1. Introduction
Atherosclerosis is a primary pathological mechanism of vascular diseases, takes a key role in diabetes mellitus (DM), and is associated with cancers [
1,
2,
3,
4]. Additionally, atherosclerosis is the leading cause of vascular diseases, including acute cerebral infarction (ACI) and acute myocardial infarction (AMI) [
5]. And patients with DM face an increased risk of accelerated atherosclerosis, leading to various vascular diseases owing to factors such as hyperglycemia, dyslipidemia, and hypertension (HT) [
3]. Numerous genetic regulatory mechanisms share similarities with atherosclerosis in the context of tumors. For example, p53 and its induction of apoptosis are involved in various malignancies—including lung, breast, gastric, and esophageal cancers [
6], and contribute to myocardial ischemia, post-ischemic cardiac remodeling, and coronary atherosclerosis [
7]. Therefore, a common biomarker for most—if not all—atherosclerosis-related diseases, including ACI, AMI, DM, and cancers, is possible.
Autoantibodies are antibodies produced by the immune system against the body’s own proteins or antigens. Normally, the immune system recognizes self-antigens as “self” and does not mount an immune response against them. However, if the immune system fails to recognize self-antigens, it starts to produce autoantibodies, which can contribute to tissue damage and disease pathology [
8,
9]. Autoantibodies have been implicated in various autoimmune diseases, cancers, atherosclerosis, and DM, and they can also serve as biomarkers for disease diagnosis [
8,
9,
10]. Previously, we searched for antibody markers using serological identification through cDNA expression cloning (SEREX) and protein array methods. Our findings highlighted potential biomarkers for stroke including DIDO1, CPSF2, FOXJ2, DNAJC2, ALDOA, FH4, ASXL2, AP3D1, SERPINE1, and TSTD2 [
11,
12,
13,
14,
15,
16,
17]. Likewise, we identified LRPAP1, CFL1, WDR1, and striatin 4 as potential markers for digestive tract tumors [
18,
19,
20,
21].
In the present study, Jumonji C-domain-containing 6 (JMJD6) was identified by SEREX using sera from patients with unstable angina pectoris (UAP). The JMJD6 is a member of the JmjC domain-containing family and has emerged as a promising target for various diseases, including breast cancer, prostate cancer, and Alzheimer's disease [
22,
23,
24,
25]. JMJD6 functions as a protein demethylase and hydroxylase, influencing RNA splicing, lipid metabolism, and apoptosis [
24,
26,
27,
28]. Despite ample evidence supporting the pivotal role of JMJD6 in the treatment and prognosis of patients with cancer [
23,
24] and its relevance in managing ischemic cardiovascular diseases [
26], a noticeable lack of research is observed regarding autoantibodies against JMJD6. Herein, we investigated serum anti-JMJD6 antibodies (s-JMJD6-Abs) in patients with atherosclerosis-related diseases and proposed s-JMJD6-Abs as a potential biomarker for ischemic stroke, AMI, DM, and cancers.
2. Results
2.1. Recognition of JMJD6 by serum antibodies from patients with UAP
This study comprehensively screened antigens recognized by SEREX using serum IgG antibodies from patients with UAP; the screening was conducted thrice. Additionally, one of the antigens identified was the region of 1075–1536 of the JMJD6 gene (accession no. NM_015167). To further investigate its potential as a disease marker, we expressed GST-fused JMJD6 protein in Escherichia coli (E. coli) and purified it using affinity chromatography. The purified protein was then used as an antigen to evaluate the serum antibody levels.
2.2. Elevation of the levels of serum antibodies against JMJD6 in patients with central nervous system diseases
We investigated the levels of s-JMJD6-Abs in healthy donors (HDs) and patients with chronic cerebral infarction (CCI), ACI, transient ischemic attack (TIA), asymptomatic cerebral infraction (asympt-CI), and deep and subcortical white matter hyperintensity (DSWMH). The s-JMJD6-Ab levels significantly increased in patients with such conditions as compared with those in HDs (
Figure 1a). When we employed a cutoff value equivalent to the HDs’ average value plus two standard deviations (SDs), the positive rates of s-JMJD6-Abs in HDs and patients with CCI, ACI, TIA, asympt-CI, and DSWMH were 3.2%, 16.9%, 19.0%, 13.0%, 10.5%, and 8.6%, respectively (
Table 1). The ability of JMJD6 antibody markers to indicate the presence of CCI, ACI, TIA, asympt-CI, and DSWMH was evaluated by receiver operating characteristic (ROC) analysis. As a result, the area under the curve (AUC) values for s-JMJD6-Abs were 0.691, 0.708, 0.689, 0.709, and 0.594, respectively (
Figure 1b–f).
2.3. Analysis of correlations between JMJD6 antibody levels and clinical parameters in the Sawara Hospital cohort
To explore the potential correlations between s-JMJD6-Ab levels and clinical parameters in the Sawara Hospital cohort, we compared the s-JMJD6-Ab levels among participants with different characteristics by using the Mann–Whitney
U test. Specifically, we compared the s-JMJD6-Ab levels between males and females, body mass index (BMI) < 25 kg/m
2 and BMI ≥ 25 kg/m
2, and the presence and absence of DM, HT, cardiovascular diseases (CVD), lipidemia, habitual smoking, and alcohol intake (
Figure 1g–n). The s-JMJD6-Ab levels were significantly higher in those with DM, HT, and CVD than in those without these diseases (
P < 0.01,
P < 0.0001, and
P < 0.01, respectively,
Figure 1i–k). In addition, smokers exhibited increased levels of s-JMJD6-Ab compared with nonsmokers (
P < 0.001,
Figure 1m); of note, smoking habit is a significant factor for vascular diseases [
26]. However, other categories showed no significant difference in s-JMJD6-Ab levels (
Figure 1g, h, l, n). Taken together, the s-JMJD6-Ab levels may be a biomarker for DM, HT, and CVD, as well as for smoking habit in the Sawara Hospital cohort.
Moreover, the relationships between the JMJD6 antibody levels and various clinical variables in the Sawara Hospital cohort were investigated using Spearman’s correlation analyses.
Table 2 presents the correlation coefficients (Rho) and
P-values for patients’ hematological examination results, smoking status, alcohol consumption status, age, sex, height, weight, BMI, and intima-media thickness (IMT) of the carotid artery. The JMJD6 antibody levels positively correlated with age (Rho = 0.3173,
P < 0.0001), maximum IMT (Rho = 0.2761,
P < 0.0001), alkaline phosphatase (Rho = 0.0989,
P = 0.0059), C-reactive protein (Rho = 0.1370,
P = 0.0007), blood glucose (Rho = 0.1343,
P = 0.0002), blood pressure (Rho = 0.0946,
P = 0.0238), and smoking duration (Rho = 0.1515,
P < 0.0001). In contrast, the JMJD6 antibody levels negatively correlated with height (Rho = −0.1291,
P = 0.0002), weight (Rho = −0.1388,
P < 0.0001), BMI (Rho = −0.0797,
P = 0.0215), albumin/globulin ratio (Rho = −0.1357,
P = 0.0001), choline esterase (Rho = −0.1352,
P = 0.0006), total protein (Rho = −0.1581,
P < 0.0001), albumin (Rho = −0.2019,
P < 0.0001), total cholesterol, (Rho = −0.1239,
P = 0.0008), triglyceride (Rho = −0.0989,
P = 0.0173), and red blood cell count (Rho = −0.1118,
P = 0.0012) (
Table 2). Overall, the JMJD6 antibody levels may be associated with certain vascular risk factors such as age, IMT, blood pressure, and blood glucose. Therefore, the JMJD6 antibody levels have potential links to vascular diseases in the Sawara Hospital cohort.
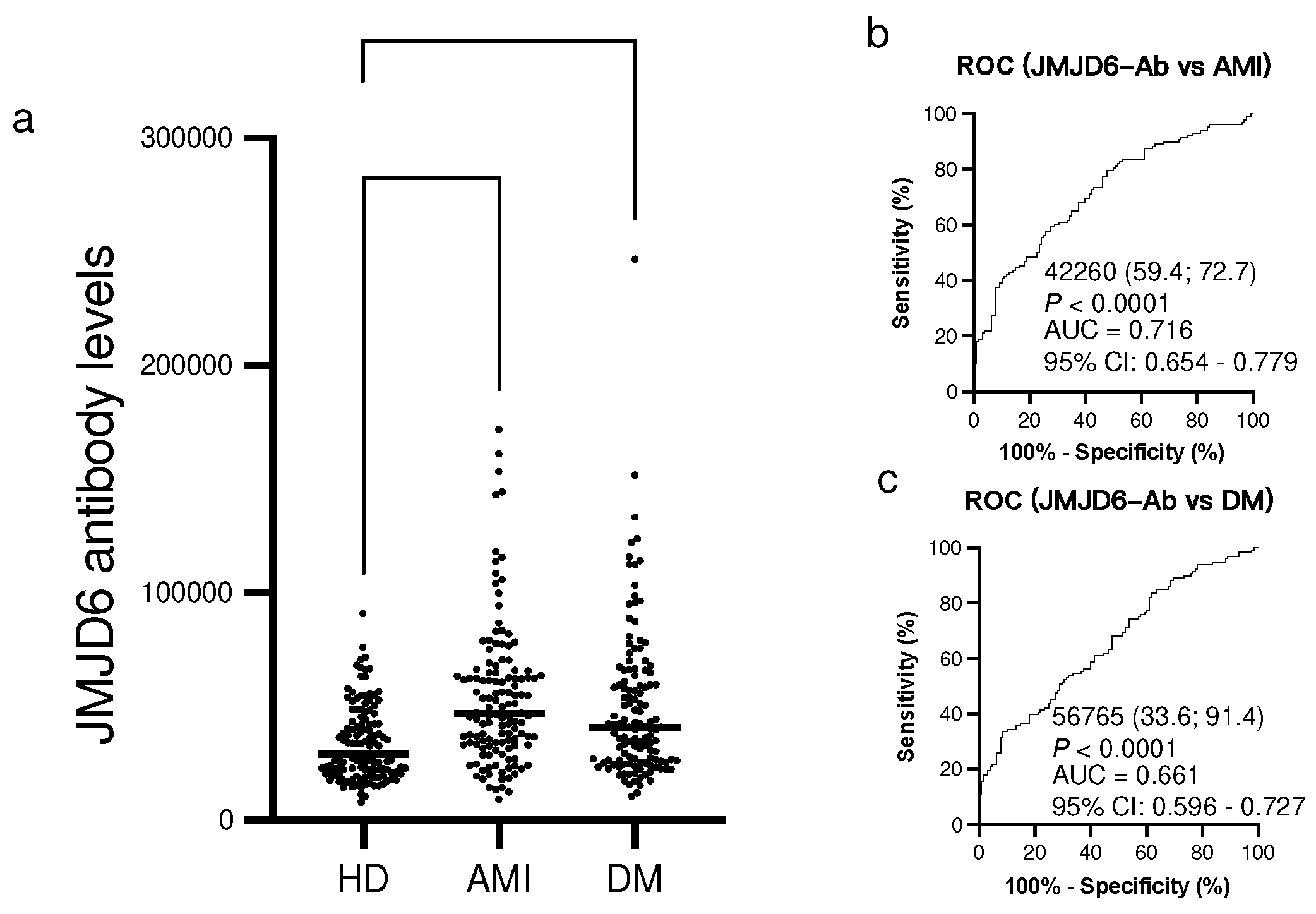
2.4. Elevation of s-JMJD6-Ab levels in patients with AMI and DM
Using Amplified luminescence proximity homogeneous assay-linked immunosorbent assay (AlphaLISA), we examined the s-JMJD6-Ab levels in age-matched HDs and patients with AMI and DM. The s-JMJD6-Ab levels were significantly higher in patients with AMI and DM than in HDs (
Figure 2a). When using the cutoff values for JMJD6-Ab levels defined as two SDs above the average s-JMJD6-Ab level in HDs, the positivity rates of s-JMJD6-Ab were 21.9% in patients with AMI and 21.1% in patients with DM (
Table 3). The ROC analysis revealed that the AUCs for s-JMJD6-Ab levels in patients with AMI and DM were 0.716 and 0.661, respectively (
Figure 2b,c), indicating a strong association of s-JMJD6-Ab levels with AMI and DM.
2.5. Elevated s-JMJD6-Ab levels in patients with cancers
Considering previous reports about the correlation between JMJD6 and cancers, we conducted further investigations using serum samples from patients with EC, gastric cancer (GC), lung cancer (LC), and mammary cancer (MC). The s-JMJD6-Ab levels were significantly higher in these patients compared with those in HDs (
Figure 3a and
Table 4). The positive rates of JMJD6-Ab were highest in patients with EC and GC (32.8% and 30.2%, respectively) (
Table 4). In addition, the AUCs of s-JMJD6-Abs were 0.721 and 0.707 for EC and GC, respectively (
Figure 3b and c), and 0.585 and 0.588 for LC and MC, respectively (
Figure 3d, e). The s-JMJD6-Ab levels may be more closely associated with EC and GC than other cancer types. Thus, the s-JMJD6-Ab levels could serve as a useful biomarker for detecting EC, GC, LC, and MC—especially EC and GC.
Subsequently, we examined the relationship between the s-JMJD6-Ab levels and the survival outcomes of patients with EC and GC. Patients with EC and GC were categorized into positive and negative groups based on the cutoff values obtained from X-tile software. In patients with EC, the prognosis of the s-JMJD6-Ab-negative group was significantly better than that of the positive group (
Figure 3f). Conversely, no significant difference was noted between the two groups in patients with GC (
Figure 3g). Therefore, the JMJD6-Ab levels can predict prognosis in patients with EC but not GC.
2.6. Combinatorial prognosis prediction by combining the s-JMJD6-Ab levels with PD-L1 and p53-Ab
Given that serum PD-L1 levels were also reported to be associated with EC prognosis [
27], we examined the prognostic effects of PD-L1 alone or in combination with s-JMJD6-Abs in EC specimens. We established a cutoff value of PD-L1 to obtain the lowest
P-value in survival analysis using X-tile software. The prognosis of the PD-L1-positive group was significantly more unfavorable than that of the PD-L1-negative group (
P = 0.0007,
Figure 3h). Moreover, the prognosis significantly differed between the s-JMJD6-Ab-negative/PD-L1-positive group and the s-JMJD6-Ab-positive/PD-L1-negative group (
P < 0.0001,
Figure 3i). Thus, the JMJD6 antibody levels have a potential association with PD-L1 expression, highlighting the significance of their interaction in patient survival.
Serum anti-p53 antibody is also one of the typical tumor markers [
28,
29]. We then analyzed the levels of p53-Abs and JMJD6-Abs to assess their prognostic implications for patients with EC. The results revealed no statistically significant difference between the p53-Ab-positive and p53-Ab-negative groups (
P = 0.2925,
Figure 3j). Next, we evaluated the predictive value of the combination of p53-Ab and JMJD6-Ab levels for EC prognosis. Notably, patients with EC in the JMJD6-Ab-positive/p53-Ab-negative subgroup exhibited a more favorable prognosis than those in the JMJD6-Ab-negative/p53-Ab-positive subgroup (
P = 0.0295,
Figure 3k). Therefore, JMJD6-Abs has a potential connection with p53-Abs, with implications for patients’ survival outcomes.
3. Discussion
This study investigated the relationship between the levels of s-JMJD6-Ab and various atherosclerosis-related diseases, including ischemic stroke, DM, AMI, and cancers. First, the levels of s-JMJD6-Ab in patients with CCI, ACI, TIA, asympt-CI, DSWMH, AMI, DM, EC, GC, LC, and MC were considerably higher than those in HDs (
Figure 1a,
Figure 2a and
Figure 3a). The levels of s-JMJD6-Ab showed a slight but significant elevation in patients with asympt-CI and DSWMH, suggesting a potential association with the early stages of ischemic stroke. Asympt-CI and DSWMH are recognized as risk factors and/or early symptoms for future stroke [
30,
31]. Therefore, s-JMJD6-Abs can be used to predict patients with a high early-stage cerebral infarction risk.
Sawara Hospital cohort showed that s-JMJD6-Ab levels were well correlated with IMT of the carotid artery (
Table 2). This suggests that s-JMJD6-Abs primarily reflect the progression of atherosclerosis and, consequently, are associated with ischemic stroke, AMI, and DM. When comparing two groups of clinical parameters using the Mann–Whitney
U test, s-JMJD6-Ab levels were significantly higher in subjects with HT or a history of habitual smoking compared with those without these risk factors(
Figure 1j and m). The HT and smoking are typical risk factors for cancer and vascular diseases [
4], which aligns with the elevated s-JMJD6-Ab levels in patients with cancer (
Figure 3a). In addition, individuals with DM or CVD showed higher s-JMJD6-Ab levels than those without these conditions in the Sawara Hospital cohort (
Figure 1i, k), consistent with the results in
Figure 2a, which compares HDs with patients with AMI and DM. Although dyslipidemia is closely linked to atherosclerosis, there was no significant difference between the lipidemia+ and lipidemia- groups in the Sawara Hospital cohort (
Figure 1l). Furthermore, s-JMJD6-Ab levels were inversely correlated with total cholesterol and triglycerides (
Table 2). These results could be influenced by medication, i.e., most Japanese patients suspected of having dyslipidemia or atherosclerosis are taking cholesterol-lowering statin drugs [
32].
One of the primary mechanisms underlying the increase in s-JMJD6-Abs is believed to result from cellular overexpression and subsequent tissue damage, leading to the release of antigen proteins into the bloodstream and triggering an immune response [
14,
33,
34]. For instance, the antigenic proteins of other atherosclerosis autoantibody markers, DIDO1-Abs, CPSF2-Abs, and FOXJ2-Abs, were highly expressed in carotid atherosclerotic plaques [
12]. Similarly, cancer autoantibody biomarkers, such as TROP2-Abs, SLC2A1-Abs, and ING1-Abs, were associated with high antigen expression in cancer tissues [
35,
36,
37]. Thus, the elevated levels of s-JMJD6-Abs may indicate increased JMJD6 expression in patients with atherosclerosis-related diseases. Then, what is the role of JMJD6? JMJD6 can promote apoptosis and autophagy by protein demethylation and hydroxylation in cancers and vascular diseases [
38,
39], which might account for the role in cancer cells and atherosclerotic plaque.
The JMJD6-Ab levels can be used to predict the prognosis of patients with EC (
Figure 3f). We then analyzed the relationship between s-JMJD6-Ab and other biomarkers in the prognosis of patients with EC. Our survival analysis showed that the levels of s-JMJD6-Abs and PD-L1 were individually useful in predicting EC prognosis (
Figure 3f, h) and that their combination exhibited further prominent difference (
Figure 3i). These results suggested that JMJD6 may be functionally related to PD-L1 in EC cells. Cancer cells can escape and spread owing to the inhibition of cell apoptosis by PD-L1 [
34]. By contrast, JMJD6 is a phosphatidylserine receptor, and a monoclonal anti-JMJD6 antibody inhibited the phagocytosis of phosphatidylserine-expressing apoptotic cells [
40]. Thus, s-JMJD6-Abs and PD-L1 have opposite effects on apoptosis, which could be reflected in their levels with respect to prognosis.
Next, we combined p53-Ab and JMJD6-Ab to predict the survival of patients with EC. The combination of s-JMJD6-Ab-positivity and p53-Ab-negativity showed a better prognosis of EC (
Figure 3k) despite the fact that the p53-Ab level alone exhibited no significant difference between the positive and negative groups (
Figure 3j). Elevated p53-Abs are observed in patients with p53-mutated cancers, primarily owing to the low expression of the p53-target gene, MDM2, and the high expression of mutated p53 proteins [
29,
41]. Thus, s-JMJD6-Abs may play a tumor-promoting role in p53-mutated cancers while potentially exhibiting a tumor-suppressive function in cancers harboring wild-type p53, which may also regulate apoptosis. In summary, the combination of s-JMJD6-Ab and s-p53-Ab could be a sensitive biomarker for prognostic assessment in patients with EC.
4. Materials and Methods
4.1. Patient and control sera
Serum samples were collected from HDs and patients with CCI, ACI, TIA, asympt-CI, and DSWMH from Chiba Prefectural Sawara Hospital. The stroke subtypes were determined according to the criteria of the Trial of Org 10172 in the Acute Stroke Treatment classification system [
42], and ACI includes large-artery atherosclerosis and small-artery occlusion (lacune). For comparisons with TIA and ACI, serum samples from HDs were obtained from patients without abnormalities in cranial magnetic resonance imaging. In addition, the sera of patients with AMI were obtained from Kyoto University Hospital, and those of patients with UAP or DM were collected from Chiba University Hospital. The serum samples of patients with TIA, ACI, AMI, and UAP were obtained within 2 weeks after disease onset. The Department of Surgery in Toho University Hospital collected sera from patients with EC, GC, LC, and MC preoperatively between June 2010 and February 2016, and patients with EC or GC were followed up until July 2018 or death.
All sera were collected from patients with written informed consent. Each serum sample was centrifuged at 3000g for 10 min, and the supernatant was stored at −80°C before using to avoid the repeated freezing/thawing of samples. This study was approved by the Local Ethical Review Board of the Chiba University, Graduate School of Medicine (approval no.: 2018-320), and the Ethics Committee of Toho University Graduate School of Medicine (nos. A18103_A17052_A16035_A16001_26095_25024_24038_ 22047, M21038_20197_19213), as well as by the review boards of the participating hospitals.
4.2. SEREX screening of the expressed recombinant proteins using a human cDNA library
We utilized a modified method for immunoscreening. To identify clones that displayed immunoreactivity against the serum IgG antibodies in patients with UAP, we employed a human aortic endothelial cell cDNA library from the Uni-ZAP XR Premade Library (Stratagene, La Jolla, CA). Then, we infected Escherichia coli XL1-Blue MRF' with Uni-ZAP XR phage and induced the expression of resident cDNAs by blotting infected bacteria onto nitrocellulose membranes (NitroBind, Osmonics, Minnetonka, MN) pretreated with 10 mM isopropyl-β-D-thiogalactopyranoside (IPTG) (Wako Pure Chemicals, Osaka, Japan) for 30 min.
Next, we washed the membranes with TBS-T [20 mM Tris–HCl (pH 7.5), 0.15 M NaCl, and 0.05% Tween-20], and blocked nonspecific binding by incubating them with 1% protease-free bovine serum albumin (Nacalai Tesque, Inc., Kyoto, Japan) in TBS-T for 1 h. The membranes were then incubated overnight with 1:2000 diluted sera. After three washes with TBS-T, these membranes were washed for 1 h with 1:5000 diluted alkaline phosphatase-conjugated goat antihuman IgG (Jackson ImmunoReseach Laboratories, West Grove, PA).
By incubating the membranes in a color development solution [100 mM Tris–HCl (pH 9.5), 100 mM NaCl, and 5 mM MgCl2], which contained 0.15 mg/mL 5-bromo-4-chloro-3-indolylphospate (Wako Pure Chemicals) and 0.3 mg/mL nitro blue tetrazolium (Wako Pure Chemicals), we detected positive reactions. To obtain monoclonality, we recloned positive clones twice further, as described above.
4.3. Sequence analysis of isolated cDNAs
To convert monoclonalized phage cDNA clones, we utilized ExAssist helper phage (Stratagene) for in vivo excision, resulting in pBluescript phagemid formation. The plasmid DNA was obtained from the E. coli SOLR strains that had been transformed with the phagemids. After sequencing the inserted cDNAs, we conducted homologous analysis using a public database provided by the National Center for Biotechnology Information (
https://blast.ncbi.nlm.nih.gov/Blast.cgi).
4.4. Expression and purification of JMJD6 protein
To generate the expression plasmids for glutathione S-transferase (GST)-fused JMJD6 protein, we integrated JMJD6 cDNA sequence between 1075 and 1834 into the EcoRI/XhoI site of pGEX-4T-1 vector (Cytiva, Marlborough, MA), as previously described [
43].
Moreover, E. coli BL-21 cells transformed with the pGEX-4T-1 clone were cultured in 200 mL of Luria-Bertani broth and treated with 0.1 mM IPTG for 3 h. The cells were then collected and lysed by sonication in BugBuster Master Mix (Novagen, San Diego, CA), followed by centrifugation at 13,000 ×g and 4°C for 10 min. GST-tagged JMJD6 proteins were purified by Glutathione Sepharose column chromatography (GE Healthcare Life Sciences) and dialyzed as previously described [
44,
45].
4.5. Amplified luminescence proximity homogeneous assay-linked immunosorbent assay
AlphaLISA was conducted in 384-well microtiter plates (white opaque OptiPlate™, Revvity, Waltham, MA) containing 2.5 µL of 1:100-diluted serum with either 2.5 µL of GST or GST-JMJD6 proteins (10 µg/mL) in AlphaLISA buffer (25 mM N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid, pH 7.4, 0.1% casein, 0.5% Triton X-100, 1 mg/mL dextran-500, and 0.05% ProClin-300). The reaction mixture was incubated at room temperature for 6–8 h and added with antihuman IgG-conjugated acceptor beads (2.5 µL at 40 µg/mL) and glutathione-conjugated donor beads (2.5 µL at 40 µg/mL). The mixture was then incubated at room temperature in the dark for 7–28 days. The chemical emissions (Alpha photon counts) were measured using an EnSpire Alpha microplate reader (Revvity), as described previously [
11,
46]. We calculated specific reactions by subtracting the emission counts of the GST control from the counts of GST-JMJD6 fusion protein.
Furthermore, we measured the serum levels of PD-L1 by using a commercially available enzyme-linked immunosorbent assay (ELISA) kit for PD-L1 (R&D Systems, Minneapolis, MN) [
27] and examined anti-p53 antibody (p53-Ab) levels by using anti-p53 EIA Kit II (MESACUP anti-p53 Test; Medical & Biological Laboratories, Nagoya, Japan), as reported previously [
47]. The cutoff value of PD-L1 was 66.6 pg/mL, which produced the lowest
P-value in the survival analysis, and that of p53-Ab was 1.3 IU/mL according to a previous report [
47].
4.6. Statistical analysis
Our statistical analysis was based on the standard procedures in biomedical research. We utilized the Mann–Whitney U test and the Kruskal–Wallis test (Mann–Whitney U test with Bonferroni correction) to determine significant differences between two groups and between three or more groups, respectively. Correlations were calculated using Spearman’s correlation analysis and logistic regression analysis. All statistical data were analyzed using GraphPad Prism 5 (GraphPad Software, Inc.). To assess the predictive values of the putative disease markers, we conducted ROC curve analysis and then set cutoff values to maximize the sums of sensitivity and specificity. Patient survival was evaluated using the Kaplan–Meier method, and survival differences were compared between groups by using the log-rank test; the cutoff values were identified by X-tile software (Version 3.6.1; Yale University, New Haven, CT). All statistical tests were two-tailed, and P-values less than 0.05 were considered statistically significant. These analytical methods ensured a reliable and rigorous statistical analysis of the study results.
5. Conclusions
Measuring JMJD6 autoantibodies may serve as a potential diagnostic biomarker for atherosclerosis-related diseases such as ACI, AMI, DM, and solid cancers. The s-JMJD6-Ab can be used to predict the prognosis of patients with EC. Additionally, combining JMJD6 autoantibodies with PD-L1 or p53-Ab levels may provide a more accurate assessment of prognosis in patients with EC.
Author Contributions
Conceptualization, B.S.Z, T.H, E.N, M.T, S.Y., K.Yok, Y.K, H.K, H.S, Y.I; Methodology, B.S.Z, X.M.Z; Software, B.S.Z; Validation, B.S.Z, T.H, E.N; Formal Analysis, B.S.Z, Y.Y, T.Mat, A.H; Investigation, B.S.Z, X.M.Z, H.W, M.I, K.Yos, M.O, S.M, T.Mac, H.Y, H.T, T.H; Resources, E.N; Data Curation, B.S.Z, X.M.Z, H.W, S.Y.L, M.K, T.H; Writing—Original Draft Preparation, B.S.Z, M.I, S.Y.L, M.K, T.H; Writing—Review and Editing, B.S.Z, M.I, K.Yos, M.O, S.M, T.Mac, H.Y, H.T, E.N, M.T, K.Yok, Y.K, H.K, H.S, Y.I, Y.Y, A.H, T.H; Supervision, E.N, M.T, K.Yok, Y.K, H.K, H.S, Y.I; Project Administration, T.H; Funding Acquisition, Y.I., E.N., M.Tac., M.O., Y.Y., K.Yok., T.Mat., T.H. Authorship must be limited to those who have contributed substantially to the work reported.
Funding
This research was funded by research grants from the Japan Science and Technology Agency (Exploratory Research No. 14657335) and JSPS KAKENHI (grant nos. 17K16626, 19K09451, 20H03449, 20K07810, 20K08445, 20K17953, 21K19437, 22K07273, 22K09227 and 22K19428).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the Chiba University, Graduate School of Medicine (approval no. 2018-320), and the Ethics Committee of Toho University Graduate School of Medicine (nos. A18103_A17052_A16035_A16001_26095_25024_24038_22047, M21038_20197_19213), as well as by the review boards of the participating hospitals.
Informed Consent Statement
Serum or plasma was collected from participants who had provided informed consent by following the protocols approved by their institutional ethical committees.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We are grateful to Prof. Masaki Takiguchi (Chiba University) and Drs. Go Tomiyoshi, Natsuko Shinmen, Rika Nakamura (Research Development Center, Fujikura Kasei Co.) for supporting this research.
Conflicts of Interest
The present study was performed in collaboration with Fujikura Kasei Co., Ltd., and Hideyuki Kuroda is an employee of Fujikura Kasei Co., Ltd. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- González-Salvatierra, S.; García-Fontana, C.; Lacal, J.; Andújar-Vera, F.; Martínez-Heredia, L.; Sanabria-de la Torre, R.; Ferrer-Millán, M.; Moratalla-Aranda, E.; Muñoz-Torres, M.; García-Fontana, B. , Cardioprotective function of sclerostin by reducing calcium deposition, proliferation, and apoptosis in human vascular smooth muscle cells. Cardiovasc Diabetol 2023, 22, 301. [Google Scholar] [CrossRef] [PubMed]
- Den Hartigh, L. J. , Conjugated linoleic acid effects on cancer, obesity, and atherosclerosis: a review of pre-clinical and human trials with current perspectives. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Shalash, M. A. M.; Rohoma, K. H.; Kandil, N. S.; Abdel Mohsen, M. A.; Taha, A. A. F. , Serum sclerostin level and its relation to subclinical atherosclerosis in subjects with type 2 diabetes. J Diabetes Complications 2019, 33, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Koene, R. J.; Prizment, A. E.; Blaes, A.; Konety, S. H. , Shared risk factors in cardiovascular disease and cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef]
- Lu, L. Q.; Tian, J.; Luo, X. J.; Peng, J. Targeting the pathways of regulated necrosis: a potential strategy for alleviation of cardio-cerebrovascular injury. Cell Mol Life Sci 2021, 78, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, S. Q.; Wang, C.; Chang, L. M.; Zhou, K. R.; Wang, J. W.; Ke, Y.; Yang, D. X.; Shi, H. G.; Wang, R.; Shi, X. L.; et al. , Geridonin and paclitaxel act synergistically to inhibit the proliferation of gastric cancer cells through ROS-mediated regulation of the PTEN/PI3K/Akt pathway. Oncotarget 2016, 7, 72990–73002. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, H.; Gao, J.; Liu, Y.; Li, J.; Wang, J. , Molecular machinery and interplay of apoptosis and autophagy in coronary heart disease. J Mol Cell Cardiol 2019, 136, 27–41. [Google Scholar] [CrossRef]
- Sexauer, D.; Gray, E.; Zaenker, P. , Tumour- associated autoantibodies as prognostic cancer biomarkers- a review. Autoimmun Rev 2022, 21, 103041–10. [Google Scholar] [CrossRef]
- Kang, E. H.; Ha, Y. J.; Lee, Y. J. , Autoantibody biomarkers in rheumatic diseases. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Wasserfall, C. H.; Atkinson, M. A. , Autoantibody markers for the diagnosis and prediction of type 1 diabetes. Autoimmunity Reviews 2006, 5, 424–428. [Google Scholar] [CrossRef]
- Hiwasa, T.; Wang, H.; Goto, K.; Mine, S.; Machida, T.; Kobayashi, E.; Yoshida, Y.; Adachi, A.; Matsutani, T.; Sata, M.; et al. , Serum anti-DIDO1, anti-CPSF2, and anti-FOXJ2 antibodies as predictive risk markers for acute ischemic stroke. BMC Med 2021, 19, 131. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Zhang, X. M.; Wang, H.; Machida, T.; Mine, S.; Kobayashi, E.; Adachi, A.; Matsutani, T.; Kamitsukasa, I.; Wada, T.; et al. , Elevated levels of autoantibodies against DNAJC2 in sera of patients with atherosclerotic diseases. Heliyon 2020, 6, e04661. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, H.; Zhang, X. M.; Goto, K. I.; Kobayashi, E.; Yoshida, Y.; Adachi, A.; Matsutani, T.; Iwadate, Y.; Mine, S.; et al. , Association of serum levels of antibodies against ALDOA and FH4 with transient ischemic attack and cerebral infarction. BMC Neurol 2021, 21, 274. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Y.; Yoshida, Y.; Kobayashi, E.; Adachi, A.; Hirono, S.; Matsutani, T.; Mine, S.; Machida, T.; Ohno, M.; Nishi, E.; et al. , Association between serum anti-ASXL2 antibody levels and acute ischemic stroke, acute myocardial infarction, diabetes mellitus, chronic kidney disease and digestive organ cancer, and their possible association with atherosclerosis and hypertension. Int J Mol Med 2020, 46, 1274–1288. [Google Scholar] [CrossRef]
- Li, S.-Y.; Yoshida, Y.; Kobayashi, E.; Kubota, M.; Matsutani, T.; Mine, S.; Machida, T.; Maezawa, Y.; Takemoto, M.; Yokote, K.; et al. , Serum anti-AP3D1 antibodies are risk factors for acute ischemic stroke related with atherosclerosis. Scientific Reports 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Kubota, M.; Yoshida, Y.; Kobayashi, E.; Matsutani, T.; Li, S. Y.; Zhang, B. S.; Mine, S.; Machida, T.; Takizawa, H.; Hiwasa, T.; et al. , Serum anti-SERPINE1 antibody as a potential biomarker of acute cerebral infarction. Sci Rep 2021, 11, 21772. [Google Scholar] [CrossRef]
- Kubota, M.; Zhang, B. S.; Li, S. Y.; Yoshida, Y.; Wang, H.; Adachi, A.; Matsutani, T.; Mine, S.; Machida, T.; Kamitsukasa, I.; et al. , Serum anti-TSTD2 antibody as a biomarker for atherosclerosis-induced ischemic stroke and chronic kidney disease. Med Int (Lond) 2023, 3, 4. [Google Scholar] [CrossRef]
- Sumazaki, M.; Shimada, H.; Ito, M.; Shiratori, F.; Kobayashi, E.; Yoshida, Y.; Adachi, A.; Matsutani, T.; Iwadate, Y.; Mine, S.; et al. , Serum anti-LRPAP1 is a common biomarker for digestive organ cancers and atherosclerotic diseases. Cancer Sci 2020, 111, 4453–4464. [Google Scholar] [CrossRef]
- Ito, M.; Hiwasa, T.; Oshima, Y.; Yajima, S.; Suzuki, T.; Nanami, T.; Sumazaki, M.; Shiratori, F.; Funahashi, K.; Takizawa, H.; et al. , Identification of serum anti-striatin 4 antibodies as a common marker for esophageal cancer and other solid cancers. Molecular and Clinical Oncology 2021, 15, 237. [Google Scholar] [CrossRef]
- Ito, M.; Yajima, S.; Suzuki, T.; Oshima, Y.; Nanami, T.; Sumazaki, M.; Shiratori, F.; Wang, H.; Hu, L.; Takizawa, H.; et al. , The combination of positive anti-WDR1 antibodies with negative anti-CFL1 antibodies in serum is a poor prognostic factor for patients with esophageal carcinoma. Med Int (Lond) 2023, 3, 11. [Google Scholar] [CrossRef]
- Hu, L.; Liu, J.; Shimada, H.; Ito, M.; Sugimoto, K.; Hiwasa, T.; Zhou, Q.; Li, J.; Shen, S.; Wang, H. , Serum anti-BRAT1 is a common molecular biomarker for gastrointestinal cancers and atherosclerosis. Front Oncol 2022, 12, 870086. [Google Scholar] [CrossRef]
- Vangimalla, S. S.; Ganesan, M.; Kharbanda, K. K.; Osna, N. A. , Bifunctional enzyme JMJD6 contributes to multiple disease pathogenesis: new twist on the old story. Biomolecules 2017, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Cioni, B.; Ratti, S.; Piva, A.; Tripodi, I.; Milani, M.; Menichetti, F.; Langella, T.; Botti, L.; De Cecco, L.; Chiodoni, C.; et al. , JMJD6 shapes a pro-tumor microenvironment via ANXA1-dependent macrophage polarization in breast cancer. Mol Cancer Res 2023, 21, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Xu, J.; Tong, D. , Targeting the JMJD6/TGF-β axis in prostate cancer by immunotherapy: a potential treatment based on RNA splicing. Medical Hypotheses 2023, 171, 111018. [Google Scholar] [CrossRef]
- Merchant, J. P.; Zhu, K.; Henrion, M. Y. R.; Zaidi, S. S. A.; Lau, B.; Moein, S.; Alamprese, M. L.; Pearse, R. V.; Bennett, D. A.; Ertekin-Taner, N.; et al. , Predictive network analysis identifies JMJD6 and other potential key drivers in Alzheimer’s disease. Communications Biology 2023, 6, 503. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S. C.; Mason, A. M.; Bäck, M.; Klarin, D.; Damrauer, S. M.; Michaëlsson, K.; Burgess, S. , Genetic predisposition to smoking in relation to 14 cardiovascular diseases. Eur Heart J 2020, 41, 3304–3310. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Yajima, S.; Suzuki, T.; Oshima, Y.; Nanami, T.; Sumazaki, M.; Shiratori, F.; Funahashi, K.; Tochigi, N.; Shimada, H. , High serum PD-L1 level is a poor prognostic biomarker in surgically treated esophageal cancer. Cancer Med 2020, 9, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Nanami, T.; Hoshino, I.; Shiratori, F.; Yajima, S.; Oshima, Y.; Suzuki, T.; Ito, M.; Hiwasa, T.; Kuwajima, A.; et al. , Presence of serum RalA and serum p53 autoantibodies in 1833 patients with various types of cancers. Int J Clin Oncol 2022, 27, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tan, Q.; Song, Y.; Shi, Y.; Han, X. , Anti-p53 autoantibody in blood as a diagnostic biomarker for colorectal cancer: A meta-analysis. Scand J Immunol 2020, 91, e12829. [Google Scholar] [CrossRef]
- Lee, K. O.; Woo, M. H.; Chung, D.; Choi, J. W.; Kim, N. K.; Kim, O. J.; Oh, S. H. , Differential impact of plasma homocysteine levels on the periventricular and subcortical white matter hyperintensities on the brain. Front Neurol 2019, 10, 1174. [Google Scholar] [CrossRef]
- Kumakura, H.; Kanai, H.; Matsuo, Y.; Iwasaki, T.; Ichikawa, S. , Asymptomatic cerebral infarction is a predictor of long-term survival and vascular or limb events in peripheral arterial disease. Eur Heart J Qual Care Clin Outcomes 2019, 5, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Ogawa, H. , "Just make it lower" is an alternative strategy of lipid-lowering therapy with statins in Japanese patients: LDL-cholesterol: the lower, the better; is it true for Asians? Circ J 2010, 74, 1731–1741. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Liu, H.; Hill, R.; Chen, C.; Hong, X.; Crawford, F.; Kingsley, M.; Zhang, Q.; Liu, X.; Chen, Z.; et al. , JMJD6 cleaves MePCE to release positive transcription elongation factor b (P-TEFb) in higher eukaryotes. Elife 2020, 9, e53930. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhao, R.; Xia, W.; Chang, C. W.; You, Y.; Hsu, J. M.; Nie, L.; Chen, Y.; Wang, Y. C.; Liu, C.; et al. , PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat Cell Biol 2020, 22, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Shimada, H.; Ochiai, T.; Kuboshima, M.; Kuroiwa, N.; Okazumi, S.; Matsubara, H.; Nomura, F.; Takiguchi, M.; Hiwasa, T. , Serological identification of TROP2 by recombinant cDNA expression cloning using sera of patients with esophageal squamous cell carcinoma. Int J Cancer 2004, 112, 1029–1035. [Google Scholar] [CrossRef]
- Kuboshima, M.; Shimada, H.; Liu, T. L.; Nakashima, K.; Nomura, F.; Takiguchi, M.; Hiwasa, T.; Ochiai, T. , Identification of a novel SEREX antigen, SLC2A1/GLUT1, in esophageal squamous cell carcinoma. Int J Oncol 2006, 28, 463–468. [Google Scholar] [CrossRef]
- Arasawa, T.; Hiwasa, T.; Kagaya, A.; Maruyama, T.; Uesato, M.; Kano, M.; Kobayashi, S.; Takizawa, H.; Iwase, K.; Nomura, F.; et al. , Analysis of patients with colorectal cancer shows a specific increase in serum anti-ING1 autoantibody levels. BMC Cancer 2023, 23, 356. [Google Scholar] [CrossRef]
- Liu, Y.; Long, Y. H.; Wang, S. Q.; Zhang, Y. Y.; Li, Y. F.; Mi, J. S.; Yu, C. H.; Li, D. Y.; Zhang, J. H.; Zhang, X. J. , JMJD6 regulates histone H2A.X phosphorylation and promotes autophagy in triple-negative breast cancer cells via a novel tyrosine kinase activity. Oncogene 2019, 38, 980–997. [Google Scholar] [CrossRef]
- Chen, S.; Wang, M.; Lu, T.; Liu, Y.; Hong, W.; He, X.; Cheng, Y.; Liu, J.; Wei, Y.; Wei, X. , JMJD6 in tumor-associated macrophage regulates macrophage polarization and cancer progression via STAT3/IL-10 axis. Oncogene 2023, 42, 2737–2750. [Google Scholar] [CrossRef]
- Fadok, V. A.; Bratton, D. L.; Rose, D. M.; Pearson, A.; Ezekewitz, R. A.; Henson, P. M. , A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 2000, 405, 85–90. [Google Scholar] [CrossRef]
- Goh, A. M.; Coffill, C. R.; Lane, D. P. , The role of mutant p53 in human cancer. J Pathol 2011, 223, 116–126. [Google Scholar] [CrossRef]
- Adams, H. P., Jr.; Bendixen, B. H.; Kappelle, L. J.; Biller, J.; Love, B. B.; Gordon, D. L.; Marsh, E. E. , 3rd, Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Yoshida, K.; Hiwasa, T.; Ito, M.; Ushigome, M.; Takizawa, H.; Li, S. Y.; Zhang, B. S.; Iwadate, Y.; Funahashi, K.; Shimada, H. , Prognostic and diagnostic significance of preoperative Jumonji domain-containing 6 antibodies in colorectal cancer. Oncol Lett 2023, 25, 127. [Google Scholar] [CrossRef] [PubMed]
- Matsutani, T.; Hiwasa, T.; Takiguchi, M.; Oide, T.; Kunimatsu, M.; Saeki, N.; Iwadate, Y. , Autologous antibody to src-homology 3-domain GRB2-like 1 specifically increases in the sera of patients with low-grade gliomas. J Exp Clin Cancer Res 2012, 31, 85. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, X. M.; Tomiyoshi, G.; Nakamura, R.; Shinmen, N.; Kuroda, H.; Kimura, R.; Mine, S.; Kamitsukasa, I.; Wada, T.; et al. , Association of serum levels of antibodies against MMP1, CBX1, and CBX5 with transient ischemic attack and cerebral infarction. Oncotarget 2018, 9, 5600–5613. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Hiwasa, T.; Oshima, Y.; Yajima, S.; Suzuki, T.; Nanami, T.; Sumazaki, M.; Shiratori, F.; Funahashi, K.; Li, S. Y.; et al. , Association of serum anti-PCSK9 antibody levels with favorable postoperative prognosis in esophageal cancer. Front Oncol 2021, 11, 708039. [Google Scholar] [CrossRef]
- Shimada, H.; Ochiai, T.; Nomura, F. , Titration of serum p53 antibodies in 1,085 patients with various types of malignant tumors: a multiinstitutional analysis by the Japan p53 Antibody Research Group. Cancer 2003, 97, 682–689. [Google Scholar] [CrossRef]
Figure 1.
Comparison of the levels of serum anti-JMJD6 antibodies (s-JMJD6-Abs) in healthy donors (HDs) and patients with chronic cerebral infarction (CCI), acute cerebral infarction (ACI), transient ischemic attack (TIA), asymptomatic ischemic stroke (asympt-CI), and deep and subcortical white matter hyperintensity (DSWMH). (
a) The figure shows the scatter dot plot of the levels of s-JMJD6-Abs examined using amplified luminescence proximity homogeneous assay-linked immunosorbent assay (AlphaLISA). The bars represent the average. The
P-values were calculated using the Kruskal–Wallis test (Mann–Whitney
U test with Bonferroni correction applied); *
P < 0.05; ****P < 0.0001.
Table 1 summarizes the exact values. (
b–f) The abilities of s-JMJD6-Ab in detecting CCI (b), ACI (c), TIA (d), asympt-CI (e), and DSWMH (f) were assessed by receiver operating characteristic (ROC) curve analysis. The numbers in the figures indicate the cutoff values for marker levels, and the numbers in parentheses indicate sensitivity (left) and specificity (right).
P-values, areas under the curve (AUCs), and 95% confidence intervals (95% CI) are also shown. (
g–n) The associations of s-JMJD6-Ab levels with sex (g), body mass index (BMI) (h), diabetes mellitus (DM) (i), hypertension (HT) (j), cardiovascular diseases (CVD) (k), lipidemia (l), smoking (m), and alcohol drinking (n) were examined in the Sawara Hospital cohort. The
P-values were calculated using the Mann–Whitney
U test; **
P < 0.01; ***
P < 0.001; ****
P < 0.0001; ns, not significant.
Figure 1.
Comparison of the levels of serum anti-JMJD6 antibodies (s-JMJD6-Abs) in healthy donors (HDs) and patients with chronic cerebral infarction (CCI), acute cerebral infarction (ACI), transient ischemic attack (TIA), asymptomatic ischemic stroke (asympt-CI), and deep and subcortical white matter hyperintensity (DSWMH). (
a) The figure shows the scatter dot plot of the levels of s-JMJD6-Abs examined using amplified luminescence proximity homogeneous assay-linked immunosorbent assay (AlphaLISA). The bars represent the average. The
P-values were calculated using the Kruskal–Wallis test (Mann–Whitney
U test with Bonferroni correction applied); *
P < 0.05; ****P < 0.0001.
Table 1 summarizes the exact values. (
b–f) The abilities of s-JMJD6-Ab in detecting CCI (b), ACI (c), TIA (d), asympt-CI (e), and DSWMH (f) were assessed by receiver operating characteristic (ROC) curve analysis. The numbers in the figures indicate the cutoff values for marker levels, and the numbers in parentheses indicate sensitivity (left) and specificity (right).
P-values, areas under the curve (AUCs), and 95% confidence intervals (95% CI) are also shown. (
g–n) The associations of s-JMJD6-Ab levels with sex (g), body mass index (BMI) (h), diabetes mellitus (DM) (i), hypertension (HT) (j), cardiovascular diseases (CVD) (k), lipidemia (l), smoking (m), and alcohol drinking (n) were examined in the Sawara Hospital cohort. The
P-values were calculated using the Mann–Whitney
U test; **
P < 0.01; ***
P < 0.001; ****
P < 0.0001; ns, not significant.
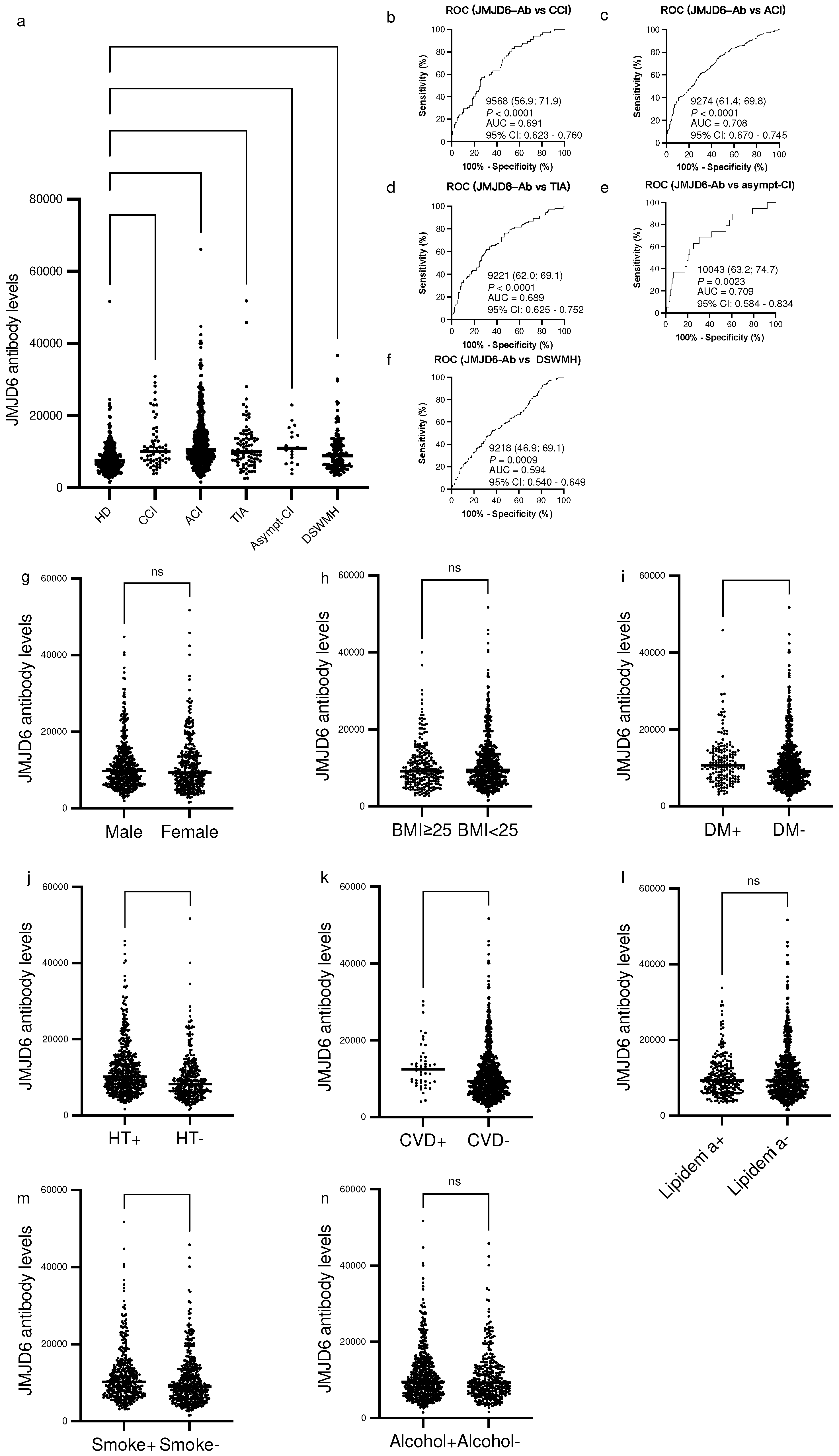
Figure 2.
Comparison of the levels of serum anti-JMJD6 antibodies (s-JMJD6-Abs) in healthy donors (HDs) and patients with acute myocardial infarction (AMI) and diabetes mellitus (DM). (a) The figure shows the levels of s-JMJD6-Abs examined by amplified luminescence proximity homogeneous assay-linked immunosorbent assay (AlphaLISA). (b,c) Antibody levels are represented by Alpha photon counts and shown in a scatter dot plot; the horizontal lines represent medians, and the dots represent outliers. The P-values were calculated using the Kruskal–Wallis test. The ability of s-JMJD6-Ab levels to detect AMI (b) and DM (c) was assessed by receiver operating characteristic (ROC) curve analysis. The numbers in the figures are the area under the curve (AUC), specificity, sensitivity, and cutoff values for the marker levels.
Figure 2.
Comparison of the levels of serum anti-JMJD6 antibodies (s-JMJD6-Abs) in healthy donors (HDs) and patients with acute myocardial infarction (AMI) and diabetes mellitus (DM). (a) The figure shows the levels of s-JMJD6-Abs examined by amplified luminescence proximity homogeneous assay-linked immunosorbent assay (AlphaLISA). (b,c) Antibody levels are represented by Alpha photon counts and shown in a scatter dot plot; the horizontal lines represent medians, and the dots represent outliers. The P-values were calculated using the Kruskal–Wallis test. The ability of s-JMJD6-Ab levels to detect AMI (b) and DM (c) was assessed by receiver operating characteristic (ROC) curve analysis. The numbers in the figures are the area under the curve (AUC), specificity, sensitivity, and cutoff values for the marker levels.
Figure 3.
Comparison of the levels of serum anti-JMJD6 antibodies (s-JMJD6-Abs) in healthy donors (HDs) and patients with esophageal cancer (EC), gastric cancer (GC), lung cancer (LC), and mammary cancer (MC). (a) The figure shows the levels of s-JMJD6-Abs examined by amplified luminescence proximity homogeneous assay-linked immunosorbent assay (AlphaLISA). Antibody levels are represented by Alpha photon counts and shown in a scatter dot plot; the horizontal lines represent medians, and the dots represent outliers. The P-values were calculated using the Kruskal–Wallis test. (b–e) The ability of s-JMJD6-Ab levels to detect EC (b), GC (c), LC (d), and MC (e) was assessed by receiver operating characteristic (ROC) curve analysis. The numbers in the figures are the area under the curve (AUC), specificity, sensitivity, and cutoff values for the JMJD6-Ab levels. (f,g) The overall survival in patients with EC and GC between the s-JMJD6-Ab-positive (JMJD6-Ab+) and s-JMJD6-Ab-negative (JMJD6-Ab−) groups (P = 0.0290, P = 0.0970; f, g) was compared and analyzed by log-rank test. (h,i) The overall survival of patients was stratified by their PD-L1 status (positive or negative) alone and in combination with the s-JMJD6-Ab levels (positive or negative), respectively. (j,k) Panels j and k illustrate the survival outcomes of patients categorized according to their p53-Ab status, both individually and in conjunction with their s-JMJD6-Ab levels (positive or negative).
Figure 3.
Comparison of the levels of serum anti-JMJD6 antibodies (s-JMJD6-Abs) in healthy donors (HDs) and patients with esophageal cancer (EC), gastric cancer (GC), lung cancer (LC), and mammary cancer (MC). (a) The figure shows the levels of s-JMJD6-Abs examined by amplified luminescence proximity homogeneous assay-linked immunosorbent assay (AlphaLISA). Antibody levels are represented by Alpha photon counts and shown in a scatter dot plot; the horizontal lines represent medians, and the dots represent outliers. The P-values were calculated using the Kruskal–Wallis test. (b–e) The ability of s-JMJD6-Ab levels to detect EC (b), GC (c), LC (d), and MC (e) was assessed by receiver operating characteristic (ROC) curve analysis. The numbers in the figures are the area under the curve (AUC), specificity, sensitivity, and cutoff values for the JMJD6-Ab levels. (f,g) The overall survival in patients with EC and GC between the s-JMJD6-Ab-positive (JMJD6-Ab+) and s-JMJD6-Ab-negative (JMJD6-Ab−) groups (P = 0.0290, P = 0.0970; f, g) was compared and analyzed by log-rank test. (h,i) The overall survival of patients was stratified by their PD-L1 status (positive or negative) alone and in combination with the s-JMJD6-Ab levels (positive or negative), respectively. (j,k) Panels j and k illustrate the survival outcomes of patients categorized according to their p53-Ab status, both individually and in conjunction with their s-JMJD6-Ab levels (positive or negative).
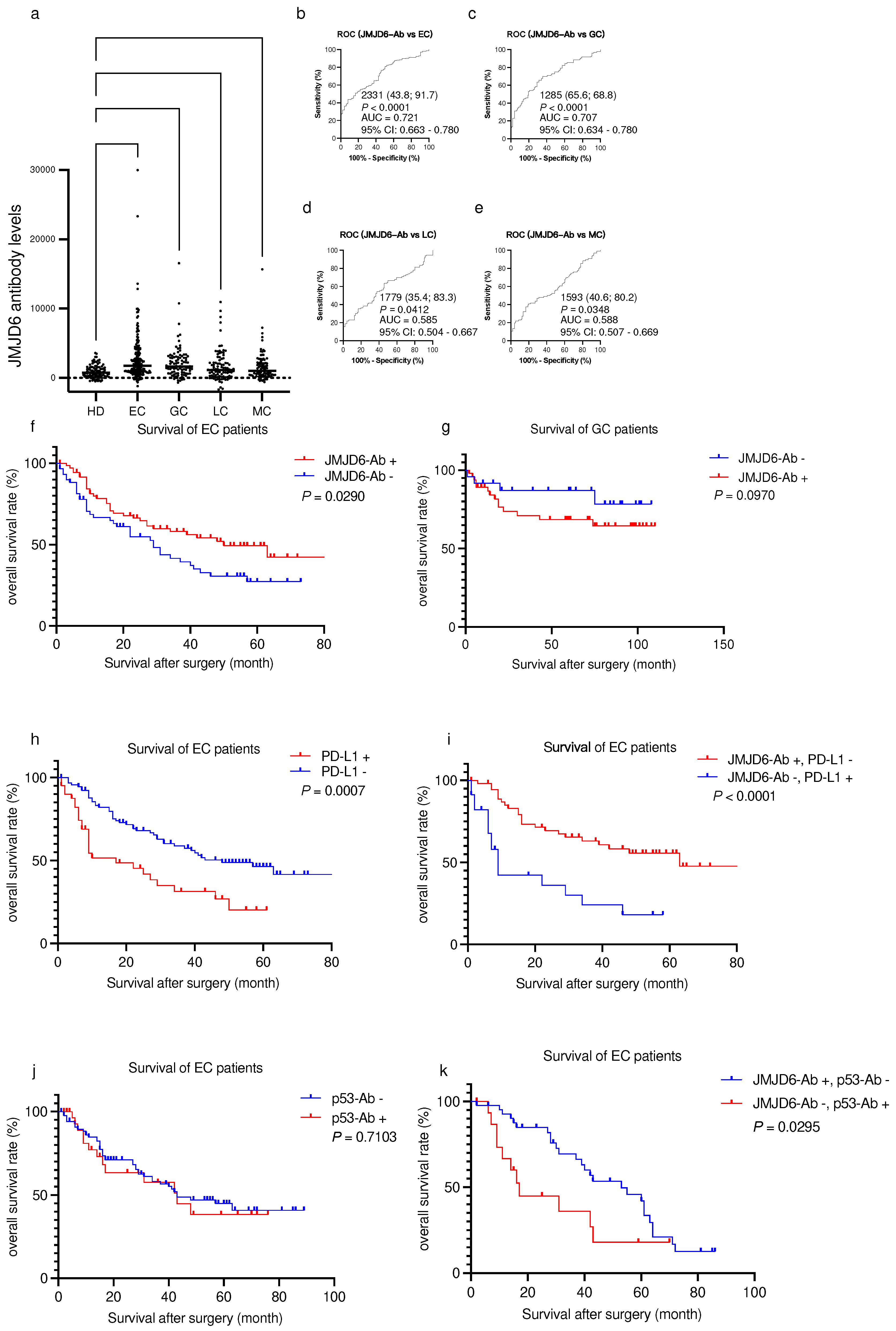
Table 1.
Comparing the serum anti-JMJD6 antibody levels between healthy donors and patients with chronic cerebral infarction (CCI), acute cerebral infarction (ACI), transient ischemic attack (TIA), asymptomatic ischemic stroke (Asympt-CI), and deep and subcortical white matter hyperintensity (DSWMH).
Table 1.
Comparing the serum anti-JMJD6 antibody levels between healthy donors and patients with chronic cerebral infarction (CCI), acute cerebral infarction (ACI), transient ischemic attack (TIA), asymptomatic ischemic stroke (Asympt-CI), and deep and subcortical white matter hyperintensity (DSWMH).
Sample information
(s-JMJD6-Ab) |
HD |
CCI |
ACI |
TIA |
asympt-CI |
DSWMH |
| Total sample number |
285 |
65 |
464 |
92 |
19 |
162 |
| Male/female |
188/97 |
47/18 |
271/193 |
55/37 |
13/6 |
88/74 |
| Age, years (average ± SD) |
52.3 ± 11.7 |
73.2 ± 9.3 |
75.5 ± 11.5 |
70.2 ± 11.6 |
67.7 ± 11.2 |
66.7 ± 10.0 |
| |
| Patient group |
Type of value |
s-JMJD6-Ab |
| HD |
Average |
8359 |
| |
SD |
4741 |
| |
Cutoff value |
17842 |
| |
Positive no. |
9 |
| |
Positive rate (%) |
3.2% |
| CCI |
Average |
11972 |
| |
SD |
6511 |
| |
Positive no. |
11 |
| |
Positive rate (%) |
16.9% |
| |
P-value (vs. HD) |
< 0.0001 |
| ACI |
Average |
12814 |
| |
SD |
7711 |
| |
Positive no. |
88 |
| |
Positive rate (%) |
19.0% |
| |
P-value (vs. HD) |
< 0.0001 |
| TIA |
Average |
11934 |
| |
SD |
7532 |
| |
Positive no. |
12 |
| |
Positive rate (%) |
13.0% |
| |
P-value (vs. HD) |
< 0.0001 |
| asympt-CI |
Average |
11632 |
| |
SD |
5077 |
| |
Positive no. |
2 |
| |
Positive rate (%) |
10.5% |
| |
P-value (vs. HD) |
0.0128 |
| DSWMH |
Average |
9897 |
| |
SD |
5320 |
| |
Positive no. |
14 |
| |
Positive rate (%) |
8.6% |
| |
P-value (vs. HD) |
0.0025 |
Table 2.
Spearman correlation analysis between the serum anti-JMJD6 antibody levels and clinical features.
Table 2.
Spearman correlation analysis between the serum anti-JMJD6 antibody levels and clinical features.
| Parameter |
Rho value |
95% confidence interval |
P-value |
Number of pairs |
| Age |
0.3173 |
0.2532 to 0.3786 |
<0.0001 |
839 |
| Height (cm) |
−0.1291 |
−0.1972 to −0.0597 |
0.0002 |
833 |
| Weight (kg) |
−0.1388 |
−0.2066 to −0.0696 |
<0.0001 |
836 |
| BMI |
−0.0797 |
−0.1488 to −0.0098 |
0.0215 |
832 |
| IMT (r) |
0.2507 |
0.1744 to 0.3240 |
<0.0001 |
640 |
| IMT (l) |
0.2661 |
0.1904 to 0.3386 |
<0.0001 |
641 |
| max IMT |
0.2761 |
0.2009 to 0.3481 |
<0.0001 |
642 |
| A/G |
−0.1357 |
−0.2047 to −0.0653 |
0.0001 |
808 |
| AST |
0.0421 |
−0.0278 to 0.1116 |
0.2238 |
836 |
| ALT |
−0.0180 |
−0.0877 to 0.0520 |
0.6045 |
835 |
| ALP |
0.0989 |
0.0266 to 0.1701 |
0.0059 |
775 |
| LDH |
0.0541 |
−0.0169 to 0.1245 |
0.1243 |
810 |
| tBil |
−0.0469 |
−0.1171 to 0.0237 |
0.1801 |
818 |
| CHE |
−0.1352 |
−0.2129 to −0.0558 |
0.0006 |
636 |
| gamma-GTP |
0.0389 |
−0.0334 to 0.1107 |
0.2778 |
782 |
| TP |
−0.1581 |
−0.2264 to −0.0883 |
<0.0001 |
812 |
| ALB |
−0.2019 |
−0.2686 to −0.1334 |
<0.0001 |
821 |
| BUN |
0.0442 |
−0.0258 to 0.1137 |
0.2022 |
834 |
| Creatinin |
0.0213 |
−0.0488 to 0.0912 |
0.5399 |
830 |
| eGFR |
−0.0540 |
−0.1274 to 0.0200 |
0.1406 |
746 |
| UA |
−0.0093 |
−0.0909 to 0.0723 |
0.8176 |
612 |
| AMY |
−0.0430 |
−0.1313 to 0.0459 |
0.3288 |
517 |
| T-CHO |
−0.1239 |
−0.1966 to −0.0498 |
0.0008 |
733 |
| HDL-c |
−0.0222 |
−0.1087 to 0.0648 |
0.6072 |
541 |
| TG |
−0.0989 |
−0.1813 to −0.0152 |
0.0173 |
579 |
| Na |
0.0093 |
−0.0611 to 0.0797 |
0.7892 |
821 |
| K |
−0.0573 |
−0.1272 to 0.01320 |
0.1009 |
821 |
| Cl |
0.0418 |
−0.0287 to 0.1119 |
0.2312 |
821 |
| CRP |
0.1370 |
0.0555 to 0.2167 |
0.0007 |
604 |
| WBC |
0.0702 |
0.0003 to 0.1394 |
0.0426 |
834 |
| RBC |
−0.1118 |
−0.1802 to −0.0422 |
0.0012 |
834 |
| HGB |
−0.0830 |
−0.1520 to −0.0132 |
0.0165 |
834 |
| HCT |
−0.0793 |
−0.1484 to −0.0095 |
0.0220 |
834 |
| MCV |
0.0879 |
0.0181 to 0.1568 |
0.0111 |
834 |
| MCH |
0.0781 |
0.0083 to 0.1472 |
0.0241 |
834 |
| MCHC |
−0.0308 |
−0.1004 to 0.0392 |
0.3750 |
834 |
| RDW |
0.0836 |
0.0138 to 0.1526 |
0.0158 |
834 |
| PLT |
−0.0966 |
−0.1653 to −0.0269 |
0.0052 |
834 |
| MPV |
−0.0109 |
−0.0807 to 0.0591 |
0.7540 |
834 |
| PCT |
−0.0956 |
−0.1644 to −0.0259 |
0.0057 |
834 |
| PDW |
−0.0470 |
−0.1165 to 0.0230 |
0.1751 |
834 |
| BS |
0.1343 |
0.0622 to 0.2049 |
0.0002 |
772 |
| HbA1c |
0.0571 |
−0.0225 to 0.1360 |
0.1474 |
645 |
| HbA1 |
0.0749 |
−0.0201 to 0.1685 |
0.1116 |
453 |
| BP |
0.0946 |
0.0102 to 0.1776 |
0.0238 |
571 |
| Smoking period |
0.1515 |
0.0761 to 0.2253 |
<0.0001 |
699 |
| Alcohol Freq |
0.0449 |
−0.0319 to 0.1212 |
0.2382 |
692 |
Table 3.
Comparing the serum anti-JMJD6 antibody levels between healthy donors and patients with acute myocardial infarction or diabetes mellitus.
Table 3.
Comparing the serum anti-JMJD6 antibody levels between healthy donors and patients with acute myocardial infarction or diabetes mellitus.
Sample information
(s-JMJD6-Ab) |
HD |
AMI |
DM |
| Total sample number |
128 |
128 |
128 |
| Male/female |
72/56 |
105/23 |
72/56 |
| Age, years (average ± SD) |
58.3 ± 5.6 |
58.2 ± 8.5 |
58.5 ± 9.2 |
| Patient group |
Type of value |
s-JMJD6-Ab |
| HD |
Average |
33844 |
| |
SD |
16513 |
| |
Cutoff value |
66869 |
| |
Positive no. |
6 |
| |
Positive rate (%) |
4.7% |
| AMI |
Average |
53889 |
| |
SD |
31263 |
| |
Positive no. |
28 |
| |
Positive rate (%) |
21.9% |
| |
P-value (vs. HD) |
< 0.0001 |
| DM |
Average |
50092 |
| |
SD |
33699 |
| |
Positive no. |
27 |
| |
Positive rate (%) |
21.1% |
| |
P-value (vs. HD) |
< 0.0001 |
Table 4.
Comparing the serum anti-JMJD6 antibody levels between healthy donors and patients with certain cancers.
Table 4.
Comparing the serum anti-JMJD6 antibody levels between healthy donors and patients with certain cancers.
Sample information
(s-JMJD6-Ab) |
HD |
EC |
GC |
LC |
MC |
| Total sample number |
96 |
192 |
96 |
96 |
96 |
| Male/female |
51/45 |
155/37 |
68/28 |
42/54 |
58/38 |
| Age, years (average ± SD) |
57.9 ± 6.0 |
67.4 ± 9.8 |
68.7 ± 10.6 |
60.9 ± 13.3 |
68.1 ± 9.6 |
|
| Patient group |
Type of value |
s-JMJD6-Ab |
| HD |
Average |
930 |
| |
SD |
932 |
| |
Cutoff value |
2793 |
| |
Positive no. |
4 |
| |
Positive rate (%) |
4.2% |
| EC |
Average |
2802 |
| |
SD |
3591 |
| |
Positive no. |
63 |
| |
Positive rate (%) |
32.8% |
| |
P-value (vs. HD) |
< 0.0001 |
| GC |
Average |
2115 |
| |
SD |
2418 |
| |
Positive no. |
29 |
| |
Positive rate (%) |
30.2% |
| |
P-value (vs. HD) |
< 0.0001 |
| LC |
Average |
1664 |
| |
SD |
2193 |
| |
Positive no. |
20 |
| |
Positive rate (%) |
20.8% |
| |
P-value (vs. HD) |
0.0031 |
| MC |
Average |
1599 |
| |
SD |
2117 |
| |
Positive no. |
15 |
| |
Positive rate (%) |
15.6% |
| |
P-value (vs. HD) |
0.0054 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).