Preprint
Article
Field Shaping for Transcutaneous Stimulation of Peripheral Nerves
Altmetrics
Downloads
140
Views
90
Comments
0
This version is not peer-reviewed
Neurorehabilitation in Movement Disorders and Neurodegenerative Diseases
Submitted:
17 January 2024
Posted:
17 January 2024
You are already at the latest version
Alerts
Abstract
Transcutaneous nerve stimulation has several neuromodulatory applications, including pain management, sensory restoration, and motor recovery. Transcutaneous stimulation delivered via electrode arrays offers ease of use and reconfigurability through non-invasive means. Also, employing electrode arrays allows regulation of the permeated electric fields for greater control over the stimulation performance. Field shaping involves the regulation of permeated electric fields and is a well-established practice for deep brain stimulation; however, its applicability to peripheral nerve stimulation still needs exploration. Adopting such techniques can help to overcome current limitations with transcutaneous stimulation, like poor targetability, early onset of fatigue, and perceived discomfort. This article provides a perspective on the applicability of field-shaping techniques in improving the overall stimulation outcome.
Keywords:
Subject: Engineering - Bioengineering
Background
Transcutaneous stimulation via electrode arrays is becoming prevalent for personalized and home-based rehabilitation as they offer non-invasiveness, ease of use, reusability, and reconfigurability [1,2,3]. They facilitate the modulation of nerve activity by activating or inhibiting nerve function for motor recovery, pain management, and sensory restoration. The field is also seeing advances with wearable neuroprostheses utilizing electrode arrays for assistive and therapeutic interventions [4,5,6]. Electrodes within an array have innate electric field interactions that permeate through tissue layers to excite the target region. For highly selective stimulation, these electric fields must be confined to the target areas [7]; however, regulating these fields can be arduous with transcutaneous delivery. The resulting charge spillage can activate the neighboring regions. Due to poor control, applications of electrical stimulation to motor function [2,8,9] still face challenges, including co-contraction of neighboring muscles due to poor targetability, discomfort, and rapid onset of fatigue due to synchronous activation. Often, sophisticated stimulation techniques are explored to improve the overall outcome.
Field shaping is a technique that involves the regulation of complex electric fields to facilitate targeted stimulation [10]. This is achieved through control over the shape, size, and location of active electrodes in an array, and the stimulation waveform. Ultimately, generating complex spatial stimulation patterns allows targeting narrower regions than those stimulated using traditional monopolar strategies. This technique is widely used for near-field stimulation, applied to deep brain stimulation (DBS), visual and auditory prostheses. These applications deploy segmented electrodes or electrodes with multi-contact designs to facilitate the formation of complex stimulation patterns [11]. Applications involving near-field stimulation embed electrodes directly in the neural tissue. However, for transcutaneous stimulation, the electric field permeation is also influenced by the properties of tissue layers in addition to electrode properties.
Integrating field shaping into clinical DBS systems facilitated customized stimulation delivery for improved therapeutic efficacy [10,12]. For spinal cord stimulation (SCS), cathodal steering enabled the targeting of neural tissues for pain relief [13,14]. Modeling and analyzing these electric fields help optimize stimulation protocols, tailoring electrode configurations, intensities, and duration to improve the clinical outcome of SCS [15]. Similarly, utilizing electrode arrays and optimizing separatrices avoided crosstalk and facilitated the electric field into target volumes for ophthalmology applications [16]. Also, optimal electrode settings and stimulus parameters modulated the retina observation surface electric field distribution [17]. Theoretical and physical modeling studies indicate that field-shaping strategies effectively stimulate narrow regions of the auditory nerve [18]. Current steering also improved the spectral resolution and pitch perception with cochlear implants [19]. Advances in high-density electrode arrays may provide a low-power, high-resolution alternative to current steering with contemporary cochlear arrays [20].
As mentioned above, field-shaping and electrode design advances have improved the status quo of near-field stimulation. Similarly, complex stimulation patterns from shaping the electric fields can be leveraged for transcutaneous stimulation. Specifically, for applications involving motor function, these novel stimulation strategies can be explored to improve spatial selectivity, targetability, comfort, regulation of stimulation-induced fatigue, and compensation of electrode displacement. With this aim, this article provides a perspective on the applicability of field-shaping to transcutaneous stimulation delivered via electrode arrays.
Computational Modeling to Study Field Shaping
Computational models are often relied upon to study electric field distribution, as assessing them is arduous in an experimental setting. These models have helped with characterizing electrodes for DBS, retinal and auditory prostheses [12,15,17]. Similarly, such models are widely used to study transcutaneous stimulation, including the prediction of sensory and motor thresholds [21,22], assessment of stimulation protocols [23] and improvements to electrode designs [24,25]. Furthermore, single-stage models that simultaneously capture field distributions in a volume of tissue layers and the resulting neuronal response can further advance the applicability of field shaping to transcutaneous stimulation [26]. The model predicted current density and electric field distributions help derive metrics for stimulation selectivity, comfort, and safety. As in Table 1, these metrics can quantify the overall stimulation performance when assessing several field-shaping strategies.
Field Shaping by Modifying Electrode Properties
Electric field modulation can be facilitated by electrode morphology or configuration pattern. Field shaping is commonly done using several return configurations and current steering methods. Activation of several electrodes simultaneously modifies the shape of the electric field. This is due to the summation effect from two or more simultaneous electric fields. Such multipolar configurations improve upon traditional monopolar stimulation [31,32,33]. These electric fields can be further modified by altering the parameters of the stimulation waveform. Furthermore, the geometric, chemical, mechanical, and physical configuration of electrodes also influences the stimulation performance [34]. While several parameters provide leverage over the modulation of electric fields, studies that apply field-shaping strategies to transcutaneous stimulation are still scarce.
Figure 1 depicts the framework for implementing and assessing field-shaping techniques for transcutaneous stimulation. The stimulation outcome is evaluated for selectivity, comfort, and safety by varying the electrode properties, configuration, and stimulation parameters. Existing studies that modify these input parameters to improve stimulation outcomes are discussed in the following sections.
Several studies have assessed the influence of electrode size for transcutaneous stimulation using computational models. Smaller electrodes tend to be more selective but are prone to discomfort. However, the choice of electrode size also comes down to muscle targets. A study by [35] recommended larger electrodes for more comfortable stimulation. It required a lower amplitude to simulate larger muscles like quadriceps, hamstrings, and gluteal muscles. Since forearm muscles are tightly packed, the use of smaller surface area electrodes is preferred for selective activation [2,36,37]. Nevertheless, small surface area electrodes are discomforting due to their high current density profiles [9,21]. Small electrodes are comfortable for thin fat layers and superficial nerves, and larger electrodes are more comfortable for thicker fat layers and deeper nerves [38]. Also, small electrodes give more granularity with electrode arrays, and their shapes can be altered dynamically.
Each electrode geometry can have a unique current density and field distribution profile [39]. Thus, by customizing the electrode geometry, the AV can be regulated. Commercial multi-layered electrodes are bulky and have a large form factor. Hence, they tend to constrain most forearm movements and can be far from desirable. Achieving comfortable stimulation by changing the surface geometry emphasizes the potential for simple, single-layered electrodes. Also, for transcranial stimulation, concentric and ring-like geometries performed better than conventional electrodes [40,41]. Studies assessing the influence of electrode geometries for cardiac pacing and neurostimulation have reported several advances using high-perimeter electrodes. The study by [30] used model-based analysis and experimentation to demonstrate that electrode geometries and their underlying current distribution are important for selectivity and comfort. And have identified suitable electrode geometries that offered better stimulation performance than conventional circular electrodes.
The electrode-skin impedance and the electrode properties primarily steer the current density distributions on the electrode surface [42,43]. Stimulation electrodes have regions of high current density along their periphery. These regions can cause inefficient stimulation and mild inflammatory responses [9,44]. Also, the penetration of high-density current into the dermo-epidermal regions induces pain sensation [21]. The electrode surface has to be modified to achieve optimal current distribution [9,37,45] or by adding current redistribution layers [46,47,48]. Notably, electrode arrays are also influenced by the presence of hydrogel [49]. The performance of conductive hydrogels that are used to redistribute the currents is highly non-linear, subjective, and deteriorates over time [50] [51,52]. As the stimulation efficacy and focus are affected by hydrogel resistivity, a modeling study suggests using a high-resistivity hydrogel interface layer only for short periods [50]. Alternatively, wet textile electrodes have also shown similar stimulation performance to conventional electrodes [53].
Dry carbon-based electrodes that are conformable also have good stimulation performance and are suitable for wearable electrode arrays [6]. The study computationally assessed different materials and how their conductivities affected the electric field distribution.
Controlling the magnitude, polarity, and number of active contacts introduces additional control over the shape of the electric field. Several studies have assessed the influence of electrode size, shape, and configuration on the permeated electric field [30,39,54].
An ideal electrode configuration must confine the charges to targeted regions and elicit a submaximal response. Also, when considering optimal electrode configurations, in addition to leveraging overlapping electric fields for larger areas of activation, their effect on muscle fatigue must be considered. When considering multiple electrodes, there is a slight increase in fatigue conditions compared to single electrodes [55]. Still, this can be mitigated with multi-array electrode configurations through asynchronous stimulation. Using multiple current sources is an effective means of stimulation [56]. Similarly, stimulation delivered via multi-pad electrodes delayed the occurrence of fatigue [57]. Additionally, the use of multipolar configuration can help to compensate for shifts in electrode displacement [7,58,59].
If the two stimulation electrodes have the same surface area, the electric field generated between them and its consequent effect on the excitability of a target motor point is influenced by the inter-electrode distance (IED). A consensus is that selectivity and muscle recruitment increase with a decrease in IED. However, for large muscles such as the quadriceps or tibialis anterior, muscle recruitment increased with IED due to the availability of multiple motor points along the path of the charge flow. Many fibers can be activated by choosing optimal inter-electrode distances[55]. For larger electrodes, [60] demonstrated that a large volume of tissues was active with a small inter-electrode distance. However, the inter-electrode distance must be larger for small electrodes to activate large volumes of tissues. Another study indicated that smaller inter-electrode distances would result in more focal stimulation when applied to areas with low subcutaneous fat [61]. Nevertheless, guidelines in choosing appropriate electrode configurations for a specific muscle to elicit desired contraction levels are limited. Despite the inter-subject variability due to nerve depth and fat thickness, studies have achieved the desired muscle response with suitable electrode configurations that facilitated selective field generation. Optimization algorithms can help to identify suitable electrode locations and configurations to evoke the target response [59,62,63].
Electrode arrays have inherent challenges with setup time, strategies to identify suitable virtual electrode configurations, reducing discomfort, and user integration. Also, the inter-subject variability due to never depth, fat thickness, and other intrinsic factors influence the overall stimulation outcome. Hence, it remains an optimization problem to identify suitable electrode combinations and stimulation parameters to customize stimulation delivery. Still, advances in electronics and switching circuits are improving the control of electrode arrays with multiple current sources. Also, the calibration time with electrode arrays can be simplified by automated algorithms [59]. Moreover, field-shaping strategies can improve transcutaneous stimulation when combined with advances in stimulation techniques, electrode configurations, material properties, and interface layers.
Conclusion
Field shaping allows for regulating permeated electric fields and is a well-established practice for deep brain stimulation; however, its applicability to peripheral nerve stimulation still needs exploration. Adopting such techniques can help to overcome current limitations with transcutaneous stimulation, like poor targetability, early onset of fatigue, and perceived discomfort.
References
- N. M. Malešević et al., ‘A multi-pad electrode based functional electrical stimulation system for restoration of grasp’, J Neuroeng Rehabil, vol. 9, no. 1, p. 66, 2012. [CrossRef]
- A.D. Koutsou, J. C. Moreno, A. J. del Ama, E. Rocon, and J. L. Pons, ‘Advances in selective activation of muscles for non-invasive motor neuroprostheses’, J Neuroeng Rehabil, vol. 13, no. 1, p. 56, 2016. [CrossRef]
- D. A. Friedenberg et al., ‘Neuroprosthetic-enabled control of graded arm muscle contraction in a paralyzed human’, Sci Rep, vol. 7, no. 1, pp. 1–10, 2017. [CrossRef]
- H. Usman, Y. Zhou, B. Metcalfe, and D. Zhang, ‘A Functional Electrical Stimulation System of High-Density Electrodes with Auto-Calibration for Optimal Selectivity’, IEEE Sens J, vol. 1748, no. c, pp. 1–1, 2020. [CrossRef]
- A. Crema, N. Malešević, I. Furfaro, F. Raschellà, A. Pedrocchi, and S. Micera, ‘A Wearable Multi-Site System for NMES-Based Hand Function Restoration’, IEEE Transactions on Neural Systems and Rehabilitation Engineering, vol. 26, no. 2, pp. 428–440, 2018. [CrossRef]
- N. Ravichandran, M. Y. Teo, A. Mcdaid, and K. Aw, ‘Conformable Electrode Arrays for Wearable Neuroprostheses’, Sensors, vol. 23, no. 6, p. 2982, Mar. 2023. [CrossRef]
- N. RaviChandran, K. C. Aw, and A. McDaid, ‘Characterizing the Motor Points of Forearm Muscles for Dexterous Neuroprostheses’, IEEE Trans Biomed Eng, vol. 67, no. 1, pp. 50–59, Jan. 2020. [CrossRef]
- H. Zhou et al., ‘Stimulating the Comfort of Textile Electrodes in Wearable Neuromuscular Electrical Stimulation’, Sensors, vol. 15, no. 7, pp. 17241–17257, Jul. 2015. [CrossRef]
- T. Keller and A. Kuhn, ‘Electrodes for transcutaneous (surface) electrical stimulation’, Journal of Automatic Control, vol. 18, no. 2, pp. 35–45, 2008. [CrossRef]
- C. R. Butson and C. C. McIntyre, ‘Current steering to control the volume of tissue activated during deep brain stimulation’, Brain Stimul, vol. 1, no. 1, pp. 7–15, Jan. 2008. [CrossRef]
- J. S. Brittain and H. Cagnan, ‘Recent Trends in the Use of Electrical Neuromodulation in Parkinson’s Disease’, Current Behavioral Neuroscience Reports, vol. 5, no. 2. Springer, pp. 170–178, Jun. 01, 2018. [CrossRef]
- M. Parazzini et al., ‘A computational model of the electric field distribution due to regional personalized or nonpersonalized electrodes to select transcranial electric stimulation target’, IEEE Trans Biomed Eng, vol. 64, no. 1, pp. 184–195, Jan. 2017. [CrossRef]
- L. Manola, J. Holsheimer, P. H. Veltink, K. Bradley, and D. Peterson, ‘Theoretical Investigation Into Longitudinal Cathodal Field Steering in Spinal Cord Stimulation’, Neuromodulation: Technology at the Neural Interface, vol. 10, no. 2, pp. 120–132, Apr. 2007. [CrossRef]
- L. N. Mishra, G. Kulkarni, and M. Gadgil, ‘A novel current steering method for targeted spinal cord stimulation’, Frontiers in Pain Research, vol. 4, p. 1028368, Feb. 2023. [CrossRef]
- M. Guidetti et al., ‘Modeling Electric Fields in Transcutaneous Spinal Direct Current Stimulation: A Clinical Perspective’, Biomedicines, vol. 11, no. 5. MDPI, May 01, 2023. [CrossRef]
- ‘Electric Field Shaping Via Separatrices For Focused Electric Retinal Stimulation Via Retinal Implants’.
- Z. Lu et al., ‘An in-silico analysis of retinal electric field distribution induced by different electrode design of trans-corneal electrical stimulation’, J Neural Eng, vol. 19, no. 5, p. 055004, Oct. 2022. [CrossRef]
- B. H. Bonham and L. M. Litvak, ‘Current focusing and steering: Modeling, physiology, and psychophysics’, Hear Res, vol. 242, no. 1–2, p. 141, Aug. 2008. [CrossRef]
- C.-C. Wu and X. Luo, ‘Current Steering with Partial Tripolar Stimulation Mode in Cochlear Implants’, Journal of the Association for Research in Otolaryngology, vol. 14, no. 2, pp. 213–231, Apr. 2013. [CrossRef]
- J. D. Falcone and P. T. Bhatti, ‘Current steering and current focusing with a high-density intracochlear electrode array’, in 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, IEEE, Aug. 2011, pp. 1049–1052. [CrossRef]
- C. D. Mørch, K. Hennings, and O. K. Andersen, ‘Estimating nerve excitation thresholds to cutaneous electrical stimulation by finite element modeling combined with a stochastic branching nerve fiber model’, Med Biol Eng Comput, vol. 49, no. 4, pp. 385–395, Apr. 2011. [CrossRef]
- J. L. Gaines, K. E. Finn, J. P. Slopsema, L. A. Heyboer, and K. H. Polasek, ‘A model of motor and sensory axon activation in the median nerve using surface electrical stimulation’, J Comput Neurosci, vol. 45, no. 1, pp. 29–43, Aug. 2018. [CrossRef]
- S. Agotici, K. Masani, and P. B. Yoo, ‘Computational Study on Spatially Distributed Sequential Stimulation for Fatigue Resistant Neuromuscular Electrical Stimulation’, IEEE Transactions on Neural Systems and Rehabilitation Engineering, vol. 29, pp. 2578–2586, 2021. [CrossRef]
- A. Kuhn, T. Keller, S. Micera, and M. Morari, ‘Array electrode design for transcutaneous electrical stimulation: A simulation study’, Med Eng Phys, vol. 31, no. 8, pp. 945–951, 2009. [CrossRef]
- N. RaviChandran, M. Y. Teo, K. Aw, and A. McDaid, ‘Design of Transcutaneous Stimulation Electrodes for Wearable Neuroprostheses’, IEEE Transactions on Neural Systems and Rehabilitation Engineering, vol. 28, no. 7, pp. 1651–1660, Jul. 2020. [CrossRef]
- N. Ravichandran, J. Hope, K. Aw, and A. Mcdaid, ‘Modeling the excitation of nerve axons under transcutaneous stimulation’, Comput Biol Med, vol. 165, p. 107463, Sep. 2023. [CrossRef]
- C. R. Butson, S. E. Cooper, J. M. Henderson, and C. C. Mcintyre, ‘Patient-Specific Analysis of the Volume of Tissue Activated During Deep Brain Stimulation’. [CrossRef]
- K. Gunalan, B. Howell, and C. C. McIntyre, ‘Quantifying axonal responses in patient-specific models of subthalamic deep brain stimulation’, Neuroimage, vol. 172, no. January, pp. 263–277, 2018. [CrossRef]
- G. Duffley, D. N. Anderson, J. Vorwerk, A. D. Dorval, and C. R. Butson, ‘Evaluation of methodologies for computing the deep brain stimulation volume of tissue activated’, J Neural Eng, vol. 16, no. 6, Oct. 2019. [CrossRef]
- N. Ravichandran, M. Y. Teo, K. Aw, and A. McDaid, ‘Design of Transcutaneous Stimulation Electrodes for Wearable Neuroprostheses’, IEEE Transactions on Neural Systems and Rehabilitation Engineering, vol. 28, no. 7, pp. 1651–1660, Jul. 2020. [CrossRef]
- C. C. Wu and X. Luo, ‘Current steering with partial tripolar stimulation mode in cochlear implants’, JARO - Journal of the Association for Research in Otolaryngology, vol. 14, no. 2, pp. 213–231, Apr. 2013. [CrossRef]
- B. H. Bonham and L. M. Litvak, ‘Current focusing and steering: Modeling, physiology, and psychophysics’, Hear Res, vol. 242, no. 1–2, pp. 141–153, Aug. 2008. [CrossRef]
- V. Valente, A. Demosthenous, and R. Bayford, ‘A tripolar current-steering stimulator ASIC for field shaping in deep brain stimulation’, IEEE Trans Biomed Circuits Syst, vol. 6, no. 3, pp. 197–207, 2012. [CrossRef]
- J. Krishnan, R. Joseph, M. C. Vayalappil, S. Krishnan, and A. Kishore, ‘A Review on Implantable Neuroelectrodes’, Crit Rev Biomed Eng, vol. 52, no. 1, pp. 21–39, 2024. [CrossRef]
- J. Flodin, R. Juthberg, and P. W. Ackermann, ‘Effects of electrode size and placement on comfort and efficiency during low-intensity neuromuscular electrical stimulation of quadriceps, hamstrings and gluteal muscles’, BMC Sports Sci Med Rehabil, vol. 14, no. 1, Dec. 2022. [CrossRef]
- J. D. Gomez-Tames, J. Gonzalez, and W. Yu, ‘A simulation study: Effect of the inter-electrode distance, electrode size and shape in Transcutaneous Electrical Stimulation’, in Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, 2012, pp. 3576–3579. [CrossRef]
- A. Kuhn, T. Keller, M. Lawrence, and M. Morari, ‘The influence of electrode size on selectivity and comfort in transcutaneous electrical stimulation of the forearm’, IEEE Transactions on Neural Systems and Rehabilitation Engineering, vol. 18, no. 3, pp. 255–262, 2010. [CrossRef]
- A. Kuhn, T. Keller, M. Lawrence, and M. Morari, ‘The Influence of Electrode Size on Selectivity and Comfort in Transcutaneous Electrical Stimulation of the Forearm’, IEEE Transactions on Neural Systems and Rehabilitation Engineering, vol. 18, no. 3, pp. 255–262, Jun. 2010. [CrossRef]
- N. RaviChandran, K. C. Aw, and A. McDaid, ‘Influence of Electrode Geometry on Selectivity and Comfort for Functional Electrical Stimulation’, in The International Functional Electrical Stimulation Society (IFESS), RehabWeek, Toronto, Canada: IFESS, 2019. [CrossRef]
- A. Datta, V. Bansal, J. Diaz, J. Patel, D. Reato, and M. Bikson, ‘Gyri-precise head model of transcranial direct current stimulation: Improved spatial focality using a ring electrode versus conventional rectangular pad’, Brain Stimul, vol. 2, no. 4, pp. 201-207.e1, Oct. 2009. [CrossRef]
- M. Bortoletto, C. Rodella, R. Salvador, P. C. Miranda, and C. Miniussi, ‘Reduced Current Spread by Concentric Electrodes in Transcranial Electrical Stimulation (tES)’, Brain Stimul, vol. 9, no. 4, pp. 525–528, 2016. [CrossRef]
- V. T. Krasteva and S. P. Papazov, ‘Estimation of current density distribution under electrodes for external defibrillation.’, Biomed Eng Online, vol. 1, no. 1, p. 7, Dec. 2002. [CrossRef]
- A. M. Sagi-Dolev, D. Prutchi, and R. H. Nathan, ‘Three-dimensional current density distribution under surface stimulation electrodes’, Med Biol Eng Comput, vol. 33, no. 3, pp. 403–408, 1995. [CrossRef]
- A. Patriciu, K. Yoshida, J. J. Struijk, T. P. DeMonte, M. L. G. Joy, and H. Stodkilde-Jorgensen, ‘Current Density Imaging and Electrically Induced Skin Burns Under Surface Electrodes’, IEEE Trans Biomed Eng, vol. 52, no. 12, pp. 2024–2031, Dec. 2005. [CrossRef]
- C. Alon, G. Kantor, and H. S. Ho, ‘Effects of Electrode Size on Basic Excitatory Responses and on Selected Stimulus Parameters’, Journal of Orthopaedic & Sports Physical Therapy, vol. 20, no. 1, pp. 29–35, Jul. 1994. [CrossRef]
- S. Papazov, K. Brandiski, and I. Daskalov, ‘Optimization of the defibrillation current density in the heart region by a two-layer segmented electrode’, J Med Eng Technol, vol. 25, no. 1, pp. 28–33, 2001. [CrossRef]
- P. F. Meyer, P. D. Gadsby, D. Van Sickle, W. E. Schoenlein, K. S. Foster, and G. P. Graber, ‘Impedance-gradient electrode reduces skin irritation induced by transthoracic defibrillation’, Med Biol Eng Comput, vol. 43, no. 2, pp. 225–229, 2005. [CrossRef]
- N. Sha, L. P. J. Kenney, B. W. Heller, A. T. Barker, D. Howard, and W. Wang, ‘The effect of the impedance of a thin hydrogel electrode on sensation during functional electrical stimulation’, Med Eng Phys, vol. 30, no. 6, pp. 739–746, 2008. [CrossRef]
- A. Kuhn, T. Keller, S. Micera, and M. Morari, ‘Array electrode design for transcutaneous electrical stimulation: A simulation study’, Med Eng Phys, vol. 31, no. 8, pp. 945–951, Oct. 2009. [CrossRef]
- G. Cooper, A. T. Barker, B. W. Heller, T. Good, L. P. J. Kenney, and D. Howard, ‘The use of hydrogel as an electrode-skin interface for electrode array FES applications’, Med Eng Phys, vol. 33, no. 8, pp. 967–972, Oct. 2011. [CrossRef]
- E. McAdams, ‘Biomedical Electrodes For Biopotential Monitoring and Electrostimulation’, in Bio-Medical CMOS ICs, H.-J. Yoo and C. van Hoof, Eds., in Integrated Circuits and Systems. , Boston, MA: Springer US, 2011, pp. 31–124. [CrossRef]
- G. Cooper, A. T. Barker, B. W. Heller, T. Good, L. P. J. Kenney, and D. Howard, ‘The use of hydrogel as an electrode-skin interface for electrode array FES applications’, Med Eng Phys, vol. 33, no. 8, pp. 967–972, 2011. [CrossRef]
- H. Zhou et al., ‘Stimulating the comfort of textile electrodes in wearable neuromuscular electrical stimulation’, Sensors (Switzerland), vol. 15, no. 7, pp. 17241–17257, Jul. 2015. [CrossRef]
- J. Gómez-Tames, J. González, and W. Yu, ‘Influence of different geometric representations of the volume conductor on nerve activation during electrical stimulation’, Comput Math Methods Med, vol. 2014, 2014. [CrossRef]
- J. H. K. Kim, M. L. Trew, A. J. Pullan, and O. Röhrle, ‘Simulating a dual-array electrode configuration to investigate the influence of skeletal muscle fatigue following functional electrical stimulation’, Comput Biol Med, vol. 42, no. 9, pp. 915–924, 2012. [CrossRef]
- C. D. Solomons, M. Slovak, B. Heller, and A. T. Barker, ‘Reducing the sensation of electrical stimulation with dry electrodes by using an array of constant current sources’, Med Eng Phys, vol. 51, pp. 91–95, 2018. [CrossRef]
- N. M. Malešević, L. Z. Popović, L. Schwirtlich, and D. B. Popović, ‘Distributed low-frequency functional electrical stimulation delays muscle fatigue compared to conventional stimulation’, Muscle Nerve, vol. 42, no. 4, pp. 556–562, Oct. 2010. [CrossRef]
- N. RaviChandran, K. Aw, and A. McDaid, ‘Electrophysiologically-identified motor points of forearm muscles’, IEEE Dataport, 2019. [CrossRef]
- N. RaviChandran, K. Aw, and A. McDaid, ‘Automatic calibration of electrode arrays for dexterous neuroprostheses: a review’, Biomed Phys Eng Express, vol. 9, no. 5, p. 052001, Sep. 2023. [CrossRef]
- J. David Gomez Tames, J. Gonzalez, and W. Yu, A Simulation Study: Effect of the Inter-Electrode Distance, Electrode Size and Shape in Transcutaneous Electrical Stimulation. 2012. [CrossRef]
- E. P. Doheny, B. M. Caulfield, C. M. Minogue, and M. M. Lowery, ‘Effect of subcutaneous fat thickness and surface electrode configuration during neuromuscular electrical stimulation’, Med Eng Phys, vol. 32, no. 5, pp. 468–474, Jun. 2010. [CrossRef]
- L. Parisi and N. RaviChandran, ‘Evolutionary Denoising-Based Machine Learning for Detecting Knee Disorders’, Neural Process Lett, vol. 52, no. 3, pp. 2565–2581, Dec. 2020. [CrossRef]
- L. Parisi, N. RaviChandran, and M. Lanzillotta, ‘Artificial Intelligence for Clinical Gait Diagnostics of Knee Osteoarthritis: An Evidence - based Review and Analysis’, TechRxiv. Accessed: Apr. 16, 2021. [Online]. Available: https://www.techrxiv.org/doi/full/10.36227/techrxiv.11786511.v1. [CrossRef]
Figure 1.
A computational modeling framework to evaluate field-shaping strategies for transcutaneous stimulation.
Figure 1.
A computational modeling framework to evaluate field-shaping strategies for transcutaneous stimulation.
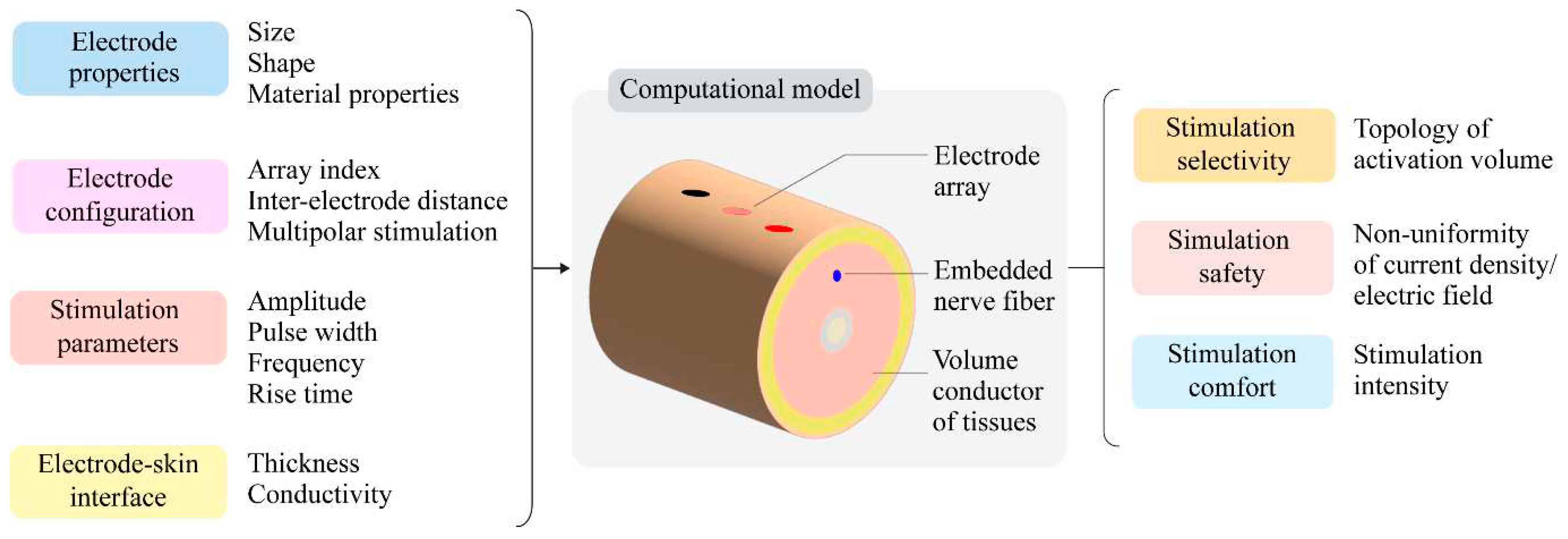
Table 1.
Metrics to evaluate the stimulation performance.
| Selectivity of stimulation | Activation volume¥ | Surface area Surface eccentricity Depth Volume Surface-to-volume ratio |
| Safety of stimulation | Non-uniformity coefficient |
Non-uniformity of current density Non-uniformity of electric field |
| Stimulation intensity | Stimulation amplitude | |
| Stimulation comfort | Stimulation intensity | Sensory threshold |
| Stimulation amplitude |
¥ Assuming a hemiellipsoid topology represents a highly selective activation volume when targeting deep nerve fibers Most studies report the topology of the electric field as activation volume (AV). The AV has been strongly coupled with clinical benefits for neurostimulation applications [27]. It represents a lobe of tissues under the active electrode with the same electric field potential (iso-surfaces) that signifies potential areas of neuronal excitability. Each AV is unique to fiber diameter, stimulus duration, and electrode configuration (shape, size, and inter-electrode distance) [28,29]. To quantify the electrode’s selectivity, the surface area, surface eccentricity, depth, and topological volume of the iso-surface are derived. An AV with a lower surface-to-volume ratio (SA:V) tends to avoid charge spillage and is highly selective. Additionally, the current density profiles must be analyzed to estimate the stimulation safety. During transcutaneous stimulation, regions of high current density with high voltage gradients must be avoided. Thus, the non-uniformity coefficient of the current density and electric field can be used to quantify stimulation safety [30]. Lastly, as complemented by experimental values, metrics like stimulation amplitude and sensory threshold can qualitatively assess simulation comfort [30].
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated







