Submitted:
15 January 2024
Posted:
16 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Materials
2.2. Preparation of TKS rubber reference samples
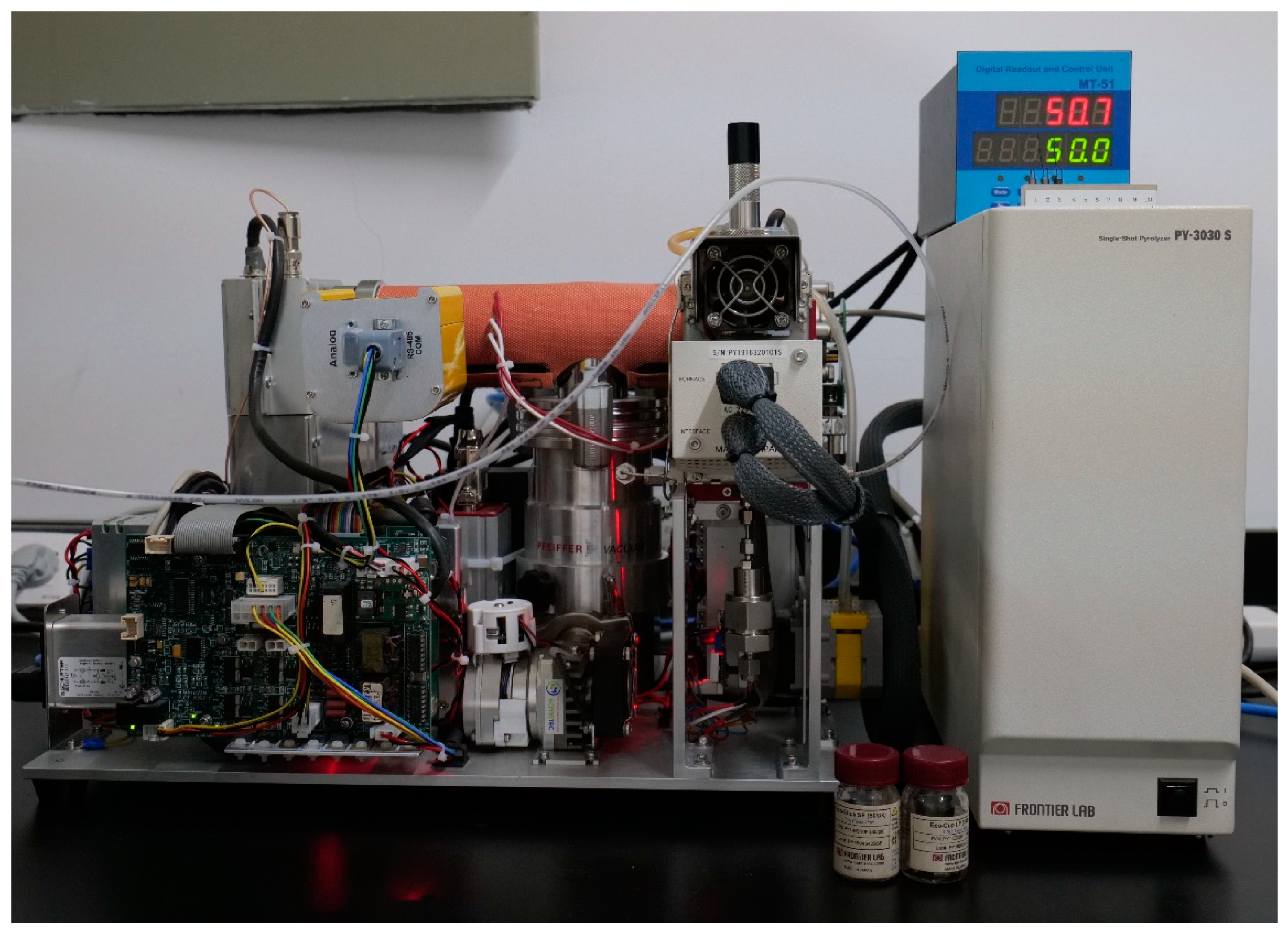
2.3. Experimental apparatus
3. Results and discussion
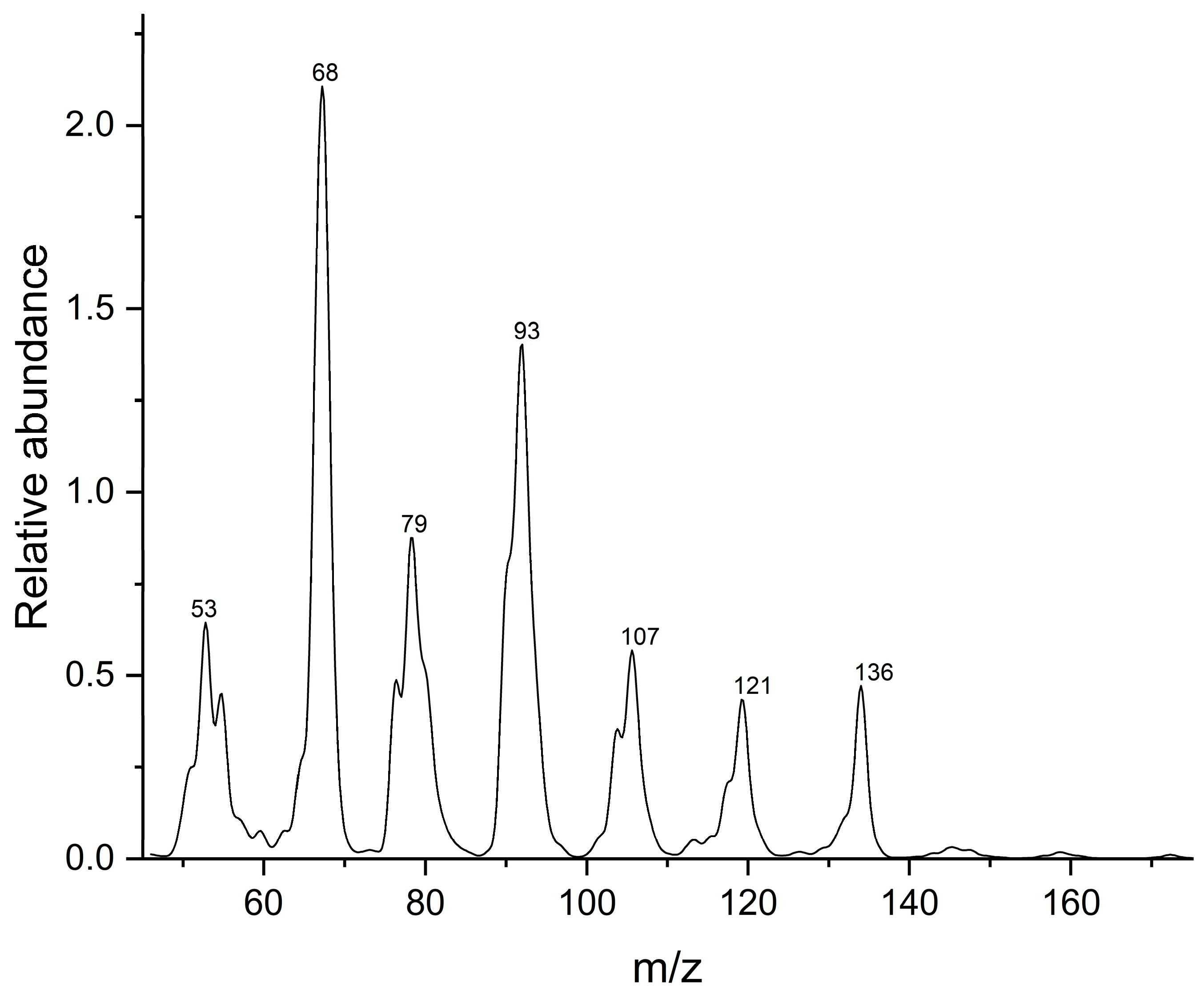
3.1. Detection principle
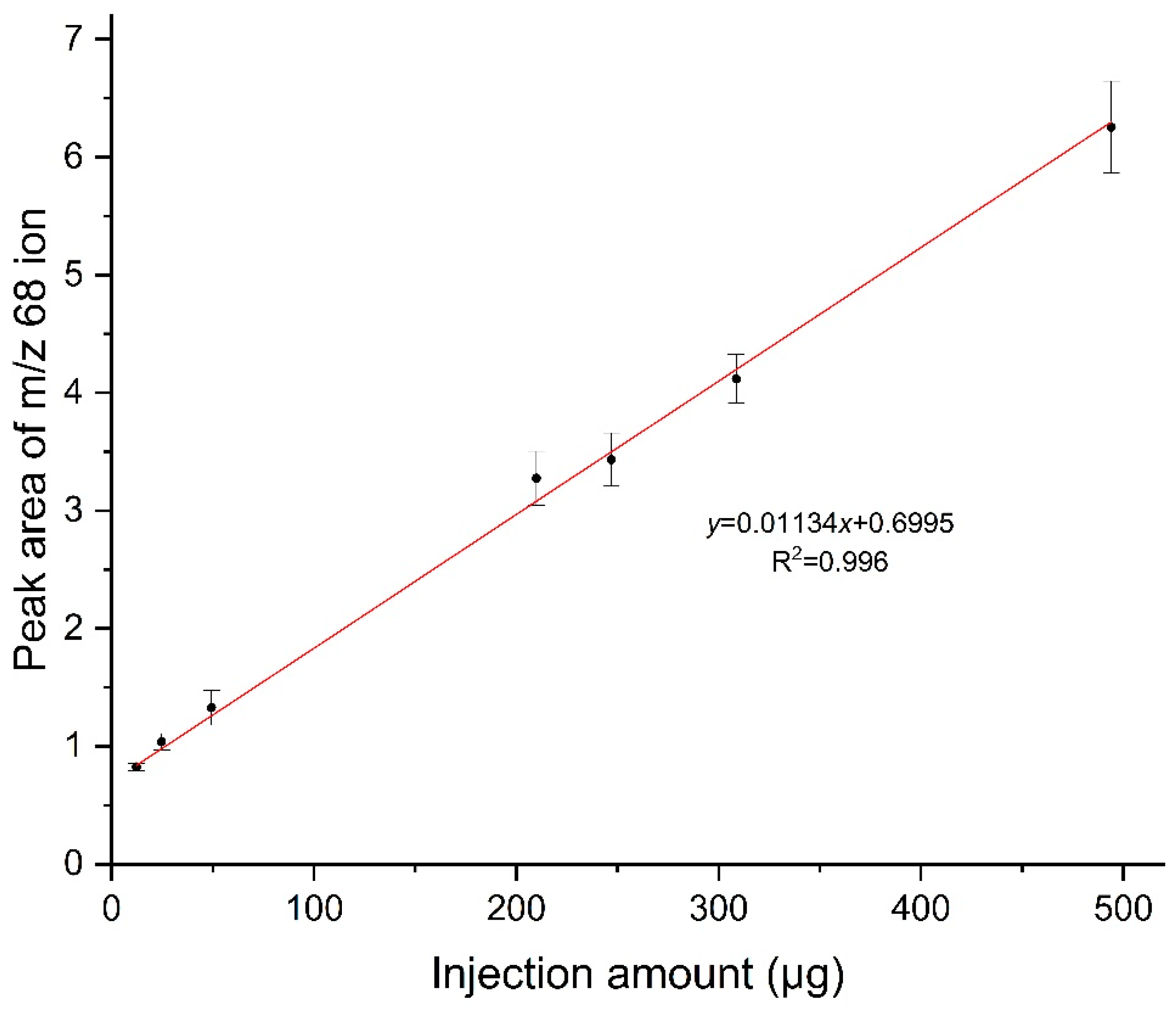
3.2. Establish standard quantitative curve
3.3. Limit of detection and recovery experiment
3.4. Comparison with other methods
4. Conclusions
Acknowledgments
References
- Cornish, K. Similarities and Differences in Rubber Biochemistry among Plant Species. Phytochemistry 2001, 57, 1123–1134. [Google Scholar] [CrossRef]
- Boonmahitthisud, A.; Boonkerd, K. Sustainable Development of Natural Rubber and Its Environmentally Friendly Composites. Curr. Opin. Green Sustain. Chem. 2021, 28, 100446. [Google Scholar] [CrossRef]
- Mooibroek, H.; Cornish, K. Alternative Sources of Natural Rubber. Appl. Microbiol. Biotechnol. 2000, 53, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Olsen, K.M.; Li, L.-F. Rooting for New Sources of Natural Rubber. Natl. Sci. Rev. 2018, 5, 89–89. [Google Scholar] [CrossRef]
- Krotkov, G. A Review of Literature onTaraxacum Koksaghyz Rod. Bot. Rev. 1945, 11, 417–461. [Google Scholar] [CrossRef]
- Whalen, M.; McMahan, C.; Shintani, D. Development of Crops to Produce Industrially Useful Natural Rubber. In Isoprenoid Synthesis in Plants and Microorganisms: New Concepts and Experimental Approaches; Bach, T.J., Rohmer, M., Eds.; Springer New York: New York, NY, 2013; pp. 329–345. ISBN 978-1-4614-4063-5. [Google Scholar]
- Lin, T.; Xu, X.; Ruan, J.; Liu, S.; Wu, S.; Shao, X.; Wang, X.; Gan, L.; Qin, B.; Yang, Y.; et al. Genome Analysis of Taraxacum Kok-Saghyz Rodin Provides New Insights into Rubber Biosynthesis. Natl. Sci. Rev. 2018, 5, 78–87. [Google Scholar] [CrossRef]
- Arias, M.; Herrero, J.; Ricobaraza, M.; Hernández, M.; Ritter, E. Evaluation of Root Biomass, Rubber and Inulincontents in Nine Taraxacum Koksaghyz Rodin Populations. Ind. Crops Prod. 2016, 83, 316–321. [Google Scholar] [CrossRef]
- Zhang, N.; Guo, T.; Ma, X.; Liu, J.; Dong, Y.; Zhang, J. Rational Rubber Extraction and Simultaneous Determination of Rubber Content and Molecular Weight Distribution in Taraxacum Kok-Saghyz Rodin by Size-Exclusion Chromatography. Chromatographia 2019, 82, 1459–1466. [Google Scholar] [CrossRef]
- Kreuzberger, M.; Hahn, T.; Zibek, S.; Schiemann, J.; Thiele, K. Seasonal Pattern of Biomass and Rubber and Inulin of Wild Russian Dandelion (Taraxacum Koksaghyz L. Rodin) under Experimental Field Conditions. Eur. J. Agron. 2016, 80, 66–77. [Google Scholar] [CrossRef]
- Hodgson-Kratky, K.J.; Stoffyn, O.M.; Wolyn, D.J. Recurrent Selection for Rubber Yield in Russian Dandelion. J. Am. Soc. Hortic. Sci. 2017, 142, 470–475. [Google Scholar] [CrossRef]
- Whalen, M.; McMahan, C.; Shintani, D. Development of Crops to Produce Industrially Useful Natural Rubber. Isoprenoid Synth. Plants Microorg. New Concepts Exp. Approaches 2013, 329–345. [Google Scholar] [CrossRef]
- Stolze, A.; Wanke, A.; van Deenen, N.; Geyer, R.; Prüfer, D.; Schulze Gronover, C. Development of Rubber-enriched Dandelion Varieties by Metabolic Engineering of the Inulin Pathway. Plant Biotechnol. J. 2017, 15, 740–753. [Google Scholar] [CrossRef]
- Buranov, A.U.; Elmuradov, B.J. Extraction and Characterization of Latex and Natural Rubber from Rubber-Bearing Plants. J. Agric. Food Chem. 2010, 58, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Nurthen, E.J.; McCleary, B.V.; Milthorpe, P.L.; Whitworth, J. Wayne. Modified Soxhlet Procedure for the Quantification of Resin and Rubber Content of Guayule. Anal. Chem. 1986, 58, 448–453. [Google Scholar] [CrossRef]
- Salvucci, M.E.; Coffelt, T.A.; Cornish, K. Improved Methods for Extraction and Quantification of Resin and Rubber from Guayule. Ind. Crops Prod. 2009, 30, 9–16. [Google Scholar] [CrossRef]
- Li, Z.G.; Cheng, P. Determination of Rubber Content in Qing Rubber Grass by Alkali Boiling Method - Part I: Perennial Grass Roots in Xinjiang. Chem. World 1954, 04, 168–169. [Google Scholar] [CrossRef]
- Guo, T.; Liu, Y.; Wei, Y.; Ma, X.; Fan, Q.; Ni, J.; Yin, Z.; Liu, J.; Wang, S.; Dong, Y.; et al. Simultaneous Qualitation and Quantitation of Natural Trans-1,4-Polyisoprene from Eucommia Ulmoides Oliver by Gel Permeation Chromatography (GPC). J. Chromatogr. B 2015, 1004, 17–22. [Google Scholar] [CrossRef]
- Wang, H.; Fan, X.; Yu, H.; Li, J.; Cui, X.; Xu, X. Characterization of Natural Rubber Concerning Its Components and Molecular Weight in Taraxacum Kok-saghyz Rodin Using Pyrolysis Gas Chromatography-mass Spectrometry. J. Sep. Sci. 2023, 46, 2201041. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zhang, M.; Gao, S.; Wang, L.; Zhang, J.; Huang, Z.; Dong, Y. Quantitative Detection of Natural Rubber Content in Eucommia Ulmoides by Portable Pyrolysis-Membrane Inlet Mass Spectrometry. Molecules 2023, 28, 3330. [Google Scholar] [CrossRef]
- Li, T.; Su, J.; Wang, C.; Watanabe, A.; Teramae, N.; Ohtani, H.; Wang, K. Advances in the Development and Application of Analytical Pyrolysis in Biomass Research: A Review. Energy Convers. Manag. 2022, 271, 116302. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Yu, K.; Li, N.; Liu, Y.; Liu, X.; Zhang, H.; Yang, B.; Wu, W.; Gao, J. Rapid Monitoring Approach for Microplastics Using Portable Pyrolysis-Mass Spectrometry. Anal. Chem. 2020, 92, 4656–4662. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, S.; Ohtani, H.; Watanabe, C. Pyrolysis-GC/MS Data Book of Synthetic Polymers: Pyrograms, Thermograms and MS of Pyrolyzates; Elsevier, 2011; ISBN 0-444-53893-3. [Google Scholar]
- Takeno, S.; Bamba, T.; Nakazawa, Y.; Fukusaki, E.; Okazawa, A.; Kobayashi, A. High-Throughput and Highly Sensitive Analysis Method for Polyisoprene in Plants by Pyrolysis-Gas Chromatography/Mass Spectrometry. Biosci. Biotechnol. Biochem. 2010, 74, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Takeno, S.; Bamba, T.; Nakazawa, Y.; Fukusaki, E.; Okazawa, A.; Kobayashi, A. Quantification of Trans-1, 4-Polyisoprene in Eucommia Ulmoides by Fourier Transform Infrared Spectroscopy and Pyrolysis-Gas Chromatography/Mass Spectrometry. J. Biosci. Bioeng. 2008, 105, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Tianyang, G.; Qing, Z.; Qiang, M.; Yiyang, D.; Jichuan, Z. Determination of Natural Rubber Content in Taraxacum Kok- Saghyz by Pyrolysis Gas Chromatography-Mass Spectrometry. 2020, 22, 43-48.
- Sen Gupla, P.K. Ghosh, Premamoy. Spectrophotometric Method for the Determination of Natural Rubber Hydrocarbon. Anal. Chem. 1966, 38, 505–505. [Google Scholar] [CrossRef]
- Smith, M.K. Viscometric Method for Determining Rubber Content in Guayule (Parthenium Argentatum Gray). J. Agric. Food Chem. 1985, 33, 928–931. [Google Scholar] [CrossRef]
- Fanning, R.; Bekkedahl, N. Quantitative Determination of Natural Rubber Hydrocarbon by Refractive Index Measurements, Rubber Chem. Technol. 1952, 25, 680–688. [Google Scholar] [CrossRef]
- Takeno, S.; Bamba, T.; Nakazawa, Y.; Fukusaki, E.; Okazawa, A.; Kobayashi, A. Quantification of Trans-1,4-Polyisoprene in Eucommia Ulmoides by Fourier Transform Infrared Spectroscopy and Pyrolysis-Gas Chromatography/Mass Spectrometry. J. Biosci. Bioeng. 2008, 105, 355–359. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, S.-K.; Dong, Y.-Y.; Ma, X.; Li, J.-R.; Guo, M.-M.; Zhang, J.-C. Fast Determination of the Rubber Content in Taraxacum Kok-Saghyz Fresh Biomass Using Portable Near-Infrared Spectroscopy and Pyrolysis–Gas Chromatography. J. Anal. Test. 2022, 6, 393–400. [Google Scholar] [CrossRef]
- Visintainer, J.; Beebe, D.H.; Myers, J.W.; Hirst, R.C. Determination of the Rubber Content in Guayule Bushes by Carbon-13 Nuclear Magnetic Resonance Spectrometry. Anal. Chem. 1981, 53, 1570–1572. [Google Scholar] [CrossRef]
| TKS spiked amount, µg | Confidence interval for peak area mean, 95% | Quantity recovered, µg | Recovery, % | RSD, % |
| 50.00 | 1.60±0.11 | 53.92±7.36 | 107.83 | 2.70 |
| 150.00 | 2.86±0.28 | 139.82±19.14 | 93.21 | 3.93 |
| 250.00 | 4.60±0.27 | 259.08±18.68 | 103.63 | 2.39 |
| Method | Equipment | Detection capability | Sample Type Suitability | References |
|---|---|---|---|---|
| Solvent Extraction Method | Grinding, stirring, chemical extraction | Flow method (99.5%) and Blender method (approximately 75%) can be obtained Purity of latex. | TKS, Scorzonera tau-saghyz, Scorzonera Uzbekistanica | [14] |
| Soxhlet Extractor Method | Soxhlet extractor | Estimate of the variance for each variety mean 0.069 % rubber | Guayule | [15] |
| Accelerated Solvent Extraction | Ball mill grinding, evaporative light scattering | Linear range 0 to 2 mg/ml. R2 = 0.995. |
Guayule | [16] |
| Alkali Boiling Method | Alkali boiling and water milling | Rough estimate with <10% variance | TKS | [17] |
| Bromination Method | Spectrophotometric analysis with brominating solution | Variance within 3% | Natural rubber latex | [27] |
| Viscometry Method | Viscometry with temperature control | Linear correlation with weight (R2 = 0.94) and IR (R2 =0.99) methods | Guayule plant material | [28] |
| Refractive Index Method | Refractive index measurement | Average deviation of 0.29% | Natural rubber latex | [29] |
| Gel Chromatography | Size Exclusion Chromatography | LOD is 0.58 mg/mL for leaves and 0.47 mg/mL for fruit; the linear range is 2–10 mg/mL for leaves and 0.5–10 mg/mL for fruit. |
Eucommia leaves and fruits | [18] |
| IR Method | Fourier Transform Infrared Spectroscopy |
LOD =12.5 µg, Linear range 25-200 µg |
Eucommia rubber | [30] |
| NIR Method | Portable fiber optic NIR spectrometer | R2= 0.95, ratio of performance to deviation (RPD)= 5.54 | TKS fresh roots | [31] |
| NMR Method | Carbon-13 Nuclear Magnetic Resonance | ±1.0% absolute accuracy, LOD 0.5% by weight | Guayule | [32] |
| PY-GC-MS Method | Thermal pyrolysis-gas chromatography-mass spectrometry detection | LOD=2.603 mg/g Linear ranged from 1.20% ±0.20% to 8.61% ±0.28% |
TKS | [26] |
| PY-MS Method | Thermal pyrolysis and mass spectrometry | LOD=0.639 µg/mg. Linear range 10-500 µg/mg RSD ≤ 3.39% |
TKS and theoretically other rubber-producing plants | This article |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





