1. Introduction
Antinuclear antibodies (ANAs) are a group of autoantibodies that recognize and target nuclear macromolecules and their complexes [
1]. Due to its high sensitivity, ANA testing is utilized as screening tool for many autoimmune diseases, such as mixed connective tissue disease, systemic sclerosis, primary Sjogren’s syndrome, autoimmune hepatitis, and systemic lupus erythematosus (SLE) [
2,
3,
4,
5,
6,
7,
8,
9,
10]. The 2019 EULAR/ACR classification of SLE requires an ANA titer of ≥1:80 (Hep-2 Indirect immunofluorescence or equivalent test) as the entry criteria since sensitivity nears 100% [
11,
12]. Furthermore, while most SLE patients are ANA-positive, most ANA-positive patients do not have SLE [
13].
ANA testing alone is therefore never sufficient for a definitive diagnosis due to its lack of specificity. In addition to other autoimmune diseases, its prevalence is common in the general population and increases with age. Approximately 14% of Americans 12 years and older have an ANA titer ≥1:80 [
14]; studies in other countries estimate the prevalence range from 7 to 18% [
15,
16,
17]. On the other hand, the presence of anti-double-stranded DNA antibodies (anti-dsDNA) is highly specific for SLE and is weighted significantly for diagnosis. The 2019 EULAR/ACR criteria require a score of 10 or higher to be to diagnostic for SLE; a positive anti-dsDNA test alone counts as 6 points [
11]. Approximately 60-83% of SLE patients have been found to have detectable levels of anti-dsDNA at some point during their illness [
18,
19,
20], and specificity of anti-dsDNA ranges from 88% to 99% depending on the testing method (acceptable by EULAR/ACR standards) used [
21,
22,
23]. One landmark cohort study found that 55% of SLE patients (n=130) exhibited elevated anti-dsDNA levels up to 9.3 years before diagnosis [
24].
There are several methods to detect ANA, most notably the indirect immunofluorescence ANA-IIF and the ELISA technique. The American and Europeans rheumatology Societies (ACR and EULAR) currently endorse ANA-IIF for ANA detection [
25]. ANAs are assayed using various techniques, with the gold standard being the fluorescent ANA [
26,
27]. ANA testing with ELISA was introduced to save time and improve objectivity.
As laboratory-based antibody testing continues to be emphasized in the diagnostic process, point-of-care tests (POCT) have become increasingly useful for early diagnosis, rapid diagnosis, and diagnostic efficiency. POCT exhibit convenience, cost-effectiveness, and accurate/reliable results, whether utilized in a laboratory, clinical, or nonprofessional setting. The benefits extend to both the provider and the patient. Providers save time by obtaining quicker results and can therefore make timely medical decisions [
28,
29]. A comprehensive review of current and emerging POCT has recently been published [
30]. In many cases, skilled technicians are not even needed, and the performance of the test can be delegated to the patient [
31]. This provides patients with the opportunity to play a more active role in their health, providing similar or better clinical outcomes than professional care management.
One emerging POCT technology is the vertical flow assay (VFA), which was developed in response to the need for rapid testing and multiplex detection that the lateral flow assay (LFA) falls short in [
32,
33,
34,
35]. As reviewed, VFA exhibit faster turnaround time, higher multiplex capability and high sample volume capacity characteristics than LFA [
36]. LFA utilizes horizontal sample flow on a test strip [
37,
38,
39]. Therefore, the multiplexing capabilities are hindered by the nature of horizontal flow and the physical shape of the test strip. The VFA addresses these issues by utilizing downward flow. Gravity assists in the capillary action of fluids, which helps yield faster results when compared to lateral flow [
40,
41].
Quicker and more convenient detection methods are needed to achieve earlier diagnoses of SLE, as this can have a significant impact on survival and quality of life [
42,
43]. Anti-dsDNA levels are often used to track the progression and severity of SLE since they correlate closely with the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) [
44,
45,
46]. Furthermore, SLE patients who experience significant increases in serum anti-dsDNA upon diagnosis are more likely to have renal disease, and surges in anti-dsDNA titers are predictive of severe lupus flares [
47,
48,
49,
50,
51]. Utilizing VFA to rapidly measure ANA and anti-dsDNA levels may thus have a profound impact on clinical management of SLE. This work is designed to explore this possibility.
2. Materials and Methods
2.1. Design of the VFA
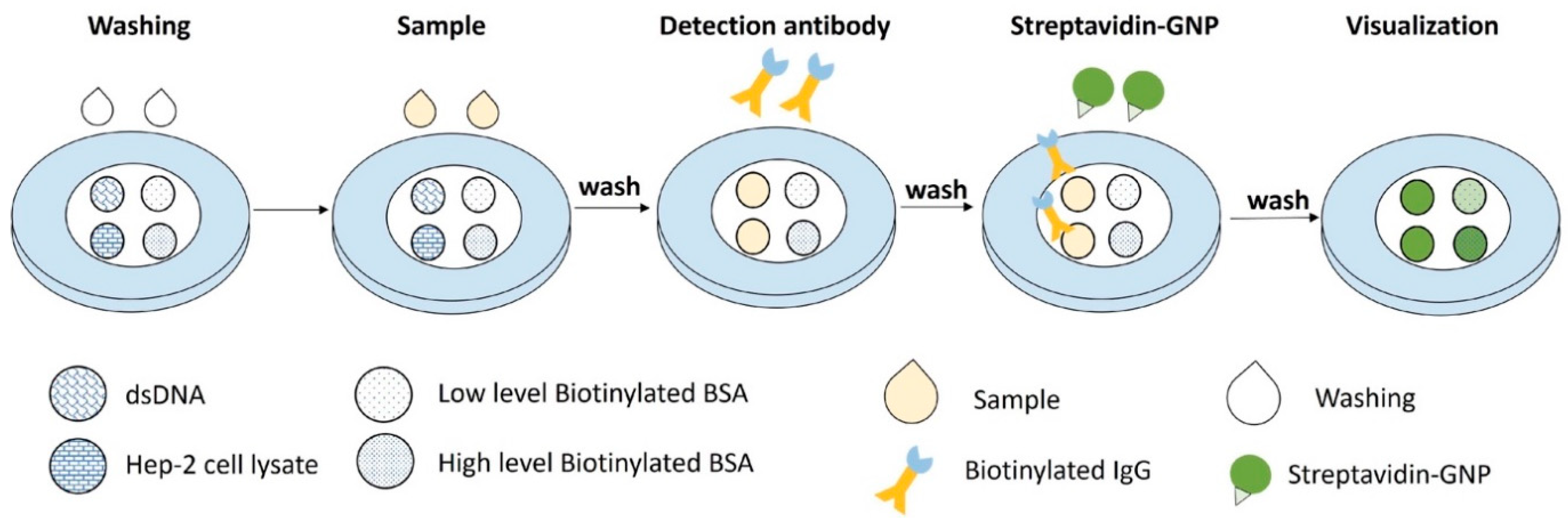
The general principle of the VFA setup is as follows: the sample containing anti-dsDNA and ANA autoantibodies bind to their respective capture antigen substrate (dsDNA and nuclear antigens) on a nitrocellulose membrane (NCM) and forms an antigen-antibody immuno-complex. Biotinylated anti-human IgG is then added to bind with the anti-dsDNA or ANAs. Finally, streptavidin-conjugated gold nanoparticles (GNP) are added to bind with the anti-IgG, displaying a green colorimetric signal on the NCM. Between each step, the membrane is washed to remove unbound materials and to prevent nonspecific binding, as shown in
Figure 1.
The rapid vertical flow immunoassay development system and universal buffer were purchased from Cytodiagnostics. Bovine serum albumin (BSA), Tween-20, Sodium chloride (NaCl), Tris-HCl, Phosphate Buffer Saline (PBS, pH 7.4), Polyethylene glycol average mol wt 6000 (PEG 6000), Polyvinylpyrrolidone average mol wt 40,000 (PVP40, Sigma) were purchased from Sigma and diluted in MilliQ water and then filtered by 0.2 µm filter (Pall Science). dsDNA (Sigma), Hep-2 cell lysate (source of nuclear antigens for ANA testing; Novus Biologicals), BSA- Bovine AGE-BSA Biotinylated Protein (R&D system), human anti-dsDNA standard plasma (708 IU/ml, PSG Lot ID: 20400), biotinylated anti-IgG (Abcam), 150 nm streptavidin-conjugated GNP (Nanocomposix) were purchased for VFA development. In addition, a double-Stranded DNA (dsDNA) IgG ELISA kit and ANA Screen ELISA kit were purchased from ORIGENE for assay validation. mBSA (methylated bovine serum albumin, Sigma Aldrich), anti-IgG horseradish peroxidase (Abcam), Gelatin (Sigma Aldrich), Ethylenediaminetetraacetic acid (EDTA, fisher), and TMB (3,3',5,5'-Tetramethylbenzidine, Thermo Fisher) were purchased for in-house ELISA validation.
2.2. Test Samples
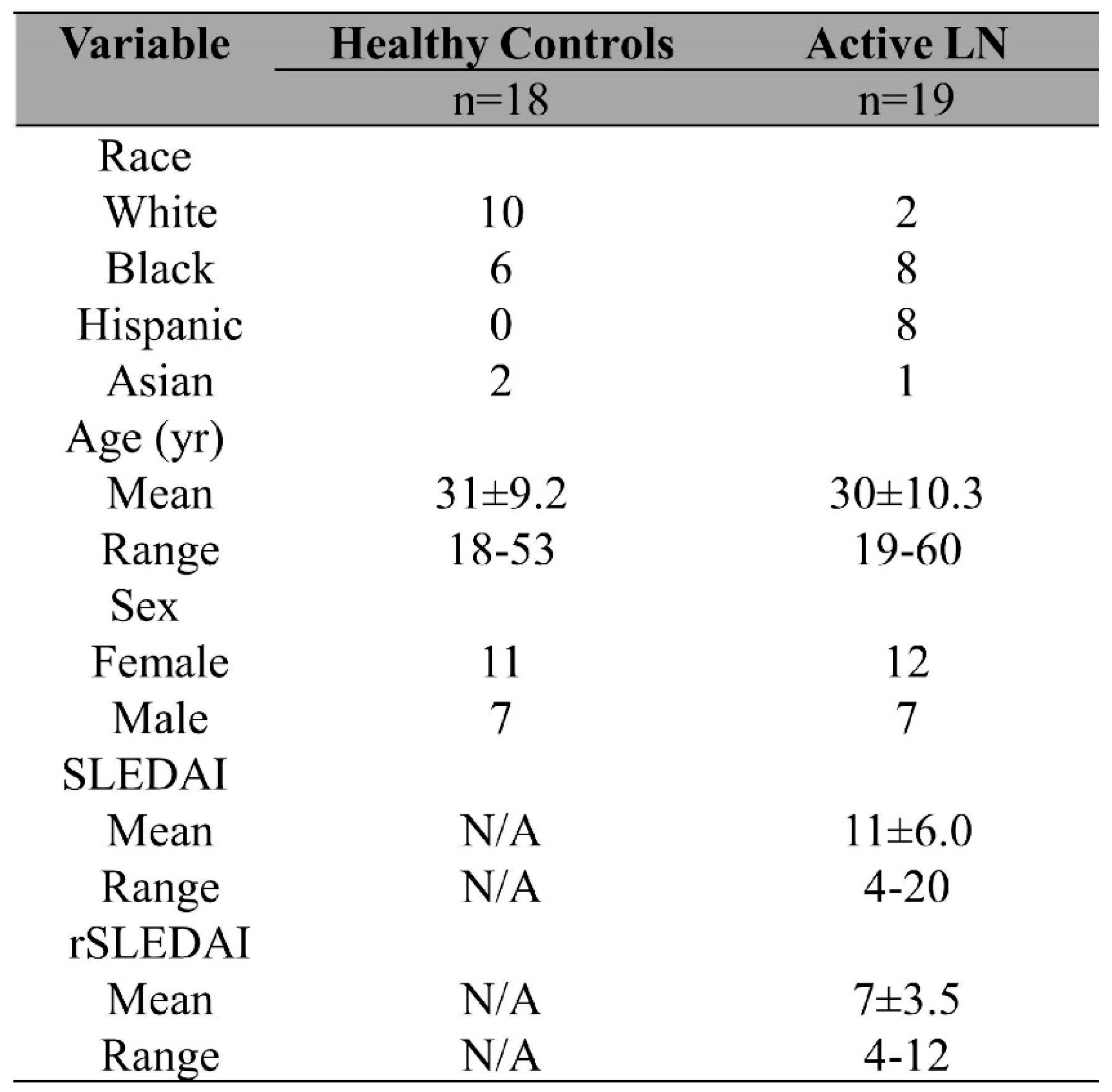
`Only archived de-identified samples were used for the study. Prior to adding to the archive, all samples were obtained with written informed consent. Archived de-identified serum samples (19 active LN, 1 inactive SLE, and 2 SLE without valid demographics) were obtained from UT Southwestern Medical Center (Dallas, TX, USA). All SLE patients met the 2012 Systemic Lupus International Collaborating Clinics SLE classification criteria. Additionally, active renal SLE (lupus nephritis, LN) activity was evaluated based on a subset of the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) 2000 criteria, designated the renal Systemic Lupus Erythematosus Disease Activity Index (rSLEDAI). Any rSLEDAI score >0 was considered as active LN. The healthy controls (HC) were volunteers with no self-reported conditions. This study was approved by the Institutional Review Boards at UT Southwestern Medical Center and the University of Houston. The demographic information of the serum samples is detailed in
Table 1. Serum samples were aliquoted and stored at -20 °C. There was no additional centrifugation before applying samples onto the VFA.
In addition to the above subjects, one inactive SLE subject was also included in the testing panel. SLEDAI: Systemic Lupus Erythematosus Disease Activity Index ; rSLEDAI: renal SLEDAI; LN: lupus nephritis.
2.3. Comparison with ELISA
All clinical samples tested by VFA were also tested using a commercial ELISA. Following manufacturer instructions, the plates were then transferred to a microplate reader (ELX808, BioTek Instruments, Winooski, VT, USA) and optical density was measured at 450 nm.
2.4. Statistical analysis
The images were first inspected by naked eye, then captured using an iPhone 12, and then analyzed by Image J. Dose-response curves were generated using Excel 2007, while the comparison between the VFA and commercial ELISA were plotted and analyzed using GraphPad Prism 5 (GraphPad, San Diego, CA). Biomarker group comparisons by VFA and commercial ELISA were analyzed using the Mann–Whitney U-test as datasets were not normally distributed. One-way ANOVA was used to analyze the LoD and linearity (r2) metrics for VFA performance evaluation. ROC curves were plotted with the area under the curve to demonstrate the discriminative power of the biomarker as measured by VFA and ELISA. The limit of detection (LoD) was the lowest concentration that can be detected, denoted by the sum of the mean of the blank (n=2) and three times the standard deviation of the blank.
3. Results
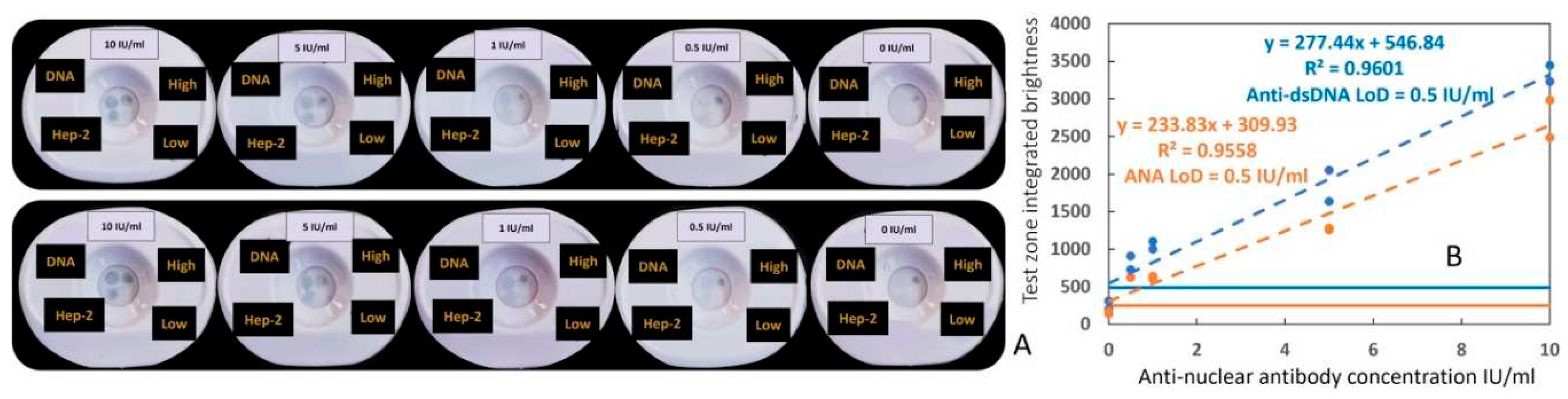
3.1. Detection of antinuclear antibody using spiked human anti-dsDNA positive control standard and the duplex VFA
A human anti-dsDNA positive control standard, which can bind dsDNA and Hep-2 cell (nuclear antigen) lysates, was used as an internal standard to engineer the rapid vertical flow immunoassay development system to detect anti-dsDNA and ANAs using gold nanoparticles (GNP). To develop the duplex VFA we first optimized the antigen concentration (Hep-2 cell lysates, dsDNA), biotinylated detection antibody and streptavidin conjugated GNP concentrations, clinical sample dilution factor and buffer components, as shown in
Supplementary Figure S1. Later, following assay protocol-1 (
supplementary method-1), positive samples were prepared by serially diluting a human anti-dsDNA positive control standard (ANA+ & αDNA+), while assay diluent was used as the negative control. Each test was run in duplicate. As shown in
Figure 2A,B, serial dilution of positive standard demonstrated a corresponding decrease in αDNA and ANA test zones signal intensity whereas the “High” and “Low” controls zones maintained their respective intensities. For the detection of both antibodies, the duplex assay showed a limit of detection (LoD) of 0.5 IU/ml and a reportable range from 0.5 IU/ml to 10 IU/ml.
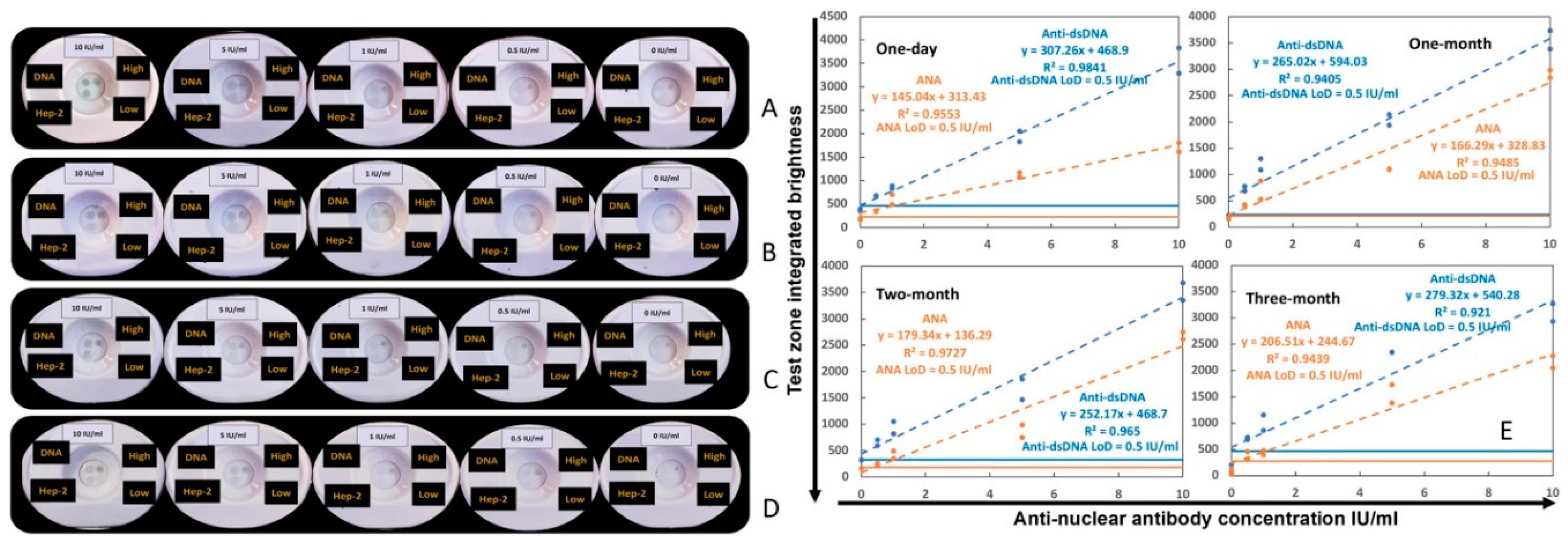
3.2. Impact of storage duration on the performance of the ANA-αDNA VFA
The stability of the duplex VFA was evaluated as per assay protocol-1 (
supplementary method-1) through storage in a desiccator at room temperature over three months. After one-day, one-month, two-month, and three-month storage, the same standard curve as above was run by serially diluting human anti-dsDNA positive control standard (ANA+ & αDNA+). In
Figure 3A–D, the images of four sets of standard curves are shown in order of increasing storage time. The linearity and LoD of the four sets of serial dilutions are shown in
Figure 3E. One-way ANOVA nonparametric test was used to compare the linearity and LoD of the four sets of data, and no significant differences was found between the storage conditions (data not shown), indicating that storage time had no impact on VFA performance.
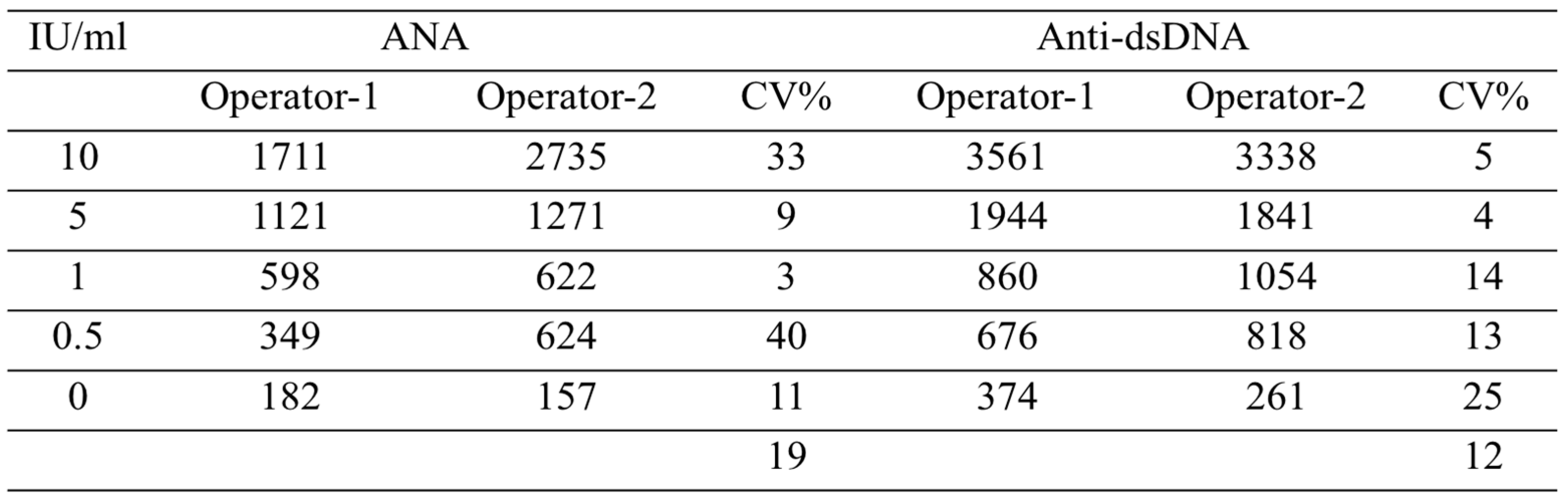
3.3. Inter-operator coefficient of variation in operating the duplex ANA-αDNA VFA
To verify the reproducibility of running and reading the duplex ANA-αDNA VFA, two operators ran the same serial dilution using the ANA-αDNA VFA, and the inter-operator coefficient of variation (CV) was calculated for each concentration point, as shown in
Table 2. The average inter-operator CV was 12.3% for reading anti-dsDNA levels and 19% for reading ANA levels.
3.4. Testing clinical serum samples using the duplex ANA-αDNA VFA and ELISA
To facilitate running of clinical samples, an all-purpose GNP diluent-2 was identified that can be used for diluting the serum sample, detection Ab and GNP (
Figure S2). Serum autoantibody levels in 22 SLE and 18 HC serum samples were assayed using the duplex ANA-αDNA VFA using assay protocol 2 (
supplementary method-2), images shown in (
Figure S3). The observer score (OS) of each assay was recorded based on
supporting method-3 (Figure S4) by three researchers through visual intensity, scores shown in
Supplementary Figure S5.
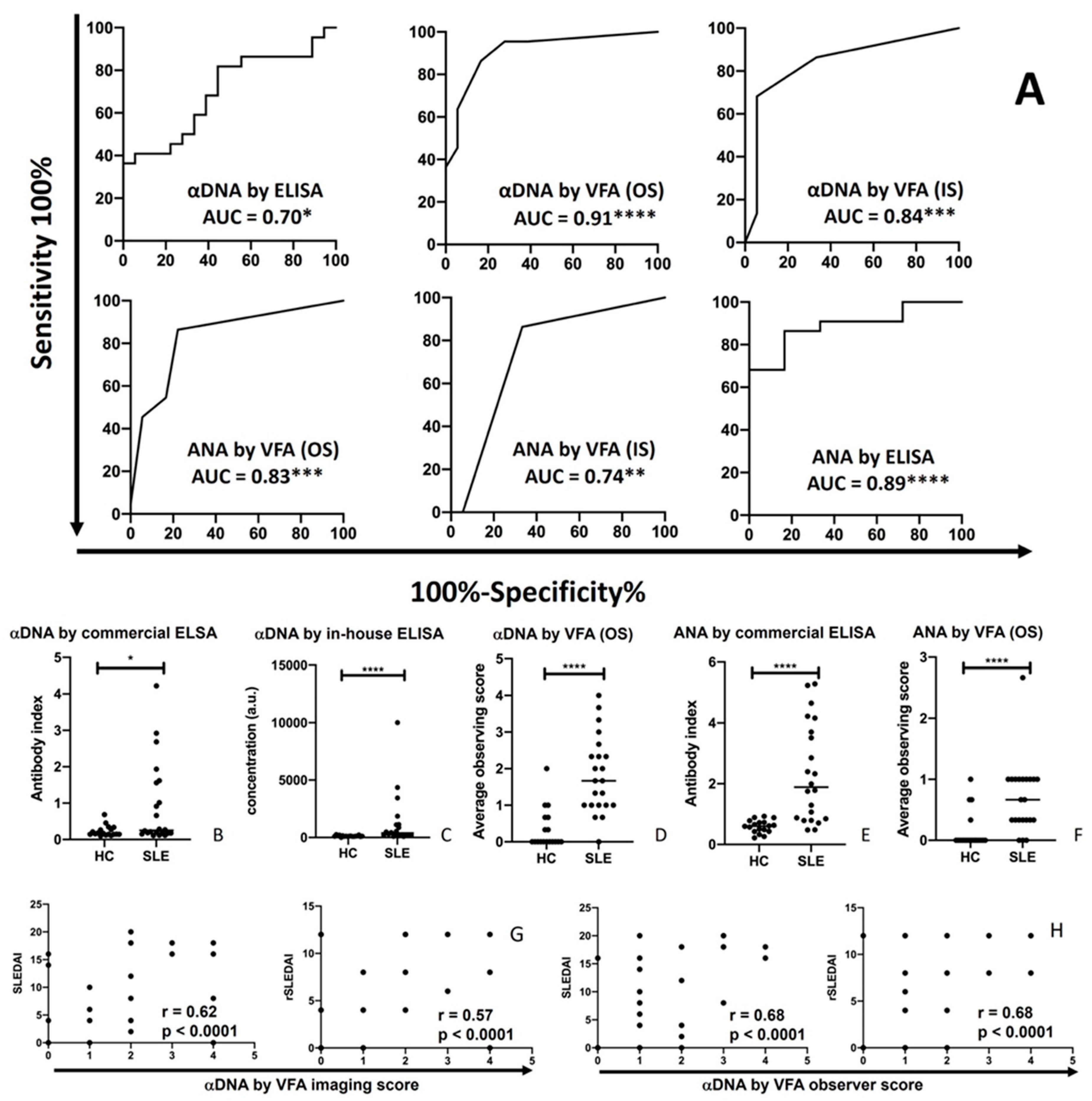
The same serum samples from 22 SLE patients and 18 healthy controls tested by VFA were also evaluated using commercial ELISA assays for anti-dsDNA and ANA detection. The OS and IS of the duplex ANA-αDNA VFA showed good discriminating power for distinguishing SLE patients from HC, as did the commercial ELISA, based on ROC AUC analysis, as shown in
Figure 4A. The αDNA levels in SLE patients were significantly higher than in healthy control when assayed by ELISA (p<0.05,
Figure 4B,C), or by VFA (p<0.0001,
Figure 4D). ANAs in SLE patients were also significantly higher than in healthy control when assayed by ELISA (p<0.0001,
Figure 4E), in good agreement with the measurement of ANA by VFA (p<0.0001, Figure 5F). Similar findings were observed when we used an in-house αDNA ELISA, as shown.
The SLEDAI and rSLEDAI indices in the 22 SLE patients (19 active LN, 1 inactive SLE, and 2 patients without valid SLEDAI or rSLEDAI value) exhibited significant Pearson correlation with serum αDNA levels as assayed by VFA (OS or IS), with correlation coefficients typically exceeding 0.6 (
Figure 4G-H), alluding to their potential clinical utility.
4. Discussion
There is clearly an unmet need for identifying improved biomarkers for SLE and LN, as reviewed elsewhere [
52]. In addition, it is also imperative to design better ways to detect these biomarkers and to enable patients to self-monitor their disease from the comfort of their home. Technologies that allow for point of care (POC) monitoring of disease are certainly needed. One such approach is a recently described LFA method for monitoring urinary disease biomarker ALCAM and urine normalizer HVEM [
39], in contrast to conventional ELISA for the same biomarker’s detection [
53,
54]. As opposed to POC devices for detecting protein biomarkers, there are currently no reports of POC devices for monitoring autoantibodies in SLE/LN. Given that anti-dsDNA and ANA autoantibodies are highly associated with lupus [
11,
12,
18,
19,
20,
21,
22,
23,
24], and can even be predictive of disease activity in SLE/LN [
44,
45,
46,
47,
48,
49,
50,
51,
55] it is important to engineer POC approaches to make this feasible. The reported work meets this unmet need.
Two POC technologies were considered, namely the LFA and the VFA. The latter was selected in view of its several advantages including faster turnaround time, higher multiplex capability and potentially higher sensitivity [
36]. Furthermore, we proceeded to design a duplex VFA capable of detecting two of the most important diagnostic autoantibodies in SLE (anti-dsDNA and ANA) within a single assay kit. The engineered duplex VFA displayed minimal background signal for the negative control and brightly colored positive zones, varying linearly with serum antibody concentrations. The duplex VFA showed a limit of detection of 0.5 IU/ml for both autoantibody targets and demonstrated good inter-operator reproducibility as well as storage stability up to the maximum period tested, 3 months. With actual clinical samples, the engineered duplex ANA-αDNA VFA displayed a diagnostic power of 0.84 by observer score in distinguishing SLE sera from HC sera, comparable with the gold standard, ELISA. Moreover, the ANA and anti-dsDNA levels measured using the engineered VFA correlated significantly with both the SLEDAI and renal SLEDAI disease activity indices. Of note, the VFA enabled duplex detection of both ANA and αDNA within 10 min, compared to the gold standard ELISA, which is single-plex and requires several days for completion in clinical laboratories. Moreover, an ELISA assay can only be performed in a laboratory setting, using equipment such as readers and washers, by a trained technician. In contrast the 10-minute turnaround time of the duplex VFA enables POC testing in primary care clinics and even home testing.
This duplex VFA can potentially be used in primary care clinics for screening of patients where rheumatic diseases are considered as a differential. Since a large fraction of autoimmune rheumatic diseases are documented to be ANA positive, this point of care test (which can be completed in 10 minutes) will enable the clinician to triage patients for confirmatory autoantibody panel testing or for referrals to a rheumatologist. On the other hand, new patients and/or high risk subjects (e.g., first degree relatives of SLE patients) who test positive for ANA and anti-dsDNA may be selected for confirmatory tests for clinching a diagnosis of SLE. The transition from ANA seropositivity to anti-DNA seropositivity can also be captured using this duplex VFA, particularly in pre-SLE or pre-clinical SLE patients. Moreover, the correlation of the assayed antibodies with SLEDAI and rSLEDAI also suggests that this duplex VFA may enable routine monitoring of disease activity in a patient with SLE. In fact, regular home-based monitoring is also feasible using either a drop of blood or saliva, as we have reported elsewhere [
56,
57].
Several aspects of this study and the VFA design could be enhanced. The sample size could be expanded even further to include a larger volume of participants, with well-organized cohorts. The engineered duplex VFA serves as an illustration of point-of-care (POC) testing utilizing serum samples. For scenarios in which a centrifuge is unavailable, the assay can be integrated with the application of Cleanascite, a reagent designed to selectively remove lipids, fats, impurities, and other cellular debris from samples. Alternatively, the Folch and Bligh and Dyer methods can be employed as substitutes. Nevertheless, a notable advantage of the VFA lies in its substantial multiplexing capability. As new disease diagnostic biomarkers emerge in the forthcoming years, this established protocol and point-of-care testing approach can be readily extended to encompass these additional markers, along with disease progression inflammatory biomarkers such as interleukins and vimentin [
58,
59,
60].
5. Conclusions
Commonly utilized methods to detect SLE-associated antibodies such as immunofluorescence or ELISA are time consuming and resource intensive. The engineered duplex VFA test reported here is formulated based on the sandwich immunoassay principle. For the first time, we have engineered a remarkably specific vertical flow-based point-of-care test for the rapid screening for rheumatic disease and systemic lupus erythematosus. To the best of our knowledge, no published work exists on the rapid detection of ANA and αDNA via VFA. In this study, the duplex ANA-αDNA VFA demonstrated substantial concordance with conventional ELISA in terms of discriminating SLE, and exhibited strong agreement with the SLEDAI and rSLEDAI in terms of diagnosing SLE activity. As a result, this point-of-care test holds promise for effectively testing or screening a large number of subjects rapidly and delivering more precise estimates of ANA and/or SLE prevalence, particularly in resource-limited settings.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Optimization of protocols for αDNA VFA and ANA VFA; Figure S2: Optimization of duplex ANA-αDNA VFA for clinical samples; Figure S3: Images of 40 clinical serum samples (22 SLE and 18 HC) tested using the duplex ANA-αDNA VFA; Figure S4: ANA-αDNA VFA reference and test zone semiquantitative reporting; Figure S5: Observer score and imaging score of 40 clinical serum samples tested using the ANA-αDNA VFA.
Author Contributions
Conceptualization, R.L. and C.M.; methodology, R.L.; H.A., and D.W. software, R.L.; validation, R.L.; H.A., and D.W.; formal analysis, R.L..; investigation, R.L. and C.M..; resources, R.S., and C.M.; data curation, R.L.; H.A., and D.W.; writing—original draft preparation, R.L., D.W., H.A., and C.M., writing—review and editing, R.L., H.A., J.A., and C.M..; visualization, R.L.; H.A., and D.W.; supervision, C.M.; project administration, R.L. and C.M.; funding acquisition, C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the NIH, grant number R01AR074096.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Boards of the JHU School of Medicine and the University of Houston.
Data Availability Statement
Data are available upon reasonable request.
Acknowledgments
The authors acknowledge all participants and patients of this study. Contributors CM conceived the study. RL, DW, HA undertook experiments needed for this study, recorded OS of the assay and data analysis. RW, DW, HA, NT and CM wrote the manuscript. All authors read and approved the manuscript. The authors would like to thank Richard Willson, Katerina Kourentzi, and Binh Vu for their technical guidance on point of care diagnostics including image analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pisetsky DS. Antinuclear antibody testing — misunderstood or misbegotten? Nat Rev Rheumatol (2017) 13:495–502. [CrossRef]
- Bossuyt, X., De Langhe, E., Borghi, M. O., & Meroni, P. L. (2020). Understanding and interpreting antinuclear antibody tests in systemic rheumatic diseases. Nature Reviews Rheumatology, 16(12), 715–726. [CrossRef]
- Pisetsky, D. S. (2012). Antinuclear Antibodies in Rheumatic Disease: A Proposal for a Function-Based Classification. Scandinavian Journal of Immunology, 76(3), 223–228. [CrossRef]
- Tan, E. M., Feltkamp, T. E. W., Smolen, J. S., Butcher, B., Dawkins, R., Fritzler, M. J., Gordon, T., Hardin, J. A., Kalden, J. R., Lahita, R. G., Maini, R. N., McDougal, J. S., Rothfield, N. F., Smeenk, R. J., Takasaki, Y., Wiik, A., Wilson, M. R., & Koziol, J. A. (1997). Range of antinuclear antibodies in “healthy” individuals. Arthritis & Rheumatism, 40(9), 1601–1611. [CrossRef]
- Bennett RM. Mixed connective tissue disease and other overlap syndromes. In: Textbook of Rheumatology, 7th ed, Kelley W, Harris EDJ, Ruddy SH, Sledge G (Eds), WB Saunders, 2004. p.1241.
- Kavanaugh, A., Tomar, R., Reveille, J., Solomon, D. H., & Homburger, H. A. (2000). Guidelines for Clinical Use of the Antinuclear Antibody Test and Tests for Specific Autoantibodies to Nuclear Antigens. In Archives of Pathology & Laboratory Medicine (Vol. 124, Issue 1, pp. 71–81). Archives of Pathology and Laboratory Medicine. [CrossRef]
- Hernández-Molina G, Nuñez-Alvarez C, Avila-Casado C, Llorente L, Hernández-Hernández C, Calderillo ML, Marroquín V, Recillas-Gispert C, Romero-Díaz J, Sánchez-Guerrero J. Usefulness of IgA Anti-α-fodrin Antibodies in Combination with Rheumatoid Factor and/or Antinuclear Antibodies as Substitute Immunological Criterion in Sjögren Syndrome with Negative Anti-SSA/SSB Antibodies. J Rheumatol. 2016 Oct;43(10):1852-1857. Epub 2016 Aug 1. PMID: 27481899. [CrossRef]
- Delgado, J., Vodonos, A., Malnick, S., Kriger, O., Wilkof-Segev, R., Delgado, B., Novack, V., Rosenthal, A., Menachem, Y., Melzer, E., & Fich, A. (2013). Autoimmune hepatitis in southern Israel: A 15-year multicenter study. In Journal of Digestive Diseases (Vol. 14, Issue 11, pp. 611–618). Wiley. [CrossRef]
- Alvarez, F., Berg, P. A., Bianchi, F. B., Bianchi, L., Burroughs, A. K., Cancado, E. L., Chapman, R. W., Cooksley, W. G. E., Czaja, A. J., Desmet, V. J., Donaldson, P. T., Eddleston, A. L. W. F., Fainboim, L., Heathcote, J., Homberg, J.-C., Hoofnagle, J. H., Kakumu, S., Krawitt, E. L., Mackay, I. R., … Zeniya, M. (1999). International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. In Journal of Hepatology (Vol. 31, Issue 5, pp. 929–938). Elsevier BV. [CrossRef]
- Al-Chalabi, T., Boccato, S., Portmann, B. C., McFarlane, I. G., & Heneghan, M. A. (2006). Autoimmune hepatitis (AIH) in the elderly: A systematic retrospective analysis of a large group of consecutive patients with definite AIH followed at a tertiary referral centre. In Journal of Hepatology (Vol. 45, Issue 4, pp. 575–583). Elsevier BV. [CrossRef]
- Aringer, M., Costenbader, K., Daikh, D., Brinks, R., Mosca, M., Ramsey-Goldman, R., Smolen, J. S., Wofsy, D., Boumpas, D. T., Kamen, D. L., Jayne, D., Cervera, R., Costedoat-Chalumeau, N., Diamond, B., Gladman, D. D., Hahn, B., Hiepe, F., Jacobsen, S., Khanna, D., Johnson, S. R. (2019). 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Annals of the Rheumatic Diseases, 78(9), 1151–1159. [CrossRef]
- Aringer, M., Brinks, R., Dörner, T., Daikh, D., Mosca, M., Ramsey-Goldman, R., Smolen, J. S., Wofsy, D., Boumpas, D. T., Kamen, D. L., Jayne, D., Cervera, R., Costedoat-Chalumeau, N., Diamond, B., Gladman, D. D., Hahn, B., Hiepe, F., Jacobsen, S., Khanna, D., … Johnson, S. R. (2021). European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) SLE classification criteria item performance. In Annals of the Rheumatic Diseases (Vol. 80, Issue 6, pp. 775–781). BMJ. [CrossRef]
- Egner, W. (2000). The use of laboratory tests in the diagnosis of SLE. Journal of Clinical Pathology, 53(6), 424–432. [CrossRef]
- Satoh M, Chan EK, Ho LA, Rose KM, Parks CG, Cohn RD, Jusko TA, Walker NJ, Germolec DR, Whitt IZ, Crockett PW, Pauley BA, Chan JY, Ross SJ, Birnbaum LS, Zeldin DC, Miller FW. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum. 2012 Jul;64(7):2319-27. PMID: 22237992; PMCID: PMC3330150. [CrossRef]
- Selmi, C., Ceribelli, A., Generali, E., Scirè, C. A., Alborghetti, F., Colloredo, G., Porrati, L., Achenza, M. I. S., De Santis, M., Cavaciocchi, F., Massarotti, M., Isailovic, N., Paleari, V., Invernizzi, P., Matthias, T., Zucchi, A., & Meroni, P. L. (2016). Serum antinuclear and extractable nuclear antigen antibody prevalence and associated morbidity and mortality in the general population over 15years. In Autoimmunity Reviews (Vol. 15, Issue 2, pp. 162–166). Elsevier BV. [CrossRef]
- Shovman, O., Gilburd, B., Barzilai, O., Shinar, E., larida, B., Zandman-Goddard, G., Binder, S. R., & Shoenfeld, Y. (2005). Evaluation of the BioPlex™ 2200 ANA Screen: Analysis of 510 Healthy Subjects: Incidence of Natural/Predictive Autoantibodies. In Annals of the New York Academy of Sciences (Vol. 1050, Issue 1, pp. 380–388). Wiley. [CrossRef]
- Mariz, H.A., Sato, E.I., Barbosa, S.H., Rodrigues, S.H., Dellavance, A. and Andrade, L.E.C. (2011), Pattern on the antinuclear antibody–HEp-2 test is a critical parameter for discriminating antinuclear antibody–positive healthy individuals and patients with autoimmune rheumatic diseases. Arthritis & Rheumatism, 63: 191-200. [CrossRef]
- Hahn, B. H. (1998). Antibodies to DNA. In F. H. Epstein (Ed.), New England Journal of Medicine (Vol. 338, Issue 19, pp. 1359–1368). Massachusetts Medical Society. [CrossRef]
- Isenberg, D. A., Manson, J. J., Ehrenstein, M. R., & Rahman, A. (2007). Fifty years of anti-ds DNA antibodies: are we approaching journey’s end? In Rheumatology (Vol. 46, Issue 7, pp. 1052–1056). Oxford University Press (OUP). [CrossRef]
- Lou, H., Wojciak-Stothard, B., Ruseva, M.M. et al. Autoantibody-dependent amplification of inflammation in SLE. Cell Death Dis 11, 729 (2020). [CrossRef]
- Orme, M. E., Voreck, A., Aksouh, R., & Schreurs, M. W. J. (2022). Anti-dsDNA Testing Specificity for Systemic Lupus Erythematosus: A Systematic Review. In The Journal of Applied Laboratory Medicine (Vol. 7, Issue 1, pp. 221–239). Oxford University Press (OUP). [CrossRef]
- Orme, M. E., Voreck, A., Aksouh, R., Ramsey-Goldman, R., & Schreurs, M. W. J. (2021). Systematic review of anti-dsDNA testing for systemic lupus erythematosus: A meta-analysis of the diagnostic test specificity of an anti-dsDNA fluorescence enzyme immunoassay. In Autoimmunity Reviews (Vol. 20, Issue 11, p. 102943). Elsevier BV. [CrossRef]
- Wichainun R, Kasitanon N, Wangkaew S, Hongsongkiat S, Sukitawut W, Louthrenoo W. Sensitivity and specificity of ANA and anti-dsDNA in the diagnosis of systemic lupus erythematosus: a comparison using control sera obtained from healthy individuals and patients with multiple medical problems. Asian Pac J Allergy Immunol. 2013 Dec;31(4):292-8. [CrossRef] [PubMed]
- Arbuckle, M. R., James, J. A., Kohlhase, K. F., Rubertone, M. V., Dennis, G. J., & Harley, J. B. (2001). Development of Anti-dsDNA Autoantibodies Prior to Clinical Diagnosis of Systemic Lupus Erythematosus. Scandinavian Journal of Immunology, 54(1–2), 211–219. [CrossRef]
- Alsaed OS, Alamlih LI, Al-Radideh O, Chandra P, Alemadi S, Al-Allaf A-W. Clinical utility of ANA-ELISA vs ANA-immunofluorescence in connective tissue diseases. Sci Rep (2021) 11:8229. [CrossRef]
- Meroni PL, Schur PH. ANA screening: an old test with new recommendations. Ann Rheum Dis (2010) 69:1420–1422. [CrossRef]
- Colglazier CL, Sutej PG. Laboratory Testing in the Rheumatic Diseases: A Practical Review. South Med J (2005) 98:185–191. [CrossRef]
- Kendall, J., Reeves, B., & Clancy, M. (1998). Point of care testing: randomised controlled trial of clinical outcome. BMJ, 316(7137), 1052–1057. [CrossRef]
- Lewandrowski, K., Flood, J., Finn, C., Tannous, B., Farris, A. B., Benzerx, T. I., & Lee-Lewandrowski, E. (2008). Implementation of Point-of-Care Rapid Urine Testing for Drugs of Abuse in the Emergency Department of an Academic Medical Center. American Journal of Clinical Pathology, 129(5), 796–801. [CrossRef]
- Lei R, Huo R, Mohan C. Current and emerging trends in point-of-care urinalysis tests. Expert Rev Mol Diagn. 2020 Jan;20(1):69-84. Epub 2019 Dec 12. PMID: 31795785; PMCID: PMC7365142. [CrossRef]
- Gubala, V., Harris, L. F., Ricco, A. J., Tan, M. X., & Williams, D. E. (2011). Point of Care Diagnostics: Status and Future. Analytical Chemistry, 84(2), 487–515. [CrossRef]
- Jiang, N., Ahmed, R., Damayantharan, M., Ünal, B., Butt, H., & Yetisen, A. K. (2019). Lateral and Vertical Flow Assays for Point-of-Care Diagnostics. Advanced Healthcare Materials, 8(14), 1900244. [CrossRef]
- Schonhorn, J. E., Fernandes, S. C., Rajaratnam, A., Deraney, R. N., Rolland, J. P., & Mace, C. R. (2014). A device architecture for three-dimensional, patterned paper immunoassays. Lab Chip, 14(24), 4653–4658. [CrossRef]
- Oh, Y. K., Joung, H.-A., Kim, S., & Kim, M.-G. (2013). Vertical flow immunoassay (VFA) biosensor for a rapid one-step immunoassay. Lab on a Chip, 13(5), 768. [CrossRef]
- Lee, D., & Lee, J. H. (2020). Paper-Based Biosensors with Lateral/Vertical Flow Assay. In Bioanalysis (pp. 115–136). Springer Singapore. [CrossRef]
- Lei, R.; Wang, D.; Arain, H.; Mohan, C. Design of Gold Nanoparticle Vertical Flow Assays for Point-of-Care Testing. Diagnostics 2022, 12, 1107. [CrossRef]
- Li, J., & Macdonald, J. (2016). Multiplexed lateral flow biosensors: Technological advances for radically improving point-of-care diagnoses. Biosensors and Bioelectronics, 83, 177–192. [CrossRef]
- Vu BV, Lei R, Mohan C, Kourentzi K, Willson RC. Flash Characterization of Smartphones Used in Point-of-Care Diagnostics. Biosensors. 2022; 12(12):1060. [CrossRef]
- Lei, R., Vu, B., Kourentzi, K., Soomro, S., Danthanarayana, A. N., Brgoch, J., Nadimpalli, S., Petri, M., Mohan, C., & Willson, R. C. (2022). A novel technology for home monitoring of lupus nephritis that tracks the pathogenic urine biomarker ALCAM. Frontiers in Immunology, 13, 1044743. [CrossRef]
- Prajapati, A., Verma, N., & Pandya, A. (2020). Highly sensitive vertical flow based point-of-care immunokit for rapid and early detection of human CRP as a cardiovascular risk factor. Biomedical Microdevices, 22(2). [CrossRef]
- Lei R, Arain H, Obaid M, Sabhnani N, Mohan C. Ultra-Sensitive and Semi-Quantitative Vertical Flow Assay for the Rapid Detection of Interleukin-6 in Inflammatory Diseases. Biosensors. 2022; 12(9):756. [CrossRef]
- Doria, A., Zen, M., Canova, M., Bettio, S., Bassi, N., Nalotto, L., Rampudda, M., Ghirardello, A., & Iaccarino, L. (2010). SLE diagnosis and treatment: When early is early. Autoimmunity Reviews, 10(1), 55–60. [CrossRef]
- Doria, A., Arienti, S., Rampudda, M., Canova, M., Tonon, M., & Sarzi-Puttini, P. (2008). Preventive strategies in systemic lupus erythematosus. Autoimmunity Reviews, 7(3), 192–197. [CrossRef]
- Koelmeyer, R., Nim, H. T., Nikpour, M., Sun, Y. B., Kao, A., Guenther, O., … Hoi, A. (2020). High disease activity status suggests more severe disease and damage accrual in systemic lupus erythematosus. Lupus Science & Medicine, 7(1), e000372. [CrossRef]
- Bentow, C., Lakos, G., Martis, P., Wahl, E., Garcia, M., Viñas, O., … Mahler, M. (2016). International multi-center evaluation of a novel chemiluminescence assay for the detection of anti-dsDNA antibodies. Lupus, 25(8), 864–872. [CrossRef]
- Andrejevic, S., Jeremic, I., Sefik-Bukilica, M., Nikolic, M., Stojimirovic, B., & Bonaci-Nikolic, B. (2013). Immunoserological parameters in SLE: high-avidity anti-dsDNA detected by ELISA are the most closely associated with the disease activity. Clinical Rheumatology, 32(11), 1619–1626. [CrossRef]
- Pan, N., Amigues, I., Lyman, S., Duculan, R., Aziz, F., Crow, M., & Kirou, K. (2013). A surge in anti-dsDNA titer predicts a severe lupus flare within six months. Lupus, 23(3), 293–298. [CrossRef]
- Narayanan, K., Marwaha, V., Shanmuganandan, K., & Shankar, S. (2010). Correlation between Systemic Lupus Erythematosus Disease Activity Index, C3, C4 and Anti-dsDNA Antibodies. Medical Journal Armed Forces India, 66(2), 102–107. [CrossRef]
- Wang, X., & Xia, Y. (2019). Anti-double Stranded DNA Antibodies: Origin, Pathogenicity, and Targeted Therapies. In Frontiers in Immunology (Vol. 10). Frontiers Media SA. [CrossRef]
- Manson JJ, Ma A, Rogers P, Mason LJ, Berden JH, van der Vlag J, D'Cruz DP, Isenberg DA, Rahman A. Relationship between anti-dsDNA, anti-nucleosome and anti-alpha-actinin antibodies and markers of renal disease in patients with lupus nephritis: a prospective longitudinal study. Arthritis Res Ther. 2009 Oct 14;11(5):R154. PMID: 19828047; PMCID: PMC2787270. [CrossRef]
- Sherer, Y., Gorstein, A., Fritzler, M. J., & Shoenfeld, Y. (2004). Autoantibody explosion in systemic lupus erythematosus: More than 100 different antibodies found in SLE patients. In Seminars in Arthritis and Rheumatism (Vol. 34, Issue 2, pp. 501–537). Elsevier BV. [CrossRef]
- Mok, C. C., & Mohan, C. (2021). Urinary Biomarkers in Lupus Nephritis: Are We There Yet?. Arthritis & rheumatology (Hoboken, N.J.), 73(2), 194–196. [CrossRef]
- Soomro S, Stanley S, Lei R, Saxena R, Petri M, Mohan C. Comprehensive Urinomic Identification of Protein Alternatives to Creatinine Normalization for Diagnostic Assessment of Lupus Nephritis. Front Immunol. 2022;13:853778. Published 2022 Jun 14. [CrossRef]
- Rongwei Lei, Nga Thai, et al. Analytical validation of urine ALCAM ELISA as a test for lupus nephritis. Expert Review of Molecular Diagnostics. [CrossRef]
- Li, H., Zheng, Y., Chen, L., & Lin, S. (2022). High titers of antinuclear antibody and the presence of multiple autoantibodies are highly suggestive of systemic lupus erythematosus. Scientific reports, 12(1), 1687. [CrossRef]
- Zhang, T., Du, Y., Wu, Q., Li, H., Nguyen, T., Gidley, G., Duran, V., Goldman, D., Petri, M., & Mohan, C. (2022). Salivary anti-nuclear antibody (ANA) mirrors serum ANA in systemic lupus erythematosus. Arthritis research & therapy, 24(1), 3. [CrossRef]
- Stanescu, I. I., Calenic, B., Dima, A., Gugoasa, L. A., Balanescu, E., Stefan-van Staden, R. I., Baicus, C., Badita, D. G., & Greabu, M. (2018). Salivary biomarkers of inflammation in systemic lupus erythematosus. Annals of anatomy = Anatomischer Anzeiger : official organ of the Anatomische Gesellschaft, 219, 89–93. [CrossRef]
- Zhang, H., & Gomika Udugamasooriya, D. (2022). Optimization of a cell surface vimentin binding peptoid to extract antagonist effect on lung cancer cells. Bioorganic chemistry, 129, 106113. [CrossRef]
- Zhang, H., & Udugamasooriya, D. G. (2023). Linker optimization and activity validation of a cell surface vimentin targeted homo-dimeric peptoid antagonist for lung cancer stem cells. Bioorganic & Medicinal Chemistry, 117560. [CrossRef]
- Shukla, S. P., Zhang, H., Fang, B., Minna, J. D., & Gomika Udugamasooriya, D. (2022). Unbiased peptoid cell screen identifies a peptoid targeting newly appeared cell surface vimentin on tumor transformed early lung cancer cells. Bioorganic & medicinal chemistry, 58, 116673. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).