Submitted:
17 January 2024
Posted:
18 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Antimicrobial resistance (AMR)
2.1. Contribution of Urinary tract infection (UTI) To AMR
2.2. AMR and Pregnancy
3. The Urinary Bladder
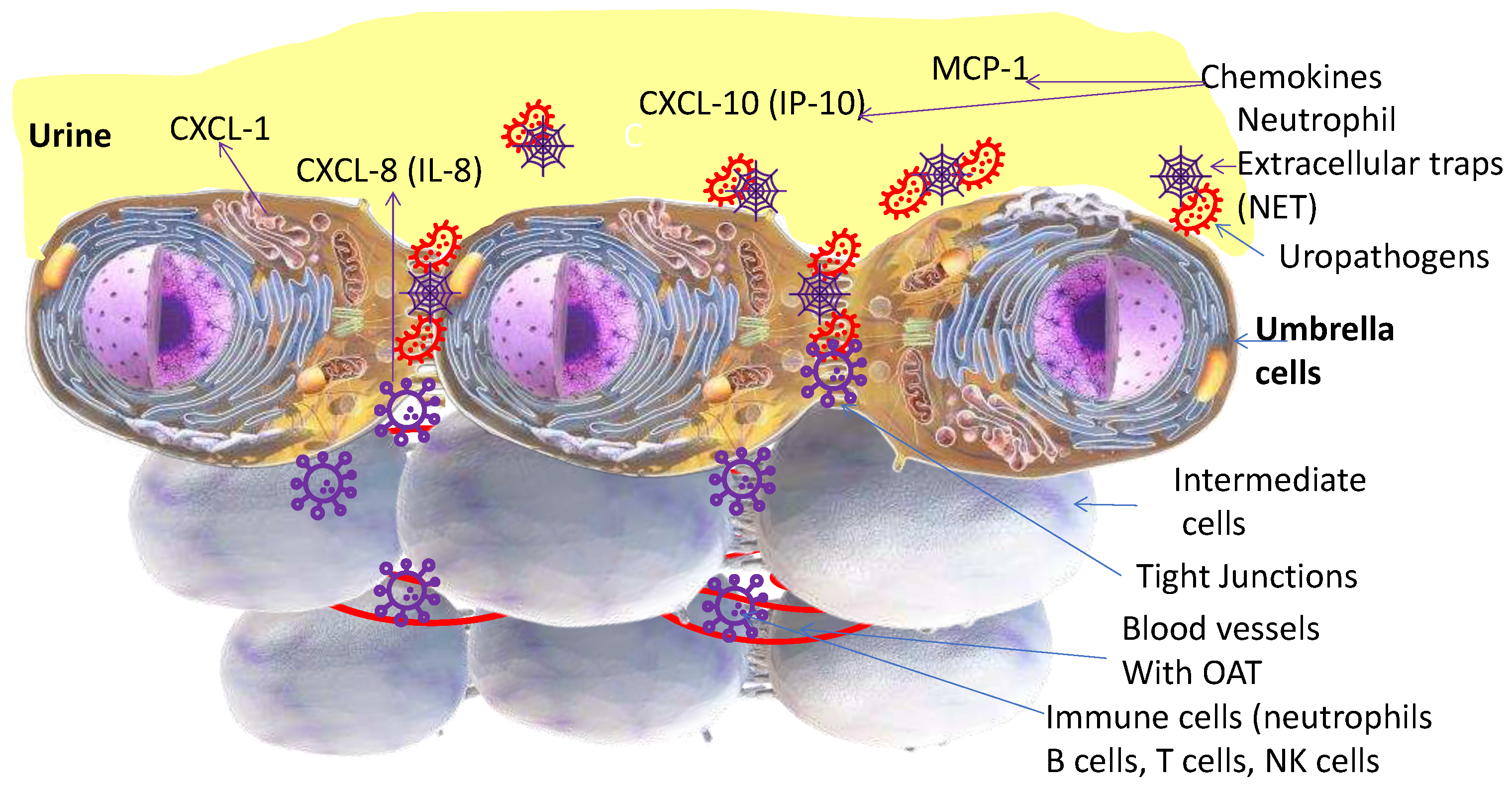
3.1. Neutrophils
4. Cystitis Etiology and Treatment
4.1. SWOT of Oral Antimicrobial Therapy (OAT) for UTI
- A.
- The variability in initial drug concentration due to variable absorption from gut and variability in urine in-flow rate of 0.3-15mL/min (~50x difference) [33]
- B.
- C.

4.1.1. Delay and variability in urinary MIC

4.1.2. Why urine levels are higher than plasma levels of OAT?
5. Root cause analysis of OAT contribution to AMR
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nguyen, A.N.T.; Gorrell, R.; Kwok, T.; Connallon, T.; McDonald, M.J. Horizontal gene transfer facilitates the molecular reverse-evolution of antibiotic sensitivity in experimental populations of H. pylori. Nat Ecol Evol 2024. [Google Scholar] [CrossRef]
- Wong, C. Antibiotic resistance is a growing threat - is climate change making it worse? Nature 2024. [Google Scholar] [CrossRef]
- Gonze, D.; Coyte, K.Z.; Lahti, L.; Faust, K. Microbial communities as dynamical systems. Curr Opin Microbiol 2018, 44, 41–49. [Google Scholar] [CrossRef]
- Wright, E.S.; Gupta, R.; Vetsigian, K.H. Multi-stable bacterial communities exhibit extreme sensitivity to initial conditions. FEMS Microbiol Ecol 2021, 97. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.S.; Vetsigian, K.H. Stochastic exits from dormancy give rise to heavy-tailed distributions of descendants in bacterial populations. Mol Ecol 2019, 28, 3915–3928. [Google Scholar] [CrossRef]
- Zhang, K.; Potter, R.F.; Marino, J.; Muenks, C.E.; Lammers, M.G.; Dien Bard, J.; Dingle, T.C.; Humphries, R.; Westblade, L.F.; Burnham, C.A.; et al. Comparative genomics reveals the correlations of stress response genes and bacteriophages in developing antibiotic resistance of Staphylococcus saprophyticus. mSystems 2023, 8, e0069723. [Google Scholar] [CrossRef]
- Cahill, D.J.; Fry, C.H.; Foxall, P.J. Variation in urine composition in the human urinary tract: evidence of urothelial function in situ? J Urol 2003, 169, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Pribis, J.P.; Dooling, S.W.; Garcia-Villada, L.; Minnick, P.J.; Xia, J.; Liu, J.; Mei, Q.; Fitzgerald, D.M.; Herman, C.; et al. Drugging evolution of antibiotic resistance at a regulatory network hub. Sci Adv 2023, 9, eadg0188. [Google Scholar] [CrossRef] [PubMed]
- Groah, S.L.; Rounds, A.K.; Perez-Losada, M. Intravesical Lactobacillus rhamnosus GG Alters Urobiome Composition and Diversity Among People With Neurogenic Lower Urinary Tract Dysfunction. Top Spinal Cord Inj Rehabil 2023, 29, 44–57. [Google Scholar] [CrossRef]
- Hull, R.; Rudy, D.; Donovan, W.; Svanborg, C.; Wieser, I.; Stewart, C.; Darouiche, R. Urinary tract infection prophylaxis using Escherichia coli 83972 in spinal cord injured patients. J Urol 2000, 163, 872–877. [Google Scholar] [CrossRef]
- Zhang, H.L.; Perez, R.; Krishnan, J.; Lautenbach, E.; Anderson, D.J. Risk Factors for Recurrence of Community-Onset Urinary Tract Infections Caused by Extended-Spectrum Cephalosporin-Resistant Enterobacterales. Open Forum Infect Dis 2023, 10, ofad561. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, Z.; Zheng, L.; Gong, Z.; Li, Y.; Jin, Y.; Huang, Y.; Chi, M. Urinary Tract Infections Caused by Uropathogenic Escherichia coli: Mechanisms of Infection and Treatment Options. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Mengistu, D.A.; Alemu, A.; Abdukadir, A.A.; Mohammed Husen, A.; Ahmed, F.; Mohammed, B. Incidence of Urinary Tract Infection Among Patients: Systematic Review and Meta-Analysis. Inquiry 2023, 60, 469580231168746. [Google Scholar] [CrossRef] [PubMed]
- Faine, B.A.; Rech, M.A.; Vakkalanka, P.; Gross, A.; Brown, C.; Harding, S.J.; Slocum, G.; Zimmerman, D.; Zepeski, A.; Rewitzer, S.; et al. High prevalence of fluoroquinolone-resistant UTI among US emergency department patients diagnosed with urinary tract infection, 2018-2020. Acad Emerg Med 2022, 29, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Rafat, D.; Agrawal, A.; Khalid, S.; Khan, A.U.; Nawab, T.; Sultan, A. Bacterial abundance and antimicrobial resistance patterns of uropathogens among pregnant women with asymptomatic bacteriuria: Association with glycemic status. Eur J Obstet Gynecol Reprod Biol X 2024, 21, 100263. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.; Zeegers, M.P.; Varghese, G.M.; Burza, S. India's National Action Plan on Antimicrobial Resistance: a critical perspective. J Glob Antimicrob Resist 2021, 27, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Von Vietinghoff, S.; Shevchuk, O.; Dobrindt, U.; Engel, D.R.; Jorch, S.K.; Kurts, C.; Miethke, T.; Wagenlehner, F. The global burden of antimicrobial resistance - urinary tract infections. Nephrol Dial Transplant 2023. [Google Scholar] [CrossRef]
- Plough, H.H. Penicillin resistance of Staphylococcus aureus and its clinical implications. Am J Clin Pathol 1945, 15, 446–451. [Google Scholar] [CrossRef]
- Milano, A.; Sulejmani, A.; Intra, J.; Sala, M.R.; Leoni, V.; Carcione, D. Antimicrobial Resistance Trends of Escherichia coli Isolates from Outpatient and Inpatient Urinary Infections over a 20-Year Period. Microb Drug Resist 2022, 28, 63–72. [Google Scholar] [CrossRef]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 2011, 52, e103–120. [Google Scholar] [CrossRef]
- Lin, K.; Zahlanie, Y.; Ortwine, J.K.; Mang, N.S.; Wei, W.; Brown, L.S.; Prokesch, B.C. Decreased Outpatient Fluoroquinolone Prescribing Using a Multimodal Antimicrobial Stewardship Initiative. Open Forum Infect Dis 2020, 7, ofaa182. [Google Scholar] [CrossRef]
- Yu, J.Y.; McKenna, V.A.; Dumyati, G.K.; Lubowski, T.J.; Carreno, J.J. Antibiotic Prescribing in New York State Medicare Part B Beneficiaries Diagnosed With Cystitis Between 2016 and 2017. Open Forum Infect Dis 2020, 7, ofz544. [Google Scholar] [CrossRef]
- Green, S.I.; Clark, J.R.; Santos, H.H.; Weesner, K.E.; Salazar, K.C.; Aslam, S.; Campbell, J.W.; Doernberg, S.B.; Blodget, E.; Morris, M.I.; et al. A Retrospective, Observational Study of 12 Cases of Expanded-Access Customized Phage Therapy: Production, Characteristics, and Clinical Outcomes. Clin Infect Dis 2023, 77, 1079–1091. [Google Scholar] [CrossRef]
- Vazquez-Montes, M.; Fanshawe, T.R.; Stoesser, N.; Walker, A.S.; Butler, C.; Hayward, G. Epidemiology and microbiology of recurrent UTI in women in the community in Oxfordshire, UK. JAC Antimicrob Resist 2024, 6, dlad156. [Google Scholar] [CrossRef]
- Albert, X.; Huertas, I.; Pereiro, II; Sanfelix, J. ; Gosalbes, V.; Perrota, C. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev 2004, 2004, CD001209. [Google Scholar] [CrossRef]
- Gould, A.P.; Winders, H.R.; Stover, K.R.; Bookstaver, P.B.; Griffin, B.; Bland, C.M.; Eiland, L.S.; Murray, M. Less common bacterial, fungal and viral infections: review of management in the pregnant patient. Drugs Context 2021, 10. [Google Scholar] [CrossRef]
- Mohapatra, S.; Venugopal, S.J.; Kalaivani, M.; Kant, S.; Tak, V.; Panigrahy, R.; Chunchanur, S.K.; Kocher, D.; Behera, B.; Pundir, S.; et al. Antibiotic resistance of uropathogens among the community-dwelling pregnant and nonpregnant female: a step towards antibiotic stewardship. BMC Infect Dis 2022, 22, 939. [Google Scholar] [CrossRef] [PubMed]
- Shafik, A.; Ahmed, I.; El Sibai, O.; Shafik, A.A. Does the composition of voided urine reflect that of the renal pelvis? Urol Res 2006, 34, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Baumgarten, R.; van de Pol, M.H.; Deen, P.M.; van Os, C.H.; Wetzels, J.F. Dissociation between urine osmolality and urinary excretion of aquaporin-2 in healthy volunteers. Nephrol Dial Transplant 2000, 15, 1155–1161. [Google Scholar] [CrossRef]
- Ashdown, H.H. Absorption from the Mucous Membrane of the Urinary Bladder. J Anat Physiol 1887, 21, 299–324 291. [Google Scholar] [PubMed]
- Torimoto, K.; Matsushita, C.; Itami, Y.; Iwamoto, T.; Owari, T.; Gotoh, D.; Miyake, M.; Hori, S.; Nakai, Y.; Aoki, K.; et al. Assessment of bladder function for stabilizing urinary volume overnight with recording of brain waves (ABSORB study). Low Urin Tract Symptoms 2022, 14, 72–77. [Google Scholar] [CrossRef]
- Hilson, A.J.; Lewis, C.A.; Harland, S.J. The permeability of the human bladder to water assessed using tritiated water. Contrib Nephrol 1990, 79, 41–44. [Google Scholar] [CrossRef]
- Watanabe, H.; Azuma, Y. Periodical measurement of urine volume in the bladder during sleep: Temporary volume reduction suggestive of absorption. Int J Urol 2016, 23, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Vodovotz, Y.; Yoshimura, N.; Chermansky, C.J.; Fitzgerald, J.; Tyagi, P. Temporally complex inflammatory networks in an animal model reveal signatures for interstitial cystitis and bladder pain syndrome phenotype. Neurourol Urodyn 2023, 42, 1839–1848. [Google Scholar] [CrossRef]
- Tyagi, P.; Maranchie, J.; Dhir, R.; Moon, C.H.; Biatta, S.; Balasubramani, G.K.; Yoshimura, N.; Fitzgerald, J.; Chermansky, C.; Kaufman, J.; et al. Unraveling the Complexity of bladder-centric chronic pain by intravesical contrast enhanced MRI. Continence (Amst) 2023. [Google Scholar] [CrossRef]
- Singh, N.; Zabbarova, I.; Ikeda, Y.; Kanai, A.; Chermansky, C.; Yoshimura, N.; Tyagi, P. Role of hyperpolarization-activated cyclic nucleotide-gated channels in aging bladder phenotype. Life Sci 2022, 289, 120203. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Tyagi, V.; Qu, X.; Chuang, Y.C.; Kuo, H.C.; Chancellor, M. Elevated CXC chemokines in urine noninvasively discriminate OAB from UTI. Am J Physiol Renal Physiol 2016, 311, F548–554. [Google Scholar] [CrossRef] [PubMed]
- Sonn, G.A.; Jones, S.N.; Tarin, T.V.; Du, C.B.; Mach, K.E.; Jensen, K.C.; Liao, J.C. Optical biopsy of human bladder neoplasia with in vivo confocal laser endomicroscopy. J Urol 2009, 182, 1299–1305. [Google Scholar] [CrossRef]
- Gupta, D.K.; Lewis, C.E.; Varady, K.A.; Su, Y.R.; Madhur, M.S.; Lackland, D.T.; Reis, J.P.; Wang, T.J.; Lloyd-Jones, D.M.; Allen, N.B. Effect of Dietary Sodium on Blood Pressure: A Crossover Trial. JAMA 2023, 330, 2258–2266. [Google Scholar] [CrossRef]
- Russell, S.K.; Harrison, J.K.; Olson, B.S.; Lee, H.J.; O'Brien, V.P.; Xing, X.; Livny, J.; Yu, L.; Roberson, E.D.O.; Bomjan, R.; et al. Uropathogenic Escherichia coli infection-induced epithelial trained immunity impacts urinary tract disease outcome. Nat Microbiol 2023, 8, 875–888. [Google Scholar] [CrossRef]
- Eldrup, J.; Thorup, J.; Nielsen, S.L.; Hald, T.; Hainau, B. Permeability and ultrastructure of human bladder epithelium. Br J Urol 1983, 55, 488–492. [Google Scholar] [CrossRef]
- Staehelin, L.A.; Chlapowski, F.J.; Bonneville, M.A. Lumenal plasma membrane of the urinary bladder. I. Three-dimensional reconstruction from freeze-etch images. J Cell Biol 1972, 53, 73–91. [Google Scholar] [CrossRef]
- Volter, D.; Schmidt, B. The reabsorption of creatinine from the rabbit bladder. Urol Res 1975, 3, 183–186. [Google Scholar] [CrossRef]
- Islam, M.M.; Takeyama, N. Role of Neutrophil Extracellular Traps in Health and Disease Pathophysiology: Recent Insights and Advances. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Krivosikova, K.; Supcikova, N.; Gaal Kovalcikova, A.; Janko, J.; Pastorek, M.; Celec, P.; Podracka, L.; Tothova, L. Neutrophil extracellular traps in urinary tract infection. Front Pediatr 2023, 11, 1154139. [Google Scholar] [CrossRef]
- Schmidt, E.P.; Overdier, K.H.; Sun, X.; Lin, L.; Liu, X.; Yang, Y.; Ammons, L.A.; Hiller, T.D.; Suflita, M.A.; Yu, Y.; et al. Urinary Glycosaminoglycans Predict Outcomes in Septic Shock and Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2016, 194, 439–449. [Google Scholar] [CrossRef]
- Mambatta, A.K.; Jayarajan, J.; Rashme, V.L.; Harini, S.; Menon, S.; Kuppusamy, J. Reliability of dipstick assay in predicting urinary tract infection. J Family Med Prim Care 2015, 4, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Bogovic Crncic, T.; Girotto, N.; Ilic Tomas, M.; Kristofic, I.; Klobucar, S.; Baticic, L.; Curko-Cofek, B.; Sotosek, V. Innate Immunity in Autoimmune Thyroid Disease during Pregnancy. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Lurie, S.; Rahamim, E.; Piper, I.; Golan, A.; Sadan, O. Total and differential leukocyte counts percentiles in normal pregnancy. Eur J Obstet Gynecol Reprod Biol 2008, 136, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Feng, C.; Zhang, X.; Lu, J.; Zhao, Y. The Diverse Biological Functions of Neutrophils, Beyond the Defense Against Infections. Inflammation 2017, 40, 311–323. [Google Scholar] [CrossRef]
- Tyagi, P.; Motley, S.S.; Koyama, T.; Kashyap, M.; Gingrich, J.; Yoshimura, N.; Fowke, J.H. Molecular correlates in urine for the obesity and prostatic inflammation of BPH/LUTS patients. Prostate 2018, 78, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Durkin, M.J.; Schmitz, V.; Hsueh, K.; Troubh, Z.; Politi, M.C. Older adults' and caregivers' perceptions about urinary tract infection and asymptomatic bacteriuria guidelines: a qualitative exploration. Antimicrob Steward Healthc Epidemiol 2023, 3, e224. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Hitchens, T.K.; Foley, L.M.; Singh, N.; Mizoguchi, S.; Kurobe, M.; Gotoh, D.; Ogawa, T.; Minagawa, T.; Ishizuka, O.; et al. Functional and histologic imaging of urinary bladder wall after exposure to psychological stress and protamine sulfate. Sci Rep 2021, 11, 19440. [Google Scholar] [CrossRef] [PubMed]
- Veranic, P.; Jezernik, K. Succession of events in desquamation of superficial urothelial cells as a response to stress induced by prolonged constant illumination. Tissue Cell 2001, 33, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Tyagi, V.; Yoshimura, N.; Witteemer, E.; Barclay, D.; Loughran, P.A.; Zamora, R.; Vodovotz, Y. Gender-based reciprocal expression of transforming growth factor-beta1 and the inducible nitric oxide synthase in a rat model of cyclophosphamide-induced cystitis. J Inflamm (Lond) 2009, 6, 23. [Google Scholar] [CrossRef]
- Tyagi, P.; Moon, C.H.; Connell, M.; Ganguly, A.; Cho, K.J.; Tarin, T.; Dhir, R.; Sholosh, B.; Maranchie, J. Intravesical Contrast-Enhanced MRI: A Potential Tool for Bladder Cancer Surveillance and Staging. Curr Oncol 2023, 30, 4632–4647. [Google Scholar] [CrossRef]
- Asemota, A.O.; Schneider, E.B.; Mowry, E.M.; Venkatesan, A. Common comorbid and secondary conditions leading to hospitalization in multiple sclerosis patients in the United States. Clin Neurol Neurosurg 2023, 232, 107851. [Google Scholar] [CrossRef]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 2013, 110, 3507–3512. [Google Scholar] [CrossRef]
- Wang, C.; Bauckman, K.A.; Ross, A.S.B.; Symington, J.W.; Ligon, M.M.; Scholtes, G.; Kumar, A.; Chang, H.W.; Twentyman, J.; Fashemi, B.E.; et al. A non-canonical autophagy-dependent role of the ATG16L1(T300A) variant in urothelial vesicular trafficking and uropathogenic Escherichia coli persistence. Autophagy 2019, 15, 527–542. [Google Scholar] [CrossRef]
- Staerk, K.; Gronnemose, R.B.; Palarasah, Y.; Lund, L.; Andersen, T.E. Intracellular uropathogenic Escherichia coli are undetectable in urinary bladders after oral mecillinam treatment: An experimental study in a pig model of cystitis. Microb Pathog 2022, 173, 105817. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Moon, C.; Singh, N.; Connell, M.; Maranchie, J.; Chermansky, C.; Yoshimura, N.; Kaufman, J. High Resolution 3D T1-Mapping of Pig Bladder Wall by Intravesical Contrast Enhanced MRI At 3T. J Urol 2021, 206, e390. [Google Scholar] [CrossRef]
- Tartaglione, T.A.; Johnson, C.R.; Brust, P.; Opheim, K.; Hooton, T.M.; Stamm, W.E. Pharmacodynamic evaluation of ofloxacin and trimethoprim-sulfamethoxazole in vaginal fluid of women treated for acute cystitis. Antimicrob Agents Chemother 1988, 32, 1640–1643. [Google Scholar] [CrossRef] [PubMed]
- Mores, C.R.; Price, T.K.; Wolff, B.; Halverson, T.; Limeira, R.; Brubaker, L.; Mueller, E.R.; Putonti, C.; Wolfe, A.J. Genomic relatedness and clinical significance of Streptococcus mitis strains isolated from the urogenital tract of sexual partners. Microb Genom 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.T.; Han, D.E.; Lee, D.H.; Kim, J.W.; Park, H.S.; Moon, D.G.; Oh, M.M. Single-dose amikacin plus 7 days of amoxicillin/clavulanate to treat acute cystitis caused by extended-spectrum beta-lactamase-producing Escherichia coli: A retrospective cohort study. Investig Clin Urol 2021, 62, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, S.; Joo, H.S.; Duong, A.C.; Bach, T.H.; Tan, V.Y.; Chatterjee, S.S.; Cheung, G.Y.; Otto, M. How Staphylococcus aureus biofilms develop their characteristic structure. Proc Natl Acad Sci U S A 2012, 109, 1281–1286. [Google Scholar] [CrossRef]
- Avery, O.T.; Macleod, C.M.; McCarty, M. Studies on the Chemical Nature of the Substance Inducing Transformation of Pneumococcal Types : Induction of Transformation by a Desoxyribonucleic Acid Fraction Isolated from Pneumococcus Type Iii. J Exp Med 1944, 79, 137–158. [Google Scholar] [CrossRef] [PubMed]
- Duployez, C.; Loiez, C.; Cattoen, C.; Descamps, D.; Wallet, F.; Vachee, A.; Microbiologist Network from, N.-P.-d.-C. In vitro susceptibility to mecillinam of Escherichia coli strains isolated from the urine of pregnant women. Med Mal Infect 2016, 46, 436–441. [Google Scholar] [CrossRef]
- Huttner, A.; Wijma, R.A.; Stewardson, A.J.; Olearo, F.; Von Dach, E.; Harbarth, S.; Bruggemann, R.J.M.; Mouton, J.W.; Muller, A.E. The pharmacokinetics of nitrofurantoin in healthy female volunteers: a randomized crossover study. J Antimicrob Chemother 2019, 74, 1656–1661. [Google Scholar] [CrossRef]
- Hirai, T.; Shiraishi, C.; Nakai, S.; Ushiro, M.; Hanada, K.; Iwamoto, T. Population kinetic-pharmacodynamic analysis of serum potassium in patients receiving sulfamethoxazole/trimethoprim. Basic Clin Pharmacol Toxicol 2022, 131, 380–391. [Google Scholar] [CrossRef]
- Sadahiro, S.; Ishida, H.; Suzuki, T.; Ishikawa, K.; Tajima, T.; Makuuchi, H. Vesicular blood flow after ligation of the internal iliac arteries in low anterior resection or abdominoperineal resection. Dis Colon Rectum 1999, 42, 1475–1479. [Google Scholar] [CrossRef]
- Parmelee, D.J.; Walovitch, R.C.; Ouellet, H.S.; Lauffer, R.B. Preclinical evaluation of the pharmacokinetics, biodistribution, and elimination of MS-325, a blood pool agent for magnetic resonance imaging. Invest Radiol 1997, 32, 741–747. [Google Scholar] [CrossRef]
- Zhang, H. Trisodium-[(2-(R)-[(4,4-diphenylcyclohexyl)phosphono-oxymethyl]-diethylenetriaminepentaacetato)(aquo)gadolinium(III): Gadofosveset. In Molecular Imaging and Contrast Agent Database (MICAD); Bethesda (MD), 2004.
- Miodonski, A.J.; Litwin, J.A. Microvascular architecture of the human urinary bladder wall: a corrosion casting study. Anat Rec 1999, 254, 375–381. [Google Scholar] [CrossRef]
- Smith, S.G.; Griffith, B.E.; Zaharoff, D.A. Analyzing the effects of instillation volume on intravesical delivery using biphasic solute transport in a deformable geometry. Math Med Biol 2019, 36, 139–156. [Google Scholar] [CrossRef]
- Lee, P.J.; Kuo, H.C. High incidence of lower urinary tract dysfunction in women with recurrent urinary tract infections. Low Urin Tract Symptoms 2020, 12, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Ganguly, A.; Chermansky, C.; Tarin, T.V.; Yoshimura, N.; Maranchie, J. Does large volume of distribution of lidocaine masks its systemic uptake from bladder? Am J Clin Exp Urol 2023, 11, 121–135. [Google Scholar]
- Sumi, C.D.; Heffernan, A.J.; Lipman, J.; Roberts, J.A.; Sime, F.B. What Antibiotic Exposures Are Required to Suppress the Emergence of Resistance for Gram-Negative Bacteria? A Systematic Review. Clin Pharmacokinet 2019, 58, 1407–1443. [Google Scholar] [CrossRef]
- Mouhssine, M.; Al Ani, D.; Al Shibli, A.; Ghatasheh, G.; Al Amri, A.; Matta, H.; Chedid, R.; Narchi, H. Intravesical gentamicin instillation in the prevention of recurrent urinary tract infections in children with neurogenic bladder- a single-center retrospective observational study. J Pediatr Urol 2023, 19, 64 e61-64 e67. [Google Scholar] [CrossRef] [PubMed]
- Zoqlam, R.; Lazauskaite, S.; Glickman, S.; Zaitseva, L.; Ilie, P.C.; Qi, S. Emerging molecular mechanisms and genetic targets for developing novel therapeutic strategies for treating bladder diseases. Eur J Pharm Sci 2022, 173, 106167. [Google Scholar] [CrossRef]
- Alpers, D.H.; Tomkins, G.M. The Order of Induction and Deinduction of the Enzymes of the Lactose Operon in E. Coli. Proc Natl Acad Sci U S A 1965, 53, 797–802. [Google Scholar] [CrossRef]
- Alou, L.; Aguilar, L.; Sevillano, D.; Gimenez, M.J.; Cafini, F.; Valero, E.; Relano, M.T.; Prieto, J. Urine bactericidal activity against resistant Escherichia coli in an in vitro pharmacodynamic model simulating urine concentrations obtained after 2000/125 mg sustained-release co-amoxiclav and 400 mg norfloxacin administration. J Antimicrob Chemother 2006, 57, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, L.; Unadkat, J.D.; Mao, Q. Effect of pregnancy on nitrofurantoin disposition in mice. J Pharm Sci 2009, 98, 4306–4315. [Google Scholar] [CrossRef]
- Ma, Q.; Gao, Y.; Lu, J.; Liu, X.; Wang, R.; Shi, Y.; Liu, J.; Su, H. The effect of regular aerobic exercise on renal function in patients with CKD: A systematic review and meta-analysis. Front Physiol 2022, 13, 901164. [Google Scholar] [CrossRef] [PubMed]
- Ekstrand, C.; Michanek, P.; Salomonsson, M.; Tegner, C.; Pelander, L. Nitrofurantoin plasma- and urine exposure in eight healthy beagle dogs following standard nitrofurantoin dosing regimen. Res Vet Sci 2022, 152, 150–155. [Google Scholar] [CrossRef]
- Parekh, S.; Hayes, C.V.; Loader, J.; Ashiru-Oredope, D.; Hand, K.; Hicks, G.; Lecky, D. The Use of the TARGET Antibiotic Checklist to Support Antimicrobial Stewardship in England's Community Pharmacies. Antibiotics (Basel) 2023, 12. [Google Scholar] [CrossRef]
- NICE antimicrobial stewardship: right drug, dose, and time? Lancet 2015, 386, 717. [CrossRef] [PubMed]
- Morris, C.J.; Rohn, J.L.; Glickman, S.; Mansfield, K.J. Effective Treatments of UTI-Is Intravesical Therapy the Future? Pathogens 2023, 12. [Google Scholar] [CrossRef]
- Andretta, E.; Longo, R.; Balladelli, M.; Sgarabotto, C.; Sgarabotto, D. Intravesical Gentamicin: An Option for Therapy and Prophylaxis against Recurrent UTIs and Resistant Bacteria in Neurogenic Bladder Patients on Intermittent Catheterization. Antibiotics (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Bilsen, M.P.; van Uhm, J.I.M.; Stalenhoef, J.E.; van Nieuwkoop, C.; Groenwold, R.H.H.; Visser, L.G.; Lambregts, M.M.C. Intravesical aminoglycoside instillations as prophylaxis for recurrent urinary tract infection: patient satisfaction, long-term safety and efficacy. JAC Antimicrob Resist 2023, 5, dlad040. [Google Scholar] [CrossRef]
- Rieger, M.M.; Shah, N.M.; Ferrante, K.L.; Tan-Kim, J.; Jacobs, M.B.; Brubaker, L.; Alperin, M. Intraoperative Gentamicin Intravesical Instillation for Prevention of Urinary Tract Infection After Urogynecologic Surgery: A Randomized Controlled Trial. Urogynecology (Phila) 2022, 28, 825–833. [Google Scholar] [CrossRef]
- Rahnama'i, M.S.; Javan Balegh Marand, A.; Roschmann-Doose, K.; Steffens, L.; Arendsen, H.J. The efficacy and safety of intravesical chondroitin sulphate solution in recurrent urinary tract infections. BMC Urol 2022, 22, 188. [Google Scholar] [CrossRef] [PubMed]
- Gugliotta, G.; Calagna, G.; Adile, G.; Polito, S.; Saitta, S.; Speciale, P.; Palomba, S.; Perino, A.; Granese, R.; Adile, B. Is intravesical instillation of hyaluronic acid and chondroitin sulfate useful in preventing recurrent bacterial cystitis? A multicenter case control analysis. Taiwan J Obstet Gynecol 2015, 54, 537–540. [Google Scholar] [CrossRef] [PubMed]
- King, G.K.; Goodes, L.M.; Hartshorn, C.; Thavaseelan, J.; Jonescu, S.; Watts, A.; Rawlins, M.; Woodland, P.; Synnott, E.L.; Barrett, T.; et al. Intravesical hyaluronic acid with chondroitin sulphate to prevent urinary tract infection after spinal cord injury. J Spinal Cord Med 2023, 46, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Bosch, B.; Hartikainen, A.; Ronkainen, A.; Scheperjans, F.; Arkkila, P.; Satokari, R. Development of a Protocol for Anaerobic Preparation and Banking of Fecal Microbiota Transplantation Material: Evaluation of Bacterial Richness in the Cultivated Fraction. Microorganisms 2023, 11. [Google Scholar] [CrossRef]
- Frimodt-Moller, N.; Bjerrum, L. Treating urinary tract infections in the era of antibiotic resistance. Expert Rev Anti Infect Ther 2023, 21, 1301–1308. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




