1. Introduction
In the last 40-50 years, drought conditions have become more prevalent globally due to climate change (Wilhite, 2016), and further increment of drought frequency is predicted (O'Gorman and Schneider, 2009; Dai, 2011; Spinoni et al., 2018). In areas where climate change will cause more droughts in the future, the composition of forests should be gradually changed to species with greater tolerance to drought stress (Gustafson and Sturtevant, 2013). Some studies indicated that repeated droughts in the same area could cause changes in the species composition of forests towards reduced diversity and the emergence of single-species tree stands (Grossiord et al., 2015; Peters et al., 2015). On the other hand, during the last decade forest management has supported tree mixing species with various resistance and ecological traits to mitigate adverse growth effects and reduce drought stress (Steckel et al., 2020). For example, in drought-prone sites in the Mediterranean region the pine–beech mixed forest was indicated to be an effective stand model for climate change (Gonzalez et al., 2017). According to Kotlarz et al. (2018) Q. robur monocultures are more sensitive to drought than mixed stands with Q. robur as the dominant species, while having a ~20% admixture of Q. robur and P. sylvestris significantly reduced deterioration of vegetation indices during short-term drought.
Many studies have been conducted on the resistance of mixed stands to water stress during drought, focusing mainly on the hydraulic properties of trees as a reaction to drought events (Croisé et al., 2001; Nardini and Luglio, 2014; Nardini et al., 2014; Doffo et al., 2017). However, trees possess plenty of belowground adaptations (e.g., root architecture and depth) that likely interact with soil properties and soil biota to deal with drought effects (Meier and Leuschner, 2008; Phillips et al., 2016; Chitra-Tarak et al., 2018). Thus, taking into account only aboveground traits of trees without consideration of belowground traits may lead to incorrect projections of the consequences of drought. Little is known on the influence of mixing various tree species on soil hydrology, and consequently the indirect impact of trees inside mixed stands on drought prevention. The stand species composition may affect soil physical and chemical properties through litterfall, constituting the litter layer on the soil surface, which comprises the boundary between the atmosphere and mineral soil in forest ecosystems (Osman, 2013). The litter layer is the first forest soil horizon that intercepts throughfall and its properties decide how much water will evaporate, be stored, or percolate into the mineral soil after each rainfall. The hydrological importance of the litter layer results from its control over the transfer of water and energy between the sub-canopy atmosphere and the mineral soil (Schaap et al., 1997). The litter layer has a low thermal conductivity and acts as an insulator between the atmosphere and mineral soil, reducing the temperature, temperature fluctuations, and thermal gradient in the mineral soil (Bonan and Shugart, 1989). Some studies have demonstrated that litter and detritus layers can retain significant amounts of throughfall, thus affecting the flow into mineral soil and the water supply to vegetation (Mader and Lull, 1968; Walsh and Voight, 1997; Putuhena and Cordery, 1996). Greiffenhagen et al. (2006) stated that these layers contain about 20% of the total water quantity available to plants up to a depth of 1 m with high special variability. The hydrological properties of the litter layer are influenced by the type of vegetation and the stand species composition. For example, Gerrits (2010) showed that cedar litter has only about one-half of the water storage capacity (1.0 mm) of beech litter (1.8 mm). Ilek et al. (2015) stated that spruce litter (Picea abies) retains nearly three times more water than beech litter (Fagus sylvatica) and almost twice as much as fir litter (Abies alba). For pine litter (Pinus radiata) and eucalyptus litter (Eucalyptus spp.), Putuhena and Cordery (1996) recorded the water storage capacity of 2.3 and 1.4 mm, respectively. However, little is known about how the degree of mixing of forest-forming species affects the litter hydrology and how water properties of the litter layer change with time, i.e., over decomposition processes taking place in the surface soil horizon. Thus, our study aimed to evaluate 1) the effect of mixing litterfall of Scots pine and sessile oak on the water storage capacity of the litter layer, and 2) how the mixing degree of both species changes the water storage capacity and bulk density while progressing the decomposition process. So far, no research has been conducted on the relationship between the effect of mixing litterfall of various trees with water storage capacity. We analyzed how differences in the chemical composition of needles and leaves affect water retention capacity to better understand water retention dynamics in the context of mixed coniferous-deciduous forests. Knowledge concerning the influence of stand species composition on the litter hydrology is important for understanding the water cycle in forest ecosystems and can be used in forest management practice under climate change conditions.
2. Materials and methods
2.1. Study site and sampling
A field experiment was set up in the Experimental Forest of the Poznań University of Life Sciences in Murowana Goślina (Poland), in the Potasze forest district (division 73a), situated in a temperate climate area (
Figure 1a). The average annual temperature at the site is 8.5℃ and the average annual precipitation is 500 mm. Dominant canopy trees include sessile oak (
Quercus petraea [Matt.] Liebl.) and, in some places, Scots pine (
Pinus sylvestris L.)[1]. Soil on the study area has been classified as Brunic Arenosol. Research material in the form of pine needles and oak leaves was collected in uniform pine or oak fragments of the stand. Thanks to this, it was possible to collect needles or leaves from the litter layer, free from admixtures of other tree species. Needles and leaves were collected using a brush at the end of November 2020, promptly after the fall of fresh organic matter.
2.2. Experiment design
The collected pine needles and oak leaves were air-dried in laboratory conditions. Then, 90 artificial samples were prepared from them, containing litter consisting of pine needles (100% pine), oak leaves (0% pine), or needles and leaves in various degrees of mixing, i.e., with a mass fraction of pine needles of 20, 40, 60 and 80% (
Figure 1b). We prepared 18 samples for each variant involving pine needles in the litter. The samples were prepared in square plastic containers, designed so that rainwater could enter the container through the top and flow out through its bottom during the field experiment. A fine-meshed plastic mesh was placed on the holes cut in the bottom of the containers and in the covers, which on the one hand allowed water to flow freely through the samples while also preventing various types of contaminants from entering the containers, including litterfall reaching the forest floor (
Figure 1c). Each sample contained 20 g of organic matter. When weighing 20 g of needles and leaves their initial moisture content was considered, which was approximately 10% (moisture content was determined after drying a portion of the organic material at 105°C). The weighed material was first placed in beakers with water until it fell to the bottom, i.e., until it reached a density of > 1 g cm
-3 (~ 7 days). This time was considered the moment of filling the internal capillarity of needles and leaves (Ilek et al. 2019). Once this level was reached, the needles and leaves were placed in square containers and immersed in water again. Then we mixed the material under water to ensure a random distribution of needles and leaves within each container (especially in the mixed oak-pine samples). Afterwards the container was quickly pulled out of the water with the organic material at the bottom. In the next stage, containers with prepared litter samples were subjected to a gravitational drainage process lasting approximately 1 hour. After completing this process, the containers with the samples were weighed to determine the wet mass of the samples. Next, we calculated the volume of each sample by measuring the height of the sample formed in the container and multiplying the height by the surface area of the container's base. Based on these measurements, we calculated the initial water storage capacity (mm) and bulk density (g cm
-3) of the artificial litter samples (with undecomposed organic matter) according to the formulas:
where 10 is a factor of conversion into mm of H
2O.
The prepared samples were transported to the forest, where they were placed in the soil in such a way that the lids of the containers were at the level of the forest floor (
Figure 1d). After every three months of decomposition 18 samples were removed from the soil (3 samples per each variant) and transported to the laboratory. These samples were immersed in water for seven days. After removing the samples from the water and completing the gravity drainage process, we calculated the water storage capacity and bulk density of the samples at a given degree of decomposition analogously to formulas 1 and 2. We also estimated the percentage changes in water storage capacity (ΔWSC), bulk density (ΔBD), mass and volume losses of samples after each three months of decay. We assumed a maximum decomposition time of 15 months (5 sampling dates).
2.3. Chemical analyses
We determined the carbon, nitrogen content, C:N ratio, and the percentages of lignin, cellulose, extractives, and ash for pure oak and pine samples. These tests were carried out on fresh samples and at each of the five stages of decomposition. Carbon and nitrogen contents, as well as the C:N ratio were additionally determined for samples with different shares of pine needles (20-80%) at each stage of decay. Before the determination of chemical components of leaves and needles the samples were ground in a Fritsch Pulverisette 15 laboratory mill (Fritsch GmbH, Germany). Carbon and nitrogen contents were determined using the Leco analyzer (Leco, St. Joseph, MI, USA). Based on the carbon and nitrogen contents the C:N ratio was calculated. Cellulose content was determined according to Seifert’s method (Browning, 1967) using a mixture of acetyl acetone, 1.4-dioxane and hydrochloric acid to isolate cellulose. Acid-insoluble lignin content was assessed according to the T 222 om-06 standard TAPPI method (Technical Association, 2006) using 72% sulfuric acid to hydrolyze and solubilize carbohydrates. Extractives soluble in alcohol (96% ethanol) were determined according to the T 204 cm-97 standard (Technical Association, 2007), whereas ash was tested according to T 211 om-02 (Technical Association, 2002). All the chemical analyses were repeated with three replicates for each sample of leaves and needles.
2.4. Statistical analyses
The statistical analysis and associated graphics were performed in Statistica 13.3 PL (StatSoft Inc.) and the programming language R (R Core Team, 2020) in the R Studio (RStudio Team, 2020). Significant differences were tested by one-way ANOVA and post-hoc Tukey’s test after checking normality of distribution with the Shapiro–Wilk test and the equality of variance with Levene’s test. In the case of the non-parametric nature of the data, the Kruskal-Wallis test was applied. We adopted a general linear model (GLM) to investigate the effect of the initial share of pine needles in the litter layer and the time of the decomposition process on the C:N ratio, percentage changes in the bulk density and water storage capacity of the samples. Principal component analysis (PCA) was employed to examine the dependence between the share of pine needles in litter samples and their properties. All the tests were performed at a significance level of 0.05.
3. Results
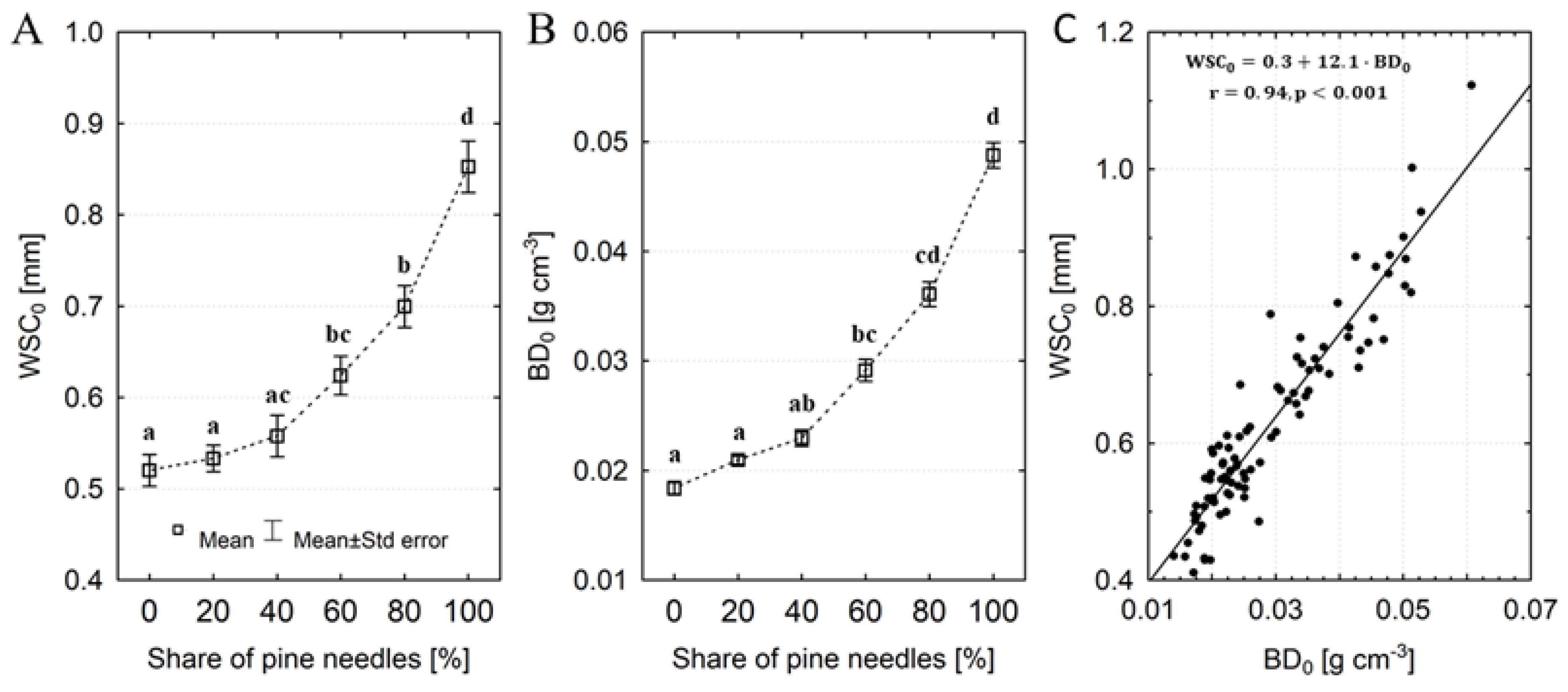
The initial water storage capacity (
WSC0) of litter samples with undecomposed organic matter ranged from 0.41 to 1.12 mm (on average 0.63 ± 0.02 mm) (
Figure 2a).
WSC0 of pure pine litter was on average 64% greater than that of pure oak litter. We observed that the 20 and 40% admixture of pine needles in the litter did not significantly increase its water capacity compared to pure oak litter. In turn, even a 20% admixture of oak leaves in the litter caused a reduction in its water storage capacity on average by ~22%. With a 40% share of oak leaves,
WSC0 of the litter was ~37% lower than that of pure pine litter (
Figure 2a). The initial bulk density (
BD0) of artificial litter samples increased with the share of pine needles (
Figure 2b).
BD0 of pure pine litter was 165% greater than that of pure oak litter samples. We observed a strong linear relationship between
WSC0 and
BD0 of litter samples (
Figure 2c).
Freshly fallen, undecomposed oak leaves and pine needles differed in their chemical properties. Pine needles contained on average 7.5% more carbon, 50% more nitrogen, 70% more cellulose, and 133% extractives than undecomposed oak leaves, which, compared to the needles, had a higher C:N ratio (on average by 45%) and contained approximately 48% and 164% more lignin and ash, respectively (
Table 1). The chemical properties of pine needles and oak leaves changed depending on their decomposition time. While the carbon content remained relatively constant in both species during the 15-month decomposition process, the nitrogen, lignin, and ash contents increased on average by ~ 92, 115, and 190% in pine and by ~ 63, 43, and 79% in oak leaves (
Table 1). The cellulose content did not change significantly in oak leaves during the decomposition process. In the case of pine, after 15 months of decomposition the cellulose content decreased by ~ 61%. In both species we also observed a decrease in C:N and extractives (greater in pine than oak) with the time of the decomposition process. Interestingly, despite the initial differences in the contents of cellulose and lignin in pine needles and oak leaves, after approximately nine months of decomposition the levels of these compounds in both species began to become similar.
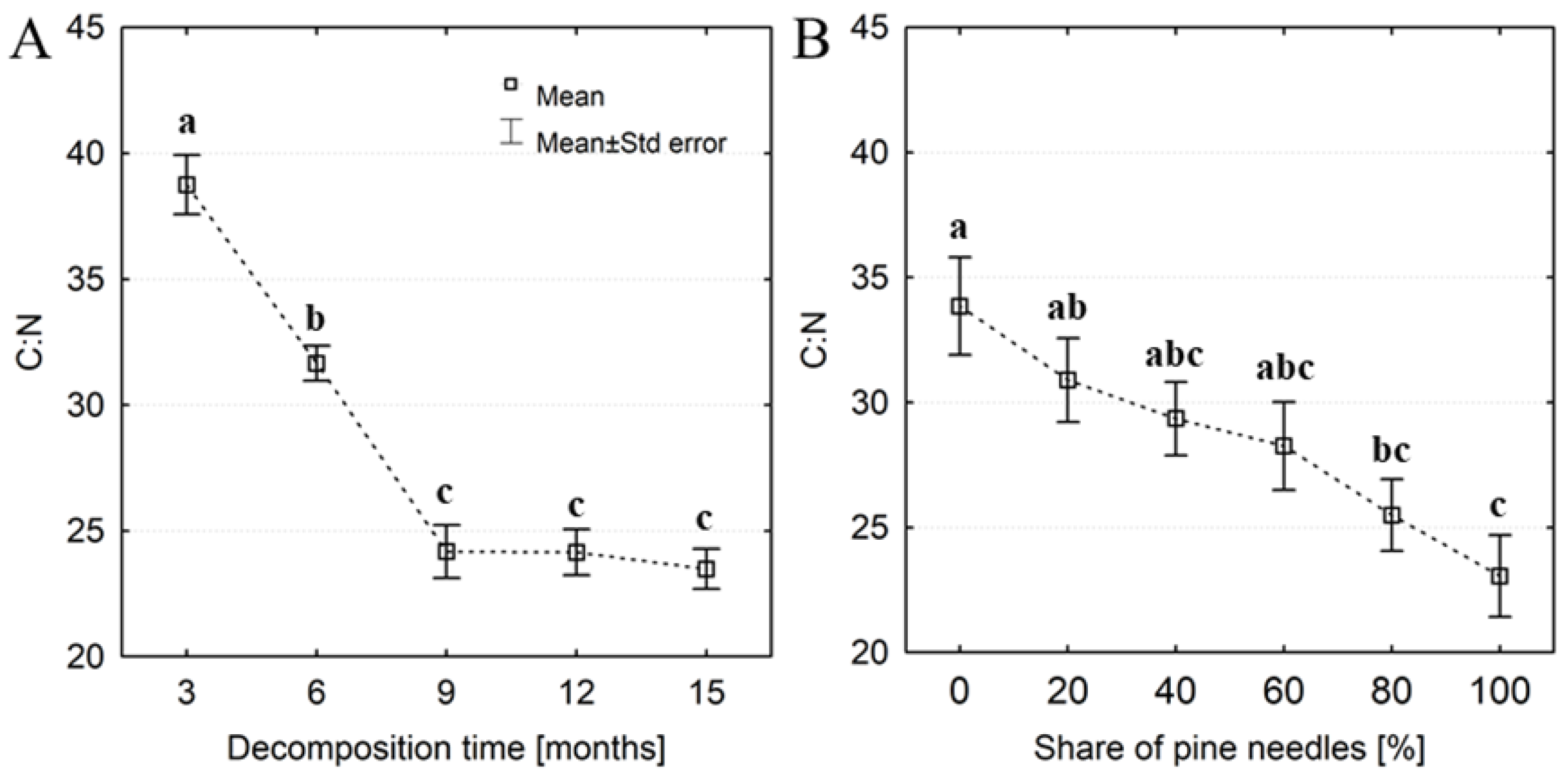
The C:N ratio of samples decreased clearly during the first months of decomposition and stabilized after approximately 9 months of decay, reaching an average of ~ 24 (
Figure 3a). We observed that C:N decreased when the initial proportion of pine needles in the samples increased, i.e., C:N of pure pine samples was approximately 32% lower than that of pure oak (
Figure 3b). The influence of decomposition time and the share of pine needles in the samples on the C:N ratio was confirmed by GLM analysis (
Table 2). The litter mass loss progressed with the decomposition process in all variants of the initial content of pine needles in the samples (
Table 3). After 15 months of decomposition the mass loss amounted to an average of 63%, with the largest loss (~ 72%) found in samples with 40% pine needles and the lowest in samples with 80 and 100% oak leaves. After the first 3 months of decomposition the lowest mass losses were recorded in samples with a predominant share of oak, while the largest (over 20%) were found in samples containing 80 and 100% of pine needles. Besides litter mass loss, we also observed changes (on average ~ 40%) in the volume of samples over the decomposition process (
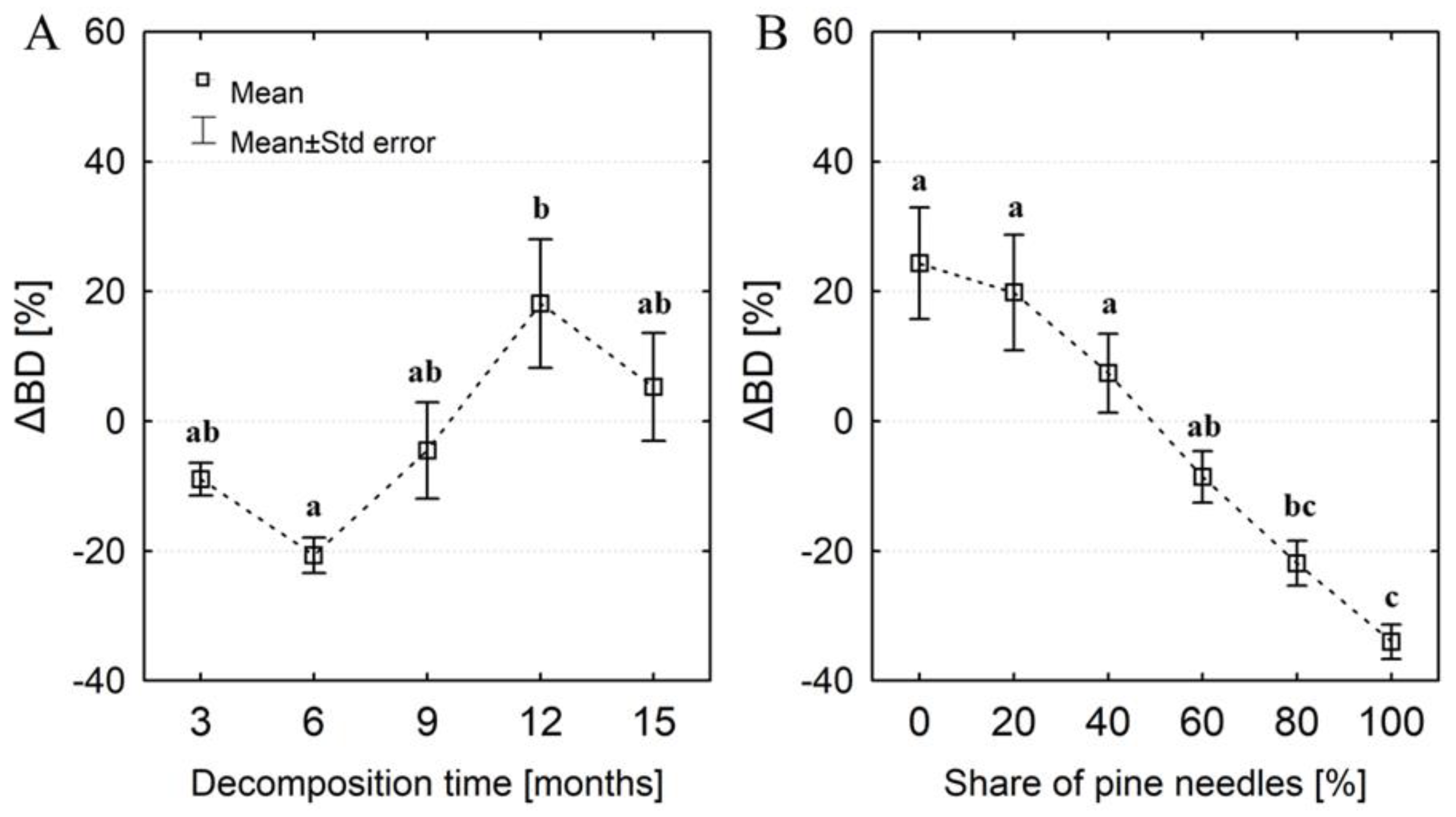
Table 3). After the first 3 months of decomposition volume losses in samples varying in the share of pine needles were below 10%. After 15 months of decay the volume loss amounted to an average of 63%, with the largest loss (>70%) found in samples with a predominant share of oak leaves (60-100%) and the lowest (~ 45%) in pure pine samples. As a result of the loss of mass and volume of samples along with the advancement of the decomposition process, there were also changes in their bulk density (ΔBD). Interestingly, throughout the entire period of the study the bulk density of the tested samples both decreased and increased. We observed that the bulk density of samples (regardless of the pine and oak mixing) in the first nine months of decay decreased on average ~11%, while after 12 and 15 months of decay the bulk density increased about 12% (
Figure 4a). In samples with a predominant pine share their density mainly decreased. In samples with a predominant proportion of oak leaves there was primarily an increase in density in relation to the initial bulk density of undecomposed litter (
Figure 4b). The GLM analysis showed that changes in the bulk density of the samples were influenced by both the decomposition time and the initial share of pine needles in the samples (
Table 2).
In general, samples with a low C:N ratio and a predominant share of oak leaves had the highest water storage capacity (WSC). The lowest WSC was found in samples with a high C:N ratio and a predominant share of pine needles (
Figure 5). We observed changes in water storage capacity (ΔWSC) in relation to the initial water storage capacity (WSC
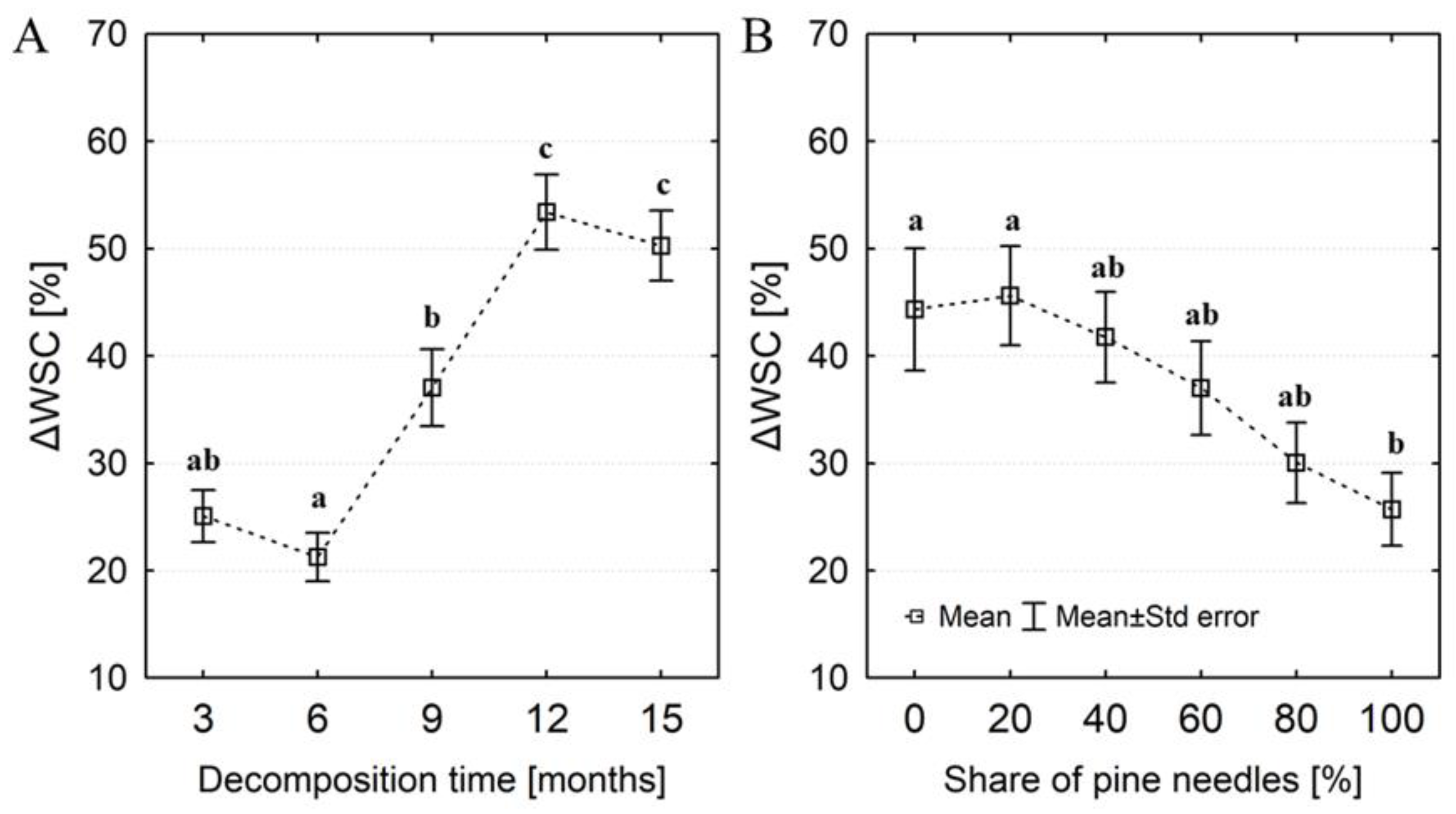
0) with the decomposition time. Regardless of the pine share, the ΔWSC increased on average by ~ 23% in the first 6 months of decay (
Figure 6a), and after 12 and 15 months the water storage capacity was over 52% greater than WSC
0. The highest increase in water storage capacity (>40%) occurred in samples with a predominant share of oak leaves, while the lowest (about 28% on average) was recorded in samples containing 80 and 100% pine needles (
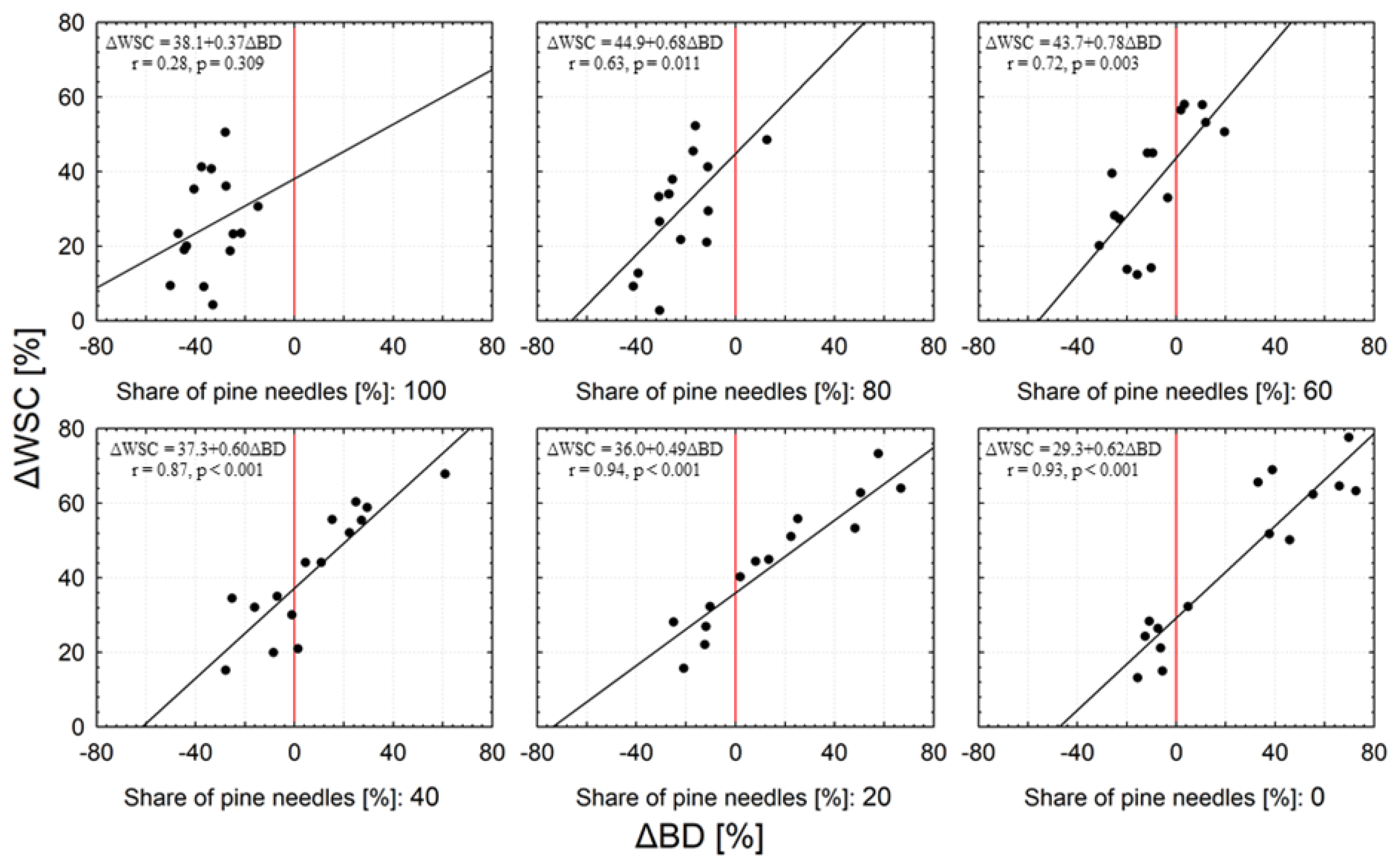
Figure 6b). The GLM analysis confirmed the influence of the initial share of pine needles and decomposition time on the ΔWSC. We found a clear linear relationship between ΔWSC and ΔBD (
Figure 7). In most samples with a predominant share of pine, their density mainly decreased, even about 50%. In turn, the bulk density of samples with a predominant proportion of oak leaves was primarily increased (even about 80%), and a decrease in density did not exceed 40%. Throughout the entire range of changes in the bulk density of the tested samples we noted an increase in water capacity compared to the WSC
0. The greatest increases in WSC (reaching 80%) were found in samples with a predominant amount of oak leaves. ΔWSC of samples with predominant pine share did not exceed 60%.
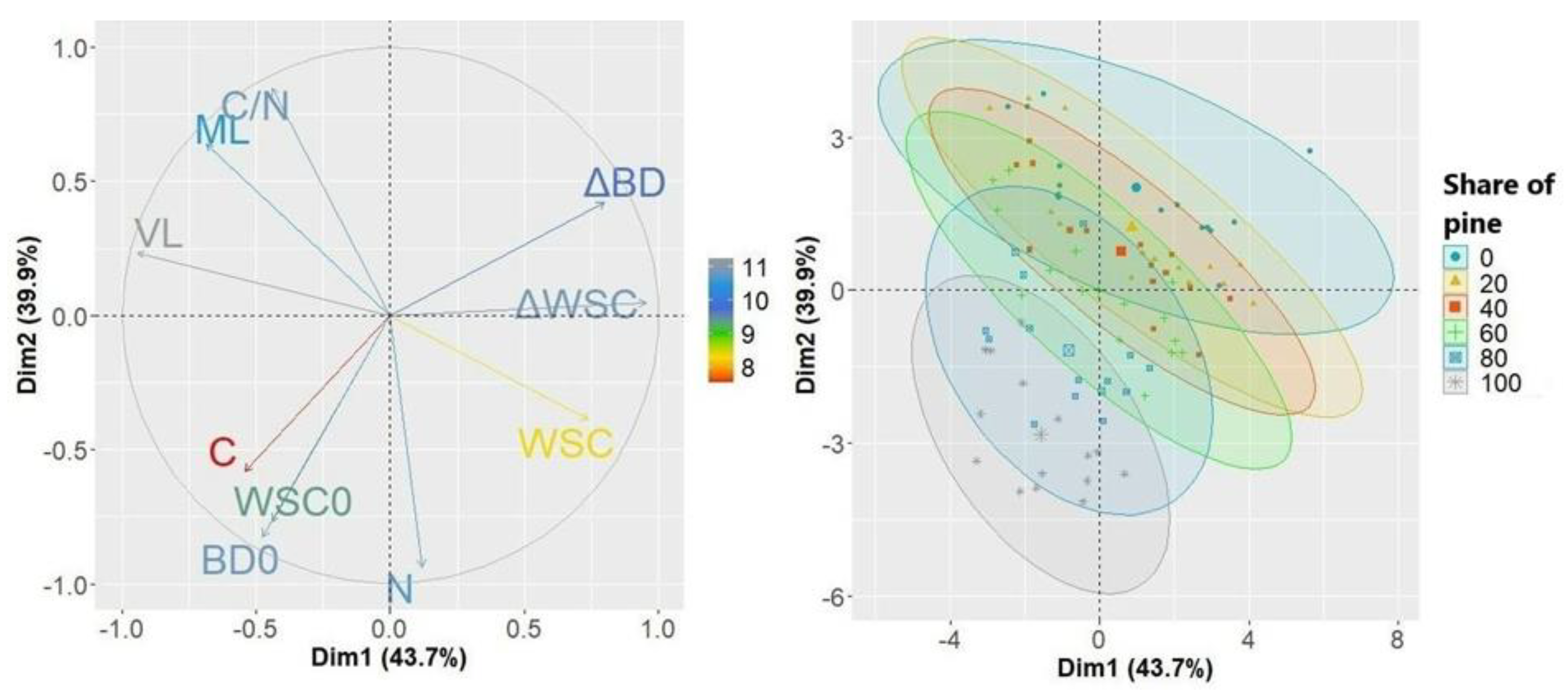
A projection of the variables on the factor plane clearly demonstrated the relationship of the share of species in the litter with their properties (
Figure 8). PCA analysis explains 84% of the variability of the examined features. Factor 1 is related to water storage capacity, while factor 2 is related to the quality of organic matter expressed by the C:N ratio. The PCA analysis confirms distinctiveness of the litter with a predominance of pine needles (80 and 100% of pine needles). These two groups were characterized by the highest initial water storage capacity, while the samples with the lowest proportion of needles were characterized by the highest changes in water storage capacity (ΔWSC) and changes in their bulk density (ΔBD).
4. Discussion
Our 15-month experiment confirmed that the degree of mixing of pine needles and oak leaves and their decomposition rate are very closely related to water storage capacity. Particular sublayers of the forest floor usually vary in their water storage capacity due to differences in bulk density, porosity, and advancement of organic matter decay, i.e., the semi-decomposed detritus layer usually has higher density and water storage capacity than the undecomposed litter layer (Zhang et al., 2006; Ilek et al. 2015). It corresponds with our results. We observed that at each stage of the decomposition process the water storage capacity of samples increased compared to undecomposed litter samples, regardless of the pine share (Figs. 6-7). The greatest increase in the water storage capacity was found in samples with a predominant share of oak (Figs. 6b and 7). It could be related to the fact that during decomposition the leaves begin to stick more closely to one another. The relatively large surface area of oak leaves may cause more water to accumulate between adjacent leaves, making it difficult to drain, which probably increased the water storage capacity of samples with a high proportion of oak leaves. The differences in the shape of individual litter components may affect the distribution of water flow channels in the forest floor (Sato et al., 2004). Thus, the presence of pine needles between the leaves may prevent the leaves from sticking to each other (especially when wet), which on the one hand reduces the water storage capacity of the litter layer and on the other hand it may result in increased water infiltration into the soil profile. Some studies indicated that the water storage capacity of the litter layer was greater in mixed stands than in pure stand types (e.g. Zheng et al., 2019; Su and Liu, 2022). Our results showed that the water storage capacity of mixed litter does not always exceed that of the pure litter layer. In the case of undecomposed litter the highest water storage capacity was recorded for pure pine litter. Admixture of the oak leaves caused a reduction of the water storage capacity (
Figure 2a). On the other hand, the pure oak undecomposed litter had the lowest water storage capacity and the admixture of pine needles did not cause a significant increase in its water storage capacity. In the case of semi-decomposed samples the lowest water storage capacity was found in pure pine samples, while the admixture of oak leaves only slightly increased the water storage capacity (
Figure 6b). The pure oak semi-decomposed samples had the highest water storage capacity and the admixture of pine did not significantly change its water storage capacity. Thus, it may be concluded that the water storage capacity of the litter layer in mixed pine-oak stands depends more on the degree of decomposition of organic matter than the proportion of mixing in the litterfall of both tree species.
The properties of detritus in the forest floor depend on the rate of decomposition processes, which in turn is important in water retention (Hashimi et al., 2023). Its degree of decay is heterogeneous both vertically (gradually increasing closer to mineral soil), and horizontally where decomposition is dependent on drivers such as climate and canopy type (Raaflaub and Valeo, 2008). The dominant factor, on which decomposition processes depend is the quality of the supplied detritus, especially litter (Lehmann and Kleber, 2015; Canessa et al., 2020; Jílkova et al., 2020). Organic matter decomposition processes include leaching, microbial colonization, and fragmentation (Lin and Webster, 2014). During detritus decomposition, microbial nutrient processes, i.e., immobilization and mineralization, play an important role in changes in detritus nutrient content and stream nutrient concentrations (Błońska et al., 2021; Gulis and Suberkropp, 2003). Our research confirmed a strong relationship between water storage capacity and the C:N ratio expressing the quality of organic matter. A high ratio corresponds to a higher organic C content and a lower N content, suggesting greater stability of organic C in the soil, while a low ratio corresponds to a lower organic C content and higher N content, indicating a greater degree of decomposition of organic matter in the soil (He et al., 2023).
The bulk density of the forest floor containing the decomposing organic matter usually increases with depth (Ilek et al., 2015). However, in our study we observed both increasing and decreasing changes in bulk density over 15 months of decay (
Figure 4 and 7). The low density of oak undecomposed litter (
Figure 2b) could have been caused by the fact that leaf litter has a large surface area and often “curls” creating air spaces within the litter layer. This produces less mass per volume than in a needled pine litter layer (Ottmar and Andreu, 2007). The increase in the density of semi-decomposed samples with a predominance of oak leaves was probably related to a lower loss of mass and a greater loss of volume in these samples during decomposition (
Table 3). In turn, the decreasing density of semi-decomposed samples with a predominant share of pine was probably related to a greater decrease in mass than in volume in the initial phase of decomposition.
In general, the water storage capacity of the forest floor is related to bulk density, i.e., the water storage capacity increases when density increases (Ilek et al., 2017). In our investigations, despite decreases in density in the initial stage of decomposition, the water storage capacity of semi-decomposed litter increased. This suggests that the hydrological properties of the litter layer are closely related not only to physical properties, but also to the change in its chemical properties during decomposition. Thus, the water storage capacity of the litter layer should also be considered at the molecular level of the structure of leaves and needles. This is because needles and leaves are chemically complex molecular structures with specific hygroscopic properties that affect their ability to water holding capacity (Percy et al., 1993; Wang et al., 2015; Beluns et al., 2022). When considering the influence of the chemical composition of leaves and needles on the water storage capacity, it should be considered that there is a different chemical structure of the outer and inner layers of leaf and needle tissue. Due to the above-mentioned chemical differences in individual layers of leaves and needles, they have probably different hygroscopic properties. As a result, degradation of individual layers of leaf and needle tissue (first external, then internal) changes the ability of water to be stored on the litter layer during its decay (Shi et al., 2011). The leaves and needles are covered with an outer layer of an extractive substance, e.g. waxes (Hanover and Reicosky, 1971; Jeffree, 2006; Muhammad et al., 2020; Grünhofer et al., 2022), which consists of hydrophobic organic compounds, mainly straight-chain aliphatic hydrocarbons (Samuels, 2008; Steinbauer et al., 2009). This composition of waxes makes them responsible for maintaining wettability of the leaf surface (Neinhuis and Barthlott, 1997). In our study a significant reduction in the percentage of extractive substances both for leaves and needles was noted after the first 3 months of their decay. A significant part of detected extractives are waxes, found in the outer layer of needle and leaf tissue (Muhammad et al., 2020). According to the literature considering wettability of leaves and needles, after removing wax from the surface of aging leaves and needles, the contact angle decreased significantly and they became easily wettable (Gou and Guo, 2019). The presence of waxes on the leaf surface tended to reduce their wettability (Brewer and Nunez, 2007). However, in the case of the tests described this significant loss of extractive substances (waxes) was not accompanied by an increase in water storage capacity. This may suggest that the extractive substances do not significantly affect the tested properties. However, it is worth focusing on the chemical components of leaves and needles that appear under the layer of waxes, lignin and cellulose. These components, due to differences in structure, differ in their hydrophobicity, e.g. cellulose is more and lignin less hydrophilic (Skaar, 1984). The sorption of moisture by each cell wall polymer not only depends on its hydrophilic nature, but also accessibility of water to the polymer’s hydroxyl groups. Most, if not all, of the hydroxyl sites in lignin are accessible to moisture. The non-crystalline portion of cellulose (approximately 40%) and the surfaces of the crystallites are accessible to moisture, whereas the crystalline part (approximately 60%) is not (Stamm, 1964; Sumi et al., 1964). Therefore, considering the water storage capacity of the litter layer including needles and leaves, attention should be paid to the content of these components. It should be noted that the content of cellulose in needles decreased gradually, with the greatest decrease observed between 6 and 9 months of decay (
Table 1). Only small changes were noted during the decomposition in the percentage of leaf cellulose. In the case of lignin a different phenomenon was observed, as the lignin content gradually increased in both needles and leaves. However, in the case of needles it can indicate the time when the greatest growth occurred (from the 3rd to the 6th month). In the case of leaves, the decline was rather constant. Finally, after 15 months of decomposition the contents of cellulose and lignin in the needles and leaves were comparable. This may indicate their similar hygroscopic properties. Differences in the hygroscopic properties of needles and leaves after decomposition can only be caused by the difference in the share of extractive substances.
5. Conclusion
This study found that the hydrological properties of litter layer depend on the proportion of pine needles and oak leaves, their decomposition time, and their chemical properties. Results revealed that the water storage capacity of the litter layer increased as it decomposed and with the proportion of oak leaves. The study suggests promoting mixed-species stands, particularly with a predominance of deciduous species, can contribute to enhanced water storage capacity and improved ecological benefits.
Author Contributions
Conceptualization, A.I. and M.Z.; methodology, A.I. and M.Z.; validation, A.I.; formal analysis, A.I., E.B. and M.Z.; investigation, A.I., E.B., K.M. and A.K.; resources, A.I., E.B., A.K. and M.Z.; data curation, A.I.; writing—original draft preparation, A.I., E.B., K.M. and M.Z.; writing—review and editing, A.I., E.B., K.M., A.K. and M.Z.; visualization, A.I. and E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Beluns, S.; Platnieks, O.; Sevcenko, J.; Jure, M.; Gaidukova, G.; Grase, L.; Gaidukovs, S. Sustainable Wax Coatings Made from Pine Needle Extraction Waste for Nanopaper Hydrophobization. Membranes 2022, 12, 537. [Google Scholar] [CrossRef] [PubMed]
- Błońska, E.; Piaszczyk, W.; Staszel, K.; Lasota, J. Enzymatic activity of soils and soil organic matter stabilization as an effect of components released from the decomposition of litter. Appl. Soil Ecol. 2020, 157, 103723. [Google Scholar] [CrossRef]
- Bonan, G.B.; Shugart, H.H. Environmental Factors and Ecological Processes in Boreal Forests. Annu. Rev. Ecol. Syst. 1989, 20, 1–28. [Google Scholar] [CrossRef]
- Brewer, C.A.; Nuñez, C.I. Patterns of Leaf Wettability along an Extreme Moisture Gradient in Western Patagonia, Argentina. Int. J. Plant Sci. 2007, 168, 555–562. [Google Scholar] [CrossRef]
- Browning BL (1967). The chemistry of Wood Interscience Publishes. New York-London-Tokyo-Sydney.
- Canessa, R.; Brink, L.v.D.; Saldaña, A.; Rios, R.S.; Hättenschwiller, S.; Mueller, C.W.; Prater, I.; Tielbörger, K.; Bader, M.Y. Relative effects of climate and litter traits on decomposition change with time, climate and trait variability. J. Ecol. 2020, 109, 447–458. [Google Scholar] [CrossRef]
- Chitra-Tarak, R.; Ruiz, L.; Dattaraja, H.S.; Kumar, M.S.M.; Riotte, J.; Suresh, H.S.; McMahon, S.M.; Sukumar, R. The roots of the drought: Hydrology and water uptake strategies mediate forest-wide demographic response to precipitation. J. Ecol. 2017, 106, 1495–1507. [Google Scholar] [CrossRef]
- Croisé, L.; Lieutier, F.; Cochard, H.; Dreyer, E. Effects of drought stress and high density stem inoculations with Leptographium wingfieldii on hydraulic properties of young Scots pine trees. Tree Physiol. 2001, 21, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Dai, A. Drought under global warming: a review. WIREs Clim. Chang. 2011, 2, 45–65, Erratum in: Clim. Change 2012, 3, 617. [Google Scholar] [CrossRef]
- Doffo, G.N.; Monteoliva, S.E.; Rodríguez, M.E.; Luquez, V.M. Physiological responses to alternative flooding and drought stress episodes in two willow (Salix spp.) clones. Can. J. For. Res. 2017, 47, 174–182. [Google Scholar] [CrossRef]
- Gerrits AMJ (2010). The Role of Interception in the Hydrological Cycle; TU Delft. Delft University of Technology 5. http://resolver.tudelft.nl/uuid:7dd2523b-2169-4e7e-992c-365d2294d02e.
- de Andrés, E.G.; Seely, B.; Blanco, J.A.; Imbert, J.B.; Lo, Y.; Castillo, F.J. Increased complementarity in water-limited environments in Scots pine and European beech mixtures under climate change. Ecohydrology 2017, 10. [Google Scholar] [CrossRef]
- Gou, X.; Guo, Z. Superhydrophobic Plant Leaves: The Variation in Surface Morphologies and Wettability during the Vegetation Period. Langmuir 2019, 35, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Greiffenhagen, A.; Wessolek, G.; Facklam, M.; Renger, M.; Stoffregen, H. Hydraulic functions and water repellency of forest floor horizons on sandy soils. Geoderma 2006, 132, 182–195. [Google Scholar] [CrossRef]
- Grossiord, C.; Forner, A.; Gessler, A.; Granier, A.; Pollastrini, M.; Valladares, F.; Bonal, D. Influence of species interactions on transpiration of Mediterranean tree species during a summer drought. Eur. J. For. Res. 2014, 134, 365–376. [Google Scholar] [CrossRef]
- Grünhofer, P.; Herzig, L.; Schreiber, L. Leaf morphology, wax composition, and residual (cuticular) transpiration of four poplar clones. Trees 2021, 36, 645–658. [Google Scholar] [CrossRef]
- Gulis, V.; Suberkropp, K. Leaf litter decomposition and microbial activity in nutrient-enriched and unaltered reaches of a headwater stream. Freshw. Biol. 2002, 48, 123–134. [Google Scholar] [CrossRef]
- Gustafson, E.J.; Sturtevant, B.R. Modeling Forest Mortality Caused by Drought Stress: Implications for Climate Change. Ecosystems 2012, 16, 60–74. [Google Scholar] [CrossRef]
- Hanover, J.W.; Reicosky, D.A. SURFACE WAX DEPOSITS ON FOLIAGE OF PICEA PUNGENS AND OTHER CONIFERS. Am. J. Bot. 1971, 58, 681–687. [Google Scholar] [CrossRef]
- Hashimi, R.; Huang, Q.; Dewi, R.K.; Nishiwaki, J.; Komatsuzaki, M. No-tillage and rye cover crop systems improve soil water retention by increasing soil organic carbon in Andosols under humid subtropical climate. Soil Tillage Res. 2023, 234. [Google Scholar] [CrossRef]
- He, J.; Chen, B.; Xu, W.; Xiang, C.; Kuang, W.; Zhao, X. Driving factors for soil C:N ratio in woody plant communities across northeastern Qinghai-Tibetan Plateau. CATENA 2023, 233. [Google Scholar] [CrossRef]
- Ilek, A.; Kucza, J.; Szostek, M. The effect of stand species composition on water storage capacity of the organic layers of forest soils. Eur. J. For. Res. 2014, 134, 187–197. [Google Scholar] [CrossRef]
- Ilek, A.; Kucza, J.; Szostek, M. The effect of the bulk density and the decomposition index of organic matter on the water storage capacity of the surface layers of forest soils. Geoderma 2016, 285, 27–34. [Google Scholar] [CrossRef]
- Ilek, A.; Szostek, M.; Kucza, J.; Stanek-Tarkowska, J.; Witek, W. The water absorbability of beech (Fagus sylvatica l.) and fir (Abies alba mill.) organic matter in the forest floor. Ann. For. Res. 2014, 62, 21–32. [Google Scholar] [CrossRef]
- Jeffree, CE. The fine structure of the plant cuticle. Annual Plant Reviews 2006, 23, 11–125. [Google Scholar]
- Jílková, V.; Straková, P.; Frouz, J. Foliage C:N ratio, stage of organic matter decomposition and interaction with soil affect microbial respiration and its response to C and N addition more than C:N changes during decomposition. Appl. Soil Ecol. 2020, 152, 103568. [Google Scholar] [CrossRef]
- Kotlarz, J.; Nasiłowska, S.A.; Rotchimmel, K.; Kubiak, K.; Kacprzak, M. Species Diversity of Oak Stands and Its Significance for Drought Resistance. Forests 2018, 9, 126. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Webster, J. Detritus decomposition and nutrient dynamics in a forested headwater stream. Ecol. Model. 2014, 293, 58–68. [Google Scholar] [CrossRef]
- Mader D L, Lull HW (1968). Depth, weight, and water storage of the forest floor in white pine stands in Massachusetts (Vol. 109). Northeastern Forest Experiment Station.
- Meier, I.C.; Leuschner, C. Belowground drought response of European beech: fine root biomass and carbon partitioning in 14 mature stands across a precipitation gradient. Glob. Chang. Biol. 2008, 14, 2081–2095. [Google Scholar] [CrossRef]
- Muhammad, S.; Wuyts, K.; Nuyts, G.; De Wael, K.; Samson, R. Characterization of epicuticular wax structures on leaves of urban plant species and its association with leaf wettability. Urban For. Urban Green. 2020, 47, 126557. [Google Scholar] [CrossRef]
- Nardini, A.; Gullo, M.A.L.; Trifilò, P.; Salleo, S. The challenge of the Mediterranean climate to plant hydraulics: Responses and adaptations. Environ. Exp. Bot. 2014, 103, 68–79. [Google Scholar] [CrossRef]
- Nardini, A.; Luglio, J. Leaf hydraulic capacity and drought vulnerability: possible trade-offs and correlations with climate across three major biomes. Funct. Ecol. 2014, 28, 810–818. [Google Scholar] [CrossRef]
- Neinhuis, C.; Barthlott, W. Characterization and Distribution of Water-repellent, Self-cleaning Plant Surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef]
- O'Gorman, P.A.; Schneider, T. The physical basis for increases in precipitation extremes in simulations of 21st-century climate change. Proc. Natl. Acad. Sci. 2009, 106, 14773–14777. [Google Scholar] [CrossRef] [PubMed]
- Osman KT, Osman KT (2013). Organic matter of forest soils. In: Forest soils. Springer, Cham: 63-76. [CrossRef]
- Ottmar R, Andreu A (2007). Litter and duff bulk densities in the southern United States. Seattle, WA: Fire and Environmental Applications team, USDA Forest Service, Joint Fire Science Program Project 04-2.
- Percy, K.; Jagels, R.; Marden, S.; McLaughlin, C.; Carlisle, J. Quantity, chemistry, and wettability of epicuticular waxes on needles of red spruce along a fog-acidity gradient. Can. J. For. Res. 1993, 23, 1472–1479. [Google Scholar] [CrossRef]
- Peters, M.P.; Iverson, L.R.; Matthews, S.N. Long-term droughtiness and drought tolerance of eastern US forests over five decades. For. Ecol. Manag. 2015, 345, 56–64. [Google Scholar] [CrossRef]
- Phillips, R.P.; Ibáñez, I.; D’orangeville, L.; Hanson, P.J.; Ryan, M.G.; McDowell, N.G. A belowground perspective on the drought sensitivity of forests: Towards improved understanding and simulation. For. Ecol. Manag. 2016, 380, 309–320. [Google Scholar] [CrossRef]
- Putuhena, W.M.; Cordery, I. Estimation of interception capacity of the forest floor. J. Hydrol. 1996, 180, 283–299. [Google Scholar] [CrossRef]
- Raaflaub, L.D.; Valeo, C. Assessing factors that influence spatial variations in duff moisture. Hydrol. Process. 2008, 22, 2874–2883. [Google Scholar] [CrossRef]
- Samuels, L.; Kunst, L.; Jetter, R. Sealing Plant Surfaces: Cuticular Wax Formation by Epidermal Cells. Annu. Rev. Plant Biol. 2008, 59, 683–707. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Kumagai, T.; Kume, A.; Otsuki, K.; Ogawa, S. Experimental analysis of moisture dynamics of litter layers—the effects of rainfall conditions and leaf shapes. Hydrol. Process. 2004, 18, 3007–3018. [Google Scholar] [CrossRef]
- Schaap, M.; Bouten, W.; Verstraten, J. Forest floor water content dynamics in a Douglas fir stand. J. Hydrol. 1997, 201, 367–383. [Google Scholar] [CrossRef]
- Shi H, Wang HX, Li YY. Wettability on plant leaf surfaces and its ecological significance. Shengtai Xuebao/Acta Ecologica Sinica 2011, 31, 4287–4298.
- Skaar C (1984). Wood-water relationships. In The Chemistry of Solid Wood; Rowell, R.M., Ed.; Advances in Chemistry Series; American Chemical Society: Washington, DC, USA; Volume 207: 127–174. [CrossRef]
- Spinoni, J.; Vogt, J.V.; Naumann, G.; Barbosa, P.; Dosio, A. Will drought events become more frequent and severe in Europe? Int. J. Climatol. 2018, 38, 1718–1736. [Google Scholar] [CrossRef]
- Stamm AJ (1964). Wood and Cellulose Science; The Ronald Press Company: New York, NY, USA; p. 549.
- Steckel, M.; del Río, M.; Heym, M.; Aldea, J.; Bielak, K.; Brazaitis, G.; Černý, J.; Coll, L.; Collet, C.; Ehbrecht, M.; et al. Species mixing reduces drought susceptibility of Scots pine (Pinus sylvestris L.) and oak (Quercus robur L., Quercus petraea (Matt.) Liebl.) – Site water supply and fertility modify the mixing effect. For. Ecol. Manag. 2020, 461. [Google Scholar] [CrossRef]
- Steinbauer, M.J.; Davies, N.W.; Gaertner, C.; Derridj, S. Epicuticular waxes and plant primary metabolites on the surfaces of juvenile Eucalyptus globulus and E. nitens (Myrtaceae) leaves. Aust. J. Bot. 2009, 57, 474–485. [Google Scholar] [CrossRef]
- Su, S.; Liu, X. The Water Storage Function of Litters and Soil in Five Typical Plantations in the Northern and Southern Mountains of Lanzhou, Northwest China. Sustainability 2022, 14, 8231. [Google Scholar] [CrossRef]
- Sumi Y, Hale R, Meyer JA, Leopold A, Ranby G. Accessibility of wood and wood carbohydrates measured with tritiated water. Tappi Journal 1964, 47, 621–624.
- Technical Association of the Pulp and Paper Industry (2002). Ash In Wood, Pulp, Paper, And Paperboard: Combustion At 525°C, T 211 om-02; Technical Association of the Pulp and Paper Industry: New York, NY, USA; p. 6.
- Technical Association of the Pulp and Paper Industry (2006). Acid Insoluble Lignin in Wood and Pulp, T 222 cm-06; Technical Association of the Pulp and Paper Industry: New York, NY, USA; p. 5.
- Technical Association of the Pulp and Paper Industry (2007). Solvent Extractives of Wood and Pulp, T 204 cm-97; Technical Association of the Pulp and Paper Industry: New York, NY, USA; p. 12.
- Walsh, R.P.D.; Voigt, P.J. Vegetation Litter: An Underestimated Variable in Hydrology and Geomorphology. J. Biogeogr. 1977, 4, 253. [Google Scholar] [CrossRef]
- Wang H, Shi H, Wang Y. The wetting of leaf surfaces and its ecological significances. Wetting and Wettability 2015, 11, 296–321. [CrossRef]
- Wilhite, D.A. Introduction: Managing drought risk in a changing climate. Clim. Res. 2016, 70, 99–102. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Zhang, Z.; Cui, H.; Lei, Y.; Wang, D.; Sui, J. Water-holding characteristics of litter in different forests at the Lianxiahe watershed. Front. For. China 2006, 1, 413–418. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, L.; Gong, W.; Yang, X.; Kang, Y. Evaluation of the Water Conservation Function of Different Forest Types in Northeastern China. Sustainability 2019, 11, 4075. [Google Scholar] [CrossRef]
Figure 1.
(A) Location of pine needles and oak leaves sampling and field experiment on the decomposition of artificial litter samples. (B) Examples of artificial pine and oak-pine litter samples prepared inside square plastic containers. (C) Top view of the lid of a plastic container with artificial litter dug into the soil. (D) View of all artificial litter samples tested for the influence of decomposition time on water storage capacity of the forest floor.
Figure 1.
(A) Location of pine needles and oak leaves sampling and field experiment on the decomposition of artificial litter samples. (B) Examples of artificial pine and oak-pine litter samples prepared inside square plastic containers. (C) Top view of the lid of a plastic container with artificial litter dug into the soil. (D) View of all artificial litter samples tested for the influence of decomposition time on water storage capacity of the forest floor.
Figure 2.
(A) Initial water storage capacity (WSC0) and (B) bulk density (BD0) of artificial litter samples prepared from fresh fallen pine needles and oak leaves, and (C) the relationship between these two variables. Different letters indicate significant differences between samples with varying shares of pine needles (Tukey’s test, p <0.05).
Figure 2.
(A) Initial water storage capacity (WSC0) and (B) bulk density (BD0) of artificial litter samples prepared from fresh fallen pine needles and oak leaves, and (C) the relationship between these two variables. Different letters indicate significant differences between samples with varying shares of pine needles (Tukey’s test, p <0.05).
Figure 3.
Changes in the C:N ratio of artificial litter samples progressing with the time of organic matter decomposition (A) and in relation to the initial share of pine needles (B). Different letters indicate significant differences in C:N ratio between decomposition times (Tukey's test, p <0.05) or shares of pine needles (Kruskal-Wallis test, p <0.05).
Figure 3.
Changes in the C:N ratio of artificial litter samples progressing with the time of organic matter decomposition (A) and in relation to the initial share of pine needles (B). Different letters indicate significant differences in C:N ratio between decomposition times (Tukey's test, p <0.05) or shares of pine needles (Kruskal-Wallis test, p <0.05).
Figure 4.
Percentage changes in bulk density (ΔBD) of artificial litter samples progressing with the time of organic matter decomposition (A) and in relation to the initial share of pine needles (B). Different letters indicate significant differences in ΔBD between decomposition times or shares of pine needles (Kruskal-Wallis test, p <0.05).
Figure 4.
Percentage changes in bulk density (ΔBD) of artificial litter samples progressing with the time of organic matter decomposition (A) and in relation to the initial share of pine needles (B). Different letters indicate significant differences in ΔBD between decomposition times or shares of pine needles (Kruskal-Wallis test, p <0.05).
Figure 5.
Dependence of water storage capacity (WSC) of the litter layer on the degree of organic matter decomposition (C:N) and the initial share of pine needles in the samples.
Figure 5.
Dependence of water storage capacity (WSC) of the litter layer on the degree of organic matter decomposition (C:N) and the initial share of pine needles in the samples.
Figure 6.
Percentage changes in water storage capacity (ΔWSC) of artificial litter samples progressing with the decomposition time (A) and in relation to the initial share of pine needles in samples (B). Different letters indicate significant differences in ΔWSC between decomposition times or shares of pine needles (Tukey's test, p < 0.05).
Figure 6.
Percentage changes in water storage capacity (ΔWSC) of artificial litter samples progressing with the decomposition time (A) and in relation to the initial share of pine needles in samples (B). Different letters indicate significant differences in ΔWSC between decomposition times or shares of pine needles (Tukey's test, p < 0.05).
Figure 7.
Dependence of percentage changes in water storage capacity (ΔWSC) of samples on changes in their bulk density (ΔBD) during the 15-month decomposition process in relation to the initial share of pine needles in the samples. The red line indicates no change in bulk density.
Figure 7.
Dependence of percentage changes in water storage capacity (ΔWSC) of samples on changes in their bulk density (ΔBD) during the 15-month decomposition process in relation to the initial share of pine needles in the samples. The red line indicates no change in bulk density.
Figure 8.
Projection of variables on the plane of the first and second PCA factors, where ML is mass loss of litter samples during decay, VL is volume loss of litter samples during decay, C is carbon content, N is nitrogen content, WSC0 is initial water storage capacity of undecomposed litter, BD0 is initial bulk density of undecomposed litter samples, WSC is the water storage capacity of semi-decomposed samples, ΔWSC is the percentage change of water storage capacity in relation to WSC0, ΔBD is the percentage change in bulk density regarding BD0.
Figure 8.
Projection of variables on the plane of the first and second PCA factors, where ML is mass loss of litter samples during decay, VL is volume loss of litter samples during decay, C is carbon content, N is nitrogen content, WSC0 is initial water storage capacity of undecomposed litter, BD0 is initial bulk density of undecomposed litter samples, WSC is the water storage capacity of semi-decomposed samples, ΔWSC is the percentage change of water storage capacity in relation to WSC0, ΔBD is the percentage change in bulk density regarding BD0.
Table 1.
Changes in chemical properties of pine needles and oak leaves progressing over the decomposition time (mean values). Different letters indicate significant differences between decomposition times (Kruskal-Wallis test, p <0.05).
Table 1.
Changes in chemical properties of pine needles and oak leaves progressing over the decomposition time (mean values). Different letters indicate significant differences between decomposition times (Kruskal-Wallis test, p <0.05).
| Variable |
C [%] |
N [%] |
C:N |
Cellulose [%] |
Lignin [%] |
Extractives [%] |
Ash [%] |
| Decomposition time [months] |
Pine |
Oak |
Pine |
Oak |
Pine |
Oak |
Pine |
Oak |
Pine |
Oak |
Pine |
Oak |
Pine |
Oak |
| 0 |
47.1a
|
43.9ab
|
1.2a
|
0.8a
|
39.9a
|
57.7a
|
44.4a
|
26.1a
|
24.2a
|
35.8a
|
30.3a
|
13.0a
|
2.2a
|
5.8a
|
| 3 |
50.0a
|
43.7ab
|
1.5ab
|
0.9ab
|
33.1ab
|
47.0ab
|
44.1a
|
24.8a
|
28.4ab
|
38.8ab
|
17.1ab
|
10.8a
|
2.5ab
|
8.1ab
|
| 6 |
50.8a
|
44.8b
|
1.8ab
|
1.2ab
|
28.2ab
|
38.9ab
|
39.9a
|
27.0a
|
36.6ab
|
42.3ab
|
19.7ab
|
9.7a
|
3.5ab
|
8.4ab
|
| 9 |
49.7a
|
43.5ab
|
2.6b
|
1.3ab
|
19.0b
|
32.7ab
|
29.7a
|
27.0a
|
47.9ab
|
45.4ab
|
13.6b
|
9.0a
|
4.4b
|
10.5ab
|
| 12 |
49.0a
|
42.0ab
|
2.5ab
|
1.4b
|
19.3ab
|
30.0b
|
27.4a
|
27.4a
|
50.7ab
|
47.4ab
|
14.8ab
|
8.6a
|
4.2ab
|
11.8b
|
| 15 |
47.8a
|
38.0a
|
2.5ab
|
1.3a
|
19.0b
|
28.4b
|
27.6a
|
26.4a
|
51.9b
|
51.1b
|
15.1ab
|
8.8a
|
4.2ab
|
10.4ab
|
Table 2.
General linear model analysis (GLM) for the C:N ratio, percentage changes in the bulk density (ΔBD) and water storage capacity (ΔWSC) of the litter layer in terms of initial bulk density and water storage capacity of artificial litter samples consisting of the share of pine needles and decomposition time. Significance effects (p <0.05) are shown in bold.
Table 2.
General linear model analysis (GLM) for the C:N ratio, percentage changes in the bulk density (ΔBD) and water storage capacity (ΔWSC) of the litter layer in terms of initial bulk density and water storage capacity of artificial litter samples consisting of the share of pine needles and decomposition time. Significance effects (p <0.05) are shown in bold.
| Variable |
C:N |
ΔBD |
ΔWSC |
| F |
p |
F |
p |
F |
p |
| Share of pine needles |
122.40 |
0.0000 |
41.58 |
0.0000 |
9.67 |
0.0000 |
| Decomposition time |
438.32 |
0.0000 |
10.34 |
0.0000 |
38.08 |
0.0000 |
| Share x Decomposition time |
3.02 |
0.0005 |
3.39 |
0.0001 |
1.79 |
0.0428 |
Table 3.
Mean percentage losses of litter mass and volume (grey color) of samples in terms of decomposition time and initial share of pine needles. Values with the same letter are not significantly different between decomposition times or shares of pine needles (Kruskal-Wallis test, p <0.05).
Table 3.
Mean percentage losses of litter mass and volume (grey color) of samples in terms of decomposition time and initial share of pine needles. Values with the same letter are not significantly different between decomposition times or shares of pine needles (Kruskal-Wallis test, p <0.05).
| Share of pine needles [%] |
Decomposition time [months] |
| 3 |
6 |
9 |
12 |
15 |
Mean |
| 0 |
8.3 |
4.4 |
33.3 |
26.9 |
50.8 |
55.6 |
56.7 |
72.7 |
55.7 |
70.3 |
40.2a |
46.0a |
| 20 |
2.5 |
7.3 |
36.7 |
23.9 |
58.3 |
64.7 |
57.5 |
66.7 |
56.7 |
79.8 |
42.3a |
45.6a |
| 40 |
7.5 |
4.8 |
39.2 |
25.7 |
62.5 |
67.1 |
60.0 |
68.8 |
71.7 |
74.0 |
48.2a |
48.1a |
| 60 |
11.7 |
4.5 |
38.3 |
22.2 |
56.7 |
51.3 |
67.5 |
69.1 |
68.3 |
65.0 |
48.5a |
40.6a |
| 80 |
20.0 |
8.1 |
37.5 |
16.2 |
57.5 |
35.7 |
68.3 |
59.3 |
65.0 |
56.3 |
49.7a |
35.1a |
| 100 |
24. 2 |
4.0 |
41.7 |
14.5 |
59.2 |
26.7 |
61.7 |
38.7 |
65.8 |
44.5 |
50.5a |
25.7a |
| Mean |
12.4a |
2.9a |
37.7a |
20.2a |
57.5b |
50.2b |
61.9b |
64.7b |
63.2b |
62.8b |
46.6 |
40.2 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).