Submitted:
15 January 2024
Posted:
16 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
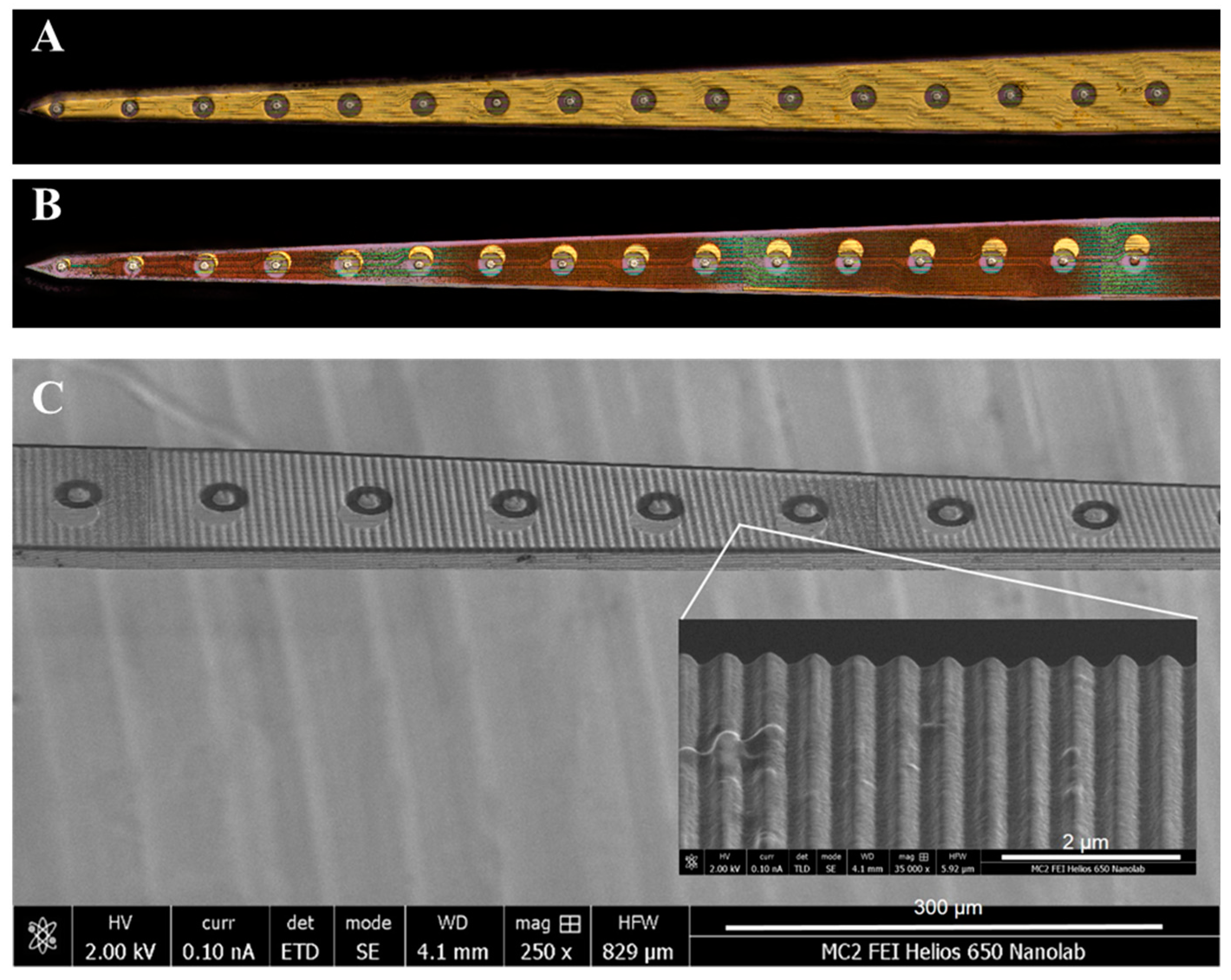
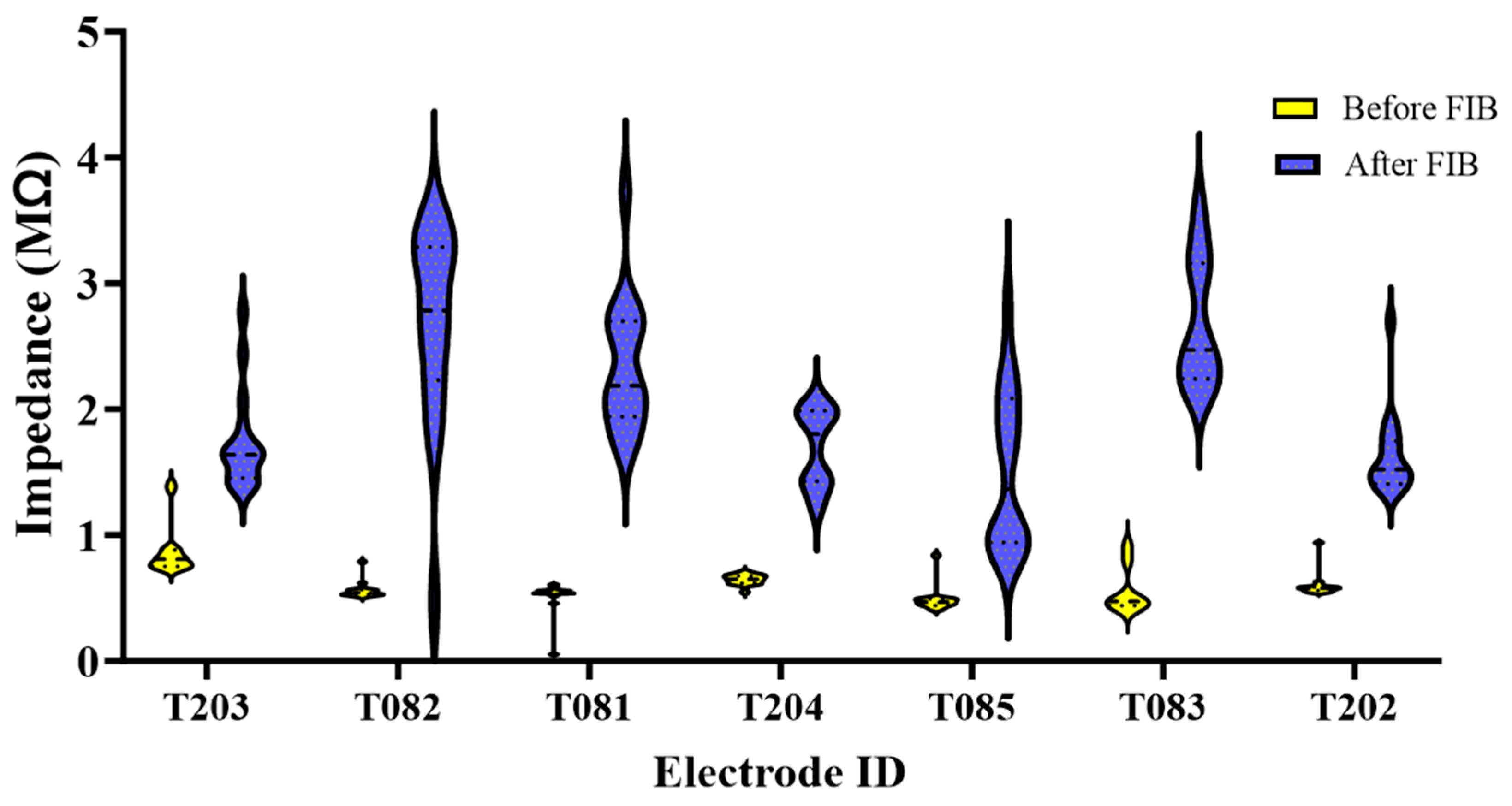
2.1. Neural Probe Manufacturing
2.2. Animals Surgeries
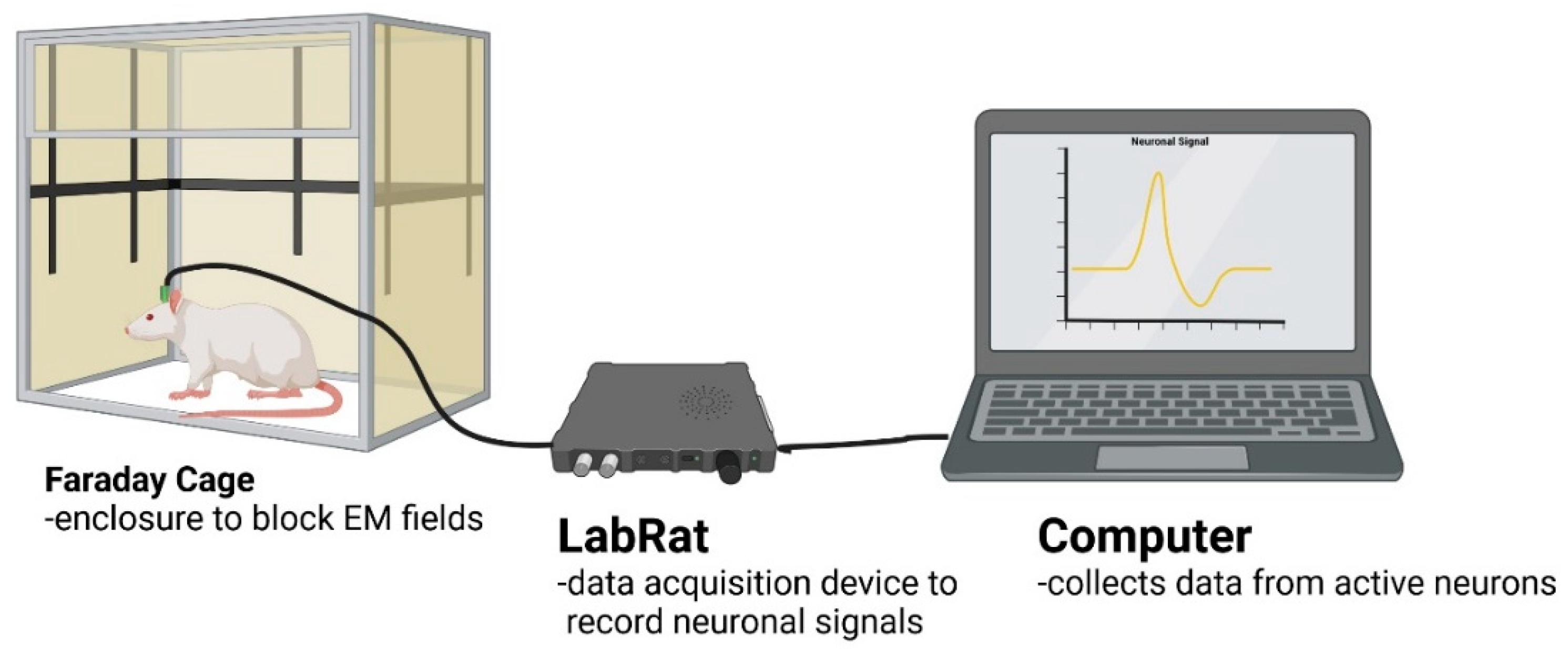
2.3. Neural Recordings
2.4. Recording Analysis
2.5. Cardiac Perfusions
2.6. RNA Isolation
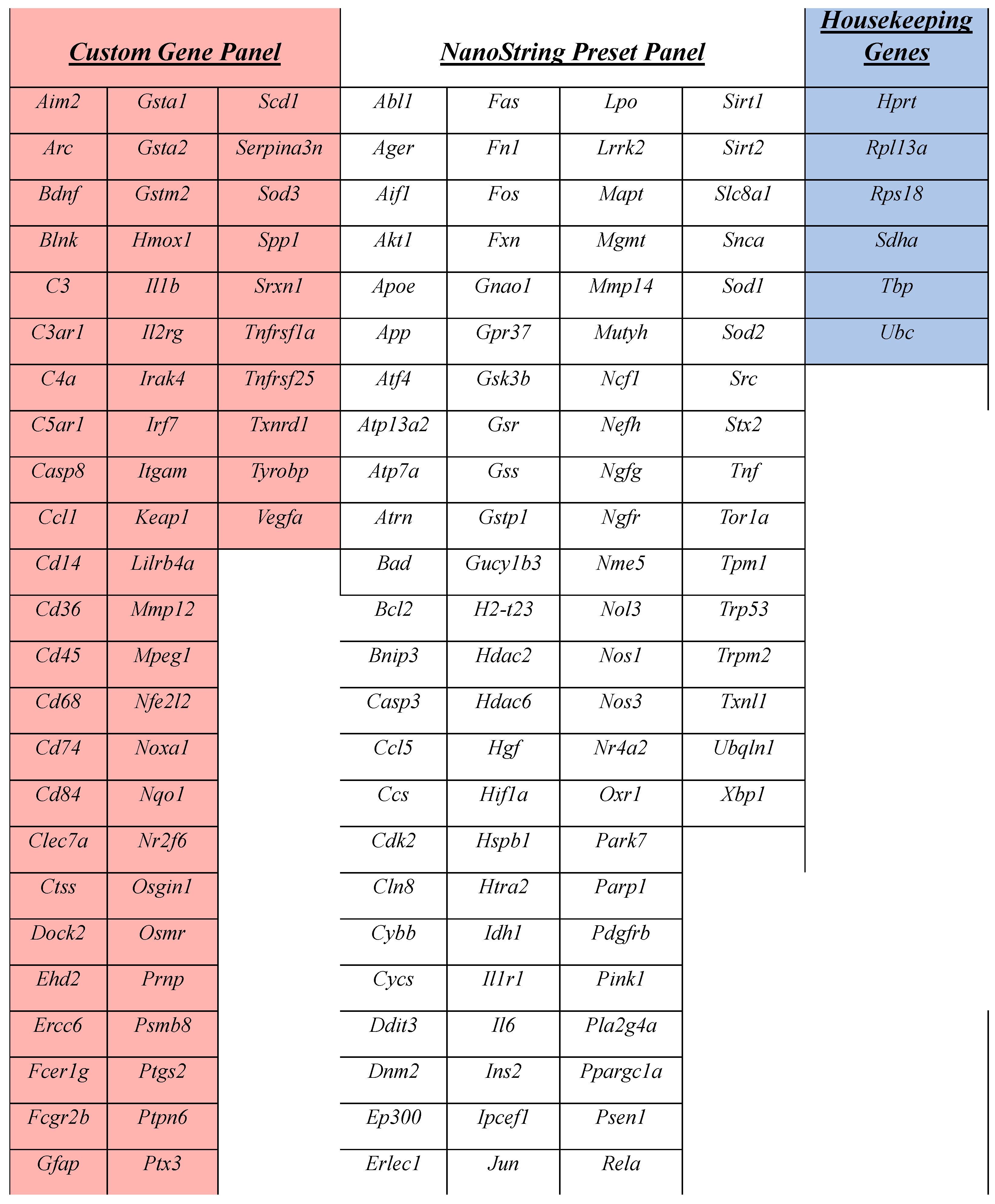
2.7. Gene Expression Assay
2.8. Data Visualization and Statistical Analysis
3. Results
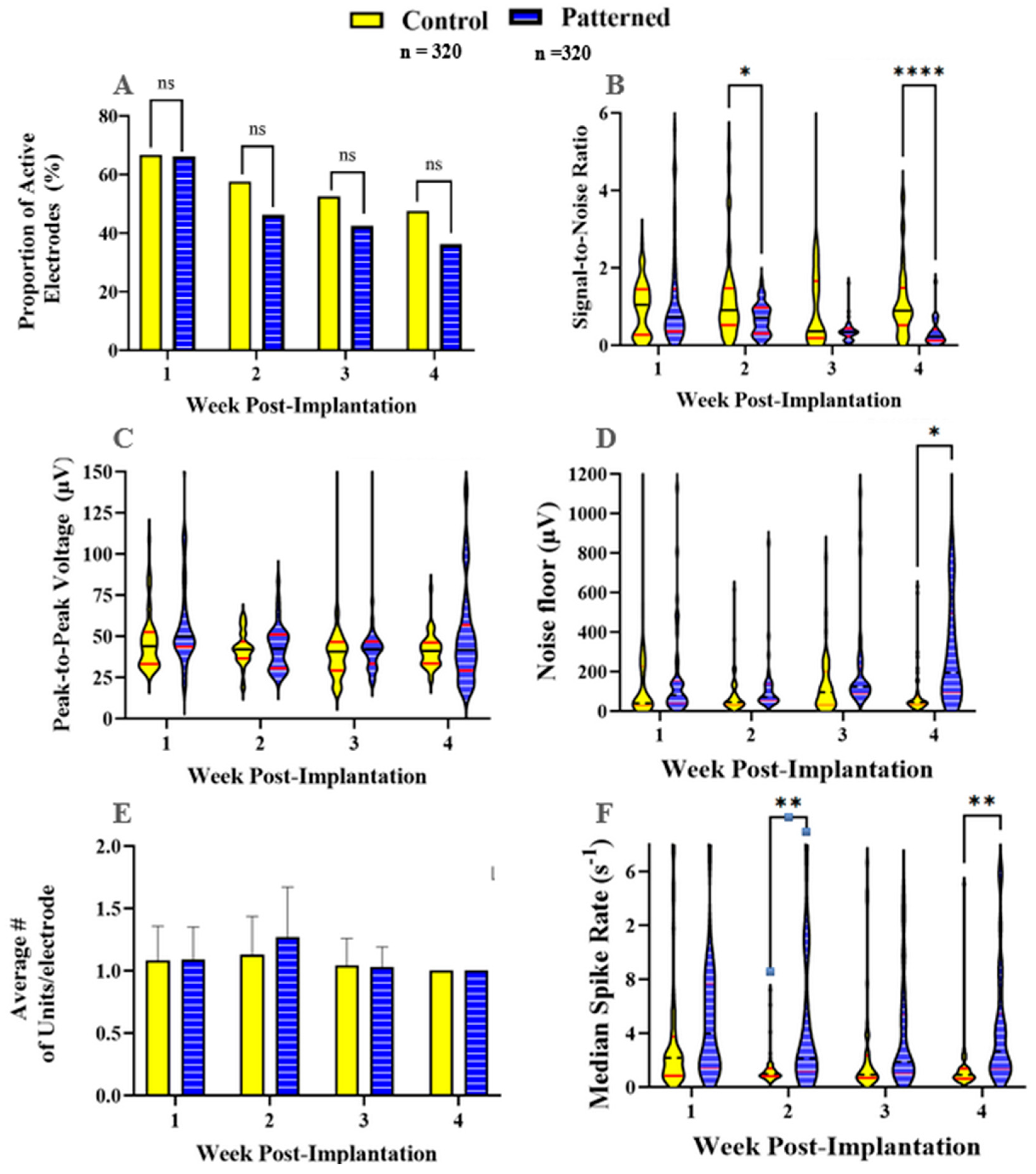
3.1. Recording Metrics
3.2. Gene Expression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Disclosures
Conflicts of Interest
References
- J. Bullard, B. C. Hutchison, J. Lee, C. A. Chestek, and P. G. Patil, "Estimating Risk for Future Intracranial, Fully Implanted, Modular Neuroprosthetic Systems: A Systematic Review of Hardware Complications in Clinical Deep Brain Stimulation and Experimental Human Intracortical Arrays," Neuromodulation, vol. 23, no. 4, pp. 411-426, Jun 2020. [CrossRef]
- J. L. Collinger et al., "High-performance neuroprosthetic control by an individual with tetraplegia," Lancet, vol. 381, no. 9866, pp. 557-64, Feb 16 2013. [CrossRef]
- B. Ajiboye, Willett, F.R., Young, D.R., Memberg, W.D., Murphy, B.A., Miller, J.P., Walter, B.L., Sweet, J.A., Hoyen, H.A., Keith, M.W., Peckham, P.H., Simeral, J.D., Donoghue, J.P., Hochberg, L.R., Kirsch, R.F., "Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: a proof-of-concept demonstration," The Lancet, vol. 398, no. 10081, pp. 1821-1830, 2017. [CrossRef]
- F. R. Willett, D. T. Avansino, L. R. Hochberg, J. M. Henderson, and K. V. Shenoy, "High-performance brain-to-text communication via handwriting," Nature, vol. 593, no. 7858, pp. 249-254, 2021/05/01 2021. [CrossRef]
- R. Kim and T. J. Sejnowski, "Strong inhibitory signaling underlies stable temporal dynamics and working memory in spiking neural networks," Nature neuroscience, vol. 24, no. 1, pp. 129-139, 2021. [CrossRef]
- Y. J. Liew, A. Pala, C. J. Whitmire, W. A. Stoy, C. R. Forest, and G. B. Stanley, "Inferring thalamocortical monosynaptic connectivity in vivo," J Neurophysiol, vol. 125, no. 6, pp. 2408-2431, 2021. [CrossRef]
- M. A. Moffitt and C. C. McIntyre, "Model-based analysis of cortical recording with silicon microelectrodes," Clinical Neurophysiology, vol. 116, no. 9, pp. 2240-2250. , 2005. [CrossRef]
- F. Dunlap, S. C. C. F. Dunlap, S. C. C. IV, E. C. Meyers, M. A. Bockbrader, and D. A. Friedenberg, "Classifying intracortical brain-machine interface signal disruptions based on system performance and applicable compensatory strategies: a review," Frontiers in Neurorobotics, vol. 14, p. 76, 2020. [CrossRef]
- S. Herwik et al., "Fabrication technology for silicon-based microprobe arrays used in acute and sub-chronic neural recording," Journal of Micromechanics and Microengineering 19, no. 7 (2009): 074008. , 2009. [CrossRef]
- K. D. Wise, "Silicon microsystems for neuroscience and neural prostheses," (in eng), IEEE Eng Med Biol Mag, vol. 24, no. 5, pp. 22-9, Sep-Oct 2005. [Online]. Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16248114. [CrossRef]
- S. M. Wellman, L. Li, Y. Yaxiaer, I. McNamara, and T. D. Y. Kozai, "Revealing Spatial and Temporal Patterns of Cell Death, Glial Proliferation, and Blood-Brain Barrier Dysfunction Around Implanted Intracortical Neural Interfaces," Front Neurosci, vol. 13, p. 493, 2019. [CrossRef]
- Bennett et al., "Neuroinflammation, oxidative stress, and blood-brain barrier (BBB) disruption in acute Utah electrode array implants and the effect of deferoxamine as an iron chelator on acute foreign body response," Biomaterials, vol. 188, pp. 144-159, Jan 2019. [CrossRef]
- J. Usoro, B. Sturgill, K. Musselman, J. R. Capadona, and J. J. Pancrazio, "On the definition of ‘chronic’ for intracortical microelectrode array applications," Micromachines, vol. 12, no. 8, p. 972, 2021.
- J. O. Usoro et al., "Influence of Implantation Depth on the Performance of Intracortical Probe Recording Sites," Micromachines (Basel), vol. 12, no. 10, Sep 27 2021. [CrossRef]
- M. Jorfi, J. L. Skousen, C. Weder, and J. R. Capadona, "Progress towards biocompatible intracortical microelectrodes for neural interfacing applications," J Neural Eng, vol. 12, no. 1, p. 011001, Feb 2015. [CrossRef]
- M. Ravikumar et al., "The Roles of Blood-derived Macrophages and Resident Microglia in the Neuroinflammatory Response to Implanted Intracortical Microelectrodes," Biomaterials, vol. S0142-9612, no. 35, pp. 8049-8064, 2014. [CrossRef]
- T. D. Y. Kozai et al., "Reduction of neurovascular damage resulting from microelectrode insertion into the cerebral cortex using in vivo two-photon mapping," Journal of neural engineering, vol. 7, no. 4, p. 046011, 2010. [CrossRef]
- R. D. Readnower et al., "Increase in blood-brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury," (in eng), Journal of Neuroscience Research, Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't vol. 88, no. 16, pp. 3530-9, Dec 2010. [CrossRef]
- Alam et al., "Cellular infiltration in traumatic brain injury," J Neuroinflammation, vol. 17, no. 1, p. 328, Nov 3 2020. [CrossRef]
- Y. Chen et al., "The role of astrocytes in oxidative stress of central nervous system: A mixed blessing," Cell Prolif, vol. 53, no. 3, p. e12781, Mar 2020. [CrossRef]
- K. Biswas, K. Alexander, and M. M. Francis, "Reactive Oxygen Species: Angels and Demons in the Life of a Neuron," NeuroSci, vol. 3, no. 1, pp. 130-145, 2022. [CrossRef]
- K. A. Guttenplan et al., "Neurotoxic Reactive Astrocytes Drive Neuronal Death after Retinal Injury," Cell Reports, vol. 31, no. 12, p. 107776, 2020/06/23/ 2020. [CrossRef]
- S. Ereifej et al., "Implantation of Neural Probes in the Brain Elicits Oxidative Stress," Frontiers in Bioengineering and Biotechnology, vol. Under Revision, 2018. [CrossRef]
- J. P. Harris et al., "Mechanically adaptive intracortical implants improve the proximity of neuronal cell bodies," J Neural Eng, vol. 8, no. 6, p. 066011, Dec 2011. [CrossRef]
- J. K. Nguyen et al., "Mechanically-compliant intracortical implants reduce the neuroinflammatory response," Journal of neural engineering, vol. 11, p. 056014, 2014. [CrossRef]
- T. Ware, D. Simon, R. L. Rennaker, and W. Voit, "Smart Polymers for Neural Interfaces," Polymer Reviews, vol. 53, no. 1, pp. 108-129, 2013. [CrossRef]
- G. Hernandez-Reynoso et al., "The Effect of MnTBAP Coatings on the Acute and Sub-Chronic Recording Performance of Planar Silicon Intracortical Microelectrode Arrays," Biomaterials, vol. 303, p. 122351, 2023. [CrossRef]
- K. A. Potter-Baker et al., "Development of Superoxide Dismutase Mimetic Surfaces to Reduce Accumulation of Reactive Oxygen Species Surrounding Intracortical Microelectrodes " Journal of Materials Chemistry B, vol. 2, pp. 2248-2258, 2014. [CrossRef]
- X. S. Zheng et al., "A superoxide scavenging coating for improving tissue response to neural implants," Acta Biomater, vol. 99, pp. 72-83, 2019. [CrossRef]
- R. Chen et al., "Poly(pro-curcumin) Materials Exhibit Dual Release Rates and Prolonged Antioxidant Activity as Thin Films and Self-Assembled Particles," Biomacromolecules, vol. 24, no. 1, pp. 294-307, Jan 9 2023. [CrossRef]
- M. Ziemba et al., "Development of a Slow-Degrading Polymerized Curcumin Coating for Intracortical Microelectrodes," ACS Appl Bio Mater, vol. 6, no. 2, pp. 806-818, Feb 20 2023. [CrossRef]
- N. M. Y. Kim, W. Schwartzman, D. Sarno, R. Wynder, G.F. Hoeferlin, Laela Gisser, J.R. Capadona, Allison Hess-Dunning. , "Fabrication Methods and Chronic In Vivo Validation of Mechanically Adaptive Microfluidic Intracortical Devices," Micromachines, vol. 14, p. 1015, 2023. [CrossRef]
- J. R. Capadona, A. J. Shoffstall, and J. J. Pancrazio, "Neuron-like neural probes," Nature materials, p. 1, 2019. [CrossRef]
- C. Xie, J. Liu, T.-M. Fu, X. Dai, W. Zhou, and C. M. Lieber, "Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes," Nature Materials, vol. 14, no. 12, pp. 1286-1292, 2015. [CrossRef]
- J. P. Seymour and D. R. Kipke, "Neural probe design for reduced tissue encapsulation in CNS," (in Eng), Biomaterials, vol. 28, no. 25, pp. 3594-607, Apr 5 2007. [CrossRef]
- G. Hong and C. M. Lieber, "Novel electrode technologies for neural recordings," Nature reviews, vol. 20, no. 6, pp. 330-345, Jun 2019. [CrossRef]
- Shoffstall and J. R. Capadona, "Prospects for a robust cortical recording interface," in Neuromodulation: Academic Press, 2018, pp. 393-413. [CrossRef]
- H. A. E. Doris Lam, Jose Cadena, Sandra K. G. Peters Ana Paula Sales,m Joanne J. Osburn, David A Soscia, Kristen S. Kulp, Elizabeth K Wheeler, Nicholar O. Fischer, "Tissue-specific extracellular matrix accelerates the formation of neural networks and communities in a neuron-glia co-culture on a multi-electrode array," Scientific Reports, 2019. [CrossRef]
- R. Kriparamanan, P. Aswath, A. Zhou, L. Tang, and K. T. Nguyen, "Nanotopography: cellular responses to nanostructured materials," J Nanosci Nanotechnol, vol. 6, no. 7, pp. 1905-19, Jul 2006. [CrossRef]
- S. Ereifej et al., "Nanopatterning effects on astrocyte reactivity," J Biomed Mater Res A, vol. 101, no. 6, pp. 1743-57, Jun 2013. [CrossRef]
- S. Ereifej, C. S. Smith, S. M. Meade, K. Chen, H. Feng, and J. R. Capadona, "The neuroinflammatory response to nanopatterning parallel grooves into the surface structure of intracortical microelectrodes," Advanced Functional Materials, vol. 28, no. 12, p. 1704420, 2018. [CrossRef]
- Y. Kim et al., "Nano-Architectural Approaches for Improved Intracortical Interface Technologies," Front Neurosci, vol. 12, p. 456, 2018. [CrossRef]
- J. Selvakumaran, J. L. Keddie, D. J. Ewins, and M. P. Hughes, "Protein adsorption on materials for recording sites on implantable microelectrodes," J. Mater. Sci. Mater. Med., vol. 19, pp. 143–151, 2008. [CrossRef]
- E. S. Ereifej, M. M. Cheng, G. Mao, and P. J. VandeVord, "Examining the inflammatory response to nanopatterned polydimethylsiloxane using organotypic brain slice methods," J Neurosci Methods, vol. 217, no. 1-2, pp. 17-25, Jul 15 2013. [CrossRef]
- W. Bedell et al., "Targeting CD14 on blood derived cells improves intracortical microelectrode performance," Biomaterials, vol. 163, pp. 163-173, 2018. [CrossRef]
- W. Bedell et al., "Understanding the Effects of Both CD14-Mediated Innate Immunity and Device/Tissue Mechanical Mismatch in the Neuroinflammatory Response to Intracortical Microelectrodes," Front Neurosci, vol. 12, p. 772, 2018. [CrossRef]
- K. Hermann, Ravikumar, M, Shoffstall, A, Ereifej, E, Kovach, K, et al, "Inhibition of the cluster of differentiation 14 innate immunity pathway with IAXO-101 improves chronic microelectrode performance," Journal of neural engineering, 2017. [CrossRef]
- K. Hermann and J. R. Capadona, "Understanding the Role of Innate Immunity in the Response to Intracortical Microelectrodes," Crit Rev Biomed Eng, vol. 46, no. 4, pp. 341-367, 2018. [CrossRef]
- K. Hermann et al., "The Role of Toll-Like Receptor 2 and 4 Innate Immunity Pathways in Intracortical Microelectrode-Induced Neuroinflammation," Front Bioeng Biotechnol, vol. 6, p. 113, 2018. [CrossRef]
- T. B. G.F. Hoeferlin, H. Olivares, J. Zhang, L.N. Druschel, B. Sturgill, M. Sobota, P. Boucher, J. Duncan, A.G. Hernandez-Reynoso, S.F. Cogan, J.J. Pancrazio, J.R. Capadona "Antioxidant Dimethyl Fumarate Temporarily but not Chronically Improves Microelectrode Performance," Micromachines, vol. 14, no. 10, p. 1902, 2023. [CrossRef]
- S. Song, L. N. Druschel, R. Chan, and J. R. Capadona, "Differential expression of genes involved in the chronic response to intracortical microelectrodes " Acta Biomaterialia, vol. Under Review, 2023. [CrossRef]
- S. Song et al., "Differential expression of genes involved in the chronic response to intracortical microelectrodes implanted in complement factor 3 depleted mice," Brain Behavior and Immunity, vol. Under Review, 2024.
- G. H.-R. B. Sturgill, L. Druschel, T.J. Smith, P.E. Boucher, G.F. Hoeferlin, T. T. Doan Thai, M.S. Jiang, J Hess, N.N. Alam, D.M. Menendez, J. Duncan, S.F. Cogan, J.P. Pancrazio*, J.R. Capadona, "Reactive Amine Functionalized Microelectrode Arrays Provide Short-Term Benefit but Long-Term Detriment to In Vivo Recording Performance," ACS Applied Bio Materials, vol. Accepted - In Press, 2024. [CrossRef]
- D. Y. Kim, N. Mueller, D. Sarno, K. Gisser, A. Hess-Dunning, J.R. Capadona, "In Vivo Validation of a Mechanically Adaptive Microfluidic Intracortical Device as a Platform for Sustain Local Drug Delivery," Frontiers in Biomaterials Science, vol. 2, p. 1279367, 2023. [CrossRef]
- N. D. S. Song, R. Chan, J.R. Capadona, "Differential expression of genes involved in the chronic response to intracortical microelectrodes," Acta Biomaterialia, vol. 1, no. 169, pp. 348-362, 2023. [CrossRef]
- S. Song, B. Regan, E. S. Ereifej, E. R. Chan, and J. R. Capadona, "Neuroinflammatory Gene Expression Analysis Reveals Pathways of Interest as Potential Targets to Improve the Recording Performance of Intracortical Microelectrodes," Cells, vol. 11, no. 15, Jul 30 2022. [CrossRef]
- H. W. Bedell, N. J. Schaub, J. R. Capadona, and E. S. Ereifej, "Differential expression of genes involved in the acute innate immune response to intracortical microelectrodes," Acta Biomater, vol. 102, pp. 205-219, Jan 15 2020. [CrossRef]
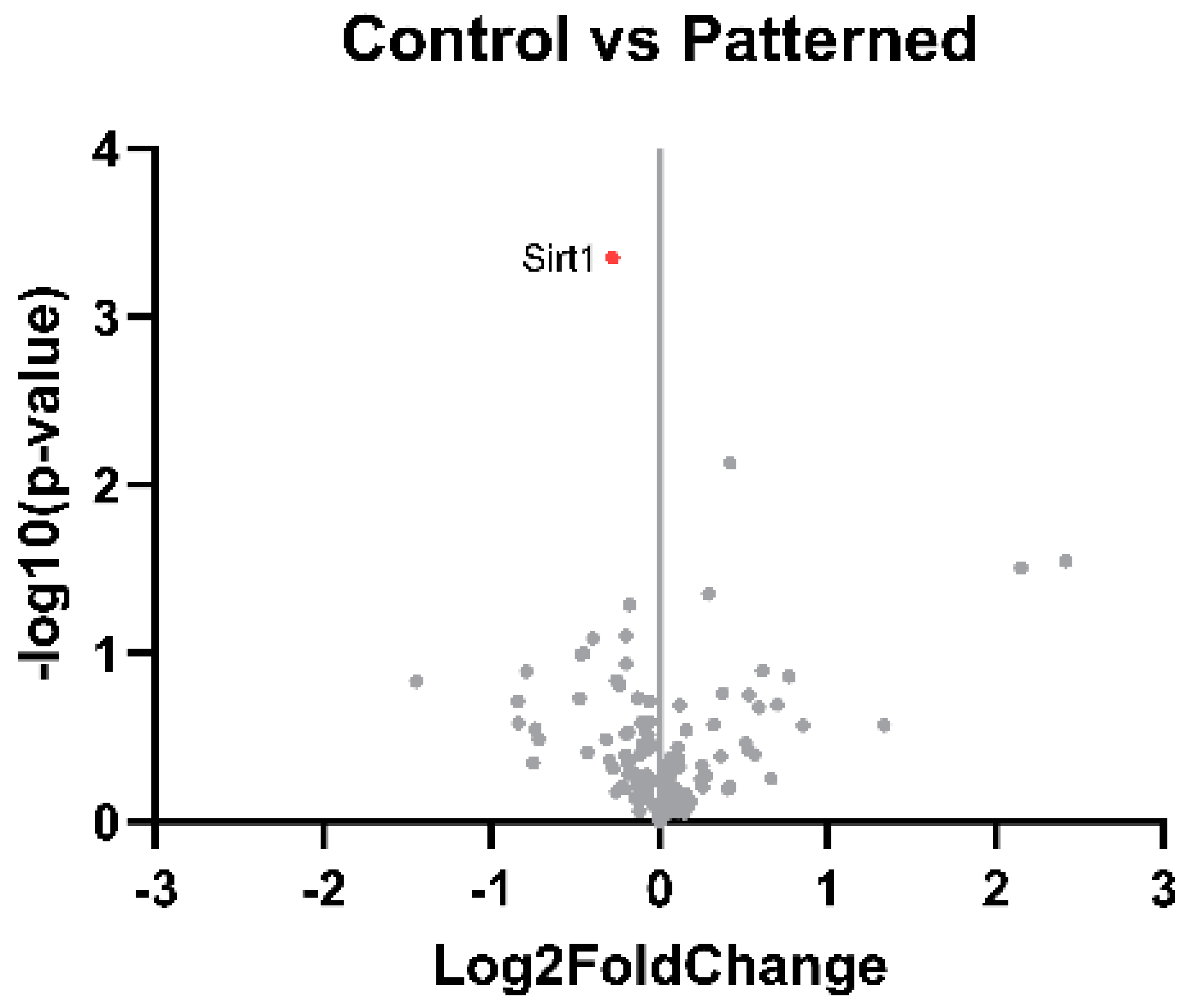
- "Sirt1 sirtuin 1 [ Rattus norvegicus (Norway rat) ]." NIH. https://www.ncbi.nlm.nih.gov/gene/309757/,%20https://www.frontiersin.org/articles/10.3389/fimmu.2022.831168/full (accessed 2023).
- J. H. Lim, Y. M. Lee, Y. S. Chun, J. Chen, J. E. Kim, and J. W. Park, "Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha," Mol Cell, vol. 38, no. 6, pp. 864-78, Jun 25 2010. [CrossRef]
- Y. Yang et al., "Regulation of SIRT1 and Its Roles in Inflammation," Front Immunol, vol. 13, p. 831168, 2022. [CrossRef]
- J. O. Usoro et al., "Influence of Implantation Depth on the Performance of Intracortical Probe Recording Sites," Micromachines, vol. 12, no. 10, 2021. [CrossRef]
- J. O. Usoro, B. S. Sturgill, K. C. Musselman, J. R. Capadona, and J. J. Pancrazio, "Intracortical Microelectrode Array Unit Yield under Chronic Conditions: A Comparative Evaluation," Micromachines, vol. 12, no. 8, 2021. [CrossRef]
- S. F. Cogan, "Neural stimulation and recording electrodes," Annu Rev Biomed Eng, vol. 10, pp. 275-309, 2008. [CrossRef]
- M. Stiller et al., "Mechanically Robust, Softening Shape Memory Polymer Probes for Intracortical Recording," Micromachines (Basel), vol. 11, no. 6, Jun 25 2020. [CrossRef]
- A. R. Chapman, Hao Chen, Marianna Stamou, Juergen Biener, Monika M. Biener, Pamela J. Lein, and Erkin Seker., " Nanoporous Gold as a Neural Interface Coating: Effects of Topography, Surface Chemistry, and Feature Size," ACS Appl Mater Inter, vol. 7, no. 13, pp. 7093–7100, 2015. [CrossRef]
- Simitzi, A. Ranella, and E. Stratakis, "Controlling the Morphology and Outgrowth of Nerve and Neuroglial Cells: The Effect of Surface Topography," Acta Biomaterialia, vol. 51, pp. 21–52, 2017. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





