Submitted:
17 January 2024
Posted:
18 January 2024
You are already at the latest version
Abstract
Keywords:
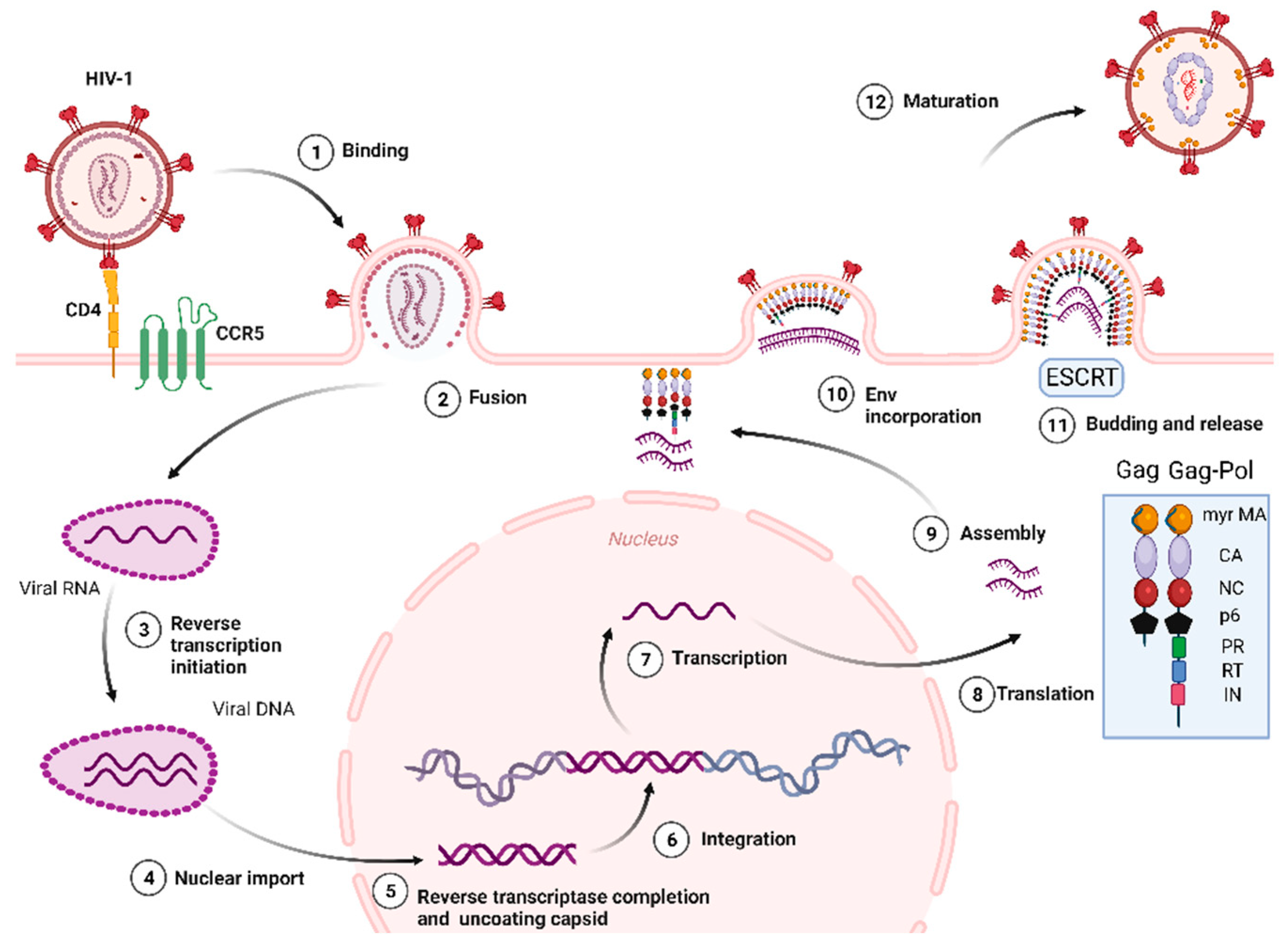
1. Introduction
2. United States Food and Drug Administration (FDA) Approved Drugs for HIV-1 Treatment
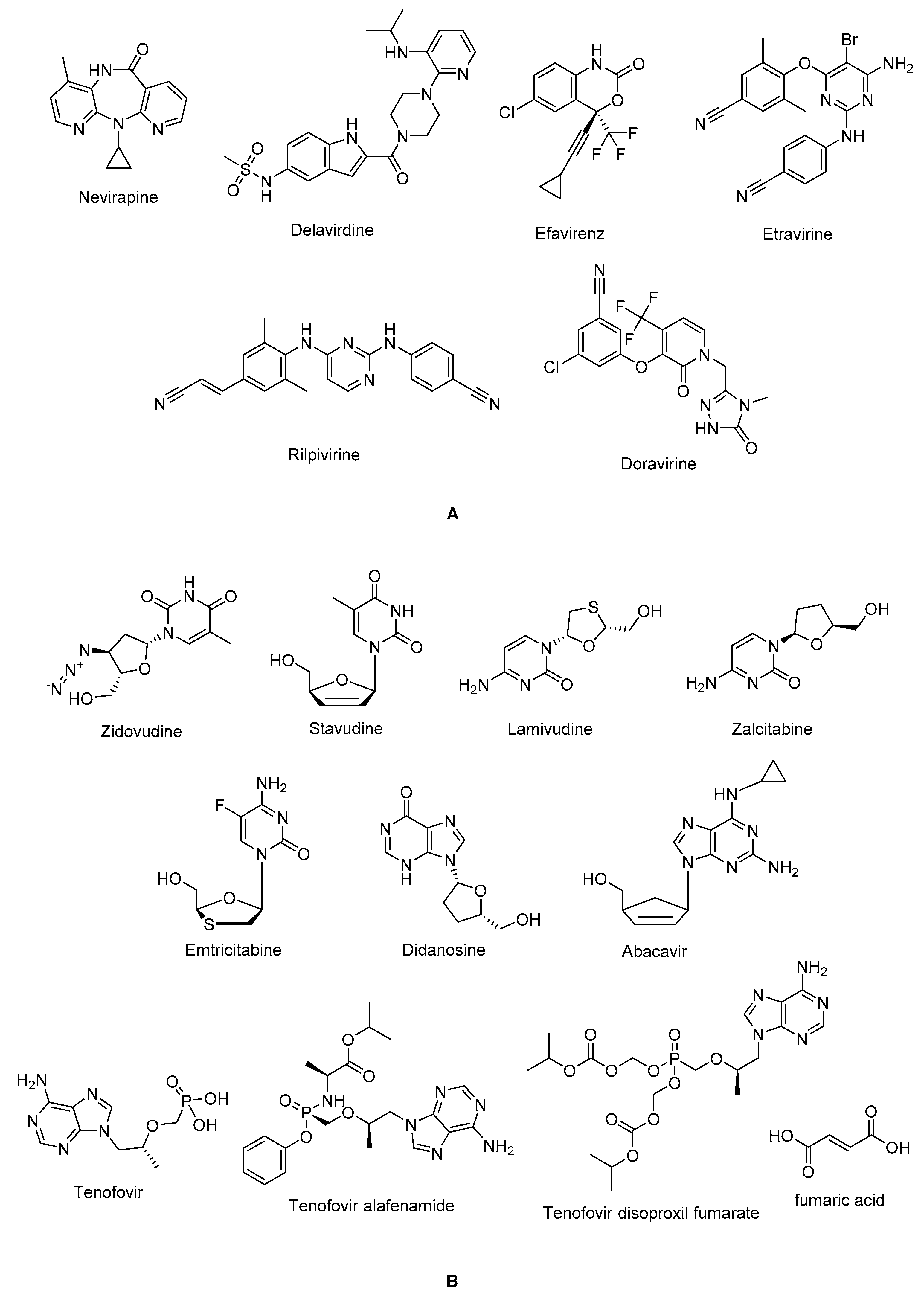
2.1. Reverse Transcriptase Inhibitors
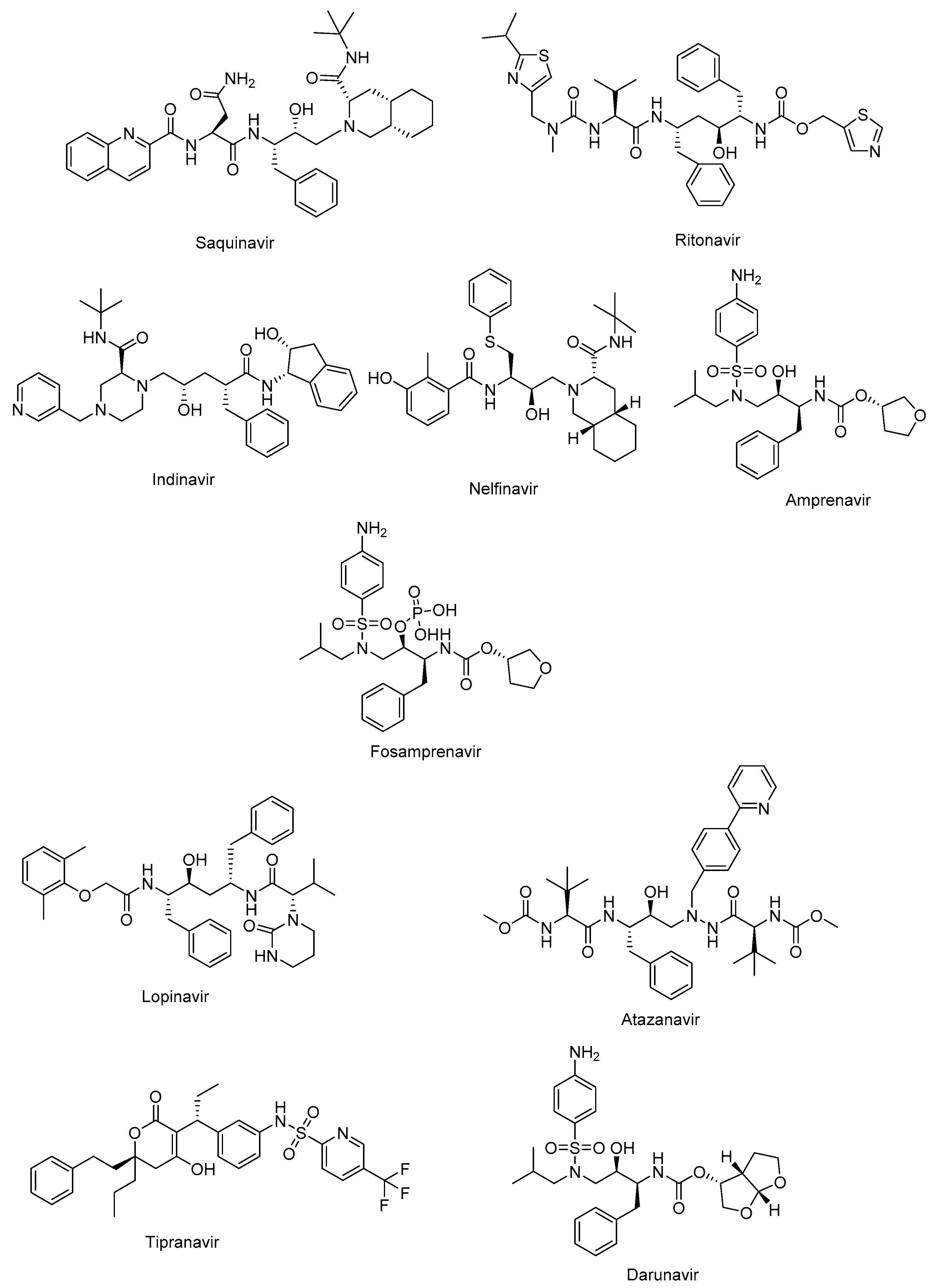
2.2. Protease Inhibitors
2.3. Fusion Inhibitors
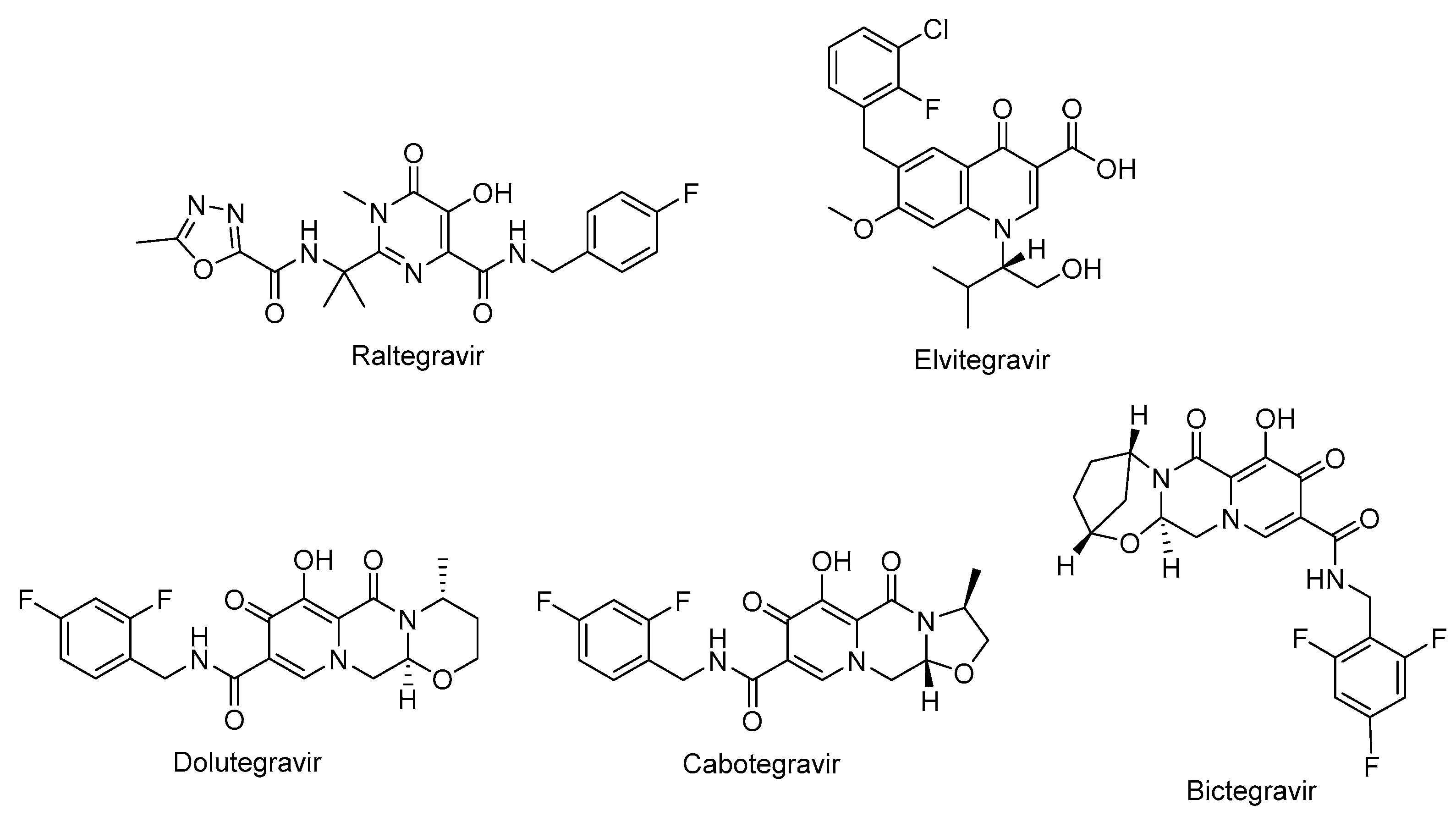
2.4. HIV-1 Integrase Inhibitors
2.5. CA Inhibitor
2.6. Pharmacokinetic Enhancers
2.7. Standard Two-Drug, Three-Drug, and Four-Drug Regimens and Fixed-Dose Combinations as cART Options
3. The Other Anti-Gag Compounds
3.1. MA Inhibitors
3.2. Maturation Inhibitors
4. Strategies for Eradication of HIV-1
4.1. “Shock and Kill (Kick and Kill)” and “Block and Lock”
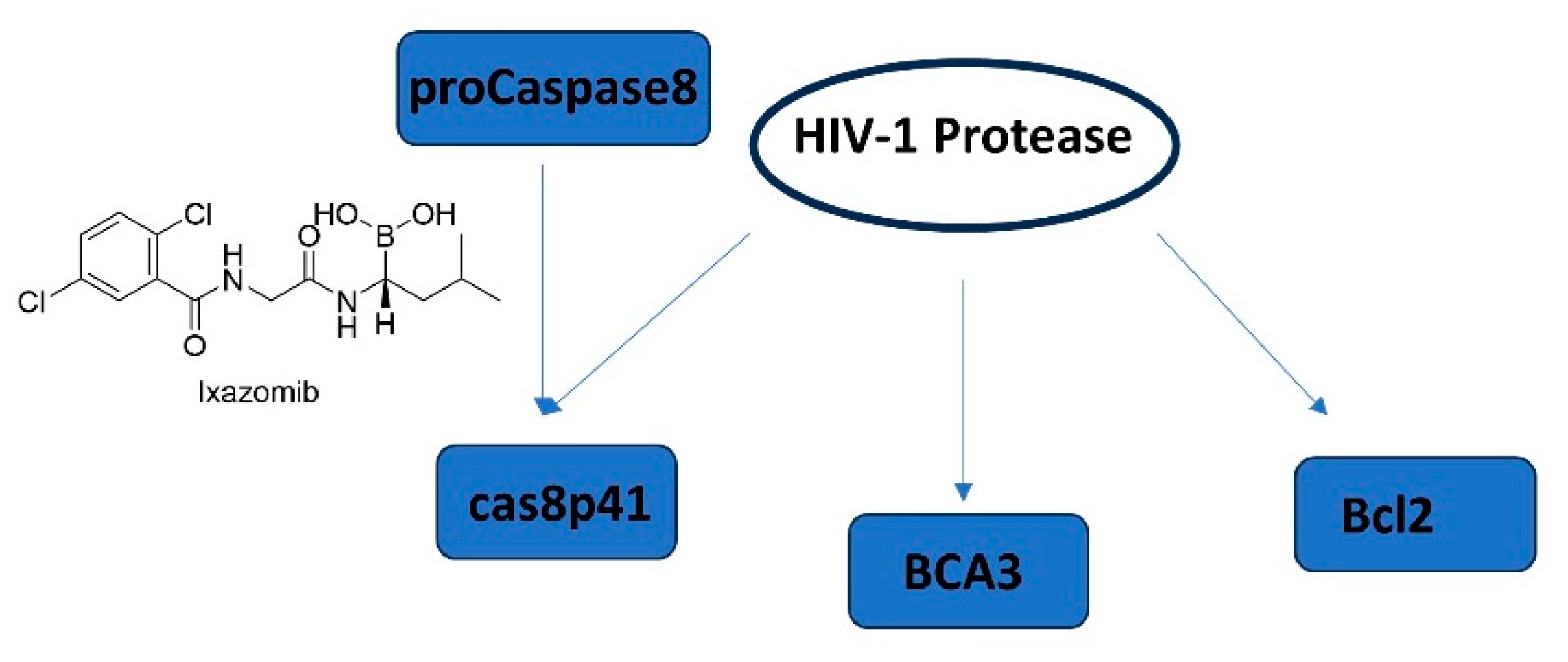
4.2. A New Approach: “Lock-in Apoptosis” and Compound L-HIPPO
4.3. Apoptosis and HIV Latency
5. Conclusions and Future Perspectives
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- German Advisory Committee Blood (Arbeitskreis Blut), Subgroup ‘Assessment of Pathogens Transmissible by Blood’. Human Immunodeficiency Virus (HIV). Transfus Med Hemother 2016, 43, 203-22. [CrossRef]
- Bbosa, N.; Kaleebu P, Ssemwanga D. HIV subtype diversity worldwide. Curr Opin HIV AIDS 2019, 14, 153-160. [CrossRef]
- Kwon, D.; Han, M.J.; Seo, K.W.; Kang, K.S. Combinatorial Strategies for Long-term Control of HIV Infection. AIDS Rev 2020, 22, 175-182. [CrossRef]
- Moranguinho, I.; Taveira, N.; Bártolo, I. Antiretroviral Treatment of HIV-2 Infection: Available Drugs Resistance Pathways, and Promising New Compounds. Int J Mol Sci 2023, 24, 5905. [CrossRef]
- 5. World Health Organization, HIV and AIDS. https://www.who.int/news-room/fact-sheets/detail/hiv-aids, (accessed on 13 July 2023).
- Summers, N.A.; Armstrong, W.S. Management of Advanced HIV Disease. Infect Dis Clin North Am 2019, 33, 743-767. [CrossRef]
- Foka, F.E.T.; Mufhandu, H.T. Current ARTs, Virologic Failure, and Implications for AIDS Management: A Systematic Review. Viruses 2023, 15, 1732. [CrossRef]
- Lehman, A.; Ellis, J.; Nalintya, E.; Bahr, N.C.; Loyse, A.; Rajasingham, R. Advanced HIV disease: A review of diagnostic and prophylactic strategies. HIV Med 2023, 24, 859-876. [CrossRef]
- Shafran, S.D.; Di Perri, G.; Esser, S.; Lelièvre, J.D.; Parczewski, M. Planning HIV therapy to prevent future comorbidities: patient years for tenofovir alafenamide. HIV Med 2019, 20, 1-16. [CrossRef]
- Siliciano, J.D.; Siliciano, R.F. In Vivo Dynamics of the Latent Reservoir for HIV-1: New Insights and Implications for Cure. Annu Rev Pathol 2022, 17, 271-294. [CrossRef]
- Gandhi, R.T.; Bedimo, R.; Hoy, J.F.; Landovitz, R.J.; Smith, D.M.; Eaton, E.F.; Lehmann, C.; Springer, S.A.; Sax, P.E.; Thompson, M.A.; Benson, C.A.; Buchbinder, S.P.; Del Rio, C.; Eron, J.J. Jr.; Günthard, H.F.; Molina, J.M.; Jacobsen, D.M.; Saag, M.S. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2022 Recommendations of the International Antiviral Society-USA Panel. JAMA 2023, 329, 63-84. [CrossRef]
- Konrad, B.P.; Taylor, D.; Conway, J.M.; Ogilvie, G.S.; Coombs, D. On the duration of the period between exposure to HIV and detectable infection. Epidemics 2017, 20, 73-83. [CrossRef]
- Bekker, L.G.; Beyrer, C.; Mgodi, N.; Lewin, S.R.; Delany-Moretlwe, S.; Taiwo, B.; Masters, M.C.; Lazarus, J.V. HIV infection. Nat Rev Dis Primers 2023, 9, 42. [CrossRef]
- van Marle, G.; Church, D.L.; van der Meer, F.; Gill, M.J. Combating the HIV reservoirs. Biotechnol Genet Eng Rev 2018, 34, 76-89. [CrossRef]
- Park, J.; Morrow, C.D. Overexpression of the gag-pol precursor from human immunodeficiency virus type 1 proviral genomes results in efficient proteolytic processing in the absence of virion production. J Virol 1991, 65, 5111-5117. [CrossRef]
- Hamard-Peron, E.; Muriaux, D. Retroviral matrix and lipids, the intimate interaction. Retrovirology 2011, 8, 15. [CrossRef]
- Mattei, S.; Schur, F.K.; Briggs, J.A. Retrovirus maturation-an extraordinary structural transformation. Curr Opin Virol 2016, 18, 27-35. [CrossRef]
- Nagata, S.; Imai, J.; Makino, G.; Tomita, M.; Kanai, A. Evolutionary Analysis of HIV-1 Pol Proteins Reveals Representative Residues for Viral Subtype Differentiation. Front Microbiol 2017, 8, 2151. [CrossRef]
- Klingler, J.; Anton, H.; Réal, E.; Zeiger, M.; Moog, C., Mély, Y.; Boutant, E. How HIV-1 Gag Manipulates Its Host Cell Proteins: A Focus on Interactors of the Nucleocapsid Domain. Viruses 2020, 12, 888. [CrossRef]
- Azimi, F.C.; Lee, J.E. Structural perspectives on HIV-1 Vif and APOBEC3 restriction factor interactions. Protein Sci 2020, 29, 391-406. [CrossRef]
- Faust, T.B.; Binning, J.M.; Gross, J.D.; Frankel, A.D. Making Sense of Multifunctional Proteins: Human Immunodeficiency Virus Type 1 Accessory and Regulatory Proteins and Connections to Transcription. Annu Rev Virol 2017, 4, 241-260. [CrossRef]
- Müller, T.G.; Zila, V.; Peters, K.; Schifferdecker, S.; Stanic, M.; Lucic, B.; Laketa, V.; Lusic, M.; Müller, B.; Kräusslich, H.G. HIV-1 uncoating by release of viral cDNA from capsid-like structures in the nucleus of infected cells. Elife 2021, 10, e64776. [CrossRef]
- Alkhatib, G. The biology of CCR5 and CXCR4. Curr Opin HIV AIDS 2009, 4, 96-103. [CrossRef]
- Sundquist, W.I.; Kräusslich, H.G. HIV-1 assembly, budding, and maturation. Cold Spring Harb Perspect Med 2012, 2, a006924. [CrossRef]
- Meng, B.; Ip, N.C.; Prestwood, L.J.; Abbink, T.E.; Lever, A.M. Evidence that the endosomal sorting complex required for transport-II (ESCRT-II) is required for efficient human immunodeficiency virus-1 (HIV-1) production. Retrovirology 2015, 12, 72. [CrossRef]
- Kleinpeter, A.B.; Freed, E.O. HIV-1 Maturation: Lessons Learned from Inhibitors. Viruses 2020, 12, 940. [CrossRef]
- Tompa, D.R.; Immanuel, A.; Srikanth, S.; Kadhirvel, S. Trends and strategies to combat viral infections: A review on FDA approved antiviral drugs. Int J Biol Macromol 2021, 172, 524-541. [CrossRef]
- Gupta, M.; Pak, A.J.; Voth, G.A. Critical mechanistic features of HIV-1 viral capsid assembly. Sci Adv 2023, 9, eadd7434. [CrossRef]
- Atta, M.G.; De Seigneux, S.; Lucas, G.M. Clinical Pharmacology in HIV Therapy. Clin J Am Soc Nephrol 2019, 14, 435-444. [CrossRef]
- Maeda, K.; Das, D.; Kobayakawa, T.; Tamamura, H.; Takeuchi, H. Discovery and Development of Anti-HIV Therapeutic Agents: Progress Towards Improved HIV Medication. Curr Top Med Chem 2019, 19, 1621-1649. [CrossRef]
- Deng, C.; Yan, H.; Wang, J.; Liu, B.; Liu, K.; Shi, Y. The anti-HIV potential of imidazole, oxazole and thiazole hybrids: A mini-review. Arab J Chem 2022, 15, 104242. [CrossRef]
- Vanangamudi, M.; Kurup, S.; Namasivayam, V. Non-nucleoside reverse transcriptase inhibitors (NNRTIs): A brief overview of clinically approved drugs and combination regimens. Curr Opin Pharmacol 2020, 54, 179-187. [CrossRef]
- Ghosn, J.; Taiwo, B.; Seedat, S.; Autran, B.; Katlama, C. HIV. Lancet 2018, 392, 685-697. [CrossRef]
- García-Deltoro, M. Rapid Initiation of Antiretroviral Therapy after HIV Diagnosis. AIDS Rev 2019, 21, 55-64. [CrossRef]
- Phanuphak, N.; Gulick, R.M. HIV treatment and prevention 2019: Current standards of care. Curr Opin HIV AIDS 2020, 15, 4-12. [CrossRef]
- Achhra, A.C.; Mwasakifwa, G.; Amin, J.; Boyd, M.A. Efficacy and safety of contemporary dual-drug antiretroviral regimens as first-line treatment or as a simplification strategy: a systematic review and meta-analysis. Lancet HIV 2016, 3, e351-e360. [CrossRef]
- Orkin, C.; Llibre, J.M.; Gallien, S.; Antinori, A.; Behrens, G.; Carr, A. Nucleoside reverse transcriptase inhibitor-reducing strategies in HIV treatment: assessing the evidence. HIV Med 2018, 19, 18-32. [CrossRef]
- Zhang, K.; Zhang, Y.; Liu, X.; Li, A.; Gao, M.; Hou, J.; Guo, C.; Zhang, T.; Wu, H.; Chen, G.; Huang, X. Three-Drug Regimens Containing Integrase Inhibitor Show Good Efficacy and Safety in Treatment-Naive Patients With HIV-1: A Bayesian Analysis. Front Pharmacol 2021, 12, 603068. [CrossRef]
- Burns, J.E.; Stöhr, W.; Kinloch-De Loes, S.; Fox, J.; Clarke, A.; Nelson, M.; Thornhill, J.; Babiker, A.; Frater, J.; Pett, S.L.; Fidler, S. Tolerability of four-drug antiretroviral combination therapy in primary HIV-1 infection. HIV Med 2021, 22, 770-774. [CrossRef]
- Flexner, C. Modern Human Immunodeficiency Virus Therapy: Progress and Prospects. Clin Pharmacol Ther 2019, 105, 61-70. [CrossRef]
- Shyr, Z.A.; Cheng, Y.S.; Lo, D.C.; Zheng, W. Drug combination therapy for emerging viral diseases. Drug Discov Today 2021, 26, 2367-2376. [CrossRef]
- Esté, J.A.; Cihlar, T. Current status and challenges of antiretroviral research and therapy. Antiviral Res 2010, 85, 25-33. [CrossRef]
- Hawkins, T. Understanding and managing the adverse effects of antiretroviral therapy. Antiviral Res 2010, 85, 201-209. [CrossRef]
- Addis, D.R.; DeBerry, J.J.; Aggarwal, S. Chronic Pain in HIV. Mol Pain 2020, 16, 1744806920927276. [CrossRef]
- Cambou, M.C.; Landovitz, R.J. Novel Antiretroviral Agents. Curr HIV/AIDS Rep 2020, 17, 118-124. [CrossRef]
- Chatzidimitriou, D.; Tsotridou, E.; Grigoropoulos, P.; Skoura, L. HIV-1: Towards understanding the nature and quantifying the latent reservoir. Acta Virol 2020, 64, 3-9. [CrossRef]
- Dubrocq, G.; Rakhmanina, N. Antiretroviral therapy interruptions: impact on HIV treatment and transmission. HIV AIDS (Auckl) 2018, 10, 91-101. [CrossRef]
- Kalidasan, V.; Theva Das, K. Lessons Learned From Failures and Success Stories of HIV Breakthroughs: Are We Getting Closer to an HIV Cure? Front Microbiol 2020, 11, 46. [CrossRef]
- Bailey, H.; Zash, R.; Rasi, V.; Thorne, C. HIV treatment in pregnancy. Lancet HIV 2018, 5, e457-e467. [CrossRef]
- Singh, K.; Marchand, B.; Kirby, K.A.; Michailidis, E.; Sarafianos, S.G. Structural Aspects of Drug Resistance and Inhibition of HIV-1 Reverse Transcriptase. Viruses 2010, 2, 606-638. [CrossRef]
- Cilento, M.E.; Kirby, K.A.; Sarafianos, S.G. Avoiding Drug Resistance in HIV Reverse Transcriptase. Chem Rev 2021, 121, 3271-3296. [CrossRef]
- Deng, C.; Yan, H.; Wang, J.; Liu, K.; Liu, B.; Shi, Y. Current scenario on non-nucleoside reverse transcriptase inhibitors (2018-present). Arab J Chem 2022, 15, 104378. [CrossRef]
- Wang, Z.; Cherukupalli, S.; Xie, M.; Wang, W.; Jiang, X.; Jia, R.; Pannecouque, C.; De Clercq, E.; Kang, D.; Zhan, P.; Liu, X. Contemporary Medicinal Chemistry Strategies for the Discovery and Development of Novel HIV-1 Non-nucleoside Reverse Transcriptase Inhibitors. J Med Chem 2022, 65, 3729-3757. [CrossRef]
- Podzamczer, D.; Fumero, E. The role of nevirapine in the treatment of HIV-1 disease. Expert Opin Pharmacother 2001, 2, 2065-78. [CrossRef]
- Ren, J.; Stammers, D.K. Structural basis for drug resistance mechanisms for non-nucleoside inhibitors of HIV reverse transcriptase. Virus Res 2008, 134, 157-70. [CrossRef]
- Costa, B.; Vale, N. Efavirenz: History, Development and Future. Biomolecules 2022, 13, 88. [CrossRef]
- Havens, J.P.; Podany, A.T.; Scarsi, K.K.; Fletcher, C.V. Clinical Pharmacokinetics and Pharmacodynamics of Etravirine: An Updated Review. Clin Pharmacokinet 2020, 59, 137-154. [CrossRef]
- Sharma, M.; Saravolatz, L.D. Rilpivirine: a new non-nucleoside reverse transcriptase inhibitor. J Antimicrob Chemother 2013, 68, 250-256. [CrossRef]
- Ferretti, F.; Boffito, M. Rilpivirine long-acting for the prevention and treatment of HIV infection. Curr Opin HIV AIDS 2018, 13, 300-307. [CrossRef]
- Mu, Y.; Pham, M.; Podany, A.T.; Cory, T.J. Evaluating emtricitabine + rilpivirine + tenofovir alafenamide in combination for the treatment of HIV-infection. Expert Opin Pharmacother 2020, 21, 389-397. [CrossRef]
- Colombier, M.A.; Molina, J.M. Doravirine: a review. Curr Opin HIV AIDS 2018, 13, 308-314. [CrossRef]
- Deeks, E.D. Doravirine: First Global Approval. Drugs 2018, 78, 1643-1650. [CrossRef]
- Pham, H.T.; Xiao, M.A.; Principe, M.A.; Wong, A.; Mesplède, T. Pharmaceutical, clinical, and resistance information on doravirine, a novel non-nucleoside reverse transcriptase inhibitor for the treatment of HIV-1 infection. Drugs Context 2020, 9, 2019-11-4. [CrossRef]
- Shin, Y.H.; Park, C.M.; Yoon, C.H. An Overview of Human Immunodeficiency Virus-1 Antiretroviral Drugs: General Principles and Current Status. Infect Chemother 2021, 53, 29-45. [CrossRef]
- Wonganan, P.; Limpanasithikul, W.; Jianmongkol, S.; Kerr, S.J.; Ruxrungtham, K. Pharmacokinetics of nucleoside/nucleotide reverse transcriptase inhibitors for the treatment and prevention of HIV infection. Expert Opin Drug Metab Toxicol 2020, 16, 551-564. [CrossRef]
- Yoshida, Y.; Honma, M.; Kimura, Y.; Abe, H. Structure, Synthesis and Inhibition Mechanism of Nucleoside Analogues as HIV-1 Reverse Transcriptase Inhibitors (NRTIs). ChemMedChem 2021, 16, 743-766. [CrossRef]
- Fraley, A.W.; Chen, D.; Johnson, K.; McLaughlin, L.W. An HIV reverse transcriptase-selective nucleoside chain terminator. J Am Chem Soc 2003, 125, 616-617. [CrossRef]
- Holec, A.D.; Mandal, S.; Prathipati, P.K.; Destache, C.J. Nucleotide Reverse Transcriptase Inhibitors: A Thorough Review, Present Status and Future Perspective as HIV Therapeutics. Curr HIV Res 2017, 15, 411-421. [CrossRef]
- Bianco, M.D.C.A.D.; Inacio Leite, D.; Silva Castelo Branco, F.; Boechat, N.; Uliassi, E.; Bolognesi, M.L.; Bastos, M.M. The Use of Zidovudine Pharmacophore in Multi-Target-Directed Ligands for AIDS Therapy. Molecules 2022, 27, 8502. [CrossRef]
- Quercia, R.; Perno, C.F.; Koteff, J.; Moore, K.; McCoig, C.; St Clair, M.; Kuritzkes, D. Twenty-Five Years of Lamivudine: Current and Future Use for the Treatment of HIV-1 Infection. J Acquir Immune Defic Syndr 2018, 78, 125-135. [CrossRef]
- Cahn, P. Emtricitabine: a new nucleoside analogue for once-daily antiretroviral therapy. Expert Opin Investig Drugs 2004, 13, 55-68. [CrossRef]
- Cusato, J.; Allegra, S.; Nicolò, A.; Calcagno, A.; D’Avolio, A. Precision medicine for HIV: where are we? Pharmacogenomics 2018, 19, 145-165. [CrossRef]
- Piliero, P.J. Pharmacokinetic properties of nucleoside/nucleotide reverse transcriptase inhibitors. J Acquir Immune Defic Syndr 2004, 37 Suppl 1, S2-S12. [CrossRef]
- Saag, M.S.; Gandhi, R.T.; Hoy, J.F.; Landovitz, R.J.; Thompson, M.A.; Sax, P.E.; Smith, D.M.; Benson, C.A.; Buchbinder, S.P.; Del Rio, C.; Eron, J.J. Jr.; Fätkenheuer, G.; Günthard, H.F.; Molina, J.M.; Jacobsen, D.M.; Volberding, P.A. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2020 Recommendations of the International Antiviral Society-USA Panel. JAMA 2020, 324, 1651-1669. [CrossRef]
- Li, G.; Wang, Y.; De Clercq, E. Approved HIV reverse transcriptase inhibitors in the past decade. Acta Pharm Sin B 2022, 12, 1567-1590. [CrossRef]
- Schinazi, R.F.; Patel, D.; Ehteshami, M. The best backbone for HIV prevention, treatment, and elimination: Emtricitabine+tenofovir. Antivir Ther 2022, 27, 13596535211067599. [CrossRef]
- Louis, J.M.; Ishima, R.; Torchia, D.A.; Weber, I.T. HIV-1 protease: structure, dynamics, and inhibition. Adv Pharmacol 2007, 55, 261-298. [CrossRef]
- Huang, L.; Chen, C. Understanding HIV-1 protease autoprocessing for novel therapeutic development. Future Med Chem 2013, 5, 1215-1229. [CrossRef]
- Konvalinka, J.; Kräusslich, H.G.; Müller, B. Retroviral proteases and their roles in virion maturation. Virology 2015, 479-480, 403-417. [CrossRef]
- Majerová, T.; Konvalinka, J. Viral proteases as therapeutic targets. Mol Aspects Med 2022, 88, 101159. [CrossRef]
- Tabler, C.O.; Wegman, S.J.; Chen, J.; Shroff, H.; Alhusaini, N.; Tilton, J.C. The HIV-1 Viral Protease Is Activated during Assembly and Budding Prior to Particle Release. J Virol 2022, 96, e0219821. [CrossRef]
- Brik, A.; Wong, C.H. HIV-1 protease: mechanism and drug discovery. Org Biomol Chem 2003, 1, 5-14. [CrossRef]
- Ghosh, A.K.; Osswald, H.L.; Prato, G. Recent Progress in the Development of HIV-1 Protease Inhibitors for the Treatment of HIV/AIDS. J Med Chem 2016, 59, 5172-5208. [CrossRef]
- Eron, J.J. HIV-1 Protease Inhibitors. Clin Infect Dis 2000, 30, S160–S170. [CrossRef]
- Lv, Z.; Chu, Y.; Wang, Y. HIV protease inhibitors: a review of molecular selectivity and toxicity. HIV AIDS (Auckl) 2015, 7, 95-104. [CrossRef]
- Weber, I.T.; Wang, Y.F.; Harrison, R.W. HIV Protease: Historical Perspective and Current Research. Viruses 2021, 13, 839. [CrossRef]
- Nascimento, A.L.C.S.; Fernandes, R.P.; Quijia, C.; Araujo, V.H.S.; Pereira, J.; Garcia, J.S.; Trevisan, M.G.; Chorilli, M. Pharmacokinetic Parameters of HIV-1 Protease Inhibitors. ChemMedChem 2020, 15, 1018-1029. [CrossRef]
- Chan-Tack, K.M.; Struble, K.A.; Birnkrant, D.B. Intracranial hemorrhage and liver-associated deaths associated with tipranavir/ritonavir: review of cases from the FDA’s Adverse Event Reporting System. AIDS Patient Care STDS 2008, 22, 843-850. [CrossRef]
- Orman, J.S.; Perry, C.M. Tipranavir: a review of its use in the management of HIV infection. Drugs 2008, 68, 1435-1463. [CrossRef]
- Spagnuolo, V.; Castagna, A.; Lazzarin, A. Darunavir for the treatment of HIV infection. Expert Opin Pharmacother 2018, 19, 1149-1163. [CrossRef]
- Squillace, N.; Bozzi, G.; Colella, E.; Gori, A.; Bandera, A. Darunavir-cobicistat-emtricitabine-tenofovir alafenamide: safety and efficacy of a protease inhibitor in the modern era. Drug Des Devel Ther 2018, 12, 3635-3643. [CrossRef]
- Doms, R.W.; Moore, J.P. HIV-1 membrane fusion: targets of opportunity. J Cell Biol 2000, 151, F9-14. [CrossRef]
- Cai, L.; Jiang, S. Development of peptide and small-molecule HIV-1 fusion inhibitors that target gp41. ChemMedChem 2010, 5, 1813-1824. [CrossRef]
- Falkenhagen, A.; Joshi, S. HIV Entry and Its Inhibition by Bifunctional Antiviral Proteins. Mol Ther Nucleic Acids 2018, 13, 347-364. [CrossRef]
- Wang, C; Cheng, S.; Zhang, Y.; Ding, Y.; Chong, H.; Xing, H.; Jiang, S.; Li, X.; Ma, L. Long-Acting HIV-1 Fusion Inhibitory Peptides and their Mechanisms of Action. Viruses 2019, 11, 811. [CrossRef]
- Xiao, T.; Cai, Y.; Chen, B. HIV-1 Entry and Membrane Fusion Inhibitors. Viruses 2021, 13, 735. [CrossRef]
- Negi, G.; Sharma, A.; Dey, M.; Dhanawat, G.; Parveen, N. Membrane attachment and fusion of HIV-1, influenza A, and SARS-CoV-2: resolving the mechanisms with biophysical methods. Biophys Rev 2022, 14, 1109-1140. [CrossRef]
- Matthews, T.; Salgo, M.; Greenberg, M.; Chung, J.; DeMasi, R.; Bolognesi, D. Enfuvirtide: The first therapy to inhibit the entry of HIV-1 into host CD4 lymphocytes. Nat Rev Drug Discov 2004, 3, 215-225. [CrossRef]
- Pang, W.; Tam, S.C.; Zheng, Y.T. Current peptide HIV type-1 fusion inhibitors. Antivir Chem Chemother 2009, 20, 1-18. [CrossRef]
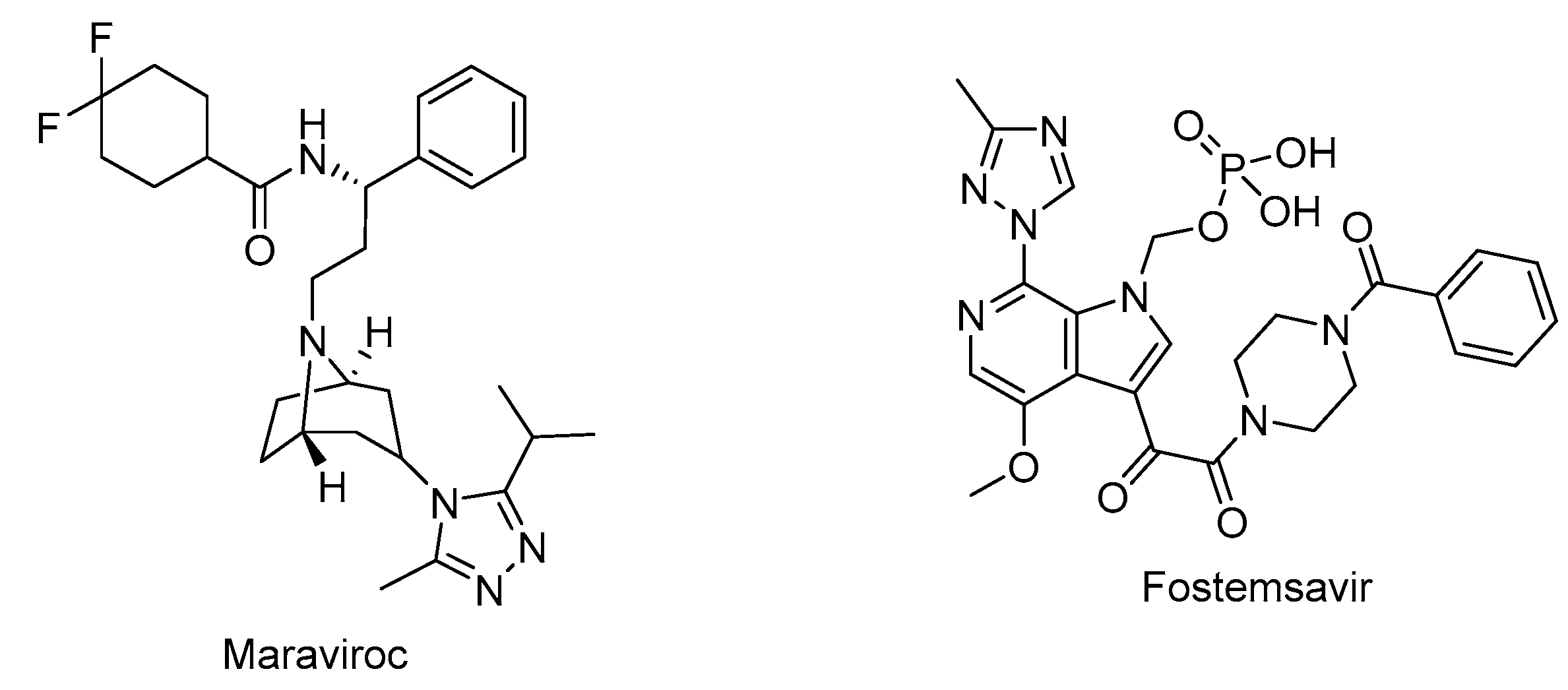
- Dorr, P.; Westby, M.; Dobbs, S.; Griffin, P.; Irvine, B.; Macartney, M.; Mori, J.; Rickett, G.; Smith-Burchnell, C.; Napier, C.; Webster, R.; Armour, D.; Price, D.; Stammen, B.; Wood, A.; Perros, M. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother 2005, 49, 4721-4732. [CrossRef]
- Tan, Q.; Zhu, Y.; Li, J.; Chen, Z.; Han, G.W.; Kufareva, I.; Li, T.; Ma, L.; Fenalti, G.; Li, J.; Zhang, W.; Xie, X.; Yang, H.; Jiang, H.; Cherezov, V.; Liu, H.; Stevens, R.C.; Zhao, Q.; Wu, B. Structure of the CCR5 chemokine receptor-HIV entry inhibitor maraviroc complex. Science 2013, 341, 1387-1390. [CrossRef]
- Kesavardhana, S.; Varadarajan, R. Stabilizing the native trimer of HIV-1 Env by destabilizing the heterodimeric interface of the gp41 postfusion six-helix bundle. J Virol 2014, 88, 9590-9604. [CrossRef]
- Woollard, S.M.; Kanmogne, G.D. Maraviroc: a review of its use in HIV infection and beyond. Drug Des Devel Ther 2015, 9, 5447-5468. [CrossRef]
- Freeman, M.M.; Seaman, M.S.; Rits-Volloch, S.; Hong, X.; Kao, C.Y.; Ho, D.D.; Chen, B. Crystal structure of HIV-1 primary receptor CD4 in complex with a potent antiviral antibody. Structure 2010, 18, 1632-1641. [CrossRef]
- Markham, A. Ibalizumab: First Global Approval. Drugs 2018, 78, 781-785. [CrossRef]
- Beccari, M.V.; Mogle, B.T.; Sidman, E.F.; Mastro, K.A.; Asiago-Reddy, E.; Kufel, W.D. Ibalizumab, a Novel Monoclonal Antibody for the Management of Multidrug-Resistant HIV-1 Infection. Antimicrob Agents Chemother 2019, 63, e00110-19. [CrossRef]
- Blair, H.A. Ibalizumab: A Review in Multidrug-Resistant HIV-1 Infection. Drugs 2020, 80, 189-196. [CrossRef]
- Chahine, E.B.; Durham, S.H. Ibalizumab: The First Monoclonal Antibody for the Treatment of HIV-1 Infection. Ann Pharmacother 2021, 55, 230-239. [CrossRef]
- Kozal, M.; Aberg, J.; Pialoux, G.; Cahn, P.; Thompson, M.; Molina, J.M.; Grinsztejn, B.; Diaz, R.; Castagna, A.; Kumar, P.; Latiff, G.; DeJesus, E.; Gummel, M.; Gartland, M.; Pierce, A.; Ackerman, P.; Llamoso, C.; Lataillade, M.; BRIGHTE Trial Team. Fostemsavir in Adults with Multidrug-Resistant HIV-1 Infection. N Engl J Med 2020, 382, 1232-1243. [CrossRef]
- Markham, A. Fostemsavir: First Approval. Drugs 2020, 80, 1485-1490. [CrossRef]
- Seval, N.; Frank, C.; Kozal, M. Fostemsavir for the treatment of HIV. Expert Rev Anti Infect Ther 2021, 19, 961-966. [CrossRef]
- Yuan, S.; Luo, Y.Q.; Zuo, J.H.; Liu, H.; Li, F.; Yu, B. New drug approvals for 2020: Synthesis and clinical applications. Eur J Med Chem 2021, 215, 113284. [CrossRef]
- Muccini, C.; Canetti, D.; Castagna, A.; Spagnuolo, V. Efficacy and Safety Profile of Fostemsavir for the Treatment of People with Human Immunodeficiency Virus-1 (HIV-1): Current Evidence and Place in Therapy. Drug Des Devel Ther 2022, 16, 297-304. [CrossRef]
- Krishnan, L.; Li, X.; Naraharisetty, H.L.; Hare, S.; Cherepanov, P.; Engelman, A. Structure-based modeling of the functional HIV-1 intasome and its inhibition. Proc Natl Acad Sci U S A 2010, 107, 15910-15915. [CrossRef]
- Engelman, A.N. Multifaceted HIV integrase functionalities and therapeutic strategies for their inhibition. J Biol Chem 2019, 294, 15137-15157. [CrossRef]
- Passos, D.O.; Li, M.; Jóźwik, I.K.; Zhao, X.Z.; Santos-Martins, D.; Yang, R.; Smith, S.J.; Jeon, Y.; Forli, S.; Hughes, S.H.; Burke, T.R. Jr.; Craigie, R.; Lyumkis, D. Structural basis for strand-transfer inhibitor binding to HIV intasomes. Science 2020, 367(6479), 810-814. [CrossRef]
- Lu, C.H.; Bednarczyk, E.M.; Catanzaro, L.M.; Shon, A.; Xu, J.C.; Ma, Q. Pharmacokinetic drug interactions of integrase strand transfer inhibitors. Curr Res Pharmacol Drug Discov 2021, 2, 100044. [CrossRef]
- Hicks, C.; Gulick, R.M. Raltegravir: the first HIV type 1 integrase inhibitor. Clin Infect Dis 2009, 48, 931-939. [CrossRef]
- Scarsi, K.K.; Havens, J.P.; Podany, A.T.; Avedissian, S.N.; Fletcher, C.V. HIV-1 Integrase Inhibitors: A Comparative Review of Efficacy and Safety. Drugs 2020, 80, 1649-1676. [CrossRef]
- Shimura, K.; Kodama, E.N. Elvitegravir: a new HIV integrase inhibitor. Antivir Chem Chemother 2009, 20, 79-85. [CrossRef]
- Johns, B.A.; Kawasuji, T.; Weatherhead, J.G.; Taishi, T.; Temelkoff, D.P.; Yoshida, H.; Akiyama, T.; Taoda, Y.; Murai, H.; Kiyama, R.; Fuji, M.; Tanimoto, N.; Jeffrey, J.; Foster, S.A.; Yoshinaga, T.; Seki, T.; Kobayashi, M.; Sato, A.; Johnson, M.N.; Garvey, E.P.; Fujiwara, T. Carbamoyl pyridone HIV-1 integrase inhibitors 3. A diastereomeric approach to chiral nonracemic tricyclic ring systems and the discovery of dolutegravir (S/GSK1349572) and (S/GSK1265744). J Med Chem 2013, 56, 5901-5916. [CrossRef]
- Fantauzzi, A.; Mezzaroma, I. Dolutegravir: clinical efficacy and role in HIV therapy. Ther Adv Chronic Dis 2014, 5, 164-177. [CrossRef]
- Scott, L.J. Dolutegravir/Lamivudine Single-Tablet Regimen: A Review in HIV-1 Infection. Drugs 2020, 80, 61-72. [CrossRef]
- Santevecchi, B.A.; Miller, S.; Childs-Kean, L.M. Doing More with Less: Review of Dolutegravir-Lamivudine, a Novel Single-Tablet Regimen for Antiretroviral-Naïve Adults With HIV-1 Infection. Ann Pharmacother 2020, 54, 1252-1259. [CrossRef]
- Max, B. Update on HIV integrase inhibitors for the treatment of HIV-1 infection. Future Virol 2019, 14, 693–709. [CrossRef]
- Sarode, I.M.; Jindal, A.B. Current status of dolutegravir delivery systems for the treatment of HIV-1 infection. J Drug Deliv Sci Technol 2022, 76, 103802. [CrossRef]
- Stellbrink, H.J.; Hoffmann, C. Cabotegravir: its potential for antiretroviral therapy and preexposure prophylaxis. Curr Opin HIV AIDS 2018, 13, 334-340. [CrossRef]
- Canetti, D.; Spagnuolo, V. An evaluation of cabotegravir for HIV treatment and prevention. Expert Opin Pharmacother 2021, 22, 403-414. [CrossRef]
- Prather, C.; Jeon, C. Cabotegravir: The first long-acting injectable for HIV pre-exposure prophylaxis. Am J Health Syst Pharm 2022, 79, 1898-1905. [CrossRef]
- Durham, S.H.; Milam, A.; Waer, D.; Chahine, E.B. Cabotegravir: The First Long-Acting Injectable for HIV Preexposure Prophylaxis. Ann Pharmacother 2023, 57, 306-316. [CrossRef]
- Liao, C.; Wang, Q. Authentic HIV-1 integrase inhibitors for the treatment of HIV-1/AIDS. In Privileged Scaffolds in Drug Discovery, Yu, B.; Li, N.; Fu, C., Eds.; Publisher: Academic Press, 2007; 154-196. doi. 10.1016/B978-0-443-18611-0.00026-7.
- Tsiang, M.; Jones, G.S.; Goldsmith, J.; Mulato, A.; Hansen, D.; Kan, E.; Tsai, L.; Bam, R.A.; Stepan, G.; Stray, K.M.; Niedziela-Majka, A.; Yant, S.R.; Yu, H.; Kukolj, G.; Cihlar, T.; Lazerwith, S.E.; White, K.L.; Jin, H. Antiviral Activity of Bictegravir (GS-9883), a Novel Potent HIV-1 Integrase Strand Transfer Inhibitor with an Improved Resistance Profile. Antimicrob Agents Chemother 2016, 60, 7086-7097. [CrossRef]
- De Clercq, E.; Zhang, Z.; Huang, J.; Zhang, M.; Li, G. Biktarvy for the treatment of HIV infection: Progress and prospects. Biochem Pharmacol 2023, 217, 115862. [CrossRef]
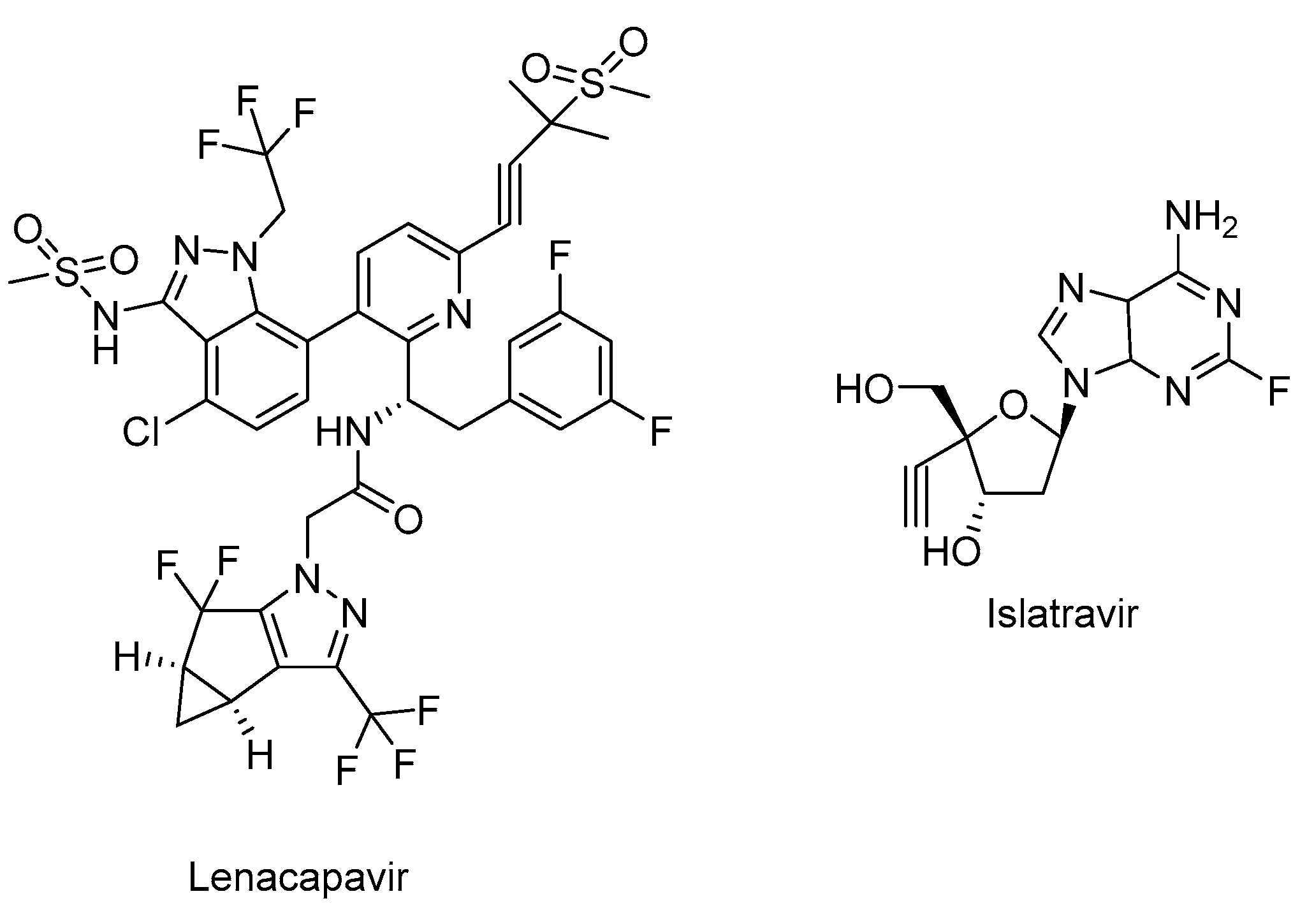
- Link, J.O.; Rhee, M.S.; Tse, W.C.; Zheng, J.; Somoza, J.R.; Rowe, W.; Begley, R.; Chiu, A.; Mulato, A.; Hansen, D.; Singer, E.; Tsai, L.K.; Bam, R.A.; Chou, C.H.; Canales, E.; Brizgys, G.; Zhang, J.R.; Li, J.; Graupe, M.; Morganelli, P.; Liu, Q.; Wu, Q.; Halcomb, R.L.; Saito, R.D.; Schroeder, S.D.; Lazerwith, S.E.; Bondy, S.; Jin, D.; Hung, M.; Novikov, N.; Liu, X.; Villaseñor, A.G.; Cannizzaro, C.E.; Hu, E.Y.; Anderson, R.L.; Appleby, T.C.; Lu, B.; Mwangi, J.; Liclican, A.; Niedziela-Majka, A.; Papalia, G.A.; Wong, M.H.; Leavitt, S.A.; Xu, Y.; Koditek, D.; Stepan, G.J.; Yu, H.; Pagratis, N.; Clancy, S.; Ahmadyar, S.; Cai, T.Z.; Sellers, S.; Wolckenhauer, S.A.; Ling, J.; Callebaut, C.; Margot, N.; Ram, R.R.; Liu, Y.P.; Hyland, R.; Sinclair, G.I.; Ruane, P.J.; Crofoot, G.E.; McDonald, C.K.; Brainard, D.M.; Lad, L.; Swaminathan, S.; Sundquist, W.I.; Sakowicz, R.; Chester, A.E.; Lee, W.E.; Daar, E.S.; Yant, S.R.; Cihlar, T. Clinical targeting of HIV capsid protein with a long-acting small molecule. Nature 2020, 584, 614-618. [CrossRef]
- Marcelin, A.G.; Charpentier, C.; Jary, A.; Perrier, M.; Margot, N.; Callebaut, C.; Calvez, V.; Descamps, D. Frequency of capsid substitutions associated with GS-6207 in vitro resistance in HIV-1 from antiretroviral-naive and -experienced patients. J Antimicrob Chemother 2020, 75, 1588-1590. [CrossRef]
- Bekerman, E.; Yant, S.R.; VanderVeen, L.; Hansen, D.; Lu, B.; Rowe, W.; Wang, K.; Callebaut, C. Long-acting lenacapavir acts as an effective preexposure prophylaxis in a rectal SHIV challenge macaque model. J Clin Invest 2023, 133, e167818. [CrossRef]
- Dzinamarira, T.; Almehmadi, M.; Alsaiari, A.A.; Allahyani, M.; Aljuaid, A.; Alsharif, A.; Khan, A.; Kamal, M.; Rabaan, A.A.; Alfaraj, A.H.; AlShehail, B.M.; Alotaibi, N.; AlShehail, S.M.; Imran, M. Highlights on the Development, Related Patents, and Prospects of Lenacapavir: The First-in-Class HIV-1 Capsid Inhibitor for the Treatment of Multi-Drug-Resistant HIV-1 Infection. Medicina (Kaunas) 2023, 59, 1041. [CrossRef]
- Subramanian, R.; Tang, J.; Zheng, J.; Lu, B.; Wang, K.; Yant, S.R.; Stepan, G.J.; Mulato, A.; Yu, H.; Schroeder, S.; Shaik, N.; Singh, R.; Wolckenhauer, S.; Chester, A.; Tse, W.C.; Chiu, A.; Rhee, M.; Cihlar, T.; Rowe, W.; Smith, B.J. Lenacapavir: A Novel, Potent, and Selective First-in-Class Inhibitor of HIV-1 Capsid Function Exhibits Optimal Pharmacokinetic Properties for a Long-Acting Injectable Antiretroviral Agent. Mol Pharm 2023, 20, 6213-6225. [CrossRef]
- Hitchcock, A.M.; Kufel, W.D.; Dwyer, K.A.M.; Sidman, E.F. Lenacapavir: A novel injectable HIV-1 capsid inhibitor. Int J Antimicrob Agents 2023, 63, 107009. [CrossRef]
- McFadden, W.M.; Snyder, A.A.; Kirby, K.A.; Tedbury, P.R.; Raj, M.; Wang, Z.; Sarafianos, S.G. Rotten to the core: antivirals targeting the HIV-1 capsid core. Retrovirology 2021, 18, 41. [CrossRef]
- Dvory-Sobol, H.; Shaik, N.; Callebaut, C.; Rhee, M.S. Lenacapavir: a first-in-class HIV-1 capsid inhibitor. Curr Opin HIV AIDS 2022, 17, 15-21. [CrossRef]
- Menéndez-Arias, L.; Delgado, R. Update and latest advances in antiretroviral therapy. Trends Pharmacol Sci 2022, 43, 16-29. [CrossRef]
- Paik, J. Lenacapavir: First Approval. Drugs 2022, 82, 1499-1504. [CrossRef]
- Segal-Maurer, S.; DeJesus, E.; Stellbrink, H.J.; Castagna, A.; Richmond, G.J.; Sinclair, G.I.; Siripassorn, K.; Ruane, P.J.; Berhe, M.; Wang, H.; Margot, N.A.; Dvory-Sobol, H.; Hyland, R.H.; Brainard, D.M.; Rhee, M.S.; Baeten, J.M.; Molina, J.M.; CAPELLA Study Investigators. Capsid Inhibition with Lenacapavir in Multidrug-Resistant HIV-1 Infection. N Engl J Med 2022, 386, 1793-1803. [CrossRef]
- Tailor, M.W.; Chahine, E.B.; Koren, D.; Sherman, E.M. Lenacapavir: A Novel Long-Acting Capsid Inhibitor for HIV. Ann Pharmacother 2023, 10600280231171375, (in press). [CrossRef]
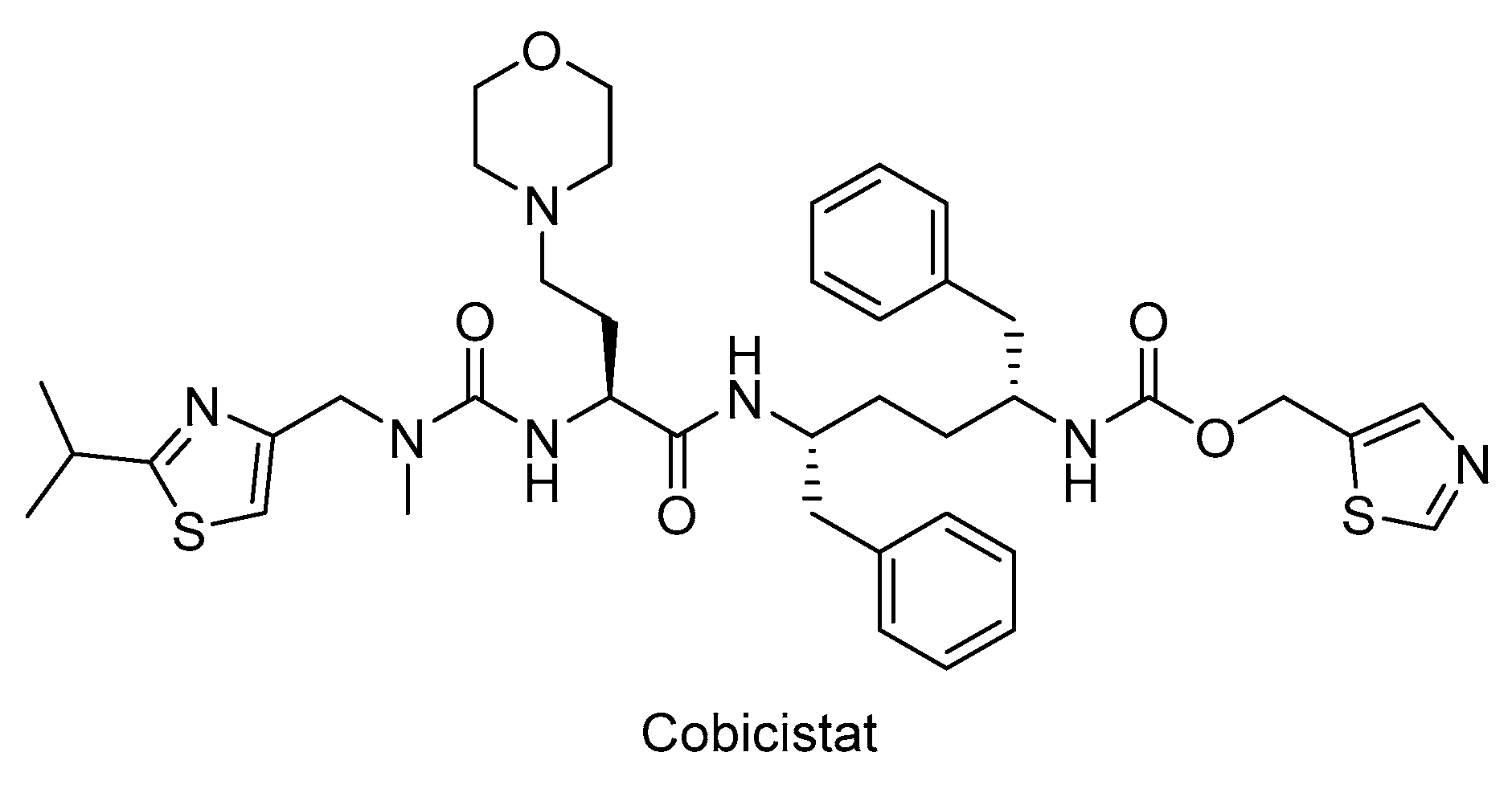
- Xu, L.; Liu, H.; Murray, B.P.; Callebaut, C.; Lee, M.S.; Hong, A.; Strickley, R.G.; Tsai, L.K.; Stray, K.M.; Wang, Y.; Rhodes, G.R.; Desai, M.C. Cobicistat (GS-9350): A Potent and Selective Inhibitor of Human CYP3A as a Novel Pharmacoenhancer. ACS Med Chem Lett 2010, 1, 209-213. [CrossRef]
- Nathan, B.; Bayley, J.; Waters, L.; Post, F.A. Cobicistat: a Novel Pharmacoenhancer for Co-Formulation with HIV Protease and Integrase Inhibitors. Infect Dis Ther 2013, 2, 111-122. [CrossRef]
- Deeks, E.D. Cobicistat: a review of its use as a pharmacokinetic enhancer of atazanavir and darunavir in patients with HIV-1 infection. Drugs 2014, 74, 195-206. [CrossRef]
- Niu, Z.X.; Wang, Y.T.; Zhang, S.N.; Li, Y.; Chen, X.B.; Wang, S.Q.; Liu, H.M. Application and synthesis of thiazole ring in clinically approved drugs. Eur J Med Chem 2023, 250, 115172. [CrossRef]
- Choudhary, M.C.; Mellors, J.W. The transformation of HIV therapy: One pill once a day. Antivir Ther 2022, 27, 13596535211062396. [CrossRef]
- Portsmouth, S.D.; Scott, C.J. The renaissance of fixed dose combinations: Combivir. Ther Clin Risk Manag 2007, 3, 579-583.
- Blair, H.A. Dolutegravir/Rilpivirine: A Review in HIV-1 Infection. Drugs 2018, 78, 1741-1750. [CrossRef]
- Hester, E.K.; Astle, K. Dolutegravir-Rilpivirine, Dual Antiretroviral Therapy for the Treatment of HIV-1 Infection. Ann Pharmacother 2019, 53, 860-866. [CrossRef]
- Markham, A. Bictegravir: First Global Approval. Drugs 2018, 78, 601-606. [CrossRef]
- Pham, H.T.; Mesplède, T. Bictegravir in a fixed-dose tablet with emtricitabine and tenofovir alafenamide for the treatment of HIV infection: pharmacology and clinical implications. Expert Opin Pharmacother 2019, 20, 385-397. [CrossRef]
- Sang, Y.; Ding, L.; Zhuang, C.; Chen, F. Design strategies for long-acting anti-HIV pharmaceuticals. Curr Opin Pharmacol 2020, 54, 158-165. [CrossRef]
- Gulick, R.M.; Ribaudo, H.J.; Shikuma, C.M.; Lalama, C.; Schackman, B.R.; Meyer, W.A.; Acosta, E.P.; Schouten, J.; Squires, K.E.; Pilcher, C.D.; Murphy, R.L.; Koletar, S.L.; Carlson, M.; Reichman, R.C.; Bastow, B.; Klingman, K.L.; Kuritzkes, D.R.; AIDS Clinical Trials Group (ACTG) A5095 Study Team. Three- vs four-drug antiretroviral regimens for the initial treatment of HIV-1 infection: a randomized controlled trial. JAMA 2006, 296, 769-781. [CrossRef]
- Moreno, S.; Perno, C.F.; Mallon, P.W.; Behrens, G.; Corbeau, P.; Routy, J.P.; Darcis, G. Two-drug vs. three-drug combinations for HIV-1: Do we have enough data to make the switch? HIV Med 2019, 20, 2-12. [CrossRef]
- Waters, L.; Church, H. Two drugs regimens for HIV. Curr Opin Infect Dis 2020, 33, 28-33. [CrossRef]
- Bares, S.H.; Scarsi, K.K. A new paradigm for antiretroviral delivery: long-acting cabotegravir and rilpivirine for the treatment and prevention of HIV. Curr Opin HIV AIDS 2022, 17, 22-31. [CrossRef]
- Brizzi, M.; Pérez, S.E.; Michienzi, S.M.; Badowski, M.E. Long-acting injectable antiretroviral therapy: will it change the future of HIV treatment? Ther Adv Infect Dis 2023, 10, 20499361221149773. [CrossRef]
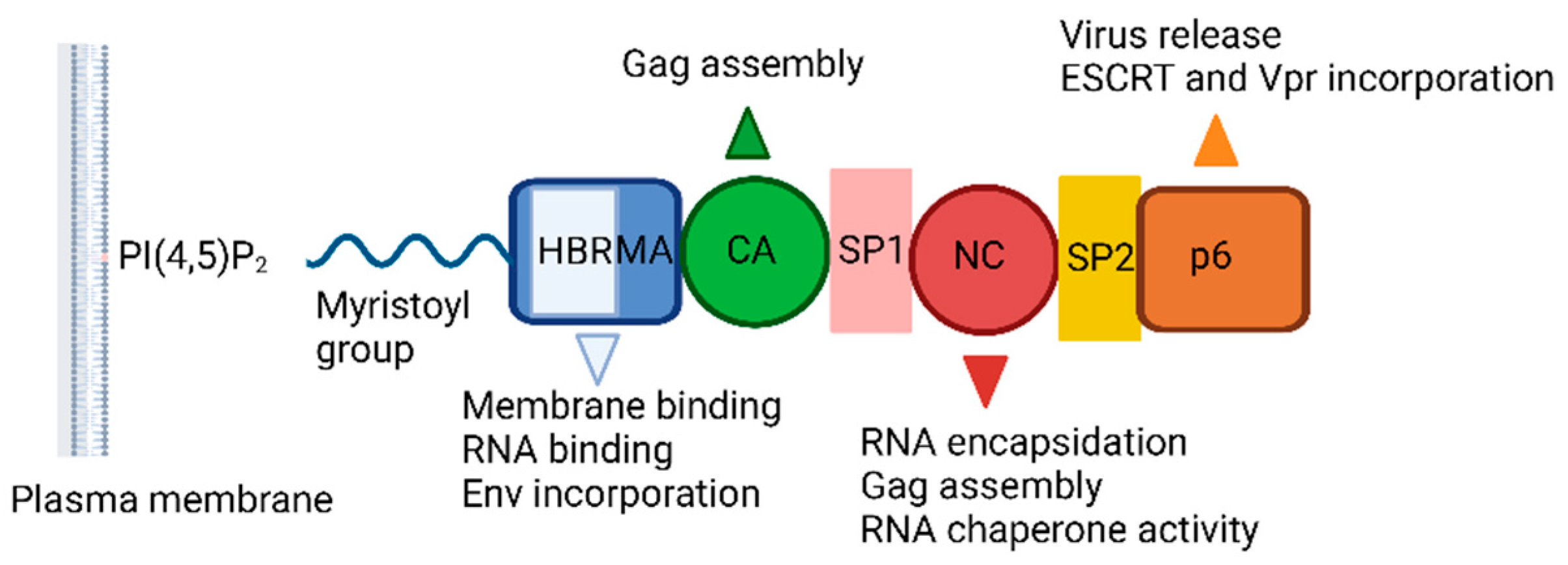
- Tedbury, P.R.; Novikova, M.; Alfadhli, A.; Hikichi, Y.; Kagiampakis, I.; KewalRamani, V.N.; Barklis, E.; Freed, E.O. HIV-1 Matrix Trimerization-Impaired Mutants Are Rescued by Matrix Substitutions That Enhance Envelope Glycoprotein Incorporation. J Virol 2019, 94, e01526-19. [CrossRef]
- Bou-Nader, C.; Muecksch, F.; Brown, J.B.; Gordon, J.M.; York, A.; Peng, C.; Ghirlando, R.; Summers, M.F.; Bieniasz, P.D.; Zhang, J. HIV-1 matrix-tRNA complex structure reveals basis for host control of Gag localization. Cell Host Microbe 2021, 29, 1421-1436.e7. [CrossRef]
- Ono, A.; Ablan, S.D.; Lockett, S.J.; Nagashima, K.; Freed, E.O. Phosphatidylinositol (4,5) bisphosphate regulates HIV-1 Gag targeting to the plasma membrane. Proc Natl Acad Sci USA 2004, 101, 14889-14894. [CrossRef]
- Larson, D.R.; Ma, Y.M.; Vogt, V.M.; Webb, W.W. Direct measurement of Gag-Gag interaction during retrovirus assembly with FRET and fluorescence correlation spectroscopy. J Cell Biol 2003, 162, 1233-44. [CrossRef]
- Kutluay, S.B.; Zang, T.; Blanco-Melo, D.; Powell, C.; Jannain, D.; Errando, M.; Bieniasz, P.D. Global changes in the RNA binding specificity of HIV-1 gag regulate virion genesis. Cell 2014, 159, 1096-1109. [CrossRef]
- Dilley, K.A.; Nikolaitchik, O.A.; Galli, A.; Burdick, R.C.; Levine, L.; Li, K.; Rein, A.; Pathak, V.K.; Hu, W.S. Interactions between HIV-1 Gag and Viral RNA Genome Enhance Virion Assembly. J Virol 2017, 91, e02319-16. [CrossRef]
- Mercredi, P.Y.; Bucca, N.; Loeliger, B.; Gaines, C.R.; Mehta, M.; Bhargava, P.; Tedbury, P.R.; Charlier, L.; Floquet, N.; Muriaux, D.; Favard, C.; Sanders, C.R.; Freed, E.O.; Marchant, J.; Summers, M.F. Structural and Molecular Determinants of Membrane Binding by the HIV-1 Matrix Protein. J Mol Biol 2016, 428, 1637-1655. [CrossRef]
- Tedbury, P.R.; Novikova, M., Ablan, S.D.; Freed, E.O. Biochemical evidence of a role for matrix trimerization in HIV-1 envelope glycoprotein incorporation. Proc Natl Acad Sci U S A 2016, 113, E182-190. [CrossRef]
- Tran, R.J.; Lalonde, M.S.; Sly, K.L.; Conboy, J.C. Mechanistic Investigation of HIV-1 Gag Association with Lipid Membranes. J Phys Chem B 2019, 123, 22, 4673-4687. [CrossRef]
- Monje-Galvan, V.; Voth, G.A. Binding mechanism of the matrix domain of HIV-1 gag on lipid membranes. Elife 2020, 9, e58621. [CrossRef]
- Murphy, R.E.; Saad, J.S. The Interplay between HIV-1 Gag Binding to the Plasma Membrane and Env Incorporation. Viruses 2020, 12, 548. [CrossRef]
- Sumner, C.; Kotani, O.; Liu, S.; Musier-Forsyth, K.; Sato, H.; Ono, A. Molecular Determinants in tRNA D-arm Required for Inhibition of HIV-1 Gag Membrane Binding. J Mol Biol 2022, 434, 167390. [CrossRef]
- Socas, L.B.P.; Ambroggio, E.E. HIV-1 Gag specificity for PIP2 is regulated by macromolecular electric properties of both protein and membrane local environments. Biochim Biophys Acta Biomembr 2023, 1865, 184157. [CrossRef]
- Tomasini, M.D.; Johnson, D.S.; Mincer, J.S.; Simon, S.M. Modeling the dynamics and kinetics of HIV-1 Gag during viral assembly. PLoS One 2018, 13, e0196133. [CrossRef]
- Novikova, M.; Adams, L.J.; Fontana, J.; Gres, A.T.; Balasubramaniam, M.; Winkler, D.C.; Kudchodkar, S.B.; Soheilian, F.; Sarafianos, S.G.; Steven, A.C.; Freed, E.O. Identification of a Structural Element in HIV-1 Gag Required for Virus Particle Assembly and Maturation. mBio 2018, 9, e01567-18. [CrossRef]
- Chen, S.; Xu, J.; Liu, M.; Rao, A.L.N.; Zandi, R.; Gill, S.S.; Mohideen, U. Investigation of HIV-1 Gag binding with RNAs and lipids using Atomic Force Microscopy. PLoS One 2020, 15, e0228036. [CrossRef]
- Roos, W.H. High-speed AFM reveals the dynamics of virus budding. Biophys J 2022, 121, 4022-4023. [CrossRef]
- Anraku, K.; Fukuda, R.; Takamune, N.; Misumi, S.; Okamoto, Y.; Otsuka, M.; Fujita, M. Highly sensitive analysis of the interaction between HIV-1 Gag and phosphoinositide derivatives based on surface plasmon resonance. Biochemistry 2010, 49, 5109–5116. [CrossRef]
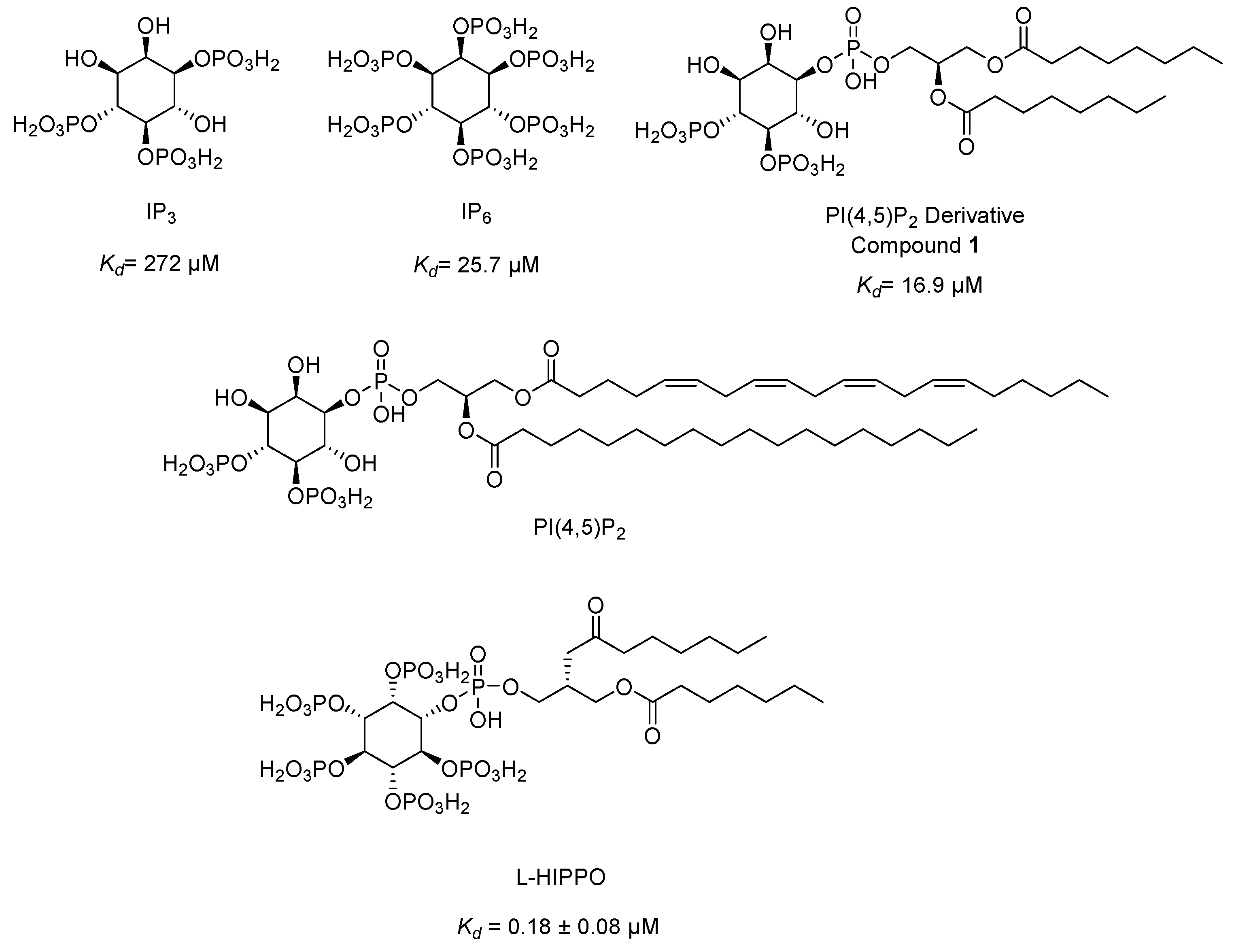
- Tateishi, H.; Anraku, K.; Koga, R.; Okamoto, Y.; Fujita, M.; Otsuka, M. Design and synthesis of lipid-coupled inositol 1,2,3,4,5,6-hexakisphosphate derivatives exhibiting high-affinity binding for the HIV-1 MA domain. Org Biomol Chem 2014, 12, 5006. [CrossRef]
- Tateishi, H.; Monde, K.; Anraku, K.; Koga, R.; Hayashi, Y.; Ciftci, H.I.; DeMirci, H.; Higashi, T.; Motoyama, K.; Arima, H.; Otsuka, M.; Fujita, M. A clue to unprecedented strategy to HIV eradication: “Lock-in and apoptosis”. Sci. Rep 2017, 7, 8957. [CrossRef]
- Ciftci, H.I.; Sierra, R.G.; Yoon, C.H.; Su, Z.; Tateishi, H.; Koga, R.; Kotaro, K.; Yumoto, F.; Senda, T.; Liang, M.; Wakatsuki, S.; Otsuka, M.; Fujita, M.; DeMirci, H. Serial Femtosecond X-Ray Diffraction of HIV-1 Gag MA-IP6 Microcrystals at Ambient Temperature. Int J Mol Sci 2019, 20, 1675. [CrossRef]
- Ciftci, H.; Tateishi, H.; Koiwai, K.; Koga, R.; Anraku, K.; Monde, K.; Dağ, Ç.; Destan, E.; Yuksel, B.; Ayan, E.; Yildirim, G.; Yigin, M.; Ertem, F.B.; Shafiei, A.; Guven, O.; Besler, S.O.; Sierra, R.G.; Yoon, C.H.; Su, Z.; Liang, M.; Acar, B.; Haliloglu, T.; Otsuka, M.; Yumoto, F.; Fujita, M.; Senda, T.; DeMirci, H. Structural insight into host plasma membrane association and assembly of HIV-1 matrix protein. Sci Rep 2021, 11, 15819. [CrossRef]
- Ciftci, H.; Sever, B.; Ayan, E.; Can, M.; DeMirci, H.; Otsuka, M.; TuYuN, A.F.; Tateishi, H.; Fujita, M. Identification of New L-Heptanoylphosphatidyl Inositol Pentakisphosphate Derivatives Targeting the Interaction with HIV-1 Gag by Molecular Modelling Studies. Pharmaceuticals (Basel) 2022, 15, 1255. [CrossRef]
- Zentner, I.; Sierra, L.J.; Fraser, A.K.; Maciunas, L.; Mankowski, M.K.; Vinnik, A.; Fedichev, P.; Ptak, R.G.; Martín-García, J.; Cocklin, S. Identification of a small-molecule inhibitor of HIV-1 assembly that targets the phosphatidylinositol (4,5)-bisphosphate binding site of the HIV-1 matrix protein. ChemMedChem 2013, 8, 426-432. [CrossRef]
- Alfadhli, A.; McNett, H.; Eccles, J.; Tsagli, S.; Noviello, C.; Sloan, R.; López, C.S.; Peyton, D.H.; Barklis, E. Analysis of small molecule ligands targeting the HIV-1 matrix protein-RNA binding site. J Biol Chem 2013, 288, 666-76. [CrossRef]
- Mattei, S.; Anders, M.; Konvalinka, J.; Kräusslich, H.G.; Briggs, J.A.; Müller, B. Induced maturation of human immunodeficiency virus. J Virol 2014, 88, 13722-13731. [CrossRef]
- Campbell, S.; Fisher, R.J.; Towler, E.M.; Fox, S.; Issaq, H.J.; Wolfe, T.; Phillips, L.R.; Rein, A. Modulation of HIV-like particle assembly in vitro by inositol phosphates. Proc Natl Acad Sci U S A 2001, 98, 10875-9. [CrossRef]
- Ricaña, C.L.; Dick, R.A. Inositol Phosphates and Retroviral Assembly: A Cellular Perspective. Viruses 2021, 13, 2516. [CrossRef]
- Poston, D.; Zang, T.; Bieniasz, P. Derivation and characterization of an HIV-1 mutant that rescues IP6 binding deficiency. Retrovirology 2021, 18, 25. [CrossRef]
- Pak, A.J.; Gupta, M.; Yeager, M.; Voth, G.A. Inositol Hexakisphosphate (IP6) Accelerates Immature HIV-1 Gag Protein Assembly toward Kinetically Trapped Morphologies. J Am Chem Soc 2022, 144, 10417-10428. [CrossRef]
- Kleinpeter, A.B.; Zhu, Y.; Mallery, D.L.; Ablan, S.D.; Chen, L.; Hardenbrook, N.; Saiardi, A.; James, L.C.; Zhang, P.; Freed, E.O. The Effect of Inositol Hexakisphosphate on HIV-1 Particle Production and Infectivity can be Modulated by Mutations that Affect the Stability of the Immature Gag Lattice. J Mol Biol 2023, 435, 168037. [CrossRef]
- Perilla, J.R.; Hadden-Perilla, J.A.; Gronenborn, A.M.; Polenova, T. Integrative structural biology of HIV-1 capsid protein assemblies: combining experiment and computation. Curr Opin Virol 2021, 48, 57-64. [CrossRef]
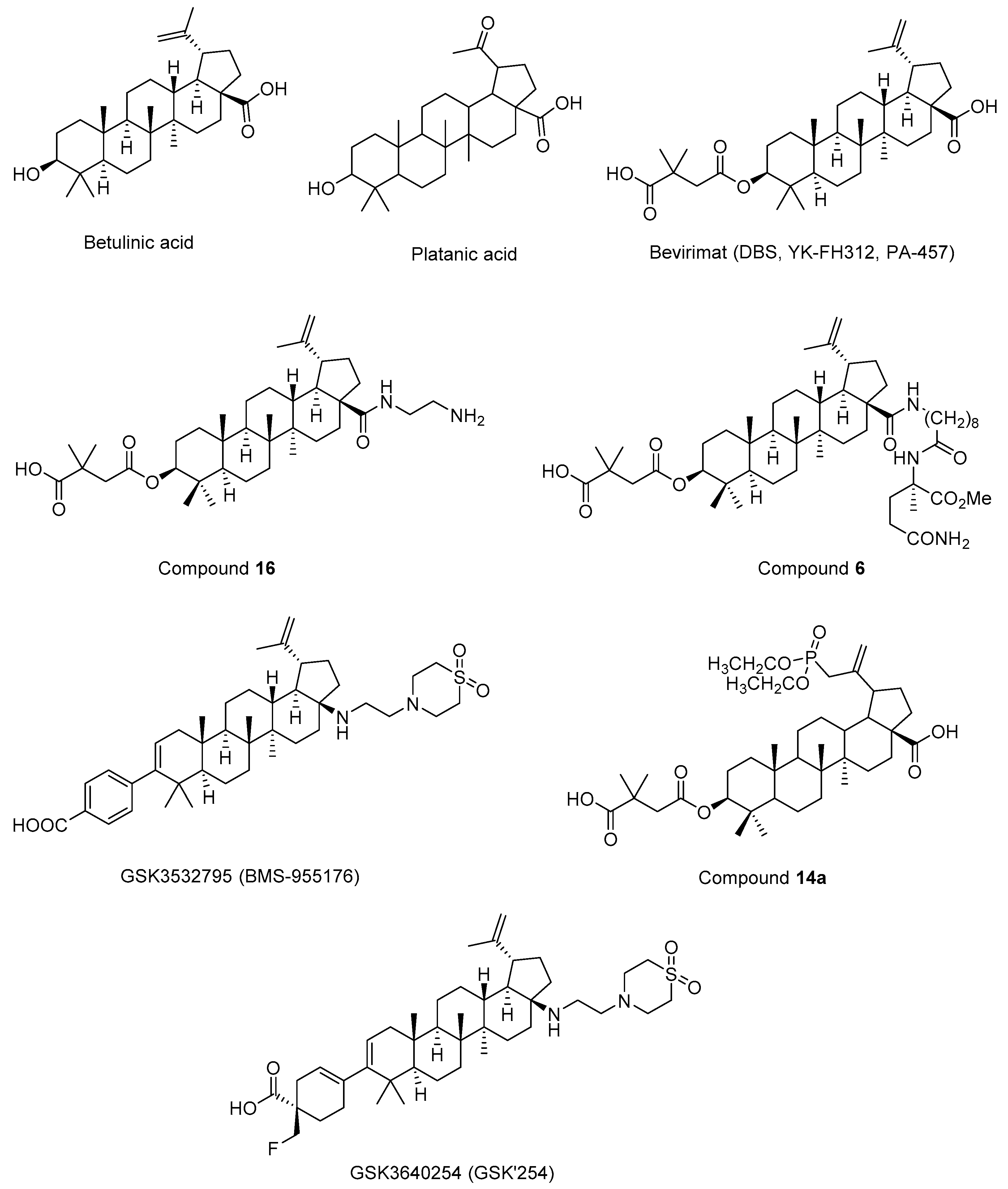
- Fujioka, T.; Kashiwada, Y.; Kilkuskie, R.E.; Cosentino, L.M.; Ballas, L.M.; Jiang, J.B.; Janzen, W.P.; Chen, I.S.; Lee, K.H. Anti-AIDS agents, 11. Betulinic acid and platanic acid as anti-HIV principles from Syzigium claviflorum, and the anti-HIV activity of structurally related triterpenoids. J Nat Prod 1994, 57, 243-247. [CrossRef]
- Kashiwada, Y.; Hashimoto, F.; Cosentino, L.M.; Chen, C.H.; Garrett, P.E.; Lee, K.H. Betulinic acid and dihydrobetulinic acid derivatives as potent anti-HIV agents. J Med Chem 1996, 39, 1016-7. [CrossRef]
- Kanamoto, T.; Kashiwada, Y.; Kanbara, K.; Gotoh, K.; Yoshimori, M.; Goto, T.; Sano, K.; Nakashima, H. Anti-human immunodeficiency virus activity of YK-FH312 (a betulinic acid derivative), a novel compound blocking viral maturation. Antimicrob Agents Chemother 2001, 45, 1225-1230. [CrossRef]
- Li, F.; Goila-Gaur, R.; Salzwedel, K.; Kilgore, N.R.; Reddick, M.; Matallana, C.; Castillo, A.; Zoumplis, D.; Martin, D.E.; Orenstein, J.M.; Allaway, G.P.; Freed, E.O.; Wild, C.T. PA-457: a potent HIV inhibitor that disrupts core condensation by targeting a late step in Gag processing. Proc Natl Acad Sci U S A 2003, 100, 13555-13560. [CrossRef]
- Adamson, C.S.; Ablan, S.D.; Boeras, I.; Goila-Gaur, R.; Soheilian, F.; Nagashima, K.; Li, F.; Salzwedel, K.; Sakalian, M.; Wild, C.T.; Freed, E.O. In vitro resistance to the human immunodeficiency virus type 1 maturation inhibitor PA-457 (Bevirimat). J Virol 2006, 80, 10957-10971. [CrossRef]
- Li, F.; Zoumplis, D.; Matallana, C.; Kilgore, N.R.; Reddick, M.; Yunus, A.S.; Adamson, C.S.; Salzwedel, K.; Martin, D.E.; Allaway, G.P.; Freed, E.O.; Wild, C.T. Determinants of activity of the HIV-1 maturation inhibitor PA-457. Virology 2006, 356, 217-224. [CrossRef]
- Sakalian, M.; McMurtrey, C.P.; Deeg, F.J.; Maloy, C.W.; Li, F.; Wild, C.T.; Salzwedel, K. 3-O-(3’,3’-dimethysuccinyl) betulinic acid inhibits maturation of the human immunodeficiency virus type 1 Gag precursor assembled in vitro. J Virol 2006, 80, 5716-22. [CrossRef]
- Zhou, J.; Chen, C.H.; Aiken, C. Human immunodeficiency virus type 1 resistance to the small molecule maturation inhibitor 3-O-(3’,3’-dimethylsuccinyl)-betulinic acid is conferred by a variety of single amino acid substitutions at the CA-SP1 cleavage site in Gag. J Virol 2006, 80, 12095-101. [CrossRef]
- Stoddart, C.A.; Joshi, P.; Sloan, B.; Bare, J.C.; Smith, P.C.; Allaway, G.P.; Wild, C.T.; Martin, D.E. Potent activity of the HIV-1 maturation inhibitor bevirimat in SCID-hu Thy/Liv mice. PLoS One 2007, 2, e1251. [CrossRef]
- Smith, P.F.; Ogundele, A.; Forrest, A.; Wilton, J.; Salzwedel, K.; Doto, J.; Allaway, G.P.; Martin, D.E. Phase I and II study of the safety, virologic effect, and pharmacokinetics/pharmacodynamics of single-dose 3-o-(3’,3’-dimethylsuccinyl)betulinic acid (bevirimat) against human immunodeficiency virus infection. Antimicrob Agents Chemother 2007, 51, 3574-3581. [CrossRef]
- Van Baelen, K.; Salzwedel, K.; Rondelez, E.; Van Eygen, V.; De Vos, S.; Verheyen, A.; Steegen, K.; Verlinden, Y.; Allaway, G.P.; Stuyver, L.J. Susceptibility of human immunodeficiency virus type 1 to the maturation inhibitor bevirimat is modulated by baseline polymorphisms in Gag spacer peptide 1. Antimicrob Agents Chemother 2009, 53, 2185-2188. [CrossRef]
- Coric, P.; Turcaud, S.; Souquet, F.; Briant, L.; Gay, B.; Royer, J.; Chazal, N.; Bouaziz, S. Synthesis and biological evaluation of a new derivative of bevirimat that targets the Gag CA-SP1 cleavage site. Eur J Med Chem 2013, 62, 453-465. [CrossRef]
- Dang, Z.; Ho, P.; Zhu, L.; Qian, K.; Lee, K.H., Huang, L.; Chen, C.H. New betulinic acid derivatives for bevirimat-resistant human immunodeficiency virus type-1. J Med Chem 2013, 56, 2029-2037. [CrossRef]
- Nowicka-Sans, B.; Protack, T.; Lin, Z.; Li, Z.; Zhang, S.; Sun, Y.; Samanta, H.; Terry, B.; Liu, Z.; Chen, Y.; Sin, N.; Sit, S.Y.; Swidorski, J.J.; Chen, J.; Venables, B.L.; Healy, M.; Meanwell, N.A.; Cockett, M.; Hanumegowda, U.; Regueiro-Ren, A.; Krystal, M.; Dicker, I.B. Identification and Characterization of BMS-955176, a Second-Generation HIV-1 Maturation Inhibitor with Improved Potency, Antiviral Spectrum, and Gag Polymorphic Coverage. Antimicrob Agents Chemother 2016, 60, 3956-3969. [CrossRef]
- Regueiro-Ren, A.; Liu, Z.; Chen, Y.; Sin, N.; Sit, S.Y.; Swidorski, J.J.; Chen, J.; Venables, B.L.; Zhu, J.; Nowicka-Sans, B.; Protack, T.; Lin, Z.; Terry, B.; Samanta, H.; Zhang, S.; Li, Z.; Beno, B.R.; Huang, X.S.; Rahematpura, S.; Parker, D.D.; Haskell, R.; Jenkins, S.; Santone, K.S.; Cockett, M.I.; Krystal, M.; Meanwell, N.A.; Hanumegowda, U.; Dicker, I.B. Discovery of BMS-955176, a Second Generation HIV-1 Maturation Inhibitor with Broad Spectrum Antiviral Activity. ACS Med Chem Lett 2016, 7, 568-572. [CrossRef]
- Regueiro-Ren, A.; Dicker, I.B.; Hanumegowda, U.; Meanwell, N.A. Second Generation Inhibitors of HIV-1 Maturation. ACS Med Chem Lett 2019, 10, 287-294. [CrossRef]
- Hwang, C.; Schürmann, D.; Sobotha, C.; Boffito, M.; Sevinsky, H.; Ray, N.; Ravindran, P.; Xiao, H.; Keicher, C.; Hüser, A.; Krystal, M.; Dicker, I.B.; Grasela, D.; Lataillade, M. Antiviral Activity, Safety, and Exposure-Response Relationships of GSK3532795, a Second-Generation Human Immunodeficiency Virus Type 1 Maturation Inhibitor, Administered as Monotherapy or in Combination With Atazanavir With or Without Ritonavir in a Phase 2a Randomized, Dose-Ranging, Controlled Trial (AI468002). Clin Infect Dis 2017, 65, 442-452. [CrossRef]
- Morales-Ramirez, J.; Bogner, J.R.; Molina, J.M.; Lombaard, J.; Dicker, I.B.; Stock, D.A.; DeGrosky, M.; Gartland, M.; Pene Dumitrescu, T.; Min, S.; Llamoso, C.; Joshi, S.R.; Lataillade, M. Safety, efficacy, and dose response of the maturation inhibitor GSK3532795 (formerly known as BMS-955176) plus tenofovir/emtricitabine once daily in treatment-naive HIV-1-infected adults: Week 24 primary analysis from a randomized Phase IIb trial. PLoS One 2018, 13, e0205368. [CrossRef]
- Chrobak, E.; Marciniec, K.; Dąbrowska, A.; Pęcak, P.; Bębenek, E.; Kadela-Tomanek, M.; Bak, A.; Jastrzębska, M.; Boryczka, S. New Phosphorus Analogs of Bevirimat: Synthesis, Evaluation of Anti-HIV-1 Activity and Molecular Docking Study. Int J Mol Sci 2019, 20, 5209. [CrossRef]
- Dicker, I.; Jeffrey, J.L., Protack, T.; Lin, Z.; Cockett, M.; Chen, Y.; Sit, S.Y.; Gartland, M.; Meanwell, N.A.; Regueiro-Ren, A.; Drexler, D.; Cantone, J.; McAuliffe, B.; Krystal, M. GSK3640254 Is a Novel HIV-1 Maturation Inhibitor with an Optimized Virology Profile. Antimicrob Agents Chemother 2022, 66, e0187621. [CrossRef]
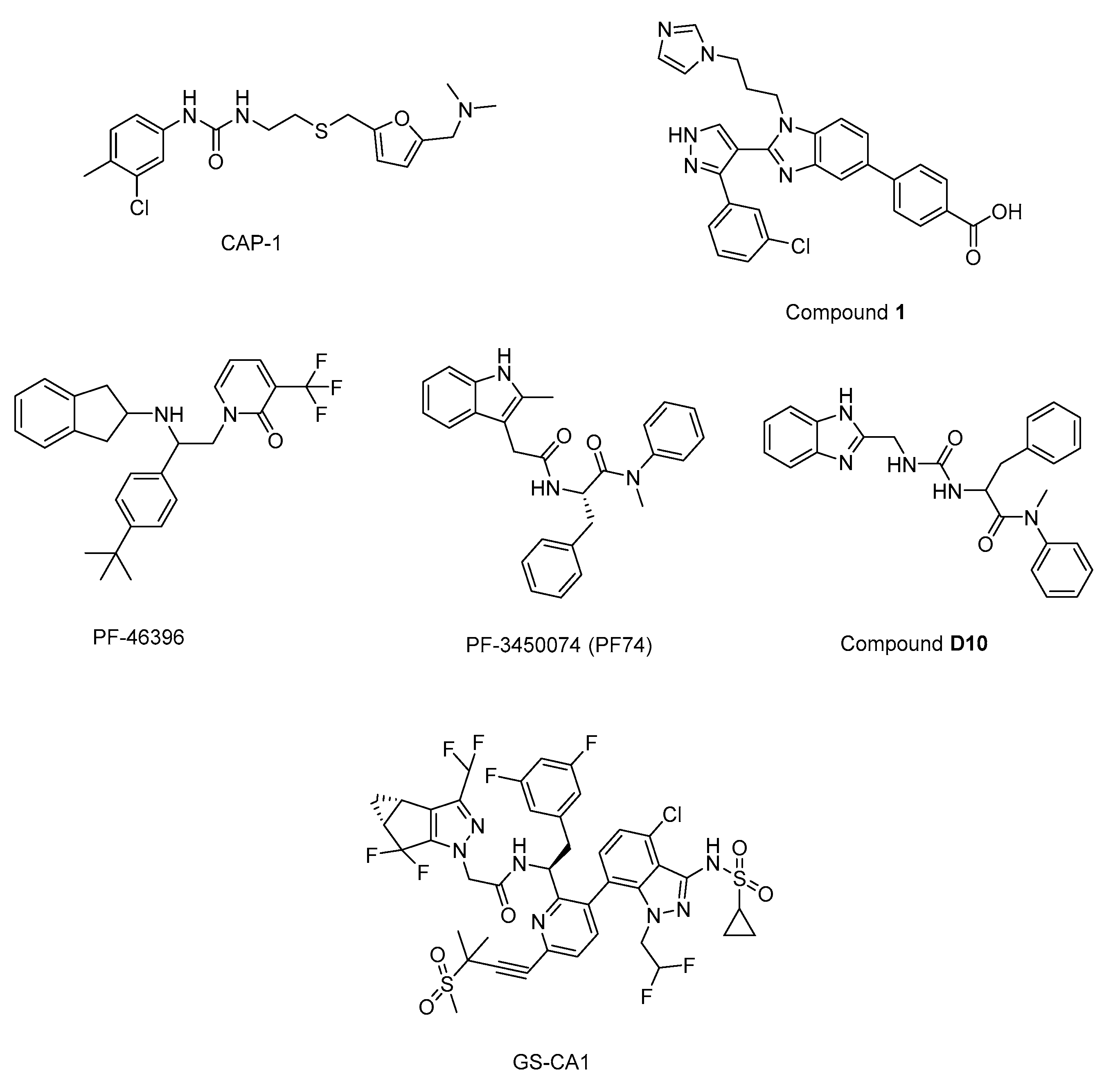
- Tang, C.; Loeliger, E.; Kinde, I.; Kyere, S.; Mayo, K.; Barklis, E.; Sun, Y.; Huang, M.; Summers, M.F. Antiviral inhibition of the HIV-1 capsid protein. J Mol Biol 2003, 327, 1013-1020. [CrossRef]
- Kelly, B.N.; Kyere, S.; Kinde, I.; Tang, C.; Howard, B.R.; Robinson, H.; Sundquist, W.I.; Summers, M.F.; Hill, C.P. Structure of the antiviral assembly inhibitor CAP-1 complex with the HIV-1 CA protein. J Mol Biol 2007, 373, 355-366. [CrossRef]
- Fader, L.D.; Bethell, R.; Bonneau, P.; Bös, M.; Bousquet, Y.; Cordingley, M.G.; Coulombe, R.; Deroy, P.; Faucher, A.M.; Gagnon, A.; Goudreau, N.; Grand-Maître, C.; Guse, I.; Hucke, O.; Kawai, S.H.; Lacoste, J.E.; Landry, S.; Lemke, C.T.; Malenfant, E.; Mason, S.; Morin, S.; O’Meara, J.; Simoneau, B.; Titolo, S.; Yoakim, C. Discovery of a 1,5-dihydrobenzo[b][1,4]diazepine-2,4-dione series of inhibitors of HIV-1 capsid assembly. Bioorg Med Chem Lett 2011, 21, 398-404. [CrossRef]
- Lemke, C.T.; Titolo, S.; von Schwedler, U.; Goudreau, N.; Mercier, J.F.; Wardrop, E.; Faucher, A.M.; Coulombe, R.; Banik, S.S.; Fader, L.; Gagnon, A.; Kawai, S.H.; Rancourt, J.; Tremblay, M.; Yoakim, C.; Simoneau, B.; Archambault, J.; Sundquist, W.I.; Mason, S.W. Distinct effects of two HIV-1 capsid assembly inhibitor families that bind the same site within the N-terminal domain of the viral CA protein. J Virol 2012, 86, 6643-6655. [CrossRef]
- Lemke, C.T.; Titolo, S.; Goudreau, N.; Faucher, A.M.; Mason, S.W.; Bonneau, P. A novel inhibitor-binding site on the HIV-1 capsid N-terminal domain leads to improved crystallization via compound-mediated dimerization. Acta Crystallogr D Biol Crystallogr 2013, 69, 1115-1123. [CrossRef]
- Tremblay, M.; Bonneau, P.; Bousquet, Y.; DeRoy, P.; Duan, J.; Duplessis, M.; Gagnon, A.; Garneau, M.; Goudreau, N.; Guse, I.; Hucke, O.; Kawai, S.H.; Lemke, C.T.; Mason, S.W.; Simoneau, B.; Surprenant, S.; Titolo, S.; Yoakim, C. Inhibition of HIV-1 capsid assembly: optimization of the antiviral potency by site selective modifications at N1, C2 and C16 of a 5-(5-furan-2-yl-pyrazol-1-yl)-1H-benzimidazole scaffold. Bioorg Med Chem Lett 2012, 22, 7512-7517. [CrossRef]
- Goudreau, N.; Lemke, C.T.; Faucher, A.M.; Grand-Maître, C.; Goulet, S.; Lacoste, J.E.; Rancourt, J.; Malenfant, E.; Mercier, J.F.; Titolo, S.; Mason, S.W. Novel inhibitor binding site discovery on HIV-1 capsid N-terminal domain by NMR and X-ray crystallography. ACS Chem Biol 2013, 8, 1074-1082. [CrossRef]
- Sticht, J.; Humbert, M.; Findlow, S.; Bodem, J.; Müller, B.; Dietrich, U.; Werner, J.; Kräusslich, H.G. A peptide inhibitor of HIV-1 assembly in vitro. Nat Struct Mol Biol 2005, 12, 671-677. [CrossRef]
- Zhang, H.; Curreli, F.; Waheed, A.A.; Mercredi, P.Y.; Mehta, M.; Bhargava, P.; Scacalossi, D.; Tong, X.; Lee, S.; Cooper, A.; Summers, M.F.; Freed, E.O.; Debnath, A.K. Dual-acting stapled peptides target both HIV-1 entry and assembly. Retrovirology 2013, 10, 136. [CrossRef]
- Blair, W.S.; Cao, J.; Fok-Seang, J.; Griffin, P.; Isaacson, J.; Jackson, R.L.; Murray, E.; Patick, A.K.; Peng, Q.; Perros, M.; Pickford, C.; Wu, H.; Butler, S.L. New small-molecule inhibitor class targeting human immunodeficiency virus type 1 virion maturation. Antimicrob Agents Chemother 2009, 53, 5080-5087. [CrossRef]
- Blair, W.S.; Pickford, C.; Irving, S.L.; Brown, D.G.; Anderson, M.; Bazin, R.; Cao, J.; Ciaramella, G.; Isaacson, J.; Jackson, L.; Hunt, R.; Kjerrstrom, A.; Nieman, J.A.; Patick, A.K.; Perros, M.; Scott, A.D.; Whitby, K.; Wu, H.; Butler, S.L. HIV capsid is a tractable target for small molecule therapeutic intervention. PLoS Pathog 2010, 6, e1001220. [CrossRef]
- Dostálková, A.; Škach, K.; Kaufman, F.; Křížová, I.; Hadravová, R.; Flegel, M.; Ruml, T.; Hrabal, R.; Rumlová, M. PF74 and Its Novel Derivatives Stabilize Hexameric Lattice of HIV-1 Mature-Like Particles. Molecules 2020, 25, 1895. [CrossRef]
- Yant, S.R.; Mulato, A.; Hansen, D.; Tse, W.C.; Niedziela-Majka, A.; Zhang, J.R., Stepan, G.J.; Jin, D.; Wong, M.H.; Perreira, J.M.; Singer, E.; Papalia, G.A.; Hu, E.Y.; Zheng, J.; Lu, B.; Schroeder, S.D.; Chou, K.; Ahmadyar, S.; Liclican, A.; Yu, H.; Novikov, N.; Paoli, E.; Gonik, D.; Ram, R.R.; Hung, M.; McDougall, W.M.; Brass, A.L.; Sundquist, W.I.; Cihlar, T.; Link, J.O. A highly potent long-acting small-molecule HIV-1 capsid inhibitor with efficacy in a humanized mouse model. Nat Med 2019, 25, 1377-1384. [CrossRef]
- Zhang, Z.; Hou, W.; Chen, S. Updates on CRISPR-based gene editing in HIV-1/AIDS therapy. Virol Sin 2022, 37, 1-10. [CrossRef]
- Stephenson, K.E.; Wagh, K.; Korber, B.; Barouch, D.H. Vaccines and Broadly Neutralizing Antibodies for HIV-1 Prevention. Annu Rev Immunol 2020, 38, 673-703. [CrossRef]
- Choudhary, M.C.; Cyktor, J.C.; Riddler, S.A. Advances in HIV-1-specific chimeric antigen receptor cells to target the HIV-1 reservoir. J Virus Erad 2022, 8, 100073. [CrossRef]
- Sun, L.; Zhang, X.; Xu, S.; Huang, T.; Song, S.; Cherukupalli, S.; Zhan, P.; Liu, X. An insight on medicinal aspects of novel HIV-1 capsid protein inhibitors. Eur J Med Chem 2021, 217, 113380. [CrossRef]
- Zhang, L.; Wei, F.; Zhang, J.; Liu, C.; López-Carrobles, N.; Liu, X.; Menéndez-Arias, L.; Zhan, P. Current medicinal chemistry strategies in the discovery of novel HIV-1 ribonuclease H inhibitors. Eur J Med Chem 2022, 243, 114760. [CrossRef]
- Passaes, C.P.; Sáez-Cirión, A. HIV cure research: advances and prospects. Virology 2014, 454-455, 340-52. [CrossRef]
- Dick, A.; Cocklin, S. Recent Advances in HIV-1 Gag Inhibitor Design and Development. Molecules 2020, 25, 1687. [CrossRef]
- Saeb, S.; Wallet, C.; Rohr, O.; Schwartz, C.; Loustau, T. Targeting and eradicating latent CNS reservoirs of HIV-1: Original strategies and new models. Biochem Pharmacol 2023, 214, 115679. [CrossRef]
- García, M.; Buzón, M.J.; Benito, J.M.; Rallón, N. Peering into the HIV reservoir. Rev Med Virol 2018, 28, e1981. [CrossRef]
- Dufour, C.; Gantner, P.; Fromentin, R.; Chomont, N. The multifaceted nature of HIV latency. J Clin Invest 2020, 130, 3381-3390. [CrossRef]
- Devanathan, A.S.; Cottrell, M.L. Pharmacology of HIV Cure: Site of Action. Clin Pharmacol Ther 2021, 109, 841-855. [CrossRef]
- Chen, J.; Zhou, T.; Zhang, Y.; Luo, S.; Chen, H.; Chen, D.; Li, C.; Li, W. The reservoir of latent HIV. Front Cell Infect Microbiol 2022, 12, 945956. [CrossRef]
- Ta, T.M.; Malik, S.; Anderson, E.M.; Jones, A.D.; Perchik, J.; Freylikh, M.; Sardo, L.; Klase, Z.A.; Izumi, T. Insights Into Persistent HIV-1 Infection and Functional Cure: Novel Capabilities and Strategies. Front Microbiol 2022, 13, 862270. [CrossRef]
- Yang, H.; Wallace, Z.; Dorrell, L. Therapeutic Targeting of HIV Reservoirs: How to Give T Cells a New Direction. Front Immunol 2018, 9, 2861. [CrossRef]
- Elsheikh, M.M.; Tang, Y.; Li, D.; Jiang, G. Deep latency: A new insight into a functional HIV cure. EBioMedicine 2019, 45, 624-629. [CrossRef]
- Cohn, L.B.; Chomont, N.; Deeks, S.G. The Biology of the HIV-1 Latent Reservoir and Implications for Cure Strategies. Cell Host Microbe 2020, 27, 519-530. [CrossRef]
- Ward, A.R.; Mota, T.M.; Jones, R.B. Immunological approaches to HIV cure. Semin Immunol 2021, 51, 101412. [CrossRef]
- Bai, R.; Lv, S.; Wu, H.; Dai, L. Insights into the HIV-1 Latent Reservoir and Strategies to Cure HIV-1 Infection. Dis Markers 2022, 2022, 6952286. [CrossRef]
- Andersen, R.J.; Ntie-Kang, F.; Tietjen, I. Natural product-derived compounds in HIV suppression, remission, and eradication strategies. Antiviral Res 2018, 158, 63-77. [CrossRef]
- Abner, E.; Jordan, A. HIV “shock and kill” therapy: In need of revision. Antiviral Res 2019, 166, 19-34. [CrossRef]
- Atkins, A.J.; Allen, A.G.; Dampier, W.; Haddad, E.K.; Nonnemacher, M.R.; Wigdahl, B. HIV-1 cure strategies: why CRISPR? Expert Opin Biol Ther 2021, 21, 781-793. [CrossRef]
- Thomas, J.; Ruggiero, A.; Paxton, W.A.; Pollakis, G. Measuring the Success of HIV-1 Cure Strategies. Front Cell Infect Microbiol 2020, 10, 134. [CrossRef]
- Kim, Y.S. Long-Acting Injectable Antiretroviral Agents for HIV Treatment and Prevention. Infect Chemother 2021, 53, 686-695. [CrossRef]
- Kula-Pacurar, A.; Rodari, A.; Darcis, G.; Van Lint, C. Shocking HIV-1 with immunomodulatory latency reversing agents. Semin Immunol 2021, 51, 101478. [CrossRef]
- Rumlová, M.; Křížová, I.; Keprová, A.; Hadravová, R.; Doležal, M.; Strohalmová, K.; Pichová, I.; Hájek, M.; Ruml, T. HIV-1 protease-induced apoptosis. Retrovirology 2014, 11, 37. [CrossRef]
- Centazzo, M.; Manganaro, L.; Alvisi, G. Cellular Targets of HIV-1 Protease: Just the Tip of the Iceberg? Viruses 2023, 15, 712. [CrossRef]
- Kim, Y.; Anderson, J.L.; Lewin, S.R. Getting the “Kill” into “Shock and Kill”: Strategies to Eliminate Latent HIV. Cell Host Microbe 2018, 23, 14-26. [CrossRef]
- Kim, J.G.; Shan, L. Beyond Inhibition: A Novel Strategy of Targeting HIV-1 Protease to Eliminate Viral Reservoirs. Viruses 2022, 14, 1179. [CrossRef]
- Tanaka, K.; Kim, Y.; Roche, M.; Lewin, S.R. The role of latency reversal in HIV cure strategies. J Med Primatol 2022, 51, 278-283. [CrossRef]
- Cummins, N.W.; Baker, J.; Chakraborty, R.; Dean, P.G.; Garcia-Rivera, E.; Krogman, A.; Kumar, S.; Kuzmichev, Y.V.; Laird, G.M.; Landay, A.; Lichterfeld, M.; Mahmood, M.; Martinson, J.; Maynes, M.; Natesampillai, S.; Rajkumar, V.; Rassadkina, Y.; Ritter, K.D.; Rivera, C.G.; Rizza, S.A.; Subramanian, K.; Tande, A.J.; Wonderlich, E.R.; Whitaker, J.A.; Zeuli, J.; Badley, A.D. Single center, open label dose escalating trial evaluating once weekly oral ixazomib in ART-suppressed, HIV positive adults and effects on HIV reservoir size in vivo. EClinicalMedicine 2021, 42, 101225. [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




