1. Introduction
Vascular diseases (arterial, venous, lymphatic and microcirculatory) have a considerable weight worldwide. All vascular patients suffer from lack of diagnosis, specialist offer, equity [
1] and the last becomes more severe in women. This review focusses on vascular diseases in women, in terms of relevance and need for a common position, coordinated with the valuable existing international actions on women’s health [
2]. Main risk factors as well as main vascular diseases are evaluated considering gender specificity.
Key points to consider on women health are related to disease as well as to availability of health education and carriers. They have a general value:
- 1-
Differences in physiophology exist between the genders, asking for more integrated and different approaches [
3]
- 2-
Epidemiological data show differences in distribution, deterioration and outcomes to be detailed and considered [
4]
- 3-
Similar diseases have different symptoms in women, often underevaluated [
2]. A delay in visiting the doctor linked to gender has been described.
- 4-
Behaviours associated with gender also involve to exposure to risk factors and attitudes to disease prevention and response.
- 5-
Some diagnostic paramenters should be validated to verify any possible differences related to gender.
- 6-
Response to therapy has been reported to be sometimes different. There is a need for proper studies and validation in women. In fact, although women′s enrollment in clinical trials is low, the conclusions are considered valid without any gender distinctions [
5].
- 7-
Health is linked to social determinants [
6,
7] and some of these are specific or have a greater weight for women.
- 8-
There is a need to guarantee equal access, development, and opportunities for women to get the best qualification, to work in the health area, becoming doctors, researchers, academics. Gender equality in science, medicine, and health contributes to gender equality in the community and gives social benefits. The UN Educational, Scientific and Cultural Organization’s Women in Science data show that, even if with a recent improvement, less than 30% of the world’s researchers are women, with relevant geographical differences [
8].
Each area of medicine can contribute in highlight its specific aspect, to enforce recommendations and action to ovecome the existing gap in gender equity.
2. Cardiovascular Risk Factors in Women
According to the World Health Organization, cardiovascular disease (CVD) is the leading cause of death among women worldwide, yet its magnitude is often underestimated. Most CVD cases can be prevented by identifying and managing modifiable cardiovascular risk factors (CVRF). There are CVRF that are common to both sexes, with specificities, and others that are unique to women.
2.1. Conventional Cardiovascular Risk Factors in Women
Aging remains an important CVRF; however, women tend to develop CVD approximately 10 years later than men, probably due to reduced CVRF exposure [
9].
In the INTERHEART study, hypertension (HTN), diabetes mellitus (DM), low physical activity, and moderate alcohol consumption exhibited a stronger association with myocardial infarction (MI) in women [
10]. A recent large-scale meta-analysis involving more than 5 million patients demonstrated that women with DM have a 30% significantly higher risk of CVD mortality and a 58% higher risk of coronary artery disease (CAD) compared to men [
11]. Data on sex-specific links between HTN and CVD risk are conflicting. HTN seems to progress more rapidly in women compared to men, starting in the third decade of life, exposing women to a higher risk of developing MI [
9,
12]. However, a large-scale meta-analysis failed to show significant sex differences in the overall cardiovascular risk associated with HTN [
13].
Female smokers face an increased risk of CAD, and their risk of developing MI is twice that of equivalent men [
14,
15]. In addition, smoking exposes women to almost 30 times greater risk of abdominal aortic aneurysm (AAA) and puts them at a higher risk of developing AAA compared to men who never smoke [
16]. These findings raise questions about the current European screening guidelines.
The SWAN study revealed dramatic increases in total cholesterol, LDL-C and (Apo)B levels related to menopause that correlate with the presence of long-term carotid plaques [
17]. In secondary prevention, women′s lipid levels remain higher, with only 51% of women achieving dyslipidemia control compared to 63% of men, differences likely attributable to lifestyle factors, adherence to medication and physical activity [
18].
In the Framingham Heart Study, obesity increased the risk of CAD by 64% in women compared to 46% in men [
19]. According to a large meta-analysis, women require higher levels of physical activity to reduce the risk of stroke compared to men [
20].
2.2. Menopause-Related Cardiovascular Risk Factors
Endogenous estrogen appears to confer a protective effect against CVD, resulting in a lower cardiovascular risk compared to men of the same age [
21]. The transition to menopause, marked by estrogen deficiency and its impact on cholesterol metabolism and atherosclerotic plaque formation, emerges as the primary risk factor for CVD in women. Notably, there is no significant difference between the impact of natural and surgical menopause [
22].
Shorter durations of estrogen exposure and the absence of estrogen′s protective CV effects during the reproductive lifespan, as seen in premature menopause (PM) and early menopause (EM) are associated with an increased risk of CVD. However, when compared to smoking, hormone therapy or Type 2 diabetes mellitus (Type 2 DM), PM and EM represent moderate yet significant risk factors for CVD. In these women, adopting a healthy lifestyle remains crucial for CVD prevention. However, more research is needed to determine whether these results are independent of menopausal hormone therapy (MHT), which can be a major confounder in the association between PM/EM and CVD [
23].
MHT appears to offer protection against CVD in younger postmenopausal women, but no significant effects on CVD outcomes are found 10 years after menopause [
24]. The International Menopause Society and the American Heart Association recommend MHT for CV prevention shortly after menopause, particularly in perimenopausal or postmenopausal women under 60, but not in women over 60 or 10 years after menopause [
25,
26]. Interestingly, some studies suggest that MHT might increase the relative risk of CAD compared to a control group during the first 2 years of treatment, but this risk diminishes between 3 and 7 years [
27]. Several post hoc analyses of the Women′s Health Initiative (WHI) data support the "timing hypothesis," which proposes that the vasoprotective effects of hormones are diminished in elderly women.
2.3. Pregnancy and Reproductive Risk Factors
Early menarche, defined as occurring before 10 years of age, is associated with elevated waist circumference, HTN, obesity, HLD, DM, and a 15% to 30% higher risk of CVD in adulthood, but the mechanisms are incompletely understood. After adjustment for other CVRF, the CVD estimates remained significant [
28,
29].
Polycystic ovary syndrome (PCOS) is associated with various CVRF, such as impaired glucose tolerance, HLD, HTN, metabolic syndrome and type 2 DM, as well as elevated subclinical markers of CVD such as coronary artery calcium, C-reactive protein levels, and carotid intima-media thickness [
30]. However, the status of PCOS as an independent CVRF for CVD remains unclear [
31,
32].
Hypertensive disorders of pregnancy (HDP) encompass chronic HTN before pregnancy, gestational HTN and preeclampsia/eclampsia. (30) During the peripartum period, women with a history of HDP have significantly higher odds of experiencing stroke, myocardial infarction, cardiomyopathy, and spontaneous coronary artery dissection. Furthermore, the risk of CVD extends well into later life [
33,
34]. A twofold CVD risk was established (RR 2.50 [95% CI, 1.43–4.37]) [
34]. The association between HDP and CVD is attenuated when adjusted for other CVRF, but persists. The causes are poorly understood [
33].
About one-third of women with gestational diabetes mellitus (GDM) will develop type 2 DM in the future (7-fold higher risk) [
35]. Women diagnosed with GDM appear to have a twice greater risk of CVD in the future compared to those without GDM (RR 1.98 [95% CI, 1.57–2.50]) [
36].
A post-hoc study by WHI found that women who breastfed for more than 12 months during their lifetime had a reduced risk of developing HTN (odds ratio [OR] 0.88, P<.001), DM (OR 0.80, P<.001), HLD (OR 0.81, P<.001), or CVD (OR 0.91, P=.008) compared to women who had never breastfed [
37].
Sex-specific risk factors are vital to assess comprehensive CVD risk in women. Further research is needed to understand their pathophysiology and mechanistic links to later-life CVD, informing precision prevention strategies. Considering the specificities of women is essential for effectively managing conventional CVRF.
3. Gender Differences in Health, Gender and Clinical Trials
Biological and gender differences affect health, diagnosis and healthcare in numerous ways. Traditionally, in medicine and healthcare the male body was considered the norm and women, the female body and its symptoms were considered deviations from this norm, with the major exception being sexual/reproductive diseases. Also, women have been, and still are, underrepresented in clinical trials, even for diseases that occur predominantly in women [
38]. Much of the underrepresentation of women in clinical trials can be dated back to the thalidomide tragedy, where women were given medication for morning sickness in pregnancy which resulted in severe birth defects [
39]. Due to perceived risk, women of reproductive age were actively discouraged from participating in clinical trials [
38], and while such discouragement is no longer a policy, women continue to be underrepresented in clinical trials.
The lack of sex and gender awareness in health research and healthcare is an ongoing issue that affects not only research, but also treatment and outcomes. Even basic awareness of the concepts of sex and gender is limited. Sex and gender are two separate terms that both impact on health; however, they tend to be conflated in medicine. A 2022 study of biomedical publications found that the term ′gender differences′ only analysed and reported biological phenomena, demonstrating how a conflation of terminology is still a frequent phenomenon in medical research [
40]. Sex refers to the biological and physiological characteristics that define men and women, boys and girls; gender is a social construct that varies and changes over time, and refers to such socially constructed characteristics of women and men, girls and boys, such as norms, behaviours, and roles [
41].
Furthermore, gender exists in a spectrum and can also be broken down into a number of categories, for example, gender identity, gender relationships, etc. [
42]. Additionally, to fully understand the impact of gender, it is important to recognise its intersection with other inequalities, and how interactions between gender, race and other categories such as sexual orientation, age, disability, etc. have an effect, and the results of these interactions in relation to power structures and oppression [
43]. All of these issues affect a person’s health, especially social determinants of health.
Failures to address sex and gender as key variables in cardiovascular research and reporting, and the resulting limited understanding of differences based on both sex and gender, have contributed to disparities in cardiovascular care and outcomes [
44]. This understanding can have grave effects on women and their health outcomes, both in cardiovascular disease and in other diseases. With cardiovascular disease, clinical guidelines have been based on data that predominantly included male study participants, and then being generalised to fit women, regardless of actual evidence of this being correct. Nicholas and colleagues describe a telling example in which a clinical trial of heart failure medications (i.e., beta-blockers, angiotensin converting enzyme inhibitors or angiotensin receptor blockers) suggested that women obtained optimal benefit from half the target dose for men and experienced an increased risk of side effects at higher doses. However, no prescription doses adjusted for sex have been established [
45]. This example, which is only one among numerous, shows that not only inadequate participation of women in clinical trials leads to several significant issues, including male-patterned inclusion criteria, sex-biased outcomes measurements, inadequate data analysis, and the missed opportunity to transfer results in clinical practice [
46], but even when differences are known, these are not always applied in practice.
There are some recent improvements that give reason for optimism in relation to increased female participation in clinical trials. The EU Clinical Trial Regulation No 536/2014 has improved clinical trial data transparency, and specifies that the subjects participating in a clinical trial should represent the population groups, such as, e.g., according to gender or age groups, that are likely to use the medicinal product. It also contains provisions for including pregnant and breastfeeding women in clinical trials, as well as a breakdown of data by sex and gender [
47]. Sosinsky et al.found that female representation in early-phase research has improved in cardiovascular clinical trials, which is good news as women have been especially underrepresented in early-phase clinical trials. However, they also found that while approximately 49% of patients with cardiovascular disease are women in the US, the average trial included only 41.9% women. As the US mandated adequate inclusion of women in trials sponsored by the National Institutes of Health (NIH) to determine sex differences already in 1993, and established the FDA’s Office of Women’s Health in 1994 [
38] they are decades ahead of Europe in efforts to implement increased female participation in clinical trials, but are still finding that women are underrepresented. Moreover, if women have been included in clinical trials, the results are generally not disaggregated or analysed for gender differences. Unless such a systematic analysis is mandatory, the future looks bleak for women.
The importance of recognising the impact of both sex and gender on health and of knowing the differences between the two in healthcare is beginning to gain ground. There is a greater appreciation of the role biological differences (sex) and socio-cultural power structures (gender) play, and both sex and gender affect health behaviour, the development of diseases, their diagnosis, management, and long-term effects of an illness [
42]. However, there is still a long way to go and there is still a continuous need to advocate and highlight the need for gender and sex awareness in health and research, and of the underrepresentation of women in clinical trials.
4. Vascular Diseases in Women
4.1. Cerebrovascular Disease
The WHO reports that, globally, there are more than 77 million people who are currently living and have had a stroke and almost two-thirds is due to ischemic stroke. Annually, 55% of all ischemic strokes occur in women and fifty-two percent of all stroke-related deaths are among women [
48]. One of the important risk factors for stroke incidence and mortality is advanced age. Men have a higher lifetime risk of ischemic stroke but, beyond the age of 85, stroke risk is higher in women [
49]. Elderly women have more severe strokes, poorer outcomes, and more difficult access to stroke care [
50]. Beyond the age of 60, changes in vascular function, such as endothelial dysfunction and arterial stiffness, are accelerated in women [
51]. Atherosclerotic carotid artery disease is one of the treatable causes of cerebrovascular disease. The Tromsø study was among the first to report sex differences in the prevalence of atherosclerotic carotid artery disease, with more women beyond the age of 75 having more carotid plaques [
52]. More contemporary data on women aged 30-79 years report that the estimated prevalence rates of increased carotid intima-media thickness, carotid plaque, and carotid stenosis are lower among women versus men: 23.2% vs. 32.1%, 17.1% vs. 25.2%, and 1.2% vs. 1.8 %, respectively [
53]. Sex differences in carotid plaque morphology and composition based on histology and non-invasive imaging have been reported. Women were found to have higher rates of stable fibrous/ fibrocalcific plaques, while men had higher rates of unstable plaques, with high-risk features such as large intraplaque hemorrhage, thin fibrous cap, large lipid core, and more inflammatory cells [
54]. The role of sex hormones in the development of atherosclerosis, plaque instability, and stroke risk has been reported. Estradiol appears to have a protective effect in women against stroke which decreases after menopause [
55] Thus, it is believed that estradiol can slow progression of atherosclerosis if hormone therapy is started soon after menopause when the endothelium is relatively healthier [
56]. Sex differences in the management of carotid disease have also been described with a greater reduction in stroke risk in women when medical management was offered in large trials of carotid stenosis trials; however, women were reported to be less likely to receive preventive medical therapy. On the other hand, women were under-represented in carotid surgery trials and only post-hoc analyses suggested that women benefited less from surgical intervention, although medical management at that time was not as optimal as what current medical treatment can offer. Furthermore, postoperative outcomes have been reported to be less favourable among women and it has been hypothesized that this may be due to smaller mean diameters of the carotid arteries in women, which may present greater technical difficulty during carotid endarterectomy [
54].
4.2. Abdominal Aortic Aneurysms
Gender-based differences in prevalence, diagnosis and management have been reported in abdominal aortic aneurysms (AAA). In a cross-sectional study of 1.5 million women and 0.8 million men tested in clinics, the overall prevalence of AAA was 0.6%, more common in men (1.5%) compared to women (0.25%), with higher prevalence in smokers. Compared to nonsmokers, the risk of AAA among those with a smoking history was 15 times greater in women (RR 15, 13.2-17.0), with significant associations in women <75 years (RR 26.4, 20.3-34.2) [
57].
The 2022 AHA/SVS [
58] and 2019 [
59] ESVS Guidelines recommend repair in unruptured aneurysms with a diameter of 5.0cm in women, with a recommendation level of class IA and class IIB, respectively. In a Swedish registry of 32,393 AAA patients, the proportion of women treated versus untreated was lower (17% versus 23%), older compared to men (76.1 [8.5] years versus 73.0 [8.5] years) and more frequently suffered from COPD. Although the median times to rupture and death were similar at approximately 2.8 years, there was a higher risk of rupture and poorer survival among untreated female patients at all time points up to 5 years after diagnosis [
60]. In a retrospective cohort of 16,386 patients with AAA, surgical repair was performed in 27% of women compared to 18% of men (p <.001). Women were more likely to undergo open surgical repair (RR 1.65; 95% CI 1.51-1.80), with open repair indicated for elective (RR, 1.82, 95% CI,1.65-1.99 and symptomatic (RR, 1.46, 95% CI, 1.15-1.81) aneurysm. In those who had endovascular repair, women were more likely to have traditional risk factors such as smoking, older age, hypertension, and a family history of AAA. A similar trend was observed with open repair.
Women presented with smaller aneurysms (mean [SD], 57 [11.7] mm vs 59 [17.7] mm in men;
P < .001). Among those where aortic anatomy data was collected during endovascular repair (EVR), aortic neck lengths and neck diameters were shorter while aorta-neck angles and neck-AAA angle were larger in women. Between 2003 and 2015, sex-specific treatment practices changed, with increased use of EVR in women over time. Women who underwent EVR were more likely to be poor candidates for open repair (p < .001). Short-term (1 year) and long-term (10 year) survival rates were poorer in women after both open and endovascular repair, although sex-based differences were more significant after the latter. Although no significant sex differences were observed with open repair for elective or symptomatic aneurysms, women who underwent open repair for ruptured AAA had a greater risk of death, which was not statistically significant with endovascular intervention [
61].
4.3. Lower Extremity Arterial Disease
Lower extremity arterial disease (LEAD), also called lower extremity peripheral artery disease (LEPAD), is the third comment on the manifestations of systemic atherosclerosis [
62]. Moreover, it is the leading cause of lower limb amputation and a major risk factor for cardiovascular mortality [
63,
64]. Women with LEAD are on average 10-20 years older than men. It is related to the loss of the protective vascular effects of estrogen, which promotes vasodilation and has antioxidant effects. General risk factors for LEAD remain similar among men and women, including smoking, hypertension, diabetes mellitus, and dyslipidemia. But in women, the duration of smoking appeared to already be a risk factor for LEAD after 10 years of smoking, compared to 30 years for men [
65]. However, other risk factors associated with LEAD in women have been shown to include obesity, osteopenia/osteoporosis, hypothyroidism, use of oral contraceptives, hormone replacement therapy, and complications associated with pregnancy (pre-eclampsia, gestational hypertension, placental abruption, and placental infarction) [
66]. In addition, diabetic women have a higher hypercoagulable state, impaired endothelium-dependent vasodilation, worse atherogenic dyslipidemia, and more metabolic syndrome than diabetic men [
67].
Recent epidemiological studies show that women have at least a similar, if not higher, prevalence of LEAD compared to men, including women from low- and middle-income countries (LMIC) and in socioeconomically disadvantaged groups. However, these data could be an underestimate, as women are often asymptomatic or have atypical symptoms compared to men, making diagnosis difficult [
68,
69]. Atypical symptoms in women can also be misinterpreted as arthritis, neuropathy, or spinal stenosis [
70].
Moreover, asymptomatic LEAD has been shown to be more common in women than in men [
66,
67,
70]. Furthermore, women also have twice the prevalence of limb-threatening ischaemia (CLTI) and more multilevel arterial occlusive disease [
71,
72]. Porras et al. [
73], in their systematic review and meta-analysis], reported that women with LEAD more often present with rest pain, while their prevalence of intermittent claudication is lower.
Women have a greater functional impairment of the lower extremities, with a shorter distance from the treadmill to intermittent claudication, a shorter maximum distance from the treadmill, and a poorer quality of life compared to men [
66,
74]. The shorter treadmill claudication distances in women with LEAD could be caused by their lower cardiopulmonary fitness and poorer self-perceived ability to climb stairs than in men. Therefore, Gardner et al. underline that women with intermittent claudication represent a subgroup of patients with LEAD who should receive high priority for exercise rehabilitation to improve physical function [
74]. Furthermore, the typical presentation of intermittent claudication in women generally occurs about 10-20 years later in age than men, and post-menopause [
75].
In some cases, in women making a diagnosis of LEAD can be a challenge. Females have a different fat distribution than males in the lower part of the body, limbs, and hips, which may hinder an accurate physical examination, including checking pulses and bruits [
76]. Additionally, duplex ultrasound may have some limitations in the examination of women. The high calcified arteries reported in women with diabetes and the elderly can cause difficulty in assessing the lumen of the artery. Moreover, the accuracy of this method might be diminished in proximal aortoiliac arterial segments in some cases of obesity. Women were also found to be more prone to adverse drug reactions of iodinated contrast media compared to men, which may limit the use of CT and contrast angiography in the diagnosis of LEAD in women. In addition, some women having ABI>1.4, as a consequence of arterial stiffness (patients with diabetes and end-stage renal disease, which occur more frequently in women) require alternative tests to diagnose unmask LEAD [
77].
Moreover, it was also reported that women with peripheral vascular diseases have low levels of knowledge and awareness about vascular diseases [
78]. There are also differences between women and men concerning the use
of guideline-directed medical therapy (GDMT)/ optimal medical treatment. Women are less likely to receive statins, antiplatelet agents, and angiotensin-converting enzyme inhibitors than men [
79,
80]. Even among high-risk patients with concurrent diabetes mellitus or chronic kidney disease, women are less likely to receive ACEi/ARB than men. Among patients with diabetes, women had poorer glycemic control than men. GDMT is less often prescribed among Black women compared with White women [
81]. Additionally, fewer women underwent surgical intervention for LEAD [
82]. They often have endovascular procedures. However, women also have greater in-hospital complications after endovascular surgery (higher rates of bleeding, vessel access site complications, haematoma, or pseudoaneurysm) [
82]. In addition, women, after endovascular intervention, also have a higher risk of myocardial infarction, dissection, amputation, and death [
83]. In the study by Hasan et al., it was shown that in women with LEAD who underwent peripheral vascular intervention (PVI), female gender was an independent predictor of major adverse cardiovascular events (MACE), mortality, non-fatal stroke, major bleeding, and higher cost. In turn, there were no significant differences in the rates of myocardial infarction, vascular complications, limb amputation, acute kidney injury, and length of stay [
84]. However, Parvar et al. showed higher mortality and MACE rates in men with LEAD despite other accepted gender disparities [
85].
A report from VASCUNET and the International Consortium of Vascular Registry showed data on open surgical revascularisation and peripheral vascular intervention (PVI) of symptomatic LEAD from 2010 to 2017 collected from administrative and registry data in the population of 11 countries. Numerous differences between women and men were observed with respect to patient age (72 vs 70 years), the proportion of octogenarians (28% vs 15%), the proportion of patients with claudication (45% vs 51%), the proportion of PVI (57% vs. 51%), and the length of hospital stay (7 days vs 6 days) [
86].
In another study, it has been shown that among claudication patients, women had a higher risk-adjusted rate of major amputation, but a lower risk of mortality. However, among CLTI patients, women had a lower risk adjusted hazard of major amputation [
87].
4.4. Vasculitis
Vasculitis is a heterogenous group of diseases defined by the presence of leucocytic inflammatory infiltrate in the vessel walls with reactive damage to the mural structures and necrosis. The endothelial injury can lead to thrombosis and ischemic tissue damage while the damage to endothelial wall can cause aneurysm and perforation with subsequent hemorrhage [
88]. The most frequently observed vasculitis in women is Takayasu Arteritis (TAK), also known as ′pulseless women′ disease, is a systemic inflammatory condition leading to damage to the medium and large arteries and their branches. It is a rare disease with a reported worldwide incidence rate of only 1 to 2 per million. It occurs predominantly in young Asian girls and women under 40 years of age. Female preponderance is characteristic of TAK (9:1). TAK is a panarteritis, but the initial site of inflammation is around the vasa vasorum and at the medioadventitial junction. In the early phase, the active inflammatory component and necrotizing character are conspicuous, with mononuclear cell infiltration (lymphocytes, histiocytes, and plasma cells) and edema. Fragmentation of elastic fibers is prominent, and giant cell granulomatous reaction and laminar medial necrosis may occur. Later, there is reactive fibrosis and increased ground substance in the intima, with overlying mural thrombus and a band of neovascularization at the intimal medial junction. Rapid or more severe inflammation leads to loss of smooth muscle cells, medial weakening, vascular dilatation, and aneurysm formation. In the healed phase, the adventitial fibrosis and scarring along with laminar medial necrosis are more impressive. The adventitial and periadventitial fibrosis observed in TAK exceeds that seen in any other inflammatory disorder of the aorta. Clinical manifestations of TAK vary depending on the stage of the disease and the vascular region involved Clinical features progress through 2 stages, although not all patients conform to this pattern:
Stage I is the “prepulseless” phase, characterized by constitutional symptoms, like fatigue, low-grade fever, night sweats, weight loss >2 kg, which are seen in <50% of patients.
Stage II is the “pulseless” phase marked by symptoms and signs of vascular inflammation (weak or missing pulses, vascular bruit, vascular tenderness) and ischemia.
Stage III is the described ′fibrotic′ stage, with complications resulting from vascular damage. Very few patients present in the third stage, and progression through all 3 stages (triphasic pattern) is observed in only 19% of the patients. Specific symptoms are related to the vessel involved and are listed in
Table 1.
The 2018 EULAR guidelines for the pharmacological treatment of TAK recommend a two-phase strategy, with a combination of conventional glucocorticoid and synthetic DMARD in phase I, and a biologic DMARD in phase II if relapse occurs [
90]. The 2021 American College of Rheumatology guidelines also recommend glucocorticoid combination therapy with DMARDs, but differ in recommending tumor necrosis factor inhibitors as an option in initial therapy; tocilizumab is recommended in refractory disease [
91]. Antiplatelet therapy is associated with a lower frequency of ischemic events but with an increase in bleeding. It is recommended in critical cerebrovascular disease or in vertebrobasilar involvement, but not routinely [
91]. Vascular lesions in TAK are responsible for most of the morbidity and mortality associated with the disease, but current guidelines favor restricting invasive therapy (open surgery or endovascular treatment) in TAK to life- or organ-threatening situations, refractory hypertension, or when patient activities are significantly affected [
89].
4.5. Vasospastic Diseases
Vascular dysregulation, the so-called vasospastic syndrome (VSS), occurs in the context of subjects who have a tendency to respond to stimuli such as coldness or emotional stress with inappropriate vasoconstriction or insufficient vasodilation in the microcirculation. People with vasospastic syndrome have cold hands and feet and abnormal vasoconstriction after local cold exposure. Women are affected more often than men. The first symptoms usually appear during puberty and eventually attenuate as subjects become older, especially after the menopause. The syndrome often seems to be inherited. The key symptoms of a person with VSS are cold hands and, sometimes, cold feet. Increased plasma ET-1 levels have been reported in patients with vascular dysregulation [
92]. There are well-established methods for diagnosing a VSS, including nailfold capillaroscopy combined with cold provocation. Raynauds syndrome, acrocyanosis and erythromelalgia are vasospastic diseases that differ with respect in prevalence, clinical picture, therapy, prognosis, and impairment of quality of life.
Raynauds syndrome occurs in 5 to 20 % of the population in Europe, is observed four times more often in women than in men and appears first at the age of 40 (3 to 80), on the average. Raynauds attacks are characterized by a paroxysmal white-blue-red or just white and blue discoloration of the fingers and toes; the attacks are induced by cold or stress, usually, cease after no more than some minutes but can also persist for hours. A distinction must be made between primary (aetiology unknown), secondary (aetiology known) and suspected secondary Raynauds syndromes (causal underlying disease suspected). There are several different therapy options of vasodilators (calcium channel blocker long acting).
Acrocyanosis is a vasospastic dystonic acral disorder that results in permanent reddish-livid discoloration, especially of the hands and feet. It is more frequent in women than in men and becomes manifest before the 25th year of age, on the average (15th to 70th year of age). A distinction is made between primary acrocyanosis without detectable underlying disease and secondary acrocyanosis with a specific underlying disease. No effective therapy for primary acrocyanosis is known, but secondary forms can sometimes be treated with vasodilators, calcium channel blockers long acting. Patients with primary and secondary
erythromelalgia, a very rare condition, sustain paroxysmal burning pain with marked reddening of the legs, feet and less often the hands. Attacks are triggered by warmth. The age of first manifestation is 40 to 55 years, but the first attacks may also occur during childhood. There are different therapeutic approaches with occasional success [
93].
4.6. Chronic Venous Insufficiency
Chronic venous insufficiency (CVI) is a vein disorder affecting millions of people every year. Women are more commonly affected than men [
94]. Chronic venous insufficiency of the lower extremities is associated with a wide clinical spectrum, ranging from asymptomatic but cosmetic problems to severe symptoms [
95,
96,
97]. This includes telangiectases, reticular veins, varicose veins, edema, pigmentation and/or eczema, lipodermatosclerosis, atrophie blanche, and venous ulceration. Abnormal venous flows of the lower extremities are observed in up to 50% of individuals, although the estimated prevalence of CVI varies depending on the population studies [
97].
Risk factors for both men and women include age, deep vein thrombosis, obesity, smoking, cancer, occupation, muscle weakness, leg injury, inactivity, family history, phlebitis, and shoegear [
98]. Pregnancy and poor biomechanics due to footwear make women more prone to chronic venous insufficiency than men.
The main pathophysiological cause of the clinical manifestation of CVI of the lower extremities is ambulatory venous hypertension, which is caused by venous valve reflux, venous flow obstruction, or both [
95,
97,
99].
To understand the pathophysiology of CVI or varicose veins, as well as their therapeutic options, such as endovenous ablations, one should know the anatomy and variations of the veins [
100,
101]. The venous system can be divided into three major components: the superficial venous system, the deep venous system, and perforating veins. The superficial veins are most frequently affected in patients with chronic venous disease [
97,
99].
Clinical features of CVI include discomfort, swelling, varicose veins, and skin changes or ulceration. Venous leg discomfort is often described as a dull ache, throbbing or heaviness, or pressure sensation after prolonged standing and is relieved by any measure that lowers venous pressure, such as elevation of the leg, compression stockings, or walking. However, leg discomfort is absent in an estimated 20% of patients with other clinical features of CVI, whereas it is the only clinical feature in approximately 10% of patients [
96]. In patients with varicose veins, tenderness could be present because of venous distension. Cutaneous changes include skin hyperpigmentation, stasis dermatitis, and ulceration. Hyperpigmentation is caused by hemosiderin deposition. Hyperpigmentation in nonvenous conditions, such as acanthosis nigricans or hemosiderosis, is more diffuse or involves other areas of the body. Lipodermatosclerosis is a type of inflammation of subcutaneous fat. A venous ulcer can be differentiated from an ischemic ulcer; ischemic ulcers are deeper than venous ulcers and often have gangrenous edges or a gangrenous base [
97,
99].
A complete history and physical examination are important to establish a proper diagnosis of CVI. Physical examination should be evaluated in the upright position to allow for maximal distension of the veins. Non-invasive and invasive diagnostic tests must assist the diagnosis. The DUS examination in patients with CVI should demonstrate both the anatomical patterns of veins and abnormalities of venous blood flow in the limbs.
All patients with signs and/or symptoms of CVI should be initially treated with conservative management. The use of compression stockings is the mainstay of conservative management. However, risk modification, such as weight reduction in an obese patient, regular walking exercise, and cessation of smoking, should also be encouraged in the patient as a conservative treatment [
97,
99].
Medical treatment has been used for decades, but there is some controversy over its exact place as a treatment modality for CVD. Venoactive drugs (VADs) are widely prescribed in some countries, but are not available in others. They can be classified into two groups: natural and synthetic drugs. The main modes of action of VADs are to decrease capillary permeability, reduce release of inflammatory mediators, or improve venous tone [
100]. Many compounds have been tested with varying success, but the most promising drugs are Ruscus extracts, micronised purified flavonoid fraction, calcium dobesilate, horse chestnut extract, hydroxyethylrutosides, red vine leaf extract, sulodexide [
97,
99].
Open surgical therapy of varicose veins with high ligation and stripping of the GSV combined with the excision of large varicose veins has been the standard of care for more than a century. During the past decade, endovenous ablation therapy has largely replaced this classic ligation and stripping.
There are two types of thermal ablation therapy: EVLA and RFA. Both are guided by ultrasound. The mechanism involves a heat generator that causes local thermal injury to the vein wall leading to thrombosis and fibrosis. EVLA and RFA showed the same safety and efficacy in terms of quality of life, occlusion, thrombophlebitis, hematoma, and recanalization after 1 year [
97,
99,
100]. The most common complication is bruising, which is observed in up to 75% of patients who receive ablation therapy. Other potential but rare complications include superficial vein thrombosis, DVT (especially EHIT), skin burns, pigmentation, and nerve injury.
Sclerotherapy is the least invasive percutaneous technique using chemical irritants to close unwanted veins. Telangiectases, reticular veins, small varicose veins, and venous segments with reflux can be treated with sclerotherapy [
97,
99].
It is important for women to be aware of chronic venous insufficiency. Venous disease of the lower extremities is a relatively common but frequently underdiagnosed medical problem. Because it is associated with a wide clinical spectrum, it is important to approach patients with suspicion of the condition. A solid understanding of the normal anatomy and function of the venous system is required to fully understand and diagnose the pathophysiology of CVI properly. Functional evaluation using DUS is essential to diagnose patients with CVI. Compression stockings are the mainstay for conservative management, but low compliance is a major hurdle for this therapy. Earlier use of venous ablation therapy should be considered in symptomatic patients.
4.7. Venous Thromboembolism
The most important acute venous pathology is venous thromboembolism (VTE), which is the combined name for deep vein thrombosis (DVT) and pulmonary embolism (PE). VTE is the third most common cardiovascular disease with an annual incidence of 100-200 per 100,000 people. The reported annual incidence rates of PE ± MVT and MVT-only cases vary from 29-78 and 45-117 per 100,000 population, respectively, according to different surveys. The prevalence of VTE increases with age, with a higher percentage in women at younger ages and in men at older ages. In women of reproductive age, VTE is a specific risk.
VTE is a major cause of morbidity and mortality during pregnancy and the postpartum period. The relative risk of VTE increases 5-fold during pregnancy and 60-fold in the puerperium (6 weeks postpartum) [
101]. Incidence of VTE increases slightly above that of the general population in the first trimester, rises to a greater degree during the third trimester,10–13 and peaks in the first two weeks after delivery. Approximately half of all pregnancy-associated VTEs occur postpartum. Pregnancy-associated risk factors for VTE include cesarean delivery, assisted reproductive technology, stillbirth, preterm birth, preeclampsia, obstetric hemorrhage and postpartum infection. Medical conditions associated antepartum or postpartum VTE include preexisting diabetes mellitus, inflammatory bowel disease, systemic lupus erythematosus, and sickle cell disease. More than half of pregnancy-related VTEs are associated with an underlying thrombophilia [
102].
In women, hormonal contraception also significantly increases the risk of VTE, especially when combined with other risk factors such as obesity, smoking or hereditary thrombophilia. Increased risk of thrombosis is also associated with ovarian stimulation therapy, in the background of which studies have demonstrated the effect of supraphysiological estrogen levels on various coagulation factors [
103]. Postmenopausal hormone replacement therapy is associated with a 2-4-fold increased risk of VTE [
104]. Patient-associated predisposing risk factors and acute triggers for VTE are listed in
Table 2.
Once the acute trigger has disappeared, the associated increased risk of thrombosis disappears, whereas persistent predisposing risk factors play an important role in the recurrence of VTE.
VTE recurs in about 30% in the 10 years following an acute event, regardless of the length of acute treatment. Independent predictors of recurrence are age, male sex, active malignancy, and neurological disease with lower limb paralysis. Other predictors of recurrence include idiopathic VTE, lupus anticoagulant or antiphospholipid antibody, antithrombin, protein C or protein S deficiency, hyperhomocysteinemia, persistently elevated plasma D-dimer in idiopathic VTE, and residual venous thrombosis [
105]. Various VTE recurrence prediction scores have been developed to assess the risk of recurrence, particularly in patients with idiopathic or cancer-related VTE. In women with idiopathic VTE, if up to one of the following risk factors was identified, the risk of recurrence of VTE was significantly lower: (1) older age (≥ 65 years), (2) obesity (BMI ≥ 30 kg/m2), (3) increased D-dimer before stopping warfarin therapy, and (4) signs of post-thrombotic syndrome. The risk of recurrence after discontinuation of anticoagulation for a combined oral contraceptive associated VTE was low and lower than after an unprovoked VTE [
106]. Persistently elevated D-dimer after discontinuation of anticoagulant therapy, age group over 50 years, male sex, and VTE unrelated to hormonal therapy (in women) increased the risk of recurrence in idiopathic VTE in the DASH prediction score [
107].
Typical symptoms of lower limb DVT include swelling, pain, redness, and dilatation of the superficial veins in the affected limb, sometimes the disease may be asymptomatic. Scoring systems (most commonly the Wells score) used to determine the probability of DVT play an important role in the diagnosis, with DVT being classified as high, medium or low probability. The "gold standard" for objective confirmation of DVT is the compression ultrasound (CUS). Pregnant patients with normal proximal CUS results should have dedicated iliac vein testing (typically Doppler/duplex ultrasonography or rarely non-contrast MRI) if they have symptoms of iliac vein DVT [
102]. For evaluation of PE computed tomography pulmonary angiography (CTPA) has become the diagnostic imaging standard. Planar lung ventilation/perfusion scintigraphy is a common test in pregnancy. Most young pregnant women do not have significant lung disease, and guidelines support the use of perfusion-only scintigraphy (i.e., no ventilation scan) in pregnant patients with normal chest radiographs to reduce maternal and fetal radiation exposure [
102]. Among the laboratory tests, an elevated (> 0.5 mg/ml) quantitative D-dimer level supports but does not confirm the diagnosis of VTE, as it can be elevated in many other conditions (post-operative, infectious, liver disease, heart failure, cancer, pregnancy, DIC).
Treatment of DVT is based on anticoagulation. The agent used in the acut phase may be subcutaneous LMWH, intravenous or subcutaneous unfractionated heparin (UFH) or subcutaneous fondaparinux. In parallel given oral vitamin K antagonist (VKA) treatment, parenteral anticoagulation should be continued until the INR is between 2 and 3 on two consecutive days, but for at least 5 days. There are also new or direct (DOAC) oral anticoagulants available for the treatment of VTE, which act by inhibiting fibrin (dabigatran) or activated factor X (apixaban, edoxaban, rivaroxaban). They have the advantage of not requiring INR control, no food interaction and significantly less drug interaction. The duration of anticoagulant treatment is known, 3 months for transient cause, at least 6 months for unknown cause, and at least 12 months for recurrent DVT. The duration of treatment is also influenced by the degree of recanalisation detectable by duplex ultrasound examination. Most of pregnancy-related VTE can be treated with low molecular weight heparin, but cases of limb- or life-threatening VTE require consideration of thrombolysis and other reperfusion therapies [
108].
Obstetricians-gynecologists, infertility and menopausal specialists have a key role in the prevention of VTE in women, to take into account the increased risk of VTE in treated women.
4.8. Lymphedema
Lymphedema is a prevalent (2-4/1000) [
109] but frequently overlooked disease caused by an imbalance between the production and reabsorption of lymphatic fluid due to disruption or overload of the lymphatic system. It can be a primary manifestation of congenitally abnormal lymphatic vessels or secondary to other conditions that damage the lymphatic vessels or lymph nodes. Although multiple factors intervene in its pathophysiology, the lymphatic endothelium expresses estrogen receptors, suggesting a role of estrogen in the lymphatic function that may explain the higher prevalence in females (60-80% of the population) [
109,
110,
111,
112].
Individuals born with agenesis or hypogenesis of the lymphatic vessels will develop lymphedema a different age, alone or within a syndrome (Noonan, Turner, etc.). Primary lymphedema is rare (1-10%) and more prevalent in females after puberty suggesting hormonal influence [
110,
113]. Penetrance is variable, and in some patients may develop after a triggering event [
114].
Worldwide is most caused by filarial infection. In developed countries, common causes of secondary lymphedema include cancer and cancer therapies, followed by obesity, infection, and inflammatory disorders. It can appear years after the initiating event. Cancer associated lymphedema is more common in females: Prevalence is high in breast cancer (13-20%) and gynecological malignancies (20-37%) compared to other malignancies equally distributed by gender [
115,
116]. The risk of lymphedema is influenced by the therapy, being higher for lymph node resection and radiation. Tamoxifen, a partial estrogen receptor agonist used in hormonal dependent breast cancer has been associated to lymphatic dysfunction and may predispose to lymphedema [
113].
Edema affecting one or both limbs is the main clinical finding. Patients describe tightness, heaviness, or discomfort. Initially, the edema is soft, pitting and improves with elevation. As lymphedema progresses, it becomes constant, and non-pitting due to cutaneous fibrosis and adipose deposition. The skin thickens, as evidenced by Stemmer′s sign (the inability to pinch the skin below the second toe) and develops hyperkeratosis with verrucose lesions. In females with breast cancer associated lymphedema, more than 60% reported that it affected their body imaging and sexuality [
117]. A common theme in patient forums revolve around finding suitable options for clothing to accommodate for the size discrepancy of the limbs, and compression garments.
Lipedema, affecting almost exclusively females, can be mistaken by lymphedema, however both conditions may overlap. Lipedema impacts quality of life, social and emotional functioning. Its diagnosis is frequently delayed, as it is the appropriate management and support [
118]. The presence of port-wine stains or limb discrepancy should raise the concern for Klippel-Trenaunay-Weber syndrome, vascular malformations or hemihypertrophy.
Diagnosis can be delayed as often body changes are attributed to obesity. Typical clinical features in the right clinical setting establish the diagnosis of lymphedema. Surveillance of patients at risk, (breast cancer, gynecological malignancies) may help reduce time lapses in diagnosis and treatment.
Objective measurements of the volume of the affected limb obtained with water displacement methods, circumferential measurements, or electronic volumetry are helpful to assess the effectiveness of therapies [
116]. Lymphoscintigraphy is commonly used to assess the lymphatic flow. It obtains serial images after administration of a radiolabeled agent distally in the affected limb.
Complex decongestive therapy is the cornerstone of lymphedema therapy regardless of the underlying reason, and its goal is to achieve and maintain maximal volume reduction in the affected limb. This multi-modality therapy is performed by a specialized therapist in multiple sessions and includes manual lymphatic drainage, multilayer bandaging, decongestive exercises, and chronic use of compression garments. Pneumatic pumps providing intermittent compression specially designed for lymphedema therapy can be used for maintenance, in addition to compression garments. These treatment modalities are time consuming and require lifelong commitment.
Surgical therapy can be considered in selected patients, directed at restoring lymphatic flow in the early stages of the disease [
116] or at reducing the limb size, in severe forms of lymphedema in addition to compression garments [
119].
Most studies focusing on quality of life and psychosocial consequences of lymphedema have been conducted in women with lymphedema after breast cancer and its treatment. Among breast cancer survivors, patients with lymphedema had significantly higher medical costs, hospitalizations, and medical visits than those without lymphedema [
120,
121]. Among female survivors of other cancers, those with lymphedema reported lower physical function and required more assistance for activities of daily living, [
122] Lifting restrictions, limited mobility, and fear of infections restrict the possibilities of actively participating in work, hobbies or sports. Other recurring themes that describe the impact of lymphedema on daily life include altered body image, sleep disturbances (avoiding the affected site, elevation of the limb during sleep), burden of self-care (economic cost, time-consuming tasks, need to alter the wardrobe), and the impact of living with a chronic disease [
123,
124].
In addition to that, patients with lymphedema are predispose to have recurrent cellulitis: 38% had at least one episode and 23% had more than one [
125]. Infections lead to progression of symptoms through lymphatic damage. Treatment of edema, prevention of injuries, and treatment of tinea pedis are recommended to decrease the risk of cellulitis [
125,
126,
127]. Rarely, women treated with radiation for breast cancer with radiation therapy can develop a rare aggressive angiosarcoma (Stewart-Treves syndrome). Skin changes should prompt a further evaluation [
128].
In conclusion, lymphedema disproportionately affects women and their physical, psychosocial, and emotional well-being. It is a chronic physical and economic burden for these patients, which deserve research and resources to prevent it, understand the challenges, and explore innovative therapies.
4.9. Pelvic Congestion Syndrome
Pelvic congestion syndrome (PCS) is a venous disorder characterized by noncyclic chronic non-cyclic pelvic pain caused by venous insufficiency predominantly associated with pelvic varicosities in women [
129]. Environmental factors such as pregnancy, obesity, jobs associated with prolonged standing or heavy lifting, some treatment interventions such as pelvic surgery and estrogen therapy, some anomalies in pelvic venous anatomy and genetic basis are risk factors involved in the pathology of PCS [
130,
131,
132].
There is a spectrum of clinical manifestations in PCS patients that can make PCS diagnosis challenging. This spectrum of clinical manifestations presents as different combinations of anatomical zones, etiology and hemodynamic disturbances. Therefore, to avoid misdiagnosis and missing rare cases, we need to consider patient symptoms based on main elements of PCS, including anatomical zones, etiology, and hemodynamic disturbances [
131,
133].
There are four anatomical zones in the abdomen and pelvis related to the pathophysiology and consequently symptoms of PCS including the left renal vein, the gonadal, internal iliac and pelvic veins, the pelvic origin extra-pelvic veins and the lower extremity veins. The pathophysiology of PCS is considered in three domains of anatomic, hemodynamic, and etiologic. Based on the SVP classification which includes symptoms (S), varices (V), and a pathophysiologic domain which is a composite anatomic-hemodynamic-etiologic domain (P), the inferior vena cava, left renal vein, gonadal veins, iliac veins and pelvic escape veins are anatomic segments of this classification. Also, the underlying pathological hemodynamic can be reflux or obstruction and the etiology can be thrombotic, non-thrombotic or congenital [
131,
133]. In addition, there are other factors involved in the pathogenesis of PCS including estrogen, inflammation, vasoactive peptides such as endothelin, calcitonin gene-related peptide (CGRP) and substance P (SP) and nociceptive mechanism, activating autonomic nervous system by sexual and non-sexual stimuli, neuropathic, and psychogenic mechanisms [
134,
135].
According to anatomical zones, there are three categories of symptoms, including venous renal symptoms, chronic pelvic pain, and extra-pelvic pain. Left renal vein compression (usually associated with nutcracker syndrome) can lead to renal venous hypertension, microhematuria or macrohematuria, and left flank or abdominal pain that is worsened by activities such as standing, sitting, or walking. Symptoms of this category are more prevalent on the left side. The second category of symptoms are characterized by chronic pelvic pain characterized as a dull unilateral or bilateral pain associated with negative cognitive, behavioral, sexual, and emotional consequences and symptoms related to lower urinary tract, sexual, bowel, pelvic floor, myofascial, or gynecologic dysfunction. Symptoms are often worse with activities such as walking and prolonged standing and improve with lying down. Symptoms of this category are sensitive but nonspecific. The third group of symptoms subdivided into symptoms localized to the external genitalia or lower extremities including reflux-related symptoms, symptoms related to posteromedial thigh, sciatic/tibial nerve varices and venous claudication associated with iliocaval venous obstruction. Also, renal hilar varices, pelvic varices and pelvic origin extra-pelvic varices are varices classification related to the anatomical zones [
129,
131,
133].
In addition to considering the clinical manifestations as a spectrum and SVP classification, the first line of diagnosis includes non-invasive approaches including pelvic ultrasound, cross-sectional computed tomography (CT) and magnetic resonance (MR) imaging, while conventional venography is the gold standard procedure for PCS diagnosis [
129,
136]. Depending on the SVP class, which is diagnosed and confirmed by imaging, there are different options for treatment, including pharmacological and hormonal therapy, endovascular therapy, and surgery [
136,
137].
PCS diagnosis is common in young women in reproductive age. Chronic pain and reduced physical activities are the main factors that affect the quality of life of PCS patients by limiting their social activity and the possibility of regular work. Furthermore, there is no explanation for the chronic pelvic pain of about 61% of patients. This fact that these patients do not know the reason for their pain makes their life harder than the group that is aware of their diagnosis [
134,
138].
In addition to social life, they have major challenges in family life as sexual challenges, infertility, and pregnancy. Symptoms such as dysmenorrhea, dysuria, and dyspareunia can affect sexual function [
138]. In addition, some case reports indicate that persistent genital arousal can be associated with PCS [
139,
140]. Infertility is another challenge for PCS patients due to ovarian varices or pelvic congestion caused by inferior vena cava obstruction [
129,
141,
142]. As mentioned below, pregnancy is a risk factor for PCS. Compression of the iliac veins by the gravid uterus, elevation of blood volume, and dilation of the ovarian and pelvic veins during pregnancy are the factors that make multiparous women prone to PCS [
129,
132,
143]. In addition, the pain that worsens during pregnancy makes pregnancy difficult for PCS patients [
144]. There is a lack of knowledge about the risk of deep vein thrombosis during pregnancy in PCS patients. These limitations and challenges in different aspects of the life of the PCS patient can affect their quality of life and induce psychological pressures that can not only affect their mental health, but also affect the circulation of the pelvis due to vasodilation [
135,
138].
Although PCS is well known as a premenopausal disease in which there is a remission of symptoms, possibly due to less estrogen and consequently decrease in vein dilation, there are postmenopausal patients who meet the PCS criteria [
143,
144,
145].
Taken together, the spectrum nature of PCS indicates the necessity of considering all of the anatomical, hemodynamical, and etiological combinations in order to avoid misdiagnosis and missing rare cases. Furthermore, interpreting PCS patient′s condition needs a holistic view for considering different aspects including neurochemical mechanisms, anatomical anomalies, psychological features, patient′s personality, job and desires, sexual function and response to sexual and non-sexual emotions and change in position.
4.10. Fibromuscular Dysplasia
According to the definition of the European consensus, fibromuscular dysplasia (FMD) is ′idiopathic, segmental, non-atherosclerotic and non-inflammatory disease of the musculature of the arterial walls, leading to stenosis of small and medium arteries′ [
146,
147].
FMD most commonly affects the cerebrovascular and renal arteries, but most of the arteries may be involved throughout the body [
147].
Histological findings show arterial fibrosis, cellular hyperplasia, and distortion of the abnormal architecture of the arterial wall. Due to a relative paucity of inflammatory cells in advanced lesions, FMD is defined as a ′non-inflammatory′ disease, but inflammation could be involved in the early stages of the disease [
148].
Since the advent of endovascular procedures, the histopathological classification has been replaced by an angiographic classification: focal or multifocal FMD. Multifocal FMD alternates areas of stenosis and dilation (“string of beads”) and is usually located in the mid and distal portion of the artery [
147,
148]. Recently, aneurysm, dissection, and arterial tortuosity have been included in the phenotype of FMD. However, a diagnosis of FAD cannot be established in the absence of focal or multifocal stenosis. Tortuosities can also be seen in the carotid, vertebral, and renal arteries. Tortuosity of the mid to distal portion of the internal carotid artery (ICA) may lead to an “S-curve”. The S curve is not specific for FMD, but its presence in individuals <70 years of age should alert the clinician [
147].
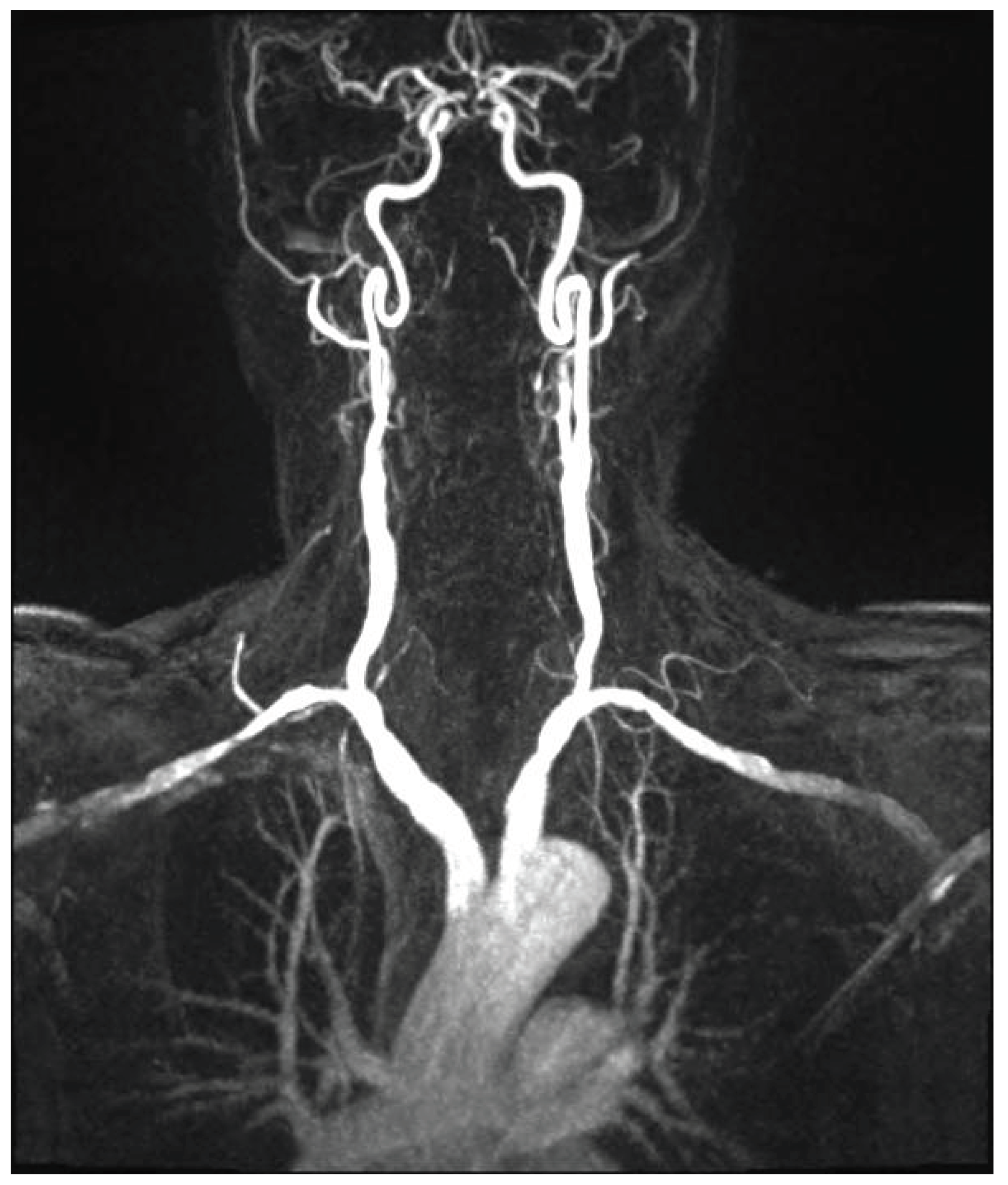
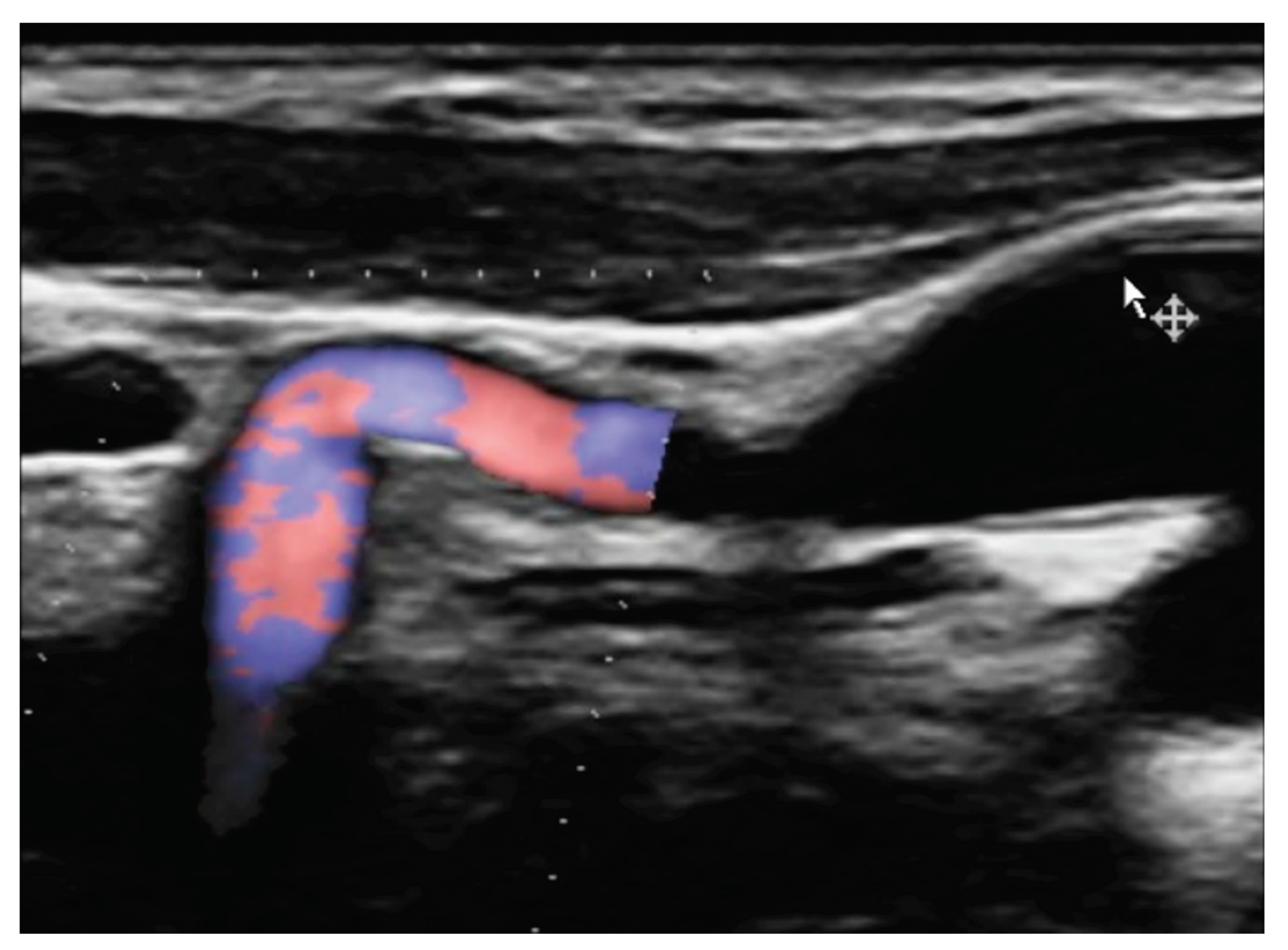
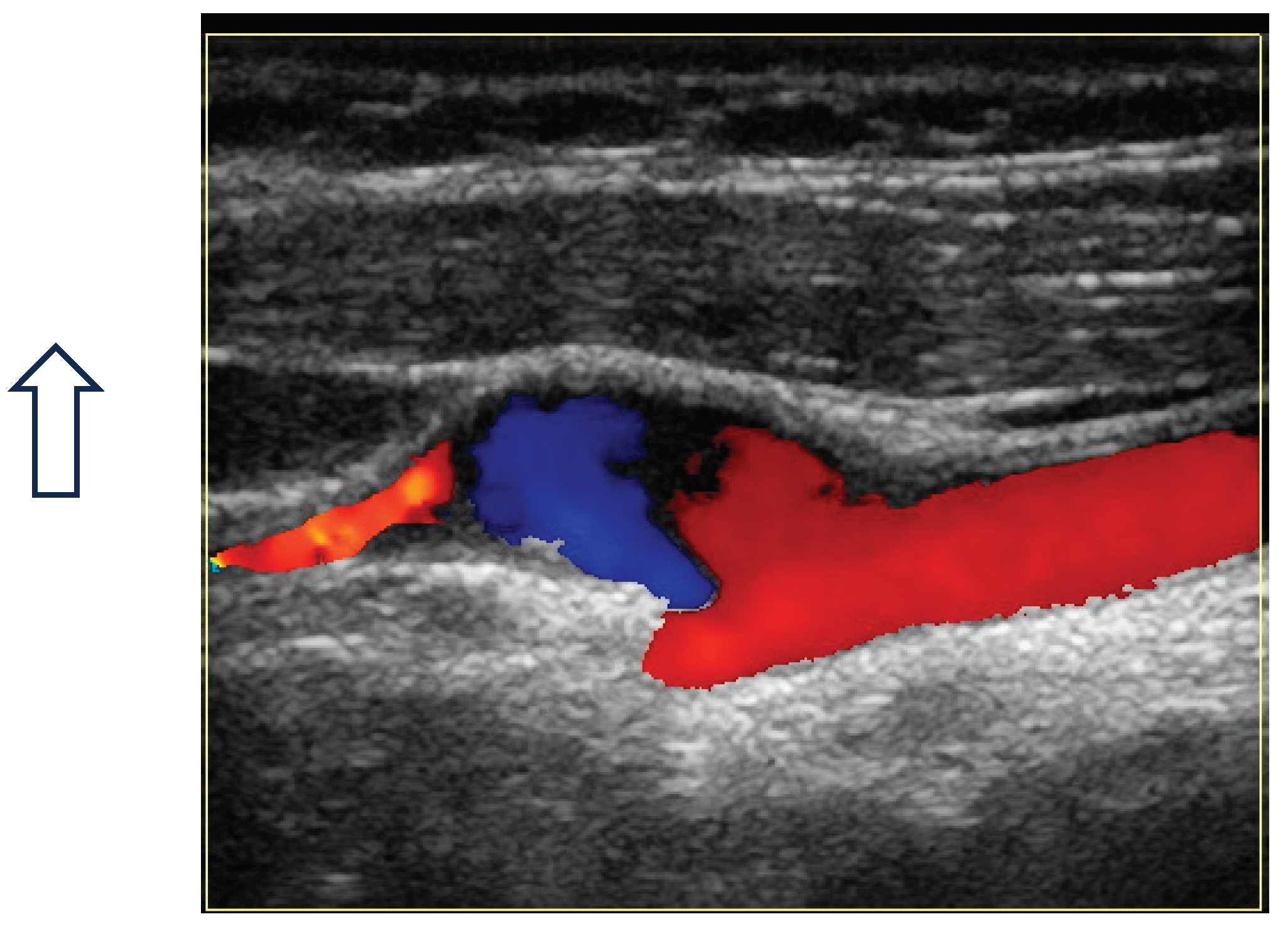
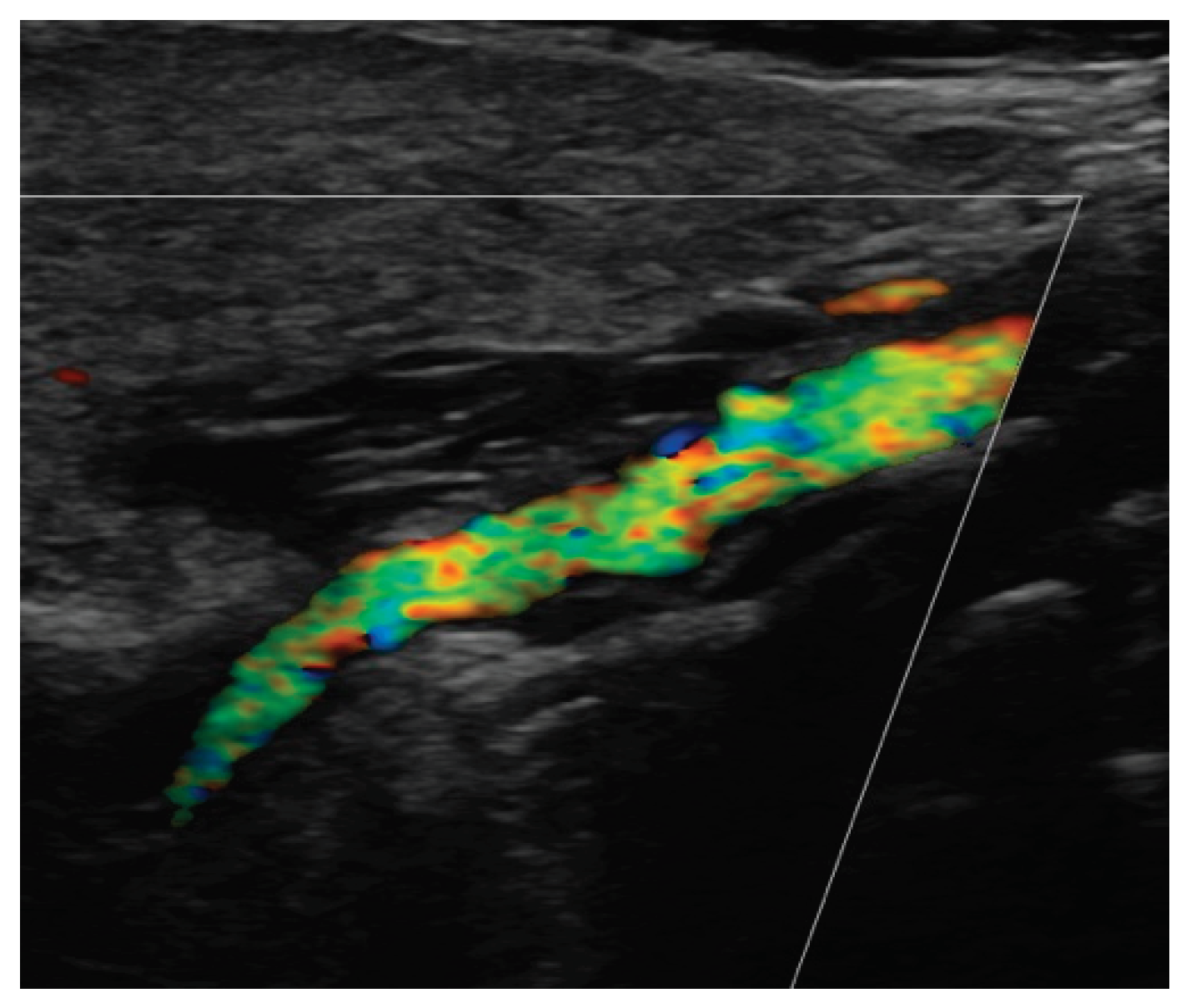
Figure 1 and
Figure 2.
4.10.1. Epidemiology, Pathogenesis and Genetics
Although approximately 82-95% of patients with FMD are women, men can also develop FMD with a higher frequency of aneurysms and dissection, especially if they are current smokers. FMD can occur at any age, but the mean age at diagnosis is 43-53 years [
148,150]. Notably, 3-6% of the patients are asymptomatic.
There are sporadic and familial forms, but only a minority of patients (1.9-7.3%) with FMD report an affected family member. The pathogenesis of FMD is poorly understood. It is linked to a combination of genetic and environmental factors. Tobacco smoking may be a potential pathogenic factor, but the data is still equivocal. The use of fluoroquinolones is associated with an increased risk of thoracic and abdominal aneurysms, and spontaneous cervical artery dissection. While waiting for specific data in FMD patients, and according to the FDA, “fluoroquinolones should not be used in patients at increased risk of aortic ruptures or dissections unless there are no other treatment options available” [
148].
Recently, a single nucleotide polymorphism rs9349379-A in the Phosphatase and Actin Regulator 1 (PHACTR1) gene has been identified as a common genetic risk variant. This risk locus is also associated with spontaneous coronary dissection (SCAD), carotid artery dissection, hypertension, and migraine headache. It should be noted that FMD, migraine, SCAD, and related diseases also have a higher prevalence in women. On the contrary, the risk locus has an inverse association with coronary atherosclerotic arterial disease, and patients with FMD appear to have fewer carotid plaques. FMD might protect them from atherosclerosis. A number of rare, exonic coding genetic variants have also been implicated in FMD. Further genetic studies are needed. Currently there is no specific genetic test for FMD and no justification for genetic testing of asymptomatic relatives of patients. But relatives should undergo clinical exam [
147,
148].
4.10.2. Renal FMD
Its prevalence is estimated between 3 and 6%. Renal arteries are involved in about 75% of FMD patients. The typical phenotype is “middle-aged white woman with personal and family history of hypertension”. Up to 90% of these women have multifocal FMD. Focal FMD is usually diagnosed before 30 years of age, with higher blood pressure and a more balanced sex distribution than multifocal FMD patients [
147]. Unlike atherosclerotic renal artery stenosis (ARAS), in patients with “string of beads” lesions, renal function and intrarenal microvascular function are usually preserved, even in the case of unilateral tight stenosis. Conversely, the impact of focal FMD is more severe but the success rate of balloon angioplasty is higher. (148) With a better spatial resolution and a better visualization of calcifications than magnetic resonance angiography (MRA), computed tomographic angiography (CTA) is the first-line imaging technique. MRA is an alternative when CTA is contraindicated. Duplex ultrasound (DUS) requires extensive expertise. Catheter based angiography may be helpful when the severity of the stenosis is difficult to assess (especially multifocal lesions) or as a control after angioplasty. It should be combined with translesional pressure gradient measurement. In experienced centers, angiography may be combined with intravascular ultrasound (IVUS) or optical coherence tomography (OCT) [
147].
4.10.3. Cerebrovascular FMD
Headaches are the most frequent, but non-specific symptoms (50-70% of patients with FMD) [
147,151]. Pulsatile tintinnus may be associated with cervical artery dissection. The prevalence of cervical artery dissection and intracranial saccular aneurysm is higher among patients with FMD, with a higher rate of neurological complications (TIA, ischemic stroke, subarachnoid hemorrhage).
Figure 3
The risk of long-term progression of FMD and the occurrence of aneurysm and dissection are not well-known [
148]. CTA and MRA are the initial imaging modalities. Catheter-based angiography must be reserved for cases that may require intervention. Carotid DUS maybe useful for surveillance, but with some drawbacks: unsatisfactory access to vertebral and carotid arteries (especially the distal cervical portion of the ICA and intracranial arteries), no validated criteria for FMD [
147].
Figure 4 and
Figure 5
4.10.4. Spontaneous Coronary Dissection (SCAD) and FMD
Acute coronary syndrome due to SCAD predominantly affects young to middle-aged women. SCAD and FMD are closely related pathologies, but clinical, radiological and genetic data suggest overlapping, instead of single disease [
148].
4.10.5. Pregnancy-Related Complications
In the FEIRI Registry, the prevalence of gestational hypertension (25%) and preterm birth (20%) was high in patients with FMD, probably related to the severity of renal FMD. However, the prevalence of preeclampsia and arterial complications was low/moderate [152].
4.11. Diabetic Angiopathy
The global burden of diabetes mellitus has increased significantly over the last several decades. The increase in diabetes incidence rate is projected to continue parallel to the increasing obesity epidemic. While young and middle-aged men show a higher prevalence of type 2 diabetes mellitus than their counterpart women, postprandial hyperglycaemia increases to a larger extent in women as they age. Consequently, women show a higher prevalence of undiagnosed diabetes after 60 years of age and of total diabetes after 70 years of age [153].
A major complication of diabetes is blood vessel disease, termed angiopathy. Patients with diabetes mellitus are at increased risk of both adverse cardiovascular and microvascular complications. Each macro- or microvascular disorder can be considered to be a tissue-specific manifestation of the glucose-driven pathogenetic processes occurring at susceptible sites in the body. Macrovascular diseases comprise coronary heart disease (CHD), peripheral arterial disease (PAD) and stroke. Microvascular complications of diabetes include diabetic retinopathy, diabetic nephropathy, and diabetic neuropathy. Recent evidence suggest sex differences in the prevalence, progression, and pathophysiology of diabetes driven macrovascular and microvascular diseases. The consequences of macrovascular complications may be greater in women. Although scarce and inconclusive, some evidence suggest that men might be at a higher risk for diabetic microvascular complications [154].
Diabetes is among the strongest risk factors for risk of future CHD. In the INTERHEART global case–control study of >27 000 individuals, diabetes mellitus was associated with a higher risk of myocardial infarction (MI) in women than any other risk factor. The association of diabetes mellitus with the risk of stroke is complex due to the variety of stroke types. Among 116316 women from the Nurses’ Health Study, aged 30 to 55 years followed for 26 years, type 1 diabetes mellitus was associated with a greater risk of ischemic and hemorrhagic strokes, while type 2 diabetes mellitus was associated with a greater risk of ischemic stroke but not hemorrhagic stroke. Recent evidence indicates that the risk of stroke is increased in diabetic patients with hyperglycemia, but not in those without hyperglycemia, suggesting an important role for metabolic disturbances in these associations [155]. Diabetes mellitus also deteriorates the outcomes of stroke and coronary heart disease. Similarly to other manifestations of atherosclerosis, diabetes is strongly linked to PAD, increasing the risk of PAD by 2 to 4 times. Unlike other forms of cardiovascular disease, diabetes does not appear to confer a higher excess risk in women compared to men for PAD [156].
Men show a faster progression of diabetic nephropathy and are more often treated with dialysis. However, during chronic dialysis treatment, diabetic women have a higher mortality risk than diabetic men. The risk of excess mortality in women appears to be impacted by age. Although several studies corroborate a higher mortality risk in older women, others suggest a higher risk among younger groups of women. Greater inflammation and oxidative stress in diabetic women with end-stage renal disease, as well as gender-related aspects regarding access to treatment and management modalities might, at least partly, explain excess mortality in diabetic women [157]
The prevalence of retinopathy in the diabetic population of the National Health and Nutrition Survey was 28.5%. Male sex, higher glycosylated hemoglobin levels, longer duration of diabetes, higher blood pressure, and insulin use were associated with the development of retinopathy [155]. The male sex has been reported to be an independent risk factor for advanced diabetic retinopathy and for the progression of the disease. However, the findings are not universal; with several other studies suggesting that the female sex is an independent risk factor for the development of diabetic retinopathy. These controversies are related to a number of factors in the studies, including age (and thus hormone levels), glycemic control, diabetes duration, and ethnic background [154].
Diabetes in men is usually diagnosed at a younger age and with lower body fat mass than in women. Women appear to have a greater burden of risk factors at the time of diagnosis of diabetes, especially obesity. Compared to men, women have a lower risk of macrovascular or microvascular disease in the absence of diabetes. The presence of diabetes confers a higher risk of vascular complications in women compared to men. Some potential explanations include the contribution of sex hormones and specific risk factors for sex. A growing body of evidence suggests that sex hormones play an important role in the regulation of cardiovascular function. In general, estrogens are considered cardioprotective and androgens are detrimental to cardiovascular health. However, recent evidence indicates the diversity and complexity of the action of sex hormones on target tissues, especially in the context of diabetes. Understanding the mechanisms of sex differences in the pathophysiology of diabetic vascular complications may contribute to personalized- and sex-specific prevention and treatment for diabetic macro- and micro-vascular disease [157].
5. Conclusions
In conclusion, women get sick differently than men. Both the symptoms of the same diseases and their course differ between women and men. In women, most vascular diseases are often atypical and asymptomatic. In addition, the resulting guidelines for the treatment of vascular diseases are based on the results of studies in which the participants were predominantly men. It has detrimental consequences for their medical care. One of the reasons for this was the assumption that the health differences between men and women were largely due only to their reproductive systems [158]. The lack of good epidemiological studies or medical trials in women means that they are diagnosed and treated like men.
It should be noted that men and women respond differently to medications. It may be related to the structure of the atherosclerotic plaque, the vessels′ reactivity, the endothelium′s functioning, and the absorption, metabolism, and distribution of chemical substances, including medications.
Furthermore, there are also differences between women and men about the use of guideline-directed medical therapy/ optimal medical treatment. Women are less likely to receive statins, antiplatelet agents, and angiotensin-converting enzyme inhibitors than men.
In addition to classic risk factors, such as hypertension, obesity, smoking, lack of physical activity, or abnormal cholesterol levels, women are exposed to additional gender-specific risks associated with changes in pregnancy (hypertension or pregnancy-induced diabetes), such as well as hormonal disorders of reproductive age (polycystic ovary syndrome, premature menopause). The presence of an additional burden, risk factors unique to women, requires intensive measures to prevent the development of vascular diseases and their effective treatment. Although most classic risk factors affect both men and women to a similar extent, increasing their chances of developing vascular disease, smoking has been shown to significantly worsen the prognosis in women.
The other issue is women′s knowledge and awareness of vascular diseases. The risk of cardiovascular events is drastically underestimated by women themselves, as well as by those around them. According to German research, more than two-thirds of women surveyed consider breast cancer to be the greatest risk and the most common cause of their fear, but only 25% of women cite cardiovascular disease in this context [159].
Therefore, to improve the medical care and treatment of women with vascular diseases, the above issues should be considered.
Author Contributions
Conceptualization Katalin Farkas, Agata Stanek, Mariella Catalano, writing - original draft preparation, Katalin Farkas, Agata Stanek, Stephanie Zbinden, Barbara Borea, Simina Ciurica, Vanessa Moore, Peggy Maguire, Maria Teresa B. Abola, Elaine B. Alajar, Antonella Marcoccia, Dilek Erer, Ana Casanegra, Hiva Sharebiani, Muriel Sprynger, Maryam Kavousi, Mariella Catalano.; writing - review and editing, Stephanie Zbinden and Agata Stanek, supervision, Muriel Sprynger and Agata Stanek. All authors have read and agreed to the published version of the manuscript. For research articles.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing does not apply to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations and acronyms
AAA: Abdominal aortic aneurysm
AHA: American Heart Association
CAD: Coronary artery disease
CUS: Compression ultrasound
CVD: Cardiovascular disease
CVI: Chronic venous insufficiency
CVRF: Cardiovascular risk factor
DM: Diabetes mellitus
DMARD: Disease-modifying antirheumatic drugs
DVT: Deep vein thrombosis
EHIT: Endovenous heat-induced thrombosis
EM: Early menopause
EVLA: Endovenous Laser Ablation
GDM: Gestational diabetes mellitus
HDP: Hypertensive disorders of pregnancy
HLD: Hyperlipidemia
HTN: Hypertension
IMS: International Menopause Society
LEAD: Lower extremity arterial disease
MHT: Menopausal hormone therapy
MI: Myocardial infarction
PAD: Peripheral arterial disease
PCOS: Polycystic ovary syndrome
PCS: Pelvic congestion syndrome
PE: Pulmonary embolism
PM: Premature menopause
RFA: Radiofrequency ablation
VAD: Venoactive drugs
VSS: Vasospastic syndrome
VTE: Venous thromboembolism
WHI: Women′s Health Initiative
References
- Lack of Equity for Vascular Patients and in Prevention of Vascular Disease-A VAS European Independent Foundation in Angiology/Vascular Medicine & International Consortium Position Paper (Under submission).
- Mauvais-Jarvis, F.; Merz, N.B.; Barnes, P.J.; Brinton, R.D.; Carrero, J.-J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
- Yerly, A.; van der Vorst, E.P.C.; Baumgartner, I.; Bernhard, S.M.; Schindewolf, M.; Döring, Y. Sex-specific and hormone-related differences in vascular remodelling in atherosclerosis. Eur. J. Clin. Investig. 2022, 53, e13885. [Google Scholar] [CrossRef]
- Shannon, G.; Jansen, M.; Williams, K.; Cáceres, C.; Motta, A.; Odhiambo, A.; Eleveld, A.; Mannell, J. Gender equality in science, medicine, and global health: where are we at and why does it matter? Lancet 2019, 393, 560–569. [Google Scholar] [CrossRef]
- Daitch, V.; Turjeman, A.; Poran, I.; Tau, N.; Ayalon-Dangur, I.; Nashashibi, J.; Yahav, D.; Paul, M.; Leibovici, L. Underrepresentation of women in randomized controlled trials: a systematic review and meta-analysis. Trials 2022, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lancet, T. Can health equity become a reality? Lancet 2008, 372, 1607. [Google Scholar] [CrossRef] [PubMed]
- Kolossváry, E.; Farkas, K.; Karahan, O.; Golledge, J.; Schernthaner, G.-H.; Karplus, T.; Bernardo, J.J.; Marschang, S.; Abola, M.T.; Heinzmann, M.; et al. The importance of socio-economic determinants of health in the care of patients with peripheral artery disease: A narrative review from VAS. Vasc. Med. 2023, 28, 241–253. [Google Scholar] [CrossRef]
- UNESCO Institute for Statistics (UIPS), June 2019. 20 June.
- George, J.; Rapsomaniki, E.; Pujades-Rodriguez, M.; Shah, A.D.; Denaxas, S.; Herrett, E.; Smeeth, L.; Timmis, A.; Hemingway, H. How Does Cardiovascular Disease First Present in Women and Men? Incidence of 12 Cardiovascular Diseases in a Contemporary Cohort of 1,937,360 People. Circ. 2015, 132, 1320–1328. [Google Scholar] [CrossRef]
- Anand, S.S.; Islam, S.; Rosengren, A.; Franzosi, M.G.; Steyn, K.; Yusufali, A.H.; Keltai, M.; Diaz, R.; Rangarajan, S.; Yusuf, S. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur. Hear. J. 2008, 29, 932–940. [Google Scholar] [CrossRef]
- Wang, Y.; O’neil, A.; Jiao, Y.; Wang, L.; Huang, J.; Lan, Y.; Zhu, Y.; Yu, C. Sex differences in the association between diabetes and risk of cardiovascular disease, cancer, and all-cause and cause-specific mortality: a systematic review and meta-analysis of 5,162,654 participants. BMC Med. 2019, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Kim, A.; Ebinger, J.E.; Niiranen, T.J.; Claggett, B.L.; Merz, C.N.B.; Cheng, S. Sex Differences in Blood Pressure Trajectories Over the Life Course. JAMA Cardiol. 2020, 5, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.A.; Huxley, R.R.; Woodward, M. Comparison of the sex-specific associations between systolic blood pressure and the risk of cardiovascular disease: a systematic review and meta-analysis of 124 cohort studies, including 1.2 million individuals. Stroke 2013, 44, 2394–2401. [Google Scholar] [CrossRef]
- Huxley, R.R.; Woodward, M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet 2011, 378, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Millett, E.R.C.; A E Peters, S.; Woodward, M. Sex differences in risk factors for myocardial infarction: cohort study of UK Biobank participants. BMJ 2018, 363, k4247. [Google Scholar] [CrossRef]
- Carter, J.L.; Morris, D.R.; Sherliker, P.; Clack, R.; Lam, K.B.H.; Halliday, A.; Clarke, R.; Lewington, S.; Bulbulia, R. Sex-Specific Associations of Vascular Risk Factors With Abdominal Aortic Aneurysm: Findings From 1.5 Million Women and 0.8 Million Men in the United States and United Kingdom. J. Am. Hear. Assoc. 2020, 9, e014748. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Greendale, G.; Crawford, S.L.; Avis, N.E.; Brooks, M.M.; Thurston, R.C.; Karvonen-Gutierrez, C.; Waetjen, L.E.; Matthews, K. The menopause transition and women′s health at midlife: a progress report from the Study of Women′s Health Across the Nation (SWAN). Menopause 2019, 26, 1213–1227. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Yang, Z. Trends in lipid profile and lipid control among survivors of stroke or myocardial infarction among US adults, 2001–2018. Front. Endocrinol. 2023, 14, 1128878. [Google Scholar] [CrossRef]
- Wilson, P.W.F.; D′Agostino, R.B.; Sullivan, L.; Parise, H.; Kannel, W.B. Overweight and Obesity as Determinants of Cardiovascular Risk. Arch. Intern. Med. 2002, 162, 1867–1872. [Google Scholar] [CrossRef]
- Diep, L.; Kwagyan, J.; Kurantsin-Mills, J.; Weir, R.; Jayam-Trouth, A. Association of Physical Activity Level and Stroke Outcomes in Men and Women: A Meta-Analysis. J. Women′s Heal. 2010, 19, 1815–1822. [Google Scholar] [CrossRef]
- Prestwood, K.M.; Unson, C.; Kulldorff, M.; Cushman, M. The Effect of Different Doses of Micronized 17ss-Estradiol on C-Reactive Protein, Interleukin-6, and Lipids in Older Women. Journals Gerontol. Ser. A 2004, 59, M827–M832. [Google Scholar] [CrossRef]
- Del Principe, D.; Ruggieri, A.; Pietraforte, D.; Villani, A.; Vitale, C.; Straface, E.; Malorni, W. The relevance of estrogen/estrogen receptor system on the gender difference in cardiovascular risk. Int. J. Cardiol. 2015, 187, 291–298. [Google Scholar] [CrossRef]
- Liu, J.; Jin, X.; Liu, W.; Chen, W.; Wang, L.; Feng, Z.; Huang, J. The risk of long-term cardiometabolic disease in women with premature or early menopause: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2023, 10, 1131251. [Google Scholar] [CrossRef]
- LaCroix, A.Z.; Chlebowski, R.T.; Manson, J.E.; Aragaki, A.K.; Johnson, K.C.; Martin, L.; Margolis, K.L.; Stefanick, M.L.; Brzyski, R.; Curb, J.D.; et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA 2011, 305, 1305–1314. [Google Scholar] [CrossRef]
- Baber, R.J.; Panay, N.; Fenton, A. 2016 IMS Recommendations on women’s midlife health and menopause hormone therapy. Climacteric 2016, 19, 109–150. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Aggarwal, B.; Beckie, T.M.; Hodis, H.N.; Johnson, A.E.; Langer, R.D.; Limacher, M.C.; Manson, J.E.; Stefanick, M.L.; Allison, M.A.; et al. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement From the American Heart Association. Circulation 2020, 142, 506–532. [Google Scholar] [CrossRef]
- Hulley, S.; Grady, D.; Bush, T.; Furberg, C.; Herrington, D.; Riggs, B.; Vittinghoff, E. for the Heart and Estrogen/progestin Replacement Study (HERS) Research Group Randomized Trial of Estrogen Plus Progestin for Secondary Prevention of Coronary Heart Disease in Postmenopausal Women. JAMA 1998, 280, 605–613. [Google Scholar] [CrossRef]
- Lakshman, R.; Forouhi, N.G.; Sharp, S.J.; Luben, R.; Bingham, S.A.; Khaw, K.-T.; Wareham, N.J.; Ong, K.K. Early Age at Menarche Associated with Cardiovascular Disease and Mortality. J. Clin. Endocrinol. Metab. 2009, 94, 4953–4960. [Google Scholar] [CrossRef]
- Canoy, D.; Beral, V.; Balkwill, A.; Wright, F.L.; Kroll, M.E.; Reeves, G.K.; Green, J.; Cairns, B.J.; S, D.; S, K.; et al. Age at Menarche and Risks of Coronary Heart and Other Vascular Diseases in a Large UK Cohort. Circ. 2015, 131, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.H.E.M.; Rosano, G.; Cifkova, R.; Chieffo, A.; van Dijken, D.; Hamoda, H.; Kunadian, V.; Laan, E.; Lambrinoudaki, I.; Maclaran, K.; et al. Cardiovascular health after menopause transition, pregnancy disorders, and other gynaecologic conditions: a consensus document from European cardiologists, gynaecologists, and endocrinologists. Eur. Heart J. 2021, 42, 967–984. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhu, Z.; Lou, H.; Zhu, G.; Huang, W.; Zhang, S.; Liu, F. Polycystic ovary syndrome (PCOS) and the risk of coronary heart disease (CHD): a meta-analysis. Oncotarget 2016, 7, 33715–33721. [Google Scholar] [CrossRef] [PubMed]
- de Groot, P.C.M.; Dekkers, O.M.; Romijn, J.A.; Dieben, S.W.M.; Helmerhorst, F.M. PCOS, coronary heart disease, stroke and the influence of obesity: a systematic review and meta-analysis. Hum. Reprod. Updat. 2011, 17, 495–500. [Google Scholar] [CrossRef]
- Haug, E.B.; Horn, J.; Markovitz, A.R.; Fraser, A.; Klykken, B.; Dalen, H.; Vatten, L.J.; Romundstad, P.R.; Rich-Edwards, J.W.; Åsvold, B.O. Association of Conventional Cardiovascular Risk Factors With Cardiovascular Disease After Hypertensive Disorders of Pregnancy. JAMA Cardiol. 2019, 4, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Haththotuwa, R.; Kwok, C.S.; Babu, A.; Kotronias, R.A.; Rushton, C.; Zaman, A.; Fryer, A.A.; Kadam, U.; Chew-Graham, C.A.; et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Qual. Outcomes 2017, 10, e003497. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L.; Casas, J.-P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Kramer, C.K.; Campbell, S.; Retnakaran, R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia 2019, 62, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.B.; Ray, R.M.; Stuebe, A.M.; Allison, M.A.; Ness, R.B.; Freiberg, M.S.; Cauley, J.A. Duration of Lactation and Risk Factors for Maternal Cardiovascular Disease. Obstetrics & Gynecology 2009, 113, 974–982. [Google Scholar] [CrossRef]
- Sosinsky, A.Z.; Rich-Edwards, J.W.; Wiley, A.; Wright, K.; Spagnolo, P.A.; Joffe, H. Enrollment of female participants in United States drug and device phase 1–3 clinical trials between 2016 and 2019. Contemp. Clin. Trials 2022, 115, 106718. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, T.S.; Teerawattananon, Y.; Tannenbaum, C.; Vijayasingham, L. Making pharmaceutical research and regulation work for women. BMJ 2020, 371, m3808. [Google Scholar] [CrossRef]
- Hurk, L.v.D.; Hiltner, S.; Oertelt-Prigione, S. Operationalization and Reporting Practices in Manuscripts Addressing Gender Differences in Biomedical Research: A Cross-Sectional Bibliographical Study. Int. J. Environ. Res. Public Heal. 2022, 19, 14299. [Google Scholar] [CrossRef]
- World Health Organisation. Gender and health. 2023. Available online: https://www.who.int/health-topics/gender#tab=tab_1 (accessed on 22 July 2023).
- Oertelt-Prigione, S. Putting gender into sex- and gender-sensitive medicine. EClinicalMedicine 2020, 20, 100305. [Google Scholar] [CrossRef]
- Davis, K. Intersectionality as buzzword: A sociology of science perspective on what makes a feminist theory successful. Fem. Theory 2008, 9, 67–85. [Google Scholar] [CrossRef]
- Mensah, G.A.; Fuster, V. Sex and Gender Differences in Cardiovascular Health. J. Am. Coll. Cardiol. 2022, 79, 1385–1387. [Google Scholar] [CrossRef]
- Nicolas, J.; Edens, M.; Vogel, B.; Mehran, R. Best Practices for Designing Informative Trials Including Women. Curr. Atheroscler. Rep. 2022, 24, 885–888. [Google Scholar] [CrossRef]
- Pilote, L.; Raparelli, V. Participation of Women in Clinical Trials: Not Yet Time to Rest on Our Laurels. J. Am. Coll. Cardiol. 2018, 71, 1970–1972. [Google Scholar] [CrossRef]
- European Commission (2023) Clinical trials - Regulation EU No 536/2014. Available online: https://health.ec.europa.eu/medicinal-products/clinical-trials/clinical-trials-regulation-eu-no-5362014_en (accessed on 22 July 2023).
- Global Burden of Disease Stroke Statistics Worldwide for the year 2019. Available online: http://ghdx.healthdata.org/gbd-results-tool.
- Seshadri, S.; A Wolf, P. Lifetime risk of stroke and dementia: current concepts, and estimates from the Framingham Study. Lancet Neurol. 2007, 6, 1106–1114. [Google Scholar] [CrossRef]
- Cordonnier, C.; Sprigg, N.; Sandset, E.C.; Pavlovic, A.; Sunnerhagen, K.S.; Caso, V.; Christensen, H.; the Women Initiative for Stroke in Europe (WISE) group. Stroke in women — from evidence to inequalities. Nat. Rev. Neurol. 2017, 13, 521–532. [Google Scholar] [CrossRef]
- Kumar, A.; McCullough, L. Cerebrovascular disease in women. Ther. Adv. Neurol. Disord. 2021, 14. [Google Scholar] [CrossRef]
- Joakimsen, O.; Bønaa, K.H.; Stensland-Bugge, E.; Jacobsen, B.K. Age and Sex Differences in the Distribution and Ultrasound Morphology of Carotid Atherosclerosis. Arter. Thromb. Vasc. Biol. 1999, 19, 3007–3013. [Google Scholar] [CrossRef]
- Song, P.; Fang, Z.; Wang, H.; Cai, Y.; Rahimi, K.; Zhu, Y.; Fowkes, F.G.R.; I Fowkes, F.J.; Rudan, I. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. Lancet Glob. Heal. 2020, 8, e721–e729. [Google Scholar] [CrossRef] [PubMed]
- Gasbarrino, K.; Di Iorio, D.; Daskalopoulou, S.S. Importance of sex and gender in ischaemic stroke and carotid atherosclerotic disease. Eur. Hear. J. 2021, 43, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.P.; Cassis, L.A.; Eghbali, M.; Reue, K.; Sandberg, K. Sex Hormones and Sex Chromosomes Cause Sex Differences in the Development of Cardiovascular Diseases. Arter. Thromb. Vasc. Biol. 2017, 37, 746–756. [Google Scholar] [CrossRef]
- Persky, R.W.; Turtzo, L.C.; McCullough, L.D. Stroke in Women: Disparities and Outcomes. Curr. Cardiol. Rep. 2010, 12, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.L.; Morris, D.R.; Sherliker, P.; Clack, R.; Lam, K.B.H.; Halliday, A.; Clarke, R.; Lewington, S.; Bulbulia, R. Sex-Specific Associations of Vascular Risk Factors With Abdominal Aortic Aneurysm: Findings From 1.5 Million Women and 0.8 Million Men in the United States and United Kingdom. J. Am. Hear. Assoc. 2020, 9, e014748. [Google Scholar] [CrossRef]
- Isselbacher, E.M.; Preventza, O.; Black, J.H.; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circ. 2022, 146, E334–E482. [Google Scholar] [CrossRef]
- Wanhainen, A.; Verzini, F.; Van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor′s Choice – European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef]
- Talvitie, M.; Stenman, M.; Roy, J.; Leander, K.; Hultgren, R. Sex Differences in Rupture Risk and Mortality in Untreated Patients With Intact Abdominal Aortic Aneurysms. J. Am. Hear. Assoc. 2021, 10, e019592. [Google Scholar] [CrossRef]
- Ramkumar, N.; Suckow, B.D.; Arya, S.; Sedrakyan, A.; Mackenzie, T.A.; Goodney, P.P.; Brown, J.R. Association of Sex With Repair Type and Long-term Mortality in Adults With Abdominal Aortic Aneurysm. JAMA Netw. Open 2020, 3, e1921240. [Google Scholar] [CrossRef]
- Olinic, D.M.; Spinu, M.; Olinic, M.; Homorodean, C.; Tataru, D.A.; Liew, A.; Schernthaner, G.H.; Stanek, A.; Fowkes, G.; Catalano, M. Epidemiology of peripheral artery disease in Europe: VAS Educational Paper. Int. Angiol. 2018, 37, 327–334. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Pawlas, N.; Cieślar, G.; Stanek, A. Chronic Lower Extremity Ischemia and Its Association with the Frailty Syndrome in Patients with Diabetes. Int. J. Environ. Res. Public Heal. 2020, 17, 9339. [Google Scholar] [CrossRef]
- Achim, A.; Stanek, A.; Homorodean, C.; Spinu, M.; Onea, H.L.; Lazăr, L.; Marc, M.; Ruzsa, Z.; Olinic, D.M. Approaches to Peripheral Artery Disease in Diabetes: Are There Any Differences? Int. J. Environ. Res. Public Heal. 2022, 19, 9801. [Google Scholar] [CrossRef] [PubMed]
- Sigvant, B.; Wiberg-Hedman, K.; Bergqvist, D.; Rolandsson, O.; Wahlberg, E. Risk factor profiles and use of cardiovascular drug prevention in women and men with peripheral arterial disease. Eur. J. Prev. Cardiol. 2009, 16, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Jelani, Q.-U.; Petrov, M.; Martinez, S.C.; Holmvang, L.; Al-Shaibi, K.; Alasnag, M. Peripheral Arterial Disease in Women: an Overview of Risk Factor Profile, Clinical Features, and Outcomes. Curr. Atheroscler. Rep. 2018, 20, 1–11. [Google Scholar] [CrossRef]
- Petrie, J.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2017, 34, 575–584. [Google Scholar] [CrossRef]
- Song, P.; Rudan, D.; Zhu, Y.; Fowkes, F.J.I.; Rahimi, K.; Fowkes, F.G.R.; Rudan, I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob. Health 2019, 7, e1020–e1030. [Google Scholar] [CrossRef]
- Kavurma, M.M.; Boccanfuso, L.; Cutmore, C.; Passam, F.; Patel, S.; Hennessy, A.; Loa, J.; A Figtree, G.; Golledge, J.; A Robinson, D.; et al. A hidden problem: peripheral artery disease in women. Eur. Hear. J. - Qual. Care Clin. Outcomes 2023, 9, 342–350. [Google Scholar] [CrossRef]
- Patel, T.; Baydoun, H.; Patel, N.K.; Tripathi, B.; Nanavaty, S.; Savani, S.; Mojadidi, M.K.; Agarwal, N.; Patel, G.; Patel, S.; et al. Peripheral Arterial Disease in Women: The Gender Effect. Cardiovasc. Revascularization Med. 2019, 21, 404–408. [Google Scholar] [CrossRef]
- Sigvant, B.; Wiberg-Hedman, K.; Bergqvist, D.; Rolandsson, O.; Andersson, B.; Persson, E.; Wahlberg, E. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J. Vasc. Surg. 2007, 45, 1185–1191. [Google Scholar] [CrossRef]
- E McCoach, C.; Armstrong, E.J.; Singh, S.; Javed, U.; Anderson, D.; Yeo, K.K.; Westin, G.G.; Hedayati, N.; A Amsterdam, E.; Laird, J.R. Gender-related variation in the clinical presentation and outcomes of critical limb ischemia. Vasc. Med. 2013, 18, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Porras, C.P.; Bots, M.L.; Teraa, M.; van Doorn, S.; Vernooij, R.W. Differences in Symptom Presentation in Women and Men with Confirmed Lower Limb Peripheral Artery Disease: A Systematic Review and Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2022, 63, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.W. Sex differences in claudication pain in subjects with peripheral arterial disease. Med. Sci. Sports Exerc. 2002, 34, 1695–1698. [Google Scholar] [CrossRef]
- Pabon, M.; Cheng, S.; Altin, S.E.; Sethi, S.S.; Nelson, M.D.; Moreau, K.L.; Hamburg, N.; Hess, C.N. Sex Differences in Peripheral Artery Disease. Circ. Res. 2022, 130, 496–511. [Google Scholar] [CrossRef]
- Patel, T.; Baydoun, H.; Patel, N.K.; Tripathi, B.; Nanavaty, S.; Savani, S.; Mojadidi, M.K.; Agarwal, N.; Patel, G.; Patel, S.; et al. Peripheral Arterial Disease in Women: The Gender Effect. Cardiovasc. Revascularization Med. 2019, 21, 404–408. [Google Scholar] [CrossRef]
- Barochiner, J.; Aparicio, L.; Waisman, G. Challenges associated with peripheral arterial disease in women. Vasc. Heal. Risk Manag. 2014, 10, 115–128. [Google Scholar] [CrossRef]
- Bush, R.L.; Kallen, M.A.; Liles, D.R.; Bates, J.T.; Petersen, L.A. Knowledge and Awareness of Peripheral Vascular Disease Are Poor Among Women at Risk for Cardiovascular Disease. J. Surg. Res. 2008, 145, 313–319. [Google Scholar] [CrossRef]
- Lanéelle, D.; Sauvet, G.; Guillaumat, J.; Trihan, J.E.; Mahé, G. Gender Differences in the Medical Treatment of Peripheral Artery Disease. J. Clin. Med. 2021, 10, 2855. [Google Scholar] [CrossRef] [PubMed]
- Pâquet, M.; Pilon, D.; Tétrault, J.-P.; Carrier, N. Protective Vascular Treatment of Patients with Peripheral Arterial Disease: Guideline Adherence According to Year, Age and Gender. Can. J. Public Heal. 2010, 101, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Canonico, M.E.; Hsia, J.; Hess, C.N.; Bonaca, M.P. Sex differences in guideline-directed medical therapy in 2021–22 among patients with peripheral artery disease. Vasc. Med. 2023, 28, 233–235. [Google Scholar] [CrossRef]
- McGinigle, K.L.; Browder, S.E.; Strassle, P.D.; Shalhub, S.; Harris, L.M.; Minc, S.D. Sex-related disparities in intervention rates and type of intervention in patients with aortic and peripheral arterial diseases in the National Inpatient Sample Database. J. Vasc. Surg. 2020, 73, 2081–2089.e7. [Google Scholar] [CrossRef]
- Altin, S.E.; Gitto, M.; Secemsky, E.A.; Rao, S.V.; Hess, C.N. Sex-Based Differences in Periprocedural Complications Following Lower Extremity Peripheral Vascular Intervention. Circ. Cardiovasc. Interv. 2022, 15, e011768. [Google Scholar] [CrossRef]
- Hassan, A.; Abugroun, A.; Daoud, H.; Mahmoud, S.; Awadalla, S.; Volgman, A.; Alonso, A. Impact of Gender Differences on Outcomes of Peripheral Artery Disease Intervention (from a Nationwide Sample). Am. J. Cardiol. 2020, 141, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Parvar, S.L.; Thiyagarajah, A.; Nerlekar, N.; King, P.; Nicholls, S.J. A systematic review and meta-analysis of gender differences in long-term mortality and cardiovascular events in peripheral artery disease. J. Vasc. Surg. 2020, 73, 1456–1465. [Google Scholar] [CrossRef]
- Behrendt, C.-A.; Sigvant, B.; Kuchenbecker, J.; Grima, M.J.; Schermerhorn, M.; Thomson, I.A.; Altreuther, M.; Setacci, C.; Svetlikov, A.; Laxdal, E.H.; et al. Editor′s Choice – International Variations and Sex Disparities in the Treatment of Peripheral Arterial Occlusive Disease: A Report from VASCUNET and the International Consortium of Vascular Registries. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 873–880. [Google Scholar] [CrossRef]
- Ramkumar, N.; Suckow, B.D.; Behrendt, C.; Mackenzie, T.A.; Sedrakyan, A.; Brown, J.R.; Goodney, P.P. Association between sex and long-term outcomes of endovascular treatment for peripheral artery disease. Catheter. Cardiovasc. Interv. 2023, 101, 877–887. [Google Scholar] [CrossRef]
- UNIREUMA Reumatologia III Edizione, Reumatologia. Per studenti e medici di medicina generale. Idelson-Gnocchi; 3° edizione (1 gennaio 2018). 2018.
- Joseph, G.; Goel, R.; Thomson, V.S.; Joseph, E.; Danda, D. Takayasu Arteritis. J. Am. Coll. Cardiol. 2023, 81, 172–186. [Google Scholar] [CrossRef]
- Hellmich, B.; Agueda, A.; Monti, S.; Buttgereit, F.; de Boysson, H.; Brouwer, E.; Cassie, R.; Cid, M.C.; Dasgupta, B.; Dejaco, C.; et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann. Rheum. Dis. 2019, 79, 19–30. [Google Scholar] [CrossRef]
- Maz, M.; Chung, S.A.; Abril, A.; Langford, C.A.; Gorelik, M.; Guyatt, G.; Archer, A.M.; Conn, D.L.; Full, K.A.; Grayson, P.C.; et al. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Giant Cell Arteritis and Takayasu Arteritis. Arthritis Rheumatol. 2021, 73, 1349–1365. [Google Scholar] [CrossRef]
- Flammer, J.; Pache, M.; Resink, T. Vasospasm, its Role in the Pathogenesis of Diseases with Particular Reference to the Eye. Prog. Retin. Eye Res. 2001, 20, 319–349. [Google Scholar] [CrossRef]
- Heidrich, H. Functional vascular diseases: Raynaud’s syndrome, acrocyanosis and erythromelalgia. Vasa 2010, 39, 33–41. [Google Scholar] [CrossRef]
- Callam, M.J. Epidemiology of varicose veins. Br. J. Surg. 1994, 81, 167–173. [Google Scholar] [CrossRef]
- Eberhardt, R.T.; Raffetto, J.D. Chronic Venous Insufficiency. Circ. 2014, 130, 333–346. [Google Scholar] [CrossRef]
- Raju, S.; Neglén, P. Chronic Venous Insufficiency and Varicose Veins. New Engl. J. Med. 2009, 360, 2319–2327. [Google Scholar] [CrossRef]
- Youn, Y.J.; Lee, J. Chronic venous insufficiency and varicose veins of the lower extremities. Korean J. Intern. Med. 2019, 34, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.G.; Evans, C.J.; Lee, A.J. Prevalence and Risk Factors of Chronic Venous Insufficiency. Angiology 2001, 52 (Suppl. 1), S5–S15. [Google Scholar] [CrossRef] [PubMed]
- De Maeseneer, M.G.; Kakkos, S.K.; Aherne, T.; Baekgaard, N.; Black, S.; Blomgren, L.; Giannoukas, A.; Gohel, M.; de Graaf, R.; Hamel-Desnos, C.; et al. Editor′s Choice – European Society for Vascular Surgery (ESVS) 2022 Clinical Practice Guidelines on the Management of Chronic Venous Disease of the Lower Limbs. Eur. J. Vasc. Endovasc. Surg. 2022, 63, 184–267. [Google Scholar] [CrossRef] [PubMed]
- Gloviczki, P.; Comerota, A.J.; Dalsing, M.C.; Eklof, B.G.; Gillespie, D.L.; Gloviczki, M.L.; Lohr, J.M.; McLafferty, R.B.; Meissner, M.H.; Murad, M.H.; et al. Society for Vascular Surgery; American Venous Forum. The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J. Vasc. Surg. 2011, 53, 2S–48S. [Google Scholar] [CrossRef] [PubMed]
- Pomp, E.R.; Lenselink, A.M.; Rosendaal, F.R.; Doggen, C.J.M. Pregnancy, the postpartum period and prothrombotic defects: risk of venous thrombosis in the MEGA study. J. Thromb. Haemost. 2008, 6, 632–637. [Google Scholar] [CrossRef]
- Maughan, B.C.M.; Marin, M.B.; Han, J.B.; Gibbins, K.J.M.; Brixey, A.G.; Caughey, A.B.; Kline, J.A.; Jarman, A.F. Venous Thromboembolism During Pregnancy and the Postpartum Period: Risk Factors, Diagnostic Testing, and Treatment. Obstet. Gynecol. Surv. 2022, 77, 433–444. [Google Scholar] [CrossRef]
- Westerlund, E.; Henriksson, P.; Wallén, H.; Hovatta, O.; Wallberg, K.R.; Antovic, A. Detection of a procoagulable state during controlled ovarian hyperstimulation for in vitro fertilization with global assays of haemostasis. Thromb. Res. 2012, 130, 649–653. [Google Scholar] [CrossRef]
- Rosendaal, F.R.; Vlieg, A.V.H.; Tanis, B.C.; Helmerhorst, F.M. Estrogens, progestogens and thrombosis. J. Thromb. Haemost. 2003, 1, 1371–1380. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.-P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Abdulrehman, J.; Elbaz, C.; Aziz, D.; Parpia, S.; Fazelzad, R.; Eischer, L.; Rodger, M.A.; Cannegieter, S.C.; Cate-Hoek, A.T.; Nagler, M.; et al. Recurrence after stopping anticoagulants in women with combined oral contraceptive-associated venous thromboembolism: A systematic review and meta-analysis. Br. J. Haematol. 2022, 199, 130–142. [Google Scholar] [CrossRef]
- Tosetto, A.; Iorio, A.; Marcucci, M.; Baglin, T.; Cushman, M.; Eichinger, S.; Palareti, G.; Poli, D.; Tait, R.C.; Douketis, J. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J. Thromb. Haemost. 2012, 10, 1019–1025. [Google Scholar] [CrossRef]
- Bates, S.M.; Rajasekhar, A.; Middeldorp, S.; McLintock, C.; Rodger, M.A.; James, A.H.; Vazquez, S.R.; Greer, I.A.; Riva, J.J.; Bhatt, M.; et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. 2018, 2, 3317–3359. [Google Scholar] [CrossRef]
- Cooper, G.; Bagnall, A. Prevalence of lymphoedema in the UK: focus on the southwest and West Midlands. Br. J. Community Nurs. 2016, 21, S6–S14. [Google Scholar] [CrossRef]
- Colmant, C.; Turpin, S.; Lambert, R.; Wong, N.; Ondrejchak, S.; Lapointe, C.; Powell, J.; Dubois, J.; McCuaig, C. Pediatric Lymphedema: Study of 180 Patients Referred to a Tertiary Lymphedema Clinic. J. Cutan. Med. Surg. 2022, 26, 502–511. [Google Scholar] [CrossRef]
- Keeley, V.; Franks, P.; Quéré, I.; Mercier, G.; Michelini, S.; Cestari, M.; Borman, P.; Hughes, A.; Clark, K.; Lisle, J.; et al. LIMPRINT in Specialist Lymphedema Services in United Kingdom, France, Italy, and Turkey. Lymphat. Res. Biol. 2019, 17, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, C.; Morfoisse, F.; Tatin, F.; Zamora, A.; Zahreddine, R.; Henrion, D.; Arnal, J.-F.; Lenfant, F.; Garmy-Susini, B. The Impact of Estrogen Receptor in Arterial and Lymphatic Vascular Diseases. Int. J. Mol. Sci. 2020, 21, 3244. [Google Scholar] [CrossRef] [PubMed]
- Morfoisse, F.; Zamora, A.; Marchaud, E.; Nougue, M.; Diallo, L.H.; David, F.; Roussel, E.; Lacazette, E.; Prats, A.-C.; Tatin, F.; et al. Sex Hormones in Lymphedema. Cancers 2021, 13, 530. [Google Scholar] [CrossRef] [PubMed]
- Brouillard, P.; Witte, M.H.; Erickson, R.P.; Damstra, R.J.; Becker, C.; Quéré, I.; Vikkula, M. Primary lymphoedema. Nat. Rev. Dis. Prim. 2021, 7, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Cormier, J.N.; Askew, R.L.; Mungovan, K.S.; Xing, Y.; Ross, M.I.; Armer, J.M. Lymphedema beyond breast cancer: a systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer 2010, 116, 5138–5149. [Google Scholar] [CrossRef]
- Rockson, S.G.; Keeley, V.; Kilbreath, S.; Szuba, A.; Towers, A. Cancer-associated secondary lymphoedema. Nat. Rev. Dis. Prim. 2019, 5, 22. [Google Scholar] [CrossRef]
- Hoyle, E.; Kilbreath, S.; Dylke, E. Body image and sexuality concerns in women with breast cancer-related lymphedema: a cross-sectional study. Support. Care Cancer 2022, 30, 3917–3924. [Google Scholar] [CrossRef]
- Falck, J.; Rolander, B.; Nygårdh, A.; Jonasson, L.-L.; Mårtensson, J. Women with lipoedema: a national survey on their health, health-related quality of life, and sense of coherence. BMC Women′s Heal. 2022, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Boyages, J.; Kastanias, K.; Koelmeyer, L.A.; Winch, C.J.; Lam, T.C.; Sherman, K.A.; Munnoch, D.A.; Brorson, H.; Ngo, Q.D.; Heydon-White, A.; et al. Liposuction for Advanced Lymphedema: A Multidisciplinary Approach for Complete Reduction of Arm and Leg Swelling. Ann. Surg. Oncol. 2015, 22, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-C.T.; Xu, Y.; Cormier, J.N.; Giordano, S.; Ridner, S.H.; Buchholz, T.A.; Perkins, G.H.; Elting, L.S. Incidence, Treatment Costs, and Complications of Lymphedema After Breast Cancer Among Women of Working Age: A 2-Year Follow-Up Study. J. Clin. Oncol. 2009, 27, 2007–2014. [Google Scholar] [CrossRef] [PubMed]
- Ridner, S.H. The Psycho-Social Impact of Lymphedema. Lymphat. Res. Biol. 2009, 7, 109–112. [Google Scholar] [CrossRef]
- Zhang, X.; McLaughlin, E.M.; Krok-Schoen, J.L.; Naughton, M.; Bernardo, B.M.; Cheville, A.; Allison, M.; Stefanick, M.; Bea, J.W.; Paskett, E.D. Association of Lower Extremity Lymphedema With Physical Functioning and Activities of Daily Living Among Older Survivors of Colorectal, Endometrial, and Ovarian Cancer. JAMA Netw. Open 2022, 5, e221671. [Google Scholar] [CrossRef]
- Paskett, E.D.; Stark, N.; Stark, N. Lymphedema: Knowledge, Treatment, and Impact Among Breast Cancer Survivors. Breast J. 2000, 6, 373–378. [Google Scholar] [CrossRef]
- Carter, B.J. Women′s experiences of lymphedema. Oncol Nurs Forum. 1997, 24, 875–882. [Google Scholar] [PubMed]
- Vignes, S.; Poizeau, F.; Dupuy, A. Cellulitis risk factors for patients with primary or secondary lymphedema. J. Vasc. Surgery: Venous Lymphat. Disord. 2021, 10, 179–185.e1. [Google Scholar] [CrossRef]
- Dupuy, A.; Benchikhi, H.; Roujeau, J.-C.; Bernard, P.; Vaillant, L.; Chosidow, O.; Sassolas, B.; Guillaume, J.-C.; Grob, J.-J.; Bastuji-Garin, S. Risk factors for erysipelas of the leg (cellulitis): case-control study. BMJ 1999, 318, 1591–1594. [Google Scholar] [CrossRef]
- Webb, E.; Neeman, T.; Bowden, F.J.; Gaida, J.; Mumford, V.; Bissett, B. Compression Therapy to Prevent Recurrent Cellulitis of the Leg. New Engl. J. Med. 2020, 383, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.J.; Mufti, A.; Sachdeva, M.; Lytvyn, Y.; Zabihi-Pour, D.; Zaaroura, H.; Yeung, J. Stewart-Treves syndrome and other cutaneous malignancies in the context of chronic lymphedema: a systematic review. Int. J. Dermatol. 2021, 61, 62–70. [Google Scholar] [CrossRef]
- Galea, M.; Brincat, M.R.; Calleja-Agius, J. A review of the pathophysiology and evidence-based management of varicoceles and pelvic congestion syndrome. Hum. Fertil. 2023, 26, 1597–1608. [Google Scholar] [CrossRef]
- Phillips, D.; Deipolyi, A.R.; Hesketh, R.L.; Midia, M.; Oklu, R. Pelvic Congestion Syndrome: Etiology of Pain, Diagnosis, and Clinical Management. J. Vasc. Interv. Radiol. 2014, 25, 725–733. [Google Scholar] [CrossRef]
- Meissner, M.H.; Khilnani, N.M.; Labropoulos, N.; Gasparis, A.P.; Gibson, K.; Greiner, M.; Learman, L.A.; Atashroo, D.; Lurie, F.; Passman, M.A.; et al. The Symptoms-Varices-Pathophysiology classification of pelvic venous disorders: A report of the American Vein & Lymphatic Society International Working Group on Pelvic Venous Disorders. Phlebol. J. Venous Dis. 2021, 36, 342–360. [Google Scholar] [CrossRef]
- Kaufman, C.; Little, N.A. Pelvic Congestion Syndrome: A Missed Opportunity. Indian J. Radiol. Imaging 2021, 31, 539–544. [Google Scholar] [CrossRef]
- Rezaei-Kalantari, K.; Fahrni, G.; Rotzinger, D.C.; Qanadli, S.D. Insights into pelvic venous disorders. Front. Cardiovasc. Med. 2023, 10, 1102063. [Google Scholar] [CrossRef]
- Gavrilov, S.G.; Vassilieva, G.Y.; Vasilev, I.M.; Grishenkova, A.S. The role of vasoactive neuropeptides in the genesis of venous pelvic pain: A review. Phlebol. J. Venous Dis. 2019, 35, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.C. Vascular congestion and hyperemia; their effect on structure and function in the female reproductive system. Am. J. Obstet. Gynecol. 1949, 57, 211–230. [Google Scholar] [CrossRef]
- Bendek, B.; Afuape, N.; Banks, E.; Desai, N.A. Comprehensive review of pelvic congestion syndrome: causes, symptoms, treatment options. Curr. Opin. Obstet. Gynecol. 2020, 32, 237–242. [Google Scholar] [CrossRef]
- Rizer, M.; Alexander, R.; Sharpe, E.; Rochon, P.J.; Brown, C.L. Pelvic Congestion Syndrome: Systematic Review of Treatment Success. Semin. Interv. Radiol. 2018, 35, 035–040. [Google Scholar] [CrossRef]
- Lazarashvili, Z.; Antignani, P.L.; Leal Monedero, J. Pelvic congestion syndrome: Prevalence and quality of life. Phlebolymphology. 2016, 23, 123–126. [Google Scholar]
- Thorne, C.; Stuckey, B. CASE REPORT: Pelvic Congestion Syndrome Presenting as Persistent Genital Arousal: A Case Report. J. Sex. Med. 2008, 5, 504–508. [Google Scholar] [CrossRef]
- Inal, M.; Bilgili, M.Y.K.; Sahin, S. Nutcracker Syndrome Accompanying Pelvic Congestion Syndrome: Color Doppler Sonography and Multislice CT Findings: A Case Report. Iran. J. Radiol. 2014, 11, e11075. [Google Scholar] [CrossRef]
- Sharma, A.; Eapen, C.E. Pelvic congestion needs attention in infertile women with Budd-Chiari syndrome. Indian J. Gastroenterol. 2023, 42, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, L.; Han, X. The Effect of a Subsequent Pregnancy After Ovarian Vein Embolization in Patients with Infertility Caused by Pelvic Congestion Syndrome. Acad. Radiol. 2019, 26, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Liddle, A.D.; Davies, A.H. Pelvic congestion syndrome: chronic pelvic pain caused by ovarian and internal iliac varices. Phlebol. J. Venous Dis. 2007, 22, 100–104. [Google Scholar] [CrossRef]
- Potla, N.; Veluri, S.-C.; Stead, T.S.; Dubey, J.; Ganti, L. Pelvic Congestion Syndrome in a Postmenopausal Female. Cureus 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Bartl, T.; Wolf, F.; Dadak, C. Pelvic congestion syndrome (PCS) as a pathology of postmenopausal women: a case report with literature review. BMC Women′s Heal. 2021, 21, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Persu, A.; Giavarini, A.; Touzé, E.; Januszewicz, A.; Sapoval, M.; Azizi, M.; Barral, X.; Jeunemaitre, X.; Morganti, A.; Plouin, P.-F.; et al. European consensus on the diagnosis and management of fibromuscular dysplasia. J. Hypertens. 2014, 32, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Gornik, H.L.; Persu, A.; Adlam, D.; Aparicio, L.S.; Azizi, M.; Boulanger, M.; Bruno, R.M.; de Leeuw, P.; Fendrikova-Mahlay, N.; Froehlich, J.; et al. First International Consensus on the diagnosis and management of fibromuscular dysplasia. Vasc. Med. 2019, 24, 164–189. [Google Scholar] [CrossRef]
- Persu, A.; Dobrowolski, P.; Gornik, H.L.; Olin, J.W.; Adlam, D.; Azizi, M.; Boutouyrie, P.; Bruno, R.M.; Boulanger, M.; Demoulin, J.-B.; et al. Current progress in clinical, molecular, and genetic aspects of adult fibromuscular dysplasia. Cardiovasc. Res. 2021, 118, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.S.; Lau, J.F.; Godbold, J.; Gustavson, S.; Olin, J.W. The S curve: A novel morphological finding in the internal carotid artery in patients with fibromuscular dysplasia. Vasc. Med. 2014, 19, 356–362. [Google Scholar] [CrossRef]
- Kim, E.S.; Olin, J.W.; Froehlich, J.B.; Gu, X.; Bacharach, J.M.; Gray, B.H.; Jaff, M.R.; Katzen, B.T.; Kline-Rogers, E.; Mace, P.D.; et al. Clinical manifestations of fibromuscular dysplasia vary by patient sex: a report of the United States registry for fibromuscular dysplasia. J. Am. Coll. Cardiol. 2013, 62, 2026–2028. [Google Scholar] [CrossRef]
- Wells, B.J.; Modi, R.D.; Gu, X.; Bumpus, S.M.; Swan, K.; Froehlich, J.B.; Gray, B.H.; Southerland, A.M.; Kim, E.S.; Mahlay, N.F.; et al. Clinical associations of headaches among patients with fibromuscular dysplasia: A Report from the US Registry for Fibromuscular Dysplasia. Vasc. Med. 2020, 25, 348–350. [Google Scholar] [CrossRef]
- Pappaccogli, M.; Prejbisz, A.; Ciurică, S.; Bruno, R.M.; Aniszczuk-Hybiak, A.; Bracalente, I.; De Backer, T.; Debiève, F.; Delmotte, P.; Di Monaco, S.; et al. Pregnancy-Related Complications in Patients With Fibromuscular Dysplasia: A Report From the European/International Fibromuscular Dysplasia Registry. Hypertension. Hypertension 2020, 76, 545–553. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Leutner, M.; Harreiter, J. Sex differences in type 2 diabetes. Diabetologia 2023, 66, 986–1002. [Google Scholar] [CrossRef]
- Maric-Bilkan, C. Sex differences in micro- and macro-vascular complications of diabetes mellitus. Clin. Sci. 2017, 131, 833–846. [Google Scholar] [CrossRef]
- Scherer, P.E.; Hill, J.A. Obesity, Diabetes, and Cardiovascular Diseases. Circ. Res. 2016, 118, 1703–1705. [Google Scholar] [CrossRef]
- Chase-Vilchez, A.Z.; Chan, I.H.Y.; Peters, S.A.E.; Woodward, M. Diabetes as a risk factor for incident peripheral arterial disease in women compared to men: a systematic review and meta-analysis. Cardiovasc. Diabetol. 2020, 19, 1–11. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef]
- Available online: https://cordis.europa.eu/article/id/27270-exclusion-from-clinical-trials-harming-womens-health/pl (accessed on 5 August 2023).
- Kendel, F.; Sieverding, M. The Impact of Gender and Age on Cardiovascular Health in Germany. In Gender, Health and Ageing; Backes, G.M., Lasch, V., Reimann, K., Eds.; VS Verlag für Sozialwissenschaften, 2006. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).