1. Introduction
Selenium (Se) was a metalloid element discovered by a Swedish chemist Jacob Berzelius in 1818. Its name comes from the Greek meaning of "Selene" for the goddess of the moon [
1]. Se was considered an essential element for plants and mammals. This non-metallic element plays an important role in improving human function and preventing cell damage by producing selenoprotein [
2]. Se compounds also participate in the neutralization of heavy metals by directly or indirectly binding to ions, preventing them from causing damage to healthy cells [
3]. The inorganic states of Se commonly present in nature include selenides (Se
2-), selenite (SeO
32-), and selenate (SeO
42-), and its two main organic forms are selenocysteine (SeCys) and selenomethylamine (SeMet), which form various Se proteins. In a reducing environment, the oxygen anion of Se is converted into a typical red elemental state, which significantly reduces its toxicity. The elemental form can be further reduced to organic selenides [
1,
4]. Although Se is essential to the human body, on the other hand, excessive intake can increase its accumulation in tissues and exhibit toxicity. Because its inorganic ions in tissues can cause oxidative stress. Organic components such as SeCys or SeMet may be mismatched during translation, leading to disordered protein synthesis [
5].
The margin between Se deficiency and its toxicity is very thin. The ideal intake of Se recommended by the WHO in 2009 was 50-55 μg per day in a human diet based on individual weight [
6]. Some reports also indicated that the minimum intake of dietary Se was 55 µg per day and the maximum limit was 400 µg. Excessive intake of Se by adults can lead to severe Se poisoning [
1]. Se oxide anions are the most active form of Se in nature, which are easily ingested by animals and humans from contaminated water streams, and even indirectly ingested by plants irrigated with Se-rich water, leading to many health hazards [
7]. On the contrary, SeNPs exhibit high biological activity and adsorption potential due to their interactions with protein chemical groups. Therefore, SeNPs were widely used in medical diagnosis, dietary supplements, cancer treatment, and environmental biotechnology [
8,
9]. SeNPs can be generated through chemical, physical, or biological methods, and their physicochemical properties depend on the conversion method [
10]. The use of physical and chemical methods to produce nanoparticles releases toxic and dangerous chemicals, which have low biocompatibility and limit their application [
11]. From previous research results, it can be seen that synthesizing SeNPs using biological methods is safe, inexpensive, and simple.
B. subtilis is a probiotic that does not pose any environmental safety risks during the Se (IV) reduction process. According to reports, this bacterium can convert Se into SeNPs by inducing detoxification systems rather than alienating electron transfer. [
11,
12].
Se, as an essential trace element in the human body, is closely related to human health and diseases. Organic Se is much higher in terms of bioavailability and safety than inorganic Se, and SeNPs has advantages in toxicity and reactivity compared to other forms of organic Se. This study used high concentration Na2SeO3 stress and pathogenic microbial confrontation culture to screen and isolate Se rich probiotics from fermented mature forage samples from a Se rich dairy cow breeding base in Hong'an County, Hubei Province; One Se rich B. subtilis strain was screened by combining morphology, physiology, biochemistry, and 16S rDNA sequence analysis. Optimization of the growth conditions for the production of SeNPs by modified Bacillus through biosynthesis methods, and systematic characterization and analysis of SeNPs using XPS, SEM-EDS, and TEM. Furthermore, B. subtilis containing SeNPs was added as probiotics to sheep feed to preliminarily explore the effects of SeNPs on physiological and biochemical indicators and Se content in various tissues of sheep.
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
Se rich probiotics were isolated from forage samples from the Se rich dairy cow breeding base in Hong'an County (latitude 31°13′ N, longitude 114°48′ E), Hubei Province, China, using dual screening of high concentration Na2SeO3 stress and pathogenic microbial confrontation culture. And combined with morphology, physiology, biochemistry, and 16S rDNA sequence to identify Se rich probiotic strain L11. LB medium: Tryptone 10 g/L, Yeast extract 5 g/L, Sodium chloride 10g/L, adjust the pH of the medium to 7.2. B. subtilis universal culture medium: 20 g/L glucose, 15 g/L peptone, 5 g/L NaCl, 0.5 g/L beef extract. Na2SeO3 was purchased from Sigma (Darmstadt, Germany) in the experiment.
2.2. Identification of Se Tolerant Strains
To characterize the colony morphology, strain L11 was cultured in LB for 48 hours, and the crystal violet staining of the strain was observed under a 100x optical microscope for microscopic observation of cell morphology. Physiological and biochemical testing shall be carried out in accordance with the Bergey Bacteriological Testing Manual [
13] (
Table S1).
Using 16S rDNA gene for molecular biology identification of L11 strain, the PCR amplification primers were L11F (5 '- AGAGTTTGATCCTGGCTCAG-3')/L11R (5 '- TACGACTTAACCCCAATCGC-3') [
14]. The PCR amplification conditions are as follows: 94 °C for 5 minutes, 94 °C for 1 minute, 56 °C for 20 seconds, 72 °C for 60 seconds, 30 cycles, and finally extended at 72 °C for 10 minutes. The 16S rDNA gene sequence of L11 strain was obtained through PCR amplification and compared with the sequences obtained in the NCBI database [
15].
2.3. Determination of L11 Reducing Na2SeO3 Activity and Culture Conditions
To determine the tolerance of L11 to selenite, strain L11 was inoculated into fresh LB medium containing 0 mM, 2 mM, 5 mM, 10 mM, and 15 mM Na
2SeO
3 (1% v/v). Monitor the light absorption value of the culture at 600 nm using a microbial growth analyzer (Bioscreen CMBR., Turku, Finland). Incubate the plate at 37 °C for 72 hours while continuously shaking (200 rpm), and measure the absorbance every 1 hour [
16].
Optimize and screen the reduction conditions of L11 strain through response surface analysis. Three single factors, temperature, pH, and rotational speed, were set as independent variables, and the reduction rate of Na2SeO3 was used as the response value. The final processing software of the experiment was Design Expert version 12 (Stat-Ease, Jinshan District, ShangHai, China), and Box Behnken single factor data and quadratic regression equation were used for analysis. The results of ANOVA analysis for data extraction showed significant differences in p<0.01 and significant differences in 0.01<p<0.05. The optimal cultivation conditions are selected by observing the interaction of various factors on the reduction rate of Na2SeO3.
2.4. Preliminary Cell Localization of SeNPs
L11 strain was cultured until the exponential growth stage, and the bacterial solution was centrifuged for 10 minutes (4 °C, 12000 × g) to collect cell culture. The precipitate was washed twice with 0.9% NaCl and wash it again before protein extraction. Finally, the corresponding periplasmic, cytoplasmic, membrane, and cell wall components were extracted using the method described by Lampis et al [
17].
2.5. Preparation of SeNPs
To obtain SeNPs, the L11 strain was cultured overnight in LB medium containing 4.0 mM selenite (180 rpm, 37 °C). After incubation, the L11 culture was centrifuge at 12000 g for 10 min and collect sediment. The precipitate was rinsed twice with 0.9% NaCl, and then resuspended in 20 mL of Tris-Cl buffer (50 mM, pH 8.2). Then, the sample was subjected to ultrasonic treatment and SeNPs was harvested as described by Wang et al [
18].
2.6. Characterization and Analysis of L11 Produced SeNPs
Scanning electron microscopy (SEM) analysis. Incubate the L11 strain culture in LB medium containing 4 mM Na2SeO3 at 37 °C for 24 hours and wash it three times with 0.9% NaCl. The precipitate was embedded in a precooled fixed solution and fixed overnight at 4 °C. The Hitachi SU 8010 microscope was used for observing strain L11 and the distribution of SeNPs.
Transmission electron microscopy (TEM) analysis. L11 strain grown for 24 hours in LB medium with or without Na2SeO3 was subjected to mild centrifugation (5000×g, 5 minutes) to collect the culture. Mix the precipitate with pre cooled fixed solution (0.1 M phosphate buffer (PBS), 2% glutaraldehyde, pH 7.4) and fix at 4 °C for 10 minutes; Subsequently, L11 strain was centrifuged for 3 minutes (5000 × g). The supernatant was fixed overnight at 4 °C and examined using Hitachi HT-7700 (Tokyo, Japan) TEM at 80.0 KV.
The morphology and chemical elements that constitute SeNPs were identified through SEM and EDS analysis. Before analysis, the SeNPs suspension was dried using a vacuum freeze-drying system (SJIA-10N, Ningbo Yinzhou Sjia Laboratory Equipment Co., Ltd., Yinzhou, China). Then, dry SeNPs were examined using SEM and energy dispersive X-ray spectroscopy (Hitachi S4800, Tokyo, Japan).
The separated SeNPs was analyzed using Zeta potential (ZP) and size distribution [
2]. SeNPs were dispersed in deionized water, sonicated for 10 minutes, and then approximately 0.5 mL of suspension was transferred to a colorimetric dish impregnated with a cell kit for particle size distribution ZP analysis.
2.7. Sheep Feeding Experiment
The experimental sheep were divided into a control group and an experimental group, with 3 parallel replicates in each group, and 10 sheep were selected for each replicate. The specific operation is as follows: Based on the principle of consistency in breed, parity, age, and physical condition, select healthy sheep (without special requirements for sheep breeds) for the experiment. The feeding method is consistent with the routine, with the only difference being that the experimental group's feed is supplemented with SeNPs solid bacterial agents at a concentration of 2 g/25 kg. After 40 days, the serum immune indicators and Se content in various tissues of the control and experimental groups were measured.
2.8. Determination of Physiological and Biochemical Indicators in Sheep Blood
On the 40th day of the experiment, EDTA-K2 anticoagulant tubes were used to collect jugular vein blood from the experimental group and the control group of sheep, and the cell contents were quickly measured using a fully automated blood analyzer. The content of red blood cells (RBC), white blood cells (WBC), and lymphocytes in sheep jugular vein blood was determined according to the method of Alagawan et al [
19]
Part of the blood was centrifuged for 10 minutes (3000g), and the supernatant was frozen to -20 °C. The antioxidant indicators of sheep serum are operated according to the instructions on the IgG, SOD, MDA, T-AOC, GSH-Px detection kit. The above test kits were all purchased from Wuhan Leibelide Technology Co., Ltd (Wuhan, Hubei, China), including blood IgG antibody test kits (batch number 201809), SOD (superoxide dismutase) kit (batch number 201809), MDA (malondialdehyde) kit (batch number 201809), T-AOC (total antioxidant capacity) kit (batch number 201809), GSH-Px (glutathione peroxidase) kit (batch number 201809).
2.9. Statistic Analysis
Excel 2021 v2212 (Microsoft, Raymond, WA, USA) and SPSS v22.0 (IBM, Amonk, NY, USA) were used for the processing and analysis of all data. The data between treatment groups were compared using Duncan’s test, with p< 0.05 indicating statistical significance. Three biological replicates were measured for each group of processed data. The data analysis results were plotted using Origin 2019 software (Microcal Software,Northamptonshire,MA,USA). The phylogenetic tree of L11 strain 16S rDNA was constructed by MEGA version 7.0 with a neighbor-joining (NJ) method and measured by bootstrap analysis with 1000 replicates.
3. Results and Discussion
3.1. Isolation and Identification of Strain L11
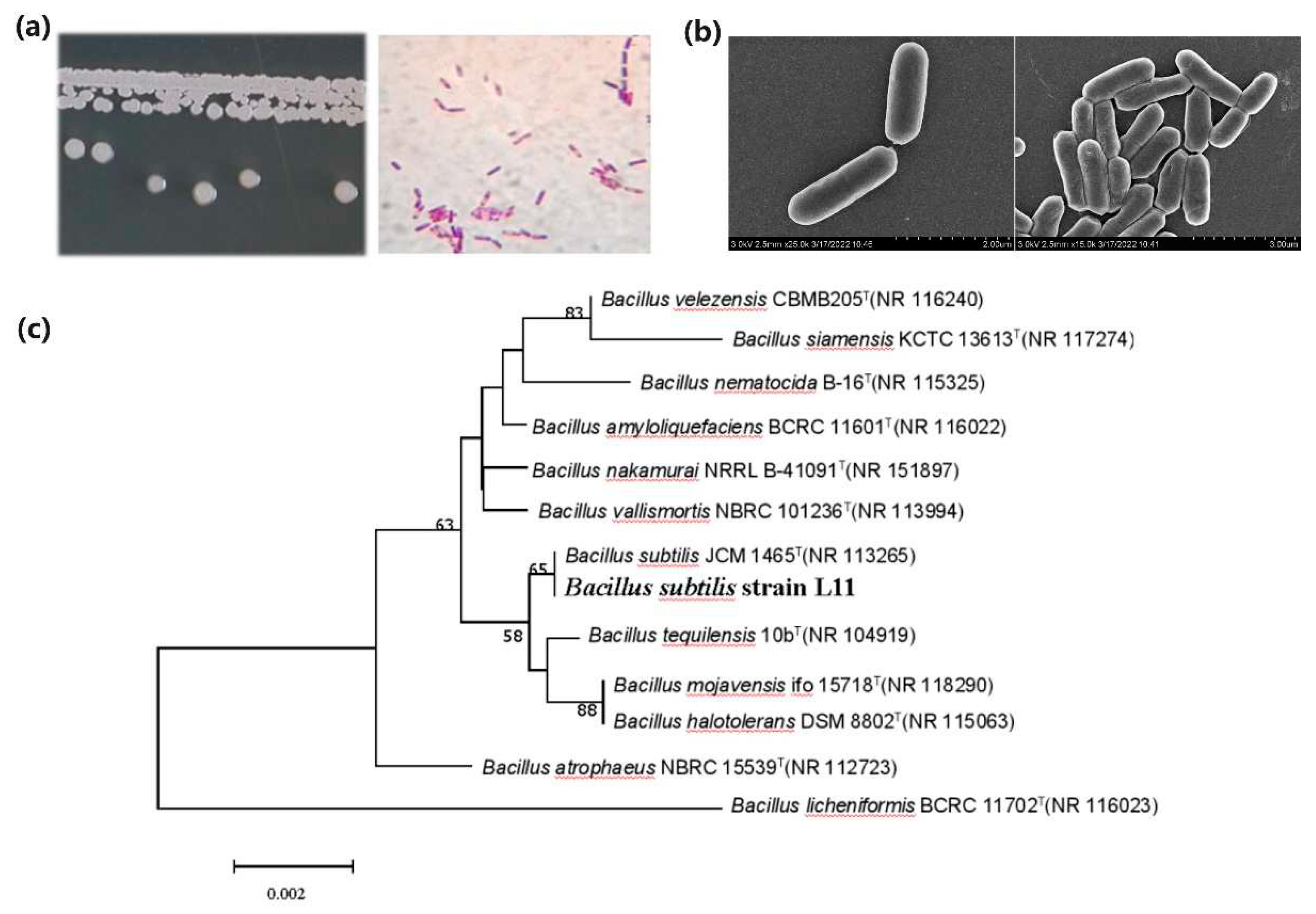
The individual cell of L11 is rod-shaped, and the two ends of the cell are relatively flat (
Figure 1a). The colony of L11 is circular, with irregular edges and a smooth and moist surface. The colony is opaque and slightly grayish white, with a diameter of about 1-2 mm. It is Gram positive and produces spores, with spores growing in the middle (
Figure 1b).
Table S1 shows the Biolog GENIII identification of L11 strain, which was compared with the data in the Biolog strain database. The results showed that after 16 hours of cultivation, the SIM (Similarity) value between L11 and
B. subtilis was 0.921, and the DIS was 4.246. The likelihood of L11 being identified as
B. subtilis was 100%.
After sequencing, the 16S rDNA of L11 strain was submitted to the GenBank database for BLAST analysis. The results showed that the genus Bacillus had the highest homology with L11. The 16S rDNA sequence of L11 was compared with several strains with higher homology in the database, and multiple sequence alignments were performed using ClusterX. A phylogenetic tree was constructed using the Neighbour Joining method in MEGA 7.0 software (
Figure 1c). From the phylogenetic tree, it can be seen that L11 is clustered in a branch with
B. subtilis JCM1465T, and its 16S rDNA sequence shares 98% homology with JCM1465T. This result supports the preliminary identification results mentioned above. Based on the morphological, physiological and biochemical characteristics of L11 and the sequence analysis of 16S rDNA, L11 was identified as
B. subtilis.
3.2. Optimization of Growth Conditions for L11 Strain
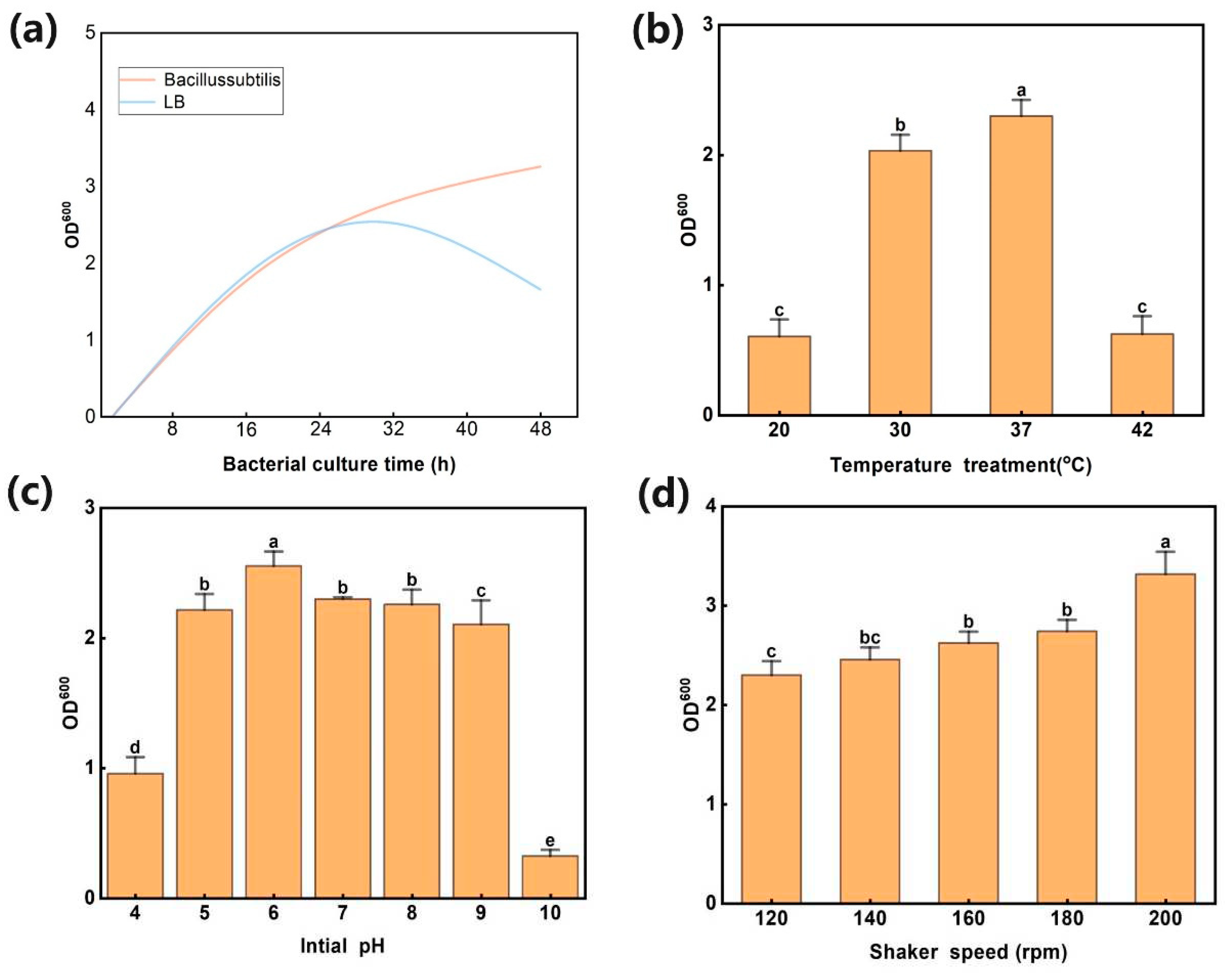
In the universal culture medium of Bacillus, L11 reached its maximum bacterial concentration at 25-26 hours, and after 30 hours, it showed a slow downward trend. In LB medium, the growth rate of L11 showed a rapid growth trend from 0 to 40 hours, and after 40 hours, the growth rate of L11 slowed down. Overall, the growth status of L11 in LB medium is better than that in Bacillus universal medium (
Figure 2a). Under the conditions of 30-37 °C, the growth concentration of L11 strain showed an upward trend. Among them, L11 has the best growth state at 37 °C (
Figure 2b). In 37 °C, 120 rpm, and LB media, the growth concentration of L11 was maintained within a stable range when the pH value was between 5-9, and the growth status of L11 was optimal at pH=6 (
Figure 2c). Under the optimal cultivation conditions mentioned above, the faster the shaking culture speed, the higher the growth concentration of L11 strain. When the shaking speed was 200 rpm, the concentration of L11 solution reaches its maximum (
Figure 2d). Therefore, the optimal growth conditions for L11 strain can be set as LB medium, oscillating culture at 37 °C, pH=6, and 200 rpm.
3.3. Optimization of Synthesis Conditions for SeNPs by L11
In order to determine the Na
2SeO
3 concentration and maximum reduction rate that L11 strain can tolerate, different concentrations of Na
2SeO
3 cultivation conditions were designed under the optimal growth conditions of L11. The results showed that when the final concentration of Na
2SeO
3 was 5-15 mmol/L, the Se content and Se reduction rate in the culture medium rapidly decreased with the increase of concentration. When the concentration was 10 and 15mmol/L, strain L11 hardly produces SeNPs, and the growth of L11 is also inhibited. It can be seen that the optimal cultivation concentration of Na
2SeO
3 is not within the range of 5-15 mmol/L (
Table S2). When the concentration of Na
2SeO
3 was 4 mmol/L and the cultivation time was 48 hours, the Se reduction rate of strain L11 reached 72%. When the cultivation time was 72 hours, the reduction rate reached 73.5%. It indicates that only 1.5% of Na
2SeO
3 was reduced within 48-72 hours, so the optimal cultivation time was selected as 48 hours (
Table S3). When the Se concentration in the culture medium is high, the detoxification process of microorganisms is activated, producing reduced Se0. By changing the color, it is possible to preliminarily determine the reduced Se produced by microorganisms. When amorphous Se0 is formed, the cell color is red; When crystalline Se
0 forms, the cell color turns gray, indicating that toxic and colorless selenite has been converted into non-toxic SeNPs [
20].
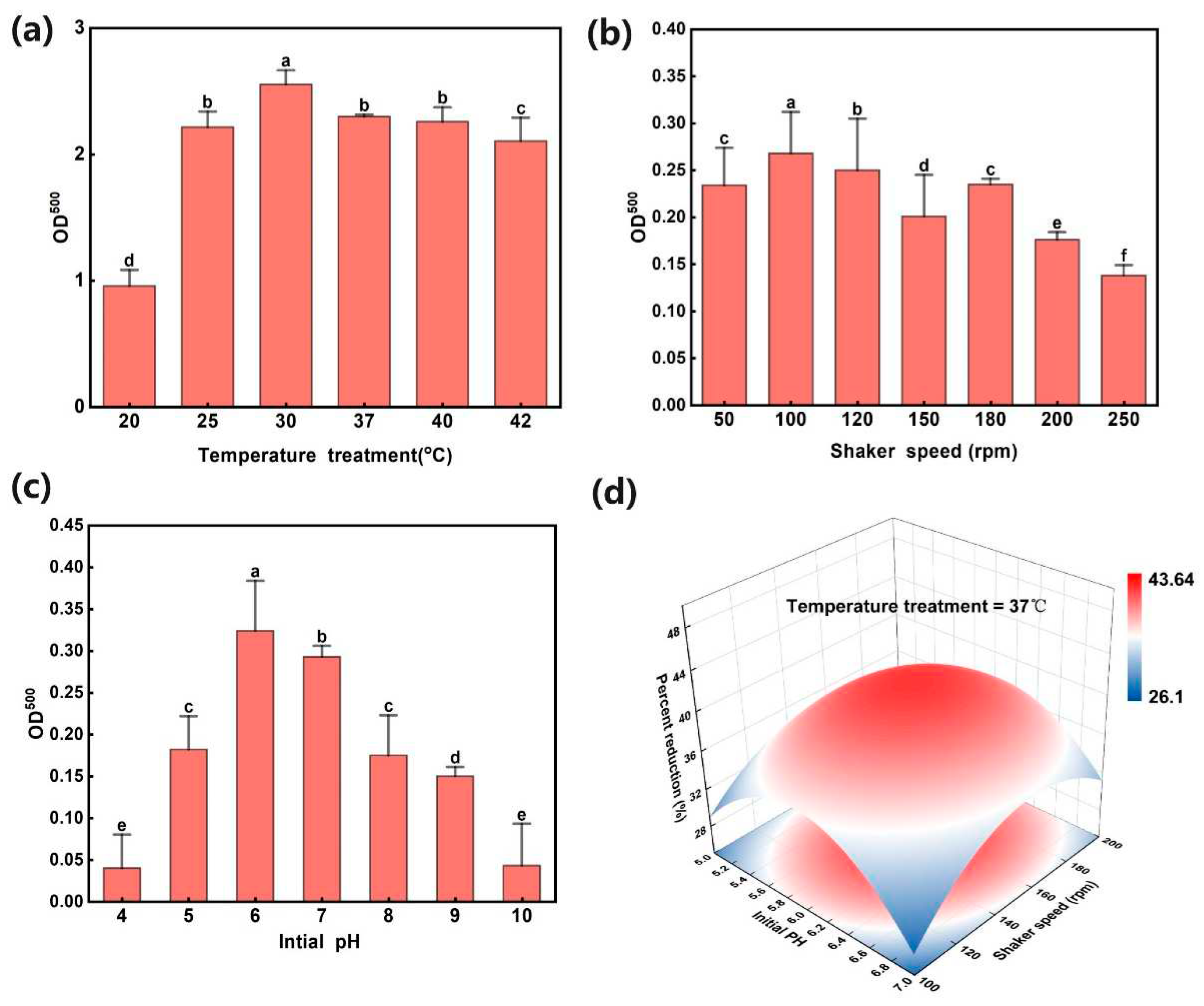
The cultivation at different temperatures affects the efficiency of L11 in converting SeNPs (
Figure 3a). When the temperature is within the range of 20-37 °C, the amount of SeNPs transformed by strain L11 increases with increasing temperature. When the temperature is within the range of 37-42 °C, the conversion rate of SeNPs begins to decrease with increasing temperature. Therefore, the most suitable temperature for strain L11 to convert SeNPs is 30-37 °C, which is consistent with the optimal temperature for L11 growth. The concentration of strain L11 at 42 °C was higher than that at 20 °C, indicating that the addition of Na
2SeO
3 to the culture medium improved the strain's high-temperature resistance to a certain extent, but the production of SeNPs decreased. When the cultivation temperature is significantly higher than the optimal growth temperature, bacterial growth is inhibited. When the cultivation temperature is lower than the optimal growth temperature of bacteria, their metabolic activity is inhibited [
21].
As the oscillation speed of the culture medium increases, the conversion rate of SeNPs shows a trend of first increasing and then decreasing; When the rotational speed is 100 rpm, the conversion rate of SeNPs reaches its maximum value, and then the conversion rate begins to decrease as the rotational speed increases (
Figure 3b). Therefore, a culture medium speed of 100 rpm is more conducive to the reduction of selenite by L11 strain.
The results in
Figure 3c indicate that as the initial pH value of the culture medium increases, the conversion rate of strain L11 reducing selenite shows a trend of first increasing and then decreasing. When the initial pH of the culture medium is 6.0, the conversion rate reaches its maximum value, which may be related to the optimal growth pH of L11 being 6.0.
In order to further analyze the comprehensive effects of three factors on the biosynthesis of SeNPs in L11, a three factor and three level response surface analysis was set up in the experiment, and 17 sets of experiments were designed. The experimental results showed that when the pH of the culture medium was 6, the temperature was set at 37 °C, and the rotational speed was 150 rpm, strain L11 had the highest reduction rate of Na2SeO3.
3.4. L11 Subcellular Localization Analysis of SeNPs
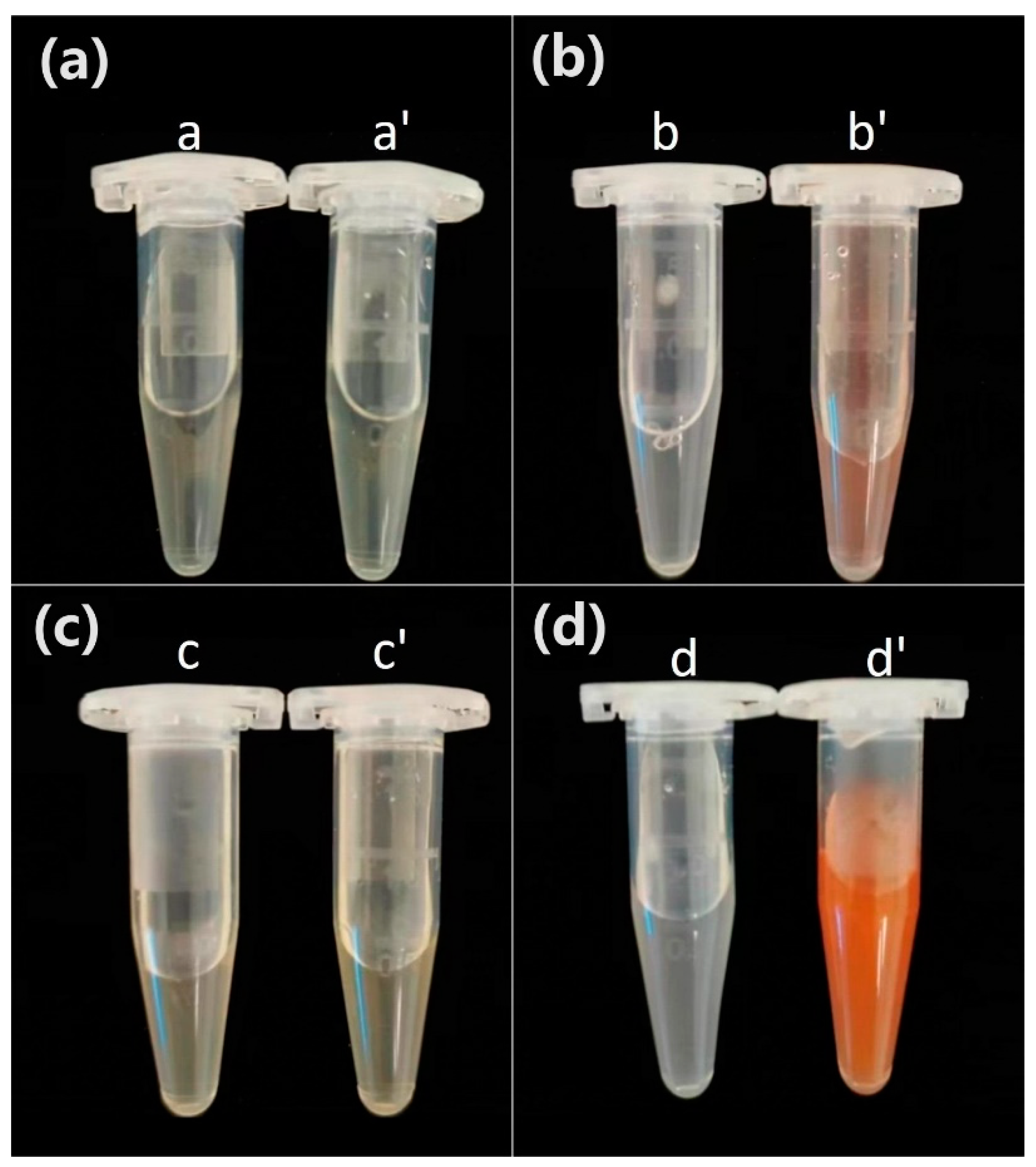
To analyze the localization of SeNPs produced by L11 strain in cells, proteins from different parts of L11 cells were isolated and their content in different protein components was displayed. As shown in
Figure 4, with the addition of Na
2SeO
3 in the culture medium, the periplasmic space proteins of L11 cells showed a light red color, while the crude extract of cell membrane and cell wall proteins showed a red color, while the control group showed colorless secretion proteins, cell membrane and cell wall proteins. The results indicate that the reduction of Na
2SeO
3 by
B. subtilis L11 to produce red SeNPs mainly occurs in the periplasmic space, cell membrane, and cell wall. Microorganisms will utilize high valence Se (Se
4+or Se
6+) in the environment as electron acceptors to convert it into SeNPs. Some microorganisms convert high valence Se into elemental Se particles outside the cell, while others convert sodium selenate or Na
2SeO
3 into elemental SeNPs within the cell, and then use special mechanisms to expel intracellular SeNPs out of the cell [
22].
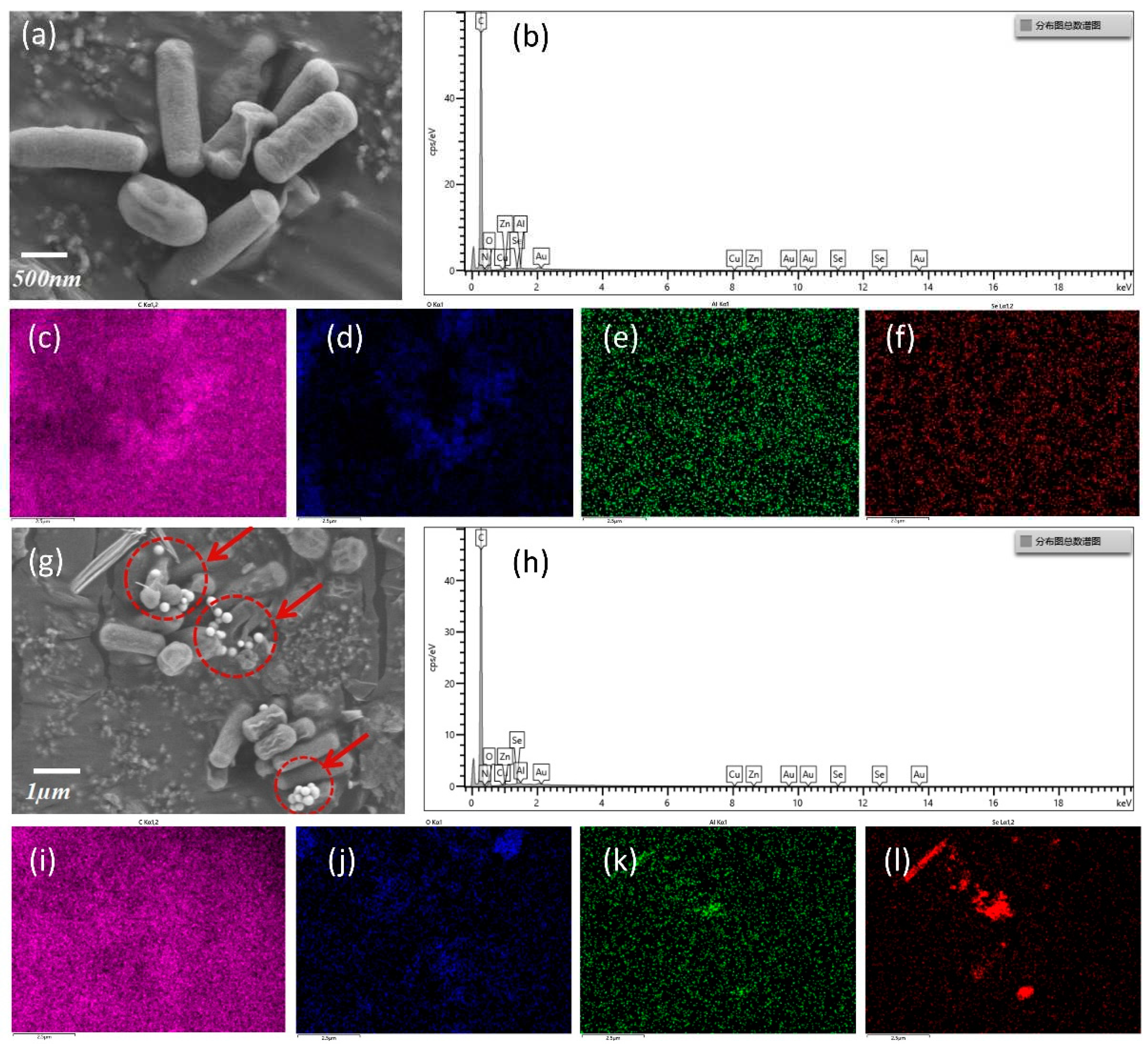
3.5. Analysis of SEM and EDS Spectra of L11 Strain Producing SeNPs
Inoculate strain L11 into LB medium without or with Na
2SeO
3 added for cultivation. After 48 hours, centrifuge and collect the precipitates to obtain the culture. After immobilization, the culture was observed by SEM and EDS analysis. The biosynthesis results of SeNPs are shown in
Figure 5.
Figure 5a–f show the SEM observation results and EDS test results of the culture without adding Na
2SeO
3, while
Figure 5g–l show the corresponding experimental group test results. Comparing the scanning electron microscopy images of cultures in
Figure 5a and
Figure 5g, it can be seen that there is a large amount of spherical particulate matter clustered around the L11 cells of the experimental group, while there is no particulate matter around the cells of the control group.
EDS analysis of the elemental composition of the samples in
Figure 5a,g showed that the experimental group culture contained Se, while the control group did not; The mass fraction and atomic number fraction of some elements in this area are shown in
Table 1. In the control group, the mass fraction and atomic number fraction of Se element are both 0, while in the experimental group, the mass fraction and atomic number fraction of Se element are 0.47% and 0.07%, respectively. The elemental distribution analysis results (
Figure 5c-f and
Figure 5i-l) further confirmed that Se only existed in the experimental group, and the scanning electron microscopy (
Figure 5g) was consistent with the EDS (
Figure 5l) results of Se distribution. The above results confirm that the particles gathered around cells contain Se, and the particle size ranges from 50 to 200 nm. Some studies have shown that the size of SeNPs plays a major role in their biological activity. Usually, smaller particles are more effective than larger particles [
23,
24]. Smeller SeNPs increase their biological activity by enhancing the action of thioredoxin reductase and selenidase peroxidase [
25]. In addition, the toxicity of smaller SeNPs is much lower than that of larger ones [
26].
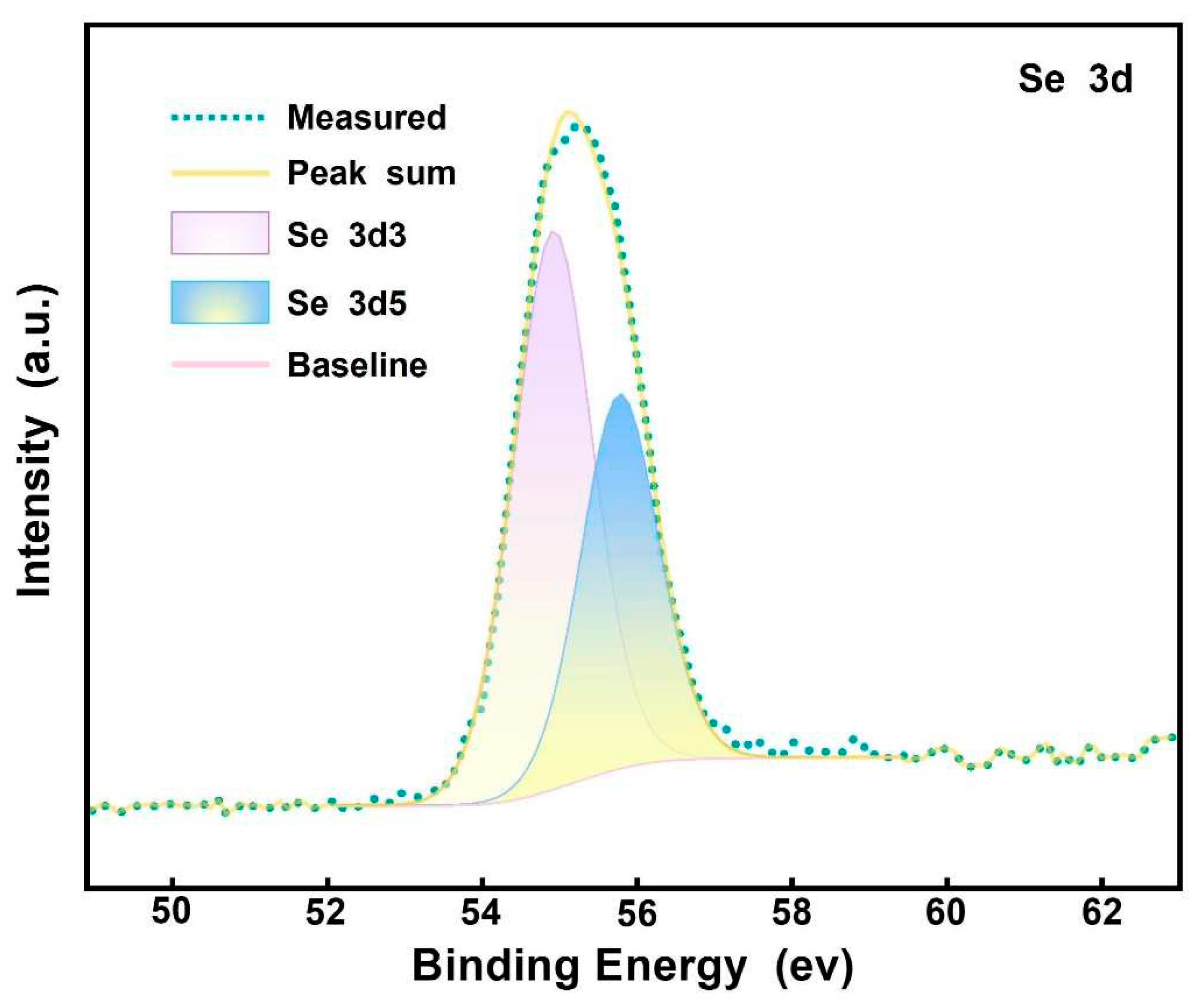
3.6. XPS Analysis of SeNPs as a Reduction Product of L11
The L11 culture containing Na
2SeO
3 was centrifuged, PBS rinsed, and centrifuged to precipitate. After freeze-drying, the reduced products were analyzed by XPS scanning. In order to accurately verify the valence state of Se obtained by L11 reduction of Na
2SeO
3, XPS Peak software was used to fit the Se 3d peak. The results showed that the peak of Se 3d5 was at 55.49 eV, while the peak of Se 3d3 was at 56.24 eV (
Figure 6). According to the report by Han et al. [
27], the Se 3d peak binding energy of Se (IV) was greater than 58.0 eV, with elemental Se ranging from 54.6 to 57.5 eV, and Se compounds ranging from 52.8 to 55.7 eV; In present study, the binding energies of Se 3d5 and Se 3d3 were between 54.6 and 57.5 eV reported by Han [
27], indicating that the particulate matter in this study was elemental Se. Based on the above EDS analysis results, the particulate matter gathered around L11 cells is biological SeNPs, which is reduced by
B. subtilis L11 to elemental Se.
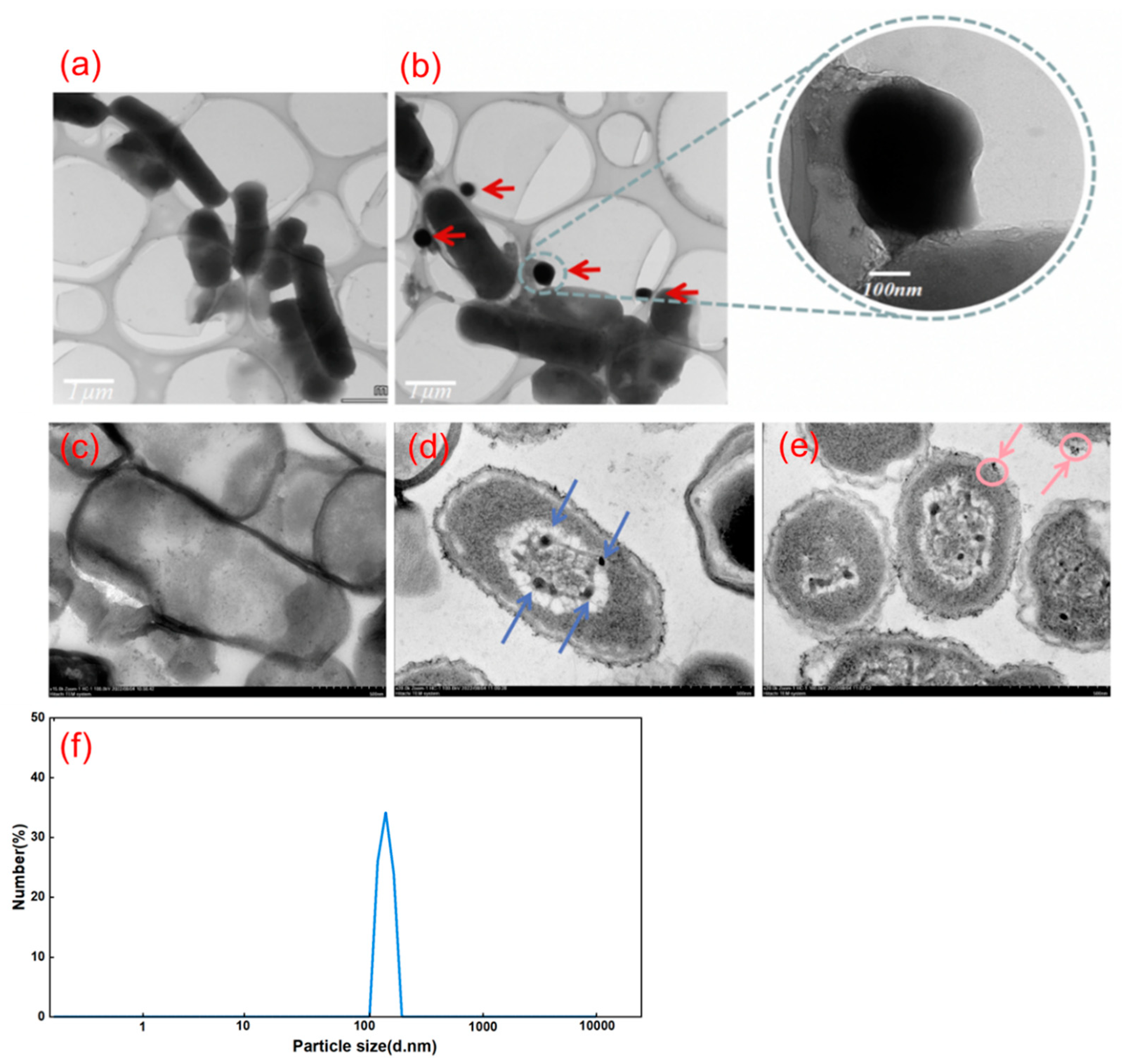
3.7. TEM Characterization and Particle Size Analysis of SeNPs Produced by L11
The TEM observation results indicate that L11 produces spherical nanoparticles of Se (
Figure 7), with particle sizes mainly distributed between 100-200 nm. In the ultra-thin sections of
B. subtilis L11 cultured without adding Na
2SeO
3, no significant SeNPs particles were observed both intracellular and extracellular (
Figure 7a, c). In the ultra-thin section of L11 culture with Na
2SeO
3 added, both intracellular and extracellular SeNPs are present (
Figure 7b, d), and extracellular SeNPs are also expelled from the field of view (
Figure 7b, e). In the cell localization experiment of L11 transformation to produce SeNPs mentioned above, it was found that red substances were present in both the periplasmic space proteins and the rough extract of the cell membrane cytoplasm (
Figure 4). Electron microscopy analysis of the reduced product confirmed that this product was SeNPs, which indicates that L11 produces SeNPs synthesized within L11 cells and then released into the extracellular space (
Figure 7d, e). The size of SeNPs produced by L11 was slightly smaller than that in
B. niabensis [
28], and the shape of the SeNPs obtained by reducing selenite by the
A. brasilense strain is basically the same [
29]. The smaller the size of SeNPs, the higher its inhibitory effect on
E. coli,
P. aeruginosa, and
S. aureus [
1].
Some bacteria rely on the non-specific selenite reductase system in the periplasmic space to reduce selenite to elemental Se. The red SeNPs generated during the reduction process accumulates in the cytoplasm and extracellular space to form SeNPs [
22]. The preliminary cell localization and TEM images of L11 transformation to produce SeNPs can confirm that the reduction of Na
2SeO
3 by L11 strain to generate SeNPs is synthesized within the cytoplasm and transported to the extracellular space. This is consistent with the synthesis of SeNPs by
Ochrobactrum sp. MPV1, but different from the way
Thauera selenati synthesizes SeNPs in the cytoplasm and transports it out of the cell [
22,
30,
31].
3.8. Preliminary Study on the Application of L11 to Produce SeNPs
In order to preliminarily analyze the application of L11 strain in livestock and poultry, this study added SeNPs rich L11 solid bacterial agent to the conventional feed of sheep, and fed it according to the conventional feeding method of sheep. The experimental results showed that the IgG content, T-AOC (total antioxidant capacity), GSH-Px (glutathione peroxidase), and SOD (superoxide dismutase) content of the experimental group were significantly higher than those of the control group (P<0.01), while the white blood cell and lymphocyte content, MDA (malondialdehyde) content were significantly lower (P<0.01) than those of the control group, and the difference in red blood cell content was not significant (P>0.05) (
Table 2). The experimental results indicate that adding SeNPs rich L11 bacterial agent to feed can significantly improve the immunity of sheep. Compared with the control group, broilers supplemented with selenium rich
B. subtilis not only gained weight, but also increased enzyme activities such as GPx, CAT, POD, and plasma levels of IL-2, IL-4, and IgG, while plasma MDA levels decreased [
32]. After oral administration, some
B. subtilis strains can colonize the intestinal mucosa of animals, optimize the composition of intestinal microbiota, and effectively stimulate immunity and metabolism. These can enhance the animal's resistance to intestinal diseases, stress, and clearance of pathogens [
33]. Supplementing a certain amount of Se can regulate gastrointestinal metabolism and antioxidant performance. In addition, Se can enhance immune cell defense against pathogens that cause infection [
34]. In addition, supplementing Se can regulate the bacterial composition in the gastrointestinal tract, thereby promoting physical health [
35,
36]. Yang et al. prepared a Se rich
B. subtilis strain and added it to the diet of broilers. By colonizing and regulating the gut microbiota population, the production performance and immunity of broilers were improved [
37].
The results in
Table 3 indicate that the Se content in the neck muscles, liver, and spleen tissues of sheep in the experimental group supplemented with L11 Se rich nanobacteria in the feed was significantly higher than that in the control group (0.01<P<0.05), and the Se content in the lungs did not reach a significant level (P>0.05). Therefore, adding SeNPs rich L11 bacterial agent to feed can increase the total Se content in livestock muscles and viscera.
4. Conclusions
SeNPs, as an essential micronutrient for the human body, are becoming increasingly important in treatment, agriculture, nutritional supplements, and antibacterial drugs. This study screened a strain L11 that produces SeNPs, and screened and optimized its optimal culture conditions and Na2SeO3 concentration for producing SeNPs. Subcellular component analysis shows that L11 mainly produces SeNPs through transformation in the periplasmic space, cell membrane, and cell wall of cells. XPS, SEM-EDS, and TEM analysis were used to demonstrate that the particles produced by L11 are SeNPs with a diameter of 50 to 200 nm. The chemical properties and composition of the particle surface were determined. The application of L11 SeNPs complex in sheep feed can significantly enhance the antioxidant enzyme activity and immunity of sheep, and increase the Se content in the neck muscles, liver, and spleen tissues of sheep. The safety of Bacillus and the characteristics of L11 make it possible for future use in fields such as food nutrition supplementation, medical treatment, selenium biofortification, agriculture, and biological antibacterial agents.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: The identification results of endophytic bacteria L11; Table S2: The first step screening of Na2SeO3 culture concentration for L11 strain; Table S3: The second step screening of Na2SeO3 culture concentration for L11 strain.
Author Contributions
Investigation, Formal analysis, Methodology, Software, Data curation, W.G.; Methodology, Software, Formal analysis, L.W.; Software, Formal analysis, Visualization, X.S.; Funding acquisition, Investigation, C.X.; Validation, Investigation, S.Y.; Experiment, Resources, Investigation, Writing review & Editing, L.L.; Conceptualization, Resources, Writing-original draft, Supervision, H.C.
Funding
The research was funded by the Horizontal science and technology of Enshi Se-De Bioengineering Co., Ltd., grant number se1-202102.
Institutional Review Board Statement
The work related to animal testing in this study has been approved by the Ethics Committee of the School of Modern Industry for Selenium Science and Engineering, Wuhan Light Industry University. The approval code was WHSE20230021.
Data Availability Statement
The data used to support the findings of this study can be made available by the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nikam, P.B.; Salunkhe, J.D.; Minkina, T.; Rajput, V.D.; Kim, B.S.; Patil, S.V. , A review on green synthesis and recent applications of red nano Selenium. Results in Chemistry 2022, 4, 100581. [Google Scholar] [CrossRef]
- Ullah, A.; Yin, X.; Wang, F.; Xu, B.; Mirani, Z.A.; Xu, B.; Chan, M.W.; Ali, A.; Usman, M.; Ali, N.; Naveed, M. , Biosynthesis of selenium nanoparticles (via Bacillus subtilis BSN313), and their isolation, characterization, and bioactivities. Molecules 2021, 26, 5559. [Google Scholar] [CrossRef]
- Nayak, V.; Singh, K.R.B.; Singh, A.K.; Singh, R.P. , Potentialities of selenium nanoparticles in biomedical science. New Journal of Chemistry 2021, 45, 2849–2878. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Yang, K.; Liu, M.; Qi, Y.; Zhang, T.; Fan, M.; Wei, X. , Antibacterial activity of selenium-enriched lactic acid bacteria against common food-borne pathogens in vitro. Journal of Dairy Science 2018, 101, 1930–1942. [Google Scholar] [CrossRef] [PubMed]
- Quinn, C.F.; El Mehdawi, A.F.; Pilon-Smits, E.A.H. Ecology of Selenium in Plants. In Selenium in plants: Molecular, Physiological, Ecological and Evolutionary Aspects; Pilon-Smits, E.A.H., Winkel, L.H.E., Lin, Z.-Q., Eds.; Springer International Publishing: Cham, 2017; pp. 177–188. [Google Scholar]
- Gupta, M.; Gupta, S. , An overview of selenium uptake, metabolism, and toxicity in plants. Frontiers in plant science 2017, 7, 2074. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, M.; Schmidt, S.; Winter, J. , Formation of Se (0) nanoparticles by duganella sp. and agrobacterium sp. isolated from Se-laden soil of North-East Punjab, India. Microbial Cell Factories 2012, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Shoeibi, S.; Mozdziak, P.; Golkar-Narenji, A. , Biogenesis of Selenium Nanoparticles Using Green Chemistry. Topics in Current Chemistry 2017, 375, 88. [Google Scholar] [CrossRef] [PubMed]
- Khoei, N.S.; Lampis, S.; Zonaro, E.; Yrjälä, K.; Bernardi, P.; Vallini, G. , Insights into selenite reduction and biogenesis of elemental selenium nanoparticles by two environmental isolates of Burkholderia fungorum. New Biotechnology 2017, 34, 1–11. [Google Scholar] [CrossRef]
- Ikram, M.; Javed, B.; Raja, N.I.; Mashwani, Z.R. , Biomedical potential of plant-based selenium nanoparticles: a comprehensive review on therapeutic and mechanistic aspects. International Journal of Nanomedicine 2021, 2021, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Saeed, K.; Fatemeh, H. , Nano-bio selenium synthesized by Bacillus subtilis modulates broiler performance, intestinal morphology and microbiota, and expression of tight junction’s proteins. Biological Trace Element Research 2022, 200, 1811–1825. [Google Scholar] [CrossRef]
- Jia, H.; Huang, S.; Cheng, S.; Zhang, X.; Chen, X.; Zhang, Y.; Wang, J.; Wu, L. , Novel mechanisms of selenite reduction in Bacillus subtilis 168:Confirmation of multiple-pathway mediated remediation based on transcriptome analysis. Journal of Hazardous Materials 2022, 433, 128834. [Google Scholar] [CrossRef]
- Bergey, D.H. , Bergey's manual of determinative bacteriology. 9 ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 1994. [Google Scholar]
- Janda, J.M.; Abbott, S.L. , 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls. Journal of clinical microbiology 2007, 45, 2761–2764. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. , Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. International journal of systematic and evolutionary microbiology 2017, 67, 1613. [Google Scholar] [CrossRef]
- Avendaño, R.; Chaves, N.; Fuentes, P.; Sánchez, E.; Jiménez, J.I.; Chavarría, M. , Production of selenium nanoparticles in Pseudomonas putida KT2440. Scientific reports 2016, 6, 37155. [Google Scholar] [CrossRef]
- Lampis, S.; Zonaro, E.; Bertolini, C.; Cecconi, D.; Monti, F.; Micaroni, M.; Turner, R.J.; Butler, C.S.; Vallini, G. , Selenite biotransformation and detoxification by Stenotrophomonas maltophilia SeITE02: novel clues on the route to bacterial biogenesis of selenium nanoparticles. Journal of Hazardous Materials 2017, 324, 3–14. [Google Scholar] [CrossRef]
- Wang, Y.; Shu, X.; Zhou, Q.; Fan, T.; Wang, T.; Chen, X.; Li, M.; Ma, Y.; Ni, J.; Hou, J. , Selenite reduction and the biogenesis of selenium nanoparticles by Alcaligenes faecalis Se03 isolated from the gut of Monochamus alternatus (Coleoptera: Cerambycidae). International Journal of Molecular Sciences 2018, 19, 2799. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Madkour, M.; El-Saadony, M.T.; Reda, F.M. , Paenibacillus polymyxa (LM31) as a new feed additive: Antioxidant and antimicrobial activity and its effects on growth, blood biochemistry, and intestinal bacterial populations of growing Japanese quail. Animal Feed Science and Technology 2021, 276, 114920. [Google Scholar] [CrossRef]
- Xia, S.; Chen, L.; Liang, J. , Enriched selenium and its effects on growth and biochemical composition in Lactobacillus bulgaricus. Journal of Agricultural and Food Chemistry 2007, 55, 2413–2417. [Google Scholar] [CrossRef] [PubMed]
- Morales, G.; Llorente, I.; Montesinos, E.; Moragrega, C. , A model for predicting Xanthomonas arboricola pv. pruni growth as a function of temperature. PLOS ONE 2017, 12, e0177583. [Google Scholar] [CrossRef] [PubMed]
- Fesharaki, P.J.; Nazari, P.; Shakibaie, M.; Rezaie, S.; Banoee, M.; Abdollahi, M.; Shahverdi, A.R. , Biosynthesis of selenium nanoparticles using Klebsiella pneumoniae and their recovery by a simple sterilization process. Brazilian Journal of Microbiology 2010. [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. , Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environmental health perspectives 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Jia, X.; Li, N.; Chen, J. , A subchronic toxicity study of elemental Nano-Se in Sprague-Dawley rats. Life Sciences 2005, 76, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Kojouri, G.A.; Sadeghian, S.; Mohebbi, A.; Mokhber Dezfouli, M.R. , The effects of oral consumption of selenium nanoparticles on chemotactic and respiratory burst activities of neutrophils in comparison with sodium selenite in sheep. Biological Trace Element Research 2012, 146, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Baltić, M. Ž.; Dokmanović Starčević, M.; Bašić, M.; Zenunović, A.; Ivanović, J.; Marković, R.; Janjić, J.; Mahmutović, H. , Effects of selenium yeast level in diet on carcass and meat quality, tissue selenium distribution and glutathione peroxidase activity in ducks. Animal Feed Science and Technology 2015, 210, 225–233. [Google Scholar] [CrossRef]
- Han, D.S.; Batchelor, B.; Abdel-Wahab, A. , XPS analysis of sorption of selenium(IV) and selenium(VI) to mackinawite (FeS). Environmental Progress & Sustainable Energy 2013, 32, 84–93. [Google Scholar] [CrossRef]
- Al-Hagar, O.E.A.; Abol-Fotouh, D.; Abdelkhalek, E.S.; Abo Elsoud, M.M.; Sidkey, N.M. , Bacillus niabensis OAB2: Outstanding bio-factory of selenium nanoparticles. Materials Chemistry and Physics 2021, 273, 125147. [Google Scholar] [CrossRef]
- Tugarova, A.V.; Mamchenkova, P.V.; Khanadeev, V.A.; Kamnev, A.A. , Selenite reduction by the rhizobacterium Azospirillum brasilense, synthesis of extracellular selenium nanoparticles and their characterization. New Biotechnology 2020, 58, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Piacenza, E.; Presentato, A.; Bardelli, M.; Lampis, S.; Vallini, G.; Turner, R.J. , Influence of Bacterial Physiology on Processing of Selenite, Biogenesis of Nanomaterials and Their Thermodynamic Stability. Molecules 2019, 24, 2532. [Google Scholar] [CrossRef]
- Schröder, I.; Rech, S.; Krafft, T.; Macy, J.M. , Purification and characterization of the selenate reductase from Thauera selenatis. Journal of Biological Chemistry 1997, 272, 23765–23768. [Google Scholar] [CrossRef]
- Qiu, H.; Gao, S.; Hou, L.; Li, A.; Zhu, L.-q.; Dong, J.; Chen, F. , Selenium-enriched Bacillus subtilis Improves Growth Performance, Antioxidant Capacity, Immune Status, and Gut Health of Broiler Chickens. Biological Trace Element Research, 2023. [Google Scholar] [CrossRef]
- Dudonné, S.; Varin, T.V.; Anhê, F.F.; Dubé, P.; Roy, D.; Pilon, G.; Marette, A.; Levy, É.; Jacquot, C.; Urdaci, M. , Modulatory effects of a cranberry extract co-supplementation with Bacillus subtilis CU1 probiotic on phenolic compounds bioavailability and gut microbiota composition in high-fat diet-fed mice. Pharma Nutrition 2015, 3, 89–100. [Google Scholar] [CrossRef]
- Gan, F.; Hu, Z.; Huang, Y.; Xue, H.; Huang, D.; Qian, G.; Hu, J.; Chen, X.; Wang, T.; Huang, K. , Overexpression of pig selenoprotein S blocks OTA-induced promotion of PCV2 replication by inhibiting oxidative stress and p38 phosphorylation in PK15 cells. Oncotarget 2016, 7, 20469. [Google Scholar] [CrossRef] [PubMed]
- Pereira, F.C.; Wasmund, K.; Cobankovic, I.; Jehmlich, N.; Herbold, C.W.; Lee, K.S.; Sziranyi, B.; Vesely, C.; Decker, T.; Stocker, R. , Rational design of a microbial consortium of mucosal sugar utilizers reduces Clostridiodes difficile colonization. Nature Communications 2020, 11, 5104. [Google Scholar] [CrossRef] [PubMed]
- Kasaikina, M.V.; Kravtsova, M.A.; Lee, B.C.; Seravalli, J.; Peterson, D.A.; Walter, J.; Legge, R.; Benson, A.K.; Hatfield, D.L.; Gladyshev, V.N. , Dietary selenium affects host selenoproteome expression by influencing the gut microbiota. The FASEB Journal 2011, 25, 2492. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, J.; Huang, K.; Liu, Q.; Liu, G.; Xu, X.; Zhang, H.; Zhu, M. , Selenium-enriched Bacillus subtilis yb-114246 improved growth and immunity of broiler chickens through modified ileal bacterial composition. Scientific Reports 2021, 11, 21690. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).