1. Introduction
Mealybugs (Hemiptera: Pseudococcidae) are important plant pests worldwide [
1]. They are small sap-sucking insects, reproduce quickly, and have a large population [
2]. Once harmed, it will lead to leaf yellowing, defoliation, reduced plant growth, even the entire plant withering and dying. Indirectly, they can also damage plants as vectors of plant diseases. In addition, the honeydew secreted by scale insects contaminates host plants and breeds sooty mold, seriously reducing the yield and quality of fruit, affecting the greening effect of street trees and flowers. On the other hand, mealybugs are small and live in seclusion, which can easily spread to new areas with the transportation of seedlings, leading to outbreaks and disasters. Therefore, many mealybugs are regarded as quarantine objects, and quarantine measures are strictly implemented to prevent their transmission and spread [
3].
Pseudococcus viburni is a worldwide species, found in 60 countries [
4], also commonly known as the obscure mealybug, previously recorded in Brazil only in the states of Minas Gerais, Rio de Janeiro, and São Paulo [
5,
6,
7]. It is possible that
P. viburni was brought into Europe on potatoes from South America via the Canary Islands in the sixteenth century [
8]. The mealybug was first described from France, as
Dactylopius viburni (Signoret, 1875). This species was spread from its area of origin early, by human transport of infested plants; subsequently, this has made it difficult to work out its area of origin.
P. viburni has a very wide host range, nearly 91 plant families and 259 genera [
4], including important economic crops such as apples, citrus, grapes and tomatoes, as well as papaya and potatoes [
9].
Climate is considered to be the main factor affecting species large-scale distribution [
10,
11]. Predicting species’ geographical distribution and its response to climate change is of great significance for biodiversity conservation and sustainable ecosystems development [
12]. The potential impact of climate change on the predicted range and distribution of invasive species is well-recognized; research efforts focus mainly on the spatial characteristics of species distribution, in order to facilitate management and protection [
13,
14].
MaxEnt (maximum entropy) is a machine learning model used to find the probability of maximum entropy distribution, which can be used to predict the potential distribution of target species that meet the maximum entropy under various conditions [
15]. This software uses appearance information and requires only a small amount of species distribution data to predict the suitable habitat. Specifically, MaxEnt predicts the potential distribution by analyzing the location data of the target species (a dependent variable) as a function of environmental variables. Distribution data is generally occurrence data obtained through field investigation or literature and network, and the existing information such as land cover, forest type, ecological zone, distance, geographical characteristics, and climate data can be used as the source of environmental variables [
16].
In this current study, the MaxEnt software was employed to predict the potential geographical distribution range of P. viburni of the world under different climate change scenarios. The main goals were 1) to identify the appropriate environmental factors affecting the potential distribution of the species; 2) to observe the trends of suitable habitat range under climate change background; 3) to provide a theoretical reference for the relevant departments to formulate corresponding quarantine measures and ensure agriculture safety.
2. Materials and Methods
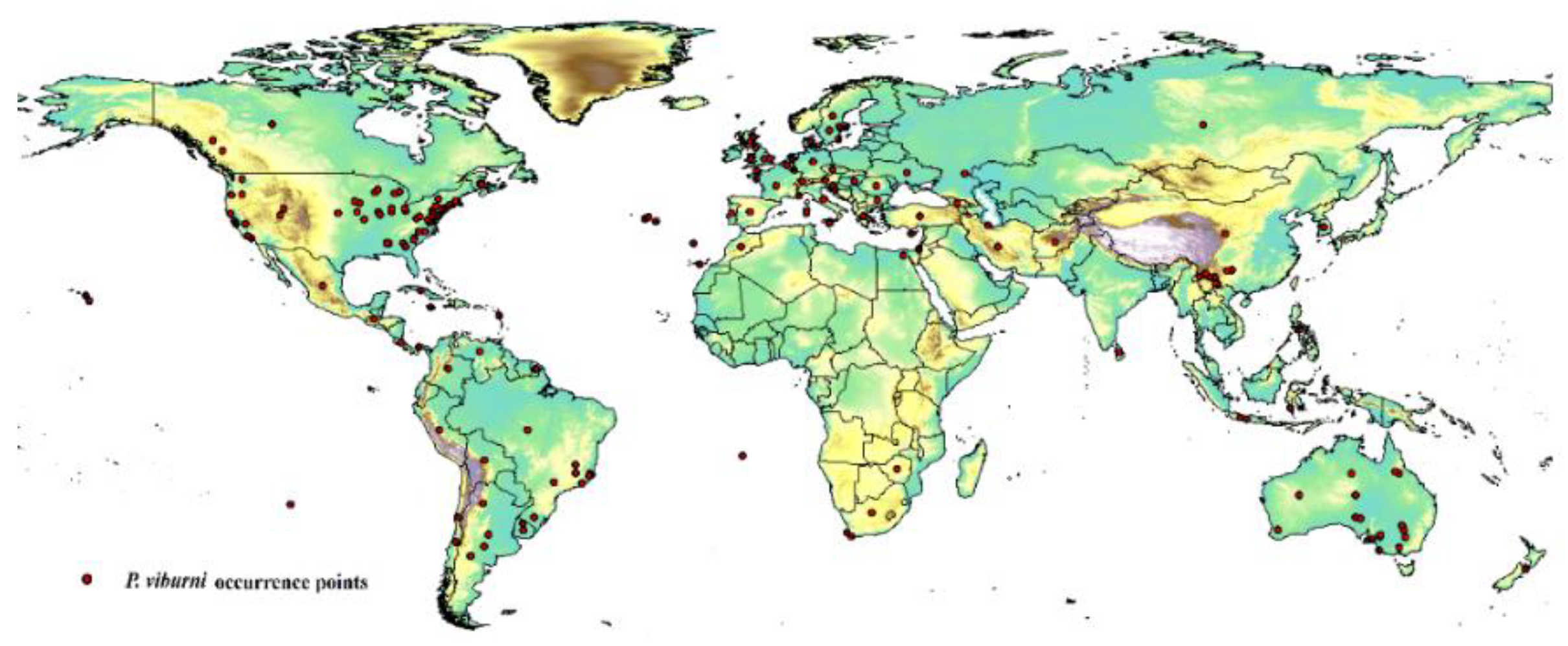
2.1. Data on Occurrence of the Species
The occurrence data of
P. viburni was primarily obtained from the Global Biological Diversity Information Facility (GBIF:
https://www.gbif.org/) and the Centre for Agriculture and Biosciences International (CABI,
https://www.cabi.org/). To reduce the impact of bias on the MaxEnt software, the R package "DISMO" was used to screen and delete distribution points with duplicate occurrences and potential errors [
17]. The method called “Fishnet” was used to create a small grid with a side length of 5 km to examine the remaining distribution points, and then randomly select location points in the grid with multiple distribution points. This work was conducted in ArcGIS 10.2 (ESRI, Redlands, CA, USA,
http://www.esri.com/). After filtering the data, 202 location points were obtained that were used in the final analysis, as shown in
Figure 1 and
Table S1.
2.2. Selection and Comparison of Environmental Variables
The environmental variables used in this study were climate variables, which were obtained from the World Environment Variable database (WorldClim 1.4;
http://www.worldclim.org) [
18]. The data included 19 bioclimatic variables (Bio1-Bio19) (
Table 1) of the current and future scenarios, the spatial resolution was 2.5 arc-minute (~ 5 km). For future climatic conditions, we used four different representative concentration pathways (RCPs), RCP2.6, RCP4.5, RCP6.0 and RCP8.5 of two future periods i.e., 2050s and 2070s. Different concentration pathways represent different radiative forcings and different CO
2 equivalent concentrations that affect the life and physiological activities of insects. For each representative concentration pathway, three different global climate models (GCMs) were selected, including HADGEM2-AO, BCC-CSM1-1 and MIROC5, to assess their future potential distribution.
According to several of the previous studies, highly correlated variables will affect the accuracy of the prediction results of species distribution models [
19]. In order to avoid excessive fitting of the model, a Pearson correlation analysis was used to screen the environmental variables with low correlation but high significance [
20]. To extract the value of each distribution point and conduct pairwise analysis on environmental factors using SPSS19, variables with a correlation larger than |0.8| were excluded (
Table S2), and finally Bio2, Bio8, Bio15, Bio18, and Bio19 were screened out for the next model analysis.
2.3. Model Construction
The MaxEnt software (version 3.4.1) was used in this study to map the potential distribution of
P. viburni [
15]. Generally, the default settings of MaxEnt software generate overfitting models [
21]. Therefore, Feature Class (FC) and Regular Multiplier (RM) were used to optimize the model. The characteristic type represents the different conversions of the coordinated variables [
22], including linear (L), multiplication (P), hinge (H), threshold (T), and Quadratic (Q) [
23]. RM can reduce the model overfitting. We used the R package "ENMeval" [
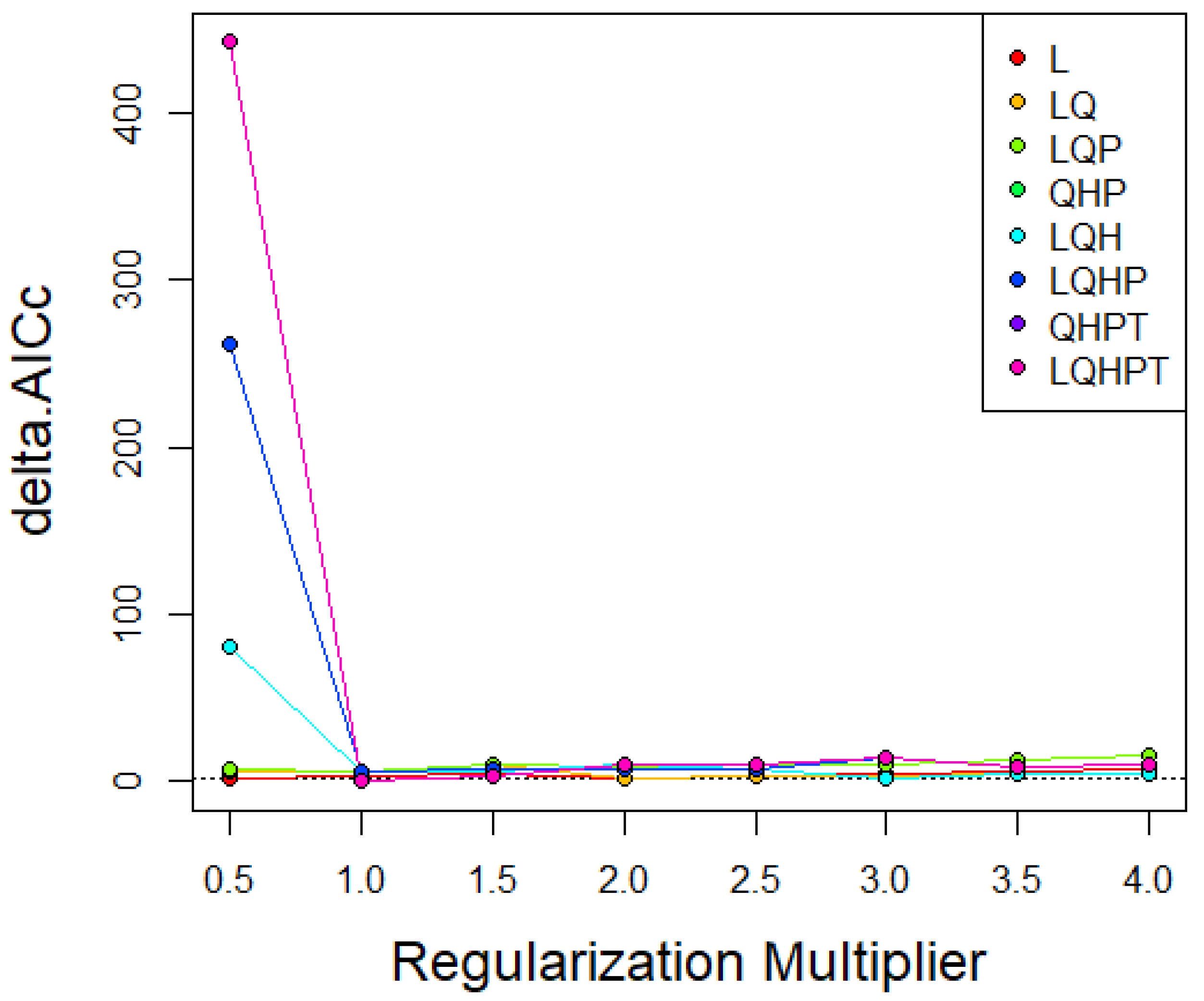
24] to test whether the parameters were over-fitted, and selected the combination of multiplication and elements based on these results [
25]. The RM value range was 0.5 to 4.0 in increments of 0.5, and the FC had eight different combinations (L, LQ, LQP, QHP, LQH, LQHP, QHPT, LQHPT). The ‘checkerboard2’ approach was applied by calculating the standardized Akaike information criterion coefficient (AICc), and the lowest delta AICc scores were selected to run the final MaxEnt models [
26].
In the research of
P. viburni, the best parameters of the FC and RM were set to QPHT and 1, respectively (
Figure 2,
Table S3). In model analysis, MaxEnt uses logical output results. A 10-fold cross-validation was used to run MaxEnt to prevent random errors [
27] based on species occurrence data and environmental variables. In this study, the same threshold was employed to define the suitable and unsuitable habitats for this species. For the prediction of future habitat areas, the average values obtained from the three global climate models (GCMs) were used to construct the distribution map of habitat areas.
2.4. Identification of Potential Habitat Threshold
Based on a logistic output format, MaxEnt produced a worldwide prediction map, which provided a continuous habitat suitability index ranging from 0 (unsuitable) to 1 (highly suitable). A 10
th percentile training presence logistic threshold (0.3062) was used to perform suitability level (defining suitable and unsuitable areas) classification on the predicted results [
28]. Suitable habitat areas were divided into four levels: 1) unsuitable habitats where suitability area was below threshold; 2) threshold – 0.4, which represented low habitat suitability, 3) 0.4 – 0.6 threshold represented moderate habitat suitability area,and 4) 0.6 – 1 threshold represented highly habitat suitability area.
2.5. Model Evaluation
Several indicators were applied to evaluate MaxEnt models including the receiver operating characteristic (AUC), kappa statistic (Kappa), the true skill statistic (TSS), and the partial AUC (pAUC) [
29]. Among them, AUC is the most widely used one, despite many disadvantages. For example, it can neither provide information about the spatial distribution of model errors, nor equally weight omission and commission errors [
26,
30]. In this study, partial receiver operating characteristic (pROC) metric approach was chosen to evaluate the performance of the model. The Niche toolbox (
http://shiny.conabio.gob.mx:3838/nichetoolb2/) was used for this processing, and parameters were set up to be assessed with 1000 repetitions and E = 0.05 [
31].
3. Results
3.1. Model Performance Evaluation
Species distribution model is a powerful tool for predicting the potential distribution areas of species, which can fully store the relationship between species distribution and environmental factors. This work predicted the habitats suitable for
P. viburni using species distribution modeling based on the known occurrence points of the species and climatic factors (including current and different model climatic variables). The results about performance of the model have been proved by pROC tests in order to have more accurate prediction potential (
Figure S1). The mean value for partial AUC at 0.05 was 0.74998 for
P. viburni (P < 0.001).
3.2. Climate Variables
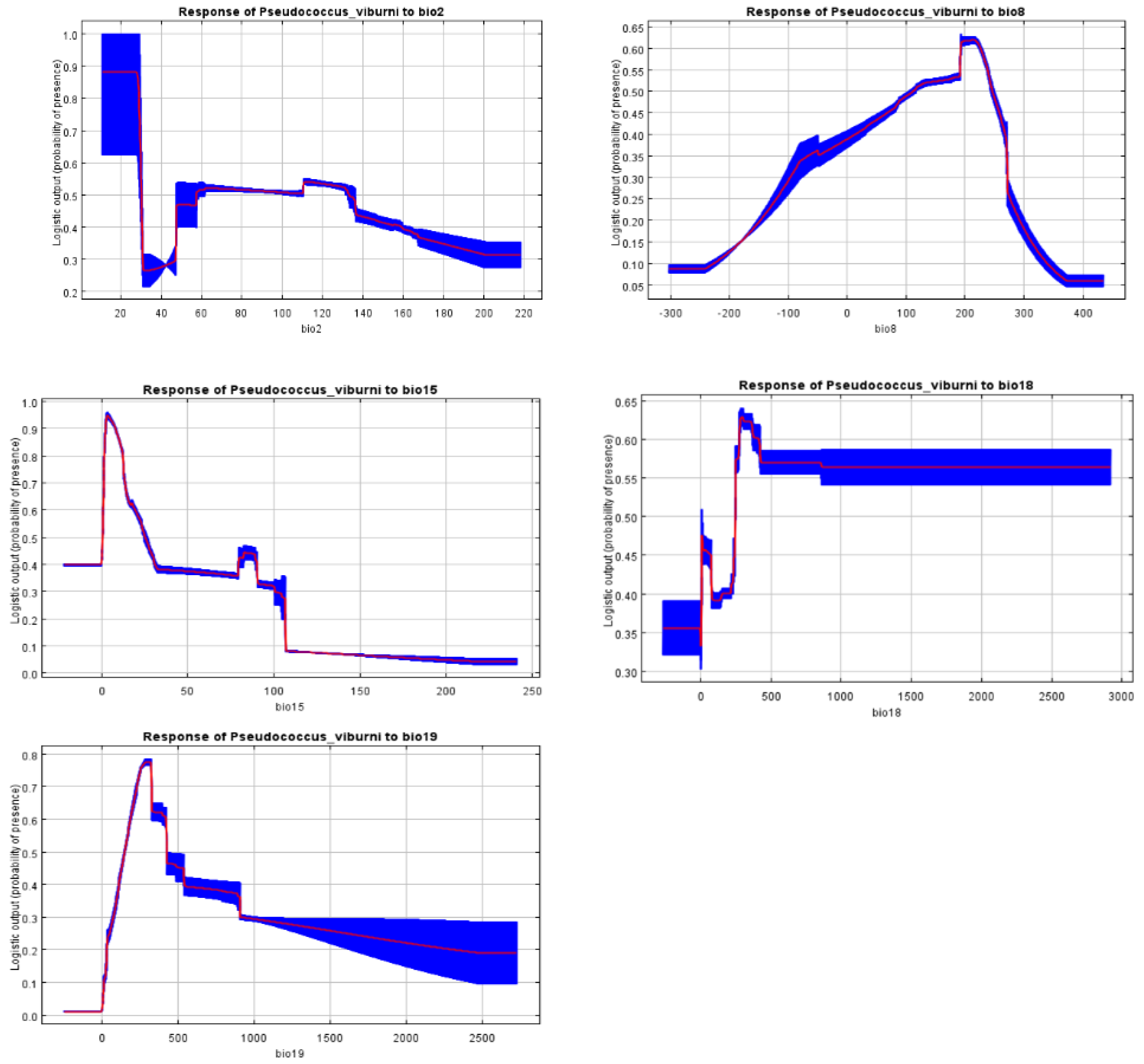
The model results showed that precipitation index was the most important factor affecting the geographical distribution of P. viburni. Further, it showed that the Precipitation of Coldest Quarter (Bio19) contributed the most to the model, accounting for about 70.7%; Precipitation seasonality (Bio15) and Mean temperature of the wettest quarter (Bio8) contributed 12.6% and 10.8% to the model, respectively.
3.2. Response Curves
The response curves indicated that how the climatic suitability of
P. viburni was changed with the five selected environmental variables (
Figure 3). Based on these response curves, climate variables associated with habitat suitability areas were 1-2.5 °C for Bio2, 19-24 °C for Bio8, 0-24 for Bio15, 250-400 mm for Bio18 and 200-400 mm for Bio19.
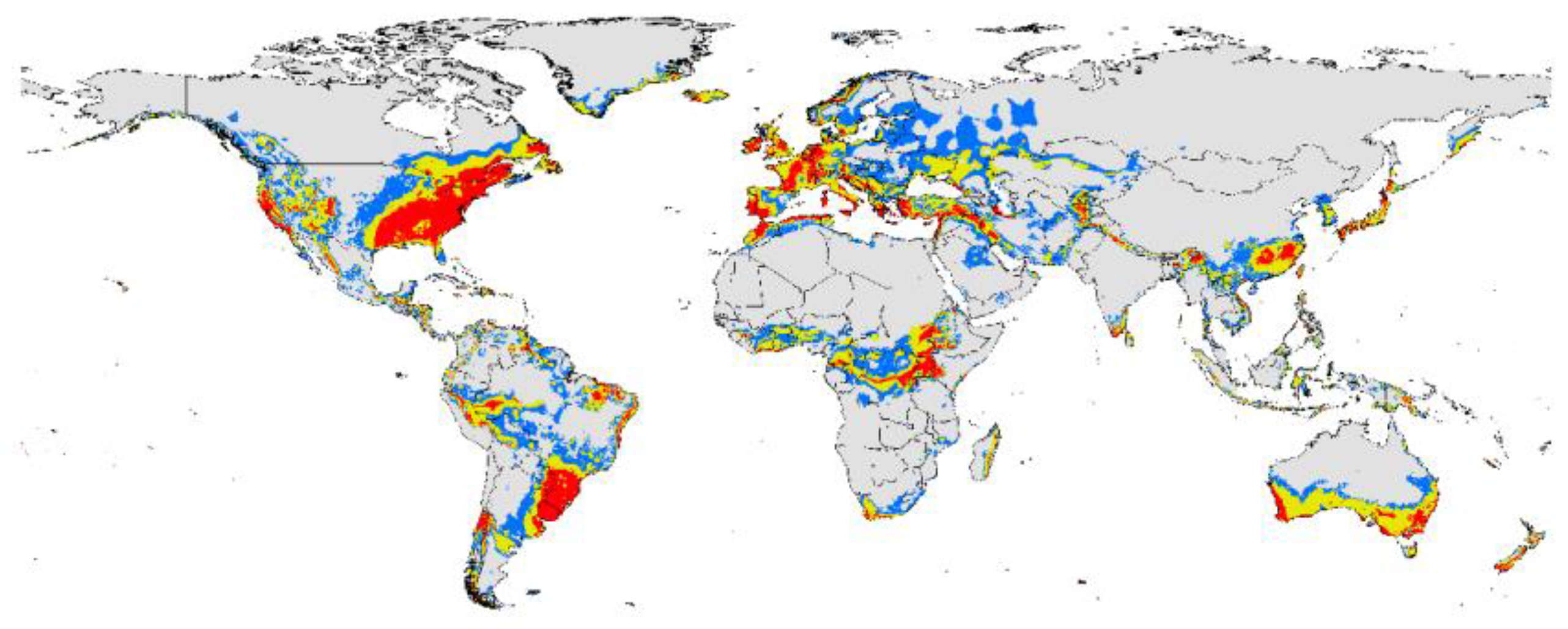
3.3. Current Suitable Area
The potential distribution map of
P. viburni was developed based on current speciation data and climate variables. Through the natural interval, the potential distribution range of
P. viburni was divided into three levels (
Figure 4). The highly suitable areas of
P. viburni: In North America, it has a distribution in most parts of the southeastern coast and parts of the western coast of North America as well as scattered in other regions of the United States, Canada, and Mexico. In South America, it is concentrated in most of southern Brazil, all of Uruguay, central Chile, and the border of Brazil, Uruguay, Argentina, and Paraguay. It is scattered in other regions of South America. In the European continent, mainly it is distributed along the Mediterranean and Atlantic coasts. In mainland Asia, it is mainly distributed in provinces such as Hunan, Anhui, Jiangxi, Fujian, and Taiwan in China, as well as scattered along the coast of Japan and in areas near China, including Afghanistan, Pakistan, India, Nepal, Bangladesh, Bhutan, Myanmar, Laos, and Vietnam. Some coastal areas in Indonesia and near the Kuril Islands in Russia also exhibit its distribution. In the African continent, it is mainly distributed near the equator, and slightly distributed in southern South Africa, along the eastern coast of Madagascar, and in central Mozambique. In mainland Australia, the pest is mainly distributed in the southern region of Australia and spans most parts of New Zealand.
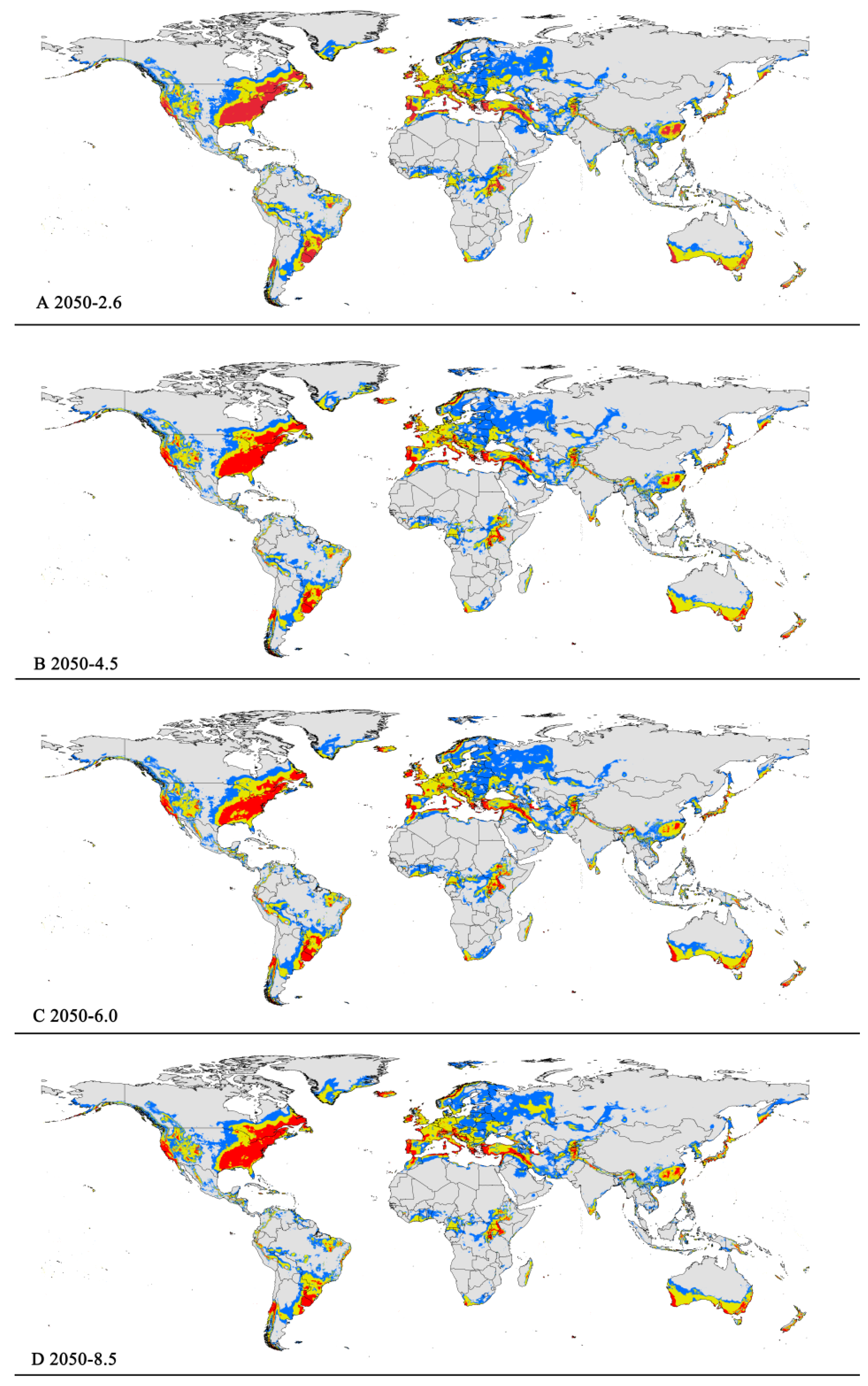
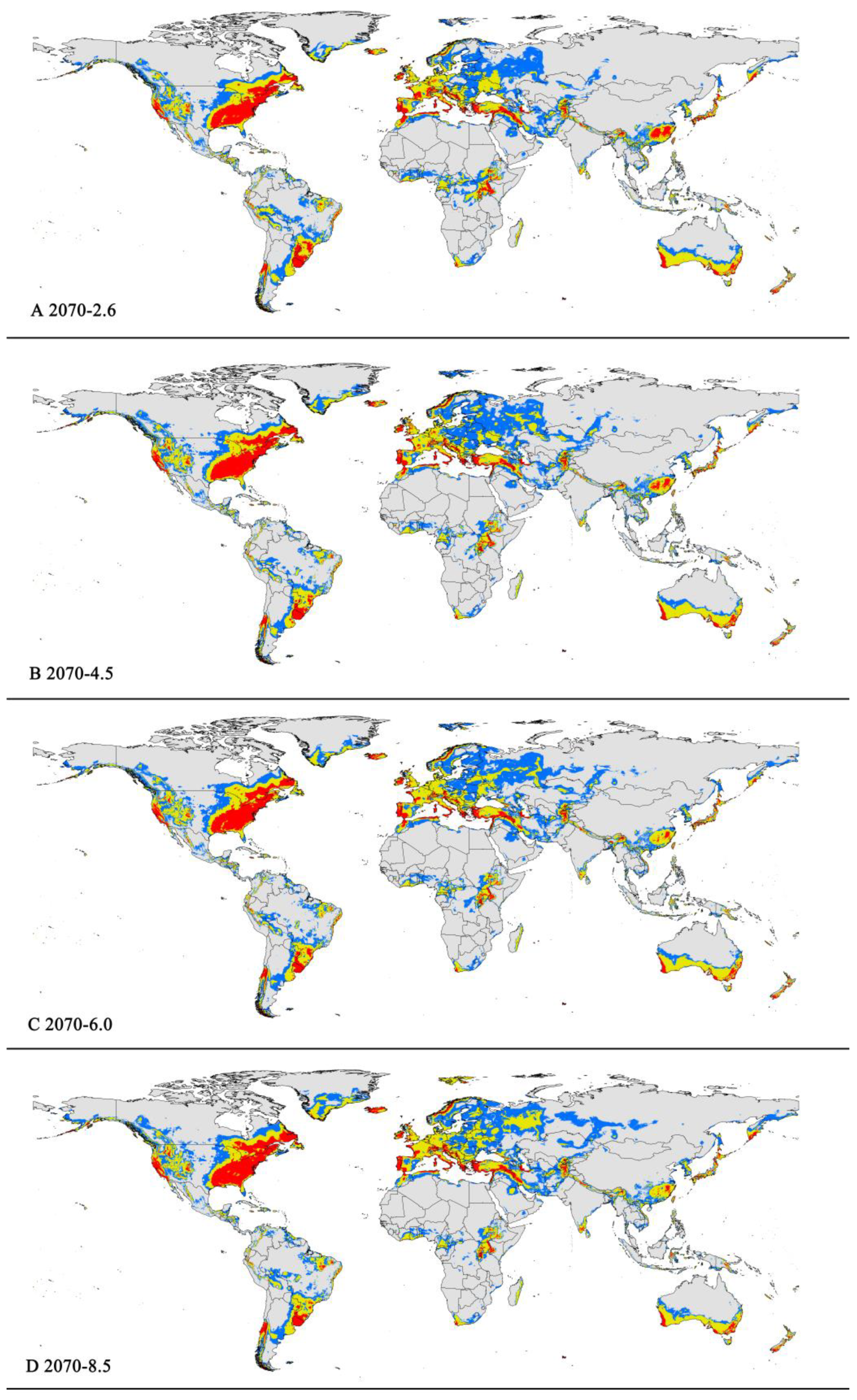
3.4. Future Potential Distribution
The potentially suitable areas for
P. viburni under future different scenarios (
Figure 5 and
Figure 6) were partially different from the current climatic conditions. The future climate scenario when compared with the current climate, the suitable areas showed different trends. From a global perspective, the highly suitable area showed a descending trend under any of the future climate scenarios. When comparing the high suitability area values for all other climate scenarios with the current suitability area value, the decrease rate was more than 10%. In the RCP6.0-2050 and RCP6.0-2070 climatic scenarios, the area is -0.98×10
7 km
2 and -1.00×10
7 km
2, respectively (
Figure 5C and
Figure 6C and
Table 3), and the decrease rate was 20.68% and 19.39%, respectively.
However, in the low and moderate suitability areas, they all showed noticeable expansion. For moderately suitability areas, the prediction of MaxEnt about gain in area under the future climate scenario combination RCP 8.5-2070 showed an increase of nearly 2.83 × 10
7 km
2 (a 12.27% expansion in the currently suitable habitat areas) (
Figure 6D and
Table 3). Except under the RCP4.5-2050 climate scenario, there was a slight decrease (3.50%), and under other climate scenarios, they all indicated a slight rise (1.17%-5.19%) (
Table 3). For low suitability area, under the RCP6.0-2050, RCP6.0-2070, and RCP8.5-2070 climate scenarios, the area increased to -3.29 × 10
7 km
2, -3.20 × 10
7 km
2, and -3.22 × 10
7 km
2, respectively, with growth rates of 24.75%, 21.55%, and 22.21%, respectively.
In summary, the total area of suitable habitat areas will tend to increase in the future. Under the RCP8.5-2070 climate scenario, the area of suitable habitat reached a maximum of 7.16 × 107 km2 i.e., an increase of 11.84% compared to the current.
4. Discussion
Currently, there has been a wide use of modeling to predict species distribution in disciplines such as ecology, biogeography, weed science and conservation biology [
32]. Particularly, regarding biological invasions, many researchers have been applying species distribution models to predict the potential distribution of invasive species subsequently using research results to strengthen the monitoring of relevant countries and regions [
33]. Compared to other models, Maxent software gained more popularity due to its stable performance and simple user interface [
34]. In the results of this study, the Maxent method predicted the potential distribution of
P. viburni based on the occurrence data of
P. viburni and environmental variables under current and future climatic conditions in the world. The results based on pROC test showed that the model had high performance — the prediction results of the model were much better than those of the random model.
The research on biological aspects of
P. viburni has been insufficient. The pest is found on all parts of a host plant [
4]. Franco et al. [
35] described a method for studying female fertility. The average lifespan of adult males is 87.7 ± 3.7 h and sexual maturity in adult males lasts approximately 45 h and is manifested by the maximal growth of waxy caudal filaments [
36]. If the environmental conditions are suitable, the female can complete development in about a month and can lay up to 200 eggs [
37]. These studies show that the growth and development of
P. viburni is related to temperature and the development cycle spans over a period of around one year.
The response curve in this study also reflected the relationship between P. viburni and relevant environmental variables including the precipitation of the coldest quarter (Bio19), precipitation seasonality (Bio15), mean temperature of the wettest quarter (Bio8), mean diurnal range (Bio2) and precipitation of the warmest quarter (Bio18). The total area of suitable habitat is likely to increase in future under different climate scenarios. Hence, the geographical areas that are supposed to provide suitable habitats for the pest in future should be focused for the future monitoring of the pest.
Under the current climatic conditions, the pest is mainly distributed in the Hunan, Anhui, Jiangxi, Fujian, and Taiwan provinces in China. Currently, only Guizhou in China has records [
38]. In the wake of global change, there is a possibility that the
P. viburni may be introduced to environments that are conducive for its growth and development. This introduction will have disastrous impacts by providing the pest with opportunities to colonize and spread due to the lack of natural enemies in the introduced range. Even though the highly suitable area is small, the moderately and marginally suitable areas are large, indicating that
P. viburni still poses an invasion risk in China. The moderately and marginally suitable areas are mainly located in South China, the quarantine measures at ports in these areas should be strengthened to prevent the invasion of
P. viburni.
In the recent past, the main components of climate change have been global warming, drought, increased CO
2 levels in the atmosphere and increase in frequency of extreme weather events (IPCC, 2014). The impact of climate change on insects is particularly important as it can directly or indirectly affect their populations and distribution [
26]. Hence, there is a need to keep a check on dispersal dynamics of invasive species under climate change. In this context, this study predicted the potential suitable areas of
P. viburni under the future climate scenario, and compared it with the suitable areas under the current climatic conditions. The results showed that the total suitable area will increase in the future climate conditions. Therefore, under the future climate scenarios, the risk of
P. viburni invasion will increase. This is required to strengthen the monitoring and quarantine measures at ports in the southern region of China to strictly prevent the invasion of
P. viburni.
Based on the theory of maximum entropy, the MaxEnt model was implemented using only two variables, i.e., occurrence information and corresponding environmental variables, yet the prediction results of the model in this study were relatively good. [
39]. In other words, the accuracy of the model based on Maxent was mainly affected by two factors: (i) occurrence data and (ii) environmental variables [
40]. According to previous studies, areas for which more distribution data is available tend to be overpredicted, while areas with fewer or no distribution records will be underpredicted. The current model is based only on some abiotic factors, which limits the accuracy of the potential distribution range of species under climate change. Other factors will also affect the results of the model, such as the background area (also known as “research area”), dispersal capacity [
41], land use [
42], and the host’s distribution range. Further, in the similar studies, some important factors are often ignored, and the data required by the model is simplified resulting in errors in the predicted results and there is a huge difference between prediction and reality. Because different researchers apply different sample numbers and parameters to the model, even predictions for the same species may yield diverse outcomes, giving varied species representation [
43].
This study provides important information about the potential distribution of P. viburni under the climate change conditions. Moreover, this work also provides an important theoretical basis for the monitoring and control of P. viburni. Further, when more data on the species distribution becomes available, niche models will better predict the potential impact of climate change on this species’ distribution.
5. Conclusions
This study used the distribution data of P. viburni and environmental factors to predict potentially suitable regions for invasion of this species under different climate scenarios. The results suggested that precipitation of the coldest quarter (Bio19), precipitation seasonality (Bio15), and mean temperature of the wettest quarter (Bio8) were the most important environmental factors determining the distribution of P. viburni. Under the current climate conditions, its potential suitable areas are mainly located in southern China, the whole of Japan, North American continent (especially the eastern part of the United States), the southwestern part of South America, the Mediterranean coast, most of Europe, the central part of Africa i.e., south of the Sahara Desert and most of the southern coast of Australia. The total area of suitable habitat is likely to show an increased trend under the future climatic conditions.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: Partial AUC Values and Graphics, null model (red distribution), distribution of expectations created via bootstrapping replacement of 50% of the total available points and 1000 resampling replicates (blue distribution); Table S1: Distribution sites for P. viburni; Table S2: Pearson’s correlation coefficients matrix of P. viburni; Table S3: ENMeval results for P. viburni from SDMs.
Author Contributions
Conceptualization, W.J. and J.W.; methodology, M.M.; software, W.J.; validation, W.J., B.C. and J.W.; formal analysis, W.J. and Z.H; investigation, M.M.; resources, J.W.; data curation, J.W.; writing—original draft preparation, W.J.; writing—review and editing, J.W.; visualization, M.M.; project administration, W.J.; funding acquisition, J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (No. 32100370), Science and Technology Innovation Funds of Shanxi Agricultural University (2020BQ79), the Excellent Doctoral Award of Shanxi Province for Scientific Research Project (SXBYKY2021024) and Science and Technology Innovation Projects of Universities in Shanxi Province (2021L097).
Acknowledgments
We would like to thank Yunyun Lu for their technical support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Franco, J.C,; Zada, A.; Menel, Z. 2009. Novel approaches for the management of mealybug pests. In: Ishaaya I, Horowitz AR (eds.) Biorational Control of Arthropod Pests. Springer, Dordrecht, 2009, 233-278.
- Thancharoen, A.; Lankawe, S.; Moonjuntha, P.; Wongphanuwat, T.; Sangtongpraow, B.; Ngoenklan, R.; Kittipadakul, P.; Wyckhuys, K.A.G. Effective biological control of an invasive mealybug pest enhances root yield in cassava. J. Pest Sci. 2018, 91, 1199–1211. [Google Scholar] [CrossRef]
- Miller, D.R.; Miller, G.L.; Watson, G.W. Invasive species of mealybugs (Hemiptera: Pseudococcidae) and their threat to U.S. Agriculture. Proc. Entomol. Soc. Wash. 2002, 104, 825–836. [Google Scholar]
- García Morales, M.; Denno, B.D.; Miller, D.R.; Miller, G.L.; Ben-Dov, Y.; Hardy., N.B. ScaleNet: A literature-based model of scale insect biology and systematics. Database 2016. [Google Scholar] [CrossRef]
- Hambleton, E.J. Notas sobre Pseudococcinae de importancia economica no Brasil com a descripção de quatro especies novas. (In Portuguese). Archivos do Instituto Biologico, São Paulo. 1935, 6, 105–120. [Google Scholar]
- Lepage, H.S. Catálogo dos Coccídeos do Brasil. (In Portuguese). Revista do Museu Paulista São Paulo 1938, 23, 327–491. [Google Scholar]
- Williams, D.J.; Granara de Willink, M.C. Mealybugs of Central and South America. CAB International, London, England, 1992. 635 pp.
- Charles, J.G. 2011. Using parasitoids to infer a native range for the obscure mealybug, Pseudococcus viburni, in South America. BioControl 2011, 56, 155–161. [Google Scholar] [CrossRef]
- Culik, M.P.; Gullan, P.J. A new pest of tomato and other records of mealybugs (Hemiptera: Pseudococcidae) from Espirito Santo, Brazil. Zootaxa 2005, 964, 1–8. [Google Scholar] [CrossRef]
- Poortinga, W.; Whitmarsh, L.; Steg, L.; Böhm, G.; Fisher, S. Climate change perceptions and their individual-level determinants: A cross-European analysis. Glob Environ Change. 2019, 55, 25–35. [Google Scholar] [CrossRef]
- Hossain, M.S.; Arshad, M.; Qian, L.; Kächele, H.; Khan, I.; Islam, M.D.I.; Mahboob, M.G.; 2020. Climate change impacts on farmland value in Bangladesh. Ecological Indicators 2020, 112, 106181. [Google Scholar] [CrossRef]
- Cao, C.Y.; Tao, J. Predicting the Areas of Suitable Distribution for Zelkova serrata in China under Climate Change. Sustainability. 2021, 13, 1493. [Google Scholar] [CrossRef]
- Broennimann, O.; Treier, U.A.; Müller-Schärer, H.; Thuiller, W.; Peterson, A.T.; Guisan, A. Evidence of climatic niche shift during biological invasion. Ecol Lett. 2007, 10, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, G., Blanchon, D., Foote, H., Pollonais, C., Mosee, A., 2015. Queensland fruit fly invasion of New Zealand: Predicting area suitability under future climate change scenarios. Perspectives in Biosecurity Research Series 2015, 2, 1–13.
- Phillips, S.J.; Anderson, R.P.; Schapire, R. E. Maximum entropy modeling of species geographic distributions. Ecol Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography. 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Hijmans, R.J,; Phillips, S,; Leathwick, J,; Elith, J. 2021. Dismo: Species distribution modeling, R package version 1.3 -5.
- Hijmans, R.J.; Cameron, S.E,; Parra, J.L,; Jones, P.G.; Jarvis, A, Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 2005, 25, 195–204. [CrossRef]
- Thapa, A.; Wu, B.; Hu, Y.B.; Nie, Y.G.; Singh, P.B.; Khatiwada, R.R.; Yan, L.; Gu, X.D.; Wei, F.W. Predicting the potential distribution of the endangered red panda across its entire range using MaxEnt modeling. Ecol. Evol. 2018, 8, 10542–10544. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.Y.; Chen, C.; Zhao, Q.; Wei, J.F.; Zhang, H.F. Identifying potentially climatic suitability areas for Arma custos (Hemiptera: Pentatomidae) in China under climate change. Insects 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Radosavljevic A, Anderson RP. Making better MaxEnt models of species distributions: Complexity, overfitting and evaluation. J Biogeo. 2014, 47, 629–643. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Zhao, Q.; Cheng, L.F.; Zhao, L.; Zhang, H.F.; Wei, J.F. The Potential Global Distribution of the White Peach Scale Pseudaulacaspis pentagona (Targioni Tozzetti) under Climate Change. Forests. 2020, 11, 192. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudik, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Kass, J.M.; Muscarella, R.; Galante, P.J.; Bohl, C.L.; Pinilla-Buitrago, G.E.; Boria, R.A.; Soley-Guardia, M.; Anderson, R.P. ENMeval 2.0: Redesigned for customizable and reproducible modeling of species’ niches and distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- Qin, Z.; Zhang, J.E.; Ditommaso, A.; Wang, R.L.; Wu, R.S. Predicting invasions of Wedelia trilobata (L.) Hitchc. with Maxent and GARP models. J Plant Res. 2015, 128, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.F.; Peng, L.F.; He, Z.Q.; Lu, Y.Y.; Wang, F. Potential distribution of two invasive pineapple pests under climate change. Pest Manag Sci. 2020, 76, 1652–1663. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, D.; Guo, G.; Hu, Y.H.; Wei, J.F.; Liu, J.Z. Species delimitation of the Dermacentor ticks based on phylogenetic clustering and niche modeling. PeerJ. 2019, 7, e6911. [Google Scholar] [CrossRef] [PubMed]
- Morato, T. González-Irusta, J.M. Dominguez-Carrió, C. Wei, C.L. Davies, A. Sweetman, A.K. Taranto, G.H. Beazley, L. García-Alegre, A.et al. Climate-induced changes in the suitable habitat of cold-water corals and commercially important deep-sea fishes in the North Atlantic. Global Change Biol. 2020, 26, 2181–2202. [Google Scholar]
- Shabani, F.; Kumar, L.; Ahmadi, M. Assessing Accuracy Methods of Species Distribution Models: AUC, Specificity, Sensitivity and the True Skill Statistic. Glob. J. Hum. Soc. Sci. 2018, 18, 6–18. [Google Scholar]
- Lobo, J.M.; Jiménez-Valverde, A.; Real, R. AUC: A misleading measure of the performance of predictive distribution models. Glob Ecol Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Osorio-Olvera, L. Lira-Noriega, A. Soberón, J. Townsend Peterson, A. Falconi, M. Contreras-Díaz, R.G.; Martínez-Meyer, E.; Barve, V.; Barve, N. ntbox: An R package with graphical user interface for modeling and evaluating multidimensional ecological niches. Methods Ecol Evol. 2020, 11, 1199–1206. [Google Scholar] [CrossRef]
- Guisan, A. Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, M.C.; Weltzin, J.F. Ecological niche models and the geography of biological invasions: A review and a novel application. In: Inderjit (eds) Invasive Plants: Ecological and Agricultural Aspects. Birkhäuser Basel. 2005, 2005, 45–46. [Google Scholar]
- Byeon, D.; Jung, S.; Lee, W.H. Review of CLIMEX and MaxEnt for studying species distribution in South Korea. J Asia Pac Biodivers. 2018, 11, 325–333. [Google Scholar] [CrossRef]
- Franco, J.C.; Pereira, R.C. Metodo para o estudo da biologia reprodutiva de especies oviparas da familia Pseudococcidae (Homoptera: Coccoidea). Actas do Congresso Iberico de Entomologia. 1992, 5 (Suppl. 3), 291–298. [Google Scholar]
- Mendel, Z. Protasov, A. Jasrotia, P. Borges da Silva, E. Levi-Zada, A. Franco, J.C. Sexual maturation and aging of adult male mealybug (Hemiptera: Pseudococcidae). B Entomol Res. 2012, 102, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Pacheco da Silva, V.C. Nondillo, A. Galzer, E.C.W. Garcia, M.S. Botton, M.I. Effect of host plants on the development, survivorship, and reproduction of Pseudococcus viburni (Hemiptera: Pseudococcidae). Fla Entomol. 2017, 100, 718–724. [Google Scholar] [CrossRef]
- Wang, Y.S.; Zhou, P.; Tian, H.; Wan, F.H.; Zhang, G.F. First Record of the Invasive Pest Pseudococcus jackbeardsleyi (Hemiptera: Pseudococcidae) on the Chinese Mainland and Its Rapid Identification Based on Species-Specific Polymerase Chain Reaction. J Econo. Entomo. 2018, 111, 2120–2128. [Google Scholar] [CrossRef] [PubMed]
- Ji, W., Gao, G., Wei, J.F., Potential Global Distribution of Daktulosphaira vitifoliae under Climate Change Based on MaxEnt. Insects 2021, 12, 347. [CrossRef]
- Kramer-Schadt, S.; Niedballa, J.; Pilgrim, J.D.; Schröder, B.; Lindenborn, J.; Reinfelder, V.; Stillfried, M.; Heckmann, I.; Scharf, A.K.; Augeri, D.M.; Cheyne, S.M.; Hearn, A.J.; Ross, J.; Macdonald, D.W.; Mathai, J.; Eaton, J.; Marshall, A.J.; Semiadi, G.; Rustam, R.; Bernard, H.; Alfred, R.; Samejima, H.; Duckworth, J.W.; Breitenmoser-Wuersten, C.; Belant, J.L.; Hofer, H.; Wilting, A. The importance of correcting for sampling bias in MaxEnt species distribution models. Diversity Distrib. 2013, 19, 1366–1379. [Google Scholar] [CrossRef]
- Mothes, C.C.; Stroud, J.T.; Clements, S.L.; Searcy, C.A. Evaluating ecological niche model accuracy in predicting biotic invasions using South Florida's exotic lizard community. J Biogeogr. 2019, 46, 432–441. [Google Scholar] [CrossRef]
- Yang, X.Q.; Kushwaha, S.P.S.; Saran, S.; Xu, J.; Roy, P.S. 2013. Maxent modeling for predicting the potential distribution of medicinal plant, Justicia adhatoda L. in Lesser Himalayan foothills. Ecol Eng. 2013, 51, 83–87. [Google Scholar] [CrossRef]
- Li, Y.C.; Li, M.Y.; Li, C.; Liu, Z.Z. Optimized Maxent Model Predictions of Climate Change Impacts on the Suitable Distribution of Cunninghamia lanceolata in China. Forests. 2020, 11, 302. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).