Introduction
Since the launch of the Global Polio Eradication
Initiative (GPEI) in 1988, five of the six World Health Organization (WHO)
regions have been certified wild poliovirus free [1].
The last case of WPV2 was detected in 1999 with the global certification of
eradication in 2015 [2] and the last case of
WPV3 was detected in 2012 and WPV3 was certified eradicated in in 2019 [3]. Only type 1 wild poliovirus (WPV1) is remaining
and that too there are only two endemic countries – Afghanistan and Pakistan [4]. Until 15th July 2023, Pakistan had
reported only 1 case [5] while Afghanistan had
reported 5 cases [6] of WPV1. No cases of
WPV1 were reported from Nigeria since 2016 [7].
But there was importation in Mozambique from Pakistan and it reported 8 WPV1
cases in 2022 [8].
The GPEI Endgame Strategic Plan (2022-2026)
outlined to strengthen plans to achieve and sustain polio-free world with the
main focus to cut outbreak response time, increasing vaccine demand,
transforming campaign effectiveness, increasing access to vaccines In
inaccessible areas, and transitioning towards government ownership [9]. The oral poliovirus vaccine (OPV) contains
attenuated live poliovirus which risks mutation and reversion to virulence [10]; thus, OPV withdrawal will eliminate the risk
of circulating vaccine-derived poliovirus (cVDPV) and vaccine-associated
paralytic polio (VAPP). All OPV will be ultimately stopped and replaced with
Inactivated Poliovirus vaccine (IPV) [11]. The
type 2 component of trivalent OPV (tOPV) causes >90% VDPVs, and nearly 40%
of VAPP cases [12]. The
first milestone of the Endgame Strategic Plan was a globally synchronized
switch from tOPV to bivalent OPV (bOPV containing types 1 and 3 Sabin
poliovirus), which was implemented in April 2016, with the withdrawal of type 2
component from the immunization program [13].
To mitigate the risk of type 2 immunity, IPV was gradually introduced in
routine immunization (RI) at or after 14 weeks of age in all OPV-using
countries [13]. IPV, unlike OPV, does not
replicate in the gut, therefore it induces lower levels of intestinal immunity.
However, most importantly, IPV given to children primed with OPV is known to
provide a good boosting effect in immunity to all poliovirus serotypes [14,15]. Thus, adding IPV to bOPV schedule could
supplement the immunity for types 1 and 3, while providing a good type 2
baseline immunity to guard against cVDPV2. Since WPV3 has also been eradicated,
it prompts the possibility to remove type 3 component in bOPV in the future.
This could prompt a switch from bOPV to mOPV1— on a similar rationale as the
switch from tOPV to bOPV. This will reduce the burden of cVDPVs and VAPPs
emerging from type 3 vaccine virus as well. Even if the program does not seem
to have an appetite for another switch, higher mOPV1 immunogenicity may justify
its use in one form or the other to stop or minimize WPV1 or VDPV1.
India, a highly populated country, contributed to
over 60% of all global polio cases until 2009. The country was certified polio
free in 2014, since the last case was reported in 2011 [16]. However, India’s neighboring countries,
Afghanistan and Pakistan, continue to report circulation of WPV1 [17,18]. Therefore, it is essential to maintain very
high population immunity through continued polio vaccination in India, that
will reduce the risk of cVDPVs as well.
The EPI schedule in India includes 4 doses of bOPV
at birth, 6, 10 and 14 weeks combined with IPV. IPV introduction in India moved
through serial changes, first one full dose at 14 weeks, then two fractional
doses at 6 and 14 weeks and with the latest revision, three fractional doses of
IPV at 6, 14 weeks and 9 months.
This study in India provides immunological data of
bOPV-IPV and its substitution with mOPV1-IPV in RI and could support moving
forward in the planned steps of the Endgame Strategy subsequent to the
tOPV-bOPV switch. The primary objective of this study was to compare
immunogenicity against poliovirus type 1 by bOPV and mOPV1 in the routine EPI
schedule along with a dose of IPV at week 14 contact, and assessing the
immunity provided by three doses of standalone IPV in the EPI schedule of
India. The secondary objectives of the study included: (1) assessment of gains
in immunity for poliovirus types 2 and 3 with one dose of IPV at
DTP3 contact, (2) seroconversion for poliovirus types 2 and 3 with 2 doses of
IPV added to mOPV1 schedule, (3) seroconversion or gains in titre of poliovirus
neutralizing antibodies with a booster dose of IPV at 18 weeks, and (4)
interference and immunogenicity of pentavalent antigens (DTP-HepB-Hib) when
administered concomitantly with bOPV, mOPV1 and IPV in EPI schedule.
Methods
This was a three-arm, multi-center, open label, and
randomized controlled trial between May 2016 to January 2017 at three medical
institutions in India. The study protocol followed good
clinical practice standards; ethical clearance was obtained from the WHO
Ethical Review Committee at Geneva and by the Institutional Review Board (IRB)
of the participating institutions in India. The trial was approved for
implementation by the Drugs Controller General-India (DCGI) and registered with
the Clinical Trial Registry of India, with the number CTRI/2016/04/006826.
Enrolment of study Participants
The parents or legally acceptable
representative (LAR) of the study participants underwent an informed consent process
including pre-natal consent for cord blood collection as well as a written
consent for study enrolment if the eligibility criteria were met. Eligibility
criteria included a full-term pregnancy, ≥2.5kg
birth weight, ≥9 APGAR score at 5 minutes, and residence at an accessible
distance from the hospital (≤30 km). A difficult labour
or postpartum complication, a suspected medical condition or congenital defect,
immunodeficiency, thrombocytopenia led to the exclusion from the study. The
participants were enrolled in the study from birth till 22 weeks. Only study
participants, presenting a complete set of data as per the protocol analysis
were included in the final analysis.
Study Design
Infants born in the selected medical institutions
were enrolled within 24 hours of birth into one of the study arms: A
(bOPV+IPV), B (mOPV+IPV) or C (IPV only). The study design is summarized in
Table 1. Children assigned to the EPI schedule
(Arm A) received doses of bOPV at birth, 6, 10, and 14 weeks. Study
participants assigned to Arm B received mOPV1 at birth, 6, 10 and 14 weeks.
Children in Arm C received three doses of IPV at 6, 10, and 14 weeks. In
addition, children in all arms also received a dose of IPV at 18 weeks. All
study arms were administered with pentavalent vaccine (DTP-HepB-Hib) at 6, 10
and 14 weeks in addition to the study-specific polio vaccines.
The infants were randomly allocated to the study arms using a centrally labeled, stratified, computer-generated, permuted block randomization (with blocks of sizes 2, 4 or 6) allocated equally to all three arms. The investigator assigned the study arm allocation as per the randomization allocation envelope prepared by the statistician from the sponsor who was not involved in the study. The random sequence was generated using SAS 9.4 [
19]. Neither the parent nor the investigator had any discretion to opt for a particular study arm. Immediately after birth, under sterile conditions, 3.0 ml. cord blood was collected from the placental side of the umbilical cord for regular tests and potentially for testing polio antibodies. After informed consent from parents and confirming eligibility criteria of the infant, the infant was vaccinated by a trained nurse as per the study arm. The study participants attended the follow-up visits at 6, 10, 14 and 22 weeks to undergo the outlined vaccination/blood withdrawal program. The adverse and severe adverse events were recorded and presented in all three arms.
Sample Processing and Analysis-Serum specimens from the study participants were stored in a deep freezer (≤20ºC) and were transported in cold-chain to the laboratory for analysis at the ICMR-National Institute of Virology (NIV), Mumbai or Pune Unit, Maharashtra, India. The serum specimens were tested using the standard microneutralization assay [
20] for detection of neutralizing antibodies against all three types. Poliovirus types 1 and 3 microneutralization assay was performed at NIV Mumbai unit, type 2 microneutralization was done in NIV, Pune, India. In addition, serum specimens were also tested for antibodies to components of pentavalent vaccine at Panacea Biotec Ltd. Drug discovery research (DDR) laboratory at Mohali.
Seropositivity was defined as the presence of neutralizing antibodies which is a reciprocal titer of ≥ 8. For children with detectable antibodies, seroconversion was defined as the four-fold increase over the expected decline of maternally-derived antibodies (half-life assumed to be 28 days) at that time-point [
21]. If there was a change in the antibody titer, from non-detectable (reciprocal titer < 8) to detectable titer (≥ 8), it was also considered as seroconversion. The analysis was performed separately for serotypes 1, 2 and 3.
Administered Vaccines
The bOPV and IPV vaccines used in this trial were from market lots of vaccines manufactured at Panacea Biotec Ltd. and Sanofi Pasteur Pvt Ltd., India, respectively. The mOPV1 vaccines were specifically manufactured by Panacea Biotec Ltd. (Delhi, India) for the purpose of this trial. Bivalent type 1 + 3 oral poliovirus vaccine (bOPV) contained at least 106.0 CCID50 of Sabin poliovirus type 1 and at least 105.8 CCID50 of Sabin poliovirus type 3 and Monovalent oral poliovirus vaccine type 1 (mOPV1) contained at least 106.0 CCID50 of Sabin-strain poliovirus type 1 Inactivated poliovirus vaccine (IPV) formulated to contain 40-8-32 D-antigen potency. The conventional dose of bOPV and mOPV1 is 2 drops orally and 0.5 ml of IPV is given intramuscularly.
Statistical Analysis
A WHO collaborative study conducted during 2013-14 in India has shown 99% seroconversion for poliovirus type 1 in the group that received bOPV with IPV at 14 weeks [
15]. Assuming cumulative seroconversion of 99%, with an equivalence margin of 5%, power of 99%, alpha level of 1%, the sample size was calculated to be 172 in each arm. Considering 20% dropouts and attrition due to some other reasons, it was decided to study 200 children in each arm, making a total of 600 children across all three study sites.
The difference in percentage points of the cumulative seroconversion with 95% confidence limits for the difference was obtained for poliovirus types 1 only. For equivalence in cumulative seroconversion to be shown, the 95% confidence interval of the difference was supposed to include 0. Per-protocol analysis was used for the analysis of the outcomes. Children whose antibody titers were above or equal to 1448 at the baseline, were excluded.
Distribution of demographic variables and titers at baseline were summarized in all three arms by providing the frequencies with percentages for categorical demographic variables, while titers were presented using median with bootstrap18 95% CI obtained from 10.000 bootstrap samples for all three serotypes. The comparison of seroconversion across the arms was assessed using Chi-square test and the comparison of doses across the arms was made using Cochrane-Armitage test. P value < 0.05 was considered to be statistically significant. R 3.4.3 and SAS 9.4 were used for analyzing the data.
Results
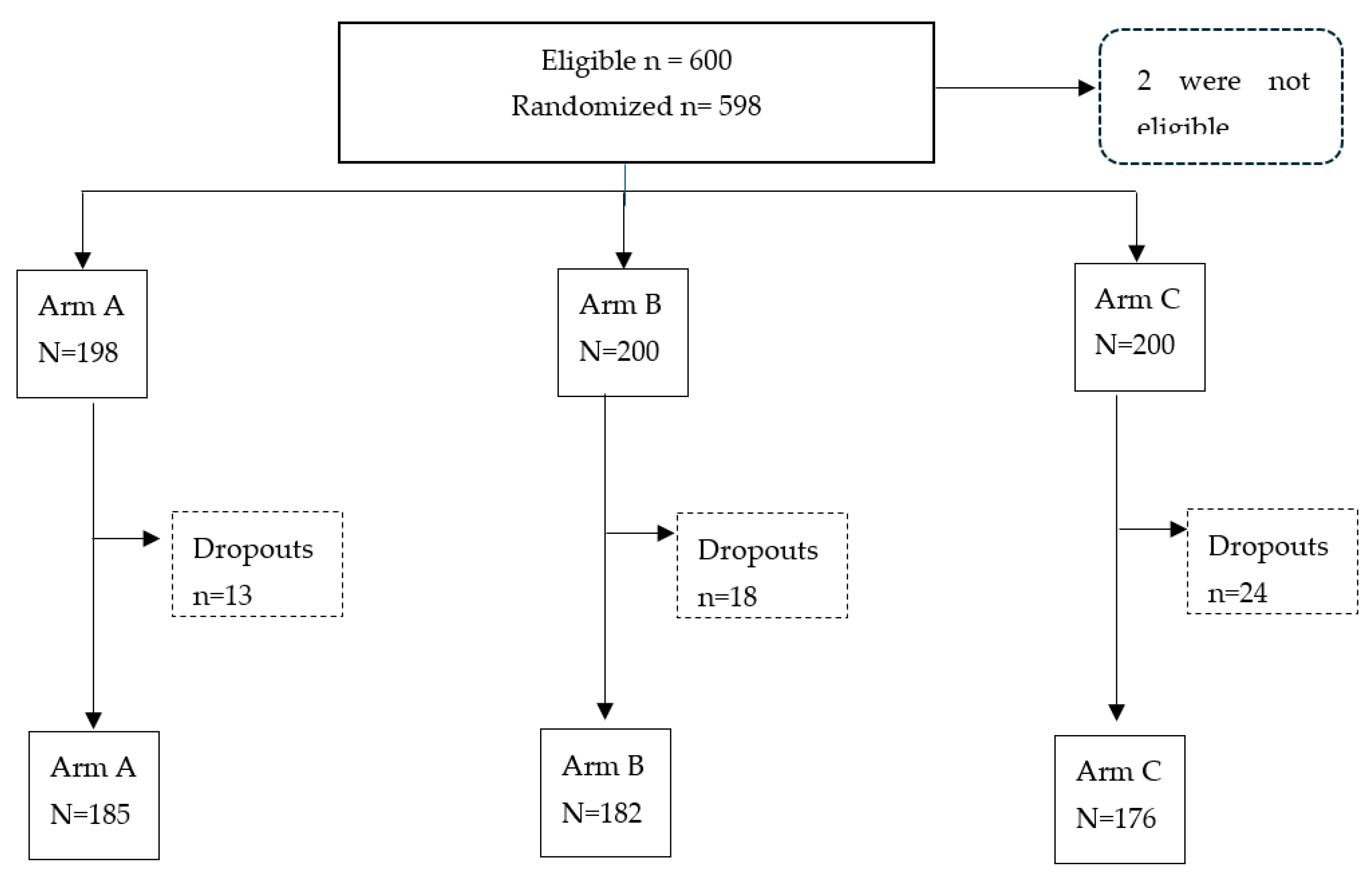
A total of 600 participants were enrolled, as shown in the consort flowchart (
Figure 1). In two cases cord blood could not be collected or the amount was insufficient for testing. Therefore, 598 study participants were subjected to randomization. The randomization resulted in 185 participants in Arm A, 182 in Arm B and 176 in Arm C after an 8.7% drop-out rate.
Baseline Characteristics
The baseline characteristics of the study participants are provided in
Table 2. The socio-demographic variables, like religion and father’s educational level, were comparable across the three arms. There were slightly more female children in Arm A and a similar proportion in the other two arms. The baseline seroprevalence levels and median titers were not significantly different across the study arms in all three serotypes.
Seroconversion
Seroconversion with 95% confidence limits, expressed as percentage, are presented in
Table 3. The cumulative seroconversion rates at 14 weeks in Arm A and B (having received 3 doses of bOPV and mOPV1, respectively) were 96.8% (93.1-98.5) and 98.4% (95-3-99.4) for type 1 poliovirus (PV) (p=0.323). At 18 weeks, after 4 doses of respective vaccine and 1 IPV dose, seroconversion was 99.5% (97.0-99.9) and 100% (97.9-100) for Arm A and B respectively. The percentage point difference in seroconversion between Arms A and B (Arm B – Arm A) for serotype 1 was 0.5% (-0.51-1.5), indicating that Arm B was equivalent to Arm A with an equivalence margin of 5%. For type 3 PV, seroconversion rates at 18 weeks were 100% (98-0.100.0) in Arm A and 81.9% (75.6-86.8) in Arm B (p<0.001).
When the infants received one dose of IPV at week 14 contact, the type 2 seroconversion increased in all three arms: Arm A: 21.6% (16.3-28.1) at 14 weeks (3bOPV) to 80.0% (73.7-85.1) (4bOPV+IPV) at 18 (p value <0.001); Arm B: 22.0% (16.6-28.5) at 14 weeks (3mOPV1) to 76.9 (70.3-82.4) (4mOPV1+IPV) at 18 weeks (p value <0.001); Arm C: 69.3% (62.1-75.6) (2IPV) to 93.2% (88.5-96.1) (3IPV) for serotype 2 (p value < 0.001).
The IPV stand-alone schedule (Arm C) at 14 weeks, having received two doses of IPV, showed 84.7% (78.6-89.4) seroconversion against type 1 poliovirus. At 18 weeks, this seroconversion rate significantly increased to 96.0% (92.0-98.1) with an additional dose of IPV (p<0.01). Type 2 seroconversion also increased significantly from 14 to 18 weeks [69.3% (62.1-75.6) vs 93.2% (88.5-96.1], [p<0.001). Finally, seroconversion against type 3 poliovirus was already high at 14 weeks with 94.3% (89.9-96.9); the third dose boosted seroconversion even further to 99.4% (96.9-99.9) (p value = 0.01). At 22 weeks, the only IPV schedule (Arm C) received four doses of IPV. The seroconversion rates for the three serotypes with four doses of IPV at week 22 was 97.2% (93.5-98.9), 96.6% (92.8-98.4) and 100% (97.9-100), for serotypes 1, 2 and 3 respectively. The seroconversion rates at 18 weeks were not statistically significant in any of the three serotypes compared to week 22 (p value = 0.771, 0.226, 1.000 respectively).
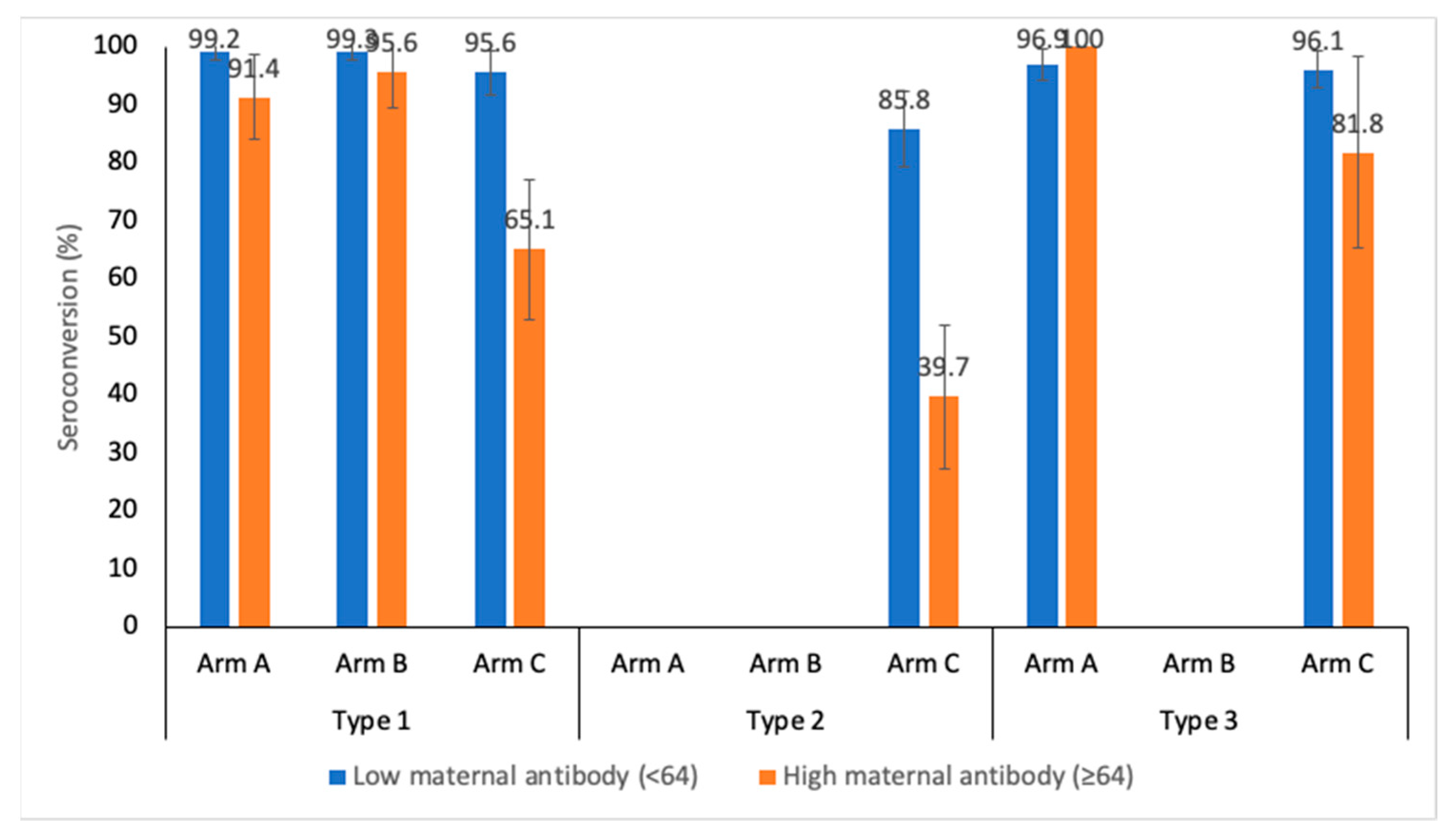
The immunogenic response against the level of maternal antibodies (baseline titers) was analyzed for bOPV, mOPV1 and IPV arms at week 14. The maternal antibody levels were categorized to low (<64) and high (≥64).
Figure 2 shows the seroconversion for the three PV serotypes stratified by the level of maternal antibodies. Serotype 1 seroconversion at week 14 in Arm A, after 3 doses of bOPV, is 99.2% (95.7-99.9) and 91.4% (81.4-96.3) for low and high maternal antibody levels, respectively (p value= 0.006). Serotype seroconversion after administering 3 doses of mOPV1 in Arm A is 99.3% (96.0-99.9) and 95.6% (85.2-98.8) for low and high maternal antibody levels, respectively (P value = 0.088). In the IPV stand-alone arm, the serotype 1 seroconversion rates were 95.6% (90.1-98.1) and 65.1% (52.8-75.7) (p value< 0.001) in low and high maternal antibody strata, respectively. The type 2 component was present only in Arm C (IPV schedule). The seroconversion against type 2 poliovirus was found to be 85.8% (78.2-91.1) and 39.7% (28.5-52.0) for low and high maternal antibodies, respectively (p value < 0.001). For type 3 PV, the seroconversion rates were 96.9% (92.9-98.7) and 100% (7.4-20.2) (p value = 0.382) in the bOPV schedule (Arm A); the IPV schedule (Arm C) had seroconversion rates of 96.1% (91.8-98.2) and 81.8% (61.5-92.7) (P value = 0.007).
Immune response of concomitant EasyFive TT
The immune responses of these component antigens were tested by ELISA. The antibody threshold was ≥0.1 IU/ml, ≥40 IU/ml, ≥0.1 IU/ml. ≥0.15 IU/ml and ≥40 IU/ml for Diphtheria, Pertussis, Tetanus, Hemophilus Influenza type b, and Hepatitis B respectively. The sero-protection for all components of the Easyfive-TT at 18 weeks was 93.3% (90.7-95.2), 58.8% (54.7-62.9), 99.5% (98.3-99.9), 99.5% (98.3-99.9) and 97.8% (93.7-99.5) for Diphtheria, Pertussis, Tetanus, Hemophilus Influenza type b, and Hepatitis B respectively. The post-vaccination Geometric Mean Titers (GMT) was higher than pre-vaccination for all antigens as shown in the
Table 5.
Discussion
There is enough evidence from the literature that bOPV or mOPV1 are more immunogenic than tOPV [
22] for the respective serotypes. mOPV1 has been found to be more immunogenic than bOPV for type 1 in some studies but this difference was not statistically significant in other studies [
23]. Our trial also demonstrated that 3 doses of mOPV1 were similar to 3 doses of bOPV against poliovirus type 1. Moreover, the median antibody titres for serotype 1 were equivalent for both bOPV and mOPV1 recipients [724 (574-818) vs 724 (724-724), p=0.203] thereby demonstrating that mOPV1 is just as efficacious as bOPV in conferring type 1 poliovirus immunity, as depicted in another study [
23].
After three vaccine doses, IPV has a 96% seroconversion rate compared to 98.4% for mOPV1 and 96.8% for bOPV for serotype 1. This shows a comparable effect of all three vaccines in conferring type 1 immunity at the same number of doses. Type 1 and 3 median antibody titres increased to 1149 and 724, respectively, in bOPV recipients. Type 1 seroconversion is raised to 100% and median antibody titres to a maximum of 1149 in mOPV1 recipients.
Addition of one dose of IPV to bOPV (Arm A) and mOPV1 (Arm B) schedules provides 60-70% seroconversion to type 2, which increases to 97% with addition of the second dose. IPV also closes immunity gap against types 1 and 3 as demonstrated in some previous studies [
24,
25].
Immunogenicity of IPV schedule
3-dose IPV schedule at -6, -10 and -14 weeks provided high immunogenicity of ≥90% against all serotypes. Similar findings were reported in trials from Cuba and Philippines with 3 doses of IPV administered at 6, 10 and 14 weeks of age of a child – Cuba trial: minimum of 95% seroconversion rate against types 1 and 3; Philippines trial: 99% seroconversion rates against all three types [
26]. Addition of another dose of IPV closed immunity gaps further for all three serotypes. Moreover, at the close of the study, IPV recipients demonstrate high levels of median antibody titres in all PV serotypes (PV1: 228 (181-269), PV2: 114 (91-144) and PV3: 362 (287-410)).
The timing of vaccine administration is important due to the interference with maternal antibodies. High levels of maternal antibodies (baseline titres >64) versus low levels show an especially significant detrimental effect on seroconversion rates in the IPV study arm (95.6% vs 65.1% for type 1, 85.8% vs 39.7% for type 2 and 96.1% and 81.8%). This supports the rationale to inoculate a child with IPV the latest possible after birth to account for maternal antibody waning. In fact, various studies have shown that a delayed administration in a 2-, 4- and 6-month schedule is more immunogenic than a 6-, 10- and 14-weeks schedule [
27,
28].
Concomitant vaccines
Administration of concomitant vaccines did not interfere with OPV/IPV immunogenicity in this trial.
From our trial it is confirmed that bOPV and mOPV1 are highly immunogenic against the respective serotypes. Addition of IPV fills the immunity gap well for the remaining types and boosts the OPV effects. IPV only schedule in early age should be a 3-dose or 4-dose schedule and the same could apply to the combination vaccine like wP-Hexavalent schedule.
Since WPV3 is globally eradicated and WPV1 continuing, replacing bOPV with mOPV1 could be one of the options for the program. The results from this study provide a basis for the future of the GPEI strategy regarding withdrawal of all oral poliovirus vaccines and the instatement of IPV or combination vaccines in routine vaccination.
Limitations
The major limitation of the present study is that unexpected type 2 seroconversion was observed in Arms A and B at 14 weeks when no type 2 containing vaccine was received by the participants till that time. Some inadvertent exposure of type 2-containing poliovirus vaccines or undetected type 2 vaccine-derived poliovirus cannot be ruled out, but both seem very unlikely. There was very limited availability of IPV in India during the study implementation phase and absence of detection of poliovirus type 2 from case and environmental surveillance is least likely in presence of sensitive surveillance in the country. We tried to investigate the reasons for unexplained type 2 seroconversion, no specific reasons could be elucidated.
Additionally, type 2 neutralization had to be conducted in different facility, however, the testing protocols were similar for all 3 serotypes.
Conclusions
This study allows to build further confidence in the steps required in the polio end game subsequent to the tOPV-bOPV switch. This study shows high efficacy in all proposed vaccine schedules to evoke high seroconversion rates and median antibody titres. Thus, each arm can be observed as the next phases of the polio eradication endgame: bOPV+IPV in the post switch era, mOPV1+IPV for WPV1 eradication and IPV-alone after certification of WPV eradication. VDPVs could be dealt with novel oral poliovirus vaccines with or without IPV for outbreak response.
Funding
The study was funded by WHO grant from Rotary Research Award 63948 and 65163
Acknowledgments
We acknowledge the very close support and guidance of World Health Organization (HQ, Geneva, SEAR and Country Office, India) in the study. We deeply appreciate the contribution of the laboratory staff at the Enterovirus Research Center (ERC Mumbai, now NIV Mumbai Unit), especially Uma Nalavade, Deepa Sharma and Sneha Rane. We also thank the NPSP-WHO India staff who supported the study. We are grateful to all research staff at the three study institutes for meticulously conducting the study. We deeply appreciate the staff from Panacea Biotec. Ltd. for their support in regulatory submission, field implementation, data management and analysis; especially Sachin Panwar and Manoj Jangir.
References
- World Health Organization. Global Polio Eradication Initiative. Semiannual report on the progress against the Polio Eradication and Endgame Strategic Plan, Geneva, Switzerland. [Internet]. 2017 [cited 2019 Jan 23]. Available from: http://polioeradication.org/wp-content/uploads/2017/12/WHO-Polio-Donor-Report-january-june-2017-web-30112017.pdf.
- GPEI-Global eradication of wild poliovirus type 2 declared [Internet]. [cited 2019 Jan 23]. Available from: http://polioeradication.org/news-post/global-eradication-of-wild-poliovirus-type-2-declared/.
- Kew, O.M.; Cochi, S.L.; Jafari, H.S.; Wassilak, S.G.F.; Mast, E.E.; Diop, O.M.; Tangermann, R.H.; Armstrong, G.L. ; Centers for Disease Control and Prevention (CDC) Possible eradication of wild poliovirus type 3--worldwide, 2012. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 1031–1033. [Google Scholar] [PubMed]
- GPEI-Endemic Countries [Internet]. [cited 2019 Jul 22]. Available from: http://polioeradication.org/where-we-work/polio-endemic-countries/.
- GPEI-Pakistan [Internet]. [cited 2023 Jul 26]. Available from: https://polioeradication.org/where-we-work/pakistan/.
- GPEI-Afghanistan [Internet]. [cited 2023 Jul 26]. Available from: https://polioeradication.org/where-we-work/afghanistan/.
- Ekwebelem, O.C.; Nnorom-Dike, O.V.; Aborode, A.T.; Ekwebelem, N.C.; Aleke, J.C.; Ofielu, E.S. Eradication of wild poliovirus in Nigeria: Lessons learnt. Public Health Pract. 2021, 2, 100144. [Google Scholar] [CrossRef]
- GPEI-Mozambique [Internet]. [cited 2023 Jul 17]. Available from: https://polioeradication.org/where-we-work/mozambique/.
- GPEI-GPEI Strategy 2022-2026 [Internet]. [cited 2023 Jul 17]. Available from: https://polioeradication.org/gpei-strategy-2022-2026/.
- Macklin, G.R.; O’Reilly, K.M.; Grassly, N.C.; Edmunds, W.J.; Mach, O.; Santhana Gopala Krishnan, R.; Voorman, A.; Vertefeuille, J.F.; Abdelwahab, J.; Gumede, N.; et al. Evolving epidemiology of poliovirus serotype 2 following withdrawal of the serotype 2 oral poliovirus vaccine. Science 2020, 368, 401–405. [Google Scholar] [CrossRef] [PubMed]
- GPEI-Polio Eradication and Endgame Strategic Plan 2013–2018 [Internet]. [cited 2023 Jul 17]. Available from: https://polioeradication.org/who-we-are/strategic-plan-2013-2018/.
- Garon, J.; Seib, K.; Orenstein, W.A.; Ramirez Gonzalez, A.; Chang Blanc, D.; Zaffran, M.; Patel, M. Polio endgame: the global switch from tOPV to bOPV. Expert Rev. Vaccines 2016, 15, 693–708. [Google Scholar] [CrossRef] [PubMed]
- GPEI-Global synchronisation and the switch [Internet]. [cited 2019 Jan 23]. Available from: http://polioeradication.org/news-post/global-synchronisation-and-the-switch/.
- Estívariz, C.F.; Jafari, H.; Sutter, R.W.; John, T.J.; Jain, V.; Agarwal, A.; Verma, H.; Pallansch, M.A.; Singh, A.P.; Guirguis, S.; et al. Immunogenicity of supplemental doses of poliovirus vaccine for children aged 6-9 months in Moradabad, India: a community-based, randomised controlled trial. Lancet Infect. Dis. 2012, 12, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Sutter, R.W.; Bahl, S.; Deshpande, J.M.; Verma, H.; Ahmad, M.; Venugopal, P.; Rao, J.V.; Agarkhedkar, S.; Lalwani, S.K.; Kunwar, A.; et al. Immunogenicity of a new routine vaccination schedule for global poliomyelitis prevention: an open-label, randomised controlled trial. Lancet 2015, 386, 2413–2421. [Google Scholar] [CrossRef] [PubMed]
- John, T.J.; Vashishtha, V.M. Eradicating poliomyelitis: India’s journey from hyperendemic to polio-free status. Indian J. Med. Res. 2013, 137, 881–894. [Google Scholar] [PubMed]
- GPEI-Afghanistan [Internet]. [cited 2019 Feb 8]. Available from: http://polioeradication.org/where-we-work/afghanistan/.
- GPEI-Pakistan [Internet]. [cited 2019 Feb 8]. Available from: http://polioeradication.org/where-we-work/pakistan/.
- SAS Institute Inc. 2014. SAS 9.4 Language Reference: Concepts, Third Edition. Cary, NC: SAS Institute Inc. ISBN 978-I-62959-308-1.
- Weldon, W.C.; Oberste, M.S.; Pallansch, M.A. Standardized Methods for Detection of Poliovirus Antibodies. Methods Mol. Biol. Clifton NJ 2016, 1387, 145–176. [Google Scholar] [CrossRef]
- Cáceres, V.M.; Sutter, R.W. Sabin monovalent oral polio vaccines: review of past experiences and their potential use after polio eradication. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2001, 33, 531–541. [Google Scholar] [CrossRef]
- John, T.J.; Jain, H.; Ravishankar, K.; Amaresh, A.; Verma, H.; Deshpande, J.; Pallansch, M.A.; Singh, A.P.; Sreevatsava, M.; Burton, A.; et al. Monovalent type 1 oral poliovirus vaccine among infants in India: report of two randomized double-blind controlled clinical trials. Vaccine 2011, 29, 5793–5801. [Google Scholar] [CrossRef] [PubMed]
- Mir, F.; Quadri, F.; Mach, O.; Ahmed, I.; Bhatti, Z.; Khan, A.; Rehman, N. ur; Durry, E.; Salama, M.; Oberste, S.M.; et al. Monovalent type-1 oral poliovirus vaccine given at short intervals in Pakistan: a randomised controlled, four-arm, open-label, non-inferiority trial. Lancet Infect. Dis. 2015, 15, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Huyen, D.T.T.; Anh, D.D.; Trung, N.T.; Hong, D.T.; Thanh, T.T.; Truong, L.N.; Jeyaseelan, V.; Lopez Cavestany, R.; Hendley, W.S.; Mainou, B.A.; et al. Inactivated Poliovirus Vaccine Closing the Type 2 Immunity Gap in Vietnam. J. Pediatr. Infect. Dis. Soc. 2022, 11, 413–416. [Google Scholar] [CrossRef]
- Tagbo, B.N.; Verma, H.; Mahmud, Z.M.; Ernest, K.; Nnani, R.O.; Chukwubike, C.; Craig, K.T.; Hamisu, A.; Weldon, W.C.; Oberste, S.M.; et al. Randomized Controlled Clinical Trial of Bivalent Oral Poliovirus Vaccine and Inactivated Poliovirus Vaccine in Nigerian Children. J. Infect. Dis. 2022, 226, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L.C.; Carlos, J.C.; Gatchalian, S.R.; Montellano, M.E.B.; Tabora, C.F.C.B.; Thierry-Carstensen, B.; Tingskov, P.N.; Sørensen, C.; Wachmann, H.; Bandyopadhyay, A.S.; et al. Immunogenicity and safety of an adjuvanted inactivated polio vaccine, IPV-Al, compared to standard IPV: A phase 3 observer-blinded, randomised, controlled trial in infants vaccinated at 6, 10, 14 weeks and 9 months of age. Vaccine 2020, 38, 530–538. [Google Scholar] [CrossRef] [PubMed]
- WHO Collaborative Study. Combined immunization of infants with oral and inactivated poliovirus vaccines: results of a randomized trial in The Gambia, Oman, and Thailand. WHO Collaborative Study Group on Oral and Inactivated Poliovirus Vaccines. Bull. World Health Organ. 1996, 74, 253–268. [Google Scholar]
- Cohen-Abbo, A.; Culley, B.S.; Reed, G.W.; Sannella, E.C.; Mace, R.L.; Robertson, S.E.; Wright, P.F. Seroresponse to trivalent oral poliovirus vaccine as a function of dosage interval. Pediatr. Infect. Dis. J. 1995, 14, 100–106. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).