1. Introduction
Hemopathogens are relatively frequent in dogs (
Canis lupus familiaris) causing different infectious diseases with variable clinical signs and outcomes. Most hemopathogens are protozoa transmitted by vectors (fleas, ticks and insects). Among them,
Leishmania infantum (Kinetoplastida: Trypanosomatidae) is an important hemopathogenic protozoan that causes canine leishmaniasis (CanL). Infected dogs are reservoirs of
L. infantum which can be further transmitted to the humans, resulting in human visceral leishmaniasis (HVL) [
1,
2]. In addition,
L. infantum can also be transmitted by insects, highlighting the permissive phlebotomine species
Lutzomyia longipalpis in South America. This insect is a tropical / subtropical sand fly commonly detected in the Central and North from Brazil, but less frequently in the southernmost regions of the country [
3].
Environmental and cultural conditions are associated with infection prevalence in canine and human hosts, and both diseases (CanL and HVL) present a high diversity of frequency in Brazilian regions [
3]. Due to the absence of CanL and HVL and the lack of circulation of
Lu. longipalpis, these diseases were considered imported cases in southern Brazil until to the early 2000s. The first CanL autochthonous case was reported in São Borja, a city bordering the province of Corrientes, Argentina, in 2008 [
4]. Other studies detect CanL in geographical regions of Argentina bordering Brazil as well as in cities from the Brazilian southernmost states (Rio Grande do Sul and Santa Catarina) few years later [5-8]. The recent widespread dispersion of
Lu. longipalpis in South Brazil was attributed to its great capacity to adapt to different ecological niches, highlighting urban environments [
3]. More recent studies are also raising the hypothesis that other insect could be vectoring
L. infantum in Brazil [
9,
10].
The epidemiological role of dogs with CanL in the transmission of
L. infantum to humans is even greater and more concerning in urban areas, as these reservoir animals are in close contact with people. Amastigotes from this protozoa present in the skin of infected dogs can be transmitted to the sandflies vectors and after to humans [
11,
12]. In many Brazilian cities, CanL outbreaks were reported to precede the onset of HVL cases [
13,
14].
CanL natural history can course from a total absence of symptoms to a severe clinical syndrome [
15]. The most frequent clinical manifestations are cutaneous (alopecia, dermatitis, onychogryphosis) and may be seen along with other clinical symptoms or pathological abnormalities. CanL signs unrelated to cutaneous lesions include ocular alterations, epistaxis, lameness, vascular and neurological disorders [
16]. In the final stage of the disease, many organs are affected, most animals present cachexia and death is result of renal or hepatic failure [
15].
L. infantum infection has been routinely diagnosed using serological tests recommended by the Brazilian Ministry of Health in the Visceral Leishmaniasis Control Program (PCLV,
Programa de Controle da Leishmaniose Visceral) [
17]. Currently, the complete diagnosis procedure includes a rapid chromatographic immunoassay (dual-path platform - DPP®) as screening test, and the enzyme-linked immunosorbent assay (ELISA) as confirmatory test. As serological tests have been questioned, especially regarding the accuracy, PCR based assays have also been included for a more comprehensive diagnosis [
18].
In the present study, several CanL autochthonous cases detected in the municipality of Uruguaiana, Rio Grande Do Sul state, were investigated. The main objectives were to confirm the increasing incidence of CanL in the urban area as well as to investigate the clinical signs of this disease in the infected urban dogs.
2. Materials and Methods
2.1. Study area
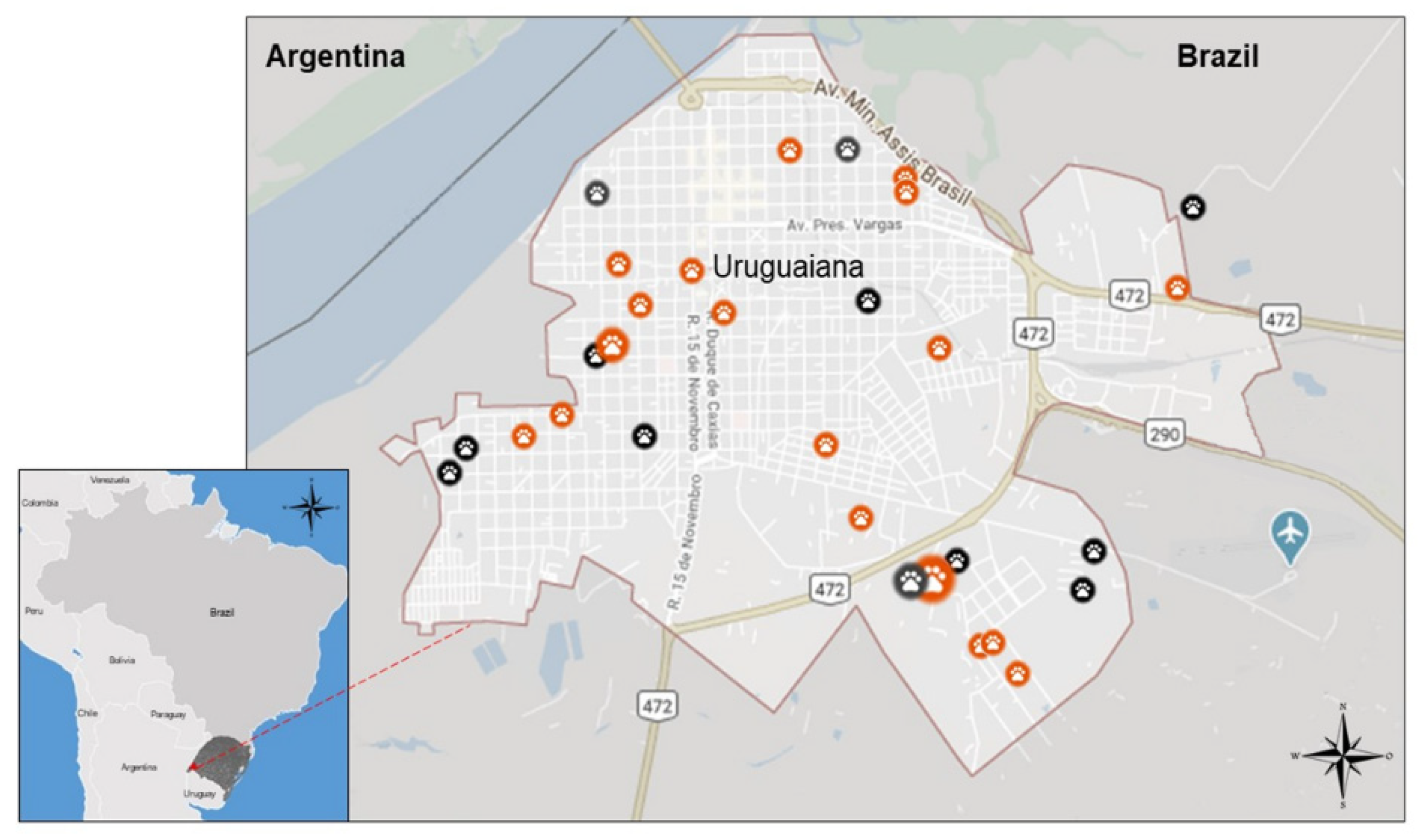
The study was conducted in the urban perimeter of Uruguaiana (Latitude -29.7495, Longitude -57.0882, 29° 44′ 58″ South, 57° 5′ 18″ West), Rio Grande do Sul state, South Brazil. It is a city located on the western border of this Brazilian southernmost state with Argentina, separated by the Uruguay River from the city of Paso de Los Libres in the province of Corrientes (
Figure 1). The municipality occupies an area of 5,702.1 km2 (2022) and the local population is 126,766 inhabitants in total (2021), according to the latest estimate by the Brazilian Institute of Geography and Statistic [
19].
2.2. Animal sampling
The animal population sampling was obtained from dogs attended by a veterinary service in the urban area of the municipality of Uruguaiana (Rio Grande do Sul, Brazil) from August 2019 to February 2020. Blood samples were collected from 51 domiciled and / or community animals suspected having CanL. The inclusion criteria for dogs in the study were to present two or more clinical signs (such as weight loss, eye injuries, skin disorders, apathy) suggesting CanL in the anamnesis and / or in the clinical examination performed by the veterinarian. Demographic and general information were provided by the pet’s tutors answering an epidemiological survey form.
The whole study was approved by the Ethics Committee on the Use of Animals (CEUA) of the Lutheran University of Brazil (CEUA-ULBRA nº 2017/355).
2.3. Laboratory analysis
Blood samples from the animals were collected by venipuncture and stored in two aliquots of around 1mL, one in a tube without anticoagulant (serum) for the immunological tests and another in a sterile tube containing EDTA (whole blood) for the real-time PCR (qPCR). The tubes for the immunological tests were kept refrigerated until processing and the others (for DNA analyses) were stored at -20ºC until the molecular detection procedure.
The rapid screening test immunochromatographic TR DPP® (DPP) was performed according the manufacturer instructions (Biomanguinhos-FIOCRUZ, Rio de Janeiro, Brazil). Positive samples in the DPP test were sent to a confirmatory ELISA assay (Biomanguinhos-FIOCRUZ, Rio de Janeiro, Brazil) performed at a reference laboratory (Laboratorio Central do Rio Grande do Sul / LACEN-RS, Porto Alegre, RS, Brazil).
The qPCR detection of L. infantum was carried out with commercial reagents. DNA was extracted by the silica adsorption methodology using the NewGene® Prep and PreAmp commercial reagents according manufacturer instructions (Simbios Biotecnologia, Cachoeirinha, RS, Brazil). L. infantum DNA was specifically detected by qPCR with LVCAmp NewGene® reagents (Simbios Biotecnologia, Cachoeirinha, RS, Brazil). All reactions were performed on the StepOnePlus ™ real-time PCR System (Applied Biosystems, Palo Alto, USA) with the following amplification conditions: a cycle of 95 ° C for 3 min, followed by 40 cycles at 95 ° C for 15 s and 60 ° C for 1 min. Negative and positive DNA L. infantum controls were used in all runs. A standard curve with 10 fold dilutions of the DNA positive control was carried out in some analyses to estimate the parasitemia. The positive result values were expressed in cycle thresholds (Cts) and parasites/mL.
2.3. Statistical analysis
All data analysis were conducted with IBM SPSS (version 23.0) and RStudio (version 4.2.3 version). The demographic data and clinical signs were represented by their frequencies (absolute and relative). For continuous data, normality was assessed using the Anderson–Darling test. Bivariate analyzes were performed to evaluate the association between categorical variables and the outcome. Absolute and relative frequencies were estimated for categorical data using Pearson's chi-square test or exact Fisher test. The pre-established significance level for the 5% alpha error, bilateral and p<0.05 were considered significant.
3. Results
3.1. Overall CanL prevalence and epidemiological data
A total of 51 dogs (approximately 10.2% of the attended animals) were considered suspect to have CanL in the clinical exam by the Veterinarians in Uruguaiana in the period of seven months of the study. These suspect CanL positive dogs were obtained in several locations in the city (
Figure 1). Laboratorial tests demonstrated that 31 out of the 51 (60.8%) dogs were positive for
L. infantum in at least one laboratorial detection method, while 20 (39.2%) were negative in immunological and molecular assays. A total of 22 (43.1%) dogs were positive in both rapid DPP and qPCR methods, throughout 6 (11.8%) were positive only in the DPP test and 3 (5.9%) only in the qPCR (
Table 1). All 6 positive samples in the DPP test were also positive in the confirmatory ELISA. In addition, the parasitemia was evaluated in the 25 (49.0%) qPCR positive samples, ranging from 3 to 52,700 parasites/mL of blood.
The general epidemiological data demonstrated the occurrence of males (n=29; 56.9%) and females (n=22; 43.1%), pure (n=9; 17.6%) and mixed (n=41; 80.4%) breeds, and young (n=23; 45.1%), adult (n=15; 29.4%) and elderly (n=11; 21.6%) in all examined animals. Among the 31 animals positive for CanL, 18 (58.1%) were males and 13 (41.9%) females. In addition, 12 (38.7%) were puppies (0-2 years old), 11 (35.5%) were adults (3-5 years old) and 6 (19.4%) were elderly dogs (>6 years old). Further, animals with short fur and that live with other dogs represented 90.3% (28) of the positive CanL (
Table 2).
3.2. Clinical signs
Different clinical signs were observed in the animals, including lymphadenopathy (n=26), dull hair coat (n=26), alopecia (n=22), desquamation (n=20), eye lesion (n=20), loss (n=17), onychogryphosis (n=16), weight muscle atrophy (n=10), hyperkeratosis (n=10), skin ulcer (n=10), apathy (n=6), hepatomegaly (n=5), and diarrhea (n=2). Among the clinical signs commonly observed in animals with visceral leishmaniasis, desquamation and onychogryphosis were frequently observed (in 64.5% and 51.6% positive animals, respectively), with p value <0.05 (
Table 3).
4. Discussion
Visceral leishmaniasis is considered a neglected disease, endemic in many Brazilian regions. This situation is still the subject of discussion in southern Brazil, as
L. infantum has not yet been detected in several cities. In the urban perimeters of the cities, dogs are the main reservoirs of the etiological protozoan
L. infantum. Furthermore, these animals can present CanL and play an important role in the epidemiology of this very health concerning zoonosis [
13].
In the present study, 51 dogs were considered suspect having CanL in the clinical exam by Veterinarians in the period of seven months in Uruguaiana, a city bordering Argentina. The convenience sampling obtained here presented different genders, breeds and ages of dogs as well as it included animals living in different neighborhoods in the urban perimeter of the city. Thirty-one (60.8%) of these 51 animals were confirmed to be positive for
L. infantum. A previous survey had already reported a high frequency of CanL, since it was observed more than 10% of dog samples with antibodies against
L. infantum between 2009 and 2010, with some neighborhoods in the city with higher frequencies than others [
20]. In view of this concerning epidemiological situation, previous studies have already investigated other animal species with leishmaniosis, including wild and domestic mammals [
21,
22].
The current study also highlights the detection of
L. infantum with different methods. Indirect immunological methods evaluate the occurrence of antibodies against this parasite in the animal body fluids, while direct tests detect
L. infantum nucleic acids (mainly DNA). The use of these different assays (immunological and molecular) is still not officially recommended, but this rule must be revised. A more accurate diagnosis could be reached evaluating the antigenic response (detecting antibodies with rapid test and/or ELISA) and the presence of the parasite by PCR [
17]. Noteworthy, the whole diagnostic performance is influenced by the time of infection, sample collection, vaccination status, immunosuppression diseases and other factors. Therefore, the use of two or more methods, including PCR, would be very welcome for a definitive diagnostic [
18,
23]. In addition, qPCR is a useful method to report the parasites load. This information has been proved to be necessary to monitor dogs with CanL as well as to evaluate the response to medicaments [24-26].
Also, the clinical exam is very important to identify CanL in the dogs in an endemic region. The main clinical signs of the infected animals observed here were enlarged lymph nodes (82.4%) and dull coat (64.7%), but weight loss and skin diseases were also important clinical manifestations. Skin lesions were detected mainly in the ears (where there is less hair), being preferable for mosquitoes [
15,
16]. Skin peeling and onychogryphosis were respectively observed 3.4 and 4.3 times more often in dogs with leishmaniasis than in uninfected animals. Onychogryphosis is a clinical manifestation highly correlated with CanL, especially when associated with other cutaneous findings such as exfoliative dermatitis, as observed in this study. Although approximately half of the dogs with leishmaniasis presented onychogryphosis in this study, this clinical sign has already been described in up to 70% of dogs with this disease [
27,
28,
29,
30,
31]. The complete pathogenesis is not completely known, but excessive nail growth has been associated with more severe parasitism [
30,
32,
33]. The frequent observation of cutaneous findings (onychogriphosis and skin scaling) in this study highlights that the observation of these main clinical manifestations are strong indications of CanL in this endemic region. Veterinarians should pay attention to these specific clinical signs to diagnose new cases.
The results of the present study also reinforce the increasing prevalence of
L. infantum in the Brazil South region. The first cases in this region were preceded by CanL and HVL cases in the most tropical regions of Argentina that border South Brazil [
34,
35]. This geographic region has been considered the entry point for this pathogen in southern Brazil, which is now spreading to other cities in the state of Rio Grande do Sul. HVL cases were already detected in the Rio Grande do Sul state last years [
36]. Therefore, more effective prevention methods are necessary to control the dissemination of
L. infantum to human and animals in all-southernmost states from Brazil.
5. Conclusions
CanL was detected in 31 out of 51 (60.8%) domestic dogs with clinical signs suggestive of this disease in the urban area of Uruguaiana city. These data demonstrate the recent expansion of L. infantum in urban dogs from this city in last years, reinforcing the introduction of this parasite in southern Brazil by the Argentinian border. It also highlights the importance of detecting dogs with CanL by clinical and laboratorial evaluation to prevent more widespread L. infantum dissemination in other dogs and humans.
Author Contributions
Conceptualization, A.P.F., P.F.S. and V.R.L.; methodology, A.P.F., V.P.S., F.G.O.G.; software, V.R.Z.B.P; validation, L.S.M and V.R.Z.B.P.; formal analysis, A.P.F., V.P.S. and V.R.L.; investigation, A.P.F., V.P.S., F.G.O.G. and N.I.; resources, A.F.S and A.S.K.F.; data curation, L.S.M., N.I. and V.R.L.; writing—original draft preparation, A.P.F., V.P.S. and V.R.L.; writing—review and editing, L.S.M., V.R.Z.B.P. and V.R.L.; supervision, A.F.S and A.S.K.F.; project administration, V.R.L.; funding acquisition, A.S.K.F and V.R.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee on the Use of Animals (CEUA) of the Lutheran University of Brazil (CEUA-ULBRA nº 2017/355).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dantas-Torres, F. Canine vector-borne diseases in Brazil. Parasit. Vectors. 2008, 1(1), 25. [CrossRef]
- Travi, B.L., Cordeiro-da-Silva, A., Dantas-Torres, F., Miró, G. Canine visceral leishmaniasis: Diagnosis and management of the reservoir living among us. PLoS Negl. Trop. Dis. 2018, 12(1), e0006082. [CrossRef]
- Andrade-Filho, J.D., Scholte, R.G.C., Amaral, A.L.G., Shimabukuro, P.H.F., Carvalho, O.S., Caldeira, R.L. Occurrence and probability maps of Lutzomyia longipalpis and Lutzomyia cruzi (Diptera: Psychodidae: Phlebotominae) in Brazil. J. Med. Entomol. 2017, 54(5), 1430-1434. [CrossRef]
- Azevedo, J.S.C., Esmeraldino, A.T., Ávila, V.P.F., Witz, M.I., Fischer, C.D.B., Tartarotti, A.L. Leishmaniose visceral canina autóctone no município de São Borja, Rio Grande do Sul, Brasil: relato de caso. Veterinária em Foco, 2009, 7(1), 52-61.
- Acardi, S.A., Liotta, D.J, Santini, M.S., Romagosa, C.M., Salomón, O.D. Detection of Leishmania infantum in naturally infected Lutzomyia longipalpis (Diptera: Psychodidae: Phlebotominae) and Canis familiaris in Misiones, Argentina: the first report of a PCR-RFLP and sequencing-based confirmation assay. Mem. Inst. Oswaldo Cruz. 2010, 105(6), 796-9. [CrossRef]
- Acosta, L., Díaz, R., Torres, P., Silva, G., Ramos, M., Fattore, G., et al. Identification of Leishmania infantum in Puerto Iguazú, Misiones, Argentina. Rev. Inst. Med. Trop. São Paulo. 2015, 57,175-6. [CrossRef]
- Rêgo, F.D., Souza, G.D., Miranda, J.B., Peixoto, L.V., Andrade-Filho, J.D. Potential Vectors of Leishmania Parasites in a Recent Focus of Visceral Leishmaniasis in Neighborhoods of Porto Alegre, State of Rio Grande do Sul, Brazil. J. Med. Entomol. 2020, 57(4), 1286-1292. [CrossRef]
- Pinto, A.O., Carvalho, D., Frizzo, C., Lopes, K., Tessari, G.B., Catecati, T., et al. First case of canine visceral leishmaniasis in the midwestern of Santa Catarina State, Brazil. Braz. J. Biol. 2021, 82, e241162. [CrossRef]
- Guimarães, V.C., Pruzinova, K., Sadlova, J., Volfova, V., Myskova, J., Filho, S.P., Volf, P. Lutzomyia migonei is a permissive vector competent for Leishmania infantum. Parasit Vectors. 2016, 9, 159. [CrossRef]
- Galvis-Ovallos, F., Ueta, A.E., Marques, G.O., Sarmento, A.M.C., Araujo, G., Sandoval, C., et al. Detection of Pintomyia fischeri (Diptera: Psychodidae) with Leishmania infantum (Trypanosomatida: Trypanosomatidae) Promastigotes in a Focus of Visceral Leishmaniasis in Brazil. J. Med. Entomol. 2021, 58(2), 830-836. [CrossRef]
- Carvalho Junior, C.G., Teixeira Neto, R.G., Lopes, V.V., Belo, V.S., Alves, N.R., de Paula, T.B., et al. Parasitism and inflammation in ear skin and in genital tissues of symptomatic and asymptomatic male dogs with visceral leishmaniasis. Parasitol Res. 2017, 116(3), 987-995. [CrossRef]
- Lopes, J.V., Michalsky, É.M., Pereira, N.C.L., Paula, A.J.V., Souza, A.G.M., Pinheiro, L.C., et al. Canine visceral leishmaniasis in area with recent Leishmania transmission: prevalence, diagnosis, and molecular identification of the infecting species. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200141. [CrossRef]
- De Araújo, V.E., Pinheiro, L.C., Almeida, M.C., de Menezes, F.C., Morais, M.H., Reis, I.A., et al. Relative risk of visceral leishmaniasis in Brazil: a spatial analysis in urban area. PLoS Negl. Trop. Dis. 2013, 7(11), e2540. [CrossRef]
- Belo, V.S., Werneck, G.L., Barbosa, D.S., Simões, T.C., Nascimento, B.W., da Silva, E.S., Struchiner, C.J. Factors associated with visceral leishmaniasis in the americas: a systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2013, 7(4), e2182. [CrossRef]
- Alvar, J., Cañavate, C., Molina, R., Moreno, J., Nieto, J. Canine leishmaniasis. Adv. Parasitol. 2004, 57, 1-88. [CrossRef]
- Pennisi, M.G., Cardoso, L., Baneth, G., Bourdeau, P., Koutinas, A., Miró, G., et al. LeishVet update and recommendations on feline leishmaniosis. Parasit Vectors. 2015, 8, 302. [CrossRef]
- Brasil - Ministério da Saúde (MS). Manual de Vigilância e controle da Leishmaniose Visceral [Internet]. 2014, 122 p. Available from: https://bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_controle_leishmaniose_visceral_1edicao.pdf.
- Sevá, A.D.P., Brandão, A.P.D., Godoy, S.N., Soares, R.M., Langoni, H., Rodrigues, B.C., et al. Investigation of canine visceral leishmaniasis in a non-endemic area in Brazil and the comparison of serological and molecular diagnostic tests. Rev. Soc. Bras. Med. Trop. 2021, 54, e01822021. [CrossRef]
- IBGE – Instituto Brasileiro de Geografia e Estatística, 2023. Uruguaiana. Obtained in https://www.ibge.gov.br/cidades-e-estados/rs/uruguaiana.html [April 08, 2023].
- Massia, L.I., Lamadril, R.D.Q., Wellicks, J.R., Bittencourt, R.A., Bittencourt, D.G., Marques, G.D., et al. Leishmaniose visceral canina em três bairros de Uruguaiana - RS. Vigil. Sanit. Debate. 2016, 4(1), 113–119. [CrossRef]
- Escobar, T.A., Dowich, G., Dos Santos, T.P., Zuravski, L., Duarte, C.A., Lübeck, I., Manfredini, V. Assessment of Leishmania infantum infection in equine populations in a canine visceral leishmaniosis transmission area. BMC Vet. Res. 2019, 15(1), 381. [CrossRef]
- Pradella, G.D., Escobar, T.A., Santos, T.P.D., Vargas, R.C., Góss, G.C., Ferrareze, P.A.G., et al. PCR-RLFP characterization of Leishmania spp. in domestic animals from the south-western border of Brazil. Rev. Bras. Parasitol. Vet. 2022, 31(3), e005222. [CrossRef]
- Garay, A.F.G., Fraenkel, S., Diaz, J.J.A.R., Recalde, O.D.S., Gómez, M.C.V., Riquelme, J.A.M., et al. Sensitivity comparison for the Leishmania spp. detection in different canine tissues using PCR-HRM. Rev. Soc. Bras. Med. Trop. 2022, 55, e0069-2022. [CrossRef]
- Paltrinieri, S., Gradoni, L., Roura, X., Zatelli, A., Zini, E. Laboratory tests for diagnosing and monitoring canine leishmaniasis. Vet. Clin. Pathol. 2016, 45(4), 552-578. [CrossRef]
- Solano-Gallego, L., Di Filippo, L., Ordeix, L., Planellas, M., Roura, X., Altet, L., et al. Early reduction of Leishmania infantum-specific antibodies and blood parasitemia during treatment in dogs with moderate or severe disease. Parasit. Vectors. 2016, 9(1), 235. [CrossRef]
- Pereira, D.C.A., Teixeira-Neto, R.G., Lopes, V.V., Pena, H.P., Paz, G.F., Custodio, C.H.X., et al. Development of quantitative PCR and digital PCR for the quantification of Leishmania infantum in dogs. Mol. Cell. Biochem. 2023, Feb 15. [CrossRef]
- Teixeira Neto, R.G., Giunchetti, R.C., Carneiro, C.M., de Almeida, R.W.V, Coura-Vital, W., Quaresma, P. F., et al. Relationship of Leishmania-specific IgG levels and IgG avidity with parasite density and clinical signs in canine leishmaniasis. Vet. Parasitol., 2010, 169(3-4), 248-257. [CrossRef]
- Galán-Relaño, Á., Maldonado, A., Gómez-Gascón, L., Tarradas, C., Astorga, R. J., Luque, I., Huerta, B. Pre-test probability and likelihood ratios for clinical findings in canine leishmaniasis. Transbound Emerg Dis. 2022, 69(6), 3540-3547. [CrossRef]
- Oliveira, M.R., Neto, M.B.O., Bezerra, T.L. et al. Canine leishmaniasis in an endemic region, Northeastern Brazil: a comparative study with four groups of animals. Parasitol. Res. 2021, 120, 3915–3923. [CrossRef]
- Silva, K.R.D., Mendonça, V.R.R.D., Silva, K.M., Nascimento, L.F.M.D., Mendes-Sousa, A.F., Pinho, F.A.D., et al. Scoring clinical signs can help diagnose canine visceral leishmaniasis in a highly endemic area in Brazil. Mem. Inst. Oswaldo Cruz. 2017, 112, 53-63. [CrossRef]
- Koutinas, A.F., Carlotti, D.N., Koutinas, C., Papadogiannakis, E.I., Spanakos, G.K., Saridomichelakis, M.N. Claw histopathology and parasitic load in natural cases of canine leishmaniosis associated with Leishmania infantum. Vet. Dermatol. 2010, 21(6), 572-577. [CrossRef]
- Koutinas, A.F., Koutinas, C.K. Pathologic mechanisms underlying the clinical findings in canine leishmaniosis due to Leishmania infantum/chagasi. Vet. Parasitol., 2014, 51(2), 527-538. [CrossRef]
- LeishVet - LeishVet guidelines for the practical management of canine and feline leishmaniosis: a brief for the practicing veterinarian. 2023. Obtained in https://www.leishvet.org/wp-content/uploads/2023/01/ALIVE-dec22-web-EN.pdf [December 27, 2023].
- Diaz, R.G., Salvatierra, K.A., Silva, G.A., Deschutter, E.J., Bornay-Llinares, F.J., Acosta, L. First molecular characterization of Leishmania infantum species in patients infected with visceral leishmaniasis in Misiones province, Argentina. Biomedica. 2019, 39,405-414. [CrossRef]
- Lamattina, D., Berrozpe, P.E., Casas, N., Moya, S.L., Giuliani, M.G., Costa, S.A., et al. Twice upon a time: The progression of canine visceral leishmaniasis in an Argentinean city. PLoS ONE. 2019, 14, e0219395. [CrossRef]
- Rio Grande do Sul – Secretaria da Saúde (MS). Leishmaniose Visceral Humana – Situação Epidemiológica / dados [Internet]. 2023. Available from: https://www.cevs.rs.gov.br/lvh-situacao-epidemiologica-dados.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).