Submitted:
15 January 2024
Posted:
16 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012 Apr;123(4):465–72. [CrossRef]
- Gajjar A, Bowers DC, Karajannis MA, Leary S, Witt H, Gottardo NG. Pediatric Brain Tumors: Innovative Genomic Information Is Transforming the Diagnostic and Clinical Landscape. J Clin Oncol. 2015 Sep 20;33(27):2986–98. [CrossRef]
- Gupta T, Shirsat N, Jalali R. Molecular Subgrouping of Medulloblastoma: Impact Upon Research and Clinical Practice. Curr Pediatr Rev. 2015;11(2):106–19. [CrossRef]
- Northcott PA, Robinson GW, Kratz CP, Mabbott DJ, Pomeroy SL, Clifford SC, et al. Medulloblastoma. Nature Reviews Disease Primers [Internet]. 2019 Feb 14;5(1):11. Available from: https://doi.org/10.1038/s41572-019-0063-6. [CrossRef]
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016 Jun;131(6):803–20. [CrossRef]
- Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021 Aug 2;23(8):1231–51. [CrossRef]
- Gupta T, Sarkar C, Rajshekhar V, Chatterjee S, Shirsat N, Muzumdar D, et al. Indian Society of Neuro-Oncology consensus guidelines for the contemporary management of medulloblastoma. Neurol India. 2017 Apr;65(2):315–32. [CrossRef]
- Lazow MA, Palmer JD, Fouladi M, Salloum R. Medulloblastoma in the Modern Era: Review of Contemporary Trials, Molecular Advances, and Updates in Management. Neurotherapeutics. 2022 Oct;19(6):1733-51. [CrossRef]
- Mushtaq N, Ul Ain R, Hamid SA, Bouffet E. Evolution of Systemic Therapy in Medulloblastoma Including Irradiation-Sparing Approaches. Diagnostics [Internet]. 2023 Jan [cited 2023 Dec 18];13(24):3680. Available from: https://www.mdpi.com/2075-4418/13/24/3680. [CrossRef]
- Zeltzer PM, Boyett JM, Finlay JL, Albright AL, Rorke LB, Milstein JM, et al. Metastasis Stage, Adjuvant Treatment, and Residual Tumor Are Prognostic Factors for Medulloblastoma in Children: Conclusions From the Children’s Cancer Group 921 Randomized Phase III Study. JCO [Internet]. 1999 Mar [cited 2023 Dec 20];17(3):832–832. Available from: https://ascopubs.org/doi/10.1200/JCO.1999.17.3.832. [CrossRef]
- Packer RJ, Gajjar A, Vezina G, Rorke-Adams L, Burger PC, Robertson PL, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006 Sep 1;24(25):4202–8. [CrossRef]
- Michalski JM, Janss AJ, Vezina LG, Smith KS, Billups CA, Burger PC, et al. Children’s Oncology Group Phase III Trial of Reduced-Dose and Reduced-Volume Radiotherapy With Chemotherapy for Newly Diagnosed Average-Risk Medulloblastoma. J Clin Oncol. 2021 Aug 20;39(24):2685–97. [CrossRef]
- Gajjar A, Robinson GW, Smith KS, Lin T, Merchant TE, Chintagumpala M, et al. Outcomes by Clinical and Molecular Features in Children With Medulloblastoma Treated With Risk-Adapted Therapy: Results of an International Phase III Trial (SJMB03). J Clin Oncol. 2021 Mar 1;39(7):822–35. [CrossRef]
- Bouffet E. Management of high-risk medulloblastoma. Neurochirurgie. 2021 Feb;67(1):61–8. [CrossRef]
- Ramaswamy V, Remke M, Bouffet E, Bailey S, Clifford SC, Doz F, et al. Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol. 2016 Jun;131(6):821–31. [CrossRef]
- Fossati P, Ricardi U, Orecchia R. Pediatric medulloblastoma: toxicity of current treatment and potential role of protontherapy. Cancer Treat Rev. 2009 Feb;35(1):79–96. [CrossRef]
- Salloum R, Chen Y, Yasui Y, Packer R, Leisenring W, Wells E, et al. Late Morbidity and Mortality Among Medulloblastoma Survivors Diagnosed Across Three Decades: A Report From the Childhood Cancer Survivor Study. J Clin Oncol. 2019 Mar 20;37(9):731–40. [CrossRef]
- Kool M, Korshunov A, Remke M, Jones DTW, Schlanstein M, Northcott PA, et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012 Apr;123(4):473–84. [CrossRef]
- Thompson EM, Ashley D, Landi D. Current medulloblastoma subgroup specific clinical trials. Transl Pediatr. 2020 Apr;9(2):157–62. [CrossRef]
- Kunder R, Jalali R, Sridhar E, Moiyadi A, Goel N, Goel A, et al. Real-time PCR assay based on the differential expression of microRNAs and protein-coding genes for molecular classification of formalin-fixed paraffin embedded medulloblastomas. Neuro Oncol. 2013 Dec;15(12):1644–51. [CrossRef]
- Gupta T, Pervez S, Dasgupta A, Chatterjee A, Epari S, Chinnaswamy G, et al. Omission of Upfront Craniospinal Irradiation in Patients with Low-Risk WNT-Pathway Medulloblastoma Is Associated with Unacceptably High Risk of Neuraxial Failure. Clin Cancer Res. 2022 Oct 3;28(19):4180–5.
- Nobre L, Zapotocky M, Khan S, Fukuoka K, Fonseca A, McKeown T, et al. Pattern of Relapse and Treatment Response in WNT-Activated Medulloblastoma. Cell Rep Med. 2020 Jun 23;1(3):100038. [CrossRef]
- Goschzik T, Mynarek M, Doerner E, Schenk A, Spier I, Warmuth-Metz M, et al. Genetic alterations of TP53 and OTX2 indicate increased risk of relapse in WNT medulloblastomas. Acta Neuropathol. 2022 Dec;144(6):1143–56.
- Cavalli FMG, Remke M, Rampasek L, Peacock J, Shih DJH, Luu B, et al. Intertumoral Heterogeneity within Medulloblastoma Subgroups. Cancer Cell. 2017 Jun 12;31(6):737-754.e6. [CrossRef]
- Schwalbe EC, Lindsey JC, Nakjang S, Crosier S, Smith AJ, Hicks D, et al. Novel molecular subgroups for clinical classification and outcome prediction in childhood medulloblastoma: a cohort study. The Lancet Oncology [Internet]. 2017 Jul 1 [cited 2024 Jan 8];18(7):958–71. Available from: https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(17)30243-7/fulltext. [CrossRef]
- Northcott PA, Buchhalter I, Morrissy AS, Hovestadt V, Weischenfeldt J, Ehrenberger T, et al. The whole-genome landscape of medulloblastoma subtypes. Nature [Internet]. 2017 Jul [cited 2021 Jun 26];547(7663):311–7. Available from: https://www.nature.com/articles/nature22973. [CrossRef]
- Hovestadt V, Ayrault O, Swartling FJ, Robinson GW, Pfister SM, Northcott PA. Medulloblastomics revisited: biological and clinical insights from thousands of patients. Nat Rev Cancer [Internet]. 2020 Jan [cited 2024 Jan 8];20(1):42–56. Available from: https://www.nature.com/articles/s41568-019-0223-8. [CrossRef]
- Helgager J, Pytel P, Vasudevaraja V, Lee EQ, Snuderl M, Iorgulescu JB, et al. WNT-Activated Medulloblastomas With Hybrid Molecular Subtypes. JCO Precis Oncol. 2020;4:PO.19.00332. [CrossRef]
- Ryan SL, Schwalbe EC, Cole M, Lu Y, Lusher ME, Megahed H, et al. MYC family amplification and clinical risk-factors interact to predict an extremely poor prognosis in childhood medulloblastoma. Acta Neuropathol [Internet]. 2012 Apr 1 [cited 2023 Dec 25];123(4):501–13. Available from: https://doi.org/10.1007/s00401-011-0923-y. [CrossRef]
- Park AK, Lee SJ, Phi JH, Wang KC, Kim DG, Cho BK, et al. Prognostic classification of pediatric medulloblastoma based on chromosome 17p loss, expression of MYCC and MYCN, and Wnt pathway activation. Neuro Oncol. 2012 Feb;14(2):203–14. [CrossRef]
- Green S, Hoover T, Doss D, Davidow K, Walter AW, Cottrell CE, et al. WNT-activated, MYC-amplified medulloblastoma displaying intratumoural heterogeneity. Neuropathology and Applied Neurobiology [Internet]. [cited 2023 Dec 25];n/a(n/a):e12945. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/nan.12945. [CrossRef]
- Hartley R, Phoenix TN. MYC promotes aggressive growth and metastasis of a WNT-medulloblastoma mouse model. Dev Neurosci. 2023 Aug 5; [CrossRef]
- Korshunov A, Remke M, Werft W, Benner A, Ryzhova M, Witt H, et al. Adult and pediatric medulloblastomas are genetically distinct and require different algorithms for molecular risk stratification. J Clin Oncol. 2010 Jun 20;28(18):3054–60. [CrossRef]
- Wooley JR, Penas-Prado M. Pediatric versus Adult Medulloblastoma: Towards a Definition That Goes beyond Age. Cancers (Basel). 2021 Dec 16;13(24):6313.
- Li Q, Dai Z, Cao Y, Wang L. Comparing children and adults with medulloblastoma: a SEER based analysis. Oncotarget [Internet]. 2018 Jul 10 [cited 2024 Jan 8];9(53):30189–98. Available from: https://www.oncotarget.com/article/23773/. [CrossRef]
- Remke M, Hielscher T, Northcott PA, Witt H, Ryzhova M, Wittmann A, et al. Adult medulloblastoma comprises three major molecular variants. J Clin Oncol. 2011 Jul 1;29(19):2717–23. [CrossRef]
- Wong Wong GCH, Li KKW, Wang WW, Liu APY, Huang QJ, Chan AKY, et al. Clinical and mutational profiles of adult medulloblastoma groups. Acta Neuropathol Commun. 2020 Nov 10;8(1):191.
- Coltin H, Sundaresan L, Smith KS, Skowron P, Massimi L, Eberhart CG, et al. Subgroup and subtype-specific outcomes in adult medulloblastoma. Acta Neuropathol. 2021 Aug 18; [CrossRef]
- Patil R, Gupta T, Maitre M, Dasgupta A, Sahay A, Epari S, et al. Clinical Audit of Survival Outcomes and Prognostic Factors in Adolescents and Adults with Medulloblastoma. Journal of Adolescent and Young Adult Oncology [Internet]. 2021 Apr 23 [cited 2021 Oct 6]; Available from: https://www.liebertpub.com/doi/abs/10.1089/jayao.2021.0034. [CrossRef]
- Mani S, Chatterjee A, Dasgupta A, Shirsat N, Epari S, Chinnaswamy G, et al. WNT-pathway medulloblastoma: what constitutes low-risk and how low can one go? Oncotarget [Internet]. 2023 Jan 12 [cited 2023 Mar 7];14:105–10. Available from: https://www.oncotarget.com/article/28360/text/.
- Ramaswamy V, Remke M, Bouffet E, Faria CC, Perreault S, Cho YJ, et al. Recurrence patterns across medulloblastoma subgroups: an integrated clinical and molecular analysis. Lancet Oncol. 2013 Nov;14(12):1200–7. [CrossRef]
- Sabel M, Fleischhack G, Tippelt S, Gustafsson G, Doz F, Kortmann R, et al. Relapse patterns and outcome after relapse in standard risk medulloblastoma: a report from the HIT-SIOP-PNET4 study. J Neurooncol. 2016 Sep;129(3):515–24. [CrossRef]
- Hill RM, Richardson S, Schwalbe EC, Hicks D, Lindsey JC, Crosier S, et al. Time, pattern, and outcome of medulloblastoma relapse and their association with tumour biology at diagnosis and therapy: a multicentre cohort study. Lancet Child Adolesc Health. 2020 Dec;4(12):865–74. [CrossRef]
- Hill RM, Plasschaert SLA, Timmermann B, Dufour C, Aquilina K, Avula S, et al. Relapsed Medulloblastoma in Pre-Irradiated Patients: Current Practice for Diagnostics and Treatment. Cancers [Internet]. 2022 Jan [cited 2024 Jan 8];14(1):126. Available from: https://www.mdpi.com/2072-6694/14/1/126. [CrossRef]
- Pietsch T, Schmidt R, Remke M, Korshunov A, Hovestadt V, Jones DTW, et al. Prognostic significance of clinical, histopathological, and molecular characteristics of medulloblastomas in the prospective HIT2000 multicenter clinical trial cohort. Acta Neuropathol. 2014 Jul;128(1):137–49.
- Von Bueren AO, Kortmann RD, von Hoff K, Friedrich C, Mynarek M, Müller K, et al. Treatment of Children and Adolescents With Metastatic Medulloblastoma and Prognostic Relevance of Clinical and Biologic Parameters. J Clin Oncol. 2016 Dec;34(34):4151–60. [CrossRef]
- Leary SES, Packer RJ, Li Y, Billups CA, Smith KS, Jaju A, et al. Efficacy of Carboplatin and Isotretinoin in Children With High-risk Medulloblastoma: A Randomized Clinical Trial From the Children’s Oncology Group. JAMA Oncol. 2021 Sep 1;7(9):1313–21.
- Dufour C, Foulon S, Geoffray A, Masliah-Planchon J, Figarella-Branger D, Bernier-Chastagner V, et al. Prognostic relevance of clinical and molecular risk factors in children with high-risk medulloblastoma treated in the phase II trial PNET HR+5. Neuro Oncol. 2021 Jul 1;23(7):1163–72. [CrossRef]
- Cohen KJ, Munjapara V, Aguilera D, Castellino RC, Stapleton SL, Landi D, et al. A Pilot Study Omitting Radiation in the Treatment of Children with Newly Diagnosed Wnt-Activated Medulloblastoma. Clin Cancer Res. 2023 Dec 15;29(24):5031–7. [CrossRef]
- Gottardo NG, Gajjar A. Verschlimmbesserung: Craniospinal Radiotherapy Is Essential in WNT Medulloblastoma Patients. Clinical Cancer Research [Internet]. 2023 Dec 15 [cited 2024 Jan 8];29(24):4996–8. Available from: https://doi.org/10.1158/1078-0432.CCR-23-2331. [CrossRef]
| Characteristics | Number of patients (%) |
| Median age (inter-quartile range) at diagnosis | 12 years (9-18 years) |
| Gender Male Female |
44 (65.7%) 23 (34.3%) |
| Post-operative residual tumor (n=57) <1.5cm2 ≥1.5cm2 |
47 (82.4%) 10 (17.6%) |
| Metastastic status at diagnosis (n=62) Non-metastatic (M0) Metastatic disease (M+) |
57 (91.9%) 05 (08.1%) |
| Conventional risk-stratification (n=55) Average-risk High-risk |
39 (70.9%) 16 (29.1%) |
| Histological subtype Medulloblastoma (not otherwise specified) Classic Desmoplastic Large-cell/Anaplastic |
22 (32.8%) 41 (61.2%) 03 (04.5%) 01 (01.5%) |
| Time interval from surgery to adjuvant radiotherapy (n=40) ≤6 weeks >6 weeks |
17 (42.5%) 23 (57.5%) |
| Craniospinal irradiation dose (n=54) 23.4-26Gy$ 35-36Gy |
23 (42.6%) 31 (57.4%) |
| Craniospinal irradiation technique (n=39) Conventional radiotherapy Three-dimensional conformal radiotherapy Intensity modulated radiation therapy |
01 (02.6%) 16 (41.0%) 22 (56.4%) |
| Adjuvant systemic chemotherapy (n=54) Yes No |
39 (72.2%) 15 (27.8%) |
| Cumulative cyclophosphamide dose (n=39) ≤12mg/m2 >12mg/m2 |
11 (28.2%) 28 (71.8%) |
| Sr No. |
Age (years) /Gender |
Stage | CSI dose at initial diagnosis | Pattern of first failure | PFS | Salvage therapy at relapse | Final Outcome | OS |
|---|---|---|---|---|---|---|---|---|
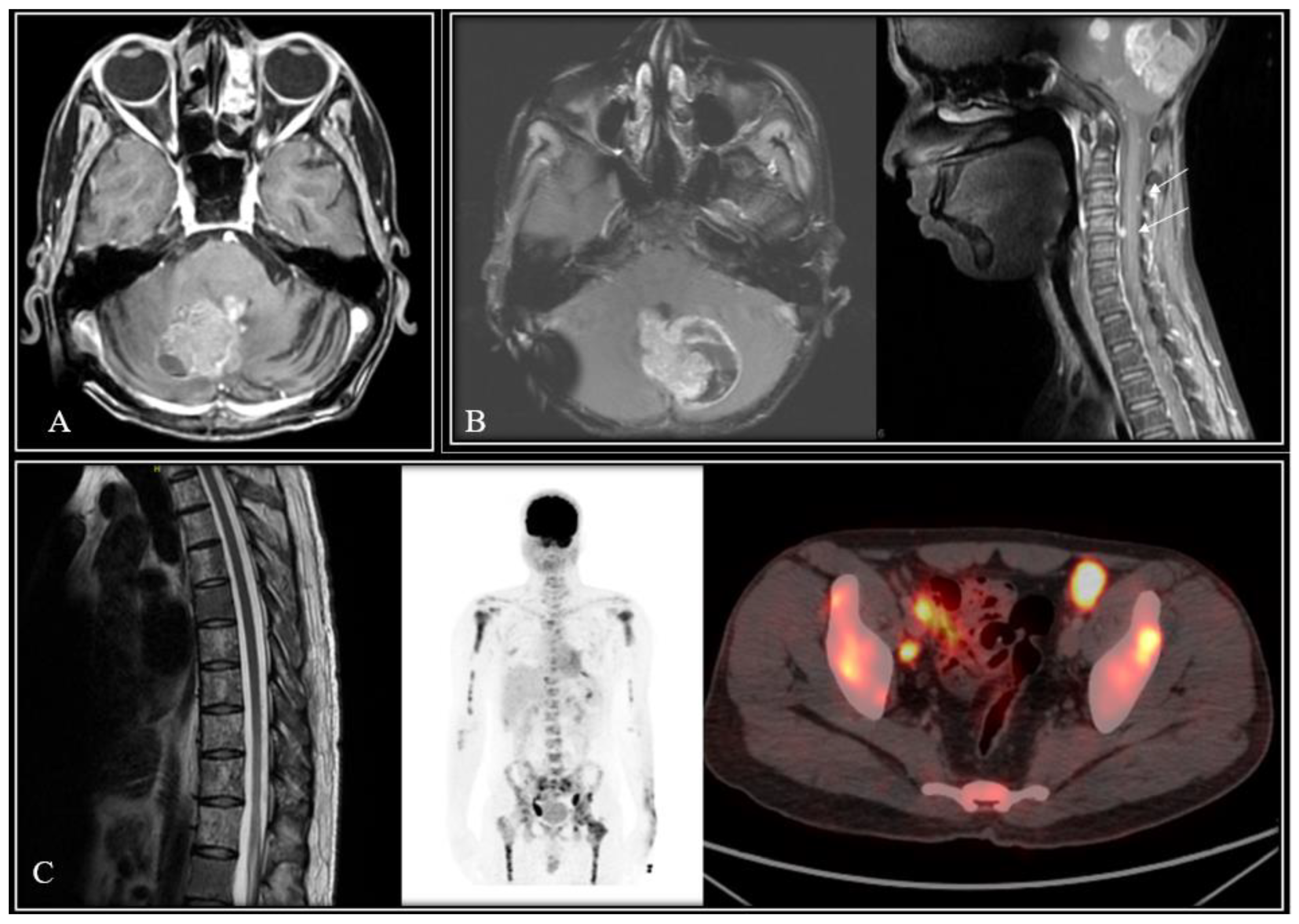
| W1 | 9/Male | Non-Metastatic | 36Gy/18fx | Tumor-bed recurrence | 25-months | Re-RT & chemotherapy | Died of disease | 80-months |
| W2 | 11/Male | Non-metastatic | 26Gy/13fx | Tumor-bed relapse plus metastases in brainstem, temporal lobe & spine | 37-months | Re-CSI (36Gy/36fx) & chemotherapy | Died of disease | 66-months |
| W3 | 13/Female | Non-metastatic | 35Gy/21fx | Leptomeningeal dissemination | 25-months | Best supportive care | Died of disease | 25-months |
| W4 | 10/Male | Non-metastatic | 14.4Gy/8fx (Incomplete RT) | Leptomeningeal dissemination |
61-months | Best supportive care | Died of disease | 62-months |
| W5 | 27/Male | Non-metastatic | 35Gy/21fx | Leptomeningeal dissemination | 58-months | Best supportive care | Died of disease | 60-months |
| W6 | 14/Male | Metastatic (frontal horn lesion) | 35Gy/21fx | No evidence of disease progression/failure | 15-months | Not applicable | Died of toxicity | 15-months |
| W7 | 9/Male | Non-metastatic | 36Gy/18fx | Tumor-bed recurrence | 56-months | Re-surgery | Died of disease | 60-months |
| W8 | 22/Male | Non-metastatic | 35Gy/21fx | Extra-neural metastases | 83-months | Chemotherapy | Alive with disease | Not applicable |
| W9 | 15/Male | Non-metastatic | Not known | Tumor-bed relapse plus metastases in frontal horn & multiple spinal metastases | 59-months | Re-surgery | Died of disease | 67-months |
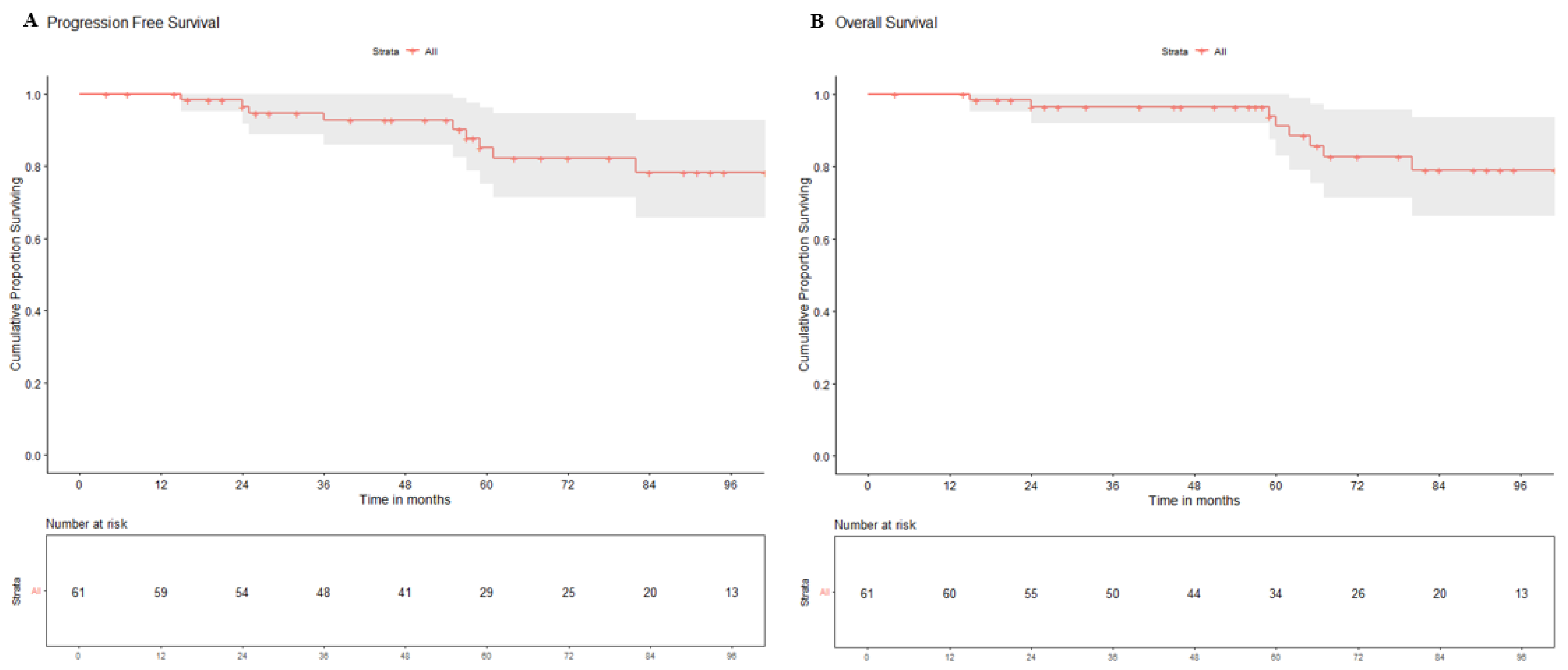
| Variables | Category | 5-year PFS (95%CI) | p-value | 5 years OS (95%CI) | p-value |
|---|---|---|---|---|---|
|
Gender |
Male | 86.0% (73.8-100%) | 0.480 | 92.6% (83.1-100%) | 0.440 |
| Female | 93.3% (81.5-100%) | 93.7% (82.6-100%) | |||
| Age at diagnosis | Child (≤16-years) | 83.5% (83.0-100%) | 0.722 | 89.5% (87.5-100%) | 0.323 |
| Adult (>16-years) | 90.0% (68.0-100%) | 90.9% (72.0-100%) | |||
|
Residual disease |
<1.5 cm2 | 84.5% (72.6-98.4%) | 0.250 | 90.7% (80.9-100%) | 0.260 |
| ≥1.5 cm2 | 100% (NE) | 100% (NE) | |||
|
Metastatic status |
Non-metastatic (M0) | 89.1% (79.1-100%) | 0.320 | 94.2% (86.4-100%) | 0.270 |
| Metastatic (M+) | 80.0% (51.6-100%) | 80.0% (51.6-100%) | |||
|
Risk-stratification |
Average-risk | 93.3% (81.5-100%) | 0.560 | 100% (NE) | 0.640 |
| High-risk | 81.2% (63.9-100%) | 87.9% (73.5-100%) | |||
|
Time interval (Surgery to RT) |
≤42 days | 87.3% (72.4-100%) | 0.500 | 94.1% (83.6-100%) | 0.440 |
| >42 days | 89.4% (76.7-100%) | 94.7% (85.2-100%) | |||
|
Dose of CSI |
Low-dose (14.4-26Gy) | 94.1% (83.0-100%) | 0.441 | 100% (NE) | 0.698 |
| High-dose (35-40Gy) | 81.0% (68.1-97.0%) | 84.6% (72.0-100%) | |||
|
Adjuvant chemotherapy |
No | 91.6% (77.2-100%) | 0.990 | 100% (NE) | 0.940 |
| Yes | 84.3% (70.6-100%) | 87.8% (75.2-100%) | |||
|
Cyclophosphamide dose |
<12gm/m2 | 92.8% (80.3-100%) | 0.970 | 100% (NE) | 0.970 |
| ≥12gm/m2 | 88.7% (77.4-100%) | 92.6% (83.2-100%) |
| Trial Identity “[Ref]” & Registration |
Risk category | WNT-MB patients | WNT-MB failures | Patterns of relapse | 5-year EFS/PFS | 5-year OS | ||
|---|---|---|---|---|---|---|---|---|
| Local | Metastatic | Combined | ||||||
| HIT 2000 “[45]” NCT00303810 |
Non-metastatic (average-risk) |
15 | 0 | 0 | 0 | 0 | 100% | 100% |
| SIOP PNET-4 “[42]” NCT01351870 |
Average-risk | 58 | 8 | 2 | 4 | 2 | 91% | 95% |
| HIT 2000 “[46]” NCT00303810 |
Metastatic (high-risk) |
4 | 0 | 0 | 0 | 0 | 100% | 100% |
| COG ACNS 0331 “[12]” NCT00085735 |
Average-risk | 64 | 4 | 4 | 0 | 0 | 93.3% | 95.5% |
| SJMB-03 “[13]” NCT00085202 |
Average-risk & high-risk | 46 | 0 | 0 | 0 | 0 | 100% | 100% |
| COG ACNS 0332 “[47]” NCT00392327 |
High-risk | 14 | 1 | 0 | 0 | 1 | 92.9% | 100% |
| SIOP PNET 5 HR+ “[48]” NCT00936156 |
High-risk | 3 | 0 | 0 | 0 | 0 | 100% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





