1. Introduction
Dysferlin, a ferlin family protein, is a structural high molecular weight protein (230 kD) highly expressed in skeletal muscle and heart.
The ferlin family of proteins has been implicated in fusion events in muscle, including myoblast fusion, vesicle trafficking and membrane repair [
1]
. Mutations in the dysferlin gene in humans cause LGMD2B/R2, OMIM
# 253601 or Miyoshi myopathy (MMD1), OMIM
# 254130.
Skeletal muscles are composed of different proportions of fiber types according to the expression of myosin heavy chain (MyHC) isoforms [
2,
3]. Muscle fibers differ also in their metabolic profile (oxidative, glycolytic, and oxidative-glycolytic) [
4]. Rat skeletal muscles are composed of four fiber types: slow type-1, fast type-2a, fast type-2b and fast type-2x fibers. Mixed fiber types, in which several myosin heavy chains co-express, also exist. According to the metabolic profile, type-1 fibers are oxidative and type-2 fibers are glycolytic or oxidative-glycolytic [
4]. Muscles with predominant slow or fast fiber type exist as slow rat soleus (SOL) and fast rat extensor digitorum longus (EDL) muscle [
5,
6].
To the best of our knowledge, the expression of Dysferlin in rat slow or fast muscle has not been studied so far. The only study addressing differential expression of Dysferlin in muscle fibers was done in humans: Single-fiber proteomics demonstrated that several muscle proteins, including Dysferlin, are selectively enriched in specific types [
7]. The purpose of this study was to investigate the expression of Dysferlin by Western blotting in fast and slow rat muscles. Since Dysferlin is a sarcolemmal "
repair" protein, a hypothesis of differential Dysferlin expression in muscles with different contractile properties was made. The topic is also relevant for LGMD2B/2R and MMD1. Thus far, selective muscle involvement has not been explained in dysferlinopathies or human muscular dystrophies in general. It is well known that distal soleus is involved initially in MMD1 and proximal vastus lateralis and biceps brachii later in the course of the disease [
8]. The principal conclusion of our study in rats is that Dysferlin is differentially expressed in slow SOL and fast EDL. The amount of Dysferlin detected by Western blotting correlated with the myosin heavy chain composition studied by immunofluorescence and PAGE.
2. Materials and Methods
2.1. Experimental animals
Four adult Wistar rats (rattus norvegicus, RccHan: WIST) were used for this study, two males (41-43 weeks of age) and two females (40-42 weeks). The rats were retired breeders housed at the Medical Experimental Center of the Faculty of Medicine in Ljubljana. The rats were maintained under barrier conditions (microbiological monitoring results- SPF according to the FELASA recommendations) at 21-24C temperature, 55+/- relative humidity, and a 12:12h dark/light cycle (7a.m.-7p.m. light). Rats received autoclavable water and breeding rodent diet (Altromin, Germany, 1314 forti) at libitum. The study was conducted under the EU legislation (Directive 2010/63 EU) and permission issued by the Veterinary Administration (Administration for Food Safety, Veterinary Sector and Plant Protection) of the Republic of Slovenia (permission number U344401-13/20021/4). The euthanasia was performed by CO2 and the EDL and SOL muscles were removed bilaterally in each animal. In total, 8 EDL muscles and 8 SOL muscles were analyzed.
2.2. Western blotting
Total muscle homogenates were prepared as described previously [
9]. Electrophoresis was carried under a constant voltage of 150 V on 6% polyacrylamide gels for two hours in a Mini-Protean Tetra Vertical Electrophoresis Cell (Biorad). Twenty µg of total proteins were loaded per well. Western blotting was carried for one hour at a constant voltage of 50V, followed by high-intensity transfer, 1A constant, for half an hour, both with cooling in Criterion blotter (Biorad). Immunoblot was developed by Leica Dysferlin antibodies, and used in a dilution of 1:1000, with a one-hour incubation time, at room temperature. Secondary, goat anti-mouse, peroxidase labeled antibodies (Biorad) were applied in a dilution of 1:20.000 for one hour. The immune reaction was developed by ECL Prime Western Blotting Detection Reagent (Amersham). Chemiluminescence was captured by Fusion FX (Vilber). The size and intensity of Dysferlin bands, as well post-transfer Myosin (not separated into individual MyHCs), were quantified using a computer program for gel analysis “ELFO for Image Tool 2.00” (ViDiTo, Košice, Slovakia). Post-transfer Myosin was stained by a ready - to-use solution of Coomassie G-250 (Invitrogen). Rations Dysferlin/post-transfer Myosin
(hereafter referred to as Dysferlin/ Myosin) were calculated for each muscle.
2.3. PAGE of MyHC isoforms
Electrophoresis of MyHC isoforms was carried out as described previously [
10] with the modification (decreased cross-linking agent concentrations) [
11]. The gels were silver stained according to the published protocol [
12]. Individual MyHC bands were identified according to the electrophoretic mobility: the fastest band between 250 and 200 kD was recognized as MyHC-1 and the slowest as MyHC-2a. The band below MyHC-2a was identified as MyHC-2x and the intermediate band between MyHC-2x and MyHC-1 as MyHC-2b [
10,
11,
13]). Individual bands of MyHC isoforms were quantified by “ELFO for Image Tool 2.00” (ViDiTo, Košice, Slovakia) and expressed in percentages.
2.4. Immunofluorescence of MyHC isoforms
Immunohistochemistry of MyHC isoforms was performed as described previously [
14,
15], with the exception of the mixtures of primary antibodies against MyHC-1 (BA-D5), -2a (SC-71) and -2b (BF-F3), which were applied on single tissue sections, followed by the mixture of three isotype specific secondary antibodies labeled with three different fluorochromes (Alexa-Fluor 350, -546 and -488) [
16] and viewed under a fluorescence microscope (Nikon). Type 1, type-2a and type 2b fibers were identified according to the specific color. Fibers that were not stained by antibodies against MyHC-1, -2a and -2b were classified as type-2x fibers.
2.5. Image analysis
A computer-assisted system [
17] with manual delineation of fiber outlines in one muscle section captured under different wavelengths appropriate for specific fluorochrome, was used to perform fiber classification and quantitative analysis for the following: (i) numerical proportions of fiber types; (ii) area proportions of fiber types; and (iii) fiber diameters. Muscle fibers were classified into pure fibers (type-1, -2a, -2b and -2x), expressing either MyHC-1, -2a, -2b or -2x, and hybrid fibers co-expressing two adult MyHC isoforms (type-2a/2b, -1/2a, and -1/2b. Fibers co-expressing more than two adult myosin isoforms (e.g., -1/2a/2b) could not be reliably estimated [
16] owing to there being no specific color shade. Since antibodies against MyHC- 2x were not used, hybrid fibers co-expressing MyHC-2x could not be estimated. On average, approximately 250 muscle fibers were analyzed for each muscle sample.
2.6. Histochemistry
The activity of succinate dehydrogenase (SDH) was demonstrated in EDL and SOL muscles, as described [
18].
2.7. Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 25. All data are presented as the mean ± standard deviation (SD). Significance was determined by the Student's t-test. The correlation between Dysferlin/Myosin ratio and MyHC isoforms was estimated by the Pearson correlation coefficient. The level of significance was set at p < 0.05.
3. Results
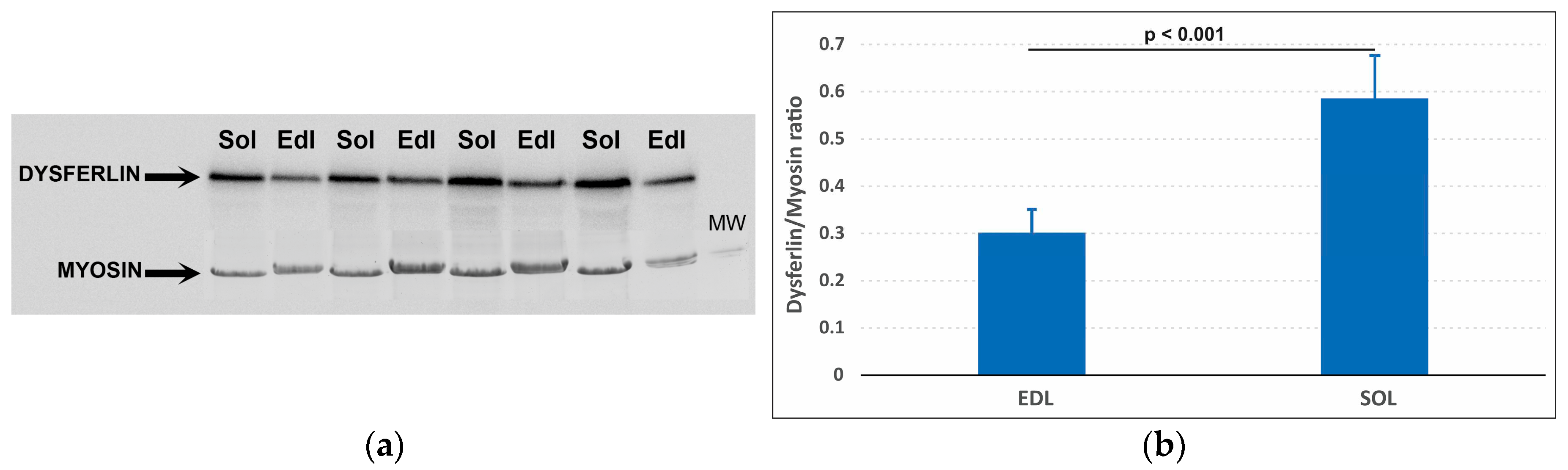
3.1. Dysferlin Western blotting
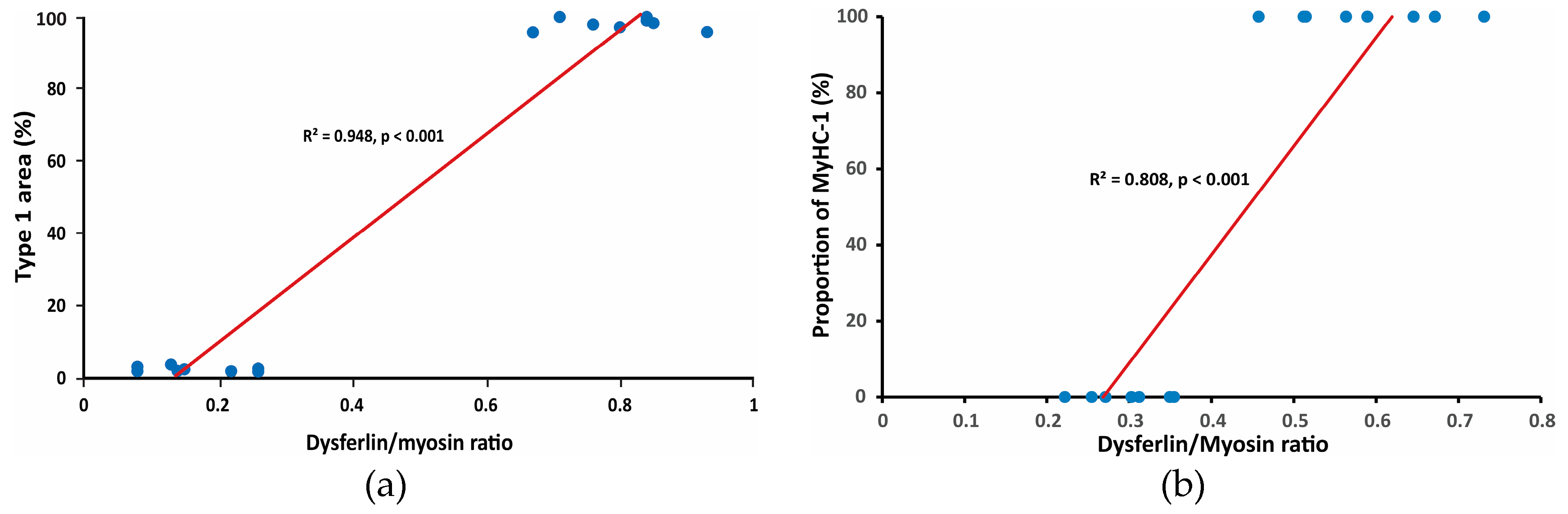
Dysferlin/Myosin ratio was significantly higher (p<0.001) in SOL than in EDL (
Figure 1).
The data indicate that Dysferlin is differentially expressed in rat EDL and SOL.
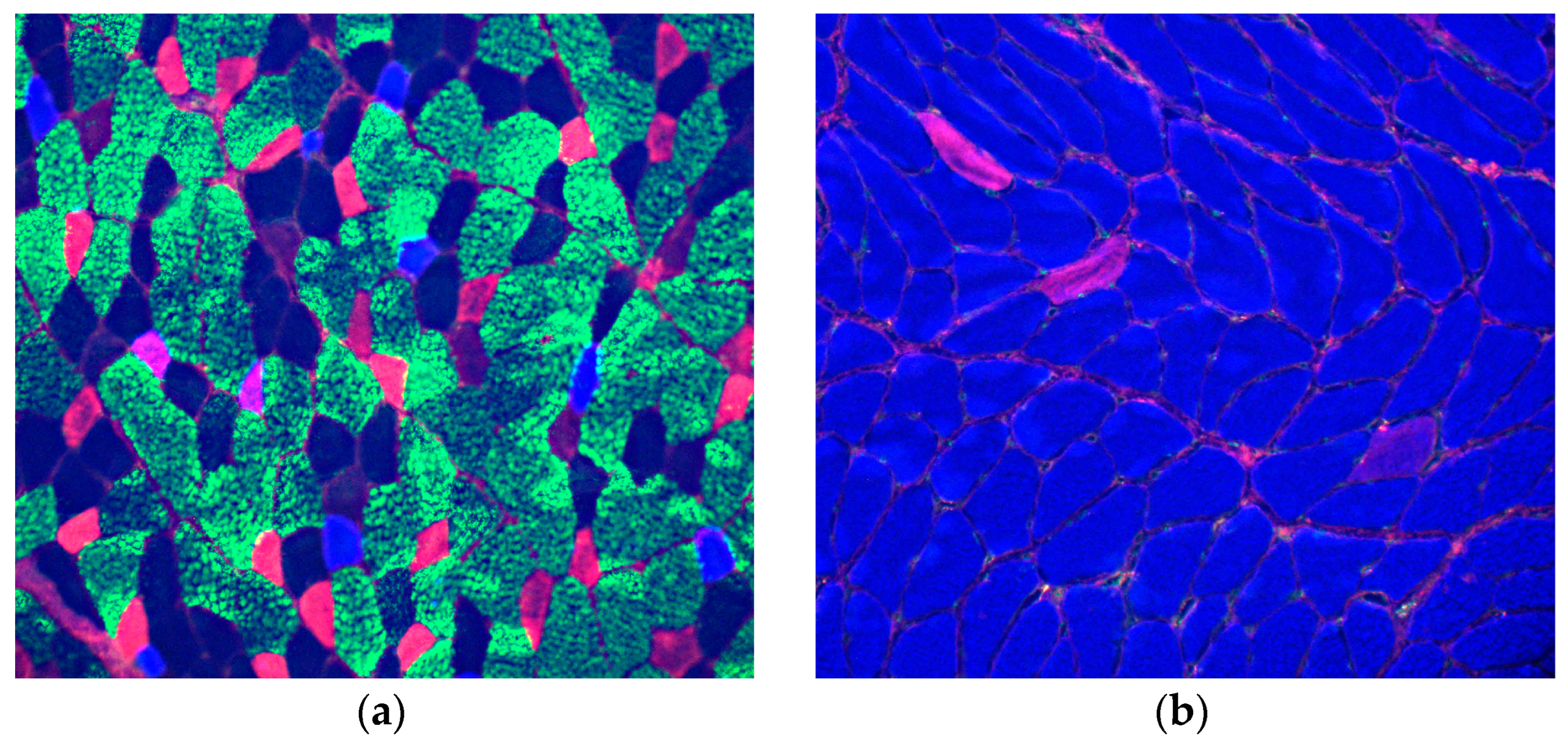
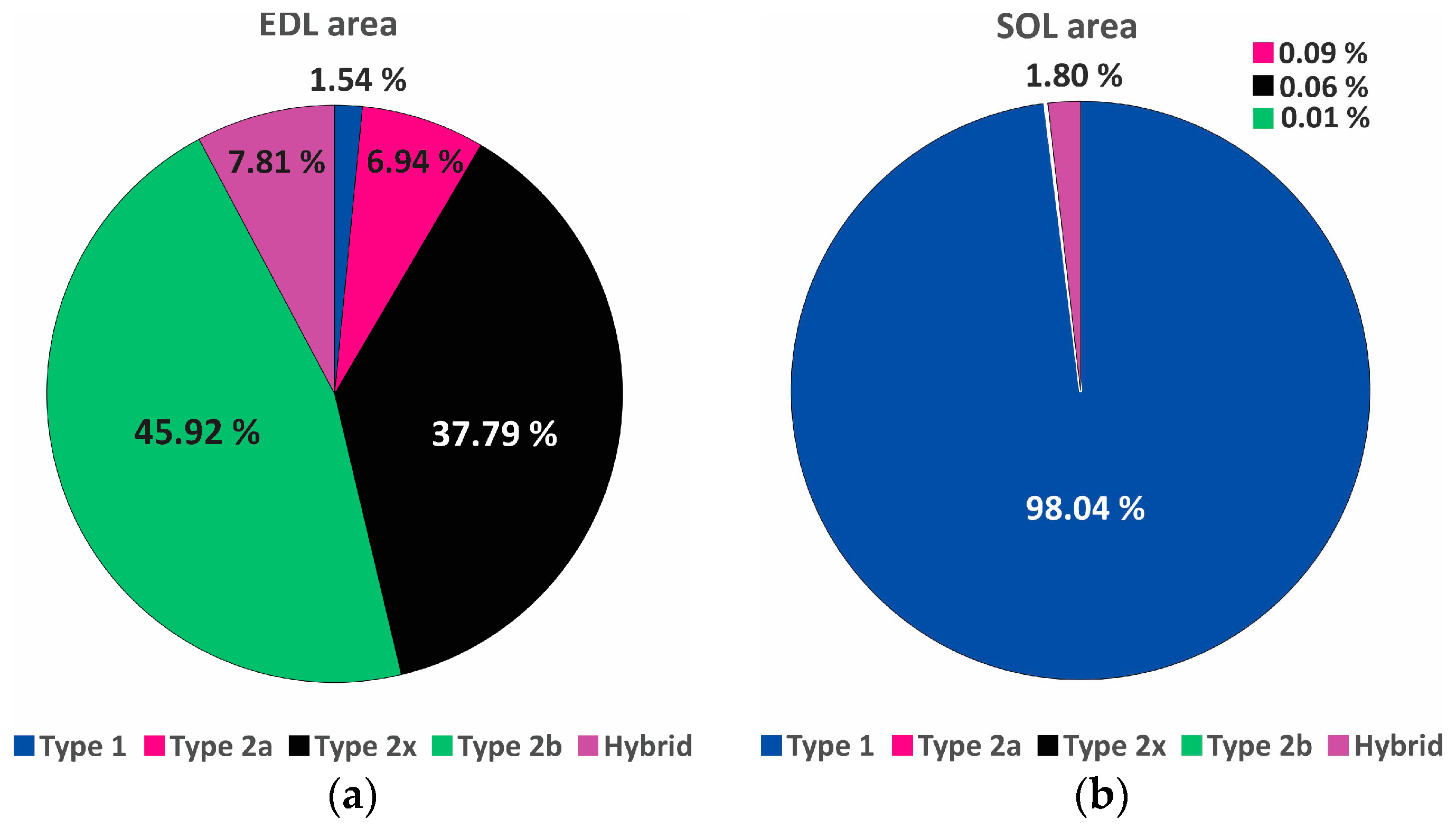
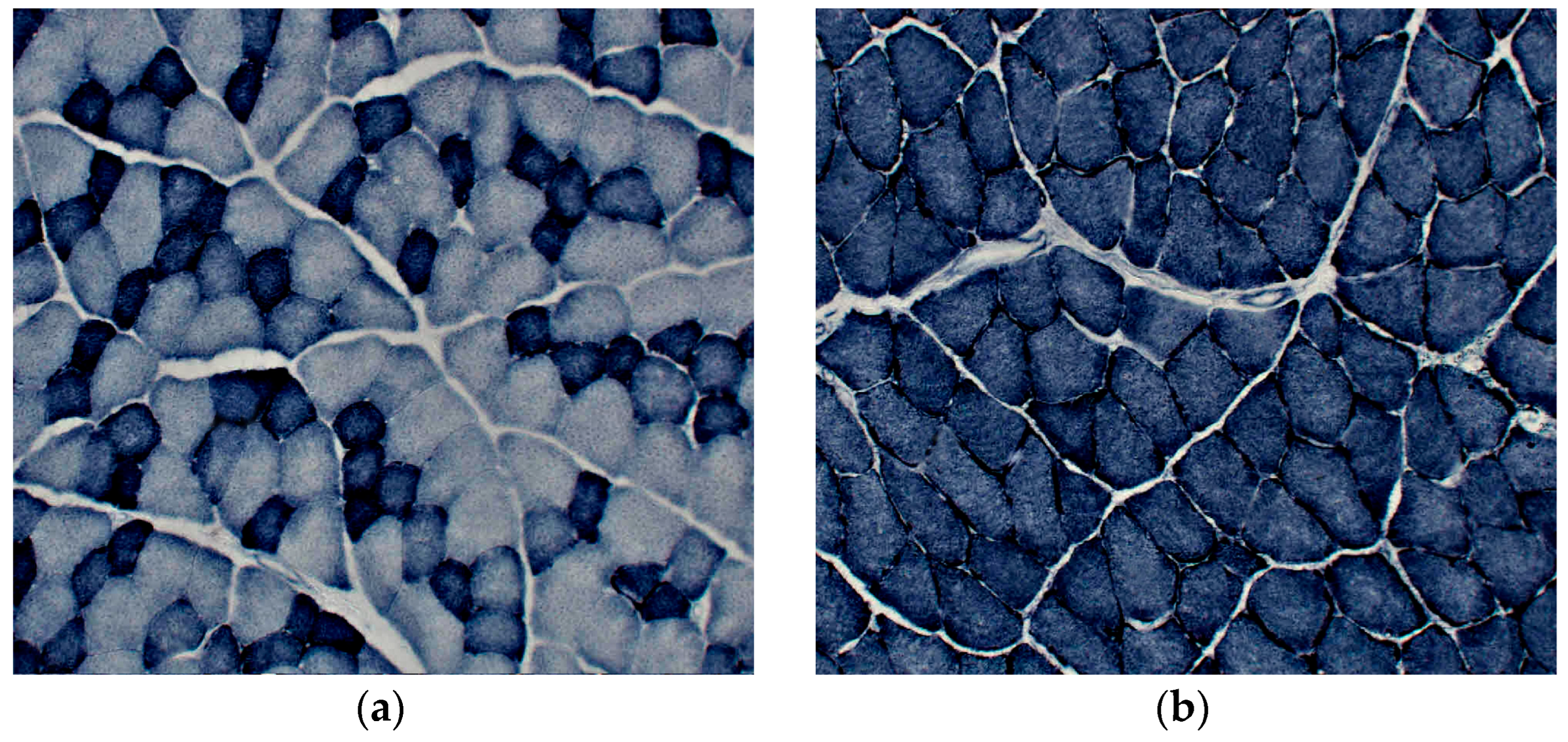
Fiber type composition of EDL and SOL muscle was estimated by immunofluorescence of MyHCs (
Figure 2 and 3) and PAGE of MyHCs (
Figure 4).
Morphometric data confirmed the visual impression that EDL is composed predominantly of type-2 (fast fibers) and that SOL is nearly exclusively of type-1 (slow fibers) (
Figure 3).
Immunofluorescence of MyHCs demonstrated typical composition of EDL with a preponderance of type-2 (fast fibers) and SOL with a preponderance of type-1 (slow) fibers.
Quantitative data of MyHC composition were obtained by PAGE of MyHC isoforms (
Figure 4).
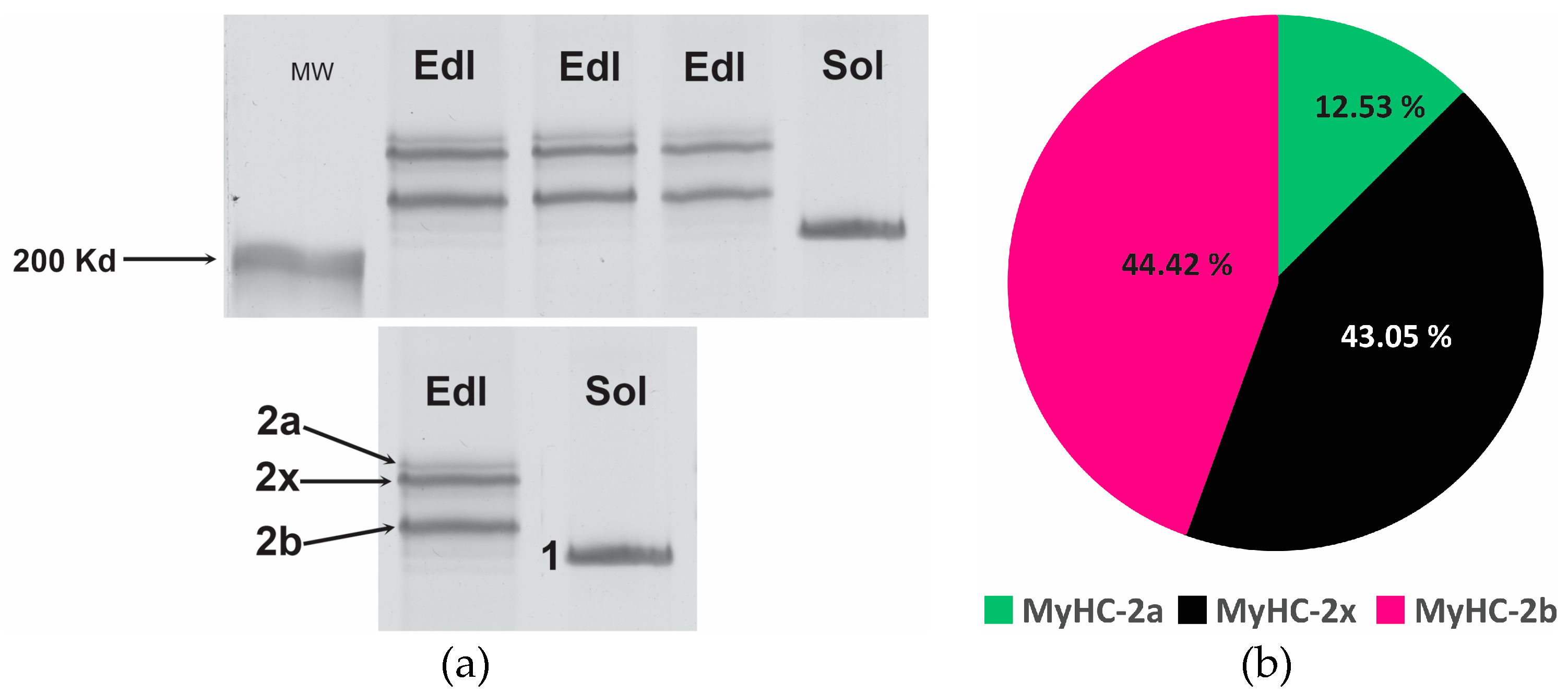
Figure 4.
PAGE of myosin heavy chain isoforms (MyHCs) in: (a) extensor digitorum longus (EDL) and soleus (SOL) muscle of rat number 2; different quantities of proteins were loaded per well (from right to left - 1µg, 1µg and 0.7µg for EDL) and 0.7µg for SOL; the gels were silver stained; and (b) Proportions of individual MyHC isoforms in EDL (MyHC- 2a, -2b, -2x): mean percentages for 8 EDLs; the hardly visible band in EDL at the height of MyHC-1 and was not taken into account in the calculations; average proportion of MyHC-1 in eight SOLs was 100% (the chart is not displayed).
Figure 4.
PAGE of myosin heavy chain isoforms (MyHCs) in: (a) extensor digitorum longus (EDL) and soleus (SOL) muscle of rat number 2; different quantities of proteins were loaded per well (from right to left - 1µg, 1µg and 0.7µg for EDL) and 0.7µg for SOL; the gels were silver stained; and (b) Proportions of individual MyHC isoforms in EDL (MyHC- 2a, -2b, -2x): mean percentages for 8 EDLs; the hardly visible band in EDL at the height of MyHC-1 and was not taken into account in the calculations; average proportion of MyHC-1 in eight SOLs was 100% (the chart is not displayed).
The composition of EDL and SOL, determined by proportions of MyHCs by PAGE, is as expected for slow SOL and fast EDL. Average proportion of MyHC-1 in eight SOLs was 100%. In eight EDLs the proportions of MyHC- 2b and -2x was similar (both about 44%) and the proportion of MyHC-2a was about 12%. The results of PAGE of MyHCs are in accordance with the results of immunofluorescence.
Oxidative capacity of EDL and SOL was demonstrated by the histochemistry of the SDH activity (
Figure 5).
SDH activity was higher in SOL than in EDL which also had smaller fibers with high SDH activity to SOL muscle. (The quantitative data for fiber diameters are in the supplementary material,
Figure S2.)
Correlation coefficients were calculated for Dysferlin quantity and fiber types (for subtypes - supplementary material) and for Dysferlin quantity and MyHC isoforms (
Figure 6).
There is a high correlation between the composition of muscles and Dysferlin quantity (for additional correlation diagrams, see supplementary material,
Figures S3 and S4).
4. Discussion
Characterization of rat SOL and rat EDL was done by immunofluorescence and PAGE of MyHCs. The results are identical to those previously reported for these muscles [
13]: SOL is a slow muscle and EDL is a fast muscle. Immunofluorescence demonstrates "in situ" distribution of MyHC isoforms in individual muscle fibers and detects smaller amounts of proteins than PAGE. This explains the exclusive presence of MyHC-1 in SOLs detected by PAGE, despite some type-2a fibers or hybrid type1/2a fibers being demonstrated by immunofluorescence. Since an antibody to MyHc-2x was not applied, hybrid type 2X fibers could not be identified. The proportions of hybrid fibers in the investigated muscles are rather low and not relevant for the main purpose of the study.
The main purpose of the study was Dysferlin analysis. We have demonstrated by Western blotting that Dysferlin is differentially expressed in slow SOL and fast EDL muscle of rats. In slow SOL, which is composed nearly exclusively of type-1 fibers, Dysferlin is more abundant than in fast EDL muscle. The single study which addresses the differential Dysferlin expression was done on human muscle [
7] using a different methodology –- proteomic analysis of single muscle fibers. The authors discovered that proteins enriched in human type-2x fibers compared to type-2a and type-1 fibers include Dysferlin, among other proteins. Since type-2x fibers are fast contracting [
19], this is different from our results, which showed more abundant Dysferlin in slow SOL than in fast EDL. Single-fiber proteomics provide relevant information regarding the distribution of specific proteins in individual fiber types, which is not the case if the proteomics of mixed muscles are studied in total muscle homogenates. However, we studied muscle predominantly composed of type-1 or type-2 fibers, slow SOL, and fast EDL, and conclude that methodological differences could not explain the different results for humans and rats.
Different sizes of muscle fibers in the investigated muscle, (SOL fibers are larger than EDL fibers), may partly explain the differences. During contraction, especially concentric, even healthy muscle fibers can be damaged [
20,
21]. Sarcolemmal stress during force development is at higher level in large fibers due to the decreased surface to volume ratio [
22]. If SOL fibers are more stressed than EDL physiologically, resulting in more abundant sarcolemmal damage, more repair machinery, including Dysferlin, may be an adaptation. It would be of interest to investigate other components for the sarcolemmal repair in SOL and EDL. In the study of 36 different human muscles the authors [
23] concluded that the fiber diameters of type-1 and type-2 fibers of the majority of muscles showed no significant differences. This means that fiber size in human muscle is not relevant for Dysferlin quantity.
It remains unclear whether different locomotion patterns (e.g., rats jump more frequently and with a higher range than humans) would provide rats with more abundant machinery for sarcolemmal repair in SOL, and so this requires further study.
Interestingly, the Dysferlin amount not only positively correlated with the proportion of type-1 fibers (and negatively correlated with the proportion of type-2 fibers) but was also "correlated" to the activity of SDH, which is a mitochondrial enzyme. (Oxidative capacity was not quantified and consequently correlation coefficients could not be calculated; the conclusion is based on qualitative observation.) In slow SOL, large fibers displayed high intensity of SDH activity while in fast EDL, small fibers displayed high intensity of SDH. This would indirectly suggest that the function of Dysferlin is beyond myoblast fusion, vesicle trafficking, and sarcolemmal repair, and may be also metabolic.
5. Conclusions
We propose that Dysferlin may have a metabolic role in addition to its involvement in the repair mechanism. Dysferlin, a ferlin family protein, is a structural high molecular weight protein (230 kD) highly expressed in skeletal muscle and heart. The ferlin family of proteins has been implicated in fusion events in muscle, including myoblast fusion, vesicle trafficking, and membrane repair. It is likely they have a different expression and role in fast and slow muscle fibers.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, s2, s3 and s4 Figure S1: Dysferlin in extensor digitorum longus (EDL) and soleus (SOL) muscles of the third and fourth rats; Figure S2: Mean fiber diameters (
x̄ ± SD) of oxidative fibers (with high, low, and intermediate activity). Figure S3: Correlation between composition of muscles, estimated by immunofluorescence of MyHCs and Dysferlin quantity; Figure S4: Correlation between composition of muscles, estimated by PAGE of MyHCs, and Dysferlin quantity;
Figure S1: Dysferlin in extensor digitorum longus (EDL) and soleus (SOL) muscles of the third and fourth rats: upper panel: Dysferlin by Western blotting: SOLs have more intensive bands than EDLs, similar to the first and second rats; MW - molecular weight marker; lower panel: corresponding post-transfer Myosin stained by Coomassie R-250.
Figure S2: Mean fiber diameters (
x̄ ± SD) of extensor digitorum longus (EDL) fibers with low oxidative (EDL-LO), intermediate oxidative (EDL-IO) and high oxidative (EDL-HO) activity and of soleus (SOL) fibers. SOL is composed of highly oxidative fibers (SOL-HO) which are larger than EDL-HO fibers (p<0.001). SOL-HO fibers are also larger than EDL-IO fibers (p<0.001).
Figure S3: Correlation between composition of muscles, estimated by immunofluorescence of MyHCs and Dysferlin quantity: (
a) for type-2 fibers; (
b) for type-2a fibers; (
c) for type-2b fibers; and (
d) for type-2x fibers. A high negative correlation exists between type-2 area and subtypes of type-2 fibers area and Dysferlin quantity.
Figure S4: Correlation between composition of muscles, estimated by PAGE of MyHCs, and Dysferlin quantity: (
a) for all fast MyHCs (MyHC-2a, -2b, -2x taken together); (
b) for MyHC-2a; (
c) for MyHC-2b; and (
d) for MyHC-2x. A high negative correlation exists between all fast MyHCs taken together and subtypes of fast MyHCs and Dysferlin quantity.
Author Contributions
Conceptualization, C.A. and M.M.; methodology, M.M. and C.A.; sotware, M.M.; validation, M.M. and C.A.; formal analysis, M.M. and C.A.; investigation, M.M.; resources, M.M.; data curation, M.M.; writing—original draft preparation, M.M.; writing—review and editing, C.A. and M.M.; visualization, M.M.; supervision, C.A.; project administration, M.M.; funding acquisition, M.M. Both authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ARIS - Slovenian Research and Innovation Agency, grant number P3-0043 to M.M.; The APC were not applicable (free waiver to M.M.).
Institutional Review Board Statement
The study was conducted under the EU legislation (Directive 2010/63 EU) and permission issued by the Veterinary Administration (Administration for Food Safety, Veterinary Sector and Plant Protection) of the Republic of Slovenia (permission number U344401-13/20021/4).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting reported results are available on written request at the authors.
Acknowledgments
The authors thank Mrs. Andreja Vidmar, Mr. Marko Slak and Mrs. Majda Črnak-Maasarani for technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Posey, A.D.; Demonbreun A.; McNally E.M. Ferlin proteins in myoblast fusion and muscle growth. Curr Top Dev Biol 2011, 96, 203-230. [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol Rev 2011, 91, 1447-1531. [CrossRef]
- Schiaffino, S.; Muscle fiber type diversity revealed by anti-myosin heavy chain antibodies. FEBS J 2018, 285, 3688-3694. [CrossRef]
- Edström, L.; Kugelberg E. Histochemical composition, distribution of fibres and fatiguability of single motor units. Anterior tibial muscle of the rat. J Neurol Neurosurg Psychiatry 1968, 31, 424-433. [CrossRef]
- Close, R. Properties of motor units in fast and slow skeletal muscles of the rat. J Physiol 1967, 193, 45-55. [CrossRef]
- Schiaffino, S.; Hanzlíková V.; Pierobon S. Relations between structure and function in rat skeletal muscle fibers. J Cell Biol 1970, 47, 107-119. [CrossRef]
- Murgia, M.; Nogara, L.; Baraldo, M.; Reggiani, C.; Mann, M.; Schiaffino, S. Protein profile of fiber types in human skeletal muscle: a single-fiber proteomics study. Skelet Muscle 2021, 11, 24. [CrossRef]
- Fanin, M.; Angelini, C. Progress and challenges in diagnosis of dysferlinopathy. Muscle Nerve 2016, 54, 821-835.
- Fanin, M.; Nascimbeni, A.C.; Fulizio, L.; Trevisan, C.P.; Meznaric-Petrusa, M., Angelini, C. Loss of calpain-3 autocatalytic activity in LGMD2A patients with normal protein expression. Am J Pathol 2003, 163, 1929-1936. [CrossRef]
- Talmadge, R.J.; Roy, R.R. Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol (1985) 1993, 75, 2337-2340. [CrossRef]
- Mizunoya, W.; Wakamatsu, J.; Tatsumi, R.; Ikeuchi, Y. Protocol for high-resolution separation of rodent myosin heavy chain isoforms in a mini-gel electrophoresis system. Anal Biochem 2008, 377, 111-113. [CrossRef]
- Blum, H., Beier, H.; Gross, H. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 1987, 8, 93-99 doi:org/10.1002/elps.1150080203.
- Smerdu, V.; Soukup, T. Demonstration of myosin heavy chain isoforms in rat and humans: the specificity of seven available monoclonal antibodies used in immunohistochemical and immunoblotting methods. Eur J Histochem 2008, 52, 179-190. [CrossRef]
- Meznaric, M.; Eržen, I.; Karen, P.; Cvetko, E. Effect of ageing on the myosin heavy chain composition of the human sternocleidomastoid muscle. Ann Anat 2018, 216, 95-99. [CrossRef]
- Meznaric, M.; Čarni A., Characterisation of flexor digitorum profundus, flexor digitorum superficialis and extensor digitorum communis by electrophoresis and immunohistochemical analysis of myosin heavy chain isoforms in older men. Ann Anat 2020, 227, 151412. [CrossRef]
- Čebašek, V.; Ribarič, S. Changes in local capillarity of pure and hybrid MyHC muscle fiber types after nerve injury in rat extensor digitorum longus muscle (EDL). Histochem Cell Biol 2019, 152, 89-107.
- Karen, P., Števanec, M.; Smerdu, V.; Cvetko, E.; Kubínová, L.; Eržen, I. Software for muscle fibre type classification and analysis. Eur J Histochem 2009, 53, 87-95. [CrossRef]
- Dubowitz, V.; Sewry, C. Histological and histochemical stains and reactions. In Muscle Biopsy A Practical Approach, 3rd ed.; Dubowitz, V., Editor; Saunders Elsevier: China, 2007; 21-39.
- Larsson, L.; Moss, R.L. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol, 1993, 472, 595-614. [CrossRef]
- Stauber, W.T. Eccentric action of muscles: physiology, injury, and adaptation. Exerc Sport Sci Rev 1989, 17, 157-185.
- McNeil, P.L.; Khakee, R. Disruptions of muscle fiber plasma membranes. Role in exercise-induced damage. Am J Pathol 1992, 140, 1097-1109.
- Petrof, B.J.; Stedman, H.H.; Shrager, J.B.; Eby, J.; Sweeney, H.L.; Kelly, A.M. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci U S A 1993, 90, 3710-3714. [CrossRef]
- Polgar, J.; Johnson M.A.; Weightman, D.; Appleton, D. Data on fibre size in thirty-six human muscles. An autopsy study. J Neurol Sci 1973, 19, 307-318. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).