Submitted:
12 January 2024
Posted:
17 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Animal trypanosomiases: a global problem
2.1. The globalisation of animal trypanosomiasis
2.2. Economic Importance of Animal Trypanosomiasis
2.3. Control of Animal Trypanosomiasis
3. Veterinary trypanocides: mechanisms of action and resistance
3.1. Diamidines
3.2. Phenanthridines
3.3. Suramin
3.4. Melaminophenyl arsenicals
3.5. Quinapyramine
4. Multidrug resistance and cross-resistance between trypanocides
5. Epidemiology of veterinary trypanocide resistance: incidence and spread in the field
| Country | Animal host | Trypanosoma spp | Drug | Diagnostic | Reference |
|---|---|---|---|---|---|
| Nigeria | Cattle | T. congolense | Homidium chloride | Test in cattle | Jones-Davies and Folkers, 1966 |
| Cattle | T. congolense | DA, ISM | Test in cattle | Na’Isa, 1967 | |
| Cattle | T. congolense | Homidium chloride | Test in mice | Joshua, 1988 | |
| Cattle | T. b brucei | Homidium chloride, DA | Test in mice | Joshua, 1988 | |
| Dog | T. b brucei | DA, pentamidine | Test in rats | Anene et al., 2006 | |
| Cattle | T. vivax | Homidium chloride | Test in sheep | Ogbaje et al., 2015 | |
| Cattle | T. vivax | ISM, DA, Homidium chloride | Test in sheep | Ogbaje et al., 2015 | |
| Dog | T. congolense, T. b brucei | DA, ISM | Test in mice | Obi et al., 2022 | |
| Ivory Coast | Cattle | T. congolense, T. vivax | Trypamidium, Homidium | (Küpper and Wolters, 1983) | |
| Burkina Faso/ Upper Volta | Cattle | T. congolense | DA, ISM | Test in mice | Authie et al., 1984 |
| T. congolense | ISM | Test in mice | Pinder and Authie, 1984 | ||
| Cattle | T. congolense | DA, ISM, Homidium | Test in goats and cattle | Clausen et al., 1992 | |
| Cattle | T. vivax | DA, ISM | Test in cattle | Sow et al., 2012 | |
| Cattle | T. congolense | ISM | DIIT, Standard mouse test | Knoppe et al., 2006 | |
| Senegal | Cattle | T. vivax | DA, Ethidium | Test in goat | Diaité et al., 1997 |
| Somalia | cattle | T. congolense | DA, ISM, | Test in mice and cattle | Ainanshe et al., 1992 |
| T. vivax | ISM, homidium and quinapyramine | Schönefeld et al., 1987 | |||
| Kenya | T. vivax | ISM, homidium and quinapyramine | Schönefeld et al., 1987 | ||
| Camel | T. evansi | ISM, homidium and quinapyramine | Single dose RCT in mice | Mdachi et al., 2023 | |
| Camel | T. evansi | Quinapyramine prosalt, melarsomine, ISM | Test in mice | Waitumbi et al., 1994 | |
| Cattle | T. congolense | DA, Homidium | Test in mice | Okello et al., 2022a | |
| Tanzania | Cattle | T. congolense | DA | Test in mice and cattle | Mbwambo, Mella and Lekaki, 1988 |
| Uganda | Cattle | T. brucei, T. vivax | Homidium | Test in mice, goats cattle | Olila et al., 2002 |
| Zambia | Cattle | T. brucei, T. congolense, T. vivax | ISM, DA | Test in mice and cattle | Sinyangwe et al., 2004 |
| Sudan | Cattle | T. brucei, T. congolense, T. vivax | Homidium bromide, ISM, DA | Test in goats | Mohamed-Ahmed et al., 1992 |
| Cattle | T. brucei, T. congolense, T. vivax | Homidium bromide | Test in sheep, goats and cattle | Abdel Gadir et al., 1981 | |
| Camel | T. evansi | Suramin, QPR | hypoxanthine incorporation test, in vivo drug sensitivity test, mouse | El Rayah et al., 1999 | |
| Ethiopia | Cattle | T. congolense | DA, ISM, Homidium | Block treatment, mouse test, BCT | Mulugeta et al., 1997 |
| cattle | T. congolense | DA, ISM | Block treatment, single dose mouse test | Afewerk et al., 2000 | |
| Donkeys | T. congolense | ISM | BCT, mice | Assefa and Abebe, 2001 | |
| Cattle | T. congolense | DA, ISM | In vivo test in mice and cattle | Chaka and Abebe, 2003 | |
| Cattle, goats | T. vivax | DA, ISM | Block treatment (goats) | W/yohannes et al., 2010 | |
| Cattle | T. vivax | DA, ISM | Block treatment (cattle) | Dagnachew et al., 2015 | |
| Cattle | T.congolense, T. vivax | DA, ISM | Block treatment (cattle) | Dagnachew et al., 2017 | |
| Cattle | T.congolense, T. brucei, T. vivax | DA, ISM | Block treatment (cattle) | Degneh et al., 2019 | |
| Mozambique | cattle | T. congolense | DA, ISM, Homidium | Multi-dose mouse test, BCT | Jamal et al., 2005 |
| Cattle | T. congolense | DA, ISM | Block treatment in cattle | Mulandane et al., 2018 | |
| Cameroun | cattle | T. congolense, T. b. brucei | DA, ISM | PCR-RFLP, AS-PCR, single-dose mouse test | Mamoudou et al., 2008 |
| Zambia, Zimbabwe, South Africa | Buffalo, tsetse fly | DA | PCR, MC, Mouse test | Chitanga et al., 2011 | |
| Mali | Cattle | T. congolense, T, vivax | DA, ISM | MIC | Mungube et al., 2012 |
| Togo | Togo | T. vivax | DA, ISM | Test in goat | Boma et al., 2022 |
| French Guyana | Cattle | T. vivax | DIM | Test in cattle and sheep | Desquesnes et al., 1995 |
| China | Buffalo, horse, mule, camel | T. evansi | Suramin and Quinapyramine prosalt | Growth inhibition test, single-dose mouse test | Zhou et al., 2004 |
| Buffaloes, horses, camels, mules | T. evansi | Quinapyramine prosalt | Liao and Shen, 2010 | ||
| Philippines | Water buffaloes | T. evansi | Likely P2/AT1-related drugs | PCR showed P2/AT1 deletion | Mingala et al., 2019 |
6. Strategies to combat the challenge of trypanocide resistance
6.1. Tackling substandard trypanocides
6.2. Rational drug use
7. Optimising use of the existing trypanocides
7.1. Adjustment of dosage and dosage regimen
7.2. Enhancement of drug delivery
7.3. Sanative pair
7.4. Combination therapy
7.5. Use of vitamins and immune modulators
7.6. Use of drug Resistance Modulators
8. Drug Repurposing
9. Avoiding cross-resistance in new trypanocides
10. Conclusion
Acknowledgments
References
- Abdel Gadir, F. , Osman, O.M., Abdalla, H.S., Abdel Razig, M.T., 1981. Ethidium bromide-resistant trypanosomes in southern Darfur. Sudan J. Vet. Res. 3, 63–65.
- Adegoke, O. Adebayo, Chukwu I. Johnpaul, Anichukwu Faith Nnenna, 2018. Effect of Vitamin E on glutathione reductase and reduced glutathione levels of male wistar albino rats infected with Trypanosoma brucei brucei. Int. J. Biomed. Adv. Res. 9, 371–375.
- Afewerk, Y. , Clausen, P.-H., Abebe, G., Tilahun, G., Mehlitz, D., 2000. Multiple-drug resistant Trypanosoma congolense populations in village cattle of Metekel district, north-west Ethiopia. Acta Trop. 76, 231–238.
- Afework, Y. , Mäser, P., Etschmann, B., von Samson-Himmelstjerna, G., Zessin, K.-H., Clausen, P.-H., 2006. Rapid identification of isometamidium-resistant stocks of Trypanosoma b. brucei by PCR–RFLP. Parasitol. Res. 99, 253–261.
- Ainanshe, O.A. , Jennings, F.W., Holmes, P.H., 1992. Isolation of drug-resistant strains of Trypanosoma congolense from the Lower Shabelle Region of southern Somalia. Trop. Anim. Health Prod. 24, 65–73.
- Akama, T. , Zhang, Y.-K., Freund, Y.R., Berry, P., Lee, J., Easom, E.E., Jacobs, R.T., Plattner, J.J., Witty, M.J., Peter, R., Rowan, T.G., Gillingwater, K., Brun, R., Nare, B., Mercer, L., Xu, M., Wang, J., Liang, H., 2018. Identification of a 4-fluorobenzyl l-valinate amide benzoxaborole (AN11736) as a potential development candidate for the treatment of Animal African Trypanosomiasis (AAT). Bioorg. Med. Chem. Lett. 28, 6–10.
- Al-Salabi, M.I. , Wallace, L.J., Luscher, A., Maser, P., Candlish, D., Rodenko, B., Gould, M.K., Jabeen, I., Ajith, S.N., De Koning, H.P., 2007. Molecular interactions underlying the unusually high adenosine affinity of a novel Trypanosoma brucei nucleoside transporter. Mol. Pharmacol. 71, 921–929.
- Aldfer, M.M. , AlSiari, T.A., Elati, H.A.A., Natto, M.J., Alfayez, I.A., Campagnaro, G.D., Sani, B., Burchmore, R.J.S., Diallinas, G., De Koning, H.P., 2022. Nucleoside Transport and Nucleobase Uptake Null Mutants in Leishmania mexicana for the Routine Expression and Characterization of Purine and Pyrimidine Transporters. Int. J. Mol. Sci. 23, 8139.
- Alghamdi, A.H. , Munday, J.C., Campagnaro, G.D., Gurvic, D., Svensson, F., Okpara, C.E., Kumar, A., Quintana, J., Abril, M.E.M., Milić, P., Watson, L., Paape, D., Settimo, L., Dimitriou, A., Wielinska, J., Smart, G., Anderson, L.F., Woodley, C.M., Kelly, S.P.Y., Ibrahim, H.M.S., Hulpia, F., Al-Salabi, M.I., Eze, A.A., Sprenger, T., Teka, I.A., Gudin, S., Weyand, S., Field, M., Dardonville, C., Tidwell, R.R., Carrington, M., O’neill, P., Boykin, D.W., Zachariae, U., De Koning, H.P., 2020. Positively selected modifications in the pore of TBAQP2 allow pentamidine to enter Trypanosoma brucei. eLife 9, e56416.
- Alibu, V.P. , Richter, C., Voncken, F., Marti, G., Shahi, S., Renggli, C.K., Seebeck, T., Brun, R., Clayton, C., 2006. The role of Trypanosoma brucei MRPA in melarsoprol susceptibility. Mol. Biochem. Parasitol. 146, 38–44.
- Allen, C.L. , 2003. Clathrin-mediated endocytosis is essential in Trypanosoma brucei. EMBO J. 22, 4991–5002.
- Alsford, S. , Eckert, S., Baker, N., Glover, L., Sanchez-Flores, A., Leung, K.F., Turner, D.J., Field, M.C., Berriman, M., Horn, D., 2012. High-throughput decoding of antitrypanosomal drug efficacy and resistance. Nature 482, 232–236.
- Anene, B.M. , Onah, D.N., Nawa, Y., 2001. Drug resistance in pathogenic African trypanosomes: what hopes for the future? Vet. Parasitol. 96, 83–100.
- Anene, B.M. , Ezeokonkwo, R.C., Mmesirionye, T.I., Tettey, J.N.A., Brock, J.M., Barrett, M.P., De Koning, H.P., 2006. A diminazene-resistant strain of Trypanosoma brucei brucei isolated from a dog is cross-resistant to pentamidine in experimentally infected albino rats. Parasitology 132, 127–133.
- Anene, B.M. , Ross, C.A., Anika, S.M., Chukwu, C.C., 1996. Trypanocidal resistance in Trypanosoma evansi in vitro: effects of verapamil, cyproheptidine, desipramine and chlorpromazine alone and in combination with trypanocides. Vet. Parasitol. 62, 43–50.
- Apted, F.I.C. , 1970. Treatment of human trypanosomiasis. In: Mulligan, H.W. (Ed.), The African Trypanosomiases. George Allen and Unwin Ltd, pp. 684–710.
- Ardelli, B.F. , Woo, P.T.K., 2001. The in vitro effects of isometamidium chloride (Samorin) on the piscine hemoflagellate Cryptobia salmositica (Kinetoplastida, Bodonina). J. Parasitol. 87, 194–202.
- Arellano-Sota, C. , Arellano-Sota, C., 1988. Biology, ecology, and control of the vampire bat. Rev. Infect. Dis. 10, S615–S619.
- Arias, J.L. , Unciti-Broceta, J.D., Maceira, J., del Castillo, T., Hernández-Quero, J., Magez, S., Soriano, M., García-Salcedo, J.A., 2015. Nanobody conjugated PLGA nanoparticles for active targeting of African Trypanosomiasis. J. Control. Release 197, 190–198.
- Asghari, M.M. , Rassouli, M., 2022. First identification of Trypanosoma vivax among camels (Camelus dromedarius) in Yazd, central Iran, jointly with Trypanosoma evansi. Parasitol. Int. 86, 102450.
- Assefa, E. , Abebe, G., 2001. Drug-resistant Trypanosoma congolense in naturally infected donkeys in north Omo Zone, southern Ethiopia. Vet. Parasitol. 99, 261–271.
- Assefa, S. , Shibeshi, W., 2018. Drug resistance in African animal trypanosomes: a review. African J. Microbiol. Res. 12, 380–386.
- Autheman, D. , Crosnier, C., Clare, S., Goulding, D.A., Brandt, C., Harcourt, K., Tolley, C., Galaway, F., Khushu, M., Ong, H., Romero-Ramirez, A., Duffy, C.W., Jackson, A.P., Wright, G.J., 2021. An invariant Trypanosoma vivax vaccine antigen induces protective immunity. Nature 595, 96–100.
- Authie, E. , Somda, B., Dossama, M., Toutou, P., 1984. [Demonstration of resistance to trypanocides among the species of Trypanosoma congolense recently isolated in Burkina]. Rev. Elev. Med. Vet. Pays Trop. 37 Spec No, 219–235.
- Bacchi, C.J. , Lambros, C., Ellenbogen, B.B., Penkovsky, L.N., Sullivan, W., Eyinna, E.E., Hutner, S.H., 1975. Drug-Resistant Leptomonas : Cross-Resistance in Trypanocide-Resistant Clones. Antimicrob. Agents Chemother. 8, 688–692.
- Baker, N. , De Koning, H.P., Mäser, P., Horn, D., 2013. Drug resistance in African trypanosomiasis: the melarsoprol and pentamidine story. Trends Parasitol. 29, 110–118.
- Baker, N. , Glover, L., Munday, J.C., Aguinaga Andrés, D., Barrett, M.P., De Koning, H.P., Horn, D., 2012. Aquaglyceroporin 2 controls susceptibility to melarsoprol and pentamidine in African trypanosomes. Proc. Natl. Acad. Sci. 109, 10996–11001.
- Baker, N. , Hamilton, G., Wilkes, J.M., Hutchinson, S., Barrett, M.P., Horn, D., 2015. Vacuolar ATPase depletion affects mitochondrial ATPase function, kinetoplast dependency, and drug sensitivity in trypanosomes. Proc. Natl. Acad. Sci. 112, 9112–9117.
- Barr, S.C. , 2009. Canine Chagas’ Disease (American Trypanosomiasis) in North America. Vet. Clin. North Am. Small Anim. Pract. 39, 1055–1064.
- Barrett, M.P. , Burchmore, R.J.S., Stich, A., Lazzari, J.O., Frasch, A.C., Cazzulo, J.J., Krishna, S., 2003. The trypanosomiases. In: Lancet. pp. 1469–1480.
- Barrett, M.P. , Zhang, Z.Q., Denise, H., Giroud, C. and Baltz, T., 1995. A diamidine-resistant Trypanosoma equiperdum clone contains a P2 purine transporter with reduced substrate affinity. Mol. Biochem. Parasitol. 73, 223–229.
- Bengaly, Z. , Vitouley, S.H., Somda, M.B., Zongo, A., Têko-Agbo, A., Cecchi, G., Adam, Y., Sidibé, I., Bayala, B., Belem, A.M.G., Van Den Abbeele, J., Delespaux, V., 2018. Drug quality analysis of isometamidium chloride hydrochloride and diminazene diaceturate used for the treatment of African animal trypanosomosis in West Africa. BMC Vet. Res. 14, 361.
- Berg, M. , Kohl, L., Van der Veken, P., Joossens, J., Al-Salabi, M.I., Castagna, V., Giannese, F., Cos, P., Versées, W., Steyaert, J., Grellier, P., Haemers, A., Degano, M., Maes, L., De Koning, H.P., Augustyns, K., 2010. Evaluation of Nucleoside Hydrolase Inhibitors for Treatment of African Trypanosomiasis. Antimicrob. Agents Chemother. 54, 1900–1908.
- Berg, S.S. , 1960. Structure of Isometamidium (M. and B. 4180A), 7-m-Amidinophenyldiazoamino-2-amino-10-ethyl-9-phenylphenanthridinium Chloride Hydrochloride, the Red Isomer present in Metamidium. Nature 188, 1106–1107.
- Carter, N.S. , Barrett, M.P., De Koning, H.P. 1999. A drug resistance determinant from Trypanosoma brucei. Trends Microbiol. 7, 469–471.
- Berger, B. J, Carter, N.S., Fairlamb, A.H. 1995. Characterisation of pentamidine-resistant Trypanosoma brucei brucei. Mol. Biochem. Parasitol. 69, 289–298.
- Berger, B.J. , Fairlamb, A.H., 1994. Properties of melarsamine hydrochloride (Cymelarsan) in aqueous solution. Antimicrob. Agents Chemother. 38, 1298–1302.
- Birhanu, H. , Gebrehiwot, T., Goddeeris, B.M., Büscher, P., Van Reet, N., 2016. New Trypanosoma evansi Type B Isolates from Ethiopian Dromedary Camels. PLoS Negl. Trop. Dis. 10, e0004556.
- Bisser, S. , N’Siesi, F., Lejon, V., Preux, P., Van Nieuwenhove, S., Miaka Mia Bilenge, C., Büscher, P., 2007. Equivalence Trial of Melarsoprol and Nifurtimox Monotherapy and Combination Therapy for the Treatment of Second-Stage Trypanosoma brucei gambiense Sleeping Sickness. J. Infect. Dis. 195, 322–329.
- Boibessot, I. , Turner, C.M.R., Watson, D.G., Goldie, E., Connel, G., McIntosh, A., Grant, M.H., Skellern, G.G., 2002. Metabolism and distribution of phenanthridine trypanocides in Trypanosoma brucei. Acta Trop. 84, 219–228.
- Boma, S. , Vitouley, S.H., Somda, M.B., Bengaly, Z., Houaga, I., Lombo, Y., Tchamdja, E., Dayo, G.-K., 2022. In vivo analysis of trypanocidal drug resistance in sahelian goats infected by Trypanosoma vivax strains collected in northern Togo. Vet. Parasitol. 306, 109723.
- Brack, C. , Delain, E., 1975. Electron-microscopic mapping of AT-rich regions and of E. coli RNA polymerase-binding sites on the circular kinetoplast DNA of Trypanosoma cruzi. J. Cell Sci. 17, 287–306.
- Brack, C. , Delain, E., Riou, G., Festy, B., 1972. Molecular organization of the kinetoplast DNA of Trypanosoma cruzi treated with berenil, a DNA interacting drug. J. Ultrastruct. Res. 39, 568–579.
- Bridges, D.J. , Gould, M.K., Nerima, B., Mäser, P., Burchmore, R.J.S., De Koning, H.P., 2007. Loss of the High-Affinity Pentamidine Transporter Is Responsible for High Levels of Cross-Resistance between Arsenical and Diamidine Drugs in African Trypanosomes. Mol. Pharmacol. 71, 1098–1108.
- Browning, C. , Gilmour, W., 1913. Bactericidal action and chemical constitution with special reference to basic benzol derivatives. J. Pathol. Bacteriol. 18, 144–145.
- Browning, C.H. , 1949. Chemotherapy of T. congolense Infections with Phenanthridine Compounds : Biological Aspects. Nature 163, 590–591.
- Browning, C.H. , Morgan, G.T., Robb, J.V.M., Walls, L.P., 1938. The trypanocidal action of certain phenanthridinium compounds. J. Pathol. Bacteriol. 46, 203–204.
- Brun, R. , Hecker, H., Lun, Z.R., 1998. Trypanosoma evansi, 79.
- Brun, R. , Lun, Z.-R., 1994. Drug sensitivity of Chinese Trypanosoma evansi and Trypanosoma equiperdum isolates. Vet. Parasitol. 52, 37–46.
- Burri, C. , Brun, R., 2003. Eflornithine for the treatment of human African trypanosomiasis. Parasitol. Res. 90, S49–S52.
- Carruthers, L. V. , Munday, J.C., Ebiloma, G.U., Steketee, P., Jayaraman, S., Campagnaro, G.D., Ungogo, M.A., Lemgruber, L., Donachie, A., Rowan, T.G., Peter, R., Morrison, L.J., Barrett, M.P., De Koning, H.P., 2021. Diminazene resistance in Trypanosoma congolense is not caused by reduced transport capacity but associated with reduced mitochondrial membrane potential. Mol. Microbiol. 116, 564–588.
- Carter, N.S. , Berger, B.J., Fairlamb, A.H., 1995. Uptake of diamidine drugs by the P2 nucleoside transporter in melarsen- sensitive and -resistant Trypanosoma brucei brucei. J. Biol. Chem. 270, 28153–28157.
- Carter, N.S. , Fairlamb, A.H., 1993. Arsenical-resistant trypanosomes lack an unusual adenosine transporter. Nature 361, 173–176.
- Catley, A. , Leyland, T., 2001. Community participation and the delivery of veterinary services in Africa. Prev. Vet. Med. 49, 95–113.
- Chaka, H. , Abebe, G., 2003. Drug resistant trypanosomes: a threat to cattle production in the Southwest of Ethiopia. Rev. Elev. Med. Vet. Pays Trop. 56, 33–3.
- Cheesman, M. , Ilanko, A., Blonk, B., Cock, I., 2017. Developing new antimicrobial therapies: Are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacogn. Rev. 11, 57–72.
- Chello, P.L. , Jaffe, J.J., 1972. Isolation, Partial Purification, and Properties of Thymidine Kinase from Trypanosoma (Trypanozoon) brucei rhodesiense. J. Parasitol. 58, 298–305.
- Chitanga, S. , Marcotty, T., Namangala, B., Van den Bossche, P., Van Den Abbeele, J., Delespaux, V., 2011. High Prevalence of Drug Resistance in Animal Trypanosomes without a History of Drug Exposure. PLoS Negl. Trop. Dis. 5, e1454.
- Chusri, S. , Villanueva, I., Voravuthikunchai, S.P., Davies, J., 2009. Enhancing antibiotic activity: a strategy to control Acinetobacter infections. J. Antimicrob. Chemother. 64, 1203–1211.
- Clausen, P.-H. , Bauer, B., Zessin, K.-H., Diall, O., Bocoum, Z., Sidibe, I., Affognon, H., Waibel, H., Grace, D., Randolph, T., 2010. Preventing and Containing Trypanocide Resistance in the Cotton Zone of West Africa. Transbound. Emerg. Dis. 57, 28–32.
- Clausen, P.-H. , Sidibe, I., Kabore´, I., Bauer, B., 1992. Development of multiple drug resistance of Trypanosoma congolense in Zebu cattle under high natural tsetse fly challenge in the pastoral zone of Samorogouan, Burkina Faso. Acta Trop. 51, 229–236.
- Coelho, A.C. , Beverley, S.M., Cotrim, P.C., 2003. Functional genetic identification of PRP1, an ABC transporter superfamily member conferring pentamidine resistance in Leishmania major. Mol. Biochem. Parasitol. 130, 83–90.
- Coppens, I. , Opperdoes, F.R., Courtoy, P.J., Baudhuin, P., 1987. Receptor-Mediated Endocytosis in the Bloodstream Form of Trypanosoma brucei 1. J. Protozool. 34, 465–473.
- Dagnachew, S. , Terefe, G., Abebe, G., Barry, D., McCulloch, R., Goddeeris, B., 2015. In vivo experimental drug resistance study in Trypanosoma vivax isolates from tsetse infested and non-tsetse infested areas of Northwest Ethiopia. Acta Trop. 146, 95–100.
- Dagnachew, S. , Tsegaye, B., Awukew, A., Tilahun, M., Ashenafi, H., Rowan, T., Abebe, G., Barry, D.J., Terefe, G., Goddeeris, B.M., 2017. Prevalence of bovine trypanosomosis and assessment of trypanocidal drug resistance in tsetse infested and non-tsetse infested areas of Northwest Ethiopia. Parasite Epidemiol. Control 2, 40–49.
- Dame, D.A. , Schmidt, C.H., 1970. The Sterile-Male Technique Against Tsetse Flies, Glossina Spp. Bull. Entomol. Soc. Am. 16, 24–30.
- Damper, D, Patton, C.L., 1976. Pentamidine transport and sensitivity in brucei-group trypanosomes. J. Protozool. 23, 349–56.
- Damper, Dianne, Patton, C.L., 1976. Pentamidine transport in Trypanosoma brucei—Kinetics and specificity. Biochem. Pharmacol. 25, 271–276.
- Das, B.P. , Boykin, D.W., 1977. Synthesis and antiprotozoal activity of 2,5-bis(4-guanylphenyl)furans. J. Med. Chem. 20, 531–536.
- Davey, D.G. , 1950. Experiments with “antrycide” in the Sudan and East Africa. Trans. R. Soc. Trop. Med. Hyg. 43, 583–616.
- Dávila, A.M.R. , Silva, R.A.M.S., 2006. Animal Trypanosomiasis in South America: Current Status, Partnership, and Information Technology. Ann. N. Y. Acad. Sci. 916, 199–212.
- Davkharbayar, B. , Davaasuren, B., Narantsatsral, S., Battur, B., Punsantsogvoo, M., Battsetseg, B., Mizushima, D., Inoue, N., Suganuma, K., 2020. Treatment Efficiency of Combination Therapy With Diminazene Aceturate and Quinapyramine Sulfate in a Horse With Dourine. J. Equine Vet. Sci. 87, 102905.
- De Deken, R. , Geerts, S., Kageruka, P., Ceulemans, F., Brandt, J., Schacht, E., Pascucci, C., Lootens, C., 1989. Chemoprophylaxis of trypanosomiasis, due to Trypanosoma (Nannomonas) congolense, in rabbits using a slow release device containing homidium bromide. Ann. Soc. Belg. Med. Trop. (1920). 69, 291–296.
- De Koning, H.P. , 2001b. Transporters in African trypanosomes: role in drug action and resistance. Int. J. Parasitol. 31, 512–522.
- De Koning, H.P. , 2017. Drug resistance in protozoan parasites. Emerg. Top. Life Sci. 1, 627–632.
- De Koning, H. , 2020. The Drugs of Sleeping Sickness: Their Mechanisms of Action and Resistance, and a Brief History. Trop. Med. Infect. Dis. 5, 14.
- De Koning, H.P. , 2001a. Uptake of Pentamidine in Trypanosoma brucei brucei is Mediated by Three Distinct Transporters: Implications for Cross-Resistance with Arsenicals. Mol. Pharmacol. 59, 586–592.
- De Koning, H.P. , Anderson, L.F., Stewart, M., Burchmore, R.J.S., Wallace, L.J.M., Barrett, M.P., 2004. The Trypanocide Diminazene Aceturate Is Accumulated Predominantly through the TbAT1 Purine Transporter: Additional Insights on Diamidine Resistance in African Trypanosomes. Antimicrob. Agents Chemother. 48, 1515–1519.
- De Koning, H.P. , Jarvis, S.M., 1999. Adenosine Transporters in Bloodstream Forms of Trypanosoma brucei brucei : Substrate Recognition Motifs and Affinity for Trypanocidal Drugs. Mol. Pharmacol. 56, 1162–1170.
- De Koning, H.P. , Jarvis, S.M., 2001. Uptake of pentamidine in Trypanosoma brucei brucei is mediated by the P2 adenosine transporter and at least one novel, unrelated transporter. Acta Trop. 80, 245–250.
- De Koning, H.P. , MacLeod, A., Barrett, M.P., Cover, B., Jarvis, S.M., 2000. Further evidence for a link between melarsoprol resistance and P2 transporter function in African trypanosomes. Mol. Biochem. Parasitol. 106, 181–185.
- de Noya, B.A. , González, O.N., 2015. An ecological overview on the factors that drives to Trypanosoma cruzi oral transmission. Acta Trop. 151, 94–102.
- Degneh, E. , Ashenafi, H., Kassa, T., Kebede, N., Shibeshi, W., Asres, K., Terefe, G., 2019. Trypanocidal drug resistance: A threat to animal health and production in Gidami district of Kellem Wollega Zone, Oromia Regional State, Western Ethiopia. Prev. Vet. Med. 168, 103–107.
- Delespaux, V. , Chitanga, S., Geysen, D., Goethals, A., Van den Bossche, P., Geerts, S., 2006. SSCP analysis of the P2 purine transporter TcoAT1 gene of Trypanosoma congolense leads to a simple PCR-RFLP test allowing the rapid identification of diminazene resistant stocks. Acta Trop. 100, 96–102.
- Delespaux, V. , De Koning, H., 2007. Drugs and drug resistance in African trypanosomiasis. Drug Resist. Updat. 10, 30–50.
- Delespaux, V. , Geerts, S., Brandt, J., Elyn, R., Eisler, M.C., 2002. Monitoring the correct use of isometamidium by farmers and veterinary assistants in Eastern Province of Zambia using the isometamidium-ELISA. Vet. Parasitol. 110, 117–122.
- Delespaux, V. , Geysen, D., Geerts, A.S., 2007. Point mutations in mitochondrial topoisomerase enzymes of Trypanosoma congolense are not involved in isometamidium resistance. Mol. Biochem. Parasitol. 151, 137–140.
- Delespaux, V. , Geysen, D., Majiwa, P.A.O., Geerts, S., 2005. Identification of a genetic marker for isometamidium chloride resistance in Trypanosoma congolense. Int. J. Parasitol. 35, 235–243.
- Delespaux, V. , Geysen, D., Van den Bossche, P., Geerts, S., 2008. Molecular tools for the rapid detection of drug resistance in animal trypanosomes. Trends Parasitol. 24, 236–42.
- Delespaux, V. , Vitouley, H.S., Marcotty, T., Speybroeck, N., Berkvens, D., Roy, K., Geerts, S., Van den Bossche, P., 2010. Chemosensitization of Trypanosoma congolense Strains Resistant to Isometamidium Chloride by Tetracyclines and Enrofloxacin. PLoS Negl. Trop. Dis. 4, e828.
- Desquesnes, M. , (de) La Rocque, S., Peregrine, A.S., 1995. French guyanan stock of Trypanosoma vivax resistant to diminazene aceturate but sensitive to isometamidium chloride. Acta Trop. 60, 133–136.
- Desquesnes, M. , Dargantes, A., Lai, D.-H., Lun, Z.-R., Holzmuller, P., Jittapalapong, S., 2013. Trypanosoma evansi and Surra: A Review and Perspectives on Transmission, Epidemiology and Control, Impact, and Zoonotic Aspects. Biomed Res. Int. 2013, 1–20.
- Desquesnes, M. , Dia, M.L., 2003. Trypanosoma vivax: mechanical transmission in cattle by one of the most common African tabanids, Atylotus agrestis. Exp. Parasitol. 103, 35–43.
- Desquesnes, M. , Gonzatti, M., Sazmand, A., Thévenon, S., Bossard, G., Boulangé, A., Gimonneau, G., Truc, P., Herder, S., Ravel, S., Sereno, D., Jamonneau, V., Jittapalapong, S., Jacquiet, P., Solano, P., Berthier, D., 2022. A review on the diagnosis of animal trypanosomoses. Parasit. Vectors 15, 64.
- Diaité, A. , Seye, M., Mane, A., Ndiaye, T., Seye, M.M., 1997. Detection and characterisation of trypanosome strains supposedly resistant to trypanocidal drugs In Senegal. In: Workshop on Epidemiological Tools for Monitoring Trypanosomosis and Tsetse Control Programmes. p. IAEA-TECDOC--925.
- Dickie, E.A. , Giordani, F., Gould, M.K., Mäser, P., Burri, C., Mottram, J.C., Rao, S.P.S., Barrett, M.P., 2020. New Drugs for Human African Trypanosomiasis: A Twenty First Century Success Story. Trop. Med. Infect. Dis. 5, 29.
- Ding, D. , Zhao, Y., Meng, Q., Xie, D., Nare, B., Chen, D., Bacchi, C.J., Yarlett, N., Zhang, Y.-K., Hernandez, V., Xia, Y., Freund, Y., Abdulla, M., Ang, K.-H., Ratnam, J., McKerrow, J.H., Jacobs, R.T., Zhou, H., Plattner, J.J., 2010. Discovery of Novel Benzoxaborole-Based Potent Antitrypanosomal Agents. ACS Med. Chem. Lett. 1, 165–169.
- DNDi (2023) EMA gives positive opinion to Fexinidazole Winthrop as first oral treatment of acute form of sleeping sickness (rhodesiense) found in East and Southern Africa. Press release, available at https://dndi.org/press-releases/2023/ema-gives-positive-opinion-fexinidazole-winthrop-first-oral-treatment-sleeping-sickness-rhodesiense/ (accessed 16/12/2023).
- Doherty, G.J. , McMahon, H.T., 2009. Mechanisms of Endocytosis. Annu. Rev. Biochem. 78, 857–902.
- Dressel, J. 1961. The discovery of Germanin by Oskar Dressel and Richard Kothe. J. Chem. Educ. 38, 620.
- Dukes, P. , 1984. Arsenic and old taxa: subspeciation and drug sensitivity in Trypanosoma brucei. Trans. R. Soc. Trop. Med. Hyg. 78, 711–725.
- Dwinger, R.H. , Agyemang, K., Snow, W.F., Rawlings, P., Leperre, P., Bah, M.L., 1994. Productivity of trypanotolerant cattle kept under traditional management conditions in the Gambia. Vet. Q. 16, 81–86.
- Eghianruwa, K.I. , Oridupa, O.A., 2018. Chemotherapeutic control of trypanosomosis - A review of past measures, current status and future trends. Vet. Arh. 88, 245–270.
- Eisler, M.C. , Brandt, J., Bauer, B., Clausen, P.-H., Delespaux, V., Holmes, P.H., Ilemobade, A., Machila, N., Mbwambo, H., McDermott, J., Mehlitz, D., Murilla, G., Ndung’u, J.M., Peregrine, A.S., Sidibé, I., Sinyangwe, L., Geerts, S., 2001. Standardised tests in mice and cattle for the detection of drug resistance in tsetse-transmitted trypanosomes of African domestic cattle. Vet. Parasitol. 97, 171–183.
- Eke, I.G. , Ezeh, I.O., Ezeudu, T.A., Eze, U.U., Anaga, A.O., Onyeyili, P.A., 2017. Anti-trypanosomal activity of secnidazole in vitro and in vivo. Trop. J. Pharm. Res. 16, 535–541.
- Eke, I.G. , Ezeh, I.O., Ezeudu, T.A., Eze, U.U., Anaga, A.O., Onyeyili, P.A., 2020. Efficacy of secnidazole-diminazene aceturate combination therapy in the late treatment of Trypanosoma brucei brucei infection in dogs. Brazilian J. Pharm. Sci. 56.
- El Rayah, I.E. , Kaminsky, R., Schmid, C., El Malik, K.H., 1999. Drug resistance in Sudanese Trypanosoma evansi. Vet. Parasitol. 80, 281–287.
- Elelu, N. , 2017. Assessment of veterinary drug retail outlets in two rural areas of Kwara state, north-central Nigeria. Sokoto J. Vet. Sci. 15, 54.
- Ewins, A.J. 1944. Chemotherapy in Tropical Medicine: A lecture delivered before the Chemical Society on th, 1944. J. Chem. Soc., 1944, 351–355. 17 February.
- Eze, A.A. , Gould, M.K., Munday, J.C., Tagoe, D.N.A., Stelmanis, V., Schnaufer, A., De Koning, H.P., 2016. Reduced Mitochondrial Membrane Potential Is a Late Adaptation of Trypanosoma brucei brucei to Isometamidium Preceded by Mutations in the γ Subunit of the F1Fo-ATPase. PLoS Negl. Trop. Dis. 10, e0004791.
- Eze, A.A. , Igoli, J., Gray, A.I., Skellern, G.G., De Koning, H.P., 2019. The individual components of commercial isometamidium do not possess stronger trypanocidal activity than the mixture, nor bypass isometamidium resistance. Int. J. Parasitol. Drugs Drug Resist. 9, 54–58.
- Ezeh, I.O. , Ugwu, E.N., Enemuo, O. V., Obi, C.F., Iheagwam, C.N., Ezeokonkwo, R.C., Onah, D.N., 2016. Efficacy of repeated doses of diminazene aceturate (Dinazene®) in the treatment of experimental Trypanosoma brucei infection of Albino rats. Iran. J. Vet. Res. 17, 124–129.
- Fairlamb, A.H. , Bowman, I.B.R., 1980. Uptake of the trypanocidal drug suramin by bloodstream forms of Trypanosoma brucei and its effect on respiration and growth rate in vivo. Mol. Biochem. Parasitol. 1, 315–333.
- Fairlamb, A.H. , Henderson, G.B., Cerami, A., 1989. Trypanothione is the primary target for arsenical drugs against African trypanosomes. Proc. Natl. Acad. Sci. 86, 2607–2611.
- Fairlamb, A.H. , Horn, D., 2018. Melarsoprol Resistance in African Trypanosomiasis. Trends Parasitol. 34, 481–492.
- Farikou, O. , Njiokou, F., Mbida Mbida, J.A., Njitchouang, G.R., Djeunga, H.N., Asonganyi, T., Simarro, P.P., Cuny, G., Geiger, A., 2010. Tripartite interactions between tsetse flies, Sodalis glossinidius and trypanosomes—An epidemiological approach in two historical human African trypanosomiasis foci in Cameroon. Infect. Genet. Evol. 10, 115–121.
- Fetene, E. , Leta, S., Regassa, F., Büscher, P., 2021. Global distribution, host range and prevalence of Trypanosoma vivax: a systematic review and meta-analysis. Parasit. Vectors 14, 80.
- Fidalgo, L.M. , Gille, L., 2011. Mitochondria and Trypanosomatids: Targets and Drugs. Pharm. Res. 28, 2758–2770.
- Foil, L.D. , Hogsette, J.A., 1994. Biology and control of tabanids, stable flies and horn flies. Rev. Sci. Tech. l’OIE 13, 1125–1158.
- Food and Agricultural Organisation (FAO), 2012. Alliance to combat black market in counterfeit veterinary drugs.
- Ford, J. , 1970. Control by destruction of the larger fauna. In: Mulligan, H.W. (Ed.), The African Trypanosomiases. George Allen and Unwin Ltd, London, pp. 557–563.
- Ford, J. , Nash, T.A.M., Welch, J. R., 1970. Control by clearing of vegetation. In: Mulligan, H.W. (Ed.), The African Trypanosomiases. George Allen and Unwin Ltd, London, pp. 543–556.
- Fourneau, E. Tréfouel, J., Vallée, J., 1924. Recherches de chimiothérapie dans la série du Bayer 205. Urées des acides aminobenzoylaminonaphthaléniques. Ann Inst Pasteur 38, 81–114.
- Franco, J. , Scarone, L., Comini, M.A., 2018a. Drugs and Drug Resistance in African and American Trypanosomiasis. In: Annual Reports in Medicinal Chemistry. Pp. 97–133.
- Franco, J.R. , Cecchi, G., Priotto, G., Paone, M., Diarra, A., Grout, L., Simarro, P.P., Zhao, W., Argaw, D., 2018b. Monitoring the elimination of human African trypanosomiasis: Update to 2016. PloS Negl. Trop. Dis. 12, e0006890.
- Friedheim, E.A. , 1949. Mel B in the treatment of human trypanosomiasis. Am. J. Trop. Med. Hyg. 29(2), 173–180.
- Friedheim, E.A.H. , 1948. Melarsen Oxide in the Treatment of Human Trypanosomiasis. Ann. Trop. Med. Parasitol. 42, 357–363.
- Friedheim, E.A.H. , 1949. Mel B in the Treatment of Human Trypanosomiasis 1,2. Am. J. Trop. Med. Hyg. S1-29, 173–180.
- Friedheim, E.A.H. , 1951. Mel B in the Treatment of Tryparsamide Resistant T. Gambiense Sleeping Sickness: Observations on Drug Resistance in the Trypanosomes of the French Cameroun 1,2. Am. J. Trop. Med. Hyg. S1-31, 218–235.
- Gadelha, C. , Zhang, W., Chamberlain, J.W., Chait, B.T., Wickstead, B., Field, M.C., 2015. Architecture of a Host–Parasite Interface: Complex Targeting Mechanisms Revealed Through Proteomics. Mol. Cell. Proteomics 14, 1911–1926.
- Garcia, M.N. , Woc-Colburn, L., Aguilar, D., Hotez, P.J. and Murray, K.O., 2015. Historical perspectives on the epidemiology of human Chagas disease in Texas and recommendations for enhanced understanding of clinical Chagas disease in the Southern United States. PLoS Negl. Trop. Dis. 9, e0003981.
- Garcia-Salcedo, J.A. , Unciti-Broceta, J.D., Valverde-Pozo, J., Soriano, M., 2016. New Approaches to Overcome Transport Related Drug Resistance in Trypanosomatid Parasites. Front. Pharmacol. 7, 351.
- Geerts, S. , Holmes, P.H., Eisler, M.C., Diall, O., 2001. African bovine trypanosomiasis: the problem of drug resistance. Trends Parasitol. 17, 25–28.
- Geiser, F. , Lüscher, A., De Koning, H.P., Seebeck, T., Mäser, P., 2005. Molecular Pharmacology of Adenosine Transport in Trypanosoma brucei : P1/P2 Revisited. Mol. Pharmacol. 68, 589–595.
- Gill, B.S. , 1971. Resistance of Trypanosoma evansi to quinapyramine, suramin, stilbamidine and tryparsamide and analysis of cross-resistance. Trans. R. Soc. Trop. Med. Hyg. 65, 352–357.
- Giordani, F. , Khalaf, A.I., Gillingwater, K., Munday, J.C., De Koning, H.P., Suckling, C.J., Barrett, M.P., Scott, F.J., 2019. Novel Minor Groove Binders Cure Animal African Trypanosomiasis in an in Vivo Mouse Model. J. Med. Chem. 62, 3021–3035.
- Giordani, F. , Paape, D., Vincent, I.M., Pountain, A.W., Fernández-Cortés, F., Rico, E., Zhang, N., Morrison, L.J., Freund, Y., Witty, M.J., Peter, R., Edwards, D.Y., Wilkes, J.M., van der Hooft, J.J.J., Regnault, C., Read, K.D., Horn, D., Field, M.C., Barrett, M.P., 2020. Veterinary trypanocidal benzoxaboroles are peptidase-activated prodrugs. PLOS Pathog. 16, e1008932.
- Giordani, Morrison, L.J., Rowan, T.G., De Koning, H.P., Barrett, M.P., 2016. The animal trypanosomiases and their chemotherapy: a review. Parasitology 143, 1862–1889.
- Gizaw, Y. , Megersa, M., Fayera, T., 2017. Dourine: a neglected disease of equids. Trop. Anim. Health Prod. 49, 887–897.
- Gonzatti, M.I. , González-Baradat, B., Aso, P.M., Reyna-Bello, A., 2014. Trypanosoma (Duttonella) vivax and Typanosomosis in Latin America: Secadera/Huequera/Cacho Hueco. In: Trypanosomes and Trypanosomiasis. Springer Vienna, Vienna, pp. 261–285.
- Gooding, R.H. , Krafsur, E.S., 2005. Tsetse genetics: Contributions to Biology, Systematics, and Control of Tsetse Flies. Annu. Rev. Entomol. 50, 101–123.
- Gould, M.K. , Schnaufer, A., 2014. Independence from Kinetoplast DNA Maintenance and Expression Is Associated with Multidrug Resistance in Trypanosoma brucei In Vitro. Antimicrob. Agents Chemother. 58, 2925–2928.
- Grace, D. , Randolph, T., Diall, O., Clausen, P.-H., 2008. Training farmers in rational drug-use improves their management of cattle trypanosomosis: A cluster-randomised trial in south Mali. Prev. Vet. Med. 83, 83–97.
- Graf, F.E. , Baker, N., Munday, J.C., De Koning, H.P., Horn, D., Mäser, P., 2015. Chimerization at the AQP2-AQP3 locus is the genetic basis of melarsoprol-pentamidine cross-resistance in clinical Trypanosoma brucei gambiense isolates. Int. J. Parasitol. Drugs Drug Resist. 5, 65–68.
- Graf, F.E. , Ludin, P., Arquint, C., Schmidt, R.S., Schaub, N., Kunz Renggli, C., Munday, J.C., Krezdorn, J., Baker, N., Horn, D., Balmer, O., Caccone, A., De Koning, H.P., Mäser, P., 2016. Comparative genomics of drug resistance in Trypanosoma brucei rhodesiense. Cell. Mol. Life Sci. 73, 3387–3400.
- Graf, F.E. , Ludin, P., Wenzler, T., Kaiser, M., Brun, R., Pyana, P.P., Büscher, P., De Koning, H.P., Horn, D., Mäser, P., 2013. Aquaporin 2 Mutations in Trypanosoma brucei gambiense Field Isolates Correlate with Decreased Susceptibility to Pentamidine and Melarsoprol. PloS Negl. Trop. Dis. 7, e2475.
- Gresh, N. , Pullman, B., 1984. A theoretical study of the nonintercalative binding of berenil and stilbamidine to double-stranded (dA-dT)n oligomers. Mol. Pharmacol. 25, 452–8.
- Hamill, L.C. , Kaare, M.T., Welburn, S.C., Picozzi, K., 2013. Domestic pigs as potential reservoirs of human and animal trypanosomiasis in Northern Tanzania. Parasit. Vectors 6, 322.
- Hawking, F. , 1958. The Action of Berenil on Trypanosomes, Including Strains Resistant to Antrycide and to Stilbamidine. J. Comp. Pathol. Ther. 68, 295–299.
- Hawking, F. , 1963. Drug-resistance of Trypanosoma congolense and other trypanosomes to quinapyramine, phenanthridines, Berenil and other compounds in mice. Ann. Trop. Med. Parasitol. 57, 262–282.
- Hawking, F. , Sen, A.B., 1960. The trypanocidal action of homidium, quinapyramine and suramin. Br. J. Pharmacol. Chemother. 15, 567–570.
- Hébert, L. , Guitton, E., Madeline, A., Géraud, T., Zientara, S., Laugier, C., Hans, A., Büscher, P., Cauchard, J., Petry, S., 2018. Melarsomine hydrochloride (Cymelarsan®) fails to cure horses with Trypanosoma equiperdum OVI parasites in their cerebrospinal fluid. Vet. Parasitol. 264, 47–51.
- Herrera, H.M. , Dávila, A.M.R., Norek, A., Abreu, U.G., Souza, S.S., D’andrea, P.S., Jansen, A.M., 2004. Enzootiology of Trypanosoma evansi in pantanal, Brazil. Vet. Parasitol. 125, 263–275.
- Holmes, P.H. , 1997. New approaches to the integrated control of trypanosomosis. Vet. Parasitol. 71, 121–135.
- Horn, D. , 2014. Antigenic variation in African trypanosomes. Mol. Biochem. Parasitol. 195, 123–129.
- Hulpia, F. , Bouton, J., Campagnaro, G.D., Alfayez, I.A., Mabille, D., Maes, L., De Koning, H.P., Caljon, G., Van Calenbergh, S., 2020a. C6–O-alkylated 7-deazainosine nucleoside analogues: Discovery of potent and selective anti-sleeping sickness agents. Eur. J. Med. Chem. 188, 112018.
- Hulpia, F. , Campagnaro, G.D., Alzahrani, K.J., Alfayez, I.A., Ungogo, M.A., Mabille, D., Maes, L., De Koning, H.P., Caljon, G., Van Calenbergh, S., 2020b. Structure–Activity Relationship Exploration of 3′-Deoxy-7-deazapurine Nucleoside Analogues as Anti- Trypanosoma brucei Agents. ACS Infect. Dis. 6, 2045–2056.
- Hulpia, F. , Mabille, D., Campagnaro, G.D., Schumann, G., Maes, L., Roditi, I., Hofer, A., De Koning, H.P., Caljon, G., Van Calenbergh, S., 2019. Combining tubercidin and cordycepin scaffolds results in highly active candidates to treat late-stage sleeping sickness. Nat. Commun. 10, 5564.
- Igoli, J.O. , Blackburn, G., Gray, A.I., Sutcliffe, O.B., Watson, D.G., Euerby, M.R., Skellern, G.G., 2015. Chromatographic and spectroscopic analysis of the components present in the phenanthridinium trypanocidal agent isometamidium. Anal. Bioanal. Chem. 407, 1171–1180.
- Ijomanta, M.K. , Ugochukwu, C.I., Ugochukwu, E.I., 2016. Comparative effects of graded doses of diaminanzine ace-turate and fixed doses of levamisole in the treatment of albino mice experimentally infected with Trypanosoma brucei brucei. J. Exp. Appl. Anim. Sci. 2, 59–70.
- Jackson, A.P. , Allison, H.C., Barry, J.D., Field, M.C., Hertz-Fowler, C., Berriman, M., 2013. A Cell-surface Phylome for African Trypanosomes. PloS Negl. Trop. Dis. 7, e2121.
- Jacobi, E.A. , 2010. Das Schlafkrankheitsmedikament Germanin als Propagandainstrument: Rezeption in Literatur und Film zur Zeit des Nationalsozialismus. Wurzbg Medizinhist Mitt 29, 43–72.
- Jacobs, R.T. , Nare, B., Wring, S.A., Orr, M.D., Chen, D., Sligar, J.M., Jenks, M.X., Noe, R.A., Bowling, T.S., Mercer, L.T., Rewerts, C., Gaukel, E., Owens, J., Parham, R., Randolph, R., Beaudet, B., Bacchi, C.J., Yarlett, N., Plattner, J.J., Freund, Y., Ding, C., Akama, T., Zhang, Y.-K., Brun, R., Kaiser, M., Scandale, I., Don, R., 2011. SCYX-7158, an Orally-Active Benzoxaborole for the Treatment of Stage 2 Human African Trypanosomiasis. PloS Negl. Trop. Dis. 5, e1151.
- Jacobs, W.A. , Heidelberger, M., 1919. Aromatic arsenic compounds. V. N-substituted glycylarsanilic acids. J. Am. Chem. Soc. 41, 1809–1821.
- Jamal, R. , Shimogawara, R., Yamamoto, K., Ohta, N., 2016. Anti-trypanosome effects of nutritional supplements and vitamin D3: in vitro and in vivo efficacy against Trypanosoma brucei brucei. Trop. Med. Health 44, 26.
- Jamal, S. , Sigauque, I., Macuamule, C., Neves, L., Marcotty, T., Penzhorn, B.L., Van den Bossche, P., 2005. The susceptibility of Trypanosoma congolense isolated in Zambézia Province, Mozambique, to isometamidium chloride, diminazene aceturate and homidium chloride. Onderstepoort J Vet Res 72.
- Jennings, F.W. , 1990. Future prospects for the chemotherapy of human trypanosomiasis. Trans. R. Soc. Trop. Med. Hyg. 84, 618–621.
- Jennings, F.W. , 1993. Combination chemotherapy of CNS trypanosomiasis. Acta Trop. 54, 205–213.
- Jones-Davies, W.J. , Folkers, C., 1966. The prevalence of homidium-resistant strains of trypanosomes in cattle in northern Nigeria. Bull. Epizoot. Dis. Afr. 14, 65–72.
- Joshua, R.A. , 1988. Drug resistance in recent isolates of Trypanosoma brucei and Trypanosoma congolense. Rev. Elev. Med. Vet. Pays Trop. 41, 359–64.
- Kabayo, J.P. , 2002. Aiming to eliminate tsetse from Africa. Trends Parasitol. 18, 473–475.
- Kaminsky, R. , Gumm, I.D., Zweygarth, E., Chuma, F., 1990. A drug incubation infectivity test (DIIT) for assessing resistance in trypanosomes. Vet. Parasitol. 34, 335–343.
- Kaminsky, R. , Schmid, C., Lun, Z.R., 1997. Susceptibility of dyskinetoplastic Trypanosoma evansi and T. equiperdum to isometamidium chloride. Parasitol. Res. 83, 816–818.
- Kaminsky, R. , Zweygarth, E., 1991. The effect of verapamil alone and in combination with trypanocides on multidrug-resistant Trypanosoma brucei brucei. Acta Trop. 49, 215–225.
- Kasozi, K.I. , MacLeod, E.T., Waiswa, C., Mahero, M., Ntulume, I., Welburn, S.C., 2022. Systematic Review and Meta-Analysis on Knowledge Attitude and Practices on African Animal Trypanocide Resistance. Trop. Med. Infect. Dis. 7, 205.
- Kaur, J. , Dey, C.S., 2000. Putative P-Glycoprotein Expression in Arsenite-Resistant Leishmania donovani Down-Regulated by Verapamil. Biochem. Biophys. Res. Commun. 271, 615–619.
- Kazibwe, A.J.N. , Nerima, B., De Koning, H.P., Mäser, P., Barrett, M.P., Matovu, E., 2009. Genotypic Status of the TbAT1/P2 Adenosine Transporter of Trypanosoma brucei gambiense Isolates from Northwestern Uganda following Melarsoprol Withdrawal. PloS Negl. Trop. Dis. 3, e523.
- Keiser, J. , 2000. Investigations of the metabolites of the trypanocidal drug melarsoprol. Clin. Pharmacol. Ther. 67, 478–488.
- Kim, J. ,Álvarez-Rodríguez, A.,Li, Z., Radwanska, M., Magez, S., 2023. Recent progress in the detection of Surra, a neglected disease caused by Trypanosoma evansi with a One Health impact in large parts of the tropic and sub-tropic world. Microorganisms (in press).
- Kinabo, L.D.B. , Bogan, J. A., 1988. The pharmacology of isometamidium. J. Vet. Pharmacol. Ther. 11, 233–245.
- King, H. , Lourie, E.M., Yorke W. 1937. New trypanocidal substances. 1360. [Google Scholar]
- Kingsley, P. , 2015. Inscrutable medicines and marginal markets: tackling substandard veterinary drugs in Nigeria. Pastoralism 5, 2.
- Klug, D.M. , Gelb, M.H., Pollastri, M.P., 2016. Repurposing strategies for tropical disease drug discovery. Bioorg. Med. Chem. Lett. 26, 2569–2576.
- Knoppe, T.N. , Bauer, B., McDermott, J.J., Peregrine, A.S., Mehlitz, D., Clausen, P.-H., 2006. Isometamidium sensitivity of Trypanosoma congolense stocks from cattle in West Africa tested in mice and the drug incubation infectivity test. Acta Trop. 97, 108–116.
- Kristjanson, P.M. , Swallow, B.M., Rowlands, G.J., Kruska, R.L., de Leeuw, P.N., 1999. Measuring the costs of African animal trypanosomosis, the potential benefits of control and returns to research. Agric. Syst. 59, 79–98.
- Kroubi, M. , Daulouede, S., Karembe, H., Jallouli, Y., Howsam, M., Mossalayi, D., Vincendeau, P., Betbeder, D., 2010. Development of a nanoparticulate formulation of diminazene to treat African trypanosomiasis. Nanotechnology 21, 505102.
- Kroubi, M. , Karembe, H., Betbeder, D., 2011. Drug delivery systems in the treatment of African trypanosomiasis infections. Expert Opin. Drug Deliv. 8, 735–747.
- Kumar, R. , Jain, S., Kumar, Saroj, Sethi, K., Kumar, Sanjay, Tripathi, B.N., 2017. Impact estimation of animal trypanosomosis (surra) on livestock productivity in India using simulation model: Current and future perspective. Vet. Parasitol. Reg. Stud. Reports 10, 1–12.
- Küpper, W. , Wolters, M., 1983. Observation on drug resistance of Trypanosoma (Nannomonas) congolense and Trypanosoma (Duttonella) vivax in cattle at a feedlot in the Northern Ivory Coast. Tropenmed. Parasitol. 34, 203–5.
- Kuriakose, S. , Muleme, H.M., Onyilagha, C., Singh, R., Jia, P., Uzonna, J.E., 2012. Diminazene Aceturate (Berenil) Modulates the Host Cellular and Inflammatory Responses to Trypanosoma congolense Infection. PloS One 7, e48696.
- Lane, A.N. , Jenkins, T.C., Brown, T., Neidle, S., 1991. Interaction of berenil with the EcoRI dodecamer d(CGCGAATTCGCG)2 in solution studied by NMR. Biochemistry 30, 1372–1385.
- Larson, S. , Carter, M., Hovel-Miner, G., 2021. Effects of trypanocidal drugs on DNA synthesis: new insights into melarsoprol growth inhibition. Parasitology 148, 1143–1150.
- Laveran, A. , 1904. The action of human serum on certain pathogenic Trypanosomes; action of arsenious acid upon Trypanosoma gambiense. Ann. Mag. Nat. Hist. Zool. Bot. Geol. 13, 401–403.
- Laveran, A. , Mesnil, F., 1902. Recherches sur le traitement et la prévention du nagana. Ann. Inst. Pasteur (Paris). 16, 786–817.
- Leach, T.M. , Roberts, C.J., 1981. Present status of chemotherapy and chemoprophylaxis of animal trypanosomiasis in the eastern hemisphere. Pharmacol. Ther. 13, 91–147.
- Lee, B.Y. , Bacon, K.M., Bottazzi, M.E., Hotez, P.J., 2013. Global economic burden of Chagas disease: a computational simulation model. Lancet Infect. Dis. 13, 342–348.
- Lejon, V. , 2003. Neuro-inflammatory risk factors for treatment failure in “early second stage” sleeping sickness patients treated with Pentamidine. J. Neuroimmunol. 144, 132–138.
- Liao, D. , Shen, J., 2010. Studies of quinapyramine-resistance of Trypanosoma brucei evansi in China. Acta Trop. 116, 173–177.
- Lidani, K.C.F. , Andrade, F.A., Bavia, L., Damasceno, F.S., Beltrame, M.H., Messias-Reason, I.J., Sandri, T.L., 2019. Chagas Disease: From Discovery to a Worldwide Health Problem. Front. Public Heal. 7, 166.
- Liebenehm, S. , Bett, B., Verdugo, C., Said, M., 2016. Optimal Drug Control under Risk of Drug Resistance – The Case of African Animal Trypanosomosis. J. Agric. Econ. 67, 510–533.
- Lin, C. , Hulpia, F., da Silva, C.F., Batista, D.D.G.J., Van Hecke, K., Maes, L., Caljon, G., Soeiro, M.D.N.C., Van Calenbergh, S., 2019. Discovery of Pyrrolo[2,3- b ]pyridine (1,7-Dideazapurine) Nucleoside Analogues as Anti- Trypanosoma cruzi Agents. J. Med. Chem. 62, 8847–8865.
- Livingstone, D. , 1858. Arsenic as a Remedy for the Tsetse Bite. BMJ s4-1, 360–361.
- Lourie, E.M. , Yorke, W., 1937. Studies in Chemotherapy. XVI. The Trypanocidal Action of Synthalin. Ann. Trop. Med. Parasitol. 31, 435–445.
- Lourie, E.M. , Yorke, W., 1939. Studies in Chemotherapy XXI. The Trypanocidal Action of Certain Aromatic Diamidines.. Ann. Trop. Med. Parasitol. 33, 289–304.
- Luckins, A.G. , Boid, R., Rae, P., Mahmoud, M.M., el Malik, K.H., Gray, A.R., 1979. Serodiagnosis of infection withTrypanosoma evansi in camels in the Sudan. Trop. Anim. Health Prod. 11, 1–12.
- Lüscher, A. , Nerima, B., Mäser, P., 2006. Combined contribution of TbAT1 and TbMRPA to drug resistance in Trypanosoma brucei. Mol. Biochem. Parasitol. 150, 364–366.
- Mabille, D. , Ilbeigi, K., Hendrickx, S., Ungogo, M.A., Hulpia, F., Lin, C., Maes, L., De Koning, H.P., Van Calenbergh, S., Caljon, G., 2022. Nucleoside analogues for the treatment of animal trypanosomiasis. Int. J. Parasitol. Drugs Drug Resist. 19, 21–30.
- Machila, N. , Wanyangu, S.W., McDermott, J., Welburn, S.C., Maudlin, I., Eisler, M.C., 2003. Cattle owners’ perceptions of African bovine trypanosomiasis and its control in Busia and Kwale Districts of Kenya. Acta Trop. 86, 25–34.
- Maclennan, K. , Jones-Davies, W., 1967. The occurrence of a berenil-resistant Trypanosoma congolense strain in Northern Nigeria. Vet. Rec. 80, 389–390.
- MacLennan, K.J.R. , 1968. Some recent findings concerning the use of Berenil for the treatment of bovine trypanosomiasis in Northern Nigeria. Trans. R. Soc. Trop. Med. Hyg. 62, 139–140.
- Magez, S. , Pinto Torres, J.E., Oh, S., Radwanska, M., 2021. Salivarian Trypanosomes Have Adopted Intricate Host-Pathogen Interaction Mechanisms That Ensure Survival in Plain Sight of the Adaptive Immune System. Pathogens 10, 679.
- Mamoudou, A. , Delespaux, V., Chepnda, V., Hachimou, Z., Andrikaye, J.P., Zoli, A., Geerts, S., 2008. Assessment of the occurrence of trypanocidal drug resistance in trypanosomes of naturally infected cattle in the Adamaoua region of Cameroon using the standard mouse test and molecular tools. Acta Trop. 106, 115–118.
- Manna, P.T. , Gadelha, C., Puttick, A.E., Field, M.C., 2015. ENTH and ANTH domain proteins participate in AP2-independent clathrin-mediated endocytosis. J. Cell Sci. 128, 2130–2142.
- Martin, S.K. , Oduola, A.M.J., Milhous, W.K., 1987. Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science. 235, 899–901.
- Mäser, P. , Sütterlin, C., Kralli, A., Kaminsky, R., 1999. A Nucleoside Transporter from Trypanosoma brucei Involved in Drug Resistance. Science. 285, 242–244.
- Mathis, A.M. , Holman, J.L., Sturk, L.M., Ismail, M.A., Boykin, D.W., Tidwell, R.R., Hall, J.E., 2006. Accumulation and Intracellular Distribution of Antitrypanosomal Diamidine Compounds DB75 and DB820 in African Trypanosomes. Antimicrob. Agents Chemother. 50, 2185–2191.
- Matovu, E. , Geiser, F., Schneider, V., Mäser, P., Enyaru, J.C.K., Kaminsky, R., Gallati, S., Seebeck, T., 2001. Genetic variants of the TbAT1 adenosine transporter from African trypanosomes in relapse infections following melarsoprol therapy. Mol. Biochem. Parasitol. 117, 73–81.
- Matovu, E. , Stewart, M.L., Geiser, F., Brun, R., Mäser, P., Wallace, L.J.M., Burchmore, R.J., Enyaru, J.C.K., Barrett, M.P., Kaminsky, R., Seebeck, T., De Koning, H.P., 2003. Mechanisms of arsenical and diamidine uptake and resistance in Trypanosoma brucei. Eukaryot. Cell 2, 1003–1008.
- Matusevicius, M. , Corey, R., Gragera, M., Yamashita, K., Sprenger, T., Ungogo, M.A., Blaza, J., Castro-Hartmann, P., Chirgadze, D., Chaitania, V.S., Afanasyev, P., Melero, R., Warshamanage, Carazo, J.M., Carrington, M., Blundell, T., Murshudov, G., Stansfeld, P., Sansom, M., De Koning, H.P., Tate, C.G., Weyand, S.N., 2023. Insights from aquaporin structures into drug-resistant sleeping sickness. Nat Comm., submitted.
- Maudlin, I. , 2006. African trypanosomiasis. Ann. Trop. Med. Parasitol. 100, 679–701.
- Mbwambo, H.A. , Mella, P.N., Lekaki, K.A., 1988. Berenil (diminazene aceturate)-resistant Trypanosoma congolense in cattle under natural tsetse challenge at Kibaha, Tanzania. Acta Trop. 45, 239–44.
- Mdachi, R.E. , Ogolla, K.O., Auma, J.E., Wamwiri, F.N., Kurgat, R.K., Wanjala, K.B., Mugunieri, L.G., Alusi, P.M., Chemuliti, J.K., Mukiria, P.W., Okoth, S.O., 2023. Variation of sensitivity of Trypanosoma evansi isolates from Isiolo and Marsabit counties of Kenya to locally available trypanocidal drugs. PloS One 18, e0281180.
- Melaku, A. , Birasa, B., 2013. Drugs and Drug Resistance in African Animal Trypanosomosis : A Review. Eur. J. Appl. Sci. 5, 82–89.
- Mills, R.M. , 2020. Chagas disease: Epidemiology and barriers to treatment. Am. J. Med. 133, 1262–1265.
- Mingala, C.N. , Pasag, A.C.P., Salinas, M.B.S., Balbin, M.M., Villanueva, M.A., 2019. Characterization of drug resistance-associated TevAT1 gene of Trypanosoma evansi from Philippine water buffaloes (Bubalus bubalis). Ann. Parasitol. 65, 381–386.
- Mizushima, D. , Amgalanbaatar, T., Davaasuren, B., Kayano, M., Naransatsral, S., Myagmarsuren, P., Otgonsuren, D., Enkhtaivan, B., Davkharbayar, B., Mungun-Ochir, B., Baatarjargal, P., Nyamdolgor, U., Soyolmaa, G., Altanchimeg, A., Zoljargal, M., Nguyen, T.-T., Battsetseg, B., Battur, B., Inoue, N., Yokoyama, N., Suganuma, K., 2020. Nationwide serological surveillance of non-tsetse-transmitted horse trypanosomoses in Mongolia. Parasite Epidemiol. Control 10, e00158.
- Mohamed-Ahmed, M.M. , Rahman, A.H., Abdel Karim, E.I., 1992. Multiple drug-resistant bovine trypanosomes in South Darfur Province, Sudan. Trop. Anim. Health Prod. 24, 179–181.
- Molefe, N.I. , Yamasaki, S., Macalanda, A.M.C., Suganuma, K., Watanabe, K., Xuan, X., Inoue, N., 2017. Oral administration of azithromycin ameliorates trypanosomosis in Trypanosoma congolense-infected mice. Parasitol. Res. 116, 2407–2415.
- Morrison, L.J. , Vezza, L., Rowan, T., Hope, J.C., 2016. Animal African Trypanosomiasis: Time to Increase Focus on Clinically Relevant Parasite and Host Species. Trends Parasitol. 32, 599–607.
- Moti, Y. , De Deken, R., Thys, E., Van Den Abbeele, J., Duchateau, L., Delespaux, V., 2015. PCR and microsatellite analysis of diminazene aceturate resistance of bovine trypanosomes correlated to knowledge, attitude and practice of livestock keepers in South-Western Ethiopia. Acta Trop. 146, 45–52.
- Mulandane, F.C. , Fafetine, J., Van Den Abbeele, J., Clausen, P.-H., Hoppenheit, A., Cecchi, G., Oosthuizen, M., Delespaux, V., Neves, L., 2018. Resistance to trypanocidal drugs in cattle populations of Zambezia Province, Mozambique. Parasitol. Res. 117, 429–436.
- Mulugeta, W. , Wilkes, J., Mulatu, W., Majiwa, P.A.O., Masake, R., Peregrine, A.S., 1997. Long-term occurrence of Trypanosoma congolense resistant to diminazene, isometamidium and homidium in cattle at Ghibe, Ethiopia. Acta Trop. 64, 205–217.
- Munday, J.C. , Eze, A.A., Baker, N., Glover, L., Clucas, C., Aguinaga Andres, D., Natto, M.J., Teka, I.A., McDonald, J., Lee, R.S., Graf, F.E., Ludin, P., Burchmore, R.J.S., Turner, C.M.R., Tait, A., MacLeod, A., Maser, P., Barrett, M.P., Horn, D., De Koning, H.P., 2014. Trypanosoma brucei, 69.
- Munday, J.C. , Rojas López, K.E., Eze, A.A., Delespaux, V., Van Den Abbeele, J., Rowan, T., Barrett, M.P., Morrison, L.J., De Koning, H.P., 2013. Functional expression of TcoAT1 reveals it to be a P1-type nucleoside transporter with no capacity for diminazene uptake. Int. J. Parasitol. Drugs Drug Resist. 3, 69–76.
- Munday, J.C. , Settimo, L., De Koning, H.P., 2015a. Transport proteins determine drug sensitivity and resistance in a protozoan parasite, Trypanosoma brucei. Front. Pharmacol. 6, 32.
- Munday, J.C. , Tagoe, D.N.A., Eze, A.A., Krezdorn, J.A.M., Rojas López, K.E., Alkhaldi, A.A.M., McDonald, F., Still, J., AlzahraFni, K.J., Settimo, L., De Koning, H.P., 2015b. Functional analysis of drug resistance-associated mutations in the Trypanosoma brucei adenosine transporter 1 (TbAT1) and the proposal of a structural model for the protein. Mol. Microbiol. 96, 887–900.
- Mungube, E.O. , Vitouley, H.S., Allegye-Cudjoe, E., Diall, O., Boucoum, Z., Diarra, B., Sanogo, Y., Randolph, T., Bauer, B., Zessin, K.-H., Clausen, P.-H., 2012. Detection of multiple drug-resistant Trypanosoma congolense populations in village cattle of south-east Mali. Parasit. Vectors 5, 155.
- Mutugi, M.W. , Boid, R., Luckins, A.G., 1994. Experimental induction of suramin-resistance in cloned and uncloned stocks of Trypanosoma evansi using immunosuppressed and immunocompetent mice. Trop. Med. Parasitol. 45, 232–6.
- Na’Isa, B.K. , 1967. Follow-up of a survey on the prevalence of homidium-resistant strains of trypanosomes in cattle in Northern Nigeria and drug cross-resistance tests on the strains with Samorin and Berenil. Bull. Epizoot. Dis. Afr. 15, 231–41.
- Namangala, B. , Odongo, S., 2014. Animal African Trypanosomosis in Sub-Saharan Africa and Beyond African Borders. In: Trypanosomes and Trypanosomiasis. Springer Vienna, Vienna, pp. 239–260.
- Napier, L.E. , Gupta, P.C. Sen, 1942. A Peculiar Neurological Sequel to Administration of 4: 4’-Diamidino-Diphenyl-Ethylene (M.&B. 744). Ind. Med. Gaz.
- Ndoutamia, G. , Moloo, S.K., Murphy, N.B., Peregrine, A.S., 1993. Derivation and characterization of a quinapyramine-resistant clone of Trypanosoma congolense. Antimicrob. Agents Chemother. 37, 1163–1166.
- Neal, R.A. , van Bueren, J., McCoy, N.G., Iwobi, M., 1989. Reversal of drug resistance in Trypanosoma cruzi and Leishmania donovani by verapamil. Trans. R. Soc. Trop. Med. Hyg. 83, 197–198.
- Nerima, B. , Matovu, E., Lubega, G.W., Enyaru, J.C.K., 2007a. Detection of mutant P2 adenosine transporter (TbAT1) gene in Trypanosoma brucei gambiense isolates from northwest Uganda using allele-specific polymerase chain reaction. Trop. Med. Int. Heal. 12, 1361–1368.
- Newton, B.A. , 1964. Mechanisms of Action of Phenanthridine and Aminoquinaldine Trypanocides. In: Advances in Chemotheraphy. Elsevier, pp. 35–83.
- Njiokou, F. , Nimpaye, H., Simo, G., Njitchouang, G.R., Asonganyi, T., Cuny, G., Herder, S., 2010. Domestic animals as potential reservoir hosts of Trypanosoma brucei gambiense in sleeping sickness foci in Cameroon. Parasite 17, 61–66.
- Obi, C.F. , Okpala, M.I., Ezeh, I.O., Onyeabor, A., Ezeokonkwo, R.C., 2022. Drug-resistant trypanosome isolates populations in dogs in Enugu North Senatorial Zone, Southeastern Nigeria. Parasitol. Res. 121, 423–431.
- Ogbaje, C.I. , Lawal, I.A., Ajanusi, O.J., 2015. Sensitivity of Nigerian field isolates of Trypanosoma vivax and Trypanosoma congolense to commonly available trypanocides. Asian Pacific J. Trop. Dis. 5, 214–218.
- Oguejiofor, C. , Ochiogu, I., Umeoduagu, C., 2010. Increasing doses of diminazene aceturate: adverse reproductive effects in female Wistar rats. Asian Pac. J. Trop. Med. 3, 887–889.
- OIE, 2013. Dourine (Infection with Trypanosoma equiperdum) Aetiology Epidemiology Diagnosis Prevention and Control References. (https://www.woah.org/fileadmin/Home/eng/Animal_Health_in_the_World/docs/pdf/Disease_cards/DOURINE.pdf). Accesed last on Dec. 10th, 2023.
- Okello, I. , Mafie, E., Eastwood, G., Nzalawahe, J., Mboera, L.E.G., 2022a. African Animal Trypanosomiasis: A Systematic Review on Prevalence, Risk Factors and Drug Resistance in Sub-Saharan Africa. J. Med. Entomol. 59, 1099–1143.
- Okello, I. , Mafie, E., Nzalawahe, J., Eastwood, G., Mboera, L.E.G., Hakizimana, J.N., Ogola, K., 2022b. Trypanosoma Congolense Resistant to Trypanocidal Drugs Homidium and Diminazene and their Molecular Characterization in Lambwe, Kenya. Acta Parasitol.
- Olila, D. , McDermott, J.J., Eisler, M.C., Mitema, E.S., Patzelt, R.J., Clausen, P.-H., Poetzsch, C.J., Zessin, K.-H., Mehlitz, D., Peregrine, A.S., 2002. Drug sensitivity of trypanosome populations from cattle in a peri-urban dairy production system in Uganda. Acta Trop. 84, 19–30.
- Oliveira, C.B. , Rigo, L.A., Rosa, L.D., Gressler, L.T., Zimmermann, C.E.P., Ourique, A.F., Da Silva, A.S., Miletti, L.C., Beck, R.C.R., Monteiro, S.G., 2014. Liposomes produced by reverse phase evaporation: in vitro and in vivo efficacy of diminazene aceturate against Trypanosoma evansi. Parasitology 141, 761–769.
- Omoja, V. , Anaga, A., Obidike, I., Ihedioha, T., Umeakuana, P., Mhomga, L., Asuzu, I., Anika, S., 2011. The effects of combination of methanolic leaf extract of Azadirachta indica and diminazene diaceturate in the treatment of experimental Trypanosoma brucei brucei infection in rats. Asian Pac. J. Trop. Med. 4, 337–341.
- Ooi, C.-P. , Schuster, S., Cren-Travaillé, C., Bertiaux, E., Cosson, A., Goyard, S., Perrot, S., Rotureau, B., 2016. The Cyclical Development of Trypanosoma vivax in the Tsetse Fly Involves an Asymmetric Division. Front. Cell. Infect. Microbiol. 6, 115.
- Ormerod, W.E. , 1952. A study of resistance to antrycide in a strain of Trypanosoma equiperdum. Br. J. Pharmacol. Chemother. 7, 674–684.
- Ormerod, W.E. , 1951a. The mode of action of antrycide. Br. J. Pharmacol. Chemother. 6, 325–333.
- Ormerod, W.E. , 1951b. A study of basophilic inclusion bodies produced by chemotherapeutic agents in trypanosomes. Br. J. Pharmacol. Chemother. 6, 334–341.
- Osman, A.S. , Jennings, F.W., Holmes, P.H., 1992. The rapid development of drug-resistance by Trypanosoma evansi in immunosuppressed mice. Acta Trop. 50, 249–257.
- Otesile, E.B. , Tabel, H., 1987. Enhanced Resistance of Highly Susceptible Balb/c Mice to Infection with Trypanosoma congolense after Infection and Cure. J. Parasitol. 73, 947.
- Paine, M.F. , Wang, M.Z., Generaux, C.N., Boykin, D.W., Wilson, W.D., De Koning, H.P., Olson, C.A., Pohlig, G., Burri, C., Brun, R., Murilla, G.A., Thuita, J.K., Barrett, M.P., Tidwell, R.R., 2010. Diamidines for human African trypanosomiasis. Curr. Opin. Investig. Drugs 11, 876–83.
- Pal, A. , Hall, B.S., Field, M.C., 2002. Evidence for a non-LDL-mediated entry route for the trypanocidal drug suramin in Trypanosoma brucei. Mol. Biochem. Parasitol. 122, 217–221.
- Pascucci, I. , Di Provvido, A., Cammà, C., Di Francesco, G., Calistri, P., Tittarelli, M., Ferri, N., Scacchia, M., Caporale, V., 2013. Diagnosis of dourine in outbreaks in Italy. Vet. Parasitol. 193, 30–38.
- Pearce, L. , 1921. Studies on the treatment of human trypanosomiasis with tryparsamide (the sodium salt of n-phenylglycineamide-p-arsonic acid). J. Exp. Med. 34, 1–104.
- Peregrine, A.S. , 1994. Chemotherapy and delivery systems: haemoparasites. Vet. Parasitol. 54, 223–248.
- Peregrine, A.S. , Gray, M.A., Moloo, S.K., 1997. Cross-resistance associated with development of resistance to isometamidium in a clone of Trypanosoma congolense. Antimicrob. Agents Chemother. 41, 1604–1606.
- Peregrine, A.S. , Mamman, M., 1993. Pharmacology of diminazene: a review. Acta Trop. 54, 185–203.
- Pinder, M. , Authie, E., 1984. The appearance of isometamidium resistant Trypanosoma congolense in West Africa. Acta Trop. 41, 247–52.
- Pohlig, G. , Bernhard, S.C., Blum, J., Burri, C., Mpanya, A., Lubaki, J.-P.F., Mpoto, A.M., Munungu, B.F., N’tombe, P.M., Deo, G.K.M., Mutantu, P.N., Kuikumbi, F.M., Mintwo, A.F., Munungi, A.K., Dala, A., Macharia, S., Bilenge, C.M.M., Mesu, V.K.B.K., Franco, J.R., Dituvanga, N.D., Tidwell, R.R., Olson, C.A., 2016. Efficacy and Safety of Pafuramidine versus Pentamidine Maleate for Treatment of First Stage Sleeping Sickness in a Randomized, Comparator-Controlled, International Phase 3 Clinical Trial. PloS Negl. Trop. Dis. 10, e0004363.
- Potts, W.H. , Jackson, C.H.N., 1952. The Shinyanga game destruction experiment. Bull. Entomol. Res. 53, 365–374.
- Priotto, G. , Kasparian, S., Mutombo, W., Ngouama, D., Ghorashian, S., Arnold, U., Ghabri, S., Baudin, E., Buard, V., Kazadi-Kyanza, S., Ilunga, M., Mutangala, W., Pohlig, G., Schmid, C., Karunakara, U., Torreele, E., Kande, V., 2009. Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: a multicentre, randomised, phase III, non-inferiority trial. Lancet 374, 56–64.
- Puente, V. , Demaria, A., Frank, F.M., Batlle, A., Lombardo, M.E., 2018. Anti-parasitic effect of vitamin C alone and in combination with benznidazole against Trypanosoma cruzi. PloS Negl. Trop. Dis. 12, e0006764.
- Pumhom, P. , Morand, S., Tran, A., Jittapalapong, S., Desquesnes, M., 2015. Trypanosoma from rodents as potential source of infection in human-shaped landscapes of South-East Asia. Vet. Parasitol. 208, 174–180.
- Pushpakom, S. , Iorio, F., Eyers, P.A., Escott, K.J., Hopper, S., Wells, A., Doig, A., Guilliams, T., Latimer, J., McNamee, C., Norris, A., Sanseau, P., Cavalla, D., Pirmohamed, M., 2019. Drug repurposing: progress, challenges and recommendations. Nat. Rev. Drug Discov. 18, 41–58.
- Pyana Pati, P. , Van Reet, N., Mumba Ngoyi, D., Ngay Lukusa, I., Karhemere Bin Shamamba, S., Büscher, P., 2014. Melarsoprol Sensitivity Profile of Trypanosoma brucei gambiense Isolates from Cured and Relapsed Sleeping Sickness Patients from the Democratic Republic of the Congo. PloS Negl. Trop. Dis. 8, e3212.
- Quintana, J.F. , Bueren-Calabuig, J., Zuccotto, F., De Koning, H.P., Horn, D., Field, M.C., 2020. Instability of aquaglyceroporin (AQP) 2 contributes to drug resistance in Trypanosoma brucei. PloS Negl. Trop. Dis. 14, e0008458.
- Quintana, J.F. , Pino, R.C. Del, Yamada, K., Zhang, N., 2018. Adaptation and Therapeutic Exploitation of the Plasma Membrane of African Trypanosomes. Genes (Basel). 9, 368.
- Ramirez, A.I.R. , 2020. Antigen discovery in Trypanosoma vivax. University of Liverpool.
- Rani, R. , Narasimhan, B., Varma, R.S., Kumar, R., 2021. Naphthoquinone derivatives exhibit apoptosis-like effect and anti-trypanosomal activity against Trypanosoma evansi. Vet. Parasitol. 290, 109367.
- Ranjbarian, F. , Vodnala, M., Alzahrani, K.J.H., Ebiloma, G.U., De Koning, H.P., Hofer, A., 2017. 9-(2′-Deoxy-2′-Fluoro-β-D-Arabinofuranosyl) Adenine Is a Potent Antitrypanosomal Adenosine Analogue That Circumvents Transport-Related Drug Resistance. Antimicrob. Agents Chemother. 61.
- Reigada, C. , Sayé, M., Valera-Vera, E., Miranda, M.R., Pereira, C.A., 2019. Repurposing of terconazole as an anti Trypanosoma cruzi agent. Heliyon 5, e01947.
- Richards, S. , Morrison, L.J., Torr, S.J., Barrett, M.P., Manangwa, O., Mramba, F., Auty, H., 2021. Pharma to farmer: field challenges of optimizing trypanocide use in African animal trypanosomiasis. Trends Parasitol. 37, 831–843.
- Riou, G. , Benard, J., 1980. Berenil induces the complete loss of kinetoplast DNA sequences in Trypanosoma equiperdum. Biochem. Biophys. Res. Commun. 96, 350–354.
- Robertson, J. , Ungogo, M.A., Aldfer, M.M., Lemgruber, L., McWhinnie, F.S., Bode, B.E., Jones, K.L., Watson, A.J.B., De Koning, H.P., Burley, G.A., 2021. Direct, Late-Stage Mono- N -arylation of Pentamidine: Method Development, Mechanistic Insight, and Expedient Access to Novel Antiparastitics against Diamidine-Resistant Parasites. ChemMedChem 16, 3396–3401.
- Rodgers, J. , Jones, A., Gibaud, S., Bradley, B., McCabe, C., Barrett, M.P., Gettinby, G., Kennedy, P.G.E., 2011. Melarsoprol Cyclodextrin Inclusion Complexes as Promising Oral Candidates for the Treatment of Human African Trypanosomiasis. PloS Negl. Trop. Dis. 5, e1308.
- Ross, C.A. , Barns, A.M., 1996. Alteration to one of three adenosine transporters is associated with resistance to Cymelarsan in Trypanosoma evansi. Parasitol. Res. 82, 183–188.
- Rottenberg, M.E. , Masocha, W., Ferella, M., Petitto-Assis, F., Goto, H., Kristensson, K., McCaffrey, R., Wigzell, H., 2005. Treatment of African Trypanosomiasis with Cordycepin and Adenosine Deaminase Inhibitors in a Mouse Model. J. Infect. Dis. 192, 1658–1665.
- Rowlands, G.J. , Leak, S.G.A., Peregrine, A.S., Nagda, S.M., Mulatu, W., D’Ieteren, G.D.M., 2001. The incidence of new and the prevalence and persistence of recurrent trypanosome infections in cattle in southwest Ethiopia exposed to a high challenge with drug-resistant parasites. Acta Trop. 79, 149–163.
- Roy Chowdhury, A. , Bakshi, R., Wang, J., Yildirir, G., Liu, B., Pappas-Brown, V., Tolun, G., Griffith, J.D., Shapiro, T.A., Jensen, R.E., Englund, P.T., 2010. The Killing of African Trypanosomes by Ethidium Bromide. PloS Pathog. 6, e1001226.
- Rurangirwa, F.R. , Tabel, H., Losos, G.J., Tizard, I.R., 1979. Suppression of antibody response to Leptospira biflexa and Brucella abortus and recovery from immunosuppression after Berenil treatment. Infect. Immun. 26, 822–826.
- Sanderson, L. , Dogruel, M., Rodgers, J., De Koning, H.P., Thomas, S.A., 2009. Pentamidine Movement across the Murine Blood-Brain and Blood-Cerebrospinal Fluid Barriers: Effect of Trypanosome Infection, Combination Therapy, P-Glycoprotein, and Multidrug Resistance-Associated Protein. J. Pharmacol. Exp. Ther. 329, 967–977.
- Schad, G.J. , Allanson, A., Mackay, S.P., Cannavan, A., Tettey, J.N.A., 2008. Development and validation of an improved HPLC method for the control of potentially counterfeit isometamidium products. J. Pharm. Biomed. Anal. 46, 45–51.
- Schillinger, D. , Röttcher, D., 1986. Treatment of camels for Trypanosoma brucei evansi infection (surra). World Anim Rev 60, 32.
- Schoenbach, E.B. , Greenspan, E.M., 1948. The pharmacology, mode of action and therapeutic potentialities of stilbamidine, pentamidine, propamidine and other aromatic diamidines—a review. Medicine (Baltimore). 27, 327–377.
- Schönefeld, A. , Röttcher, D., Moloo, S.K., 1987. The sensitivity to trypanocidal drugs of Trypanosoma vivax isolated in Kenya and Somalia. Trop. Med. Parasitol. 38, 177–80.
- Scott, A.G. , Tait, A., Michael, C., Turner, R., 1996. Characterisation of clones lines of Trypanosoma brucei expressing stable resistance to MelCy and suramin. Acta Trop. 60, 251–262.
- Scott, A.G. , Tait, A., Turner, C.M.R., 1997. Trypanosoma brucei:Lack of Cross-resistance to Melarsoprolin Vitroby Cymelarsan-Resistant Parasites. Exp. Parasitol. 86, 181–190.
- Scott, F.J. , Khalaf, A.I., Giordani, F., Wong, P.E., Duffy, S., Barrett, M., Avery, V.M., Suckling, C.J., 2016. An evaluation of Minor Groove Binders as anti-Trypanosoma brucei brucei therapeutics. Eur. J. Med. Chem. 116, 116–125.
- Seidl, A. , Dávila, A.M.R., Silva, R.A.M.S., 1999. Estimated Financial Impact of Trypanosoma vivax on the Brazilian Pantanal and Bolivian Lowlands. Mem. Inst. Oswaldo Cruz 94, 269–272.
- Sekhar, G.N. , Watson, C.P., Fidanboylu, M., Sanderson, L., Thomas, S.A., 2014. Delivery of Antihuman African Trypanosomiasis Drugs Across the Blood-Brain and Blood-CSF Barriers. In: Advances in Pharmacology. Pp. 245–275.
- Shahi, S.K. , Krauth-Siegel, R.L., Clayton, C.E., 2002. Overexpression of the putative thiol conjugate transporter TbMRPA causes melarsoprol resistance in Trypanosoma brucei. Mol. Microbiol. 43, 1129–1138.
- Shapiro, T.A. , Englund, P.T., 1990. Selective cleavage of kinetoplast DNA minicircles promoted by antitrypanosomal drugs. Proc. Natl. Acad. Sci. 87, 950–954.
- Shaw, A.P.M. , Cecchi, G., Wint, G.R.W., Mattioli, R.C., Robinson, T.P., 2014. Mapping the economic benefits to livestock keepers from intervening against bovine trypanosomosis in Eastern Africa. Prev. Vet. Med. 113, 197–210.
- Shikanai Yasuda, M.A. , 2022. Emerging and reemerging forms of Trypanosoma cruzi transmission. Mem. Inst. Oswaldo Cruz. 117, e210033.
- Singaro, J.R.S. , Yapo, F.B., Doua, F., Miezan, T.W., Baltz, T., 1996. The Efficacy of Pentamidine in the Treatment of Early-Late Stage Trypanosoma brucei gambiense Trypanosomiasis. Am. J. Trop. Med. Hyg. 55, 586–588.
- Sinyangwe, L. , Delespaux, V., Brandt, J., Geerts, S., Mubanga, J., Machila, N., Holmes, P.H., Eisler, M.C., 2004. Trypanocidal drug resistance in eastern province of Zambia. Vet. Parasitol. 119, 125–135.
- Sneader, W. , 2005. Drug Discovery, Drug Discovery: A History. John Wiley and Sons Ltd, Chichester, England.
- Sonibare, A. , Famuyide, I., Takeet, M., Oyewusi, I., Egbetade, A., Abapka, S., Adediran, O., Solanke, A., 2016. Diminazene-resistant Trypanosoma vivax in West African Dwarf lamb, south-west, Nigeria: A case report. Niger. J. Parasitol. 37, 62.
- Sow, A. , Sidibé, I., Bengaly, Z., Marcotty, T., Séré, M., Diallo, A., Vitouley, H.S., Nebié, R.L., Ouédraogo, M., Akoda, G.K., Van den Bossche, P., Van Den Abbeele, J., De Deken, R., Delespaux, V., 2012. Field detection of resistance to isometamidium chloride and diminazene aceturate in Trypanosoma vivax from the region of the Boucle du Mouhoun in Burkina Faso. Vet. Parasitol. 187, 105–111.
- Steketee, P.C. , Dickie, E.A., Iremonger, J., Crouch, K., Paxton, E., Jayaraman, S., Alfituri, O.A., Awuah-Mensah, G., Ritchie, R., Schnaufer, A., Rowan, T., De Koning, H.P., Gadelha, C., Wickstead, B., Barrett, M.P., Morrison, L.J., 2021. Divergent metabolism between Trypanosoma congolense and Trypanosoma brucei results in differential sensitivity to metabolic inhibition. PLOS Pathog. 17, e1009734.
- Steketee, P.C. , Vincent, I.M., Achcar, F., Giordani, F., Kim, D.-H., Creek, D.J., Freund, Y., Jacobs, R., Rattigan, K., Horn, D., Field, M.C., MacLeod, A., Barrett, M.P., 2018. Benzoxaborole treatment perturbs S-adenosyl-L-methionine metabolism in Trypanosoma brucei. PloS Negl. Trop. Dis. 12, e0006450.
- Stephen, L.E. , 1958. Suramin Complexes. Ann. Trop. Med. Parasitol. 52, 417–426.
- Stephen, L.E. , 1962. Suramin Complexes. Ann. Trop. Med. Parasitol. 56, 406–414.
- Stevens, L. , Dorn, P.L., Schmidt, J.O., Klotz, J.H., Lucero, D., Klotz, S.A., 2011. Kissing Bugs. The Vectors of Chagas. In: Advances in Parasitology. Pp. 169–192.
- Steverding, D. , 2010. The development of drugs for treatment of sleeping sickness: a historical review. Parasit. Vectors 3, 15.
- Steverding, D. , Antoszczak, M., Huczyński, A., 2016. In vitro activity of salinomycin and monensin derivatives against Trypanosoma brucei. Parasit. Vectors 9, 409.
- Steverding, D. , Troeberg L., 2024. 100 years since the publication of the suramin formulation. Parasitol Res. 123:11.
- Stewart, M.L. , Burchmore, R.J.S., Clucas, C., Hertz-Fowler, C., Brooks, K., Tait, A., MacLeod, A., Turner, C.M.R., De Koning, H.P., Wong, P.E., Barrett, M.P., 2010. Multiple Genetic Mechanisms Lead to Loss of Functional TbAT1 Expression in Drug-Resistant Trypanosomes. Eukaryot. Cell 9, 336–343.
- Stewart, M.L. , Krishna, S., Burchmore, R.J.S., Brun, R., De Koning, H.P., Boykin, D.W., Tidwell, R.R., Ed Hall, J., Barrett, M.P., 2005. Detection of arsenical drug resistance in Trypanosoma brucei with a simple fluorescence test. Lancet 366, 486–487.
- Stijlemans, B. , De Baetselier, P., Caljon, G., Van Den Abbeele, J., Van Ginderachter, J.A., Magez, S., 2017. Nanobodies As Tools to Understand, Diagnose, and Treat African Trypanosomiasis. Front. Immunol. 8, 724.
- Strober, W. , 2015. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 111, A3.B.1–A3.B.3.
- Suganuma, K. , N’Da, D.D., Watanabe, K., Tanaka, Y., Mossaad, E., Elata, A., Inoue, N., Kawazu, S., 2022. Therapeutic Efficacy of Orally Administered Nitrofurantoin against Animal African Trypanosomosis Caused by Trypanosoma congolense Infection. Pathogens 11, 331.
- Summers, J.M. , Hawkins, S.E., 1978. The effect of antrycide of patterns of RNA synthesis in Amoeba discoides. J. Cell Sci. 30, 237–250.
- Suswam, E.A. , Taylor, D.W., Ross, C.A., Martin, R.J., 2001. Changes in properties of adenosine transporters in Trypanosoma evansi and modes of selection of resistance to the melaminophenyl arsenical drug, Mel Cy. Vet. Parasitol. 102, 193–208.
- Sutcliffe, O.B. , Skellern, G.G., Araya, F., Cannavan, A., Sasanya, J.J., Dungu, B., Van Gool, F., Munstermann, S., Mattioli, R.C., 2014. Animal trypanosomosis: making quality control of trypanocidal drugs possible. Rev. Sci. Tech. L’OIE 33, 813–830.
- Sutherland, C.S. , Stone, C.M., Steinmann, P., Tanner, M., Tediosi, F., 2017. Seeing beyond 2020: an economic evaluation of contemporary and emerging strategies for elimination of Trypanosoma brucei gambiense. Lancet Glob. Heal. 5, e69–e79.
- Sutherland, I.A. , Holmes, P.H., 1993. Alterations in drug transport in resistant Trypanosoma congolense. Acta Trop. 54, 271–278.
- Sutherland, I.A. , Mounsey, A., Eisler, M., Holmes, P.H., 1992a. Kinetic modelling of isometamidium chloride (Samorin) uptake by Trypanosoma congolense. Parasitology 105, 91–95.
- Sutherland, I.A. , Mounsey, A., Holmes, P.H., 1992b. Transport of isometamidium (Samorin) by drug-resistant and drug-sensitive Trypanosoma congolense. Parasitology 104, 461–467.
- Tarello, W. , 2005. Trypanosoma evansi infection in three cats. Rev. Med. Vet. (Toulouse). 156, 133–134.
- Taylor, K. , Authié, E.M.-L., 2004. Pathogenesis of animal trypanosomiasis. CAB International, Wallingford, UK.
- Tchamdja, E. , Clausen, P.-H., Kulo, A.E., Batawui, K., Bauer, B., Den Abbeele, J. Van, Delespaux, V., Hoppenheit, A., 2019. How rational drug use reduces trypanosome infections in cattle in chemo-resistance hot-spot villages of northern Togo. Acta Trop. 190, 159–165.
- Tchamdja, E. , Kulo, A.E., Akoda, K., Teko-Agbo, A., Assoumy, A.M., Niang, E.M.M., Batawui, K., Adomefa, K., Bankolé, A.A., Kombiagou, K., Hoppenheit, A., Clausen, P.-H., Mattioli, R.C., Peter, R., Napier, G.B., De Deken, R., Marcotty, T., Van Den Abbeele, J., Delespaux, V., 2016. Drug quality analysis through high performance liquid chromatography of isometamidium chloride hydrochloride and diminazene diaceturate purchased from official and unofficial sources in Northern Togo. Prev. Vet. Med. 126, 151–158.
- Teka, I.A. , Kazibwe, A.J.N., El-Sabbagh, N., Al-Salabi, M.I., Ward, C.P., Eze, A.A., Munday, J.C., Mäser, P., Matovu, E., Barrett, M.P., De Koning, H.P., 2011. The Diamidine Diminazene Aceturate Is a Substrate for the High-Affinity Pentamidine Transporter: Implications for the Development of High Resistance Levels in Trypanosomes. Mol. Pharmacol. 80, 110–116.
- Thomas, J.A. , Baker, N., Hutchinson, S., Dominicus, C., Trenaman, A., Glover, L., Alsford, S., Horn, D., 2018. Insights into antitrypanosomal drug mode-of-action from cytology-based profiling. PloS Negl. Trop. Dis. 12, e0006980.
- Thuita, J.K. , Wolf, K.K., Murilla, G.A., Bridges, A.S., Boykin, D.W., Mutuku, J.N., Liu, Q., Jones, S.K., Gem, C.O., Ching, S. and Tidwell, R.R., 2015. Chemotherapy of second stage human African trypanosomiasis: comparison between the parenteral diamidine DB829 and its oral prodrug DB868 in vervet monkeys. PLoS Negl. Trop. Dis. 9, e0003409.
- Tihon, E. , Imamura, H., Van den Broeck, F., Vermeiren, L., Dujardin, J.-C., Van Den Abbeele, J., 2017. Genomic analysis of Isometamidium Chloride resistance in Trypanosoma congolense. Int. J. Parasitol. Drugs Drug Resist. 7, 350–361.
- Touratier, L. , 1992. Eleventh International meeting on Trypanosoma evansi : report of the working group. Rev. Sci. Tech. l’OIE 11, 275–304.
- Travis, A.S. , 1991. A hundred years of chemotherapy 1891-1991. Biochem. (Lond). 13, 9–12.
- Truc, P. , Büscher, P., Cuny, G., Gonzatti, M.I., Jannin, J., Joshi, P., Juyal, P., Lun, Z.-R., Mattioli, R., Pays, E., Simarro, P.P., Teixeira, M.M.G., Touratier, L., Vincendeau, P., Desquesnes, M., 2013. Atypical human infections by animal trypanosomes. PloS Negl. Trop. Dis. 7, e2256.
- Truc, P. , Jamonneau, V., N’Guessan, P., N’Dri, L., Diallo, P.B., Cuny, G., 1998. Trypanosoma brucei, m: T. congolense; 92.
- Tsuruo, T. , Iida, H., Nojiri, M., Tsukagoshi, S., Sakurai, Y., 1983. Circumvention of vincristine and Adriamycin resistance in vitro and in vivo by calcium influx blockers. Cancer Res. 43, 2905–10.
- Tsuruo, T. , lida, H., Tsukagoshi, S., Sakurai, Y., 1981. Overcoming of Vincristine Resistance in p388 Leukemia in Vivo and in Vitro Through Enhanced Cytotoxicity of Vincristine and Vinblastine by Verapamil. Cancer Res. 41, 1967–1972.
- Umar, I.A. , Rumah, B.L., Kamla, S.L., Jobin, Ibrahim, Isah, M.A., 2008. Effects of intraperitoneal administration of vitamins C and E or A and E combinations on the severity of Trypanosoma brucei brucei infection in rats. African J. Biochem. Res. 2, 88–91.
- Umeakuana, P.U. , Gibson, W., Ezeokonkwo, R.C., Anene, B.M, 2019. Identification of Trypanosoma brucei gambiense in naturally infected dogs in Nigeria. Parasit. Vectors 12, 420.
- Unciti-Broceta, J.D. , Arias, J.L., Maceira, J., Soriano, M., Ortiz-González, M., Hernández-Quero, J., Muñóz-Torres, M., De Koning, H.P., Magez, S., Garcia-Salcedo, J.A., 2015. Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African Trypanosomiasis. PLOS Pathog. 11, e1004942.
- Ungogo, M.A. , Aldfer, M.M., Natto, M.J., Zhuang, H., Chisholm, R., Walsh, K., Mcgee, M., Ilbeigi, K., Asseri, J.I., Burchmore, R.J.S., Caljon, G., Van, S., De Koning, H.P., 2023. Cloning and characterization of Trypanosoma congolense and T. vivax nucleoside transporters reveal the potential of P1-type carriers for the discovery of broad-spectrum nucleoside-based therapeutics against Animal African trypanosomiasis. Int. J. Mol. Sci. 24, 3144.
- Ungogo, M.A. , Campagnaro, G.D., Alghamdi, A.H., Natto, M.J., De Koning, H.P., 2022. Differences in Transporters Rather than Drug Targets Are the Principal Determinants of the Different Innate Sensitivities of Trypanosoma congolense and Trypanozoon Subgenus Trypanosomes to Diamidines and Melaminophenyl Arsenicals. Int. J. Mol. Sci. 23, 2844.
- Uzonna, J.E. , Kaushik, R.S., Gordon, J.R., Tabel, H., 1999. Cytokines and antibody responses during Trypanosoma congolense infections in two inbred mouse strains that differ in resistance. Parasite Immunol. 21, 57–71.
- Van den Bossche, P. , Doran, M., Connor, R.J., 2000. An analysis of trypanocidal drug use in the Eastern Province of Zambia. Acta Trop. 75, 247–258.
- Van Hoof, L. , Henrard, C., Peel, E., 1944. Pentamidine in the prevention and treatment of trypanosomiasis. Trans. R. Soc. Trop. Med. Hyg. 37, 271–280.
- Vanhollebeke, B. , Truc, P., Poelvoorde, P., Pays, A., Joshi, P.P., Katti, R., Jannin, J.G., Pays, E., 2006. Human Trypanosoma evansi Infection Linked to a Lack of Apolipoprotein L-I. N. Engl. J. Med. 355, 2752–2756.
- Vansterkenburg, E.L.M. , Coppens, I., Wilting, J., Bos, O.J.M., Fischer, M.J.E., Janssen, L.H.M., Opperdoes, F.R., 1993. The uptake of the trypanocidal drug suramin in combination with low-density lipoproteins by Trypanosoma brucei and its possible mode of action. Acta Trop. 54, 237–250.
- Vitouley, H.S. , Mungube, E.O., Allegye-Cudjoe, E., Diall, O., Bocoum, Z., Diarra, B., Randolph, T.F., Bauer, B., Clausen, P.-H., Geysen, D., Sidibe, I., Bengaly, Z., Van den Bossche, P., Delespaux, V., 2011. Improved PCR-RFLP for the Detection of Diminazene Resistance in Trypanosoma congolense under Field Conditions Using Filter Papers for Sample Storage. PloS Negl. Trop. Dis. 5, e1223.
- Vodnala, S.K. , Lundbäck, T., Yeheskieli, E., Sjöberg, B., Gustavsson, A.-L., Svensson, R., Olivera, G.C., Eze, A.A., De Koning, H.P., Hammarström, L.G.J., Rottenberg, M.E., 2013. Structure–Activity Relationships of Synthetic Cordycepin Analogues as Experimental Therapeutics for African Trypanosomiasis. J. Med. Chem. 56, 9861–9873.
- Vreysen, M.J.B. , Seck, M.T., Sall, B., Bouyer, J., 2013. Tsetse flies: Their biology and control using area-wide integrated pest management approaches. J. Invertebr. Pathol. 112, S15–S25.
- W/yohannes, D. , Kebede, E., Abebe, G., 2010. Study on the assessment of drug resistance on Trypanosoma vivax in Tselemti woreda, Tigray, Ethiopia. Ethiop. Vet. J. 14, 15–30.
- Wainwright, M. , 2010. Dyes, trypanosomiasis and DNA: a historical and critical review. Biotech. Histochem. 85, 341–354.
- Waitumbi, J.N. , Murphy, N.B., Peregrine, A.S., 1994. Genotype and drug-resistance phenotype of Trypanosoma evansi isolated from camels in northern Kenya. Ann. Trop. Med. Parasitol. 88, 677–683.
- Wall, R.J. , Rico, E., Lukac, I., Zuccotto, F., Elg, S., Gilbert, I.H., Freund, Y., Alley, M.R.K., Field, M.C., Wyllie, S., Horn, D., 2018. Clinical and veterinary trypanocidal benzoxaboroles target CPSF3. Proc. Natl. Acad. Sci. 115, 9616–9621.
- Wangwe, I.I. , Wamwenje, S.A., Mirieri, C., Masila, N.M., Wambua, L., Kulohoma, B.W., 2019. Modelling appropriate use of trypanocides to restrict wide-spread multi-drug resistance during chemotherapy of animal African trypanosomiasis. Parasitology 146, 774–780.
- Ward, C.P. , Wong, P.E., Burchmore, R.J., De Koning, H.P., Barrett, M.P., 2011. Trypanocidal Furamidine Analogues: Influence of Pyridine Nitrogens on Trypanocidal Activity, Transport Kinetics, and Resistance Patterns. Antimicrob. Agents Chemother. 55, 2352–2361.
- Watkins, T.I. , Woolfe, G., 1952. Effect of Changing the Quaternizing Group on the Trypanocidal Activity of Dimidium Bromide. Nature 169, 506–506.
- Whiteside, E.F. , 1962. Interactions between drugs, trypanosoomes and cattle in the field. In: Drugs, Parasites and Hosts. In: Goodwin, L.G., Nimmo-Smith.J., R.H., Churchill, A. (Eds.), Drugs, Parasites and Hosts. London, pp. 116–141.
- Wiedemar, N. , Graf, F.E., Zwyer, M., Ndomba, E., Kunz Renggli, C., Cal, M., Schmidt, R.S., Wenzler, T., Mäser, P., 2018. Beyond immune escape: a variant surface glycoprotein causes suramin resistance in Trypanosoma brucei. Mol. Microbiol. 107, 57–67.
- Wiedemar, N. , Hauser, D.A., Mäser, P., 2020. 100 Years of Suramin. Antimicrob. Agents Chemother. 64(3), e01168-19.
- Wiedemar, N. , Zwyer, M., Zoltner, M., Cal, M., Field, M.C., Mäser, P., 2019. Expression of a specific variant surface glycoprotein has a major impact on suramin sensitivity and endocytosis in Trypanosoma brucei. FASEB BioAdvances 1, 595–608.
- Wilkes, J.M. , Mulugeta, W., Wells, C., Peregrine, A.S., 1997. Modulation of mitochondrial electrical potential: a candidate mechanism for drug resistance in African trypanosomes. Biochem. J. 326, 755–761.
- Wilkes, J.M. , Peregrine, A.S., Zilberstein, D., 1995. The accumulation and compartmentalization of isometamidium chloride in Trypanosoma congolense, monitored by its intrinsic fluorescence. Biochem. J. 312, 319–327.
- Wilkowsky, S.E. , 2018. Trypanosoma. In: Parasitic Protozoa of Farm Animals and Pets. Springer International Publishing, Cham, pp. 271–287.
- Williamson, J. , 1957. Suramin complexes. I. Prophylactic activity against Trypanosoma congolense in small animals. Ann Trop Med Parasitol 51, 440–456.
- Williamson, J. , 1970. Review of chemotherapeutic and chemotherapeutic agents. In: Mulligan, H.W. (Ed.), The African Trypanosomiases. George Allen and Unwin Ltd, London, pp. 684–710.
- Williamson, J. , Desowitz, R.S., 1956. Prophylactic Activity of Suramin Complexes in Animal Trypanosomiasis. Nature 177, 1074–1075.
- Williamson, J. , March, J.C., Scott-Finnigan, T.J., 1982. Drug synergy in experimental African trypanosomiasis. Tropenmed. Parasitol. 33, 76–82.
- Williamson, J. , Rollo, I.M., 1959. Drug resistance in trypanosomes; cross-resistance analyses. Br. J. Pharmacol. Chemother. 14, 423–430.
- Willson, M. , Callens, M., Kuntz, D.A., Perié, J., Opperdoes, F.R., 1993. Synthesis and activity of inhibitors highly specific for the glycolytic enzymes from Trypanosoma brucei. Mol. Biochem. Parasitol. 59, 201–210.
- Witola, W.H. , Inoue, N., Ohashi, K., Onuma, M., 2004. RNA-interference silencing of the adenosine transporter-1 gene in Trypanosoma evansi confers resistance to diminazene aceturate. Exp. Parasitol. 107, 47–57.
- World Health Organization (WHO), 2013. Control and surveillance of human African trypanosomiasis. World Health Organ. Tech. Rep. Ser. 1–237.
- Wragg, W.R. , Washbourn, K., Brown, K.N., Hill, J., 1958. Metamidium: a New Trypanocidal Drug. Nature 182, 1005–1006.
- Wring, S. , Gaukel, E., Nare, B., Jacobs, R., Beaudet, B., Bowling, T., Mercer, L., Bacchi, C., Yarlett, N., Randolph, R., Parham, R., Rewerts, C., Platner, J., Don, R., 2014. Pharmacokinetics and pharmacodynamics utilizing unbound target tissue exposure as part of a disposition-based rationale for lead optimization of benzoxaboroles in the treatment of Stage 2 Human African Trypanosomiasis. Parasitology 141, 104–118.
- Yongsheng, Y. , Yongchun, O., Chengmai, R., Yuanguo, C. and Fenqin, Z., 1996. Trypanocidal value of liposomal diminazene in experimental Trypanosoma brucei evansi infection in mice. Vet. Parasitol. 61, 349–352.
- Yaro, M. , Munyard, K.A., Stear, M.J., Groth, D.M., 2016. Combatting African Animal Trypanosomiasis (AAT) in livestock: The potential role of trypanotolerance. Vet. Parasitol. 225, 43–52.
- Yesufu, H.M. , 1971. Experimental transmission of Trypanosoma gambiense to domestic animals. Ann. Trop. Med. Parasitol. 65, 341–347.
- Yorke, W. , Adams, A.R.D., Murgatroyd, F., 1929. Studies in Chemotherapy I. A Method for Maintaining Pathogenic Trypanosomes Alive in Vitro at 37° C. for 24 Hours. Ann. Trop. Med. Parasitol. 23, 501–518.
- Zeelen, J. , van Straaten, M., Verdi, J., Hempelmann, A., Hashemi, H., Perez, K., Jeffrey, P.D., Hälg, S., Wiedemar, N., Mäser, P., Papavasiliou, F.N., Stebbins, C.E., 2021. Structure of trypanosome coat protein VSGsur and function in suramin resistance. Nat. Microbiol. 6, 392–400.
- Zhang, Z.Q. , Giroud, C., Baltz, T., 1993. Trypanosoma evansi: In Vivo and in Vitro Determination of Trypanocide Resistance Profiles. Exp. Parasitol. 77, 387–394.
- Zhou, J. , Shen, J., Liao, D., Zhou, Y., Lin, J., 2004. Resistance to drug by different isolates Trypanosoma evansi in China. Acta Trop. 90, 271–275.
- Zimmermann, S. , Hall, L., Riley, S., Sørensen, J., Amaro, R.E., Schnaufer, A., 2016. A novel high-throughput activity assay for the Trypanosoma brucei editosome enzyme REL1 and other RNA ligases. Nucleic Acids Res. 44, e24–e24.
- Zoltner, M. , Campagnaro, G.D., Taleva, G., Burrell, A., Cerone, M., Leung, K.-F., Achcar, F., Horn, D., Vaughan, S., Gadelha, C., Zíková, A., Barrett, M.P., De Koning, H.P., Field, M.C., 2020. Suramin exposure alters cellular metabolism and mitochondrial energy production in African trypanosomes. J. Biol. Chem. 295, 8331–8347.
- Zoltner, M. , Horn, D., De Koning, H.P., Field, M.C., 2016. Exploiting the Achilles’ heel of membrane trafficking in trypanosomes. Curr. Opin. Microbiol. 34, 97–103.
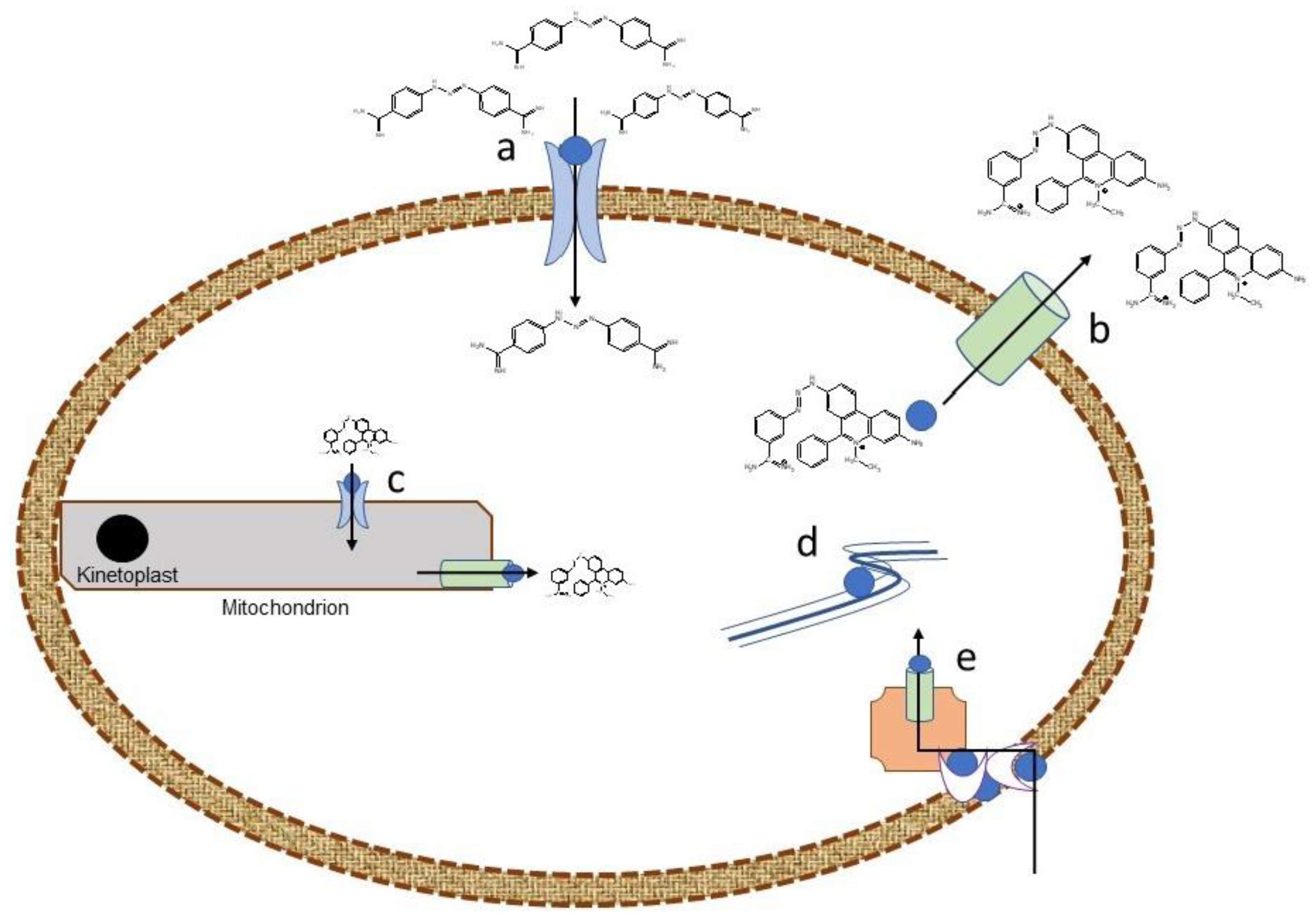
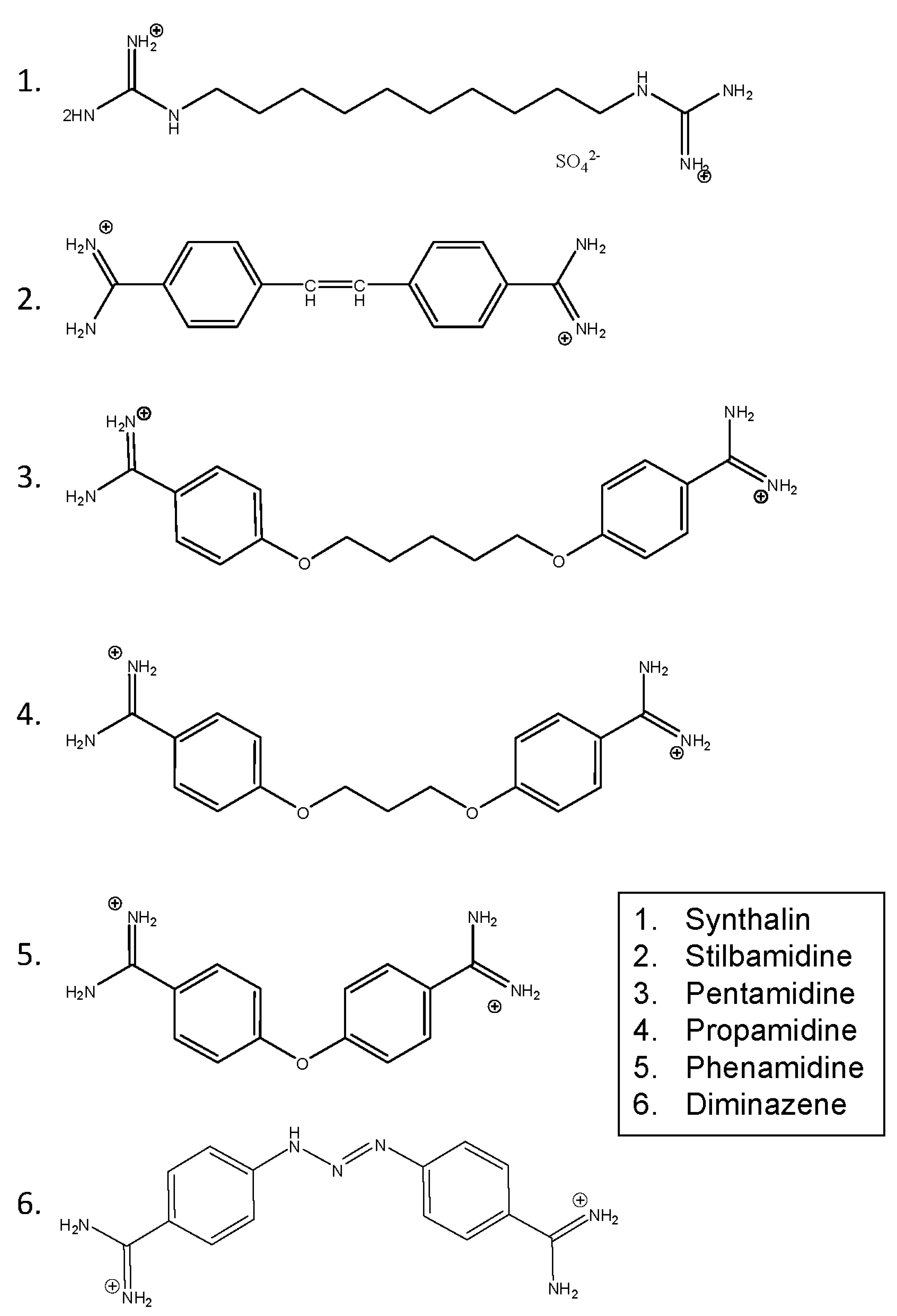
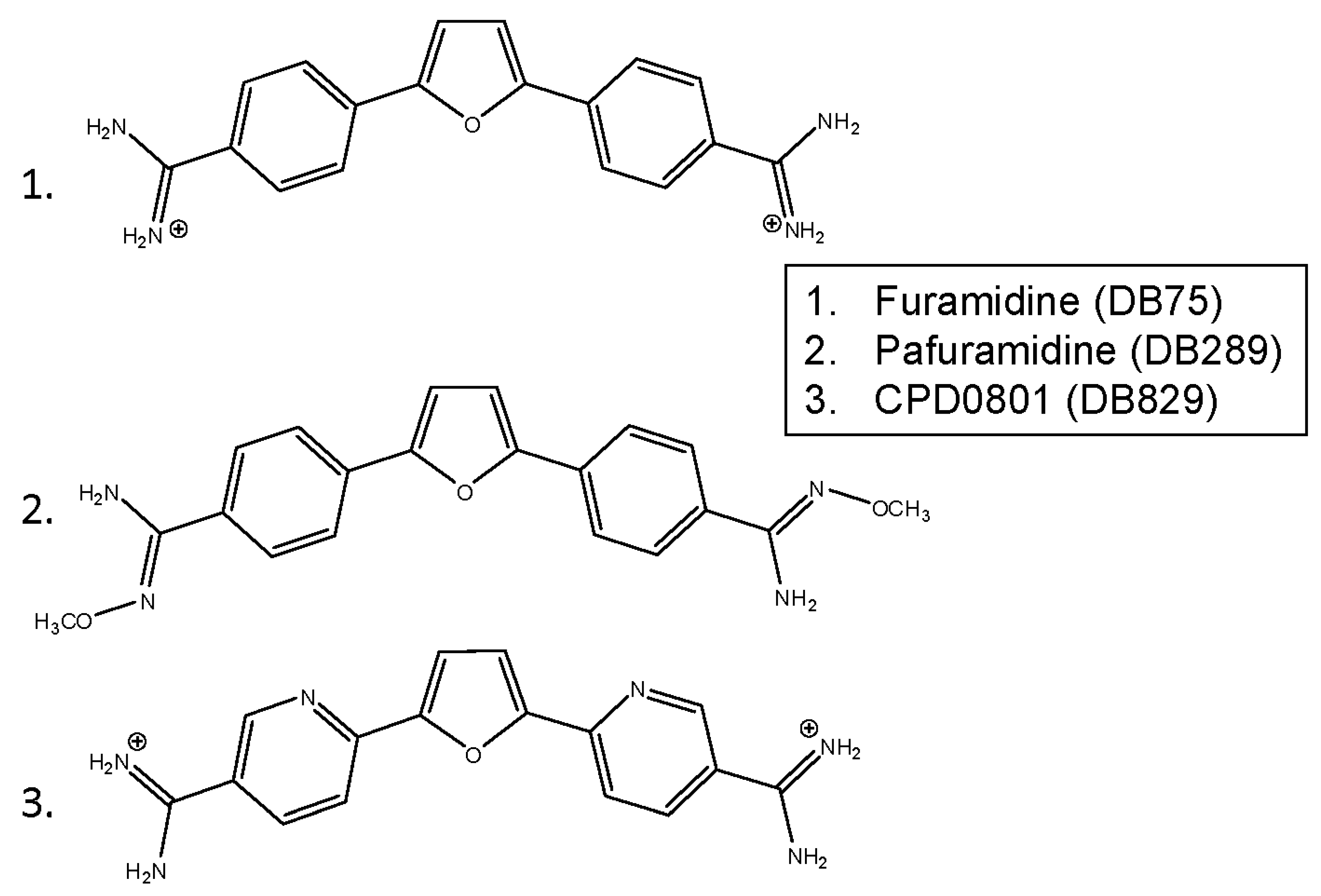
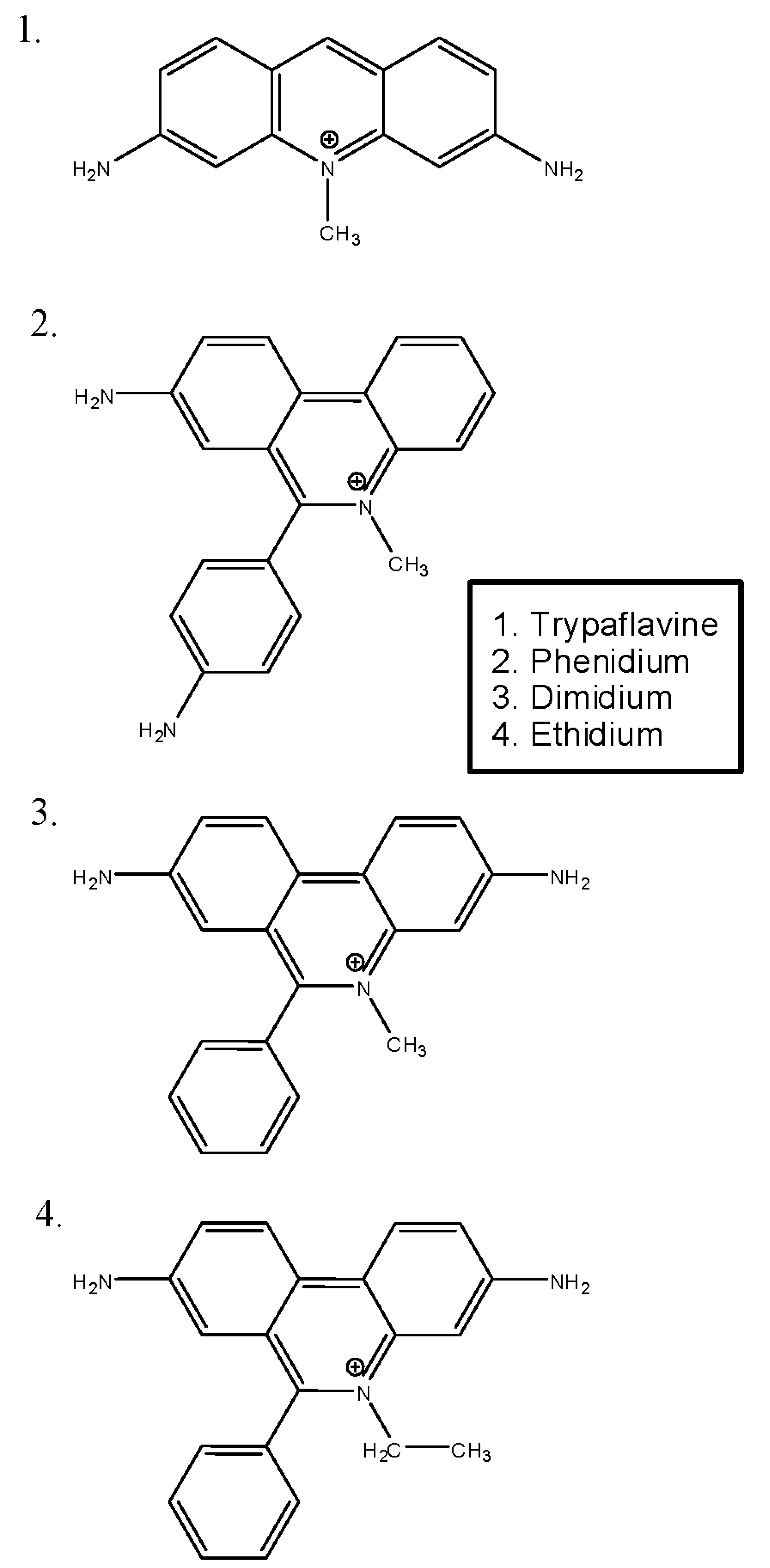
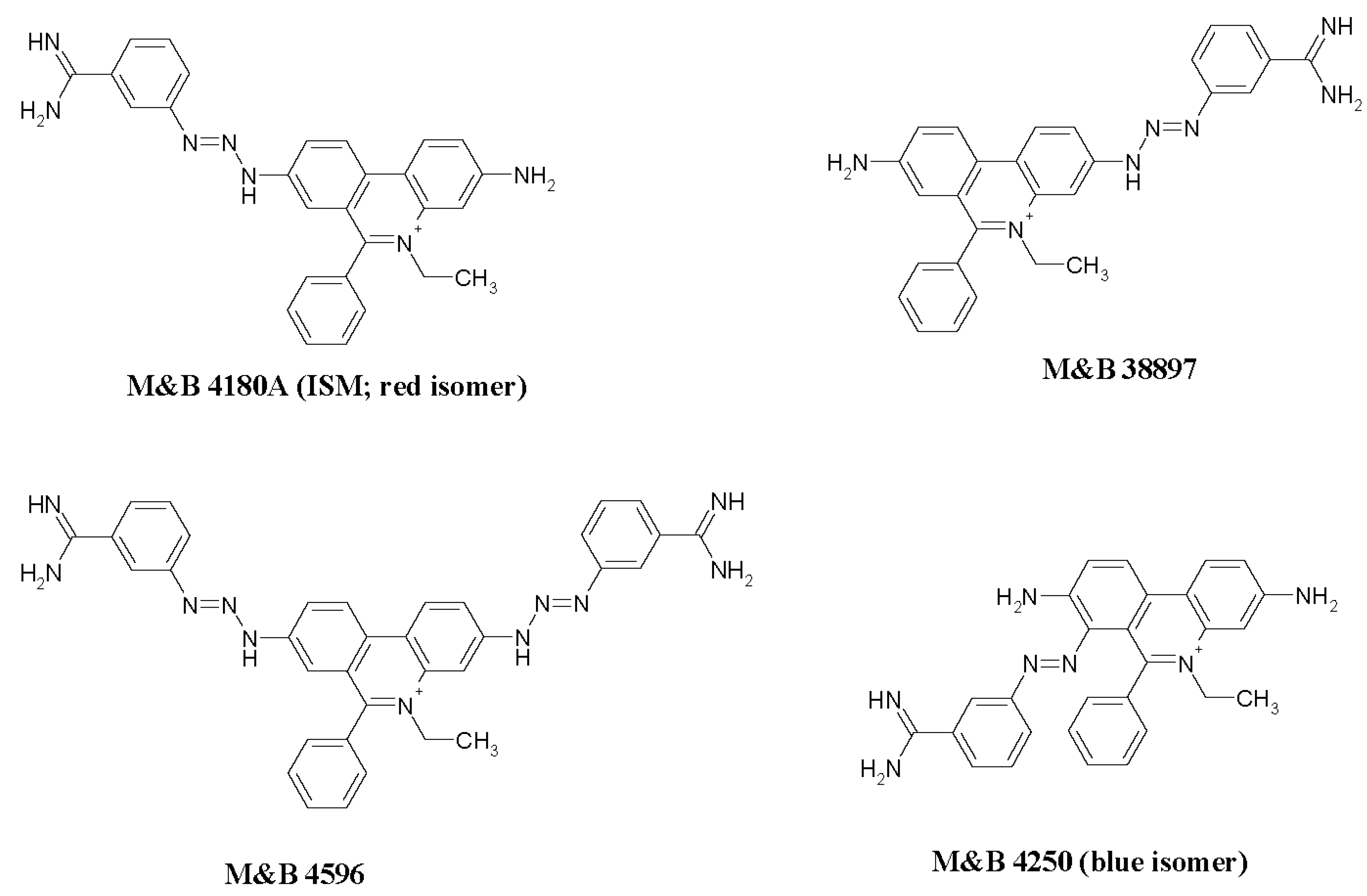
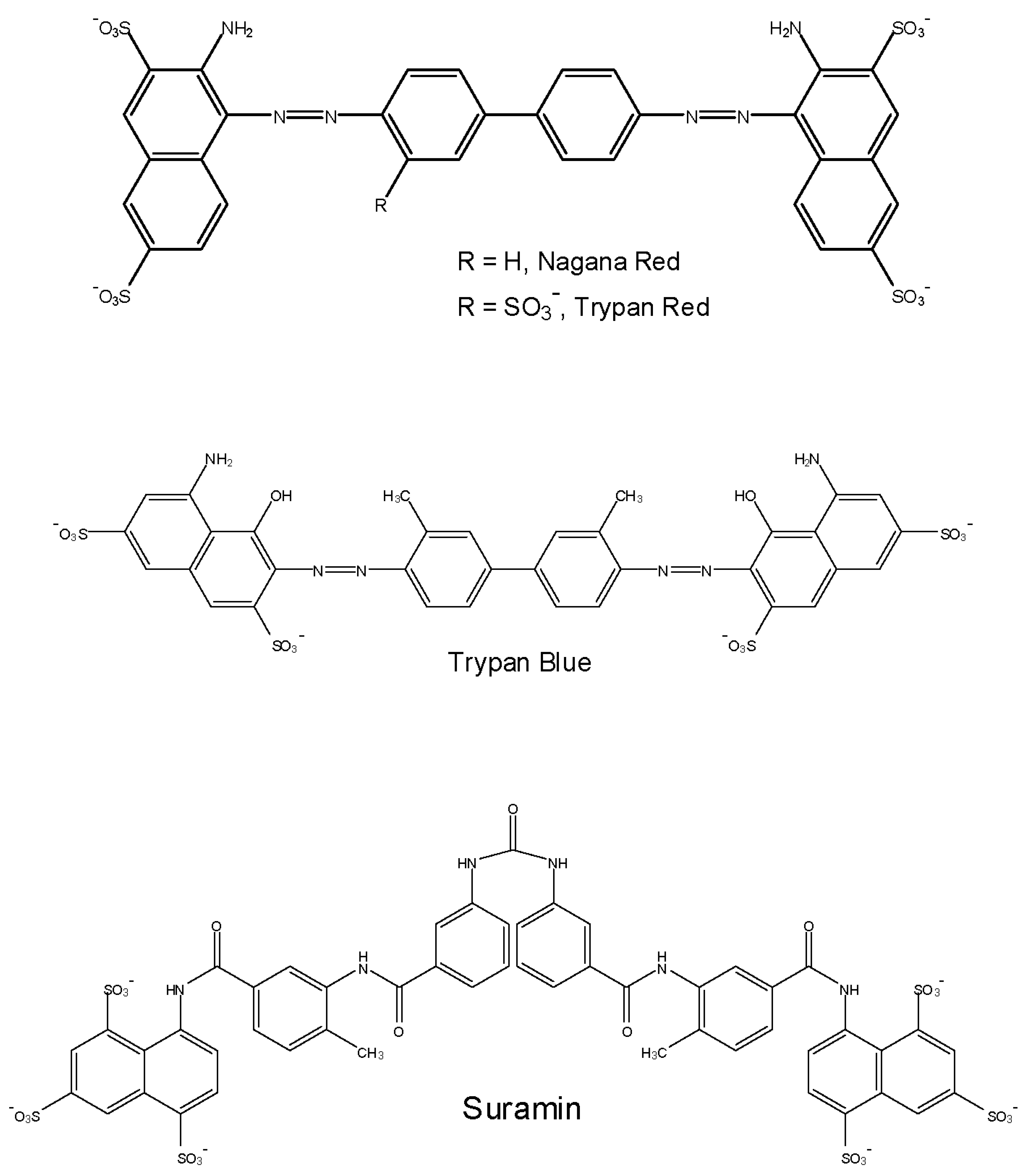
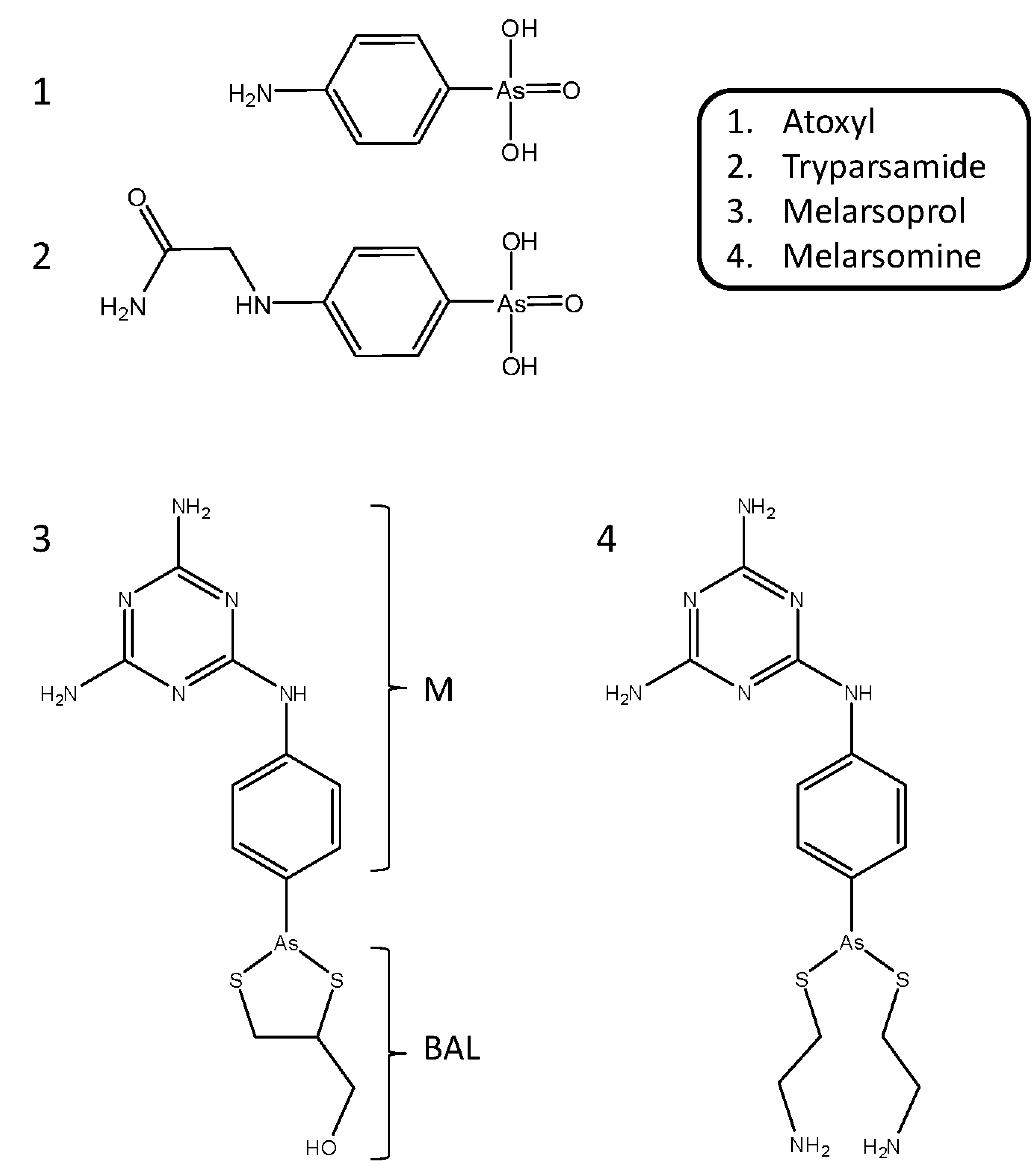
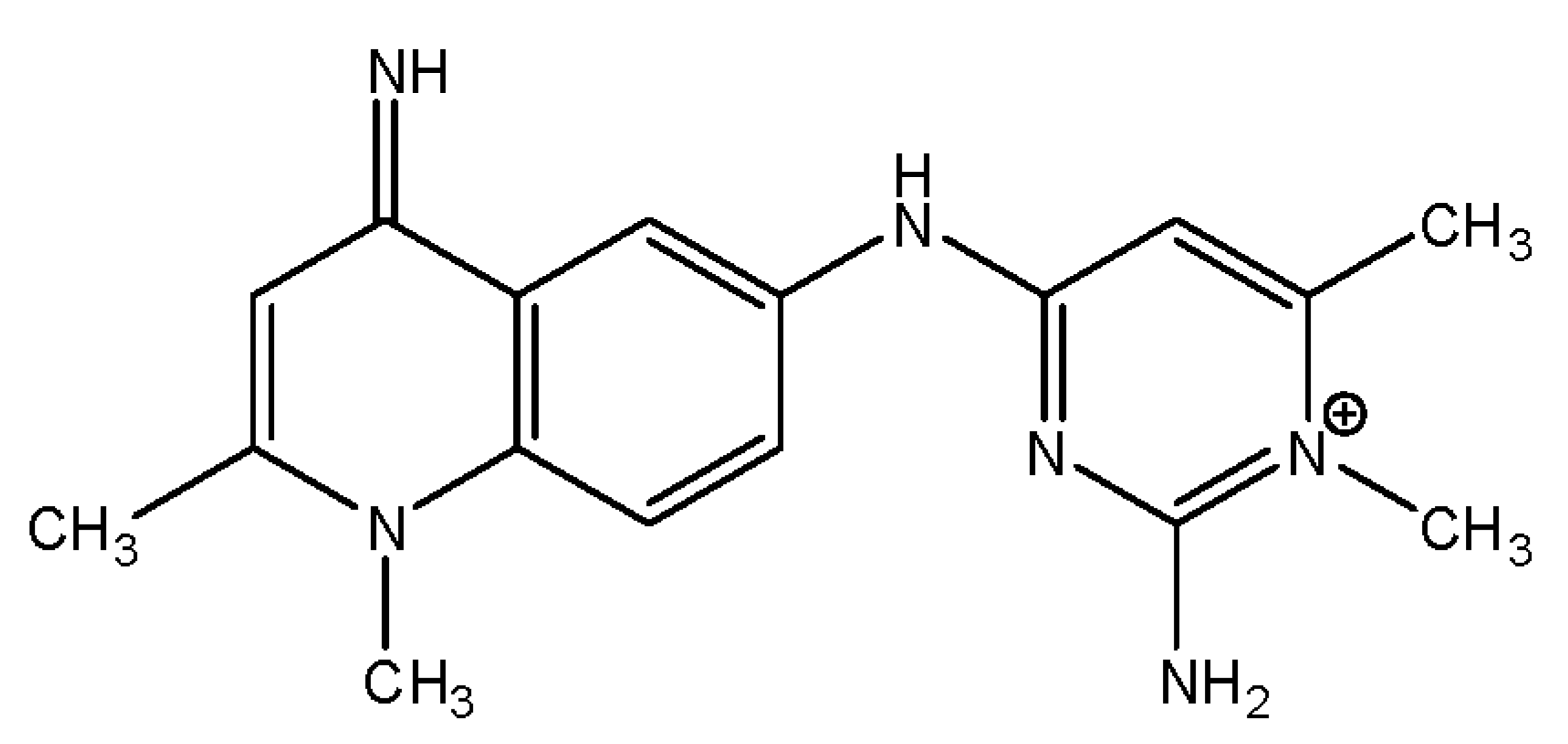
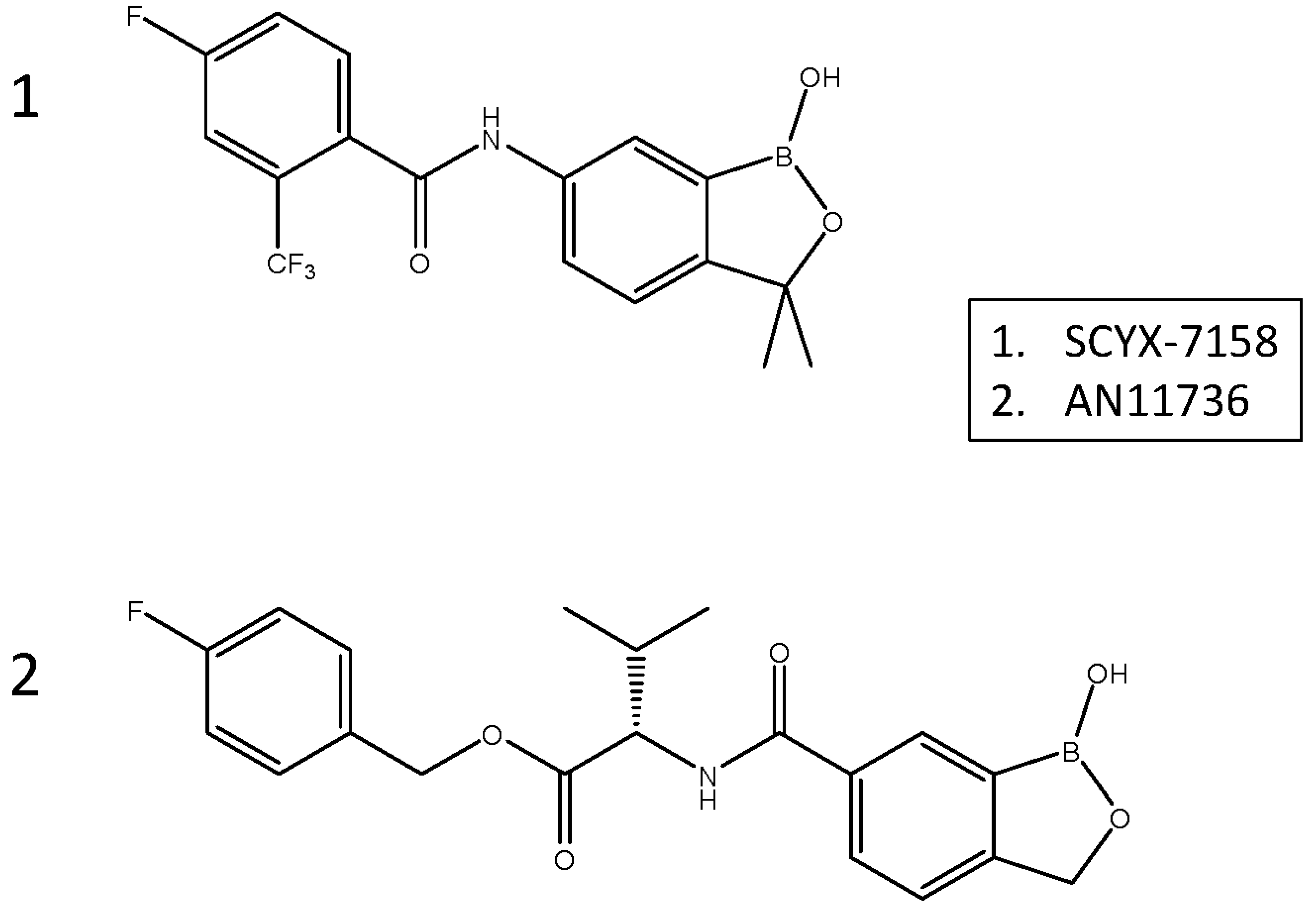
| Drug resistance developed | Species | Type of study | Drugs Cross-resistant to | Drugs not cross-resistant to | Reference |
|---|---|---|---|---|---|
| Melarsomine | T. evansi | In vivo (mice) | Diminazene and pentamidine | Suramin, isometamidium | Osman et al., 1992 |
| T. evansi | In vitro | Diminazene and Arsobal | Suramin and antrycide | Ross and Barn, 1996 | |
| T. brucei | In vivo (mice) | Melarsoprol | Suramin | Scott et al., 1996 | |
| T. brucei | In vivo (mice) | Melarsoprol (in vivo only), trimelarsen, melarsen oxide diminazene, pentamidine | Melarsoprol in vitro | Scott et al., 1997 | |
| T. evansi | In vitro and in vivo | Downregulation of diminazene transporter P2/AT1 | Suswam et al., 2001 | ||
| Tryparsamide | T. evansi | In vivo | Oxophenarsine | All others | Gill, 1971 |
| Diminazene | T. evansi | In vivo | Melarsomine | Zhang et al., 1993 | |
| T. brucei | In vitro | Pentamidine, furamidine, and melarsomine | Isometamidium, AN7973 and SCYX7158. | Teka et al., 2011 | |
| T. congolense | In vitro | -- | Isometamidium, AN7973 and SCYX7158. | Carruthers et al., 2021 | |
| Stilbamidine (diamidine) | T. evansi | In vivo | Oxophenarsine (aromatic arsenical), Melarsoprol, MsB, acriflavine, tryparsamide, diminazene, and metamidium | Quinapyramine, suramin | Gill, 1971 |
| Isometamidium | T. congolense | Diminazene, homidium chloride, quinapyramine sulphate | Diminazene | Peregrine et al., 1997 | |
| T. brucei | In vitro | Ethidium bromide, diminazene, and pentamidine | - | Eze et al., 2016 | |
| T. congolense | In vivo | - | - | Tihon et al., 2017 | |
| Suramin | T. evansi | In vivo | ---- | Oxophenarsine (aromatic arsenical), Melarsoprol, MsB, acriflavine, tryparsamide, diminazene, metamidium, quinapyramine | Gill, 1971 |
| T. evansi | In vivo | ---- | --- | Mutugi et al., 1994) | |
| T. brucei | In vivo | ---- | Melarsomine | Scott et al., 1996 | |
| T. brucei | In vivo | Trypan blue | Melarsoprol, Pentamidine | Wiedemar et al., 2018 | |
| Quinapyramine | T. equiperdum | In vivo | Dimidium and stilbamidine | Suramin | Ormerod et al., 1952 |
| T. evansi | In vivo | None | Oxophenarsine (aromatic arsenical), Melarsoprol, MsB, acriflavine, tryparsamide, diminazene, metamidium, suramin | Gill, 1971 | |
| T. congolense | In vivo (mice) | Diminazene, isometamidium, homidium | - | Ndoutamia et al., 1993 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





