1. Introduction
The vast majority of drugs launched on the market over the recent 2 to 3 decades, exist as single enantiomers (or are achiral), this stems from the fact that for chiral molecules one enantiomer brings the therapeutic benefit whilst the other has no effect, or can have deleterious effects, as is the case for thalidomide (commercialized i.e. as Contergan
®) administered in the 1960’s as a racemate to pregnant women, where the (
R)-enantiomer is a sedative and the (
S)-enantiomer a teratogen in a number of countries [
1]. [It must be pointed out that in the special case of thalidomide, just administering the (
R)-isomer to patients would not suffice from a safety point of view as the molecule is sterically unstable under physiological conditions leading to bio-inversion and formation of the undesired enantiomer]. The current market for single enantiomer drugs is more than
$300 billion. Many stereoselective methods are available to make single-enantiomer chiral drugs, but the most promising by far, is via asymmetric catalysis. Many important and even block-buster drugs have been obtained using this methodology [
2,
3]. Asymmetric catalytic synthesis can be achieved through three principally distinct methods; metal-based catalysis, organocatalysis, and biocatalysis. Although catalysis in general is a green methodology, the overarching considerations in all these approaches is the: 1) cost of the catalyst, 2) catalytic activity throughout the reaction and 3) recycling the catalyst. There are many solutions; the catalyst can be immobilized, and traditionally this was achieved by attachment to an appropriate solid support [
4], and then used either under batch conditions or in continuous flow. Under these conditions the reactions are heterogenous and entail certain disadvantages (support type, pore size, stability of catalyst, immobilisation method, existence of linker, interactions between the solid support and the components), this can be overcome using liquids to immobilize the catalysts, the most common being ionic liquids [
5], or Biphasic fluorous liquids [
6], but the most recent is the Deep Eutectic Solvent (DES) category [
7]. DESs have emerged as very useful liquids for many applications, but their most striking contribution is in the field of catalysis, particularly biocatalysis [
7] and organocatalysis [
8]. What is a DES? A DES is a liquid that is obtained when a hydrogen bond acceptor (HBA), like a quaternary ammonium salt is mixed with a hydrogen bond donor (HBD) [
9]. Their melting points are lower than that of their components and they readily mix with water, but gratifyingly are immiscible with most organic solvents. DESs are recyclable, reusable, stable, non-flammable, biodegradable and non-toxic mixtures. They have properties similar to ionic liquids, but unlike ionic liquids they are non-toxic and are not biodegradable. One other significant advantage is their solubility properties, and ability to stabilize transition states, particularly for organocatalyzed reactions. Some informative reviews on the subject have been published by the Alicante/Perugia groups [
8], Del Amo [
9], and by Domínguez de Maria [
10].
In this review, we will look at the use of promising asymmetric catalytic reactions that lead to Active Pharmaceutical Ingredients (APIs) that have been successfully conducted in NADESs.
3. Asymmetric Organocatalysis in DESs
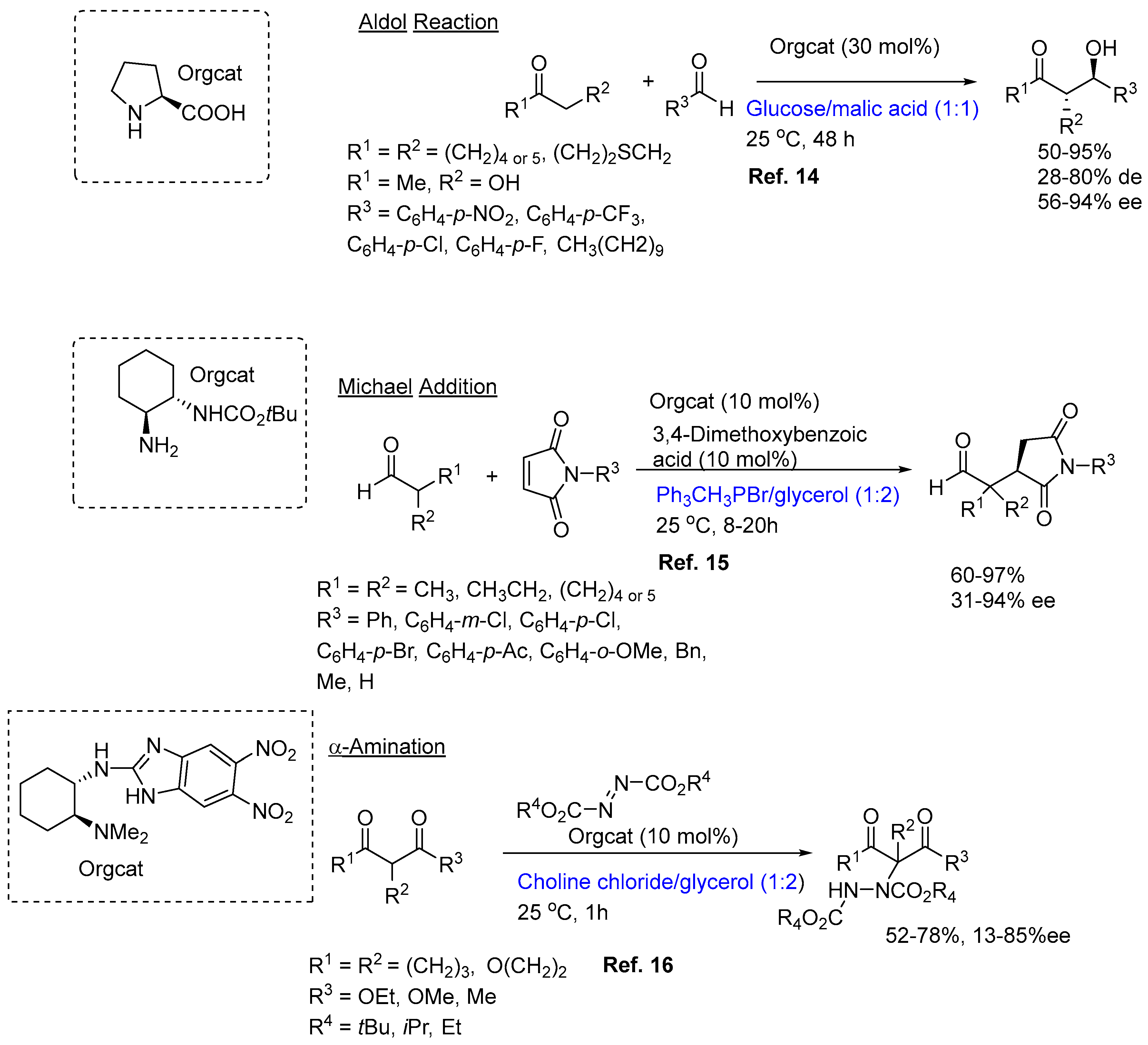
The area of organocatalysis is perhaps the newest member to the field of asymmetric catalysis. Although various types of organocatalytic transformations have been used over the last 100 years, including the landmark Hajos-Parrish-Wiechert-Eder-Sauer intramolecular Aldol reaction used as an inspiration in the 70s to make drug molecules. It, however, only became an important catalytic tool two decades ago with the developments of the 2022 Nobelists in chemistry, Ben List and David MacMillan [
13]. More recently asymmetric organocatalysis has been hooked up with DESs. The 2021 review by the Alicante/Perugia group provides a number of interesting examples on the application of asymmetric organocatalysis in NADESs for accessing some key target molecules (
Figure 1) [
8]. Most of the reactions that have been studied to-date fall into the very limited categories of: Aldol condensation, Michael addition and α-aminations, as well as a report on an integrated biocatalytic/organocatalytic sequence that provides chiral 1,3-diols with yields of up to 70% and 99% ee. In fact, prior to 2016 there were no reports on the application of NADES to support organocatalysis [
10] and the expectations were very high.
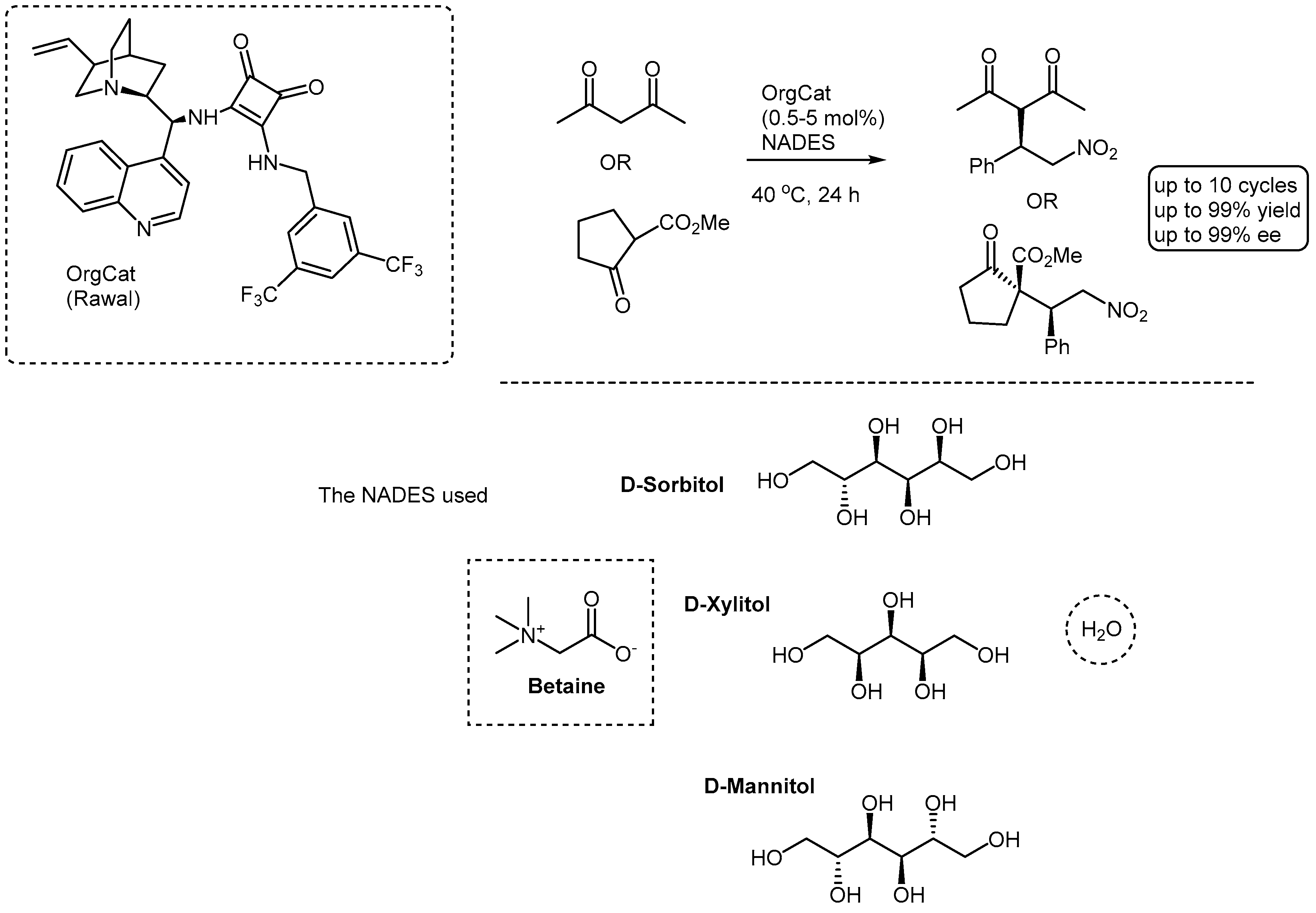
More recently, on the back of some preliminary work on the use of an immobilized cinchona-squaramide organocatalyst (immobilized on size-controlled porous glass beads) for key Michael additions of acetylactetone and methyl 2-oxocyclopentane-1-carboxylate to nitrostyrene [17], we investigated the same reactions but using NADES [18]. The results were highly enlightening, affording the Michael adducts in very high yields and stereoselectivities (
Scheme 1). Yields of up to 99% and enantioselectivities of up to 99% ee could be achieved after 24h at 40
oC (the reaction had to be run at this temperature to maintain the NADES in the liquid form and as viscous as possible. Three types of where NADES were investigated: Betaine/Sorbitol/Water; Betaine/Xylitol/Water and Betaine/Mannitol/Water. It was with the former that the best results were achieved, this was presumed to be due to the structural characteristics of the sorbitol. In the interest of recyclability, several reaction cycles were conducted with the same NADES and organocatalyst, in the case of methyl 2-oxocyclopentane-1-carboxylate up to 10 cycles could be successfully performed without any significant drop in yield and stereoselectivities.
One other question is the role of the NADES solvent in organocatalyzed reactions. Our recent experience in the Michael reaction is that the solvent, or components of the solvent compete with the organocatalyst. For example, the Michael reaction with methyl 2-oxocyclopentane-1-carboxylate occurred in Betaine/Sorbitol/Water with a yield of 99% and a diastereoselectivity of 58% d.e. without any catalyst present. Some good support for this conjecture came from our DFT calculations, which showed that there was a lowering of the reaction transition state by the sorbitol component [18]. This was assumed to be due to key H-bonding between the sorbitol and the reaction transition state complex. Even stronger support came from the observation that some one of the reactions in the Betaine/Sorbitol/Water was highly enantioselective (for acetylacetone) 75% ee. This phenomenon is not uncommon, and in fact both base and acid catalysed reactions have been successfully executed in NADES (reactions like, Perkin, Knoevenagel and Aldol condensations) [
10]. This discussion naturally leads to the role of chiral NADES as a medium for conducting asymmetric organocatalysis. A good example (however, maybe a touch biased) was the use of various L-proline (as HBA) derived NADES, that were used to support an asymmetric Michael reaction that involved butan-2-one and nitrostyrene [19]. Amongst the NADES that were tested, the best performers consisted of diethyleneglycol/Proline (3/1) (92%,
syn/anti 85/15, ee 26/rac) and glycolic acid/proline (3/1) (15%,
syn/anti 60/40, ee 86/95). This exciting aspect was also reviewed in 2022 by Tang and Yao [20], but they alerted us to the paucity of examples currently known and decreases in enantioselectivity due to NADES recycling.
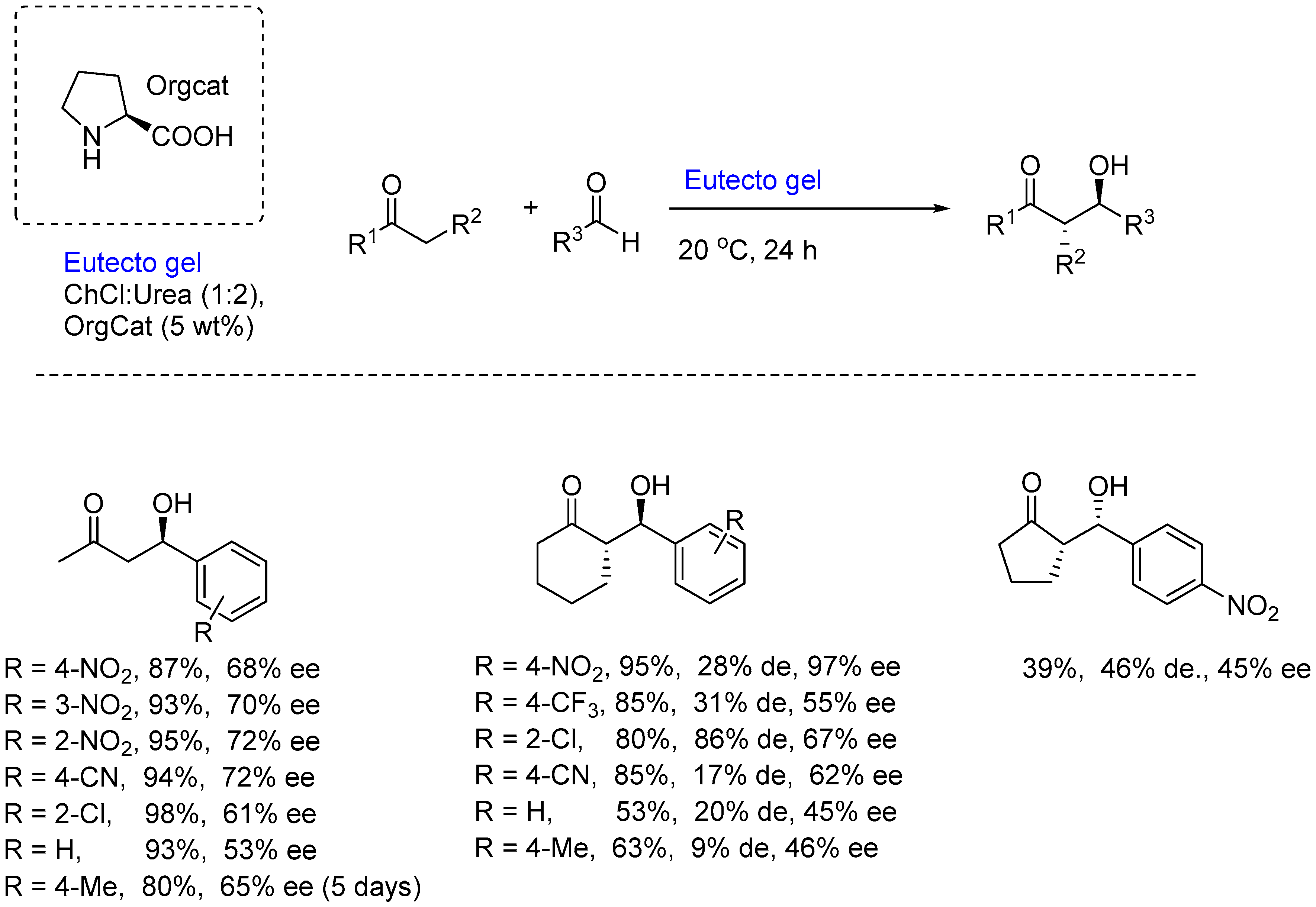
A more recent development has been with eutectogels, and their use in asymmetric organocatalysis. In 2021, the group of Ramón and D’Anna, reported on the application of these materials for asymmetric Aldol reactions [21]. These materials have been categorized as “catalytic soft materials” and are a combination of Low Molecular Weight Gelators (LMWGs) and NADES. They were designed to act as specific organized vessels to perform organocatalytic reactions. In this work, a variety of canonical amino acids, like proline, cysteine and asparagine, etc (all L) were used for a variety of Aldol reactions between ketones and aldehydes – having initially acetone and
p-nitrobenzaldehyde as the benchmark (
Scheme 2). Initial studies to determine the best LMWG and NADES combination, using the reaction of acetone with
p-nitrobenzaldehyde indicated that when the gel derived from using L-proline (5 wt%) in the NADES, ChCl/Urea (1:2) at 20
oC for 24h, the aldol product was obtained in 87% yield and 68% ee (
Scheme 2). The scope of the reaction was evaluated, and in some cases enantioselectivities of up to 95% could be achieved, although in those cases where diastereomers were formed, the diastereoselectivities were only moderate. Michael additions were also conducted, using isobutyraldehyde or maleimide with
trans-β-nitrostyrene, a base additive or an acid additive and 5 mol% proline, and unfortunately the yields were very poor.
Overall, this is a very promising enabling technology that has much potential to offer for the synthesis of biologically active compounds but is still in its infancy.
4. Biocatalysis in DESs
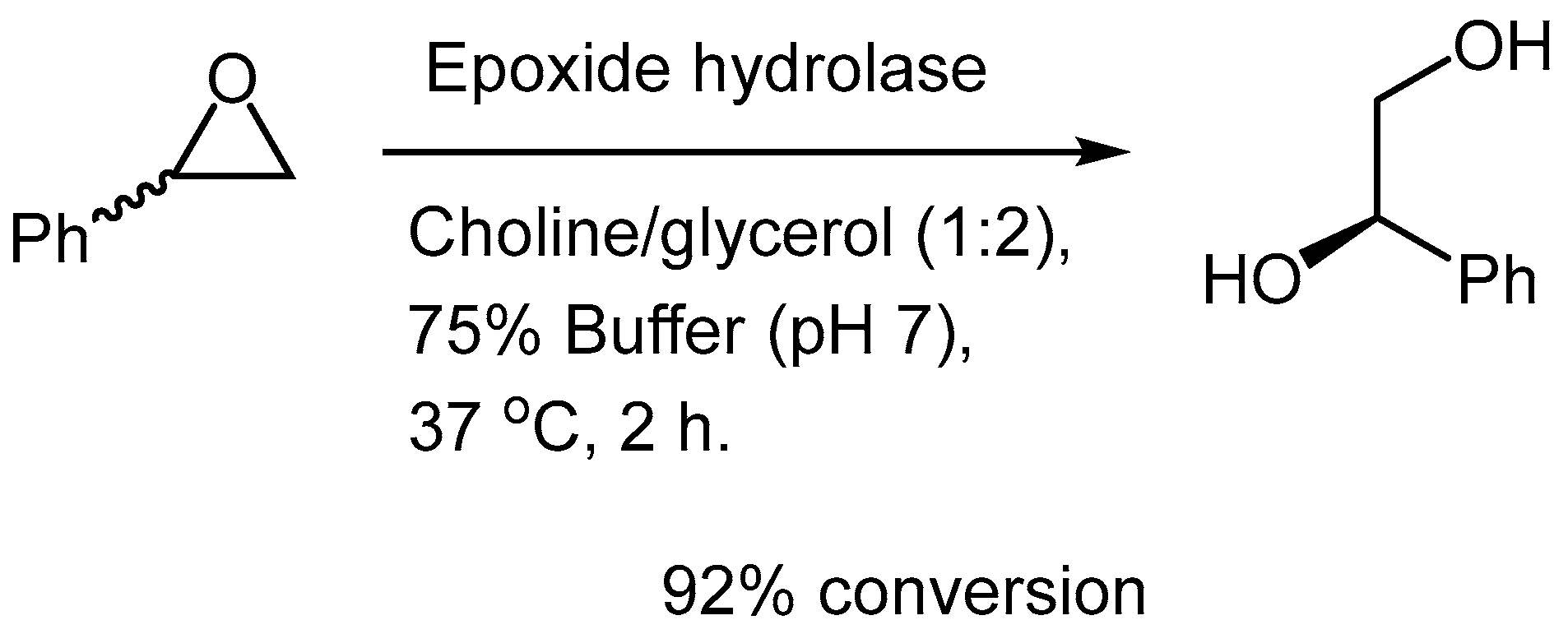
The long-lasting paradigm that enzymes could actually function in a strictly aqueous environment was finally debunked in the mid-1980s by Klibanov and coworkers when they showed that the reactions could be run in organic solvents [22,23]. Since then, it has been shown by numerous examples how efficient biocatalytic reactions can be performed in “unconventional” systems such as “classical” organic solvents, room temperature ionic liquids (RTIL), supercritical fluids and of course NADES [24]. The latter category, along with RTILs, have brought several advantages for conducting enzyme-driven transformations, having enjoyed enormous attention as being broadly useful in organic chemistry. The ground making work on the use of NADES in biocatalysis, was made by Kazlauskas group in 2008, where they demonstrated that it was entirely feasible to run biocatalysis in these nonconventional systems, as was showcased by different biotransformations relying on hydrolases (CALB type) as catalytic species in a variety of choline chloride based NADES [25].
Scheme 3.
Pioneering work from Kazlauskas’ group on deracemization of styrene oxide.
Scheme 3.
Pioneering work from Kazlauskas’ group on deracemization of styrene oxide.
This pioneering work showed the far superior performance in the NADES chosen to conduct this ring-opening hydrolysis, with 92% conversion in comparison to <5% in “standard” aqueous buffer. Unfortunately, the enantiopurity or the direction of the enantioselectivity (stereochemical configuration of the product) was not addressed by the authors.
They have also been used successfully in trans-esterification reactions. Several articles disclose the use of glucose and several acyl donors for enzymatic transesterifications in DESs. For example, in 2015, Pöhnlein et al. reported the transesterification of glucose with vinyl hexanoate using immobilized CalB (Novozym 435) in different DESs [26]. Ester products were obtained in the reactions that used both ChCl/urea and ChCl/glucose DESs. The beauty of this approach was that in one case the NADES behaved as the solvent and the substrate. In 2020 the same two hydrophilic DESs were reported for the transesterification of glucose with vinyl decanoate [27].
The same group also explored, for the first time, the use of a hydrophobic DES (menthol/ decanoic acid) as a suitable reaction medium for sugar ester production [28]. The yields obtained with these NADES were significantly higher than with hydrophilic NADESs. Different reaction parameters were investigated to optimize the synthesis further. 20 milligrams/ mL iCalB and 0.5 M glucose resulted in the highest initial reaction velocity for the esterification reaction, and the highest initial reaction velocity was observed with 1.5 M glucose in the transesterification reaction. The enzyme was reusable for at least five cycles without significant loss of activity.
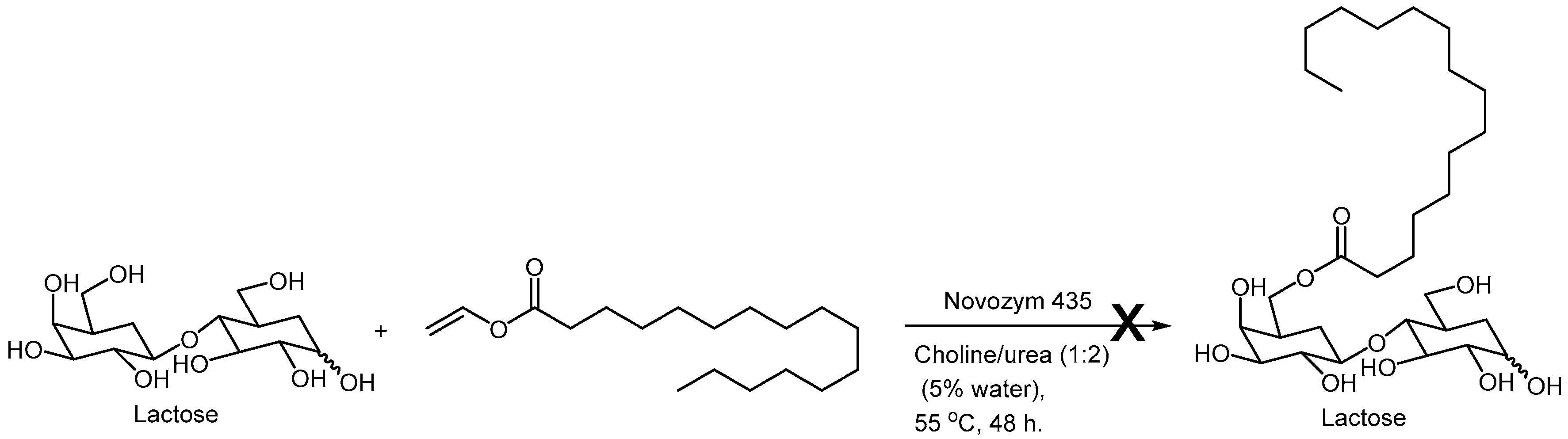
On the downside, a recent study from Ubialli’s group addressed the problems involved in the synthesis of sugar fatty acid esters from dissacharides, like lactose using choline chloride based NADES [29]. They encountered difficulties in conducting this transformation using standard Novozym lipases (
Scheme 4). An important point was raised by these authors, where they correctly recognized that the NADES does not behave as a simple solvent, but in fact its individual components interact with the reaction system constituents. (“Overall, the conclusion is that DESs are not innocent solvents because they interact with the substrates and the catalysts and influence the reaction outcome (or directly hamper it”). They also liked the choice of DES to the choice of catalyst for a reaction, particularly a chiral catalyst, where there is no such thing as a “one-size-fits-all” solution. “Rather than being a simple “one-size-fits-all” solution, incorporating a DES in a reaction needs a profound study for each particular case, starting from the genuine motivation to use them (solubility, sustainability, etc.), considering the enzyme and substrate compatibility, and eventually the ease of a downstream unit”.
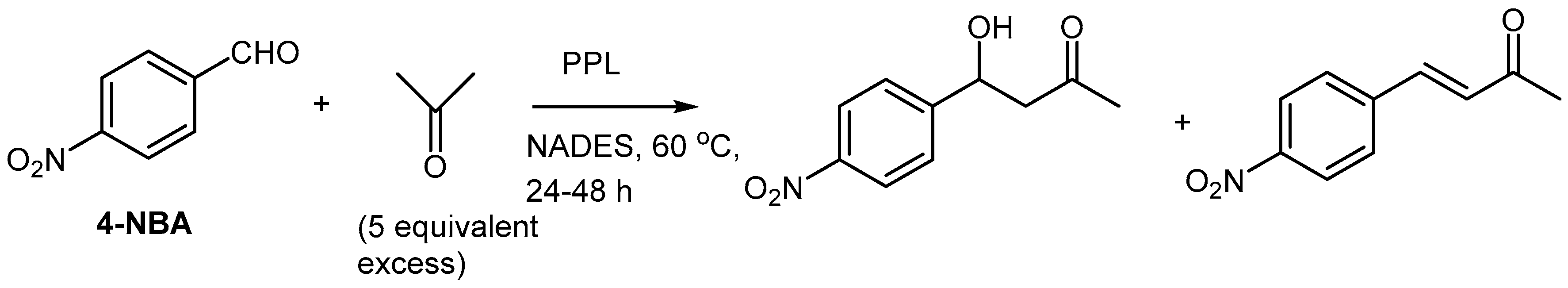
The enzyme porcine pancreas lipase (PPL) was used in the enzymatic catalysis of the aldol reaction condensation of
p-nitrobenzaldehyde and acetone in the same NADES ChCl/glycerol as described above (
Scheme 5) [30].
It was noted that the product composition could be directly correlated with the choice of DES, differing in their hydrophobicity. The initial reaction velocity of the aldol addition was higher in the hydrophilic NADES, ChCl:Gly (1:1.5). Unfortunately, this DES was limited by the solubility for the nitrobenzaldehyde (4-NBA) substrate. To overcome this problem, a NADES consisting of tetrabutylammonium chloride:4-NBA (2.2:1.5) was prepared and evaluated. The 4-NBA containing DES exhibited the highest solubility for 4-NBA. However, the fastest reaction with a final yield of 1296.5 mM, or 71% yield and 82% conversion after 32 h, was achieved when no NADES was used (olvent-less reaction conditions) and improving the productivity up to 40.5 mM h−1 compared to reactions performed in NADES. An enone side-product was obtained in most cases, but particularly for the more hydrophilic NADES, like ChCl/Glycerol. BSA-catalyzed aldol additions were also investigated but did not exhibit specificity neither for aldol nor olefin formation.
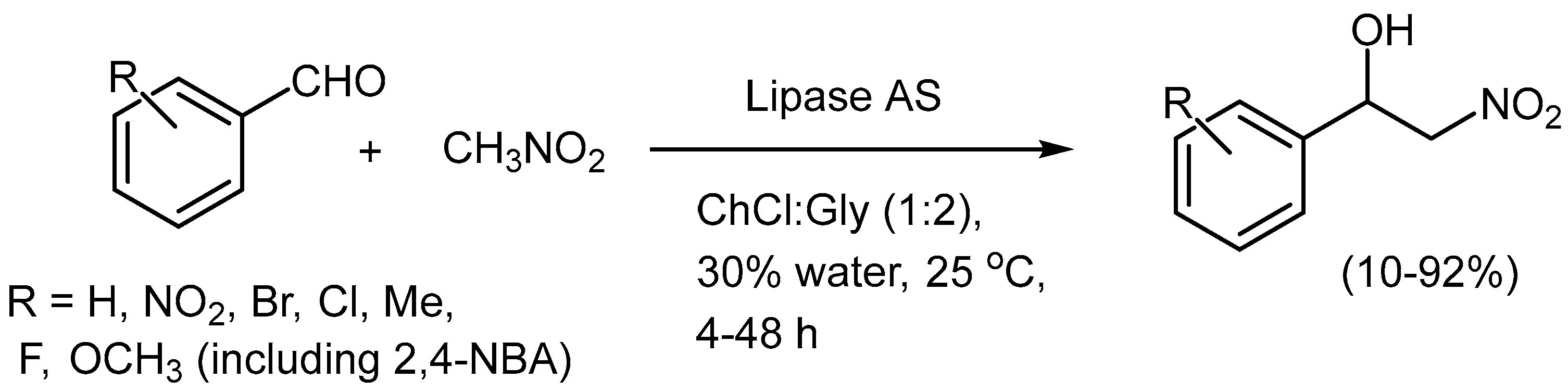
The Henry reaction has also been explored in NADES. The Henry-reaction is simple, but is useful from a pharmaceutical point of view due to the presence of a vicinal benzylic chiral alcohol and a nitro group when aldehydes are used and nitro-amino compounds when imines are used (
aza-Henry variant). The useful lipase-catalysed (
Aspergillus niger [lipase AS “Amano”]) C-C bond forming Henry reaction between nitromethane and carbonyl acceptors (4-NBA and other aromatic aldehydes) has been demonstrated to proceed in an excellent manner in DESs such as ChCl: glycerol (1:2). By the addition of H
2O up to 30 vol-% it was found that the lipase activity could be improved and under optimized conditions, a best yield of >92% was achieved (
Scheme 6) [31].
Interestingly, when the aza-Henry reaction was conducted between benzaldehyde N-tosyl imine and nitromethane in ChCl:Gly (1:2) the product was obtained in 17% yield after 40 h, proving that the NADES could catalyse the reaction. This is a similar observation to one of ours for a Michael addition reaction [18]. The reactions with the Lipase present gave slightly better yields (up to 39%). The reaction enantioselectivity was not evaluated.
The examples showcased here are convincing evidence of the influence that NADESs in their various compositions have on the outcomes of different biocatalytic processes. Moreover, the main message to be transmitted in these examples is that by carefully choosing the most appropriate NADES we can get the most out of the enzymatic reaction at hand.
5. Metal-Based Asymmetric Catalysis (MBAC) in DESs
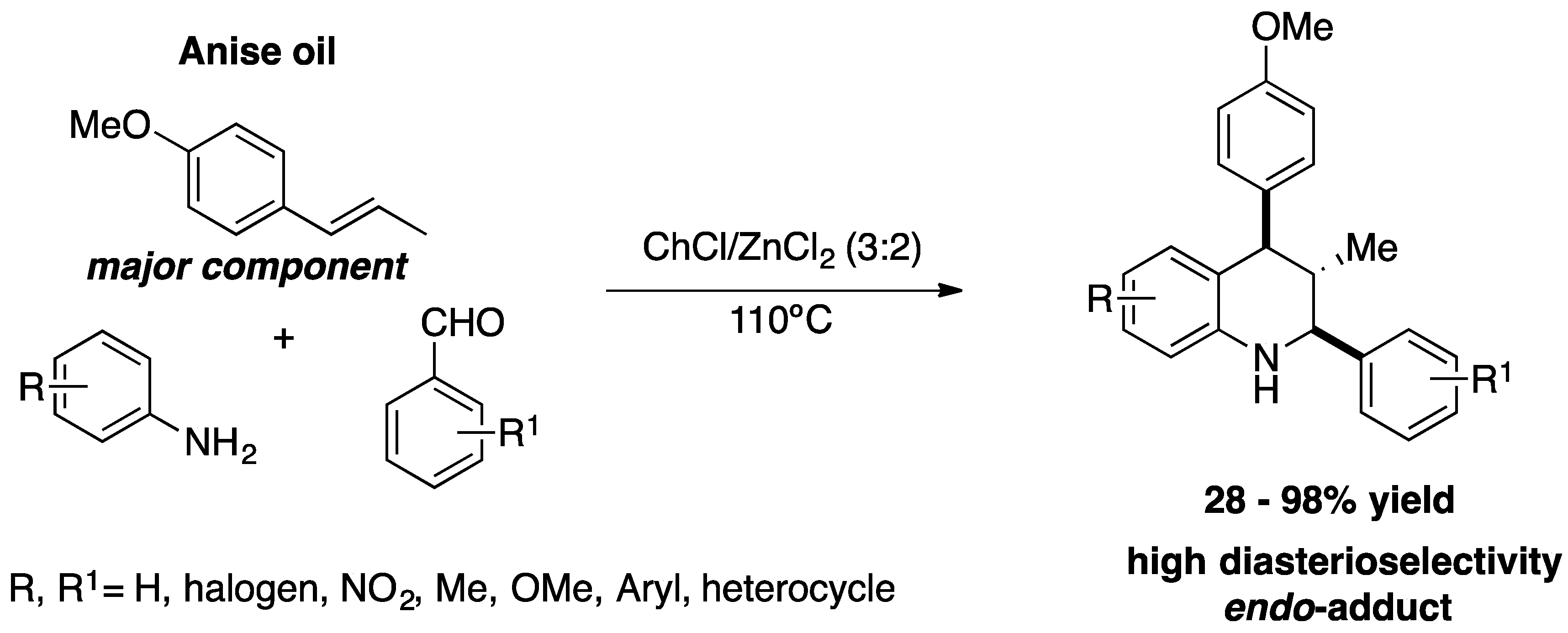
Metal-based asymmetric catalysis is very important for the preparation of bioactive chiral compounds in the pharmaceutical industry, but the toxicity of heavy metals and the volatile organic solvents jeopardises its sustainability [32]. The use of DESs in asymmetric metal-based catalysis is the way to make these reactions more sustainable, since both the solvent and the metal-catalyst can be reused several times. So far, DESs have shown their versatility in reactions based on transition-metals, such as: Suzuki-Miyaura coupling, Hiyama, Heck, aminocarbonylations, Sonogashira, Pd-catalyzed C-S bond formation, among others. In most of these reactions the DESs performed well, in that they gave very good to excellent yields and it was possible to reuse the solvents and the catalysts several times while maintaining the activity [33,34]. Furthermore, these reactions are part of the synthetic pathway for various bioactive compounds [35]. Despite its extraordinary performance in metal-based catalysis reactions, its application in asymmetric catalytic synthesis based on metals is rare. So far, only a few examples have been reported in the literature and the metals have been used in stoichiometric quantities rather than catalytic. Peñaranda-Gómez
et al. reported a sustainable and diastereoselective method for the preparation of tetrahydroquinoline derivatives from star anise oil, using a mixture of choline chloride (ChCl)/zinc chloride as DES (
Scheme 7)[36]. This diastereoselective synthesis is a multicomponent inverse electron-demand aza Diels−Alder reaction, carried out with anise oil (the main component is
trans-anethole), anilines and aromatic aldehydes in ChCl/ZnCl
2 mixtures (3:2) at 110 ºC for 3h, providing a total of 22 tetrahydroquinoline derivatives in low to excellent yields with high diastereoselectivity [36]. The diastereoisomers formed have the
cis-(2e,4e) configuration, which was determined by NMR analysis. In addition, DESs can be recycled for 3 cycles, maintaining their activity. The DESs in this reaction act as a solvent and catalyst and are crucial to the reaction. It should be emphasised that tetrahydroquinoline derivatives are value-added compounds in the field of medicinal chemistry. DSC determined the best temperature and composition of DESs for this multicomponent reaction.
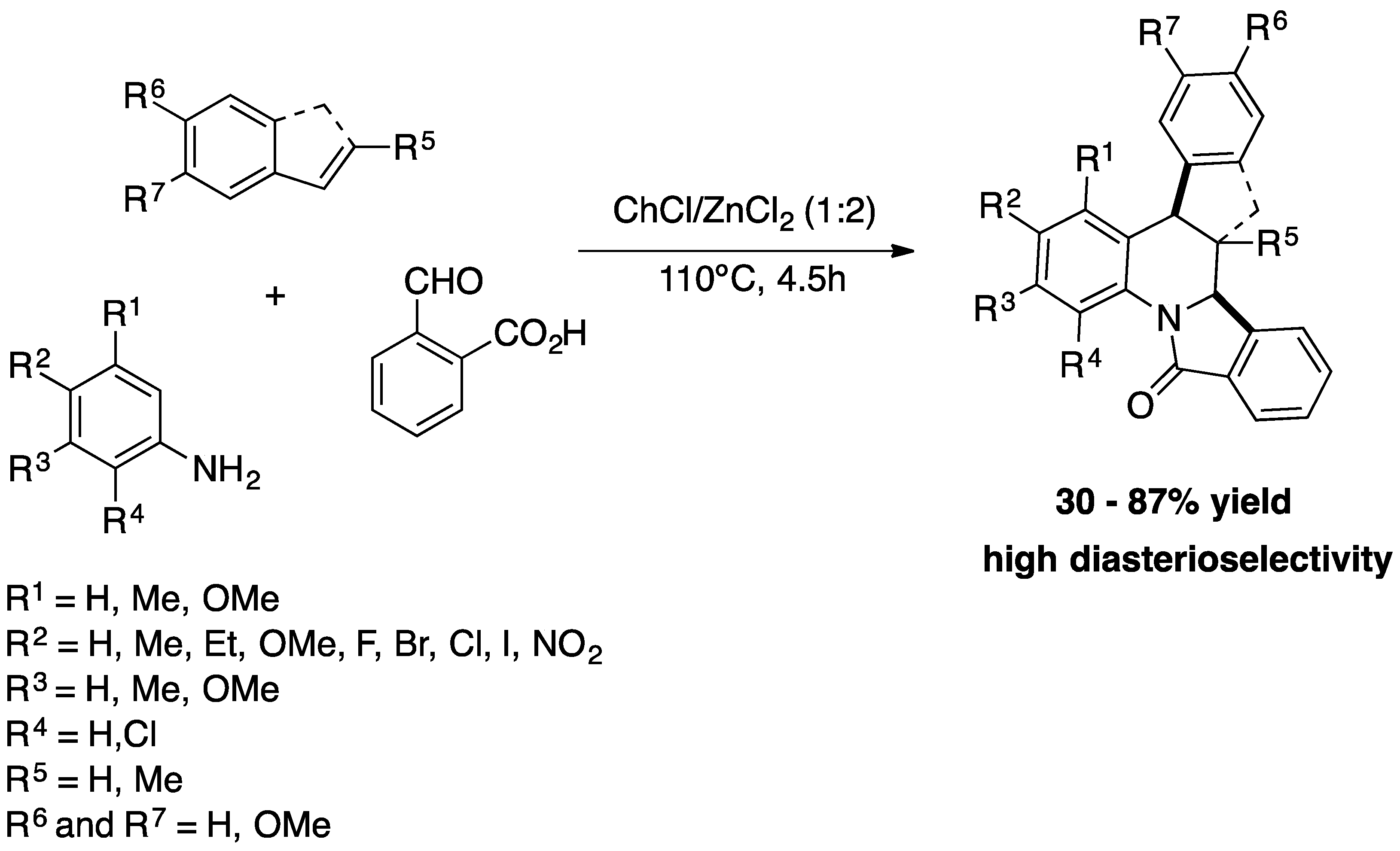
Recently, Sanabria-Sánchez
et al. developed a multicomponent, diastereoselective, one-pot Povarov reaction in DESs for the preparation of a wide variety of dihydroisoindolo[2,1-a]quinolin-11-ones derivatives. Similar to the methodology described by Peñaranda-Gómez
et al. the DESs act as solvent and catalyst. Various DESs consisting of Lewis or Bronsted acids were studied, such as ChCl/ZnCl
2, Urea/ZnCl
2, ChCl/MgCl
2, ChCl/SnCl
2, ChCl/APTS, ChCl/CF
3SO
3H and ZnCl
2/ethylene glycol and reaction conditions to choose the best system, ChCl/ZnCl
2 (1:2) at 110 ºC for 4.5h (
Scheme 8) [37]. Twenty dihydroisoindolo[2,1-a]quinoline-11-one derivatives were prepared with yields of up to 87% and high diastereoselectivities, by reacting a wide variety of anilines, alkenes (
trans-anethole, methyl eugenol and indene) and 2-formylbenzoic acid in ChCl/ZnCl
2 (1:2) at 110 ºC for 4.5h.
1H NMR analysis of the compounds confirmed the production of a single diastereoisomer, the
6,6a-trans isomer. In addition, the DES mixture could be reused for six cycles without loss of activity.
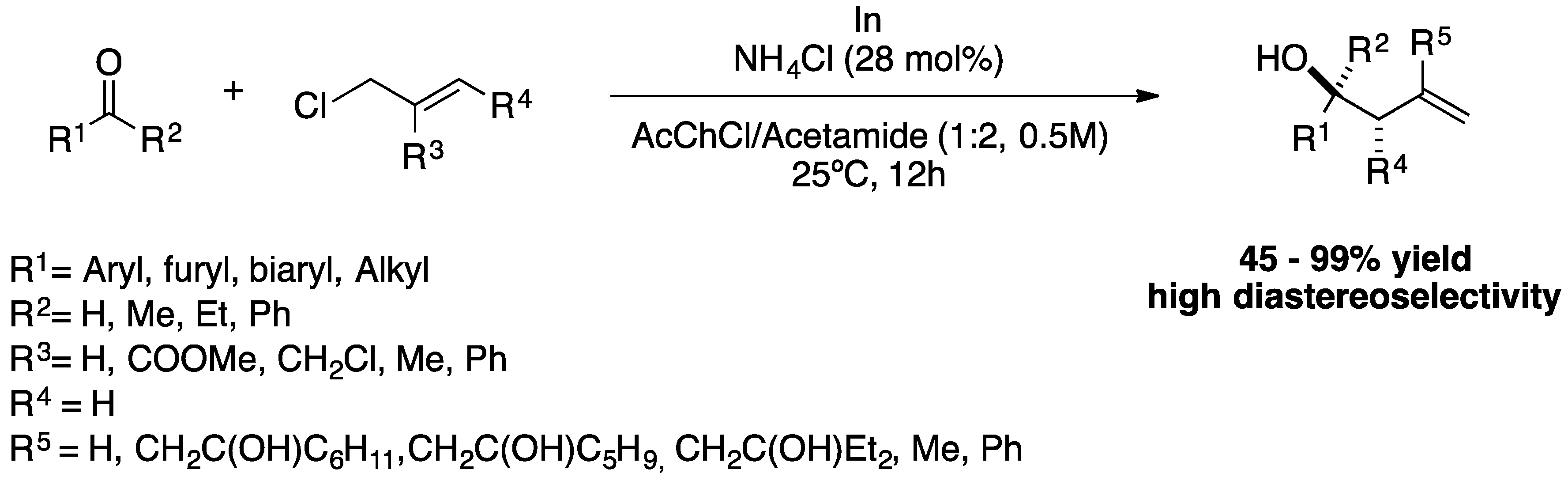
The development of more sustainable methods for the preparation of homoallylic alcohols is of great interest to the pharmaceutical industry. González-Gallardo
et al. reported the allylation of various carbonyl compounds via indium organometallic and stable and desiccable allyl chloride derivatives in DESs. Several DESs were studied for this reaction, such as: choline chloride (ChCl):ethylene glycol (1:2), acetylcholine chloride (AcChCl):acetamide (1:2), ChCl:glycerol (1:2), ChCl:urea (1:2), MePPh
3Br:glycerol (1:2), ChCl:L-malic acid (1:1), ChCl:D-galactose (5:2). To the reactions carried out with the DESs ChCl:ethylene glycol (1:2) and AcChCl:acetamide (1:2) were added ammonium salts (NH
4Cl or NH
4OAc) for stabilizes the species of In, furnishing the desired product with excellent yields. Once both of the DES gave excellent yields the AcChCl:acetamide were selected as a solvent due it’s lower toxicity [38]. Twenty homoallyl alcohol derivatives were prepared in moderate to excellent yields and with high diastereoselectivities through the Barbier allylation of a wide range of carbonyl derivatives and chloroallyl substrates mediated by indium (stabilised by NH
4Cl) in DESs at 25 ºC for 12h (
Scheme 9). The recyclability of DESs and NH
4Cl was studied over 6 cycles, but they only maintain their performance over 4 cycles. In any case, it can be emphasised that DESs perform well in organometallic reactions, making these reactions more sustainable due to their lower toxicity and good recyclability.
Another promising approach is the chemoenzymatic cascade, which combines transition metal catalysis and biocatalysis for the asymmetric synthesis of bioactive molecules [39]. Basically, metal-enzymatic cascades and sequential transformations consist primarily of the role of metal species (Pd, Ru, Au, Ir and Fe) in catalysing various organic reactions (C-C couplings, isomerisations, hydrations...) and their combination with stereospecific enzymes, including alcohol dehydrogenases, alcohol oxidases, aldolases, amine dehydrogenases, amino acid dehydrogenases, among others. The use of DESs in metal-enzymatic cascades and sequential transformations is rare, but the few results that have been reported are very promising for the pharmaceutical industry.
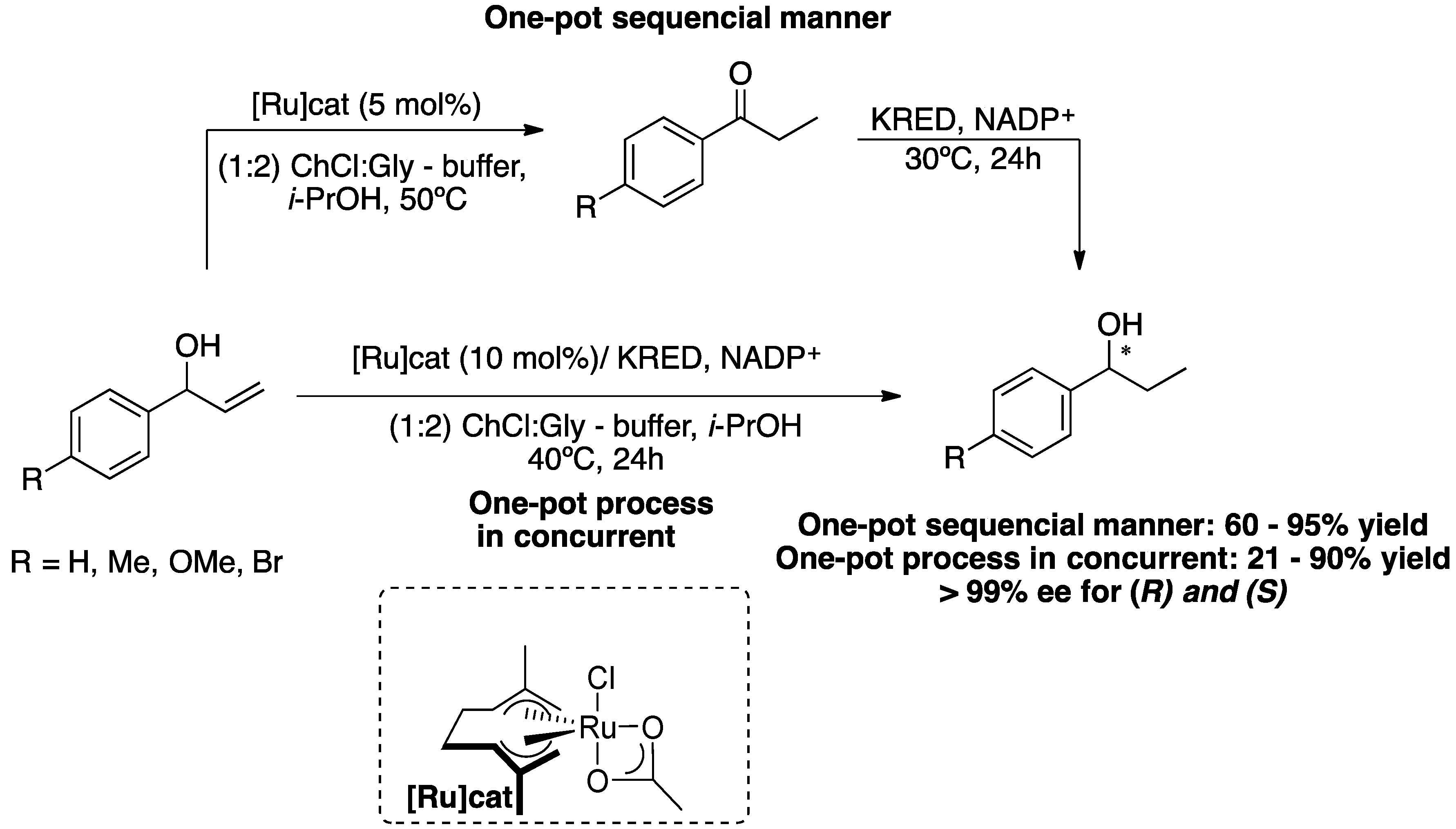
The first successful example of the integration of metal and biological catalysis in DESs-buffer mixtures was reported in 2018 [40]. This chemoenzymatic cascade consists of the ruthenium-catalysed isomerisation reaction of allylic alcohols to an α, β-saturated ketone, followed by enantioselective enzymatic reduction promoted by ketoreductases (KREDs), providing enantiopure alcohols (
Scheme 10). This chemoenzymatic cascade was carried out using two different approaches, a sequential reaction (one-pot in two steps) and a simultaneous (concurrent) reaction (one-pot in one step). The choice of solvent mixture, based on DESs and buffers, was made carefully, making it possible for both reactions to take place without inhibiting the metal catalyst or the enzyme. The best mixture of DESs was ChCl/glycerol (1:2) or ChCl/sorbitol (1:1) for enantioselective bioreduction, however, for the chemoenzymatic reactions, the first mixture of DESs was chosen due to the high viscosity of sorbitol-based DES, even at 40 ºC. In the case of the sequential approach (one-pot in two stages), the first stage involves the Ru-catalysed isomerisation of racemic allylic alcohols in DESs buffer,
i-PrOH at 50 ºC, after consumption of the starting material, for the second stage the temperature was reduced to 30 ºC and KRED and its cofactor (NADP
+), obtaining the desired chiral alcohols in good to excellent yields and high enantioselectivities. With regard to the second approach, the simultaneous reaction (one-pot in one-step), both catalysts were added at the start of the reaction, for this reason the enzyme showed poor stability in the aqueous buffer, resulting in incomplete reactions, providing the desired product mixed with unreacted ketone and, in some cases, allyl alcohol was also obtained, indicating slow isomerisation. However, five chiral saturated alcohols were prepared in low to excellent yields and with excellent enantioselectivities via the chemoenzymatic reaction of allylic alcohols catalysed by KRED and Ru complex (10 % mol) at 40 °C in ChCl/Gly (1:2)-buffer (4:1) (
Scheme 10).
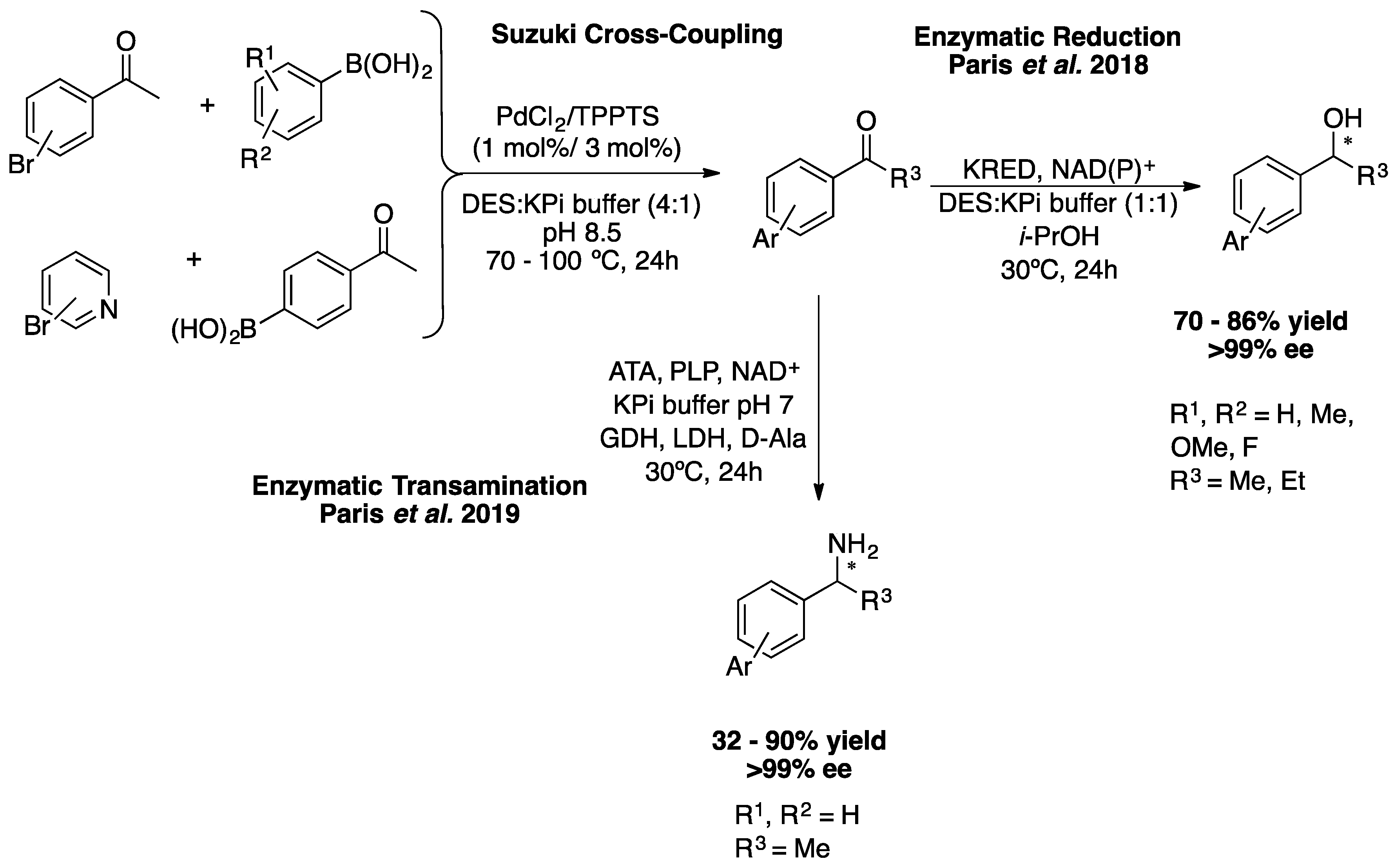
In 2018, Paris
et al. described the first application of DESs as a solvent in Suzuki coupling followed by bioreduction for the preparation of enantiopure biaryl alcohols in very good yields (up to 86%) and excellent ee (> 99%) (
Scheme 11) [41]. The two-step method consists of the palladium-catalysed Suzuki coupling of 4′-haloacetophenones with arylboronic acids at 70-100 °C in a choline chloride:glycerol (Gly) buffer mixture, followed by the enzymatic reduction of the ketone formed catalysed by alcohol dehydrogenases at 30 °C. Separately, both the palladium-catalysed coupling reactions and the bioreductions were previously carried out in mixtures of DES and DES:buffer. Subsequently, the reaction conditions were optimised with regard to the choice of components and the proportion of DES mixture; the proportion of DES and buffer and also the substrate concentration. Using these ChCl/Gly (1:2)-buffer (4:1) conditions at 100 °C for the Suzuki coupling step at 200 mM subtract, it was possible to prepare 10 biaryl ketone derivatives in high yields. In addition, the reaction conditions for enzymatic reduction were optimised, requiring dilution to 75 mM with a buffer containing
i-PrOH, ketonareductases (KRED) and cofactor (NADP
+) to establish efficient bioreduction of the transiently formed ketone. Using these conditions, it was possible to reduce a wide variety of substrates. Furthermore, using the stereocomplementary ADH enzymes of
Lactobacillus kefir and
Rhodococcus ruber, it is possible to obtain (
R)- and (
S)-enantiopure biaryl alcohols.
Later, the same groups of Gröger and González-Sabín reported the preparation of enantipure biaryl amines through a chemoenzymatic cascate in DESs-buffer mixture, which consists of a palladium-catalysed cross-coupling reaction followed by enantioselective enzymatic amination (
Scheme 11) [42]. As described above, the first step was the optimization of the reaction conditions by choosing the most suitable solvent for both reactions to take place in excellent yields. The best DESs (co-solvent) for this chemoenzymatic cascade was (1:2) ChCl/Gly. In addition, biocatalysis using amine transaminases (ATAs) in DESs has not been reposted before. The reactions were carried out according to the schematic representation (
Scheme 11), the Pd-catalysed step was carried out at 200 mM substrate loading in a DES:buffer mixture (4:1) and the biocatalysis step at 25 mM before dilution to a buffer containing 10% w/w DES due to enzyme stability problems. Five (
R)-biaryl amines were prepared using this methodology in low to very good yields with excellent enantioselectivities (>99% ee) [42].
The preparation of chiral compounds through asymmetric catalytic syntheses carried out in DESs has not been widely explored so far, perhaps because, in general, chiral metal complexes (consisting of a chiral ligand coordinated to a metal) are probably very sensitive to the aqueous medium and end up decomposing; another aspect is that most DESs contain other components that coordinate with the metal, giving rise to racemic products. An alternative is to resort to the chemoenzymatic cascade or the use of organocatalysts for the preparation of chiral compounds, since both approaches have shown very promising results using DESs as reaction media.
6. Conclusions
In this review we have looked at recent developments in the use of NADES in asymmetric catalysis, from the point of view of metal-based catalysis, organocatalysis and biocatalysis. By far, perhaps for historical reasons this alternative solvent system has been used more frequently in biocatalysis than other catalytic reactions. Despite the great potential of this alternative medium, in the case of the number of asymmetric reactions so far examined have been limited, being restricted mainly to Aldol, Michael, α-aminations, asymmetric hydrolysis and some others.
With regard to the use of DESs in metal-based catalysis for the preparation of chiral bioactive compounds, we can see that it has been very little explored. However, the few examples in the literature have shown that DESs help to stabilise catalysts and could also be recycled many times, maintaining their activity. Chemoenzymatic cascade reactions are another alternative approach for the enantioselective preparation of bioactive compounds in which NADESs have been successfully used.
Many challenges and opportunities remain, the most intriguing of which is their application as both solvent and catalyst, to provide single enantiomer or scalemic products without the requirement for the addition of a chiral catalyst, as our group previously showed for a simple organocatalyzed Michael reaction [18].
The effect of the NADES on the reaction system was briefly discussed in this report, and it would be honest to say that NADES are “non-innocent” solvents. This is something that will need to be seriously addressed, so that the advantages of NADES can be maximized for efficient and selective chemical synthesis. The problem with restricted mass-transfer is also an issue that will need to be carefully resolved or at least improved as we go forward.
As far as we are aware there have been no reports on the use of NADES in photoredox and continuous flow processes, due to certain practical issues, but one can predict that such applications will be inevitable in the near future.
On a final note, we expect that there will be further and more frequent applications of NADES in stereoselective transformations in the coming years.