Introduction
Childhood overweight and obesity is one of the major concerns of health systems around the world. The prevalence of overweight and obesity between children and adolescents aged 5-19 increase from 4% in 1975 to 18% in 2016 (over 340 million children and adolescents aged 5-19). Children under the age of 5 years overweight or obese were estimated to be 38.2 million in 2019 (1).
Antibiotic treatment in the early days of life has been associated with abdominal fat accumulation in the following 6 to 24 months in humans. (2,3) Antibiotics are drugs that have the capacity to eliminate or inhibit bacterial growth, and are used to treat neonatal infections, which are primarily bacterial in origin, as pneumonia, sepsis, and meningitis. (4)
The antibiotics use is high around the world in Neonatal Intensive Care Units (NICU), where it is estimated that it represents 25% of used drugs due to the risk of infection development that premature neonates are exposed to (5, 6). With a low expression of co-stimulatory molecules in the immune system and without immunological memory, neonatal exposure to pathogens before, during, or after birth favours the establishment of an infection with the risk of developing early or late neonatal sepsis, which is often lethal (7).
Antibiotics used in NICUs are classified by the World Health Organization (WHO), based on the Access, Watch, Reserve, (AWaRe) Essential Medicines List for Children and their use. The antibiotics of the first level of access belong to restricted-spectrum, such as Ampicillin and Amikacin. Observation antibiotics, such as Meropenem and Cefotaxime are broad-spectrum, and the reserved group classification refers to those antibiotics used in the multidrug resistant infection treatment (8).
While antibiotic use is essential, it can be regulated and targeted by culture, antibiograms, and new techniques such as sequencing, which allow rapid, highly sensitive, and specific identification of bacterial species (9). However, the recovery rate from blood culture microorganisms is still less than 17%, which does not allow specific management (8), Mortality is estimated to increase 7% every hour due to delayed initiation of antimicrobial therapy in critically ill patients (10), making it extremely important to provide rapid targeted therapy to the causative microorganism.
The antimicrobial mechanism of action, pharmacokinetics, distribution, elimination, toxicity and side effects in the body are particular characteristics of each antibiotic, which are considered during the election (11, 12). The last few years have been particularly important in terms of understanding how the patient’s age influences the effects that will be observed. The antimicrobial action extends, as a secondary effect, to the microbiota of the neonatal patient, affecting the diversity of colonizing microorganisms, particularly Bifidobacterium, an abundant microorganism during lactation, decrease and increase abundance of Klebsiella and Enterococcus spp.; the modification is detected up to 24 months after exposure to antibiotics (13, 14), a situation that is associated with the subsequent development of obesity.
Most of the current studies do not identify the specific antibiotic associated with microbiome and weight changes; however, the mechanism of action of each antibiotic varies (11, 12), it is important to associate the type and magnitude of a side effect to the antibiotic used. Moreover, genetic background, feeding, and ambient variations in human patients make difficult to compare the effects of different antibiotics in such heterogeneous conditions. The advantage offered by an animal model, is the antibiotic comparison in similar, controlled conditions.
In order to compare side effects of antibiotics frequently used in neonatal intensive care, a newborn rat model in a controlled environment was chosen to evaluate the individual effect of three treatments preferably used in NICUs, upon anthropometric and biochemical adult-equivalent changes.
Material and Methods
Ethical statement: The protocol was approved by Research, Ethical and CICUAL Commitees of Instituto Nacional de Perinatología (INPer), under the registration number of 212250-3100331.
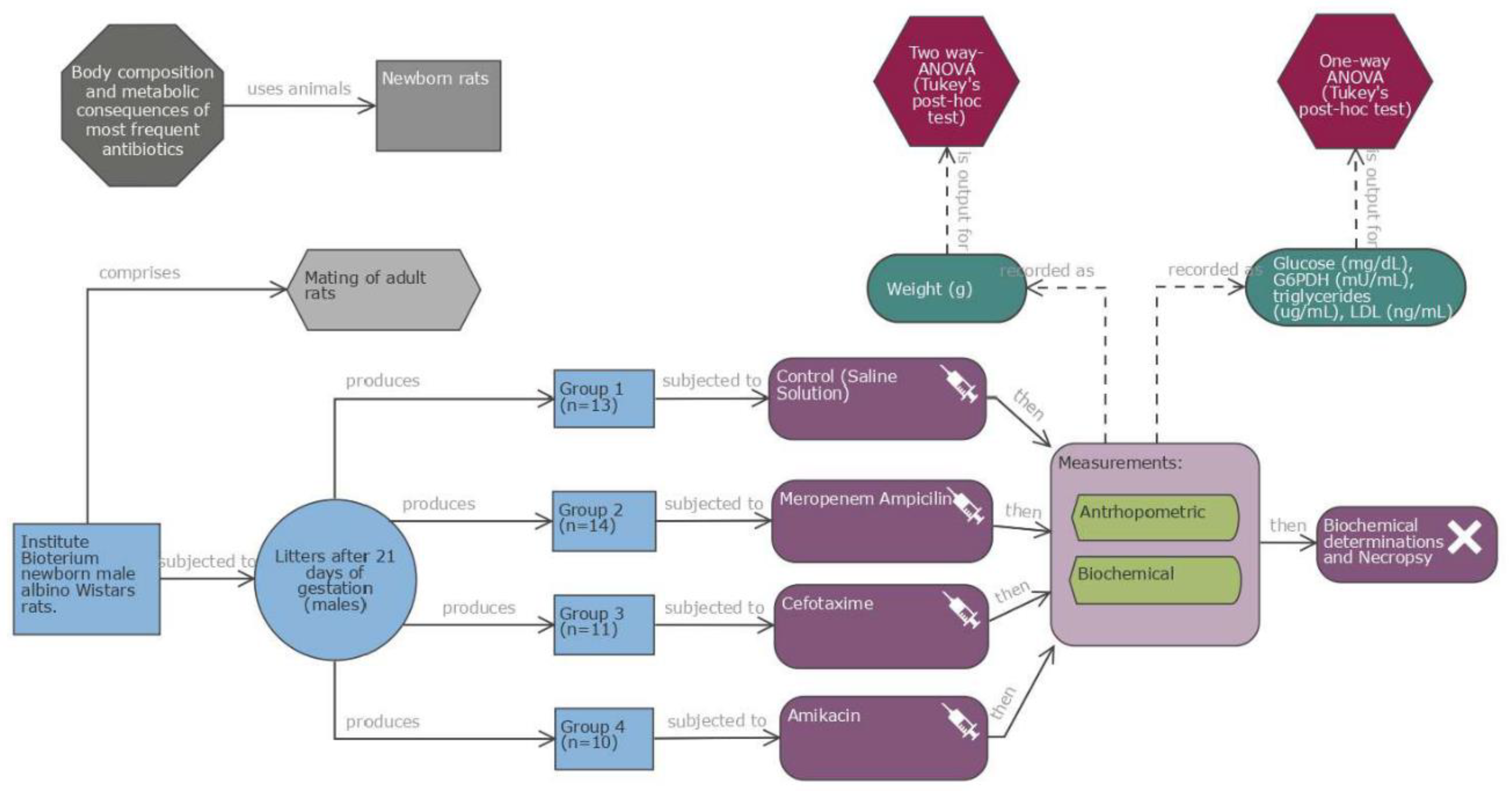
Study design: Experimental study in newborn male albino Wistar rats (one day old, weight 6.4-7.5 g) from the facilities of Instituto Nacional de Perinatología. The rat litters were kept with a rat mother in a cage, fed with mother’s milk, in a temperature-controlled environment (22-24 °C), with 55-60% humidity under a 12h light-dark cycle for 7 days throughout the experimentation. Four groups (14 rats each) were formed. Newborn rats were randomly allocated. Antibiotic doses were administered intraperitoneally (IP) during five days, starting on the first day of life. Doses were prepared individually according to weight (approximate volume of 50 to 150 microliters in each dose). Rats were weaned at 21 days, weighted weekly until the age of 28 days, when they were sacrificed, after 12h of fasting. Blood was collected by cardiac puncture and separated into serum and whole cells. Serum was frozen at -70 °C until use. Rats were sacrificed by CO
2 inhalation and cervical dislocation; each rat was necropsied; the study diagram was showed in the
Figure 1.
Antibiotic dosing. Four antibiotics were tested, two of them in combination Ampicillin/Meropenem (Access/Watch groups) 100 μg/g every 12 h /10 μg/g every 12 h (each of these two antibiotics was adjusted to the required dosages based on weight); Cefotaxime 200 μg/g every 24 h; (Watch group) and Amikacin 15 μg/g every 24 h (Access group). Equivalent doses to those used in human newborns in the NICU were used. Main characteristics of the antibiotics are described in
Table 1. For the control group physiological Saline Solution was administered in similar volume (μL) IP every 24 h. The antibiotics administrated ever 24 h were given in the mornings at 7 am and those with an indication of every 12 h had an additional administration at 7 pm.
Rat measurements.
Rats were weighed each day on an electronic scale (Acculab, VI-200, 11722, NY, USA), calibrated between all measurements. Rats were weaned at 21 days of life, when rat mother was removed, and experimental rats remain in the cage. The rats were allowed ad libitum access to food and water for an additional week, then, sacrificed followed by biochemical determinations as well as necropsy. Rat ELISA (CUSABIO, Wuhan, Hubei Providence, China. CP: 430223) was used according to the brand instructions.
Glucose (GL44 Blood glucose monitor, mg/dL), Rat Glucose-6-phosphate 1-deshydrogenase (G6PD) Cat. No. CSB-EL009121RA reported in mU/mL, Rat Triglyceride (TG) Cat. No. CSB-E11705r, reported in μg/mL, Rat low density lipoprotein cholesterol (LDL-C) Cat. No. CSB-E16561r reported in ng/mL.
Sample Size calculation
Sample size calculation was performed based on the outcome of weight gain at 28 days form birth evaluated by ANOVA model, a comparison between 4 study groups was consider with 95% reliability (α=0.05), a statistical power of 80% (β=0.20) and a grade effect size (Cohen’s f=0.45). The minimum size calculation per study group was 14 experimental units. Sample size calculation was performed in R software version 4.3.2 with the “pwr” package.
Statistical analysis
Data of the present study were analysed using GraphPad Prism v.9.1. The data was presented as mean and standard deviation (SD). Weight gain was calculated as the difference between the weight at 28 days and the weight at baseline. The comparison of weights between study groups, at each week from Birth, was carried out by means of a two-way ANOVA (Antibiotics and age of the rat), and post-hoc tests were carried out with the Tukey’s test for multiple comparison and by Benjamini, Krieger and Yekutieli test (q values) for controlling the false discovery rate.. For comparisons of biochemical parameters and weight gain on day 28, a one-way Brown-Forsythe ANOVA (G&PDH and LDL) or a Kruskal-Wallis test (Glucose and Triglycerides) were use. The election of the model was based throughout residuals analyses. Multiple comparisons between groups were performed by Dunett’s T3 post-hoc test in the ANOVA models and by Dunn’s post-hoc test in the Kruskal-Wallis models. The values of biochemical parameters were plotted in box and whisker plots.
Differences were considered statistically significant where p-value was p<0.05.
Results
Initially, 56 rats were included in the study. Some died during the experimental manipulation, remaining 13 in the control group, 14 in the Meropenem/Ampicillin group, 11 in the Cefotaxime group, and 10 in the Amikacin group. One day old male newborn rats were homogeneous on anthropometric measures (
Table 2). Weight values of each study group at baseline 7, 14, 21 and 28 days are shown in
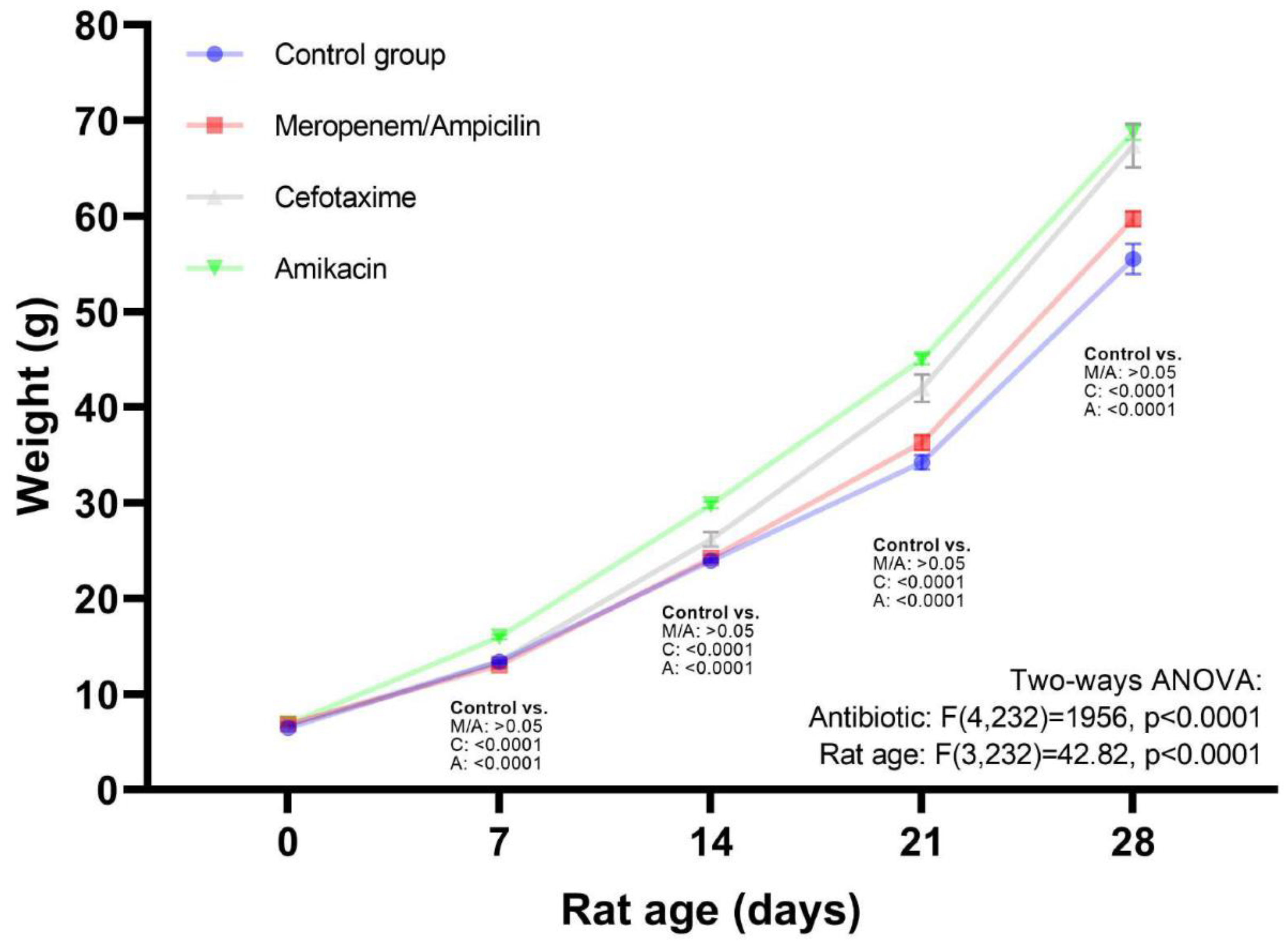
Table 3. Weight gain at 28 days of the follow up were as follows: Control: 47.38 g (SD:6.17), Meropenem/Ampicillin: 52.63 g (SD:2.93), Cefotaxime: 62.66 g (SD:5.53), and Amikacin: 61.88 g (SD:2.42). On day 28, comparing the antibiotics; the Cefotaxime (p<0.0001) and Amikacin (p<0.0001) groups showed a significant difference with respect to the control group, but not with respect to the Meropenem/Ampicillin group (p=0.19), Cefotaxime (p=0.0002) and Amikacin (p<0.0001) showed a statistical difference with respect of Meropenem/Ampicillin, but were not statistically different between them (p=0.99).
Figure 2 shows the overall effect of treatment with the various antibiotics during the follow-up period. Multiple comparison test in ANOVA model showed that weight in all weeks was higher in the Cefotaxime and Amikacin groups compared to the control group and the Meropenem/Ampicillin group did not show significant differences with respect to the control group. In
Table 2, the false discovery rate test (q-values) suggests that all study groups were different from the control group at 21 and 28 days.
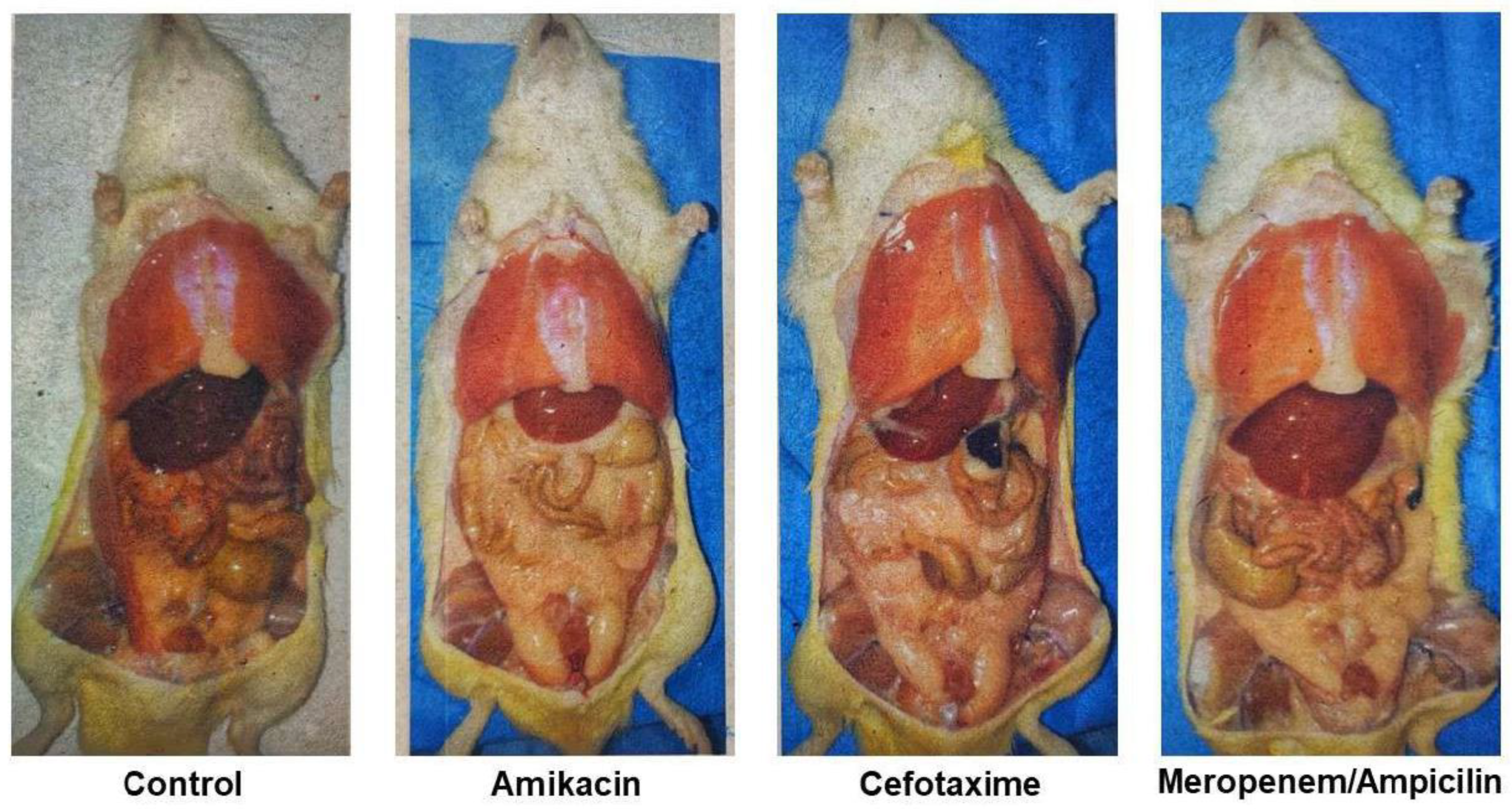
The accumulation of abdominal fat at the age of 28 days in representative animals is presented in
Figure 3, it can be seen that fat accumulation was greater in rats treated with antibiotics.
The distributions and comparisons between the study groups of the biochemical parameters are shown in
Figure 4 and
Figure 5.
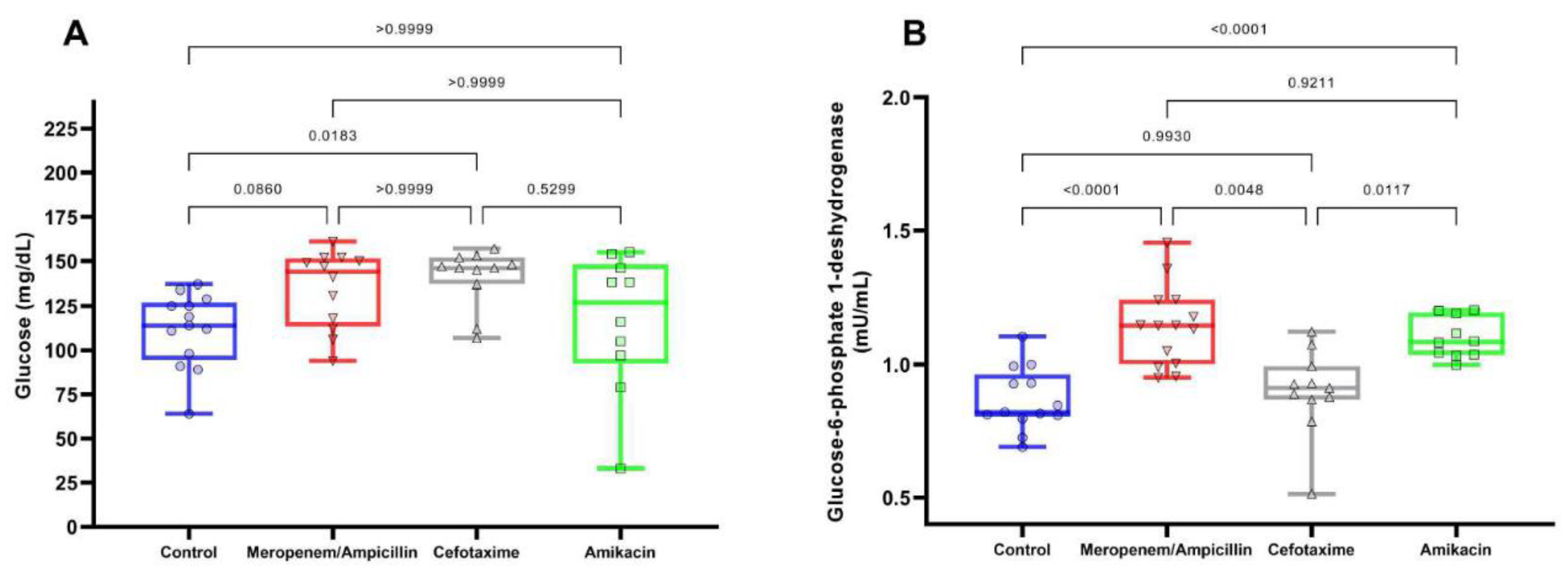
Figure 4 shows the values of glucose (Fig. 4A) and G6PDH (Fig. 4B) on day 28. It was observed that glucose levels are similar between the different study groups (Control: 111.38 [SD: 20.93], Meropenem/Ampicillin: 134.42 [SD: 21.76], Cefotaxime: 140 [SD: 16.38] and Amikacin: 116 [SD: 38.83 ]), only a difference was observed between the Cefotaxime group with respect to the control (p=0.02), while the levels of G6PDH, an important enzyme for catalysis of NADPH production, which protects cells against oxidative stress, presented greater differences were between the study groups (Control: 0.87 [SD: 0.12], Meropenem/Ampicillin: 1.14 [SD: 0.15], Cefotaxime: 0.899 [SD: 0.16], Amikacin: 1.1 [SD: 0.08]), being Meropenem/Ampicillin group and Amikacin group, greater than the control group (Both: p<0.0001), and the Cefotaxime group (p=0.005 and p=0.01, respectively).
In reference to lipids (
Figure 5), triglycerides (Fig. 5A) showed the tendency to be higher in the Cefotaxime group (88.5 [SD: 8.8]), and Meropenem/Ampicillin (84 [SD: 7.2]), than in the Amikacin group (80.5 [SD: 0.8]) and control group (79.8 [SD: 1.07]), however, only Cefotaxime group has statistically significant differences in comparison with control group (p=0.03). On the other hand, the levels of LDL values (Fig. 5B) increased with the use of Cefotaxime (113 [SD: 77.9], p=0.04) and Amikacin (138.5 [SD: 96.8] p=0.03), in comparison to the control group (30 [SD: 41.2]) which were not significantly different than Meropenem/Ampicillin (81.5 [SD:60.4]).
Discussion
In this experimental study we aimed to compare side effects of antibiotics frequently used in neonatal intensive care throughout and animal model. We observed an increased weight gain by fat accumulation, and a deficient management regulation of carbohydrates and lipids in antibiotic-treated rats at birth. The effect was not homogeneous for the antibiotics tested, perhaps related to differences in their individual mechanisms of action, elimination, dosage, and toxicity patterns. The model allows this precision in the observations, otherwise difficult to achieve in a heterogeneous population, such as the human one. Weight differences were observed since the first week of life. According to the physical growth assessment curves of male Wistar rats with reference values of body weight (g) as a function of chronological age (days), the weight obtained at day 28 did not exceed the percentile 97, falling outside the obesity parameter for chronological age (15), however, it is within the overweigh range.
Weight gain, both in humans and in animal models, has been associated with changes in the microbiota. It is interesting to note that the group with the lowest weight gain was Meropenem/Ampicillin.
A study, which determines the microbiota after antibiotic treatment in young adult mice, reports taxonomic changes in the microbiome, changes in copies of key genes involved in carbohydrates metabolism and alterations in the regulation of hepatic lipid and cholesterol metabolism (16). The gut microbiota modulates blood lipids, including cholesterol levels (17), which could explain why antibiotics use increases triglycerides in Cefotaxime and Meropenem/Ampicillin treated groups, an effect that was not observed in Amikacin-treated rats, perhaps due to differences in the microorganism’s sensitivity to the antibiotic. Despite statistically significant differences in triglycerides, the range of values was comparable to that reported in a prior study (18), although it should be noted that the lipid levels in that study were not measured in newborn rats.
LDL cholesterol level increased in the Amikacin and Cefotaxime treated groups with very large differences with respect to the control group. The values observed in the control group in our study were comparable to those reported in a prior study (18), thereby reflecting that large differences in the antibiotic-treated groups are likely of clinical relevance. Long-term trajectories of lipids after antibiotic treatment should be studied to further characterize their clinical relevance.
The concentrations used for each antibiotic tested differ because the therapeutic doses are different. The lowest concentration used in this study was for Amikacin, and it was the antibiotic without effect on triglycerides and glucose levels, perhaps, revealing an effect exerted on the organs involved in carbohydrates and lipids metabolism. In this regard, it must be taken into account that, at the time of treatment, most of the organs are under development. Particularly, in the rat model, with multiple gestation and immature breeds at birth, short gestation, rapid maturity, and short adult life, known as altricial species, organs are under development at birth. For example, pancreatic β-cell neogenesis continues throughout neonatal life, on postnatal days 1-2 the total islet mass doubles, on days 4-10 the rate of islet neogenesis is higher than the rate of islet growth. At weaning, the pancreas is fully developed (19). Compared to humans, changes in pancreas maturity occur in the last weeks of pregnancy (20), with the pancreas expanding during the neonatal period (21). Prematurity disrupts normal pancreatic development by precipitating events, giving place to incomplete expansion of pancreatic β-cells and lower number of islets, making it difficult for the pancreas to respond to the demand of glucose regulation later in adulthood. Under this comparison, the experimental period of this work corresponds with human prematurity, meaning that the use of antibiotic in this period may increase the risk of developing diabetes, although, it depends on the type of antibiotic used. Despite the statistically significant differences among groups, none of the observed mean values were in the range of diabetes (>200-300 mg/dl) (22, 23).
According to the equivalence parameters between human and rat lifespan (24), the antibiotic administration time was longer than usual, and the follow-up time was relatively short, corresponding to two years of human life. Most studies, which associate antibiotic treatment with metabolic changes, do not distinguish between the gestational ages of the babies during treatment. When full-term babies were studied for the effect on weight gain after antibiotic treatment on neonatal period, researchers found no weight gain, not even after transplanting the microbiota of treated babies, which also lose weight (25).
It is important to mention that the results of the tests for false discovery rate showed differences with respect to the multiple comparisons of weights throughout the follow-up of the study, indicating that the weights between study groups at baseline and at 7 days are similar between study groups, and from 21 days onwards all groups were different from each other. Cefotaxime and Amikacin groups had the highest weight values. The use of q values is more useful in this type of statistical analysis in which multiple comparisons were made to try to avoid committing type 1 error.
Limitations of this work were the lack of quantitative fat measurements when the animals were necropsied. Furthermore, it is important to mention that when performing the cardiac puncture to take the blood, there is a high risk of a failure in the process, with less opportunity to take the sample, or just less volume sample, which explains the differences on samples numbers for biochemical analysis between the groups. Another important limitation observed in our study was the loss of mice throughout the follow-up, causing the minimum calculated sample size not to be attained. Nonetheless, statistical evaluations of each statistical model were performed to verify that the model assumptions were adequate for comparisons.
The knowledge of metabolic alteration under antibiotic treatment in newborns is an important step. Future observations and experiments could be directed to mitigate them, for instance 1) restoring a normal microbiota by providing it as soon as possible after antibiotic treatment, 2) providing protective substances for organ development, such as those of lipid origin (Vitamin E) used to protect neonatal rats from Amikacin (26), and 3) preferring the use of antibiotics with lower therapeutic doses to minimize damages, trying to protect postnatal developing organs.
In the other hand, it is important to take into consideration the impact of antibiotics in food-producing farm animals, which impose a positive pressure for the emergence of antibiotic-resistant bacteria (AMR) (27). Furthermore, AMR genes can be transmitted to humans through the consumption of meat-harboring-resistant bacteria, as in the case of ESKAPE bacteria (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species), being pigs one of the biggest reservoirs of AMR (28).
Experimental studies in animal models as well as in humans have shown that different types of antibiotics might influence the development of obesity among children and that in effect they have the ability to alter the human gut microbiota (obesity-related dysbiosis) (29-32). Therefore, the limitation of the use of certain broad spectrum antibiotics in children might reduce the burden of the obesity epidemic and gut microbiota-related diseases.
Many questions that remain unanswered from this study, such as the reversibility of the observed effects, the timing of damage, the dose dependence of damage, and the impact of antibiotics on postnatal organ development compared to the effect on the microbiome. Most of all, it is crucial to understand that obesity is not only the result of imbalance between the calorie intake and expenditure, but also that it could be triggered by external events coming from something as basic as preconception care in terms of the having enough knowledge on how to follow a healthy life style.
In conclusion, the weight gain observed with antibiotic treatments in the first days after birth was confirmed in the rat model, with values in the overweight range, but not of obesity. Weight gain was lower with Meropenem/Ampicillin than with Amikacin and Cefotaxime. Increased glucose levels not meeting the criteria for diabetes were found in the Cefotaxime group with respect to control. Large increases in LDL were observed in the Cefotaxime and Amikacin groups, compared to control, which were larger than those in previous studies. Overall, these changes could be mediated by disruptions of organogenesis or the early-life microbiome, which are known to have long-term impacts on weight and metabolic parameters. Longer follow-up studies are needed to address these changes.
Acknowledgments
This work was supported by Instituto Nacional de Perinatologia under the registration number of 212250-3100331.
References
- World Health Organization. Obesity and Overweight. World Health Organization (2021). https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed 24 October 2023).
- Turta O, Rautava S. Antibiotics, obesity and the link to microbes - what are we doing to our children? BMC Med. (2016) 14:57, 1-6. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed 24 October 2023). [CrossRef]
- Cox LM, & Blaser MJ. Antibiotics in early life and obesity. Nat Rev End (2015) 11:3, 182–190. [CrossRef]
- World Health Organization. Newborn health. World Health Organization (2023). https://www.who.int/teams/maternal-newborn-child-adolescent- health-and-ageing/newborn-health/newborn-infections (accessed 24 October 2023).
- Rahman AE, Hossain AT, Zaman S Bin, Salim N, K.C A, Day LT, et al. Antibiotic use for inpatient newborn care with suspected infection: EN-BIRTH multi-country validation study. BMC Pregnancy Childbirth. (2021) 1;21.
- Ting JY, & Shah PS. Antibiotic stewardship in neonates: Challenges and opportunities.Translational Pediatrics (2020) 9:198–201. [CrossRef]
- Segura-Cervantes E, Mancilla-Ramírez J, González-Canudas J, Alba E, Santillán-Ballesteros R, Morales-Barquet D, Sandoval-Plata G, et al. Inflammatory Response in Preterm and Very Preterm Newborns with Sepsis. Mediators of Inflammation 2016. [CrossRef]
- Prusakov P, Goff DA, Wozniak PS, Cassim A, Scipion CEA, Urzúa S, Ronchi A, et al. A global point prevalence survey of antimicrobial use in neonatal intensive care units: The no-more-antibiotics and resistance (NO-MAS-R) study. EClinicalMedicine (2021) 32. [CrossRef]
- Cortese F, Scicchitano P, Gesualdo M, Filaninno A, de Giorgi E, Schettini F, et al. Early and Late Infections in Newborns: Where Do We Stand? A Review. Pediatrics and Neonatology (2016). 57:265–273. [CrossRef]
- Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Critical Care Med (2006) 34:1589–1596. [CrossRef]
- Butranova OI, Ushkalova EA, Zyryanov SK, & Chenkurov MS. Developmental Pharmacokinetics of Antibiotics Used in Neonatal ICU: Focus on Preterm Infants. Biomedicines. (2023) 11:940. [CrossRef]
- Rivera-Chaparro ND, Cohen-Wolkowiez M and Greenberg RG. ‘Dosing antibiotics in neonates: Review of the pharmacokinetic data’. Future Microbiology. (2017) 12:1001–1016. [CrossRef]
- Kwon Y, Cho YS, Lee YM, Kim SJ, Bae J, & Jeong SJ. Changes to Gut Microbiota Following Systemic Antibiotic Administration in Infants. Antibiotics. 2022; 11, 470. [CrossRef]
- Reyman M, van Houten MA, Watson RL, Chu MLJN, Arp K, de Waal WJ, et al. Effects of early-life antibiotics on the developing infant gut microbiome and resistome: a randomized trial. Nature Communications. (2022) 13:1. [CrossRef]
- Cossio-Bolaños M, Campos RG, Vitoria RV, Hochmuller Fogaça RT, de Arruda M. Reference curves for assessing the physical growth of male Wistar rats. Nutr Hosp. (2013) 28:2151–2156.
- Cho I, Yamanishi S, Cox L, Methé BA, Zavadil J, Li K, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. (2012) 488:621–626. [CrossRef]
- Le Roy T, Lécuyer E, Chassaing B, Rhimi M, Lhomme M, Boudebbouze S, et al. The intestinal microbiota regulates host cholesterol homeostasis. BMC Biol. (2019) 17:1.
- Aberare OL, Okuonghae P, Mukoro N, et al. Triglycerides, total cholesterol, high density lipoprotein cholesterol and low density lipoprotein cholesterol in rats exposed to premium motor spirit fumes. N Am J Med Sci. 2011;3(6):277-280. [CrossRef]
- O’Dowd JF, & Stocker CJ. Endocrine pancreatic development: Impact of obesity and diet. Frontiers in Physiology. (2013) 4:170. :. [CrossRef]
- Bloomfield, FH. Impact of prematurity for pancreatic islet and beta-cell development. Journal of Endocrinology. 2018; 238, R161–R171. [Google Scholar] [CrossRef]
- Jacovetti C & Regazzi, R. Mechanisms Underlying the Expansion and Functional Maturation of β-Cells in Newborns: Impact of the Nutritional Environment. Int J Mol Sci. (2022) 23:2096. [CrossRef]
- Rehman HU, Ullah K, Rasool A, Manzoor R, Yuan Y, Tareen AM, et al. Comparative impact of streptozotocin on altering normal glucose homeostasis in diabetic rats compared to normoglycemic rats. Sci Rep. 2023 May 16;13(1):7921. [CrossRef]
- Hikmah Nuzulu, Shita AD, Maulana H. View of diabetic blood glucose level profile with stratified dose streptozotocin (SD-STZ) and multi low dose streptozotocin (MLD-STZ) induction methods. (s/f). The Journal of Tropical Life, 5(2015). doi:11594/jtls.5.1.%x.
- Quinn, R. Comparing rat’s to human’s age: How old is my rat in people years? Nutrition. (2005) 21:775–777). [CrossRef]
- Uzan-Yulzari A, Turta O, Belogolovski A, Ziv O, Kunz C, Perschbacher S, et al. ‘Neonatal antibiotic exposure impairs child growth during the first six years of life by perturbing intestinal microbial colonization’, Nature Communications. (2021) 12:443. doi:1038/s41467-020-20495-4.
- Kara A, Cetin H, Oktem F, Ciris IM, Altuntas I, & Kaya S. Amikacin induced renal damage and the role of the antioxidants on neonatal rats. Renal Failure. (2016) 38:671–677. [CrossRef]
- Conceição S, Queiroga MC, Laranjo M. Antimicrobial Resistance in Bacteria from Meat and Meat Products: A One Health Perspective. Microorganisms. 2023 Oct 17;11(10):2581. [CrossRef]
- Tang KL, Caffrey NP, Nóbrega DB, Cork SC, Ronksley PE, Barkema HW, Polachek AJ et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis. Lancet Planet Health. 2017 Nov;1(8):e316-e327. [CrossRef]
- Gerber JS, Bryan M, Ross RK, Daymont C, Parks EP, Localio AR, et. al. Antibiotic Exposure During the First 6 Months of Life and Weight Gain During Childhood. JAMA 2016, 315, 1258–1265. [CrossRef]
- Ternak, G. , Edel, Z., Feiszt, Z., Szekeres, J., Visegrady, B. and Wittmann, I. Selective Association between Cephalosporin, Quinolone, Macrolide, and Penicillin Antibiotic Consumption and Childhood Obesity in Europe. 2015, Health, 7, 1306-1314. [CrossRef]
- Ternák G, Németh M, Rozanovic M, Márovics G, Bogár L. “Growth-Promoting Effect" of Antibiotic Use Could Explain the Global Obesity Pandemic: A European Survey. Antibiotics (Basel). 2022 Sep 28;11(10):1321. [CrossRef]
- Becker J, Schüpbach-Regula G, Steiner A, Perreten V, Wüthrich D, Hausherr A, et al. Effects of the novel concept 'outdoor veal calf' on antimicrobial use, mortality and weight gain in Switzerland. (2020). Preventive veterinary medicine, 176, 104907. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).