1. Introduction
In our country, rapidly moving towards a super-aging society, the number of patients with cognitive impairment (CI) is increasing rapidly along with the increase in the number of those with chronic kidney disease (CKD). In recent years, it has become evident that CKD patients have a higher frequency of CI and various studies have been conducted to explore the mechanisms, relationships, and strategies concerning CKD and CI. Although many findings have been presented, numerous problems remain unresolved. On the other hand, it has been shown that cerebral atrophy progresses rapidly in patients with CKD, especially in dialysis patients. Brain atrophy is significantly associated with CI, and atrophy of the frontal lobe and hippocampus is thought to be associated with CI in those patients.
This article provides an overview of CI, brain atrophy, and their relationship, and also outlines the factors associated with CI, mechanisms of CI in CKD, and strategies for addressing CI in CKD.
2. Brain Atrophy in Patients with CKD
2.1. Brain Atrophy in Patients with Non-Dialysis Dependent CKD (ND)
Recently, the relationship between urinary protein and renal function levels and brain volume was examined by MRI in 8,630 general population in Japan, and it was reported that the higher the urinary albumin excretion and the lower the estimated glomerular filtration rate (eGFR), the lower the whole brain volume and the faster the progression of brain atrophy [
1].
2.2. Brain Atrophy in Patients on Hemodialysis (HD)
In dialysis patients, the frequency of brain atrophy is high even at a young age, and it is thought that the mechanism cannot be explained by aging alone. Yoshimitsu et al. [
2] evaluated brain atrophy in 55 HD patients and 35 healthy subjects using ventricular-brain ratio (VBR) quantified from MRI images, and reported that the VBR of HD patients was significantly greater in every 10 years of age in the 30s to 60s age range.
2.3. Brain Atrophy in Patients on peritoneal Dialysis (PD)
In recent years, it has been reported that brain gray matter volume (GMV) and GMV ratio (GMR) decrease with age, but white matter volume (WMV) and WMV ratio (WMR) remain unchanged, as revealed through the analysis of brain MRI using statistical parametric mapping (SPM) [
3]. Subsequently, we conducted an analysis using SPM on brain MRI data from patients with ND, PD, and HD, comparing both brain volume and the rate of change [
4,
5].
2.3.1. Comparison between PD and ND Patients
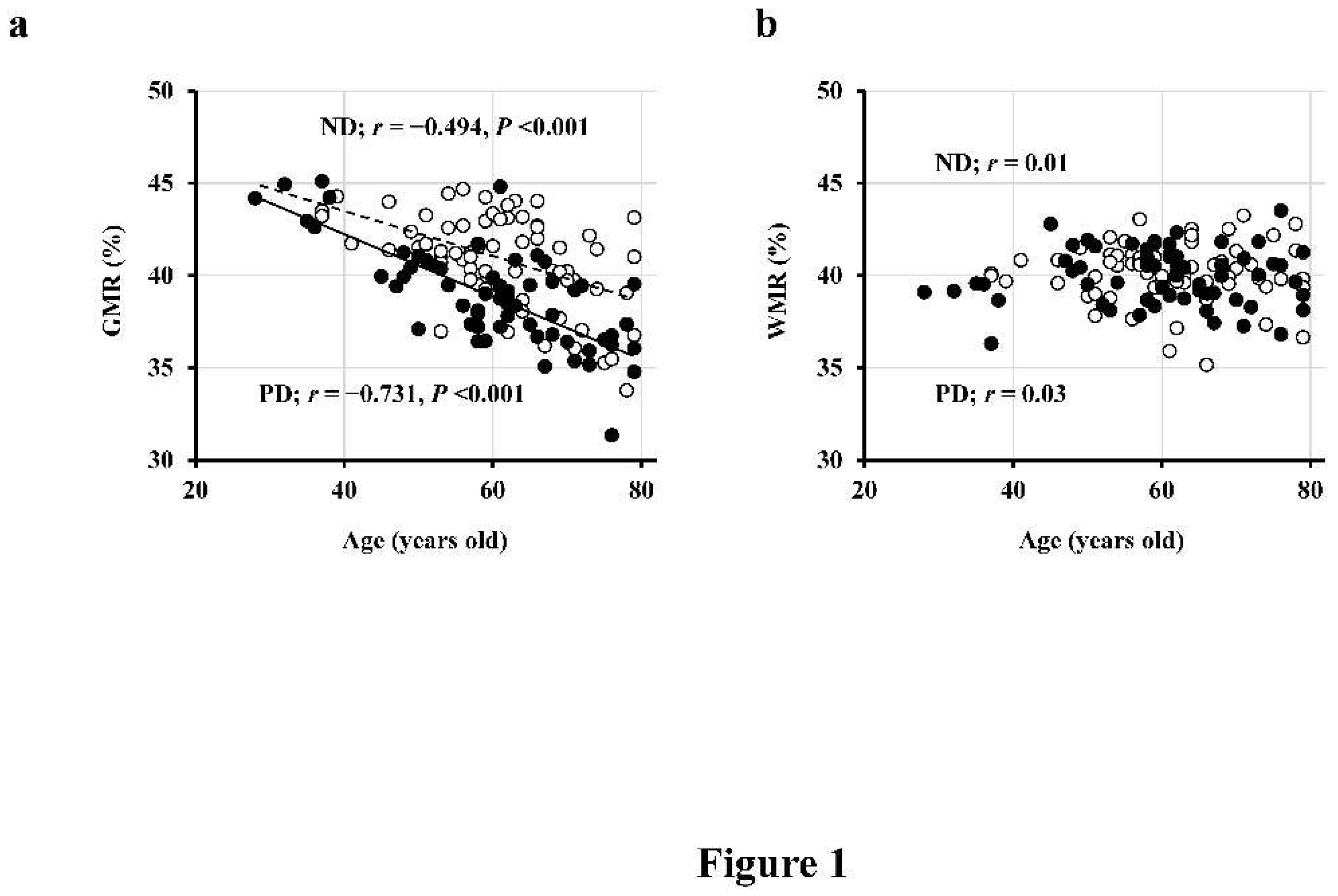
First, in a cross-sectional study involving 69 patients with ND (ages 61 ± 10 years, 37/32 males and females, eGFR 39 ± 12 mL/min/1.73 m
2) and 62 PD patients (ages 60 ± 12 years, 41/21 males and females), we observed a significant negative correlation between GMR and age (
Figure 1a), whereas WMR did not exhibit a correlation with age (
Figure 1b) [
4]. Moreover, the regression line was lower in PD patients compared to ND patients (indicating smaller GMR in PD patients at the same age), and this difference further increased with age (
Figure 1a) [
4].
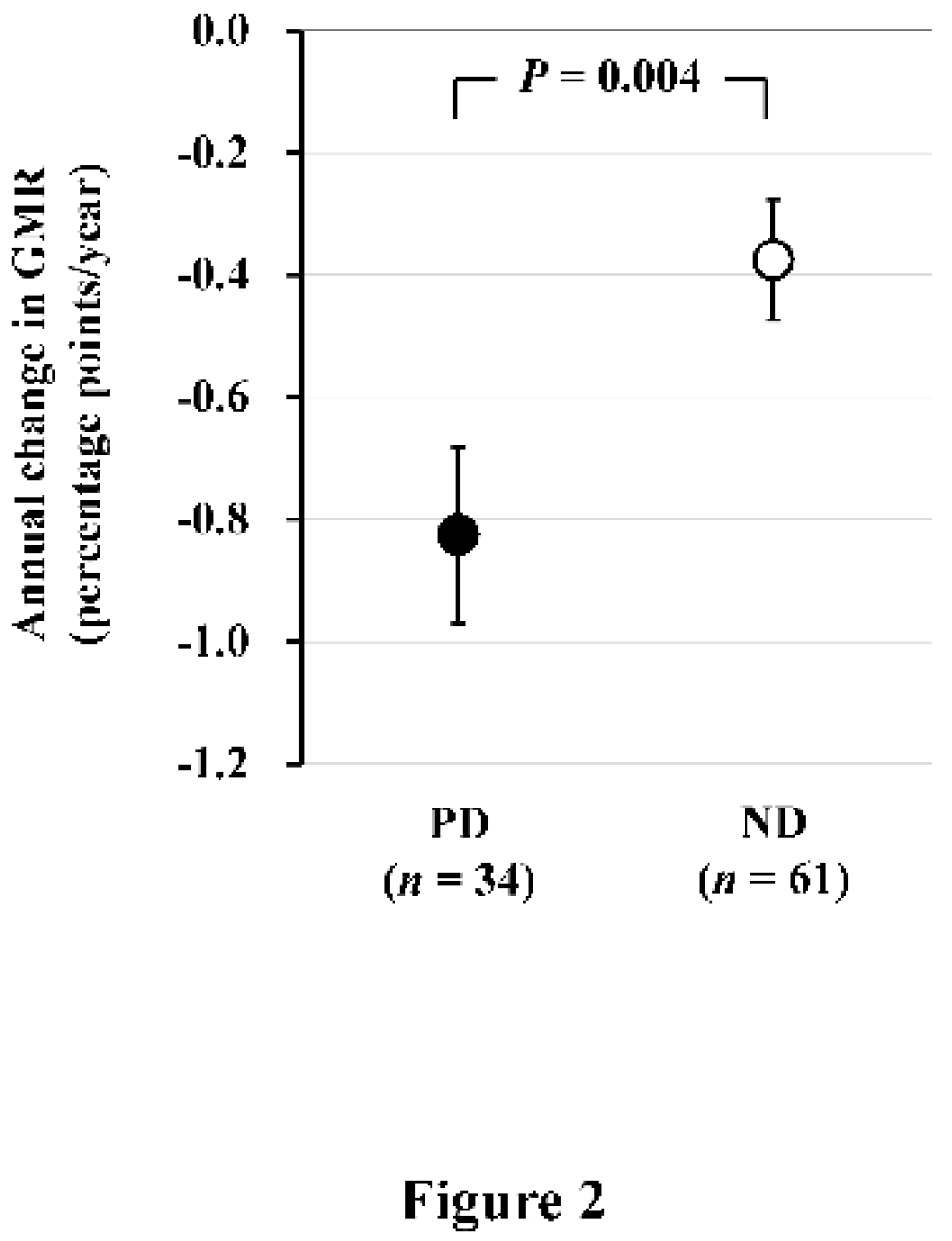
In a subsequent longitudinal study involving 61 ND patients (age 61 ± 10 years, 32/29 males and females, eGFR 39 ± 12 mL/min/1.73 m
2) and 34 PD patients (age 60 ± 11 years, 21/13 males and females) who underwent brain MRI examinations after a 2-year interval, we found that the annual change in GMR (AC-GMR) was −0.38 ± 0.10 percentage-points/year in ND patients and −0.83 ± 0.14 percentage-points/year in PD patients. This indicates that brain atrophy progressed more than 2 times faster in PD patients compared to ND patients (
Figure 2) [
4]. According to a report on general healthy subjects, GMR decreases with age at a rate of 0.2–0.3 percentage-points/year, suggesting that brain atrophy progresses 3 times more rapidly in PD patients than in healthy subjects.
2.3.2. Comparison between PD and HD Patients
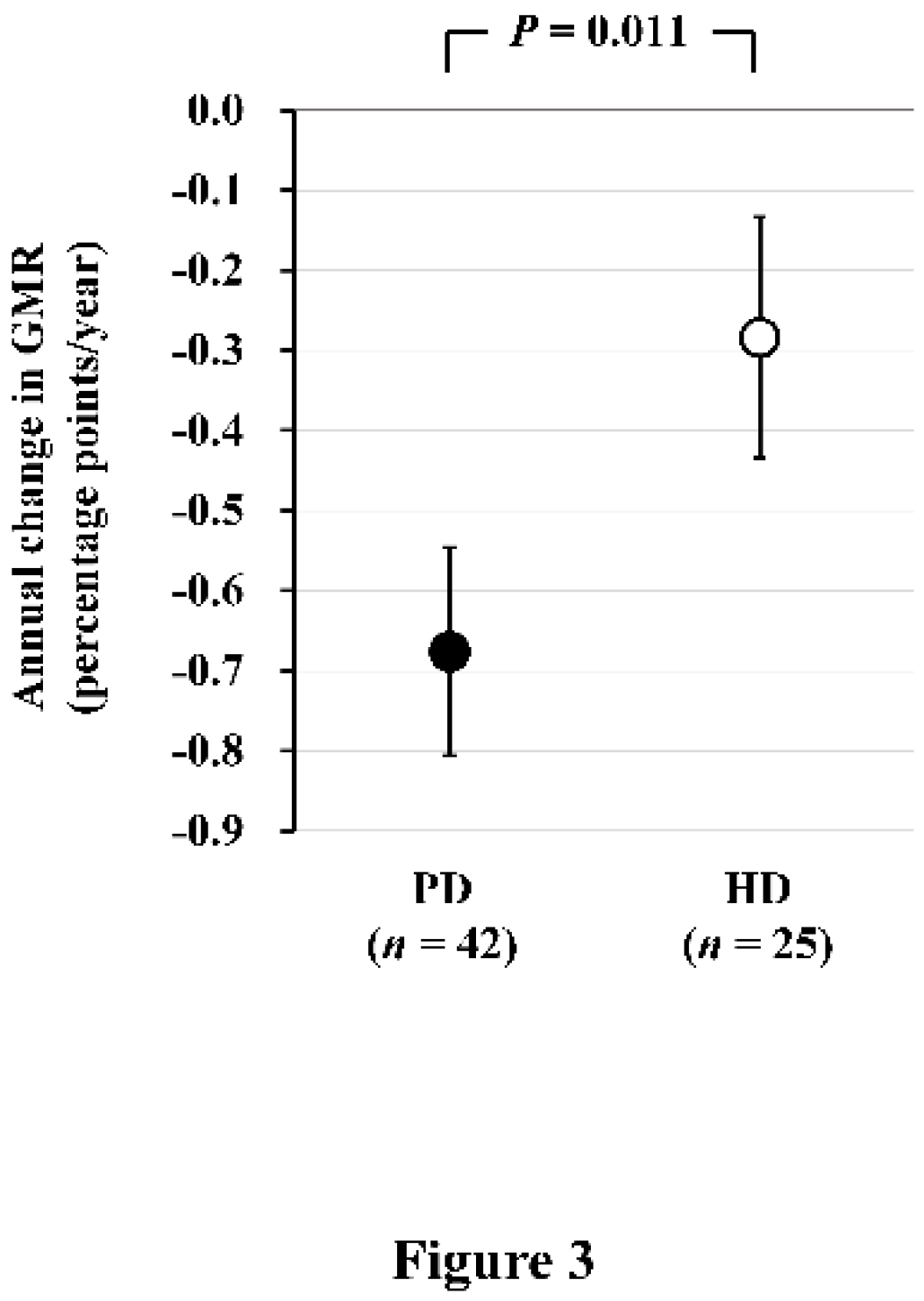
Next, we conducted a comparison of brain atrophy between PD and HD patients. Seventy-three PD and 34 HD patients who underwent brain MRI were recruited for a cross-sectional analysis. Among them, 42 PD and 25 HD patients who underwent a second brain MRI after 2 years were recruited for a longitudinal analysis. In the cross-sectional analysis, GMR was significantly lower in PD patients than HD patients [least square mean (LSM): 39.2% vs. 40.0%,
P = 0.018). AC-GMR was significantly greater in PD patients than HD patients, and this difference remained statistically significant even after adjusting for potential confounding factors (LSM: −0.68 vs. −0.28 percentage-points/year,
P = 0.011) (
Figure 3) [
5]. This study provides evidence of a more accelerated progression of brain atrophy in PD patients compared to HD patients.
3. Relationship between Brain Atrophy and Cognitive Function in CKD Patients
We conducted a trail making test (TMT) in 95 ND patients who underwent MRI and examined the relationship between TMT scores and brain volume. We demonstrated a significant negative correlation between TMT scores and GMR, and this significance persisted even after adjusting for confounding factors such as age, sex, diabetes mellitus, eGFR, educational history, systolic blood pressure, smoking and alcohol consumption, hemoglobin level, history of cardiovascular disease, and amount of urinary protein (
Table 1) [
6].
More interestingly, when the brain was partitioned into four regions (frontal lobe, temporal lobe, parietal lobe, and occipital lobe), and the correlation between GMR and TMT scores in each region was investigated, a significant negative correlation was observed in the frontal and temporal lobes even after adjusting for multivariable variables. No correlation was found in the parietal and occipital lobes. This suggests that the atrophy of the frontal and temporal lobes contributes to the decline in frontal lobe function (executive function), aligning with our initial hypothesis.
4. Factors and Pathophysiology Related to CI in Patients with CKD
Several factors contribute to CI in CKD patients, encompassing classical factors such as age, race (black), diabetes, hypertension, and cardiovascular disease as classical factors, and albuminuria, kidney dysfunction, anemia, oxidative stress, malnutrition and inflammation, and uremic toxins [
7]. The types of dementia, in order of frequency, include Alzheimer's dementia, vascular dementia, and Lewy body dementia. Notably, vascular dementia is more prevalent among patients with CKD [
8].
4.1. Albuminuria and Kidney Dysfunction
We investigated the relationship between CKD and the onset of dementia in the Hisayama Study, revealing significant associations of albuminuria and kidney dysfunction with incident dementia. Albuminuria demonstrated associations with both Alzheimer's dementia and vascular dementia. In contrast, kidney dysfunction, as indicated by an eGFR of <60 mL/min/1.73 m
2 was specifically associated with vascular dementia but not linked to the development of Alzheimer's dementia [
9].
On the other hand, Viggiano et al.'s review article10) demonstrates a correlation between mild CI (MCI) and eGFR, with the frequency of MCI increasing as eGFR decreases. Moreover, when examined by age, the frequency of MCI increases with aging in all patients, including the normal control group. However, while the difference in frequency based on the presence of CKD is extremely significant, the variations in frequency among ND, HD, and PD patients are minimal, with almost no difference observed in those under 40 years old. Beyond the age of 40, the distinctions among these patient groups become more pronounced with aging (HD > PD > ND) [
10].
Recently, in the Suita study involving 6,215 subjects in Japan, it was reported that the odds ratio (95% confidence interval) of MCI for the Mini-Mental State Examination (MMSE) score <26 was significantly higher at 1.49 (1.22–1.83) and 2.35 (1.69–3.26) for in participants with eGFR 45–59.9 mL/min/1.73 m
2 and <45 mL/min/1.73 m
2, respectively, compared to those with eGFR >60 mL/min/1.73 m
2 [
11].
4.2. Sleep Duration and Sleep Quality
Recent reports [
12,
13,
14,
15] indicate an association between sleep duration, sleep quality, and CI in patients with CKD. Among ND patients, CI is linked to sleep apnea syndrome and sleeping longer than 9 hours [
12,
13]. In PD patients, narcolepsy is correlated with lower modified MMSE scores [
14]. Furthermore, an analysis of 2286 cases of United States Renal Data System (USRDS) data revealed an association between sleep disturbances and Kidney Disease Quality of Life Cognitive Function (KDQOL-CF) scores in HD patients [
15].
4.3. Frailty
Recently, the association between Frailty and CI has been documented in ND, HD, PD patients and kidney transplant recipients [
16,
17,
18,
19,
20,
21,
22,
23]. It has been suggested that the mechanisms underlining this relationship involve an elevated concentration of Aβ in cerebrospinal fluid and increased Aβ deposition in the brain, attributed to reduced physical activity. Liang et al. [
24] have also reported that among 69 older adults with normal cognitive function, those who were physically active and met or exceeded the exercise recommendations by the American Heart Association exhibited significantly lower levels of Aβ deposition, as measured with positron emission tomography, and higher levels of Aβ42 in the cerebrospinal fluid compared to inactive individuals who did not meet the recommendation. Alternatively, other animal studies have demonstrated that physical training enhances angiogenesis, synaptogenesis and neurogenesis, particularly in the hippocampus and gyrus dentatus, and initiates the upregulation of numerous neurotrophic factors in the brain [
25,
26], especially in hippocampus [
27,
28].
An elevation of BDNF concentrations is liked to an increase in hippocampal size and an enhancement of spatial memory and learning performance [
29]. Insulin-like growth factor-1 (IGF-1) has been demonstrated to boost BDNF signaling in response to activity stimulation. The neurogenesis induced by exercise in the rat hippocampus is inhibited by injection of a serum that blocks IGF-1 from leaving the bloodstream and entering the cerebrospinal fluid [
30]. IGF-1 also contributes greatly to the exercise-induced effects of BDNF on recall [
31]. Neuronal uptake of IGF-1 is stimulated by exercise, and these neurons then show signs of activity and increase their expression of BDNF [
32].
5. Mechanisms of Cognitive Impairment in CKD
5.1. Atherosclerosis and Cerebrovascular Disease
Cerebrovascular disease in CKD primarily arises from a synergistic interplay between classical and non-classical mechanisms. Classical risk factors and mechanisms encompass hypertension, diabetes, atrial fibrillation, carotid artery disease, heart failure, obesity, and dyslipidemia, all frequently comorbid in CKD. Non-classical risk factors, including chronic inflammation, uremic toxins, reactive oxygen radicals, anemia, and bone mineral disorders, are believed to contribute to the risk of cerebrovascular and CI by inducing vascular injury and endothelial dysfunction.33) Uremia is considered to promote atherosclerosis through protein carbamylation and contribution to dyslipidemia.
Tasmoc et al. [
34] investigated the association between pulse wave velocity (PWV), reflecting arterial stiffness, and cognitive function (TMT, MMSE, etc.) in a study involving 72 HD patients. They reported a significant correlation between elevated PWV values and CI. Additionally, in the COPE study [
35], a prospective multicenter cohort study in the Netherlands involving 85 elderly CKD patients including ND and dialysis patients, the relationship between PWV measured by MRI and cognitive function was examined. The study found significant correlations between PWV and all aspects of memory, executive function, and psychomotor speed. Particularly in executive function, a significant association persisted even after adjusting for age, gender, and education.
On the other hand, in the PACE study of 330 dialysis patient [
36], it was reported that the augmentation index (AI) and central pulse pressure (cPP), which more notably reflect systemic artery stiffness than PWV, were significantly associated with CI compared to PWV. Recently, Nishimura et al. [
37] reported in a study of 100 HD patients (mean age 67.9 years old, mean history of dialysis 7.3 years) that those with an ankle brachial index (ABI) ≥1.06 (
n = 69) had significantly higher Montreal Cognitive Assessment (MoCA) scores (25.5 ± 3.9 vs. 22.3 ± 4.6) compared to patients with an ABI <1.06 (
n = 31). Moreover, ABI and MoCA showed a significant positive correlation in multiple regression analysis.
These studies suggest that arterial stiffness may be associated with CI and could represent the underlying mechanism for incident CI in elderly CKD patients.
5.2. Hypotension and Decrease of Regional Cerebral Blood Flow during HD
In HD patients, there are specific factors related to HD that influence the risk of cerebrovascular accidents, including cerebral hypoperfusion, enhanced arteriosclerosis, and blood pressure fluctuations [
38]. The mean flow velocity of cerebral arteries has been shown to decrease significantly during HD, leading to transient cerebral ischemia and ischemic white matter lesions over time [
39].
One of the factors associated with CI in HD is a rapid decrease in blood pressure during HD. Mizumasa et al. [
40] conducted a three-year longitudinal study examining the association of rapid hypotension during HD with brain ischemia and brain atrophy using brain MRI. The study revealed a positive association between the total number of rapid hypotension episodes during the HD sessions and the number of lacunar infarctions, along with the degree of frontal lobe atrophy over the three-year period.
In addition, the relationship between decreased regional cerebral blood flow and CI has been reported. Kobayashi et al. [
41] examined the cognitive function of rCBF and MMSE measured by SPECT in 54 HD patients, and reported that rCBF in the middle cerebral artery perfusion region was significantly lower than in other areas in patients with reduced MMSE.
It has also been reported that cerebral oxygen saturation (rSO
2) decreases during HD as observed in near-infrared spectroscopy (NIRS) [
42]. Malik et al. [
43] reported that rSO
2 decreased most significantly at 35 min after the initiation of HD. More recently, MacEwen et al. [
44] highlighted that the decrease in rSO
2 was strongly influenced by the reduction in blood pressure during HD. They found that a decrease in mean blood pressure by 10 mmHg during HD increased the risk of cerebral ischemia (rSO
2 decreased by 15% or more) by 3%, and the risk rose rapidly when the mean blood pressure dropped below 60 mmHg.
Recently, there has been extensive research on the relationship between cerebral ischemia and CI in dialysis patients The extent of reduction in mean cerebral blood flow velocity during HD correlates with the degree of cognitive decline and the deterioration of white matter lesions after 12 months. Additionally, the rSO2 of the left frontal lobe, measured by NIRS, is significantly correlated with MoCA score. Furthermore, there has been reported correlation between mixed venous oxygen saturation and MoCA score in the left internal cerebral vein [
45,
46,
47].
5.3. Oxidative Stress
In order to elucidate the mechanism of CI in CKD, we conducted the following experiments using CKD mice created through 5/6 nephrectomy. At 8 weeks post-modeling, we performed the water maze test and evaluated the learning function of CKD mice compared to sham-operated control mice. Subsequently, we conducted pathological and immunohistological examinations using the extracted brains. In the hippocampus of CKD mice, degenerated cells with nuclear condensation (pyknotic cells) appeared along with the accumulation of 8-hydroxy-2'-deoxyguanosine (8-OHdG). The learning ability of CKD mice was significantly decreased in the water maze test. Conversely, in CKD mice administered with an antioxidant (Tempol), the accumulation of 8-OHdG and pyknotic cells in the hippocampus was minimal, and the results of the water maze test were equivalent to those of the control mice (
Figure 4) [
48]. These findings suggest that oxidative stress associated with CKD plays a significant role in neuronal damage in the brain and the decline of learning ability in CI in CKD.
5.4. Insoluble Tau Protein
Recently, Matsuki et al. [
49] demonstrated impaired memory function in CKD mice. The researchers extracted the hippocampus from both CKD and healthy mice, performing a proteomic analysis by partitioning the hippocampus into soluble and insoluble fractions through salting and salting out. The results indicated increased levels of insoluble tau protein and RNA splicing-related proteins in the brains of CKD mice, akin to findings in Alzheimer's disease. The study further revealed elevated levels of insoluble phosphorylated tau protein in the hippocampus and cerebral cortex of CKD mice, along with increased immunoglobulin heavy chains. This suggests that the dysfunction of the blood-brain barrier (BBB) enhances substance permeability. Additionally, a multivariable logistic regression analysis of 980 CKD patients, considering CI as the objective variable, identified elevated blood urea nitrogen and low nutritional status as strong risk factors for dementia. This implies that the accumulation of urea and other uremic substances is secondarily associated with CI rather than the pure kidney filtration function itself.
6. Measures for CI in Patients with CKD
Interventions aimed at reducing the incidence of CI in patients with CKD include management of classical cardiovascular risk factors, using renin-angiotensin system (RAS) inhibitors, maintaining strict blood pressure management, incorporating cognitive training, engaging in regular exercise, and more [
50].
6.1. RAS inhibitors
When the angiotensin II receptor antagonist telmisartan was administered to mice in the CKD model through 5/6 nephrectomy, both the accumulation of 8-OHdG in the brain hippocampus and the decline in learning capacity were suppressed, similar to the effects observed with Tempol administration. [
51] Clinically, a meta-analysis has demonstrated the inhibitory effect of RAS inhibitors on Alzheimer's disease and age-related cognitive decline [
52].
6.2. Strict Antihypertensive Management
It has been suggested that strict antihypertensive management may suppress the onset of dementia. In the SPRINT-MIND Study,53) a randomized controlled trial (RCT) comparing the incidence of CI between the intensive management group, with a target systolic blood pressure of less than 120 mmHg, and the standard control group with a target of less than 140 mmHg, among hypertensive patients aged 50 years and older with no history of diabetes or stroke, the incidence rate of suspected dementia, set as a primary endpoint, was 7.2 and 8.6 cases per 1,000 person-years in the intensive management group and in the standard management group, respectively [hazard ratio (HR) 0.83, 95% confidence interval (CI) 0.67–1.04, P = 0.10]. Although the difference did not reach statistical significance, there was a trend towards a lower incidence in the intensive management group. Furthermore, the study demonstrated that the risks of MCI (HR 0.81, 95% CI 0.69–0.9, P = 0.007) and the composite outcome of MCI and suspected dementia (HR 0.85, 95% CI:0.74–0.97, P = 0.01) as secondary endpoints were significantly lower in the intensive management group.
6.3. Management of Anemia
It has been revealed that treatment of anemia with recombinant human erythropoietin (rHuEPO) improves brain function. In a study by Grimm et al.54), involving 15 chronic HD patients, the event-related potential P300 of brain waves was measured. They reported a significant shortening of the peak latency of P300 following the improvement in anemia with rHuEPO (hematocrit 22.7% → 30.6%), indicating an observed enhancement in higher cognitive functions. Additionally, Singh et al. [
55] reported similar effects in both ND and dialysis patients with CKD.
6.4. Exercise Therapy
In recent years, concerns regarding frailty have emerged among elderly dialysis patients. This condition is characterized by a decline in skeletal muscle mass and strength, the onset of sarcopenia, and a decline in overall physical and cognitive functions associated with aging. This frailty contributes to compromised daily functioning and the manifestation of physical and mental vulnerabilities. Consequently, the importance of exercise therapy in addressing these challenges has gained recognition. Exercise therapy has been reported not only to enhance physical function but also to contribute to the improvement in cognitive function. Manfredini et al. [
56] conducted a study involving 296 dialysis patients, randomly assigning them to a group performing 10 min of walking exercise three times a week at home (exercise group) or a control group without exercise. After six months, they evaluated the 6-minute walking test, sit-to-stand test, and the KDQOL-SF scale to measure the quality of life (QOL) in kidney disease patients. The results indicated a significant extension in walking distance and a noteworthy reduction in sit-to-stand time in the exercise group compared to the control group. Additionally, the cognitive function score in the KDQOL-SF was significantly improved in the exercise group. Furthermore, in a recent study, Otobe et al. [
57] investigated 53 outpatient cases at CKD stages G3–G4. The patients were randomly assigned to an exercise group (
n = 27), where group exercise training occurred once a week at a facility, coupled with self-directed exercise at home at least twice a week for 24 weeks, and a control group (
n = 26). The study aimed to compare changes in cognitive function between the two groups. The results demonstrated that, in comparison to the control group, the exercise group exhibited significant improvements in both delayed and immediate recall scores on the Wechsler Memory Scale-Revised Logical Memory.
In an RCT by McAdams-DeMarco et al. [
58], not only physical exercise during HD using an ergometer but also cognitive training (brain training games using tablets) demonstrated an improvement in cognitive function. The study reported a significant reduction in the TMT time, indicating a positive effect on cognitive enhancement. Recently, Liu et al. [
59] conducted a meta-analysis of these RCTs. The findings indicated that exercise during or between HD sessions significantly improved cognitive function in HD patients. Notably, the effects were particularly significant when the duration was 30 min or more, performed at least three times a week, and continued for a minimum of 16 weeks.
6.5. Interventions Targeting Multiple Factors such as Lifestyle Improvement
Dementia is a multifactorial condition influenced by various factors, and it is considered crucial to simultaneously address interventions targeting multiple factors. In the FINGER study conducted in Finland [
60], individuals at risk of developing dementia, such as those with MCI, were randomly assigned to a lifestyle improvement group receiving interventions in exercise, diet, cognitive training, and vascular risk management, or a control group. The study aimed to investigate changes in cognitive function. As a result, cognitive function significantly improved in the lifestyle improvement group, showing a remarkable 25% enhancement compared to the control group. Moreover, significant improvements were observed in executive function and processing speed, with 83% and 150% improvements in executive function and processing speed, respectively. A trend toward improvement was also noted in memory function. Given the historical difficulty in preventing dementia for individuals at high risk, this study has made a significant impact on dementia researchers. At the Alzheimer's Disease International Conference in 2019, ten risk factors for dementia (ApoE ε4, education, hearing loss, hypertension, obesity, smoking, depression, diabetes, reduced physical activity, social isolation) and nine protective factors (excluding ApoE ε4) were unveiled for prevention [
61].
6.6. Extension of Dialysis Session Time
It has been suggested that extended HD sessions may contribute to an improvement in cognitive function. Ok et al.62) conducted a prospective, case-controlled study involving 247 patients who consented to 8-hour HD sessions three times a week and a control group consisted of 247 patients receiving 4-hour HD sessions three times a week, matched for age, gender, diabetes mellitus, and dialysis history over a 12-month period. The study compared prognosis, cognitive function, QOL, etc., revealing a significant improvement in memory function in the long-hour HD group. Conversely, Chertow et al. [
63] reported in the FHN Trial that a frequent HD six times a week did not result in improved cognitive function.
6.7. Decrease in Dialysate Temperature
Eldehni et al. [
64] reported in an RCT that reducing the dialysate temperature by 0.5°C can inhibit the progression of cerebral white matter lesions over one year, and a meta-analysis has also shown that low-temperature HD suppresses the risk of lowering blood pressure during HD [
65]. Currently, an RCT is being conducted to investigate the effect of suppressing the decline in cognitive function by lowering the dialysate temperature [
66]
6.8. Prevention and Measures against Lowering Blood Pressure and Cerebral Ischemia during and after HD
To prevent a drop in blood pressure during HD, it is crucial to set dry weights appropriately and minimized the rate of fluid removal per hour. Diabetic patients, in particular, exhibit severe systemic arteriosclerosis, along with stenotic and occlusive lesions in the intra- and extracranial main arteries. They are more susceptible to hypotension during HD and orthostatic hypotension after HD. In such cases, fluid and blood pressure management becomes exceptionally important due to impaired autoregulation of the brain, increasing the risk of hemodynamic cerebrovascular accidents.67) Nevertheless, vasopressor administration may be effective in cases where blood pressure decreases during dialysis. Fujisaki et al. [
68] reported that the reduction in cerebral blood flow resulting from orthostatic hypotension after HD can be prevented by administration of vasopressor drugs such as midodrine hydrochloride and droxidopa. Notably, droxidopa exhibits a remarkable effect in retaining cerebral blood flow. The mechanism involves the dilation of cerebral blood vessels by activating adrenergic receptors, particularly β receptors that cross the BBB, in addition to inducing peripheral vasoconstriction. It is speculated that this mechanism contributes to the preservation of cerebral blood flow.
6.9. PD
It has been reported that cognitive function is better preserved in PD compared to HD. According to the statistical survey conducted by the Japanese Society for Dialysis Therapy at the end of 2018, the frequency of dementia was 12.68% in HD patients and 5.62% in PD patients, indicating a lower occurrence of dementia in PD than HD patients. [
69] While this difference is believed to be primarily due to biases in the choice of treatment, observational studies adjusted for patients’ background [
70,
71] and meta-analyses [
72,
73] have reported a lower risk of CI in PD compared to HD. Thus, the possibility that this factor contributes to the observed difference cannot be ruled out.
6.10. KTx
Cognitive function has been reported to improve with KTx; Chhabra et al. [
74] a study measuring P300 in 20 KTx recipients aged 18–50 years before and 3 months after KTx. They demonstrated that P300 latency, which was prolonged before KTx, shortened to a level comparable to healthy subjects after KTx, and reported that cognitive function improved with improvement in anemia. On the other hand, Joshee et al. [
75] compared cognitive function between pre- and post-KTx, KTx and non-KTx patients, and KTx recipients and healthy subjects. They reported an improvement in cognitive function after KTx but noted that it did not reach the level observed in healthy subjects.
6.11. Valerian
As an agonist of the adenosine A1 receptor, valerian inhibits cholinergic transmission, enhances the frequency intensity of delta, theta, and alpha waves in the frontal cortex, and exerts a sedative effect. Samaei et al. [
76] examined the impact of valerian on cognitive function in a double-blind crossover RCT and reported an improvement effect in MMSE scores. However, no changes were observed in brain wave patterns.
6.12. Melatonin
More recently, a study involving 102 HD patients examined the impact of melatonin administration on sleep quality (PSQI) and changes in MoCA were investigated [
77]. The patients were divided into a melatonin group, receiving melatonin, and a control group without melatonin. Significant improvements in both PSQI and MoCA scores were observed in the melatonin group, whereas no significant differences were noted in the control group before and after the study period [
77].
7. Conclusions
Numerous challenges lie ahead in the issues of CKD and CI, including elucidating mechanisms, establishing preventive and therapeutic approaches, creating guidelines for medical practices related to environmental considerations for dementia in dialysis patients, and developing legal frameworks for withholding, withdrawing or discontinuation of dialysis. In the rapidly aging society, the issues of CKD and CI are expected to become increasingly significant. Early preventive efforts are deemed crucial, and a thorough understanding of the interrelationship between CKD and CI is essential to implement appropriate measures. Continuous research efforts are anticipated to accumulate both foundational and clinical evidence, leading to the establishment of feasible and effective preventive and therapeutic strategies in real-world clinical settings.
Author Contributions
All authors contributed to the conceptualization, writing, original draft preparation, review, and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI (Grant Number 18K08213).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Kyushu University (#23-112).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in our cited studies.
Data Availability Statement
Not applicable.
Acknowledgments
The authors appreciate the members of the Observational Study on Cerebro- and Cardiovascular Complication in Non-Dialysis-Dependent, Hemodialysis, and Peritoneal Dialysis Patients with Chronic Kidney Disease (VCOHP Study) investigators for their helpful enrollment of patients to this study, and the staff of the Advanced Preventive Medical Center in Kyushu University Hospital for their kind cooperation.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the study design; collection, analyses, or interpretation of data; writing of the manuscript; or decision to publish the results.
References
- Maki, K.; Ohara, T.; Hata, J.; Shibata, M.; Hirabayashi, N.; Honda, T.; Sakata, S.; Furuta, Y.; Akiyama, M.; Yamasaki, K.; et al.; Japan Prospective Studies Collaboration for Aging and Dementia (JPSC-AD) study group CKD, Brain Atrophy, and White Matter Lesion Volume: The Japan Prospective Studies Collaboration for Aging and Dementia. Kidney Med. 2022, 5, 100593. [Google Scholar] [CrossRef] [PubMed]
- Yoshimitsu, T.; Hirakata, H.; Fujii, K.; Kanai, H.; Hirakata, E.; Higashi, H.; Kubo, M.; Tanaka, H.; Shinozaki, M.; Katafuchi, R.; et al. Cerebral ischemia as a causative mechanism for rapid progression of brain atrophy in chronic hemodialysis patients. Clin. Nephrol. 2000, 53, 445–451. [Google Scholar] [PubMed]
- Taki, Y.; Goto, R.; Evans, A.; Zijdenbos, A.; Neelin, P.; Lerch, J.; Sato, K.; Ono, S.; Kinomura, S.; Nakagawa, M.; et al. Voxel-based morphometry of human brain with age and cerebrovascular risk factors. Neurobiol. Aging 2004, 25, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Tsuruya, K.; Yoshida, H.; Kuroki, Y.; Nagata, M.; Mizumasa, T.; Mitsuiki, K.; Yoshiura, T.; Hirakawa, M.; Kanai, H.; Hori, K.; et al. Brain atrophy in peritoneal dialysis and CKD stages 3-5, a cross-sectional and longitudinal study. Am. J. Kidney Dis. 2015, 65, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Tsuruya, K.; Yoshida, H.; Yamada, S.; Haruyama, N.; Tanaka, S.; Tsuchimoto, A.; Eriguchi, M.; Fujisaki, K.; Torisu, K.; Nakano, T. More rapid progression of brain atrophy in patients on peritoneal dialysis compared with hemodialysis: The VCOHP Study. Hypertens. Res. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Tsuruya, K.; Yoshida, H.; Haruyama, N.; Fujisaki, K.; Hirakata, H.; Kitazono, T. Clinical Significance of Fronto-Temporal Gray Matter Atrophy in Executive Dysfunction in Patients with Chronic Kidney Disease: The VCOHP Study. PLoS ONE 2015, 10, e0143706. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Kiernan, M.C.; Murray, A.; Rosner, M.H.; Ronco, C. Kidney-brain crosstalk in the acute and chronic setting. Nat. Rev. Nephrol. 2015, 11, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Fukunishi, I.; Kitaoka, T.; Shirai, T.; Kino, K.; Kanematsu, E.; Sato, Y. Psychiatric disorders among patients undergoing hemodialysis therapy. Nephron 2002, 91, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Takae, K.; Hata, J.; Ohara, T.; Yoshida, D.; Shibata, M.; Mukai, N.; Hirakawa, Y.; Kishimoto, H.; Tsuruya, K.; Kitazono, T.; et al. Albuminuria Increases the Risks for Both Alzheimer Disease and Vascular Dementia in Community-Dwelling Japanese Elderly: The Hisayama Study. J. Am. Heart Assoc. 2018, 7, e006693. [Google Scholar] [CrossRef] [PubMed]
- Viggiano, D.; Wagner, C.A.; Martino, G.; Nedergaard, M.; Zoccali, C.; Unwin, R.; Capasso, G. Mechanisms of cognitive dysfunction in CKD. Nat. Rev. Nephrol. 2020, 16, 452–469. [Google Scholar] [CrossRef] [PubMed]
- Arafa, A.; Kawachi, H.; Matsumoto, C.; Teramoto, M.; Yasui, Y.; Kato, Y.; Matsuo, M.; Nakao, Y.M.; Kashima, R.; Kokubo, Y. The association between the estimated glomerular filtration rate and cognitive impairment: The Suita Study. Hypertens. Res. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Kang, E.W.; Abdel-Kader, K.; Yabes, J.; Glover, K.; Unruh, M. Association of sleep-disordered breathing with cognitive dysfunction in CKD stages 4-5. Am. J. Kidney Dis. 2012, 60, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Warsame, F.; Chu, N.M.; Hong, J.; Mathur, A.; Crews, D.C.; Bayliss, G.; Segev, D.L.; McAdams-DeMarco, M.A. Sleep duration and cognitive function among older adults with chronic kidney disease: Results from the National Health and Nutrition Examination Survey (2011-2014). Nephrol. Dial. Transplant. 2023, 38, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Yang, Z.; Wang, J.; Xiong, Z.; Liao, J.; Hao, L.; Liu, G.; Ren, Y.; Wang, Q.; et al. Sleep Disorders and Cognitive Impairment in Peritoneal Dialysis: A Multicenter Prospective Cohort Study. Kidney Blood Press. Res. 2019, 44, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Kutner, N.G.; Zhang, R.; Huang, Y.; Bliwise, D.L. Association of sleep difficulty with Kidney Disease Quality of Life cognitive function score reported by patients who recently started dialysis. Clin. J. Am. Soc. Nephrol. 2007, 2, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Otobe, Y.; Hiraki, K.; Hotta, C.; Nishizawa, H.; Izawa, K.P.; Taki, Y.; Imai, N.; Sakurada, T.; Shibagaki, Y. Mild cognitive impairment in older adults with pre-dialysis patients with chronic kidney disease: Prevalence and association with physical function. Nephrology (Carlton) 2019, 24, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Otobe, Y.; Hiraki, K.; Hotta, C.; Izawa, K.P.; Sakurada, T.; Shibagaki, Y. The impact of the combination of kidney and physical function on cognitive decline over 2 years in older adults with pre-dialysis chronic kidney disease. Clin. Exp. Nephrol. 2019, 23, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Coppolino, G.; Bolignano, D.; Gareri, P.; Ruberto, C.; Andreucci, M.; Ruotolo, G.; Rocca, M.; Castagna, A. Kidney function and cognitive decline in frail elderly: Two faces of the same coin? Int. Urol. Nephrol. 2018, 50, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- McAdams-DeMarco, M.A.; Tan, J.; Salter, M.L.; Gross, A.; Meoni, L.A.; Jaar, B.G.; Kao, W.H.; Parekh, R.S.; Segev, D.L.; Sozio, S.M. Frailty and Cognitive Function in Incident Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2015, 10, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Song, M.K.; Ward, S.E.; Bair, E.; Weiner, L.J.; Bridgman, J.C.; Hladik, G.A.; Gilet, C.A. Patient-reported cognitive functioning and daily functioning in chronic dialysis patients. Hemodial. Int. 2015, 19, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Lin, J.; Cao, P.; Chen, J.; Zhou, T.; Yang, R.; Lu, S.; Yu, X.; Yang, X. Prevalence and Prognosis of Coexisting Frailty and Cognitive Impairment in Patients on Continuous Ambulatory Peritoneal Dialysis. Sci. Rep. 2018, 8, 17305. [Google Scholar] [CrossRef] [PubMed]
- Chu, N.M.; Gross, A.L.; Shaffer, A.A.; Haugen, C.E.; Norman, S.P.; Xue, Q.L.; Sharrett, A.R.; Carlson, M.C.; Bandeen-Roche, K.; Segev, D.L.; et al. Frailty and Changes in Cognitive Function after Kidney Transplantation. J. Am. Soc. Nephrol. 2019, 30, 336–345. [Google Scholar] [CrossRef]
- Hidaka, S.; Nishimiura, A.; Hirata, M.; Ishioka, K.; Ohtake, T.; Oka, M.; Tamura, T.; Shibata, K.; Nishihara, M.; Kuji, T.; et al. Prevalence of mild cognitive impairment and its association with handgrip strength in patients on hemodialysis. Sci. Rep. 2022, 12, 3850. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.Y.; Mintun, M.A.; Fagan, A.M.; Goate, A.M.; Bugg, J.M.; Holtzman, D.M.; Morris, J.C.; Head, D. Exercise and Alzheimer's disease biomarkers in cognitively normal older adults. Ann. Neurol. 2010, 68, 311–318. [Google Scholar] [CrossRef]
- Vaynman, S.; Gomez-Pinilla, F. Revenge of the "sit": How lifestyle impacts neuronal and cognitive health through molecular systems that interface energy metabolism with neuronal plasticity. J. Neurosci. Res. 2006, 84, 699–715. [Google Scholar] [CrossRef] [PubMed]
- Cotman, C.W.; Berchtold, N.C.; Christie, L.A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007, 30, 464–472. [Google Scholar] [CrossRef] [PubMed]
- van Praag, H.; Christie, B.R.; Sejnowski, T.J.; Gage, F.H. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. USA 1999, 96, 13427–13431. [Google Scholar] [CrossRef] [PubMed]
- van Praag, H.; Kempermann, G.; Gage, H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999, 2, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Tyler, W.J.; Alonso, M.; Bramham, C.R.; Pozzo-Miller, L,D. From acquisition to consolidation: On the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn. Mem. 2002, 9, 224–237. [Google Scholar] [CrossRef] [PubMed]
- Trejo, J.L.; Carro, E.; Torres-Aleman, I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. 2001, 21, 1628–1634. [Google Scholar] [CrossRef]
- Ding, Q.; Vaynman, S.; Akhavan, M.; Ying, Z.; Gomez-Pinilla, F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience 2006, 140, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Carro, E.; Nunez, A.; Busiguina, S.; Torres-Aleman, I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J. Neurosci. 2000, 20, 2926–2933. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.M.; Ademi, Z.; Doehner, W.; Lip, G.Y.H.; Mark, P.; Toyoda, K.; Wong, C.X.; Sarnak, M.; Cheung, M.; Herzog, C.A.; et al. Chronic Kidney Disease and Cerebrovascular Disease: Consensus and Guidance From a KDIGO Controversies Conference. Stroke 2021, 52, e328–e346. [Google Scholar] [CrossRef] [PubMed]
- Tasmoc, A.; Donciu, M.D.; Veisa, G.; Nistor, I.; Covic, A. Increased arterial stiffness predicts cognitive impairment in hemodialysis patients. Hemodial. Int. 2016, 20, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, L.E.; Trompet, S.; Jukema, J.W.; Kroft, L.J.M.; de, Bresser, J. ; van Osch, M.J.P.; Hammer, S.; Witjes, M.N.; van Buren, M.; Mooijaart, S.P. Association of cardiovascular structure and function with cerebrovascular changes and cognitive function in older patients with end-stage renal disease. Aging (Albany NY) 2020, 12, 1496–1511. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.D.; Meoni, L.A.; Jaar, B.G.; Shafi, T.; Linda, *!!! REPLACE !!!*; Kao, W.H.; Estrella, M.M.; Parekh, R.; Sozio, S.M. Association of Arterial Stiffness and Central Pressure With Cognitive Function in Incident Hemodialysis Patients: The PACE Study. Kidney Int. Rep. 2017, 2, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Hidaka, S.; Kawaguchi, T.; Matsukawa, A. Relationship between Lower Extremity Peripheral Arterial Disease and Mild Cognitive Impairment in Hemodialysis Patients. J. Clin. Med. 2023, 12, 2145. [Google Scholar] [CrossRef] [PubMed]
- Findlay, M.D.; Dawson, J.; Dickie, D.A.; Forbes, K.P.; McGlynn, D.; Quinn, T.; Mark, P.B. Investigating the relationship between cerebral blood flow and cognitive function in hemodialysis patients. J. Am. Soc. Nephrol. 2019, 30, 147–158. [Google Scholar] [CrossRef] [PubMed]
- MacEwen, C.; Sutherland, S.; Daly, J.; Pugh, C.; Tarassenko, L. Relationship between hypotension and cerebral ischemia during hemodialysis. J. Am. Soc. Nephrol. 2017, 28, 2511–2520. [Google Scholar] [CrossRef] [PubMed]
- Mizumasa, T.; Hirakata, H.; Yoshimitsu, T.; Hirakata, E.; Kubo, M.; Kashiwagi, M.; Tanaka, H.; Kanai, H.; Fujimi, S.; Iida, M. Dialysis-related hypotension as a cause of progressive frontal lobe atrophy in chronic hemodialysis patients: A 3-year prospective study. Nephron Clin. Pract. 2004, 97, c23–c30. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Mochida, Y.; Ishioka, K.; Oka, M.; Maesato, K.; Moriya, H.; Hidaka, S.; Ohtake, T. The effects of blood pressure and the renin-angiotensin-aldosterone system on regional cerebral blood flow and cognitive impairment in dialysis patients. Hypertens. Res. 2014, 37, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, H.; Ookawara, S.; Tabei, K. Aggravation of Cerebral Oxygenation due to Intradialytic Hypotension Induced by Blood Volume Reduction During Hemodialysis: A Case Report. Ther. Apher. Dial. 2015, 19, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.; Kudlicka, J.; Lachmanova, J.; Valerianova, A.; Rocinova, K.; Bartkova, M.; Tesar, V. Tissue ischemia worsens during hemodialysis in end-stage renal disease patients. J. Vasc. Access 2017, 18, 47–51. [Google Scholar] [CrossRef] [PubMed]
- MacEwen, C.; Watkinson, P.; Tarassenko, L.; Pugh, C. Relationship between Hypotension and Cerebral Ischemia during Hemodialysis. J. Am. Soc. Nephrol. 2017, 28, 2511–2520. [Google Scholar] [CrossRef] [PubMed]
- Findlay, M.D.; Dawson, J.; Dickie, D.A.; Forbes, K.P.; McGlynn, D.; Quinn, T.; Mark, P.B. Investigating the Relationship between Cerebral Blood Flow and Cognitive Function in Hemodialysis Patients. J. Am. Soc. Nephrol. 2019, 30, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Kovarova, L.; Valerianova, A.; Kmentova, T.; Lachmanova, J.; Hladinova, Z.; Malik, J. Low Cerebral Oxygenation Is Associated with Cognitive Impairment in Chronic Hemodialysis Patients. Nephron 2018, 139, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Chai, C.; Wang, H.; Chu, Z.; Li, J.; Qian, T.; Mark Haacke, E.; Xia, S.; Shen, W. Reduced regional cerebral venous oxygen saturation is a risk factor for the cognitive impairment in hemodialysis patients: A quantitative susceptibility mapping study. Brain Imaging Behav. 2020, 14, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Fujisaki, K.; Tsuruya, K.; Yamato, M.; Toyonaga, J.; Noguchi, H.; Nakano, T.; Taniguchi, M.; Tokumoto, M.; Hirakata, H.; Kitazono, T. Cerebral oxidative stress induces spatial working memory dysfunction in uremic mice: Neuroprotective effect of tempol. Nephrol. Dial. Transplant. 2014, 29, 529–538. [Google Scholar] [CrossRef]
- Matsuki, H.; Mandai, S.; Shiwaku, H.; Koide, T.; Takahashi, N.; Yanagi, T.; Inaba, S.; Ida, S.; Fujiki, T.; Mori, Y.; et al. Chronic kidney disease causes blood-brain barrier breakdown via urea-activated matrix metalloproteinase-2 and insolubility of tau protein. Aging (Albany NY) 2023, 15, 10972–10995. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.A.; Weiner, D.E.; Sarnak, M.J. Cognitive Impairment in CKD: Pathophysiology, Management, and Prevention. Am. J. Kidney Dis. 2019, 74, 782–790. [Google Scholar] [CrossRef]
- Haruyama, N.; Fujisaki, K.; Yamato, M.; Eriguchi, M.; Noguchi, H.; Torisu, K.; Tsuruya, K.; Kitazono, T. Improvement in spatial memory dysfunction by telmisartan through reduction of brain angiotensin II and oxidative stress in experimental uremic mice. Life Sci. 2014, 113, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Wang, H.F.; Wang, X.; Li, J.; Xing, C.M. The association of renin-angiotensin system blockade use with the risks of cognitive impairment of aging and Alzheimer's disease: A meta-analysis. J. Clin. Neurosci. 2016, 33, 32–38. [Google Scholar] [CrossRef]
- SPRINT MIND Investigators for the SPRINT Research Group. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA 2019, 321, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Grimm, G.; Stockenhuber, F.; Schneeweiss, B.; Madl, C.; Zeitlhofer, J.; Schneider, B. Improvement of brain function in hemodialysis patients treated with erythropoietin. Kidney Int. 1990, 38, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; Sahni, V.; Wadhwa, A.; Garg, S.; Bajaj, S.K.; Kohli, R.; Agarwal, S.K. Effect of improvement in anemia on electroneurophysiological markers (P300) of cognitive dysfunction in chronic kidney disease. Hemodial. Int. 2006, 10, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, F.; Mallamaci, F.; D'Arrigo, G.; Baggetta, R.; Bolignano, D.; Torino, C.; Lamberti, N.; Bertoli, S.; Ciurlino, D.; Rocca-Rey, L.; et al. Exercise in Patients on Dialysis: A Multicenter, Randomized Clinical Trial. J. Am. Soc. Nephrol. 2017, 28, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Otobe, Y.; Yamada, M.; Hiraki, K.; Onari, S.; Taki, Y.; Sumi, H.; Hachisuka, R.; Han, W.; Takahashi, M.; Suzuki, M.; et al. Physical Exercise Improves Cognitive Function in Older Adults with Stage 3-4 Chronic Kidney Disease: A Randomized Controlled Trial. Am. J. Nephrol. 2021, 52, 929–939. [Google Scholar] [CrossRef] [PubMed]
- McAdams-DeMarco, M.A.; Konel, J.; Warsame, F.; Ying, H.; González Fernández, M.; Carlson, M.C.; Fine, D.M.; Appel, L.J.; Segev, D.L. Intradialytic Cognitive and Exercise Training May Preserve Cognitive Function. Kidney Int. Rep. 2017, 3, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Song, Y.; Zhao, D.; Zhan, M. Effect of exercise on cognitive impairment in patients undergoing haemodialyses: A systematic review and meta-analysis of randomised controlled trials. J. Ren. Care 2022, 48, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T.; et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2019, 390, 2673–2734. [Google Scholar] [CrossRef] [PubMed]
- Ok, E.; Duman, S.; Asci, G.; Tumuklu, M.; Onen, *!!! REPLACE !!!*; Sertoz, O.; Kayikcioglu, M.; Toz, H.; Adam, S.M.; Yilmaz, M.; Tonbul, H.Z.; et al. Long Dialysis Study Group. Comparison of 4- and 8-h dialysis sessions in thrice-weekly in-centre haemodialysis: A prospective, case-controlled study. Nephrol. Dial. Transplant. 2011, 26, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Chertow, G.M.; Levin, N.W.; Beck, G.J.; Depner, T.A.; Eggers, P.W.; Gassman, J.J.; Gorodetskaya, I.; Greene, T.; James, S.; Larive, B.; et al.; FHN Trial Group In-center hemodialysis six times per week versus three times per week. N. Engl. J. Med. 2010, 363, 2287–2300. [Google Scholar] [PubMed]
- Eldehni, M.T.; Odudu, A.; McIntyre, C.W. Randomized clinical trial of dialysate cooling and effects on brain white matter. J. Am. Soc. Nephrol. 2015, 26, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, R.A.; Bdair, F.; Akl, E.A.; Garg, A.X.; Thiessen-Philbrook, H.; Salameh, H.; Kisra, S.; Nesrallah, G.; Al-Jaishi, A.; Patel, P.; et al. Effect of Lowering the Dialysate Temperature in Chronic Hemodialysis: A Systematic Review and Meta-Analysis. Clin. J. Am. Soc. Nephrol. 2016, 11, 442–457. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, I.; Odudu, A.; Baharani, J.; Fergusson, N.; Griffiths, H.; Harrison, J.; Maruff, P.; Thomas, G.N.; Woodhall, G.; Youseff, S.; et al. Evaluation of the effect of Cooled HaEmodialysis on Cognitive function in patients suffering with end-stage KidnEy Disease (E-CHECKED): Feasibility randomised control trial protocol. Trials 2020, 21, 820. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Davis, S.C.; Truijen, J.; Stok, W.J.; Secher, N.H.; van Lieshout, J.J. Intensive blood pressure control affects cerebral blood flow in type 2 diabetes mellitus patients. Hypertension 2011, 57, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Fujisaki, K.; Kanai, H.; Hirakata, H.; Nakamura, S.; Koga, Y.; Hattori, F.; Iida, M. Midodrine hydrochloride and L-threo-3,4-dihydroxy-phenylserine preserve cerebral blood flow in hemodialysis patients with orthostatic hypotension. Ther. Apher. Dial. 2007, 11, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Nitta, K.; Nakai, S.; Masakane, I.; Hanafusa1, N.; Goto, S.; Taniguchi, M.; Hasegawa, T.; Wada, A.; Hamano, T.; Hoshino, J.; et al.; on behalf of Japanese Society for Dialysis Therapy Renal Data Registry Committee Annual dialysis data report of the 2018 JSDT Renal Data Registry: Dementia, performance status, and exercise habits. Ren. Replace. Ther. 2021, 7, 41. [Google Scholar] [CrossRef]
- Wolfgram, D.F.; Szabo, A.; Murray, A.M.; Whittle, J. Risk of dementia in peritoneal dialysis patients compared with hemodialysis patients. Perit. Dial. Int. 2015, 35, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Neumann, D.; Mau, W.; Wienke, A.; Girndt, M. Peritoneal dialysis is associated with better cognitive function than hemodialysis over a one-year course. Kidney Int. 2018, 93, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Guo, X.; Xia, X.; Yu, H.; Li, X.; Jiang, A. The comparison of cognitive function and risk of dementia in CKD patients under peritoneal dialysis and hemodialysis: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2019, 98, e14390. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Soliman, K.; Mohamed, M.M.; Daoud, A.; Shafiq, T.; Fülöp, T.; Baharani, J. The effects of dialysis modality choice on cognitive functions in patients with end-stage renal failure: A systematic review and meta-analysis. Int. Urol. Nephrol. 2021, 53, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, Y.K.; Sood, S.; Rathi, O.; Mahajan, S. Effect of renal transplantation on cognitive function in hemodialysis patients: A longitudinal study. Int. Urol. Nephrol. 2017, 49, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Joshee, P.; Wood, A.G.; Wood, E.R.; Grunfeld, E.A. Meta-analysis of cognitive functioning in patients following kidney transplantation. Nephrol. Dial. Transplant. 2018, 33, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Samaei, A.; Nobahar, M.; Hydarinia-Naieni, Z.; Ebrahimian, A.A.; Tammadon, M.R.; Ghorbani, R.; Vafaei, A.A. Effect of valerian on cognitive disorders and electroencephalography in hemodialysis patients: A randomized, cross over, double-blind clinical trial. BMC Nephrol. 2018, 19, 379. [Google Scholar] [CrossRef] [PubMed]
- Marzieh, S.H.; Jafari, H.; Shorofi, S.A.; Setareh, J.; Moosazadeh, M.; Espahbodi, F.; Saeedi, M.; Arbon, P.; Ghorbani Vajargah, P.; Karkhah, S. The effect of melatonin on sleep quality and cognitive function of individuals undergoing hemodialysis. Sleep Med. 2023, 111, 105–110. [Google Scholar] [PubMed]
Figure 1.
Inverse association of GMR, but not WMR, with age. The association of GMR (a), but not WMR (b), with age in both PD (closed circles; n = 62) and ND (open circles; n = 69) patients are seen. Abbreviations: GMR, gray matter volume ratio; ND, non-dialysis-dependent chronic kidney disease; PD, peritoneal dialysis; WMR, white matter volume ratio. Reproduced from Ref. 4.
Figure 1.
Inverse association of GMR, but not WMR, with age. The association of GMR (a), but not WMR (b), with age in both PD (closed circles; n = 62) and ND (open circles; n = 69) patients are seen. Abbreviations: GMR, gray matter volume ratio; ND, non-dialysis-dependent chronic kidney disease; PD, peritoneal dialysis; WMR, white matter volume ratio. Reproduced from Ref. 4.
Figure 2.
Comparison of the annual change in GMR between PD and ND patients. Annual change in GMR, as determined by subtraction of baseline GMR from GMR after 2 years, is significantly greater in PD patients than in ND patients. Data are least square mean ± standard error. Abbreviations: GMR, gray matter volume ratio; ND, non-dialysis-dependent chronic kidney disease; PD, peritoneal dialysis. Reproduced from Ref. 4.
Figure 2.
Comparison of the annual change in GMR between PD and ND patients. Annual change in GMR, as determined by subtraction of baseline GMR from GMR after 2 years, is significantly greater in PD patients than in ND patients. Data are least square mean ± standard error. Abbreviations: GMR, gray matter volume ratio; ND, non-dialysis-dependent chronic kidney disease; PD, peritoneal dialysis. Reproduced from Ref. 4.
Figure 3.
Comparison of the annual change in GMR between PD and HD patients. Annual change in GMR, as determined by subtraction of baseline GMR from GMR after 2 years, is significantly greater in PD patients than in HD patients. Data are least square mean ± standard error. Abbreviations: GMR, gray matter volume ratio; HD, hemodialysis; PD, peritoneal dialysis. Reproduced from Ref. 5.
Figure 3.
Comparison of the annual change in GMR between PD and HD patients. Annual change in GMR, as determined by subtraction of baseline GMR from GMR after 2 years, is significantly greater in PD patients than in HD patients. Data are least square mean ± standard error. Abbreviations: GMR, gray matter volume ratio; HD, hemodialysis; PD, peritoneal dialysis. Reproduced from Ref. 5.
Figure 4.
Effect of tempol on prevention of uremia-induced spatial working memory dysfunction and inhibition of 8-OHdG accumulation in the hippocampal CA3 region. (a) The numbers of errors during radial arm water maze test on the fifth day in Cont-Veh (white triangle), CKD-Veh (black triangle), Cont-TMP (white circle) and CKD-TMP (black circle) mice are shown. The number of errors in CKD-TMP mice is significantly decreased to levels similar to those observed in control mice, and significantly lower than CKD-Veh mice. The ends of the box represent the upper and lower quartiles; thus the box spans the interquartile range. The median is marked by a vertical line inside the box. The two lines outside the box that extend to the highest and lowest observations represent the whiskers. *P <0.05 versus the other three groups. (b) Effect of TMP on prevention of oxidative DNA damage generation. Representative microphotographs of 8-OHdG immunostaining in the hippocampal CA3 region from each group are shown. Magnification: ×200. Quantitative analysis of 8-OHdG-positive neurons in the hippocampal CA3 region is shown. 8-OHdG immunoreactivity in the hippocampal CA3 region is significantly higher in CKD-Veh mice than TMP-treated CKD mice. The ends of the box represent the upper and lower quartiles; thus, the box spans the interquartile range. The median is marked by a vertical line inside the box. The two lines outside the box that extend to the highest and lowest observations represent the whiskers. *P <0.05 versus Cont-Veh mice. †P <0.05 versus CKD-Veh mice. Abbreviations: CA3, cornu ammonis 3; CKD, chronic kidney disease; Cont, control; 8-OHdG, 8-hydroxy-2'-deoxyguanosine; TMP, tempol; Veh, vehicle. Reproduced from Ref. 48.
Figure 4.
Effect of tempol on prevention of uremia-induced spatial working memory dysfunction and inhibition of 8-OHdG accumulation in the hippocampal CA3 region. (a) The numbers of errors during radial arm water maze test on the fifth day in Cont-Veh (white triangle), CKD-Veh (black triangle), Cont-TMP (white circle) and CKD-TMP (black circle) mice are shown. The number of errors in CKD-TMP mice is significantly decreased to levels similar to those observed in control mice, and significantly lower than CKD-Veh mice. The ends of the box represent the upper and lower quartiles; thus the box spans the interquartile range. The median is marked by a vertical line inside the box. The two lines outside the box that extend to the highest and lowest observations represent the whiskers. *P <0.05 versus the other three groups. (b) Effect of TMP on prevention of oxidative DNA damage generation. Representative microphotographs of 8-OHdG immunostaining in the hippocampal CA3 region from each group are shown. Magnification: ×200. Quantitative analysis of 8-OHdG-positive neurons in the hippocampal CA3 region is shown. 8-OHdG immunoreactivity in the hippocampal CA3 region is significantly higher in CKD-Veh mice than TMP-treated CKD mice. The ends of the box represent the upper and lower quartiles; thus, the box spans the interquartile range. The median is marked by a vertical line inside the box. The two lines outside the box that extend to the highest and lowest observations represent the whiskers. *P <0.05 versus Cont-Veh mice. †P <0.05 versus CKD-Veh mice. Abbreviations: CA3, cornu ammonis 3; CKD, chronic kidney disease; Cont, control; 8-OHdG, 8-hydroxy-2'-deoxyguanosine; TMP, tempol; Veh, vehicle. Reproduced from Ref. 48.
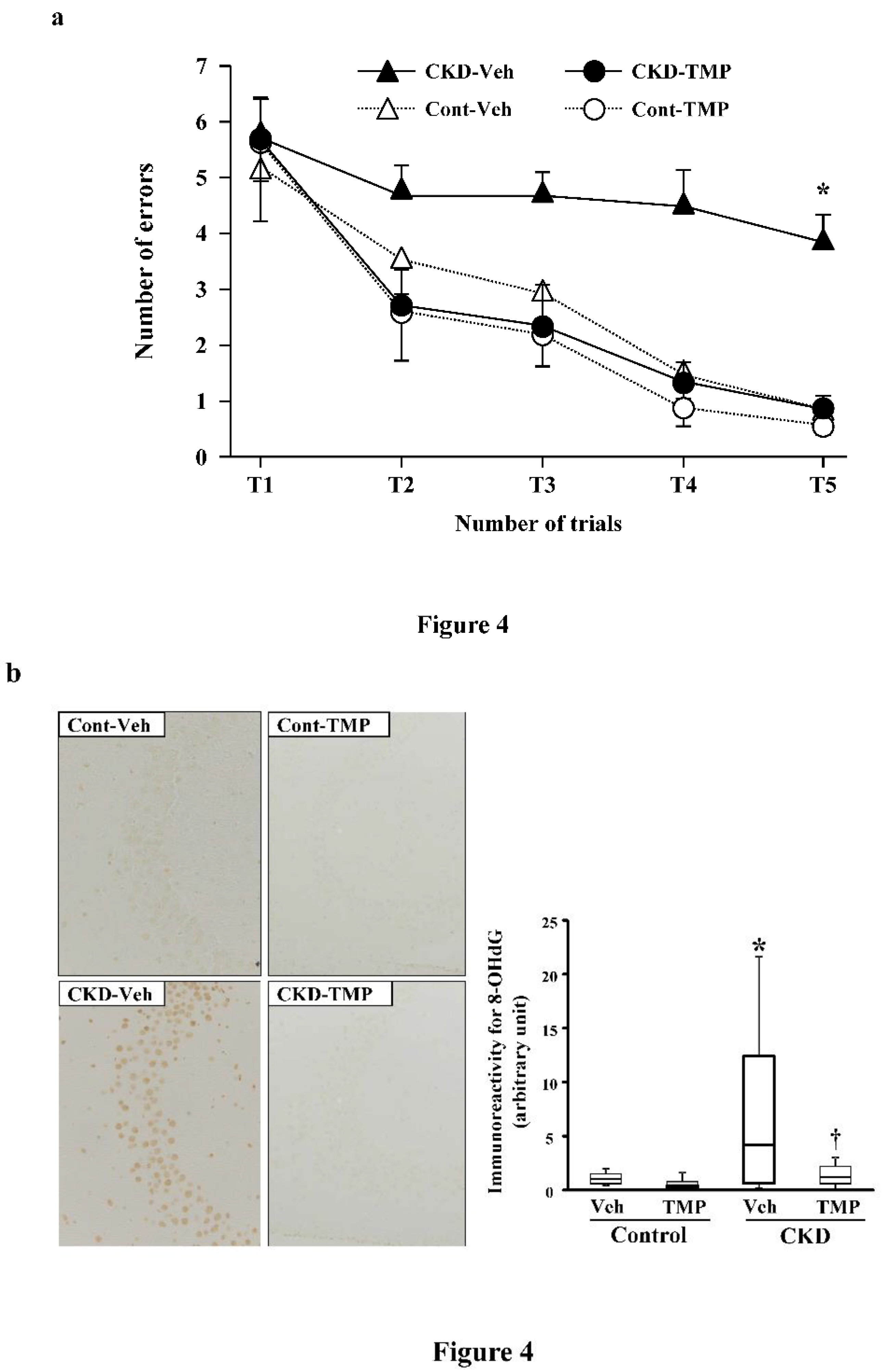
Table 1.
Univariable and multivariable-adjusted regression analyses of correlation between whole-brain GMR and TMT scores in all participants.
Table 1.
Univariable and multivariable-adjusted regression analyses of correlation between whole-brain GMR and TMT scores in all participants.
| |
|
TMT-A |
TMT-B |
ΔTMT |
| Univariable analysis |
Standardized β |
−0.490 |
−0.516 |
−0.476 |
| |
P |
<0.001 |
<0.001 |
<0.001 |
| Model I |
Standardized β |
−0.442 |
−0.467 |
−0.432 |
| |
P |
<0.001 |
<0.001 |
<0.001 |
| Model II |
Standardized β |
−0.394 |
−0.423 |
−0.393 |
| |
P |
0.002 |
<0.001 |
0.003 |
| Model III |
Standardized β |
−0.349 |
−0.362 |
−0.332 |
| |
P |
0.012 |
0.006 |
0.013 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).