Submitted:
15 January 2024
Posted:
16 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of g-C3N4 (u-CNB) and O-Doped g-C3N4 (O-CNS) Samples
2.2.1. Preparation of g-C3N4 (u-CNB)
2.2.2. Preparation of O-Doped g-C3N4 (O-CNS)
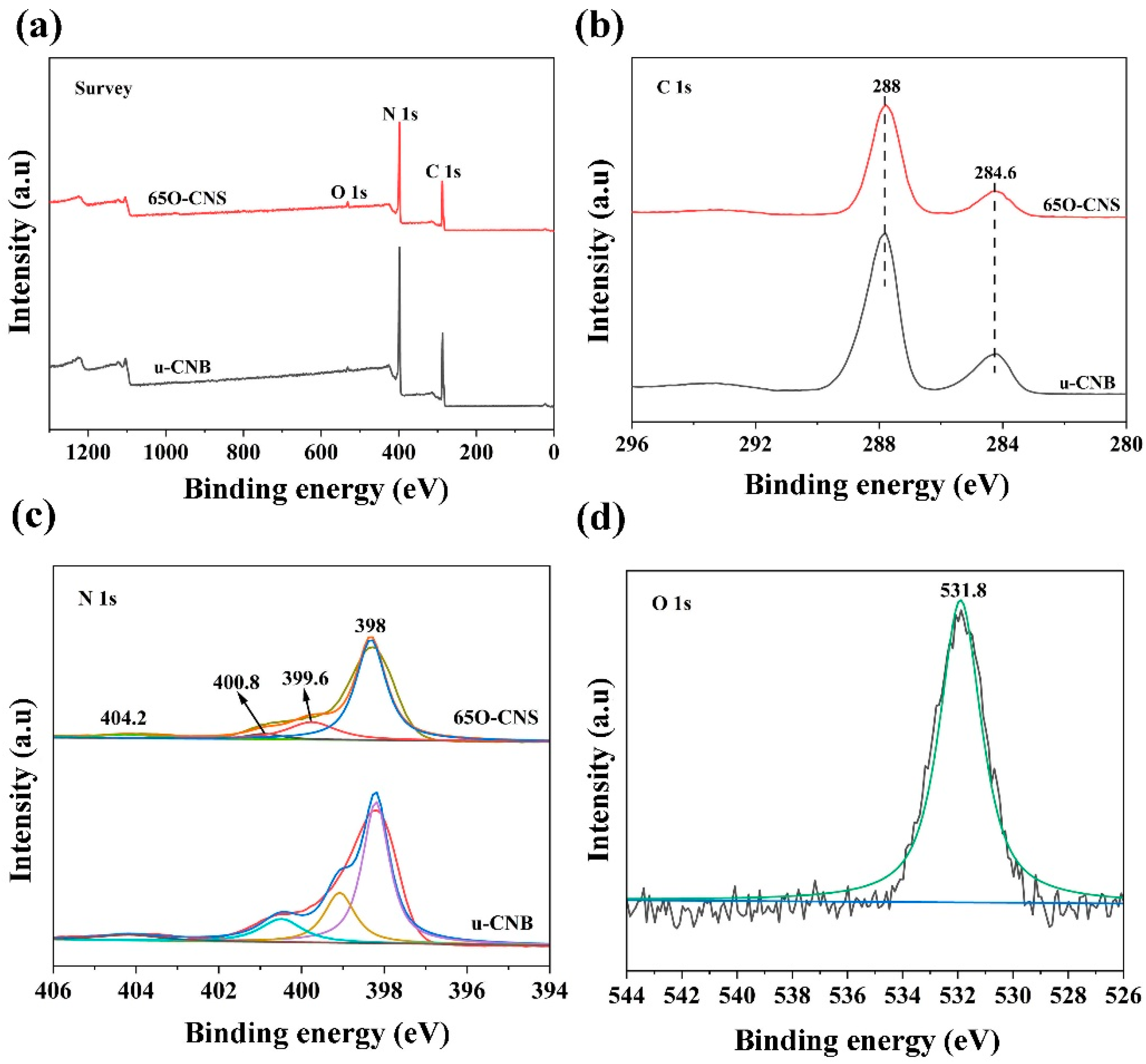
2.3. Characterization
2.4. Photoelectrochemical Measurements
2.5. Photocatalytic Hydrogen Evolution Experiments
3. Results
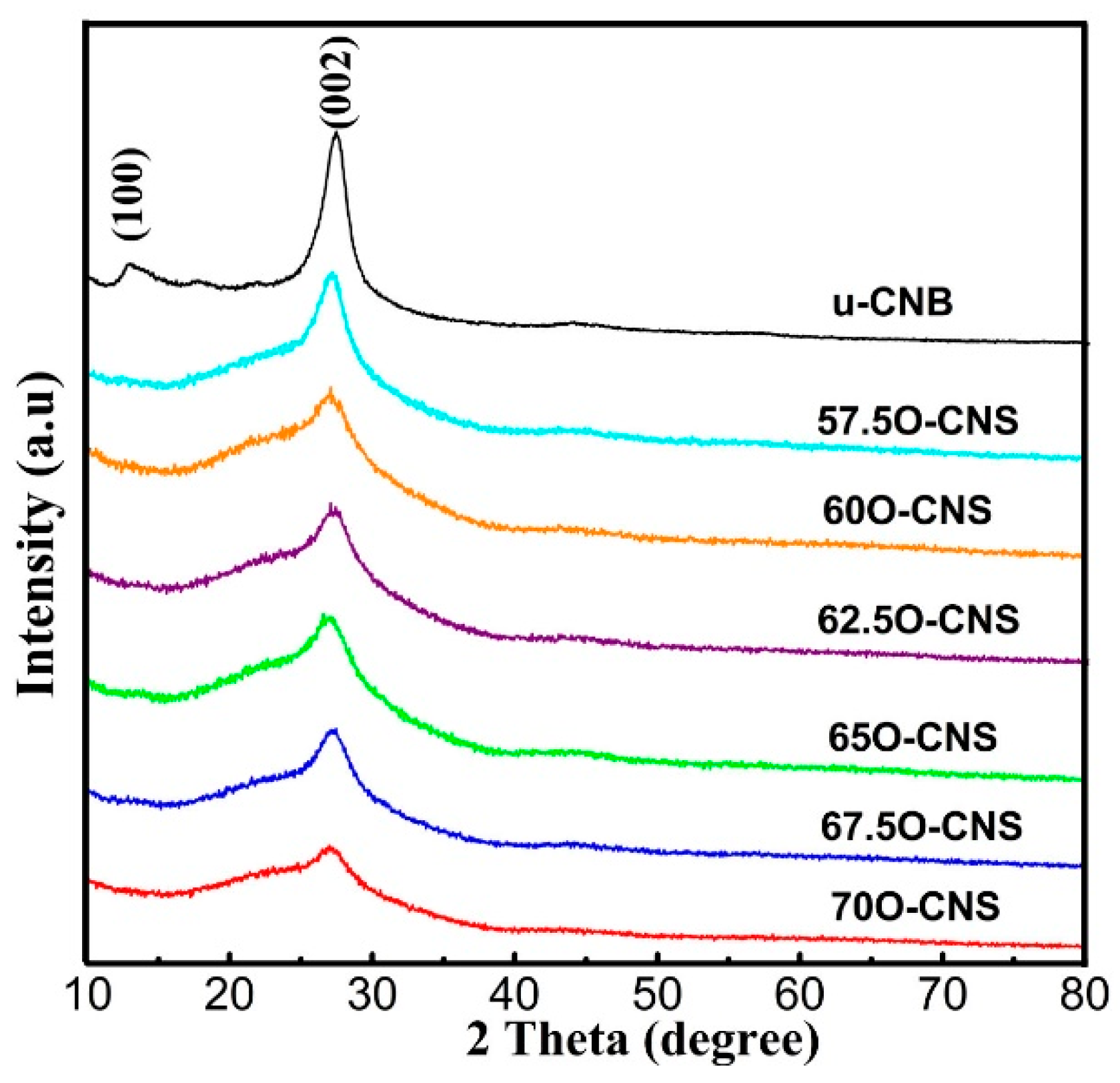
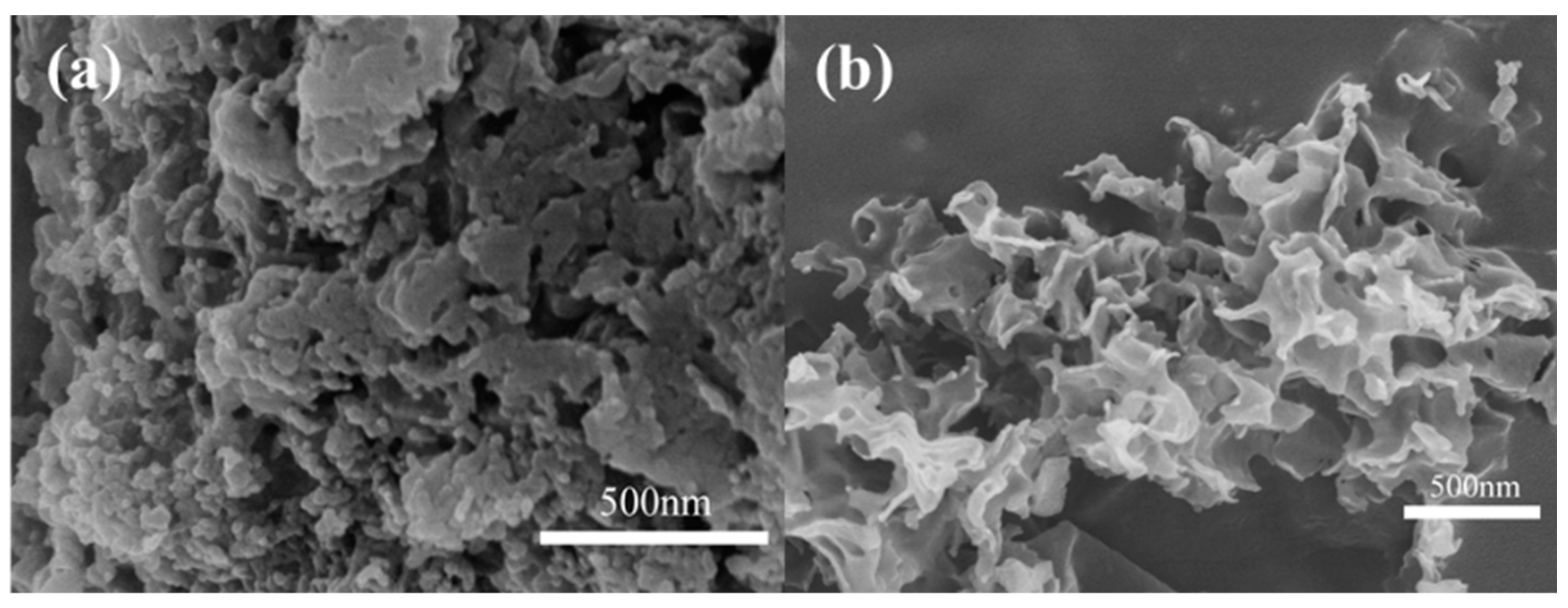
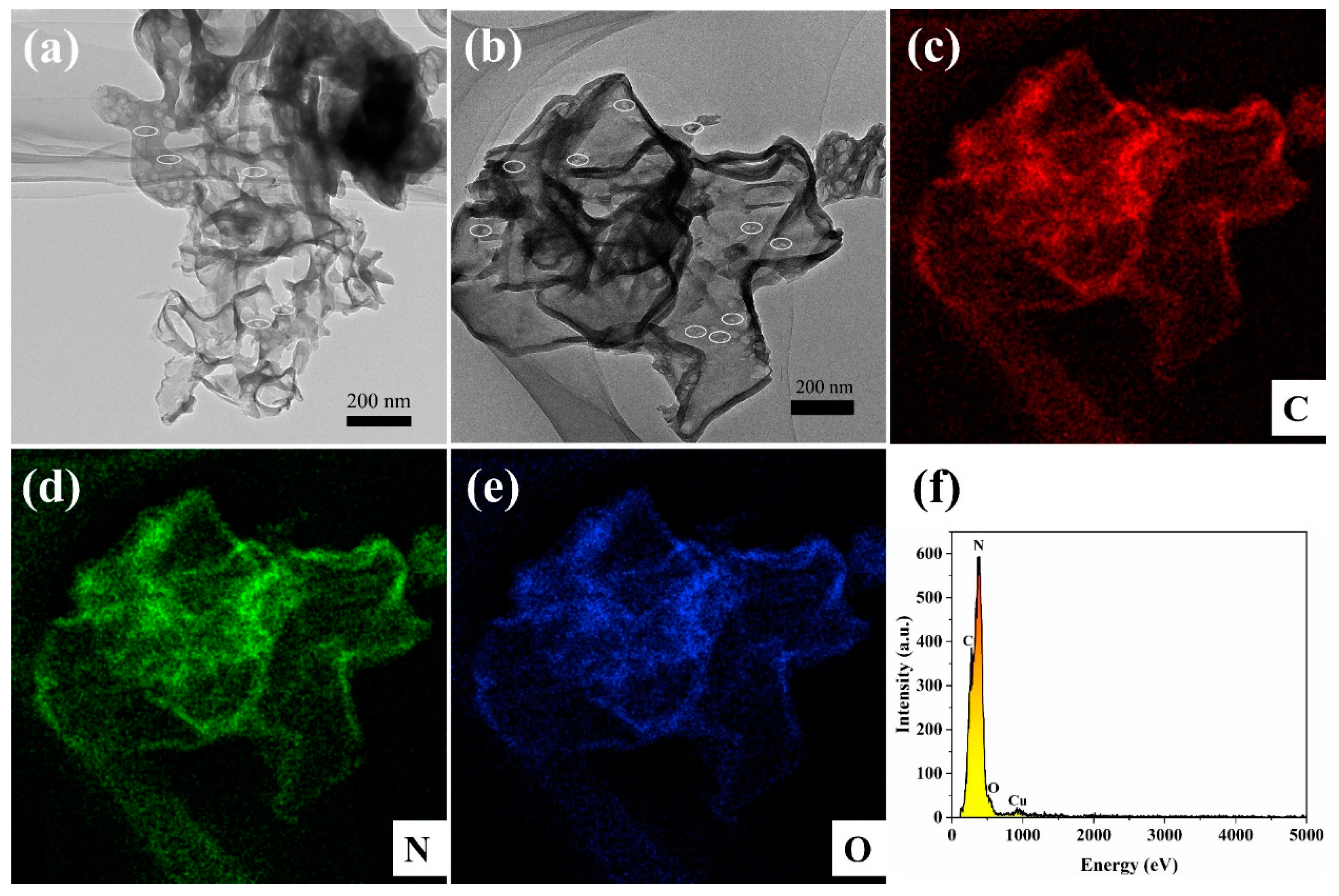
3.1. Structure and Morphology
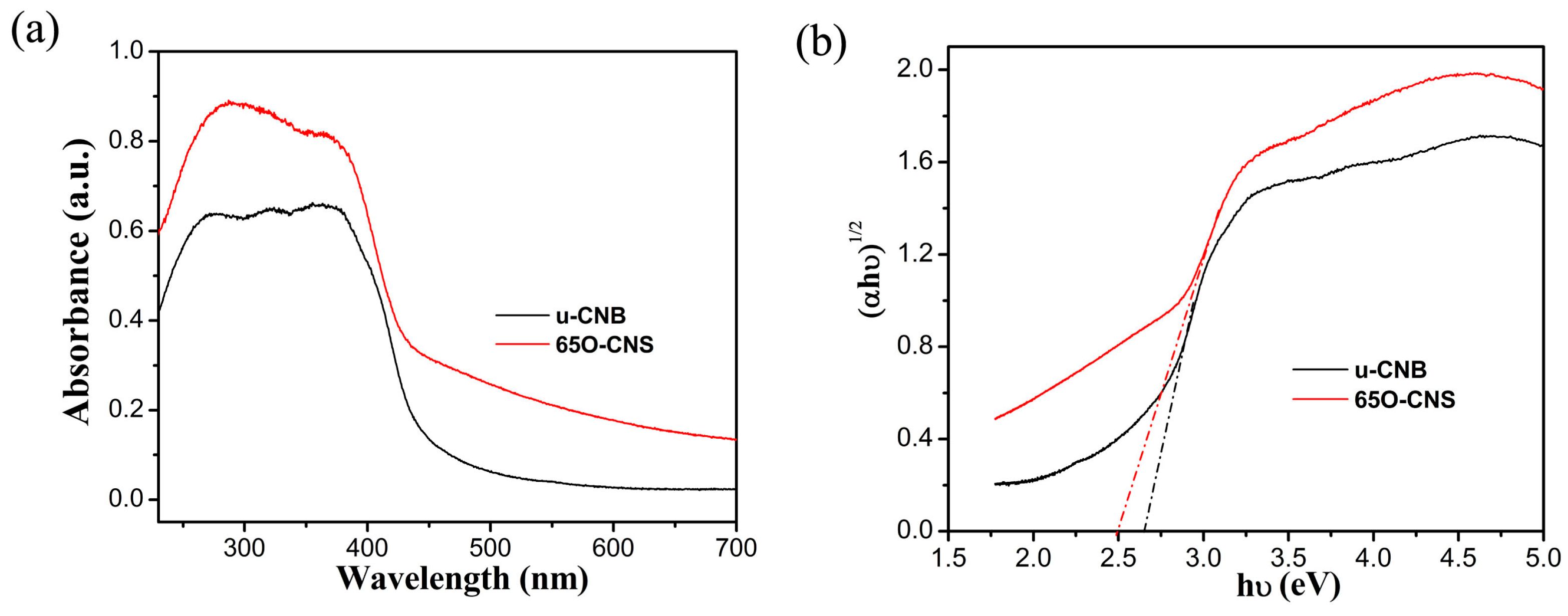
3.2. Specific Surface Area and Optical Properties
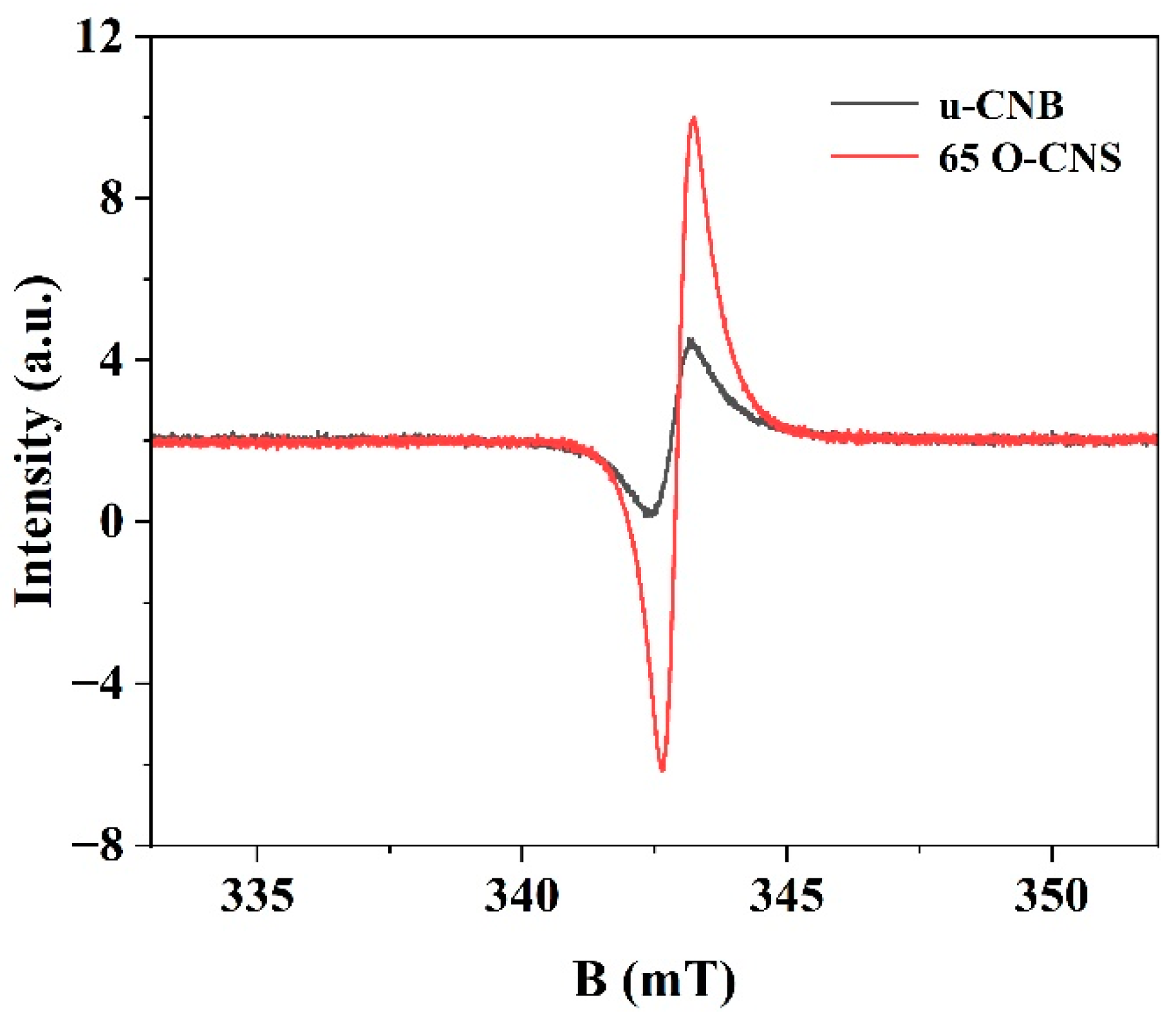
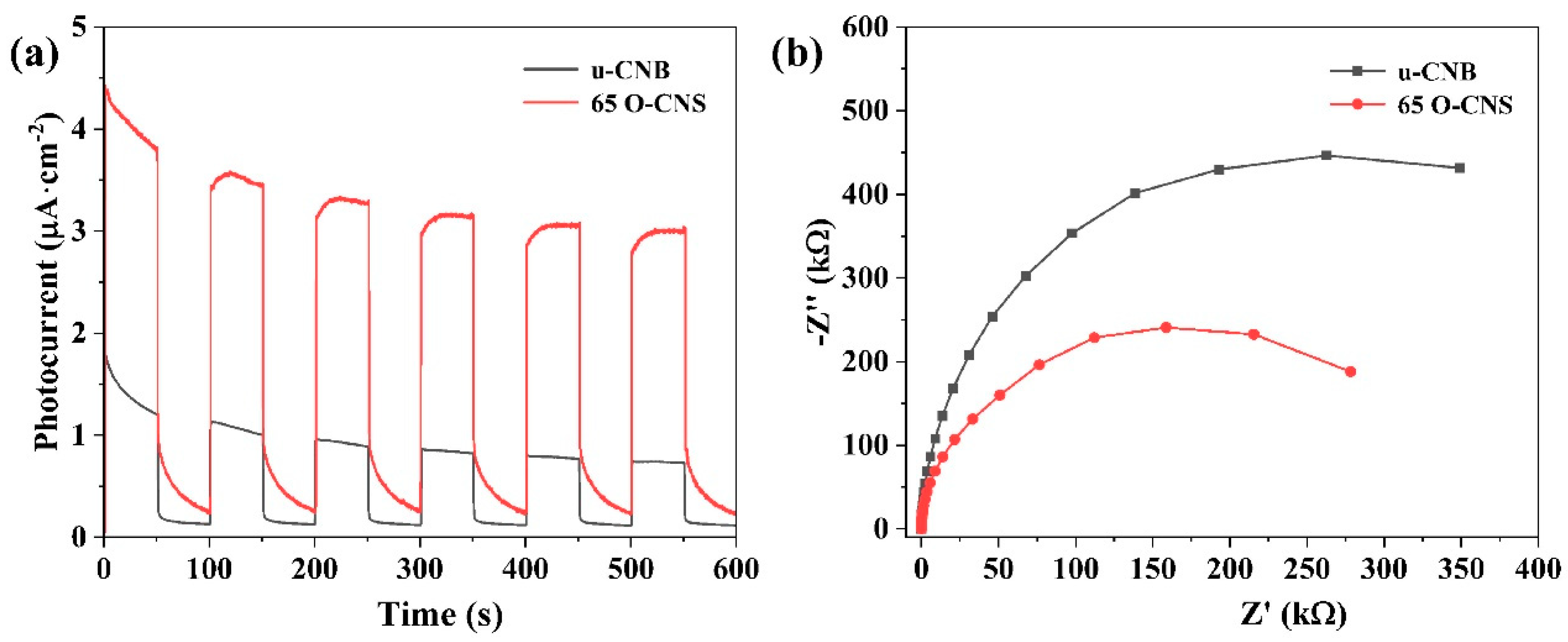
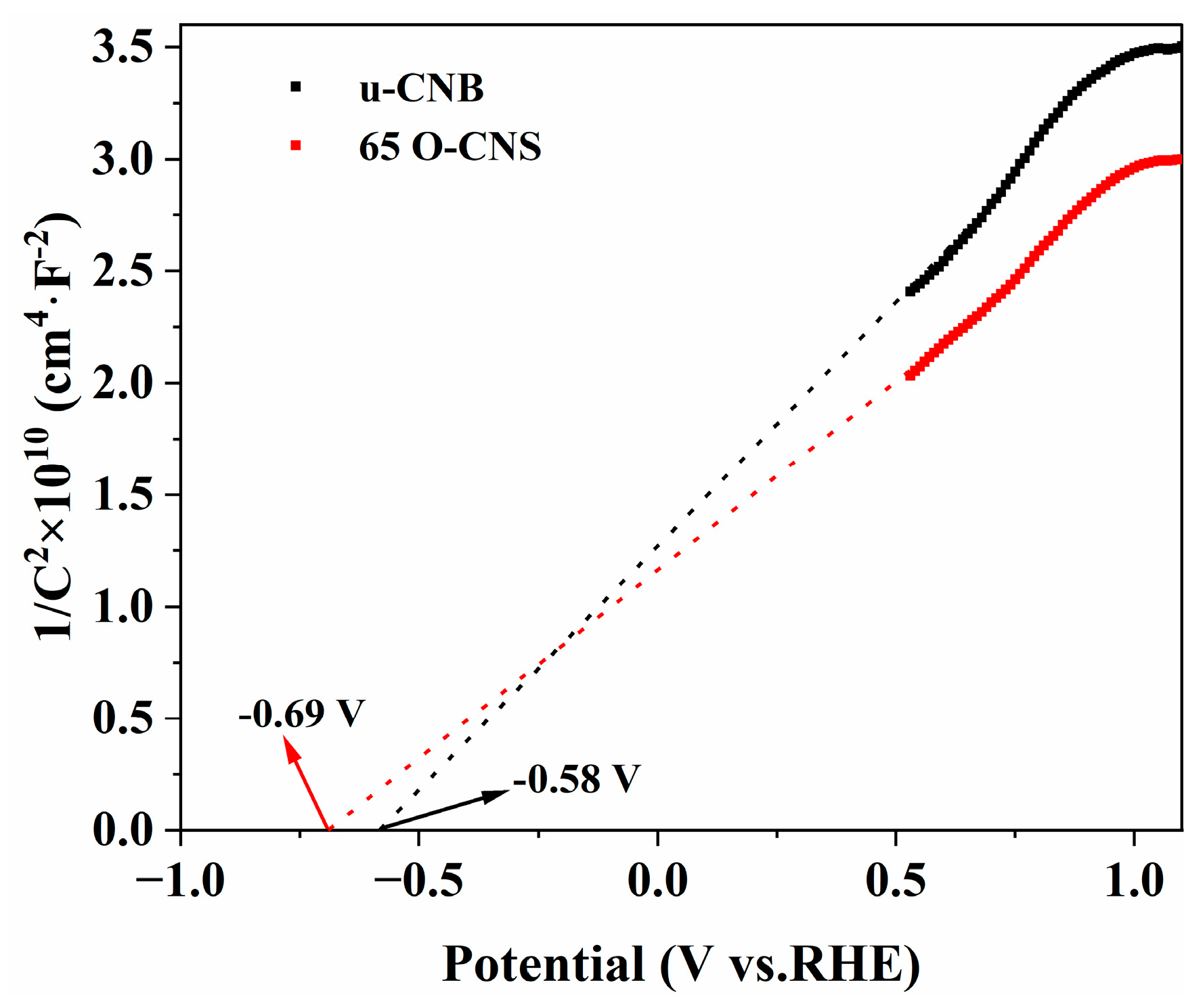
3.3. Electron Paramagnetic Resonance and Electrochemical Measurements
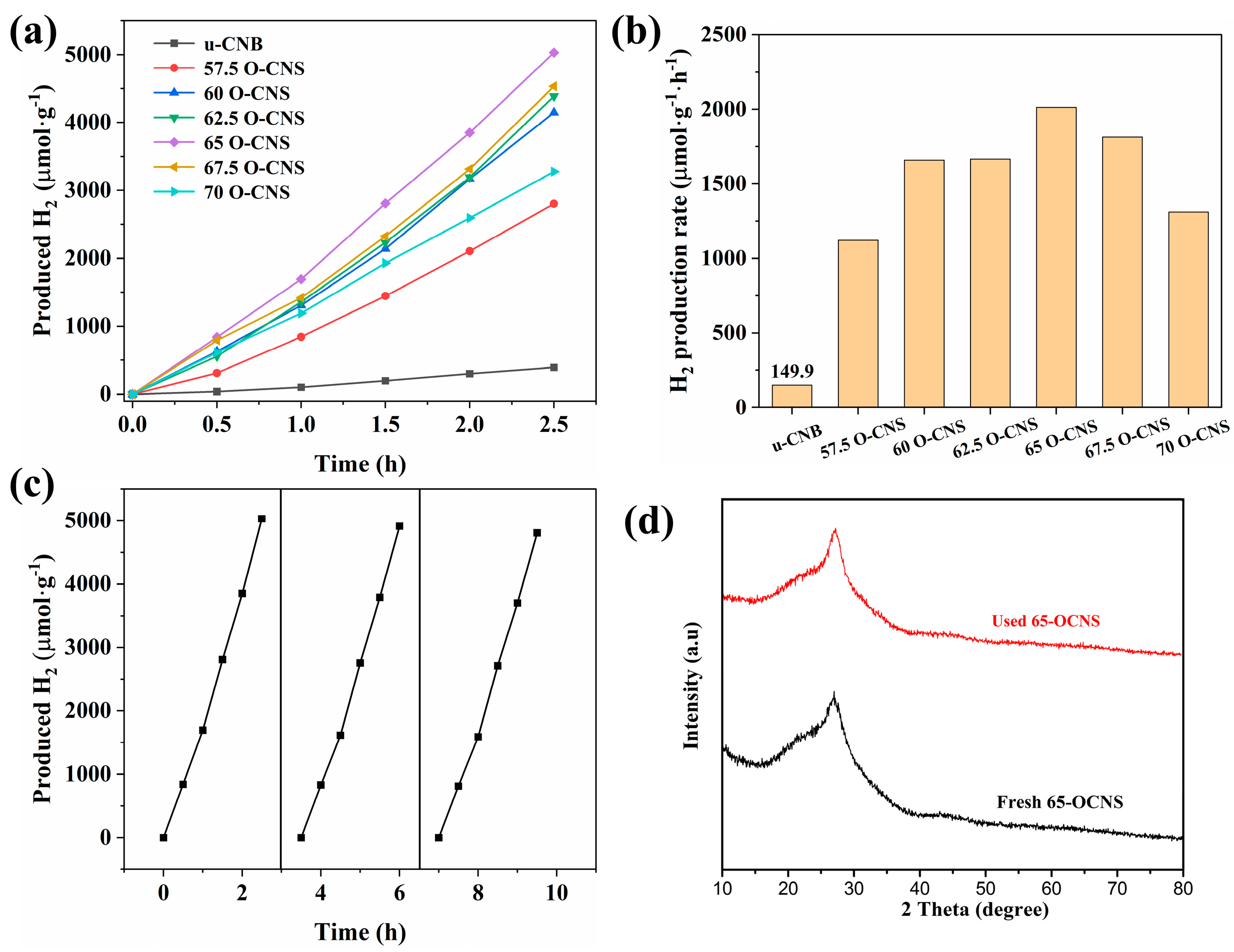
3.3. Photocatalytic Hydrogen Production Performance
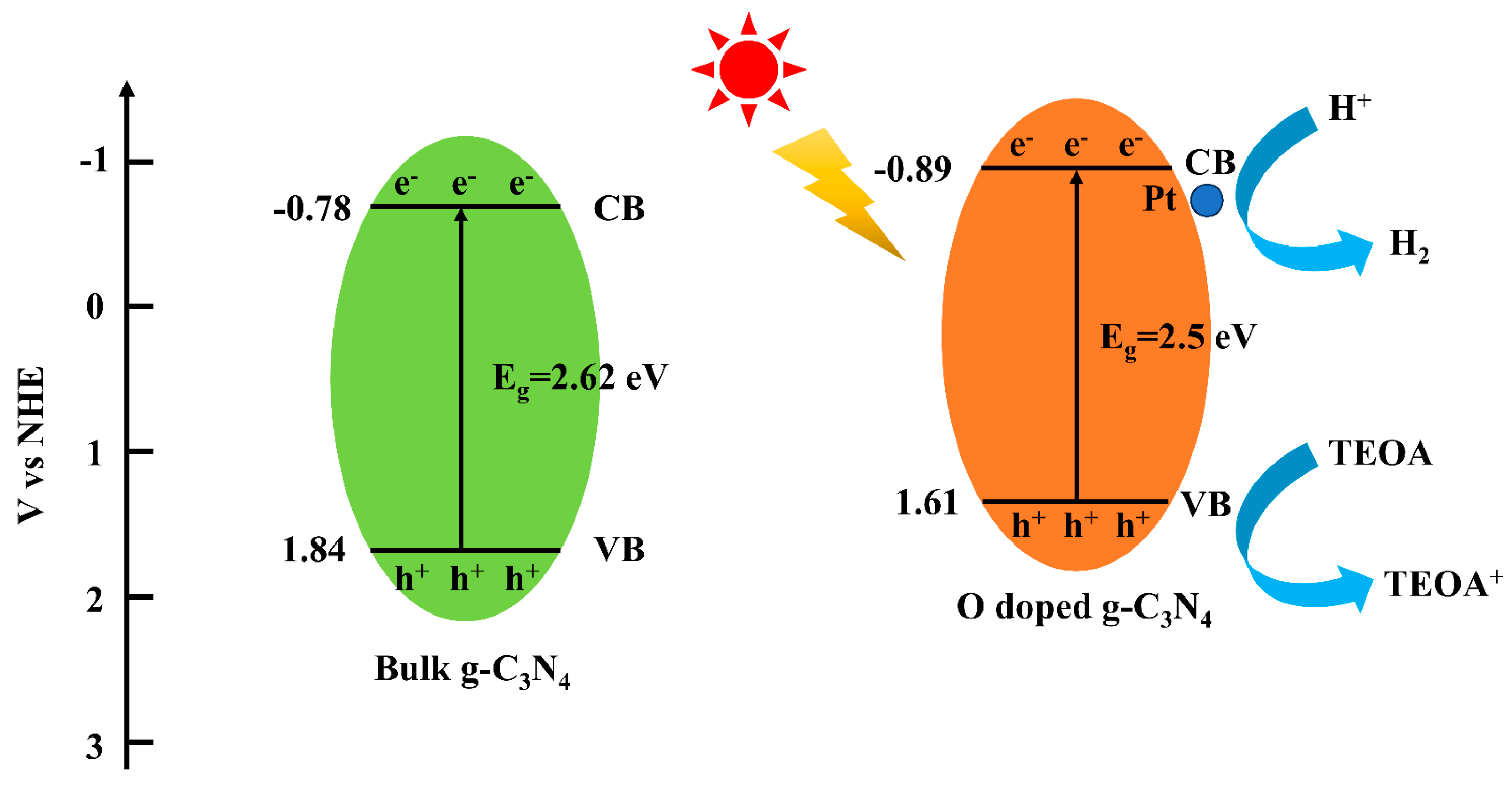
3.4. Photocatalytic Mechanism
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Takata, T.; Jiang, J.; Sakata, Y.; Nakabayashi, M.; Shibata, N.; Nandal, V.; Domen, K. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 2020, 581, 411–414. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, J.; He, B.B.; Wang, H.W.; Wang, R.; Gong, Y.S. In-situ construction of coral-like porous P-doped g-C3N4 tubes with hybrid 1D/2D architecture and high efficient photocatalytic hydrogen evolution. Appl. Catal. B: Environ. 2019, 241, 159–166. [Google Scholar] [CrossRef]
- Liang, Q.; Zhang, C.J.; Xu, S.; Zhou, M.; Zhou, Y.T.; Li, Z.Y. In situ growth of CdS quantum dots on phosphorus-doped carbon nitride hollow tubes as active 0D/1D heterostructures for photocatalytic hydrogen evolution. J. Colloid Interf. Sci. 2020, 577, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.B.; Yuan, X.Z.; Zeng, G.M.; Chen, X.H.; Wu, Z.; Liang, J.; Zhang, J.; Wang, H.; Wang, H. Phosphorus-and sulfur-codoped g-C3N4: facile preparation, mechanism insight, and application as efficient photocatalyst for tetracycline and methyl orange degradation under visible light irradiation. ACS Sustain. Chem. Eng. 2017, 5, 5831–5841. [Google Scholar] [CrossRef]
- Li, Y.; Gu, M.; Zhang, X.M.; Fan, J.J.; Lv, K.L.; Carabineiro, S.A.; Dong, F. 2D g-C3N4 for advancement of photo-generated carrier dynamics: status and challenges. Mater. Today 2020, 41, 270–303. [Google Scholar] [CrossRef]
- Yu, X.N.; Ng, S.F.; Putri, L.K.; Tan, L.L.; Mohamed, A.R.; Ong, W.J. Point-defect engineering: leveraging imperfections in graphitic carbon nitride (g-C3N4) photocatalysts toward artificial photosynthesis. Small 2021, 17, 2006851. [Google Scholar] [CrossRef]
- Liu, X.L.; Ma, R.; Zhuang, L.; Hu, B.W.; Chen, J.R.; Liu, X.Y.; Wang, X.K. Recent developments of doped g-C3N4 photocatalysts for the degradation of organic pollutants. Crit. Rev. Env. Sci. Tec. 2021, 51, 751–790. [Google Scholar] [CrossRef]
- He, F.; Wang, Z.X.; Li, Y.X.; Peng, S.Q.; Liu, B. The nonmetal modulation of composition and morphology of g-C3N4-based photocatalysts. Appl. Catal. B: Environ. 2020, 269, 118828. [Google Scholar] [CrossRef]
- Samanta, S.; Martha, S.; Parida, K. Facile Synthesis of Au/g-C3N4 Nanocomposites: An inorganic/organic hybrid plasmonic photocatalyst with enhanced hydrogen gas evolution under visible-light irradiation. Chemcatchem 2014, 6, 1453–1462. [Google Scholar] [CrossRef]
- Tan, M.X.; Ma, Y.; Yu, C.Y.; Luan, Q.J.; Li, J.J.; Liu, C.B.; Dong, W.J.; Su, Y.J.; Qiao, L.J.; Gao, L.; Lu, Q.P.; Bai, Y. Boosting photocatalytic hydrogen production via interfacial engineering on 2D ultrathin Z-Scheme ZnIn2S4/g-C3N4 heterojunction. Adv. Funct. Mater. 2022, 32, 2111740. [Google Scholar] [CrossRef]
- Deonikar, V.G.; Reddy, K.K.; Chunge, W-J.; Kim, H. Facile synthesis of Ag3PO4/g-C3N4 composites in various solvent systems with tuned morphologies and their efficient photocatalytic activity for multi-dye degradation. J Photoch. Photobio. A Chem. 2019, 368, 168–181. [Google Scholar] [CrossRef]
- Cagdas, Y.; Sule, E-E. Solar light-responsive α-Fe2O3/CdS/g-C3N4 ternary photocatalyst for photocatalytic hydrogen production and photodegradation of methylene blue. J. Alloy Compd. 2022, 908, 164584. [Google Scholar] [CrossRef]
- Shi, Y.X.; Li, L.L.; Sun, H.R.; Xu, Z.; Cai, Y.; Shi, W.L.; Guo, F.; Du, X. Engineering ultrathin oxygen-doped g-C3N4 nanosheet for boosted photoredox catalytic activity based on a facile thermal gas-shocking exfoliation effect. Sep. Purif. Technol. 2022, 292, 121038. [Google Scholar] [CrossRef]
- Jia, X.W.; Li, Y.F.; Liu, X.C.; Yu, X.D.; Wang, C.; Shi, Z.; Xing, Y. Highly crystalline sulfur and oxygen co-doped g-C3N4 nanosheets as an advanced photocatalyst for efficient hydrogen generation. Catal. Sci. Technol. 2022, 12, 5136–5142. [Google Scholar] [CrossRef]
- Tang, H.; Xia, Z.H.; Chen, R.; Liu, Q.Q.; Zhou, T.H. Oxygen doped g-C3N4 with nitrogen vacancy for enhanced photocatalytic hydrogen evolution. Chem-Asian J. 2020, 15, 3456–3461. [Google Scholar] [CrossRef]
- Saka, C. Surface modification with oxygen doping of g-C3N4 nanoparticles by carbon vacancy for efficient dehydrogenation of sodium borohydride in methanol. Fuel 2022, 310, 122444. [Google Scholar] [CrossRef]
- Ta, X.M.C.; Daiyan, R.; Nguyen, T.K.A.; Amal, R.; Tran-Phu, T.; Tricoli, A. Alternatives to water photooxidation for photoelectrochemical solar energy conversion and green H2 production. Adv. Energy Mater. 2022, 12, 2201358. [Google Scholar] [CrossRef]
- Jia, T.K.; Fu, F.; Li, J.; Deng, Z.; Long, F.; Yu, D.S.; Cui, Q.; Wang, W.M. Rational construction of direct Z-scheme SnS/g-C3N4 hybrid photocatalyst for significant enhancement of visible-light photocatalytic activity. Appl. Surf. Sci. 2020, 499, 143941. [Google Scholar] [CrossRef]
- Mo, Z.; Xu, H.; Chen, Z.G.; She, X.J.; Song, Y.H.; Wu, J.J.; Yan, P.C.; Xu, L.; Lei, Y.C.; Yuan, S.Q. Self-assembled synthesis of defect-engineered graphitic carbon nitride nanotubes for efficient conversion of solar energy. Appl. Catal. B Environ. 2018, 225, 154–161. [Google Scholar] [CrossRef]
- Wang, D.D.; Li, Y.H.; Yu, B.; Li, H.J.; Jiang, W.; Deng, X.; Wen, Y.; Liu, C.B.; Che, G.B. Improved visible-light driven photocatalysis by loading Au onto C3N4 nanorods for degradation of RhB and reduction of CO2. Adv. Powder Technol. 2021, 32, 1653–1662. [Google Scholar] [CrossRef]
- Li, J.H.; Shen, B.; Hong, Z.H.; Lin, B.Z.; Gao, B.F.; Chen, Y.L. A facile approach to synthesize novel oxygen-doped g-C3N4 with superior visible-light photoreactivity. Chem. Commun. 2012, 48, 12017–12019. [Google Scholar] [CrossRef]
- Pham, X.N.; Nguyen, H.T.; Pham, T.N.; Nguyen, T.T.B.; Nguyen, M.B.; Tran, V.T.T.; Doan, H.V. Green synthesis of H-ZSM-5 zeolite-anchored O-doped g-C3N4 for photodegradation of Reactive Red 195 (RR 195) under solar light. J. Taiwan Inst. Chem. E. 2020, 114, 91–102. [Google Scholar] [CrossRef]
- Doan, H.V.; Nguyen, H.T.; Ting, V.P.; Guan, S.; Eloi, J.C.; Hall, S.R.; Pham, X.N. Improved photodegradation of anionic dyes using a complex graphitic carbon nitride and iron-based metal-organic framework material. Faraday Discuss. 2021, 231, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gu, P.C.; Ma, R.; Luo, C.T.; Wen, T.; Zhao, G.X.; Cheng, W.C.; Wang, X.K. Recent developments in fabrication and structure regulation of visible-light-driven g-C3N4-based photocatalysts towards water purification: a critical review. Catal. Today 2019, 335, 65–77. [Google Scholar] [CrossRef]
- Hu, S.Z.; Ma, L.; You, J.G.; Li, F.Y.; Fan, Z.P.; Wang, F.; Liu, D.; Gui, J.Z. A simple and efficient method to prepare a phosphorus modified g-C3N4 visible light photocatalyst. Rsc Adv. 2014, 4, 21657–21663. [Google Scholar] [CrossRef]
- Long, X.; Feng, C.; Yang, S.; Ding, D.; Feng, J.; Liu, M.; Chen, Y.; Tan, J.; Peng, X.; Shi, J. Oxygen doped graphitic carbon nitride with regulatable local electron density and band structure for improved photocatalytic degradation of bisphenol A. Chem. Eng. J. 2022, 435, 134835. [Google Scholar] [CrossRef]
- Li, K.X.; Zeng, Z.X.; Yan, L.S.; Luo, S.L.; Luo, X.B.; Huo, M.X.; Guo, Y.H. Fabrication of platinum-deposited carbon nitridenanotubes by a one-step solvothermal treatment strategy and their efficient visible-light photocatalytic activity. Appl. Catal. B Enviro. 2015, 165, 428–437. [Google Scholar] [CrossRef]
- Cheng, C.; Zong, S.C.; Shi, J.W.; Xue, F.; Zhang, Y.Z.; Guan, X.J.; Zheng, B.T.; Deng, J.K.; Guo, L.J. Facile preparation of nanosized MoP as cocatalyst coupled with g-C3N4 by surface bonding state for enhanced photocatalytic hydrogen production. Appl. Catal. B Environ. 2020, 265, 118620. [Google Scholar] [CrossRef]
- Zhang, F.; Xie, F.; Zhu, S.; Liu, J.; Zhang, J.; Mei, S.; Zhao, W. A novel photofunctional g-C3N4/Ag3PO4 bulk heterojunction for decolorization of RhB. Chem. Eng. J. 2013, 228, 435–441. [Google Scholar] [CrossRef]
- Lin, X.H.; Wu, Y.; Xiang, J.; He, D.; Li, S. Elucidation of mesopore-organic molecules interactions in mesoporous TiO2 photocatalysts to improve photocatalytic activity. Appl. Catal. B Environ. 2016, 199, 64–74. [Google Scholar] [CrossRef]
- Jiang, W.; Zong, X.; An, L.; Hua, S.; Miao, X.; Luan, S.; Wen, Y.; Tao, F.F.; Sun, Z. Consciously constructing heterojunction or direct Z-Scheme photocatalysts by regulating electron flow direction. ACS Catal. 2018, 8, 2209–2217. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, B.; Shan, C.; Zhang, W.M.; Dionysiou, D.D.; Pan, B.C. Roles of oxygen-containing functional groups of O-doped g-C3N4 in catalytic ozonation: Quantitative relationship and first-principles investigation. App. Catal. B Environ. 2021, 292, 120155. [Google Scholar] [CrossRef]
- Sun, D.W.; Long, C.C.; Huang, J.H. Highly dispersed platinum-anchored g-C3N4 nanotubes for photocatalytic hydrogen generation. Int. J. Hydrogen Energ. 2023, 48, 943–952. [Google Scholar] [CrossRef]
- Wu, X.; Li, D.; Luo, B.; Chen, B.; Huang, Y.; Yu, T.; Shi, W. Molecular-level insights on NIR-driven photocatalytic H2 generation with ultrathin porous S-doped g-C3N4 nanosheets. Appl. Catal. B: Environ. 2023, 325, 122292. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, J.; Yu, L.; Peng, J.; Song, Z.; Xiong, Z.; Zhai, T. Tailoring advanced N-defective and S-doped g-C3N4 for photocatalytic H2 evolution. Small 2023, 19, 2301116. [Google Scholar] [CrossRef]
- Yang, B.; Han, J.; Zhang, Q.; Liao, G.; Cheng, W.; Ge, G.; Jia, X. Carbon defective g-C3N4 thin-wall tubes for drastic improvement of photocatalytic H2 production. Carbon 2023, 202, 348–357. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhao, S.; Zhang, Y.W.; Fang, J.S.; Zhou, Y.M.; Yuan, S.H.; Zhang, C.; Chen, W.X. One-pot synthesis of K-doped g-C3N4 nanosheets with enhanced photocatalytic hydrogen production under visible-light irradiation. Appl. Surf. Sci. 2018, 440, 258–265. [Google Scholar] [CrossRef]
- Li, X.H.; Li, Y.J.; Zhu, P.F.; Jin, Z.L. Integrating Co3O4 with ZnIn2S4 p-n heterojunction for efficient photocatalytic hydrogen production. Int. J. Energ. Res. 2022, 46, 15589–15601. [Google Scholar] [CrossRef]
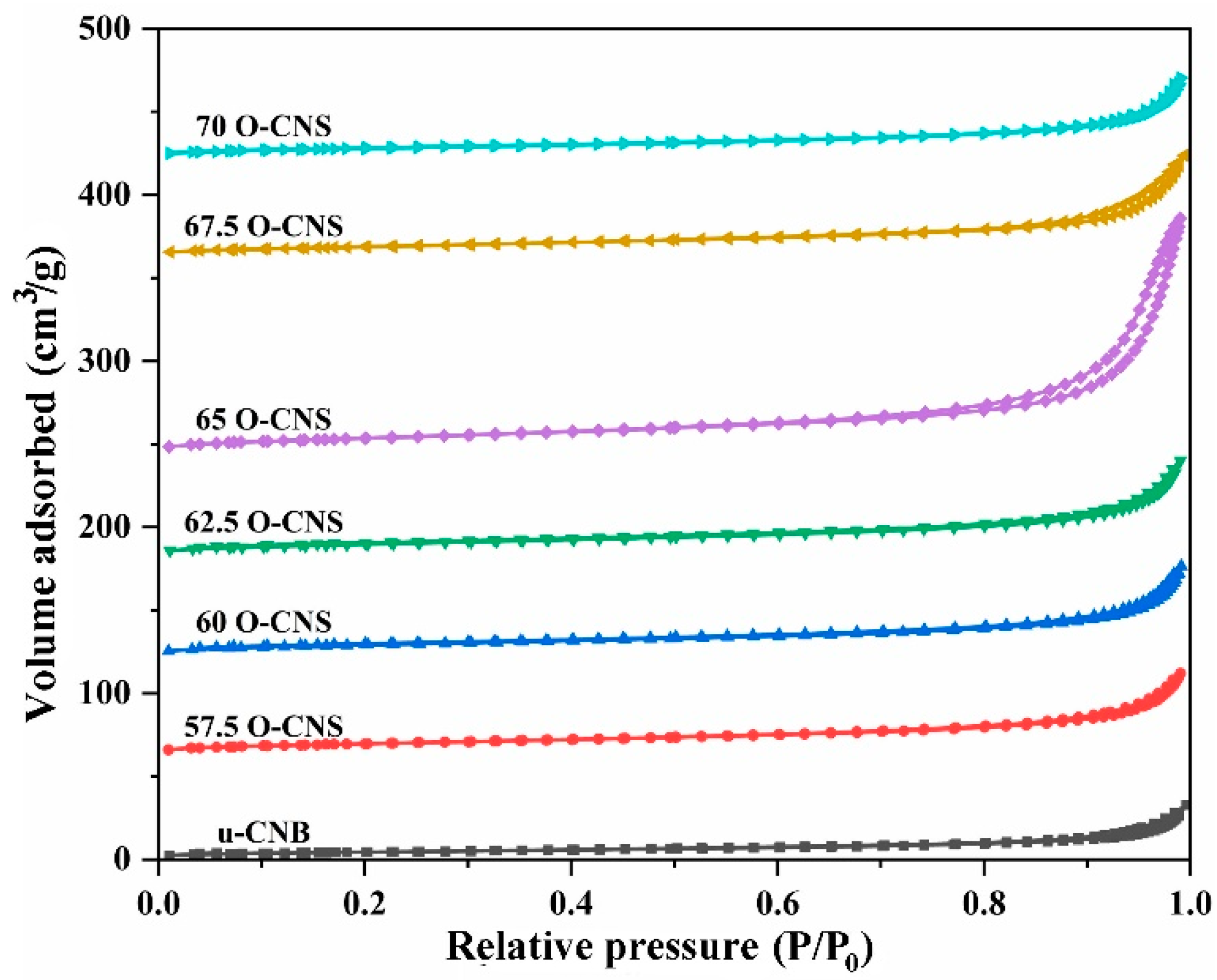
| Samples | BET (m2·g-1) | Average Pore Width (nm) |
Pore Volume (cm3·g-1) |
|---|---|---|---|
| u-CNB | 13.9 | 9.125 | 0.0567 |
| 57.5 O-CNS | 33.8 | 27.256 | 0.327 |
| 60 O-CNS | 32.4 | 34.321 | 0.465 |
| 62.5 O-CNS | 34.5 | 40.539 | 0.544 |
| 65 O-CNS | 48.2 | 43.246 | 0.583 |
| 67.5 O-CNS | 31.3 | 46.784 | 0.614 |
| 70 O-CNS | 28.8 | 49.653 | 0.638 |
| Samples | Light Source | Reactant Solution | Hydrogen Evolution (μmol h-1 g-1) |
Reference |
|---|---|---|---|---|
| O-doped g-C3N4 nanosheets | 300W Xe Lamp (λ > 420 nm) | 80 mL water+20 mL TEOA, 1% H2PtCl6 | 2012.9 | This work |
| Pt/g-C3N4 nanotube | 300W Xe Lamp (λ > 420 nm) | TEOA aqueous solution (100 mL, 10 vol%) | 5304 | Ref [33] |
| S-doped g-C3N4 | 300W Xe Lamp (λ > 420 nm) | 50 mL aqueous TEOA solutions (10 vol%), 2% Pt | 161.32 | Ref [34] |
| Oxygen-doped g-C3N4 sheets | 300 W Xenon lamp (λ > 420 nm) | 100 mL aqueous solution containing 10 vol% TEOA, 3% Pt | 2200 | Ref [15] |
| N-Defective and S-Doped g-C3N4 | 300 W Xe lamp (λ > 420 nm) | 10 mL TEOA+ 90 mL deionized water, 3% Pt | 5651.5 | Ref [35] |
| Carbon defective g-C3N4 | 300 W Xe lamp (λ > 420 nm) | 100 mL of 10 vol% TEOA aqueous solution, 1% Pt | 1534 | Ref [36] |
| K-doped g-C3N4 | 300 W Xe lamp (λ > 400 nm) | 100 mL containing 10 vol % TEOA, 3% Pt | 1337.2 | Ref [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





