1. Introduction
In acidic soils, such as Andisol and Ultisol, the presence of toxic levels of aluminum (Al) is often associated with low phosphorus (P) bioavailability. This is because the acidity of the soil causes Al to be solubilized into its ionic form, Al3+, a highly toxic form of Al that can exert toxicity on living organisms, such as plants and microorganisms. In addition, Al3+ can form insoluble complexes with orthophosphate anions (Pi), the labile form of P that is readily assimilated by plants. This binding of Pi limits the availability of P to plants, which can lead to P deficiency. In addition, an important fraction of the total soil P is integrated into complex organic molecules, which are not immediately bioavailable to plants. Therefore, both Al toxicity and P deficiency are both important constraints for plant growth in acidic soils.
Plant growth promoting bacteria (PGPB) are a group of bacteria that can enhance crop growth and yields by alleviating the negative effects of environmental stresses and nutrient deficiencies [
1,
2,
3,
4]. PGPB support plants through both direct mechanisms, such as active promotion of plant growth, and indirect mechanisms, such as protection from growth inhibitors [
5]. Phosphobacteria are those PGPB have intrinsic mechanisms to both solubilize inorganic forms of P (Pi) or/and mineralize organic forms of P (Po) contained in soils. Therefore, phosphobacteria are capable to convert the scarcely available soil P complexes into bioavailable phosphates, which can be uptake by plants and used in its nutrition [
9]. Some of these phosphobacteria have also been shown to be Al-tolerant and effective in increasing plant tolerance to Al stress. Therefore, phosphobacteria have been proposed as potential biofertilizers in acidic soils.
We previously isolated three phosphobacteria strains:
Enterobacter sp. RJAL6 (reclassified as
Serratia sp. RJAL6),
Klebsiella sp. RCJ4, and
Enterobacter sp. 198. These remarkable microbes exhibit significant plant growth-promoting (PGP) activity towards wheat [
1], ryegrass [
3,
10], and/or avocado [
2] under environmental stresses and phosphorus (P) deficiency. Notably, these strains are tolerant to high Al concentrations (10 mM), solubilize Pi, mineralize Po forms through the release of organic acids and the enzymatic activity of phosphatases, respectively, and produce auxins and siderophores [
11]. These characteristics make them potential bioinoculants for improving P bioavailability in acidic soils with high Al content.
Although these bacteria have shown promise as biofertilizers for plants grown in acidic soils with low P availability and high Al concentrations, the genetic machinery involved in these processes is not fully understood. In this study, we performed genome-based taxonomy and comparative genomic analysis of the three phosphobacteria strains to identify the genes associated with key mechanisms for P uptake, Al tolerance and plant growth promotion.
2. Materials and Methods
2.1. Isolation and culture of the strains
The strains RJAL6 and RCJ4, were isolated in a previous study from the rhizosphere of ryegrass (
Lolium perenne) grown in an Andisol (39°06′12″ S-72°37′42″ W; soil pH of 5.3) amended with cattle dung manure as described by Mora et al., (2017) [
10]. Meanwhile, the strain 198 was previously isolated from the rhizosphere of avocado trees (32°50′59″ S-71°00′08″ W; soil pH of 5.95) as described by Barra et al., (2016) [
1]. These particular strains were chosen for our study because they are tolerant to Al (10 mM), can solubilize Pi and mineralize Po forms by releasing organic acids and intracellular and extracellular phosphatases, produce siderophores and auxins, can use ACC as their sole nitrogen source, and promote plant growth in wheat, ryegrass, and avocado under environmental stresses or P deficiency. The strains were maintained on Luria-Bertani agar at 30°C and conserved as bacterial suspensions in 30% v/v glycerol at 80°C until their use.
2.2. Sequencing and assembly of the phosphobacteria genomes
For the genomic DNA extraction, bacterial strains were grown overnight in 10 mL of LB broth at 30 °C in a rotatory shaker with constant agitation (120 rpm). Bacterial cells were collected by centrifugation at 3,000 × g for 4 min and the total gDNA was extracted using the Dneasy UltraClean® Microbial kit (Qiagen, Inc.) according to the manufacturer’s instructions. The quantity and quality of the extracted DNA was determined using a MultiskanTM GO Microplate Spectrophotometer (Thermo Fisher Scientific Inc.). The whole genomes of the strains were
de novo sequenced using Illumina HiSeq 2500 platform in Macrogen Inc. (Seoul, Republic of Korea) with 100 bp paired-end reads. The DNA libraries were prepared using a TruSeq Stranded mRNA LT Sample Prep Kit (Illumina, San Diego, CA, USA) according to the manufacturer's protocol. The draft genome sequences were assembled using Abyss 2.0 [
12] and deposited in GeneBank database under the accession numbers GCA_009025585 (198), GCA_009025755 (RCJ4) and GCA_009025565 (RJAL6).
2.3. Genome annotation and comparisons
The draft genome sequences were annotated through RAST server using the default pipeline [
13]. Complete 16S rRNA gene sequences (>1500 bp) were extracted from the annotated genome sequences and compared against those of validly named species available in EzBioCloud portal by BLAST [
14]. The Overall relatedness genome indices (OGRIs), as average nucleotide identity (ANI) and digital DNA-DNA hybridization (dDDH) between the draft genome sequences of the strains from this study and their closest phylogenetic relatives were calculated using OrthoANIu Calculator web tool [
15] and Genome-to-Genome Distance Calculator (GGDC) web tool [
16]. The genome-based phylogeny was inferred from the distances calculated with the genome BLAST distance method [
17] using the Type Strain Genome Server (TYGS) pipeline [
18].
The gene clusters encoding the enzymes involved on the biosynthesis of plant-growth promoting (PGP) molecules and mechanisms were manually mapped and annotated on the draft genome sequences of the strains using ARTEMIS [
19]. The ORFs annotation was performed based on protein domain sequence similarity comparisons against the Conserved Domains Database (CDD) considering criteria of GC reading-frame content [
20].
3. Results and Discussion
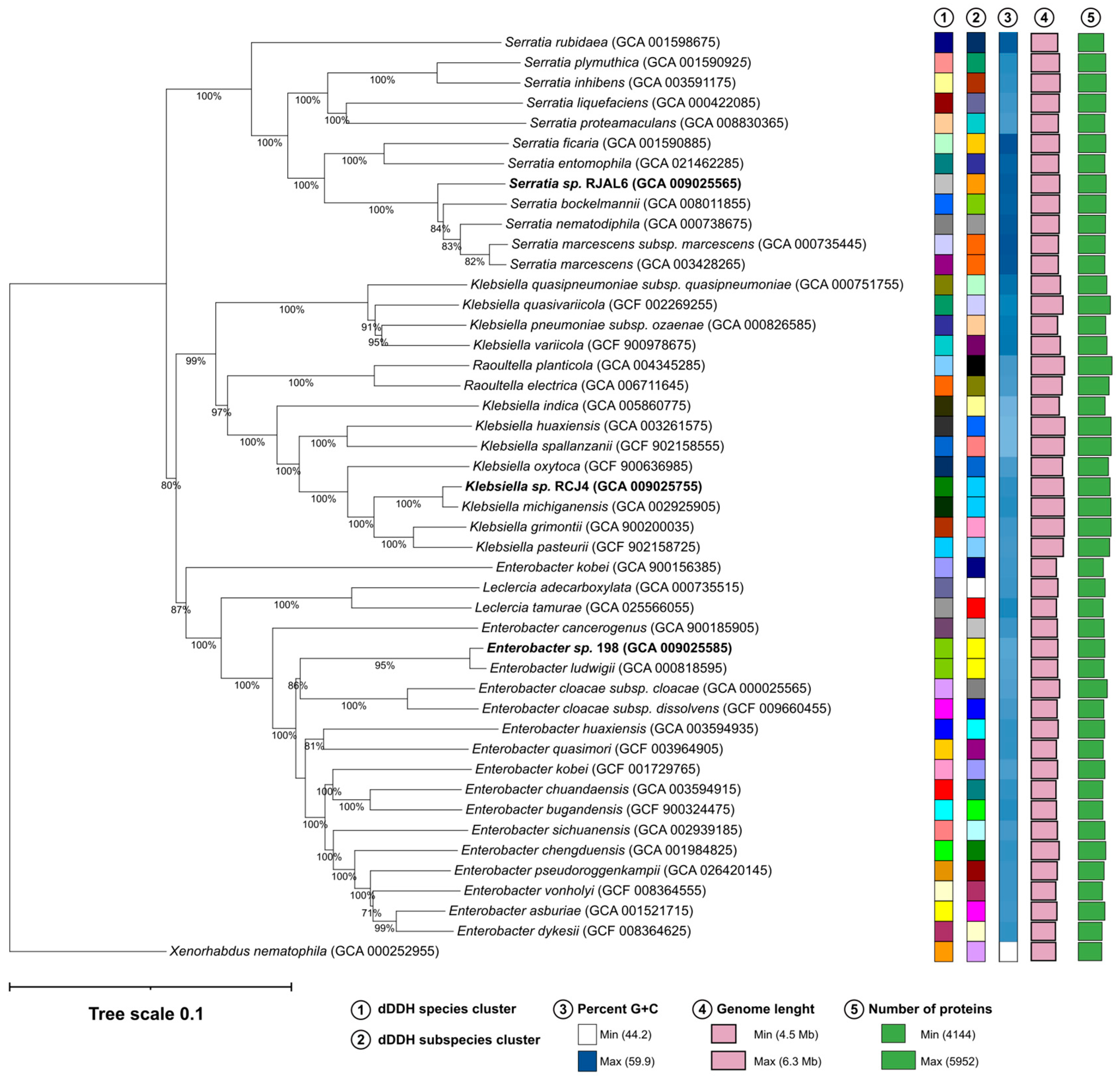
3.1. Genome based taxonomy
The complete 16 rRNA gene sequences (>1500 bp) extracted from the draft genome sequences of strains 198 and RJAL6 were found to be similar to Enterobacter ludwigii EN-119T (99.9%) and Serratia nematodiphila DSM 21420T (99.6%), respectively. Meanwhile the 16 rRNA gene sequence of the strain RCJ4 showed 100% of similarity with the sequences of Klebsiella pasteurii SPARK 836 C1T and Klebsiella michiganensis W14T.
The genome-based phylogeny showed on its distribution to the strain 198 positioned into a well-defined subclade with
Enterobacter ludwigii DSM 16688
T (GCA 000818595), with a bootstrap value of 95% (
Figure 1). The strain RCJ4 occupied a well-supported subclade with the genome sequences of
Klebsiella michiganensis DSM 25444
T (GCA 002925905), with a bootstrap value of 100%. The strain RJAL6 (previously identified as
Enterobacter sp.) form a well-supported distinct branch with a bootstrap value of 100%, closely related to
Serratia bockelmanii S3
T (GCA 008011855) and next to the subclade housing
S. nematodiphila,
S. marescens and
S. marescens subs. marescens (
Figure 1). The genomic sizes for the strains 198 and RCJ4 were 5.0 and 6.1 Mb, almost similar to the genome lengths of their phylogenetic neighbours
E. ludwigii DSM 16688
T (4.9 Mb) and
K. michiganensis DSM 25444
T (6.2 Mb) (
Figure 1). In terms of the G+C content, strains 198 and RCJ4 showed values 54.5% and 55.9%, almost identical to the values shown by
E. ludwigii DSM 16688
T (54.6%) and
K. michiganensis DSM 25444
T (56.0%) (
Figure 1). The strain RJAL6 showed a genome length of 5.3 Mb with a G+C content of 59.4%, differing in size with
S. nematodiphila DSM 21420
T (5.2 Mb) and in G+C content with
S. bockelmanii S3
T (59.0%) (
Figure 1). The draft genome sequences of strains 198 and RCJ4 showed dDDH values of 91.3% and 89.6% with its closest phylogenomic neighbours
Enterobacter ludwigii EN-119
T and
Klebsiella michiganensis 25444
T confirming their assignments to these described species. The dDDH value between the draft genome sequence of RJAL6 and its closest relatives
S. nematodiphila and
S. bockelmanii was 62.7% for both of them, below the 70% cut-off value used for a novel species designation [
21]. These results were in line with the OrthoANIu value of 95.3% obtained from the comparisons between RJAL6 with
S. nematodiphila and
S. bockelmanii respectively, accomplishing with the 95-96% threshold also used for prokaryotic species delimitation [
22]. These results strongly suggest to the strain
Serratia sp. RJAL6 candidate for a novel species.
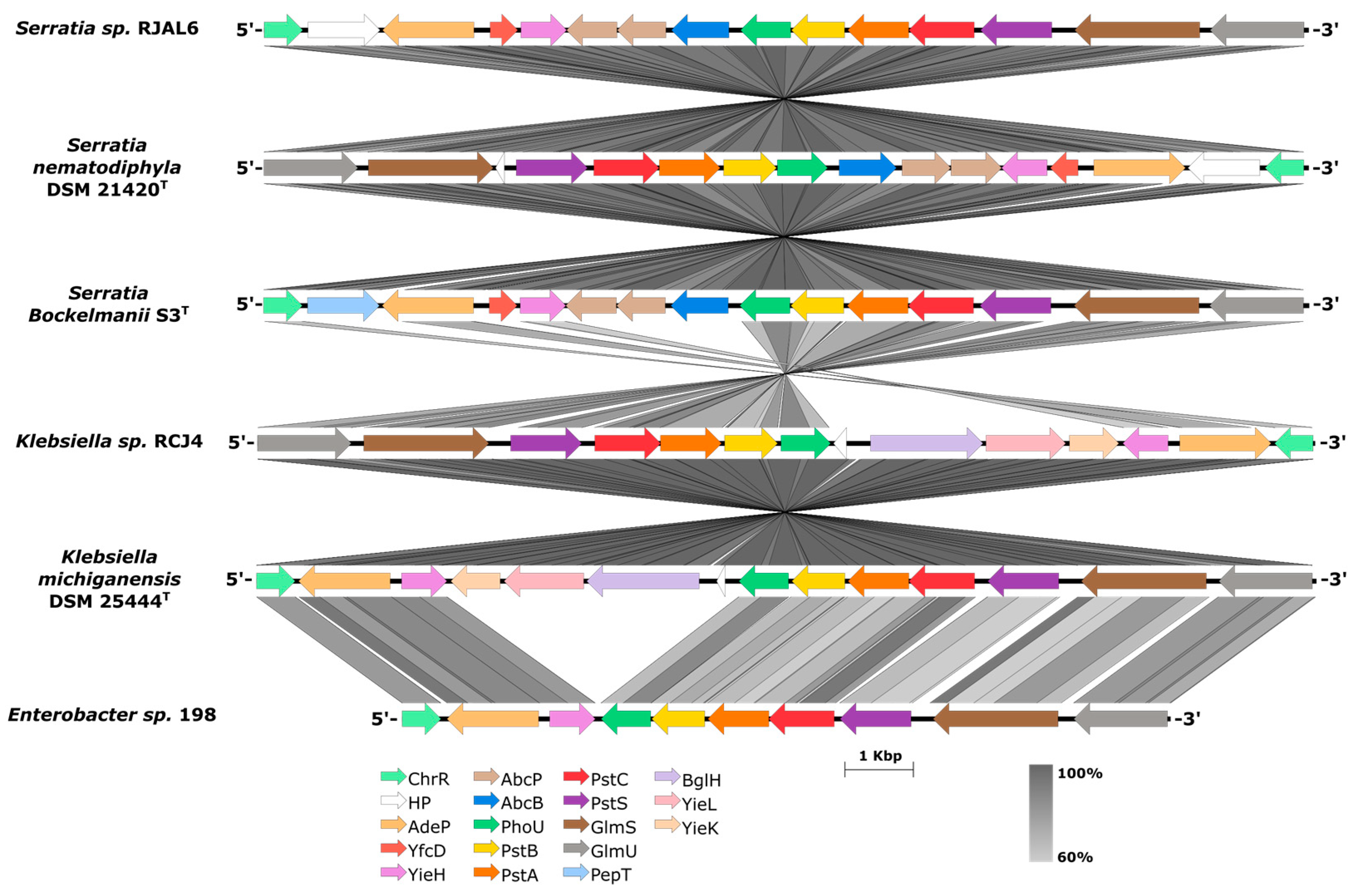
3.2. Phosphate uptake regulation genes
The draft genome sequences of
Enterobacter sp. 198,
Klebsiella sp. RCJ4 and the candidate for a novel species
Serratia sp. RJAL6, presented all the genes of the PhoU-
PstSCAB operon (
Figure 2) for the uptake and transport of Pi when cells undergo Pi starvation [
23]. The
pst operon (Phosphate specific transport) is a system constituted of 4 proteins, a periplasmic Pi binding protein PstS, the two integral membrane proteins PstC and PstA involved in the translocation of the Pi through the inner membrane and the peripherical ATPase membrane protein PstB which supports energy to the system [
24]. The additional PhoU protein would negatively regulates the signalling transmission and modulates the activity of
pstSCAB preventing excess of Pi import [
25]. Representative phylogenomic neighbours as
S. bockelmanii,
S. nematodiphila and
K. michiganensis also presented the complete PhoU-
PstSCAB operon (
Figure 2) evidencing the higher degree of conservation of this regulation system between PGP phosphobacteria species.
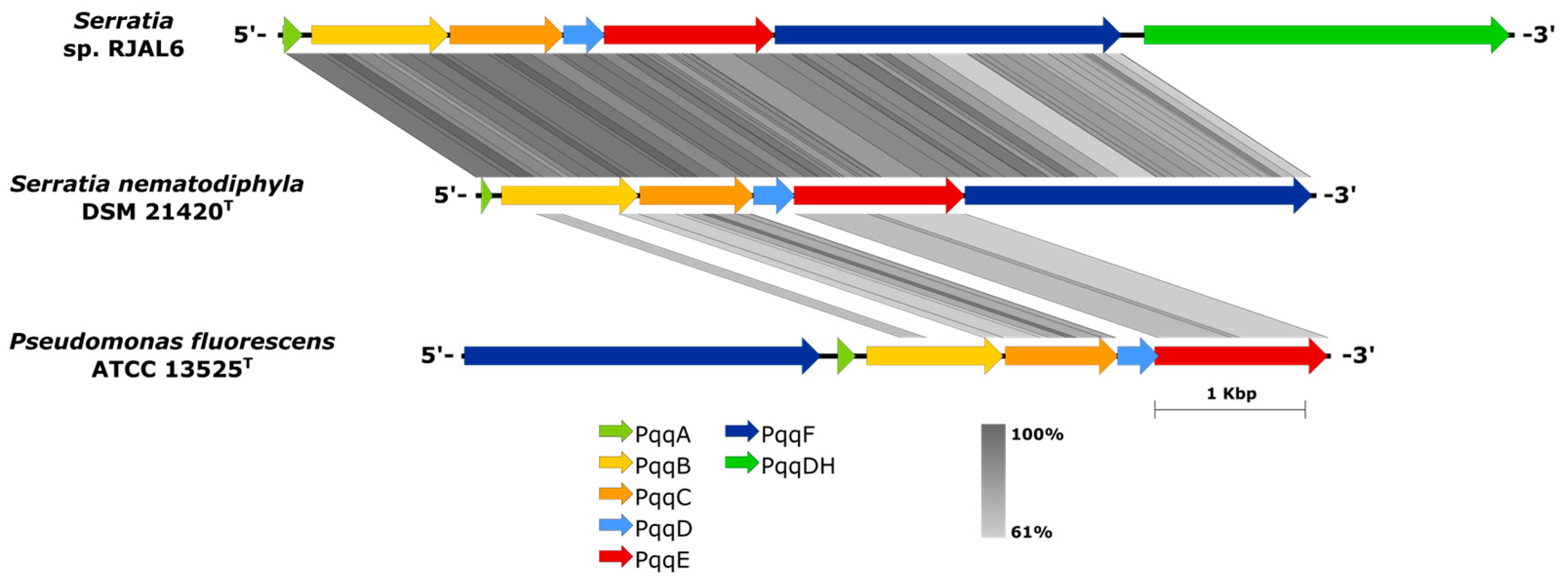
3.3. Gluconic acid production (Pqq genes)
Complete
pqqABCDEF operon for the biosynthesis of pyrroloquinoline quinone (PQQ) was present only in the draft genome sequence of
Serratia sp. RJAL6 (
Figure 3). Although little is known about the biosynthetic mechanisms by which the pyrroloquinoline quinone is produced, it has been well reported that
pqqA encodes for the precursor peptide for this coenzyme [
26]. Some chemical reactions have been hypothesized for the conversion of the precursor peptide
pqqA to the final PQQ but only the final step on the conversion from the intermediary 3a-(2-amino-2-carboxyethyl)-4,5-dioxo-4,5,6,7,8,9-hexahydroquinoline-7,9-dicarboxylic acid (AHQQ) by
pqqC encoding oxidase has been experimentally confirmed [
27]. In addition, the
pqqABCDEF operon found on
Serratia sp. RJAL6 includes an additional
Pqq gene that encodes for a pyrroloquinoline quinone-dependent periplasmic glucose dehydrogenase (
Figure 3), which would be the responsible for the conversion from glucose to gluconic acid [
8]. The gluconic acid is an organic acid produced by PGPB, which works as a chelator for cations bound to phosphate while acts reducing the pH, leading to the conversion of PO
43- to bioavailable sources of P for plants (HPO
42− and H
2PO
4-) [
28,
29].
3.4. Tryptophan-dependant auxin biosynthesis
The three phosphobacteria in this study were previously described to produce indole-3-acetic acid (IAA), the first described member of the phytohormones auxins [
30]. Several pathways to produce IAA have been described in bacteria, being most of them derived from the tryptophan biosynthetic pathway [
31]. The tryptophan biosynthetic pathway is well conserved among kingdoms and it is mediated by the enzymatic machinery encoded by the
trpEGDCFBA gene cluster [
32], which was present in the genome sequences of the three phosphobacteria described in this study (
Figure S1). The cluster comprising the anthranilate synthase subunits TrpE and TrpG, involved on the conversion of chorismate to anthranilate, an anthranilate-phosphoribosyl transferase TrpD which insert a phosphoribosyl phyrophosphate (PPi) to yield N-(5'-phosphoribosyl)-anthranilate, a bifunctional phosphoribosyl-anthranilate isomerase/indoleglycerol phosphate synthase TrpCF which catalyse the isomerization and subsequent conversion to indole 3-glycerol phosphate and the tryptophan synthase subunits TrpB and TrpA that converts indole 3-glycerol phosphate into L-tryptophan [
32]. Despite the accessorial enzymes involved on the conversion of tryptophan to IAA were not grouped along with or detected in the genomic neighbourhood of the
trpEGDCFBA gene cluster, the genomes of the three strains contained the
ipdC gene encoding for indole-pyruvate decarboxylase, a key enzyme in the synthesis of IAA from tryptophan through the intermediary indole pyruvate (IPyA) [
33].
3.5. Secondary metabolites
Isonitrile secondary metabolites, characterized by a nitrogen-carbon triple bond (-N≡C) group, display diverse bioactivities [
34]. Their biosynthesis in
Serratia sp. RJAL6 relies on the isonitrile synthase encoded by the isnA gene, which utilizes L-tryptophan or tyrosine as primary amino donors and ribulose-5-phosphate as the carbonyl donor. A subsequent oxidative decarboxylation step is then mediated by a Fe(II)/α-KG-dioxygenase encoded by the isnB gene [
35]. Genomic analysis revealed the presence of both these genes in
Serratia sp. RJAL6, a signature shared with its phylogenomic neighbors
S. bockelmanii and
S. nematodiphila (
Supplementary Figure S2). Furthermore, a glycosyltransferase gene, potentially involved in sugar conjugation as in rhabduscin biosynthesis, was also identified, strengthening the evidence for isonitrile production in this strain [
36].
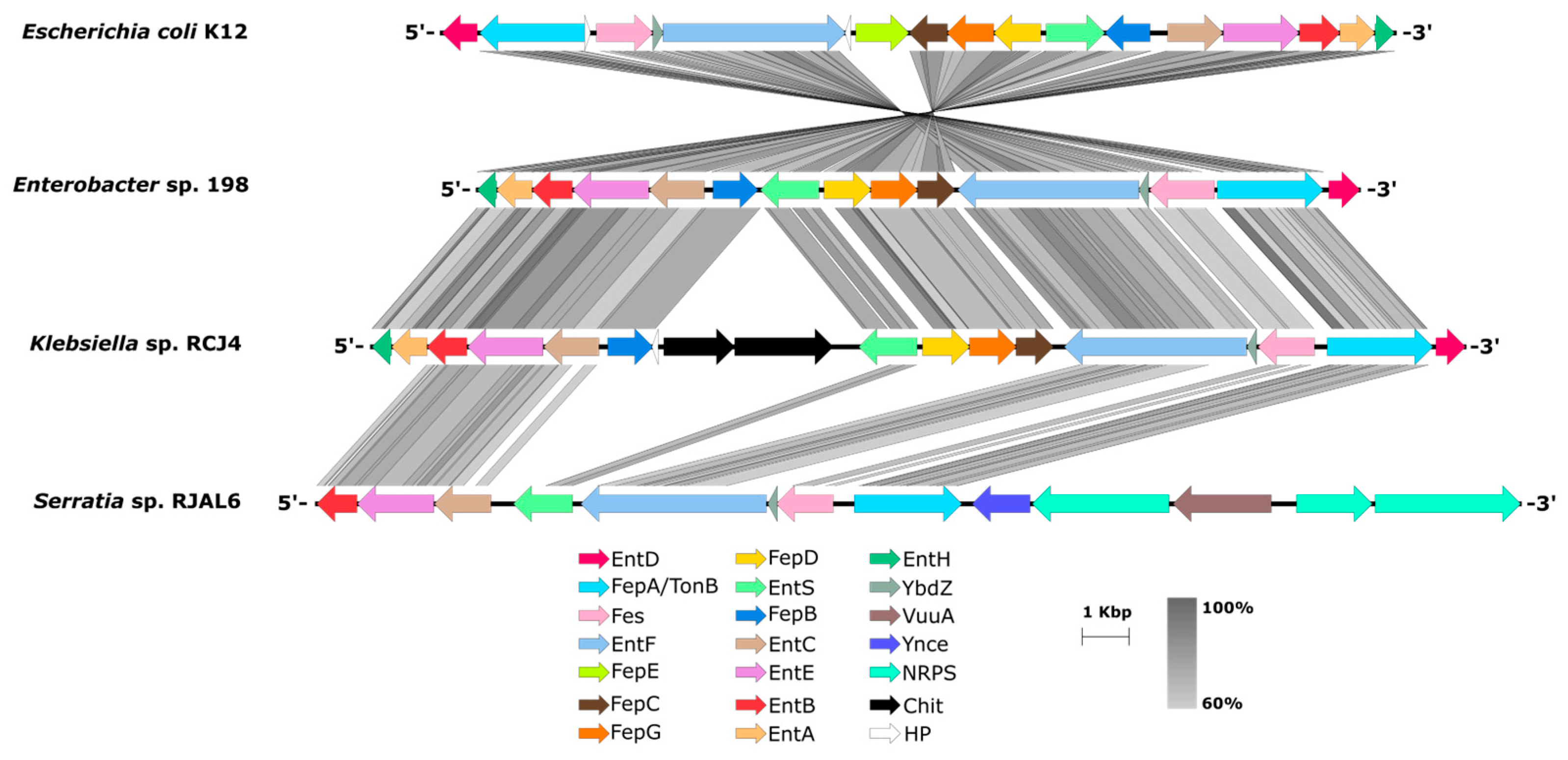
The three strains also evidenced the presence of the siderophores biosynthetic gene cluster, particularly the
ent gene cluster encoding enzymatic machinery for enterobactin production also encoded in
Escherichia coli K12 (
Figure 4). The enterobactin is a low molecular weight molecule capable to chelate ferric iron Fe
+3 with high affinity to deliver it to the cells [
37]. The biosynthesis of this compound initiates with the synthesis of 2,3-dihydroxybenzoate (DHB) from chorismate by the action of the 2,3-dihydro-2,3-dihydroxybenzoate dehydrogenase EntA, the isochorismatase EntB and the isochorismate synthase EntC to then being activated by the 2,3-dihydroxybenzoate-AMP ligase EntE [
38]. EntF is a non-ribosomal peptide synthase (NRPS), which it works catalysing the formation of DHB-L-Ser and additional NRPSs works polymerizing three of this units to then cyclize the trimer forming the lactone structure [
39]. The 4’-phosphopantetheinyl transferase EntD, required for the activation of EntB and EntF thiolation domains [
37] as well as the proofreading thiosterase EntH involved in the hydrolysis of thioester bond between EntB and wrongly charged molecules [
40] are also required for the biosynthesis process (
Figure 4). Interestingly, the genes
entA,
entH and
entD were not observed only in the syntenic subregion of the
ent gene cluster for the genomes of RJAL6 (
Figure 4) sharing this genomic feature with other
Serratia species as
S. bockelmanii,
S. nematodiphila and
S. marescens (NCBI genome database: GCA 008011855, GCA 000738675, GCA 003428265, respectively). Furthermore, a membrane exporter EntS mostly located between
ent-
fep (
Figure 4) is responsible for enterobactin secretion to the extracellular space [
41].
Following downstream in the genome sequences of the strains 198 and RCJ4 showed the
fepBDGCA genes comprising the ferric-enterobactin transport system (
Figure 4). The Fep transport system consist in a FepA, also known as TonB, outer membrane receptor for enterobactin, a FepB periplasmic protein, a FepC ATP-binding protein and two inner-membrane proteins FepD and FepG [
42]. Although strain RJAL6 only showed
fepA gene within the
ent genomic subregion (
Figure 4), the remaining
fepBDGC genes were also found in other regions of the genome (NCBI protein database: KAB5499428-KAB5499430 and KAB5499529). Once Fe
+3-enterobactin complex is formed and incorporated to the cell, the Fes esterase is responsible for releasing the iron from enterobactin into bacterial cytoplasm [
43] to be used for the cells.
In acidic soils, characterized for toxic Al
+3 levels, resident bacteria face a constant threat of Al toxicity. A recent proteomic investigation revealed an adaptation employed by Al-tolerant strains 198, RCJ4, and RJAL6 [
44]. These resilient bacteria exhibit a targeted upregulation of proteins crucial for iron (Fe) uptake and trafficking upon Al³⁺ exposure. The observed overproduction encompasses siderophore precursors, siderophore receptors, and Fe-siderophore transporters. This intriguing response suggests a countermeasure directed at mitigating potential disruption of intracellular Fe homeostasis, a phenomenon potentially caused by the competitive binding affinity of Al³⁺ for intracellular Fe³⁺ binding sites, leading to a putative "structural iron deficiency"[
44]. Remarkably, the siderophores produced by these bacteria are versatile, and while their primary function is Fe acquisition, they readily form stable chelates with other cations like Al
+3, creating extracellular Al-siderophore complexes [
10,
45]. This strategy would reduce cellular uptake of the toxic Al
+3, acting as a protective shield against its harmful effects.
Bacillus megaterium exemplifies this solution. When confronted with high Al and limited Fe, it ramps up production of two specific siderophores, schizokinen and Ndeoxyschizokinen, that likely hinder passive Al uptake [
46]. Therefore, the presence of Al responsive iron uptake genes, combined with versatile metal chelation mechanisms involving siderophores, emerges as a pivotal survival strategy for acid soils-adapted phosphobacteria.
4. Conclusions
Whole-genome sequencing of strains RJAL6, RCJ4, and 198 unveiled a compelling genetic repertoire underpinning their remarkable efficacy as plant growth-promoting bacteria (PGPB). All three strains harbor genes encoding the enzymatic apparatus for efficient phosphate solubilization, transport, and assimilation. This arsenal includes precursors for gluconic acid biosynthesis, a key organic acid implicated in chelating and mobilizing recalcitrant soil phosphates. In addition, these strains own the genes encoding the metabolic pathways to produce secondary metabolites such as the siderophore enterobactin and isonitrile molecules for the case of the strain RJAL6. All the strains are genetically benefited with the metabolic pathways to produce plant growth promotion hormones such as auxins or indole-3-acetic acid (IAA). The strain RJAL6 could be distinguished from its closest phylogenetic neighbors Serratia bockelmannii and Serratia nematodiphila by its genomic features and therefore it merits to be assigned to a novel species. This opens the range of further research applied to characterize the physiological, biochemical and genetic features of this strain and its prospective role as a novel PGPB.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Synteny of the trpEGDCFBA gene cluster sub-region in the genome sequences of the strains from this study.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, P.J.B-E., M.D-T. and P.D-C.; methodology, P.J.B-E., C.C-A. and G.L.; validation, P.J.B-E., M.D-T., M.d.l.L.M. and P.D-C; formal analysis, C.C-A., C.P. and P.J.B-E.; investigation, , C.C-A. and P.J.B-E.,; resources, P.J.B-E., M.D-T. and P.D-C.; data curation, P.J.B-E., C.C-A. and G.L.; writing—original draft preparation, C.C-A. and P.J.B-E.; writing—review and editing, P.J.B-E., M.D-T., C.P., M.d.l.L.M. and P.D-C; visualization, P.J.B-E., C.C-A. and G.L.; supervision, P.J.B-E.; project administration, P.J.B-E.; funding acquisition, P.J.B-E., M.D-T. and P.D-C.. All authors have read and agreed to the published version of the manuscript.” Please turn to the CRediT taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
Funding
This research was funded by the Agencia Nacional de Investigación y Desarrollo (ANID) of Chilean government through FONDECYT initiation project No. 11200377 (P.J.B.-E.) and FONDECYT Regular Projects No. 1241293 (P.J.B.-E.); 1210684 (M.D.-T.); 1201196 (P.D.-C.) and 1230084 (M.d.l.L.M.). This study was also funding by Proyecto REDES ETAPA INICIAL, Convocatoria 2017, REDI170334 (P.J.B.-E.), by Concurso Anillos de Investigación en Áreas Temáticas, ANID ATE220038 (P.D.-C., M.D.-T., P.J.B.-E.) and by Universidad de La Frontera (DiUFRO), Proyectos de Investigación Vinculados a la Red Nexer No. DNX22-0009 (PJB) and DNX22-0005 (P.D.-C).
Acknowledgments
The authors acknowledge to Scientific and Technological Bioresource Nucleus of Universidad de La Frontera (BIOREN–UFRO) and Service Management Analytical Research and Training Center (SmartC-BIOREN).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barra, P. J., Inostroza, N. G., Acuña, J. J., Mora, M. L., Crowley, D. E., Jorquera, M. A. (2016). Formulation of bacterial consortia from avocado (Persea americana Mill.) and their effect on growth, biomass and superoxide dismutase activity of wheat seedlings under salt stress. Appl. Soil Ecol. 102, 80–91. [CrossRef]
- Barra, P. J., Inostroza, N. G., Mora, M. L., Crowley, D. E., Jorquera, M. A. (2017). Bacterial consortia inoculation mitigates the water shortage and salt stress in an avocado (Persea americana Mill.) nursery. Appl. Soil Ecol. 111, 39–47. [CrossRef]
- Barra, P. J., Pontigo, S., Delgado, M., Parra–Almuna, L., Duran, P., Valentine, A. J., Jorquera, M. L., Mora, M. L. (2019). Phosphobacteria inoculation enhances the benefit of P–fertilization on Lolium perenne in soils contrasting in P–availability. Soil Biol. Biochem. 136, 107516. [CrossRef]
- Inostroza, N.G., Barra, P.J., Wick, L.Y., Mora, M.L., Jorquera, M.A. (2017). Effect of rhizobacterial consortia from undisturbed arid- and agro-ecosystems on wheat growth under different conditions. Lett. Appl. Microbiol. 64, 158–163. [CrossRef]
- Glick, B. R. (2012). Plant growth-promoting bacteria: mechanisms and applications. Scientifica, 2012, 963401. [CrossRef]
- Ali, S. and Glick, B. R. (2019). Plant–bacterial interactions in management of plant growth under abiotic stresses. In New and Future Developments in Microbial Biotechnology and Bioengineering, 21–45. Amsterdam, the Netherlands: Elsevier. [CrossRef]
- Singh, J. S., and Gupta, V. K. (2018). Soil microbial biomass: A key soil driver in management of ecosystem functioning. Sci. Total Environ., 634, 497–500. [CrossRef]
- Castagno, L. N., Sannazzaro, A. I., Gonzalez, M. E., Pieckenstain, F. L., Estrella, M. J. (2021). Phospkhobacteria as key actors to overcome phosphorus deficiency in plants. Ann Appl Biol. 2021; 178: 256– 267. [CrossRef]
- Viruel, E., Lucca, M. E., Siñeriz, F. (2011). Plant growth promotion traits of phosphobacteria isolated from Puna, Argentina. Archives of microbiology, 193(7), 489–496. [CrossRef]
- Mora, M. L., Demanet, R., Acuña J., Viscardi, S., Jorquera, M., Rengel, Z., Durán, P. (2017). Aluminum-tolerant bacteria improve the plant growth and phosphorus content in ryegrass grown in a volcanic soil amended with cattle dung manure. Appl. Soil Ecol. 115, 19–26. [CrossRef]
- Barra, P. J., Viscardi, S., Jorquera, M. A., Duran, P. A., Valentine, A. J., Mora, M. L. (2018). Understanding the Strategies to Overcome Phosphorus-Deficiency and Aluminum-Toxicity by Ryegrass Endophytic and Rhizosphere Phosphobacteria. Frontiers in microbiology, 9, 1155. [CrossRef]
- Jackman, S. D., Vandervalk, B. P., Mohamadi, H., Chu, J., Yeo, S., Hammond, S. A., Jahesh, G., Khan, H., Coombe, L., Warren, R. L., Birol, I. (2017). ABySS 2.0: resource-efficient assembly of large genomes using a Bloom filter. Genome research, 27(5), 768–777. [CrossRef]
- Aziz, R.K., Devoid, S., Disz, T., Edwards, R.A., Henry, C.S., Olsen, G.J., Olson., R., Overbeek, R., Parello, B., Pusch, G.D., Stevens, R.L., Vonstein, V., Xia, F. (2012). SEED Servers: High-Performance Access to the SEED Genomes, Annotations, and Metabolic Models. Plos one. 7(10), e48053. [CrossRef]
- Yoon, S. H., Ha, S. M., Lim, J., Kwon, S., Chun, J. (2017a). A largescale evaluation of algorithms to calculate average nucleotide identity. Antonie van Leeuwenhoek. 110, 1281–1286. [CrossRef]
- Yoon, S. H., Ha, S. M., Kwon, S., Lim, J., Kim, Y., Seo, H., and Chun, J. (2017b). Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. International journal of systematic and evolutionary microbiology, 67(5), 1613–1617. [CrossRef]
- Meier-Kolthoff, J.P., Auch, A.F., Klenk, H-P., Göker, M. (2013). Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 14, 60. [CrossRef]
- Auch, A. F., Henz, S. R., Holland, B. R., Göker, M. (2006). Genome BLAST distance phylogenies inferred from whole plastid and whole mitochondrion genome sequences. BMC Bioinform.7, 350. [CrossRef]
- Meier-Kolthoff, J.P. and Göker, M. (2019). TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat Commun. 10, 2182. [CrossRef]
- Berriman, M., and Rutherford, K. M. (2003). Viewing and annotating sequence data with Artemis. Brief. Bioinform. 4, 124–132. [CrossRef]
- Bibb, M. J., Findlay, P. R., Johnson, M. W. (1984). The relationship between base composition and codon usage in bacterial genes and its use for the simple and reliable identification of protein-coding sequences. Gene 30, 157–166. [CrossRef]
- Meier-Kolthoff, J.P., Hahnke, R.L., Petersen, J., Scheuner, C., Michael, V., Fiebig, A., Rohde, C., Rohde, M., Fartmann, B., Goodwin, L.A., Chertkov, O., Reddy, T., Pati, A., Ivanova, N.N., Markowitz, V., Kyrpides, N.C., Woyke, T., Göker, M., Klenk, H-P. (2014). Complete genome sequence of DSM 30083T, the type strain (U5/41T) of Escherichia coli, and a proposal for delineating subspecies in microbial taxonomy. Stand Genomic Sci. 9, 2. [CrossRef]
- Richter, M. and Rosselló-Móra, R. (2009). Shifting the genomic gold standard for the prokaryotic species definition. Proc. Natl. Acad. Sci. U.S.A., 106(45), 19126–19131. [CrossRef]
- Rao, N. N., and Torriani, A. (1990). Molecular aspects of phosphate transport in Escherichia coli. Mol. Microbiol., 4(7), 1083–1090. [CrossRef]
- Aguena, M., Yagil, E. Spira, B. (2002). Transcriptional analysis of the pst operon of Escherichia coli. Mol. Genet. Genom., 268(4), 518–524. [CrossRef]
- Gardner, S. G., McCleary, W. R. (2019). Control of the phoBR Regulon in Escherichia coli. EcoSal Plus, 8(2). [CrossRef]
- Puehringer, S., Metlitzky, M. Schwarzenbacher, R. (2008). The pyrroloquinoline quinone biosynthesis pathway revisited: a structural approach. BMC biochemistry, 9, 8. [CrossRef]
- Klinman, J. P., and Bonnot, F. (2014). Intrigues and intricacies of the biosynthetic pathways for the enzymatic quinocofactors: PQQ, TTQ, CTQ, TPQ, and LTQ. Chem. Rev., 114(8), 4343–4365. [CrossRef]
- Chen, Y. P., Rekha, P. D., Arun, A. B., Shen, F. T., Lai, W. A., Young, C. C. (2006). Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl. Soil Ecol., 34, 33–41. [CrossRef]
- Prabhu, N., Borkar, S., Garg, S. (2019). Phosphate solubilization by microorganisms. In Advances in Biological Science Research, 161–176. Amsterdam, the Netherlands: Elsevier. [CrossRef]
- Kogl, F. and Kostermans, D. (1934). Hetero-auxin als Stoffwechselprodukt niederer pflanzlicher Organismen. Isolierung aus Hefe.13. Mitteilung uber pflanzliche Wachstumsstoffe. Hoppe-Seyler´s Zeitschrift fur physiologische Chemie, 228:113-121. [CrossRef]
- Morffy, N. and Strader, L. C. (2020). Old Town Roads: routes of auxin biosynthesis across kingdoms. Curr. Opin. Plant Biol., 55, 21–27. [CrossRef]
- Kagan, J., Sharon, I., Beja, O., Kuhn, J. C. (2008). The tryptophan pathway genes of the Sargasso Sea metagenome: new operon structures and the prevalence of non-operon organization. Genome Biol., 9(1), R20. [CrossRef]
- Khan, A. R., Park, G. S., Asaf, S., Hong, S. J., Jung, B. K., Shin, J. H. (2017). Complete genome analysis of Serratia marcescens RSC-14: A plant growth-promoting bacterium that alleviates cadmium stress in host plants. PloS one, 12(2), e0171534. [CrossRef]
- Chen, T.-Y., Chen, J., Tang, Y., Zhou, J., Guo, Y., Chang, W.-C. (2021), Current Understanding toward Isonitrile Group Biosynthesis and Mechanism. Chin. J. Chem., 39: 463-472. [CrossRef]
- Brady, S. F., and Clardy, J. (2005). Cloning and heterologous expression of isocyanide biosynthetic genes from environmental DNA. Angewandte Chemie (International ed. in English), 44(43), 7063–7065. [CrossRef]
- Crawford, J. M., Kontnik, R., Clardy, J. (2010). Regulating alternative lifestyles in entomopathogenic bacteria. Curr. Biol. 20(1), 69–74. [CrossRef]
- Reitz, Z. L., Sandy, M., Butler, A. (2017). Biosynthetic considerations of triscatechol siderophores framed on serine and threonine macrolactone scaffolds. Metallomics, 9(7), 824–839. [CrossRef]
- Rusnak, F., Faraci, W. S., Walsh, C. T. (1989). Subcloning, expression, and purification of the enterobactin biosynthetic enzyme 2,3-dihydroxybenzoate-AMP ligase: demonstration of enzyme-bound (2,3-dihydroxybenzoyl) adenylate product. Biochemistry, 28(17), 6827–6835. [CrossRef]
- Zane, H. K., Naka, H., Rosconi, F., Sandy, M., Haygood, M. G., Butler, A. (2014). Biosynthesis of amphi-enterobactin siderophores by Vibrio harveyi BAA-1116: identification of a bifunctional nonribosomal peptide synthetase condensation domain. J. Am. Chem. Soc. 136(15), 5615–5618. [CrossRef]
- Leduc, D., Battesti, A., Bouveret, E. (2007). The hotdog thioesterase EntH (YbdB) plays a role in vivo in optimal enterobactin biosynthesis by interacting with the ArCP domain of EntB. Journal of bacteriology, 189(19), 7112–7126. [CrossRef]
- Furrer, J. L., Sanders, D. N., Hook-Barnard, I. G., McIntosh, M. A. (2002). Export of the siderophore enterobactin in Escherichia coli: involvement of a 43 kDa membrane exporter. Mol. Microbiol., 44(5), 1225–1234. [CrossRef]
- Chenault, S. S., and Earhart, C. F. (1991). Organization of genes encoding membrane proteins of the Escherichia coli ferrienterobactin permease. Mol. Microbiol., 5(6), 1405–1413. [CrossRef]
- Caza, M., Garénaux, A., Lépine, F., Dozois, C. M. (2015). Catecholate siderophore esterases Fes, IroD and IroE are required for salmochelins secretion following utilization, but only IroD contributes to virulence of extra-intestinal pathogenic Escherichia coli. Mol. Microbiol., 97(4), 717–732. [CrossRef]
- Barra, P. J., Duran, P., Delgado, M., Viscardi, S., Claverol, S., Larama, G., Dumont, M., Mora, M. L. (2023). Proteomic response to phosphorus deficiency and aluminum stress of three aluminum-tolerant phosphobacteria isolated from acidic soils. iScience, 26(10), 107910. [CrossRef]
- Schalk, I. J., Hannauer, M., Braud, A. (2011). New roles for bacterial siderophores in metal transport and tolerance. Environ. Microbiol., 13(11), 2844–2854. [CrossRef]
- Hu, X., and Boyer, G. L. (1996). Siderophore-Mediated Aluminum Uptake by Bacillus megaterium ATCC 19213. Appl. Environ. Microbiol., 62(11), 4044–4048. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).