1. Introduction
Respiratory distress syndrome (RDS) is the most common respiratory disease in premature infants and is attributed to anatomical and functional immaturity of the neonatal lungs. The severity of RDS is inversely correlated with the degree of prematurity, and diagnosis is based on clinical manifestations, blood gas analysis, and imaging. Classically, neonates with RDS are treated with oxygen and mechanical ventilatory support (invasive or non-invasive), but exogenous surfactant administration is the cornerstone of treatment [
1]. Despite all of the progress in perinatal medicine and neonatology, RDS is still frequently observed in very preterm infants [
2], and is associated with severe complications including intraventricular haemorrhage, air-leak syndromes, bronchopulmonary dysplasia [
1], and mortality [
3].
The accurate prediction and prompt diagnosis of RDS is of paramount importance, but its development and evolution after birth is difficult to anticipate. Despite the numerous studies performed during the last decades, no clinical or biological factor has been found to predict RDS early and accurately. Additionally, the classical clinical description of RDS has changed over the years, as its management has significantly evolved with the use of antenatal corticosteroids and the early initiation of nasal continuous positive pressure (CPAP) in the delivery room [
4]. It should also be noted that when common clinical indicators of RDS development are able to be used, disease progression has likely already occurred. At this point, the failure of supporting treatments (most commonly CPAP failure) is imminent and is associated with significant morbidity and mortality [
5].
During the last years, oxygen requirement after birth has been proposed to guide surfactant replacement in preterm infants. According to the latest (2023) European Consensus Guidelines on the management of RDS, infants with RDS should be given rescue surfactant early in the course of the disease—more specifically, those infants requiring a fraction of inspired oxygen (FiO
2) > 0.30 while on a CPAP pressure of at least 6 cm H
2O [
6]. Lung ultrasound has also recently been proposed as a means of early identification of infants with RDS to help guide exogenous surfactant therapy [
7].
Several laboratory techniques have also been used to evaluate surfactant activity in the amniotic or gastric fluid. These techniques are either quantitative (lecithin/sphingomyelin ratio, phosphatidylglycerol levels, and lamellar body counts) or qualitative (stable microbubble and surfactant adsorption tests), and are able to provide a more personalized management of neonates with RDS [
8,
9].
In this context, gastric fluid could potentially be an excellent biological specimen for the detection of candidate biomarkers related to lung maturation, and to help guide the management of RDS. The use of advanced bio-analytical approaches, such as metabolomics, may play a key role in achieving this goal [
10]. Metabolomic analysis of the amniotic fluid has been used for both the prediction of preterm delivery and bronchopulmonary dysplasia [
11]. Recently, gastric fluid metabolites were found to predict survival in severe prematurity [
12].
The purpose of the present study was to investigate whether metabolomic analysis of gastric fluid obtained early after birth in infants born before 32 weeks’ gestation, could identify differences in intermediate metabolites between infants who require rescue surfactant therapy, and those who do not require replacement therapy, and to assess if any of these metabolites could serve as an indicator of the need for surfactant therapy in very preterm infants with RDS.
2. Materials – Methods
2.1.1. Study design and population
This sub-study is part of a prospective, single center, investigation on the role of metabolomics for the prediction of neonatal outcomes in very preterm infants (born at <32 weeks’ gestational) conducted from March 1
st, 2017, to December 31
st, 2020. Results describing the role of gastric fluid and urine metabolomic analysis obtained shortly after birth for the prediction of survival in very preterm infants have recently been published [
12].
Herein, we analyzed data of the gastric fluid metabolomic analysis focusing on the need for surfactant replacement therapy for RDS. As previously described [
12], we excluded neonates who were outborn; had known congenital infections, anomalies, or inborn errors of metabolisms; received chest compressions; and infants with missing gastric samples or those without parental consent. In the present study, infants who were given surfactant prior to the sampling of the gastric fluid, either in the delivery room or very early after admission to the NICU, were also not included in the analysis.
We recorded variables related to neonatal demographical characteristics (gestational age, birth weight, sex and being small for gestational age), pregnancy (maternal age, multiple gestation, chorioamnionitis, hypertension/pregnancy-induced hypertension, antenatal corticosteroids), delivery mode, status after birth (Apgar scores at 1 and 5 min), intubation [in the delivery room (DR) and during the first 3 days of life], and receipt of exogenous surfactant in the NICU setting for the management of RDS (mode of administration and total doses). Neonates with blood culture confirmed early-onset sepsis were recorded as well.
Cases were defined as neonates who were treated with exogenous surfactant for RDS, whereas the control group was made up of infants with mild or no RDS who did not receive surfactant. In our NICU, in line with the 2016 and 2019 updated European Consensus guidelines [
13,
14], infants with clinical signs of RDS [including increased oxygen requirements (FiO
2 >0.30) while on CPAP of at least 6 cm H
2O], and compatible radiographic findings, were given rescue surfactant (poractant alpha) using either less invasive surfactant administration techniques, or following endotracheal intubation and invasive mechanical ventilation. All infants were started on caffeine citrate from the first day of life.
2.1.2. Sampling
Gastric fluid samples were collected from the enrolled neonates with a thin gastric tube during the first hour of their life. Samples were stored at − 80 °C until the metabolomic analysis.
2.1.3. Outcomes of the study
The main outcomes of this sub-study were: 1) to identify differences in the gastric metabolites in very preterm infants with RDS who received surfactant replacement compared to a control group of infants with mild or no RDS who did not receive surfactant; and 2) to evaluate the possible role of gastric fluids metabolites, alone or in combination with clinical variables, as indicators of the need for surfactant replacement therapy in preterm infants with RDS.
2.2. Analytical Techniques
2.2.1. Sample Preparation
All samples were thawed at room temperature before analysis. Initially, 50 μL of gastric fluid sample were mixed with 10 μL of myristic acid-d7 (internal standard, IS) and 50 μL of ice-cold MeOH (−20 °C). The mixture was vortexed for 2 minutes and then centrifuged at 10,000 rpm for 15 minutes. Subsequently, 70 μL of the clear supernatant were transferred into a clear Eppendorf tube and evaporated under vacuum. Twenty-five microliters of MeOX 2% pyridine were added, followed by vortexing for 2 min and heating for 90 minutes at 30 °C. Then, 50 μL of MSTFA, 1% TMCS were added, and the sample was again heated for an additional 30 minutes at 37 °C. Finally, 10 μL of pentadecane (injection standard at 100 mg/L) were introduced, and the sample was subjected to GC-MS analysis.
A quality control (QC) sample was also prepared by mixing equal volumes of all analyzed real gastric samples (pooled sample). QC samples were analyzed at the beginning of the analytical batch and every ten real gastric fluid samples to ensure analytical performance.
2.2.2. GC-MS Analysis
GC-MS analysis was performed in an Agilent 7890A GC-MS system (Agilent Technologies, Santa Clara, CA, USA), equipped with a CTC autosampler and a PTV injector. Gas chromatography was performed on a 30 m HP-5 ms UI (Agilent J&W) capillary column (film thickness of 0.25 mm; I.D. of 0.25 µm), while back-flush elution was carried out in a 1.5 m deactivated column with a film thickness of 0.18 mm. Initial column temperature was set at 60 °C for 1 min and then increased to 300 °C at a 10 °C/min rate. The temperature was maintained at 300 °C for 6 min. Total run time was 30 min, followed by a 12 min back-flush run and a solvent delay at 6 min. Helium (99.999%) was used as the carrier gas at a flow rate of 3 mL/min, and injection volume was set at 1 μL. Splitless mode injection was performed at the PTV injector, where temperature was increased from 270 °C to 350 °C. MS was operated at electron impact ionization mode (EI; 70 eV). Ion source and transfer line temperatures were set at 230 °C and 250 °C, respectively. All mass spectra were acquired in full scan mode between 50 and 600 amu.
2.3. Statistical analysis
The Kolmogorov-Smirnov test was used to assess the normality assumption. Continuous variables were described using the mean and standard deviation (SD) or median and interquartile range (IQR) according to the normality assumption. Frequencies (percentage) were used to describe categorical variables. The independent samples t-test and the Mann-Whitney U test were used to compare continuous variables between the two groups. The Pearson X2 test was used for the association between dichotomous variables. Crude and adjusted Odds ratios (ORs), and 95% Confidence Intervals (CI) for the prediction of receipt of surfactant, were calculated from univariate and multivariate logistic regression, respectively, followed by ROC/AUC calculation. An alpha-level <0.2 was selected as a cut-off for variable removal in the automated model selection and backward elimination was preferred. All the statistical tests were two–sided and the level of significance was set at a=0.05. Data analysis was carried out using IBM SPSS Statistics, version 29.0 (IBM Corp., Armonk, N.Y., USA).
Gas Chromatography-Mass Spectrometry (GC-MS) data obtained from all analyzed samples were processed using MSD CHEMSTATION software. The Free online Assignment Validator and Integrator (GAVIN) script for MATLAB (MathWorks, Natick, MA) was employed for peak integration, complementing AMDIS for peak deconvolution and identification. Metabolite identification was based on Agilent Fiehn library and NIST17 MS library (mainlib library), with a minimum match factor of 50%.
3. Results
3.1. Characteristics of the study population
Seventy-three neonates were analyzed, 43 cases and 30 controls. The perinatal-neonatal characteristics of the studied neonates are shown in
Table 1. Compared to controls, cases had significantly lower gestational age, birth weight as well as Apgar scores at 1 and 5 min, respectively. Moreover, cases were significantly more often intubated after delivery and received invasive mechanical ventilation in the NICU during the first three days after birth compared to infants in the control group (
Table 1). In cases, an average of 1.8 (0.8) surfactant doses were instilled, intratracheally, through an endotracheal tube (n=19, 44.2%) or a catheter (n=17, 39.5%) whereas in 7 infants (16.3%) both approaches were used. No infant requiring intubation was given prophylactic surfactant in the DR. One of the neonates treated with surfactant developed early-onset sepsis.
3.3. Differences in gastric fluid samples
A detailed description of gastric fluid metabolites in neonates including the differences between cases and controls is presented in Supp.
Table 1. Statistically significant differences were observed in specific metabolites, namely L-proline (p=0.018), L-glycine (p=0.036), N-acetyl- L-serine (p=0.036) and L-threonine (p=0.038), all of which were increased in cases compared to controls (
Table 2). However, despite the observed differences, the AUC values obtained (
Table 2) indicate that none of the metabolites alone could accurately predict the need for surfactant replacement.
3.4. Predictors of the need for surfactant replacement therapy
3.4.1. Univariate analysis
Gestational age, Apgar scores at 1 and 5 minutes, and intubation in the DR were significant predictors of the need for surfactant treatment in univariate analysis. Both gestational age and Apgar scores were considered negative predictors, associated with decreased probability for surfactant need for every 1 unit increase in their size [gestational age, OR 0.70, 95%CI 0.54-0.91, p=0.009, Apgar score at 1 min, OR 0.70, 95% CI 0.511-0.94, p=0.002 and Apgar score at 5 min, OR 0.45, 95% CI 0.22-0.91, p=0.026] (Supp.
Table 2). In contrast, intubation in the DR, L-glycine and acetyl-L-serine were respectively associated with an 8.59-fold (OR 8.59, 95%CI 2.26-32.62, p=0.002), 1.147-fold (OR 1.147, 95%CI 1.01-1.30, p=0.029) and 1.143-fold (OR 1.143, 95%CI 1.01-1.29, p=0.027) increase in the probability of receiving surfactant therapy.
3.4.2. Multivariable analysis
Two multivariable models were constructed: a model including only significant clinical variables from univariate analysis, and a model with the combination of above clinical variables and gastric fluid metabolites (Supp.
Table 3). In the first model, intubation in the DR remained the most potent predictor of receipt of surfactant (OR 8.12, p=0.002, 95% CI 2.14-31.75), demonstrating an AUC value of 0.69 (95% CI 0.57-0.81). When gastric fluid metabolites were incorporated into the multivariable model, the most accurate prediction for the receipt of surfactant therapy was accomplished with the inclusion of six variables: GA, Apgar scores at 1 and 5 minutes, intubation in the DR, acetyl-L-serine and L-glycine. Among them, intubation in the DR and acetyl-L-serine were found to be the most significant, posing an 8.12-fold and 1.13-fold increased risk for surfactant receipt (
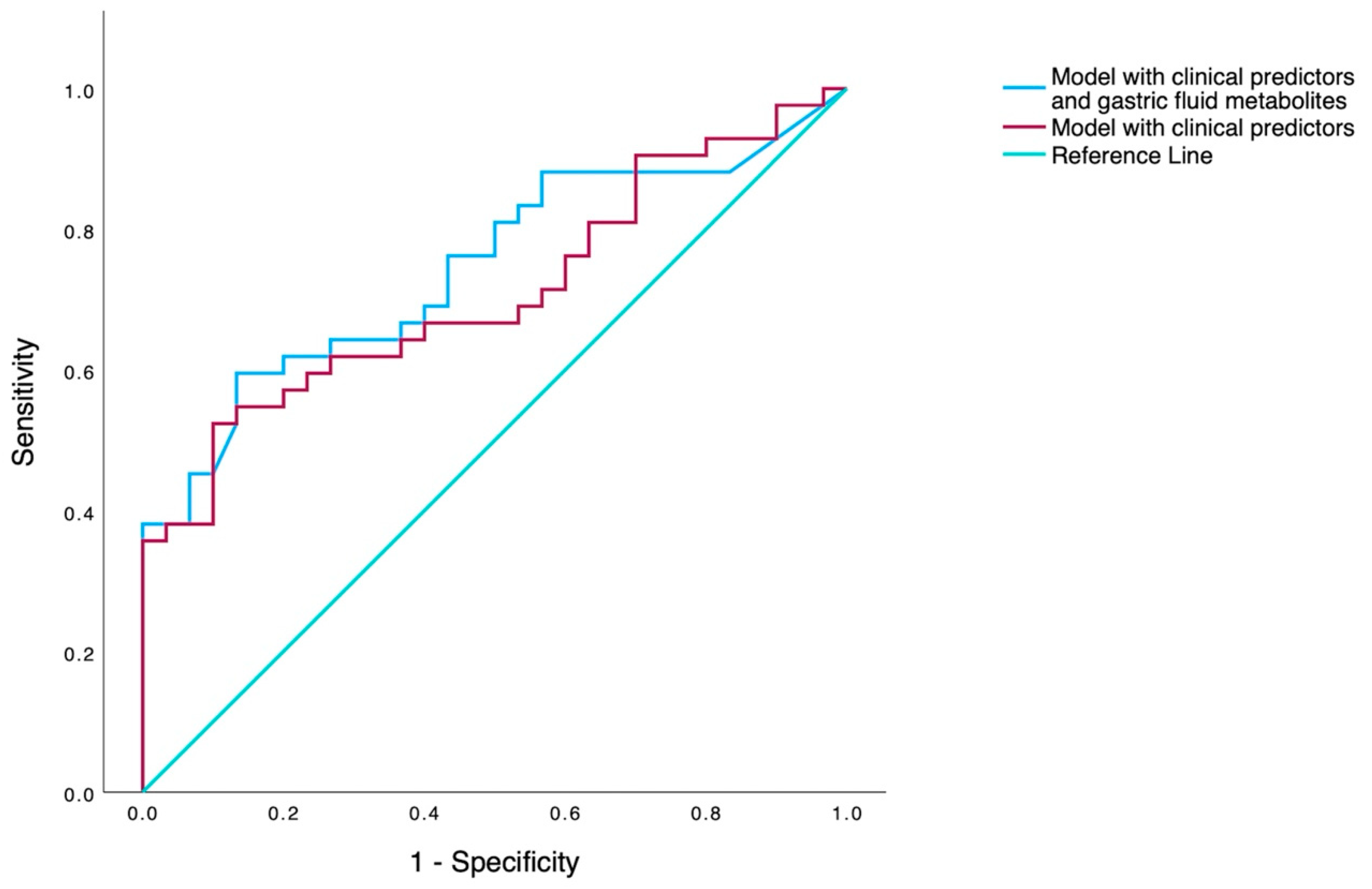
Table 3), and the model demonstrated an AUC value of 0.76 (95% CI 0.64-0.86) (
Figure 1.).
4. Discussion
In this prospective study, we evaluated whether targeted metabolomic analysis (GC-MS) of the gastric fluid in preterm neonates, obtained within the first hour of life, could predict the need for exogenous surfactant administration for the treatment RDS. Significant differences were observed between the two groups in gastric fluid metabolites (L-proline, L-glycine, L-threonine and acetyl-L-serine) and in perinatal-neonatal parameters. Of the metabolites, none could solely predict the need for surfactant replacement with high accuracy, whereas multivariate analysis revealed intubation in the DR as the most potent clinical predictor of surfactant receipt (OR 8.12, p=0.002, 95%CI 2.14-31.75), demonstrating an AUC value of 0.69 (95%CI 0.57-0.81). However, the integration of metabolomic (L-glycine and acetyl-L-serine) and clinical data (gestational age, Apgar scores at 1 and 5 minutes, and intubation in the DR) allowed the construction of a model with good accuracy AUC 0.76 (95% CI 0.64-0.86) in predicting surfactant replacement therapy.
4.1. Biochemical role and origin of the identified metabolites
Very few studies have applied metabolomics in preterm infants with RDS to investigate their metabolic profile, and possibly identify metabolic alterations/pathways associated with the pathophysiology of RDS. In these studies, bronchoalveolar lavage fluid (BALF) [
15,
16] and urine were used as specimens [
17]. In the present study, gastric fluid was the biofluid of interest, in which four amino acids were found to be significantly higher in infants treated with surfactant compared to controls.
Nevertheless, the source and role of the above metabolites are difficult to be interpreted. They may represent biomarkers produced in the gastrointestinal tract of the newborn preterm infants. On the other hand, the gastric fluid obtained that early after birth may still contain some quantity of amniotic fluid. Fetuses swallow large amounts of amniotic fluid every day. After birth, this is gradually emptied from the stomach due to gastric secretion and feeding [
18]. Therefore, they could be metabolic products derived from the amniotic fluid, as glycine, proline, threonine and serine have all been measured the human amniotic fluid [
19,
20]. L-glycine is produced from L-serine, a nonessential amino acid with a wide range of cellular functions including the biosynthesis of proteins, sphingolipids, phospholipids (including phosphatidylserine), and folate [
21]. Notably, L-glycine is involved in collagen production [
22] being, thus, crucial for the structural integrity of various organs, including the lungs. Threonine is another essential amino acid related to glycine and serine [
23]. It is also used in the biosynthesis of proteins, playing a critical role in the maintenance of intestinal mucosal integrity and barrier function [
24]. In a recent study of metabolomics, Metwaly et al. reported significantly reduced serine, glycine and threonine levels in the serum of critically ill adult patients with acute respiratory distress syndrome compared to ventilated controls, suggesting the serine-glycine metabolic pathway to have a dominant involvement in the disease pathophysiology. To explain these metabolic derangements, the authors constructed the so called “folate hypothesis” which, in brief, suggests that dysfunctional folate metabolism leads to low serine (the precursor to several amino acids) and disrupts mitochondrial redox homeostasis causing oxidative stress injury. Moreover, low folate hampers cellular regeneration through impaired synthesis of pyrimidines and purines [
25]. Proline is a non-essential amino acid with key roles in protein structure and function, and in the maintenance of cellular redox homeostasis. It is either obtained through food or can be produced de novo within the cells from protein structures, with collagen being a notable source [
26].
Regarding L-serine, it should be highlighted that we identified acetyl-L-serine, an acetylated derivative of the specific amino acid. Interestingly, this metabolite could be of microbial origin. For instance, it may be synthesized by
Escherichia Coli (E.Coli), a common microorganism involved in intra-uterine infection and preterm delivery, especially at very early gestational ages [
27], such as our study population. As previously documented, in a two-step process, serine acetyltransferase of
E. Coli catalyzes the O-acetylation of serine to O-acetyl-L-serine, in which sulphur is next assimilated leading to the synthesis of cysteine [
28]. In our case however, we were unable to differentiate whether it was the N- or O-acetyl-L-serine. Moreover, there was no difference in incidence of maternal chorioamnionitis or early-onset sepsis (both caused by bacterial infection) between the study groups to further support this speculation. Had we conducted microbiological studies of the obtained gastric fluid, we could more confidently explain the association of the gut micro-organisms with the identified acetyl-L-serine.
In any case, none of the above gastric fluid metabolites could accurately serve as sole predictors for the need for surfactant treatment in infants with RDS, nor were we able to identify any metabolite directly or indirectly associated with surfactant production and its metabolism. Yet, in another recent study, in which a similar approach was applied for the management of preterm infants with RDS, lamellar body count measured in the gastric aspirates, was found to have a moderate reliability to detect CPAP failure (AUC 0.703). Notably, the reliability of lamellar body count to predict CPAP failure was even smaller (AUC 0.314) in the subgroup of neonates born at ≤ 32 weeks’ gestation [
29].
4.2. Clinical parameters to prognosticate surfactant replacement therapy
It is well recognized that the incidence and severity of RDS are inversely related to lung immaturity and, therefore, to gestational age and birth-weight. In a study involving extremely preterm infants, surfactant was given in 99.4% and 61.4% of cases born at 22 and 28 weeks’ gestation, respectively [
30]. We found intubation in the DR to be the most significant clinical predictor of surfactant use. Reasons for endotracheal intubation
of preterm infants immediately after birth most often include resuscitation and provision of invasive mechanical ventilation [
2]
as well as surfactant administration [
6]
. More immature infants with RDS, especially those with early, severe disease, fail to initiate and establish adequate respiration after delivery, explaining thus resuscitation measures and lower Apgar scores. In a recent, prospective observational, study involving 153 preterm infants (26 to 34 weeks gestation) with RDS who were managed with CPAP and/or surfactant, gestation < 32 weeks, no antenatal corticosteroids, hypothermia on admission, Apgar score < 3 at 1 minute, and Silverman-Andersen score (an indicator of respiratory distress severity) greater than 2 at 2 hours were found to be the significant factors for predicting surfactant requirement in multivariate regression analysis [
31]. Notably, none of the study infants who required intubation after birth were given prophylactic surfactant in the DR.
4.3. Integration of metabolomic and clinical data
Unlike previously conducted relevant investigations in preterm infants, we explored the role of the significant metabolites along with other significant clinical parameters as predictors for the need for surfactant. Studies have shown that integration of metabolomic and clinical data improve the accuracy of statistical models in predicting patient outcomes [
32,
33]. As concluded in a recent systematic review and network meta-analysis of 53 studies, in which the clinical decision thresholds for surfactant administration in preterm infants were evaluated, further research into optimizing surfactant treatment for RDS should be performed, with a focus on practical surrogates of disease severity including infants’ gestational age, co-morbidities and predisposing factors, modality and level of respiratory support, as well as FiO
2 [
34].
As a matter of fact, the combination of metabolomics (L-glycine and acetyl-L-serine) and clinical data (gestational age, Apgar scores at 1 and 5 minutes, and intubation in the DR) allowed the construction of a model with improved accuracy (AUC 0.76) in predicting the need for surfactant replacement. Notably, as described above, in our center the criterion of an increased oxygen requirement (FiO
2 >0.30) while on CPAP, was important in clinical decision making to administer surfactant. Still, the performance of our model is suboptimal compared to the FiO
2-0.3 threshold (AUC 0.83) [
5], lung radiograph (AUC 0.80), or ultrasound (AUC 0.95) [
9].
4.4. Advantages and limitations
To the best of our knowledge, this is the first study to evaluate targeted metabolomic-based data for the identification of intermediate metabolites in the gastric fluid that could possibly be used as predictors of the need for exogenous surfactant in very preterm infants with RDS. Previous investigations (using mass spectrometry) only measured specific surfactant compounds (e.g., lecithin, sphingomyelin, phosphatidylglycerol, and surfactant protein A) in gastric aspirates as indicators of lung maturation [
8]. However, there are disadvantages in our study as well. The fact that this investigation was limited to one center, and therefore the small sample size, might have precluded the identification of other gastric fluid biomarkers with higher predictive accuracy. Similarly, we were unable to evaluate the effect of perinatal-neonatal parameters (e.g., the subgroup of extremely preterm infants, maternal chorioamnionitis, etc.) on metabolites. Overall, despite the development of a good predictive model incorporating clinical parameters and metabolites, we believe that it offers limited advantage compared to the currently used clinical indicators in the management of preterm infants with RDS.
5. Conclusion
In conclusion, metabolomic analysis of gastric fluid aspirates obtained immediately after birth highlighted acetyl-L-serine as a possible biomarker, but metabolite profiling alone was not sufficient to identify preterm neonates with RDS requiring exogenous surfactant. The integration of metabolomic and clinical data improved the accuracy in predicting the need for surfactant replacement therapy. Despite these promising results, further research is required to explore the role of gastric fluid metabolomics in the diagnosis and management, not only of RDS, but also of other neonatal medical conditions and outcomes. With the future development of diagnostic kits that are easy-to-use in the NICU setting, metabolomics holds promise for the significant advancement in the optimal, and individualized management of the sick neonate.
References
- Warren, J.B.; Anderson, J.M. Core Concepts: Respiratory Distress Syndrome. NeoReviews 2009, 10, e351–e361. [CrossRef]
- Moya, F.R.; Mazela, J.; Shore, P.M.; Simonson, S.G.; Segal, R.; Simmons, P.D.; Gregory, T.J.; Guardia, C.G.; Varga, J.R.; Finer, N.N.; et al. Prospective Observational Study of Early Respiratory Management in Preterm Neonates Less than 35 Weeks of Gestation. BMC Pediatr. 2019, 19, 147. [CrossRef]
- Guinsburg, R.; de Almeida, M.F.B.; de Castro, J.S.; Silveira, R.C.; Caldas, J.P. de S.; Fiori, H.H.; do Vale, M.S.; Abdallah, V.O.S.; Cardoso, L.E.M.B.; Alves Filho, N.; et al. Death or Survival with Major Morbidity in VLBW Infants Born at Brazilian Neonatal Research Network Centers. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2016, 29, 1005–1009. [CrossRef]
- Jobe, A.H. What Is RDS in 2012? Early Hum. Dev. 2012, 88 Suppl 2, S42-44. [CrossRef]
- Dargaville, P.A.; Aiyappan, A.; De Paoli, A.G.; Dalton, R.G.B.; Kuschel, C.A.; Kamlin, C.O.; Orsini, F.; Carlin, J.B.; Davis, P.G. Continuous Positive Airway Pressure Failure in Preterm Infants: Incidence, Predictors and Consequences. Neonatology 2013, 104, 8–14. [CrossRef]
- Sweet, D.G.; Carnielli, V.P.; Greisen, G.; Hallman, M.; Klebermass-Schrehof, K.; Ozek, E.; Te Pas, A.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome: 2022 Update. Neonatology 2023, 120, 3–23. [CrossRef]
- Rodriguez-Fanjul, J.; Jordan, I.; Balaguer, M.; Batista-Muñoz, A.; Ramon, M.; Bobillo-Perez, S. Early Surfactant Replacement Guided by Lung Ultrasound in Preterm Newborns with RDS: The ULTRASURF Randomised Controlled Trial. Eur. J. Pediatr. 2020, 179, 1913–1920. [CrossRef]
- Autilio, C. Techniques to Evaluate Surfactant Activity for a Personalized Therapy of RDS Neonates. Biomed. J. 2021, 44, 671–677. [CrossRef]
- De Luca, D.; Autilio, C.; Pezza, L.; Shankar-Aguilera, S.; Tingay, D.G.; Carnielli, V.P. Personalized Medicine for the Management of RDS in Preterm Neonates. Neonatology 2021, 1–12. [CrossRef]
- Le Gouellec, A.; Plazy, C.; Toussaint, B. What Clinical Metabolomics Will Bring to the Medicine of Tomorrow. Front. Anal. Sci. 2023, 3. [CrossRef]
- Baraldi, E.; Giordano, G.; Stocchero, M.; Moschino, L.; Zaramella, P.; Tran, M.R.; Carraro, S.; Romero, R.; Gervasi, M.T. Untargeted Metabolomic Analysis of Amniotic Fluid in the Prediction of Preterm Delivery and Bronchopulmonary Dysplasia. PloS One 2016, 11, e0164211. [CrossRef]
- Besiri, K.; Begou, O.; Deda, O.; Bataka, E.; Nakas, C.; Gika, H.; Kontou, A.; Agakidou, E.; Sarafidis, K. A Cohort Study of Gastric Fluid and Urine Metabolomics for the Prediction of Survival in Severe Prematurity. Metabolites 2023, 13, 708. [CrossRef]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Plavka, R.; Saugstad, O.D.; Simeoni, U.; Speer, C.P.; Vento, M.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome - 2016 Update. Neonatology 2017, 111, 107–125. [CrossRef]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Te Pas, A.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; Simeoni, U.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome - 2019 Update. Neonatology 2019, 115, 432–450. [CrossRef]
- Fabiano, A.; Gazzolo, D.; Zimmermann, L.J.I.; Gavilanes, A.W.D.; Paolillo, P.; Fanos, V.; Caboni, P.; Barberini, L.; Noto, A.; Atzori, L. Metabolomic Analysis of Bronchoalveolar Lavage Fluid in Preterm Infants Complicated by Respiratory Distress Syndrome: Preliminary Results. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2011, 24 Suppl 2, 55–58. [CrossRef]
- Giambelluca, S.; Verlato, G.; Simonato, M.; Vedovelli, L.; Bonadies, L.; Najdekr, L.; Dunn, W.B.; Carnielli, V.P.; Cogo, P. Chorioamnionitis Alters Lung Surfactant Lipidome in Newborns with Respiratory Distress Syndrome. Pediatr. Res. 2021, 90, 1039–1043. [CrossRef]
- Christopoulou, I.; Kostopoulou, E.; Matzarapi, K.; Chasapi, S.A.; Spyroulias, G.A.; Varvarigou, A. Identification of Novel Biomarkers in Late Preterm Neonates with Respiratory Distress Syndrome (RDS) Using Urinary Metabolomic Analysis. Metabolites 2023, 13, 644. [CrossRef]
- Widström, A.M.; Christensson, K.; Ransjö-Arvidson, A.B.; Matthiesen, A.S.; Winberg, J.; Uvnäs-Moberg, K. Gastric Aspirates of Newborn Infants: pH, Volume and Levels of Gastrin- and Somatostatin-like Immunoreactivity. Acta Paediatr. Scand. 1988, 77, 502–508. [CrossRef]
- Scott, C.R.; Teng, C.C.; Sagerson, R.N.; Nelson, T. Amino Acids in Amniotic Fluid: Changes in Concentrations during the First Half of Pregnancy. Pediatr. Res. 1972, 6, 659–663. [CrossRef]
- Sano, M.; Nagura, H.; Ueno, S.; Nakashima, A. Amino Acid Composition of Amniotic Fluid during the Perinatal Period Reflects Mother’s Fat and Carbohydrate Intake. Nutrients 2021, 13, 2136. [CrossRef]
- Holeček, M. Serine Metabolism in Health and Disease and as a Conditionally Essential Amino Acid. Nutrients 2022, 14, 1987. [CrossRef]
- de Paz-Lugo, P.; Lupiáñez, J.A.; Meléndez-Hevia, E. High Glycine Concentration Increases Collagen Synthesis by Articular Chondrocytes in Vitro: Acute Glycine Deficiency Could Be an Important Cause of Osteoarthritis. Amino Acids 2018, 50, 1357–1365. [CrossRef]
- Ghosh, S. Metabolomic Studies for Metabolic Alterations Induced by Non-Steroidal Anti-Inflammatory Drugs: Mini Review. Biomolecules 2021, 11, 1456. [CrossRef]
- Mao, X.; Zeng, X.; Qiao, S.; Wu, G.; Li, D. Specific Roles of Threonine in Intestinal Mucosal Integrity and Barrier Function. Front. Biosci. Elite Ed. 2011, 3, 1192–1200. [CrossRef]
- Metwaly, S.; Côté, A.; Donnelly, S.J.; Banoei, M.M.; Lee, C.H.; Andonegui, G.; Yipp, B.G.; Vogel, H.J.; Fiehn, O.; Winston, B.W. ARDS Metabolic Fingerprints: Characterization, Benchmarking, and Potential Mechanistic Interpretation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L79–L90. [CrossRef]
- Vettore, L.A.; Westbrook, R.L.; Tennant, D.A. Proline Metabolism and Redox; Maintaining a Balance in Health and Disease. Amino Acids 2021, 53, 1779–1788. [CrossRef]
- Cools, P. The Role of Escherichia Coli in Reproductive Health: State of the Art. Res. Microbiol. 2017, 168, 892–901. [CrossRef]
- Hindson, V.J. Serine Acetyltransferase of Escherichia Coli: Substrate Specificity and Feedback Control by Cysteine. Biochem. J. 2003, 375, 745–752. [CrossRef]
- Raschetti, R.; Centorrino, R.; Letamendia, E.; Benachi, A.; Marfaing-Koka, A.; De Luca, D. Estimation of Early Life Endogenous Surfactant Pool and CPAP Failure in Preterm Neonates with RDS. Respir. Res. 2019, 20, 75. [CrossRef]
- Bell, E.F.; Hintz, S.R.; Hansen, N.I.; Bann, C.M.; Wyckoff, M.H.; DeMauro, S.B.; Walsh, M.C.; Vohr, B.R.; Stoll, B.J.; Carlo, W.A.; et al. Mortality, In-Hospital Morbidity, Care Practices, and 2-Year Outcomes for Extremely Preterm Infants in the US, 2013-2018. JAMA 2022, 327, 1–16. [CrossRef]
- Nanda, D.; Nangia, S.; Thukral, A.; Yadav, C.P. A New Clinical Respiratory Distress Score for Surfactant Therapy in Preterm Infants with Respiratory Distress. Eur. J. Pediatr. 2020, 179, 603–610. [CrossRef]
- Acharjee, A.; Hazeldine, J.; Bazarova, A.; Deenadayalu, L.; Zhang, J.; Bentley, C.; Russ, D.; Lord, J.M.; Gkoutos, G.V.; Young, S.P.; et al. Integration of Metabolomic and Clinical Data Improves the Prediction of Intensive Care Unit Length of Stay Following Major Traumatic Injury. Metabolites 2021, 12, 29. [CrossRef]
- Rhee, E.P.; Clish, C.B.; Ghorbani, A.; Larson, M.G.; Elmariah, S.; McCabe, E.; Yang, Q.; Cheng, S.; Pierce, K.; Deik, A.; et al. A Combined Epidemiologic and Metabolomic Approach Improves CKD Prediction. J. Am. Soc. Nephrol. 2013, 24, 1330. [CrossRef]
- Ramaswamy, V.V.; Bandyopadhyay, T.; Abiramalatha, T.; Pullattayil S, A.K.; Szczapa, T.; Wright, C.J.; Roehr, C.C. Clinical Decision Thresholds for Surfactant Administration in Preterm Infants: A Systematic Review and Network Meta-Analysis. EClinicalMedicine 2023, 62, 102097. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).