Submitted:
17 January 2024
Posted:
17 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
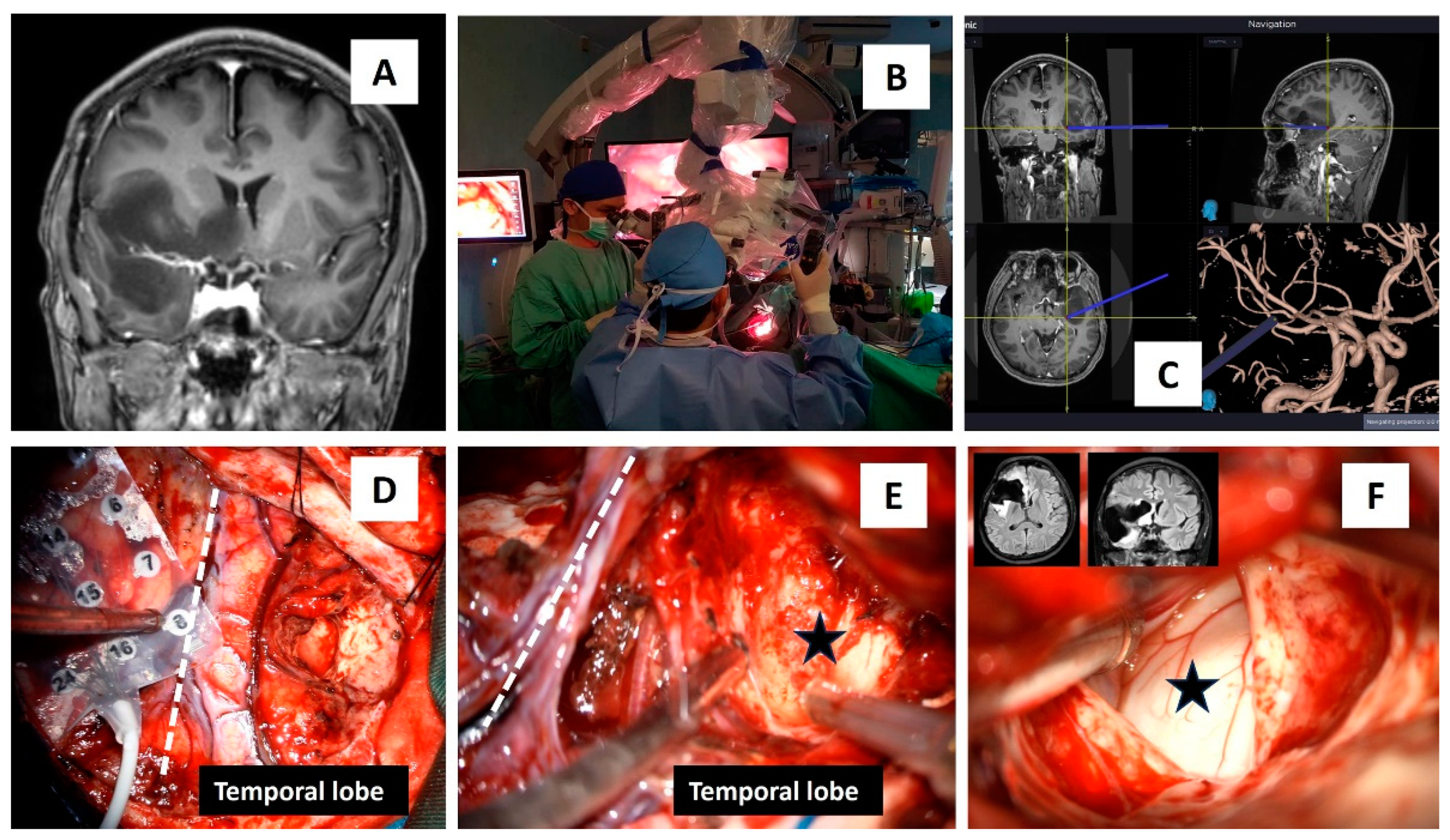
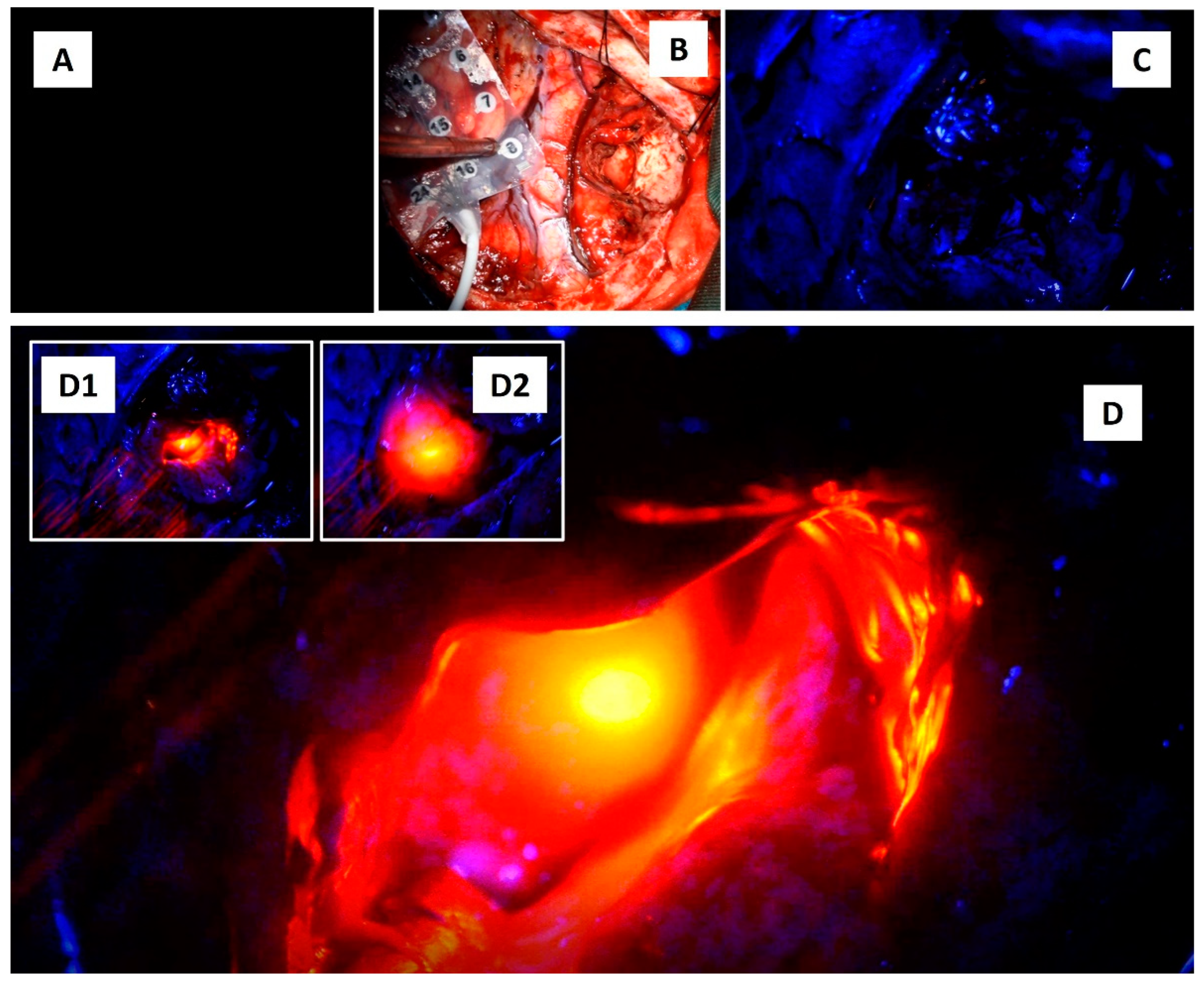
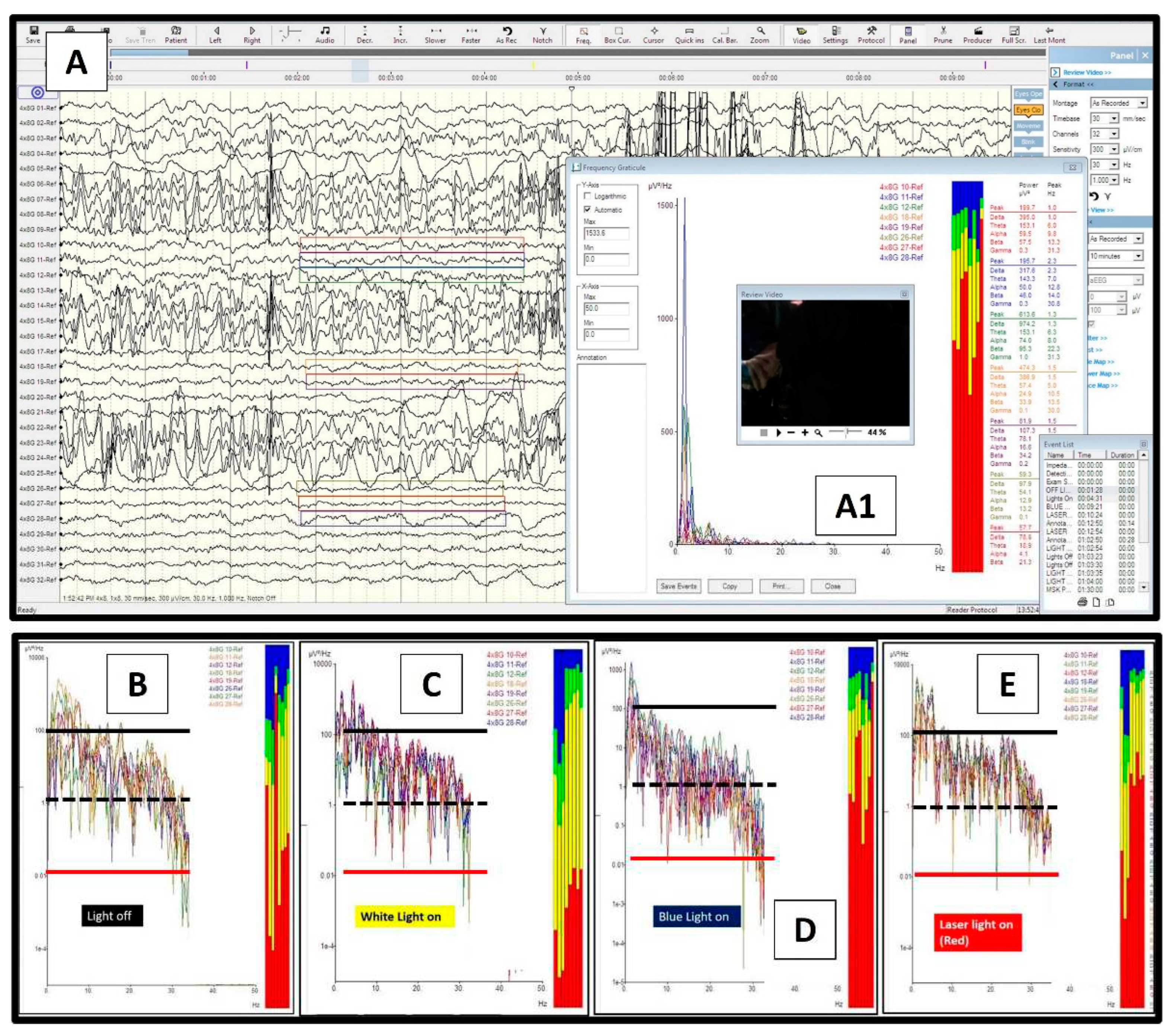
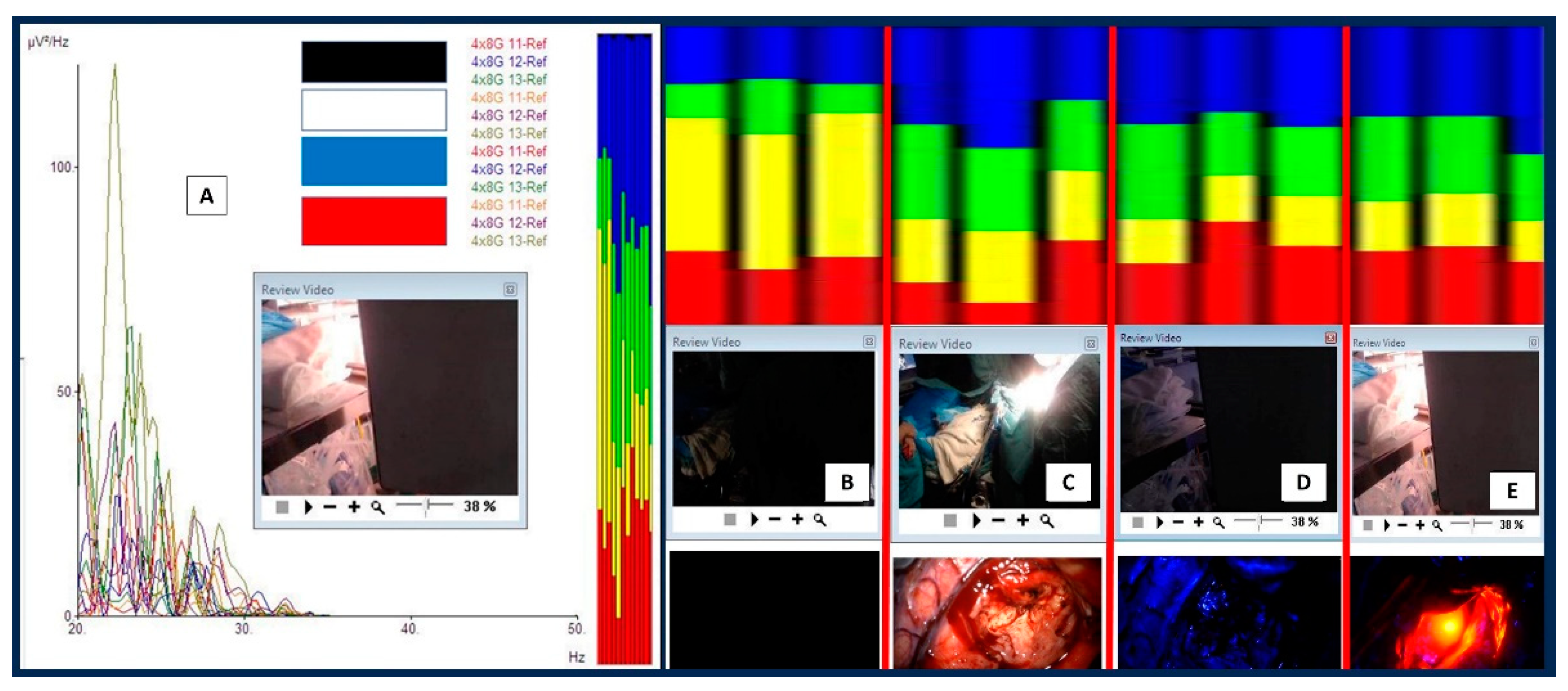
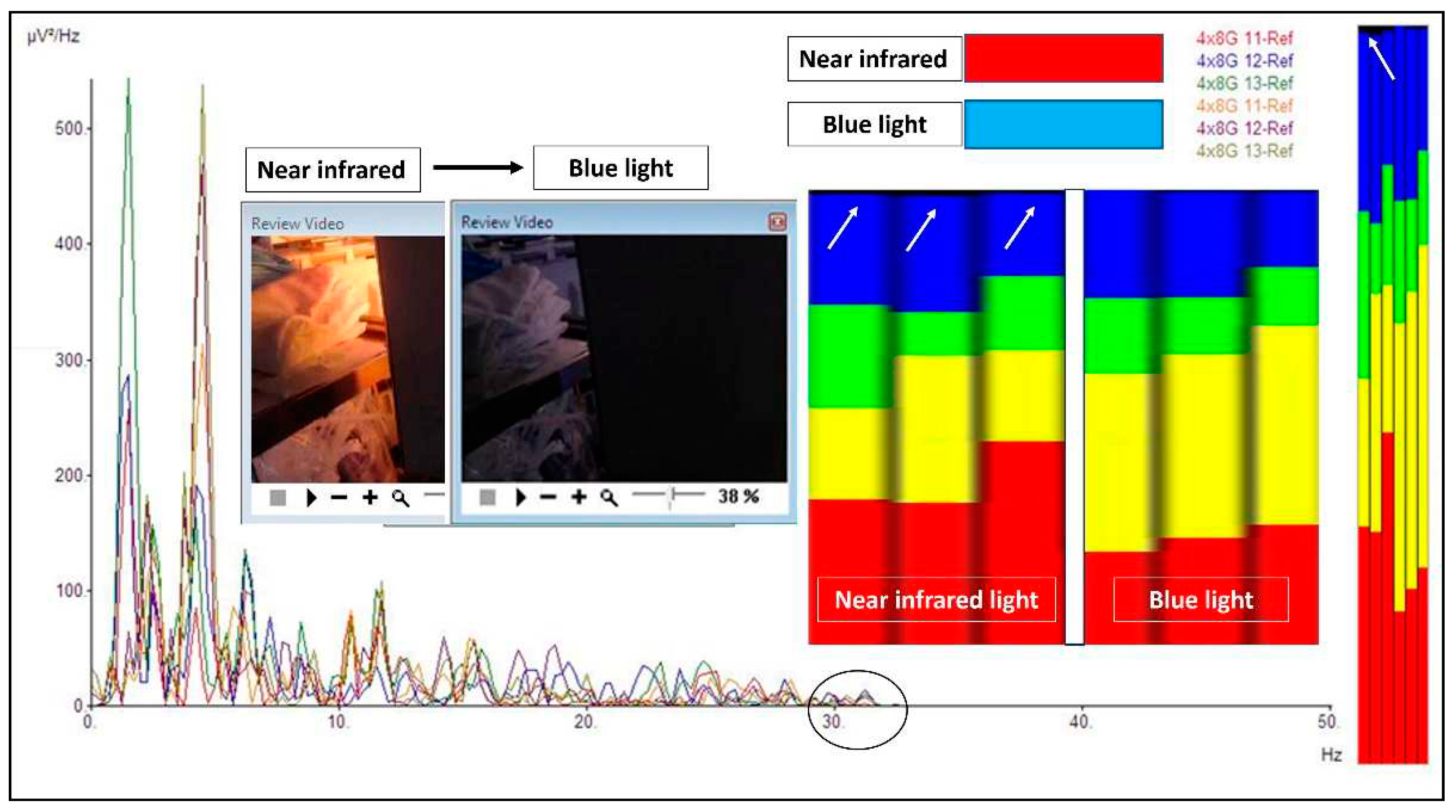
2. Case report: findings during awake brain surgery on marked and rapid brainwave changes when the brain was directly exposed to different wavelengths of light
3. Discussion
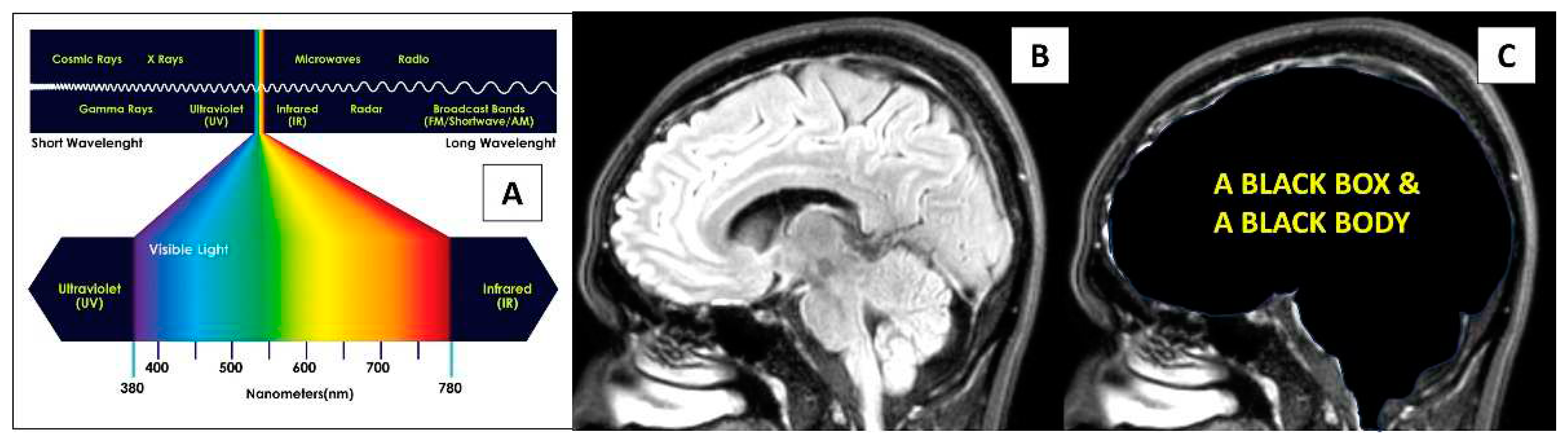
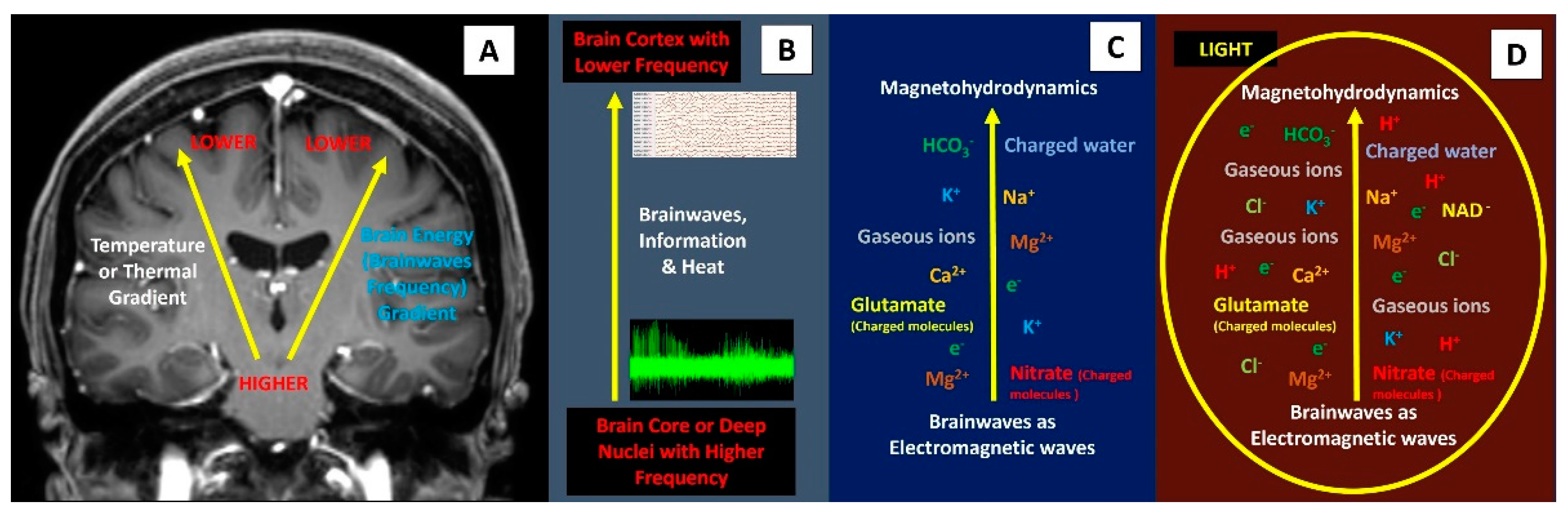
Physics perspective on direct light energy onto the brain
Photobiomodulation (PBM) and medical application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wyse, C. A. , Selman, C., Page, M. M., Coogan, A. N., & Hazlerigg, D. G. (2011). Circadian desynchrony and metabolic dysfunction; did light pollution make us fat?. Medical hypotheses, 77(6), 1139–1144. [CrossRef]
- Yu, B. , & Wang, C. Y. (2022). Osteoporosis and periodontal diseases - An update on their association and mechanistic links. Periodontology 2000, 89(1), 99–113. [CrossRef]
- Schulz, P., & Steimer, T. (2009). Neurobiology of circadian systems. CNS drugs, 23 Suppl 2, 3–13. [CrossRef]
- Walker, W. H. 2nd., Walton, J. C., DeVries, A. C., & Nelson, R. J. (2020). Circadian rhythm disruption and mental health. Translational psychiatry, 10(1), 28. [CrossRef]
- Wirz-Justice, A., Skene, D. J., & Münch, M. (2021). The relevance of daylight for humans. Biochemical pharmacology, 191, 114304. [CrossRef] [PubMed]
- Ballestero, M. F. M. , Furlanetti, L. L., Augusto, L. P., Chaves, P. H. C., Santos, M. V., & de Oliveira, R. S. (2019). Decompressive craniectomy for severe traumatic brain injury in children: analysis of long-term neuropsychological impairment and review of the literature. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery, 35(9), 1507–1515. [CrossRef]
- Yang, X. F. , Wen, L., Shen, F., Li, G., Lou, R., Liu, W. G., et al. (2008). Surgical complications secondary to decompressive craniectomy in patients with a head injury: a series of 108 consecutive cases. Acta neurochirurgica, 150(12), 1241–1248. [CrossRef]
- Woo, P. Y. M. , Mak, C. H. K., Mak, H. K. F., & Tsang, A. C. O. (2020). Neurocognitive recovery and global cerebral perfusion improvement after cranioplasty in chronic sinking skin flap syndrome of 18 years: Case report using arterial spin labelling magnetic resonance perfusion imaging. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia, 77, 213–217. [CrossRef]
- Idris, Z. , Mustapha, M., & Abdullah, J. M. (2014). Microgravity environment and compensatory: Decompensatory phases for intracranial hypertension form new perspectives to explain mechanism underlying communicating hydrocephalus and its related disorders. Asian journal of neurosurgery, 9(1), 7–13. [CrossRef]
- Tekatas, A. , & Mungen, B. (2013). Migraine headache triggered specifically by sunlight: report of 16 cases. European neurology, 70(5-6), 263–266. [CrossRef]
- Lema, A.K. , & Anbesu, E.W. (2022). Computer vision syndrome and its determinants: A systematic review and meta-analysis. SAGE Open Med, 10:20503121221142402. [CrossRef] [PubMed] [PubMed Central]
- Naeser, M. A. , Zafonte, R., Krengel, M. H., Martin, P. I., Frazier, J., Hamblin, M. R., et al. (2014). Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: open-protocol study. Journal of neurotrauma, 31(11), 1008–1017. [CrossRef]
- Salehpour, F. , Mahmoudi, J., Kamari, F., Sadigh-Eteghad, S., Rasta, S. H., & Hamblin, M. R. (2018). Brain Photobiomodulation Therapy: a Narrative Review. Mol Neurobiol, 55(8):6601-6636. [CrossRef] [PubMed] [PubMed Central]
- Salehpour, F. , Gholipour-Khalili, S., Farajdokht, F., Kamari, F., Walski, T., Hamblin, M.R., et al. (2020). Therapeutic potential of intranasal photobiomodulation therapy for neurological and neuropsychiatric disorders: a narrative review. Rev Neurosci, 31(3):269-286. [CrossRef] [PubMed] [PubMed Central]
- Abijo, A., Lee, C. Y., Huang, C. Y., Ho, P. C., & Tsai, K. J. (2023). The Beneficial Role of Photobiomodulation in Neurodegenerative Diseases. Biomedicines, 11(7), 1828. [CrossRef]
- Semyachkina-Glushkovskaya, O. , Abdurashitov, A., Klimova, M., Dubrovsky, A., Shirokov, A., Fomin, A., et al. (2020). Photostimulation of cerebral and peripheral lymphatic functions. Translational Biophotonics, 2(1-2), p.e201900036. [CrossRef]
- Rab, A. S. , Polino, E., Man, Z. X., Ba An, N., Xia, Y. J., Spagnolo, N., et al. (2017). Entanglement of photons in their dual wave-particle nature. Nature communications, 8(1), 915. [CrossRef]
- Collell, G. , & Fauquet, J. (2015). Brain activity and cognition: a connection from thermodynamics and information theory. Frontiers in psychology, 6, 818. [CrossRef]
- Hayward, J. N., & Baker, M. A. (1969). A comparative study of the role of the cerebral arterial blood in the regulation of brain temperature in five mammals. Brain research, 16(2), 417–440. [CrossRef]
- Wang, H. , Wang, B., Normoyle, K. P., Jackson, K., Spitler, K., Sharrock, M. F., et al. (2014). Brain temperature, and its fundamental properties: a review for clinical neuroscientists. Front Neurosci, 8:307. [CrossRef] [PubMed] [PubMed Central]
- Idris, Z. , Yee, A. S., Wan Hassan, W. M. N., Hassan, M. H., Ab Mukmin, L., Mohamed Zain, K.A., et al. (2023). Clinical outcomes and thermodynamics aspect of direct brain cooling in severe head injury. Surg Neurol Int,14:158. [CrossRef] [PubMed] [PubMed Central]
- Józef, R. Z. (2005). Physical Plasma Switchability in the Brain, Electromagnetic Biology and Medicine, 24:3, 273-281. [CrossRef]
- Nakada, T. (2009). Neuroscience of water molecules: a salute to Professor Linus Carl Pauling. Cytotechnology 59, 145–152.
- Nicholson, C. , & Hrabětová, S. (2017). Brain Extracellular Space: The Final Frontier of Neuroscience. Biophysical journal, 113(10), 2133–2142. [CrossRef]
- Lei, Y. , Han, H., Yuan, F., Javeed, A., & Zhao, Y. (2017). The brain interstitial system: Anatomy, modeling, in vivo measurement, and applications. Progress in neurobiology, 157, 230–246. [CrossRef]
- Purushotham, S. S. , & Buskila, Y. (2023). Astrocytic modulation of neuronal signalling. Frontiers in network physiology, 3, 1205544. [CrossRef] [PubMed]
- James, E. , Jake, B., Frederik, L., Vindy, T., Ian, H., Emily, H., et al. (2023). Illuminating the brain: Revealing brain biochemistry with synchrotron X-ray spectromicroscopy. Journal of Electron Spectroscopy and Related Phenomena, 266, 147355. [CrossRef]
- Bruggeman, P.J. , Kushner, M. J., Locke, B. R., Gardeniers J. G. E., Graham, W. G., Graves, D. B., et al. (2016). Plasma–liquid interactions: a review and roadmap. Plasma Sources Sci. Technol, 25, 053002. DOI 10.1088/0963-0252/25/5/053002.
- Peng, Y. , Alsagri, A. S., Afrand, M., & Moradi, R. (2019). A numerical simulation for magnetohydrodynamic nanofluid flow and heat transfer in rotating horizontal annulus with thermal radiation. RSC advances, 9(39), 22185–22197. [CrossRef] [PubMed]
- Maccaferri, N. , Zubritskaya, I., Razdolski, I., Chioar, I-A., Belotelov, V., Kapaklis, V., et al. (2020). Nanoscale magnetophotonics. J Appl Phys, 127(8):1– 30. [CrossRef]
- Suzuki, K. , Yamada, K., Nakada, K., Suzuki, Y., Watanabe, M., Kwee, I. L., et al. (2017). MRI characteristics of the glia limitans externa: A 7T study. Magnetic resonance imaging, 44, 140–145. [CrossRef]
- Zhang, Y. , Guo, S., Sun, M., Mariniello, L., Tozzi, A., & Zhao, X. (2022). Front Waves of Chemical Reactions and Travelling Waves of Neural Activity. Journal of NeuroPhilosophy, 1(2). [CrossRef]
- Andersen, S. S. , Jackson, A. D., & Heimburg, T. (2009). Towards a thermodynamic theory of nerve pulse propagation. Progress in neurobiology, 88(2), 104–113. [CrossRef] [PubMed]
- Drukarch, B. , Wilhelmus, M. M. M., & Shrivastava, S. (2021). The thermodynamic theory of action potential propagation: a sound basis for unification of the physics of nerve impulses. Reviews in the neurosciences, 33(3), 285–302. [CrossRef]
- Idris, Z. , Zakaria, Z., Yee, A. S., Fitzrol, D. N., Ghani, A. R. I., Abdullah J. M., et al. (2021). Quantum and Electromagnetic Fields in Our Universe and Brain: A New Perspective to Comprehend Brain Function. Brain Sci,11(5):558. [CrossRef] [PubMed] [PubMed Central]
- Idris, Z. (2020). Quantum Physics Perspective on Electromagnetic and Quantum Fields Inside the Brain. The Malaysian journal of medical sciences:MJMS, 27(1), 1–5. [CrossRef]
- Idris, Z. , Yee, A. S., Wan Hassan, W. M. N., Hassan, M. H., Mohd Zain, K. A., & Abdul Manaf, A. (2022). A Clinical Test for a Newly Developed Direct Brain Cooling System for the Injured Brain and Pattern of Cortical Brainwaves in Cooling, Noncooling, and Dead Brain. Therapeutic hypothermia and temperature management, 12(2), 103–114. [CrossRef]
- Schiffer, F. , Johnston, A. L., Ravichandran, C., Polcari, A., Teicher, M. H., Webb, R. H., et al. (2009). Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: a pilot study of 10 patients with major depression and anxiety. Behavioral and brain functions : BBF, 5, 46. [CrossRef]
- Hamblin, M. R. (2016). Shining light on the head: Photobiomodulation for brain disorders. BBA clinical, 6, 113–124. [CrossRef]
- Peoples, C. , Spana, S., Ashkan, K., Benabid, A. L., Stone, J., Baker, G. E., et al. (2012). Photobiomodulation enhances nigral dopaminergic cell survival in a chronic MPTP mouse model of Parkinson's disease. Parkinsonism & related disorders, 18(5), 469–476. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




