You are currently viewing a beta version of our website. If you spot anything unusual, kindly let us know.
Preprint
Article
Integrating a Fundus Camera with High Frequency Ultrasound for Precise Ocular Lesion Assessment
Altmetrics
Downloads
124
Views
65
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Abstract
Ultrasound A-scan is an important tool for quantitative assessment of ocular lesions. However, its usability is limited by difficulty to accurately localize the ultrasound probe to a lesion of interest. In this study, a transparent LiNbO3 single crystal ultrasound transducer was fabricated, and integrated with a widefield fundus camera to guide the ultrasound local position. The novel fundus camera guided ultrasound probe was validated for in vivo measurement of rat eyes. Anterior and posterior segments of the rat eye could be unambiguously differentiated with the fundus photography guided ultrasound measurement. A model eye was also used to verify the clinical potential of the prototype device.
Keywords:
Subject: Engineering - Bioengineering
1. Introduction
Choroidal nevi are benign ocular tumors that are estimated to be present in 5% of adults over 40 years of age in the United States [1]. A small fraction of these lesions transforms into malignant lesions called uveal melanoma, which affect 6 non-Hispanic white individuals per million and at a lower rate in other races. However, due to being situated in the delicate structures of the eye, intraocular tumor biopsy and subsequent pathological examination are performed for diagnostic purposes only in exceptional cases where the ocular oncologist is unable to make a conclusive diagnosis after clinical examination and systemic evaluation [2]. After diagnosis of uveal melanoma, patient typically undergo enucleation or globe sparing radiation therapy that can severely affect the vision [3]. Early diagnosis of these malignant lesions can avoid enucleation and significantly preserve the patient’s eyesight [4,5]. Furthermore, even with successful local disease control, 50% of the patients diagnosed uveal melanoma develop metastatic disease, with a high rate of subsequent mortality [3]. Therefore, early detection of malignancy is of paramount importance to preserve eyesight and improve survival in patients with uveal melanoma.
The evaluation of a suspected ocular tumor is performed with dilated fundus examination and multi-modal imaging to accurately identify benign and malignant lesions including choroidal nevi, uveal melanoma, choroidal metastasis, circumscribed choroidal hemangioma, choroidal osteoma, and retinoblastoma. With the advent of widefield and ultra-widefield fundus cameras [6,7,8], identifying suspected lesions even in the periphery of the retina is possible. Fundus images provide critical information, such as the size, location, color, presence of orange pigment, and presence of overlying drusen. [2,9]. However, traditional fundus photography lacks depth-resolved information and typically shows the superficial layer information which is not enough to determine other important characteristics of an ocular tumor. Spectral-domain optical coherence tomography with enhanced depth-resolved imaging (EDI SD-OCT) capability has shown promise to accurately illustrate the intrinsic optical characteristics of ocular lesions [10]. Despite being able to distinguish lesions from normal tissue, the low penetration capability of OCT limits its usage to only very thin lesions, typically less than 1 mm in thickness, especially in melanocytic lesions [11]. Imaging techniques such as fluorescein angiography (FA), indocyanine green angiography (ICGA), and fundus autofluorescence (FAF) can provide valuable information such as changes in the retinal pigmented epithelium (RPE) overlying ocular lesions, tumor vasculature, and associated vascular leakage.[1,2,12,13] However, these imaging modalities do not provide sufficient information related to the tumor depth, and their standalone diagnostic accuracy is relatively low.
Ultrasound imaging of the eye has been a longstanding imaging technique due to its excellent tissue penetration capability, non-invasive nature, and ability to differentiate tissues with different echogenicity [14,15]. Experimental research with different ultrasound transducer materials and frequencies is also gaining popularity for non-invasive imaging of the eye [16,17,18]. Usage of ultrasound is important in many subspecialities of ophthalmology including cataract surgery planning, glaucoma, vitreoretinal surgery, uveitis, oculoplastic procedures, and emergency medicine in addition to ocular oncology where it serves a critical purpose of diagnosing and monitoring ocular tumors, such as uveal melanoma [2,19,20]. For imaging the posterior retina, both a one-dimensional amplitude scan (A-scan) and a two-dimensional brightness scan (B-scan) are used [14]. The B-scan can be used to observe anterior and posterior segments of the ocular lesion as well as the axial and lateral dimensions of the eye. Although one-dimensional vector A-scan could be derived from a B-scan image, its usability for quantitative measurement of lesion thickness is limited, as the logarithmic amplification used to generate the B-scan image prevents the quantitative analysis needed for accurate lesion echogenicity analysis [21]. The logarithmic amplification increases the dynamic range, however, reduces the sensitivity, which further prevents the differentiation between tissues where the variation of echogenicity is minimal. Moreover, the focused beam used in the B-scan probe further complicates the process of computational echogenicity analysis of ocular lesions [21]. A specifically designed A-scan probe called standardized A-scan with non-focused parallel beam and S-shaped amplification was optimized for eye bio-microscopy [22,23] and has been an important part of many ophthalmology clinics ever since. Standardized A-scan has become the gold standard for calculating the apical height of ocular lesions, specifically because of its enhanced penetration capability and ability to differentiate the lesion border from the scleral tissue [14,15,21]. Several quantifiable parameters other than the apical height have been extracted out of A-scan ultrasound, such as median internal reflectivity (%) of a lesion, heterogenous/homogenous echogenicity, number of internal reflectivity peaks inside a lesion, sound attenuation pattern (angle ĸ) to facilitate an accurate clinical diagnosis [14,19,24]. The internal reflectivity and sound attenuation patterns are well established for differentiating choroidal melanoma from other lesions such as choroidal nevus, retinoblastoma, circumscribed choroidal hemangioma, choroidal metastasis [19,24,25]. Given that treated choroidal melanomas show a considerable rise in median internal reflectivity, the A-scan ultrasound may also be useful in assessing the effectiveness of treatment [14].
Despite the promise, ultrasound A-scan comes with a set of challenges. Precise guidance of the A-scan line towards the lesion requires an experienced operator, especially when the lesion is relatively small. Inaccurate guidance could lead to erroneous measurement of critical parameters such as apical height and internal reflectivity. Furthermore, the growth of benign lesions such as choroidal nevi could indicate its transformation towards malignant choroidal melanoma [26], and clinicians often observe the nevus size over a long period of time. However, monitoring the apical height from the exact same spot over a prolonged period is nearly impossible because of the absence of A-scan guidance. Therefore, accurate guidance an of A-scan ultrasound probe is highly desirable for quantitative analysis, management, and documentation of ocular lesions.
In this article, we demonstrated the feasibility of using a fundus camera to accurately guide an ultrasound A-scan probe in the posterior region of the eye. A widefield fundus camera integrated with a transparent LiNbO3 single-crystal ultrasound transducer facilitated the guidance of ultrasound localization. In vivo experiments with rat eyes demonstrated the ability of the prototype to simultaneously gather fundus images and A-scans of the eye. The resultant A-scan clearly differentiated the anterior cornea, the eye lens, and the posterior retina. The optical performance of the imager was also examined with a model human eye and compared with the performance of the same imager but with a glass window replacing the transparent transducer. The resultant images show that the imager with a transparent transducer can clearly visualize the retina and can be readily implemented in clinical settings.
2. Materials and Methods
2.1. Design of the transparent ultrasound probe
Based on the transparent application for the transducer, the transparent lithium niobate (LNO) single crystal was selected as the core component for the ultrasound transducer fabrication. A Krimboltz, Leedom, and Mattaei (KLM) transducer equivalent circuit model-based modeling software (PiezoCAD, Sonic Concepts, Woodinville, WA, USA) was applied to simulate and optimize the electrical impedance and pulse-echo performance of the designed transducer (Figure 3). To ensure the transparency for light penetration, the colorless parylene film (Parylene C, Specialty Coating Systems, Indianapolis, IN, USA) with acoustic impedance of 2.5MRayl was chosen as the matching layer for acoustic impedance compensation, and the transparent epoxy (EPO-TEK 301, Epoxy Technology, Inc., Billerica, MA, USA) with acoustic impedance of 3.05 MRayl was determined as backing layer for absorbing reflected ultrasound and penetrating optical source. The designed parameters of the transducer are summarized and listed in Table 1.
2.2. Fabrication of the transparent ultrasound probe
A 40 MHz transparent LNO disk wafer (Boston Piezo-Optics, Inc., Bellingham, MA, USA) with a size of 5 mm x 5 mm was purchased and manufactured for this study. Both sides of the LNO were sputtered indium tin oxide (ITO), a transparent electrode, by the sputtering system (NSC-3000 Sputter Coater, Nano-Master, Inc., Austin, TX, USA). Afterward, the LNO with electrodes was shielded by the brass housing and connected with one side of the double-shield coaxial cable, and the other side of the cable was connected with the Sub-Miniature version A (SMA) connector for ultrasound driving system connection. Degassed epoxy (EPO-TEK 301) was poured into the brass housing to form the backing layer. After 3 hours of curing for the epoxy, the Au/Cr electrode (100 nm / 50 nm) was sputtered to conduct the ITO edge of the LNO and brass housing for ground connection. Finally, the parylene film was coated on the surface of the LNO as a matching layer and water-proof layer, then, the fabrication of the transparent transducer was finished and was further characterized and tested. Figure 1a illustrates the schematic diagram of the transparent ultrasound probe. The transducer has 5 mm X 5 mm of clear penetration window. Figure 1b shows the finished cylinder-type transparent ultrasound transducer. The letters printed on the background could easily be read, further indicating the transparency of the transducer.
2.3. Testing the performance of the transparent ultrasound probe
The electrical impedance and phase spectrum of the fabricated transparent transducer were measured by an impedance analyzer (HP 4294A, Agilent Technologies, Santa Clara, CA, USA). The pulse-echo performance of the transparent transducer was measured by the set-up detailed in previous work [27]. The transducer was immersed into the water tank, and the quartz plate was applied as a reflector for reflecting generated ultrasound. The transducer excited by pulser-receiver (Panametrics 5900PR, Olympus NDT Inc., Waltham, MA, USA) with 1 µJ energy per pulse transmitted and received the ultrasound. Theoretically, the -6 dB axial resolution (Raxial) can be determined as Raxial = λ/(2BW) [27], whereas λ is the wavelength of the ultrasound in water, and BW is the -6 dB bandwidth of the transducer. Therefore, the -6 dB axial resolution can be estimated as 40 µm. The high-resolution contact lens-based transducer will better fit the fundus camera for ocular imaging.
2.4. Experimental setup
Figure 2a illustrates the schematic diagram of the experimental setup for in vivo retinal imaging of rat eyes. The light source is a visible light LED (M530L4, Thorlabs Inc., Newton, NJ, USA) with a center wavelength of 530 nm and a full-width half maximum (FWHM) of 35 nm. For albino rats, the contrast of the retinal vasculature is poor when visualized with red and NIR light, and the UV portion of the spectrum is heavily attenuated by the ocular lens. Therefore, the choice of wavelength in the green portion of the spectrum was made as it shows the retinal vessels with excellent contrast [28]. An optical fiber (MHP550L02, Thorlabs Inc., Newton, NJ, USA) coupled the LED light to the eyelid of the rat and the end of the fiber was fixed to the eyelid with tape. The optical power at the tip of the fiber was measured to be 5 mW. The imaging system consisted of the transparent ultrasound probe as the frontal element, followed by an ophthalmic lens (L1) (f= 11 mm) (Volk Digital Series Wide Field lens, Volk, Mentor, OH, USA), a relay lens (L2) (f= -50 mm, LC1715, Thorlabs Inc., Newton, NJ, USA), and a camera lens (L3) (12 fixed focal length lens, 33-303, Edmund Optics Inc., Barrington, NJ). The working distance of L1 was chosen with the thickness of the transparent ultrasound probe in mind, so that, when the transducer touches the cornea properly, a retinal image is created by L1 at the retina conjugate plane (RCP) and this image is relayed to the camera sensor (GS3-U3-41S4M-C, FLIR Integrated Imaging Solutions Inc., Richmond, Canada) by L2 and L3. Figure 2b shows a photographic illustration of the imaging system.
Furthermore, we investigated how the transparency of the ultrasound probe might affect the image quality during human eye imaging. In order to do that, we imaged an eye model (OEMI-7, Ocular Instruments, Bellevue, Washington, USA) first with the proposed imaging system. Then the ultrasound transducer was replaced with a glass window (WG10530, Thorlabs Inc., Newton, NJ, USA). The glass window is similar in thickness to the ultrasound transducer, but optically neutral. Ultrasound signal was generated and received by a pulser receiver (Panametrics 5900PR, Olympus NDT Inc., Waltham, MA, USA) and a custom-made MATLAB script was created to visualize and perform adequate filtering on the signal. A custom-made LABVIEW interface was created to control image acquisition.
2.5. Animal preparation
The Association of Research in Vision and Ophthalmology's guidelines for the ethical use of animals in ophthalmic and visual science research were followed in all aspects of the experimental protocols and related animal care. The related experimental protocol was approved by the University of Illinois Chicago's (UIC) Animal Care Committee. The study employed a sample of eleven-month-old male Sprague Dawley rats (N = 2). The rats were acquired from Charles River Laboratories (Wilmington, MA) and housed within UIC’s Biology Resource Lab. After the rats were anesthetized, the pupil was dilated using a drop of 1% tropicamide ophthalmic solution. A lubricant ocular gel (GenTeal, Alcon Laboratories, Fort Worth, TX, USA) was applied to the whole anterior eye to keep the eye surface hydrated throughout the experiment. The eye gel also served as a coupling medium between the transparent ultrasound probe and the eye to ensure the propagation of the ultrasound waves. An animal holder was used to keep the rat steady and keep the head of the rat immobilized. The animal holder facilitated five degrees of freedom position controls.
3. Results
Figure 1a-b illustrates the electrical impedance and phase spectrum of the fabricated transparent transducer. The resonant frequency (fr) and anti-resonant frequency (fa) were located as 32, and 41, respectively, which is close to the simulation results. Hence, the effective electromechanical coupling coefficient (keff) of the transducer, describing the conversion efficiency between electrical energy and mechanical energy, is calculated as 0.62 by the following equation (1) [27].
The electric impedance magnitude of the fabricated transducer is in the range of 30 to 40 Ω around the resonance frequency of the transducer, approximately closing to match the ideal electrical impedance of 50 Ω. The pulse-echo performance of the transducer is illustrated in Figure (c-d). The result shows the central frequency of the transducer as 37 MHz, and the -6 db fractional bandwidth as 30%, which are in good agreement with the simulation results (40 MHz).
Figure 3.
(a) simulated and (b) measured electrical impedance and phase spectrum of the transducer. (c) simulated and (d) measured pulse-echo performance of the transducer.
Figure 3.
(a) simulated and (b) measured electrical impedance and phase spectrum of the transducer. (c) simulated and (d) measured pulse-echo performance of the transducer.
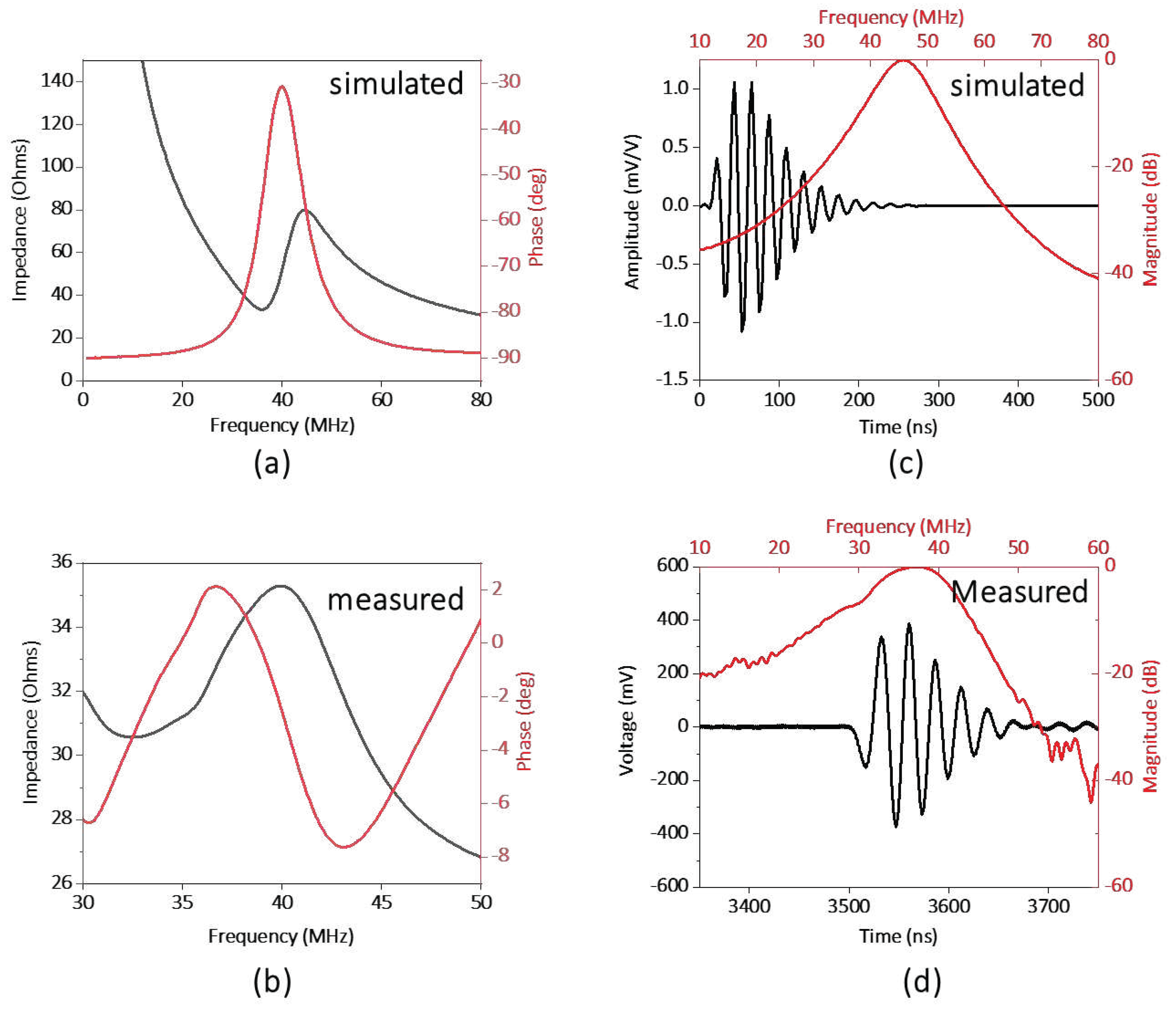
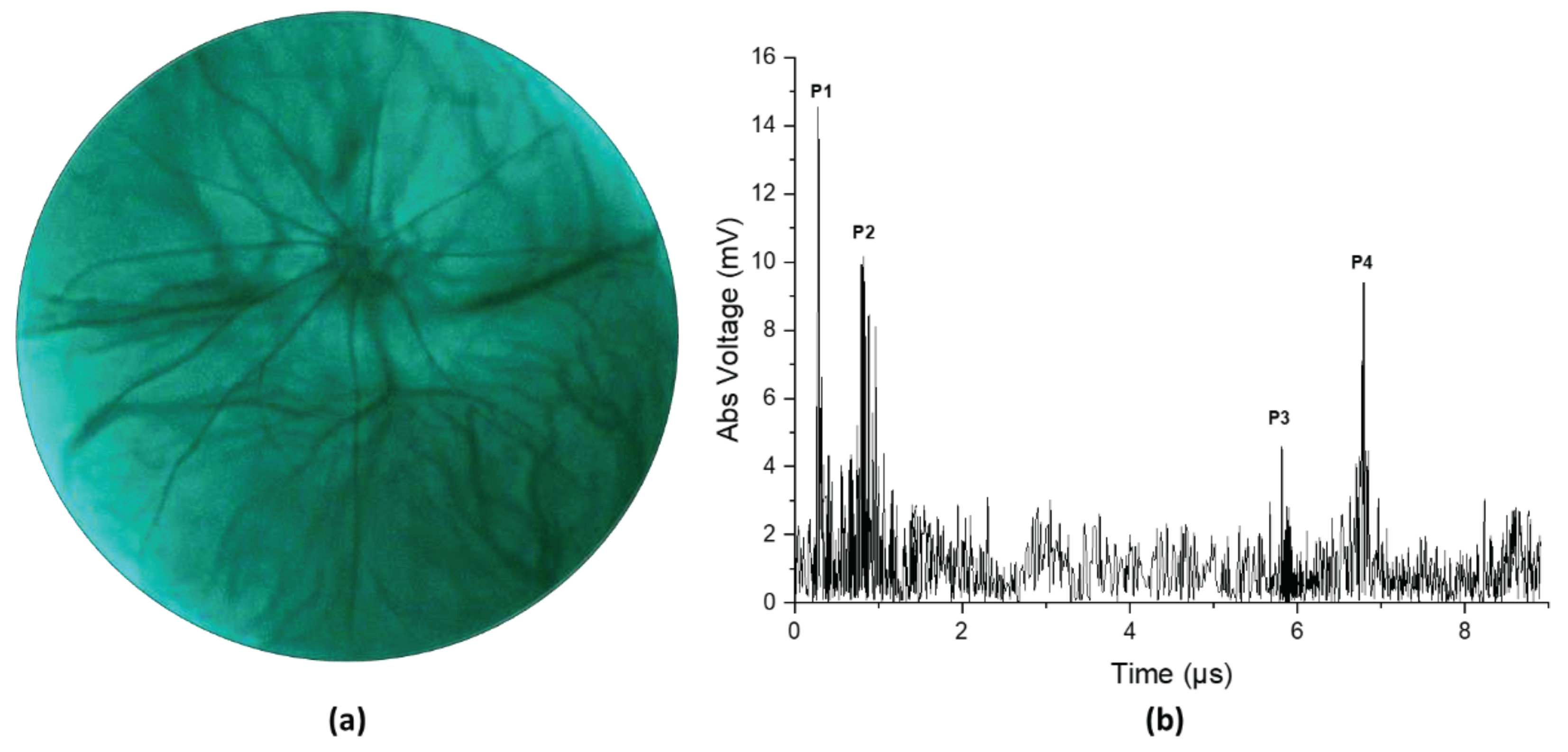
Figure 4 illustrates the result from an in vivo experiment in a rat eye. Figure 4a shows the fundus image and Figure 4b shows the corresponding ultrasound pulse-echo image. The acquired pulse echo signal constitutes both positive and negative voltage. However, to ensure similarity with the clinical ocular ultrasound pulse-echo signal, we illustrated the absolute voltage instead of the real voltage. It is evident from Figure 4a that the retinal and choroidal vessels as well as the optic nerve of the rat retina are clearly imaged in the fundus photograph. The ultrasound data shows four distinct peaks corresponding to four anatomical locations inside the eye, namely the cornea (P1), the anterior capsule of the crystalline lens (P2), the posterior capsule of the crystalline lens (P3), and the retina (P4). The axial length of the eye could be approximated from the time interval between the corneal peak and the retinal peak, given that we know the speed of sound in the eye. The speed of sound in aqueous humor and vitreous humor is around 1532 m/s, whereas the speed of sound inside the ocular lens is around 1641 m/s [29]. For conservative estimation, we assume that the speed of sound inside the whole eye is 1532 m/s. We find the axial length of the eye using the following equation,
Where is the time interval between the corneal peak and the retinal peak, is the speed of ultrasound. The axial length was calculated to be 4.902 mm, which is close to the axial length reported by other studies on a similar strain of rats measured by OCT [30]. The slight mismatch in axial length from the reported value could be attributed to the fact that the lens encompasses more than 60% of the volume of the eye in rats [31]. So, our simple approximation of a constant velocity would not be sufficient for calculating the axial length correctly. However, for the human eye, the lens is small relative to the whole eye, so even a simple approximation could give us a nearly accurate result. Furthermore, the velocity of sound in different human eye tissues and their interfaces are well characterized [32].
Figure 4.
(a) Fundus image taken from in vivo imaging on a rat eye. (b) Ultrasound pulse-echo data gathered from the same rat eye.
Figure 4.
(a) Fundus image taken from in vivo imaging on a rat eye. (b) Ultrasound pulse-echo data gathered from the same rat eye.
The effect of the transparent transducer on the imaging performance of the system is illustrated in Figure 5. a illustrates an image of the retina of an eye model taken with the proposed prototype. Figure 5b illustrates an image of the retina of the same eye model taken after replacing the transparent transducer with an optically neutral glass window. The glass window was chosen in such a way that it didn’t change the magnification of imaging and only the difference in imaging performance would be highlighted. The structures present in the eye model, namely the blood vessels, the optic nerve head, and the pigmented ocular lesions on the superior and inferior retina could be easily identified in both images. The mean RGB intensity of the images taken with the transparent transducer and the glass window are 121 and 135, showing the illumination efficiency of the device is not severely affected by the transparent transducer. The ocular vessel branches present in the model eye are clearly seen in both images. However, the smaller vessels that constitute the vessel branches could be differentiated in the image taken with the glass window, whereas the image taken with the transparent transducer doesn’t resolve the smaller vessels in the vessel branches clearly. That being said, the quality of the image taken with the transparent transducer should be sufficient to accurately guide the ultrasound signal to ocular lesions, which is the goal of the prototype.
4. Discussion
In summary, we demonstrated the feasibility of using a fundus camera to guide the local position of an ultrasound transducer to detect pulse-echo signals from the posterior segment of the eye. A high frequency transparent ultrasound transducer was used to facilitate simultaneous fundus and ultrasound imaging. In vivo testing was performed on rats with our prototype and simultaneous fundus image and ultrasound A-scan were gathered. Furthermore, the optical performance and image quality of the prototype on the human eye were examined with a model eye. In addition to providing objective guidance for ultrasound imaging, the fundus image would also provide valuable information such as the presence of orange pigment or drusen overlying an ocular tumor, which are important features in the diagnosis of melanocytic choroidal tumors [1,26,33].
For this feasibility study, we utilized a high-frequency ultrasound probe with a center frequency of 40 MHz in order to differentiate the cornea, the ocular lens, and the retina clearly in the rat eye. The rat retina is much thinner than the human retina [34], and needs high-frequency ultrasound signal to differentiate different ocular structures properly. The -6 dB axial resolution of the system was calculated to be 40 μm, which is reasonable for imagining the eye of the rat. On the other hand, the attenuation of ultrasound waves significantly increases with the operating frequency, reducing the penetration depth. The smaller axial length of a rat [29] eye ( around 7 mm) helps to acquire signals from the retina with reasonable quality. For the human eye, the attenuation at this frequency makes the acquisition of signal from the retina impossible. Therefore, we were not able to conduct ultrasound measurements from the human eye with this preliminary light-ultrasound prototype. However, the primary purpose of this study was to demonstrate the feasibility of using fundus imaging as the guidance for ocular ultrasound and we demonstrated that clearly with an in vivo experiment on rat eyes. Ultrasound probes used in ophthalmology clinics generally have a center frequency between 7 MHz-20 MHz [15] to compensate for the attenuation to enhance the penetration depth. We are currently pursuing a transparent ultrasound transducer with a frequency similar to the clinical ophthalmic ultrasound systems for future studies of human eyes.
The ultrasound transducer used in this study is a 5 mm x 5 mm square-shaped transducer. The limited lateral resolution from the transducer means that the echo signal received by the transducer comes from a larger area inside the eye, which in turn makes the signal noise prone. Notably, the signal coming from the posterior of the crystalline lens (peak P3 in Figure 4b) has a relatively weaker signal strength. This could be attributed to the large beam width and the acute curvature of the posterior section of the rat lens as a portion of the beam reaches the border while the rest is still traveling inside the lens, creating an averaging effect.
An eye model was used to verify the effect that the transparent ultrasound probe had on the image quality (Figure 5). For the actual human eye, factors such as the pupil size, and eye movement could affect the result. Therefore, the model eye was a better choice for accurate comparison. To deliver the light inside the model eye, a small hole was drilled 5 mm away from the cornea of the model eye and a small optical fiber was inserted which delivered the illumination inside the eye.
For in vivo testing in rat eyes, the retina was illuminated through the eyelid, which is contrary to the conventional method of both delivering the illumination and receiving the retinal reflection through the pupil. This method has recently been proposed to acquire widefield retinal images even with limited pupil size in human eyes [35]. However, the added complexity of having a separate illumination path would increase the complexity of fundus image-guided ultrasound acquisition in patients. Miniaturized indirect illumination-based fundus imagers have shown promise in acquiring non-mydriatic widefield fundus images without the complexity of conventional pupillary ring illumination or a separate illumination system. A simple solution to the pupil limitation without requiring a separate illumination system is provided by miniature indirect illumination, which offers a different approach towards the development of widefield portable fundus cameras [36,37]. In our future prototype, we plan to include this illumination method to construct a compact, portable, widefield fundus image-guided ultrasound probe to accurately extract ultrasound information from ocular lesions in human patients.
5. Conclusions
A fundus image-guided ultrasound probe was demonstrated for accurate measurement of ultrasound pulse-echo signal from the posterior segment of the eye. In vivo imaging of rat eyes demonstrated the feasibility of capturing retinal images and ultrasound measurements simultaneously. The optical performance and the image quality of the prototype were further validated using a model eye to demonstrate potential usability in humans.
6. Patents
Pending patent application for multimodal fundus-ultrasound eye imager.
Author Contributions
Conceptualization, Michael Heiferman, Qifa Zhou and Xincheng Yao; Data curation, Alfa Rossi and Yushun Zeng; Formal analysis, Chen Gong, Mojtaba Rahimi and Xin Sun; Investigation, Alfa Rossi, Yushun Zeng, Mojtaba Rahimi, Chen Gong, Mohammad Soleimani, Ali Djalilian and Xin Sun; Methodology, Alfa Rossi, Yushun Zeng and Mojtaba Rahimi; Resources, Mohammad Soleimani, Ali Djalilian and Mark Humayun; Software, Alfa Rossi, Taeyoon Son and Yushun Zeng; Supervision, Qifa Zhou and Xincheng Yao; Validation, Taeyoon Son, Michael Heiferman and Mark Humayun; Visualization, Alfa Rossi, Taeyoon Son and Yushun Zeng; Writing – original draft, Alfa Rossi and Yushun Zeng; Writing – review & editing, Qifa Zhou, Mohammad Soleimani, Ali Djalilian, Mark Humayun and Xincheng Yao.
Funding
This work was supported by the National Institutes of Health (NIH) under grant R01EY028662, R01EY030126, and R01EY035084.
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care Committee of University of Illinois Chicago (protocol code: 23-068 and date of approval: 7/11/2023).
Data Availability Statement
The data presented in this study is available within the article.
Acknowledgments
The authors thank Tara Nguyen and Jiwang Chen for helping with animal preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chien, J.L.; Sioufi, K.; Surakiatchanukul, T.; Shields, J.A.; Shields, C.L. Choroidal nevus: a review of prevalence, features, genetics, risks, and outcomes. Current opinion in ophthalmology 2017, 28, 228–237. [Google Scholar] [CrossRef]
- Solnik, M.; Paduszyńska, N.; Czarnecka, A.M.; Synoradzki, K.J.; Yousef, Y.A.; Chorągiewicz, T.; Rejdak, R.; Toro, M.D.; Zweifel, S.; Dyndor, K.; et al. Imaging of Uveal Melanoma-Current Standard and Methods in Development. Cancers 2022, 14. [Google Scholar] [CrossRef]
- Krantz, B.A.; Dave, N.; Komatsubara, K.M.; Marr, B.P.; Carvajal, R.D. Uveal melanoma: epidemiology, etiology, and treatment of primary disease. Clinical ophthalmology (Auckland, N.Z.) 2017, 11, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Gálvez, C.C.; Ordaz-Favila, J.C.; Villar-Calvo, V.M.; Cancino-Marentes, M.E.; Bosch-Canto, V. Retinoblastoma: Review and new insights. Frontiers in oncology 2022, 12, 963780. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Furuta, M.; Berman, E.L.; Zahler, J.D.; Hoberman, D.M.; Dinh, D.H.; Mashayekhi, A.; Shields, J.A. Choroidal nevus transformation into melanoma: analysis of 2514 consecutive cases. Archives of ophthalmology (Chicago, Ill.: 1960). 2009, 127, 981–987. [Google Scholar] [PubMed]
- Yao, X.; Son, T.; Ma, J. Developing portable widefield fundus camera for teleophthalmology: Technical challenges and potential solutions. Experimental biology and medicine (Maywood, N.J.) 2022, 247, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Nagiel, A.; Lalane, R.A.; Sadda, S.R.; Schwartz, S.D. ULTRA-WIDEFIELD FUNDUS IMAGING: A Review of Clinical Applications and Future Trends. Retina (Philadelphia, Pa.) 2016, 36, 660–678. [Google Scholar] [CrossRef]
- Quinn, N.; Csincsik, L.; Flynn, E.; Curcio, C.A.; Kiss, S.; Sadda, S.R.; Hogg, R.; Peto, T.; Lengyel, I. The clinical relevance of visualising the peripheral retina. Progress in Retinal and Eye Research 2019, 68, 83–109. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, A. Choroidal melanoma. Oman journal of ophthalmology 2012, 5, 3–9. [Google Scholar] [CrossRef]
- Torres, V.L.; Brugnoni, N.; Kaiser, P.K.; Singh, A.D. Optical coherence tomography enhanced depth imaging of choroidal tumors. American journal of ophthalmology 2011, 151, 586–593. [Google Scholar] [CrossRef]
- Say, E.A.; Shah, S.U.; Ferenczy, S.; Shields, C.L. Optical coherence tomography of retinal and choroidal tumors. Journal of ophthalmology 2011, 2011, 385058. [Google Scholar] [CrossRef]
- Gündüz, K. Gündüz, K.; Yeşiltaş; YS. Diagnostic Techniques: Angiography. in Singh, A.D., and Damato, B.E. (Eds.): ‘Clinical Ophthalmic Oncology: Basic Principles’ (Springer International Publishing, 2019), pp. 209–234.
- Midena, E.; Frizziero, L.; Pilotto, E.; Parrozzani, R. Diagnostic Techniques: Autofluorescence. in Singh, A.D., and Damato, B.E. (Eds.): ‘Clinical Ophthalmic Oncology: Basic Principles’ (Springer International Publishing, 2019), pp. 257-270.
- Lorek, B.H.; Aronow, M.E.; Singh, A.D. Diagnostic Techniques: Ultrasonography. in Singh, A.D., and Damato, B.E. (Eds.): ‘Clinical Ophthalmic Oncology: Basic Principles’ (Springer International Publishing, 2019), pp. 271–293.
- Kadakia, A.; Zhang, J.; Yao, X.; Zhou, Q.; Heiferman, M.J. Ultrasound in ocular oncology: Technical advances, clinical applications, and limitations. Experimental biology and medicine (Maywood, N.J.) 2023, 248, 371–379. [Google Scholar] [CrossRef]
- Safari, A.; Zhou, Q.; Zeng, Y.; Leber, J.D. Advances in development of Pb-free piezoelectric materials for transducer applications. Japanese Journal of Applied Physics 2023, 62, SJ0801. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, L.; Chen, R.; Li, R.; Kang, H.; Zeng, Y.; Yan, Y.; Priya, S.; Zhou, Q. Design and Fabrication of 15-MHz Ultrasonic Transducers Based on a Textured Pb(Mg1/3Nb2/3)O3-Pb(Zr, Ti)O3 Ceramic. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control 2022, 69, 3095–3101. [Google Scholar] [CrossRef]
- Li, R.; Zeng, Y.; Sun, X.-x.; Li, C.; Li, R.; Zheng, T.; Jiang, L.; Wu, J. Multidimensional synergy-induced high piezoelectricity and reliability KNN piezoceramics for high-frequency ultrasonic transducers. Science China Materials 2023, 66, 686–695. [Google Scholar] [CrossRef]
- Fonkeu, Y.; Singh, N.; Hayden-Loreck, B.; Singh, A.D. Diagnostic A-Scan of Choroidal Melanoma: Automated Quantification of Parameters. Ocular oncology and pathology 2019, 5, 350–357. [Google Scholar] [CrossRef]
- Silverman, R.H. Focused ultrasound in ophthalmology. Clinical ophthalmology (Auckland, N.Z.) 2016, 10, 1865–1875. [Google Scholar] [CrossRef] [PubMed]
- Chopdar, A.; Aung, T. Multimodal retinal imaging. (JP Medical Ltd, 2014) 2014.
- Ossoinig, K.C. Standardized echography: basic principles, clinical applications, and results. International ophthalmology clinics 1979, 19, 127–210. [Google Scholar] [CrossRef] [PubMed]
- Ossoinig, K.C. Quantitative echography--the basis of tissue differentiation. Journal of clinical ultrasound: JCU 1974, 2, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Fonkeu, Y.; Lorek, B.H.; Singh, A.D. Diagnostic A-Scan of Choroidal Tumors: Comparison of Quantified Parameters. Ocular oncology and pathology 2019, 5, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Campagnoli, T.R.; Medina, C.A.; Singh, A.D. CHOROIDAL MELANOMA INITIALLY TREATED AS HEMANGIOMA: DIAGNOSTIC AND THERAPEUTIC CONSIDERATIONS. Retinal cases & brief reports 2016, 10, 175–182. [Google Scholar]
- ‘Factors Predictive of Growth and Treatment of Small Choroidal Melanoma: COMS Report No. 5. Archives of Ophthalmology 1997, 115, 1537–1544. [Google Scholar]
- Chen, R.; Jiang, L.; Zhang, T.; Matsuoka, T.; Yamazaki, M.; Qian, X.; Lu, G.; Safari, A.; Zhu, J.; Shung, K.K.; et al. Eco-Friendly Highly Sensitive Transducers Based on a New KNN–NTK–FM Lead-Free Piezoelectric Ceramic for High-Frequency Biomedical Ultrasonic Imaging Applications. IEEE Transactions on Biomedical Engineering 2019, 66, 1580–1587. [Google Scholar] [CrossRef]
- Alterini, T.; Díaz-Doutón, F.; Burgos-Fernández, F.J.; González, L.; Mateo, C.; Vilaseca, M. Fast visible and extended near-infrared multispectral fundus camera. Journal of biomedical optics 2019, 24, 1–7. [Google Scholar]
- Lozano, D.C.; Twa, M.D. Development of a Rat Schematic Eye From In Vivo Biometry and the Correction of Lateral Magnification in SD-OCT Imaging. Investigative Ophthalmology & Visual Science 2013, 54, 6446–6455. [Google Scholar]
- Wu, Y.; Luo, X.; Feng, Y.; Yang, J.; Fan, H.; Cen, X.; Li, W. Comparison of the accuracy of axial length measurement by different imaging methods in Sprague Dawley rats. Frontiers in Neuroscience, 2023, 16. 2023. [Google Scholar]
- Remtulla, S.; Hallett, P.E. A schematic eye for the mouse, and comparisons with the rat. Vision research 1985, 25, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, K.J. Ultrasound velocities for axial eye length measurement. Journal of cataract and refractive surgery 1994, 20, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Shields, J.A.; Kiratli, H.; De Potter, P.; Cater, J.R. Risk factors for growth and metastasis of small choroidal melanocytic lesions. Ophthalmology 1995, 102, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, C.L.; Kim, A.Y.; Kashani, A.H. Normative Retinal Thicknesses in Common Animal Models of Eye Disease Using Spectral Domain Optical Coherence Tomography. in Editor (Ed.)^(Eds.): ‘Book Normative Retinal Thicknesses in Common Animal Models of Eye Disease Using Spectral Domain Optical Coherence Tomography’ (Springer International Publishing, 2018, edn.), pp. 157–166.
- Toslak, D.; Thapa, D.; Chen, Y.; Erol, M.K.; Paul Chan, R.V.; Yao, X. Trans-palpebral illumination: an approach for wide-angle fundus photography without the need for pupil dilation. Opt. Lett. 2016, 41, 2688–2691. [Google Scholar] [CrossRef]
- Rossi, A.; Rahimi, M.; Le, D.; Son, T.; Heiferman, M.J.; Chan, R.V.P.; Yao, X. Portable widefield fundus camera with high dynamic range imaging capability. Biomedical optics express 2023, 14, 906–917. [Google Scholar] [CrossRef]
- Toslak, D.; Liu, C.; Alam, M.N.; Yao, X. Near-infrared light-guided miniaturized indirect ophthalmoscopy for nonmydriatic wide-field fundus photography. Opt. Lett. 2018, 43, 2551–2554. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
(a) The schematic diagram of the transparent transducer. (b) The fabricated transparent transducer with connections. G: ground; W: wire.
Figure 1.
(a) The schematic diagram of the transparent transducer. (b) The fabricated transparent transducer with connections. G: ground; W: wire.
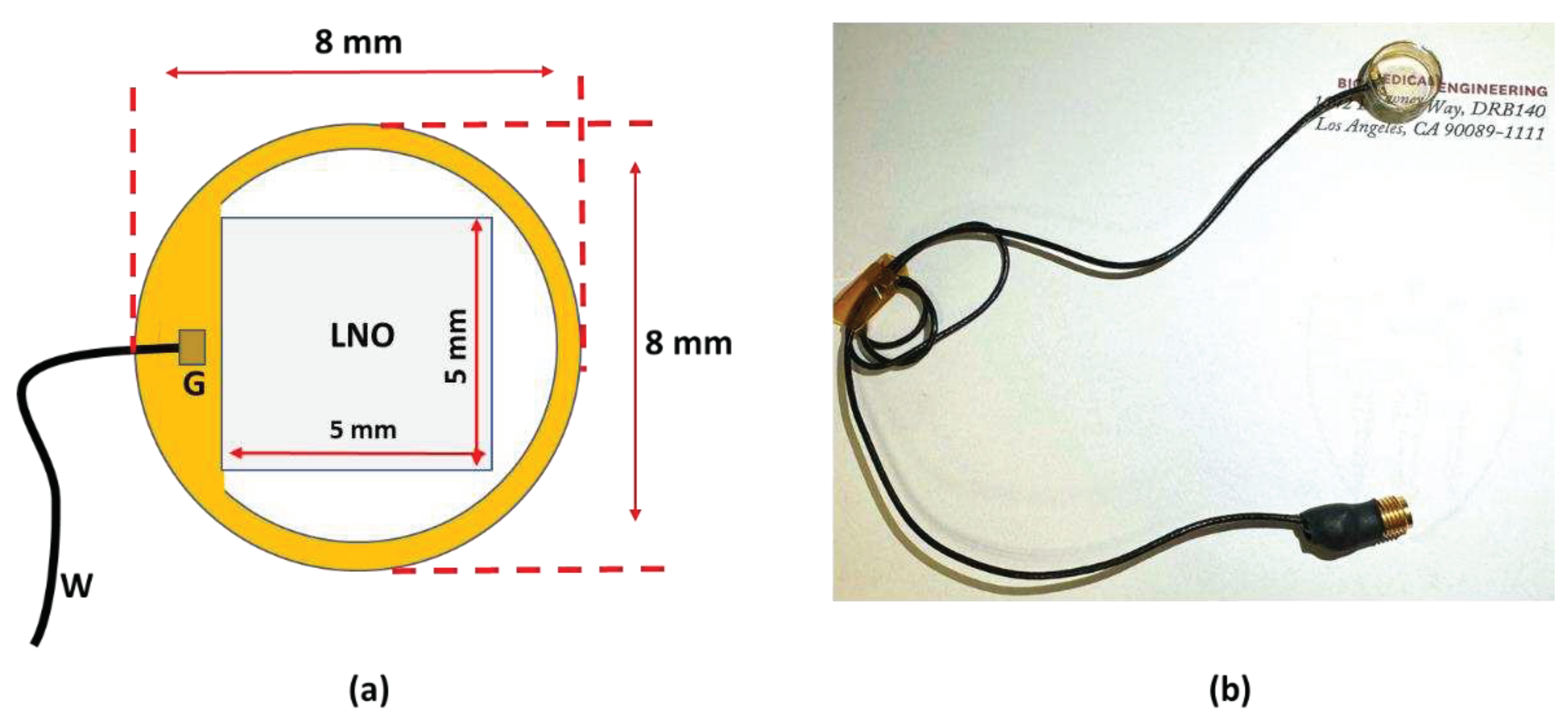
Figure 2.
(a) The schematic illustration of the experimental setup. (b) Photographic illustration of the imaging system. RCP: Retina conjugate plane.
Figure 2.
(a) The schematic illustration of the experimental setup. (b) Photographic illustration of the imaging system. RCP: Retina conjugate plane.
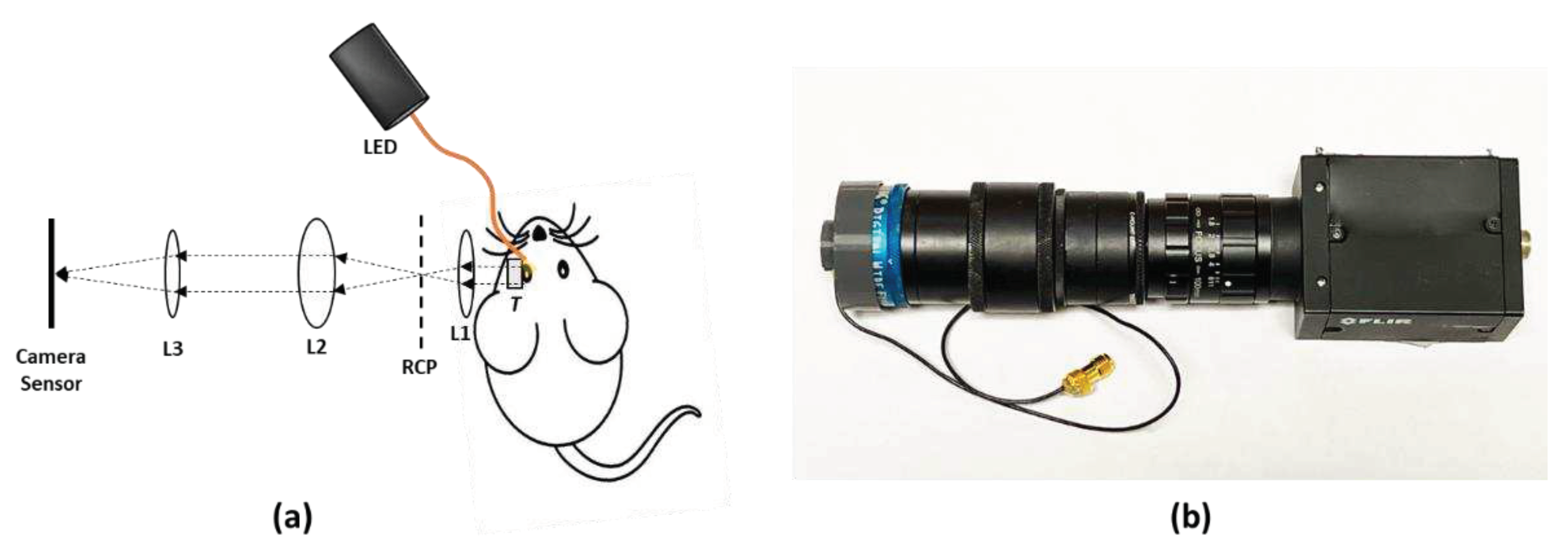
Figure 5.
(a) Image of the retina of an eye model taken with the prototype. (b) Image of the same model eye taken after replacing the ultrasound transducer in the prototype with a glass window.
Figure 5.
(a) Image of the retina of an eye model taken with the prototype. (b) Image of the same model eye taken after replacing the ultrasound transducer in the prototype with a glass window.
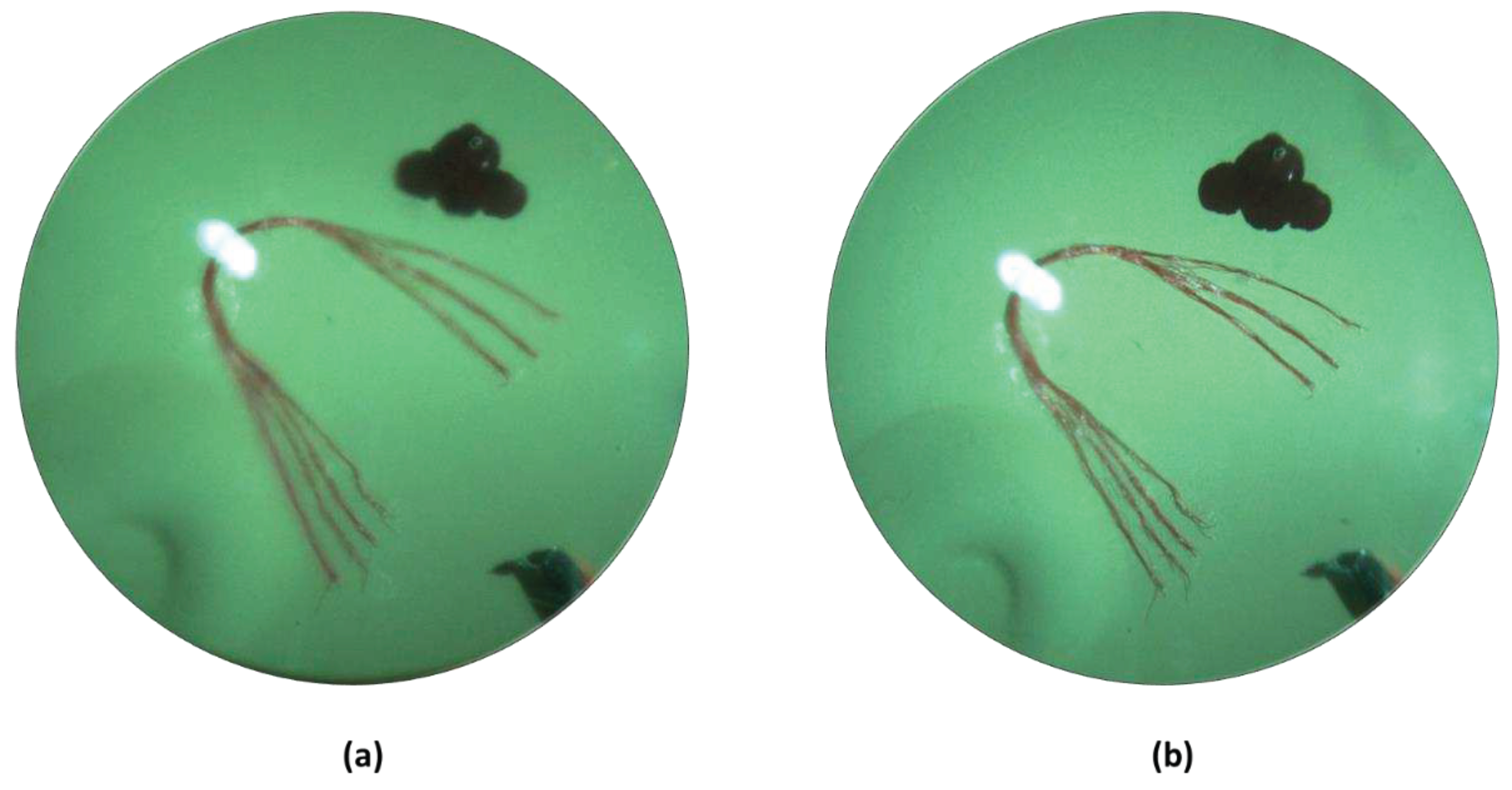
Table 1.
Design parameters of the transparent ultrasound transducer.
| Parameter | Value |
|---|---|
| Center frequency | 40 MHz |
| Surface area | 5 mm x 5 mm |
| Lithium Niobate (LNO) thickness | 70 µm |
| Matching layer (Parylene) thickness | 10 µm |
| Backing layer (Epo-Tek 301) thickness | 5 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Submitted:
15 January 2024
Posted:
16 January 2024
You are already at the latest version
Alerts
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
15 January 2024
Posted:
16 January 2024
You are already at the latest version
Alerts
Abstract
Ultrasound A-scan is an important tool for quantitative assessment of ocular lesions. However, its usability is limited by difficulty to accurately localize the ultrasound probe to a lesion of interest. In this study, a transparent LiNbO3 single crystal ultrasound transducer was fabricated, and integrated with a widefield fundus camera to guide the ultrasound local position. The novel fundus camera guided ultrasound probe was validated for in vivo measurement of rat eyes. Anterior and posterior segments of the rat eye could be unambiguously differentiated with the fundus photography guided ultrasound measurement. A model eye was also used to verify the clinical potential of the prototype device.
Keywords:
Subject: Engineering - Bioengineering
1. Introduction
Choroidal nevi are benign ocular tumors that are estimated to be present in 5% of adults over 40 years of age in the United States [1]. A small fraction of these lesions transforms into malignant lesions called uveal melanoma, which affect 6 non-Hispanic white individuals per million and at a lower rate in other races. However, due to being situated in the delicate structures of the eye, intraocular tumor biopsy and subsequent pathological examination are performed for diagnostic purposes only in exceptional cases where the ocular oncologist is unable to make a conclusive diagnosis after clinical examination and systemic evaluation [2]. After diagnosis of uveal melanoma, patient typically undergo enucleation or globe sparing radiation therapy that can severely affect the vision [3]. Early diagnosis of these malignant lesions can avoid enucleation and significantly preserve the patient’s eyesight [4,5]. Furthermore, even with successful local disease control, 50% of the patients diagnosed uveal melanoma develop metastatic disease, with a high rate of subsequent mortality [3]. Therefore, early detection of malignancy is of paramount importance to preserve eyesight and improve survival in patients with uveal melanoma.
The evaluation of a suspected ocular tumor is performed with dilated fundus examination and multi-modal imaging to accurately identify benign and malignant lesions including choroidal nevi, uveal melanoma, choroidal metastasis, circumscribed choroidal hemangioma, choroidal osteoma, and retinoblastoma. With the advent of widefield and ultra-widefield fundus cameras [6,7,8], identifying suspected lesions even in the periphery of the retina is possible. Fundus images provide critical information, such as the size, location, color, presence of orange pigment, and presence of overlying drusen. [2,9]. However, traditional fundus photography lacks depth-resolved information and typically shows the superficial layer information which is not enough to determine other important characteristics of an ocular tumor. Spectral-domain optical coherence tomography with enhanced depth-resolved imaging (EDI SD-OCT) capability has shown promise to accurately illustrate the intrinsic optical characteristics of ocular lesions [10]. Despite being able to distinguish lesions from normal tissue, the low penetration capability of OCT limits its usage to only very thin lesions, typically less than 1 mm in thickness, especially in melanocytic lesions [11]. Imaging techniques such as fluorescein angiography (FA), indocyanine green angiography (ICGA), and fundus autofluorescence (FAF) can provide valuable information such as changes in the retinal pigmented epithelium (RPE) overlying ocular lesions, tumor vasculature, and associated vascular leakage.[1,2,12,13] However, these imaging modalities do not provide sufficient information related to the tumor depth, and their standalone diagnostic accuracy is relatively low.
Ultrasound imaging of the eye has been a longstanding imaging technique due to its excellent tissue penetration capability, non-invasive nature, and ability to differentiate tissues with different echogenicity [14,15]. Experimental research with different ultrasound transducer materials and frequencies is also gaining popularity for non-invasive imaging of the eye [16,17,18]. Usage of ultrasound is important in many subspecialities of ophthalmology including cataract surgery planning, glaucoma, vitreoretinal surgery, uveitis, oculoplastic procedures, and emergency medicine in addition to ocular oncology where it serves a critical purpose of diagnosing and monitoring ocular tumors, such as uveal melanoma [2,19,20]. For imaging the posterior retina, both a one-dimensional amplitude scan (A-scan) and a two-dimensional brightness scan (B-scan) are used [14]. The B-scan can be used to observe anterior and posterior segments of the ocular lesion as well as the axial and lateral dimensions of the eye. Although one-dimensional vector A-scan could be derived from a B-scan image, its usability for quantitative measurement of lesion thickness is limited, as the logarithmic amplification used to generate the B-scan image prevents the quantitative analysis needed for accurate lesion echogenicity analysis [21]. The logarithmic amplification increases the dynamic range, however, reduces the sensitivity, which further prevents the differentiation between tissues where the variation of echogenicity is minimal. Moreover, the focused beam used in the B-scan probe further complicates the process of computational echogenicity analysis of ocular lesions [21]. A specifically designed A-scan probe called standardized A-scan with non-focused parallel beam and S-shaped amplification was optimized for eye bio-microscopy [22,23] and has been an important part of many ophthalmology clinics ever since. Standardized A-scan has become the gold standard for calculating the apical height of ocular lesions, specifically because of its enhanced penetration capability and ability to differentiate the lesion border from the scleral tissue [14,15,21]. Several quantifiable parameters other than the apical height have been extracted out of A-scan ultrasound, such as median internal reflectivity (%) of a lesion, heterogenous/homogenous echogenicity, number of internal reflectivity peaks inside a lesion, sound attenuation pattern (angle ĸ) to facilitate an accurate clinical diagnosis [14,19,24]. The internal reflectivity and sound attenuation patterns are well established for differentiating choroidal melanoma from other lesions such as choroidal nevus, retinoblastoma, circumscribed choroidal hemangioma, choroidal metastasis [19,24,25]. Given that treated choroidal melanomas show a considerable rise in median internal reflectivity, the A-scan ultrasound may also be useful in assessing the effectiveness of treatment [14].
Despite the promise, ultrasound A-scan comes with a set of challenges. Precise guidance of the A-scan line towards the lesion requires an experienced operator, especially when the lesion is relatively small. Inaccurate guidance could lead to erroneous measurement of critical parameters such as apical height and internal reflectivity. Furthermore, the growth of benign lesions such as choroidal nevi could indicate its transformation towards malignant choroidal melanoma [26], and clinicians often observe the nevus size over a long period of time. However, monitoring the apical height from the exact same spot over a prolonged period is nearly impossible because of the absence of A-scan guidance. Therefore, accurate guidance an of A-scan ultrasound probe is highly desirable for quantitative analysis, management, and documentation of ocular lesions.
In this article, we demonstrated the feasibility of using a fundus camera to accurately guide an ultrasound A-scan probe in the posterior region of the eye. A widefield fundus camera integrated with a transparent LiNbO3 single-crystal ultrasound transducer facilitated the guidance of ultrasound localization. In vivo experiments with rat eyes demonstrated the ability of the prototype to simultaneously gather fundus images and A-scans of the eye. The resultant A-scan clearly differentiated the anterior cornea, the eye lens, and the posterior retina. The optical performance of the imager was also examined with a model human eye and compared with the performance of the same imager but with a glass window replacing the transparent transducer. The resultant images show that the imager with a transparent transducer can clearly visualize the retina and can be readily implemented in clinical settings.
2. Materials and Methods
2.1. Design of the transparent ultrasound probe
Based on the transparent application for the transducer, the transparent lithium niobate (LNO) single crystal was selected as the core component for the ultrasound transducer fabrication. A Krimboltz, Leedom, and Mattaei (KLM) transducer equivalent circuit model-based modeling software (PiezoCAD, Sonic Concepts, Woodinville, WA, USA) was applied to simulate and optimize the electrical impedance and pulse-echo performance of the designed transducer (Figure 3). To ensure the transparency for light penetration, the colorless parylene film (Parylene C, Specialty Coating Systems, Indianapolis, IN, USA) with acoustic impedance of 2.5MRayl was chosen as the matching layer for acoustic impedance compensation, and the transparent epoxy (EPO-TEK 301, Epoxy Technology, Inc., Billerica, MA, USA) with acoustic impedance of 3.05 MRayl was determined as backing layer for absorbing reflected ultrasound and penetrating optical source. The designed parameters of the transducer are summarized and listed in Table 1.
2.2. Fabrication of the transparent ultrasound probe
A 40 MHz transparent LNO disk wafer (Boston Piezo-Optics, Inc., Bellingham, MA, USA) with a size of 5 mm x 5 mm was purchased and manufactured for this study. Both sides of the LNO were sputtered indium tin oxide (ITO), a transparent electrode, by the sputtering system (NSC-3000 Sputter Coater, Nano-Master, Inc., Austin, TX, USA). Afterward, the LNO with electrodes was shielded by the brass housing and connected with one side of the double-shield coaxial cable, and the other side of the cable was connected with the Sub-Miniature version A (SMA) connector for ultrasound driving system connection. Degassed epoxy (EPO-TEK 301) was poured into the brass housing to form the backing layer. After 3 hours of curing for the epoxy, the Au/Cr electrode (100 nm / 50 nm) was sputtered to conduct the ITO edge of the LNO and brass housing for ground connection. Finally, the parylene film was coated on the surface of the LNO as a matching layer and water-proof layer, then, the fabrication of the transparent transducer was finished and was further characterized and tested. Figure 1a illustrates the schematic diagram of the transparent ultrasound probe. The transducer has 5 mm X 5 mm of clear penetration window. Figure 1b shows the finished cylinder-type transparent ultrasound transducer. The letters printed on the background could easily be read, further indicating the transparency of the transducer.
2.3. Testing the performance of the transparent ultrasound probe
The electrical impedance and phase spectrum of the fabricated transparent transducer were measured by an impedance analyzer (HP 4294A, Agilent Technologies, Santa Clara, CA, USA). The pulse-echo performance of the transparent transducer was measured by the set-up detailed in previous work [27]. The transducer was immersed into the water tank, and the quartz plate was applied as a reflector for reflecting generated ultrasound. The transducer excited by pulser-receiver (Panametrics 5900PR, Olympus NDT Inc., Waltham, MA, USA) with 1 µJ energy per pulse transmitted and received the ultrasound. Theoretically, the -6 dB axial resolution (Raxial) can be determined as Raxial = λ/(2BW) [27], whereas λ is the wavelength of the ultrasound in water, and BW is the -6 dB bandwidth of the transducer. Therefore, the -6 dB axial resolution can be estimated as 40 µm. The high-resolution contact lens-based transducer will better fit the fundus camera for ocular imaging.
2.4. Experimental setup
Figure 2a illustrates the schematic diagram of the experimental setup for in vivo retinal imaging of rat eyes. The light source is a visible light LED (M530L4, Thorlabs Inc., Newton, NJ, USA) with a center wavelength of 530 nm and a full-width half maximum (FWHM) of 35 nm. For albino rats, the contrast of the retinal vasculature is poor when visualized with red and NIR light, and the UV portion of the spectrum is heavily attenuated by the ocular lens. Therefore, the choice of wavelength in the green portion of the spectrum was made as it shows the retinal vessels with excellent contrast [28]. An optical fiber (MHP550L02, Thorlabs Inc., Newton, NJ, USA) coupled the LED light to the eyelid of the rat and the end of the fiber was fixed to the eyelid with tape. The optical power at the tip of the fiber was measured to be 5 mW. The imaging system consisted of the transparent ultrasound probe as the frontal element, followed by an ophthalmic lens (L1) (f= 11 mm) (Volk Digital Series Wide Field lens, Volk, Mentor, OH, USA), a relay lens (L2) (f= -50 mm, LC1715, Thorlabs Inc., Newton, NJ, USA), and a camera lens (L3) (12 fixed focal length lens, 33-303, Edmund Optics Inc., Barrington, NJ). The working distance of L1 was chosen with the thickness of the transparent ultrasound probe in mind, so that, when the transducer touches the cornea properly, a retinal image is created by L1 at the retina conjugate plane (RCP) and this image is relayed to the camera sensor (GS3-U3-41S4M-C, FLIR Integrated Imaging Solutions Inc., Richmond, Canada) by L2 and L3. Figure 2b shows a photographic illustration of the imaging system.
Furthermore, we investigated how the transparency of the ultrasound probe might affect the image quality during human eye imaging. In order to do that, we imaged an eye model (OEMI-7, Ocular Instruments, Bellevue, Washington, USA) first with the proposed imaging system. Then the ultrasound transducer was replaced with a glass window (WG10530, Thorlabs Inc., Newton, NJ, USA). The glass window is similar in thickness to the ultrasound transducer, but optically neutral. Ultrasound signal was generated and received by a pulser receiver (Panametrics 5900PR, Olympus NDT Inc., Waltham, MA, USA) and a custom-made MATLAB script was created to visualize and perform adequate filtering on the signal. A custom-made LABVIEW interface was created to control image acquisition.
2.5. Animal preparation
The Association of Research in Vision and Ophthalmology's guidelines for the ethical use of animals in ophthalmic and visual science research were followed in all aspects of the experimental protocols and related animal care. The related experimental protocol was approved by the University of Illinois Chicago's (UIC) Animal Care Committee. The study employed a sample of eleven-month-old male Sprague Dawley rats (N = 2). The rats were acquired from Charles River Laboratories (Wilmington, MA) and housed within UIC’s Biology Resource Lab. After the rats were anesthetized, the pupil was dilated using a drop of 1% tropicamide ophthalmic solution. A lubricant ocular gel (GenTeal, Alcon Laboratories, Fort Worth, TX, USA) was applied to the whole anterior eye to keep the eye surface hydrated throughout the experiment. The eye gel also served as a coupling medium between the transparent ultrasound probe and the eye to ensure the propagation of the ultrasound waves. An animal holder was used to keep the rat steady and keep the head of the rat immobilized. The animal holder facilitated five degrees of freedom position controls.
3. Results
Figure 1a-b illustrates the electrical impedance and phase spectrum of the fabricated transparent transducer. The resonant frequency (fr) and anti-resonant frequency (fa) were located as 32, and 41, respectively, which is close to the simulation results. Hence, the effective electromechanical coupling coefficient (keff) of the transducer, describing the conversion efficiency between electrical energy and mechanical energy, is calculated as 0.62 by the following equation (1) [27].
The electric impedance magnitude of the fabricated transducer is in the range of 30 to 40 Ω around the resonance frequency of the transducer, approximately closing to match the ideal electrical impedance of 50 Ω. The pulse-echo performance of the transducer is illustrated in Figure (c-d). The result shows the central frequency of the transducer as 37 MHz, and the -6 db fractional bandwidth as 30%, which are in good agreement with the simulation results (40 MHz).
Figure 3.
(a) simulated and (b) measured electrical impedance and phase spectrum of the transducer. (c) simulated and (d) measured pulse-echo performance of the transducer.
Figure 3.
(a) simulated and (b) measured electrical impedance and phase spectrum of the transducer. (c) simulated and (d) measured pulse-echo performance of the transducer.

Figure 4 illustrates the result from an in vivo experiment in a rat eye. Figure 4a shows the fundus image and Figure 4b shows the corresponding ultrasound pulse-echo image. The acquired pulse echo signal constitutes both positive and negative voltage. However, to ensure similarity with the clinical ocular ultrasound pulse-echo signal, we illustrated the absolute voltage instead of the real voltage. It is evident from Figure 4a that the retinal and choroidal vessels as well as the optic nerve of the rat retina are clearly imaged in the fundus photograph. The ultrasound data shows four distinct peaks corresponding to four anatomical locations inside the eye, namely the cornea (P1), the anterior capsule of the crystalline lens (P2), the posterior capsule of the crystalline lens (P3), and the retina (P4). The axial length of the eye could be approximated from the time interval between the corneal peak and the retinal peak, given that we know the speed of sound in the eye. The speed of sound in aqueous humor and vitreous humor is around 1532 m/s, whereas the speed of sound inside the ocular lens is around 1641 m/s [29]. For conservative estimation, we assume that the speed of sound inside the whole eye is 1532 m/s. We find the axial length of the eye using the following equation,
Where is the time interval between the corneal peak and the retinal peak, is the speed of ultrasound. The axial length was calculated to be 4.902 mm, which is close to the axial length reported by other studies on a similar strain of rats measured by OCT [30]. The slight mismatch in axial length from the reported value could be attributed to the fact that the lens encompasses more than 60% of the volume of the eye in rats [31]. So, our simple approximation of a constant velocity would not be sufficient for calculating the axial length correctly. However, for the human eye, the lens is small relative to the whole eye, so even a simple approximation could give us a nearly accurate result. Furthermore, the velocity of sound in different human eye tissues and their interfaces are well characterized [32].
Figure 4.
(a) Fundus image taken from in vivo imaging on a rat eye. (b) Ultrasound pulse-echo data gathered from the same rat eye.
Figure 4.
(a) Fundus image taken from in vivo imaging on a rat eye. (b) Ultrasound pulse-echo data gathered from the same rat eye.

The effect of the transparent transducer on the imaging performance of the system is illustrated in Figure 5. a illustrates an image of the retina of an eye model taken with the proposed prototype. Figure 5b illustrates an image of the retina of the same eye model taken after replacing the transparent transducer with an optically neutral glass window. The glass window was chosen in such a way that it didn’t change the magnification of imaging and only the difference in imaging performance would be highlighted. The structures present in the eye model, namely the blood vessels, the optic nerve head, and the pigmented ocular lesions on the superior and inferior retina could be easily identified in both images. The mean RGB intensity of the images taken with the transparent transducer and the glass window are 121 and 135, showing the illumination efficiency of the device is not severely affected by the transparent transducer. The ocular vessel branches present in the model eye are clearly seen in both images. However, the smaller vessels that constitute the vessel branches could be differentiated in the image taken with the glass window, whereas the image taken with the transparent transducer doesn’t resolve the smaller vessels in the vessel branches clearly. That being said, the quality of the image taken with the transparent transducer should be sufficient to accurately guide the ultrasound signal to ocular lesions, which is the goal of the prototype.
4. Discussion
In summary, we demonstrated the feasibility of using a fundus camera to guide the local position of an ultrasound transducer to detect pulse-echo signals from the posterior segment of the eye. A high frequency transparent ultrasound transducer was used to facilitate simultaneous fundus and ultrasound imaging. In vivo testing was performed on rats with our prototype and simultaneous fundus image and ultrasound A-scan were gathered. Furthermore, the optical performance and image quality of the prototype on the human eye were examined with a model eye. In addition to providing objective guidance for ultrasound imaging, the fundus image would also provide valuable information such as the presence of orange pigment or drusen overlying an ocular tumor, which are important features in the diagnosis of melanocytic choroidal tumors [1,26,33].
For this feasibility study, we utilized a high-frequency ultrasound probe with a center frequency of 40 MHz in order to differentiate the cornea, the ocular lens, and the retina clearly in the rat eye. The rat retina is much thinner than the human retina [34], and needs high-frequency ultrasound signal to differentiate different ocular structures properly. The -6 dB axial resolution of the system was calculated to be 40 μm, which is reasonable for imagining the eye of the rat. On the other hand, the attenuation of ultrasound waves significantly increases with the operating frequency, reducing the penetration depth. The smaller axial length of a rat [29] eye ( around 7 mm) helps to acquire signals from the retina with reasonable quality. For the human eye, the attenuation at this frequency makes the acquisition of signal from the retina impossible. Therefore, we were not able to conduct ultrasound measurements from the human eye with this preliminary light-ultrasound prototype. However, the primary purpose of this study was to demonstrate the feasibility of using fundus imaging as the guidance for ocular ultrasound and we demonstrated that clearly with an in vivo experiment on rat eyes. Ultrasound probes used in ophthalmology clinics generally have a center frequency between 7 MHz-20 MHz [15] to compensate for the attenuation to enhance the penetration depth. We are currently pursuing a transparent ultrasound transducer with a frequency similar to the clinical ophthalmic ultrasound systems for future studies of human eyes.
The ultrasound transducer used in this study is a 5 mm x 5 mm square-shaped transducer. The limited lateral resolution from the transducer means that the echo signal received by the transducer comes from a larger area inside the eye, which in turn makes the signal noise prone. Notably, the signal coming from the posterior of the crystalline lens (peak P3 in Figure 4b) has a relatively weaker signal strength. This could be attributed to the large beam width and the acute curvature of the posterior section of the rat lens as a portion of the beam reaches the border while the rest is still traveling inside the lens, creating an averaging effect.
An eye model was used to verify the effect that the transparent ultrasound probe had on the image quality (Figure 5). For the actual human eye, factors such as the pupil size, and eye movement could affect the result. Therefore, the model eye was a better choice for accurate comparison. To deliver the light inside the model eye, a small hole was drilled 5 mm away from the cornea of the model eye and a small optical fiber was inserted which delivered the illumination inside the eye.
For in vivo testing in rat eyes, the retina was illuminated through the eyelid, which is contrary to the conventional method of both delivering the illumination and receiving the retinal reflection through the pupil. This method has recently been proposed to acquire widefield retinal images even with limited pupil size in human eyes [35]. However, the added complexity of having a separate illumination path would increase the complexity of fundus image-guided ultrasound acquisition in patients. Miniaturized indirect illumination-based fundus imagers have shown promise in acquiring non-mydriatic widefield fundus images without the complexity of conventional pupillary ring illumination or a separate illumination system. A simple solution to the pupil limitation without requiring a separate illumination system is provided by miniature indirect illumination, which offers a different approach towards the development of widefield portable fundus cameras [36,37]. In our future prototype, we plan to include this illumination method to construct a compact, portable, widefield fundus image-guided ultrasound probe to accurately extract ultrasound information from ocular lesions in human patients.
5. Conclusions
A fundus image-guided ultrasound probe was demonstrated for accurate measurement of ultrasound pulse-echo signal from the posterior segment of the eye. In vivo imaging of rat eyes demonstrated the feasibility of capturing retinal images and ultrasound measurements simultaneously. The optical performance and the image quality of the prototype were further validated using a model eye to demonstrate potential usability in humans.
6. Patents
Pending patent application for multimodal fundus-ultrasound eye imager.
Author Contributions
Conceptualization, Michael Heiferman, Qifa Zhou and Xincheng Yao; Data curation, Alfa Rossi and Yushun Zeng; Formal analysis, Chen Gong, Mojtaba Rahimi and Xin Sun; Investigation, Alfa Rossi, Yushun Zeng, Mojtaba Rahimi, Chen Gong, Mohammad Soleimani, Ali Djalilian and Xin Sun; Methodology, Alfa Rossi, Yushun Zeng and Mojtaba Rahimi; Resources, Mohammad Soleimani, Ali Djalilian and Mark Humayun; Software, Alfa Rossi, Taeyoon Son and Yushun Zeng; Supervision, Qifa Zhou and Xincheng Yao; Validation, Taeyoon Son, Michael Heiferman and Mark Humayun; Visualization, Alfa Rossi, Taeyoon Son and Yushun Zeng; Writing – original draft, Alfa Rossi and Yushun Zeng; Writing – review & editing, Qifa Zhou, Mohammad Soleimani, Ali Djalilian, Mark Humayun and Xincheng Yao.
Funding
This work was supported by the National Institutes of Health (NIH) under grant R01EY028662, R01EY030126, and R01EY035084.
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care Committee of University of Illinois Chicago (protocol code: 23-068 and date of approval: 7/11/2023).
Data Availability Statement
The data presented in this study is available within the article.
Acknowledgments
The authors thank Tara Nguyen and Jiwang Chen for helping with animal preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chien, J.L.; Sioufi, K.; Surakiatchanukul, T.; Shields, J.A.; Shields, C.L. Choroidal nevus: a review of prevalence, features, genetics, risks, and outcomes. Current opinion in ophthalmology 2017, 28, 228–237. [Google Scholar] [CrossRef]
- Solnik, M.; Paduszyńska, N.; Czarnecka, A.M.; Synoradzki, K.J.; Yousef, Y.A.; Chorągiewicz, T.; Rejdak, R.; Toro, M.D.; Zweifel, S.; Dyndor, K.; et al. Imaging of Uveal Melanoma-Current Standard and Methods in Development. Cancers 2022, 14. [Google Scholar] [CrossRef]
- Krantz, B.A.; Dave, N.; Komatsubara, K.M.; Marr, B.P.; Carvajal, R.D. Uveal melanoma: epidemiology, etiology, and treatment of primary disease. Clinical ophthalmology (Auckland, N.Z.) 2017, 11, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Gálvez, C.C.; Ordaz-Favila, J.C.; Villar-Calvo, V.M.; Cancino-Marentes, M.E.; Bosch-Canto, V. Retinoblastoma: Review and new insights. Frontiers in oncology 2022, 12, 963780. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Furuta, M.; Berman, E.L.; Zahler, J.D.; Hoberman, D.M.; Dinh, D.H.; Mashayekhi, A.; Shields, J.A. Choroidal nevus transformation into melanoma: analysis of 2514 consecutive cases. Archives of ophthalmology (Chicago, Ill.: 1960). 2009, 127, 981–987. [Google Scholar] [PubMed]
- Yao, X.; Son, T.; Ma, J. Developing portable widefield fundus camera for teleophthalmology: Technical challenges and potential solutions. Experimental biology and medicine (Maywood, N.J.) 2022, 247, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Nagiel, A.; Lalane, R.A.; Sadda, S.R.; Schwartz, S.D. ULTRA-WIDEFIELD FUNDUS IMAGING: A Review of Clinical Applications and Future Trends. Retina (Philadelphia, Pa.) 2016, 36, 660–678. [Google Scholar] [CrossRef]
- Quinn, N.; Csincsik, L.; Flynn, E.; Curcio, C.A.; Kiss, S.; Sadda, S.R.; Hogg, R.; Peto, T.; Lengyel, I. The clinical relevance of visualising the peripheral retina. Progress in Retinal and Eye Research 2019, 68, 83–109. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, A. Choroidal melanoma. Oman journal of ophthalmology 2012, 5, 3–9. [Google Scholar] [CrossRef]
- Torres, V.L.; Brugnoni, N.; Kaiser, P.K.; Singh, A.D. Optical coherence tomography enhanced depth imaging of choroidal tumors. American journal of ophthalmology 2011, 151, 586–593. [Google Scholar] [CrossRef]
- Say, E.A.; Shah, S.U.; Ferenczy, S.; Shields, C.L. Optical coherence tomography of retinal and choroidal tumors. Journal of ophthalmology 2011, 2011, 385058. [Google Scholar] [CrossRef]
- Gündüz, K. Gündüz, K.; Yeşiltaş; YS. Diagnostic Techniques: Angiography. in Singh, A.D., and Damato, B.E. (Eds.): ‘Clinical Ophthalmic Oncology: Basic Principles’ (Springer International Publishing, 2019), pp. 209–234.
- Midena, E.; Frizziero, L.; Pilotto, E.; Parrozzani, R. Diagnostic Techniques: Autofluorescence. in Singh, A.D., and Damato, B.E. (Eds.): ‘Clinical Ophthalmic Oncology: Basic Principles’ (Springer International Publishing, 2019), pp. 257-270.
- Lorek, B.H.; Aronow, M.E.; Singh, A.D. Diagnostic Techniques: Ultrasonography. in Singh, A.D., and Damato, B.E. (Eds.): ‘Clinical Ophthalmic Oncology: Basic Principles’ (Springer International Publishing, 2019), pp. 271–293.
- Kadakia, A.; Zhang, J.; Yao, X.; Zhou, Q.; Heiferman, M.J. Ultrasound in ocular oncology: Technical advances, clinical applications, and limitations. Experimental biology and medicine (Maywood, N.J.) 2023, 248, 371–379. [Google Scholar] [CrossRef]
- Safari, A.; Zhou, Q.; Zeng, Y.; Leber, J.D. Advances in development of Pb-free piezoelectric materials for transducer applications. Japanese Journal of Applied Physics 2023, 62, SJ0801. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, L.; Chen, R.; Li, R.; Kang, H.; Zeng, Y.; Yan, Y.; Priya, S.; Zhou, Q. Design and Fabrication of 15-MHz Ultrasonic Transducers Based on a Textured Pb(Mg1/3Nb2/3)O3-Pb(Zr, Ti)O3 Ceramic. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control 2022, 69, 3095–3101. [Google Scholar] [CrossRef]
- Li, R.; Zeng, Y.; Sun, X.-x.; Li, C.; Li, R.; Zheng, T.; Jiang, L.; Wu, J. Multidimensional synergy-induced high piezoelectricity and reliability KNN piezoceramics for high-frequency ultrasonic transducers. Science China Materials 2023, 66, 686–695. [Google Scholar] [CrossRef]
- Fonkeu, Y.; Singh, N.; Hayden-Loreck, B.; Singh, A.D. Diagnostic A-Scan of Choroidal Melanoma: Automated Quantification of Parameters. Ocular oncology and pathology 2019, 5, 350–357. [Google Scholar] [CrossRef]
- Silverman, R.H. Focused ultrasound in ophthalmology. Clinical ophthalmology (Auckland, N.Z.) 2016, 10, 1865–1875. [Google Scholar] [CrossRef] [PubMed]
- Chopdar, A.; Aung, T. Multimodal retinal imaging. (JP Medical Ltd, 2014) 2014.
- Ossoinig, K.C. Standardized echography: basic principles, clinical applications, and results. International ophthalmology clinics 1979, 19, 127–210. [Google Scholar] [CrossRef] [PubMed]
- Ossoinig, K.C. Quantitative echography--the basis of tissue differentiation. Journal of clinical ultrasound: JCU 1974, 2, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Fonkeu, Y.; Lorek, B.H.; Singh, A.D. Diagnostic A-Scan of Choroidal Tumors: Comparison of Quantified Parameters. Ocular oncology and pathology 2019, 5, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Campagnoli, T.R.; Medina, C.A.; Singh, A.D. CHOROIDAL MELANOMA INITIALLY TREATED AS HEMANGIOMA: DIAGNOSTIC AND THERAPEUTIC CONSIDERATIONS. Retinal cases & brief reports 2016, 10, 175–182. [Google Scholar]
- ‘Factors Predictive of Growth and Treatment of Small Choroidal Melanoma: COMS Report No. 5. Archives of Ophthalmology 1997, 115, 1537–1544. [Google Scholar]
- Chen, R.; Jiang, L.; Zhang, T.; Matsuoka, T.; Yamazaki, M.; Qian, X.; Lu, G.; Safari, A.; Zhu, J.; Shung, K.K.; et al. Eco-Friendly Highly Sensitive Transducers Based on a New KNN–NTK–FM Lead-Free Piezoelectric Ceramic for High-Frequency Biomedical Ultrasonic Imaging Applications. IEEE Transactions on Biomedical Engineering 2019, 66, 1580–1587. [Google Scholar] [CrossRef]
- Alterini, T.; Díaz-Doutón, F.; Burgos-Fernández, F.J.; González, L.; Mateo, C.; Vilaseca, M. Fast visible and extended near-infrared multispectral fundus camera. Journal of biomedical optics 2019, 24, 1–7. [Google Scholar]
- Lozano, D.C.; Twa, M.D. Development of a Rat Schematic Eye From In Vivo Biometry and the Correction of Lateral Magnification in SD-OCT Imaging. Investigative Ophthalmology & Visual Science 2013, 54, 6446–6455. [Google Scholar]
- Wu, Y.; Luo, X.; Feng, Y.; Yang, J.; Fan, H.; Cen, X.; Li, W. Comparison of the accuracy of axial length measurement by different imaging methods in Sprague Dawley rats. Frontiers in Neuroscience, 2023, 16. 2023. [Google Scholar]
- Remtulla, S.; Hallett, P.E. A schematic eye for the mouse, and comparisons with the rat. Vision research 1985, 25, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, K.J. Ultrasound velocities for axial eye length measurement. Journal of cataract and refractive surgery 1994, 20, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Shields, J.A.; Kiratli, H.; De Potter, P.; Cater, J.R. Risk factors for growth and metastasis of small choroidal melanocytic lesions. Ophthalmology 1995, 102, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, C.L.; Kim, A.Y.; Kashani, A.H. Normative Retinal Thicknesses in Common Animal Models of Eye Disease Using Spectral Domain Optical Coherence Tomography. in Editor (Ed.)^(Eds.): ‘Book Normative Retinal Thicknesses in Common Animal Models of Eye Disease Using Spectral Domain Optical Coherence Tomography’ (Springer International Publishing, 2018, edn.), pp. 157–166.
- Toslak, D.; Thapa, D.; Chen, Y.; Erol, M.K.; Paul Chan, R.V.; Yao, X. Trans-palpebral illumination: an approach for wide-angle fundus photography without the need for pupil dilation. Opt. Lett. 2016, 41, 2688–2691. [Google Scholar] [CrossRef]
- Rossi, A.; Rahimi, M.; Le, D.; Son, T.; Heiferman, M.J.; Chan, R.V.P.; Yao, X. Portable widefield fundus camera with high dynamic range imaging capability. Biomedical optics express 2023, 14, 906–917. [Google Scholar] [CrossRef]
- Toslak, D.; Liu, C.; Alam, M.N.; Yao, X. Near-infrared light-guided miniaturized indirect ophthalmoscopy for nonmydriatic wide-field fundus photography. Opt. Lett. 2018, 43, 2551–2554. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
(a) The schematic diagram of the transparent transducer. (b) The fabricated transparent transducer with connections. G: ground; W: wire.
Figure 1.
(a) The schematic diagram of the transparent transducer. (b) The fabricated transparent transducer with connections. G: ground; W: wire.

Figure 2.
(a) The schematic illustration of the experimental setup. (b) Photographic illustration of the imaging system. RCP: Retina conjugate plane.
Figure 2.
(a) The schematic illustration of the experimental setup. (b) Photographic illustration of the imaging system. RCP: Retina conjugate plane.

Figure 5.
(a) Image of the retina of an eye model taken with the prototype. (b) Image of the same model eye taken after replacing the ultrasound transducer in the prototype with a glass window.
Figure 5.
(a) Image of the retina of an eye model taken with the prototype. (b) Image of the same model eye taken after replacing the ultrasound transducer in the prototype with a glass window.

Table 1.
Design parameters of the transparent ultrasound transducer.
| Parameter | Value |
|---|---|
| Center frequency | 40 MHz |
| Surface area | 5 mm x 5 mm |
| Lithium Niobate (LNO) thickness | 70 µm |
| Matching layer (Parylene) thickness | 10 µm |
| Backing layer (Epo-Tek 301) thickness | 5 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Preliminary Design and Evaluation of a B-Scan OCT-Guided Needle
Karen Joos
et al.
Photonics,
2014
An Intraocular Pressure Measurement Technique Based on Acoustic Radiation Force Using an Ultrasound Transducer: A Feasibility Study
Hee Lee
et al.
Sensors,
2021
Wide-Field OCT Angiography at 400 KHz Utilizing Spectral Splitting
Laurin Ginner
et al.
Photonics,
2014
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








