1. Introduction
Nowadays, the advanced development of materials based on biocompatibility and biodegradability is a long-term target for biomedical and bioengineering applications. The physicochemical characteristics of polymers have attracted tremendous attention in order to explore their potential use as natural and harmless materials [
1].
Gelatin is considered to be one of the most versatile biopolymers among natural polymers. It has a unique set of biological properties, including its safe nature, biocompatibility, biodegradability, and absence of toxicity [
2]. Thus, it is an attractive material in the drug, food, and tissue engineering sectors. The gelatin presently used in tissue engineering products are mainly of porcine or bovine origin. Nevertheless, the use of these gelatins tends to be more limited due to socio-cultural and disease associated problems [
3]. Therefore, the need of alternative sources of bovine and porcine gelatin has greatly increased. Recently, camel skin gelatin has been successfully isolated and characterized. It has been shown that camel skin gelatin has excellent physicochemical characteristics due to its mammalian source [
4]. Chitosan is a naturally occurring polymer derived by the process of alkaline hydrolysis of chitin, a common chemical present in the shells of crustaceans and the outer coverings of insects. Chitosan has garnered significant interest for its favourable characteristics, such as its compatibility with living creatures, ability to be decomposed by natural processes, and lack of harmful or toxic effects. As a result, it has found extensive use in the fields of food, drugs, and biomedicine. In many medical applications, both chitosan and gelatin have to be crosslinked in order to improve their physicochemical properties.
Recently, the incorporation of therapeutic agents into biomaterials represents a novel approach to controlling inflammation, preventing infection, and stimulating tissue regeneration [
5,
6].
Moringa oleifera (
M. oleifera) is a member of the Moringaceae family and was
called “the miracle tree” or “tree of life” in old times because of its useful effects in curing various diseases. A wide variety of therapeutic virtues have been attributed to its roots, bark, leaves, flowers, fruits, and seeds
. Phytochemical analyses have shown that the leaves of
M. oleifera contain large amounts of protein, vitamins, β-carotene, flavonoids, and minerals. In fact,
M. oleifera was widely utilised as a folk remedy to treat diabetes, malnutrition, anaemia, arthritis, and as an antiulcer [
7]. Several studies have shown that leaf extract increases human dermal fibroblast proliferation, leading to faster wound healing [
8,
9]. Further, its antibacterial,
antihypertensive, anti-inflammatory, antifungal, immunomodulatory, cardioprotective, and hepatoprotective activities have also been documented [
10,
11]. A few studies regarding the development of formulations loaded with
Moringa extract intended for wound treatment as scaffolds, films, and hydrogels have also been recently reported [
12,
13,
14].
This investigation is the initial one to the best of our knowledge regarding the use of camel skin gelatin-chitosan-moringa as a novel biomaterial (biofilm) for biomedical applications. In the current study, the potential of using this film formulation as a functional biomaterial for pharmaceuticals was investigated. The antioxidant, biocompatibility, and anti-inflammatory properties as well as the antibacterial effects of the film formulation were analysed.
2. Materials and Methods
Extraction of gelatin
The gelatin was extracted from camels’ skin as described previously [
15]. Briefly, the camel skins of
Camelus dromedarius were randomly collected from the slaughterhouse. The skins were washed thoroughly with distilled water and cut into small pieces. At room temperature, camel skin was immersed in 0.5 M NaOH with a skin/solution ratio of 1:5 (w/v) for 3 days to eliminate non-collagenous proteins. After thorough washing, the pre-treated skins were soaked in 0.1 M citric acid with a ratio of 1:5 (w/v) for 1 hour. The samples were washed again with distilled water until the pH was neutral. The final step was carried out with distilled water at a ratio of 1:5
w/v at 50°C for 6 h. The supernatant was passed through Whatman No. 4 filter paper, lyophilized, and subjected to analyses.
Polymer (G-CH-M) construction
Briefly, the chitosan film forming solution (1% w/w) was prepared by dissolving the chitosan powder (90% DD, degree of deacetylation) in a 1% w/w solution of acetic acid under magnetically stirred for 8 h at room temperature until it completely dissolved. The gelatin derived from camel skin was solubilized in water (4%) and subjected to heating at 60°C for a duration of 5-10 min. Prepared solutions are homogenized for 30 mins in order to assure their homogenization. The gelatin/chitosan/moringa solution was lyophilized for 48 h after being frozen at -80°C for 8 h. Finally, the dry films were subjected to biological analysis.
Antibacterial activity of G-CH-M biopolymer
The antibacterial activity of the G-CH-M biopolymer against both Gram-positive
Staphylococcus aureus (
S. aureus ATCC 12600) and Gram-negative
Escherichia coli (
E. coli ATCC 9637) was evaluated by the agar diffusion method [
16]. The bacteria were spread onto the LB agar petri dish with a 10
6 CFU/mL density. Wells measuring 6 mm in diameter in the LB agar were filled with 30 μl of the prepared compound. After that, the petri dishes were incubated for 24 hours at 37°C. After incubation, the diameter of the zone of inhibition was measured. For each bacterial species, experiments were performed in triplicate assays.
Liquid medium microdilution assay
The antibacterial efficacy of the sterilised biopolymer was further assessed utilising microdilution techniques [
17]. Briefly, the disinfected biopolymer (10 mg) was added to the bacterial suspensions (OD 0.1- 0.2 at 600 nm) and incubated at 37°C for 12 h, 18 h and 24 h. At each time, the absorbance value of the sample was determined at 600 nm. The bacterial inhibition percentage was determined as follows:
where, Ic and Is are the absorbance values of the control inoculum and the inoculum containing biopolymers at predetermined times, respectively.
Crystal violet biofilm assay
A static biofilm formation assay was performed in 96-well polystyrene plates as described previously, with some modifications [
18]. In brief,
E. coli and
S. aureus were cultured in LB and thinned out using fresh LB to an OD600 of 0.02. A mixture of 200 μl of the bacterial culture and 200 μl of LB broth was combined and incubated at 37°C for 24 h (stationary phase) without agitation. Plates were rinsed three times with phosphate buffered saline (PBS) the following day after LB medium was discarded. Following this, bacterial biofilms were observed by staining for 15 mins at ambient temperature with 125 μl of 0.1% crystal violet. Plates were subsequently rinsed, and the quantity of biofilm, which had been dissolved in 100 μl of 95% ethanol, was determined through OD measurement at 570 nm.
Motility assay
Motility assays against
E. coli and
S. aureus was performed using 0.5% agar LB plates containing 0.8% glucose, as previously described [
19]. Both
E. coli and
S. aureus were grown to an OD600 of 1.0 and about 100 μl of cultures were placed in motility plates using a sterilized pipette tip. The samples were added to these bacteria and then incubated at 37°C under microaerobic conditions for 18 h. Each experiment was performed using at least three independent replicates.
Antioxidant activities
DPPH radical-scavenging activity
The scavenging of 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) assays was performed as described previously [
20]. A 100 µl volume of sample solutions with varying concentrations (ranging from 0.25 to 10 mg/ml) was combined with 100 µl of ethanol and a DPPH solution (0.002%). The reaction mixture was thereafter placed in a dark environment and allowed to incubate for a duration of 60 min. The measurement of absorbance was conducted at a wavelength of 517 nm utilising a 96 well microplate reader. Ascorbic acid served as the positive control. The DPPH radical scavenging activity was assessed by employing the subsequent formula:
where Doc and Dos are the absorbances of control and sample, respectively. Each experiment was conducted in triplicate..
Ferrous chelating activity
The ferrous chelating assay was conducted according to [
21]. At ambient temperature, the mixtures were incubated for five minutes. Following this, 10 µl of a 5 mM ferrozine solution was added. The absorbance of each sample mixture was determined at 700 nm using a microplate reader following a 10-minute reaction time. Ethylene diamine-tetraacetic acid (EDTA) was used as a positive control. utilised The test was carried out in triplicate. The chelating activity was determined using the same equation for DPPH activity.
DNA Damage Protective Effect assay
The efficacy of the biopolymer in safeguarding supercoiled pHEN4 plasmid DNA against H
2O
2 was assessed using the DNA nicking assay, adhering to a marginally altered iteration of the preceding account [
22]. Concisely, The plasmid DNA of the supercoiled pHEN4 chromosome was isolated from JM109
E.
coli bacteria utilising the PureLink™ Quick Plasmid Miniprep Kit, which is commercially available from Invitrogen. At room temperature, 0.5 µg of the purified plasmid DNA was incubated with 10 µL of various irradiated samples. Following the addition of Fenton's reagents (30 mM H
2O
2, 50 µM ascorbic acid, and 80 µM FeCl
3), the mixture was incubated at 37°C for a duration of 5 min. After incubation, the protective activity of various samples against DNA damage induced by hydroxyl radicals was evaluated using a 1% (w/v) agarose gel, followed by ethidium bromide staining. Later, photographs of the composites were taken using UV light. As a control, untreated DNA was utilised to assess the DNA strand fractures.
Biocompatibility
The haemolysis tests were used to evaluate the biocompatibility between the biopolymer and erythrocytes [
23]. 800 μl of G-CH-M biopolymer at different concentrations (1 mg/ml, 3 mg/ml, 6 mg/ml, 12 mg/ml, 25 mg/ml and 50 mg/ml) were added to 200 μl of fresh rabbit whole blood (whole blood: normal saline = 8: 10). Following an hour of incubation at 37°C, the samples were centrifuged at 4000 rpm for 5 min. The absorbance of the supernatant was measured at 545 nm.. The experiments were run in triplicate. The haemolysis rate (HR) was calculated according to the following equation:
where AS, AP, and AN present the absorbance of the biopolymer at different concentrations, the positive control (distilled water) and the negative control (normal saline), respectively.
Anti-inflammatory activity
Protein denaturation technique was employed in accordance with the protocol outlined by Khat et al. [
34] to assess the anti-inflammatory activity of the G-CH-M biopolymer. Briefly, 1 ml of biopolymer in different concentrations (100, 200, 500, and 1000 μg/ml) was added to 1 ml of PBS (pH 6.4) and 1 ml of bovine serum albumin solution (5%). For protein denaturation, samples were then incubated in a water bath at 72 °C for 10 min, and cooled for 30 min at 25°C. The turbidity of the solutions (level of protein precipitation) was measured at 660 nm. The experiments were conducted in duplicate, and the mean absorbance values were recorded. Negative (without samples) controls were assayed in a similar manner.
Results
Antibacterial activities
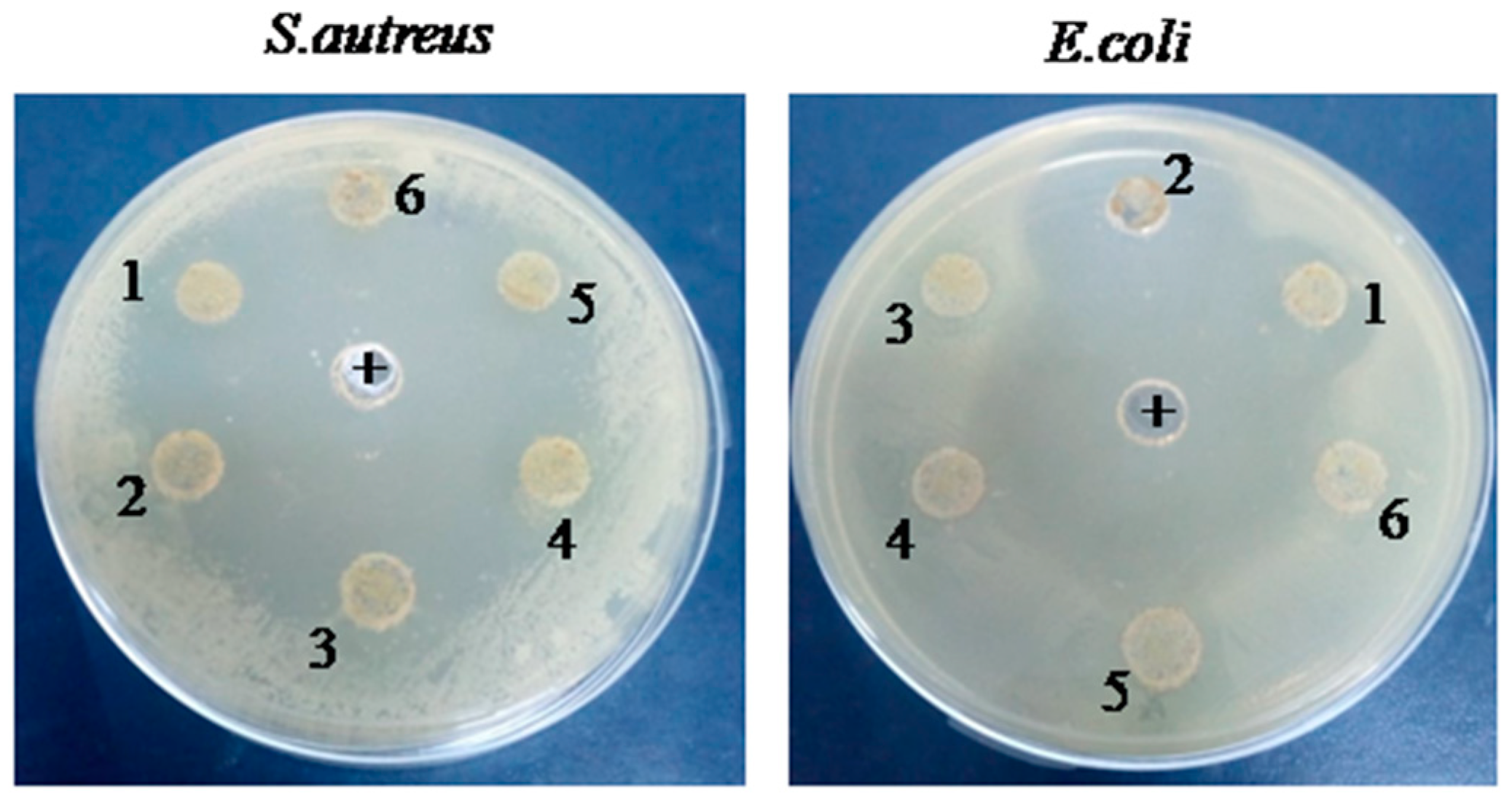
Agar well diffusion results showed that the G-CH-M biopolymer exhibited a potent inhibitory effect against tested bacteria (
Figure 1). The zone of inhibition increased with increasing concentrations of the G-CH-M biopolymer. The largest inhibition zone was found in the G-CH-M biopolymer at 15 and 20 mg/ml (wells 1 and 2). The overall results confirmed that the zone of inhibition of the biopolymer was greater in
S. aureus than in
E. coli. Furthermore, the
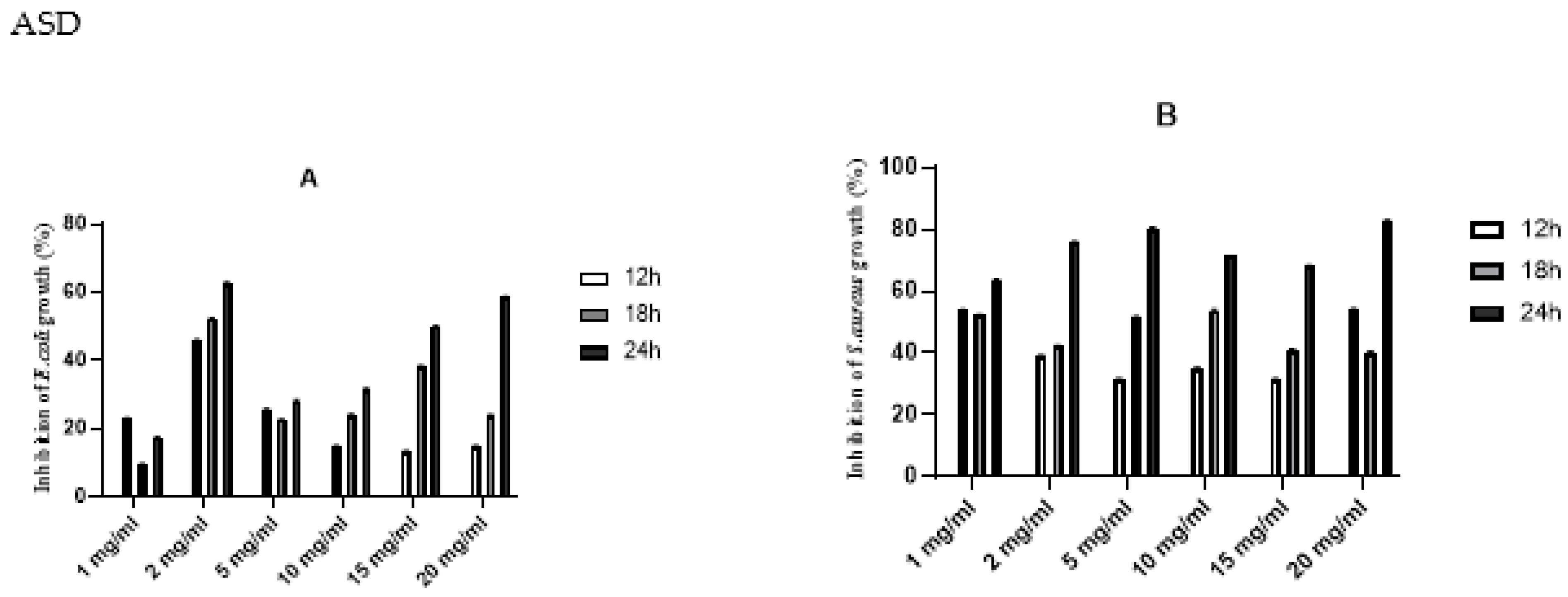
antibacterial activity using the liquid medium microdilution assay exhibited a significant increase in inhibition activity against
S. aureus after 24 h of incubation, while the biopolymer demonstrates a moderate antibacterial activity against
E. coli. (
Figure 2).
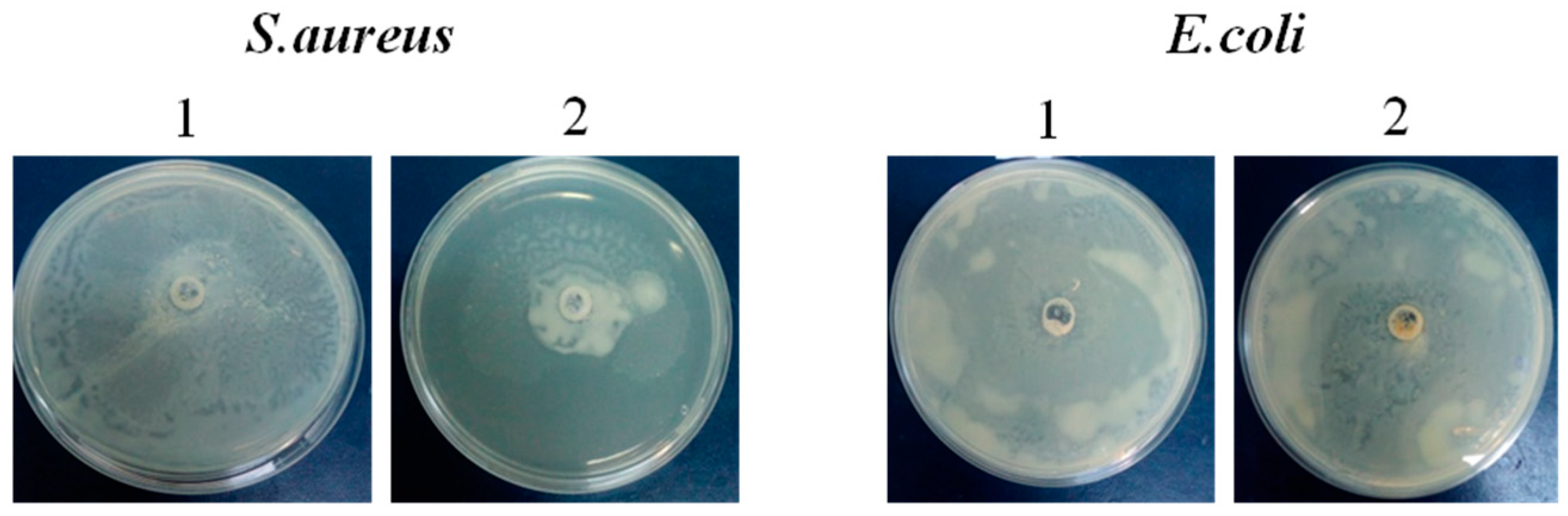
Modulation of bacteria motility
Figure 3 displays the analysis of the motilities of
S.
aureus and
E.
coli following incubation with a biopolymer. The motility of
S. aureus was significantly reduced by the biopolymer. The treatment with biopolymer shows a strong effect, as it decreased the
S. aureus colonies by 50%. In contrast, the treatment had no discernible effect on the motility of the
E.
coli strain in comparison to the control group.
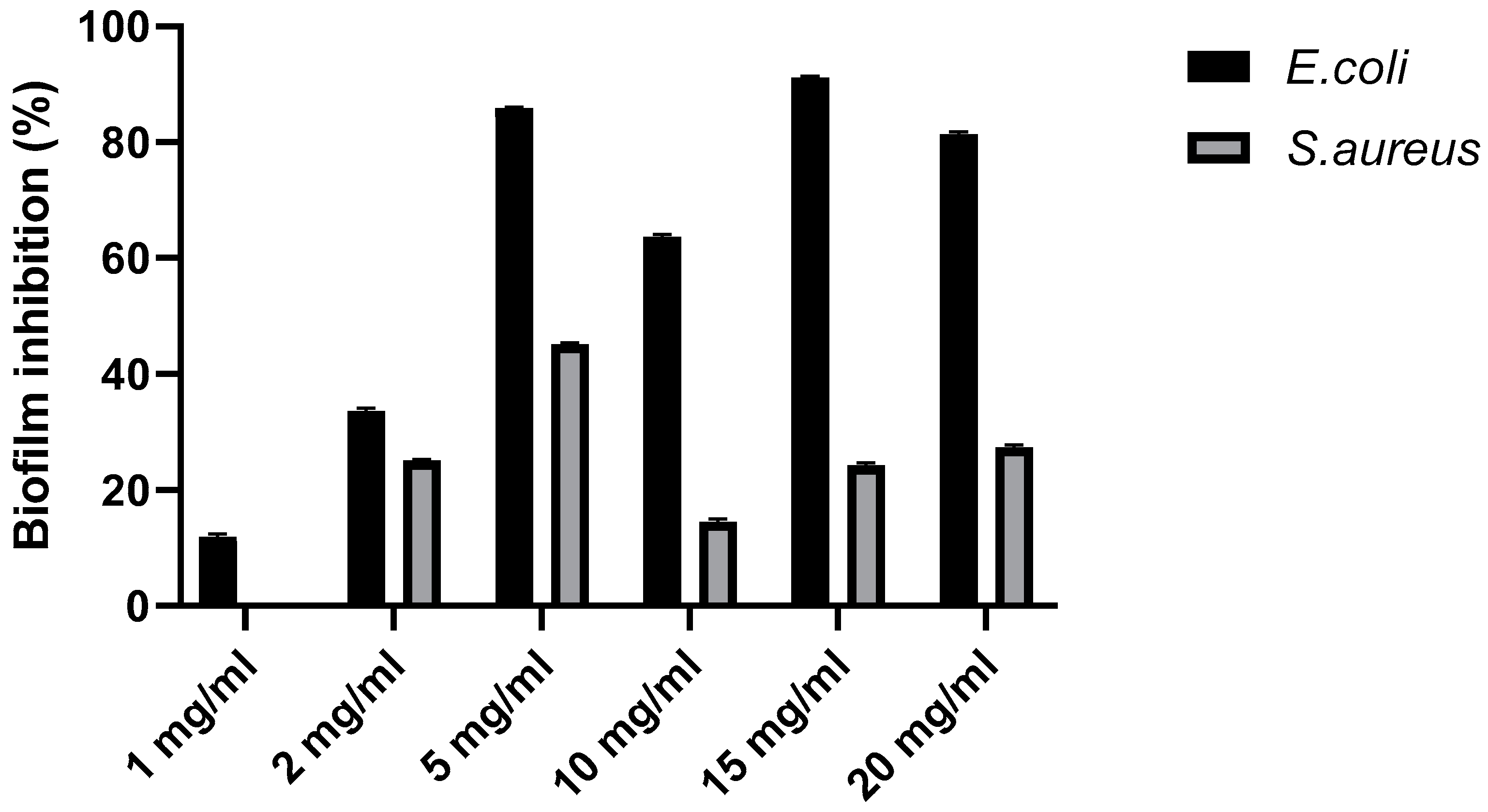
Anti-biofilm activity
The anti-biofilm effects of the biopolymer determined using the crystal violet method are presented in
Figure 4. Current finding show that biofilm enriched with moringa extract seems to inhibit the initial binding of
E. coli to the polystyrene plane. In fact, inhibition of 11.04% biofilm formation by
E. coli in the presence of biofilm was observed. However, biopolymer had no effect on the formation of biofilm by
S. aureus after incubation for 24 h.
Antioxidant activities
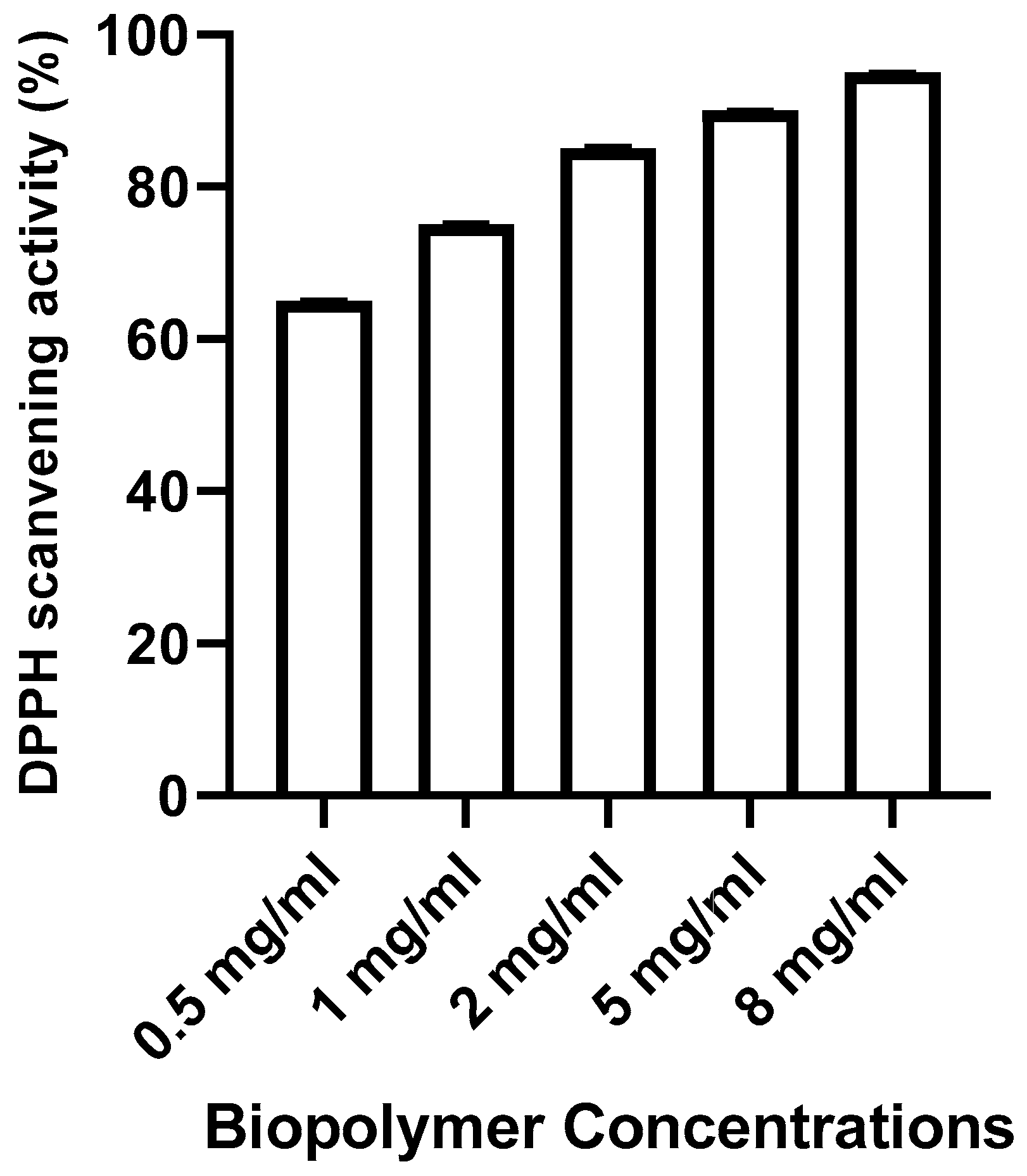
DPPH assay
The DPPH assay is often adopted to evaluate the total antioxidant power of various plants. The result of the DPPH free radical-scavenging ability of the biopolymer is shown in
Figure 5 and compared with ascorbic acid as a control standard. As can be seen from
Figure 5, the DPPH radical scavenging increased from 60% to 95% when the concentration of the biopolymer increased from 0.5 to 8 mg/ml. The results indicated that the biopolymer exhibited potential DPPH radical scavenging activity.
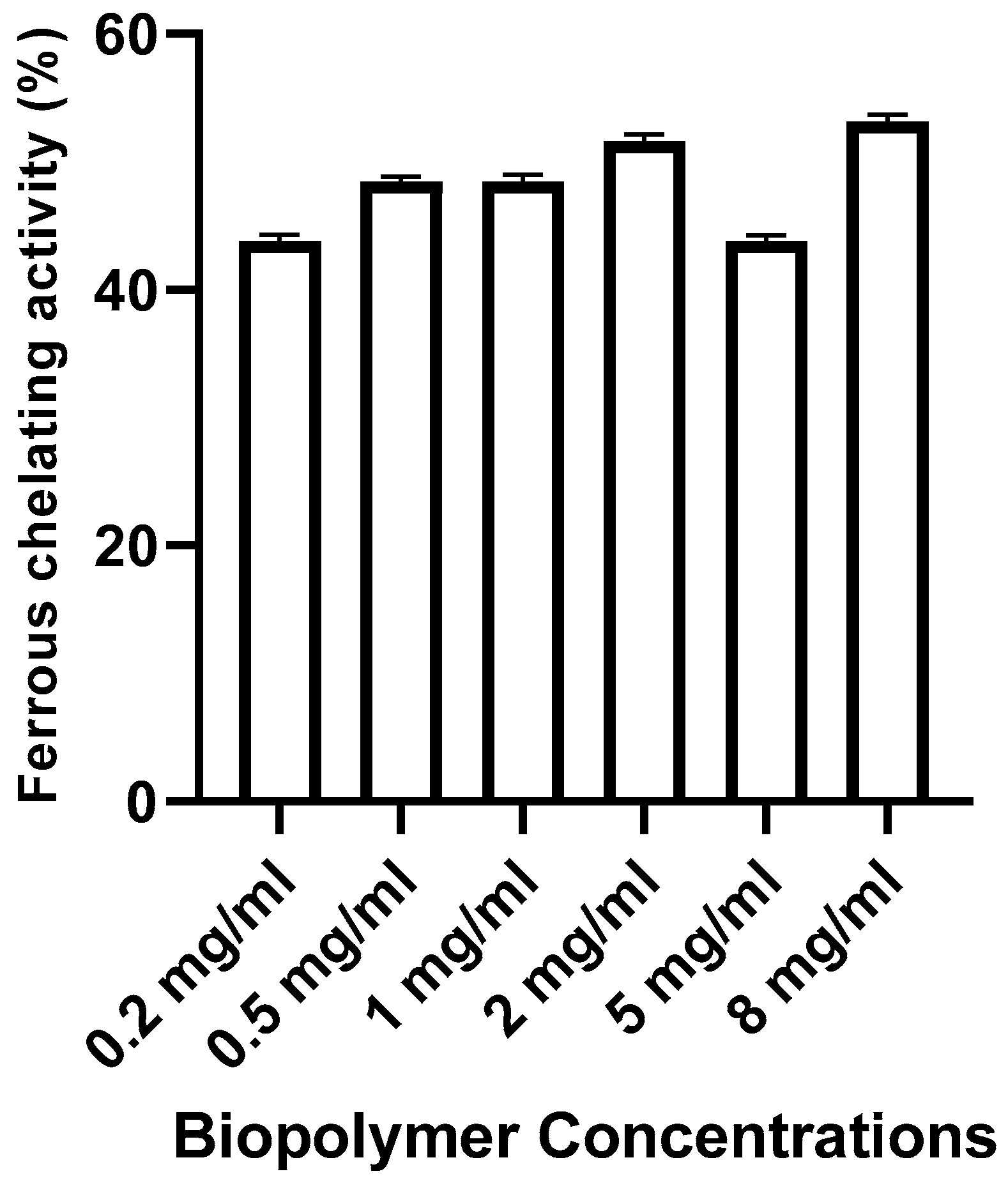
Ferrous chelating activity
As shown in
Figure 6, the metal chelating activity of the biopolymer increased with increasing concentrations used in the test. In fact, biofilm (8 mg/ml) showed the highest reducing power (53.12%).
DNA Damage Protective Effect assay
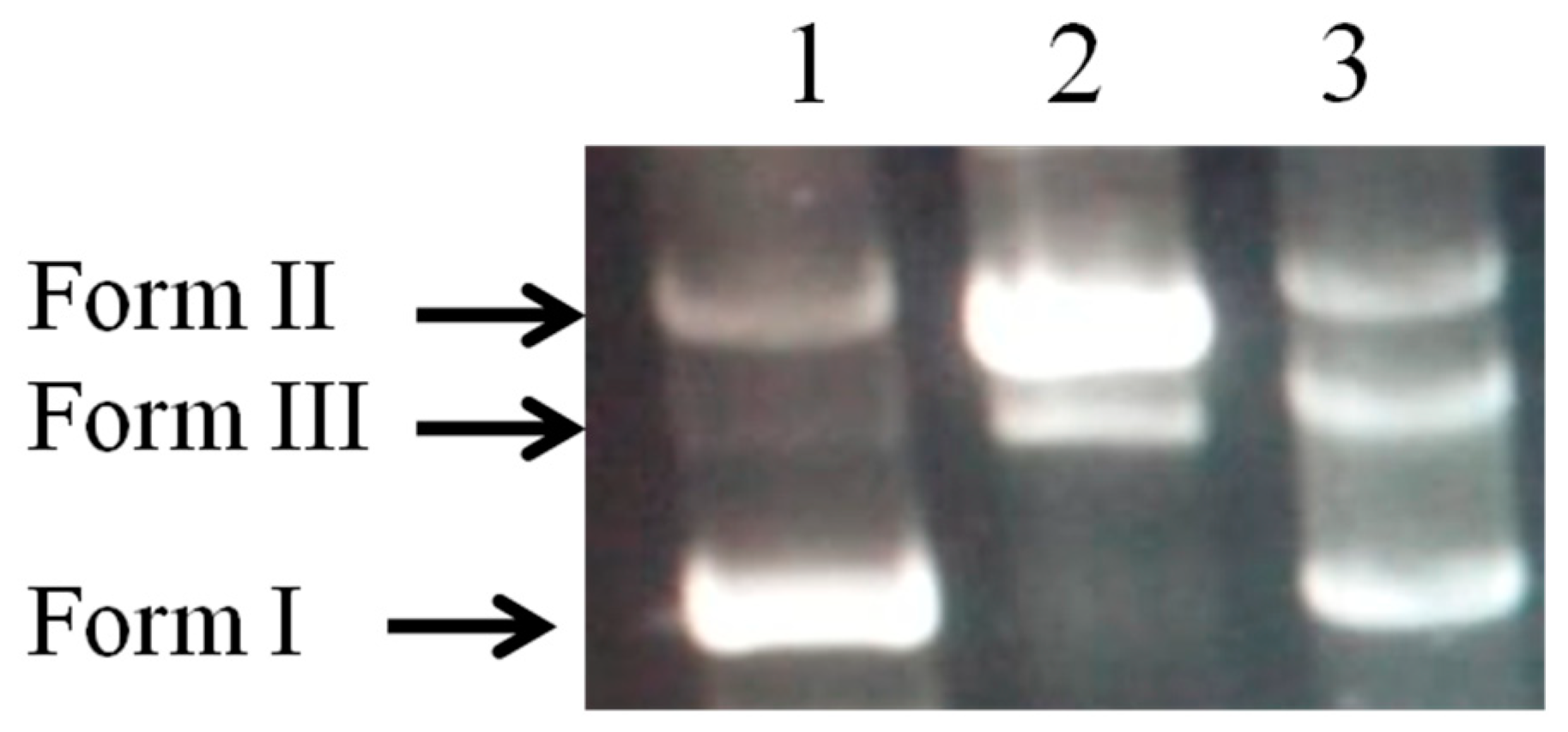
The efficiency of the biopolymer in preventing oxidative damage to DNA is shown in
Figure 7. The damage to plasmid DNA produces an open circular form and, further, a linear form of DNA. Therefore, the formation of circular and linear forms of DNA is indicative of single strand breaks and double strand breaks, respectively. The plasmid DNA was mainly of the supercoiled form (Form I) in the absence of Fe
2+ and H
2O
2 (
Figure 7, lane 1). After the addition of Fenton’s reaction mixture, the supercoiled form of DNA is converted into the circular and linear forms (
Figure 7, lane 2), which indicate that hydroxy radicals generated from iron-mediated decomposition of H
2O
2 generate both single strand and double strand DNA breaks.
Our findings unequivocally demonstrate the safeguarding effect that biofilm provides against strand disruption caused by hydroxyl radicals in a Fenton's reaction mixture (
Figure 7, Lane 3)
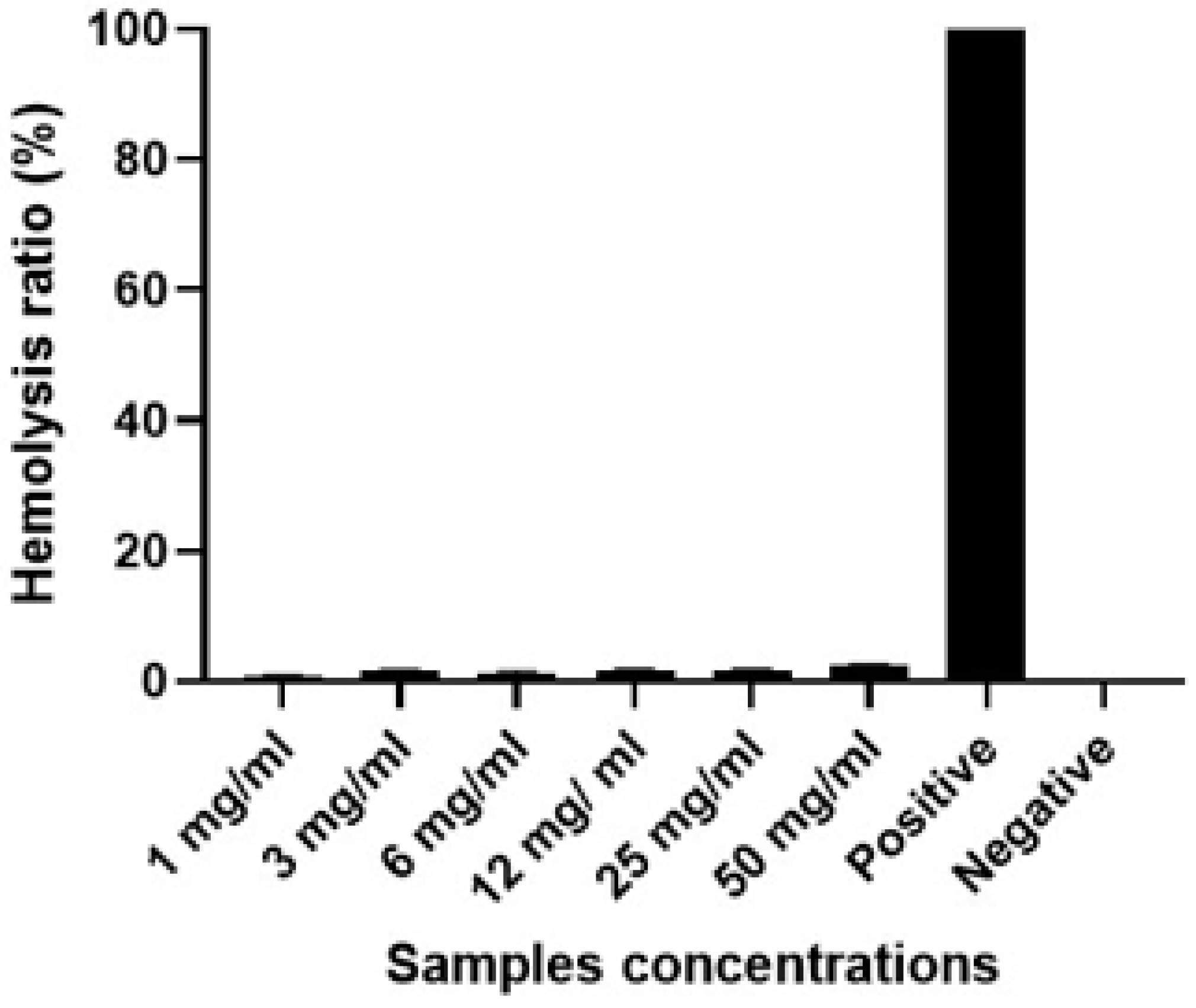
Biocompatibility test
A haemolysis test was employed to determine the erythrocyte compatibility of the G-CH-M biopolymer. The haemolysis rates of the biopolymer with varied concentrations are shown in
Figure 8. From
Figure 8, the biopolymer showed good biocompatibility with rabbit blood. According to the ISO10993-4:2002 standard and ASTM F756-00 (2000), the haemolysis rate of blood contacting materials must be lower than 5%. It was observed that haemolysis rates of biopolymer with different concentrations were all below 2%, showing a significant difference as compared with the control. The gained results are in agreement with previous studies that reported that the haemolysis degrees of chitosan and gelatin based-membranes exhibited excellent hemocompatibility.
Anti-inflammatory activity
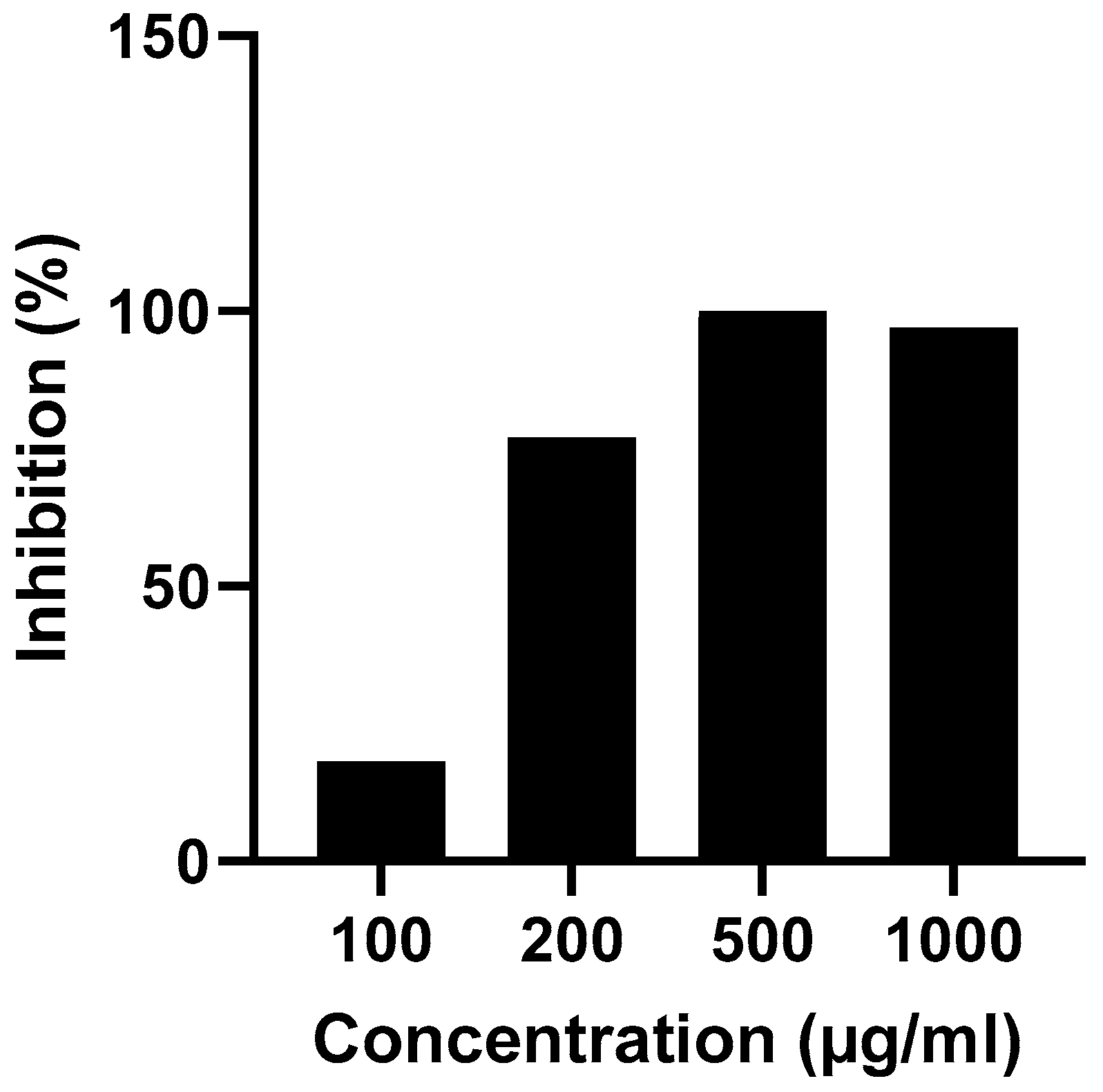
Figure 9 displays the varying concentrations of the G-CH-M biopolymer and its corresponding in vitro anti-inflammatory efficacy. Increasing the concentration of the sample resulted in higher inhibition percentage for bovine serum albumin (BSA) denaturation.
M.
oleifera is extensively utilised in traditional medicine for the treatment of inflammatory conditions.
Discussion
Infection of wound with pathogens delays the healing process and causes severe wound dehydration. Therefore, it is important to evaluate the applicability of the developed wound dressing membranes for potential use in the healthcare sector. Our result demonstrates the antibacterial activity of the fabricated biopolymer against
S. aureus and
E. coli. A significant increase in inhibition activity was observed against
S. aureus after 24 h of incubation. While the biopolymer demonstrates moderate antibacterial activity against
E. coli. The observed outcome may be ascribed to the lipopolysaccharide's (LPS) putative protective effect against Gram-negative bacteria. These results are similar to the observations of Thaya et al. [
25] and Divya et al. [
26] who demonstrated that a greater reduction in bacterial growth was observed after treatment with biopolymer enriched with gelatin-coated zinc oxide nanoparticles or alginate. Furthermore, previous studies reported that both chitosan and chitosan–gelatin membrane-forming solutions had great inhibitory effects on
E. coli and
L. monocytogenes [
27]. Similarly, Bukar et al. [
28] confirmed that the moringa leaf aqueous extract had antibacterial activity against
S. aureus,
E. coli, and
S. typhimurium. Therefore, integrating moringa leaf extract into wound dressing applications may present an intriguing prospect. This phenomenon can be attributed to the ongoing significance of natural products and organic by-product forms in the formulation of medications [
29]. Consequently, the boundless array of potential therapeutic leads presented by biological diversity is a direct consequence.
The effect of the G-CH- M biopolymer formulation was observed. The motility of
S. aureus was significantly reduced, while that of
E.
coli was not affected.. It has been reported that bacterial motility is associated with biofilm development and antibiotic resistance [
30,
31]. Importantly, our results show that inhibition of bacterial swarming was associated with the antibacterial activity of biopolymer. These findings are in agreement with previous studies that showed that bioactive compound with antimicrobial activity block swarming motility [
19].
The current study reported the antibiofilm activities of the G-CH-M biopolymer, where the inhibition of the initial attachment of
E. coli to the polystyrene surface was observed. Biofilms are associated with growth and pathogenicity. Several studies have reported that biopolymer can inhibit biofilm formation by pathogenic bacteria [
25,
30]. As mentioned above, moringa contains a variety of bioactive compounds with antimicrobial properties that affect the growth, survival, and pathogenicity of different pathogens. However, in our study, no correlation between antibacterial activity and biofilm formation was observed when the strain was exposed to the biopolymer.
This study also demonstrated that this biopolymer base has antioxidant activities and thus could be attributed to the presence of certain antioxidant compounds such as kaempferol, quercetin, and other polyphenolic molecules released from the moringa leaf extract [
31]. Moringa is widely recognised for its exceptional antioxidant properties and its ability to effectively eliminate reactive compounds. Polyphenols exert several biological roles through their antioxidant activity. Moringa contains phenolic antioxidant chemicals that can effectively shield pHEN4 plasmid DNA from harm caused by Fenton's reagents. This protection is achieved through a mechanism involving the transfer of electrons or hydrogen atoms, as previously reported [
32].
The present study indicated that G-CH-M biopolymer have good biocompatibility, and it’s in agreement with previous studies that reported that the haemolysis degrees of chitosan and gelatin based-membranes exhibited excellent hemocompatibility. Combining the results obtained from antioxidant activities and biocompatibility tests, the G-CH-M biopolymer might prevent the inflammation reaction. Our results support the traditional use of
M. oleifera to treat inflammatory disorders [
36].
Conclusions
Biopolymer prepared with camel gelatin- chitosan and enriched with moringa has promising properties of high antioxidant, and anti-inflammatory activities and good biocompatibility, which are crucial for a tissue engineering application. In addition, this study shows that the combination of moringa, camel gelatin, and chitosan has a strong impact on the motility and biofilm formation of bacteria, especially gram-positive bacteria. Therefore, this newly developed biopolymer is expected to have wide application in the field of tissue engineering, especially in wound dressings.
Author Contributions
Investigation, S.B., and M.D; resources, A.F., S.I.S., S.B., M.D., T.K., M.H., S.M.H., M.F.I. and D.J.A;; writing—original draft preparation, A.F., S.I.S., S.B., M.D., T.K., M.H., S.M.H., M.F.I. and D.J.A; writing—review and editing, A.F., S.I.S., S.B., M.D., T.K., M.H., S.M.H., M.F.I. and D.J.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to express our gratitude to Livestock and Wildlife Laboratory, Arid Lands Institute (I.R.A), University of Gabès, Médenine, Tunisia for assistance in conducting the research.
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
References
- Arfat, Y.A., Benjakul, S., Prodpran, T., Sumpavapol, P and Songtipya, P., “Properties and antimicrobial activity of fish protein isolate/fish skin gelatin film containing basil leaf essential oil and zinc oxide nanoparticles.” Food Hydrocoll., 2014, 41, 265-273. [CrossRef]
- Kuijpers, A.J., Van Wachem, P.B., van Luyn, M.J., Plantinga, J.A., Engbers, G.H., Krijgsveld, J., Zaat, S.A., Dankert, J., Feijen, J., “In vivo compatibility and degradation of cross linked gelatin gels incorporated in knitted Dacron.” J. Biomed. Mater. Res., 2000,51, 136-145.
- Gómez-Guillén, M.C., Giménez, B., López-Caballero, M.A., Montero, M.P., “Functional and bioactive properties of collagen and gelatin from alternative sources: A review.” Food Hydrocoll., 2011, 25, 1813–1827. [CrossRef]
- Al-Hassan, A. A., “Gelatin from camel skins: Extraction and characterizations.” Food Hydrocolloids, 2020, 101, 105457. [CrossRef]
- Suganya, S., Senthil Ram, T., Lakshmi, B., Giridev, V., “Herbal drug incorporated antibacterial nanofibrous mat fabricated by electrospinning: an excellent matrix for wound dressings.” J. App. Polymer Science., 2011, 121, 2893-2899. [CrossRef]
- Shan, Y.-H., Peng, L.-H., Liu, X., Chen, X., Xiong, J., Gao, J.-Q., “Silk fibroin/gelatin electrospun nanofibrous dressing functionalized with astragaloside IV induces healing and anti-scar effects on burn wound.” Inter J pharma., 2015, 479, 291-301. [CrossRef]
- Mbikay, M. “Therapeutic potential of Moringa oleifera leaves in chronic hyperglycemia and dyslipidemia: a review.” Frontiers in Pharmacology, 2012, 3, 24. [CrossRef]
- Gothai, S., Arulselvan, P., Tan, W.S., Fakurazi, S., “Wound healing properties of ethyl acetate fraction of Moringa oleifera in normal human dermal fbroblasts.” J Intercult Ethnopharmacol., 2016, 51–6. [CrossRef]
- Muhammad, AA., Pauzi, N.A., Arulselvan, P., Abas, F., Fakurazi, S., “In vitro wound healing potential and identifcation of bioactive compounds from Moringa oleifera Lam.” BioMed Res Int., 2013, ID974580, 1–10. [CrossRef]
- Saini, R. K., Sivanesan, I., and Keum, Y. S., “Phytochemicals of Moringa oleifera: a review of their nutritional, therapeutic and industrial significance.” 3 Biotech., 2016, 6, 203. [CrossRef]
- [11]. Ma, Z. F., Ahmad, J., Zhang, H., Khan, I., & Muhammad, S., “Evaluation of phytochemical and medicinal properties of Moringa (Moringa oleifera) as a potential functional food. South African Journal of Botany, 2020, 129, 40-46. [CrossRef]
- Rubio-Elizalde, I., Bernáldez-Sarabia, J., Moreno-Ulloa, A., Vilanova, C., Juárez, P., Licea- Navarro, A., Castro-Ceseña, A.B., “Scaffolds based on alginate-PEG methyl ether methacrylate-Moringa oleifera-Aloe vera for wound healing applications.” Carbohydr. Polym., 2019, 206, 455–467. [CrossRef]
- Chin, C.Y., Ng, P.Y., Ng, S.F., “Moringa oleifera standardised aqueous leaf extractloaded hydrocolloid film dressing: in vivo dermal safety and wound healing evaluation in STZ/HFD diabetic rat model.” Drug Deliv. Transl. Res., 2019, 9, 453–468. [CrossRef]
- Singh, B., Kumar, A., “Graft and crosslinked polymerization of polysaccharide gum to form hydrogel wound dressings for drug delivery applications.” Carbohydr. Res., 2020,489, 107949. [CrossRef]
- Bessalah, S., Jebahi, S., Faraz, A., Raoufi, A., Tırınk, C., Dridi, W., andMohamed Hammadi, A. W. “Effect of gamma radiation on novel gelatin extracted from camel skin for pharmaceutical application.” Pakistan J of Zoology, 2023,55(2), 819. [CrossRef]
- Berghe, V. A., and Vlietinck, A. J. “Screening methods for antibacterial and antiviral agents from higher plants.” Methods in Plant Biochemistry, 1991,6(3),47-68.
- Wang, S., Zheng, F., Huang, Y., Fang, Y., Shen, M., Zhu, M., Shi, X., “Encapsulation of amoxicillin within laponite-doped poly (lactic-co-glycolic acid) nanofibers: preparation, characterization, and antibacterial activity.” ACS Applied Materials & Interfaces., 2012, 6393-6401. [CrossRef]
- O’Toole, G.A., Kolter, R., “Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development.” Mol. Microbiol., 1998, 30, 295–304. [CrossRef]
- O’May, C., Tufenkji, N., “The Swarming omtility of Pseudomonas aeruginosa is blocked by cranberry proanthocyanidins and other tannin-containing materials.” Appl. Environ. Microbiol. 2011, 77, 3061−3067. [CrossRef]
- Bersuder, P., Hole, M., & Smith, G. “Antioxidants from a heated histidine-glucose model system. I: Investigation of the antioxidant role of histidine and isolation of antioxidants by high-performance liquid chromatography.” Journal of the American Oil Chemists' Society, 1998, 75, 181-187. [CrossRef]
- Hus, B., Coupar, I.M., Ng,K., “Antioxidant activity of hot water extract from the fruit of the Doum palm Hyphaene thebaica.” Food Chem., 2006, 98(2),317–328. [CrossRef]
- Lee, J.C., Kim, H.R., Kim, J., Jang, Y.S., 2002. “Antioxidant property of an ethanol extract of the stem of Opuntia ficus-indica var. Saboten.” J. Agric. Food Chem., 50, 6490–6496. [CrossRef]
- International, Standard Practice for Assessment of Hemolytic Properites of Materials, 2000 (n.d.).
- Williams, LAD., Connar, AO, Latore, L, Dennis O, Ringer S, Whittaker, JA, Conrad J, Vogler B, Rosner H, Kraus, W. “The in vitro anti-denaturation effects induced by natural products and non-steroidal compounds in heat treated (immunogenic) Bovine Serum Albumin (BSA) is proposed as a screening assay for the detection of anti-inflammatory compounds, without the use of animals in the early stages of the drug discovery process.” West Indian Med. J., 2008,58, 327-331.
- Thaya, R., Vaseeharan, B., Sivakamavalli, J., Iswarya, A., Govindarajan, M., Alharbi, N. S., Kadaikunnan, S., Al-Anbr, M. N., Khaled, J. M., & Benelli, G. “Synthesis of chitosan-alginate microspheres with high antimicrobial and antibiofilm activity against multi-drug resistant microbial pathogens.” Microbial Pathogenesis, 2018, 114, 17-24. [CrossRef]
- Divya, M., Vaseeharan, B., Abinaya, M., Vijayakumar, S., Govindarajan, M., Alharbi, N. S., Kadaikunnan, S., Khaled, J. M., & Benelli, G., “Biopolymer gelatin-coated zinc oxide nanoparticles showed high antibacterial, antibiofilm and anti-angiogenic activity.” Journal of Photochemistry and Photobiology B: Biology.,2018, 178, 211-218. [CrossRef]
- Matica, M. A., Aachmann, F. L., Tøndervik, A., Sletta, H., & Ostafe, V., “Chitosan as a wound dressing starting material: Antimicrobial properties and mode of action.” International Journal of Molecular Sciences., 2019, 20, 5889. [CrossRef]
- Bukar A, Uba A, Oyeyi T “Antimicrobial profile of Moringa oleifera Lam. extracts against some food-borne microorganisms.” Bayero J Pure Appl Sci., 2010, 3,43–48. [CrossRef]
- Ajose DJ, Oluwarinde BO, Abolarinwa TO, Fri J, Montso KP, Fayemi OE, Aremu AO, Ateba CN. Combating bovine mastitis in the dairy sector in an era of antimicrobial resistance: ethno-veterinary medicinal option as a viable alternative approach. Frontiers in veterinary science. 2022 Apr 4;9:800322. [CrossRef]
- Butler, M. T., Q. F. Wang, and R. M. Harshey., “Cell density and mobility protect swarming bacteria against antibiotics.” Proc. Natl. Acad. Sci. U. S. A., 2010, 107,3776–3781. [CrossRef]
- O’Toole, G., H. B. Kaplan, and R. Kolter. Biofilm formation as microbial development. Annu. Rev.” Microbiol., 2000, 54,49–79. [CrossRef]
- Vaseeharan, B., Sivakamavalli, J., & Thaya, R., “Synthesis and characterization of chitosan-ZnO composite and its antibiofilm activity against aquatic bacteria.” Journal of Composite Materials., 2015,49, 177-184. [CrossRef]
- Siddhuraju, P., Becker, K., “Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves.” J Agric Food Chem., 2003, 51,2144–2155. [CrossRef]
- Shibata, H., Sakamoto, Y., OKA, M., & KONO, Y., “Natural antioxidant, chlorogenic acid, protects against DNA breakage caused by monochloramine.” Bioscience, Biotechnology, and Biochemistry., 1999, 63, 1295-1297. [CrossRef]
- Alhakmani, F., Kumar, S., & Khan, S. A., “Estimation of total phenolic content, in–vitro antioxidant and anti–inflammatory activity of flowers of Moringa oleifera.” Asian Pacific Journal of Tropical Biomedicine., 2013, 3, 623-627. [CrossRef]
- Fard, M. T., Arulselvan, P., Karthivashan, G., Adam, S. K., Fakurazi, S., “Bioactive extract from Moringa oleifera inhibits the pro-inflammatory mediators in lipopolysaccharide stimulated macrophages.” Pharmacognosy Magazine., 2015,11, S556. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).