Submitted:
18 January 2024
Posted:
19 January 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
2. Materials and Methods
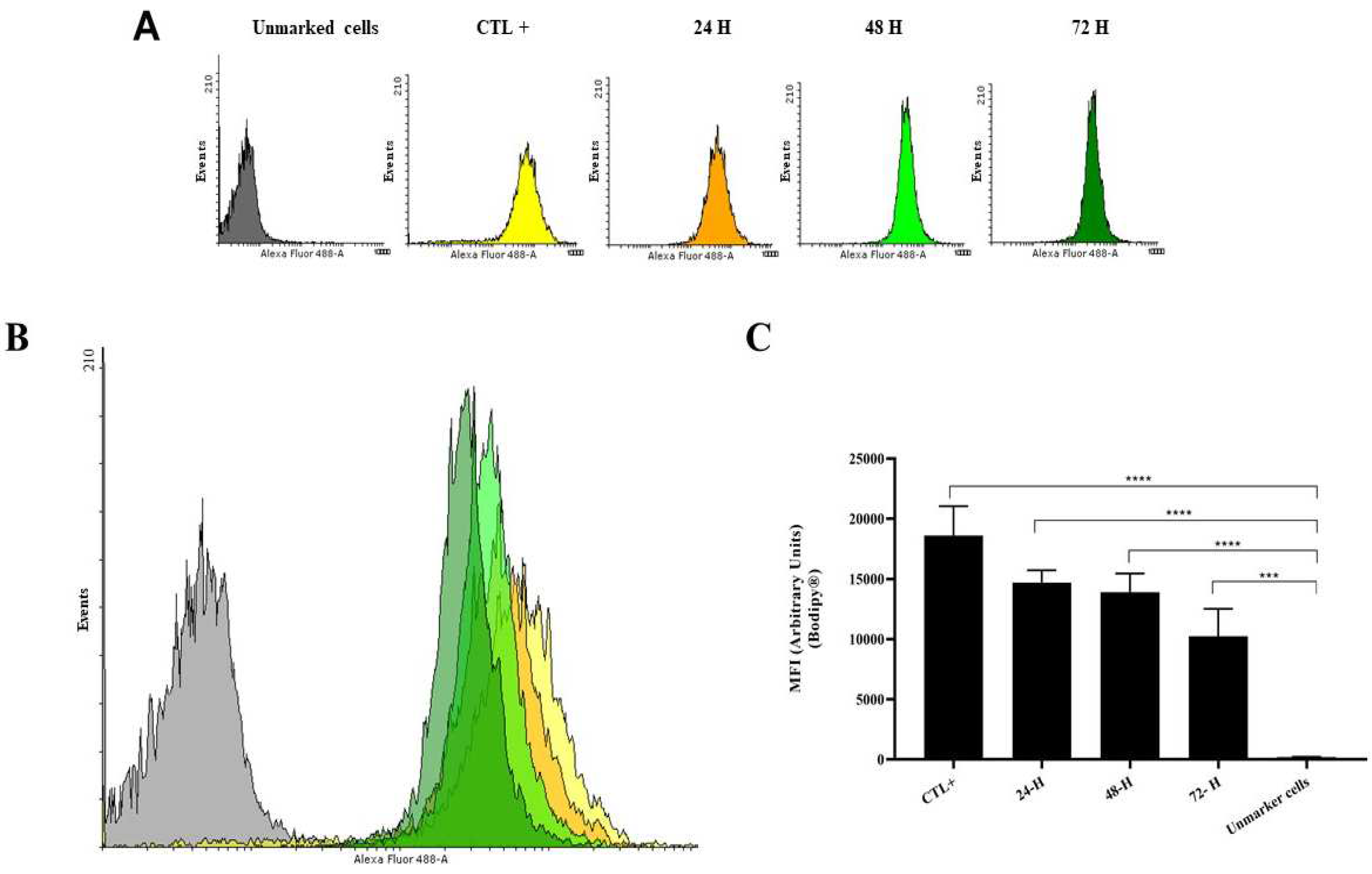
2.1. Quantification Method of Lipid Bodies in Promastigote of Leishmania (L.) Amazonensis
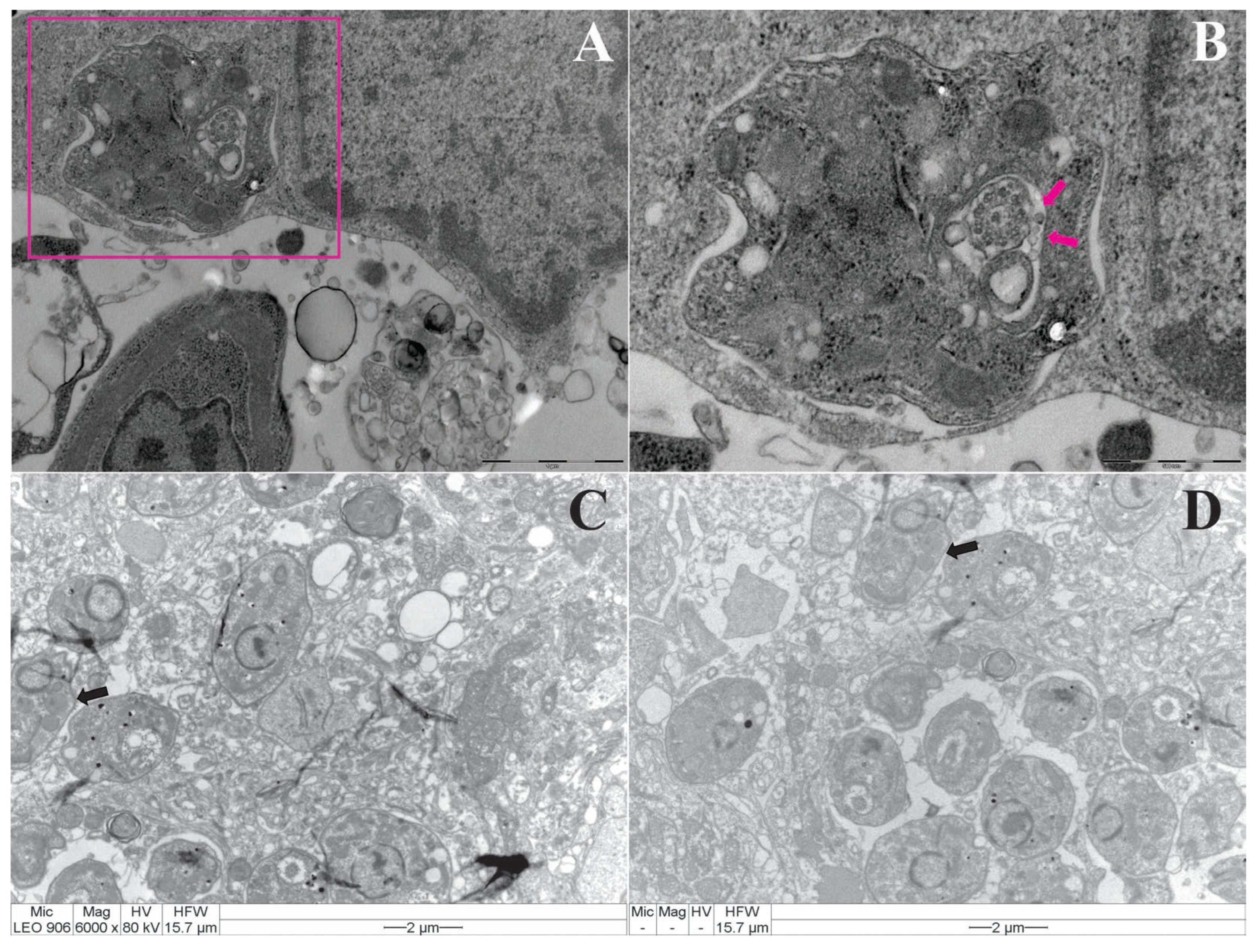
2.2. Transmission Electron Microscopy of Macrophages Infected with Leishmania (Leishmania) Amazonensis
2.3. Transmission Electron Microscopy of Tissues from Male Balb/c Mice Infected with Leishmania (Leishmania) Amazonensis
2.4. Leishmania Diagnosis: Some Findings of Natural Canine Infection
2.4.1. Careful Anamnesis, Clinical and Routine Laboratory Tests
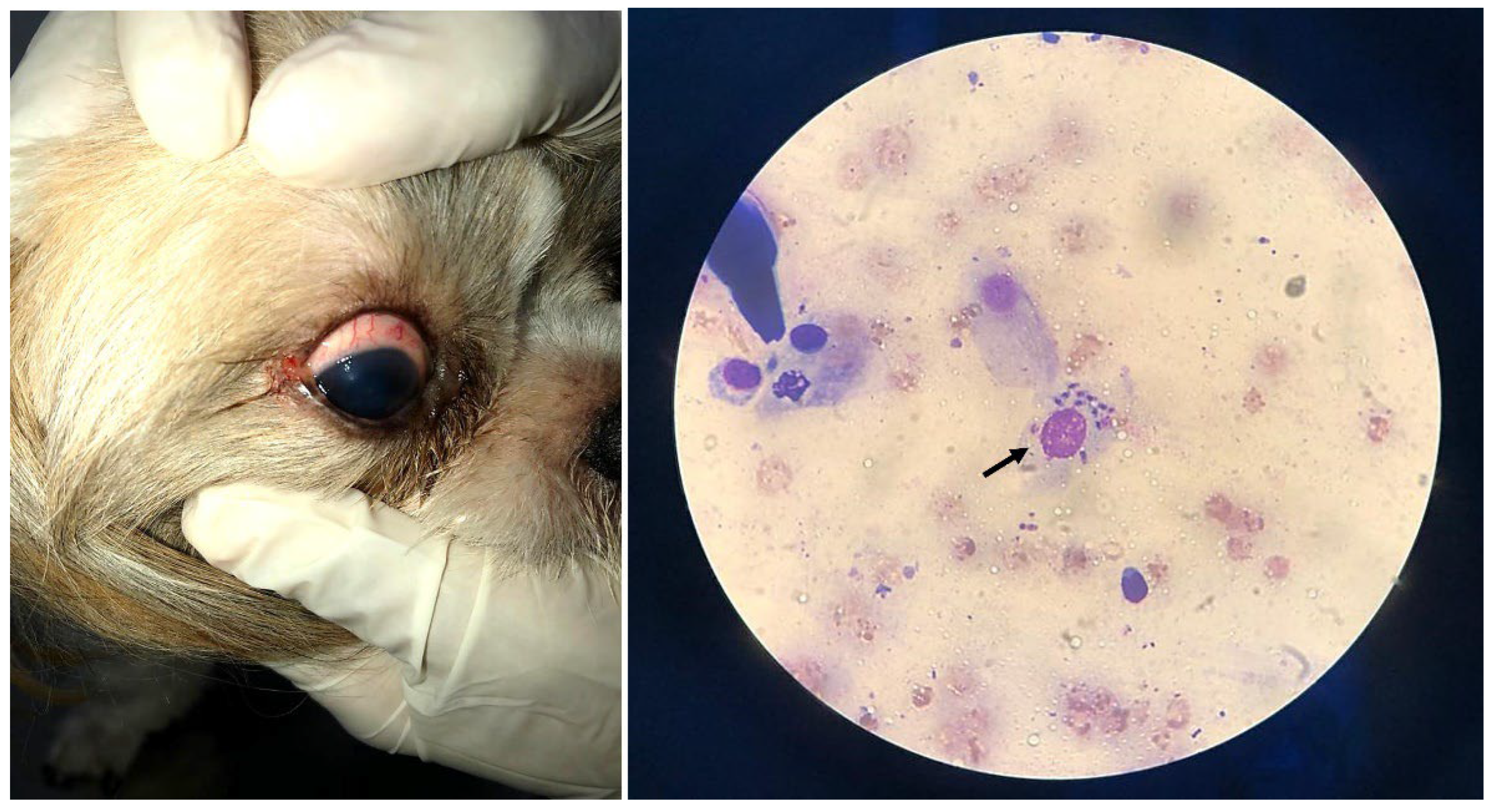
2.4.2. Impression Cytology of the Infected Ocular Surface
2.5. Molecular Analysis of Canine Clinical Samples by Polymerase Chain Reaction PCR
3. Results
3.1. Endocytosis and Exocytosis Process of Leishmania: Infected Macrophage by Promastigotes, Infected Mice Tissues by Amastigotes and the Quantification of Lipid Bodies in Promastigote of Leishmania (L.) Amazonensis
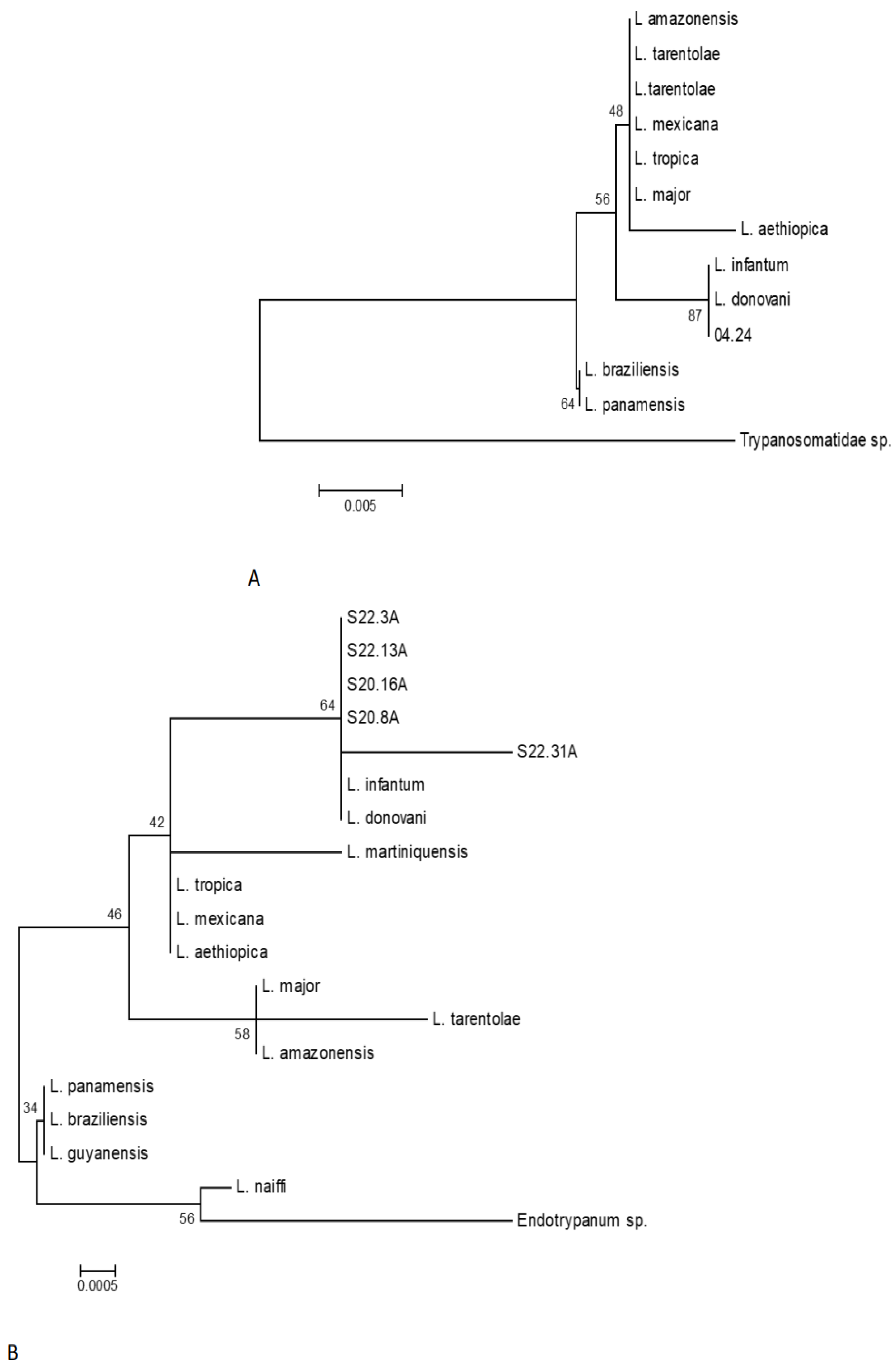
3.2. Results of PCR Tested for the Biological Samples Collected from 130 dogs of Amazonian Endemic Area and the Results Obtained from Our Case Report “Jimmy”
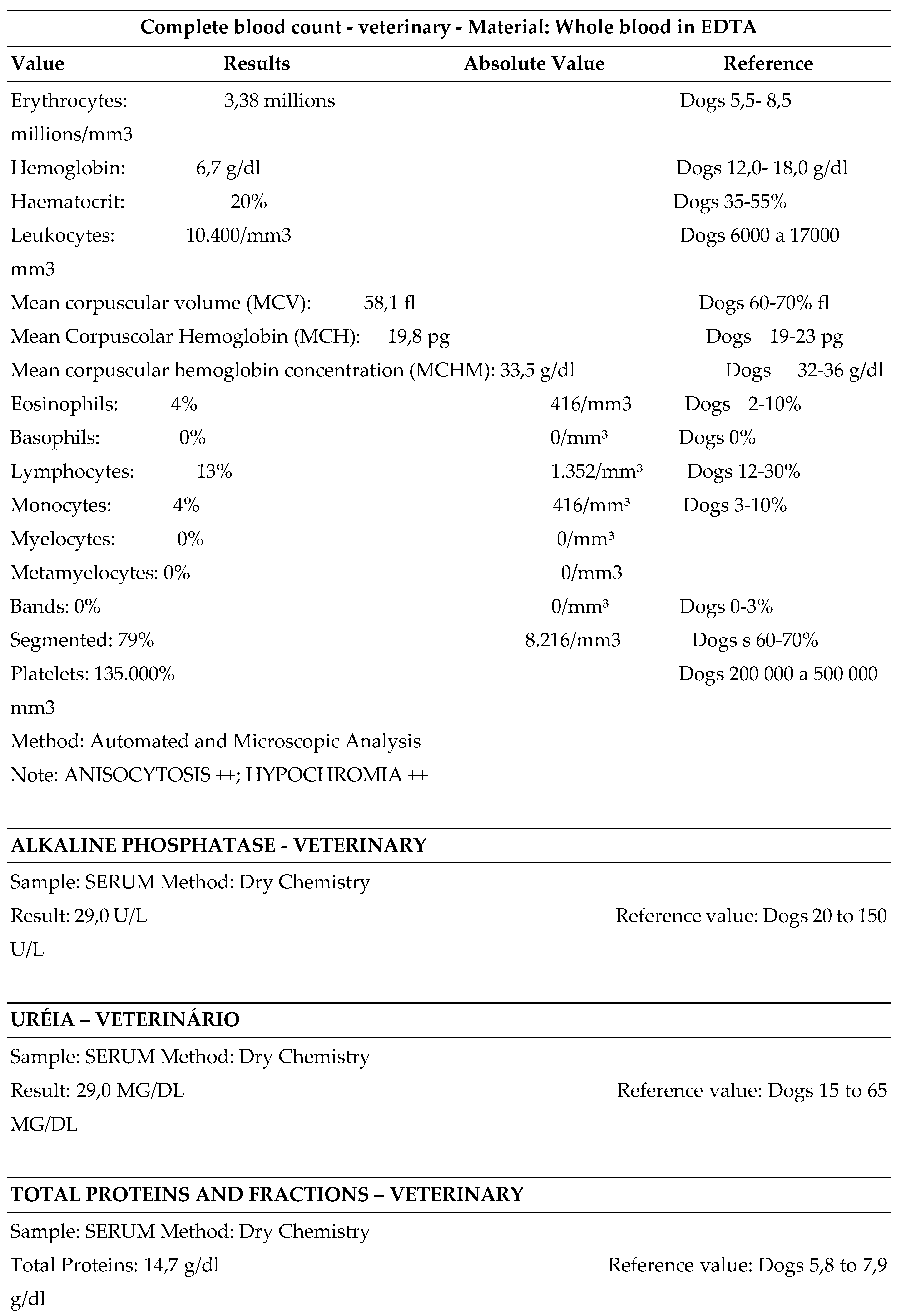
3.3. Leishmania Diagnosis: Some Findings of a Natural Canine Infection after Anamnesis, Clinical and Routine Laboratory Tests
3.4. Results of PCR for the Biological Samples Collected from “Jimmy”
- Auditive duct swabs: (PCR) Leishmania sp. NEGATIVE
- Mucous membrane of the anus swabs: (PCR) Leishmania sp. POSITIVE
- Oral Mucosa swabs: (PCR) Leishmania sp. POSITIVE
- Whole Blood*: (PCR) Anaplasma platys NEGATIVE
- Whole Blood*:(PCR) Babesia vogeli NEGATIVE
- Whole Blood*: (PCR) Ehrlichia canis NEGATIVE
- Whole Blood*: (PCR) Leishmania sp. POSITIVE
- Whole Blood*: (PCR) Mycoplasma sp. NEGATIVE
- Whole Blood*: (PCR) Rangelia vitalli NEGATIVE
- Preputial secretion swabs: (PCR) Leishmania sp. POSITIVE
- Ocular Swab: (PCR) Leishmania sp. POSITIVE
4. Discussion
5. Conclusions

Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Countries | Year of reports | Leishmaniasis cutaneous/ visceral | Other parasitic diseases | Diagnostic methods | N° of references |
|---|---|---|---|---|---|
| Brazil, Hungary, Italy, Spain, Portugal, Bulgaria | 2013, 2014, 2015, 2016 | Leishmania sp, a | babesiosis | IFAT, ELISA, PCR, DPP serological, qPCR | 70, 71 *,72,73,74,75 |
| Brazil, Iran, Albania | 2011,2013,2014, 2015,2016 | Leishmania sp, a, b | toxoplasmosis | IFAT, ELISA, PCR serological, parasitological | 71,73,76,77,80,81 |
| Brazil, Iran, Albania | 2011, 2013, 2014, 2015 | Leishmania sp a, b | neosporosis | IFAT, ELISA, PCR serological, parasitological | 71,77,78,79,80,81,82 |
| Hungary, Portugal, Albania | 2015, 2016 | Leishmania sp a | dirofilariosis | PCR serological | 72,74,77,83 |
| Countries | Year of reports | Leishmaniasis cutaneous/ visceral |
Other parasitic diseases | Diagnostic methods |
N° of references |
|---|---|---|---|---|---|
| Spain | 2012 | Leishmania sp | babesiosis | seroreactivity, PCR | 84 * |
| Brazil, Turkey** | 2011, 2013, 2014, 2015 | Leishmania sp a. b, c. d | toxoplasmosis | immunofluorescence serology, qPCR IFAT, MAT | 81,85,86,87,88,89,90 |
| Brazil | 2011, 2014 | Leishmania sp a | neosporosis | immunofluorescence | 85,88 |
| Portugal | 2015 | Leishmania sp | dirofilariosis | ELISA, DAT | 91 |
Appendix B

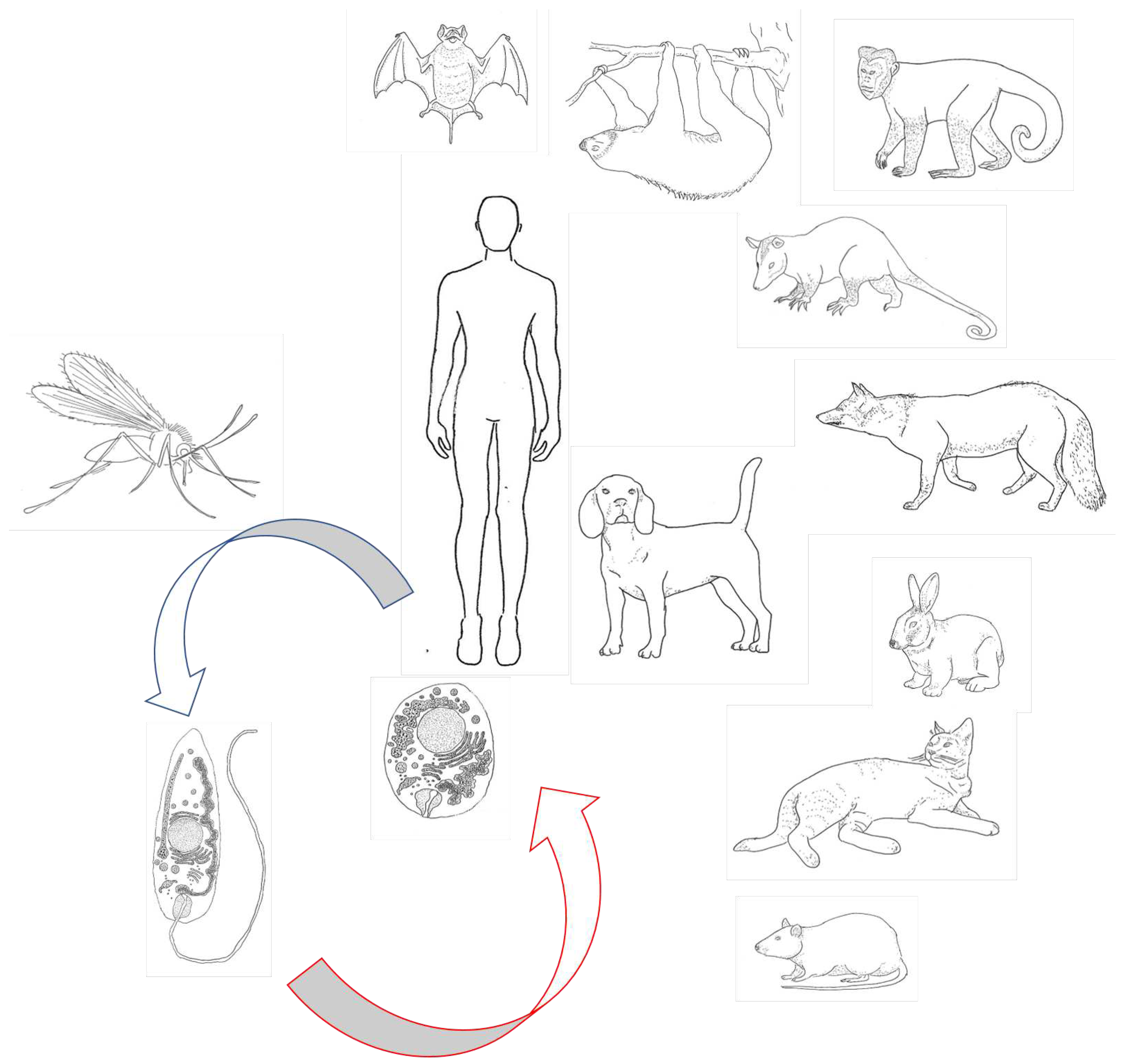
Appendix C
References
- Gabriel. M.; Galué-Parra, A.; Pereira, W.L.A.; Pedersen, K.W.; da Silva, E.O. Leishmania 360°: Guidelines for Exosomal Research. Microorganisms 2021, 9, 2081. [Google Scholar] [CrossRef]
- WHO. Control of Neglected Tropical Diseases. Available online: https://www.who.int/teams/control-ofneglected-tropical-diseases/interventions/strategies (accessed on 10 June 2023).
- Gizzarelli, M.; Bosco, A.; Manzillo, V.F.; Bongiorno, G.; Bianchi, R.; Giaquinto, D.; Fayala, N.E.H.B.; Varloud, M.; Crippa, A.; Gradoni, L.; et al. Examining the Relationship of Clinical and Laboratory Parameters With Infectiousness to Phlebotomus perniciosus and Its Potential Infectivity in Dogs With Overt Clinical Leishmaniasis. Front. Veter- Sci. 2021, 8. [Google Scholar] [CrossRef]
- Wijerathna, T.; Gunathilaka, N. Diurnal adult resting sites and breeding habitats of phlebotomine sand flies in cutaneous leishmaniasis endemic areas of Kurunegala District, Sri Lanka. Parasites Vectors 2020, 13, 1–10. [Google Scholar] [CrossRef]
- Cecílio, P.; Cordeiro-Da-Silva, A.; Oliveira, F. Sand flies: Basic information on the vectors of leishmaniasis and their interactions with Leishmania parasites. Commun. Biol. 2022, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dodd, R. Transmission of Parasites by Blood Transfusion. Vox Sang. 1998, 74, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Pangrazio, K.K.; Costa, E.A.; Amarilla, S.P.; Cino, A.G.; Silva, T.M.; Paixão, T.A.; Costa, L.F.; Dengues, E.G.; Diaz, A.A.R.; Santos, R.L. Tissue distribution of Leishmania chagasi and lesions in transplacentally infected fetuses from symptomatic and asymptomatic naturally infected bitches. Veter- Parasitol. 2009, 165, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Avila-García, M.; Farfan-Labonne, B.; Ramírez-Ramírez, A.; Mancilla-Ramírez, J.; Segura-Cervantes, E.; Galindo-Sevilla, N. Transplacental Transmission of Cutaneous Leishmania mexicana Strain in BALB/c Mice. Am. J. Trop. Med. Hyg. 2013, 89, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Karkamo, V.; Kaistinen, A.; Näreaho, A.; Dillard, K.; Vainio-Siukola, K.; Vidgrén, G.; Tuoresmäki, N.; Anttila, M. The first report of autochthonous non-vector-borne transmission of canine leishmaniosis in the Nordic countries. Acta Veter- Scand. 2014, 56, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Naucke, T.J.; Amelung, S.; Lorentz, S. First report of transmission of canine leishmaniosis through bite wounds from a naturally infected dog in Germany. Parasites Vectors 2016, 9, 256. [Google Scholar] [CrossRef]
- Miró, G.; López-Vélez, R. Clinical management of canine leishmaniosis versus human leishmaniasis due to Leishmania infantum: Putting “One Health” principles into practice. Veter- Parasitol. 2018, 254, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Miró, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. LeishVet guidelines for the practical management of canine leishmaniosis. Parasites Vectors 2011, 4, 86–86. [Google Scholar] [CrossRef]
- Herrera-Mares, A.; Guzmán-Cornejo, C.; Ulloa-García, A.; Córdoba-Aguilar, A.; la Fuente, M.C.S.-D.; Suzán, G. Mites, rodents, and pathogens: A global review for a multi-species interaction in disease ecology. Acta Trop. 2022, 232, 106509. [Google Scholar] [CrossRef]
- Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. Infectious agents and how they cause disease. Available from: https://www.ncbi.nlm.nih.
- Murray, J.L.; Connell, J.L.; Stacy, A.; Turner, K.H.; Whiteley, M. Mechanisms of synergy in polymicrobial infections. J. Microbiol. 2014, 52, 188–199. [Google Scholar] [CrossRef]
- Baneth, G. , Thamsborg, S. M., Otranto, D., Guillot, J., Blaga, R., Deplazes & P., Solano-Gallego, 2015. L. Major Parasitic Zoonoses Associated with Dogs and Cats in Europe J. Comp. Pathol. 8: 1-21.
- Vilhena, H. , Martinez-Díaz, V. L., Cardoso, L., Vieira, L., Altet, L., Francino, O., Pastor, J. & Silvestre-Ferreira, A. C. 2013. Feline vector-borne pathogens in the north and centre of Portugal Parasites & Vectors 6: 99-104.
- Dantas-Torres, F. & Otranto, D. 2014. Dogs, cats, parasites, and humans in Brazil: opening the black box Parasites & Vectors 7: 22-47.
- Futuyma, D. J. (1998). Evolutionary biology (3rd ed., p. 763). Sinauer Association, Sunderland. 15.
- Rosário, C.J.D.; Dominici, M.F.; Braga, M.D.S.C.; Lima, C.A.; Pereira, J.G.; Melo, F.A. Quantificação da IL-10 e do INF-γ em cães com ou sem sinais clínicos de infecção com Leishmania (Leishmania) chagasi. 2018, 38, 129–132. [CrossRef]
- Yakubovich, E.I.; Polischouk, A.G.; Evtushenko, V.I. Principles and Problems of Exosome Isolation from Biological Fluids. Biochem. (Moscow), Suppl. Ser. A: Membr. Cell Biol. 2022, 16, 115–126. [Google Scholar] [CrossRef]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, J.; Casari, I.; Manfredi, M.; Falasca, M. Role of lipid signalling in extracellular vesicles-mediated cell-to-cell communication. Cytokine Growth Factor Rev. 2023, 73, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Gocze, P.M.; Freeman, D.A. Factors underlying the variability of lipid droplet fluorescence in MA-10 leydig tumor cells. Cytometry 1994, 17, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Plotkowski MC, Brandão BA, de Assis MC, Feliciano LFP, Raymond B, Freitas C, Saliba AM, Zahm JM, Touqui L, Bozza PT. Lipid body mobilization in the ExoU-induced release of inflammatory mediators by airway epithelial cells. Microbial pathogenesis 45 (1): 30-37, 2008.
- Da Silva BJM, Souza-Monteiro JR, Rogez H, Crespo-López ME, do Nascimento JLM, Silva EO. Selective effects of Euterpe oleracea (açai) on Leishmania (Leishmania) amazonensis and Leishmania infantum. Biomedicine & Pharmacotherapy 97: 1613-1621, 2018c.
- Baptista-Fernandes, T.; Marques, C.; Rodrigues, O.R.; Santos-Gomes, G.M. Intra-specific variability of virulence in Leishmania infantum zymodeme MON-1 strains. Comp. Immunol. Microbiol. Infect. Dis. 2007, 30, 41–53. [Google Scholar] [CrossRef]
- Singh, R.; Joseph, A.; Umapathy, T.; Tint, N.L.; Dua, H.S. Impression cytology of the ocular surface. Br. J. Ophthalmol. 2005, 89, 1655–1659. [Google Scholar] [CrossRef] [PubMed]
- Galvão, G.R.; Gonçalves, E.C.; Moura, L.G.S.; Virgolino, R.R.; Neves, A.M.P.; Aguiar, D.C.F. First molecular description of autochthonous urban cases of canine visceral leishmaniasis in the city of Belém, Pará, Brazil. Braz. J. Biol. 2023, 83, e267617. [Google Scholar] [CrossRef]
- Galvão, G.R. , 2018. Detecção e epidemiologia molecular de Leishmania sp. em três municípios do estado do Pará. Belém: Universidade Federal do Pará, 58 p. Dissertação de Mestrado em Biologia de Agentes Infecciosos e Parasitários.
- Tizard, Ian R. Imunologia veterinária / Ian R. Tizard; By Luciana Medina, Mateus D. Luchese. - 9. ed. - Rio de Janeiro: Elsevier, 2014. [Google Scholar]
- Lipids in Host and Protozoan Parasite Interaction | Frontiers Research Topic (frontiersin.org). Available online: https://www.frontiersin.org/research-topics/28311/lipids-in-host-and-protozoan-parasite-interaction#:~:text=Elucidating%20the%20molecular%20mechanisms%20and%20strategies%20by%20which,development%20of%20novel%20antiparasitic%20drug%20targets%20and%20therapies. (accessed on 10 September 2023).
- Solano-Gallego, L.; Llull, J.; Ramos, G.; Riera, C.; Arboix, M.; Alberola, J.; Ferrer, L. The Ibizian hound presents a predominantly cellular immune response against natural Leishmania infection. Veter- Parasitol. 2000, 90, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Luis Álvarez, Pablo-Jesús Marín-García, Lola Llobat et al. Immune and genomic characterization of Ibizan hound and its relationship with Leishmania infantum infection, 02 September 2022, PREPRINT (Version 1) available at Research Square. 02 September. [CrossRef]
- Toepp, A.J.; Petersen, C.A. The balancing act: Immunology of leishmaniosis. Res. Veter- Sci. 2020, 130, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Day, M. Immune System Development in the Dog and Cat. J. Comp. Pathol. 2007, 137, S10–S15. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Hosein, S.; Blake, D.P.; Solano-Gallego, L. Insights on adaptive and innate immunity in canine leishmaniosis. Parasitology 2016, 144, 95–115. [Google Scholar] [CrossRef]
- Varadé, J.; Magadán, S.; González-Fernández. Human immunology and immunotherapy: main achievements and challenges. Cell. Mol. Immunol. 2020, 18, 805–828. [Google Scholar] [CrossRef]
- Pereira M, Valério-Bolas A, Saraiva-Marques C, Alexandre-Pires G, Pereira da Fonseca I, Santos-Gomes G. Desenvolvimento do Sistema Imunológico Canino: Do Útero ao Idoso. Vet Sci. 2019; 6 (4): 83. Publicado em 21 de outubro de 2019. [CrossRef]
- Taghipour, A.; Abdoli, A.; Ramezani, A.; Abolghazi, A.; Jahromi, M.A.M.; Maani, S.; Nejadi, S.M.H.; Rasti, S.; Shams, M.; Ghasemi, E. Leishmaniasis and Trace Element Alterations: a Systematic Review. Biol. Trace Element Res. 2021, 199, 3918–3938. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Pecoul, B.; Rijal, S.; Boehme, C.; Aksoy, S.; Malecela, M.; Tapia-Conyer, R.; Reeder, J.C. Eliminating the Neglected Tropical Diseases: Translational Science and New Technologies. PLOS Neglected Trop. Dis. 2016, 10, e0003895. [Google Scholar] [CrossRef] [PubMed]
- Neurauter, A., Kierulf, B., Kullmann, Li, A., M., Manger, I. and Pedersen, K. W. From basic research to potential clinical applications: Adapting methods for EV enrichment and analysis. Available online: (PDF) From basic research to potential clinical applications: Adapting methods for EV enrichment and analysis(researchgate.net) (accessed on 10 June 2023).
- Kullmann, A.; , Reed, B. M. & Pedersen, K. W. Available online: https://assets.thermofisher.com/TFS-Assets/BID/Application-Notes/rapid-bead-based-isolation-exosomes-app-note.pdf (accessed on 28 August 2023).
- Zhang, K. Balancing de novo synthesis and salvage of lipids by Leishmania amastigotes. Curr. Opin. Microbiol. 2021, 63, 98–103. [Google Scholar] [CrossRef]
- Shunmugam, S.; Arnold, C.-S.; Dass, S.; Katris, N.J.; Botté, C.Y. The flexibility of Apicomplexa parasites in lipid metabolism. PLOS Pathog. 2022, 18, e1010313. [Google Scholar] [CrossRef]
- Johnson, Alexander. How does cholesterol affect the fluidity of a membrane? https://scienceoxygen.
- Cargnelutti, D.E.; Borremans, C.G.; Tonelli, R.L.; Carrizo, L.C.; Salomón, M.C. Diagnosis of Leishmania infection in a nonendemic area of South America. J. Microbiol. Immunol. Infect. 2016, 49, 809–812. [Google Scholar] [CrossRef]
- Johansen, M. V., Lier T. & Sithithaworn, P. 2015. Progress in research and control of helminth infections in Asia Towards im.
- prove diagnosis of neglected zoonotic trematodes using a One Health approach Acta Tropica 141(2): 161–169.
- Al Dahouk, S. , Sprague, L. D. & Neubauer H. 2013. New developments in the diagnostic procedures for zoonotic brucellosis in humans Rev. sci. tech. Off. int. Epiz. 32(1): 177-188.
- Tavares, R. G. , Staggemeier, R., Borges, A. L. P., Rodrigues, M. T., Castelan, L. A. Vasconcelos, J., Anschau, M. E. & Spalding, S. M. 2011. Molecular techniques for the study and diagnosis of parasite infection J. Venom. Anim. Toxins incl. Trop. Dis 17(3): 239-248.
- Steinmann, P. , Utzinger, J., Du, Z. W. & Zhou, X. N. 2010. Multiparasitism: a neglected reality on global, regional and local scale. Advances in Parasitology 73: 21-50.
- Griffiths, E. C. , Pedersen, A. B., Fenton, A. & Petchey, O. L. 2014. Analysis of a summary network of coinfection in humans reveals that parasites interact most via shared resources Proc. R. Soc. B. 281: 1-9.
- Laurenti, M.D.; Rossi, C.N.; da Matta, V.L.R.; Tomokane, T.Y.; Corbett, C.E.P.; Secundino, N.F.C.; Pimenta, P.F.P.; Marcondes, M. Asymptomatic dogs are highly competent to transmit Leishmania (Leishmania) infantum chagasi to the natural vector. Veter- Parasitol. 2013, 196, 296–300. [Google Scholar] [CrossRef]
- European Scientific Counsel Companion Animal Parasites. 2011. Control of Intestinal Protozoa in dogs and cats - Guideline 6 1rd Edition. Malvern: ESCCAP. Available online: 3sbvfy71_ESCCAP_Guide_6_spanish_version_def.pdf (accessed on 10 June 2023).
- European Scientific Counsel Companion Animal Parasites. 2012. Available online: https://www.esccap.org/guidelines/ (accessed on 10 June 2023).
- Velez, R.; Gállego, M. Commercially approved vaccines for canine leishmaniosis: a review of available data on their safety and efficacy. Trop. Med. Int. Heal. 2020, 25, 540–557. [Google Scholar] [CrossRef]
- Martínez, C.R.; Ruiz, C.J. Alterations in Host Lipid Metabolism Produced During Visceral Leishmaniasis Infections. Curr. Trop. Med. Rep. 2019, 6, 250–255. [Google Scholar] [CrossRef]
- Mencke, N. 2011. The importance of canine leishmaniosis in non-endemic areas, with special emphasis on the situation in Germany. Berl Munch Tierarztl Wochenschr. 124(11-12): 434-442.
- Palatnik-De-Sousa, C.B.; Day, M.J. One Health: The global challenge of epidemic and endemic leishmaniasis. Parasites Vectors 2011, 4, 197–197. [Google Scholar] [CrossRef]
- Chargui, N. , Haouas, N., Slama, D., Gorcii, M., Jaouadi, K., Essabbah-Aguir, N., Mezhoud, H. & Babba, H. 2013. Transmission of visceral leishmaniasis in a previously non-endemic region of Tunisia: Detection of Leishmania DNA in Phlebotomus perniciosus Journal of Vector Ecology 38(1): 1-5.
- Woodhall, D. M. , Eberhard, M. L. & Parise, M. E. 2014. Neglected Parasitic Infections in the United States: Toxocariasis Am J Trop Med Hyg. 90(5): 810–813.
- Farrar, J. , White, N. J., Hotez, P. J., Junghanss, T., Lalloo, D. & Kang, G. 2014. Manson’s Tropical Diseases. A Manual of the Diseases of Warm Climates. 23rd Edition China: Elsevier Saunders.
- World Health Organization. 2012. Available online: https://www.who.int/publications-detail-redirect/WHO-HTM-NTD-2012.1 (accessed on 10 June 2023).
- Akritidis, N. Parasitic, fungal and prion zoonoses: an expanding universe of candidates for human disease. Clin. Microbiol. Infect. 2011, 17, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L. J. , van der Giessen, J. W. B., Batz, M. B., Kojima, M. & Cahill, S. 2013. Have foodborne parasites finally become a global concern? Trends in Parasitology 29(3): 101-103.
- Tangtrongsup, S. & Scorza, V. 2010. Update on the Diagnosis and Management of Giardia spp Infections in Dogs and Cats Topics in Companion Animal Medicine 25(3): 155-162.
- Bouzid, M. , Halai, K., Jeffreys, D. & Hunter, P. R. 2015. The prevalence of Giardia infection in dogs and cats, a systematic review and meta-analysis of prevalence studies from stool samples Veterinary Parasitology 207(3–4): 181–202.
- Braga, Í. A. , dos Santos, L. G. F., Ramos, D. G. S., Melo, A. L. T., Mestre, G. L. C. & de Aguiar, D. M. 2011. Occurrence of Ancylostoma in dogs, cats and public places from Andradina city, São Paulo state, Brazil Rev. Inst. Med. Trop. 53(4): 181-184.
- Spratt, D. M. 2015. Species of Angiostrongylus (Nematoda: Metastrongyloidea) in wildlife: A review International Journal for Parasitology: Parasites and Wildlife 4(2): 178–189.
- Paulan, S.d.C.; Lins, A.G.d.S.; Tenório, M.d.S.; da Silva, D.T.; Pena, H.F.d.J.; Machado, R.Z.; Gennari, S.M.; Buzetti, W.A.S. Seroprevalence rates of antibodies againstLeishmania infantum and other protozoan and rickettsial parasites in dogs. 2013, 22, 162–166. 22. [CrossRef]
- Zanette, M.F.; de Lima, V.M.F.; Laurenti, M.D.; Rossi, C.N.; Vides, J.P.; da Costa Vieira, R.F.; Biondo, A.W.; Marcondes, M. Serological cross-reactivity of Trypanosoma cruzi, Ehrlichia canis, Toxoplasma gondii, Neospora caninum and Babesia canis to Leishmania infantum chagasi tests in dogs. Rev. Soc. Bras. Med. Trop. 2014, 47, 105–107. [Google Scholar] [CrossRef]
- Willi, B. , Spiri, A. M., Meli, M. L., Grimm, F., Beatrice, L., Riond, B., Bley, T., Jordi, R., Dennler, M. & Hofmann-Lehmann, R. 2015. Clinical and molecular investigation of a canine distemper outbreak and vector-borne infections in a group of rescue dogs imported from Hungary to Switzerland. BMC Vet Res. 16(11): 154-169.
- René-Martellet, M.; Lebert, I.; Chêne, J.; Massot, R.; Leon, M.; Leal, A.; Badavelli, S.; Chalvet-Monfray, K.; Ducrot, C.; Abrial, D.; et al. Diagnosis and incidence risk of clinical canine monocytic ehrlichiosis under field conditions in Southern Europe. Parasites Vectors 2015, 8, 3–3. [Google Scholar] [CrossRef] [PubMed]
- Pantchev, N.; Schnyder, M.; Vrhovec, M.G.; Schaper, R.; Tsachev, I. Current Surveys of the Seroprevalence of Borrelia burgdorferi, Ehrlichia canis, Anaplasma phagocytophilum, Leishmania infantum, Babesia canis, Angiostrongylus vasorum and Dirofilaria immitis in Dogs in Bulgaria. Parasitol. Res. 2015, 114, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Krawczak, F.D.S.; Reis, I.A.; Da Silveira, J.A.; Avelar, D.M.; Marcelino, A.P.; Werneck, G.L.; Labruna, M.B.; Paz, G.F. Leishmania, Babesia and Ehrlichia in urban pet dogs: co-infection or cross-reaction in serological methods? Rev. da Soc. Bras. de Med. Trop. 2015, 48, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Cardinot, C. B. , Silva, J. E., Yamatogi, R. S., Nunes, C. R. M., Biondo, A. W., Vieira, R. F. & Marcondes, M. 2016. Detection of Ehrlichia canis, Babesia vogeli and Toxoplasma gondii and dna in the brain of dogs naturally infected with Leishmania infantum. Journal of Parasitology 102(2): 275-279.
- Hamel, D.; Shukullari, E.; Rapti, D.; Silaghi, C.; Pfister, K.; Rehbein, S. Parasites and vector-borne pathogens in client-owned dogs in Albania. Blood pathogens and seroprevalences of parasitic and other infectious agents. Parasitol. Res. 2015, 115, 489–499. [Google Scholar] [CrossRef]
- Lopes, M. G. , Mendonça, I. L., Fortes, K.P., Amaku, M., Pena, H. de F & Gennari, S. M. 2011. Presence of antibodies against Toxoplasma gondii, Neospora caninum and Leishmania infantum in dogs from Piauí. Rev Bras Parasitol Vet. 20(2): 111-114.
- Sakamoto, K.P.; de Melo, G.D.; Machado, G.F. T and B lymphocytes in the brains of dogs with concomitant seropositivity to three pathogenic protozoans: Leishmania chagasi, Toxoplasma gondii and Neospora caninum. BMC Res. Notes 2013, 6, 226–9. [Google Scholar] [CrossRef]
- Braga, A.R.C.; Corrêa, A.P.F.L.; Camossi, L.G.; da Silva, R.C.; Langoni, H.; Lucheis, S.B. Coinfection by Toxoplasma gondii and Leishmania spp. in domestic cats (Felis catus) in State of Mato Grosso do Sul. Rev. da Soc. Bras. de Med. Trop. 2014, 47, 796–797. [Google Scholar] [CrossRef]
- Seabra, N. M. D. , Pereira, V. F., Kuwassaki, M. V., Benassi, J. C., & Oliveira, T. M. F. D. S. 2015. Toxoplasma gondii, Neospora caninum and Leishmania spp. serology and Leishmania spp. PCR in dogs from Pirassununga, SP. Revista Brasileira de Parasitologia Veterinária 24(4): 454-458.
- Sharifdini, M. , Mohebali, M., Keshavarz, H., Hosseininejad, M., Hajjaran, H., Akhoundi, B., Foroushani, A. R., Zarei, Z. & Charehdar, S. 2011. Iran Neospora caninum and Leishmania infantum Co-Infection in Domestic Dogs (Canis familiaris) in Meshkin-Shahr District, Northwestern Iran. J Arthropod Borne Dis. 5(2): 60-68.
- Maia, C.; Coimbra, M.; Ramos, C.; Cristovão, J.M.; Cardoso, L.; Campino, L. Serological investigation of Leishmania infantum, Dirofilaria immitis and Angiostrongylus vasorum in dogs from southern Portugal. Parasites Vectors 2015, 8, 152–152. [Google Scholar] [CrossRef]
- Ayllón, T.; Diniz, P.P.V.; Breitschwerdt, E.B.; Villaescusa, A.; Rodríguez-Franco, F.; Sainz, A. Vector-Borne Diseases in Client-Owned and Stray Cats from Madrid, Spain. Vector-Borne Zoonotic Dis. 2012, 12, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Coelho, W.M.D.; Amarante, A.F.T.D.; Apolinário, J.d.C.; Coelho, N.M.D.; de Lima, V.M.F.; Perri, S.H.V.; Bresciani, K.D.S. Seroepidemiology of Toxoplasma gondii, Neospora caninum, and Leishmania spp. infections and risk factors for cats from Brazil. Parasitol. Res. 2011, 109, 1009–13. [Google Scholar] [CrossRef] [PubMed]
- Sobrinho, L.S.V.; Rossi, C.N.; Vides, J.P.; Braga, E.T.; Gomes, A.A.D.; de Lima, V.M.F.; Perri, S.H.V.; Generoso, D.; Langoni, H.; Leutenegger, C.; et al. Coinfection of Leishmania chagasi with Toxoplasma gondii, Feline Immunodeficiency Virus (FIV) and Feline Leukemia Virus (FeLV) in cats from an endemic area of zoonotic visceral leishmaniasis. Veter- Parasitol. 2012, 187, 302–306. [Google Scholar] [CrossRef]
- Cardia, D.F.F.; Camossi, L.G.; Neto, L.d.S.; Langoni, H.; Bresciani, K.D.S. Prevalence of Toxoplasma gondii and Leishmania spp. infection in cats from Brazil. Veter- Parasitol. 2013, 197, 634–637. [Google Scholar] [CrossRef]
- de Sousa, K.C.M.; Herrera, H.M.; Domingos, I.H.; Campos, J.B.V.; dos Santos, I.M.C.; Neves, H.H.; Machado, R.Z.; André, M.R. Serological detection of Toxoplasma gondii, Leishmania infantum and Neospora caninum in cats from an area endemic for leishmaniasis in Brazil. 2014, 23, 449–455. [CrossRef]
- Paşa, S.; Vardarlı, A.T.; Erol, N.; Karakuş, M.; Töz, S.; Atasoy, A.; Balcıoğlu. C.; Tuna, G.E.; Ermiş,.V.; Ertabaklar, H.; et al. Detection of Leishmania major and Leishmania tropica in domestic cats in the Ege Region of Turkey. Veter- Parasitol. 2015, 212, 389–392. [Google Scholar] [CrossRef]
- Maia, C.; Ramos, C.; Coimbra, M.; Cardoso, L.; Campino, L. Prevalence of Dirofilaria immitis antigen and antibodies to Leishmania infantum in cats from southern Portugal. Parasitol. Int. 2015, 64, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Beri, D.; Rodriguez, M.; Singh, M.; Liu, Y.; Rasquinha, G.; An, X.; Yazdanbakhsh, K.; Lobo, C.A. Identification and characterization of extracellular vesicles from red cells infected with Babesia divergens and Babesia microti. Front. Cell. Infect. Microbiol. 2022, 12, 962944. [Google Scholar] [CrossRef]
- Moumène, A.; Meyer, D.F. Ehrlichia’s molecular tricks to manipulate their host cells. Microbes Infect. 2016, 18, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Md. Nazmul Islam, Kusay Faisal Al-tabatabaie, Md. Ahasan Habib, Sheikh Sharif Iqbal, Khurram Karim Qureshi and Eid M. Al-Mutairi, Design of a Hollow-Core Photonic Crystal Fiber Based Edible Oil Sensor, Crystals 2022, 12, 1362. [CrossRef]
- Rodríguez, N.E.; Lockard, R.D.; Turcotte, E.A.; Araújo-Santos, T.; Bozza, P.T.; Borges, V.M.; Wilson, M.E. Lipid bodies accumulation in Leishmania infantum-infected C57BL/6 macrophages. Parasite Immunol. 2017, 39. [Google Scholar] [CrossRef] [PubMed]
| Gene | Methods | Oligonucleotides | Base Pair (BP) | Identification |
|---|---|---|---|---|
| SSUr | PCR | R221- 5’-GGTTCCTTTCCTGATTTACG-3’ R332 -5’-GGCCCGTAAAGGCCGAATAG-3’ |
603 | Kinetoplastid |
| Nested | R223 -5’-TCCCATCGCAACCTCGGTT-3’ R333 - 5’-AAAGCGGGCGCGGTGCTG-3’ |
490 | Leishmania sp. | |
| SSUs | PCR | S4 - 5’ GAT CCA GCT GCA GGT TCA CC 3’ S12 - 5’ GGT TGA TTC CGT CAACGG AC3’ |
520 | Trypanossomatids |
| Nested | S17 - 5’ CCA AGC TGC CCA GTA GAA T 3’ S18 - 5’ TCG GGC GGA TAA AAC ACC 3’ |
358 | Leishmania sp. | |
| kDNA | PCR | Leish 1 - 5´ AACTTTTCTGGTCCTCCGGGTAG -3´´ Leish 2 - 5’ ACCCCCAGTTTCCCGCC -´- 3 |
120 | L. infantum |
| AGE | INFECTION (%) | TOTAL | |
|---|---|---|---|
| PRESENT | ABSENT | ||
| 0 to 12 months | 14 (10.7) | 10 (7.7) | 24 |
| 1 to 8 years | 47 (36.2) | 40 (30.8) | 87 |
| Over 8 years | 7 (5.3) | 6 (4.6) | 13 |
| Not informed | 4 (3.1) | 2 (1.5) | 6 |
| Total of animals | 72 | 58 | 130 |
| INFECTION | SYMPTOMATIC (%) | ASYMPTOMATIC (%) | TOTAL |
|---|---|---|---|
| Present | 65 (50) | 7 (5.4) | 72 |
| Absent | 28 (21.5) | 30 (23.1) | 58 |
| Total | 93 | 37 | 130 |
| Biological Sample | Blood (+) | Blood (-) | Total |
|---|---|---|---|
| Swab (+) | 43 (33.1%) | 11 (8.5%) | 54 |
| Swab (-) | 15 (11.5%) | 61 (46.9%) | 76 |
| Total | 58 | 72 | 130 |
| kDNA (Minicircle) | Chromosomal (SSUr-rDNA) | |||
|---|---|---|---|---|
| Blood | Swab | Blood | Swab | |
| POSITIVE | 47 (36.2%) | 38 (29.2%) | 38 (29.2%) | 32 (24.6%) |
| NEGATIVE | 83 (63.8%) | 92 (70.8%) | 92 (70.8%) | 98 (75.4%) |
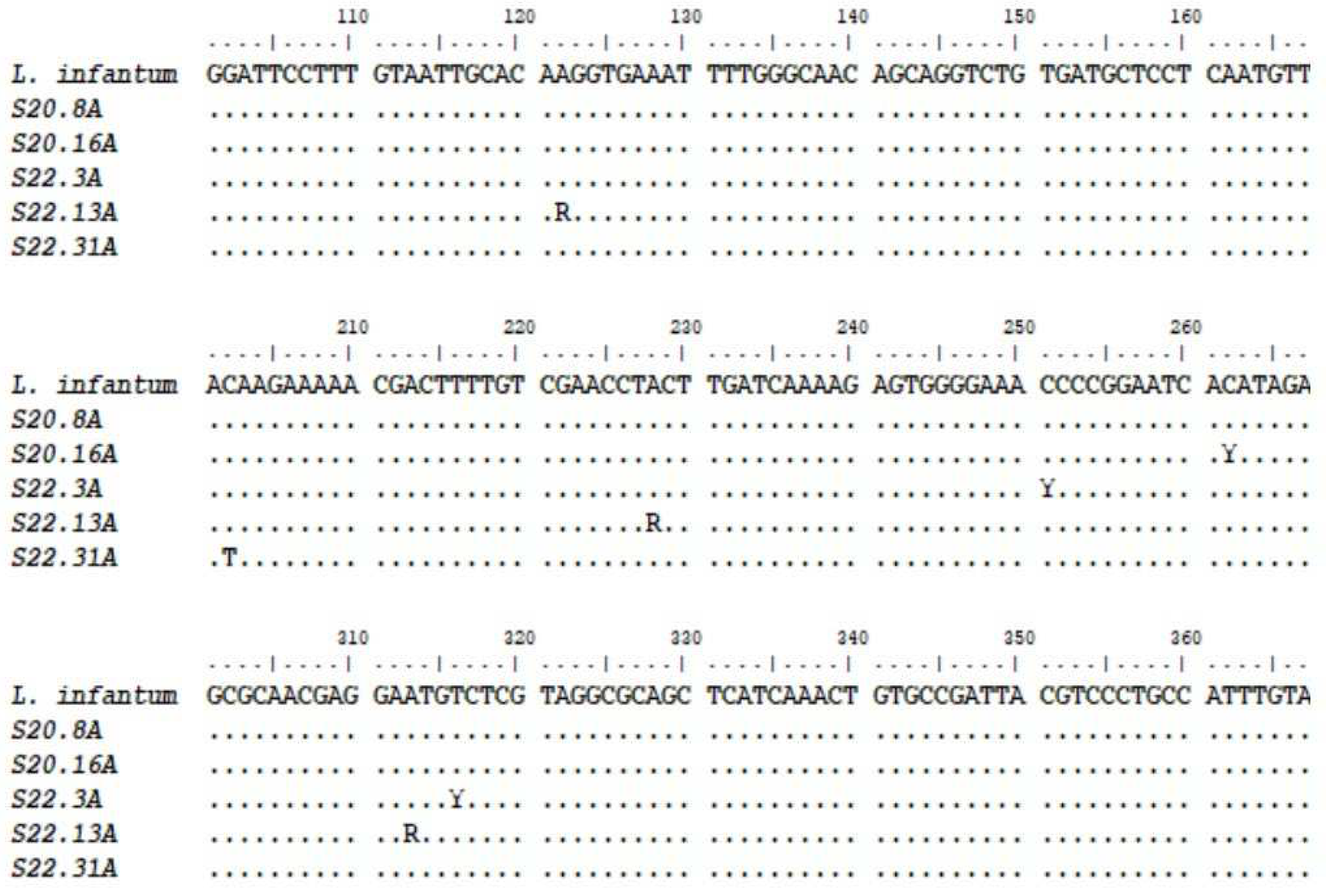
| Code | Position | Degenerate Nucleotide | Nucleotides |
|---|---|---|---|
| S20.16 | 262 | Y | C/T |
| S22.3 | 251, 316 | Y | C/T |
| S22.13 | 122, 228, 313 | R | A/G |
| S22.31 | 202 | T |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





