Submitted:
16 January 2024
Posted:
17 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Flagellar Proteins
2.1. FliC (BPSL3319)
2.2. FlgL (BPSL0281)
3. Type 3 Secretion Systems
3.1. T3SS-3 Translocation Pore Proteins
3.2. T3SS-3 Effector Proteins
4. Type 5 Secretion Systems
4.1. BimA (BPSS1492)
4.2. BpaE (BPSS0908)
4.3. BatA (BPSL2237)
5. Type 6 Secretion Systems
5.1. T6SS Shaft Proteins
5.2. TssM (BPSS1512)
6. Type IV Pili
7. General Outer Membrane Proteins: β-Barrels
7.1. BamA (BPSL2151)
7.2. OmpW1 (BPSL1552)
7.3. OpcP (BPSS0879), OpcP1 (BPSS0708), and OmpW2 (BPSL2704)
7.4. Bucl8 (BPSL1972)
8. General Outer Membrane Proteins: Lipoproteins
8.1. Omp7 (BPSL765)
8.2. Omp1 (BPSL0999)
9. ABC-Binding Cassette (ABC) Transporters
10. Miscellaneous Antigens
10.1. Omp3 (BPSL2522)
10.2. MprA (BPSS1993)
10.3. AhpC (BPSL2096)
10.4. IscA (BPSL2287), TadE (BPSL1897), and AcoD (BPSL3369)
11. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eberl, L.; Vandamme, P. Members of the genus Burkholderia: Good and bad guys. F1000Res 2016, 5, F1000 Faculty Rev–1007. [Google Scholar] [CrossRef]
- Parte, A.C.; Carbasse, J.S.; Meier-Kolthoff, J.P.; Reimer, L.C., Göker, M. List of Prokaryotic names with Standing in Nomenclature (LPSN) moves to the DSMZ. Int J Syst Evol Microbiol 2020, 70, 5607-5612. (accessed 2023 November 11). [CrossRef]
- Center for Disease Control and Prevention. Available from: https://www.selectagents.gov/sat/list.htm (accessed 2023 November 11). 11 November.
- Limmathurotsakul, D.; Golding, N.; Dance, D.A.B.; Messina, J.P.; Pigott, D.M.; Moyes, C.L.; Rolim, D.B.; Bertherat, E.; Day, N.P.J.; Peacock, S.J.; et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol 2016, 1, 15008. [Google Scholar] [CrossRef] [PubMed]
- Zlosnik, J.E.A.; Costa, P.S.; Brant, R.; Mori, P.Y.B.; Hird, T.J.; Fraenkel, M.C.; Wilcox, P.G.; Davidson, AGF, Speert DP. Mucoid and Nonmucoid Burkholderia cepacia Complex Bacteria in Cystic Fibrosis Infections. Am J Resp Crit Care Med 2011, 183(1). [CrossRef]
- Folescu, T.W.; da Costa, C.H.; Cohen, R.W.F.; Cohen, R.; da Conceição Neto, O.C.; Albano, R.M.; Marques, E.A. Burkholderia cepacia complex: Clinical course in cystic fibrosis patients. BMC Pulm Med 2015, 15, 158. [Google Scholar] [CrossRef] [PubMed]
- Häfliger, E.; Atkinson, A.; Marschall, J. Systematic review of healthcare-associated Burkholderia cepacia complex outbreaks: Presentation, causes and outbreak control. Infect Prevent Practice 2020, 2(3), 100082. [Google Scholar] [CrossRef] [PubMed]
- Pongmala, K.; Pierret, A.; Oliva, P.; Pando, A.; Davong, V.; Rattanavong, S.; Silvera, N.; Luangraj, M.; Boithias, L.; Xayyathip, K; et al. Distribution of Burkholderia pseudomallei within a 300-cm deep soil profile: Implications for environmental sampling. Sci Rep 2022, 12, 8674. [Google Scholar] [CrossRef] [PubMed]
- Merritt, A.J.; Inglis, T.J.J. The Role of Climate in the Epidemiology of Melioidosis. Curr Trop Med Resp 2017, 4(4), 185–191. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.Y.I.; Fisher, D. Earth, wind, rain, and melioidosis. Lancet Planet Health 2018, 2(8), E329–E330. [Google Scholar] [CrossRef]
- Birnie, E.; Biemond, J.J.; Wiersinga, W.J. Drivers of melioidosis endemicity: Epidemiological transition, zoonosis, and climate change. Curr Opin Infect Dis 2022, 35(3), 196–204. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention. Available from: https://emergency.cdc.gov/han/2022/han00470.asp (accessed 2023 November 11). 11 November.
- Gassiep, I.; Grey, V.; Thean, L.J.; Farquhar, D.; Clark, J.E.; Ariotti, L.; Graham, R.; Jennison, A.V.; Bergh, H.; Anuradha, S.; et al. Expanding the Geographic Boundaries of Melioidosis in Queensland, Australia. Am J Trop Med Hyg 2023, 108, 1215–1219. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Virk, H.S.; Torres, A.G.; Currie, B.J.; Peacock, S.J.; Dance, D.A.B.; Limmathurotsakul, D. Melioidosis. Nat Rev Dis Primers 2018, 4, 17107. [Google Scholar] [CrossRef]
- Singh, M.; Mahmood, M. Melioidosis: The great mimicker. J Community Hosp Intern Med Perspect 2017, 7(4), 245–247. [Google Scholar] [CrossRef]
- Khakhum, N.; Chapartegui-González, I.; Torres, A.G. Combating the great mimicker: Latest progress in the development of Burkholderia pseudomallei vaccines. Expert Rev Vaccines, 2020, 19(7), 653-660. [CrossRef]
- UpToDate. Available from: https://www.uptodate.com/contents/melioidosis-epidemiology-clinical-manifestations-and-diagnosis (accessed 2023 November 11). 11 November.
- Losada, L.; Ronning, C.M.; DeShazer, D.; Woods, D.; Fedorova, N.; Kim, H.S.; Shabalina, S.A.; Pearson, T.R.; Brinkac, L; Tan, P. ; et al. Continuing Evolution of Burkholderia mallei Through Genome Reduction and Large-Scale Rearrangements. Genome Biol Evol 2010, 2, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Rojas, J.L.G.; Schmidt, C.G.; Herskin, M.; et al. Assessment of the control measures of the category A diseases of Animal Health Law: Burkholderia mallei (Glanders). EFSA J 2022, 20(1), e07069. [Google Scholar] [CrossRef] [PubMed]
- Merck Veterinary Manual. Available from: https://www.merckvetmanual.com/generalized-conditions/glanders/glanders-in-horses-and-other-animals (accessed 2023 November 11). 11 November.
- Van Zandt, K.E.; Greer, M.T.; Gelhaus, H.C. Glanders: An overview of infection in humans. Orphanet J Rare Dis 2013, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Godoy, D.; Randle, G.; Simpson, A.J.; Aanensen, D.M.; Pitt, T.L.; Kinoshita, R.; Spratt, B.G. Multilocus Sequence Typing and Evolutionary Relationships among the Causative Agents of Melioidosis and Glanders, Burkholderia pseudomallei and Burkholderia mallei. J Clin Microbiol 2003, 41(5), 2068–2079. [Google Scholar] [CrossRef] [PubMed]
- Morales-Ruíz L.M.; Rodríguez-Cisneros, M.; Kerber-Díaz, J.C.; Rojas-Rojas, F.U.; Ibarra, J.A.; Los Santos, P.E. Burkholderia orbicola sp. nov., a novel species within the Burkholderia cepacia complex. Arch Microbiol 2022, 204(3), 178. [CrossRef]
- Center for Disease Control and Prevention. Available from: https://www.cdc.gov/hai/organisms/bcepacia.html (accessed 2023 November 11). 11 November.
- Tavares, M.; Kozak, M.; Balola, A.; Sá-Correia, I. Burkholderia cepacia Complex Bacteria: A Feared Contamination Risk in Water-Based Pharmaceutical Products. Clin Microbiol Rev 2020, 33(3), e00139–19. [Google Scholar] [CrossRef] [PubMed]
- Daccò, V.; Alicandro, G.; Consales, A.; Rosazza, C.; Sciarrabba, C.S.; Cariani, L.; Colombo, C. Cepacia syndrome in cystic fibrosis: A systematic review of the literature and possible new perspectives in treatment. Pediatr Pulmonol 2023, 58(5), 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Mahenthiralingam, E.; Urban, T.A.; Goldberg, J.B. The multifarious, multireplicon Burkholderia cepacia complex. Nat Rev Microbiol 2005, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Horsley, A.; Webb, K.; Bright-Thomas, R.; Govan, J.; Jones, A. Can early Burkholderia cepacia complex infection in cystic fibrosis be eradicated with antibiotic therapy? Front Cell Infect Microbiol 2011, 1, 18. [Google Scholar] [CrossRef]
- Lord, R.; Jones, A.M.; Horsley, A. Antibiotic treatment for Burkholderia cepacia complex in people with cystic fibrosis experiencing a pulmonary exacerbation. Cochrane Database Sys Rev 2020, 2020(4), CD009529. [Google Scholar] [CrossRef]
- LiPuma, J.J. The Changing Microbial Epidemiology in Cystic Fibrosis. Clin Microbiol Rev 2010, 23(2), 299–323. [Google Scholar] [CrossRef] [PubMed]
- Zlosnik, J.E.A.; Henry, D.A.; Hird, T.J.; Hickman, R.; Campbell, M.; Cabrera, A.; Chiavegatti, G.L.; Chilvers, M.A.; Sadarangani, M. Epidemiology of Burkholderia Infections in People with Cystic Fibrosis in Canada between 2000 and 2017. Ann Am Thorac Soc 2020, 17(12). [CrossRef]
- Pradenas, G.A.; Ross, B.N.; Torres, A.G. Burkholderia cepacia Complex Vaccines: Where Do We Go from here? Vaccines (Basel). 2016, 4(2), 10. [Google Scholar] [CrossRef]
- Grund, M.E.; Soo, J.C.; Cote, C.K.; Berisio, R.; Lukomski, S. Thinking Outside the Bug: Targeting Outer Membrane Proteins for Burkholderia Vaccines. Cells 2021, 10(3), 495. [Google Scholar] [CrossRef]
- Irudal, S.; Scoffone, V.C.; Trespidi, G.; Barbieri, G.; D’Amato, M.; Viglio, S.; Pizza, M.; Scarselli, M.; Riccardi, G.; Buroni, S. Identification by Reverse Vaccinology of Three Virulence Factors in Burkholderia cenocepacia That May Represent Ideal Vaccine Antigens. Vaccines (Basel) 2023, 11(6), 1039. [Google Scholar] [CrossRef]
- Cocorullo, M.; Chiarelli, L.R.; Stelitano, G. Improving Protection to Prevent Bacterial Infections: Preliminary Applications of Reverse Vaccinology against the Main Cystic Fibrosis Pathogens. Vaccines (Basel) 2023, 11(7), 1221. [Google Scholar] [CrossRef] [PubMed]
- Luangasanatip, N.; Flasche, S.; Dance, D.A.B.; Limmathurotsakul, D.; Currie, B.J.; Mukhopadhyay, C.; Atkins, T.; Titball, R.; Jit, M. The global impact and cost-effectiveness of a melioidosis vaccine. BMC Med 2019, 17, 129. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J Mol Biol 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform 2008, 10, 421. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2021 available from: https://www.R-project.org.
- Scholz, H.C.; Joseph, M.; Tomaso, H.; Dahouk, S.A.; Witte, A.; Kinne, J.; Hagen, R.M.; Wernery, R.; Wernery, U.; Neubauer, H. Detection of the reemerging agent Burkholderia mallei in a recent outbreak of glanders in the United Arab Emirates by a newly developed fliP-based polymerase chain reaction assay. Diag Microbiol Infect Dis 2006, 54(4), 241–247. [Google Scholar] [CrossRef]
- Chua, K.L.; Chan, Y.Y.; Gan, Y.H. Flagella Are Virulence Determinants of Burkholderia pseudomallei. Infect Immun 2003, 71(4), 1622–1629. [Google Scholar] [CrossRef]
- Chuaygud, T.; Tungpradabkul, S.; Sirisinha, S.; Chua, K.L.; Utaisincharoen, P. A role of Burkholderia pseudomallei flagella as a virulent factor. Trans R Soc Trop Med Hyg 2008, 102, Suppl 1:S140–S144. [Google Scholar] [CrossRef]
- Tomich, M.; Herfst, C.A.; Golden, J.W.; et al. Role of Flagella in Host Cell Invasion by Burkholderia cepacia. Infect Immun 2002, 70(4), 1799–1806. [Google Scholar] [CrossRef]
- Urban, T.A.; Griffith, A.; Torok, A.M.; Smolkin, M.E.; Burns, J.L.; Goldberg, J.B. Contribution of Burkholderia cenocepacia Flagella to Infectivity and Inflammation. Infect Immun 2004, 72(9). [CrossRef]
- Ceballos-Olvera, I.; Sahoo, M.; Miller, M.A.; del Barria, L.; Re, F. Inflammasome-dependent Pyroptosis and IL-18 Protect against Burkholderia pseudomallei Lung Infection while IL-1β Is Deleterious. PLoS Pathog 2011, 7(12), e1002452. [Google Scholar] [CrossRef]
- West, T.E.; Myers, N.D.; Chantratita, N.; Chierakul, W.; Limmathurotsakul, D.; Wuthiekanun, V.; Miao, E.A.; Hajjar, A.M.; Peacock, S.J.; Liggitt, H.D.; et al. NLRC4 and TLR5 Each Contribute to Host Defense in Respiratory Melioidosis. PLoS Negl Trop Dis 2014, 8(9), e3178. [Google Scholar] [CrossRef] [PubMed]
- Koosakulnirand, S.; Phokrai, P.; Jenjaroen, K.; Roberts, R.A.; Utaisincharoen, P.; Dunachie, S.J.; Brett, P.J.; Burtnick, M.N.; Chantratita, N. Immune response to recombinant Burkholderia pseudomallei FliC. PloS One 2018, 13(6), e0198906. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, K.; Dankmeyer, J.L.; Bernhards, R.C.; Fetterer, D.P.; Waag, D.M.; Worsham, P.L.; DeShazer, D. Activation of Toll-Like Receptors by Live Gram-Negative Bacterial Pathogens Reveals Mitigation of TLR4 Responses and Activation of TLR5 by Flagella. Front Cell Infect Microbiol 2021, 11, 745325. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Minamino, T. Flagella-Driven Motility of Bacteria. Biomolecules 2019, 9(7), 279. [Google Scholar] [CrossRef]
- Brett, P.J.; Mah, D.C.; Woods, D.E. Isolation and characterization of Pseudomonas pseudomallei flagellin proteins. Infect Immun 1994, 62(5), 1914–1919. [Google Scholar] [CrossRef]
- Brett, P.J.; Woods, D.E. Structural and Immunological Characterization of Burkholderia pseudomallei O-Polysaccharide–Flagellin Protein Conjugates. Infect Immun 1996, 64(7), 2824–2828. [Google Scholar] [CrossRef]
- Chen, Y.S.; Hsiao, Y.S.; Lin, H.H.; Yen, C.M.; Chen, S.C.; Chen, Y.L. Immunogenicity and anti-Burkholderia pseudomallei activity in Balb/c mice immunized with plasmid DNA encoding flagellin. Vaccine 2006, 24(6), 750–758. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Hsiao, Y.S.; Lin, H.H.; Liu, Y.; Chen, Y.L. CpG-Modified Plasmid DNA Encoding Flagellin Improves Immunogenicity and Provides Protection against Burkholderia pseudomallei Infection in BALB/c Mice. Infect Immun 2006, 74(3), 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.G.; Gregory, A.E.; Hatcher, C.L.; Vinet-Oliphant, H.; Morici, L.A.; Titball, R.W.; Roy, C.J. Protection of non-human primates against glanders with a gold nanoparticle glycoconjugate vaccine. Vaccine 2015, 33(5), 686–692. [Google Scholar] [CrossRef]
- Gregory, A.E.; Judy, B.M.; Qazi, O.; Blumentritt, C.A.; Brown, K.A.; Shaw, A.M.; Torres, A.G.; Titball, R.W. A gold nanoparticle-linked glycoconjugate vaccine against Burkholderia mallei. Nanomedicine: NBM 2015, 11(2), 447-456. [CrossRef]
- Muruato, L.A.; Tapia, D.; Hatcher, C.L.; Kalita, M.; Brett, P.J.; Gregory, A.E.; Samuel, J.E.; Titball, R.W.; Torres, A.G. Use of Reverse Vaccinology in the Design and Construction of Nanoglycoconjugate Vaccines against Burkholderia pseudomallei. Clin Vaccine Immunol 2017, 24(11), e00206–17. [Google Scholar] [CrossRef]
- Tapia, D.; Sanchez-Villamil, J.I.; Torres, A.G. Multicomponent gold nano-glycoconjugate as a highly immunogenic and protective platform against Burkholderia mallei. npj Vaccines 2020, 5, 82. [Google Scholar] [CrossRef]
- Tapia, D.; Sanchez-Villamil, J.I.; Stevenson, H.L.; Torres, A.G. Multicomponent Gold-Linked Glycoconjugate Vaccine Elicits Antigen-Specific Humoral and Mixed TH1-TH17 Immunity, Correlated with Increased Protection against Burkholderia pseudomallei. mBio 2021, 12(3), e01227–21. [Google Scholar] [CrossRef]
- Hajam, I.A.; Dar, P.A.; Shahnawaz, I.; Jaume, J.C.; Lee, J.H. Bacterial flagellin—a potent immunomodulatory agent. Exp Mol Med 2017, 49(9), e373. [Google Scholar] [CrossRef]
- Charuchaimontri, C.; Suputtamongol, Y.; Nilakul, C.; Chaowagul, W.; Chetchotisakd, P.; Lertatanasuwun, N.; Intaranongpai, S.; Brett, P.J.; Woods, D.E. Antilipopolysaccharide II: An antibody protective against fatal melioidosis. Clin Infect Dis 1999, 29(4), 813–818. [Google Scholar] [CrossRef]
- Chen, Y.S.; Shiuan, D.; Chen, S.C.; Chye, S.M.; Chen, Y.L. Recombinant Truncated Flagellin of Burkholderia pseudomallei as a Molecular Probe for Diagnosis of Melioidosis. Clin Diagn Lab Immunol 2003, 10(3), 423–425. [Google Scholar] [CrossRef] [PubMed]
- Felgner, P.L.; Kayala, M.A.; Vigil, A.; Burk, C.; Nakajima-Sasaki, R.; Pablo, J.; Molina, D.M.; Hirst, S.; Chew, J.S.W.; Wang, D.; et al. A Burkholderia pseudomallei protein microarray reveals serodiagnostic and cross-reactive antigens. PNAS 2009, 106(32), 13499–13504. [Google Scholar] [CrossRef]
- Suwannasaen, D.; Mahawantung, J.; Chaowagul, W.; Limmathurotsakul, D.; Felgner, P.L.; Davies, H.; Bancroft, G.J.; Titball, R.W.; Lertmemongkolchai, G. Human Immune Responses to Burkholderia pseudomallei Characterized by Protein Microarray Analysis. J Infect Dis. 2011, 203(7), 1002–1011. [Google Scholar] [CrossRef]
- Kohler, C.; Dunachie, S.J.; Müller, E.; Kohler, A.; Jenjaroen, K.; Teparrukkul, P.; Baier, V.; Ehricht, R.; Steinmetz, I. Rapid and Sensitive Multiplex Detection of Burkholderia pseudomallei-Specific Antibodies in Melioidosis Patients Based on a Protein Microarray Approach. PLoS Negl Trop Dis 2016, 10(7), e0004847. [Google Scholar] [CrossRef]
- Scott, A.E.; Twine, S.M.; Fulton, K.M.; Titball, R.W.; Essex-Lopresti, A.E.; Atkins, T.P.; Prior, J.L. Flagellar Glycosylation in Burkholderia pseudomallei and Burkholderia thailandensis. J Bacteriol 2011, 193(14), 3577–3587. [Google Scholar] [CrossRef]
- Hanuszkiewicz, A.; Pittock, P.; Humphries, F.; Moll, H.; Rosales, A.R.; Molinaro, A.; Moynagh, P.N.; Lajoie, G.A.; Valvano, M.A. Identification of the Flagellin Glycosylation System in Burkholderia cenocepacia and the Contribution of Glycosylated Flagellin to Evasion of Human Innate Immune Responses. J Biol Chem 2014, 289(27), 19231–19244. [Google Scholar] [CrossRef]
- DeShazer, D.; Brett, P.J.; Carlyon, R.; Woods, D.E. Mutagenesis of Burkholderia pseudomallei with Tn5-OT182: Isolation of Motility Mutants and Molecular Characterization of the Flagellin Structural Gene. J Bacteriol 1997, 179(7), 2116–2125. [Google Scholar] [CrossRef] [PubMed]
- Wikraiphat, C.; Charoensap, J.; Utaisincharoen, P.; Wongratanacheewin, S.; Taweechaisupapong, S.; Woods, D.E.; Bolscher, J.G.M.; Sirisinha, S. Comparative in vivo and in vitro analyses of putative virulence factors of Burkholderia pseudomallei using lipopolysaccharide, capsule and flagellin mutants. FEMS Immunol Med Microbiol 2009, 56(3), 253–259. [Google Scholar] [CrossRef]
- Whitlock, G.C.; Deeraksa, A.; Qazi, O.; Judy, B.M.; Taylor, K.; Propst, K.L; Duffy, A.J.; Johnson, K.; Kitto, G.B.; Brown, K.A. Protective response to subunit vaccination against intranasal Burkholderia mallei and B. pseudomallei challenge. Procedia Vaccinol 2010, 2(1). [CrossRef]
- Hales, B.A.; Morgan, J.A.W.; Hart, C.A.; Winstanley, C. Variation in Flagellin Genes and Proteins of Burkholderia cepacia. J Bacteriol 1998, 180(5), 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Musson, J.A.; Reynolds, C.J.; Rinchai, D.; Nithichanon, A.; Khaenam, P.; Favry, E.; Spink, N.; Chu, K.K.Y.; de Soyza, A.; Bancroft, G.J.; et al. CD4+ T Cell Epitopes of FliC Conserved between Strains of Burkholderia: Implications for Vaccines against Melioidosis and Cepacia Complex in Cystic Fibrosis. J Immunol 2014, 193(12), 6041–6049. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Marshall, N.C.; Rowland, J.L.; McCoy, J.M.; Worrall, L.J.; Santos, A.S.; Strynadka, N.C.J.; Finlay, B.B. Assembly, structure, function and regulation of type III secretion systems. Nat Rev Microbiol 2017, 15, 323–337. [Google Scholar] [CrossRef]
- Wagner, S.; Grin, I.; Malmsheimer, S.; Singh, N.; Torres-Vargas, C.E.; Westerhausen, S. Bacterial type III secretion systems: A complex device for the delivery of bacterial effector proteins into eukaryotic host cells. FEMS Microbiol Lett 2018, 365(19), fny20. [Google Scholar] [CrossRef]
- Goodin, J.L.; Raab, R.W.; McKown, R.L.; Coffman, G.L.; Powell, B.S.; Enama, J.T.; Ligon, J.A.; Andrews, G.P. Yersinia pestis outer membrane type III secretion protein YscC: Expression, purification, characterization, and induction of specific antiserum. Protein Expr Purif 2005, 40(1), 152–156. [Google Scholar] [CrossRef] [PubMed]
- Fasciano, A.C.; Shaban, L.; Mecsas, J. Promises and Challenges of the Type Three Secretion System-Injectisome as an Anti-Virulence Target. EcoSal Plus 2019, 8(2). [CrossRef]
- Hotinger, J.A.; May, A.E. Antibodies Inhibiting the Type III Secretion System of Gram-Negative Pathogenic Bacteria. Antibodies (Basel) 2020, 9(3). [CrossRef]
- Hotinger, J.A.; Pendergrass, H.A.; May, A.E. Molecular Targets and Strategies for Inhibition of the Bacterial Type III Secretion System (T3SS); Inhibitors Directly Binding to T3SS Components. Biomolecules 2021, 11(2), 316. [Google Scholar] [CrossRef]
- Wallner, A.; Moulin, L.; Busset, N.; Rimbault, I.; Béna, G. Genetic Diversity of Type 3 Secretion System in Burkholderia s.l. and Links With Plant Host Adaptation. Front Microbiol 2021, 12, 761215. [Google Scholar] [CrossRef] [PubMed]
- Rainbow, L.; Hart, C.A.; Winstanley, C. Distribution of type III secretion gene clusters in Burkholderia pseudomallei, B. thailandensis and B. mallei. J Med Microbiol 2002, 51(5), 374–384. [Google Scholar] [CrossRef]
- Vander Broek, C.W.; Stevens, J.M. Type III Secretion in the Melioidosis Pathogen Burkholderia pseudomallei. Front Cell Infect Microbiol 2017, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.P.; Wood, M.W.; Taylor, L.A.; Monaghan, P.; Hawes, P.; Jones, P.W.; Wallis, T.S.; Galyov, E.E. An Inv/Mxi-Spa-like type III protein secretion system in Burkholderia pseudomallei modulates intracellular behaviour of the pathogen. Molec Microbiol 2002, 46(3), 649–659. [Google Scholar] [CrossRef]
- Gutierrez, M.G.; Pfeffer, T.L.; Warawa, J.M. Type 3 Secretion System Cluster 3 Is a Critical Virulence Determinant for Lung-Specific Melioidosis. PLoS Negl Trop Dis 2015, 9(1), e3441. [Google Scholar] [CrossRef]
- Kurtz, J.R.; Petersen, H.E.; Frederick, D.R.; Morici, L.A.; McLachlan, J.B. Vaccination with a Single CD4 T Cell Peptide Epitope from a Salmonella Type III-Secreted Effector Protein Provides Protection against Lethal Infection. Infect Immun 2014, 82(6), 2424–2433. [Google Scholar] [CrossRef]
- Lee, S.J.; Benoun, J.; Sheridan, B.S.; Fogassy, Z.; Pham, O.; Pham, Q.M.; Puddington, L.; McSorley, S.J. Dual immunization with SseB/flagellin provides enhanced protection against Salmonella infection mediated by circulating memory cells. J Immunol 2017, 199(4), 1353–1361. [Google Scholar] [CrossRef]
- Xiong, X.; Jiao, J.; Gregory, A.E.; Wang, P.; Bi, Y.; Wang, X.; Jiang, Y.; Wen, B.; Portnoy, D.A.; Samuel, J.E.; et al. Identification of Coxiella burnetii CD8+ T-Cell Epitopes and Delivery by Attenuated Listeria monocytogenes as a Vaccine Vector in a C57BL/6 Mouse Model. J Infect Dis 2017, 215(10), 1580–1589. [Google Scholar] [CrossRef]
- Harley, V.S.; Dance, D.A.; Tovey, G.; McCrossan, M.V.; Drasar, B.S. An ultrastructural study of the phagocytosis of Burkholderia pseudomallei. Microbios 1998, 94(377), 35–45. [Google Scholar]
- Steele-Mortimer, O. The Salmonella-containing Vacuole – Moving with the Times. Curr Opin Microbiol 2008, 11(1), 38–45. [Google Scholar] [CrossRef]
- Allwood, E.M.; Devenish, R.J.; Prescott, M.; Adler, B.; Boyce, J.D. Strategies for Intracellular Survival of Burkholderia pseudomallei. Front Microbiol 2011, 2, 170. [Google Scholar] [CrossRef]
- Latomanski, E.A.; Newton, H.J. Interaction between autophagic vesicles and the Coxiella-containing vacuole requires CLTC (clathrin heavy chain). Autophagy 2018, 14(10), 1710–1725. [Google Scholar] [CrossRef]
- Stevens, M.P.; Haque, A.; Atkins, T.; Hill, J.; Wood, M.W.; Easton, A.; Nelson, M.; Underwood-Fowler, C.; Titball, R.W.; Bancroft, G.J.; et al. Attenuated virulence and protective efficacy of a Burkholderia pseudomallei bsa type III secretion mutant in murine models of melioidosis. Microbiol 2004, 150(8), 2669–2676. [Google Scholar] [CrossRef] [PubMed]
- Druar, C.; Yu, F.; Barnes, J.L.; Okinaka, R.T.; Chantratita, N.; Beg, S.; Stratilo, C.W.; Olive, A.J.; Soltes, G.; Russell, M.L.; et al. Evaluating Burkholderia pseudomallei Bip proteins as vaccines and Bip antibodies as detection agents. FEMS Microbiol Immunol 2007, 52(1), 78–87. [Google Scholar] [CrossRef] [PubMed]
- Pumirat, P.; Cuccui, J.; Stabler, R.A.; Stevens, J.M.; Muangsombut, V.; Singsuksawat, E.; Stevens, M.P.; Wren, B.W.; Korbsrisate, S. Global transcriptional profiling of Burkholderia pseudomallei under salt stress reveals differential effects on the Bsa type III secretion system. BMC Microbiol 2010, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Ooi, W.F.; Ong, C.; Nandi, T.; Kreisberg, J.F.; Chua, H.H.; Sun, G.; Chen, Y.; Mueller, C.; Conejero, L.; Eshaghi, M.; et al. The Condition-Dependent Transcriptional Landscape of Burkholderia pseudomallei. PLoS Genet 2013, 9(9), e1003795. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Wong, R.R.; Ghazali, A.K.; Hara, Y.; Aziz, T.N.T.; Nathan, S. Transcriptional landscape of Burkholderia pseudomallei cultured under environmental and clinical conditions. Microb Genom 2023, 9(4). [CrossRef]
- Lichtenegger, S.; Stiehler, J.; Saiger, S.; Zauner, A.; Kleinhappl, B.; Bernecker, C.; Schlenke, P.; Wagner, G.E.; Krause, K.; Gastager, M.; et al. Burkholderia pseudomallei triggers canonical inflammasome activation in a human primary macrophage-based infection model. PLoS Negl Trop Dis 2020, 14(11), e0008840. [Google Scholar] [CrossRef]
- Leyton, D.L.; Rossiter, A.E.; Henderson, I.R. From self sufficiency to dependence: Mechanisms and factors important for autotransporter biogenesis. Nat Rev Microbiol 2012, 10, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.R.; Hor, L; Pilapitiya, A. ; Luirink, J.; Paxman, J.J.; Heras, B. Phylogenetic Classification and Functional Review of Autotransporters. Front Immunol 2022, 13, 921272. [Google Scholar] [CrossRef] [PubMed]
- Adler, N.R.L.; Stevens, J.M.; Stevens, M.P.; Galyov, E.E. Autotransporters and Their Role in the Virulence of Burkholderia pseudomallei and Burkholderia mallei. Front Microbiol 2011, 2, 151. [Google Scholar] [CrossRef]
- Stevens, M.P.; Stevens, J.M.; Jeng, R.L.; Taylor, L.A.; Wood, M.W.; Hawes, P.; Monaghan, P.; Welch, M.D.; Galyov, E.E. Identification of a bacterial factor required for actin-based motility of Burkholderia pseudomallei. Mol Microbiol 2005, 56(1), 40–53. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.G.; Borst, L.; Cotter, P.A. Characterization of BcaA, a putative classical autotransporter protein in Burkholderia pseudomallei. Infect Immun 2013, 81(4), 1121–1128. [Google Scholar] [CrossRef]
- Zimmerman, S.M.; Dyke, J.S.; Jelesijevic, T.P.; Michel, F.; Lafontaine, E.R.; Hogan, R.J. Antibodies against In Vivo-Expressed Antigens Are Sufficient To Protect against Lethal Aerosol Infection with Burkholderia mallei and Burkholderia pseudomallei. Infect Immun 2017, 85(8), e00102–17. [Google Scholar] [CrossRef]
- Mil-Homens, D.; Fialho, A.M. Trimeric autotransporter adhesins in members of the Burkholderia cepacia complex: A multifunctional family of proteins implicated in virulence. Front Cell Infect Microbiol 2011, 1. [Google Scholar] [CrossRef]
- Lafontaine, E.R.; Chen, Z.; Huertas-Diaz, M.C.; Dyke, J.S.; Jelesijevic, T.P.; Michel, F.; Hogan, R.J.; He, B. The autotransporter protein BatA is a protective antigen against lethal aerosol infection with Burkholderia mallei and Burkholderia pseudomallei. Vaccine X 2019, 1, 100002. [Google Scholar] [CrossRef]
- Sitthidet, C.; Korbsrisate, S.; Layton, A.N.; Field, T.R.; Stevens, M.P.; Stevens, J.M. Identification of Motifs of Burkholderia pseudomallei BimA Required for Intracellular Motility, Actin Binding, and Actin Polymerization. J Bacteriol 2011, 193(8), 1901–1910. [Google Scholar] [CrossRef]
- Kespichayawattana, W.; Rattanachetkul, S.; Wanun, T.; Utaisincharoen, P.; Sirisinha, S. Burkholderia pseudomallei Induces Cell Fusion and Actin-Associated Membrane Protrusion: A Possible Mechanism for Cell-to-Cell Spreading. Infect Immun 2000, 68(9), 5377–5384. [Google Scholar] [CrossRef]
- Sitthidet, C.; Stevens, J.M.; Chantratita, N.; Currie, B.J.; Peacock, S.J.; Korbsrisate, S.; Stevens, M.P. Prevalence and Sequence Diversity of a Factor Required for Actin-Based Motility in Natural Populations of Burkholderia Species. J Clin Microbiol 2008, 46(7), 2418–2422. [Google Scholar] [CrossRef]
- Mukhopadhyay, C.; Kaestli, M.; Vandana, K.E.; Sushma, K.S.; Mayo, M.; Richardson, L.; Tuanyok, A.; Keim, P.; Godoy, D.; Spratt, B.G.; et al. Molecular Characterization of Clinical Burkholderia pseudomallei Isolates from India. Am J Trop Med Hyg 2011, 85(1), 121–123. [Google Scholar] [CrossRef] [PubMed]
- Corea, E.M.; de Silva, A.D.; Thevanesam, V. Melioidosis in Sri Lanka. Trop Med Infect Dis. 2018, 3(1), 22. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.L.; Fane, A.; Sarovich, D.S.; Price, E.P.; Rush, C.M.; Govan, B.L.; Parker, E.; Mayo, M.; Currie, B.J.; Ketheesan, N. Increased Neurotropic Threat from Burkholderia pseudomallei Strains with a B. mallei–like Variation in the bimA Motility Gene, Australia. Emerg Infect Dis 2017, 23(5), 740-749. [CrossRef]
- Sarovich, D.S.; Price, E.P.; Webb, J.R.; Ward, L.M.; Voutsinos, M.Y.; Tuanyok, A.; Mayo, M.; Kaestli, M.; Currie, B.J. Variable Virulence Factors in Burkholderia pseudomallei (Melioidosis) Associated with Human Disease. PLoS One 2014, 9(3), e91682. [Google Scholar] [CrossRef] [PubMed]
- Gora, H.; Hasan, T.; Smith, S.; Wilson, I.; Mayo, M.; Woerle, C.; Webb, J.R.; Currie, B.J.; Hanson, J.; Meumann, E.M. Melioidosis of the Central Nervous System: Impact of the bimABm Allele on Patient Presentation and Outcome. Clin Infect Dis 2022, ciac111. [Google Scholar] [CrossRef]
- Campos, C.G.; Byrd, M.S.; Cotter, P.A. Functional Characterization of Burkholderia pseudomallei Trimeric Autotransporters. Infect Immun 2013, 81(8), 2788–2799. [Google Scholar] [CrossRef]
- Adler, N.R.L.; Stevens, M.P.; Dean, R.E.; Saint, R.J.; Pankhania, D.; Prior, J.L.; Atkins, T.P.; Kessler, B.; Nithichanon, A.; Lertmemongkolchai, G.; et al. Systematic Mutagenesis of Genes Encoding Predicted Autotransported Proteins of Burkholderia pseudomallei Identifies Factors Mediating Virulence in Mice, Net Intracellular Replication and a Novel Protein Conferring Serum Resistance. PLoS One 2015, 10(4), e0121271. [Google Scholar] [CrossRef]
- Coulthurst, S. The Type VI secretion system: A versatile bacterial weapon. Microbiol (Reading) 2019, 165(5). [CrossRef]
- Singh, R.P.; Kumari, K. Bacterial type VI secretion system (T6SS): An evolved molecular weapon with diverse functionality. Biotech Letters 2023, 45, 309–331. [Google Scholar] [CrossRef]
- DeShazer, D. A novel contact-independent T6SS that maintains redox homeostasis via Zn2+ and Mn2+ acquisition is conserved in the Burkholderia pseudomallei complex. Microbiol Res 2019, 226, 48–54. [Google Scholar] [CrossRef]
- Toesca, I.J.; French, C.T.; Miller, J.F. The Type VI Secretion System Spike Protein VgrG5 Mediates Membrane Fusion during Intercellular Spread by Pseudomallei Group Burkholderia Species. Infect Immun 2014, 82(4), 1436–1444. [Google Scholar] [CrossRef]
- Schwarz, S.; Singh, P.; Robertson, J.D.; LeRoux, M.; Skerrett, S.J.; Goodlett, D.R.; West, T.E.; Mougous, J.D. VgrG-5 Is a Burkholderia Type VI Secretion System-Exported Protein Required for Multinucleated Giant Cell Formation and Virulence. Infect Immun 2014, 82(4), 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Kostow, N.; Welch, M.D. Plasma membrane protrusions mediate host cell–cell fusion induced by Burkholderia thailandensis. Mol Biol Cell 2022, 33(8), ar70. [Google Scholar] [CrossRef] [PubMed]
- Schell, M.A.; Ulrich, R.L.; Ribot, W.J.; Brueggemann, E.E.; Hines, H.B.; Chen, D.; Lipscomb, L.; Kim, H.S.; Mrázek, J.; Nierman, W.C.; et al. Type VI secretion is a major virulence determinant in Burkholderia mallei. Mol Microbiol 2007, 64(6), 1466–1485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ye, F.; Wang, Y.; Liu, R.; Huang, Z.; Chen, C.; Liu, L.; Kang, X.; Dong, S.; Rajaofera, M.J.N.; et al. Role of type VI secretion system protein TssJ-3 in virulence and intracellular survival of Burkholderia pseudomallei. Biochem Biophys Res Common 2023, 682(19), 397–406. [Google Scholar] [CrossRef] [PubMed]
- Spiewak, H.L.; Shastri, S.; Zhang, L.; Schwager, S.; Eberl, L.; Vergunst, A.C.; Thomas, M.S. Burkholderia cenocepacia utilizes a type VI secretion system for bacterial competition. Microbiologyopen 2019, 8(7), e00774. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; West, T.E.; Boyer, F.; Chiang, W.C.; Carl, M.A.; Hood, R.D.; Rohmer, L.; Tolker-Nielsen, T.; Skerrett, S.J.; Mougous, J.D. Burkholderia Type VI Secretion Systems Have Distinct Roles in Eukaryotic and Bacterial Cell Interactions. PLoS Pathog 2010, 6(8), e1001068. [Google Scholar] [CrossRef]
- Burtnick, M.N.; Brett, P.J.; Harding, S.V.; Ngugi, S.A.; Ribot, W.J.; Chantratita, N.; Scorpio, A.; Milne, T.S.; Dean, R.E.; Fritz, D.L.; et al. The Cluster 1 Type VI Secretion System Is a Major Virulence Determinant in Burkholderia pseudomallei. Infect Immun 2011, 79(4), 1512–1525. [Google Scholar] [CrossRef]
- Shalom, G.; Shaw, J.G.; Thomas, M.S. In vivo expression technology identifies a type VI secretion system locus in Burkholderia pseudomallei that is induced upon invasion of macrophages. Microbiol (Reading) 2007, 153, 2689–2699. [Google Scholar] [CrossRef]
- Burtnick, M.N.; DeShazer, D.; Nair, V.; Gherardini, F.C.; Brett, P.J. Burkholderia mallei Cluster 1 Type VI Secretion Mutants Exhibit Growth and Actin Polymerization Defects in RAW 264.7 Murine Macrophages. Infect Immun 2010, 78(1), 88–99. [Google Scholar] [CrossRef]
- Burtnick, M.N.; Shaffer, T.L.; Ross, B.N.; Muruato, L.A.; Sbrana, E.; DeShazer, D.; Torres, A.G.; Brett, P.J. Development of Subunit Vaccines That Provide High-Level Protection and Sterilizing Immunity against Acute Inhalational Melioidosis. Infect Immun 2018, 86(1), e00724–17. [Google Scholar] [CrossRef]
- Tran, Q.T.L.; Nguyen, H.V.; Pham, H.T.; Mai, T.V.; Nguyen, Q.H.M.; Le, D.V.; Bui, L.N.H.; Hoang, L.T.H.; Hoang, T.Q.; Trinh, T.T. Clinical Utility of Combined Whole-cell Antigen and Recombinant Hemolysis Co-regulated Protein 1-Enzyme-linked Immunosorbent Assays Reveals Underdiagnosed Cases of Melioidosis in Vietnam. Am J Trop Med Hyg 2022, 107(3), 585–591. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.E.; Berner, A.; Lipp, M.; Kohler, C.; Assig, K.; Lichtenegger, S.; Saqib, M.; Müller, E.; Trinh, T.T.; Gad, A.M. et al. Protein Microarray-Guided Development of a Highly Sensitive and Specific Dipstick Assay for Glanders Serodiagnostics. J Clin Microbiol 2023, 61(1), e01234-22. [CrossRef]
- Sengyee, S.; Yarasai, A.; Janon, R.; Morakot, C.; Ottiwet, O.; Schmidt, L.K.; West, T.E.; Burtnick, M.N.; Chantratita, C.; Brett, P.J. Melioidosis Patient Survival Correlates With Strong IFN-γ Secreting T Cell Responses Against Hcp1 and TssM. Front Immunol 2021, 12, 698303. [Google Scholar] [CrossRef]
- Biryukov, S.S.; Cote, C.K.; Klimko, C.P.; Dankmeyer, J.L.; Rill, N.O.; Shoe, J.L.; Hunter, M.; Shamsuddin, Z.; Velez, I.; Hedrick, Z.M.; et al. Evaluation of two different vaccine platforms for immunization against melioidosis and glanders. Front Microbiol 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Li, G.; Li, J.; Zheng, M.; Peng, X.; Rao, Y.; Li, M.; Zhou, R.; Rao, X. Hcp1-loaded staphylococcal membrane vesicle vaccine protects against acute melioidosis. Front Immunol 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Brunet, Y.R.; Hénin, J.; Celia, H.; Cascales, E. Type VI secretion and bacteriophage tail tubes share a common assembly pathway. EMBO Rep 2014, 15(3), 315–321. [Google Scholar] [CrossRef]
- Backmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol 2010, 10, 787–796. [Google Scholar] [CrossRef]
- Kumar, S.; Anselmo, A.C.; Banerjee, A.; Zakrewsky, M.; Mitragotri. Shape and size-dependent immune response to antigen-carrying nanoparticles. J Control Release 2015, 220(Pt A), 141-148. [CrossRef]
- Baranov, M.V.; Kumar, M.; Sacanna, S.; Thutupalli, S.; van den Bogaart, G. Modulation of Immune Responses by Particle Size and Shape. Front Immunol 2020, 11. [Google Scholar] [CrossRef]
- Shanks, J.; Burtnick, M.N.; Brett, P.J.; Waag, D.M.; Spurgers, K.N.; Ribot, W.J.; Schell, M.A.; Panchal, R.G.; Gherardini, F.C.; Wilkinson, K.D. , et al. Burkholderia mallei tssM Encodes a Putative Deubiquitinase That Is Secreted and Expressed inside Infected RAW 264.7 Murine Macrophages. Infect Immun 2009, 77(4), 1636–1648. [Google Scholar] [CrossRef]
- Tan, K.S.; Chen, Y.; Lim, T.C.; Tan, G.Y.G.; Liu, Y.; Lim, Y.T.; Macary, P.; Gan, Y.H. Suppression of Host Innate Immune Response by Burkholderia pseudomallei through the Virulence Factor TssM. J Immunol 2010, 184(9), 5160–5171. [Google Scholar] [CrossRef]
- Burtnick, M.N.; Brett, P.J.; DeShazer, D. Proteomic Analysis of the Burkholderia pseudomallei Type II Secretome Reveals Hydrolytic Enzymes, Novel Proteins, and the Deubiquitinase TssM. Infect Immun 2014, 82(8). [CrossRef]
- Jacobsen, T.; Bardiaux, B.; Francetic, O.; Izadi-Pruneyre, N.; Nilges, M. Structure and function of minor pilins of type IV pili. Med Microbiol Immunol 2020, 209(3), 301–308. [Google Scholar] [CrossRef]
- Essex-Lopresti, A.E.; Boddey, J.A.; Thomas, R.; Smith, M.P.; Hartley, M.G.; Atkins, T.; Brown, N.F.; Tsang, C.H.; Peak, I.R.A.; Hill, J; et al. A Type IV Pilin, PilA, Contributes to Adherence of Burkholderia pseudomallei and Virulence In Vivo. Infect Immun 2005, 73(2), 1260-1264. [CrossRef]
- Sangdee, K.; Waropastrakul, S.; Wongratanachewin, S.; Homchampa, P. Heterologously type IV pilus expressed proteins of Burkholderia pseudomallei is immunogenic but fails to elicit protective immunity in mice. Southeast Asian J Trop Med Public Health 2011, 42(5), 1190–1196. [Google Scholar]
- Wu, R.; Stephenson, R.; Gichaba, A.; Noinaj, N. The Big BAM Theory: An Open and Closed Case? Biochim Biophys Acta Biomembr 2020, 1862(1), 183062. [Google Scholar] [CrossRef]
- Rayes, J.E.; Rodríguez-Alonso, R.; Collet, J.F. Lipoproteins in Gram-negative bacteria: New insights into their biogenesis, subcellular targeting and functional roles. Curr Opin Microbiol 2021, 61, 25–34. [Google Scholar] [CrossRef]
- Chatorvedi, D.; Mahalakshmi, R. Transmembrane β-barrels: Evolution, folding and energetics. Biochim Biophys Acta Biomembr 2017, 1859(12), 2467–2482. [Google Scholar] [CrossRef]
- Ni, D.; Huang, Y. The Expression, Purification, and Structure Determination of BamA from E. coli. In: Buchanan, S.; Noinaj, N. (eds) The BAM Complex. Methods Mol Biol 2015, 1329, 169–178. [Google Scholar] [CrossRef]
- Mouhib, M.; Benediktsdottir, A.; Nilsson, C.S.; Chi, C.N. Influence of Detergent and Lipid Composition on Reconstituted Membrane Proteins for Structural Studies. ACS Omega 2021, 6(38), 24377–24381. [Google Scholar] [CrossRef]
- Doerner, P.A.; Sousa, M.C. Extreme Dynamics in the BamA β-Barrel Seam. Biochem 2017, 56(24), 3142–3149. [Google Scholar] [CrossRef]
- Iadanza, M.G.; Schiffrin, B.; White, P.; Watson, M.A.; Horne, J.E.; Higgins, A.J.; Calabrese, A.N.; Brockwell, D.J.; Tuma, R.; Kalli, A.C.; et al. Distortion of the bilayer and dynamics of the BAM complex in lipid nanodiscs. Commun Biol 2020, 3, 766. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Wan, K.L.; Mohamed, R.; Nathan, S. Immunization with the recombinant Burkholderia pseudomallei outer membrane protein Omp85 induces protective immunity in mice. Vaccine 2010, 28(31), 5005–5011. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Wan, K.L.; Mohamed, R.; Nathan, S. A genome level survey of Burkholderia pseudomallei immunome expressed during human infection. Microbes Infect 2008, 10(12-13), 1335-1345. [CrossRef]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res 2008, 36(Suppl_2), W5-W9. [CrossRef]
- Xu, Q.; Guo, M.; Yu, F. β-Barrel Assembly Machinery (BAM) Complex as Novel Antibacterial Drug Target. Molecules 2023, 28(9), 3758. [Google Scholar] [CrossRef] [PubMed]
- Malinverni, J.C.; Werner, J.; Kim, S.; Sklar, J.G.; Kahne, D.; Misra, R.; Silhavy, T.J. YfiO stabilizes the YaeT complex and is essential for outer membrane protein assembly in Escherichia coli. Mol Microbiol 2006, 61, 151–164. [Google Scholar] [CrossRef]
- Singh, R.; Capalash, N.; Sharma, P. Immunoprotective potential of BamA, the outer membrane protein assembly factor, against MDR Acinetobacter baumannii. Sci Rep 2017, 7, 12411. [Google Scholar] [CrossRef]
- De Araujo, A.E.V.; Conde, L.V.; da Silva, H.C. Jr; Machado, L.A.; Lara, F.A.; Chapeaurouge, A.; Pauer, H.; Hardoim, C.C.P.; Antunes, L.C.M.; Carvalho-Assef, A.P.D.; et al. Cross-reactivity and immunotherapeutic potential of BamA recombinant protein from Acinetobacter baumannii. Microbes Infect 2021, 23(4-5), 104801. [CrossRef]
- Guan, Q.; Wang, X.; Wang, X.; Teng, D.; Wang, J. In silico analysis and recombinant expression of BamA protein as a universal vaccine against Escherichia coli in mice. Apply Microbiol Biotechnol 2016, 100(11), 5089–5098. [Google Scholar] [CrossRef]
- Storek, K.M.; Auerbach, M.R.; Shi, H.; Garcia, N.K.; Sun, D.; Nickerson, N.N.; Vij, R.; Lin, Z.; Chiang, N.; Schneider, K; et al. Monoclonal antibody targeting the β-barrel assembly machine of Escherichia coli is bactericidal. PNAS 2018, 115(14), 3692-3697. [CrossRef]
- Vij, R.; Lin, Z.; Chiang, N.; Vernes, J.M.; Storek, K.M.; Park, S.; Chan, J; Meng, Y. G.; Comps-Agrar, L.; Luan, P.; et al. A targeted boost-and-sort immunization strategy using Escherichia coli BamA identifies rare growth inhibitory antibodies. Sci Rep 2018, 8, 7136. [Google Scholar] [CrossRef]
- Meng, E.C.; Goddard, T.D.; Pettersen, E.F.; Couch, G.S.; Pearson, Z.J.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Tools for structure building and analysis. Protein Sci 2023, 32(11), e4792. [Google Scholar] [CrossRef]
- Hallgren, J.; Tsirigos, K.D.; Pedersen, M.D.; Armenteros, J.J.A.; Marcatili, P.; Nielsen, H.; Krogh, A.; Winther, O. DeepTMHMM predicts alpha and beta transmembrane proteins using deep neural networks. bioRxiv 2023. [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- McClean, S.; Healy, M.E.; Collins, C.; Carberry, S.; O’Shaughnessy, L.; Dennehy, R.; Adams, Á; Kennelly, H.; Corbett, J.M.; Carty, F.; et al. Linocin and OmpW Are Involved in Attachment of the Cystic Fibrosis-Associated Pathogen Burkholderia cepacia Complex to Lung Epithelial Cells and Protect Mice against Infection. Infect Immun 2016, 84(5), 1424-1437. [CrossRef]
- Casey, W.T.; Spink, N.; Cia, F.; Collins, C.; Romano, M.; Berisio, R.; Bancroft, G.J.; McClean, S. Identification of an OmpW homologue in Burkholderia pseudomallei, a protective vaccine antigen against melioidosis. Vaccine 2016, 34(23), 2616–2621. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Cortázar, J.; Bossi, L.; Quinn, C.; Reynolds, C.J.; Butler, D.K.; Corcoran, N.; Murchú, M.Ó.; McMahon, E.; Singh, M.; Rongkard, P; et al. BpOmpW Antigen Stimulates the Necessary Protective T-Cell Responses Against Melioidosis. Front Immunol 2021, 12, 767359. [Google Scholar] [CrossRef] [PubMed]
- Siritapetawee, J.; Prinz, H.; Krittanai, C.; Sugninta, W. Expression and refolding of Omp38 from Burkholderia pseudomallei and Burkholderia thailandensis, and its function as a diffusion porin. Biochem J 2004, 384(Pt 3), 609–617. [Google Scholar] [CrossRef]
- Kiekens, S.; Sass, A.; van Nieuwerburgh, F.; Deforce, D.; Coenye, T. The Small RNA ncS35 Regulates Growth in Burkholderia cenocepacia J2315. mSphere 2018, 3(1). [CrossRef]
- Sass, A.M.; Coenye, T. The Small RNA NcS25 Regulates Biological Amine-Transporting Outer Membrane Porin BCAL3473 in Burkholderia cenocepacia. mSphere 2023, 8(2), e00083–23. [Google Scholar] [CrossRef] [PubMed]
- Podnecky, N.L.; Rhodes, K.A.; Schweizer, H.P. Efflux pump-mediated drug resistance in Burkholderia. Front Microbiol 2015, 6, 305. [Google Scholar] [CrossRef] [PubMed]
- Bachert, B.A.; Choi, S.J.; Snyder, A.K.; Rio, R.V.M.; Durney, B.C.; Holland, L.A.; Amemiya, K.; Welkos, S.L.; Bozue, J.A.; Cote, C.K.; et al. A Unique Set of the Burkholderia Collagen-Like Proteins Provides Insight into Pathogenesis, Genome Evolution and Niche Adaptation, and Infection Detection. PLoS One 2015, 10(9), e0137578. [Google Scholar] [CrossRef] [PubMed]
- Grund, M.E.; Choi, S.J.; McNitt, D.H.; Barbier, M.; Hu, G.; LaSala, P.R.; Cote, C.K.; Berisio, R.; Lukomski, S. Burkholderia collagen-like protein 8, Bucl8, is a unique outer membrane component of a putative tetrapartite efflux pump in Burkholderia pseudomallei and Burkholderia mallei. PLoS One 2020, 15(11), e0242593. [Google Scholar] [CrossRef]
- Grund, M.E.; Kramarska, E.; Choi, S.J.; McNitt, D.H.; Klimko, C.P.; Rill, N.O.; Dankmeyer, J.L.; Shoe, J.L.; Hunter, M.; Fetterer, D.P.; et al. Predictive and Experimental Immunogenicity of Burkholderia Collagen-like Protein 8-Derived Antigens. Vaccines (Basel) 2021, 9(11), 1219. [Google Scholar] [CrossRef] [PubMed]
- Grund, M.; Choi, S.J.; Powell, L.; Lukomski, S. Intranasal immunization with a Bucl8-based vaccine ameliorates bacterial burden and pathological inflammation, and promotes an IgG2a/b dominant response in an outbred mouse model of Burkholderia infection. Front Immunol 2023, 14. [Google Scholar] [CrossRef]
- Manning, P.A.; Beutin, L.; Achtman, M. Outer membrane of Escherichia coli: Properties of the F sex factor traT protein which is involved in surface exclusion. J Bacteriol 1980, 142(1), 285–294. [Google Scholar] [CrossRef]
- Perumal, N.B.; Minkley, E.G. Jr. The product of the F sex factor traT surface exclusion gene is a lipoprotein. J Biol Chem 1984, 259(9), 5357–5360. [Google Scholar] [CrossRef]
- Chamberlain, N.R.; Brandt, M.E.; Erwin, A.L.; Radolf, J.D.; Norgard, M.V. Major integral membrane protein immunogens of Treponema pallidum are proteolipids. Infect Immun 1989, 57(9), 2872–2877. [Google Scholar] [CrossRef]
- Brandt, M.E.; Riley, B.S.; Radolf, J.D.; Norgard, M.V. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect Immun 1990, 58(4), 983–991. [Google Scholar] [CrossRef]
- Wilson, M.M.; Bernstein, H.D. Surface Exposed Lipoproteins: An Emerging Secretion Phenomenon in Gram-negative Bacteria. Trends Microbiol 2016, 24(3), 198–208. [Google Scholar] [CrossRef]
- Schell, M.A.; Zhao, P.; Wells, L. Outer membrane proteome of Burkholderia pseudomallei and Burkholderia mallei from diverse growth conditions. J Proteome Res 2011, 10(5), 2417–2424. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.A.; Seixas, A.M.M.; Mandal, M.; Rodríguez-Ortega, M.J.; Leitão, J.H. Characterization of the Burkholderia cenocepacia J2315 Surface-Exposed Immunoproteome. Vaccines (Basel) 2020, 8(3), 509. [Google Scholar] [CrossRef] [PubMed]
- Teufel, F.; Armenteros, J.J.A.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat Biotechnol 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- Hara, Y.; Mohamed, R.; Nathan, S. Immunogenic Burkholderia pseudomallei Outer Membrane Proteins as Potential Candidate Vaccine Targets. PLoS One 2009, 4(8), e6496. [Google Scholar] [CrossRef]
- Champion, O.L.; Gourlay, L.J.; Scott, A.E.; Lassaux, P.; Conejero, L.; Perletti, L.; Hemsley, C.; Prior, J.; Bancroft, G.; Bolognesi, M.; et al. Immunisation with proteins expressed during chronic murine melioidosis provides enhanced protection against disease. Vaccine 2016, 34(14), 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Dyke, J.S.; Huertas-Diaz, M.C.; Michel, F.; Holladay, N.E.; Hogan, R.J.; He, B.; Lafontaine, E.R. The Peptidoglycan-associated lipoprotein Pal contributes to the virulence of Burkholderia mallei and provides protection against lethal aerosol challenge. Virulence 2020, 11(1), 1024–1040. [Google Scholar] [CrossRef]
- Gourlay, L.J.; Peri, C.; Ferrer-Navarro, M.; Conchillo-Solé, O.; Gori, A.; Rinchai, D.; Thomas, R.J.; Champion, O.L.; Michell, S.L.; Kewcharoenwong, C.; et al. Exploiting the Burkholderia pseudomallei Acute Phase Antigen BPSL2765 for Structure-Based Epitope Discovery/Design in Structural Vaccinology. Chem Biol 2013, 20(9), 1147–1156. [Google Scholar] [CrossRef]
- Michel, L.V.; Shaw, J.; MacPherson, V.; Barnard, D.; Bettinger, J.; D’Arcy, B.; Surendran, N.; Hellman, J.; Pichichero, M.E. Dual orientation of the outer membrane lipoprotein Pal in Escherichia coli. Microbiol (Reading) 2015, 161(Pt 6), 1251–1259. [Google Scholar] [CrossRef]
- Michel, L.V.; Snyder, J.; Schmidt, R.; Milillo, J.; Grimaldi, K.; Kalmeta, B.; Khan, M.N.; Sharma, S.; Wright, L.K.; Pichichero, M.E. Dual Orientation of the Outer Membrane Lipoprotein P6 of Nontypeable Haemophilus influenzae. J Bacteriol 2013, 195(14), 3252–3259. [Google Scholar] [CrossRef]
- Shinoy, M.; Dennehy, R.; Coleman, L.; Carberry, S.; Schaffer, K.; Callaghan, M.; Doyle, S.; McClean, S. Immunoproteomic Analysis of Proteins Expressed by Two Related Pathogens, Burkholderia multivorans and Burkholderia cenocepacia, during Human Infection. PLoS One 2013, 8(11), e80796. [Google Scholar] [CrossRef] [PubMed]
- Makidon, P.E.; Knowlton, J.; Groom, J.V. II; Blanco, L.P.; LiPuma, J.J.; Bielinska, A.U.; Baker, J.R. Jr. Induction of immune response to the 17 kDa OMPA Burkholderia cenocepacia polypeptide and protection against pulmonary infection in mice after nasal vaccination with an OMP nanoemulsion-based vaccine. Med Microbiol Immunol 2010, 199, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Dennehy, R.; Romano, M.; Ruggiero, A.; Mohamed, Y.F.; Dignam, S.L.; Troncoso, C.M.; Callaghan, M.; Valvano, M.A.; Berisio, R.; McClean, S. The Burkholderia cenocepacia peptidoglycan-associated lipoprotein is involved in epithelial cell attachment and elicitation of inflammation. Cell Microbiol 2016, 19(5), e12691. [Google Scholar] [CrossRef]
- Peri, C.; Gori, A.; Gagni, P.; Sola, L.; Girelli, D.; Sottotetti, S.; Cariani, L.; Chiari, M.; Cretich, M.; Colombo, G. Evolving serodiagnostics by rationally designed peptide arrays: The Burkholderia paradigm in Cystic Fibrosis. Sci Rep 2016, 6, 32873. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.J.; Vigil, A.; DeShazer, D.; Waag, D.M.; Felgner, P.; Goldberg, J.B. Distinct human antibody response to the biological warfare agent Burkholderia mallei. Virulence 2012, 3(6), 510–514. [Google Scholar] [CrossRef] [PubMed]
- Nithichanon, A.; Rinchai, D.; Buddhisa, S.; Saenmuang, P.; Kewcharoenwong, C.; Kessler, B.; Khaenam, P.; Chetchotisakd, P.; Maillere, B.; Robinson, J.; et al. Immune Control of Burkholderia pseudomallei––Common, High-Frequency T-Cell Responses to a Broad Repertoire of Immunoprevalent Epitopes. Front Immunol 2018, 9, 484. [Google Scholar] [CrossRef]
- Seixas, A.M.M.; Sousa, S.A.; Feliciano, J.R.; Gomes, S.C.; Ferreira, M.R.; Moreira, L.M.; Leitão, J.H. A Polyclonal Antibody Raised against the Burkholderia cenocepacia OmpA-like Protein BCAL2645 Impairs the Bacterium Adhesion and Invasion of Human Epithelial Cells In Vitro. Biomedicines 2021, 9(12), 1788. [Google Scholar] [CrossRef]
- Hara, Y.; Chin, C.Y.; Mohamed, R.; Puthucheary, S.D.; Nathan, S. Multiple-antigen ELISA for melioidosis - a novel approach to the improved serodiagnosis of melioidosis. BMC Infect Dis 2013, 13, 165. [Google Scholar] [CrossRef]
- Davidson, A.L.; Dassa, E.; Orelle, C.; Chen, J. Structure, Function, and Evolution of Bacterial ATP-Binding Cassette Systems. Microbiol Mol Biol Rev 2008, 72(2), 317–364. [Google Scholar] [CrossRef]
- Rees, D.C.; Johnson, E.; Lewinson, O. ABC transporters: The power to change. Nat Rev Mol Cell Biol 2009, 10, 218–227. [Google Scholar] [CrossRef]
- Garmory, H.S.; Titball, R.W. ATP-Binding Cassette Transporters Are Targets for the Development of Antibacterial Vaccines and Therapies. Infect Immun 2004, 72(12), 6757–6763. [Google Scholar] [CrossRef]
- Harland, D.N.; Dassa, E.; Titball, R.W.; Brown, K.A.; Atkins, H.S. ATP-binding cassette systems in Burkholderia pseudomallei and Burkholderia mallei. BMC Genomics 2007, 8, 83. [Google Scholar] [CrossRef]
- Harland, D.N.; Chu, K.; Haque, A.; Nelson, M.; Walker, N.J.; Sarkar-Tyson, M.; Atkins, T.P.; Moore, B.; Brown, K.A.; Bancroft, G.; et al. Identification of a LolC Homologue in Burkholderia pseudomallei, a Novel Protective Antigen for Melioidosis. Infect Immunol 2007, 75(8), 4173–4180. [Google Scholar] [CrossRef]
- Sharma, S.; Zhou, R.; Wan, L.; Feng, S.; Song, K.; Xu, C.; Li, Y.; Liao, M. Mechanism of LolCDE as a molecular extruder of bacterial triacylated lipoproteins. Nat Commun 2021, 12, 4687. [Google Scholar] [CrossRef]
- Terui, Y.; Saroj, S.D.; Sakamoto, A.; Yoshida, T.; Higashi, K.; Kurihara, S.; Suzuki, H.; Toida, T.; Kashiwagi, K.; Igarashi, K. Properties of putrescine uptake by PotFGHI and PuuP and their physiological significance in Escherichia coli. Amino Acids 2013, 46, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.C.; Blevins, T.C.; Short, S.A. Regulation of peptide transport in Escherichia coli: Induction of the trp-linked operon encoding the oligopeptide permease. J Bacteriol 1986, 165(2), 428–433. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, M.V.; Maier, R.J. Peptide Transport in Helicobacter pylori: Roles of Dpp and Opp Systems and Evidence for Additional Peptide Transporters. J Bacteriol 2007, 189(9), 3392–402. [Google Scholar] [CrossRef] [PubMed]
- Tippayawat, P.; Saenwongsa, W.; Mahawantung, J.; Suwannasaen, D.; Chetchotisakd, P.; Limmathurotsakul, D.; Peacock, S.J.; Felgner, P.L.; Atkins, H.S.; Titball, R.W.; et al. Phenotypic and Functional Characterization of Human Memory T Cell Responses to Burkholderia pseudomallei. PLoS Negl Trop Dis 2009, 3(4), e407. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.K.; Tippayawat, P.; Walker, N.J.; Harding, S.V.; Atkins, H.S.; Maillere, B.; Bancroft, G.J.; Lertmemongkolchai, G.; Altmann, D.M. CD4+ T cell immunity to the Burkholderia pseudomallei ABC transporter LolC in melioidosis. Eur J Immunol 2011, 41(1), 107–115. [Google Scholar] [CrossRef] [PubMed]
- Tippayawat, P.; Pinsiri, M.; Rinchai, D.; Riyapa, D.; Romphruk, A.; Gan, T.H.; Houghton, R.L.; Felgner, P.L.; Titball, R.W.; Stevens, M.P.; et al. Burkholderia pseudomallei Proteins Presented by Monocyte-Derived Dendritic Cells Stimulate Human Memory T Cells In Vitro. Infect Immun 2011, 79(1), 305–313. [Google Scholar] [CrossRef]
- Scott, A.E.; Burtnick, M.N.; Stokes, M.G.M.; Whelan, A.O.; Williamson, E.D.; Atkins, T.P.; Prior, J.L.; Brett, P.J. Burkholderia pseudomallei Capsular Polysaccharide Conjugates Provide Protection against Acute Melioidosis. Infect Immun 2014, 82(8), 3206–3213. [Google Scholar] [CrossRef]
- Settles, E.W.; Sonderegger, D.; Shannon, A.B.; Celona, K.R.; Lederer, R.; Yi, J.; Seavey, C.; Headley, K.; Mbegbu, M.; Harvey, M.; et al. Development and evaluation of a multiplex serodiagnostic bead assay (BurkPx) for accurate melioidosis diagnosis. PLoS Negl Trop Dis 2023, 17(2), e0011072. [Google Scholar] [CrossRef]
- Khemaissa, S.; Sagan, S.; Walrant, A. Tryptophan, an Amino-Acid Endowed with Unique Properties and Its Many Roles in Membrane Proteins. Crystals 2021, 11(9), 1032. [Google Scholar] [CrossRef]
- Sousa, S.; Morad, M.; Feliciano, J.R.; Pita, T.; Nady, S.; El-Hennamy, R.E.; Abdel-Rahman, M.; Cavaco, J.; Pereira, L.; Barreto, C.; et al. The Burkholderia cenocepacia OmpA-like protein BCAL2958: Identification, characterization, and detection of anti-BCAL2958 antibodies in serum from B. cepacia complex-infected Cystic Fibrosis patients. AMB Express 2016, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.A.; Liu, Y. Sequencing and characterization of a novel serine metalloprotease from Burkholderia pseudomallei. FEMS Microbiol Lett 2000, 192(1), 67–72. [Google Scholar] [CrossRef] [PubMed]
- Valade, E.; Thibault, F.M.; Gauthier, Y.P.; Palencia, M.; Popoff, M.Y.; Vidal, D.R. The PmlI-PmlR Quorum Sensing System in Burkholderia pseudomallei Plays a Key Role in Virulence and Modulates Production of the MprA Protease. J Bacteriol 2004, 186(8), 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.Y.; Tan, S.C.; Nathan, S. Immunogenic recombinant Burkholderia pseudomallei MprA serine protease elicits protective immunity in mice. Front Cell Infect Microbiol 2012, 2, 85. [Google Scholar] [CrossRef] [PubMed]
- Welkos, S.; Blanco, I.; Okaro, U.; Chua, J.; DeShazer, D. A DUF4148 family protein produced inside RAW264.7 cells is a critical Burkholderia pseudomallei virulence factor. Virulence 2020, 11(1), 1041–1058. [Google Scholar] [CrossRef] [PubMed]
- O'Riordan, A.A.; Morales, V.A.; Mulligan, L.; Faheem, N.; Windle, H.J.; Kelleher, D.P. Alkyl hydroperoxide reductase: A candidate Helicobacter pylori vaccine. Vaccine 2012, 30, 3876–3884. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.H.; Wang, H.F.; Nian, Z.G.; Wang, Y.D.; Zeng, Q.Y.; Zhang, G. Immunization with alkyl hydroperoxide reductase subunit C reduces Fusobacterium nucleatum load in the intestinal tract. Sci Rep 2017, 7, 10566. [Google Scholar] [CrossRef]
- Lou, H.; Li, X.; Sheng, X.; Fang, S.; Wan, S.; Sun, A.; Chen, H. Development of a Trivalent Construct Omp18/AhpC/FlgH Multi Epitope Peptide Vaccine Against Campylobacter jejuni. Front Microbiol 2022, 12. [Google Scholar] [CrossRef]
- Dunachie, S.J.; Jenjaroen, K.; Reynold, C.J.; Quigley, K.J.; Sergeant, R.; Sumonwiriya, M; Chaichana, P. ; Chumseng, S.; Ariyaprasert, P.; Lassaux, P.; et al. Infection with Burkholderia pseudomallei – immune correlates of survival in acute melioidosis. Sci Rep 2017, 7, 12143. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.; Goudet, A.; Jenjaroen, K.; Sumonwiriya, M.; Rinchai, D.; Musson, J.; Overbeek, S.; Makinde, J.; Quigley, K.; Manji, J.; et al. T Cell Immunity to the Alkyl Hydroperoxide Reductase of Burkholderia pseudomallei: A Correlate of Disease Outcome in Acute Melioidosis. J Immunol 2015, 194(10), 4814–4824. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.K.; Orne, C.E.; Shaffer, T.L.; Wilson, S.M.; Khakhum, N.; Torres, A.G.; Brett, P.J.; Burtnick, M.N. Development of Melioidosis Subunit Vaccines Using an Enzymatically Inactive Burkholderia pseudomallei AhpC. Infect Immun 2022, 90(8), e00222–22. [Google Scholar] [CrossRef] [PubMed]
- Klimko, C.P.; Shoe, J.L.; Rill, N.O.; Hunter, M.; Dankmeyer, J.L.; Talyansky, Y.; Schmidt, L.K.; Orne, C.E.; Fetterer, D.P.; Biryukov, S.S.; et al. Layered and integrated medical countermeasures against Burkholderia pseudomallei infections in C57BL/6 mice. Front Microbiol 2022, 13, 965572. [Google Scholar] [CrossRef]
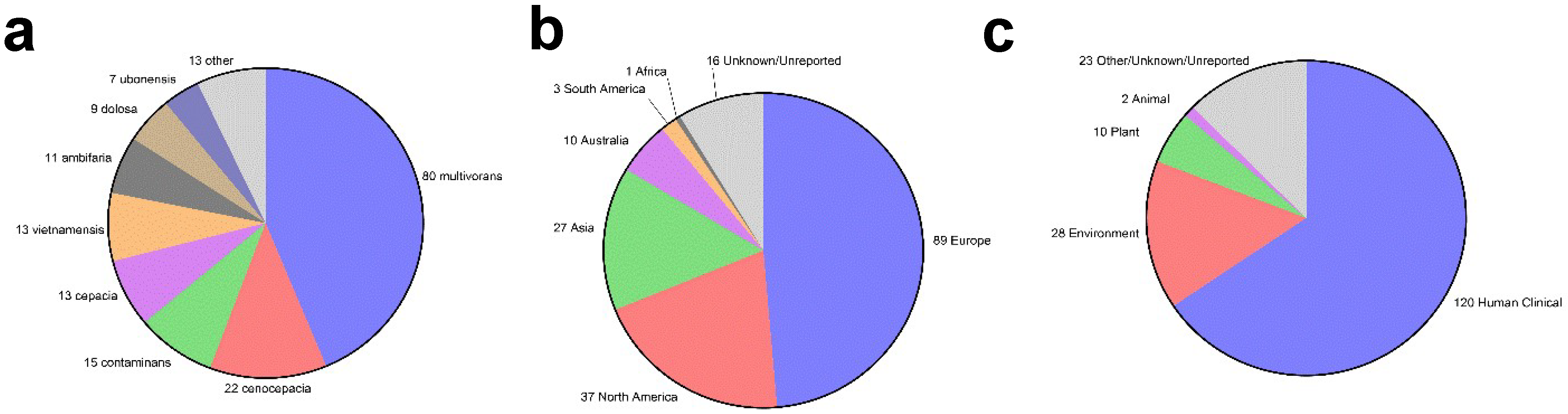
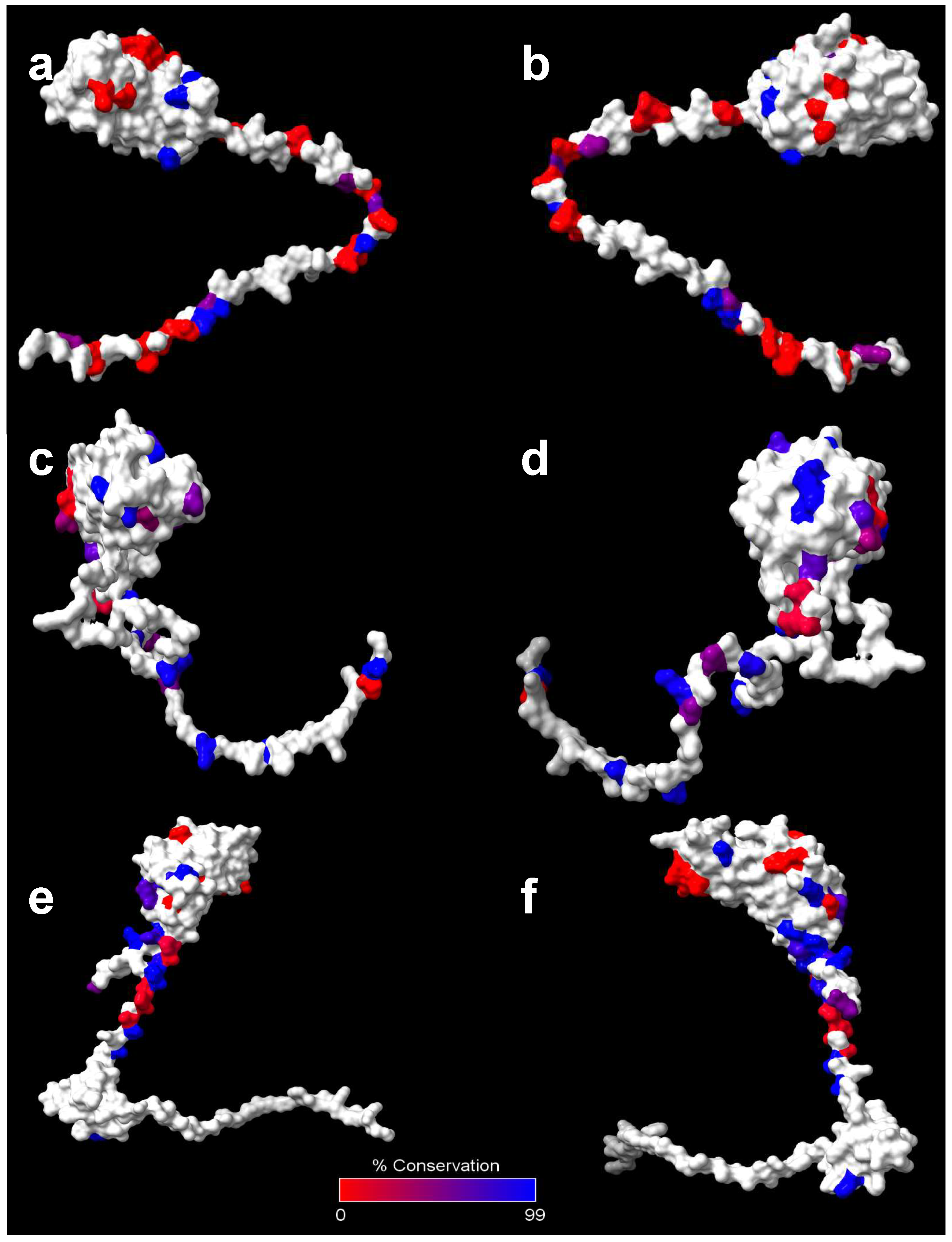
| B. pseudomallei K96243 Locus | Protein Name |
Antigen Class |
Absent in B. mallei? |
Bcc % Identity to K96243 |
Bcc % Similarity to K96243 |
Bcc % Coverage to K96243 |
% of Bcc Strains with Gene |
|---|---|---|---|---|---|---|---|
| BPSL2151 | BamA | β-barrel | 94.0 | 96.9 | 99.8 | 100 | |
| BPSL0999 | Omp1 | Lipoprotein | 93.5 | 95.9 | 99.2 | 100 | |
| BPSL2765 | Omp7 | Lipoprotein | 85.5 | 93.1 | 100 | 100 | |
| BPSL2522 | Omp3 | Other | 91.6 | 92.9 | 99.7 | 100 | |
| BPSL1552 | OmpW1 | β-barrel | 78.3 | 88.0 | 98.8 | 100 | |
| BPSS0879 | OpcP | β-barrel | 75.4 | 82.4 | 100 | 100 | |
| BPSL0281 | FlgL | Flagella | * | 67.9 | 80.8 | 100 | 100 |
| BPSL1972 | Bucl8 | β-barrel | 78.0‡ | 88.2‡ | 98.1‡ | 99 | |
| BPSS0708 | OpcP1 | β-barrel | Partially | 75.7 | 85.5 | 99.9 | 98 |
| BPSL3319 | FliC | Flagella | * | 74.0† | 84.6† | 98.2† | 59† |
| BPSL2704 | OmpW2 | β-barrel | 87.8 | 94.1 | 91.0 | 56 | |
| BPSS1593 | PilV | Pilin | Partially | 42.8 | 56.0 | 78.3 | 25 |
| BPSS1532 | BipB | T3SS | 33.2 | 53.2 | 55.6 | 5 | |
| BPSS1993 | MprA | Other | Absent | 83.3 | 89.6 | 99.3 | 4 |
| BPSS1529 | BipD | T3SS | 32.2 | 55.1 | 58.0 | 1 | |
| BPSS1531 | BipC | T3SS | 0 | 0 | 0 | 0 |
| B. pseudomallei K96243 Locus | Protein Name |
Antigen Class |
Absent in B. mallei? |
Bcc % Identity to K96243 |
Bcc % Similarity to K96243 |
Bcc % Coverage to K96243 |
% of Bcc Strains with Gene |
|---|---|---|---|---|---|---|---|
| BPSL2096 | AhpC | Other | 98.9 | 99.7 | 100 | 100 | |
| BPSL2287 | IscA | Other | 95.7 | 97.2 | 100 | 100 | |
| BPSL2277 | LolC | ABC Transporter | 93.3 | 97.1 | 100 | 100 | |
| BPSL3105 | Hcp6 | T6SS | Absent | 92.2 | 97.1 | 100 | 100 |
| BPSS0467 | PotF | ABC Transporter | Absent | 86.1 | 92.1 | 99.4 | 100 |
| BPSS2141 | OppA | ABC Transporter | 82.8 | 89.4 | 96.4 | 100 | |
| BPSL3369 | AcoD | Other | 74.3 | 84.7 | 100 | 100 | |
| BPSL1897 | TadE | Other | 48.3 | 60.7 | 92.6 | 100 | |
| BPSS0171 | Hcp4 | T6SS | 89.2 | 95.7 | 100 | 48 | |
| BPSS2098 | Hcp3 | T6SS | 93.1 | 97.7 | 100 | 16 | |
| BPSS1498 | Hcp1 | T6SS | 25.6 | 42.0 | 97.9 | 12 | |
| BPSS0099 | Hcp5 | T6SS | Absent | 88.6 | 95.1 | 100 | 8 |
| BPSS0518 | Hcp2 | T6SS | Partially | 95.6 | 98.3 | 100 | 2 |
| BPSS1512 | TssM | T6SS* | 0 | 0 | 0 | 0 | |
| BPSS1524 | BopA | T3SS | 0 | 0 | 0 | 0 |
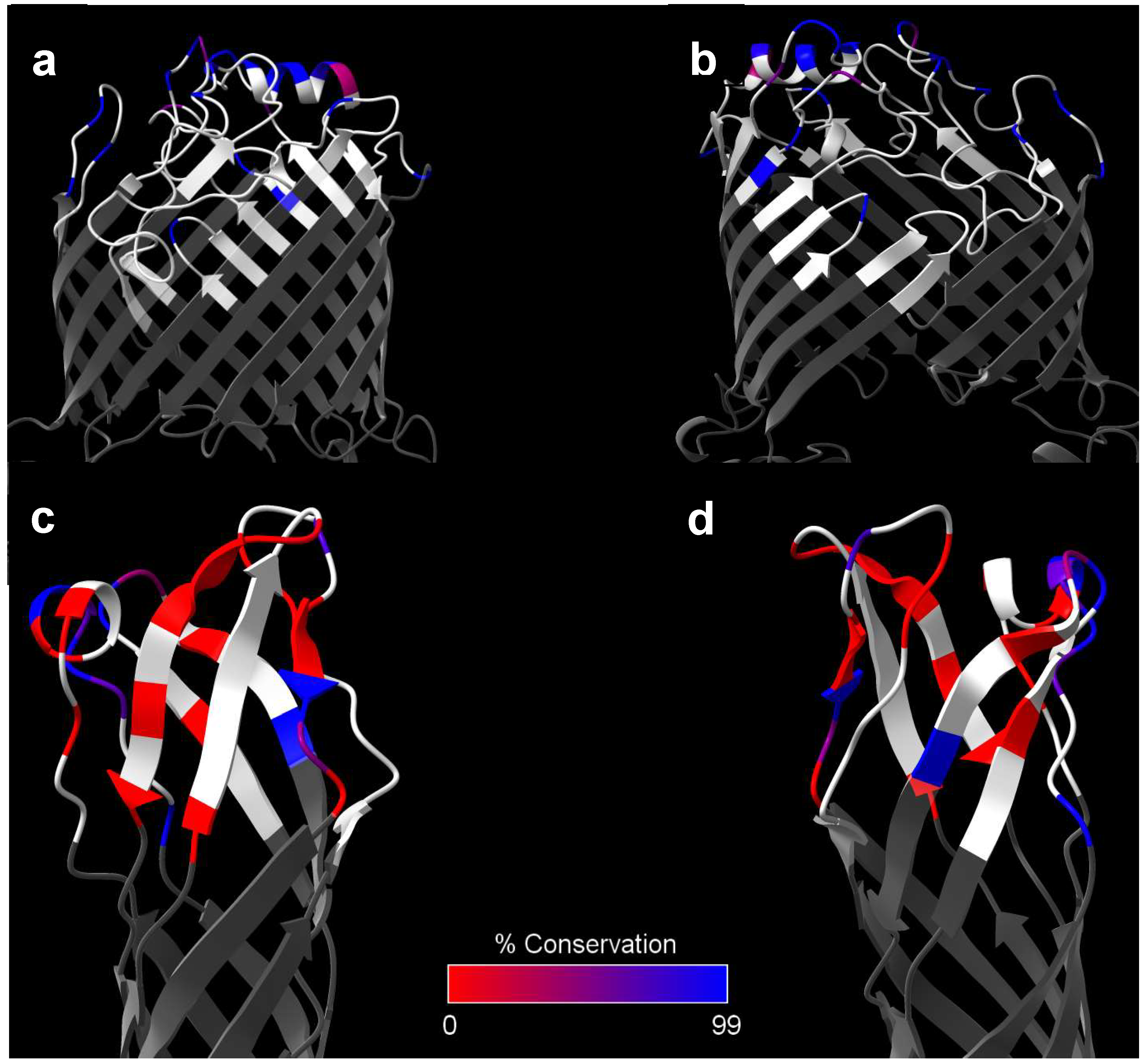
| B. pseudomallei K96243 Locus | Protein Name |
Bcc % Identity to K96243 (Full Protein) | Bcc % Identity to K96243 (Extracellular Loops) |
|---|---|---|---|
| BPSL2151 | BamA | 94.0 | 98.2 |
| BPSL2704 | OmpW2 | 87.8 | 88.9 |
| BPSL1972 | Bucl8 | 78.0 | 88.0 |
| BPSS0708 | OpcP1 | 75.7 | 69.8 |
| BPSL1552 | OmpW1 | 78.3 | 67.7 |
| BPSS0879 | OpcP | 75.4 | 59.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





