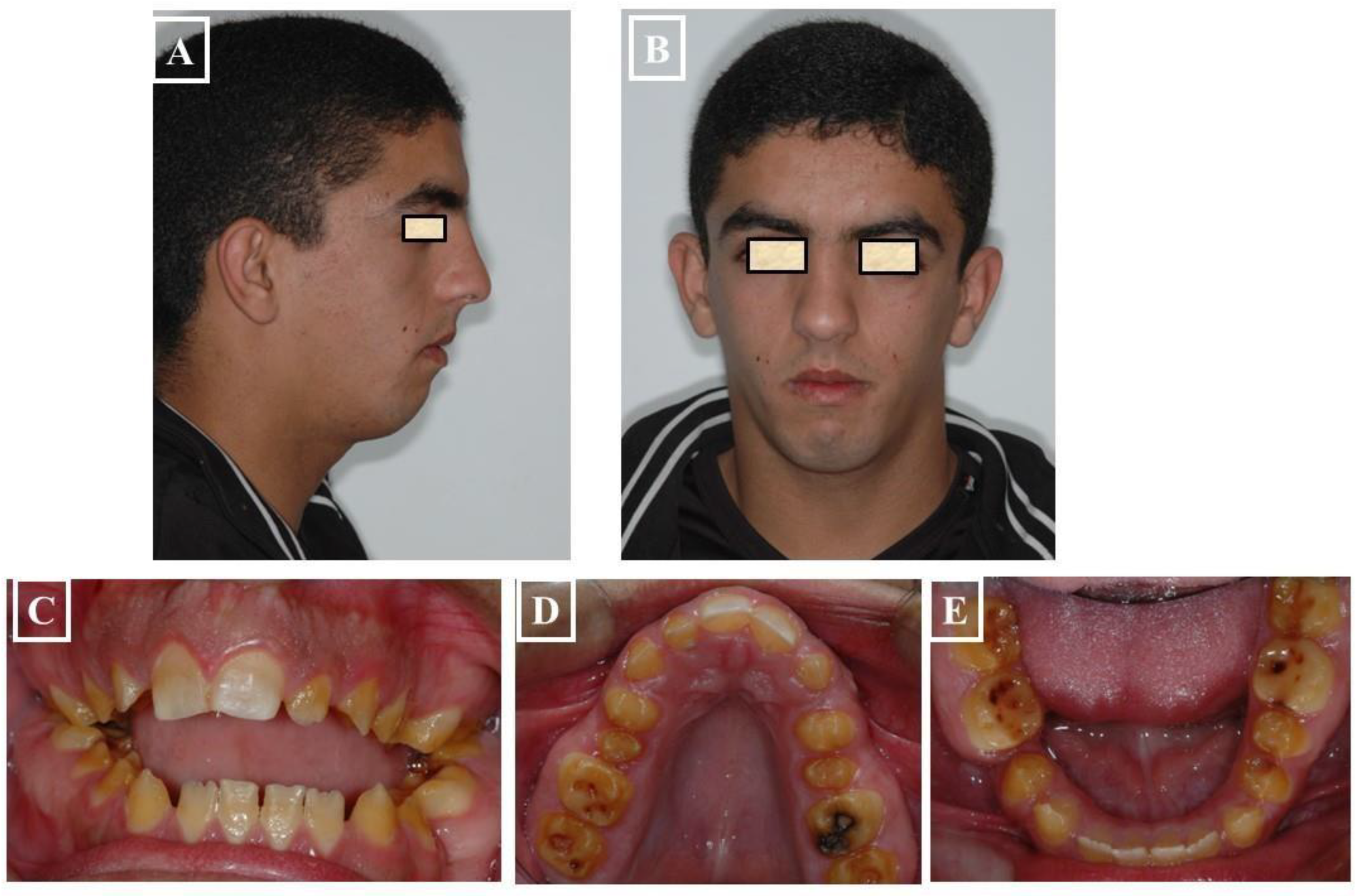
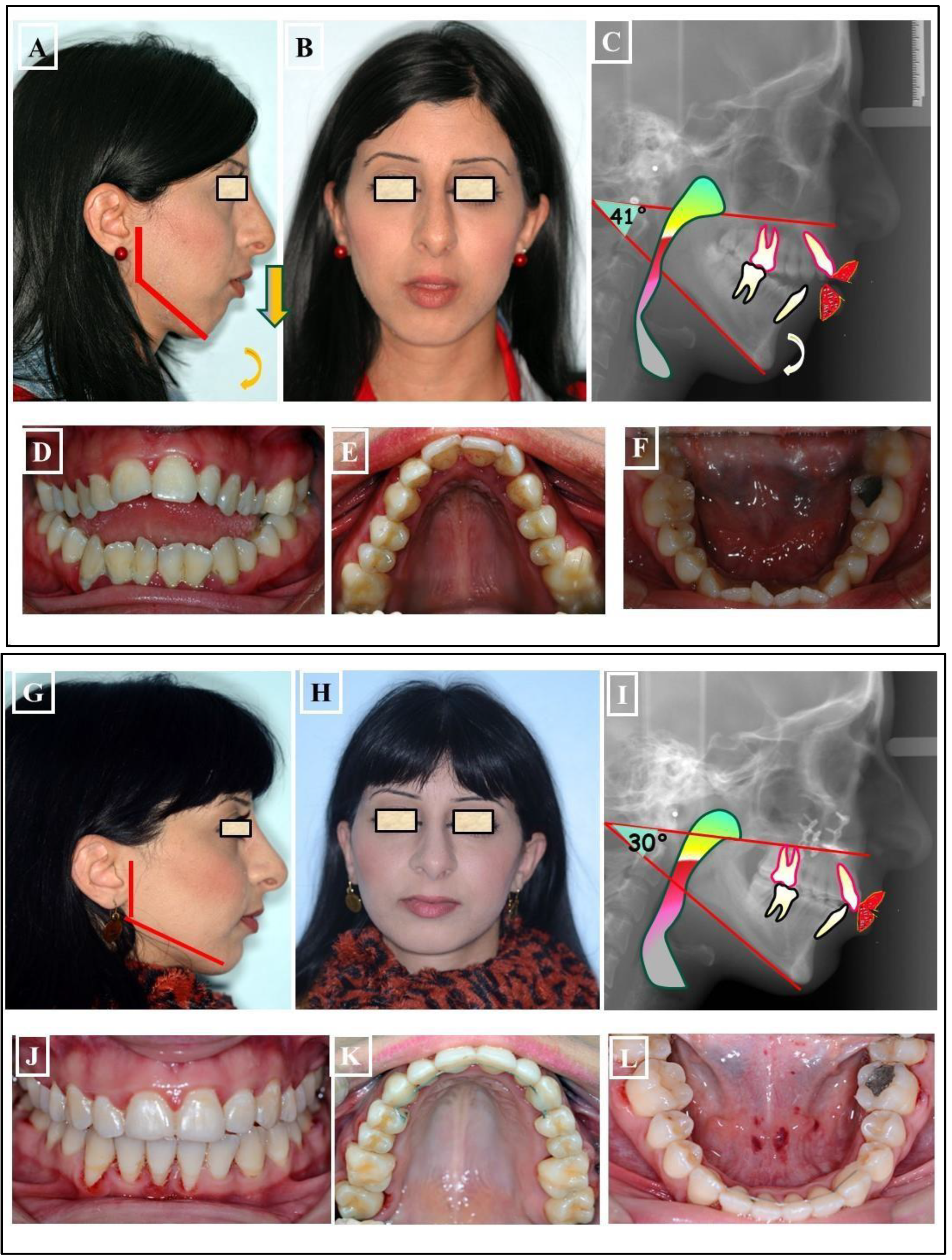
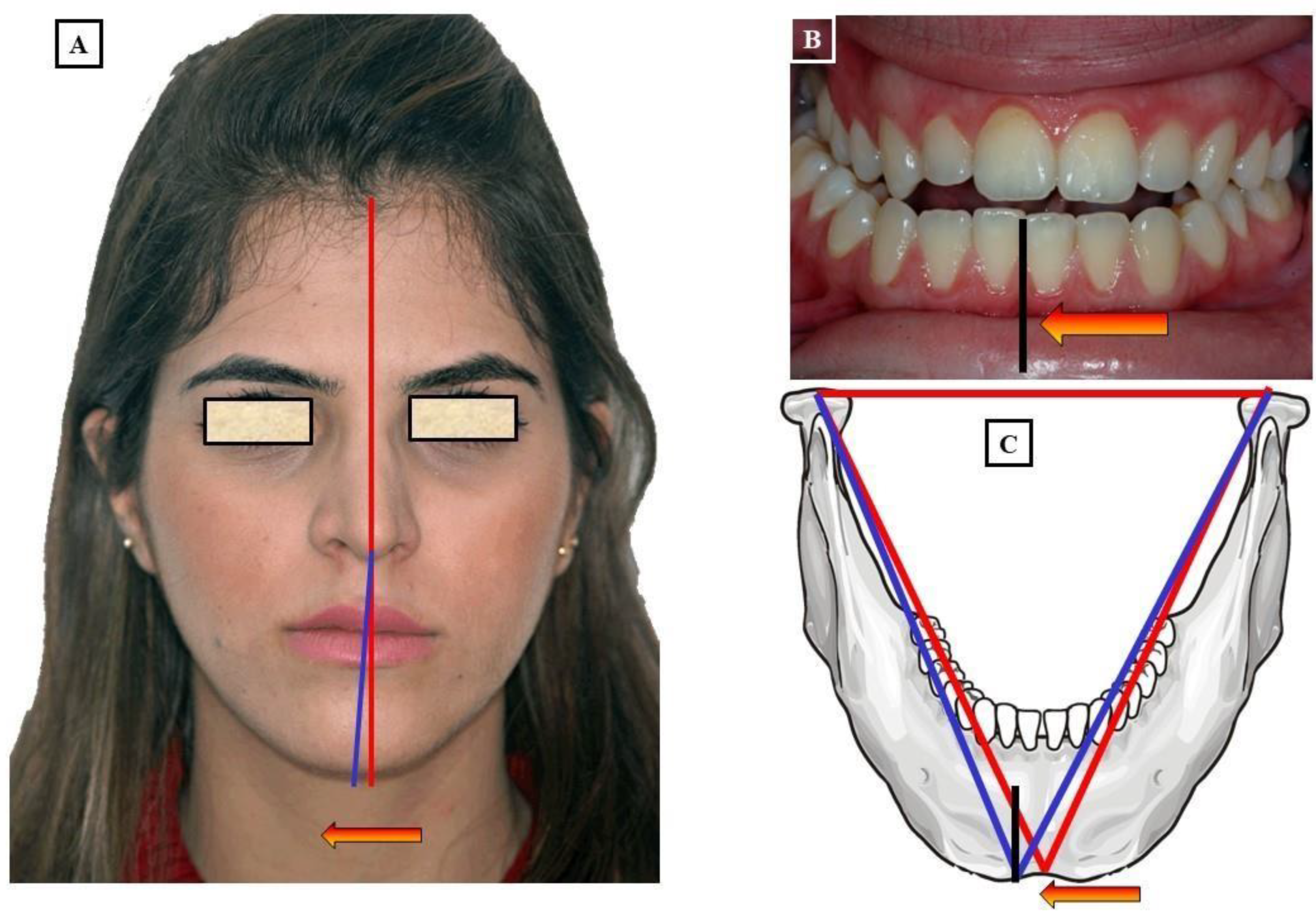
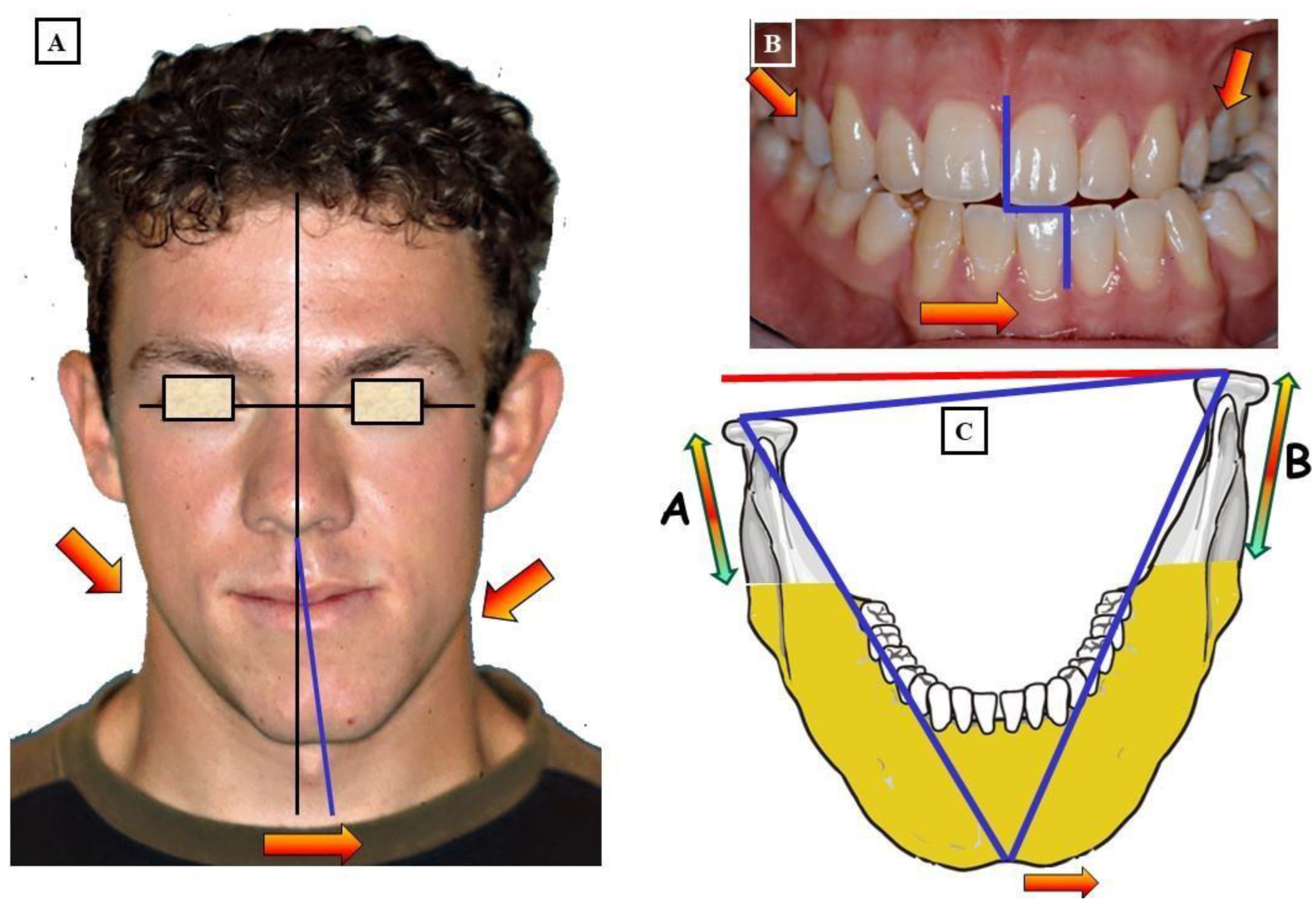
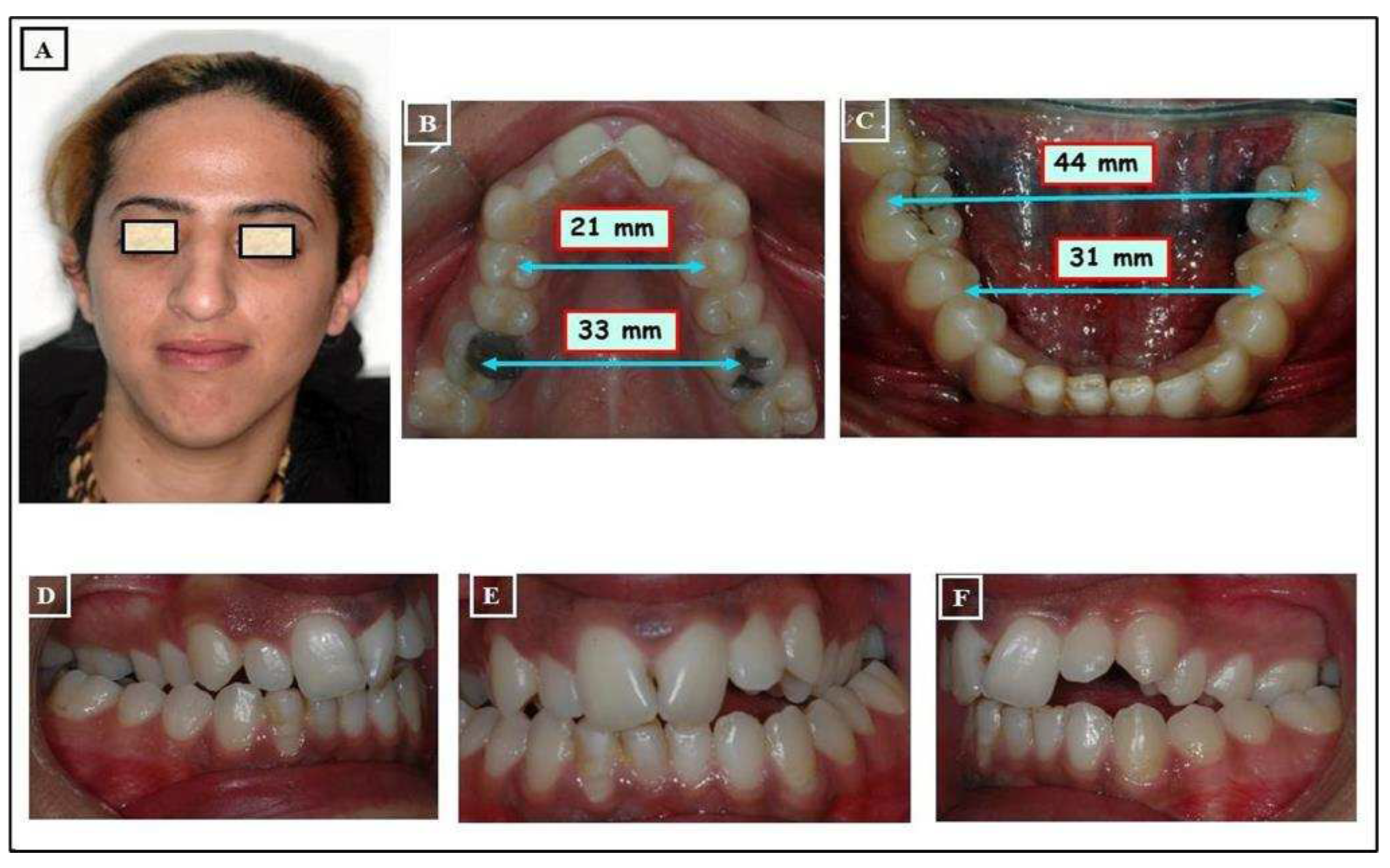
- When the transverse discrepancy is skeletal and originates from the lower jaw, facial asymmetry due to the lower jaw deviation towards the crossbite side can be observed. This asymmetry is typically a growth disorder in the condylar region of the lower jaw (FIGURE 11a-c). These growth disorders can be referred to as condylar hyperplasia when they reach a certain degree, resulting in pronounced facial asymmetry due to the lower jaw deviation to the opposite side (Figure 12A-C). In not uncommon cases, both the mandible and the maxilla are involved in this transverse discrepancy.
- Combination: In this malformation, all the above-mentioned reasons for this transverse discrepancy can be responsible (Figure 13A-D).
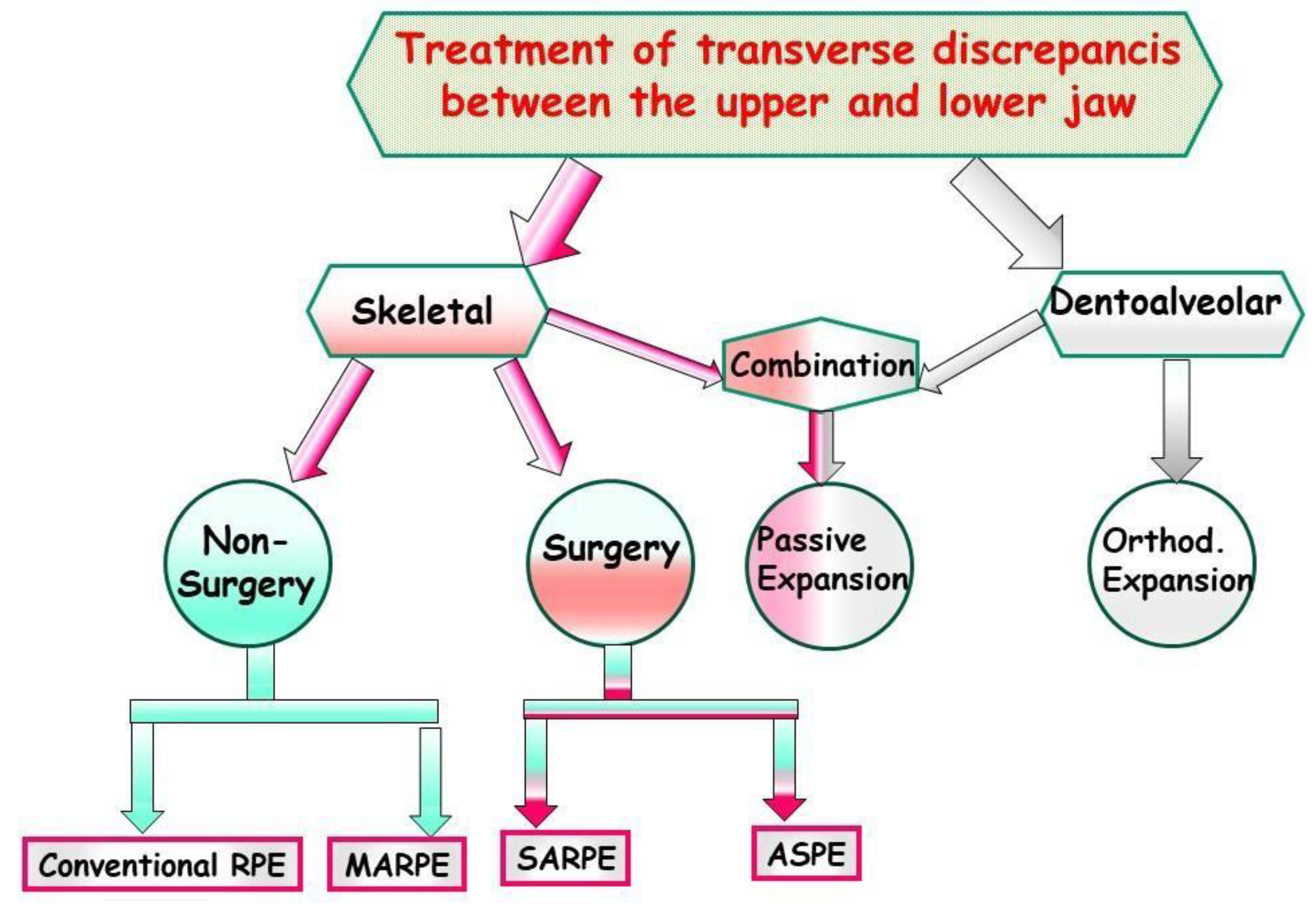
8. Management
- Conventional Rapid Maxillary Expansion (RME or RPE): A prevalent therapeutic approach for younger individuals to address maxillary transverse deficiency is Rapid Maxillary Expansion (RME). The objective of this intervention is to broaden the midpalatal suture by exerting lateral forces against the teeth and marginal alveolar bone. RME proves effective in children and adolescents before sutural closure. However, in non-growing adolescents and young adults, the success rate of maxillary expansion decreases with the closure of sutures. (Figure 18A-l).
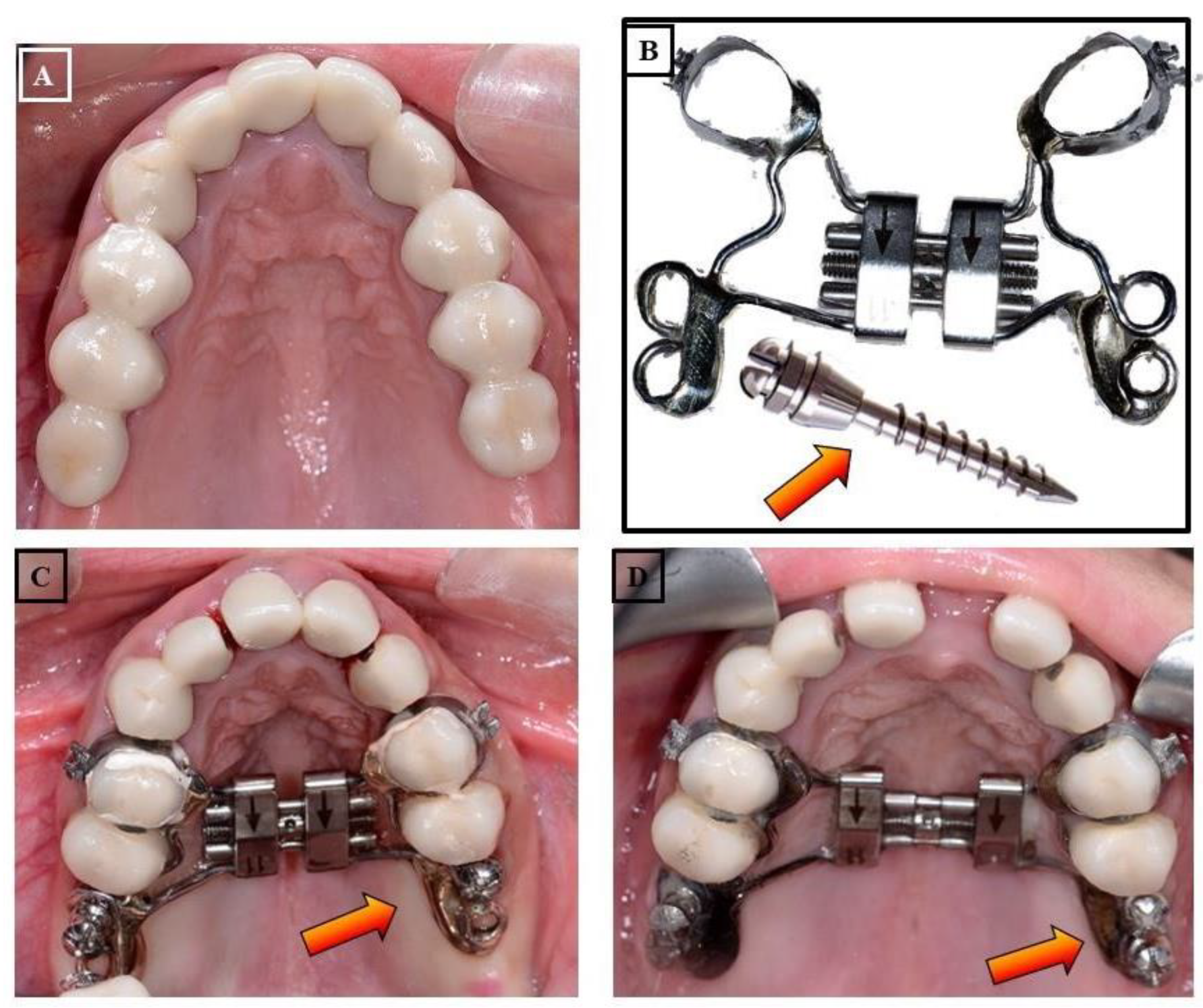
- Micro-implant Assisted Rapid Palatal Expander (MARPE): In recent years, another palatal expansion design has been developed with a jackscrew attached to the palatal vault by a temporary anchorage device (Figure 19A-D). This design is the micro-implant assisted rapid palatal expander (MARPE), used to combat undesired dental effects by achieving pure skeletal changes. MARPE is a simple modification of a conventional RPE appliance. The main difference is the incorporation of micro-implants into the palatal jackscrew to ensure expansion of the underlying basal bone, minimizing dentoalveolar tipping and expansion.
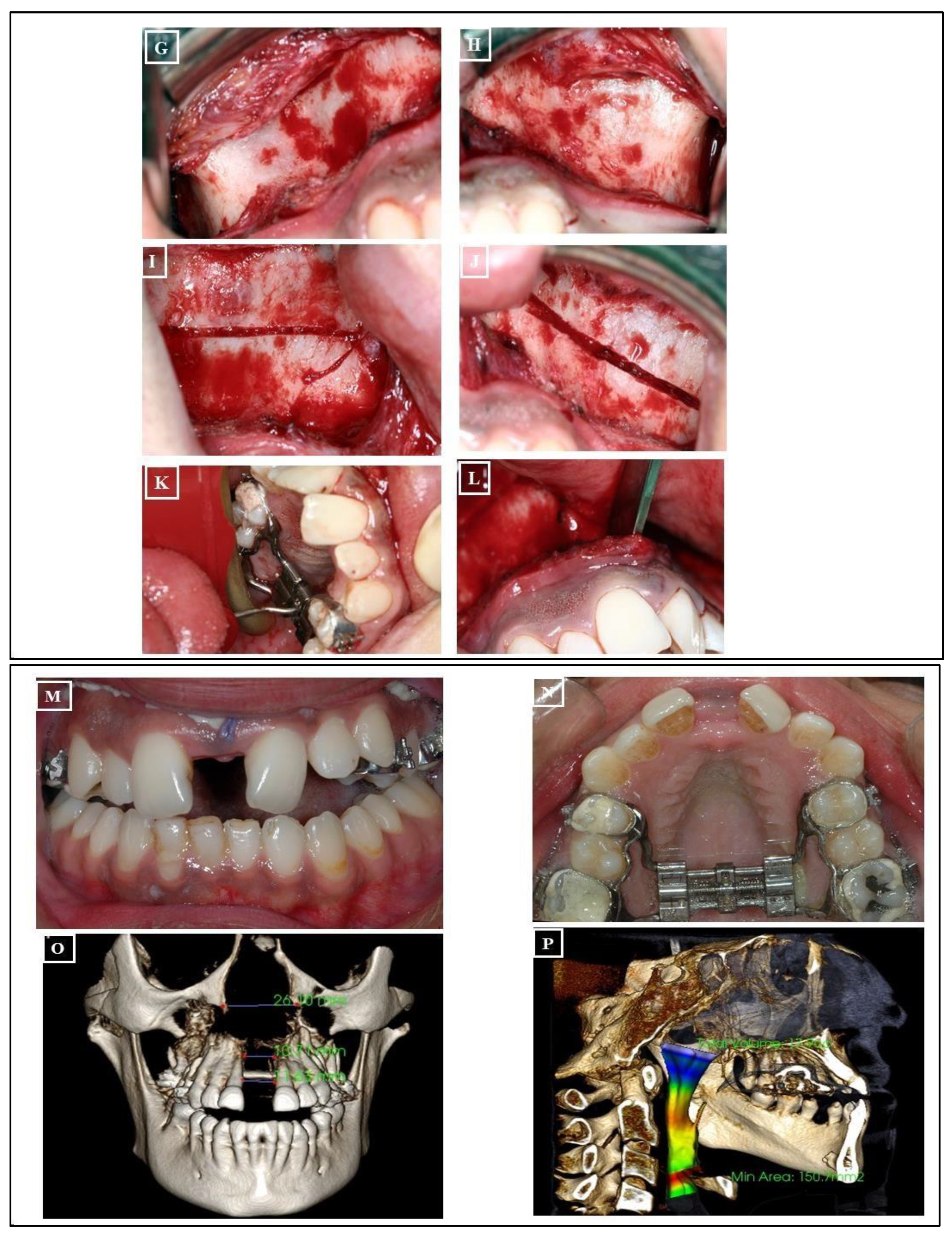
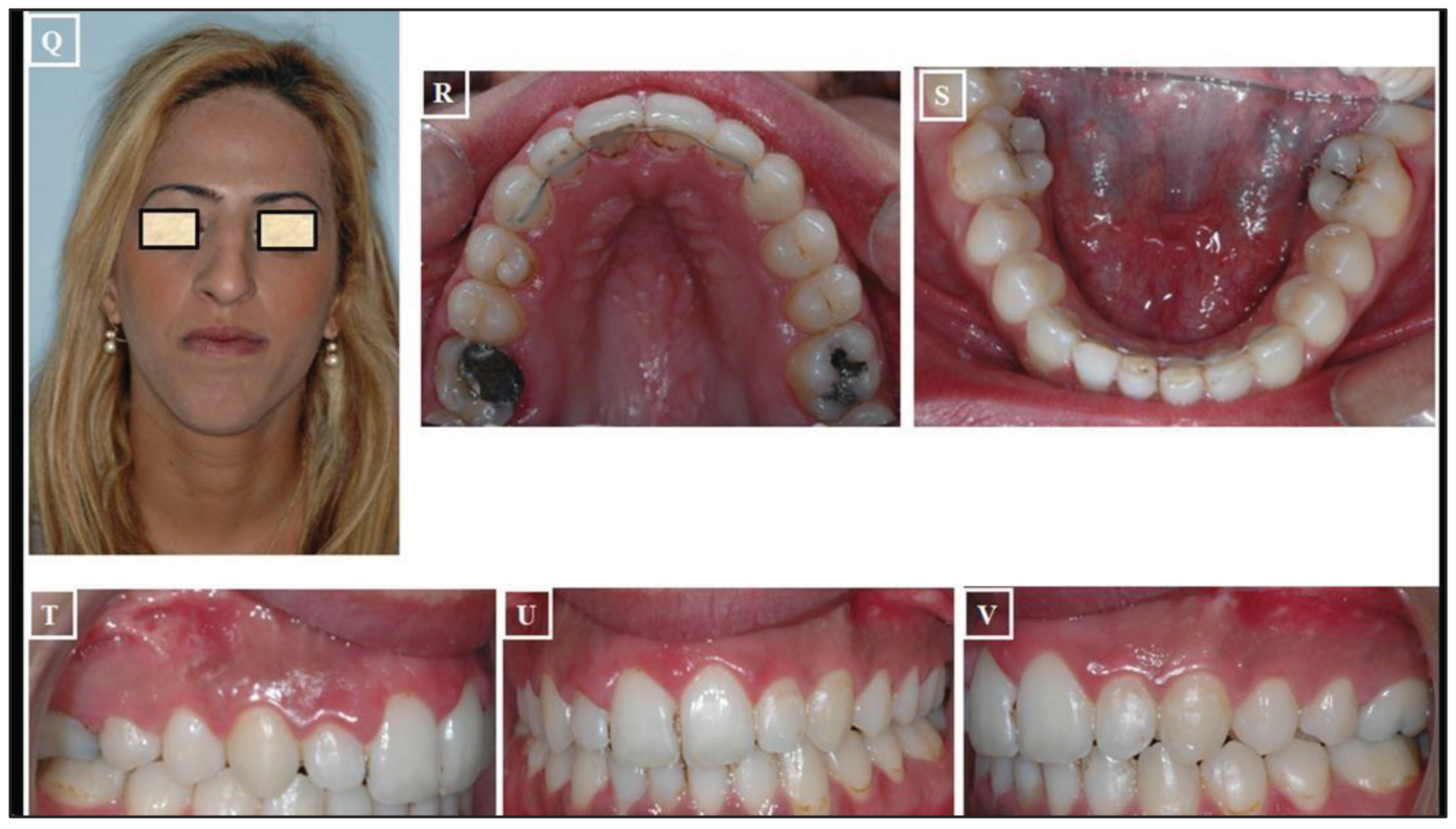
- Surgically Assisted Rapid Palatal Expansion (SARPE): The maturity level of the individual plays a significant role when assessing the impact of Rapid Maxillary Expansion (RME) on craniofacial structures. RME treatment tends to be more effective in children than in adults. Although achieving maxillary expansion in older patients is plausible, the outcomes are not as predictable or enduring. In such instances, surgically assisted RME (SARME or SARPE) is an alternative for adolescents, and for adults, SARME remains the sole option for widening the maxilla. However, complications associated with the surgical procedure and financial constraints limit the widespread applicability of this treatment among adults. The surgical approach might be advisable in patients with extreme maxillary hypoplasia requiring extensive expansion (especially if the posterior teeth incline buccally). It also might be the preferred choice for patients who have significant gingival recession with the probable dehiscences and fenestrations, and it might be beneficial for patients with sleep apnea (Figure 20A-V)
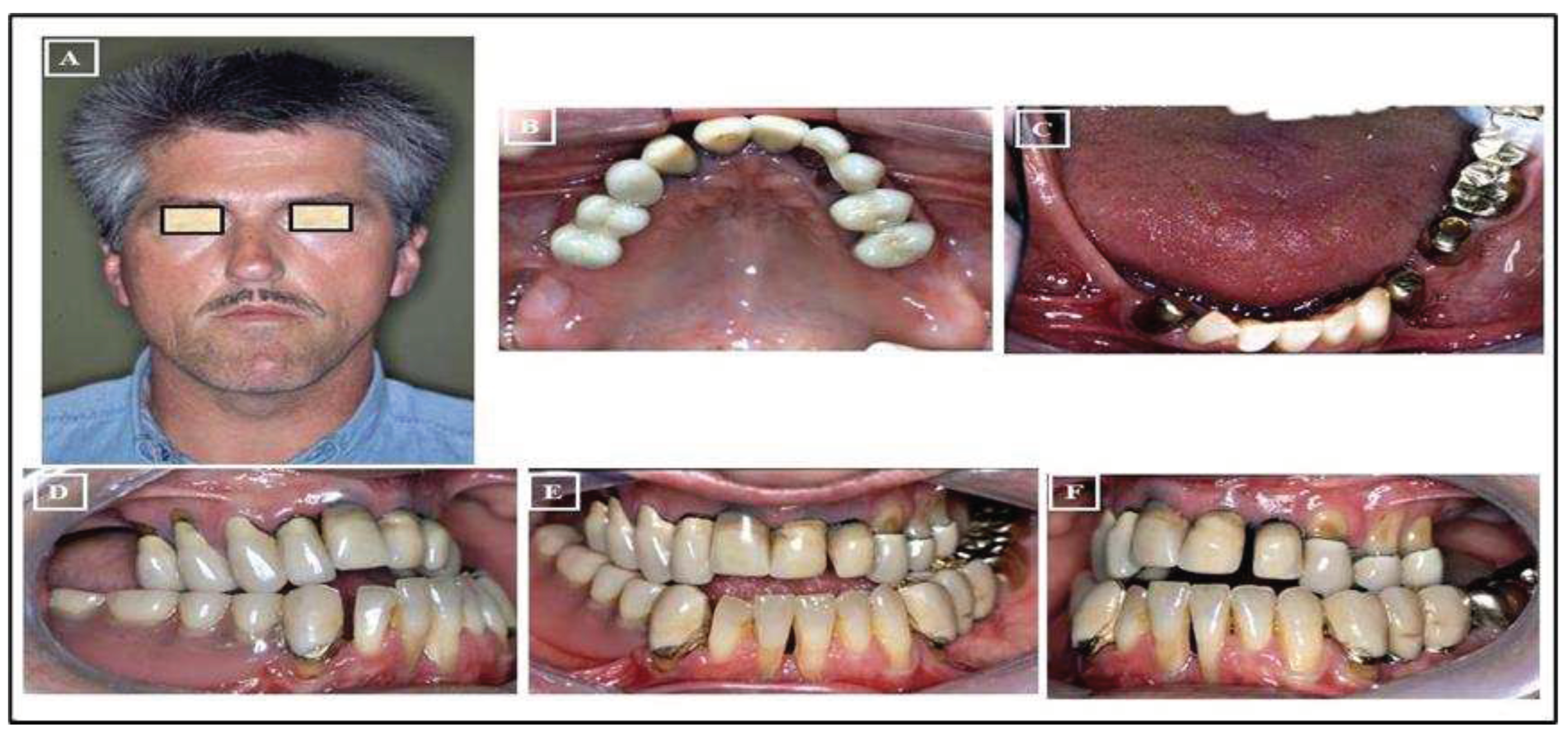
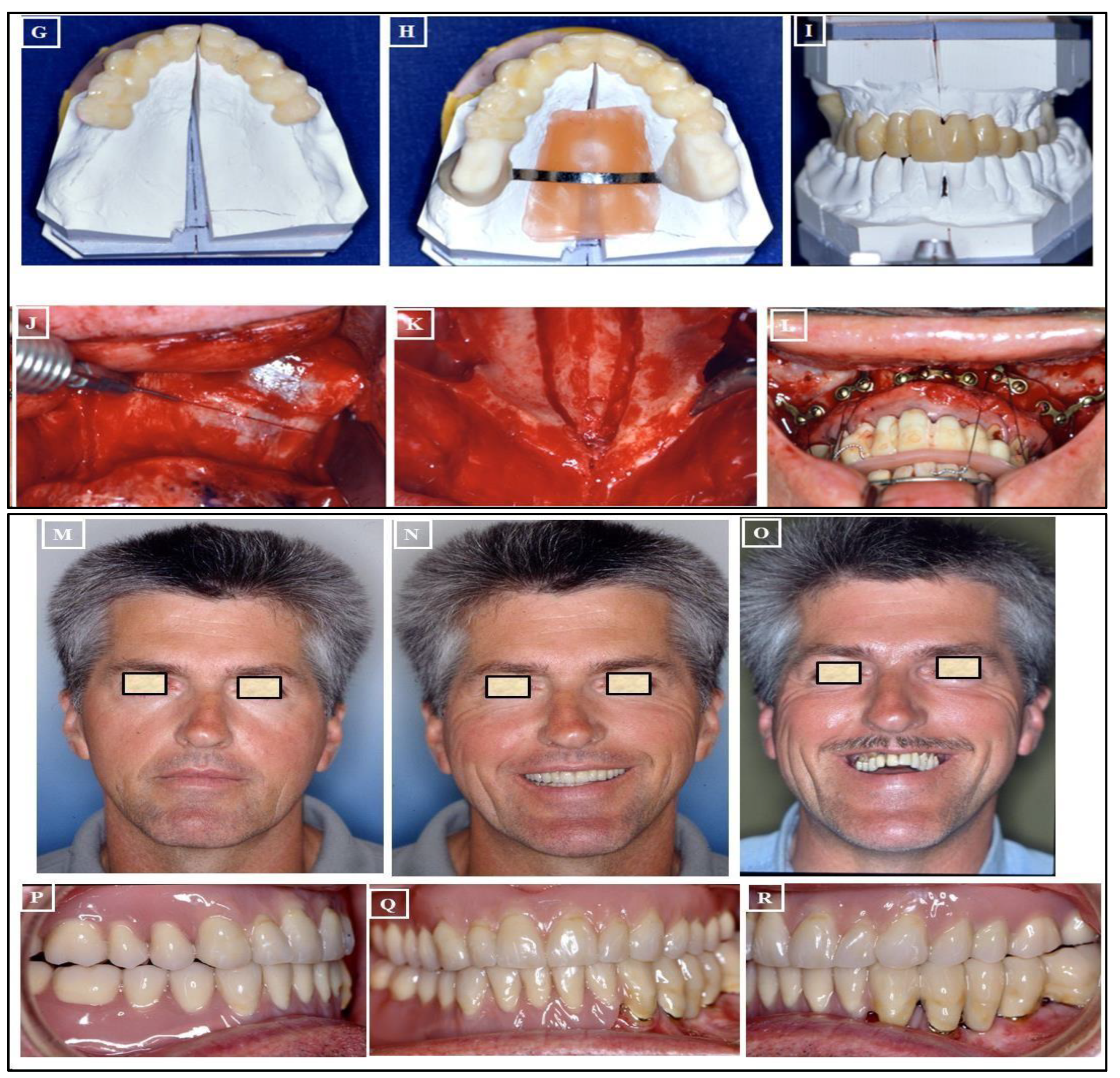
- Absolute Surgically Palatal Expansion (ASPE): This involves a surgical separation of the maxilla in the paramedian plane, not within the area of the median palatal suture, for the planned transverse expansion of the maxilla. A preoperative simulation on the surgical models is necessary for this procedure (Figure 21A-R).
9. Stability after Treatment
10. Animal Model for STD
11. Conclusions
References
- Andrews, L.F. The six keys to normal occlusion. Am. J. Orthod. 1972, 62, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Tamburrino, R.K.; Boucher, N.S.; Vanarsdall, R.L.; Secchi, A. The transverse dimension: diagnosis and relevance to functional occlusion. RWISO J 2010. [Google Scholar]
- Ricketts, R.M. Introducing Computerized Cephalometrics; Rocky Mountain Data Systems.
- Steiner, C.C. The use of cephalometrics as an aid to planning and assessing orthodontic treatment. Am. J. Orthod. 1960, 46, 721–735. [Google Scholar] [CrossRef]
- Downs, W.B. Analysis of the dentofacial profile. The Angle Orthodontist 1956. [Google Scholar]
- Andrews, L.F. Syllabus of the Andrews orthodontic philosophy.
- McNamara, J.A.; Brudon, W.L.; Kokich, V.G. Orthodontics and Dentofacial Orthopedics; 2nd, reprint ed.; Needham Press, 2001; ISBN 9780963502230.
- Vanarsdall, R.L. Transverse dimension and long-term stability. Semin. Orthod. 1999, 5, 171–180. [Google Scholar] [CrossRef]
- Cordray, F.E. Three-dimensional analysis of models articulated in the seated condylar position from a deprogrammed asymptomatic population: a prospective study. Part 1. Am. J. Orthod. Dentofacial Orthop. 2006, 129, 619–630. [Google Scholar] [CrossRef]
- Utt, T.W.; Meyers, C.E.; Wierzba, T.F.; Hondrum, S.O. A three-dimensional comparison of condylar position changes between centric relation and centric occlusion using the mandibular position indicator. Am. J. Orthod. Dentofacial Orthop. 1995, 107, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.D. Condylar axis position, as determined by the occlusion and measured by the CPI instrument, and signs and symptoms of temporomandibular dysfunction. The Angle Orthodontist 1999. [Google Scholar]
- Tamburrino, R.; Secchi, A.; Katz, S.; Pinto, A. Assessment of the three-dimensional condylar and dental positional relationships in CR-to-MIC Shifts.
- JA McNamara, J.R.; McClatchey, L.M.N. PART B: TREATMENT TIMING AND MIXED DENTITION THERAPY. Orthodontics-E-Book …, 2022. [Google Scholar]
- Bin Dakhil, N.; Bin Salamah, F. The diagnosis methods and management modalities of maxillary transverse discrepancy. Cureus 2021, 13, e20482. [Google Scholar] [CrossRef] [PubMed]
- Greco, P.M.; Jr, R.V. An evaluation of anterior temporal and masseter muscle activity in appliance therapy. The Angle …, 1999. [Google Scholar]
- Williamson, E.H.; Lundquist, D.O. Anterior guidance: its effect on electromyographic activity of the temporal and masseter muscles. J. Prosthet. Dent. 1983, 49, 816–823. [Google Scholar] [CrossRef]
- Manns, A.; Chan, C.; Miralles, R. Influence of group function and canine guidance on electromyographic activity of elevator muscles. J. Prosthet. Dent. 1987, 57, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Okano, N.; Baba, K.; Igarashi, Y. Influence of altered occlusal guidance on masticatory muscle activity during clenching. J. Oral Rehabil. 2007, 34, 679–684. [Google Scholar] [CrossRef]
- McNamara, J.A. Maxillary transverse deficiency. Am. J. Orthod. Dentofacial Orthop. 2000, 117, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Sarver, D.M.; Proffit, W.R. Special considerations in diagnosis and treatment planning.
- Harrel, S.K. Occlusal forces as a risk factor for periodontal disease. Periodontol. 2000 2003, 32, 111–117. [Google Scholar] [CrossRef]
- Davies, S. Occlusal considerations in periodontics. In A guide to good occlusal practice; BDJ Clinician’s Guides; Springer International Publishing: Cham, 2022; pp. 165–189. ISBN 978-3-030-79224-4. [Google Scholar]
- Saravanan, R. Comparative Analysis of Occlusal Force Distribution Using T-Scan in Chronic Periodontitis Patients Before and After Periodontal Therapy. Scholastic Medical Sciences 2023. [Google Scholar]
- Ricketts, R.M. Forum on the tonsile and adenoid problem in orthodontics-Respiratory obstruction syndrome. Am J Orthod 1968. [Google Scholar] [CrossRef]
- Comyn, F.; Gislason, T.; Pack, A.; Maislin, G.; Arnardottir, E.; Benediktsdottir, B.; Juliusson, S.; Einarsdottir, H.; Schwab, R. MRI comparison of craniofacial structures in sleep apneic patients. In B66. THE UPPER AIRWAY: CONTROL OF FUNCTION AND PATHOPHYSIOLOGY; American Thoracic Society, 2009; p. A3596.
- Christie, K.F.; Boucher, N.; Chung, C.-H. Effects of bonded rapid palatal expansion on the transverse dimensions of the maxilla: a cone-beam computed tomography study. Am. J. Orthod. Dentofacial Orthop. 2010, 137, S79–85. [Google Scholar] [CrossRef]
- Kiliç, N.; Oktay, H. Effects of rapid maxillary expansion on nasal breathing and some nasorespiratory and breathing problems in growing children: a literature review. Int. J. Pediatr. Otorhinolaryngol. 2008, 72, 1595–1601. [Google Scholar] [CrossRef]
- Oliveira De Felippe, N.L.; Da Silveira, A.C.; Viana, G.; Kusnoto, B.; Smith, B.; Evans, C.A. Relationship between rapid maxillary expansion and nasal cavity size and airway resistance: short- and long-term effects. Am. J. Orthod. Dentofacial Orthop. 2008, 134, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Betts, N.J. Surgically assisted maxillary expansion. Atlas of the Oral and Maxillofacial Surgery Clinics 2016, 24, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Reyneke, J.P.; Conley, R.S. Surgical/orthodontic correction of transverse maxillary discrepancies. Oral Maxillofac. Surg. Clin. North Am. 2020, 32, 53–69. [Google Scholar] [CrossRef]
- Dawson, P.E. New definition for relating occlusion to varying conditions of the temporomandibular joint. J. Prosthet. Dent. 1995, 74, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Guichet, N.F. Biologic laws governing functions of muscles that move the mandible. Part I. Occlusal programming. J. Prosthet. Dent. 1977, 37, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.D.; Southard, K.A.; Southard, T.E. Early Transverse Treatment. Semin. Orthod. 2005, 11, 130–139. [Google Scholar] [CrossRef]
- Rakosi, T.; Jonas, I.; Graber, T.M. Color atlas of dental medicine, Orthodontic-Diagnosis. … of Orthodontics …1994.
- Redmond, W.R. Digital models: a new diagnostic tool. Journal of clinical orthodontics: JCO2001.
- Haas, A.J. Rapid expansion of the maxillary dental arch and nasal cavity by opening the midpalatal suture. The Angle Orthodontist 1961. [Google Scholar]
- Marshall, S.; Dawson, D.; Southard, K.A.; Lee, A.N.; Casko, J.S.; Southard, T.E. Transverse molar movements during growth. Am. J. Orthod. Dentofacial Orthop. 2003, 124, 615–624. [Google Scholar] [CrossRef]
- Thilander, B.; Lennartsson, B. A study of children with unilateral posterior crossbite, treated and untreated, in the deciduous dentition--occlusal and skeletal characteristics of significance in predicting the long-term outcome. J. Orofac. Orthop. 2002, 63, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.D.; Bell, W.H.; Williams, C.E.; Kennedy, J.W. Control of the transverse dimension with surgery and orthodontics. Am. J. Orthod. 1980, 77, 284–306. [Google Scholar] [CrossRef] [PubMed]
- Betts, N.J.; Vanarsdall, R.L.; Barber, H.D. Diagnosis and treatment of transverse maxillary deficiency. … journal of adult …1995.
- Ricketts, R.M. Perspectives in the clinical application of cephalometrics: the first fifty years. The Angle Orthodontist 1981. [Google Scholar]
- Suri, L.; Taneja, P. Surgically assisted rapid palatal expansion: a literature review. Am. J. Orthod. Dentofacial Orthop. 2008, 133, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Macchi, A.; Carrafiello, G.; Cacciafesta, V.; Norcini, A. Three-dimensional digital modeling and setup. Am. J. Orthod. Dentofacial Orthop. 2006, 129, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Sawchuk, D.; Currie, K.; Vich, M.L.; Palomo, J.M.; Flores-Mir, C. Diagnostic methods for assessing maxillary skeletal and dental transverse deficiencies: A systematic review. Korean J. Orthod. 2016, 46, 331–342. [Google Scholar] [CrossRef]
- Haas, A.J. Palatal expansion: just the beginning of dentofacial orthopedics. Am. J. Orthod. 1970, 57, 219–255. [Google Scholar] [CrossRef] [PubMed]
- Jr, J.M.; Riolo, M.L.; Enlow, D.H. Growth of the maxillary complex in the rhesus monkey (Macaca mulatta) This study was supported in part by United States Public Health Service Grants HD-02272 …. 1976.
- Baccetti, T.; Franchi, L.; Cameron, C.G.; McNamara, J.A. Treatment timing for rapid maxillary expansion. Angle Orthod. 2001, 71, 343–350. [Google Scholar] [CrossRef]
- Angelieri, F.; Franchi, L.; Cevidanes, L.H.S.; Gonçalves, J.R.; Nieri, M.; Wolford, L.M.; McNamara, J.A. Cone beam computed tomography evaluation of midpalatal suture maturation in adults. Int. J. Oral Maxillofac. Surg. 2017, 46, 1557–1561. [Google Scholar] [CrossRef]
- Haas, A.J. The treatment of maxillary deficiency by opening the midpalatal suture. Angle Orthod. 1965, 35, 200–217. [Google Scholar] [CrossRef]
- Proffit, W.R.; Fields, H.W.; Larson, B.; Sarver, D.M. Contemporary orthodontics-e-book; books.google.com. 2018. [Google Scholar]
- Proffit, W.R.; Turvey, T.A.; Phillips, C. The hierarchy of stability and predictability in orthognathic surgery with rigid fixation: an update and extension. Head Face Med. 2007, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, Í.; Oktay, H.; Demirci, M. The effect of rapid maxillary expansion on conductive hearing loss. The Angle Orthodontist 1996. [Google Scholar]
- Bell, R.A. A review of maxillary expansion in relation to rate of expansion and patient’s age. Am. J. Orthod. 1982, 81, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Mathur, R. Maxillary Expansion. Int. J. Clin. Pediatr. Dent. 2010, 3, 139–146. [Google Scholar] [CrossRef]
- Cleall, J.F.; Bayne, D.I.; Posen, J.M.; Subtelny, J.D. Expansion of the midpalatal suture in the monkey. Angle Orthod. 1965, 35, 23–35. [Google Scholar] [CrossRef]
- Storey, E. Tissue response to the movement of bones. Am. J. Orthod. 1973, 64, 229–247. [Google Scholar] [CrossRef]
- Hicks, E.P. Slow maxillary expansion: a clinical study of the skeletal versus dental response to low-magnitude force. Am. J. Orthod. 1978, 73, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, R.J.; Ingram, A.H. Forces produced by rapid maxillary expansion: II. Forces present during treatment. The Angle Orthodontist 1964. [Google Scholar]
- Brunetto, D.P.; Sant’Anna, E.F.; Machado, A.W.; Moon, W. Non-surgical treatment of transverse deficiency in adults using Microimplant-assisted Rapid Palatal Expansion (MARPE). Dental Press J. Orthod. 2017, 22, 110–125. [Google Scholar] [CrossRef]
- Shen, T.; Zhao, B.; Wang, C.; Xiao, Y.; Han, Y.; Zhao, G.; Ke, J. Efficacy of different designs of mandibular expanders: A 3-dimensional finite element study. Am. J. Orthod. Dentofacial Orthop. 2020, 157, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Lines, P.A. Adult rapid maxillary expansion with corticotomy. Am. J. Orthod. 1975, 67, 44–56. [Google Scholar] [CrossRef]
- Bailey, L.J.; White, R.P.; Proffit, W.R.; Turvey, T.A. Segmental LeFort I osteotomy for management of transverse maxillary deficiency. J. Oral Maxillofac. Surg. 1997, 55, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Lehman, J.A.; Haas, A.J.; Haas, D.G. Surgical orthodontic correction of transverse maxillary deficiency: a simplified approach. Plast. Reconstr. Surg. 1984, 73, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Kraut, R.A. Surgically assisted rapid maxillary expansion by opening the midpalatal suture. Oral Maxillofac. Surg. 1984, 42, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.W.; Bell, W.H.; Kimbrough, O.L.; James, W.B. Osteotomy as an adjunct to rapid maxillary expansion. Am. J. Orthod. 1976, 70, 123–137. [Google Scholar] [CrossRef]
- Bays, R.A.; Greco, J.M. Surgically assisted rapid palatal expansion: an outpatient technique with long-term stability. J. Oral Maxillofac. Surg. 1992, 50, 110–3, discussion 114. [Google Scholar] [CrossRef] [PubMed]
- Koudstaal, M.J.; Poort, L.J.; van der Wal, K.G.H.; Wolvius, E.B.; Prahl-Andersen, B.; Schulten, A.J.M. Surgically assisted rapid maxillary expansion (SARME): a review of the literature. Int. J. Oral Maxillofac. Surg. 2005, 34, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Mossaz, C.F.; Byloff, F.K.; Richter, M. Unilateral and bilateral corticotomies for correction of maxillary transverse discrepancies. Eur. J. Orthod. 1992, 14, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Northway, W.M.; Meade, J.B. Surgically assisted rapid maxillary expansion: a comparison of technique, response, and stability. Angle Orthod. 1997, 67, 309–320. [Google Scholar] [CrossRef]
- Glassman, A.S.; Nahigian, S.J.; Medway, J.M.; Aronowitz, H.I. Conservative surgical orthodontic adult rapid palatal expansion: sixteen cases. Am. J. Orthod. 1984, 86, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.-H.; Woo, A.; Zagarinsky, J.; Vanarsdall, R.L.; Fonseca, R.J. Maxillary sagittal and vertical displacement induced by surgically assisted rapid palatal expansion. Am. J. Orthod. Dentofacial Orthop. 2001, 120, 144–148. [Google Scholar] [CrossRef]
- Mommaerts, M.Y. Transpalatal distraction as a method of maxillary expansion. Br. J. Oral Maxillofac. Surg. 1999, 37, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.L.; Pangrazio-Kulbersh, V.; Borgula, T.; Kaczynski, R. Stability of orthopedic and surgically assisted rapid palatal expansion over time. Am. J. Orthod. Dentofacial Orthop. 1998, 114, 638–645. [Google Scholar] [CrossRef]
- Neyt, N.M.F.; Mommaerts, M.Y.; Abeloos, J.V.S.; De Clercq, C.A.S.; Neyt, L.F. Problems, obstacles and complications with transpalatal distraction in non-congenital deformities. J Craniomaxillofac Surg 2002, 30, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Bishara, S.E.; Staley, R.N. Maxillary expansion: Clinical implications. Am. J. Orthod. Dentofacial Orthop. 1987, 91, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Mew, J. Long-term effect of rapid maxillary expansion. European journal of orthodontics 1993. [Google Scholar]
- Velázquez, P.; Benito, E.; Bravo, L.A. Rapid maxillary expansion. A study of the longterm effects. Am. J. Orthod. Dentofacial Orthop. 1996, 109, 361–367. [Google Scholar] [CrossRef]
- Pogrel, M.A.; Kaban, L.B.; Vargervik, K.; Baumrind, S. Surgically assisted rapid maxillary expansion in adults. Int. J. Adult Orthodon. Orthognath. Surg. 1992, 7, 37–41. [Google Scholar] [PubMed]
- Zahl, Chr. ; Gerlach, K. Palatinaldistraktor. Mund Kiefer Gesichtschir. 2002, 6, 446–449. [Google Scholar] [CrossRef]
- Proffit, W.R.; Turvey, T.A.; Phillips, C. Orthognathic surgery: a hierarchy of stability. Int. Adult Orthodon. Orthognath. Surg. 1996; 11, 191–204. [Google Scholar] [CrossRef]
- Woods, M.; Wiesenfeld, D.; Probert, T. Surgically-assisted maxillary expansion. Aust. Dent. J. 1997, 42, 38–42. [Google Scholar] [CrossRef]
- Wei, X.; Thomas, N.; Hatch, N.E.; Hu, M.; Liu, F. Postnatal craniofacial skeletal development of female c57bl/6ncrl mice. Front. Physiol. 2017, 8, 697. [Google Scholar] [CrossRef] [PubMed]
- Abu-Toamih-Atamni, H.J.; Lone, I.M.; Binenbaum, I.; Mott, R.; Pilalis, E.; Chatziioannou, A.; Iraqi, F.A. Mapping novel QTL and fine mapping of previously identified QTL associated with glucose tolerance using the collaborative cross mice. Mamm. Genome 2023. [Google Scholar] [CrossRef]
- Adams, D.J.; Ackert-Bicknell, C.L. Genetic regulation of bone strength: a review of animal model studies. Bonekey Rep. 2015, 4, 714. [Google Scholar] [CrossRef] [PubMed]
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis: classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016, 11, 19. [Google Scholar] [CrossRef]
- Lone, I.M.; Midlej, K.; Nun, N.B.; Iraqi, F.A. Intestinal cancer development in response to oral infection with high-fat diet-induced Type 2 diabetes (T2D) in collaborative cross mice under different host genetic background effects. Mamm. Genome 2023, 34, 56–75. [Google Scholar] [CrossRef]
- Lone, I.M.; Iraqi, F.A. Genetics of murine type 2 diabetes and comorbidities. Mamm. Genome 2022, 33, 421–436. [Google Scholar] [CrossRef]
- Durrant, C.; Tayem, H.; Yalcin, B.; Cleak, J.; Goodstadt, L.; de Villena, F.P.-M.; Mott, R.; Iraqi, F.A. Collaborative Cross mice and their power to map host susceptibility to Aspergillus fumigatus infection. Genome Res. 2011, 21, 1239–1248. [Google Scholar] [CrossRef]
- Lone, I.M.; Nun, N.B.; Ghnaim, A.; Schaefer, A.S.; Houri-Haddad, Y.; Iraqi, F.A. Highfat diet and oral infection induced type 2 diabetes and obesity development under different genetic backgrounds. Anim. Models Exp. Med. 2023, 6, 131–145. [Google Scholar] [CrossRef]
- Lone, I.M.; Zohud, O.; Nashef, A.; Kirschneck, C.; Proff, P.; Watted, N.; Iraqi, F.A. Dissecting the Complexity of Skeletal-Malocclusion-Associated Phenotypes: Mouse for the Rescue. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef]
- Yehia, R.; Lone, I.M.; Yehia, I.; Iraqi, F.A. Studying the Pharmagenomic effect of Portulaca oleracea extract on anti-diabetic therapy using the Collaborative Cross mice. Phytomedicine Plus 2023, 3, 100394. [Google Scholar] [CrossRef]
- Zohud, O.; Lone, I.M.; Midlej, K.; Obaida, A.; Masarwa, S.; Schröder, A.; Küchler, E.C.; Nashef, A.; Kassem, F.; Reiser, V.; Chaushu, G.; Mott, R.; Krohn, S.; Kirschneck, C.; Proff, P.; Watted, N.; Iraqi, F.A. Towards genetic dissection of skeletal class III malocclusion: A review of genetic variations underlying the phenotype in humans and future directions. J. Clin. Med. 2023, 12. [Google Scholar] [CrossRef]
- Lone, I.M.; Zohud, O.; Midlej, K.; Awadi, O.; Masarwa, S.; Krohn, S.; Kirschneck, C.; Proff, P.; Watted, N.; Iraqi, F.A. Narrating the Genetic Landscape of Human Class I Occlusion: A Perspective-Infused Review. J. Pers. Med. 2023, 13. [Google Scholar] [CrossRef]
- Watted, N.; Lone, I.M.; Zohud, O.; Midlej, K.; Proff, P.; Iraqi, F.A. Comprehensive Deciphering the Complexity of the Deep Bite: Insight from Animal Model to Human Subjects. J. Pers. Med. 2023, 13. [Google Scholar] [CrossRef]
- Lone, I.M.; Zohud, O.; Midlej, K.; Proff, P.; Watted, N.; Iraqi, F.A. Skeletal Class II Malocclusion: From Clinical Treatment Strategies to the Roadmap in Identifying the Genetic Bases of Development in Humans with the Support of the Collaborative Cross Mouse Population. J. Clin. Med. 2023, 12. [Google Scholar] [CrossRef]
- Ghnaim, A.; Lone, I.M.; Nun, N.B.; Iraqi, F.A. Unraveling the host genetic background effect on internal organ weight influenced by obesity and diabetes using collaborative cross mice. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




