1. Introduction
Recurrent respiratory papillomatosis (RRP) is a benign clinical condition of the respiratory tract associated with an infection caused by Human Papillomavirus (HPV), especially HPV-6 and -11. While the majority of the cases are associated with low-risk HPV genotypes, a small fraction (less than 5%) is sustained by HPV-16 and other high-risk HPV genotypes [
1]. RRP is a relative rare disease which can affect both children (Juvenile-Onset, JORRP) and adults (Adult-Onset, AORRP) with an approximately estimated incidence of 4 per 100,000 children and 2 per 100,000 adults, respectively [
2,
3,
4,
5,
6]. It is characterized by the development of multiple papillomas in the connective tissue of the upper respiratory tract, with a particular predilection for the larynx [
2]: lesions, varying in size and showing rapid growth rates, can favor voice alterations, persistent cough, and airway blockage. Respiratory symptoms can significantly impact the quality of life, affecting breathing, speaking, and overall daily functioning [
2].
In adulthood RRP can occur in individuals aged 20-40 years, whereas JORRP usually develops in children 1-4 years old, without any significant differences in incidence based on sex, race, or ethnicity [
7,
8,
9,
10]. Transmission pathways of juvenile onset and adult onset forms are likely distinct. Previous studies showed that adult onset, following sexual transmission, is more prevalent in individuals practicing oral sex and having multiple partners [
1,
5]. Conversely, JORRP is attributed to exposure to genital warts during pregnancy or delivery: a maternal history of condylomas was associated with a 200-fold increased risk, along with a higher risk during vaginal delivery in comparison with the cesarean delivery [
5]. The more frequent clinical symptom in children is hoarseness, whereas dysphonia dyspnea, dysphagia, upper respiratory tract infections and pneumonia are the most common conditions in adults [
2,
3,
5]. However, the natural course and severity of diseases can vary according to individual and virological characteristics. Being diagnosed at a young age is the most significant factor related to disease severity and progression. In fact, a diagnosis in children aged <3 years results in a risk almost 4 times higher of undergoing repeat surgery and complications, following the involvement of other anatomical sites [
2]. The ability of the virus to replicate latently in epithelial cells is the main cause of recurrence, whereas an efficient immune response can prolong the periods of remission [
2].
Prevention is based on HPV vaccination, and surgery is the first therapeutic option followed by adjuvant therapies (
e.g., interferon-α2a, antivirals) to reduce the high risk of relapse [
11,
12]. Laser excision represents the most common treatment, whereas tracheotomy may become necessary in cases of more severe disease [
5]. However, scientific evidence did not recommend a frequent administration of adjuvant therapy, because of its adverse events, which include the risk of carcinogenicity and teratogenicity [
5]. Moreover, the routine distribution of quadrivalent and nonavalent HPV vaccines (Gardasil
®), which can protect against HPV-6, -11, -16, -18, can help decrease the incidence of RRP and can be potentially therapeutic preventing recurrences [
11].
Although the anatomical sites infected by HPV are mainly larynx and trachea, malignant transformation in bronchi and lungs (<1% of all lung neoplasms) can rarely occur, representing the primary cause of death in this patient population [
9,
13,
14,
15,
16]. Malignancy, which can occur after surgical/chemotherapeutic therapies or after a relapse, within decades from disease onset [
17,
18,
19], is the consequence of translocation of fragments from the larynx to the lung tissue, after therapeutic manipulations [
20]. Moreover, several studies suggest the potential role of other individual (
i.e., age, immune response) and virological (
i.e., HPV-genotype, integration of the viral DNA into host genome) variables involved in the development of lesions, including those in the lower airway respiratory tract [
8,
10,
18]. However, scanty evidence on the mechanisms implicated in lung/pulmonary involvement and dysplasia in RRP is currently available [
8,
10,
18].
A systematic review was carried out in order to assess the prevalence of complications, including pulmonary involvement and lung tumor, and the mortality in RRP. Moreover, secondary aim was to assess the prevalence of HPV infection and of its genotypes to evaluate the role of viral types in the development and progression of cancerous lesions.
2. Materials and Methods
2.1. Search Strategy
The protocol of the present systematic review was recorded on PROSPERO (registration ID CRD42023426064). Articles focused on the prevalence of pulmonary disease and mortality in patients with RRP published on PubMed and Scopus databases were retrieved. The following strings were chosen for database search: “recurrent respiratory papillomatosis and lung tumor” and “pulmonary tumor and recurrent respiratory papillomatosis”. All studies published between 1st January 1997 and 31st December 2022 were evaluated. Reference lists of previously published reviews and selected studies were also assessed to include articles excluded by the search engines.
2.2. Study selection
All observational retrospective and prospective studies describing pulmonary involvement in RRP patients were included. Exclusion criteria were: articles written not in English language, commentaries, letters, reviews, case-reports or -series with fewer than 10 patients).
After removal of duplicates, records were independently screened by two authors (I.S. and B.D.). Following the assessment of titles and abstracts, full-texts were carefully evaluated and suitable articles were selected to be included in the systematic review. If no consensus could be reached, a third author (G.S.) was consulted to resolve the conflict.
2.3. Data extraction
The following study characteristics and outcomes were extracted: first author and title of the article; year of publication; year/s when the study was conducted; follow-up; study design; country/ies where the study was performed; demographics (i.e., sex and age); sample size; lung involvement or pulmonary lesions in JORPP and AORRP; mortality; lung tumor; type of lung involvement, HPV prevalence and HPV genotypes.
2.4. Study quality assessment
The inter-rater agreement for study selection and data extraction was ~100% and only a few inconsistencies were solved by consensus and support of a third investigator (G.S.). Guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) were adopted [
22], as well as the Scottish Intercollegiate Guidelines Network [
23] and the Joanna Briggs Institute Critical Appraisal tools (JBI) [
35] to assess the quality of the observational and experimental studies (
Table 1 and
Table 2), respectively.
2.5. Statistical analysis
Study characteristics were summarized with descriptive statistics: qualitative variables with absolute and relative (percentage) frequencies and quantitative variables with means and standard deviations (SD) or medians and interquartile range (IQR), respectively.
Forest plots were used to represent study variability with 95% confidence intervals (CI) for the following outcomes: prevalence of lung involvement in all samples and stratified by HPV-11 infection), lung tumor, and mortality. Heterogeneity was measured with the inconsistency indicator (I2), where an I2 value >50% indicated substantial heterogeneity. Fixed- or random-effects models were chosen keeping into consideration the expected between-study heterogeneity. Egger weighted regression test methods and Bias assessment plots were used to assess publication bias. A two-tailed p-value less than 0.05 was considered statistically significant. The statistical softwares StatsDirect version 3.1.12 (StatsDirect Ltd.) and STATA version 17 (StatsCorp, College Station, Texas, USA) were used.
3. Results
3.1. Study selection
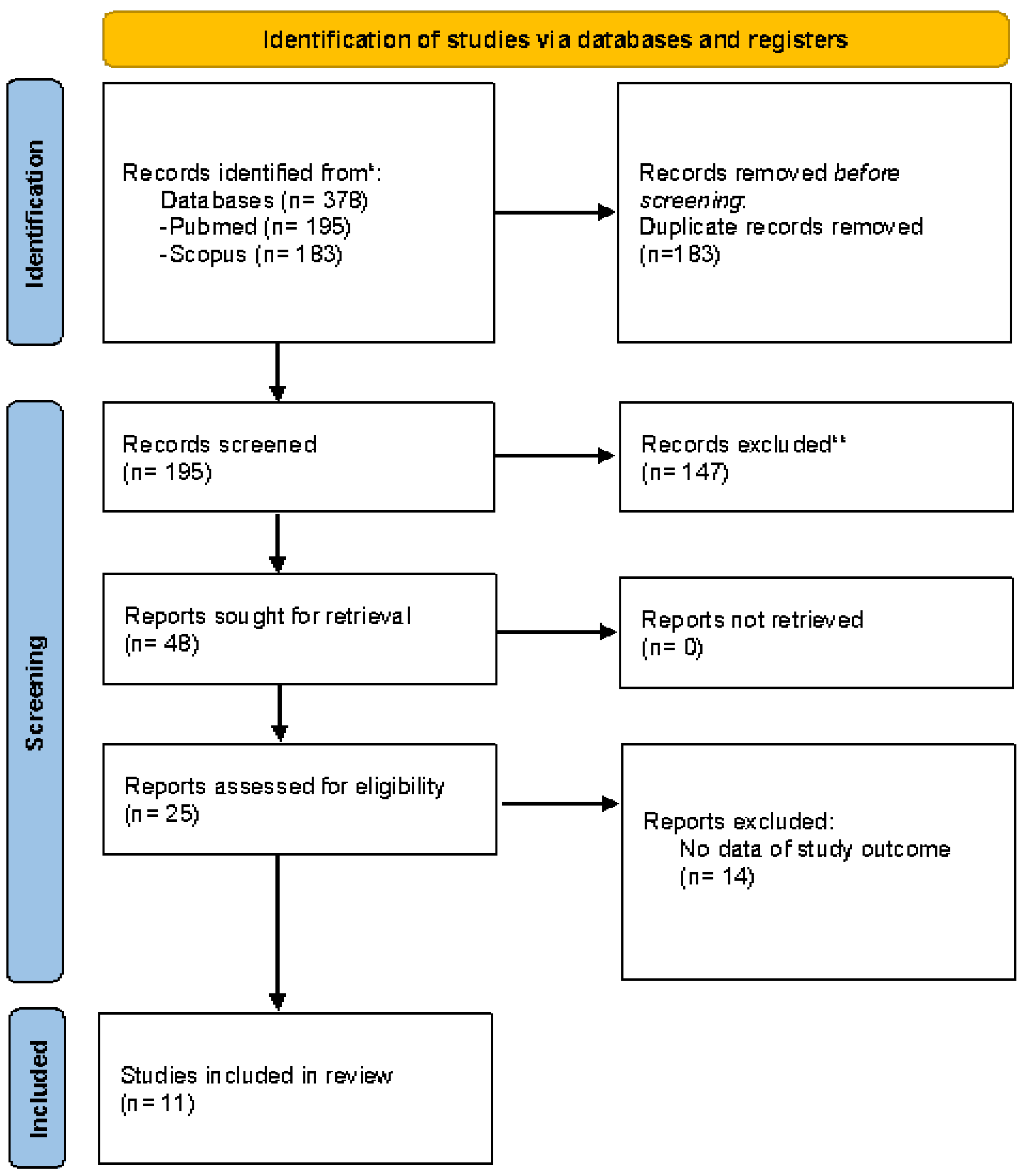
Overall, 378 records were initially found. After removal of duplicates (183; 48.4), 147 (38.8) articles were excluded as they were deemed unrelated to the primary and secondary aims. Subsequently, a total of 48 studies were screened by titles and abstracts. A total of 25 full texts were evaluated and 11 articles were selected (i.e., 44% of the records screened at the initial phase of the process). (
Figure 1).
3.2. Quality assessment
The majority of the studies (10; 90.9%) had a retrospective/observational study design. The quality assessment showed a high risk of bias in the majority of the studies (8/10, 80%) [
24,
26,
28,
29,
30,
31,
32,
33,
34], whereas only two had a moderate risk (2/10, 20%) [
25,
27] (
Table 1).
Table 1.
Checklist for cohort studies (1) according to the Scottish Intercollegiate Guidelines Network. .
Table 1.
Checklist for cohort studies (1) according to the Scottish Intercollegiate Guidelines Network. .
| Ref. |
Study |
Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
Score |
Grade of evidence (2)
|
| [24] |
Sievers C, 2021 |
No |
No |
No |
Yes |
No |
1 |
- |
| [25] |
Yang Q, 2021 |
Yes |
Yes |
No |
No |
Yes |
3 |
|
| [26] |
Karatayli-Ozgursoy S, 2016 |
Yes |
No |
No |
No |
No |
1 |
- |
| [27] |
Omland T,2014 |
Yes |
Yes |
No |
No |
Yes |
3 |
|
| [28] |
Soldatski IL,2005 |
Yes |
No |
No |
No |
Yes |
2 |
- |
| [29] |
Gerein V,2005 |
Yes |
No |
No |
Yes |
No |
2 |
- |
| [30] |
Wiatrak BJ,2004 |
Yes |
No |
No |
Yes |
No |
2 |
- |
| [31] |
Gabbott M,1997 |
Yes |
No |
No |
Yes |
No |
2 |
- |
| [33] |
Zawadzka-Głos L, 2003 |
Yes |
No |
No |
Yes |
No |
2 |
- |
| [34] |
R. Rabah, 2001 |
Yes |
No |
No |
No |
No |
1 |
- |
Only one with an “experimental-phase II” design [
32] was classified as high-risk of bias (
Table 2).
Table 2.
JBI risk of bias assessment table. Nine items per the study were evaluated and the risk of bias was calculated on the number of positive answers. Y = yes, n = no, u = unclear. The risk of bias was evaluated as high. Moderate, and low if the percentage of positive answers were respectively ≤49%, between 50% and 75%, or above 75%.
Table 2.
JBI risk of bias assessment table. Nine items per the study were evaluated and the risk of bias was calculated on the number of positive answers. Y = yes, n = no, u = unclear. The risk of bias was evaluated as high. Moderate, and low if the percentage of positive answers were respectively ≤49%, between 50% and 75%, or above 75%.
| Ref. |
First author |
Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
Q6 |
Q7 |
Q8 |
Q9 |
Overall appraisal |
| [32] |
Allen CT.; 2019 |
Yes |
Not |
Not applicable |
No |
Yes |
Yes |
Not applicable |
Yes |
Unclear |
High Risk |
3.3. Characteristics of the selected studies
Studies were published between 1997 [
31] and 2021 [
24,
25]. The recruitment period ranged from 1981 [
31] to 2020 [
24]. Study designs were mainly retrospective (7/11, 63.6%) [
25,
26,
27,
28,
31,
32,
33], followed by prospective/longitudinal (2/11, 18.1%) [
29,
30], and experimental (1/11, 9.0%) designs [
32]; Only one article did not report the design of the study (1/11, 9.0%) [
24]. A total of 8 (72.7%) studies [
25,
26,
28,
30,
31,
32,
33,
34] were monocenter, 2 (18.2%) [
27,
29] multicenter, and one (9%) [
24] did not clarify the number of clinical centers involved in the research. Studies were mainly conducted in USA (5, 45.4%) [
24,
30,
31,
32,
34], followed by China (1,9%) [
25], Norway (1,9%) [
32], Russia (1,9%) [
28], Germany (1,9%) [
29], Australia (1,9%) [
31] and Poland (1,9%) [
27] (
Table 3).
3.4. Characteristics of the study samples
The sample size ranged from 12 [
32] to 448 [
28] patients, for a total of 1,382 patients. Among the 1,278 subjects included in the analysis, a total of 950 (74.3%) [
25,
28,
30,
31,
33,
34] and 290 (22.7%) [
24,
26,
27,
32] were classified as JORRP (1-18 years) and AORRP (>18 years), respectively. Only one study did not discriminate between JORRP and AORRP, for a total of 38 patients [
29]. Information on gender was reported by 11 (100%) studies, with a total of 731 males and 535 females.
3.5. Outcomes
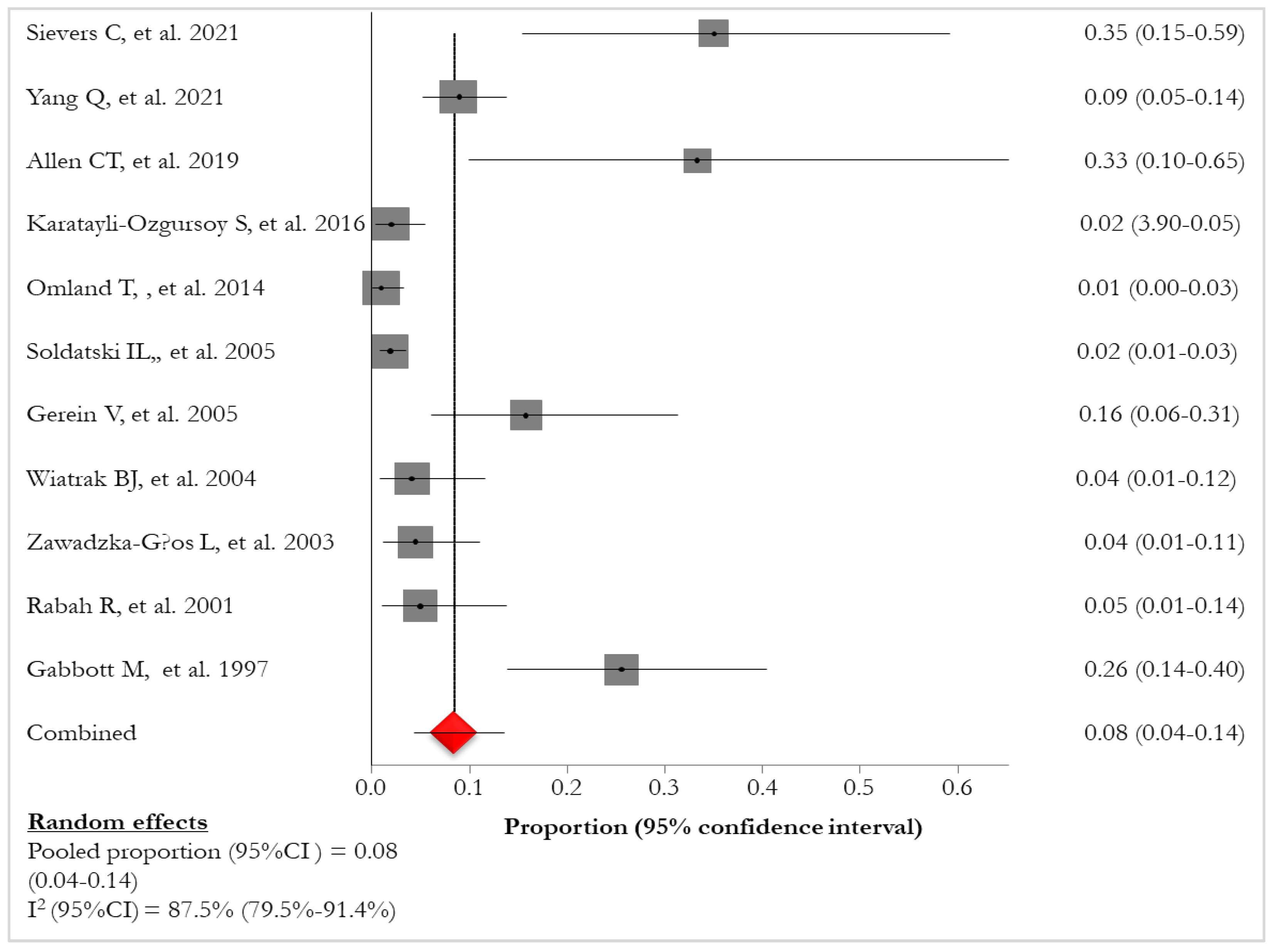
Lung involvement and/or pulmonary lesions were described with the following definitions: “lung/pulmonary involvement” [
25,
26,
31], “pulmonary spread” [
29], “pulmonary disease” [24; 33; 34], “pulmonary lesion” [
32], “pulmonary papillomatosis” [
28,
30], and “development of dysplasia” [
27]. The pooled prevalence was 8% (95% CI:4%-14%; I
2: 87.5%), ranging from 1% [
27] to 35% [
24] (
Table 4;
Figure 2).
Pooled risk difference of lung involvement between JORRP and AORRP was 5% (95% CI: -7%-18%; I
2: 85.6%) ranging from -1.1% [
32] to 4.5% [
24] (p-value: 0.41) (
Figure 3) [
24,
26,
27,
32].
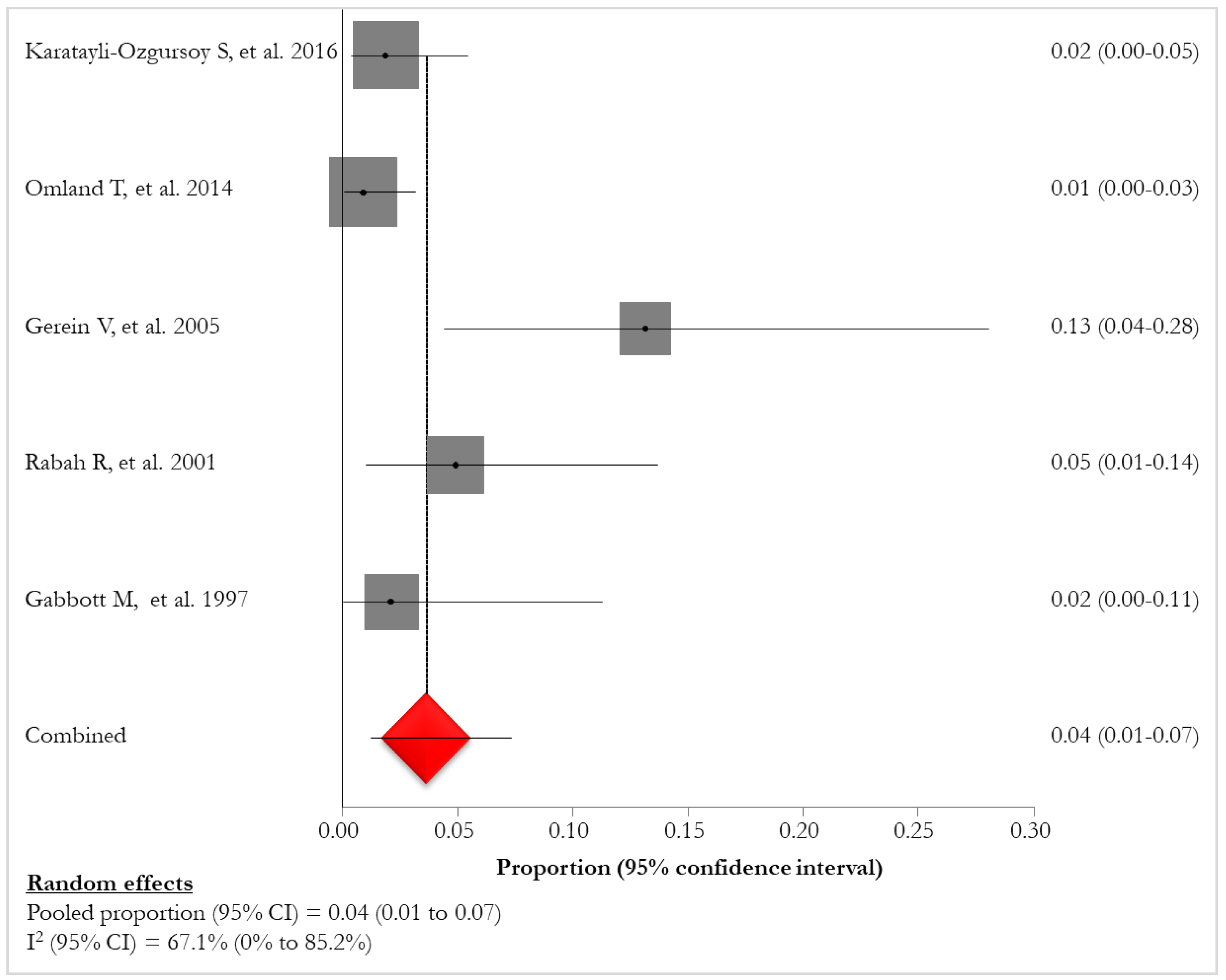
Lung tumor was reported in 5 (45.5%) studies [
26,
27,
29,
31,
34] with a pooled prevalence of 4% (95% CI:1%- 7%; I
2: 67.1%) ranging from 1% [
27] to 13% [
29] (
Figure 3); the following definitions were used to assess the prevalence of lung tumor in RRP patients: “invasive pulmonary carcinoma ex-papillomatosis” [
26], “lung carcinoma” [
27], “pulmonary malignant transformation” [
29], “pulmonary/lung squamous cell carcinoma” [
31,
34] (Tab.5, Fig. 4).
Table 5.
Prevalence of lung tumor in RRP patients [26-27, 29, 31, 34].
Table 5.
Prevalence of lung tumor in RRP patients [26-27, 29, 31, 34].
| REF |
First Author |
N° Lung tumor |
% Lung tumor |
| [26] |
Karatayli-Ozgursoy S., 2016 |
3/159 |
1.9 |
| [27] |
Omland T., 2014 |
2/224 |
0.9 |
| [29] |
Gerein V., 2005 |
5/38 |
13.1 |
| [31] |
Gabbott M., 1997 |
1/12 |
8.3 |
| [34] |
R. Rabah., 2001 |
3/61 |
4.9 |
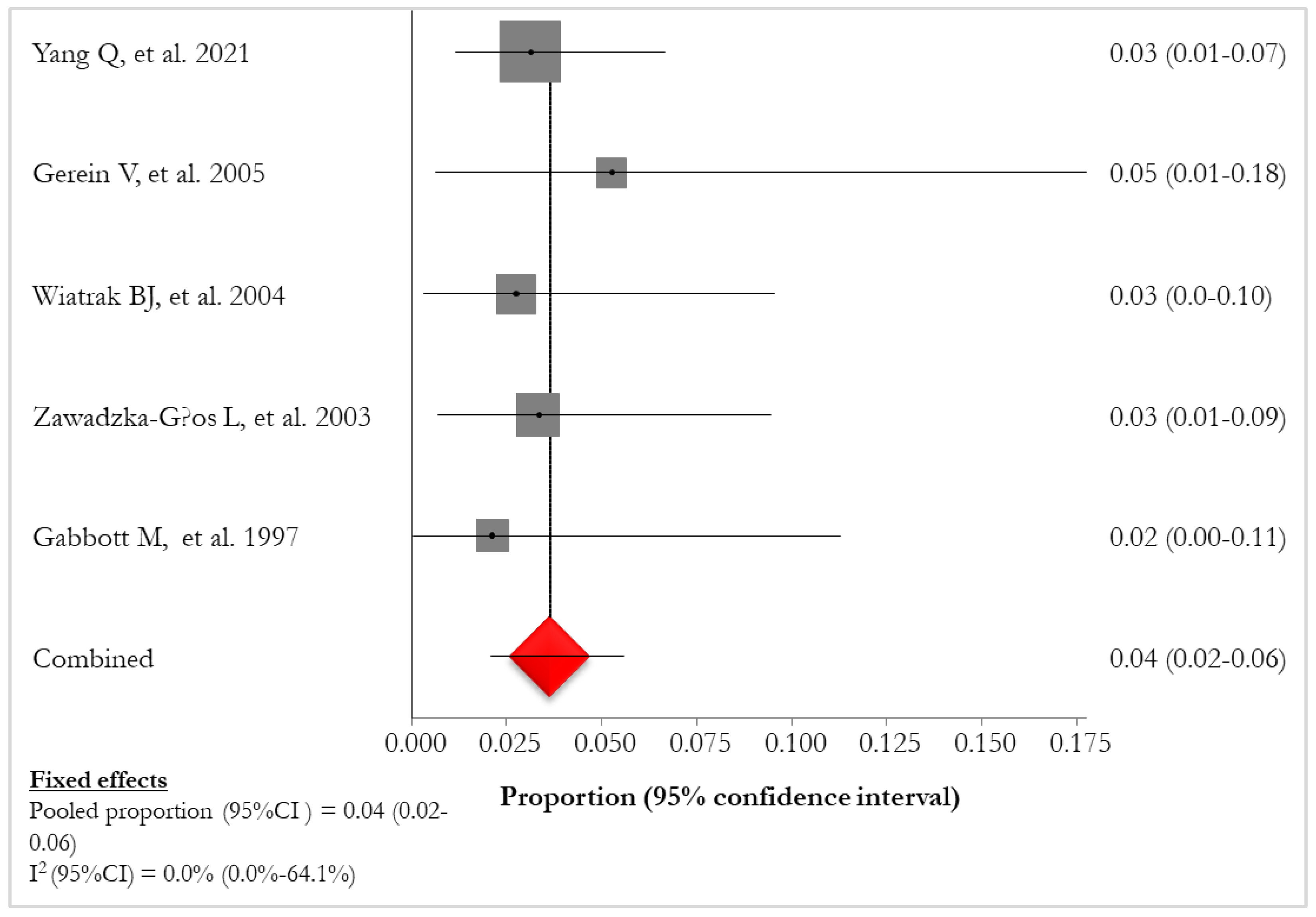
The pooled mortality in patients with lung involvement (14/42, 33,3%) [
25,
29,
30,
31,
33] was 4% (95% CI:2%-6%; I
2: 0%), ranging from 2% [
31] to 5% [
29] (
Figure 5). Mortality of lung tumor (7/17, 41,2%) was reported in 4 (36.4%) studies [
28,
29,
31,
34] (Tab. 6).
Table 6.
Frequencies and percentages of mortality in RRP patients [25, 29, 30, 31, 33].
Table 6.
Frequencies and percentages of mortality in RRP patients [25, 29, 30, 31, 33].
| REF |
First Author |
Mortality (n) |
Mortality (%) |
| [25] |
Yang Q., 2021 |
6/17 |
35.3 |
| [29] |
Gerein V., 2005 |
2/6 |
33.3 |
| [30] |
Wiatrak BJ., 2004 |
2/3 |
66.6 |
| [31] |
Gabbott M., 1997 |
1/12 |
8.3 |
| [33] |
Zawadzka-Głos L.., 2003 |
3/4 |
75.0 |
3.5.1. Prevalence of HPV-6 and -11 infections in study population
Eight (72.7%) studies [
24,
27,
28,
29,
30,
31,
32,
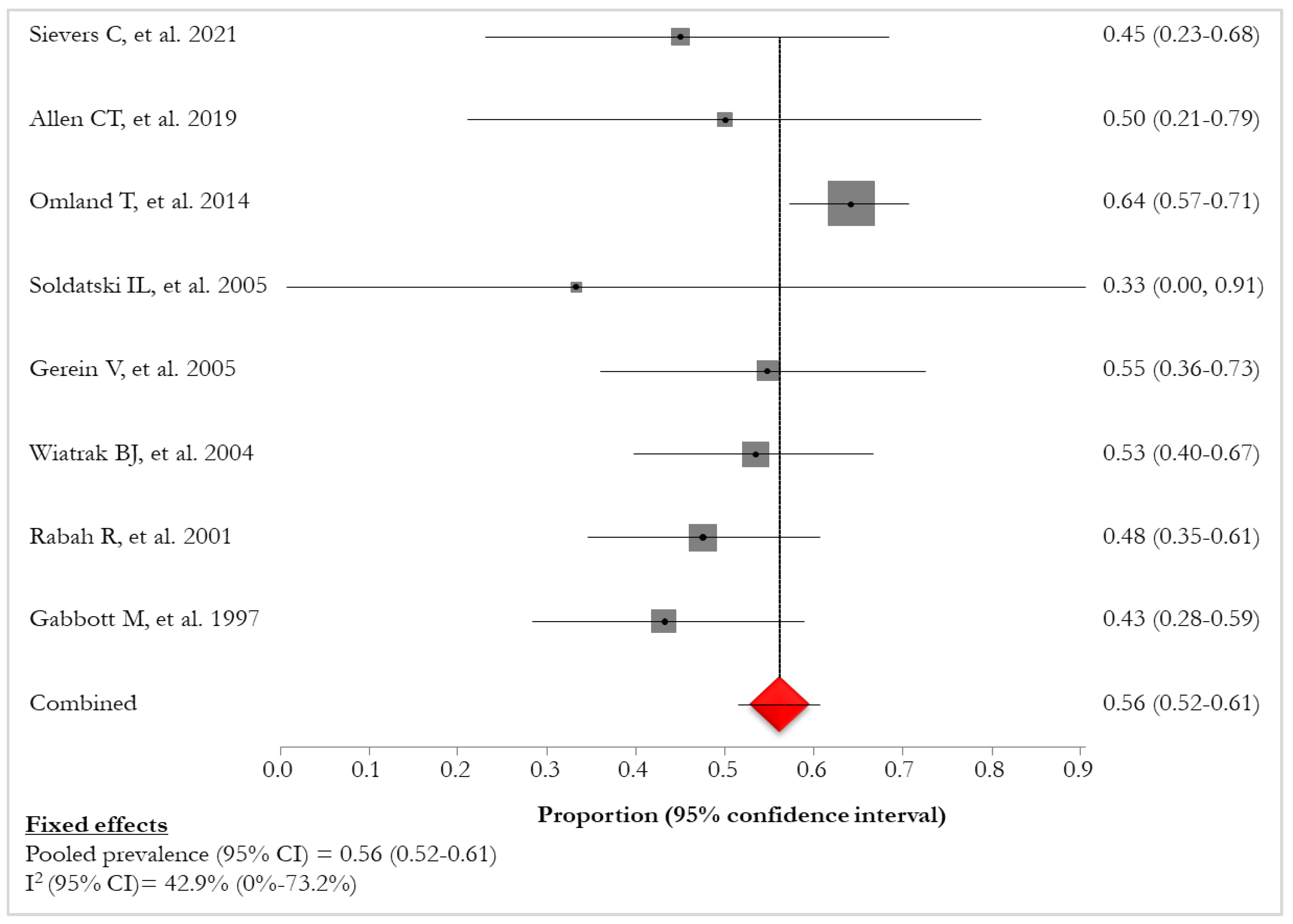
34] reported a prevalence of HPV-6 or -11 of 91% (436/480). The pooled prevalence of HPV-6 was 56% (95% CI: 52%-61%; I
2: 42.9%), ranging from 33% [
28] to 64% [
27] (
Figure 6,
Table 7).
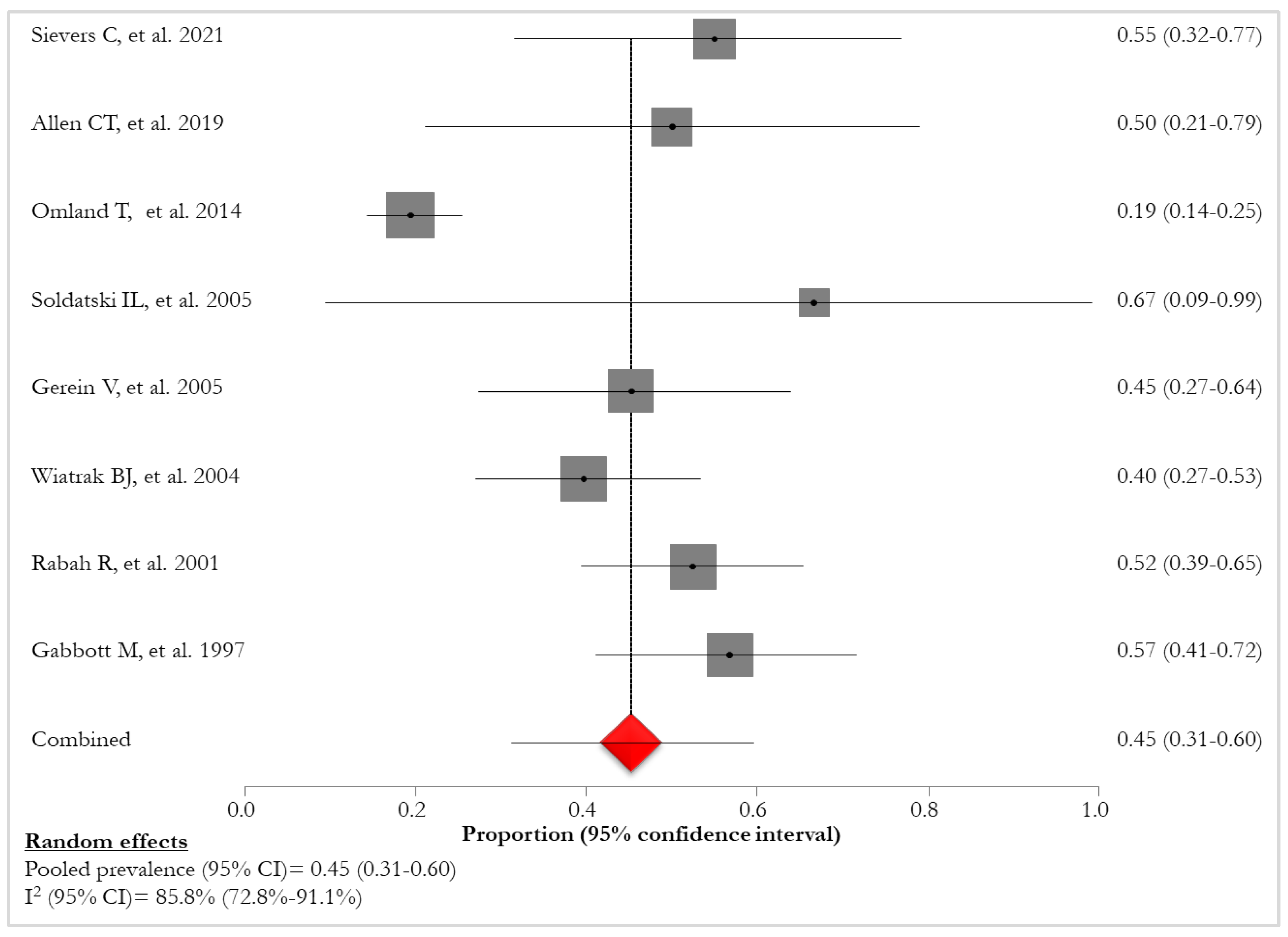
The pooled prevalence of HPV-11, reported in eight studies [
24,
27,
28,
29,
30,
31,
32,
34] was 45% (95% CI:31%-60%; I
2:85.8%), varying from 19% [
27] to 67% [
28] (
Figure 7).
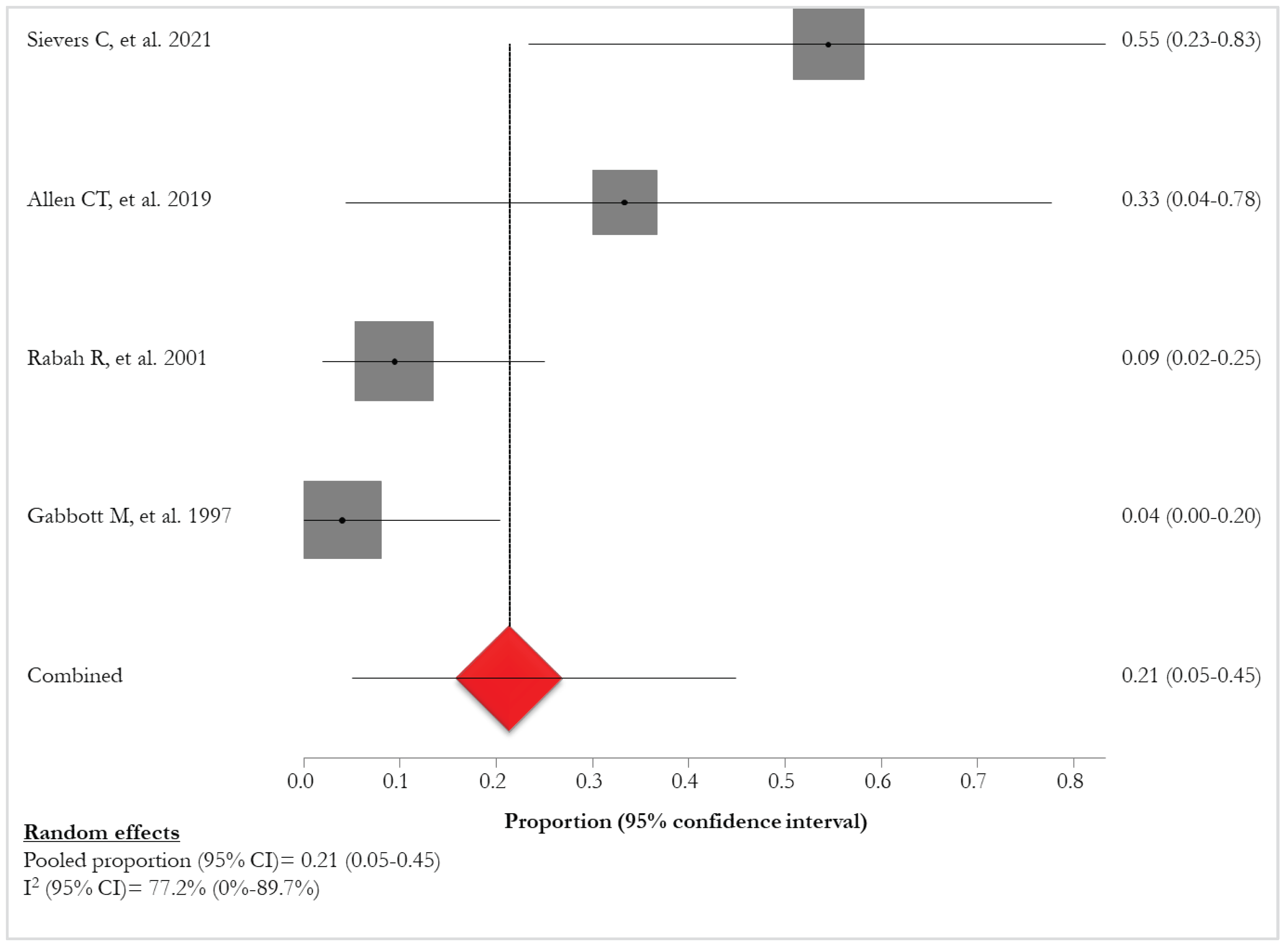
A higher prevalence of HPV-11 (4/8, 50%) [
24,
31,
32,
34] was found in patients with lung involvement in comparison with those with HPV-6 infection (2/8, 25%) [
24,
32]. The pooled proportion of HPV11-positivity of patients with lung involvement was 21% (95% CI: 5%-45%; I
2: 77.2%), ranging from 4% [
31] to 55% [
24] (
Figure 8).
4. Discussion
Pulmonary involvement in patients with RRP can be a serious and potentially severe complication. The present systematic review, despite the high in-between studies heterogeneity, mainly associated with population and study design differences, showed a prevalence of lung involvement of 8%. The estimates are partially similar to what was reported in the scientific literature. Specifically, recent reviews focusing on individuals aged less than 20 years demonstrated an estimated incidence of lung involvement equal to 3.3%, and an estimated incidence of lung tumor of 16% in the same category of patients [
18]. In agreement with our results, Pai and Colleagues (2022) reported an incidence of 8.9% of pulmonary involvement in the RRP population, with a higher risk in younger patients, in those undergoing to multiple surgical operations, in those tracheostomized, and in those with tracheal involvement [
14].
However, the variability of study-related definitions of pulmonary involvement, such as lung/pulmonary involvement, pulmonary lesions, pulmonary spread, pulmonary disease, or pulmonary papillomatosis, referring to the development and progression of pulmonary parenchymal lesions of laryngotracheal papillomatosis, could favor epidemiological bias of the estimates.
In addition, our findings highlight the absence of differences between Juvenile- and Adult-onset RRP, despite the estimated prevalence which was found to be slightly high in young patients. Notably, more studies (6/11) described lung involvement or cases of lung cancer only in JORRP, with the juvenile population size being larger than that of the adult group (950
VS. 290, respectively). Juvenile RRP patients with lung involvement may evolve in more aggressive disease, needing more frequently surgical interventions and being associated with lower treatment success rates [
18,
36]. Currently, the two most frequently prescribed drugs are Interferon and Cidofovir, although the evidence on their effectiveness in RRP patients with pulmonary involvement is poor because of the poor number of cases and the long-term follow-up needed to evaluate the recurrence rate [
18].
In agreement with previous studies, both the overall prevalence of lung tumors and mortality in JORRP and AORRP was 4% [
20,
37,
38]. The development of lung involvement, as well as further progression to cancer, could be related to several factors, such as integration of HPV in host genome and host characteristics. However, specific studies focused on the pathogenesis of complications in RRP patients were currently scanty [
39,
40] and could partially hinder the assessment of new therapeutic options.
Contrary to what was described in other HPV-related diseases, where the development of cancerous lesions is mainly driven by HR-HPV genotypes [
41,
42,
43], a significant role played by low-risk HPV genotypes is confirmed, especially for HPV 6 and HPV 11 genotypes. In fact, despite their “low risk” classification, those genotypes can trigger cellular proliferation and transformation into dysplasia and carcinoma [
44]. 8/11 studies (72.7%) detected HPV genotypes with a positivity of 91% for HPV-6 or -11: a pooled estimated prevalence of 56% and 45% were found for HPV-6 and HPV-11, respectively. Notably, considering only cases with lung involvement, the prevalence of HPV-11 infection was 21%, in agreement with previous studies, which demonstrated that infections caused by HPV-11 can cause more severe disease and an increased risk of cancer when compared with those caused by HPV 6 [
38,
45]. As highlighted in other HPV-related diseases in the recent past, in order to clarify the prognostic role of HPV genotypes and adequately estimate the prevalence of HPV infection in RRP cases, it would be the standardization of the methodology of HPV detection and typing, as well as the clarification of the mechanisms involved in the transformation of respiratory cells [
18].
Our findings underscore the need for further studies to confirm the risk of malignant transformation associated with different HPV genotypes.
Our review had several limitations, mainly related to the poor quality of the selected studies and to missing variables, which could affect the precision of the estimates. These features highlight the importance of conducting more comprehensive analysis, implying more appropriate study designs. Further longitudinal studies could provide key information on progression from RRP to pulmonary diseases, to support the development of new tools for diagnosis and treatment of infected patients.
Despite the limitations of the review, including the overall poor quality of some studies and missing variables, complexities and challenges associated with the study of pulmonary involvement in RRP should be emphasized, underscoring the need of further observational and experimental research to improve patient outcomes: studies tailored on those objectives could provide crucial insights on the progression from RRP to pulmonary diseases, favoring the identification and development of new diagnostic and treatment strategies for RRP patients.
5. Conclusions
In conclusion, lung involvement as well as progression to lung cancer in patients with RRP, represent a clinical not negligible consequence both in young and adult patients. Juvenile RRP, requiring frequent surgical interventions and being associated with low success treatment rates, is a serious threat in this patient population. The findings of the present systematic review highlight the need for more in-depth studies to elucidate the mechanisms behind the occurrence of lung involvement and explore the risk factors associated with different HPV genotypes and their interaction with host immune responses in RRP patients.
Author Contributions
Conceptualization, I.S., N.M., B.D.L. and G.S.; methodology, I.S., N.M., B.D.L. A.P. and G.S.; software, B.D.L., L.S., M.P and G.S; validation, I.S., N.M., B.D.L. A.P. and G.S.; formal analysis, B.D.L., L.S., M.P and G.; investigation, , I.S., N.M., B.D.L. and G.S.; resources, I.S., N.M., B.D.L., L.S, M.P.,I.M.; data curation, , I.S., N.M., B.D.L., L.S, M.P., A.P. and G.S.; writing—original draft preparation, , I.S., N.M., B.D.L.; writing—review and editing, , I.S., N.M., S.A, M.M. and G.S .; visualization, I.S., N.M., B.D.L., L.S., M.P, S.A., I.M, M.M, A.P. and G.S.; supervision, A.P. and G.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval were waived due to the design of the study.
Informed Consent Statement
Patient consent was waived due to the design of the study.
Data Availability Statement
The data is available in case it is requested for motivated reasons.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gillison ML, Alemany L, Snijders PJ, et al. Human papillomavirus and diseases of the upper airway: head and neck cancer and respiratory papillomatosis. Vaccine 2012; 30 Suppl 5:F34-54.
- Carifi M, Napolitano D, Morandi M, et al. Recurrent respiratory papillomatosis: current and future perspectives. Ther Clin Risk Manag 2015;11:731-8.
- Katsenos S, Becker HD. Recurrent respiratory papillomatosis: a rare chronic disease, difficult to treat, with potential to lung cancer transformation: apropos of two cases and a brief literature review. Case Rep Oncol. 2011;4(1):162-71.
- Reeves WC, Ruparelia SS, Swanson KI, et al. National registry for juvenile-onset recurrent respiratory papillomatosis. Arch Otolaryngol Head Neck Surg 2003;129(9):976-82.
- Goon P, Sonnex C, Jani P, et al. Recurrent respiratory papillomatosis: an overview of current thinking and treatment. Eur Arch Otorhinolaryngol 2008; 265(2):147-51.
- Wiatrak, BJ. Overview of recurrent respiratory papillomatosis. Curr Opin Otolaryngol Head Neck Surg 2003;11(6):433-41.
- Abramson AL, Steinberg BM, Winkler B. Laryngeal papillomatosis: clinical, histopathologic and molecular studies. Laryngoscope 1987;97(6):678-85.
- Silverberg MJ, Thorsen P, Lindeberg H, et al. Condyloma in pregnancy is strongly predictive of juvenile-onset recurrent respiratory papillomatosis. Obstet Gynecol. 2003; 101(4):645-52.
- Marchiori E, Araujo Neto Cd, et al. Laryngotracheobronchial papillomatosis: findings on computed tomography scans of the chest. J Bras Pneumol. 2008;34(12):1084-9.
- Kashima HK, Shah F, Lyles Aet al. A comparison of risk factors in juvenile-onset and adult-onset recurrent respiratory papillomatosis. Laryngoscope 1992;102(1):9-13.
- Fancello V, Melis A, Piana AF, et al. HPV Type 6 and 18 Coinfection in a Case of Adult-Onset Laryngeal Papillomatosis: Immunization with Gardasil. Case Rep Otolaryngol 2015;2015:916023.
- Ouda AM, Elsabagh AA, Elmakaty IM, et al. HPV and Recurrent Respiratory Papillomatosis: A Brief Review. Life (Basel) 2021;11(11):1279.
- Fortes HR, von Ranke FM, Escuissato DL et al. Recurrent respiratory papillomatosis: A state-of-the-art review. Respir Med 2017;126:116-121.
- Pai SI, Wasserman I, Ji YD, Gilman M, Hung YP, Faquin WC, Mino-Kenudson M, Muniappan A. Pulmonary manifestations of chronic HPV infection in patients with recurrent respiratory papillomatosis. Lancet Respir Med. 2022 Oct;10(10):997-1008. [CrossRef] [PubMed]
- Chang CH, Wang HC, Wu MT, et al. Virtual bronchoscopy for diagnosis of recurrent respiratory papillomatosis. J Formos Med Assoc 2006;105(6):508-11.
- Valdivia Padilla A, Tellez-Garcia E, Grosu H. A Case of Recurrent Respiratory Papillomatosis With Lung Involvement and Malignant Transformation. Cureus 2022;14(4):e24370.
- Gallagher TQ, Derkay CS. Recurrent respiratory papillomatosis: update 2008. Curr Opin Otolaryngol Head Neck Surg 2008;16:536–542.
- Gelinas JF, Manoukian J, Cote A. Lung involvement in juvenile onset recurrent respiratory papillomatosis: a systematic review of the literature. Int J Pediatr Otorhinolaryngol 2008;72:433–452.
- Silver RD, Rimell FL, Adams GL, et al. Diagnosis and management of pulmonary metastasis from recurrent respiratory papillomatosis. Otolaryngol Head Neck Surg 2003;129(6):622-9.
- Kramer SS, Wehunt WD, Stocker JT, Kashima H. Pulmonary manifestations of juvenile laryngotracheal papillomatosis. AJR Am J Roentgenol. 1985;144(4):687-94.
- Abramson AL, Nouri M, Mullooly V, et al. Latent Human Papillomavirus infection is comparable in the larynx and trachea. J Med Virol 2004;72(3):473-7.
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71.
- Scottish Intercollegiate Guidelines Network. SIGN 50: a guideline developer’s handbook. Edinburgh, UK: SIGN; 2014.
- Sievers C, Robbins Y, Bai K, et al. Comprehensive multiomic characterization of human papillomavirus-driven recurrent respiratory papillomatosis reveals distinct molecular subtypes. Commun Biol 2021;4(1):1416.
- Yang Q, Li Y, Ma L, et al. Long-term Outcomes of Juvenile Onset Recurrent Respiratory Papillomatosis with Pulmonary Involvement. Laryngoscope 2021;131(7):EE2277-E2283.
- Karatayli-Ozgursoy S, Bishop JA, Hillel A, et al. Risk Factors for Dysplasia in Recurrent Respiratory Papillomatosis in an Adult and Pediatric Population. Ann Otol Rhinol Laryngol 2016;125(3):235-41.
- Omland T, Lie KA, Akre H, et al. Recurrent respiratory papillomatosis: HPV genotypes and risk of high-grade laryngeal neoplasia. PLoS One 2014;9(6):e99114.
- Soldatski IL, Onufrieva EK, Steklov AM, et al Tracheal, bronchial, and pulmonary papillomatosis in children. Laryngoscope 2005;115(10):1848-54.
- Gerein V, Rastorguev E, Gerein J, et al. Incidence, age at onset, and potential reasons of malignant transformation in recurrent respiratory papillomatosis patients: 20 years experience. Otolaryngol Head Neck Surg 2005;132(3):392-4.
- Wiatrak BJ, Wiatrak DW, Broker TR, et al. Recurrent respiratory papillomatosis: a longitudinal study comparing severity associated with human papilloma viral types 6 and 11 and other risk factors in a large pediatric population. Laryngoscope 2004;114(11 Pt 2 Suppl 104):1-23.
- Gabbott M, Cossart YE, Kan A, et al. Human papillomavirus and host variables as predictors of clinical course in patients with juvenile-onset recurrent respiratory papillomatosis. J Clin Microbiol 1997;35(12):3098-103.
- Allen CT, Lee S, Norberg SM, et al. Safety and clinical activity of PD-L1 blockade in patients with aggressive recurrent respiratory papillomatosis. J Immunother Cancer 2019;7(1):119.
- Zawadzka-Głos L, Jakubowska A, Chmielik M, et al. Lower airway papillomatosis in children. Int J Pediatr Otorhinolaryngol 2003;67(10):1117-21.
- Rabah R, Lancaster WD, Thomas R, et al. Human papillomavirus-11-associated recurrent respiratory papillomatosis is more aggressive than human papillomavirus-6-associated disease. Pediatr Dev Pathol. 2001;4(1):68-72.
- Joanna Briggs Institute (JBI) Checklist for quasi-experimental studies. Available online at: https://jbi.global/sites/default/files/2019-05/JBI_Quasi-Experimental_Appraisal_Tool2017_0.pdf. Date last accessed: June 16 2023.
- Boudjemaa S, Leboulanger N, Dainese L, et al. Metastatic squamous-cell carcinoma of the lung arising in a 12-year-old boy with juvenile recurrent respiratory papillomatosis of neonatal onset. Turk Patoloji Derg 2014;30:133–13.
- Magid MS, Chen YT, Soslow R, et al. Juvenile-onset recurrent respiratory papillomatosis involving the lung: a case report and review of the literature. Pediatr Dev Pathol 1998;1:157– 63.
- Cook JR, Hill DA, Humphrey PA, et al. Squamous cell carcinoma arising in recurrent respiratory papillomatosis with pulmonary involvement: emerging common pattern of clinical features and human papillomavirus serotype association. Mod Pathol. 2000;13:914-918.
- Fleskens SA, Bergshoeff VE, Voogd AC, et al. Interobserver variability of laryngeal mucosal premalignant lesions: a histopathological evaluation. Mod Pathol. 2011;24:892-898.
- Sanchez GI, Jaramillo R, Cuello G, et al. Human papillomavirus genotype detection in recurrent respiratory papillomatosis (RRP) in Colombia. Head Neck. 2013;35:229-234.
- Bussu F, Muresu N, Crescio C, et al. Low Prevalence of HPV Related Oropharyngeal Carcinogenesis in Northern Sardinia. Cancers (Basel). 2022 Aug 30;14(17):4205. [CrossRef]
- Muresu N, Sotgiu G, Saderi L, et al. Distribution of HPV Genotypes in Patients with a Diagnosis of Anal Cancer in an Italian Region. Int J Environ Res Public Health. 2020 Jun 23;17(12):4516. [CrossRef]
- Muresu N, Sotgiu G, Saderi L, et al. Italian observational study on HPV infection, E6, and p16 expression in men with penile cancer. Virol J. 2020 Oct 22;17(1):161. [CrossRef]
- Pim D, Banks L. Interaction of viral oncoproteins with cellular target molecules: infection with high-risk vs low-risk human papillomaviruses. APMIS. 2010;118:471-493.
- Mounts P, Kashima H. Association of human papillomavirus subtype and clinical course in respiratory papillomatosis. Laryngoscope 1984;94:28-33.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).