1. Introduction
Plasmodium falciparum is responsible for over 90% of all the estimated malaria cases and deaths globally. With the emergence of resistance to artemisinin [
1], developing effective malaria vaccines against
P. falciparum has become increasingly important. The RTS, S/AS01 (Mosquirix™), a pre-erythrocytic stage malaria vaccine, suffers low efficacy with rapidly waning antibodies [
2]. The R21/Matrix-M™, another pre-erythrocytic stage malaria vaccine based on the same sporozoite antigen, was recently recommended by the WHO for malaria prevention in children [
1]. It has an efficacy above 75% in a seasonal malaria transmission setting [
3,
4]. Unless pre-erythrocytic stage malaria vaccines confer sterile immunity, effective blood-stage malaria vaccines are crucial to complement pre-erythrocytic vaccines to alleviate the burden of malaria.
Understanding naturally acquired immunity to malaria could pave the way to developing efficacious malaria vaccines. Successfully treating malaria-infected children using passively transferred immunoglobulin G (IgG) from adult residents in malaria-endemic settings suggests that antibodies are critical mediators of naturally acquired immunity to malaria [
5,
6]. To date, no unanimously accepted antibody-mediated correlate of protection against malaria exists. Studies have mainly focused on antibody levels and breadth against
P. falciparum antigens [
7]. The ability of antibodies to neutralise
P. falciparum in vitro in growth inhibition assays has been widely assessed as a correlate of protection against malaria. However, inconsistent results have been reported [
8]. Antibody-mediated functions against
P. falciparum based on their ability to recruit cells of the innate immune system by their Fc-portion have recently gained attention.
Neutrophils are the most abundant white blood cells in the human peripheral blood and are regulated as the first line of defence of the innate immune system [
9,
10]. Opsonising antibodies against
P. falciparum can recruit neutrophils by their Fc-portion, releasing reactive oxygen species (ROS) that destroy the parasite [
11]. This antibody-mediated function can be measured in an
in vitro solid-phase chemiluminescence assay called the antibody-dependent respiratory burst (ADRB), where isoluminol is used to detect extracellular ROS released by neutrophils [
12,
13]. ADRB has been correlated with protection against clinical malaria [
14].
Naturally acquired immunity to malaria is influenced by malaria transmission intensity [
15]; in areas with high malaria transmission, young children are mainly susceptible to severe malaria, while older children and adults are not. Thus, immunity to severe malaria appears to be acquired relatively quickly during the first years of life. In contrast, children and adults are at risk of clinical malaria episodes in low malaria transmission areas, and immunity appears to be acquired more slowly.
Over the past two decades, global malaria transmission trends have fluctuated. WHO estimated a decrease of 13 million malaria cases globally in 2014 compared to 2000 [
1]. Global malaria cases increased in 2015, with the most significant increase of 11 million estimated cases between 2019 and 2020 [
1]. In 2022, there was an estimated increase of 5 million cases compared to 2021 [
1]. In children, malaria antibodies and antibody-mediated functional activity generally decrease with the reduction of malaria transmission [
16,
17,
18]. Even in the context of RTS, S vaccination, antibody magnitude and antibody-mediated functional activity significantly reduce post-vaccination in children [
19,
20]. There is limited knowledge of the influence of changing malaria transmission on antibody magnitude in adults. The few studies conducted among adults mainly span one to three malaria transmission periods [
21,
22]. Whether or not functional antibody activity is maintained in adults in the context of declining malaria transmission has not been studied. To better understand naturally acquired immunity to malaria, we aim to assess ADRB in an area of declining malaria transmission over 25 years in children and adults.
2. Materials and Methods
2.1. Study area and population
Nyamisati is a rural village located in the mangrove swamps of the Rufiji River Delta in the Kibiti District of Tanzania. Several cross-sectional surveys to assess the epidemiology of malaria have been conducted in Nyamisati from 1986 to 2019, where demographic characteristics, axillary body temperature, venous blood and blood smears were collected. Reports from these studies revealed a gradual decline from hyperendemic to hypoendemic malaria transmission intensity [
23,
24]. The decrease in malaria transmission may be attributed to several factors, including a research team closely monitoring malaria from 1985-1991 and 1993-1999, where diagnosis and treatment were readily available. The team then moved, but repeated cross-sectional surveys were ongoing from 1993 to 2019, where the research team distributed bed nets during the 1999, 2010, 2016 and 2019 cross-sectional surveys. Other factors that may have contributed to the decline in malaria transmission in Nyamisati include the introduction of rapid malaria diagnosis kits, bed net distribution campaigns and the introduction of artemisinin-based combination therapy (ACT) as first-line malaria treatment, replacing sulfadoxine-pyrimethamine (SP) alone or in combination with oral quinine.
The present study is based on six cross-sectional surveys where venous blood was collected into EDTA tubes from which plasma and packed cells were separated and frozen at -20 °C and later stored at -80°C for long-term storage. The samples are from the 1993, 1994, and 1995 cross-sectional surveys, referred to as the high malaria transmission period, and from the 2016, 2018 and 2019 cross-sectional surveys, referred to as the low malaria transmission period. Individuals were selected from the high and low malaria transmission periods matched by age.
2.2. Parasite detection by microscopy
Blood from a pricked finger or EDTA tube was placed on a labelled glass slide to prepare a thin and thick smear. The slides were air-dried, and the thin smear was fixed by dipping it in absolute methanol for a few seconds. They were then stained with 10% Giemsa for 20 minutes. The slides were then rinsed with a gentle flow of buffered water until the stain was removed. They were then dried and read using a light microscope at 100x magnification using oil immersion. The slide was declared negative when no parasites were observed over 100 fields. Parasites were counted per 200 white blood cells, assuming 8000 white blood cells per microliter of blood, to estimate their density.
2.3. Parasite detection by real-time PCR
Real-time PCR was used to detect Plasmodium infection (
P. falciparum,
P. vivax,
P. ovale spp., and
P. malariae). DNA was extracted from packed cells by either a Qiagen blood mini kit (Qiagen, Germantown, MD, USA) for 1993, 1994,1995, 2018, and 2019 cross-sectional surveys or by magnetic bead separation method using a Hamilton Chemagic Star Robot (Hamilton, Bonadouz, Switzerland) for the 2016 survey. Real-time PCR was performed in an ABI Taqman 7500 or QuantStudio™ 5 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) [
25]. For each reaction, the master mix included all species-specific probes, forward primers and conserved reverse primers. The
P. ovale and
P. malariae probes (synthesised by BioSearch Technologies, Novato, CA, USA) and the
P. vivax and
P. falciparum probes (synthesised by Applied Biosystems) were each labelled with a distinct fluorophore, and, depending on the master mix, either ROX or Purple Mustang was used as the reference dye[
26]. The reaction was performed in a final volume of 25 μl per well containing 5 μl DNA (corresponding to 5 μl of whole blood), 12.5 μl of either TaqMan universal master mix or TaqMan multiplex master mix (Applied Biosystems), 0.5 μl (10 μmol/L) of the
P. falciparum-specific forward primer, 0.125 μl (10 μmol/L) of each of the other species-specific forward primers and 0.5 μl (10 μmol/L) of the reverse primer, 0.2 μl (10 μmol/L) of each species-specific probe, passive reference dye ROX or Mustang Purple and DNA/RNA-free water. The samples were run using a cut-off of 45 cycles to define positive samples, starting with 95 ˚C for 20 s, then the thermal cycles of 95 ˚C for 1 s and 60 ˚C for 20 s. Standards, negative and species-specific positive controls were included on each plate. The assay was optimised to detect all species simultaneously, with a detection limit of approximately 0.5 parasites per μl blood.
2.4. Plasmodium falciparum parasite preparation
P. falciparum parasites of the 3D7 strain were cultured with 2% haematocrit in O+ human erythrocytes in culture media (RPMI 1640 media supplemented with 30mM HEPES, 0.05mg/ml hypoxanthine, 0.025mg/ml gentamicin, 2mg/ml D-glucose, 3% Albumax II and 7.5% sodium bicarbonate) and allowed to reach 10-15% parasitemia. Using a magnetic column (Miltenyi Biotec), mature trophozoites were isolated and put back into a culture to enable their development to the schizont stage. A protease inhibitor, Epoxysuccinyl-L-leucylamido (4-guanidino) butane (E64; Sigma-Aldrich), was added to allow schizont development to mature schizonts for 6-8 h without rupture. After centrifugation at 1800 rpm for 5 minutes, the pellet was resuspended with 1ml of 1 x PBS. The number of parasitophorous vacuolar membrane-enclosed merozoite structures (PEMS) was counted using a counting chamber at 1:20 dilution in 1 x PBS. PEMS concentration was estimated using the formula below:
2.5. Neutrophils preparation
Neutrophils were isolated as previously described [
27]. Fresh whole blood from healthy Kenyan volunteers was collected in heparin vacutainer tubes. The blood was mixed 1:1 with Hanks buffered salt solution (HBSS) minus phenol red, Ca
2+ and Mg
2+ ions (Sigma-Aldrich) and layered 1:1 over Histopaque 1077 (Sigma-Aldrich) in 50-mL falcon tubes. The tubes were then centrifuged at 600xg, 20°C with no brakes. Following centrifugation, the peripheral blood mononuclear cells (PBMC) and histopaque layer were removed without disturbing the RBC pellet. The RBC pellet was resuspended with 5ml HBSS and mixed with 3% dextran (Fisher Bioreagent) by inverting the falcon tube ten times and then incubated at room temperature in the dark for one hour. The supernatant was then carefully collected without disturbing the RBC pellet. The supernatant was then centrifuged for seven minutes at 500g at 4°C. After centrifugation, the supernatant was discarded. The remaining pellet was treated with 10ml of ice-cold 0.2% NaCl for 30 seconds and stopped with 10ml of ice-cold 1.6% NaCl to lyse the residual RBC contaminant. The falcon tube was centrifuged for 7 minutes at 500g at 4°C, and the supernatant was discarded. RBC contaminant was lysed several times to achieve a clear pellet. Once a clear pellet was achieved, it was resuspended with 0.5 mL polymorphonuclear (PMN) buffer (HBSS with 0.1% BSA (Jackson ImmunoResearch), 1% D-(+) - Glucose Hybri Max (Sigma-Aldrich), sterile filtered through 0.22um syringe filter (Pall Life Sciences)). The resuspended neutrophils in the PMN buffer were kept on ice until used.
To ensure a neutrophil yield greater than 90%, 5 µL of the resulting suspension was smeared on a slide, fixed with methanol for 10 seconds, stained with 5% Giemsa for 10 minutes and observed under x400 magnification, ensuring contamination with RBCs and debris was less than 10%.
To assess neutrophil viability, 5 µL of the suspension was mixed with 45 µL trypan blue (Sigma-Aldrich) solution, and 15 µL of this was added to a hemocytometer and observed at x400 under an inverted microscope. Viable cells were identified as not permeable to trypan blue and were at 99 - 100%. The following formula was used to estimate neutrophil concentration:
The concentration of the PMN was then adjusted accordingly using PMN buffer, ensuring each well was coated with 5.0 X 105 neutrophils.
2.6. ADRB assay
The assay was adapted from Llewellyn et al., 2015 [
12]. Briefly, on each well of a Nunc
TM MaxiSorp
TM 96-well opaque plate, 100µL of 10 x 10
5 PEMS was added and incubated at room temperature overnight. Wells were washed three times with 200µL of 1xPBS per well and then blocked with 200µL Casein (Thermo Scientific) in 1xPBS per well for 1 hour at room temperature. Following a wash step, 50µL plasma samples and controls diluted 1:50 in 1xPBS were added to the plate and incubated at 37 ֯C for 1 hour. Following another washing step, 50µL of 5x10
5 neutrophils was rapidly added to each well onto the plate, followed by 50µL of isoluminol at 0.04mg/ml in 1xPBS. The plate was immediately loaded onto the Biotek synergy plate reader to be read using the Gen 5 software measuring maximum relative light unit (RLU) in each well for 1000 milliseconds every two minutes for 1 hour. On each plate, a pool of hyper-immune Kenyan adult sera was used as a positive control and to assign indexed RLU to each sample tested. Six plasma samples from malaria-naïve individuals were used as negative controls.
2.7. Data analysis
Statistical analysis was performed using R and RStudio 2023.06.0+421(R core team, version 4.1.3 (2022-03-10) [
28] and GraphPad Prism (version 9.5.1), where a p-value < 0.05 was considered statistically significant for all tests.
Age was grouped into ten categories: 0-4, 5-8, 9-12, 13-16, 17-20, 21-25, 26-30, 31-35, 36-40 and > 40 years. Fever was defined as axillary body temperature ≥ 37.5°C or febrile in the past 48 hours. Symptomatic malaria was defined as fever and P. falciparum-positive at sample collection. In contrast, asymptomatic infection was described as an absence of fever but P. falciparum-positive at sample collection. We determined the ADRB cut-off as mean plus three standard deviations indexed RLU of six negative controls. The levels of ADRB activity were grouped into three levels of responders by tertiles: high, medium, and low. The Mann-Whitney U test was used to compare differences between medians, unpaired t-test was used to compare mean ADRB activity between age groups, and Spearman’s rank correlation was used to assess correlations between ADRB activity and age.
A logistic regression model stratified by malaria transmission was fitted to investigate the association between ADRB activity and parasitemia, adjusting for age (as a continuous variable), sex and fever.
3. Results
3.1. Demographic characteristics
Plasma samples from six cross-sectional surveys in Nyamisati, three surveys during the high and three during the low malaria transmission periods, were selected for the study. The characteristics of the study participants from each malaria transmission period are presented in
Table 1. There were 329 study participants in total, 158 and 171 from the high and low malaria transmission period, respectively, none of which participated in more than one survey. A similar age distribution, with 16 years as the median age, was observed in both the high and low malaria transmission periods,
Table 1.
3.2. Malaria transmission
In both the high and low malaria transmission periods, real-time PCR was a more sensitive method of detecting Plasmodium parasites than microscopy. No participant was P. vivax positive by real-time PCR, and P. falciparum was the dominant species in both malaria transmission periods. P. falciparum prevalence by real-time PCR is presented in
Table 1.
Symptomatic malaria was limited to children under 12 years during the high malaria transmission period, while asymptomatic infections occurred in all age groups. During the low malaria transmission period, both symptomatic malaria and asymptomatic infections were reported in children and adults, Supplementary
Figure 1.
During the high malaria transmission period, there were more individuals with asymptomatic infections than symptomatic malaria. On the contrary, there was no difference in the proportion of individuals with symptomatic malaria and asymptomatic infections during the low malaria transmission period,
Table 1.
In both the high and low malaria transmission period, parasite density was higher among individuals with symptomatic malaria than those with asymptomatic infection. However, only one asymptomatic individual had detectable parasites by microscopy during the low malaria transmission period,
Table 1.
3.3. Age and malaria transmission intensity influence ADRB activity
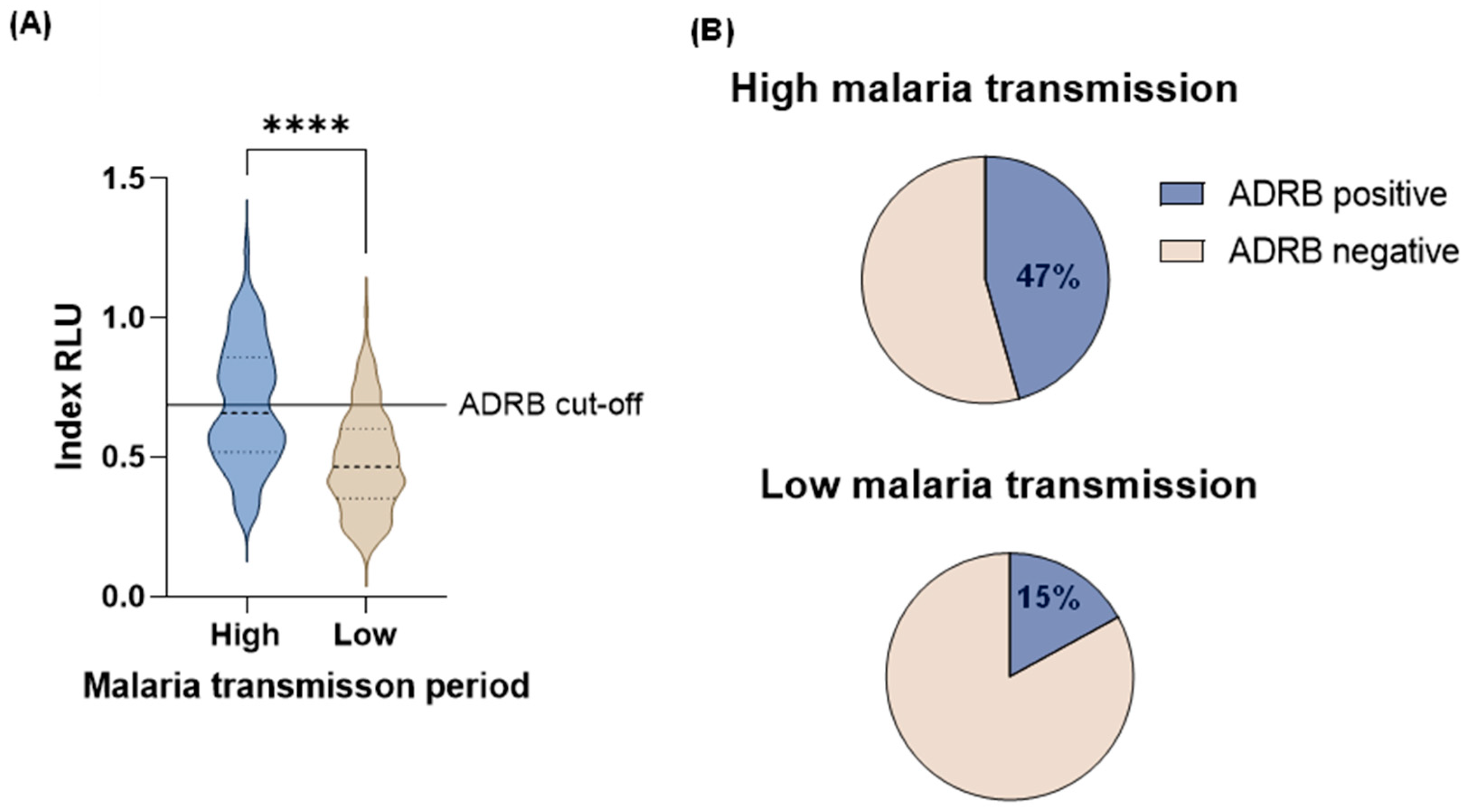
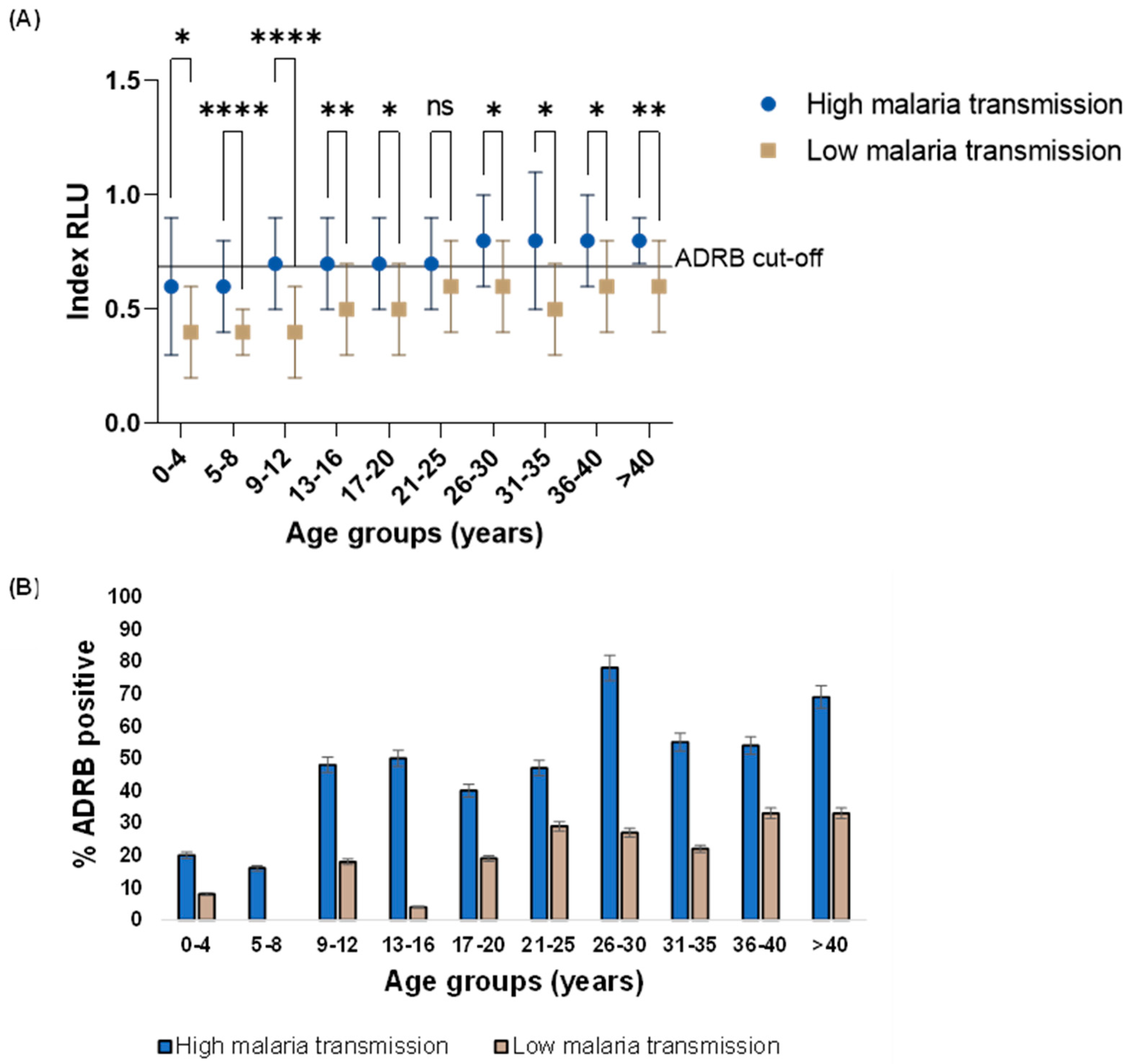
Overall, higher ADRB activity was observed in the high (median: 0.64 RLU, IQR: 0.51RLU - 0.84RLU) than in the low (median: 0.48 RLU, IQR: 0.36RLU - 0.62RLU) malaria transmission period (Mann-Whitney U test, p < 0.0001) (
Figure 1 A) this was consistent within age categories (
Figure 2 A). Only adults 21 years and above during the high malaria transmission period had mean ADRB activity above the ADRB cut-off (
Figure 2 A).
A more significant proportion of individuals were ADRB positive during the high (47%, 95% CI: 39% - 55%) than in the low (15%, 95% CI: 10% - 21%) malaria transmission period (
Figure 1 B) this was also consistent within age categories (
Figure 2 B).
There was a positive correlation between ADRB activity and age in both the high (r = 0.44, 95% CI 0.31 – 0.56, p < 0.0001) and low (r = 0.35, 95% CI 0.19 – 0.48, p < 0.0001) malaria transmission period.
3.4. Age, parasitemia and symptomatic malaria are associated with ADRB activity
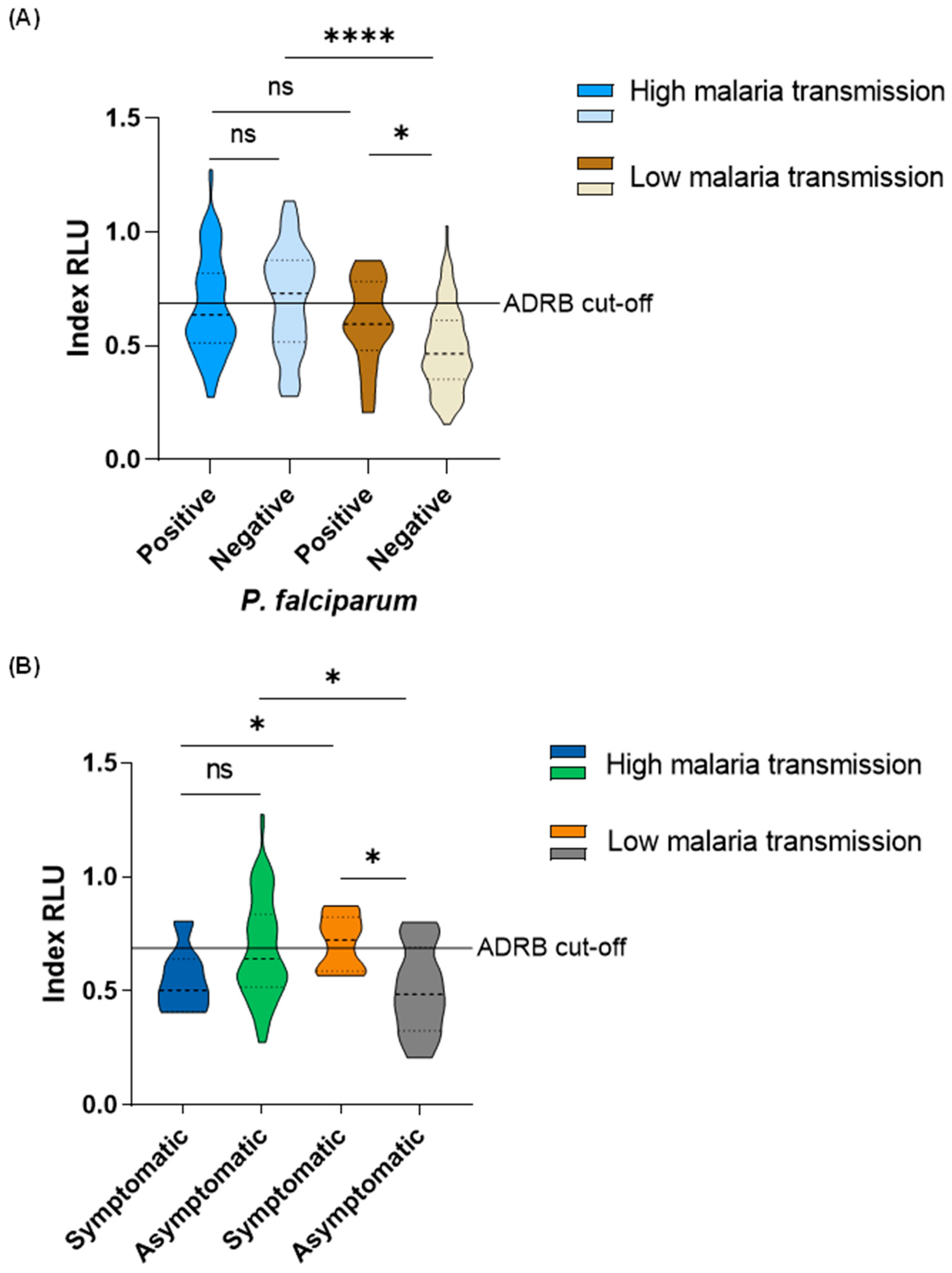
During the high malaria transmission period, there was no difference in ADRB activity between P. falciparum-positive (median: 0.64RLU, IQR: 0.51RLU - 0.82RLU) and negative (median: 0.73RLU, IQR: 0.52RLU – 0.88RLU) individuals. Interestingly, during the low malaria transmission period, ADRB activity was higher among P. falciparum-positive individuals (median: 0.60 RLU, IQR: 0.52RLU – 0.77RLU) than in P. falciparum-negative individuals (median: 0.47RLU, IQR 0.36RLU – 0.61RLU) (Mann-Whitney U test, p = 0.01). There was no difference in ADRB activity between P. falciparum-positive individuals from the high (median: 0.64 RLU, IQR: 0.51RLU - 0.82RLU) versus low (median: 0.60 RLU, IQR: 0.52RLU – 0.77RLU) malaria transmission period. ADRB activity was higher among P. falciparum-negative individuals from the high malaria transmission period (median: 0.73RLU, IQR: 0.52RLU – 0.88RLU) than those in the low malaria transmission period (median: 0.47RLU, IQR 0.36RLU – 0.61RLU) (Mann-Whitney U test, p < 0.0001) (
Figure 3 A).
There was no difference in ADRB activity between individuals with symptomatic malaria (median: 0.50RLU, IQR: 0.48RLU – 0.66RLU) and asymptomatic infections (median: 0.64RLU, IQR: 0.51RLU – 0.84RLU) in the high malaria transmission period. However, ADRB activity was higher in individuals with symptomatic malaria (median: 0.72RLU, IQR: 0.60RLU – 0.80RLU) than those with asymptomatic infections (median: 0.49RLU, IQR: 0.37RLU – 0.63RLU) (Mann-Whitney U test, p = 0.018) during the low malaria transmission period. In individuals with symptomatic malaria, higher ADRB activity was observed from those in the low (median: 0.72RLU, IQR: 0.60RLU – 0.80RLU) than those in the high (median: 0.50RLU, IQR: 0.48RLU – 0.66RLU) (Mann-Whitney U test, p = 0.041) malaria transmission period. Nevertheless, in individuals with asymptomatic infection, higher ADRB activity was observed from those in the high (median: 0.64RLU, IQR: 0.51RLU – 0.84RLU)) than those in the low (median: 0.49RLU, IQR: 0.37RLU – 0.63RLU) (Mann-Whitney U test, p = 0.014) malaria transmission period (
Figure 3 B).
To assess the association between ADRB activity (top versus low ADRB responder) and parasitemia (parasite positivity as measured by PCR), adjusting for age (as a continuous variable), sex and fever, a logistics regression model stratified by malaria transmission was fitted. The odds were significantly higher among adults than in children in both the high (OR: 1.109, 95% CI: 1.052 - 1.186, p < 0.001) and low (OR: 1.071, 95% CI: 1.038 - 1.110, p < 0.001) malaria transmission period. Also, during the low but not the high malaria transmission period, individuals who were parasite PCR-positive at the time of the survey had significantly higher odds of being ADRB’s top responders (OR: 8.273, 95% CI: 1.851 - 40.596, p = 0.006) than individuals who were parasite PCR-negative at the time of the survey (
Table 2).
4. Discussion
The development of naturally acquired immunity to P. falciparum depends on exposure to the parasite. Consequently, malaria transmission intensity influences the development of naturally acquired immunity to P. falciparum [
29,
30]. In this study, we aimed to evaluate naturally acquired immunity in an area that has experienced a gradual decline in malaria transmission over 25 years from 1993 to 2019. To accomplish this, we assessed the ability of opsonising antibodies against P. falciparum merozoites to recruit neutrophils in an in vitro solid-phase chemiluminescence assay called the antibody-dependent respiratory burst (ADRB). ADRB has a well-standardised protocol [
12] and has previously been reported as a correlate of protection against clinical malaria [
14]. Here, we measured ADRB activity in children and adults, comparing a period of high versus low malaria transmission.
Indeed, malaria transmission intensity impacted ADRB activity in Nyamisati, where we report higher ADRB activity during the high malaria transmission period than in the low malaria transmission period in children and adults, proposing that antibodies promoting ADRB activity correlate to the degree of malaria endemicity. Conforming with these results, a study comparing data from two villages in Senegal reported higher ADRB activity from the village with holoendemic malaria transmission than from the village with mesoendemic malaria transmission [
13]. A different longitudinal study assessing other antibody-mediated functions in children reported a decline in opsonic phagocytosis and maintenance of complement-fixing activity over a year [
17]. The decrease in malaria transmission intensity does not necessarily impact all antibody-mediated functions equally; other antibody-mediated functions may be maintained.
In malaria-endemic areas, an individual’s age reflects cumulative exposure to malaria [
29,
31]. In this study, age is an essential predictor of ADRB activity, and ADRB activity is positively correlated with age. This suggests that acquiring antibodies promoting ADRB activity coincides with developing naturally acquired immunity rendered by exposure. Other studies have reported this observation assessing ADRB activity [
13,
14] and other Fc-dependent antibody-mediated functions against P. falciparum [
32,
33,
34,
35]. A captivating observation was that only adults 21 years and above during the high malaria transmission period had mean ADRB activity above the ADRB cut-off. This observation may imply that as malaria transmission declined in Nyamisati, the level of naturally acquired immunity against P. falciparum also declined. To our current knowledge, it is the first time that data on antibody-mediated functional activity against P. falciparum has been reported in the context of declining malaria transmission in the same area over an extended period in children and adults. Declines in the level of immunity in a population may influence the occurrence of malaria epidemics with a significant morbidity burden. One study in Thailand implied that the decrease in P. falciparum transmission and the level of immunity might influence the emergence of artemisinin-resistant falciparum malaria [
16]. Parts of Africa have experienced a significant reduction in malaria transmission [
36,
37], and if the levels of immunity decline, malaria vaccines promoting strong antibody-mediated functions against P. falciparum will be necessary to maintain immunity.
We observed higher ADRB activity among P. falciparum-positive than negative individuals during the low but not the high malaria transmission period. This observation could be because ongoing P. falciparum infection boosted antibodies promoting ADRB activity [
38,
39]. Similar findings have been reported with antibody-mediated opsonic phagocytosis [
33] and antibody-dependent natural killer cell activity [
35] against P. falciparum in Kenyan children. Antibody boosting is not likely to significantly influence differences in ADRB activity among parasite-positive versus negative individuals in the high malaria transmission period due to constant exposure to the parasite.
The finding that symptomatic malaria during the survey was a significant predictor of higher ADRB activity during the low malaria transmission period cannot be explained. This finding contradicts longitudinal studies reporting ADRB activity against P. falciparum as a correlate of protection against clinical malaria [
13,
14]. This study is based on data from cross-sectional studies; therefore, further studies are necessary to assess the correlation between antibody-mediated functions and the risk of malaria in this cohort.
In this study, we only employed ADRB to assess the level of immunity to P. falciparum. Since there is no single correlate measure of immunity to malaria, other antibody-mediated functions could be used with ADRB to assess the level of immunity to malaria. Besides antibody-mediated functional activity, antibody subclass, specifically the cytophilic IgG1 and IgG3 [
40,
41] and antibody levels to specific P. falciparum antigens (39) have been predictors of immunity against clinical malaria. Assessing these with antibody-dependent functional activity will provide a finer understanding of immunity to malaria.
In summary, we show that in Nyamisati, an area with declining malaria transmission in Tanzania, antibodies promoting ADRB activity against P. falciparum reduce in children and adults and are influenced by exposure to the parasite. These findings propose that naturally acquired immunity to P. falciparum decreased in children and adults as malaria transmission declined. In the current era of intensified control measures to eliminate malaria, these findings imply that effective malaria vaccines will be necessary to induce and maintain effective immunity in children and adults.
Supplementary Materials
The following supporting information can be downloaded at Preprints.org. Figure S1: Median age among individuals with symptomatic malaria and asymptomatic infection in the high and low malaria transmission period
Author Contributions
Conceptualization, A.F and F.O; Data curation, D.M, J.T, R.O, V.Y, I.R, B.N and A.F; Formal analysis, D.M, K.W, I.C and P.V; Funding acquisition, A.F and F.O; Investigation, D.M, A.F and F.O; Methodology, D.M, R.O and L.N; Project administration, S.K; Resources, F.O; Software, D.M, K.W, I.C and P.V; Supervision, V.Y, S.K, B.N, A.F and F.O; Validation, D.M, J.T and R.O; Visualization, D.M and J.T; Writing – original draft, D.M; Writing – review & editing, D.M, J.T, R.O, L.N, K.W, V.Y, S.K, B.N, A.F and F.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded through Initiative to Develop Africa Research Leaders (IDeAL) grant number DEL-15-003, Swedish Research Council (VR Developmental Grant 2021-04072).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Muhimbili University of Health and Allied Sciences (MUHAS-REC-06-2023), the National Institutet for Medical Research (NIMR/HQ/R.8a/Vol.IX/4348), and the Central Ethical Review Board in Stockholm (Dnr. 00-084, 2012/1151–32, 2015_2199-32). The Kibiti district and the Nyamisati village boards permitted blood sample collection.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.
Acknowledgements
We want to thank all study participants, Nyamisati Malaria Research Group members and all who donated blood for neutrophil preparation.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the study's design, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- WHO. World Malaria Report 2023. Geneva. World Health Organisation 2023.
- Seidlein L Von, Hanboonkunupakarn B, Jittmala P. RTS, S / AS01, a vaccine targeting pre-erythrocytic stages of Plasmodium falciparum. 2017;1(6):533–7. [CrossRef]
- Datoo M.S, Natama M.H, Somé A, Traoré O, Rouamba T, Bellamy D, et al. Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso : a randomised controlled trial. Lancet. 2021;397:1809–18. [CrossRef]
- Datoo M.S, Natama H.M, Somé A, Bellamy D, Traoré O, Rouamba T, et al. Efficacy and immunogenicity of R21/Matrix-M vaccine against clinical malaria after 2 years follow-up in children in Burkina Faso: a phase 1/2b randomised controlled trial. Lancet Infect Dis. 2022;22:1728–36. [CrossRef]
- Cohen S, McGregor I.A and Carrington S. Gamma-globulin and acquired immunity to human Malaria. Nature Publishing Group. 1961;192:733-7. [CrossRef]
- Edozien J.C, Gilles H.M and Udeozo I.O.K. Adult and cord-blood gamma-globulin and immunity to malaria in Nigerians. The Lancet. 1962;951–5. [CrossRef]
- Tijani M.K, Lugaajju A, Persson K.E.M. Naturally acquired antibodies against Plasmodium falciparum: Friend or foe? Pathogens. MDPI AG; 2021;10:832. [CrossRef]
- Duncan C.J.A, Hill A.V.S, Ellis R.D. Can growth inhibition assays (GIA) predict blood-stage malaria vaccine efficacy? Christopher. Hum Vacci Immono. 2012;8:706–14. [CrossRef]
- Nauseef W.M. Neutrophils, from cradle to grave and beyond. Immunol Rev. Blackwell Publishing Ltd; 2016. p. 5–10. [CrossRef]
- Liew P.X, Kubes P. During Health and Disease. Physiol Rev. 2019;99:1223–48. [CrossRef]
- Greve B, Lehman L.G, Lell B, Luckner D, Schmidt-Ott R, Kremsner P.G. High Oxygen Radical Production Is Associated with Fast Parasite Clearance in Children with Plasmodium falciparum Malaria. J Infect Dis. 1999;179:1584-6. [CrossRef]
- Llewellyn D, Miura K, Fay M.P, Williams A.R, Murungi L.M, Shi J, et al. Standardization of the antibody-dependent respiratory burst assay with human neutrophils and Plasmodium falciparum malaria. Sci Rep. 2015;5:1–14. [CrossRef]
- Mansourou A, Joos C, Niass O, Diouf B, Tall A, et al. Improvement of the antibody-dependent respiratory burst assay for assessing protective immune responses to malaria. Open Biol. 2022;12:210288. [CrossRef]
- Joos C, Marrama L, Polson H.E.J, Corre S, Diatta A.M, Diouf B, et al. Clinical protection from falciparum malaria correlates with neutrophil respiratory bursts induced by merozoites opsonized with human serum antibodies. PLoS One. 2010;5(3):e9871. [CrossRef]
- Marsh K, Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol. 2006;28:51–60. [CrossRef]
- Ataíde R, Powell R, Moore K, McLean A, Phyo A.P, Nair S, et al. Declining Transmission and Immunity to Malaria and Emerging Artemisinin Resistance in Thailand: A Longitudinal Study. Journal of Infectious Diseases. 2017;216:723–31. [CrossRef]
- Mugyenyi C.K, Elliott S.R, Yap X.Z, Feng G, Boeuf P, Fegan G, et al. Declining Malaria Transmission Differentially Impacts the Maintenance of Humoral Immunity to Plasmodium falciparum in Children. Journal of Infectious Diseases. 2017;216:879–98. [CrossRef]
- Raghavan M, Kalantar K.L, Duarte E, Teyssier N, Takahashi S, Kung A.F, et al. Proteome-wide antigenic profiling in Ugandan cohorts identifies associations between age, exposure intensity, and responses to repeat-containing antigens in Plasmodium falciparum. Elife. 2023;12:e81401. [CrossRef]
- Kurtovic L, Agius P.A, Feng G, Drew D.R, Ubillos I, Sacarlal J, et al. Induction and decay of functional complement-fixing antibodies by the RTS, S malaria vaccine in children, and a negative impact of malaria exposure. BMC Med. 2019;17:45. [CrossRef]
- Feng G, Kurtovic L, Agius P.A, Aitken E.H, Sacarlal J, Wines B.D, et al. Induction, decay, and determinants of functional antibodies following vaccination with the RTS, S malaria vaccine in young children. BMC Med. 2022;20:289. [CrossRef]
- Dent A.E, Chelimo K, Sumba P.O, Spring M.D, Crabb B.S, Moormann A.M, et al. Temporal stability of naturally acquired immunity to merozoite surface protein-1 in Kenyan adults. Malar J. 2009;8:1–10. [CrossRef]
- Crompton P.D, Kayala M.A, Traore B, Kayentao K, Ongoiba A, Weiss G.E. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. PNAS. 2010;107:2–7. [CrossRef]
- Färnert A, Yman V, Homann M.V, Wandell G, Mhoja L, Johansson M, et al. Epidemiology of malaria in a village in the Rufiji River Delta, Tanzania : declining transmission over 25 years revealed by different parasitological metrics. Malar J. 2014;13:1–12. [CrossRef]
- Yman V, Wandell G, Mutemi D.D, Miglar A, Asghar M, Hammar U, et al. Persistent transmission of Plasmodium malaria and Plasmodium ovale species in an area of declining Plasmodium falciparum transmission in Eastern Tanzania. PLoS Negl Trop Dis. 2019;13:1–16. [CrossRef]
- Shokoples S.E, Ndao M, Kowalewska-Grochowska K, Yanow S.K. Multiplexed real-time PCR assay for discrimination of Plasmodium species with improved sensitivity for mixed infections. J Clin Microbiol. 2009;47:975–80. [CrossRef]
- Vafa Homann M, Emami SN, Yman V, Stenström C, Sondén K, Ramström H, et al. Detection of Malaria Parasites After Treatment in Travelers: A 12-month Longitudinal Study and Statistical Modelling Analysis. EBioMedicine. 2017;25:66–72. [CrossRef]
- Maqbool M, Vidyadaran S, George E, Ramasamy R. Optimisation of Laboratory Procedures for Isolating Human Peripheral Blood-Derived Neutrophils. Med J Malay. 2011;66(4):296-9.
- Posit team. RStudio: Integrated Development Environment for R. Boston, MA: Posit Software, PBC; 2023.
- Doolan D.L, Dobaño C, Baird J.K. Acquired immunity to Malaria. Clin Microbiol Rev. 2009;22:13–36. [CrossRef]
- Freya F. Immunity to malaria in an era of declining malaria transmission. Parasitology. 2016;143:139-53.
- Langhorne J, Ndungu F.M, Sponaas A.M, Marsh K. Immunity to malaria: More questions than answers. Nat Immunol. 2008;9:725–32. [CrossRef]
- Hill D.L, Eriksson E.M, Li CSN, Suen W, Chiu C.Y, Ryg- V, et al. Opsonising Antibodies to Plasmodium falciparum Merozoites Associated with Immunity to Clinical Malaria. PLoS One. 2013;8(9):e74627. [CrossRef]
- Osier F.H.A, Feng G, Boyle M.J, Langer C, Zhou J, Richards J.S, et al. Opsonic phagocytosis of Plasmodium falciparum merozoites : mechanism in human immunity and a correlate of protection against malaria. BMC Med. 2014;12:1–15. [CrossRef]
- Kurtovic L, Behet M.C, Feng G, Reiling L, Chelimo K, Dent A.E, et al. Human antibodies activate complements against Plasmodium falciparum sporozoites and are associated with protection against malaria in children. BMC Med. 2018;16:61. [CrossRef]
- Odera D.O, Tuju J, Mwai K, Nkumama I.N, Fürle K, Chege T, et al. Anti-merozoite antibodies induce natural killer cell effector function and are associated with immunity against malaria. Trans Med. 2023;eabn5993. [CrossRef]
- Bhatt S, Weiss D.J, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–11. [CrossRef]
- Nkumama I.N, O’Meara W.P, Osier F.H.A. Changes in Malaria Epidemiology in Africa and New Challenges for Elimination. Trends Parasitol. 2017;33:128–40. [CrossRef]
- White M.T, Griffin J.T, Akpogheneta O, Conway D.J, Koram K.A, Riley E.M, et al. Dynamics of the antibody response to Plasmodium falciparum infection in African children. Journal of Infectious Diseases. 2014;210:1115–22. [CrossRef]
- Akpogheneta O.J, Dunyo S, Pinder M, Conway D.J. Boosting antibody responses to Plasmodium falciparum merozoite antigens in children with highly seasonal exposure to infection. Parasite Immunol. 2010;32:296–304. [CrossRef]
- Cherif M.K, Ouédraogo O, Sanou G.S, Diarra A, Ouédraogo A, Tiono A, et al. Antibody responses to Plasmodium falciparum blood stage antigens and incidence of clinical malaria in children living in an endemic area in Burkina Faso. BMC Res Notes. 2017;10:472. [CrossRef]
- Dobaño C, Santano R, Vidal M, Jiménez A, Jairoce C, Ubillos I, et al. Differential patterns of IgG subclass responses to Plasmodium falciparum antigens in relation to malaria protection and RTS, S vaccination. Front Immunol. 2019;10:439. [CrossRef]
- Osier F.H.A, Fegan G, Polley S.D, Murungi L, Verra F, Tetteh K.K.A, et al. Breadth and Magnitude of Antibody Responses to Multiple Plasmodium falciparum Merozoite Antigens Are Associated with Protection from Clinical Malaria. American Society for Microbiology. 2008;76:2240–8. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).