1. Introduction
Cassava (Manihot esculenta Crunz) is a staple for more than 600 million people in Eastern, Western and Southern Africa (1). These regions produce and consume more than 54% of the world’s cassava, underpinning the importance of the crop to their livelihoods. The crop’s clonal nature, ability to survive in marginal soils, tolerance to drought (2) and perennial nature which allows for peace meal harvest allow for a diversity of uses for food, feed and industry, making it an important food security crop (3,4,5). However, cassava’s usefulness is limited by presence of the cyanogenic glucosides, namely linamarin and lotaustralin in the leaves and roots (6,7). Linamarine readily hydrolyzes to glucose and acetone cyanohydrin in presence of linamarase enzyme and in neutral or alkaline medium, acetone cyanohydrin decomposes to liberate cyanide and a hydrogen ion (8).
Production of hydrogen cyanide (HCN) in plants is said to be a defense mechanism against herbivores and pathogens (9,10). Total cyanogenic concentration in cassava may vary with cultivar, environmental conditions, cultural practices, and plant age. Indeed, HCN content in the range of 2 ppm to >1000 ppm has been reported (8,11). Cassava is thus classified as “sweet” if it has root HCN content < 100 ppm on wet basis and “bitter” if it has root HCN > 100 ppm (12,13). In fact, cassava with HCN content > 10 ppm on dry weight basis or > 100 ppm on fresh weight basis is considered not safe for human consumption and should undergo further processing before consumption (14).
Dietary intake of high amounts of HCN can be toxic to and/or lethal (15,16). Prolonged dietary exposure to HCN is linked to degenerative conditions of the nervous system; konzo and tropical ataxic neuropathy (17–19). Despite these risks, some communities still cultivate bitter (high HCN) varieties because they are not stolen by human or rodents (20). The rate of adoption of new crop varieties is dependent upon end user preferences (21-24).
Consumers of boiled cassava have preference for non-bitter cassava varieties (3,25,26), characterized by low HCN content. HCN content is thus a major component of cassava root quality for boiled or steamed cassava roots, a product that is desired by consumers of cassava. In the Amazon region, farmers readily classify cassava varieties into sweet, i.e. aipim, macaxera or table varieties and bitter varieties which are said to be for industry (27), while in Malawi, farmers ably classify their varieties as either “cool” or “bitter” depending on HCN levels (28). Owing to the increasing interest in boiled cassava, efforts are underway to initiate breeding for low HCN cassava varieties. However, the success of these efforts largely depends on amount of variation present and the proportion of this variation that is heritable (28). In addition to genotypic differences, variation in fresh cassava root HCN content is said to be influenced by the soil nutrient status (30) and climatic factors such as precipitation and temperature (31).
Phenotyping for HCN content of fresh cassava roots by cassava breeding programs is commonly done using any of three variations of the picrate method. These include 0 ppm to 800 ppm color scale (8), the modified picrate method (6,32) which allows for collection of semi-quantitative data using a spectrophotometer, or another modification of the picrate method suggested by (34) which follows a 1 to 9 color scale. All these methods have ontologies for data handling in the cassava breeding database; Cassavabase (35) and are thus deployed by major cassava breeding programs. Whether or not these methods provide comparable results is unknown. Furthermore, it is not known whether sufficient genetic variation exists in available germplasm to initiate long-term breeding for low HCN. The effect weather variables on HCN phenotypic expression variability within the available germplasm also remains unknown. Thus, in this study, we sought to (a) access the extent of genetic variation and heritability for HCN within the study germplasm (b) compare accuracy of three HCN phenotyping protocols (c) access the relationship between the weather variables; precipitation, temperature, and relative humidity and fresh cassava root HCN phenotypic expression.
2. Results
2.1. Variability for HCN in fresh cassava roots
Highly significant differences among clones (P < 0.001) and locations (P<0.001) were observed for all three HCN assessment methods. Furthermore, there was significant clone by environment interactions for the methods (
Table 1).
On the 1-9_Scale, fresh root HCN content ranged from 2 to 9 with a combined location average of 6.56. The highest average HCN content was recorded in Arua (7.70) followed by Serere (7.58), while Namulonge (5.45) had the lowest average HCN content (
Table 2). On the 0 to 800 scale, fresh root HCN content ranged from 65 ppm to 800 ppm with a combined location average of 430.21 ppm. The highest average HCN content (614.24 ppm) was again recorded in Arua, while the least (236.03 ppm) was again recorded in Namulonge (
Table 2). For the HCN_Spec method, fresh root HCN content ranged from 119.6 ppm to 676.3ppm with a combined location average of 504.1 ppm. However, the highest average fresh root HCN content (608.53 ppm) was recorded in Serere while the least (399.39 ppm) was recorded in Tororo. Generally, HCN observations at Namulonge were comparable to Tororo while observations for Serere were comparable to Arua (
Table 2).
Within sites, broad sense heritability ranged 0.46 to 0.54 on the 1 to 9 scale, 0.24 to 0.64 on the 0 to 800 ppm scale and 0.22 to 0.56 using the spectrophotometric method. Across locations, broad sense heritability was 0.14 for the spectrophotometric method, 0.26 for the 1 to 9 scale and 0.32 for the 0 to 800 ppm scale. The genetic component of the variation (V
g) was 0.48 for the 1 to 9 scale, 3596 for the spectrophotometric method and 13990 for the 0 to 800 scale. (
Table 2).
On the 1 – 9_Scale, Arua had clones scoring 4 to 9 with a high median value of 8. The distribution was skewed to the right (
Figure 1). In Serere scores ranged 3 to 9, with 50% of the clones scoring between 7 and 8, there by skewing the distribution to the right. In Tororo, HCN scores ranged 3 to 9 with 50% clones scoring between 5 and 6 while in Namulonge scores ranged 2 to 9, with a median of 6 and a near uniform distribution of scores across the scale (
Figure 1). However, for the 0 – 800_Scale, HCN scores ranged 100 to 800 ppm in Arua with 75% clones scoring 400 – 800 ppm, there-by skewing the distribution to the right. Similarly, in Serere, HCN scores ranged 100 to 800 ppm, with a median score slightly below 700 ppm. The distribution of scores was skewed to the right. In Namulonge and Tororo, the median HCN scores were 200 ppm and 150 ppm respectively with scores ranging from less than 50 ppm to 800 ppm. For both locations, the distribution was skewed to the left (
Figure 1). A similar trend was observed using the spectrophotometric method. Generally, Arua and Serere had high HCN values, thereby skewing the distributions to the right, while Namulonge and Tororo had uniform distributions (
Figure 1).
2.2. Accession ranking for fresh cassava root HCN content by BLUPS
BLUPS consistently ranked four of the five bitter local farmers’ clones among the top 10 for high HCN content (
Table 3). Over all, six clones; Tongolo, Nyamatia, Kazimwenge, Quinine, UG16F303P006 and UG16F158P004 were consistently ranked among the top 10 for high HCN content. On the other hand, 5 clones; UG16F290P295, UG16F293P169, UG16F057P001, UG16F001P013 and UG16F290P075 were consistently ranked among the top ten for low HCN content. Surprisingly, the local bitter farmers’ accession Edwarat was ranked among the top 10 low HCN clones by both the 1 to 9 scale and spectrophotometric methods. Overall, 5 clones were consistently selected by all methods, 6 clones by 1 to 9 scale and 0 to 800 scale and 9 clones by 1 to 9 scale and spectrophotometric method (
Table 3).
2.3. Comparison of phenotyping method performance
2.3.1. Correlation between the methods
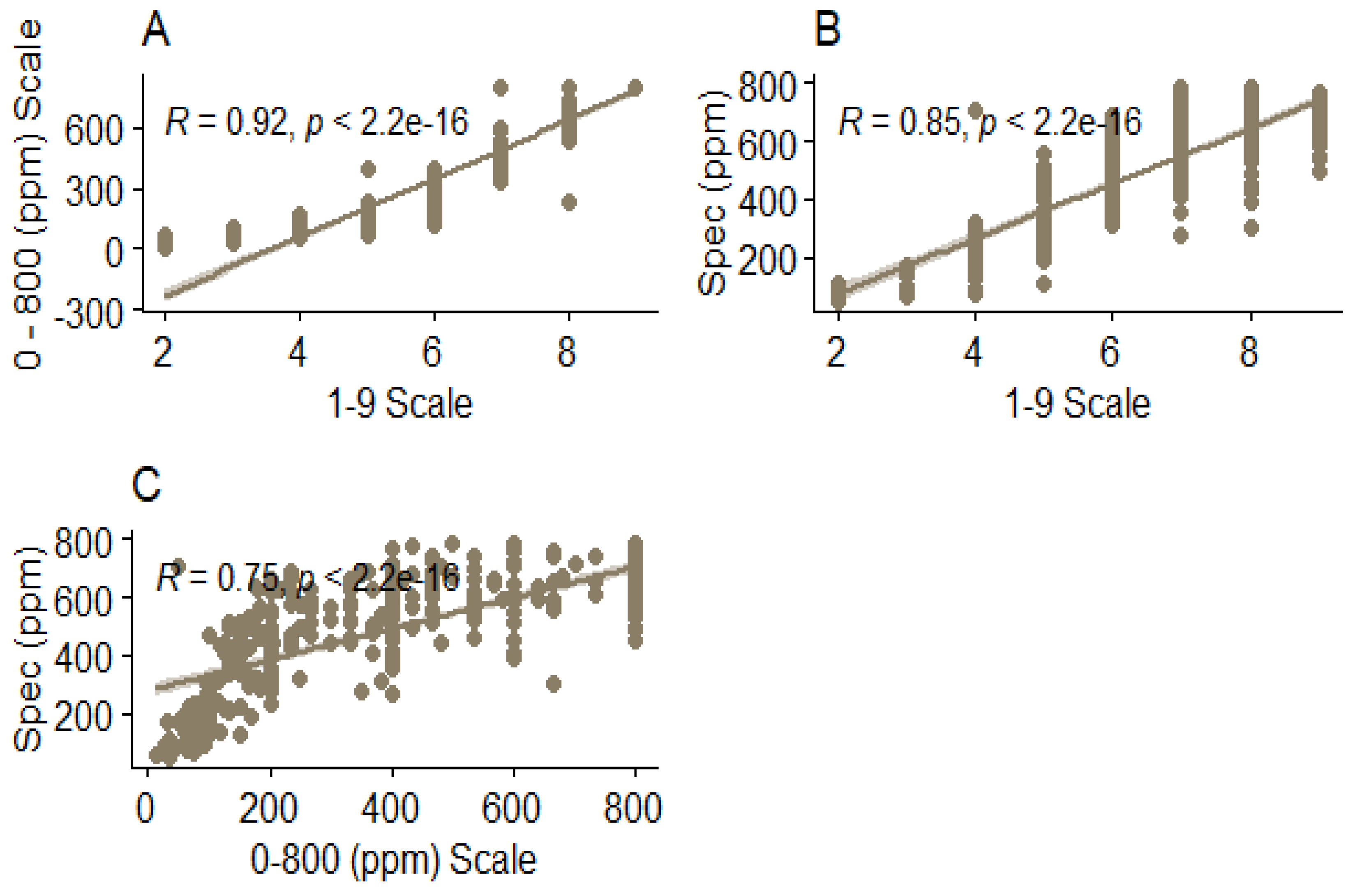
Regression analysis revealed a strong positive relationship between all the methods. The highest coefficent of determinationation (R
2 = 0.92, p < 0.001) was observed between the between the 1 to 9 scale and the 0 to 800 ppm scale (
Figure 2A). Furthermore, there was a strong positive relationship (R
2 = 0.85, p < 0.001) between the 1 to 9 scale and the spectrophotometric method (
Figure 2B) and between the 0 to 800 ppm scale and the spectrophotometric method (R
2 = 0.75, p < 0.001) (
Figure 2C).
2.3.2. Comparison of residuals
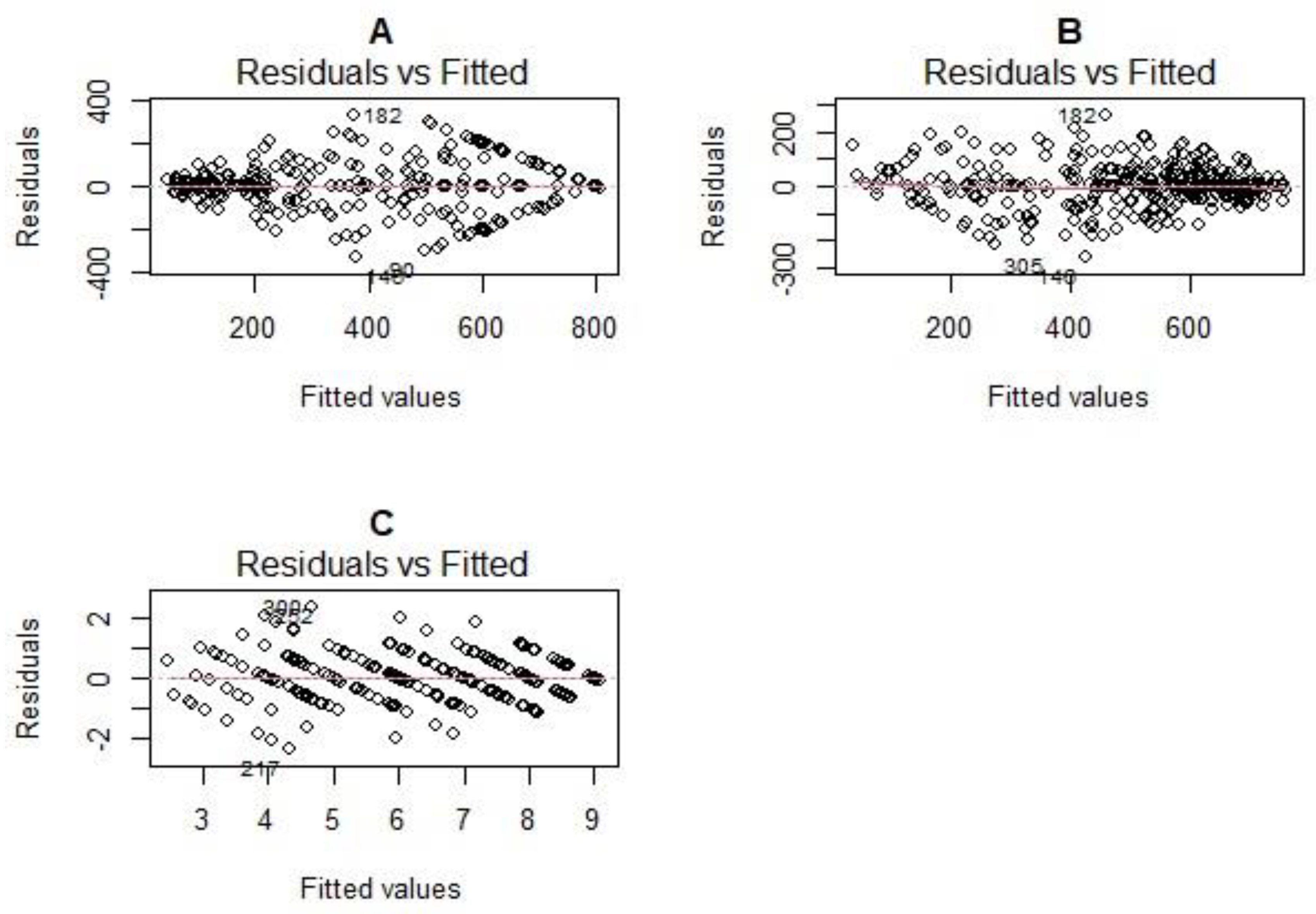
Large residuals were observed between 300 ppm and 600 ppm for both the 0 to 800 ppm scale and between 200 ppm and 400 ppm for the spectrophotometric method (
Figure 3B). Residuals were more clustered towards the zero line for higher HCN readings (more than 400 ppm) for the spectrophotometric method while for the 0 to 800 ppm scale, this pattern was observed for lower HCN scores (less than 200 ppm). There was a random distribution of residuals for the 1 to 9 scale (
Figure 3C).
For a uniform comparison of residuals for all three methods, we computed the relative standard error (RSE). For the 1 to 9 scale, RSE was 17.4%, RSE was 25.7% for the Spec method and 41.32% for the 0 to 800 scale method, indicating that the 1 to 9 scale was the most accurate, followed by the spectrophotometric method, with the 0 to 800 scale being least accurate.
2.4. Variation in weather variables at experimental test locations
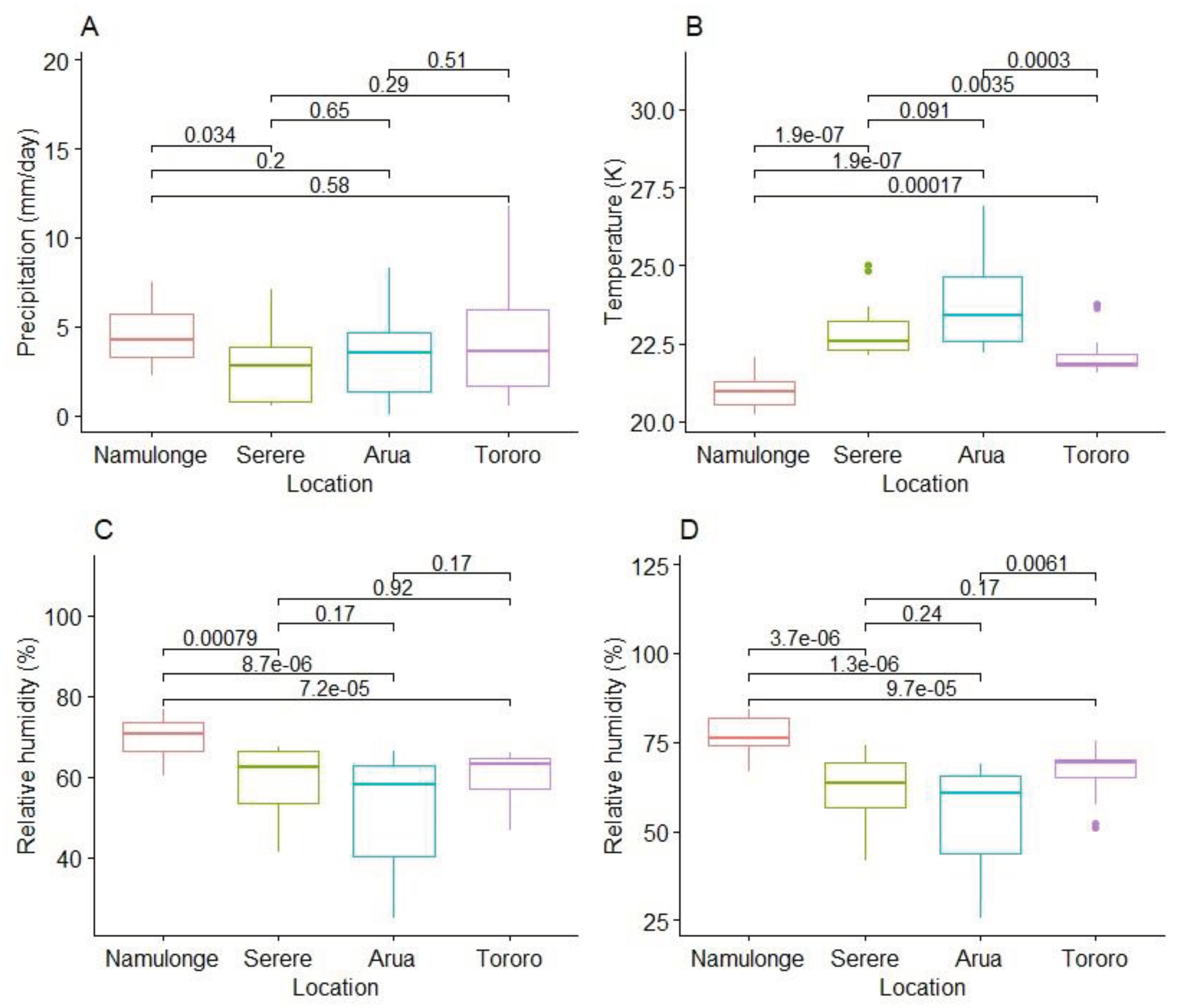
We compared locations for differences in precipitation (mm/day), average daily temperature (K) and relative humidity (%) at 12 and18 hour intervals. There was significant variation (p < 0.001) among locations for 24-hour temperature, relative humidity at 12 hours and relative humidity at 18 hours (
Figure 4). Except for precipitation, Namulonge was significantly different from all other locations. For precipitation, Namulonge received significantly higher precipitation (p = 0.034) than Serere but not was significantly different from Tororo and Arua (
Figure 4A). Namulonge had the least average 24-hour temperature (20. 97 K) followed by Tororo (22.16 K), Serere (22.99 K) and Arua (23.90 K) respectively. Arua was significantly hotter than Tororo (p = 0.0003) but did not differ from Serere (
Figure 4B). For relative humidity at 12 hours, Namulonge was high (average = 70%) and significantly different from all other locations. However, there were no significant differences between the other locations (
Figure 4C). For relative humidity at 18 hours, Namulonge had the highest relative humidity (77.03%) and was significantly different from other locations. This was followed by Tororo (66.17%) and Serere (60.98%). Arua with the least relative humidity (54.53%) was not significantly different from Serere. Serere was also not significantly different from Tororo but Tororo was significantly different from Arua (p = 0.006) (
Figure 4D).
3. Discussion
3.1. Genetic variability and heritability for HCN
A major objective of this study was to establish whether significant genetic variation exists for HCN in available germplasm, and whether different assessment methods vary in precision. Highly significant variation for HCN among clones was observed (
Table 1). Fresh root HCN content ranged 2.5 to 9 (mean 6.62) on the 1 to 9 scale, 165.2 ppm to 753.38 ppm on the 0 ppm to 800 ppm scale, and 119.50 ppm to 611.01 ppm on the HCN_Spec (
Table 1). It is thus evident that there was variability for HCN within the evaluated germplasm that comprised both local and elite landraces. This favors selection for low HCN. The ranges of HCN reported in this study are comparable to those reported in earlier studies (36–38). Based on the 1 to 9 scale, the genetic component of the variation (V
g) was 0.48, which is higher than (V
g) = 0.21 reported in African germplasm from the International Institute for Tropical Agriculture (IITA), Nigeria and much lower than in Colombian (Vg) = 1.58 and Brazilian germplasm (Vg) = 2.59 (Ogbonna et al. 2020). The higher genetic variation for HCN in Ugandan germplasm compared to IITA’s can be attributed to the deliberate inclusion of bitter landraces in the study i.e., Tongolo, Quinine, Nyamatia, Kazimwenge and Edwarat material whereas the high genetic variance reported for Brazilian and Colombian germplasm is explained by the fact that Latin America, particularly Brazil is the center of diversity of cassava. Given the large genetic variability for HCN reported for Latin American cassava clones, African cassava breeding programs targeting low HCN product profiles can enhance the diversity in their populations through germplasm exchange with their Latin American counterparts. Germplasm exchange could also be beneficial for other traits like fresh root yield, where some African cassava breeding programs are not making appreciable genetic gains (39).
A clear understanding of trait heritability informs strategies to drive genetic gains (40,41) as heritability estimates are critical to selection decisions (42). Broad sense heritability (H2) estimates were low to moderate across locations, H2 = (0.14 – 0.32). This could be explained by the large effect of environmental factors on HCN phenotypic expression. Furthermore, for all phenotyping methods, the residual variance was high, suggesting that better heritability estimates can be obtained with further optimization of the methods. These H2 estimates are however comparable to estimates previously reported for West African germplasm (H2 = 0.27) (38) and (H2 = 0.18) (37) but much lower than (H2 = 0.76 – 0.82) reported for Brazilian germplasm by the same authors using the 1 to 9 scale.
The diversity for bitter clones is maintained within African populations by farmers who selectively grow bitter varieties for various reasons (20,28,43). In Ugandan communities where communal livestock grazing is done, it is common practice for farmers to plant guard rows of bitter cassava landraces around their fields of the sweet cassava crop to” protect them from damage by livestock.”
Whereas (37) suggested 3 categorizations of HCN on the 1 to 9 scale; sweet (1- 4), intermediate (4.1-5) and bitter (5.1-9), these categories are contestable given that the levels of taste were neither tested by a trained sensory panel nor validated by a consumer panel. Thus, there is no evidence to suggest that a genotype with a score of 5.1 or 6.0 will be perceived as bitter by the consumer. Rather, we suggest 4 categories that could be of practical implications for cassava breeding; low HCN (1 – 4.0) corresponding to 0 - 100 ppm, intermediate HCN (4.1 - 5.9) corresponding to 101 - 200 ppm, high (6.0 - 7.0) corresponding to (201 - 400 ppm) and very high (7.1 – 9.0) corresponding to 401 – 900 ppm.
Thus, for breeding efforts targeting boiled products (associated with minimal processing), an average score of 1- 4 is desirable, but less than 6.0 is acceptable. For the fresh-root chewing product profile (common in Tanzania) which is associated with no processing at all, the cut-off for selection should be at 4.0. This categorization is somewhat in alignment with (44) who recommended a maximum residue limit (MRL) of 200 ppm for cyanogenic glycosides in cassava for food. For product profiles involving extensive processing like drying, milling and fermentation, the cut off may be relaxed to include clones averaging 6.0 - 7.0 given that significant amounts of cyanogens are lost during processing (45–47). However, when initial fresh root HCN concentrations are very high, traditional processing methods may not be sufficient to detoxify the roots (45), resulting in fatalities (15, 16, 48). Depending on the target product profile, different cassava breeding programs may have different cut-offs, mindful of the efficiency of traditional processing methods available and of local and international regulations on acceptable maximum residual levels of cyanide in cassava food and processed products.
3.2.Effect of the environment on fresh root HCN content
Fresh cassava root HCN content is known to vary even within roots of the same variety (10,49). These variations can in part be accounted for by differences in genotypes, climatic factors, and soils (12,18,50,51). The highest mean HCN by location was recorded in Arua (7.7) and Serere (7.58) (
Table 2). These locations also had the highest temperatures recorded during the crop growing period (23.9 K and 22.99 K) respectively and the least precipitation and relative humidity after 18 hours (Figure 5B). This is consistent with observations by (31,52) who reported that cyanide in cassava roots tended to increase in periods of drought and/or dry weather.
The higher cassava root HCN levels recorded in Arua and Serere could be explained by the effect of temperature on the crop’s physiology. Cyanide in cassava is produced from the breakdown of linamarine and lotaustraline, a process catalyzed by hydroxynitrile lyase (HNL) enzyme, which is in the leaves and stems but not in the roots (53). Increase in temperature increases rate of transpiration and sap flow in the plant, but could also increase the rate of HNL enzyme activity, thus elevating cassava root cyanide levels (53). Furthermore, temperature might influence the rate of cyanide detoxification in cassava roots via the β – cyanoaline pathway, which coverts cyanide to the amino acid asparagine. This reaction is catalyzed by β – cyanoaline synthase (CAS) enzyme, which is sensitive to heat denaturation, therefore its activity could be suppressed at high temperature (53).
Given the projected 1.2◦C – 4. 4◦C increase in temperature on the African continent between the year 2030 and 2050 (54), it is essential that cassava varieties that are high yielding, low in HCN and stable across environments be developed. However, variations in temperature and relative humidity alone may not fully explain the effect of the environment on HCN phenotypic expression. In Tanzania, farmers claim that low fertility soils, red clayey and sandy soils have a tendency to produce bitter cassava roots (30). The effect of climatic factors and soil physical (texture) and chemical composition (pH and mineral composition) ought to be studied simultaneously across seasons and locations for more compelling observations.
BLUPS offer excellent predictive ability to guide selection decisions (55–57). Five of the bitter landraces featured among the top 10 high HCN clones, while one (Edwarat) was surprisingly ranked among the top 10 low HCN clones by two of the methods (
Table 3). This clone is a landrace in a semi-arid environment in Eastern Uganda where it is said to be always bitter; thus, its name “Edwarat” which means “the bitter one.” This underscores the impact of environmental factors on fresh root HCN content. Cassava genotypes that are high yielding and low on HCN across several environments are highly desirable (50).
3.3. Accuracy of HCN phenotyping methods
The 1 to 9 scale (CV = 15.6%, H
2 = 0.26) is a more accurate and reproducible method compared to either the spectrophotometric method (CV = 24.6%, H
2 = 0.14) or the 0 to 800 scale (CV = 38.5%, H
2 = 0.32). Thus, whereas the spectrophotometric method is more accurate than the 0 to 800 scale, it is less reproducible. The 0 to 800 scale seems to be accurate for low HCN scores up to 300 ppm but is inaccurate for higher scores (
Figure 3A). The spectrophotometric method on the other hand seems less accurate for the low values (less than 400 ppm) but accurate for the high values (
Figure 3B). The large residuals the 0 to 800 scale could be due to the difficulty in distinguishing picrate papers colors and assigning the correct corresponding scores, an inherent problem associated with color scales (58). Furthermore, residuals observed when using the spectrophotometer could possibly be exacerbated by human error in time intervals colored picrate papers are eluted in water, especially when large sample numbers must be read on the spectrophotometer in a single batch. For the 1 to scale, residuals were equally spread out across the range of predicted values, suggesting a constant variance of residuals across the scale (
Figure 3C). The high coefficients of determination between the methods (R
2 = 0.75 – 0.92) suggest that one can predict phenotypes of one method, using another with a high degree of confidence since the methods yield comparable results.
For selection purposes, the 1 to 9 scale and spectrophotometric method outperformed the 0 to 800 scale as between them, they consistently selected 9 out of the top 10 low HCN clones. Given the extra requirements in sample preparation, machinery, and power, which make the spectrophotometric protocol an error prone, expensive, and lower throughput alternative, the more accurate 1 to 9 scale should be the method of choice for routine phenotyping in cassava breeding programs. At late-stage breeding trials when the population size is small and exact fresh HCN content is required to support variety release applications, the spectrophotometric method can be deployed. However, given the inherent difficulty in fresh cassava root HCN phenotyping, there is need to develop reproducible, accurate, and high throughput phenotyping protocols for HCN.
Near infrared spectroscopy (NIRS) is one such technologies that has been adopted by major cassava breeding programmes in Uganda and Nigeria for cassava root quality phenotyping (59-61) and has recently been shown to separate low and high HCN clones with high accuracy (62). Thus, cassava breeders targeting low HCN cassava varieties may find the concept of phenomic selection as recently described by (63) more appealing compared to genome based tools like marker assisted selection (MAS) given the phenotypic plasticity of the trait. Acyanogenic cassava can be produced via genetic transformation (64). However, in many African jurisdictions as is the case in Uganda, transgenics still face stiff resistance from anti-transgenics lobbyist groups and would be break through research (65,66) is largely restricted to laboratory and confined field trials. Thus, conventional breeding for low cyanogenic cassava remains the most potent avenue to safeguard the public from potential harmful effects of dietary HCN consumption.
4. Materials and Methods
4.1. Description of the study area
Variability for HCN was studied across four locations; The National Crops Resources Research Institute (NaCRRI), central Uganda, the National Semi-arid Resources Research Institute (NaSARRI), Serere in eastern Uganda, Abi Zonal Agricultural Research Institute (Abi ZARDI), Arua northern Uganda and Tororo in eastern Uganda. NaCRRI, is located at longitude 32.62717 and latitude 0.521526 and 1134 meters above sea level, characterized by a bi-modal rainfall pattern with an average annual rainfall of 1325 mm and annual average temperature of 21◦C. The soils are sandy loams. NaSARRI is located at longitude 33.54901 and latitude 1.499403 with an elevation of 1104 meters above sea level. The area is semi-arid with average annual temperatures of 26.5◦C. The soils are light sandy loams. Abi ZARDI is located at longitude 30.95 and latitude 3.08333 at an altitude of 1109 meters above sea level, experiences a bi-modal rainfall pattern with average annual rainfall of 1250 mm and average annual temperature of 24◦C. The soils are sandy loam. Finally, Tororo is located at longitude 34.0223 and latitude 0.769 with an elevation of 1278 meters above sea level. The mean annual temperature of 21◦C. The area is characterized by light sandy soils and a bi-modal rainfall pattern.
4.2. Description of study materials and field trial establishment
A set of 64 clones was used in this study. These consisted of eight bitter landraces collected from farmers’ fields across the country and 56 advanced breeding clones. These clones were selections from cycle two of genomic selection population (67). At each location, clones were established in the field in a randomized complete block design with two replicates. Each plot consisted of five rows of plants with a spacing of 1 x 1 meter, with 2-meter alleys between plots. Plots were kept weed free by hand weeding. No fertilizers and/or supplemental irrigation were applied. Further details of the trials can be found in the online open access data repository; Cassavabase at
www.cassavabase.org.
4.3. Sample selection, preparation, and data collection
4.3.1. Sample selection
At harvest (12 months after planting), three middle rows of each plot were uprooted and all harvested roots pooled together. Three uniformly sized roots were then selected and taken to the laboratory for analysis. At the laboratory, roots were washed under running water to remove soil and debris and then dried with a towel.
4.3.2. Sample preparation
For each root, the distal and proximal portions were sliced off with a kitchen knife and discarded, leaving a 10-centimeter middle portion. This portion was peeled with a kitchen knife and the parenchyma grated with a kitchen grater. The grated slices per root were wrapped together and a portion immediately weighed off into a falcon tube containing 5 ml of pH 8 phosphate buffer. A fresh picrate paper strip (1 cm wide, 3 cm long) prepared by dipping Whatman 1 filter paper (Whatman International Limited) in a solution of picric acid (0.5% w/v) in 2.5% (w/v) sodium carbonate and allowing the paper to dry at room temperature was immediately suspended above the falcon tube and the tube tightly stoppered. The tubes were kept in the dark for 16 hours at room temperature.
4.4. Data collection
4.4.1. Measurement of HCN
Three assessments for HCN were undertaken. Firstly, scores were taken of the final color of the picrate paper strip on a 1 to 9 scale with 1 and 9 representing extremes of low and high HCN content respectively (34). Secondly, scores of the picrate paper were also taken using the 0 – 800 parts per million (ppm) scale with 0 ppm and 800 ppm representing extremes of low and high HCN content respectively (8). Thirdly, the scored picrate paper was eluted in 3 milliliters of distilled water for 30 minutes and the absorbance of the resultant-colored solution read on a spectrophotometer (Jenway 6305 UV/Visible Spectrophotometer) at 510 nm against a similarly prepared blank but whose picrate paper was not exposed to the sample. To get the final HCN value in parts per million, the absorbance value of the sample was multiplied by 396 (32).
4.4.2. Weather data
Weather data were obtained for the study sites from the AgERA5 database (68) using the R package ag5Tools (69). The AgERA5 dataset provides daily surface meteorological data from 1979 at a resolution of 0.1◦ (70). Thus, daily weather data were extracted for the period September 2020 to September 2021 for the parameters; 24-hour temperature (K), relative humidity after 12hours (%), relative humidity after 18 hours (%) and precipitation (mm/day).
4.5. Data analysis
For each of the three HCN assessment methods, an independent data set was assembled. Data analysis was done in R statistical package. Analysis of variance was done to test for significance of observed differences in means of clones, locations and for the interaction of genotypes with location (GEI) at 0.05 alpha level. Means of clones and locations were compared using Tukey’s honestly significant difference test using the
HSD.test function of the
Agricolae package at 0.05 alpha level. Borad sense heritability was computed based on single site analysis and on a combination of data sets from all the sites. The
lmer function of R statistical package was used to fit the mixed linear model;
where y is the response vector of HCN for a given location, β is the vector of fixed effects and
u is the vector of random genetic effects with design matrix
Z (relating trait values to genotype, environment, and genotype by environment interaction) and ε is the error (residual). Variance components were then extracted and used for the estimation of broad sense heritability (H
2). Within locations (single trial), broad sense heritability was estimated as;
where; H
2 is the broad sense heritability, V
g is the genotypic variance, V
e is the error (residual) variance. Across locations (trials), broad sense heritability was estimated as;
where;
is the genotypic variance, V
e is the error (residual) variance,
is variance due genotype by environment interaction
Ranking of clones according to HCN content was done using best unbiased linear predictors (BLUPS), which were computed using the ranef function in R. Variance components, heritability and BLUPS were computed separately for each HCN phenotyping method. The Kruskal-Wallis test was done to test the hypothesis that the test environments did not experience different weather conditions and results plotted using the ggpubr package. Correlation analysis was performed to investigate the relationship between the HCN phenotyping methods using the package ggpairs in R.
5. Conclusions
From this study; four conclusions are apparent; 1) there is appreciable genetic diversity for HCN in locally available Ugandan germplasm 2) heritability for HCN within the study germplasm is low to moderate 3) the 1 to 9 scale is more accurate and more reproducible than either the 0 to 800 ppm scale or spectrophotometric methods 4) average daily temperature and relative humidity during crop growth influence fresh cassava root HCN phenotypic expression. This information provides frameworks for undertaking sustainable cassava breeding mindful of acceptable thresholds for HCN in developed products and the phenotypic plasticity of the trait.
Author Contributions
Conceptualization, M.K, M.B.S., E.N. and R.S.K.; methodology, M.K, E.N.; software, validation, M.B.S., E.N., and R.S.K; formal analysis, M.K.; investigation, M.K.; resources, R.S.K; data curation, F.B.N., and N.M; writing—original draft preparation, M.K.; writing—review and editing, R.S.K., P.I., A.N., A.O, J.K.B., E.W., K.S.I; visualization, M.K.; supervision, M.B.S., E.N., R.S.K.; project administration, R.S.K.; funding acquisition, R.S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Nextgen Cassava Breeding Project through a grant to Cornell University by Bill & Melinda Gates Foundation (BMGF) (Grant INV-007637) and the UK’s Foreign Common Wealth and Development Office (FCDO). Additional funding was provided by the Regional Universities Forum for Capacity Building in Agriculture (RUFORUM) through grant RU-NARO/2020/post-Doc/01 to NaCRRI.
Data Availability Statement
Data is available on the corresponding trial detail pages in an online open access repository; Cassavabase (
www.cassavabase.org).
Acknowledgments
The authors would like to acknowledge the selfless contribution of the field and laboratory technicians of the Nutrition and Bio-analytical laboratory at NaCRRI for their meticulous work in field trial maintenance and data collection. We are further grateful to the management of ABI ZARDI and NaSARRI for hosting field trials and availing their laboratory facilities for HCN phenotyping. We are also grateful to David Brown (Cornell University, Ithaca, New York, USA) for mining and curation of climatic data from the AgERA5 database.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Manyong VM, Makinde KO, Bokanga M, Whyte J. The contribution of IITA-improved cassava to food security in sub-Saharan Africa : an impact study.
- El-Sharkawy MA. Stress-Tolerant Cassava: The Role of Integrative Ecophysiology-Breeding Research in Crop Improvement. Open J Soil Sci. 2012;02(02):162–86. [CrossRef]
- Iragaba P, Hamba S, Nuwamanya E, Kanaabi M, Nanyonjo RA, Mpamire D, et al. Identification of cassava quality attributes preferred by Ugandan users along the food chain. Int J Food Sci Technol. 2021;56(3):1184–92. [CrossRef]
- Nuwamanya E, Kawuki RS. Quantification of starch physicochemical characteristics in a cassava segregating population. 2010;(May 2014). [CrossRef]
- Nanyonjo AR, Dufour D, Kawuki RS, Kyazze F, Esuma W, Wembabazi E, et al. Original article Assessment of end user traits and physicochemical qualities of cassava flour : a case of Zombo district, Uganda. 2021;1–9.
- Haque MR, Bradbury JH. Total cyanide determination of plants and foods using the picrate and acid hydrolysis methods. 2002;77:107–14. [CrossRef]
- Daniel L, Da J, Francisco C, Zelder F, Bergenståhl B, Dejmek P. Straightforward rapid spectrophotometric quantification of total cyanogenic glycosides in fresh and processed cassava products. FOOD Chem [Internet]. 2014;158:20–7. Available from: http://dx.doi.org/10.1016/j.foodchem.2014.02.066. [CrossRef]
- Egan S V, Yeoh HH, Bradbury JH. Simple picrate paper kit for determination of the cyanogenic potential of cassava flour. J Sci Food Agric. 1998;76(1):39–48.
- Zagrobelny M, Møller BL. Cyanogenic glucosides in the biological warfare between plants and insects: The Burnet moth-Birdsfoot trefoil model system. Phytochemistry [Internet]. 2011;72(13):1585–92. Available from: http://dx.doi.org/10.1016/j.phytochem.2011.02.023. [CrossRef]
- Gleadow RM, Møller BL. Cyanogenic glycosides: Synthesis, physiology, and phenotypic plasticity. Annu Rev Plant Biol. 2014;65:155–85. [CrossRef]
- Bradbury JH, Egan S V. Rapid screening assay of cyanide content of cassava. Phytochem Anal. 1992;3(2):91–4. [CrossRef]
- McKey D, Cavagnaro TR, Cliff J, Gleadow R. Chemical ecology in coupled human and natural systems: People, manioc, multitrophic interactions and global change. Chemoecology. 2010;20(2):109–33. [CrossRef]
- Wheatley CC, Ghuzel G, Zakhia N. The Nature of the Tuber. Nat Tuber. 2003;964–9.
- FAO/WHO. Book Review: Safety Evaluation of Certain Food Additives and Contaminants. Nutr Health. 2001;15(1):74–74.
- Alade Akintonwa and O. L. Tunwashe. Fatal Cyanide Poisoning from Cassava-based Meal. Hum Exp Toxicol. 1992;11:47–9. [CrossRef]
- Alitubeera PH, Eyu PK, , Benon A, Alex R, Zhu B. Outbreak of Cyanide Poisoning Caused by Consumption of Cassava Flour — [Internet]. Vol. 68. 2019. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6611475/.
- Cliff J. Konzo : From Poverty , Cassava , and Cyanogen Intake to Toxico-Nutritional Neurological Disease. PLoS Negl Trop Dis. 2011;5(6):1–8. [CrossRef]
- Cliff J, Muquingue H, Nhassico D, Nzwalo H, Bradbury JH. Konzo and continuing cyanide intoxication from cassava in Mozambique. Food Chem Toxicol [Internet]. 2011;49(3):631–5. Available from: http://dx.doi.org/10.1016/j.fct.2010.06.056. [CrossRef]
- Nhassico D, Muquingue H, Cliff J, Cumbana A, Bradbury JH. Rising African cassava production, diseases due to high cyanide intake and control measures. 2008;2049(May):2043–9. [CrossRef]
- Nakabonge G. Local varieties of cassava : conservation , cultivation and use in Uganda. Environ Dev Sustain. 2018;20(6):2427–45. [CrossRef]
- Bechoff A, Tomlins K, Fliedel G, Becerra Lopez-lavalle LA, Westby A, Hershey C, et al. Cassava traits and end-user preference: Relating traits to consumer liking, sensory perception, and genetics. Crit Rev Food Sci Nutr. 2018;58(4):547–67. [CrossRef]
- Dufour, Dominique, Hershey Clair, Hamaker RB and JL. Integrating end-user preferences into breeding programmes for roots tubers and bananas. Int J Food Sci Tech. 2021;56:1071–5. [CrossRef]
- Thiele G, Dufour D, Vernier P, Mwanga ROM, Parker ML, Schulte Geldermann E, et al. A review of varietal change in roots, tubers and bananas: consumer preferences and other drivers of adoption and implications for breeding. Int J Food Sci Technol. 2021;56(3):1076–92. [CrossRef]
- Polar V, Ashby JA, Thiele G, Tufan H. When is choice empowering? Examining gender differences in varietal adoption through case studies from sub-saharan africa. Sustain. 2021;13(7):1–19. [CrossRef]
- Takam Tchuente HN, Fongang Fouepe GH, Mbwentchou Yao DC, Mathe S, Teeken B. Varietal diversity as a lever for cassava variety development: exploring varietal complementarities in Cameroon. J Sci Food Agric. 2023;(February). [CrossRef]
- Honfozo LF, Djibril Moussa IM, Adinsi L, Bouniol A, Adetonah S, Chadare FJ, et al. Cross-approaches for advising cassava trait-preferences for boiling. Cogent Food Agric [Internet]. 2023;9(1). Available from: https://doi.org/10.1080/23311932.2023.2253716. [CrossRef]
- Bezerra C, Ferreira E, Cunha RL, Tomé J, Neto DF, Silva RDS. Chemical root traits differentiate ‘ bitter ’ and ‘ sweet ’ cassava clones from the Amazon. 2019;77–85. [CrossRef]
- Mkumbira J, Chiwona-Karltun L, Lagercrantz U, Mahungu NM, Saka J, Mhone A, et al. Classification of cassava into “bitter” and “cool” in Malawi: From farmers’ perception to characterisation by molecular markers. Euphytica. 2003;132(1):7–22. [CrossRef]
- O. B. B, S. A. I, M. A. A, M. S. A, S. Y. A, J. M. Heritability and Genetic Advance for Grain Yield and its Component Characters in Maize (Zea Mays L.). Int J Plant Res. 2012;2(5):138–45. [CrossRef]
- Imakumbili MLE, Semu E, Semoka JMR, Abass A, Mkamilo G. Soil nutrient adequacy for optimal cassava growth, implications on cyanogenic glucoside production: A case of konzo-affected Mtwara region, Tanzania. PLoS One. 2019;14(5):1–17. [CrossRef]
- Banea-Mayambu JP, Tylleskär T, Gitebo N, Matadi N, Gebre-Medhin M, Rosling H. Geographical and seasonal association between linamarin and cyanide exposure from cassava and the upper motor neurone disease konzo in former Zaire. Trop Med Int Heal. 1997;2(12):1143–51. [CrossRef]
- Meredith G Bradbury SVE and JHB. Picrate paper kits for determination of total cyanogens in cassava roots and all forms of cyanogens in cassava products. 1999.
- Haque MR, Bradbury JH. Preparation of linamarin from cassava leaves for use in a cassava cyanide kit. 2004;85:27–9. [CrossRef]
- W.M.G. Fukuda, C.L. Guevara, R. Kawuki A, Ferguson ME. Selected morphological and agronomic descriptors for the characterization of cassava [Internet]. IITA, Ibadan, Nigeria. 2010 [cited 2022 Mar 29]. Available from: https://books.google.co.ug/books?hl=en&lr=&id=-SnckHhBlEYC&oi=fnd&pg=PA1&dq=FUKUDA+2010+hydrogen+cyanide+cassava+scale&ots=_tpYNzi4sg&sig=QbPKY4T0MSnmZK6biD6Z7XzTjUQ&redir_esc=y#v=onepage&q&f=false.
- Morales N, Ogbonna AC, Ellerbrock BJ, Bauchet GJ, Tantikanjana T, Tecle IY, et al. Breedbase: a digital ecosystem for modern plant breeding. G3 Genes|Genomes|Genetics. 2022;12(April). [CrossRef]
- Dufour D, Dufour E, Tirrone G, Escobar A, Giraldo A, Sanchez T. Evaluation of highland cassava for starch production in Colombia [Abstract]. First Sci Cassava Meet Challenges New Millenium [Internet]. 2008;(November):1 p. Available from: http://ciat.catalog.cgiar.org/dbtw-wpd/exec/dbtwpub.dll.
- Torres LG, Oliveira EJ De, Ogbonna AC, Fonseca F, Simiqueli GF, Kantar MB. Can Cross-Country Genomic Predictions Be a Reasonable Strategy to Support Germplasm Exchange ? – A Case Study With Hydrogen Cyanide in Cassava. 2021;12(December). [CrossRef]
- Ogbonna AC, Braatz de Andrade LR, Rabbi IY, Mueller LA, Jorge de Oliveira E, Bauchet GJ. Large-scale genome-wide association study, using historical data, identifies conserved genetic architecture of cyanogenic glucoside content in cassava (Manihot esculenta Crantz) root. Plant J. 2021;105(3):754–70. [CrossRef]
- Manze F, Rubaihayo P, Ozimati A, Gibson P, Esuma W, Bua A, et al. Genetic Gains for Yield and Virus Disease Resistance of Cassava Varieties Developed Over the Last Eight Decades in Uganda. Front Plant Sci. 2021;12(June):1–11. [CrossRef]
- Schmidt P, Hartung J, Bennewitz J, Hans-Peter P. Heritability in plant breeding on a genotype-difference basis. Genetics. 2019;212(4):991–1008. [CrossRef]
- Giovanny EC-P. Heritability : meaning and computation Heritability : meaning and computation [Internet]. 2019. Available from: https://excellenceinbreeding.org/sites/default/files/manual/EiB-M2_Heritability_18-02-20.pdf.
- Bernardo R. Parental selection, number of breeding populations, and size of each population in inbred development. Theor Appl Genet. 2003;107(7):1252–6. [CrossRef]
- Mubalama JM, Ayagirwe RBB, Martin P, Nguezet D, Mondo JM, Irenge AB, et al. 44-52Determinants of Adoption and Farmers’ Preferences for Cassava Varieties in Kabare Territory, Eastern Democratic Republic of Congo. Am J Rural Dev [Internet]. 2019;7(2):44–52. Available from: http://pubs.sciepub.com/ajrd/7/2/1.
- Zhong Y, Xu T, Ji S, Wu X, Zhao T, Li S, et al. Effect of ultrasonic pretreatment on eliminating cyanogenic glycosides and hydrogen cyanide in cassava. Ultrason Sonochem. 2021;78. [CrossRef]
- Cardoso AP, Mirione E, Ernesto M, Massaza F, Cliff J, Rezaul Haque M, et al. Processing of cassava roots to remove cyanogens. J Food Compos Anal. 2005;18(5):451–60. [CrossRef]
- Bandna C. EFFECT OF PROCESSING ON THE CYANIDE CONTENT OF CASSAVA. 2012;2(3):947–58.
- Adewusi SRA, Akindahunsi AA. Cassava processing, consumption, and cyanide toxicity. 2015;4108(September):12–23. [CrossRef]
- CCDN. Working together to eliminate cyanide poisoning, konzo, tropic ataxic neuropathy (TAN) and neurolathyrism. 2011 Dec 18;1–4. Available from: https://biblio.ugent.be/publication/2002992/file/2003018.pdf.
- Nuwamanya E, Turyasingura C, Magumba I, Katungisa A, Alicai T. Cyanogenic Potential Variations Within Plot , Plant and Roots of Cassava Varieties Grown in the Same Environment. ProcNatlAcadSciIndiaSectBBiolSci. 2022. [CrossRef]
- Jørgensen K, Bak S, Busk PK, Sørensen C, Olsen CE, Puonti-Kaerlas J, et al. Cassava plants with a depleted cyanogenic glucoside content in leaves and tubers. Distribution of cyanogenic glucosides, their site of synthesis and transport, and blockage of the biosynthesis by RNA interference technology. Vol. 139, Plant Physiology. 2005. p. 363–74. [CrossRef]
- Njankouo Ndam Y, Mounjouenpou P, Kansci G, Kenfack MJ, Fotso Meguia MP, Natacha Ngono Eyenga NS, et al. Influence of cultivars and processing methods on the cyanide contents of cassava (Manihot esculenta Crantz) and its traditional food products. Sci African. 2019;5.
- Bokanga, Mpoko, Indira JE and AGOD. Bokanga1994 [Internet]. Acta Hortic; 1994. p. 375, 131–40. Available from: https://www.ishs.org/ishs-article/375_11.
- Zidenga T, Siritunga D, Sayre RT. Cyanogen metabolism in cassava roots: Impact on protein synthesis and root development. Front Plant Sci. 2017;8(February):1–12. [CrossRef]
- Almazroui M, Saeed F, Saeed S, Nazrul Islam M, Ismail M, Klutse NAB, et al. Projected Change in Temperature and Precipitation Over Africa from CMIP6. Earth Syst Environ [Internet]. 2020;4(3):455–75. Available from: https://doi.org/10.1007/s41748-020-00161-x. [CrossRef]
- Panter DM, Allen FL. Using best linear unbiased predictions to enhance breeding for yield in soybean: II. Selection of superior crosses from a limited number of yield trials. Crop Sci. 1995;35(2):405–10. [CrossRef]
- Piepho HP, Möhring J, Melchinger AE, Büchse A. BLUP for phenotypic selection in plant breeding and variety testing. Euphytica. 2008;161(1–2):209–28. [CrossRef]
- Molenaar H, Boehm R, Piepho HP. Phenotypic selection in ornamental breeding: It’s better to have the BLUPs than to have the BLUEs. Front Plant Sci. 2018;871(November):1–14. [CrossRef]
- Tivana LD, Da Cruz Francisco J, Zelder F, Bergenståhl B, Dejmek P. Straightforward rapid spectrophotometric quantification of total cyanogenic glycosides in fresh and processed cassava products. Food Chem [Internet]. 2014;158:20–7. Available from: http://dx.doi.org/10.1016/j.foodchem.2014.02.066. [CrossRef]
- Namakula BF, Nuwamanya E, Kanaabi M, Wembambazi E, Kawuki RS. Predicting starch content of cassava with near infrared spectroscopy in Ugandan cassava germplasm. J Near Infrared Spectrosc [Internet]. 2023;0(0):1–7. Available from: https://doi.org/10.1177/09670335231194739. [CrossRef]
- Nkouaya Mbanjo EG, Hershberger J, Peteti P, Agbona A, Ikpan A, Ogunpaimo K, et al. Predicting starch content in cassava fresh roots using near-infrared spectroscopy. Front Plant Sci. 2022;13(November):1–16. [CrossRef]
- Nuwamanya E, Enoch W, Kanaabi M, Namakula FB, Katungisa A, Lyatumi I, et al. Development and validation of near-infrared spectroscopy procedures for prediction of cassava root dry matter and amylose contents in Ugandan cassava germplasm. J Sci Food Agric. 2023. [CrossRef]
- Kanaabi M, Kayondo IS, Nandudu L, Ozimati A, Kawuki RS, Nuwamanya E, et al. Rapid analysis of hydrogen cyanide in fresh cassava roots using NIRSand machine learning algorithms : Meeting end user demand for low cyanogenic cassava. 2023;(July):1–14. [CrossRef]
- Robert P, Brault C, Rincent R, Segura V. Phenomic Selection: A New and Efficient Alternative to Genomic Selection. Methods Mol Biol. 2022;2467:397–420. [CrossRef]
- Siritunga D, Sayre RT. Generation of cyanogen-free transgenic cassava. Planta. 2003;217(3):367–73. [CrossRef]
- Magambo S, Nabatanzi A, Alicai T, Wembabazi E, Oketcho K, Nakalembe I, et al. Somatic embryo production and GFP genetic transformation in elite Ugandan cassava genotypes. Sci African [Internet]. 2024;23(December 2023):e02039. Available from: https://doi.org/10.1016/j.sciaf.2023.e02039. [CrossRef]
- Taylor N, Gaitán-Solís E, Moll T, Trauterman B, Jones T, Pranjal A, et al. A High-throughput Platform for the Production and Analysis of Transgenic Cassava (Manihot esculenta) Plants. Trop Plant Biol [Internet]. 2012 Mar 30 [cited 2024 Jan 3];5(1):127–39. Available from: https://link.springer.com/article/10.1007/s12042-012-9099-4. [CrossRef]
- Ozimati A, Kawuki R, Esuma W, Kayondo IS, Wolfe M. Training Population Optimization for Prediction of Cassava Brown Streak Disease Resistance in West African Clones. 2018. [CrossRef]
- Buontempo C, Thépaut JN, Bergeron C. Copernicus Climate Change Service. IOP Conf Ser Earth Environ Sci. 2020;509(1):10–2. [CrossRef]
- Brown D, de Sousa K, van Etten J. ag5Tools: An R package for downloading and extracting agrometeorological data from the AgERA5 database. SoftwareX [Internet]. 2023;21:101267. Available from: https://doi.org/10.1016/j.softx.2022.101267. [CrossRef]
- Boogaard H, Schubert J, De Wit A, Lazebnik J, Hutjes R, Van der Grijn G. Agrometeorological indicators from 1979 to present derived from reanalysis. Copernicus Clim Chang Serv Clim Data Store (CDS) DOI 1024381/cds6c68c9bb [Internet]. 2020 [cited 2023 Dec 31]; Available from: https://cds.climate.copernicus.eu/cdsapp#!/dataset/10.24381/cds.6c68c9bb?tab=overview.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).