1. Introduction
Free-living amoebae (FLA) are unicellular eukaryotes that can be isolated from a wide range of habitats such as soil, rivers and drinking water [1,2]. During their life cycle, FLA can alternate between two forms: a vegetative form, called the trophozoite, producing pseudopods responsible for cellular movements and feeding, and a dormant and resistant form, called the cyst, which develops when environmental conditions are hostile for the amoeba [3,4].
Some of these FLA are qualified as amphizoic, implying that they are able to exist as free-living organisms in nature, and can occasionally invade animal hosts and become pathogenic. These amphizoic FLA can cause serious ocular or cerebral pathologies. While ocular infections, called Amoebic Keratitis (AK), are caused by FLA from the genus Acanthamoeba, cerebral infections named Granulomatous Amoebic Encephalitis (GAE), Balamuthia Amoebic Encephalitis (BAE) and Primary Amoebic Meningoencephalitis (PAM) are caused by Acanthamoeba spp., Balamuthia mandrillaris and Naegleria fowleri, respectively [4,5].
As several FLA have been described to be resistant to disinfection methods of drinking water, such as chlorine, ultraviolet irradiations or ozonation [6-8], they can proliferate at different points of Drinking Water Networks (DWN) such as distribution systems or storage towers. In particular, these protozoa can develop in biofilms of DWN where they will feed on bacteria and become a major source of amoeba-resisting microorganisms in drinking water [9], and therefore a risk for human health. We have shown in a previous study, conducted in three Drinking Water Storage Tanks (DWST) of a Drinking Water Network in the Parisian area (France), that FLA were enriched in the biofilms collected at the surface of the DWST, with an amoebal density (quantity of amoebae per mL) 10 times higher than at the bottom [2]. In this work, the amoeba Acanthamoeba castellanii was detected in each DWST, and FLA were isolated only in the cyst form. The unequal distribution of amoebae in the biofilm was hypothesized to be due to vertical variation of the water level in the DWST, called the tidal range, which would allow organic matter deposition and drying on the support at each immersion/emersion cycle, and therefore promote cell adhesion and biofilm formation in upper levels of the DWST. Apart from this hypothesis, cell volumetric mass density of FLA cysts could be another factor, independent of the biofilm formation that contributes to their unequal distribution in the biofilm of the DWST. To further analyze this second hypothesis, a laboratory-scale water column mimicking a water reservoir was specifically developed in this study to analyze the vertical distribution of cysts of three different FLA species, i.e. Acanthamoeba castellanii, Vermamoeba vermiformis, and Balamuthia mandrillaris, as a function of their age.
2. Materials and Methods
2.1. Chemicals
All chemicals were purchased from Merck Laboratories (St-Quentin-Fallavier, France), except for Fetal Bovine Serum (FBS) which was provided by Thermo-Fisher (Villebon-sur-Yvette, France).
2.2. FLA culture
Acanthamoeba castellanii (ATCC 30010 strain) was grown axenically in PYG medium (ATCC medium 712) composed of 2% (w/v) peptone protein, 0.1% (w/v) yeast extract, 100 mM glucose, 4 mM MgSO4, 400 µM CaCl2, 3.4 mM sodium citrate, 2.5 mM Na2HPO4, 2.5 mM KH2PO4, 50 µM Fe (NH4)2(SO4)2, pH 6.5, following the procedure described in [10].
Vermamoeba vermiformis (ATCC 50237 strain) was cultured axenically in PYNFH medium (ATCC medium 1034) containing 1% (w/v) bacto-peptone, 1% (w/v) yeast extract, 0.1% (w/v) RNA type VI from torula yeast, 34 µM folic acid, 1.5 µM hemin, 3.5 mM Na2HPO4, 2.7 mM KH2PO4, 10% (v/v) FBS, pH 6.5, following the procedure described in [10].
Balamuthia mandrillaris (ATCC 50209 strain) was cultured in RPMI-1640 medium supplemented with 10% FBS (complete RPMI-1640 medium) and penicillin at 100U/mL and streptomycin at 100 µg/mL (Fisher Scientific, Illkirch, France) in the presence of a monolayer of Vero cells (ATCC; United States – Manassas, VA), as previously described [11]. Briefly, the culture required two steps: the first step consisted in subculturing the Vero cells at 1.105 cells/mL in complete RPMI-1640 medium for 3 days, and in a second step, the culture medium was changed gently, to avoid detachment of the Vero adherent cells, and replaced by RPMI-1640 complete medium containing B. mandrillaris.
A. castellanii and V. vermiformis were grown at 27 °C in the dark without shaking and the Vero cells and B. mandrillaris were cultivated at 37 °C with 5% of CO2 in the dark without shaking. All FLA were cultured with a starting cell density at 5.104 amoebae/mL twice a week in these conditions.
2.3. Encystment
The encystment method was the same for the three FLA used in this study, namely Acanthamoeba castellanii, Vermamoeba vermiformis and Balamuthia mandrillaris, and was adapted from the method previously developed [12]. Briefly, 15 mL of a culture of FLA at 1.106 trophozoites/mL were centrifuged at room temperature at 3000 g for 10 min, before resuspension and 3 washes in Neff buffer containing 0.1 M KCl, 8 mM MgSO4, 0.4 mM CaCl2, 1 mM NaHCO3, 20 mM Tris-HCl, pH 8.8 [13]. Amoebae were then incubated in 30 mL of Neff buffer at 27 °C for 5 days to generate cysts, and then further treated for 10 min at room temperature with 0.5 % (w/v) SDS (Sodium Dodecyl Sulfate) to eliminate the remaining trophozoite and pseudocyst forms, which are sensitive to SDS, unlike mature cysts. By comparing the number of FLA before (total FLA) and after (mature cysts) SDS treatment, we observed that ~95-100% of A. castellanii and V. vermiformis were encysted, while only ~70% of B. mandrillaris were transformed into cysts. The selected mature cysts were then washed 3 times in PAS buffer (ATCC medium 1323) containing 1 mM Na2HPO4, 1 mM KH2PO4, 16 µM MgSO4, 27 µM CaCl2, 2 mM NaCl, with a centrifugation at 3000 g for 15 min between each washing step. At this step, the cyst age was defined as 0 weeks, corresponding to the starting point of the study. The cysts were then stored at 4 °C in PAS buffer during 23 weeks, corresponding to the whole duration of the study, and used for further experiments each time point specified. The PAS buffer was changed each 4 weeks, by centrifuging the cyst suspensions at 3000 g for 15 min at 4 °C. Before adding the cysts to the laboratory-scale model of water column, the cysts were centrifuged at 3000 g for 15 min, resuspended in distilled water pre-filtered through a 0.22 µm porosity filter (Sartorius Stedim Biotech, Aubagne, France) and passed through a 25G needle (Terumo, Rueil-Malmaison, France) to avoid any cluster.
2.4. Model of water column
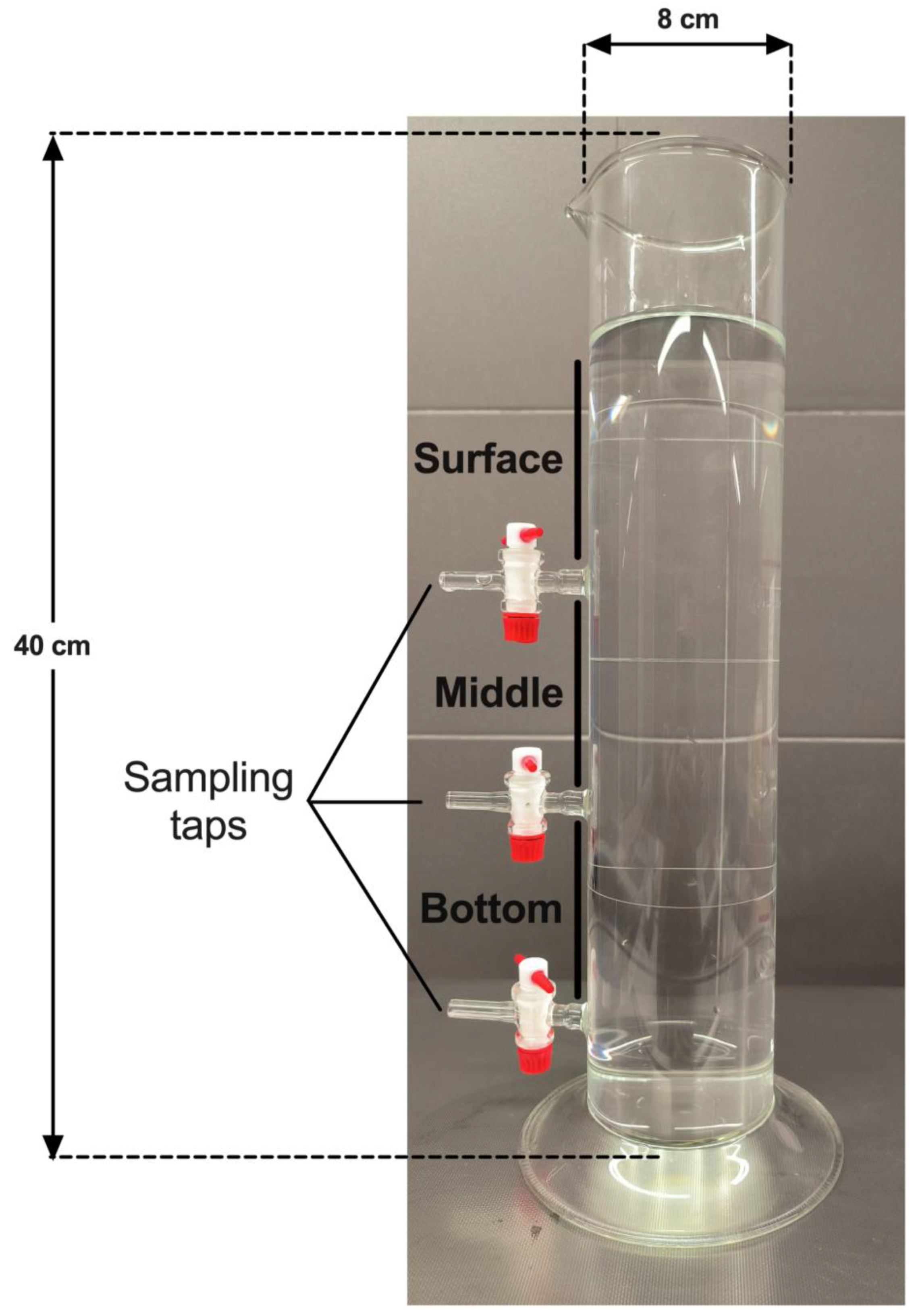
A laboratory-scale model of water reservoir was specifically designed for this study by the VERRE SCIEN-TECH facility of the Faculty of Pharmacy – Paris Saclay University (
Figure 1). The model of water column is a glass cylinder of 40 cm height and 8 cm diameter, thus containing a volume up to 2 L. The model was equipped with 3 taps installed at equal distance to each other, thus defining 3 sections, named surface, middle and bottom, allowing to collect an equal volume of 500 mL in each section. The 3 taps were installed at the bottom of each section.
2.5. Sampling
The cysts of A. castellanii, V. vermiformis or B. mandrillaris, aged from 0 to 23 weeks, were added in the water column containing 1.7 L of distilled water pre-filtered through a 0.22 µm porosity filter (Sartorius Stedim Biotech, Aubagne, France), in order to mimick in a laboratory-scale model the conditions of water storage in a DWST, in a maximal volume of 3 mL to reach the final cellular density of 5.104 cysts/mL, and the water column was further covered with parafilm and a cap of aluminium foil.
After 24 h of incubation at room temperature without agitation, 500 mL of water samples were collected at each section in the following order to minimize as much as possible water mixing between sections: surface first, then middle, then bottom. As the tap in the bottom section could not be installed at the lowest level of the column for technical reasons, it was placed at 4 cm upper to the basis of the water column, leaving a volume of 200 mL below the lowest tap. Following water sampling in each section, samples were centrifuged at 3000 g for 15 min at room temperature. The pellets were resuspended in 10 mL of pre-filtered distilled water and the cysts were counted in triplicates using a Malassez counting chamber (Fisher Scientific, Illkirch, France).
2.6. Statistical analyses
The statistical analyses of the results were carried out using the Kruskal-Wallis non-parametric test (P < 0.0001), followed by uncorrected Dunn’s multiple comparison test (P < 0.05), and using GraphPad Prism software (version 9.3.1; San Diego, CA, United States).
3. Results
The aim of this work was to compare the vertical distribution of cysts from 3 different FLA, namely Acanthamoeba castellanii, Vermamoeba vermiformis and Balamuthia mandrillaris, in a water column as a function of their age, from 0 to 23 weeks.
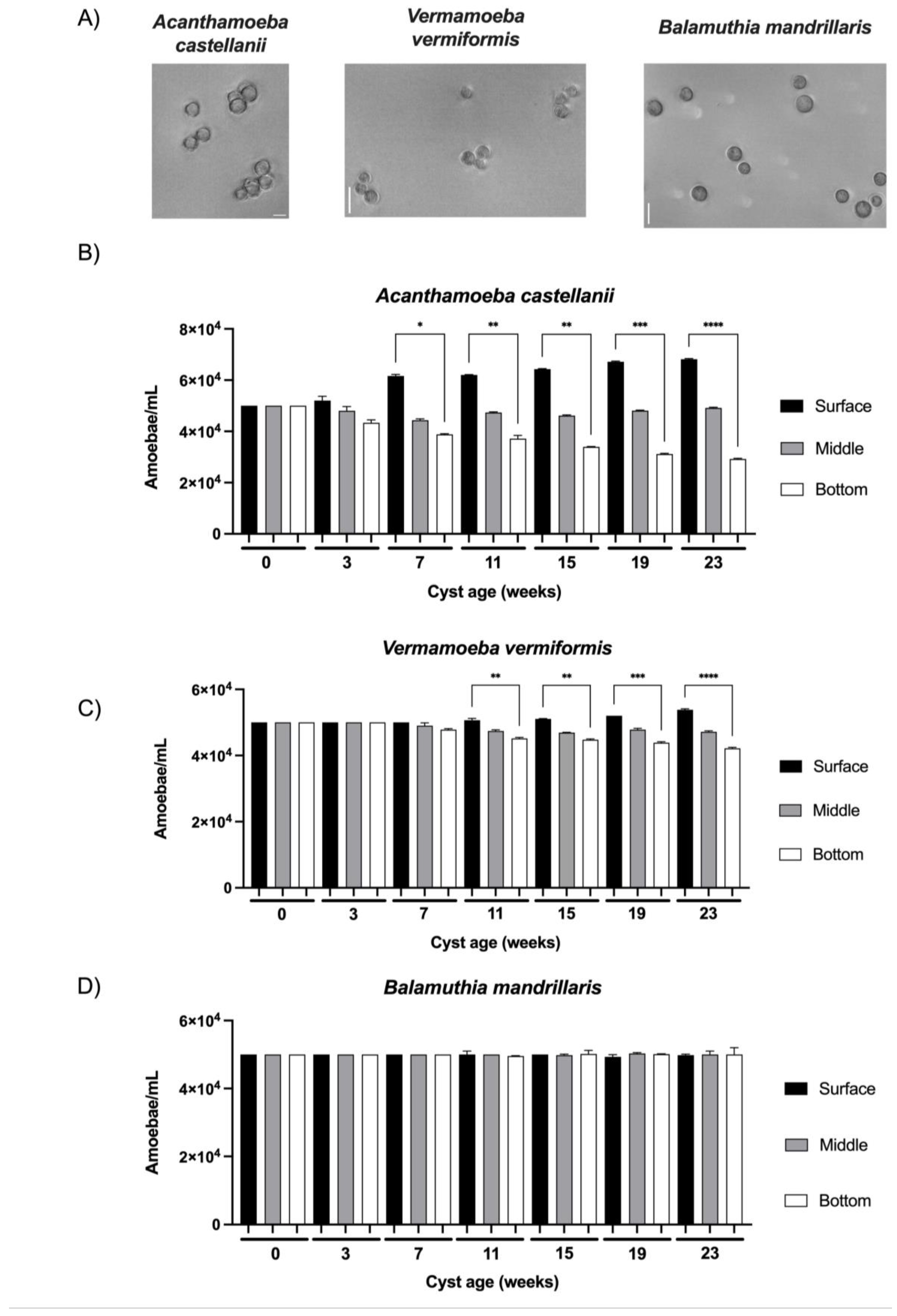
Just after their formation at the age of 0 weeks, the cysts, which have been selected based on their resistance to SDS treatment [12], developed a cell wall with characteristic layers of mature cysts of the 3 FLA species studied in the current work (
Figure 2A). At this initial time point, the cysts of
A. castellanii,
V. vermiformis and
B. mandrillaris were equally distributed in the three sections, namely surface, middle and bottom, with an amoebal density at 5.10
4 amoeba/mL after an incubation of 24h in the water column (Figures 2B, 2C and 2D). Although no significant difference was observed in cyst morphology or volume within each species of the three FLA studied for the whole duration of the study, cyst size varied substantially between FLA species:
A. castellanii from 14 to 23 µm,
V. vermiformis from 9 to 13 µm and
B. mandrillaris from 11 to 17 µm (
Figure 2A). Additionally, several variations in vertical distribution were noticed according to cyst age: at the age of 3 weeks, the cysts of
A. castellanii showed a tendency to be more abundant at the surface compared to the bottom (
Figure 2B). This tendency was confirmed when older cysts showed significant differences in amoebal density between the surface and the bottom section at 7 weeks and then increased with time:
A. castellanii cysts were from 1.6 to 2.3 times more abundant in the surface section compared to the bottom from the age of 7 weeks to 23 weeks, respectively (
Figure 2B). Concerning the cysts of
Vermamoeba vermiformis, the same tendency of unequal distribution between surface and bottom sections as a function of cyst age was observed, but with a lower intensity: a significant increase of 12% of
V. vermiformis cysts between surface and bottom sections started at 11 weeks and this difference was gradually enlarged to 27% until 23 weeks (
Figure 2C). From 0 to 23 weeks, the significant increases in cyst abundance at the surface section were also accompanied by a significant decrease of 41% and 16% for
A. castellanii and
V. vermiformis, respectively, at the bottom section (Figure 2B and 2C). Conversely, no significant differences were observed in the middle section either for
A. castellanii or
V. vermiformis as a function of cyst age during the 23 weeks of the study (Figures 2B and 2C). Furthermore, no variation of
B. mandrillaris cyst vertical distribution was observed as a function of their age (
Figure 2D): similar cyst concentrations were determined at the three levels of the water column during the whole duration of the study.
4. Discussion
In the current work, we have observed an enrichment of A. castellanii and V. vermiformis cysts, but not B. mandrillaris cysts, in the surface section of the water column during a period of 23 weeks of cyst aging, suggesting a variation of volumetric mass density of the cysts of these FLA species as a function of their age. To our knowledge, three main hypotheses may explain the differences of FLA cyst distribution observed in the present work.
Firstly, Acanthamoeba castellanii has been described to gradually loose water during encystment [14]. A persistence of this process during cyst aging, after its formation, would rather induce an increase of its volumetric mass density as more material would be accumulated in less space, and thus lead to cyst shrinkage in direct opposition to the results observed in our current work. Our results indicate that water loss would not be the factor inducing an enrichment of A. castellanii and V. vermiformis cysts in upper water levels as a function of their age.
Secondly, one could hypothesize that these differences could be attributed to variations in cyst wall composition between FLA species. Indeed, the cyst wall of the three FLA studied in the present work are different in terms of structure and biochemical composition. At the ultrastructural level, Acanthamoeba spp. and Vermamoeba spp. cyst walls are composed of two layers [14,15], while Balamuthia mandrillaris contains a supplementary coat [16]. The cyst walls of B. mandrillaris are presumably composed of proteins and cellulose, with a proportion that remains to be determined [17,18]. Additionally, the cyst walls of A. castellanii are mainly composed of proteins and carbohydrates, especially cellulose [19-21], while the cysts of V. vermiformis are composed of more than 64 % of proteins and only 4.2% of cellulose [22]. However, as the volumetric mass density of cellulose-containing molecules increases with cellulose content [23], an increase of cellulose content in FLA cyst wall during their aging would not lead to a displacement to upper levels in water. Moreover, as cellulose is the main cyst wall component in A. castellanii, but not in V. vermiformis where an enrichment in surface water levels was also observed for older cysts, the presence of this hydrophilic polymer in cyst walls does not seem to be the factor influencing FLA cyst volumetric mass density during their aging. While the hydrophobicity of proteins composing FLA cyst wall could also be considered, it is improbable that A. castellanii or V. vermiformis would synthesize more proteins, and specifically hydrophobic proteins, on their surface during cyst aging, as FLA cysts have low metabolism [24,25]. Moreover, if the SDS treatment used in our method to select mature cysts, as previously described in [12], would have introduced artifacts in cyst wall composition, these changes would probably not have evolved further as a function of FLA age. Therefore, this treatment is presumably not a main factor influencing the floatability of cysts as a function of their age.
During encystment, A. castellanii degrades some organelles including digestive vacuoles or mitochondria, as well as several macromolecules such as proteins, phospholipids, or glycogen [14]. These degradation products could then be used as a source of energy in the FLA cyst which is metabolically dormant, leading to a decrease of dry weight during cyst formation. Accordingly, in mammalian cells, different volumetric mass densities have been measured during the cell cycle of a cell line, as well as between cell lines, probably because of different quantities of biochemical components such as proteins or nucleotides [26-28]. Therefore, a third hypothesis, presumably the most plausible, could be emitted in the current study assuming that macromolecule degradation would continue after cyst formation, during their aging, leading to a decrease of their dry weight as well as their volumetric mass density, and therefore to their redistribution to upper water levels. Besides this possibility, within the cyst wall of A. castellanii, as well as V. vermiformis, the endocyst is separated from the ectocyst by a space that could contribute to cyst floating in upper levels of the water column, in opposition to B. mandrillaris, where the cyst layers are more compact [12,17]. According to our data, this process of cyst maturation after encystment would occur in A. castellanii and, to a lower extent, in V. vermiformis, but not in B. mandrillaris where no variation of FLA cyst distribution was observed as a function of their age. Therefore, besides the role of the tidal range in the enrichment of FLA in the biofilms collected in the surface of DWST [2], this phenomenon of volumetric mass density decrease as a function of cyst age would also contribute, independently of the biofilm formation, to the increase of some FLA species at the surface water level and thereafter in the biofilm. In particular, as A. castellanii and V. vermiformis, are accumulated at surface water levels as a function of their age, these FLA species should be particularly further monitored at the surface of DWSTs, as they could constitute a reservoir of pathogenic microorganisms and therefore a potential risk for human health.
In future works, as the FLA cyst age appears to influence cyst distribution in a water column, the analysis of biochemical compositions of young and old cysts of different FLA species would allow to examine more in detail the process of FLA cyst maturation after encystment, and determine its role in cyst distribution in a water column. Furthermore, the comparison of the distribution of young cysts of A. castellanii in a model of water reservoir presenting a tidal range with the dispersion of old cysts of the same FLA species in a water column privated of tidal range would allow to determine the relative importances of tidal range and FLA cyst age in the distribution of cysts in a DWST.
Acknowledgments
This work was funded by the Syndicat des Eaux d’Ile-De-France (grant N° 18HR2863) and has benefited from the facility VERRE SCIEN-TECH of the Faculté de Pharmacie – Université Paris Saclay. We are thankful to Lukas Montejo for critically reading the manuscript.
References
- Rodriguez-Zaragoza, S. Ecology of free-living amoebae. Crit. Rev. Microbiol. 1994, 20, 225–241. [CrossRef]
- Taravaud, A.; Ali, M.; Lafosse, B.; Nicolas, V.; Féliers, C.; Thibert, S.; Lévi, Y.; Loiseau, P.M.; Pomel, S. Enrichment of free-living amoebae in biofilms developed at upper water levels in drinking water storage towers: An inter-and intra-seasonal study. Sci. Total Environ. 2018, 633, 157-166. [CrossRef]
- Khan, N.A. Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol. Rev. 2006, 30, 564–595. [CrossRef]
- Lorenzo-Morales, J.; Khan, N.A.; Walochnik, J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite 2015, 22, 10. [CrossRef]
- Visvesvara, G.S.; Moura, H.; Schuster, F.L. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol. Med. Microbiol. 2007, 50, 1–26. [CrossRef]
- Storey, M.V.; Winiecka-Krusnell, J.; Ashbolt, N.J.; Stenström, T.A. The efficacy of heat and chlorine treatment against thermotolerant Acanthamoebae and Legionellae. Scand. J. Infect. Dis. 2004, 36, 656-662. [CrossRef]
- Thomas, V.; Bouchez, T.; Nicolas, V.; Robert, S.; Loret, J.F.; Lévi, Y. Amoebae in domestic water systems: resistance to disinfection treatments and implication in Legionella persistence. J. Appl. Microbiol. 2004, 97, 950-963. [CrossRef]
- Cervero-Arago, S.; Sommer, R.; Araujo R.M. Effect of UV irradiation (253.7 nm) on free Legionella and Legionella associated with its amoebae hosts. Water Res. 2014, 67, 299-309. [CrossRef]
- Thomas, V.; Loret, J.F.; Jousset, M.; Greub, G. Biodiversity and amoebae and amoebae-resisting bacteria in a drinking water treatment plant. Environ. Microbiol. 2008, 10, 2728-2745. [CrossRef]
- Fechtali-Moute, Z.; Loiseau, P.M.; Pomel, S. Stimulation of Acanthamoeba castellanii excystment by enzyme treatment and consequences on trophozoite growth. Front. Cell Dev. Biol. 2022, 10, 982897. [CrossRef]
- Schuster, F.L.; Visvesvara G.S. Axenic growth and drug sensitivity studies of Balamuthia mandrillaris, an agent of amoebic meningoencephalitis in humans and other animals. J. Clin. Microbiol. 1996, 34, 385-388. [CrossRef]
- Fouque, E.; Trouilhé, M.C.; Thomas, V.; Hartemann, P.; Rodier, M.H.; Héchard, Y. Cellular, biochemical, and molecular changes during encystment of free-living amoebae. Eukaryot. Cell 2012, 11, 382–7. [CrossRef]
- Neff, R.J.; Ray, S.A.; Benton, W.F.; Wilborn, M. Chapter 4 Induction of Synchronous Encystment (Differentiation) in Acanthamoeba sp. Methods Cell Biol. 1964, 1, 55-83. [CrossRef]
- Bowers, B.; Korn, E.D. The fine structure of Acanthamoeba castellanii (Neff strain). II. Encystment. J. Cell Biol. 1969, 41, 786-805. [CrossRef]
- Dykova, I.; Pindova, Z.; Fiala, I.; Dvorakova, H.; Machackova, B. Fish-isolated strains of Hartmannella vermiformis Page, 1967: morphology, phylogeny and molecular diagnosis of the species in tissue lesions. Folia Parasitol. 2005, 52, 295-303. [CrossRef]
- Visvesvara, G.S.; Schuster, F.L.; Martinez, A. Balamuthia mandrillaris, N. G., N. sp., agent of amebic meningoencephalitis in humans and other animals. J. Eukaryot. Microbiol. 1993, 40, 504- 514. [CrossRef]
- Siddiqui, R.; Jarroll, E.L.; Khan, N.A. Balamuthia mandrillaris: staining properties of cysts and trophozoites and the effect of 2,6-dichlorobenzonitrile and calcofluor white on encystment. J. Eukaryot. Microbiol. 2009, 56, 136-141. [CrossRef]
- Siddiqui, R.; Khan, N.A.; Jarroll, E.L. The cyst wall carbohydrate composition of Balamuthia mandrillaris. Parasitol. Res. 2009, 104, 1439–1443. [CrossRef]
- Weisman, R.A. Differentiation in Acanthamoeba castellanii. Annu. Rev. Microbiol. 1976, 30, 189–219. [CrossRef]
- Chávez-Munguía, B.; Omaña-Molina, M.; González-Lázaro, M.; González-Robles, A.; Bonilla, P.; Martínez-Palomo, A. Ultrastructural study of encystation and excystation in Acanthamoeba castellanii. J. Eukaryot. Microbiol. 2005, 52, 153–8. [CrossRef]
- Dudley, R.; Alsam, S.; Khan, N.A. Cellulose biosynthesis pathway is a potential target in the improved treatment of Acanthamoeba keratitis. Appl. Microbiol. Biotechnol. 2007, 75, 133-140. [CrossRef]
- Upadhyay, J.M.; Crow, S.; Cox, A. The cyst wall composition of Hartmannella glebae. Proc. Soc. Exp. Biol. Med. 1984, 175, 424-428. [CrossRef]
- Daicho, K.; Kobayashi, K.; Fujisawa, S.; Saito, T. Crystallinity-independent yet modification-dependent true density of nanocellulose. Biomacromolecules 2020, 21, 939-945. [CrossRef]
- Wang, Y.; Jiang, L.; Zhao, Y.; Ju, X.; Wang, L.; Jin, L.; Fine, R.D.; Li, M. Biological characteristics and pathogenicity of Acanthamoeba. Front. Microbiol. 2023, 14, 1147077. [CrossRef]
- Fouque, E.; Trouilhé, M.C.; Thomas, V.; Humeau, P.; Héchard, Y. Encystment of Vermamoeba (Hartmanella) vermiformis: effects of environmental conditions and cell concentration. Exp. Parasitol. 2014, 145, S62-S68. [CrossRef]
- Bryan, A.K.; Hecht, V.C.; Shen, W.; Payer, K.; Grover, W.H., Manalis, S.R. Measuring single cell mass, volume, and density with dual suspended microchannel resonators. Lab. Chip 2014, 14, 569-576. [CrossRef]
- Zlotek-Zlotkiewicz, E.; Monnier, S.; Cappello, G.; Le Berre, M.; Piel, M. Optical volume and mass measurements show that mammalian cells swell during mitosis. J. Cell Biol. 2015, 211, 765-774. [CrossRef]
- Miettinen, T.P.; Ly, K.S.; Lam, A.; Manalis, S.R. Single-cell monitoring of dry mass and dry mass density reveals exocytosis of cellular dry content in mitosis. eLife 2022, 11, e76664. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).