Submitted:
17 January 2024
Posted:
18 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
2.1. Soil Collection and Preparation Prior to Experiment Installation
2.2. Greenhouse Experiment
2.2.1. Experimental Design and Plant Growth Conditions
2.2.2. Soil and Plant Sampling
2.3. Sample Processing and Analytical Determinations
2.3.1. Soil Measurements
2.3.2. Plant Measurements
- % Ndfa, the proportion of plant N derived from BNF
- δ15Nref, the δ15N value of the reference plant (non-fixing)
- δ15Nfix, the δ15N value of the fixing plant (lupine)
- B, the δ15N value of a fixing plant growing in a medium without N
2.4. Statistics
3. Results
3.1. Soil Characterization
3.2. Species, Soil and Sampling Time Effects on PBray1 Concentration
| Treatment effect | DF | F Value | P > F† |
| Species | 2 | 23.05 | <0.0001 |
| Soil type | 3 | 101.56 | <0.0001 |
| Species x Soil type | 6 | 1.36 | 0.2564 |
| Sampling time | 3 | 91.85 | <0.0001 |
| Species x sampling time | 6 | 2.29 | 0.0518 |
| Soil type x sampling time | 9 | 6.66 | <0.0001 |
| Species x soil type x sampling time | 18 | 2.17 | 0.0133 |
| † Significant effects (p < 0.05) are in bold. Note: The within-subject factor was sampling time. 48, 76, 87, and 103 days after planting and the between-subject factors were species and soil type | |||
3.3. Species and Soil Type Effects on Soil pH
3.4. Aboveground Biomass and Nutritional Status: Effects of Species and Soil Type
3.4.1. Shoot Dry Weight and P and N Content
3.4.2. Plant Ca, Mg, K and Micronutrients (Fe and Mn) Concentrations in Aboveground Biomass
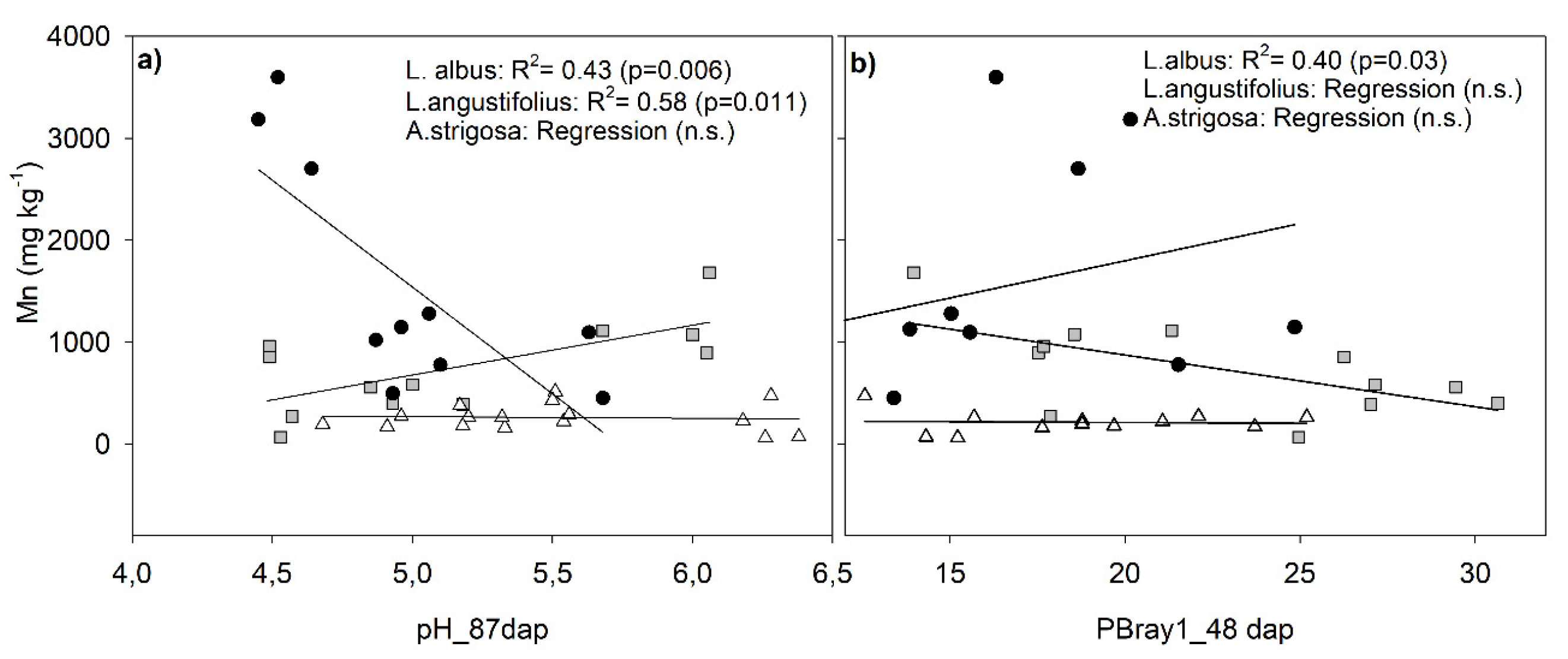
3.5. Relationships between Soil and Plant Parameters
4. Discussion
4.1. Variations in Soil PBray1 Concentration throughout the Plant Growth Cycle
4.2. Changes in Soil pH and Acidity and Their Relationship with Plant Available P
4.3. Plant Growth and Nutrition in Relation to the Soil Chemical Properties
4.3.1. Shoot Dry Weight and P and N in Shoot Biomass
4.3.2. Base Cation and Mn Concentration in Shoot Biomass
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vassallo, M. Dinámica y competencia intrasectorial en la agricultura uruguaya: los cambios en la última década. Agrociencia 2013, 17(2), 170–179. [CrossRef]
- Ernst, O. R.; Dogliotti, S.; Cadenazzi, M.; Kemanian, A. R. Shifting crop-pasture rotations to no-till annual cropping reduces soil quality and wheat yield. Field Crops Research 2018, 217, 180–187. [CrossRef]
- Ernst, O. R.; Kemanian, A. R.; Mazzilli, S. R.; Cadenazzi, M.; Dogliotti, S. Depressed attainable wheat yields under continuous annual no-till agriculture suggest declining soil productivity. Field Crops Research 2016, 186, 107–116. [CrossRef]
- 4. Ministerio de Agricultura y Pesca (MGAP). DIEA presenta los resultados de la Encuesta Agrícola “Invierno 2021”. Available online: https://www.gub.uy/ministerio-ganaderia-agricultura-pesca/datos-y-estadisticas/estadisticas/diea-presenta-resultados-encuesta-agricola-invierno-2021.
- Beretta-Blanco, A.; Pérez, O.; Carrasco-Letelier, L. Soil quality decrease over 13 years of agricultural production. Nutrient Cycling in Agroecosystems 2019, 114(1), 45–55. [CrossRef]
- 6. Ernst O, Siri-Prieto G (2009) Impact of perennial pasture and tillage systems on carbon input and soil quality indicators. Soil Tillage Res 105:260–268. [CrossRef]
- Cano, J. D.; Ernst, O. Balance aparente de fósforo en rotaciones agrícolas del litoral oeste del Uruguay 1. Informaciones Agronómicas 2005, 32, 8–11.
- Syers, J. K.; Johnston, A. E.; Curtin, D. Efficiency of soil and fertilizer phosphorus use: reconciling changing concepts of soil phosphorus behaviour with agronomic information. FAO fertilizer and plant nutrition bulletin. 2008, p 128.
- Menezes-Blackburn, D.; Giles, C.; Darch, T.; George, T. S.; Blackwell, M.; Stutter, M.; et al. Opportunities for mobilizing recalcitrant phosphorus from agricultural soils: a review. Plant and Soil 2018, 427(1–2), 5–16. [CrossRef]
- Richardson, A. E.; Barea, J. M.; McNeill, A. M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant and Soil 2009, 321(1–2), 305–339. [CrossRef]
- Kamh, M.; Abdou, M.; Chude, V.; Wiesler, F.; Horst, W. J. Mobilization of phosphorus contributes to positive rotational effects of leguminous cover crops on maize grown on soils from northern Nigeria. Journal of Plant Nutrition and Soil Science 2002, 165(5), 566–572. [CrossRef]
- Lambers, H.; Clements, J. C.; Nelson, M. N. How aphosphorus-acquisition strategy based on carboxylate exudation powers the success and agronomic potential of lupines (Lupinus,Fabaceae). American Journal of Botany 2013, 100(2), 263–288. [CrossRef]
- AyaIa, W.; Barrios, E.; Macedo, I.; Sawchik, J.; Terra, J. Scouting benefits and developing innovations in temperate grassland to sustainable agriculture production. In Sustainable use ofgrassland resources for forage production, biodiversity and environmental protection: Proceedings of23,d International Grassland. Vijay, D., Srivastava, M. K, Gupta, C. K, Malaviya, D. R., Roy, M. M., Mahanta, S. K, S., J. B., Maity, A. and P. K. G. (Eds)., Eds.; 2015; pp 208–214.
- Fontaine, S.; Abbadie, L.; Aubert, M.; Barot, S.; Bloor, J. M. G.; Derrien, D.; et al. Plant–soil synchrony in nutrient cycles: Learning from ecosystems to design sustainable agrosystems. Global Change Biology 2023, 30(1). [CrossRef]
- Kirkegaard, J.; Christen, O.; Krupinsky, J.; Layzell, D. Break crop benefits in temperate wheat production. Field Crops Research. June 3, 2008, pp 185–195. [CrossRef]
- Hallama, M.; Pekrun, C.; Lambers, H.; Kandeler, E. Hidden miners – the roles of cover crops and soil microorganisms in phosphorus cycling through agroecosystems. Plant and Soil 2019, 434(1–2), 7–45. [CrossRef]
- Mera, M.; Lizana, X. C.; Calderini, D. F. Cropping systems in environments with high yield potential of southern Chile. In Crop Physiology: Applications for Genetic Improvement and Agronomy: Second Edition; 2015; pp 111–140. [CrossRef]
- Espinoza, S.; Ovalle, C.; Zagal, E.; Matus, I.; Tay, J.; Peoples, M. B.; et al. Contribution of legumes to wheat productivity in Mediterranean environments of central Chile. Field Crops Research 2012, 133, 150–159. [CrossRef]
- Nuruzzaman, M.; Lambers, H.; Bolland, M. D. A.; Veneklaas, E. J. Phosphorus uptake by grain legumes and subsequently grown wheat at different levels of residual phosphorus fertiliser. Australian Journal of Agricultural Research 2005, 56(10), 1041–1047. [CrossRef]
- Wolko, B.; Clements, J. C.; Naganowska, B.; Nelson, M. N.; Yang, H. Lupinus; Kole, C., Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2011. [CrossRef]
- 21. Soil Survey Staff. Keys to soil taxonomy. Soil Conservation Service. 2014, p 410. <http://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs142p2_051546.pdf>.
- Nelson, D. W., & Sommers, L. E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis. D.L. Sparks, A.L. Page, P.A. Helmke, R.H. Loeppert, P.N. Soltanpour, M.A. Tabatabai, C. T. J. and M. E. S., Ed.; 1996. [CrossRef]
- Thomas, G. W. Exchangeable Cations. In Methods of Soil Analysis. Page, A., Ed.; 1983; pp 159–165. [CrossRef]
- Lajtha, K.; Driscoll, C. T.; Jarrell, W. M.; Elliott, E. T. Soil phosphorus: characterization and total element analysis. Standard Soil Methods for Long Term Ecological Research 1999, 115–143.
- Rabuffetti, A. La fertilidad del suelo y su manejo. Montevideo: Hemisferio Sur 2017.
- Keeney, D. R.; Nelson, D. W. Nitrogen-Inorganic Forms. In Methods of Soil Analysis, Agronomy Monograph 9, Part 2. Page, A., Ed.; wiley, 1983; pp 643–698. [CrossRef]
- Weatherburn, M. W. Phenol-Hypochlorite Reaction for Determination of Ammonia. Analytical Chemistry 1967, 39(8), 971–974. [CrossRef]
- Gee, G. W.; Bauder, J. W. Particle Size Analysis by Hydrometer: A Simplified Method for Routine Textural Analysis and a Sensitivity Test of Measurement Parameters. Soil Science Society of America Journal 1979, 43(5), 1004–1007. [CrossRef]
- Shearer, G.; Kohl, D. H. N2-fixation in field settings: estimations based on natural 15N abundance. Australian Journal of Plant Physiology 1987, 13(6), 699–756. [CrossRef]
- Murphy, J.; Riley, J. P. A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 1962, 27(C), 31–36. [CrossRef]
- Jones, J. B.; Case, V. W. Sampling, handling, and analyzing plant tissue samples. In Soil Testing and Plant Analysis; wiley, 2018; pp 389–427. [CrossRef]
- Team, R. C. A Language and Environment for Statistical Computing. R: A Language and Environment for Statistical Computing. 2017, p https://www.R-project.org. <http://www.r-project.org>.
- Condron, L. M.; Turner, B. L.; Cade-Menun, B. J. Chemistry and dynamics of soil organic phosphorus. Phosphorus: Agriculture and the Environment 2015, 87–121. [CrossRef]
- Hernández, J.; Zamalvide, J. Procesos de retención de fosforo por los suelos evaluados a través de parámetros de suelo y planta. Agrociencia 1998, 2, 48–63.
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. In Plant and Soil; 2001; Vol. 237, pp 173–195. [CrossRef]
- Barrow, N. J. The effects of pH on phosphate uptake from the soil. Plant and Soil 2017, 410(1–2), 401–410. [CrossRef]
- Barrow, N. J.; Hartemink, A. E. The effects of pH on nutrient availability depend on both soils and plants. Plant and Soil 2023, 487(1–2), 21–37. [CrossRef]
- Lambers, H.; Hayes, P. E.; Laliberté, E.; Oliveira, R. S.; Turner, B. L. Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends in Plant Science. Elsevier Ltd February 1, 2015, pp 83–90. [CrossRef]
- Pearse, S. J.; Veneklaas, E. J.; Cawthray, G. R.; Bolland, M. D. A.; Lambers, H. Carboxylate release of wheat, canola and 11 grain legume species as affected by phosphorus status. Plant and Soil 2006, 288(1–2), 127–139. [CrossRef]
- Watt, M.; Evans, J. R. Phosphorus acquisition from soil by white lupin (Lupinus albus L.) and soybean (Glycine max L.), species with contrasting root development. Plant and Soil 2003, 248(1–2), 271–283. [CrossRef]
- Neumann, G.; Römheld, V. Root excretion of carboxylic acids and protons in phosphorus-deficient plants. Plant and Soil 1999, 211(1), 121–130. [CrossRef]
- Shen, J.; Li, H.; Neumann, G.; Zhang, F. Nutrient uptake, cluster root formation and exudation of protons and citrate in Lupinus albus as affected by localized supply of phosphorus in a split-root system. Plant Science 2005, 168(3), 837–845. [CrossRef]
- Lambers, H.; Shane, M. W.; Cramer, M. D.; Pearse, S. J.; Veneklaas, E. J. Root structure and functioning for efficient acquisition of phosphorus: Matching morphological and physiological traits. Annals of Botany. October 2006, pp 693–713. [CrossRef]
- Brand, J. D.; Tang, C.; Rathjen, A. J. Adaptation of Lupinus angustifolius L. and L. pilosus Murr. to calcareous soils. Australian Journal of Agricultural Research 1999, 50(6), 1027–1033. [CrossRef]
- Kerley, S. J.; Shield, I. F.; Huyghe, C. Specific and genotypic variation in the nutrient content of lupin species in soils of neutral and alkaline pH. Australian Journal of Agricultural Research 2001, 52(1), 93–102. [CrossRef]
- Wang, Y.; Krogstad, T.; Clarke, J. L.; Hallama, M.; Øgaard, A. F.; Eich-Greatorex, S.; et al. Rhizosphere organic anions play a minor role in improving crop species’ ability to take up residual phosphorus (P) in agricultural soils low in P availability. Frontiers in Plant Science 2016, 7(November 2016). [CrossRef]
- Kirkby, E. A.; Le Bot, J.; Adamowicz, S.; Römheld, V. Nitrogen in physiology - An agronomic perspective and implications for the use of different nitrogen forms. Proceedings International Fertiliser Society 2009, 653, York, 1–48.
- Unkovich, M. J.; Baldock, J.; Peoples, M. B. Prospects and problems of simple linear models for estimating symbiotic N2 fixation by crop and pasture legumes. Plant and Soil 2010, 329(1), 75–89. [CrossRef]
- Unkovich, M. J.; Pate, J. S. An appraisal of recent field measurements of symbiotic N2 fixation by annual legumes. Field Crops Research 2000, 65(2–3), 211–228. [CrossRef]
- Bolland, M. D. A.; Brennan, R. F. Comparing the phosphorus requirements of wheat, lupin, and canola. Australian Journal of Agricultural Research 2008, 59(11), 983–998. [CrossRef]
- Li, H.; Shen, J.; Zhang, F.; Tang, C.; Lambers, H. Is there a critical level of shoot phosphorus concentration for cluster-root formation in Lupinus albus? Functional Plant Biology 2008, 35(4), 328–336. [CrossRef]
- Mengel, K.; Kirkby, E. A.; Kosegarten, H.; Appel, T. Principles of Plant Nutrition; Mengel, K., Kirkby, E. A., Kosegarten, H., Appel, T., Eds.; Springer Netherlands: Dordrecht, 2001. [CrossRef]
- Sulieman, S.; Tran, L. S. P. Legume Nitrogen Fixation in Soils with Low Phosphorus Availability: Adaptation and Regulatory Implication; 2017. [CrossRef]
- Pueyo, J. J.; Quiñones, M. A.; Coba de la Peña, T.; Fedorova, E. E.; Lucas, M. M. Nitrogen and Phosphorus Interplay in Lupin Root Nodules and Cluster Roots. Frontiers in Plant Science 2021, 12. [CrossRef]
- Neumann, G.; Massonneau, A.; Martinoia, E.; Römheld, V. Physiological adaptations to phosphorus deficiency during proteoid root development in white lupin. Planta 1999, 208(3), 373–382. [CrossRef]
- Fink, J. R.; Inda, A. V.; Bavaresco, J.; Barrón, V.; Torrent, J.; Bayer, C. Adsorption and desorption of phosphorus in subtropical soils as affected by management system and mineralogy. Soil and Tillage Research 2016, 155, 62–68. [CrossRef]
- Bouray, M.; Moir, J. L.; Lehto, N. J.; Condron, L. M.; Touhami, D.; Hummel, C. Soil pH effects on phosphorus mobilization in the rhizosphere of Lupinus angustifolius. Plant and Soil 2021, 469(1–2), 387–407. [CrossRef]
- Martínez-Alcalá, I.; Clemente, R.; Bernal, M. P. Metal availability and chemical properties in the rhizosphere of lupinus albus l. growing in a high-metal calcareous soil. Water, Air, and Soil Pollution 2009, 201(1–4), 283–293. [CrossRef]
- Ding, W.; Cong, W. F.; Lambers, H. Plant phosphorus-acquisition and -use strategies affect soil carbon cycling. Trends in Ecology and Evolution. 2021, pp 899–906. [CrossRef]
- Dessureault-Rompré, J.; Nowack, B.; Schulin, R.; Luster, J. Spatial and temporal variation in organic acid anion exudation and nutrient anion uptake in the rhizosphere of Lupinus albus L. Plant and Soil 2007, 301(1–2), 123–134. [CrossRef]
- Monei, N.; Hitch, M.; Heim, J.; Pourret, O.; Heilmeier, H.; Wiche, O. Effect of substrate properties and phosphorus supply on facilitating the uptake of rare earth elements (REE) in mixed culture cropping systems of Hordeum vulgare, Lupinus albus and Lupinus angustifolius. Environmental Science and Pollution Research 2022, 29(38), 57172–57189. [CrossRef]
- Pang, J.; Bansal, R.; Zhao, H.; Bohuon, E.; Lambers, H.; Ryan, M. H.; et al. The carboxylate-releasing phosphorus-mobilizing strategy can be proxied by foliar manganese concentration in a large set of chickpea germplasm under low phosphorus supply. New Phytologist 2018, 219(2), 518–529. [CrossRef]
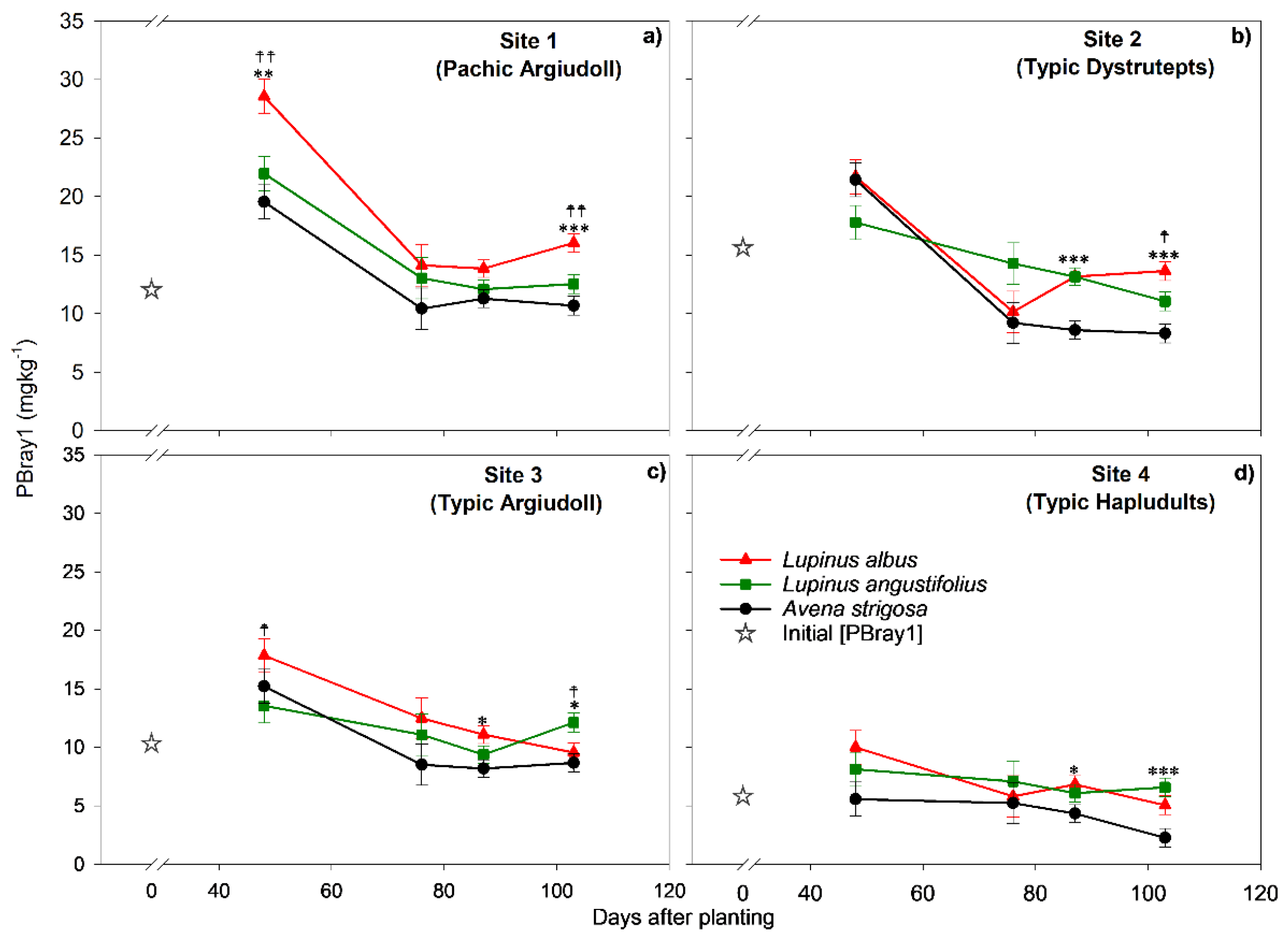
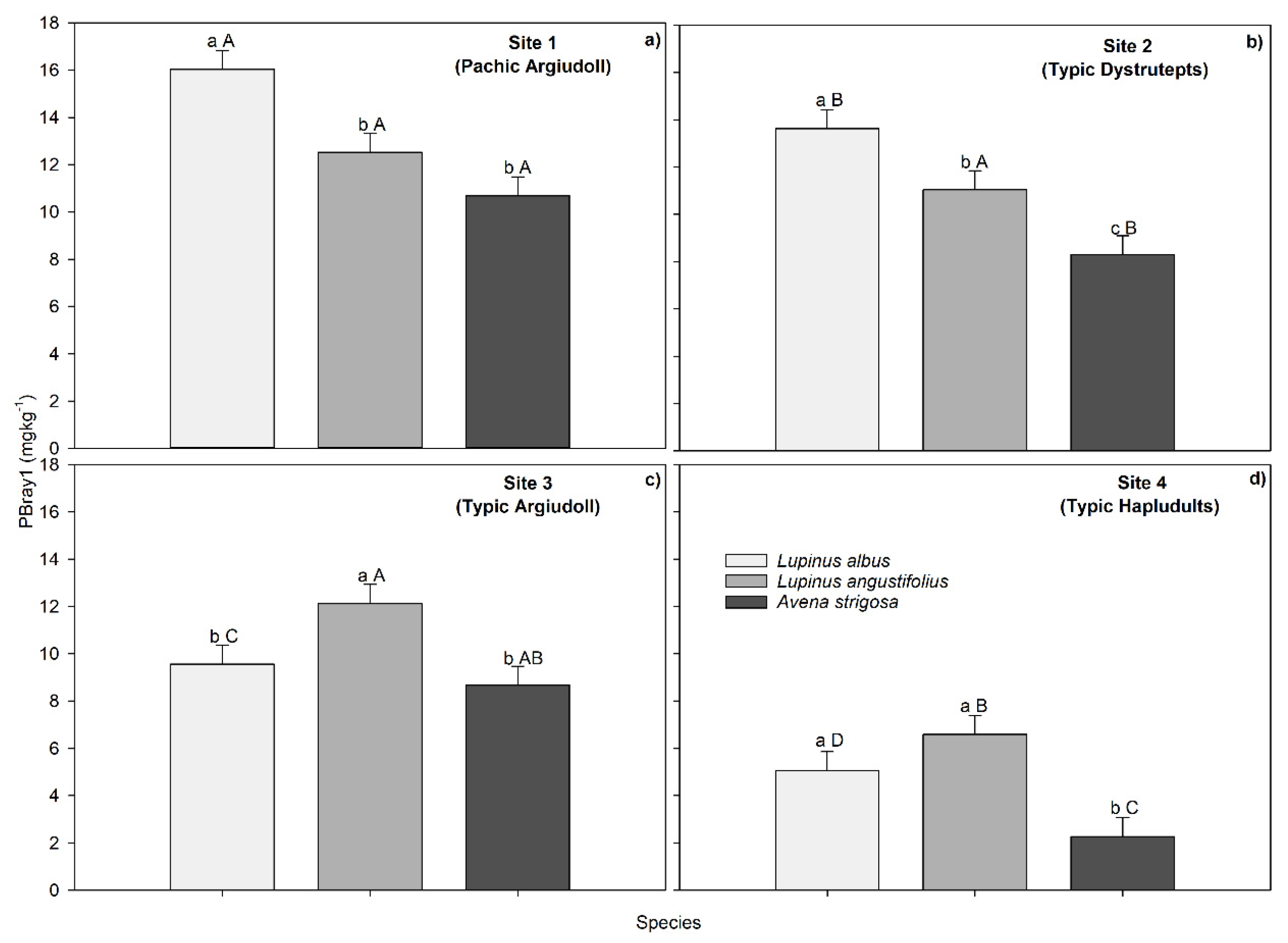
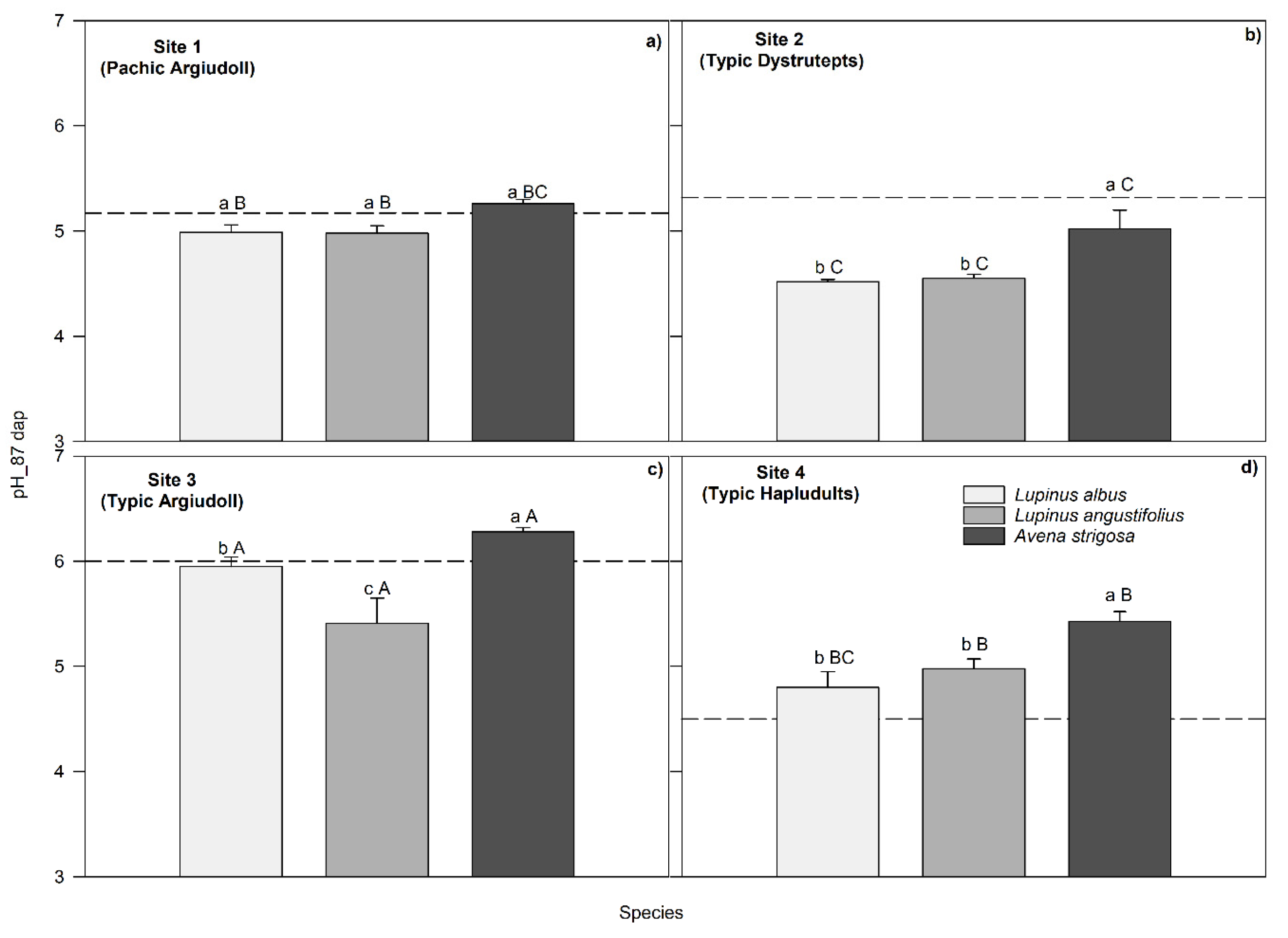
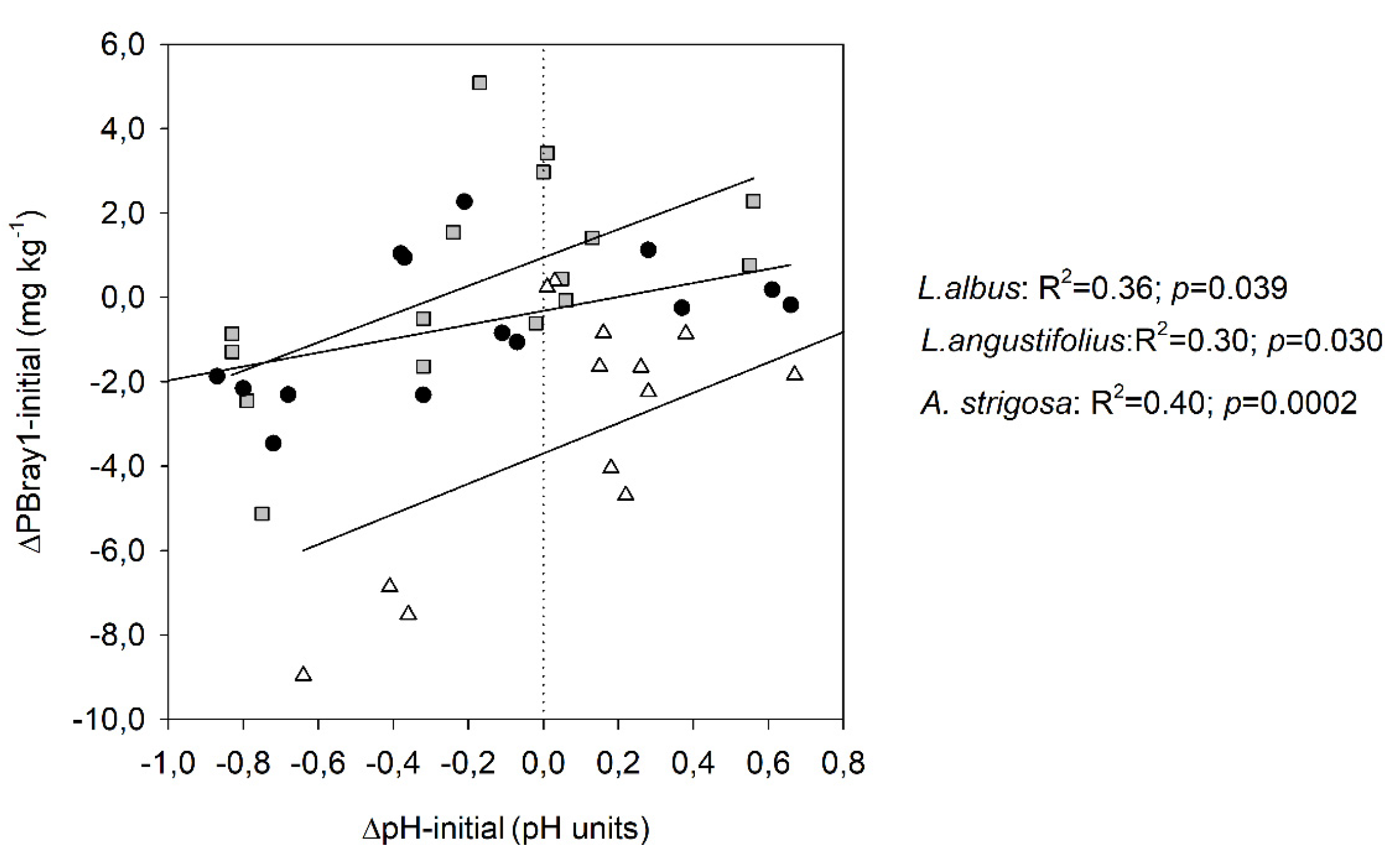
| Site (Location) | Soil type (USDA Classification system) † | Geological material | OM | Sand | Silt | Clay | Texture‡ |
| % | |||||||
| Site 1 (Colonia) | Pachic Argiudoll | Clay silt sediment/ Crystalline basement | 4.8 | 16.3 | 38.8 | 44.9 | C |
| Site 2(T. & Tres) | Typic Dystrutepts | Crystalline basement | 3.7 | 23.5 | 44.2 | 32.3 | CL |
| Site 3 (Montevideo) | Typic Argiudolls | Libertad clayey silt sediment | 1.7 | 19.3 | 52.3 | 28.4 | SiCL |
| Site 4(Rivera) | Typic Hapludults | Colluvial material (Sandy soils)/ Tacuarembó sandstones | 1.4 | 84.7 | 1.4 | 13.9 | SL |
| †USDA: source: [21]. ‡Texture: C= clay; SiCL= silty clay loam; SL= sandy loam; CL= clay loam. | |||||||
| Site (soil type†) | PBray1 | NH+4-N | NO-3-N | Ca2+ | Mg2+ | K+ | Na+ | Al3+exch. | pH |
| mg kg-1 | cmolc kg-1 | ||||||||
| Site 1 (Pachic Argiudoll) | 12.0 | 16.4 | 21.9 | 14.5 | 3.0 | 1.0 | 0.2 | 0.05 | 5.17 |
| Site 2 (Typic Dystrutepts) | 15.6 | 25.7 | 0.3 | 4.6 | 1.2 | 0.3 | 0.1 | 0.05 | 5.32 |
| Site 3 (Typic Argiudoll) | 10.3 | 7.8 | 0.9 | 17.7 | 2.8 | 1.4 | 0.2 | 0.02 | 6.00 |
| Site 4 (Typic Hapludults) | 5.8 | 10.9 | 12.1 | 1.4 | 0.5 | 0.1 | 0.1 | 0.16 | 4.50 |
| †USDA: source: [21]. | |||||||||
| Site | Species | Exchangeable Acidity | Exchangeable Aluminum | ||
| cmolc kg-1 | |||||
| 1 | Lupinus albus | 0.07 ± 0.01 | ns C | 0.01 ± 0.03 | ns C |
| Lupinus angustifolius | 0.08 ± 0.02 | ns C | 0.04 ± 0.03 | ns ns | |
| Avena strigosa | 0.07 ± 0.01 | ns BC | 0.04 ± 0.01 | ns AB | |
| 2 | Lupinus albus | 0.69 ± 0.04 | a A | 0.18 ± 0.03 | ns A |
| Lupinus angustifolius | 0.56 ± 0.09 | a A | 0.10 ± 0.03 | ns ns | |
| Avena strigosa | 0.33 ± 0.04 | b A | 0.13 ± 0.03 | ns A | |
| 3 | Lupinus albus | 0.08 ± 0.03 | ns C | 0.06 ± 0.02 | ns BC |
| Lupinus angustifolius | 0.05 ± 0.00 | ns C | 0.06 ± 0.02 | ns ns | |
| Avena strigosa | 0.03 ± 0.01 | ns C | 0.03 ± 0.03 | ns B | |
| 4 | Lupinus albus | 0.32 ± 0.08 | a B | 0.11 ± 0.03 | ns AB |
| Lupinus angustifolius | 0.23 ± 0.04 | ab B | 0.10 ± 0.03 | ns ns | |
| Avena strigosa | 0.17 ± 0.07 | b B | 0.08 ± 0.03 | ns AB | |
| Significance of treatment effect | |||||
| Species | 0.0008 | 0.6089 | |||
| Soil type | <.0001 | 0.0008 | |||
| Species*soil type | 0.0179 | 0.6339 | |||
| Significant effects (p < 0.05) are in bold. Different lowercase letters within a column indicate differences between species within each soil type, and different capital letters within a column indicate differences between soils across species being significant at a p-level of 0.05.; ns: means no significant difference. | |||||
| Site | Species | Shoot Dry Weight | Plant P Concentration | Plant N Concentration | Plant PContent | Plant NContent | |||||
| g pot-1 | mg g-1 dry weight | mg pot-1 | |||||||||
| 1 | Lupinus albus | 12.5 ± 1.5 | a A | 1.3 ± 0.1 | a AB | 29.3 ± 1.0 | a ns | 15.5 ± 1.7 | a A | 366.5 ± 46.5 | a A |
| Lupinus angustifolius | 6.7 ± 0.3 | b A | 1.4 ± 0.1 | a A | 26.1 ± 1.6 | a ns | 9.0 ± 0.6 | b A | 174.4 ± 15.3 | b A | |
| Avena strigosa | 14.5 ± 1.1 | a B | 1.2 ± 0.1 | b A | 5.7 ± 0.2 | b B | 16.8 ± 1.3 | a B | 81.9 ± 4.8 | c B | |
| 2 | Lupinus albus | 13.9 ± 1.3 | b A | 1.4 ± 0.1 | a A | 31.9 ± 1.3 | a ns | 19.5 ± 1.3 | a A | 436.9 ± 24.7 | a A |
| Lupinus angustifolius | 7.8 ± 1.1 | c A | 1.4 ± 0.1 | a A | 24.5 ± 0.4 | b ns | 10.6 ± 1.6 | b A | 189.8 ± 25.8 | b A | |
| Avena strigosa | 27.0 ± 2.7 | a A | 0.9 ± 0.0 | b B | 8.4 ± 1.0 | c AB | 23.5 ± 1.4 | a A | 222.1 ± 19.1 | b A | |
| 3 | Lupinus albus | 14.0 ± 1.3 | a A | 1.1 ± 0.1 | c B | 28.8 ± 1.9 | a ns | 15.4 ± 2.1 | a A | 402.4 ± 37.0 | a A |
| Lupinus angustifolius | 7.0 ± 1.9 | b A | 1.5 ± 0.1 | a A | 29.4 ± 1.4 | a ns | 10.8 ± 2.9 | b A | 203.6 ± 52.9 | b A | |
| Avena strigosa | 8.1 ± 0.4 | b C | 1.3 ± 0.1 | b A | 6.5 ± 0.3 | b B | 10.2 ± 0.9 | b C | 52.9 ± 3.01 | c B | |
| 4 | Lupinus albus | 6.5 ± 1.1 | b B | 0.9 ± 0.1 | a C | 27.5 ± 2.4 | a ns | 5.6±1.2 | ns B | 180.7 ± 38.6 | a B |
| Lupinus angustifolius | 1.7 ± 0.4 | c B | 0.6 ± 0.2 | b B | 30.2 ± 0.0 | a ns | 1.1±0.6 | ns B | 38.1 ± 0.0 | b B | |
| Avena strigosa | 11.0 ± 0.4 | a BC | 0.5 ± 0.0 | b C | 10.2 ± 0.6 | b A | 5.3±0.3 | ns D | 111.8 ± 2.8 | ab B | |
| Significance of treatment effect | |||||||||||
| Species | <.0001 | 0.0004 | <.0001 | <.0001 | <.0001 | ||||||
| Soil type | <.0001 | <.0001 | 0.3286 | <.0001 | <.0001 | ||||||
| Species*soil type | <.0001 | 0.0017 | 0.0088 | 0.0062 | 0.0009 | ||||||
| Significant effects (p <0.05) are in bold. Different lowercase letters within a column indicate differences between species within each soil type, and different capital letters within a column indicate differences between sites across species being significant at a p-level of 0.05; ns: means no significant difference. | |||||||||||
| Site | Species | Plant Ca Concentration | Plant Mg Concentration | Plant K Concentration | Plant Mn Concentration | Plant Fe Concentration | |||||
| mg g-1 | mg kg-1 | ||||||||||
| 1 | Lupinus albus | 5.6 ± 0.4 | b A | 1.4 ± 0.1 | b A | 14.3 ± 1.7 | ns B | 482.6 ± 51.2 | b B | 97.2 ± 12.7 | b BC |
| Lupinus angustifolius | 26.5 ± 1.6 | a ns | 4.7 ± 0.3 | a ns | 16.2 ± 0.2 | ns ns | 1067.0 ± 150.2 | a B | 214.2 ± 23.5 | a ns | |
| Avena strigosa | 3.1 ± 0.2 | c B | 1.2 ± 0.1 | c B | 15.5 ± 0.6 | ns ns | 216.7 ± 27.6 | b ns | 36.9 ± 8.6 | b B | |
| 2 | Lupinus albus | 3.9±0.4 | b B | ± 0.1 | b B | 13.7 ± 1.1 | ns B | 537.6 ± 218.2 | b B | 54.0 ± 5.5 | ab C |
| Lupinus angustifolius | 22.5±1.1 | a ns | 4.4 ± 0.2 | a ns | 14.7 ± 1.9 | ns ns | 3161.0 ± 259.1 | a A | 142.1 ± 4.8 | a ns | |
| Avena strigosa | 2.9±0.3 | b B | 1.5 ± 0.1 | b B | 13.0 ± 0.9 | ns ns | 214.9 ± 21.66 | b ns | 28.7 ± 2.6 | b B | |
| 3 | Lupinus albus | 7.2±0.1 | b AB | 2.0 ± 0.3 | b AB | 17.1 ± 0.7 | ns A | 1191.2 ± 21.51 | a A | 165.3 ± 21.5 | a AB |
| Lupinus angustifolius | 26.7±1.3 | a ns | 4.5 ± 0.2 | a ns | 17.4 ± 1.1 | ns ns | 791.9 ± 184.0 | a B | 214.6 ± 36.6 | a ns | |
| Avena strigosa | 4.4±0.6 | b AB | 1.0 ± 0.2 | b B | 15.1 ± 0.2 | ns ns | 209.8 ± 96.0 | b ns | 47.7 ± 18.7 | b B | |
| 4 | Lupinus albus | 4.3 ± 0.1 | b B | 1.2 ± 0.2 | b B | 13.2 ± 1.3 | ns B | 628.6 ± 210.0 | ab B | 189.0 ± 28.5 | b A |
| Lupinus angustifolius | 12.0 ± 0.0 | a ns | 3.1 ± 0.0 | a ns | 10.8 ± 0.0 | ns ns | 1021.5 ± 0.0 | a B | 218.6 ± 0.0 | b ns | |
| Avena strigosa | 4.1 ± 0.2 | a A | 2.9 ± 0.2 | a A | 14.7 ± 0.2 | ns ns | 403.0 ± 47.3 | b ns | 440.1 ± 88.7 | a A | |
| Significance of treatment effect | |||||||||||
| Species | <.0001 | <.0001 | 0.7014 | <.0001 | 0.0342 | ||||||
| Soil type | 0.1651 | 0.4564 | 0.0453 | <.0001 | <.0001 | ||||||
| Species*soil type | 0.0237 | 0.0026 | 0.4818 | <.0001 | <.0001 | ||||||
| Significant effects (p <0.05) are in bold. Different lowercase letters within a column indicate differences between species within each soil type, and different capital letters within a column indicate differences between sites across species being significant at a p-level of 0.05. ns: means no significant difference. | |||||||||||
| ∆pH-Initial † | PBray1-48dap | PBray1-87dap | Shoot Dry Weight | Plant N Content | Plant P Content | Plant Fe Concentration | |
| PBray1-48dap | -0.56 *** | ||||||
| PBray1-87dap | -0.61 *** | 0.76 *** | |||||
| Shoot dry weight | -0.24 | 0.39 ** | 0.06 | ||||
| Plant N content | -0.56 *** | 0.49 *** | 0.50 *** | 0.32 * | |||
| Plant P content | -0.59 *** | 0.66 *** | 0.45 ** | 0.83 *** | 0.54 *** | ||
| Plant Fe concentration | 0.53 *** | -0.57 *** | -0.42 ** | -0.36 * | -0.17 | -0.61 *** | |
| Plant Mn concentration | -0.36 * | 0.02 | 0.36 * | -0.28 | 0.14 | -0.13 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





