1. Introduction
The color of meat and meat products plays a crucial role in attracting the attention of consumers and influencing their purchasing decisions [
1]. The meat industry commonly uses nitrates and nitrites to give cured meat a distinctive color. Nitrates are reduced to nitrite by nitrate reductase-containing microorganisms. Nitrite is then converted to nitric oxide that subsequently reacts with myoglobin in meat and forms the nitrosylmyoglobin pigment. This pigment confers a reddish color to cured meat. Upon cooking, nitrite is denatured and converted into the pink pigment nitrosyl hemochrome. Additionally, nitrite contributes to flavor development, prevents the oxidation of lipids and proteins, and inhibits the growth of pathogenic bacteria such as
Clostridium botulinum [
2]. However, the use of nitrates and nitrites has raised concerns regarding the formation of carcinogenic N-nitrosamines [
3].
Alternatives for the use of pure nitrates and nitrites have gained interest for addressing the issue of N-nitrosamine formation and meeting consumer preferences for natural and clean-label products [
4]. One strategy that has been extensively studied is the use of nitrate sources such as plants or vegetables [
5,
6]. When combined with nitrate-reducing bacteria, these natural sources have been shown to facilitate color development in meat products to an extent similar to the addition of pure chemical sources [
7,
8]. Bacterial production of nitric oxide has also received attention for the reddening of meat products [
9]. These approaches represent clean-label strategies but may require additional processing steps and do not reduce the carcinogenic risk. In contrast, a strategy that avoids nitric oxide sources and subsequent N-nitrosamine carcinogenic risk involves the formation of the red pigment zinc protoporphyrin (ZnPP).
The natural formation of ZnPP was demonstrated during the processing of dry-cured hams where it conferred a characteristic reddish color in ripened hams [
10,
11]. Notably, the long elaboration period of nitrite-free dry-cured ham allowed for the progressive formation of ZnPP. However, in many cooked meat products, the elaboration process takes several hours. Therefore, an important limitation for a broad number of applications is the slow formation of ZnPP in these products. Horse meat and porcine liver have shown higher ZnPP-forming capacities than that of pork, thereby opening new possibilities for nitrite-free meat product development [
12,
13]. Consequently, an optimized process for obtaining a potential coloring ingredient with preformed ZnPP was developed to valorize porcine liver [
14]. This process involves the anaerobic incubation of pork liver homogenates (20% liver dispersed in an ascorbic acid and acetic acid aqueous solution) at pH 4.8 and 45 °C for 24 h. The resulting ingredient can be used in the formulation of several cooked products such as liver pâté [
15]. The safety of some cooked products can be guaranteed by inactivating sporulating microorganisms through sterilization. Liver pâté is often sold as a sterilized product and is, therefore, a good candidate for the study of an ingredient's thermal stability and final product characteristics.
However, several aspects must be considered to ascertain the usefulness of liver homogenates as coloring agents for nitrite-free meat product development. For instance, when forming ZnPP, we found that the liver homogenate color after incubation was brown but exhibited a red color at pH 7 and above. For this reason, the pH was adjusted to 7.5 using pyrophosphates, which are common additives used in pâté formulations to enhance the protein technological properties. However, the coloring capacity of the ingredient at normal meat product pH needs to be examined. Other important aspects to consider are the effects of antioxidant addition and sterilization treatment on ZnPP content and color. Antioxidant addition prevents pigment degradation and the generation of off-flavors in nitrite-free final products. However, the resulting ingredient is a partial liver autolysate with antioxidant properties [
16]. Thus, it may be possible to take advantage of the presence of antioxidants. Moreover, by adding the whole homogenate after incubation, the addition of water, which is normally added to the formulation of certain products, such as pâté, can be omitted. Alternatively, the ZnPP pigment, which remains insoluble at the end of the optimization process, could be concentrated 5-fold using centrifugation, thereby allowing for increased inclusion levels in meat product formulations. In reformulation processes, the replacement of the liver with this ZnPP-rich ingredient (as a whole homogenate or in the form of pellets) may also affect the texture characteristics of the final product because the protein techno-functional properties may differ from those of the native liver. Finally, the interactions between ZnPP and other matrix compounds should not be disregarded as free ZnPP binds to hemoglobin during the elaboration of hams [
10]. Therefore, different ingredient addition methods, ZnPP content and its interactions with other food components for optimal color development, and the impact of reformulation processes on texture and lipid oxidation of nitrite-free cooked meat products remain to be studied. Despite the above, it is reasonable to hypothesize that the use of ZnPP-rich ingredients from porcine liver in meat products may offer a safe, eco-friendly, and healthy choice of natural and clean-label cooked meat products and ultimately provide an alternative to nitrites.
Therefore, this study aimed to investigate the effects of reformulation processes on the color, texture, and lipid oxidation of nitrite-free sterilized pâtés formulated with and without the addition of antioxidants. The reformulation processes involved the replacement of liver with two different liver homogenates rich in ZnPP. Additionally, the solubilization of ZnPP and its binding to hemoglobin were studied in different ZnPP-rich ingredient fractions, and the proximate composition, pH, instrumental color and stability, porphyrin pigment content, lipid oxidation, volatile profile, and textural properties of the pâtés were characterized.
2. Materials and Methods
2.1. Reagents and Standards
ZnPP, protoporphyrin IX (PPIX), and equine myoglobin were purchased from Merck (Darmstadt, Germany). Porcine chlorohemin (heme) was purchased from PanReac AppliChem (Barcelona, Spain). Acetonitrile suitable for high-performance liquid chromatography (HPLC) and trifluoroacetic acid (TFA) were purchased from VWR Chemicals (Radnor, PA, USA). Distilled and ultrapure water was obtained using a Milli-Q system (Millipore, Burlington, MA, USA).
2.2. Preparation of ZnPP-Rich Ingredients
Twenty porcine livers were purchased at a local slaughterhouse (Càrniques Juià S.A., Girona, Spain) on four different days (5 livers/day). Each day, the liver veins and connective tissues were trimmed, the livers were then diced and ground together at ≤4 ºC in a meat cutter bowl to obtain four batches of liver paste, immediately vacuum-packed in aluminum bags, and then stored at −20 ºC until needed.
ZnPP-rich ingredients were obtained from porcine liver homogenates, as described by Llauger et al. (2023). Briefly, liver homogenates consisting of 20% (w/w) fine liver paste were dispersed in an aqueous solution containing ascorbic acid at a final concentration of 1000 mg/L and acetic acid at 2500 mg/L and were adjusted to pH = 4.8 with 1 M NaOH. This mixture was incubated in a 5-L capacity reactor with a continuous flow of nitrogen at 45 ºC for 24 h to obtain an autolyzed homogenate rich in ZnPP (ZnPP-H). Two different 5-L capacity productions of ZnPP-H were obtained from each batch and stored at –20 ºC until use. Finally, to obtain a homogenous ingredient, one product from each batch was mixed to obtain two 20-L replicates.
Thus, ZnPP-H could be used directly in the formulation of products as a potential coloring ingredient. However, the ZnPP pigment mainly remained in the insoluble fraction after centrifugation (5520 × g, 20 min, at 4 °C). Hence, it was possible to obtain a pellet (ZnPP-P) that could also be used in the formulation of products with a moisture content of 81%, which is similar to that of the liver. The ZnPP-H ingredient was adjusted to pH 7.5 with tetrasodium pyrophosphate (TSPP) the day before pâté preparation to obtain a good initial color. In terms of the ZnPP-P ingredient, a saturated solution of TSPP was added at a level of 5% of the pellet weight, mixed, and adjusted to pH 7.5 before centrifugation under the same conditions to remove water and obtain the final ZnPP-P ingredient. After pH adjustment, the ingredients were kept refrigerated at 4 ºC overnight.
2.3. Experimental Design and Pâté Preparation
The liver and fat used in pâté elaboration were bought at a local slaughterhouse (Càrniques Juià S.A., Girona, Spain), whereas the rest of the additives and spices were bought at Collelldevall S.L. (Banyoles, Spain). Eight experimental pâté formulations (2 kg each), including a positive control, negative control, and six formulations with ZnPP-rich ingredients, were prepared independently in duplicate in a pilot plant. The negative control formulation expressed per 100 g of prepared product was as follows: 31.2 g liver, 45 g pork back fat, 20 g water, 0.20 g black pepper, 1.6 g sodium chloride, and 2 g sodium caseinate. The same formulation was also used to elaborate the positive control with the addition of 0.015% sodium nitrite. Based on the negative control formulation, the other experimental pâté formulations were as follows: 1) 16% ZnPP-H, 16% replacement of the liver together with the complete replacement of water with ZnPP-H (i.e., 0% water + 26.2% liver + 25.0% ZnPPH of the total formulation); 2) 40% ZnPP-P, 40% replacement of the liver with ZnPP-P (i.e., 18.72% liver + 12.48% ZnPP-P of the formulation), 3) 60% ZnPP-P, 60% replacement of the liver with ZnPP-P (i.e., 12.48% liver + 18.72% ZnPP-P of the formulation). The remaining formulations were prepared with the same ZnPP-rich ingredients, but the pâtés included 0.5% sodium ascorbate (E-301) and 0.1% tocopherol (E-309) as antioxidants (A), resulting in 4) 16% ZnPP-H/A, 5) 40% ZnPP-P/A, and 6) 60% ZnPP-P/A, respectively. Considering that the ZnPP-H was prepared from a 20% liver homogenate, the 16% ZnPP-H and 16% ZnPP-H/A formulations had the same theoretical protein content as that in the controls.
On the day of preparation, the liver and fat fractions were cut into cubes. The fat was scalded for 30 min in hot water (85 ºC) and the ZnPP-rich ingredients were heated at 40 ºC in a bath. Water, caseinate, and fat were then mixed in a cutter bowl. Subsequently, the liver was mixed with the rest of the ingredients according to the different formulations and emulsified while maintaining the temperature at ≥38 ºC. Finally, the liver pâté mixture was mixed under a vacuum, manually distributed into 15 aluminum cans (7.3 Ø × 3.7 cm, ∼130 g pâté/can) until full, and then hermetically closed using a sealing machine (Talleres Ezquerra Seamers S.L., Navarra, Spain). The remaining liver pâté of each batch was immediately vacuum packed in metalized bags (oxygen permeability of 1.5 mL/m2/24 h and low water vapor permeability of 1 g/m2/24 h) and stored at −80 ºC until analysis. Finally, the pâté in the cans was sterilized in an autoclave at 112 ºC for 50 min and cooled to 35 ºC for 25 min. Liver pâtés were stored at room temperature, and three random cans of each formulation were selected for analysis 24 h after manufacture. One of the cans was used to measure the instrumental color, and the other two cans for texture analysis. The latter cans were placed in a storage cabinet at 25 ºC overnight to ensure a homogeneous temperature of the samples. After these analyses, samples corresponding to the same formulation were mixed and the pH was measured as described below. Then samples were aliquoted, vacuum-packed in metalized bags, and stored at −20 °C until chemical analyses were carried out. Different formulations were replicated.
2.4. Physicochemical Analyses: Proximate Composition, pH, and Color
The moisture content was determined according to ISO 1442:1997, until a constant weight was reached. The protein content was determined according to ISO 937:1978 based on the Kjeldahl digestion method and multiplied by a factor of 6.25. The ash content was determined according to ISO 936:1996. Crude fat content was determined using the Soxhlet method (ISO 1443:1973). The chloride content was determined according to ISO 1841-2:1996 using a potentiometric titrator (785 DMP Titrino, Metrohm AG, Herisau, Switzerland) and expressed as sodium chloride. The pH was determined using S40 SevenMulti (Mettler-Toledo SAE, Barcelona, Spain) and Inlab Solids Pro (Mettler-Toledo SAE) probes. All centesimal analyses were performed in duplicate, and six replicates were used to measure the pH value. Unless otherwise specified, the average of each formulation was considered a single measurement. Instrumental lightness (L*), redness (a*), and yellowness (b*) were measured according to the Commission International de l’Eclairage (CIE) CIELab space color on the inner surface of pâtés immediately after being cut crosswise (∼1.80 cm-thick slices), with three measurements per side using a Minolta Spectrophotometer CM-600d (Konica Minolta, Inc., Chiyoda, Tokyo, Japan). The illuminant was set to D65, with an observer angle of 10 °. The color measurements were repeated on the same surface 15 min after being cut and exposed to atmospheric conditions under white fluorescent light (approximately 900 lx) to determine color stability.
2.5. Pâté Image Acquisition
High-quality images were acquired using a photographic system that included a calibrated Canon EOS 50D digital camera (Canon Inc., Tokyo, Japan) with a picture resolution of 15.1 megapixels and an objective Canon EF-S 18-200 mm f/3.5-5.6 IS. The camera was mounted in a black closet (1.06 × 106 × 2.50 m3) with halogen lights Solux Q50MR16 CG/47/36°12 V/50 W/4700 K (Eiko Ltd., Shawnee, KS, U.S.A.). White balance was achieved using a white card (Lastolite Ltd., Leicestershire, U.K.). The camera was connected to a computer to store the images. Different pâté formulations were placed 50 cm below the camera on uniformly black surfaces. The canned products were photographed immediately after opening.
2.6. Textural Properties: Puncture and Spreadability Test
The puncture test was performed using the Texture Analyzer TA.HD Plus (Stable Micro Systems Ltd., Surrey, UK). Two cans of each formulation were tested with a cylindrical probe of 5 mm diameter at a head speed of 1 mm·s-1. The force required to sink the probe to a depth of 20 mm was determined by using a load cell weighing 5 kg. Six measurements were performed for each can. The spreadability test was carried out using a Texture Analyzer TA.XT2 (Stable Micro Systems Ltd., Surrey, England) with a spreader consisting of a conical tip submerging at a speed of 2 mm·s-1 with the shape of an inverted cone. Spreadability was expressed as the compression force required to deform the pâté by 75% using a load cell of 30 kg. Two measurements were performed for each can, and the average was considered a single measurement. All results were recorded using the Exponent software (Stable Micro Systems Ltd., Surrey, England).
2.8. Determination of ZnPP, PPIX, and Heme Pigments
The ZnPP and PPIX content was determined similarly as described elsewhere [
14]. Briefly, 2.5 g of pâté for the controls and 2.0 g for the remaining formulations were weighed in 50-mL centrifuge tubes and homogenized using an Ultra-Turrax T25 (IKA Werke GmbH & Co. KG, Staufen, Germany) for 30 sec at 13500 rpm in 10 mL of a cold solvent extraction mixture containing ethyl acetate/acetic acid/dimethyl sulfoxide (10:2:1, v/v/v) while the tube was immersed in ice. After a 20 min extraction at 4 ºC to ease the fat removal, the extracts were centrifuged (2850 × g, 20 min, 4 ºC), the supernatant was filtered through filter paper (grade 1), the filtrate was collected into amber glass tubes immersed on ice, and then finally transferred into 10-mL amber volumetric flasks. Subsequently, an aliquot (1 mL) was filtered through a 0.2-µm nylon syringe filter, and 200 µL were transferred to 96-microwell plates to read the ZnPP fluorescence with excitation set at 416 nm and emission at 588 nm, whereas PPIX fluorescence was read with an excitation set at 400 nm and emission set at 630 nm using a Varioskan Flash microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).
The total heme pigment content was determined as described by Hornsey (1956) with minor modifications. Briefly, 2.0 g of pâté was homogenized with 9 mL of 90% (v/v) aqueous acetone containing HCl (0.24 M) in triplicate. The samples were then gently magnetically stirred at 4 ºC for 20 min. After centrifugation and filtration under the conditions mentioned above, an aliquot was filtered through a 0.2-µm nylon syringe filter and injected (30 µL) into an Agilent 1100 series HPLC system (Waldbronn, Germany) coupled to a UV-Vis detector set at 400 nm to quantify the total heme. The separation of porphyrins was performed on a Synergi column (150 × 4.6 mm, 4 µm, 80 Å) from Phenomenex (Torrance, USA). The column elution was carried out at a flow rate of 1 mL/min at 35 ºC using a gradient elution method with acetonitrile-water phases containing 0.05% TFA (20:80, respectively; initial eluting conditions), thereby increasing the proportion of acetonitrile to 100% within 10 min. After the run was completed, the column re-equilibration time was 5 min. All porphyrin results are expressed as micromoles per kilogram of liver on a dry weight basis.
2.9. Lipid Hydroperoxides
Lipid hydroperoxide content was determined by reduction with ferric thiocyanate, as described previously [
18]. In duplicate, 3 g of pâté was weighed into a 50-mL Falcon tube, and then 5 mL of chloroform and 10 mL of methanol were added. The samples were then homogenized for 1 min at 9500 rpm while immersed in ice using an Ultra-Turrax T25 (IKA Werke GmbH & Co. KG, Staufen, Germany). Next, 5 mL of chloroform was added, followed by vortexing for 1 min, and 5 mL of water was added, followed by vortexing for 1 min. Then the samples were centrifuged (3000 × g, 30 min, at 4 ºC), and after removing the upper phase (aqueous phase), the lipid extract (lower phase) was filtered through filter paper (grade 1) in a 10-mL volumetric flask. Finally, 200 µL of the lipid extract was mixed with 2.8 mL of methanol/butanol (2:1, v/v), incubated at room temperature for 20 min, and the absorbance was read at 500 nm. The results were expressed as µmols of cumene hydroperoxide equivalents per kg sample.
2.10. Hexanal Content and Volatile Profile
The volatile profiles of the pâtés were determined using headspace solid-phase microextraction (HS-SPME) with a Combi PAL injector autosampler (CTC Analytics, Zwingen, Switzerland). Before analysis, a 50/30 µm Divinylbenzene/Carboxen/Polydimethylsiloxane (DVB/CAR/PDMS) Stable Flex SPME fiber (Supelco, Bellefonte, PA, USA) was preconditioned at 260 ºC for 30 min in the injector port of a gas chromatograph (GC) model 6850 coupled to a mass selective detector (MS) model 5975C VL MSD (Agilent Technologies, Santa Clara, CA, USA). For HS-SPME extraction, 1 g of the pork liver pâté was weighed in a 10-mL amber vial and 50 µL of 2-pentanone solution (Alfa Aesar,Ward Hill, MA, 99% purity) was added as an internal standard at a concentration of 60.49 µg/mL. The sample vials were incubated at 40 ºC for 20 min, and the SPME fiber was inserted into the vial and exposed to the headspace for 40 min. The SPME fiber was desorbed and maintained in the injector port for 10 min at 280 ºC in split mode (1:10). The compounds were separated in a DB-5MS capillary column (Agilent J&W Scientific; 30 m, 0.25 mm id, film thickness 1.00 µm). Helium was used as a carrier gas with a constant flow of 1 mL·min-1. The GC oven temperature program was started when the fiber was inserted into the GC injector port. The temperature was held at 40 ºC for 10 min, ramped to 200 ºC at 5 ºC·min-1, then to 280 ºC at 80 ºC·min-1, and held for 1 min, giving a total run time of 44 min. Mass spectral data were acquired in the range of 40–250 amu in scan acquisition mode, with the mas detector transfer line maintained at 280 ºC and the mas source at 230 ºC while the mass quad was at 150 ºC. Volatile compounds were tentatively identified by comparing their mass spectra with the database of the National Institute of Standards and Technology (NIST 2.0 version, Gaithersburg, MD, USA) and MassHunter software B.05.01. The results are expressed in area units per gram of sample.
2.11. Urea-Polyacrylamide Gel Electrophoresis (PAGE) of Soluble Fractions
The interactions between ZnPP-H, ZnPP-P, and hemoglobin were examined by adding a hemolyzed red blood cell (RBC) fraction to different fractions of ZnPP-rich ingredients. The RBC fraction was obtained from commercial blood from a local slaughterhouse that contained tripolyphosphate solution (0.4%, w/v) as an anticoagulant. Blood was centrifuged at 2540 × g for 15 min at 5–10 ºC and the cellular fraction was diluted 1:1 with MilliQ water. After 30 min under stirring, the RBC fraction was centrifuged at 20900 × g for 30 min at 15–20 ºC to remove erythrocytic stroma.
The soluble fractions of ZnPP-H were obtained after adjusting the pH at 4.8, 6.7, and 7.5 with 1 M NaOH and centrifuging at 5520 × g for 20 min at 4 °C. The resulting pellets at different pH were resuspended in the initial volume with distilled water and with a 5% RBC fraction aqueous solution. The ZnPP-P ingredient (pH 4.8) was resuspended at the initial volume with water and the pH was adjusted to 4.8, 6.7, and 7.5. The same ingredients were resuspended in final 1% and 5% RBC aqueous solutions. When required, the pH of the resuspended samples was readjusted to the desired pH (4.8, 6.7, or 7.5), incubated at 4 °C for 1 h, and subsequently centrifuged under the same conditions. All supernatants containing the soluble fractions were filtered through filter paper, and the soluble ZnPP species at different pH values were separated using urea-PAGE, as described previouslyby Wang et al., (2021). Briefly, filtered supernatants were individually mixed in the following proportions: 29% sample, 50% sample buffer (50 mM Tris-HCl at pH 6.8 and 8 M urea), 20% glycerol, and 1% 2-mercaptoethanol. An aliquot of the RBC fraction was used as a marker and prepared as sample with 0.2% Coomassie Brilliant Blue G (CBB) prior to analysis. The hand-cast gel was prepared using a 4.5% stacking gel (4.5% acrylamide, 4 M urea, 125 mM Tris HCl pH 6.8) and a 10% separating gel (10% acrylamide, 4 M urea, 375 mM Tris HCl pH 8.8). The electrophoresis was run at 10 mA for 30 min followed by 20 mA for approximately 180 min at 4 °C. After electrophoresis, the gel was irradiated with a 420 nm purple light-emitting diode light source (OSSV5111A, OptoSupply Co. Ltd., Hong Kong, China). Fluorescent images were captured using a digital camera equipped with a 600-nm bandpass filter (BPB-60, Fujifilm Corp., Tokyo, Japan). To detect protein bands, the gel was stained with CBB solution (0.1% CBB, 40% methanol, 10% acetic acid) for 10 min and then destained with a solution containing 10% methanol and 7% acetic acid overnight.
2.12. Statistical Analysis
Statistical analyses were performed using XLSTAT statistical software (version Lumiver, 2023; New York, USA). One-way analysis of variance (ANOVA) was used to examine whether there was a significant difference among the different pâté formulations in terms of proximate composition, chloride content, pH, instrumental color, porphyrin content, texture, and oxidation parameters. In terms of color, a series of one-way ANOVAs was performed for each exposure time (immediately after opening and after 15 min of exposure to air and light) to determine the existence of significant differences between the different formulations. Additionally, a series of one-way ANOVAs was performed for each formulation to determine the existence of significant differences between each exposure time. Likewise, a series of one-way ANOVAs was performed before and after the sterilization treatments to determine the existence of significant differences in porphyrin content between the different formulations. Additionally, a series of one-way ANOVAs was performed for each formulation to determine the existence of significant differences in porphyrin content as a result of the sterilization treatment (before and after). Significant differences among the different pâté formulations found using one-way ANOVA were evaluated using Tukey’s honest significant difference test. Statistical significance was set at p < 0.05.
4. Discussion
The decrease in protein content with increased liver replacement percentages in the formulations could be due to the higher moisture and lower protein content of ZnPP-P (81.4% and 11.7%, respectively) compared with those of porcine livers (72–75% and 19–20%, respectively) [
24,
25]. The higher the concentration of ZnPP-rich ingredients, the higher the final pH. This finding can be attributed to the addition of TSPP to attain a pH of 7.5 in the ZnPP-rich ingredients. The higher ash content of the 16% ZnPP-H and 16% ZnPP-H/A formulations compared with that of the ZnPP-P formulations could be due to the replacement of water with ZnPP-H containing dissolved salts. However, the pH range (from 6.63 to 6.87) was relatively narrow and similar to that reported in other studies [
26,
27]. Likewise, the proximate compositions of the different pâté formulations were within those described in the literature [
26,
27]. Thus, pâté reformulation with ZnPP-rich ingredients did not substantially change the proximate composition of nitrite-free pâtés.
Conversely, there were important differences in ZnPP and heme content, which were mainly explained by the partial replacement of the liver with ZnPP-rich ingredients (
Table 3). Nonetheless, in a previous study on liver homogenates, ascorbic acid addition caused a greater decrease in heme content than the decrease without it, whereas the ZnPP content increased (Llauger et al., 2023). Hence, it is also possible that ZnPP formation may have occurred, to some extent, during pâté elaboration, thereby explaining not only the ZnPP content in the controls, but also the higher ZnPP content in the 60% ZnPP-P/A formulation compared with that in the 60% ZnPP-P formulation before sterilization. In general, the sterilization treatment caused a heme content decrease which was significant in all formulations with ZnPP-rich ingredients, whereas ZnPP decrease with thermal treatment was only observed in the 40% ZnPP-P/A and 60% ZnPP-P/A formulations. This finding agrees with the reported increased stability of ZnPP extracts at thermal treatments up to 70 °C when compared with that of extracts containing nitrosyl-heme [
28]. The contents of the different porphyrins explain the pâté appearances (
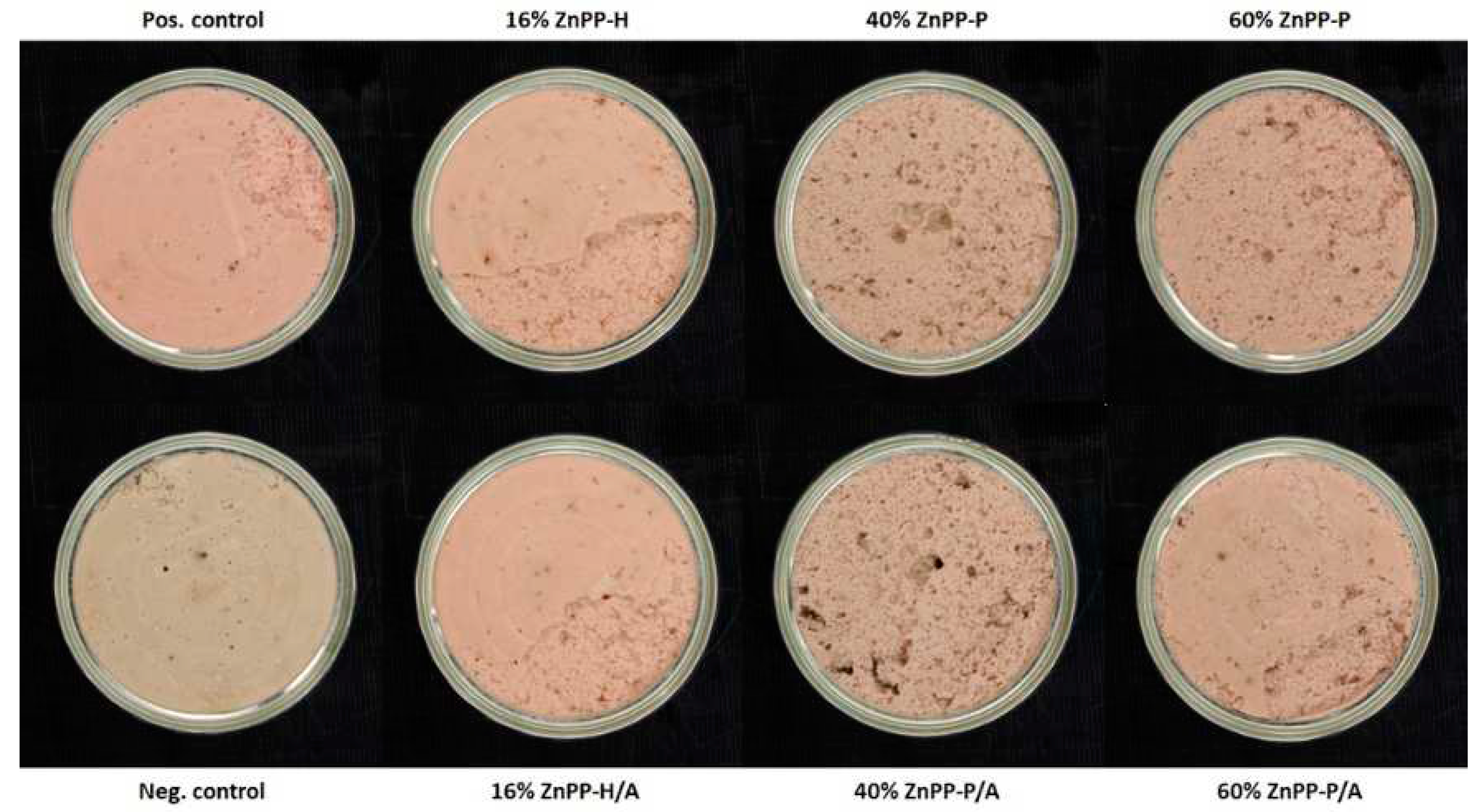
Figure 1). The pinkish color of the positive control with nitrites was due to the presence of nitrosyl-heme, whereas the dull visual appearance of the negative control could be attributed to protein denaturation and hemichrome formation. Therefore, the red color of the pâtés containing ZnPP-rich ingredients can be attributed to the presence of ZnPP, which was relatively stable under thermal treatment (
Table 3). The stability of ZnPP during sterilization treatments is of great interest for obtaining safe, red-colored, and nitrite-free meat products.
However, as shown in
Figure 1 and
Table 2 and
Table 3, not only the ZnPP content (or level of addition) but also the form of the ZnPP-rich ingredients determines the red color of the product. Interestingly, ZnPP-H addition offered an intense red color (a* value) despite having a lower ZnPP concentration than that in ZnPP-P. The observed instrumental color (L*a*b*) values of pâtés formulated with ZnPP-H were consistent with those reported in other studies using different ingredients in the formulation of liver pâtés [
20,
27,
29]. The effect of the ZnPP-H-soluble fraction on color was examined by replacing the water in the pâté formulation with the ZnPP-H supernatant obtained after centrifugation at pH 4.8 and subsequently adjusted to pH 7.5, causing little effect on the redness of the pâtés (data not shown). It is unclear whether some soluble compounds in ZnPP-H interacted with other matrix compounds and participated in the appearance of the red color. However, in dry-cured hams, the binding of ZnPP to hemoglobin has recently been reported as the main coloring pigment [
10]. Therefore, we examined the presence of soluble ZnPP at different pH values when combined with or without hemoglobin. The results suggested that the pH and interaction of ZnPP with other compounds in the food matrix, such as hemoglobin, may have contributed to the increased content of soluble and bound ZnPP. The presence of soluble ZnPP could explain the red appearance of the 16% ZnPP-H and 16% ZnPP-H/A formulations, which involved the mixing of insoluble ZnPP with a higher amount of hemoglobin from fresh liver than that in the other formulations as a consequence of the low percentage of liver replacement with ZnPP-rich ingredients in pâté formulations.
In addition to pigment thermal stability, the color stability under light and air exposure is another important feature to consider. In the negative control, air exposure had a very low blooming effect. In other formulations, porphyrin oxidation explained the observed color fading, which was measured as a loss of redness. In line with this finding, color fading has also been reported in nitrified meat products exposed to air and light, regardless of the addition of antioxidants such as ascorbate [
30]. Under our conditions, ZnPP-H addition resulted in pâtés with redness and appearance similar to that of conventional nitrified pâtés, not only immediately after opening but also after exposure to light for 15 min. This indicates that the development of nitrite-free pâtés based on ZnPP is a good alternative to nitrite in terms of color and color stability.
In all formulations, the lipid hydroperoxide content was low compared to the values reported for pâtés and other cooked meat products [
31,
32,
33]. Lipid hydroperoxides easily decompose into secondary lipid oxidation products at high temperatures, which explains their low content in pâtés after sterilization. Similar to our findings, the most abundant lipid oxidation breakdown compound reported in pâtés was hexanal [
27,
34,
35]. The elevated hexanal content in the negative control can be partly explained by the relatively high content of heme iron, a well-known potent pro-oxidant [
36]. Notably, the positive and negative controls contained similar amounts of heme before and after sterilization; however, heme was bound to nitric oxide in the positive control. Hence, the formation of nitrosyl-heme upon the addition of nitrifying agents reduces the catalytic effect of heme [
37]. However, the heme content of the negative control was similar to that of the pâtés containing ZnPP-rich ingredients, whereas the hexanal content of the pâtés containing ZnPP-rich ingredients was lower than that of the negative control (
Table 1 and
Table 3). The decreased hexanal content in the pâtés with ZnPP-rich ingredients could partly be attributed to the tendency towards low heme amounts. Alternatively, the low hexanal values could be attributed to ascorbic acid addition and formation of peptides with antioxidant properties during the ZnPP-rich ingredient preparation [
16]. This latter explanation may be the main reason for the minimized lipid oxidation progression in the 16% ZnPP-H and 16% ZnPP-H/A formulations when compared with that of the positive control and some ZnPP-P pâté formulations. The antioxidant properties of ZnPP-H could also explain why further antioxidant addition to the ZnPP-H/A formulation seemed unnecessary to prevent oxidation. Likewise, the presence of antioxidant compounds in ZnPP-P should not be disregarded, given the lack of changes in ZnPP-P pâtés after antioxidant addition and the fact that the hexanal levels of these formulations were similar to those of the positive control with added nitrites.
Notably, the negative control showed higher hexanal values than those of the positive control, which could be explained by the well-known antioxidant properties of nitrite [
37]. The progression of oxidation may have increased the hardness of the negative control by crosslinking the proteins with the oxidation products of lipids and/or proteins, resulting in hardened products during storage [
38]. Compositional factors also greatly influenced the textural properties of the pâtés [
29,
39]. In this regard, the protein contents of the 16% ZnPP-H and 16% ZnPP-H/A formulations were similar to those of the positive and negative controls, thus explaining the observed similarities in textural properties (
Table 1). However, the protein content in the 40% and 60% ZnPP-P formulations was also similar to that of the controls, and only the protein content of the 40% and 60% ZnPP-P/A formulations were slightly lower than those of the negative control. Thus, the decrease in hardness values with the amount of ZnPP-rich ingredients can be attributed to partial protein denaturation caused by the decrease in pH during the elaboration of these ingredients and the reported proteolysis that occurred during their elaboration [
16]. The degradation of native liver proteins into small proteins and peptides during the production of ZnPP-rich ingredients may explain the formation of a slightly intertwined gel network, resulting in weakened and spreadable gels. Therefore, despite the observed changes in texture, they are not considered a limiting factor for the application of ZnPP-rich ingredients, because the texture can be tailored for the development of non-nitrified pâtés through formulation and structuring processes [
40].
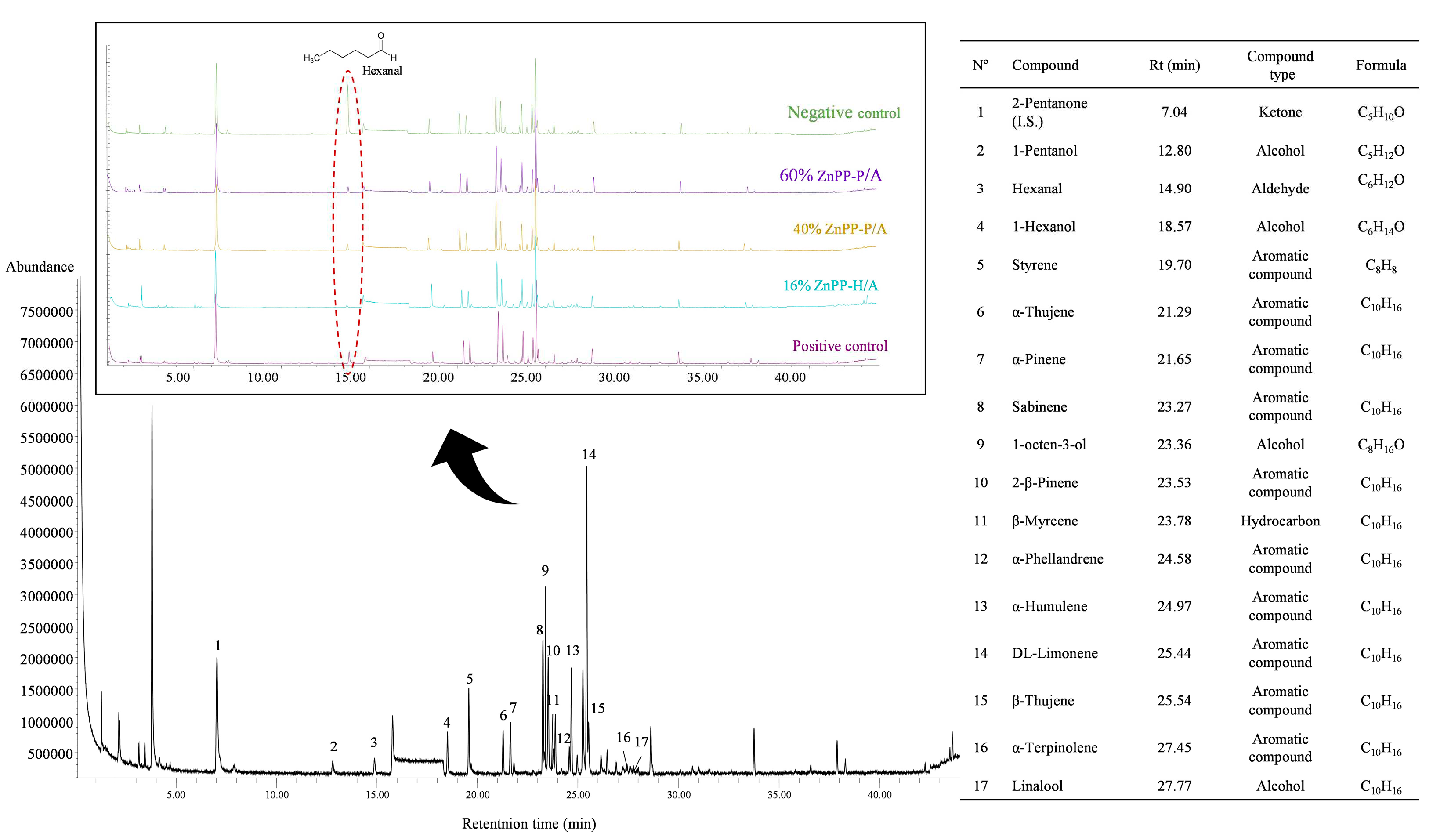
Finally, the volatile profiles agree with those observed in similar pâté formulations [
27,
34,
35,
41]. Volatile terpenes are relatively abundant and relevant because of their low threshold values that determine the aromatic characteristics of the final product. These compounds originate from spice and herb addition, which explains the absence of major differences between the different pâté formulations. Therefore, the addition of ZnPP-rich ingredients did not substantially change the pâté volatile profile when compared with that of the positive control with nitrite.
Figure 1.
Photographs of canned pork liver pâtés after sterilization treatment and storage for 24 h at room temperature. The pâté formulations include: Pos. Control, positive control with nitrites; Neg. Control, negative control without nitrites; 16% ZnPP-H, complete replacement of water and 16% of the liver with whole autolyzed homogenate rich in Zn-protoporphyrin (ZnPP-H); 40% ZnPP-P and 60% ZnPP-P, replacement of 40% and 60% of the liver, respectively, with pellet rich in Zn-protoporphyrin (ZnPP-P), which was obtained after centrifugation (5520 × g, 20 min) of the whole autolyzed homogenate. All ZnPP-H and ZnPP-P pâté formulations in which the antioxidants sodium ascorbate and tocopherol were added (i.e., 16 % ZnPP-H/A, 40 % ZnPP-P/A, and 60 % ZnPP-H/A) are also shown.
Figure 1.
Photographs of canned pork liver pâtés after sterilization treatment and storage for 24 h at room temperature. The pâté formulations include: Pos. Control, positive control with nitrites; Neg. Control, negative control without nitrites; 16% ZnPP-H, complete replacement of water and 16% of the liver with whole autolyzed homogenate rich in Zn-protoporphyrin (ZnPP-H); 40% ZnPP-P and 60% ZnPP-P, replacement of 40% and 60% of the liver, respectively, with pellet rich in Zn-protoporphyrin (ZnPP-P), which was obtained after centrifugation (5520 × g, 20 min) of the whole autolyzed homogenate. All ZnPP-H and ZnPP-P pâté formulations in which the antioxidants sodium ascorbate and tocopherol were added (i.e., 16 % ZnPP-H/A, 40 % ZnPP-P/A, and 60 % ZnPP-H/A) are also shown.
Figure 2.
Coomassie Brilliant Blue-stained (left) and fluorescent (right) images of supernatants separated using urea-polyacrylamide gel electrophoresis (PAGE). (A) Lane 1 corresponds to a hemolyzed red blood cell (RBC) fraction. Lanes 2, 3, and 4 correspond to soluble fractions of ZnPP-H obtained after centrifugation at 5520 × g for 20 min at pH 4.8, 6.7, and 7.5, respectively. Lanes 5, 6, and 7 correspond to soluble fractions from the resulting ZnPP-H pellets obtained after centrifugation at pH 4.8, 6.7, and 7.5, respectively, which were resuspended at the initial volume with water and re-adjusted to the same pH. Lanes 8, 9, and 10 correspond to soluble fractions from the resulting ZnPP-H pellets obtained after centrifugation at pH 4.8, 6.7, and 7.5, respectively, which were resuspended at the initial volume with 5% RBC fraction solution and re-adjusted to the same pH. (B) Lane 1 corresponds to an RBC fraction. Lanes 2, 3, and 4 correspond to soluble fractions of ZnPP-P obtained after centrifugation at 5520 × g for 20 min at pH 4.8, resuspended at the initial volume with water adjusted to pH 4.8, 6.7, and 7.5, respectively. Lanes 5, 6, and 7 correspond to soluble fractions of ZnPP-P resuspended at the initial volume with 1% RBC fraction aqueous solution adjusted to pH 4.8, 6.7, and 7.5, respectively. Lanes 8, 9, and 10 correspond to soluble fractions of ZnPP-P resuspended at the initial volume with 5% RBC fraction aqueous solution adjusted to pH 4.8, 6.7, and 7.5, respectively.
Figure 2.
Coomassie Brilliant Blue-stained (left) and fluorescent (right) images of supernatants separated using urea-polyacrylamide gel electrophoresis (PAGE). (A) Lane 1 corresponds to a hemolyzed red blood cell (RBC) fraction. Lanes 2, 3, and 4 correspond to soluble fractions of ZnPP-H obtained after centrifugation at 5520 × g for 20 min at pH 4.8, 6.7, and 7.5, respectively. Lanes 5, 6, and 7 correspond to soluble fractions from the resulting ZnPP-H pellets obtained after centrifugation at pH 4.8, 6.7, and 7.5, respectively, which were resuspended at the initial volume with water and re-adjusted to the same pH. Lanes 8, 9, and 10 correspond to soluble fractions from the resulting ZnPP-H pellets obtained after centrifugation at pH 4.8, 6.7, and 7.5, respectively, which were resuspended at the initial volume with 5% RBC fraction solution and re-adjusted to the same pH. (B) Lane 1 corresponds to an RBC fraction. Lanes 2, 3, and 4 correspond to soluble fractions of ZnPP-P obtained after centrifugation at 5520 × g for 20 min at pH 4.8, resuspended at the initial volume with water adjusted to pH 4.8, 6.7, and 7.5, respectively. Lanes 5, 6, and 7 correspond to soluble fractions of ZnPP-P resuspended at the initial volume with 1% RBC fraction aqueous solution adjusted to pH 4.8, 6.7, and 7.5, respectively. Lanes 8, 9, and 10 correspond to soluble fractions of ZnPP-P resuspended at the initial volume with 5% RBC fraction aqueous solution adjusted to pH 4.8, 6.7, and 7.5, respectively.
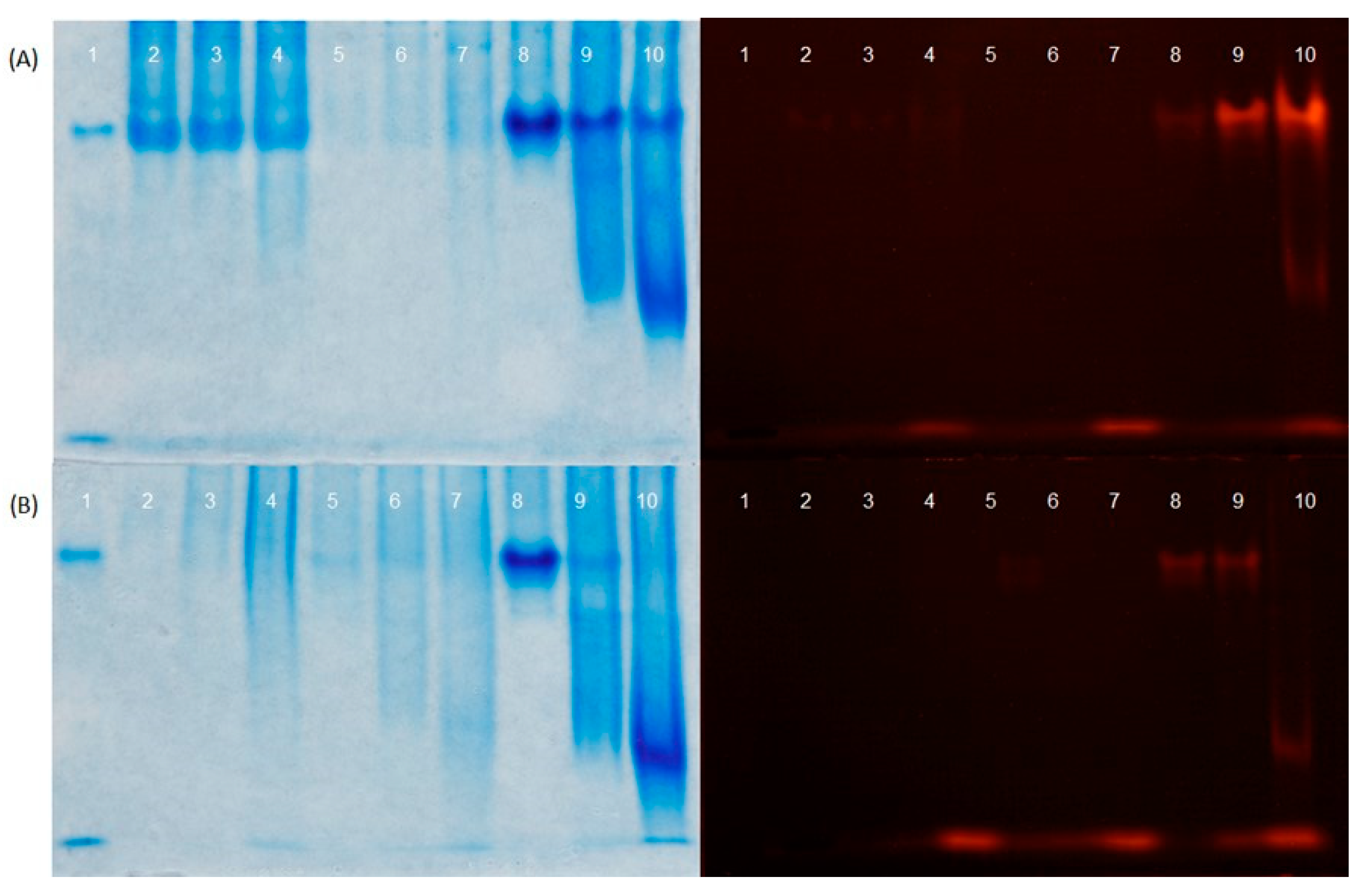
Figure 3.
Total ion chromatogram of the volatile organic compounds found during the elaboration of canned pork liver pâtés containing ingredients rich in Zn-protoporphyrin. The compound number 1 (2-pentanone) was used as an internal standard.
Figure 3.
Total ion chromatogram of the volatile organic compounds found during the elaboration of canned pork liver pâtés containing ingredients rich in Zn-protoporphyrin. The compound number 1 (2-pentanone) was used as an internal standard.
Table 1.
Proximate composition, chloride content, pH, texture properties (hardness and spreadability), lipid hydroperoxides (LHP) content, and hexanal content of liver pates with added ZnPP-rich ingredients1.
Table 1.
Proximate composition, chloride content, pH, texture properties (hardness and spreadability), lipid hydroperoxides (LHP) content, and hexanal content of liver pates with added ZnPP-rich ingredients1.
| |
Positive Control |
Negative Control |
16%
ZnPP-H |
40%
ZnPP-P |
60%
ZnPP-P |
16%
ZnPP-H/A |
40%
ZnPP-P/A |
60%
ZnPP-P/A |
| Moisture (%) |
53.7 ± 0.52 |
52.5 ± 2.50 |
53.1 ± 1.73 |
55.5 ± 1.10 |
55.9 ± 1.89 |
54.2 ± 0.42 |
54.1 ± 0.44 |
55.5 ± 1.35 |
| Fat (%) |
30.0 ± 1.39 |
30.1 ± 0.42 |
30.6 ± 1.98 |
28.3 ± 1.30 |
27.7 ± 1.47 |
29.2 ± 1.50 |
30.9 ± 2.61 |
29.3 ± 1.74 |
| Protein (%) |
10.0 ± 0.35ab
|
10.5 ± 0.24a
|
10.5 ± 0.31a
|
9.2 ± 0.15ab
|
9.3 ± 0.23ab
|
10.5 ± 0.24a
|
8.9 ± 0.26b
|
8.8 ± 0.56b
|
| Ash (%) |
2.03 ± 0.01c
|
2.03 ± 0.05c
|
2.86 ± 0.06a
|
2.29 ± 0.04b
|
2.43 ± 0.03b
|
2.85 ± 0.02a
|
2.32 ± 0.05b
|
2.43 ± 0.04b
|
| NaCl (%) |
1.65 ± 0.03 |
1.71 ± 0.05 |
1.70 ± 0.07 |
1.72 ± 0.13 |
1.67 ± 0.05 |
1.73 ± 0.05 |
1.74 ± 0.04 |
1.72 ± 0.10 |
| pH |
6.66 ± 0.01c
|
6.63 ± 0.03c
|
6.67 ± 0.02c
|
6.81 ± 0.01ab
|
6.87 ± 0.01a
|
6.68 ± 0.01c
|
6.77 ± 0.02b
|
6.87 ± 0.02a
|
| Hardness (N) |
2.0 ± 0.13b
|
2.3 ± 0.19a
|
1.8 ± 0.10c
|
1.2 ± 0.15d
|
0.8 ± 0.30f
|
1.9 ± 0.18bc
|
1.0 ± 0.18e
|
0.7 ± 0.06f
|
| Spreadability (N) |
9.8 ± 0.71b
|
11.5 ± 1.07a
|
8.8 ± 0.38b
|
6.5 ± 1.09c
|
4.5 ± 0.97d
|
9.4 ± 0.75b
|
5.4 ± 0.89cd
|
4.7 ± 0.33d
|
LHP
(µmols CHP/kg) |
16.3 ± 0.46 |
17.3 ± 2.91 |
9.2 ± 6.08 |
15.9 ± 1.39 |
17.4 ± 7.08 |
10.6 ± 6.73 |
20.4 ± 10.70 |
34.8 ± 25.89 |
| Hexanal (AU·105/g) |
25.6 ± 1.94b
|
107.5 ± 13.12a
|
6.6 ± 1.92c
|
14.3 ± 6.38bc
|
10.8 ± 3.88bc
|
6.4 ± 2.62c
|
24.9 ± 8.49b
|
23.1 ± 7.57b
|
Table 2.
Instrumental color parameters (L*: lightness; a*: red/green; b*: yellow/blue) and color stability of liver pates with added ZnPP-rich ingredients1.
Table 2.
Instrumental color parameters (L*: lightness; a*: red/green; b*: yellow/blue) and color stability of liver pates with added ZnPP-rich ingredients1.
| Formulation |
Initial time (0 min) |
|
After 15 min of exposure to light and air |
| L* |
a* |
b* |
|
L* |
a* |
b* |
| Positive Control |
59.6 ± 0.79a, x
|
9.8 ± 0.27a, x
|
14.5 ± 0.24e, y
|
|
58.5 ± 0.82a, y
|
8.8 ± 0.24a, y
|
15.6 ± 0.29d, x
|
| Negative Control |
58.1 ± 1.05ab
|
1.4 ± 0.15e, y
|
15.5 ± 0.34d, y
|
|
58.1 ± 1.18ab
|
1.8 ± 0.19e, x
|
16.5 ± 0.37c, x
|
| 16% ZnPP-H |
58.2 ± 0.76ab, x
|
7.5 ± 0.24b, x
|
18.9 ± 0.50a
|
|
56.9 ±1.13bc, y
|
6.6 ± 0.29b, y
|
19.1 ± 0.27a
|
| 40% ZnPP-P |
57.1 ± 0.82ab
|
4.9 ± 0.23d, x
|
17.3 ± 0.48c
|
|
56.6 ± 0.92c
|
4.2 ± 0.35d, y
|
17.4 ± 0.51b
|
| 60% ZnPP-P |
55.3 ± 1.60c
|
5.5 ± 0.34c, x
|
17.9 ± 0.34b
|
|
54.2 ± 1.75d
|
4.6 ± 0.33c, y
|
17.5 ± 0.75b
|
| 16% ZnPP-H/A |
59.8 ± 1.10a, x
|
7.4 ± 0.29b, x
|
19.2 ± 0.38a
|
|
58.8 ± 0.79a, y
|
6.4 ± 0.38b, y
|
19.4 ± 0.52a
|
| 40% ZnPP-P/A |
57.4 ± 1.24ab
|
4.6 ± 0.17d, x
|
16.9 ± 0.39c, y
|
|
57.6 ± 0.53abc
|
4.0 ± 0.21d, y
|
17.4 ± 0.34b, x
|
| 60% ZnPP-P/A |
58.1 ± 0.92ab, x
|
4.7 ± 0.20d, x
|
17.1 ± 0.31c, y
|
|
57.0 ± 0.79bc, y
|
3.9 ± 0.12d, y
|
17.4 ± 0.19b, x
|
Table 3.
Heme, Zn-protoporphyrin (ZnPP), and protoporphyrin IX (PPIX) content in liver pate before (PB) and after the sterilization (SP) treatment1.
Table 3.
Heme, Zn-protoporphyrin (ZnPP), and protoporphyrin IX (PPIX) content in liver pate before (PB) and after the sterilization (SP) treatment1.
| Formulation |
Heme (µmol/kg DM) |
|
ZnPP (µmol/kg DM) |
|
PPIX (µmol/kg DM) |
| PB |
SP |
|
PB |
SP |
|
PB |
SP |
| Positive control |
226 ± 20.3a
|
161 ± 30.6a
|
|
0.5 ± 0.11c
|
0.8 ± 0.11c
|
|
0.2 ± 0.03d
|
0.3 ± 0.05d
|
| Negative control |
193 ± 17.6ab
|
120 ± 28.9ab
|
|
0.4 ± 0.29c
|
1.9 ± 0.70c
|
|
0.2 ± 0.05d
|
0.4 ± 0.10d
|
| 16% ZnPP-H |
198 ± 5.8ab, x
|
88 ± 8.4b, y
|
|
23.0 ± 4.64c
|
30.2 ± 2.35c
|
|
2.6 ± 0.59d
|
2.60 ± 1.17cd
|
| 40% ZnPP-P |
181 ± 11.7ab, x
|
93 ± 3.3ab, y
|
|
122.7 ± 15.46b
|
76.6 ± 13.29ab
|
|
6.5 ± 0.83c
|
6.2 ± 2.00bcd
|
| 60% ZnPP-P |
166 ± 10.0b, x
|
79 ± 5.8b, y
|
|
175.5 ± 18.69b
|
147.7 ± 7.88a
|
|
10.7 ± 0.95ab
|
10.1 ± 2.64ab
|
| 16% ZnPP-H/A |
201 ± 14.5ab, x
|
78 ± 5.5b, y
|
|
29.2 ± 3.10c
|
28.1 ± 0.84c
|
|
3.0 ± 0.40d
|
2.8 ± 0.15cd
|
| 40% ZnPP-P/A |
172 ±10.2b, x
|
90 ± 4.2b, y
|
|
166.6 ± 17.52b, x
|
85.1 ± 6.68b, y
|
|
8.5 ± 1.02bc
|
8.4 ± 0.24abc
|
| 60% ZnPP-P/A |
156 ± 5.5b, x
|
75 ±7.3b, y
|
|
246.4 ±14.43a, x
|
132.2 ± 4.26ab, y
|
|
13.4 ± 0.89a
|
13.3 ±0.49a
|