1. Introduction
Thermal insulation materials are very important building construction materials that reduce energy consumption of a building while simultaneously providing the indoor comfort. When properly installed, thermal insulation materials significantly reduce greenhouse gas emissions including carbon dioxide (CO
2) [
1]. Therefore, development of new eco-effective thermal insulation materials is essential to comply with the climate change policy first set by the United Nations in Paris Agreement [
2] and approved by European Commission in the Green Deal [
3] as well as offset rising costs of energy resources. In the context of the Green Deal, the development of renewable lignocellulosic biomass (LCB)-based materials from agricultural residues are particularly desirable, because they contribute to a “green building” with nearly zero energy [
4]. Moreover, it was declared that the production of LCB-based materials has many of advantages such as low environmental impact, less energy consumption, low cost, low density, scalability, biodegradability and good insulation properties [
5]. However, still the share of LCB-based materials in insulation application reaches only 10%, from which the largest share is virgin/recycled wood fibers and cellulose (recycled paper) [
6]. This signifies the potential to develop and offer new products in the market.
While LCB-based materials used in thermal insulation are in demand because of their environmental friendliness, they are hygroscopic and therefore sensitive to colonization by microorganisms such as fungi and bacteria, challenging the longevity and favorable thermal properties of these materials. Depending on environmental conditions and installation method the produced LCB-based insulation can be colonized by the airborne fungal spores [
7]. Moreover, the fungi-infected insulation concerns indoor air quality that can cause health hazard of inhabits [
8]. Therefore, the durability of LCB-based thermal insulation materials also includes biological resistance that is dependent on temperature and humidity variations but is largely poorly investigated, as reviewed by Schritt and Pleissner [
6]. Water-related thermal properties of LCB-based materials are also on a high importance to be investigated [
5] because a varying humidity concerns not only to biodeterioration but also on the thermal properties [
9,
10]. The microbial quality (mold and bacteria) of industrial crops (flax, hemp, straw) which potentially could be as raw material for insulation is affected by atmospheric conditions during growing season and always present with wide diversity of molds [
11]. An investigated rigid insulation panels from rape straw and hemp shives without added fungicides showed very different mold fungi growth rates, in general reaching over 25% from the area for hemp aggregates and no visible growth for rape aggregate [
12].
Therefore, a preservation treatment of LCB raw materials is necessary to prevent or decrease the mold grow on the end product. Boric acid an borates are commonly used as biocides in commercial cellulosic thermal insulation [
13,
14]. Cellulose insulation treated with sodium polyborate showed good results precluding fungi growth for at least 124 days at high temperatures and relative humidity [
8]. The production of hemp fibers by steam explosion pulping was found as good process capable to decrease fungal contamination that is unfavorable in insulation materials [
15]. None or marginal mold growth was detected on thermal insulation boards from corn pith and sodium alginate with added 8% of boric acid [
14]. The successful antifungal activity of silver nanoparticles was demonstrated on gypsum drywall and was suggested to building materials as effective protection of indoor environments from mold development [
16]. The effect of different solvents like cold/hot water, benzene-ethanol, ethanol-ether, NaOH and HCl was investigated on mold development on bamboo timber revealing the best resistance to mold grow of 1% HCl [
17]. The surface treatment with castor oil based polyurethane resin of particleboards from sugarcane and eucalyptus wood decreased the percentage of the mold colonization area even after 12 months of natural exposure [
18]. Wood fiber insulation boards containing different mixtures of spruce and hardwoods produced in a dry process with PMDI adhesives and different additives demonstrated sufficient mold fungi resistance with surface growth rate < 50%, however the results significantly varied depending on test method [
19]. Impregnation of bleached chemi thermo mechanical pulp by hydrophobic betulin containing extractives from birch wood outer bark resulted to improved water and fungal resistance properties [
20].
The studies reviewed above highlight the importance for further research into mold fungi effect on existing and newly developed LCB-based thermal insulation products. Therefore, our study continues the research of new thermal insulation materials [
21,
22] from locally sourced and annually harvested LCB such as wheat straw, water reed and corn stalk providing results of mold fungi resistance. The research is significant due to the selected raw materials are wide available around the world and the obtained results could be useful for development and increase of LCB-based thermal insulation market share.
2. Materials and Methods
2.1. Raw LCB
Wheat straw (Triticum aestivum)—WS (grain-extracted from Limbaži district, Latvia), water reeds (Phragmites australis, whole plant harvested in winter from Puzes Lake, Ventspils district, Latvia) and corn (Zea mays) stalks (fresh, ear/grain-extracted from the farm “Pauri”, Blome, Latvia) were used in the study as locally grown raw materials. The delivered raw materials were chopped in a knife mill (CM4000, LAARMANN, Roermond, The Netherlands) to pass a sieve with openings of Ø 30 mm. The chopped LCB materials were considered to further processing.
2.2. Processing of Raw LCB
2.2.1. Steam Explosion Pulping (SEP)
Based on the previous studies [
21,
22], the chopped raw LCB was moisturized up to 80% of moisture content by immersing it in water for 24 h. After, the LCB was drained and separately treated in a home-made SE device of original construction with a 0.5 L batch reactor at the constant conditions: temperature of 230 °C, residence time of 30 s maintaining the pressure of 30 bar. After, the wet SEP was collected and manually-squeezed in a juice-like press to remove the liquid fraction.
2.2.2. Thermo-Mechanical Pulping (TMP)
TMP of chopped and soda-treated LCB was performed in a single-disc refiner Regmed MD-300 (Osasco, Brazil) at constant 1450 rpm. The soda treatment was performed by adding 4% of NaOH based on dry LCB weight and cooking in water at the proportion 1:28 for 30 min. After, the soda-treated LCB was drained and subjected to the refiner fulfilled with water (20 ℃). The duration of TMP process was constant (10 min) for all samples achieving the gap of 0.25 mm between universal plates. The resulting fiber solution was drained through a 2 mm sieve and manually-squeezed in a juice-like press.
2.2.3. Mechanical Foaming of Processed LCB
The obtained SEP and TMP materials were mechanically foamed by a self-made device through a system of two rotating cylinders (900 rpm) coupled with stainless steel wires as described in [
23]. The procedure was performed at least 3x to separate and homogenize the obtained fiber mass making it fluffy and suitable for application as loose-fill thermal insulation material.
2.2.4. Admixture of Fire Retarder and Fungicide
To prevent the investigated materials from fire and biological effects, 8% of boric acid (H3BO3, CAS: 10043-35-3; Chempur, Poland) and 7% of di-Sodium tetraborate decahydrate (Na2B4O7 × 10H2O, CAS: 1303-96-4; Chempur, Poland) were added based on dry weight of LCB. The substances first were solubilized in the hot (90 °C) water at the proportion 1:3, then, sprayed on the foamed LCB samples.
The control samples of unprocessed raw materials were prepared as well by the chopping in the knife mill to pass a sieve of Ø 20 mm and admission of the above-mentioned substances. A control sample of SEP without admixture of fire retardant and fungicide substances was prepared additionally to detect the effect of SE treatment alone on mold fungi growth.
All the prepared LCB samples were conditioned prior to mold growth test in a chamber under controlled conditions (temperature 20 ± 2 ℃ and relative humidity 60 ± 5%) until equilibrium moisture content, which was reached 9.8 ± 0.4% for raw, 7.8 ± 0.1% for SEP, and 10.3 ± 0.2% for TMP samples, respectively.
2.3. Mold Growth Tests
Mold growth tests were performed following the procedure described in Annex F of [
24] with fungal inoculates obtained from DSMZ - German Collection of Microorganisms and Cell Cultures GmbH. The following inoculates were used: A –
Trichoderma viride strain DSM 1963 (synonym ATCC 9645), B –
Chaetomium globulosum strain DSM 1962 (synomym ATCC 6205), C –
Paecilomyces variotii strain DSM 1961 (synonym ATCC 18502), D –
Talaromyces pinophilus (
Penicillium pinophilum) strain DSMZ 1944 (synonym ATCC 36839), E –
Aspergillus niger strain DSM 1957 (synonym ATCC 6275). All fungal isolates were grown on 0.5X PDA (Potato dextrose agar) for 14 days at 25 ℃ (in dark) until sporulation. Spores were collected by applying 10 mL sterile water onto the mycelial surface in each Petri dish (Ø 120 mm) and disrupting the mycelium with a sterile inoculation loop to resuspend the spores into the water. Spore suspension was passed through a column containing sterile cotton to remove fungal hyphae. Spores were counted using an automated cell counter LunaFX7 (Logos biosystems, S. Korea). 25 mL of the uncompressed loose-fill LCB test specimens were evenly placed onto filter paper (Watman, pre-wetted with 4mL sterile water) inside a Petri dish (Ø 90 mm). Four pine (
Pinus sylvestris L.) sapwood specimens (30 × 30 × 5 mm) per Petri dish were used as control sample to verify spore growth of each mold fungi. Each specimen was spray-inoculated with 10
6 spores (in 4 mL water) of a single fungal strain, and Petri dishes sealed with Parafilm. Additional to the fungi-inoculated LCB samples, sterile water was used on each processed LCB material as control for any possible microbial growth originating from the processed test materials themselves. Four replicates were prepared for each combination of material type and fungal species and incubated for 28 days at 28 ℃ (in dark), >90% RH.
2.4. Evaluation of Mold Fungal Growth
All loose-fill LCB and wood control sample specimens (
Table 1) were inspected under stereomicroscope (Leica MZ9
5) for qualitative scoring using relative scale from 0 to 5 adopted from [
25]. Representative images were taken for each specimen. The mold growth rate of each test specimen was evaluated using the scale ranking as following:
0 – no detectable growth;
1 – small growth with ~20% colonization;
2 – sparse growth with ~40% colonization;
3 – moderate growth with ~ 60% colonization;
4 – heavy growth with ~80% colonization;
5 – very heavy colonization across the entire material surface (~100%).
The factors of the influence on the mean values accomplished with standard variation of evaluated sample’s mold fungi growth rate were analyzed by one-way ANOVA at the significance level α = 0.05 [
26].
3. Results
3.1. Mould Fungal Colonization on Wood Controls
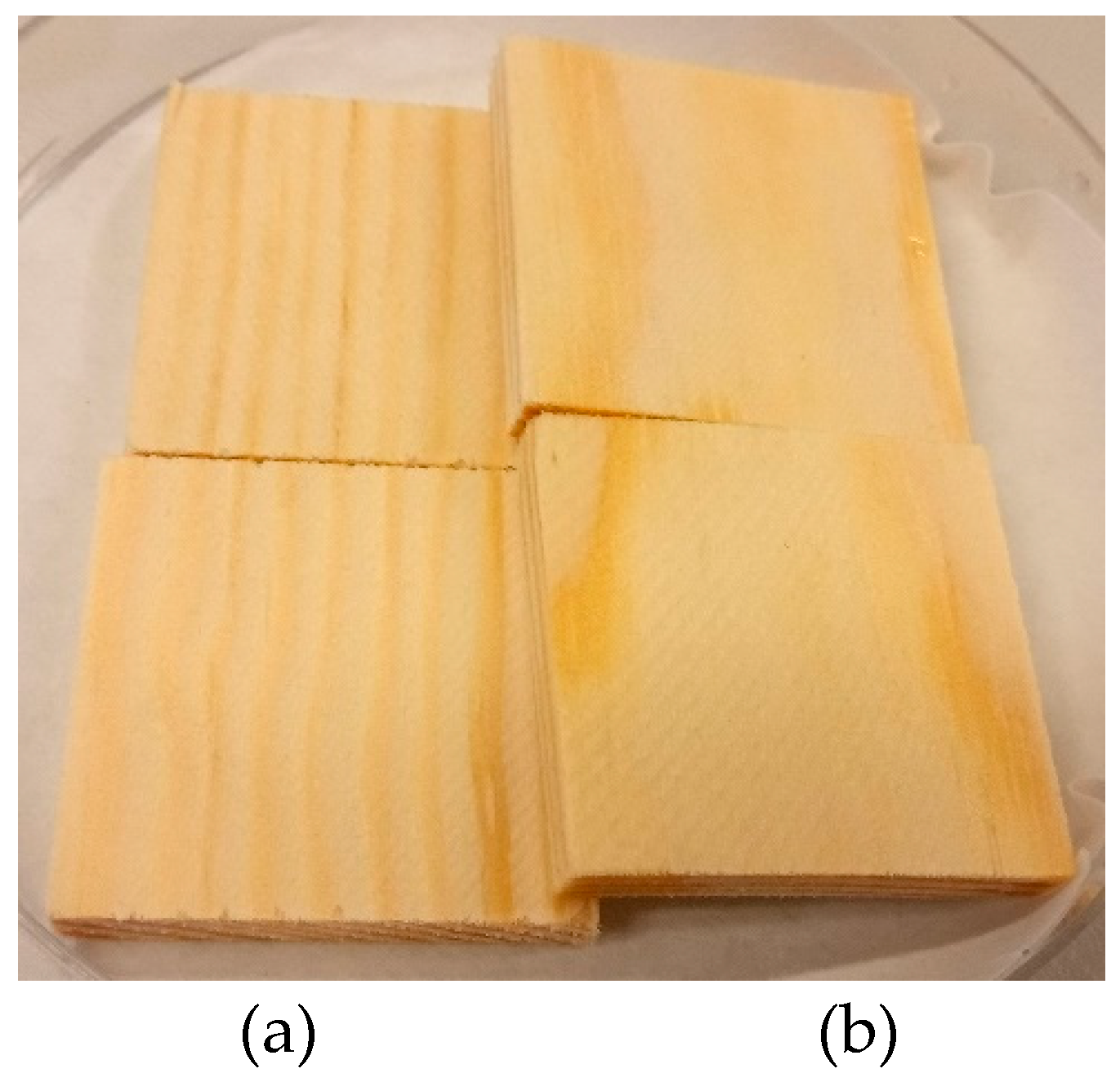
The initially delivered control specimens of pine sapwood for mold fungi tests contained different surfaces these were characterized as tangential and radial as shown in
Figure 1. Therefore, the selected fungi were inoculated on both wood sample surfaces. The results of the mold fungi growth on the wood control samples are summarized in the
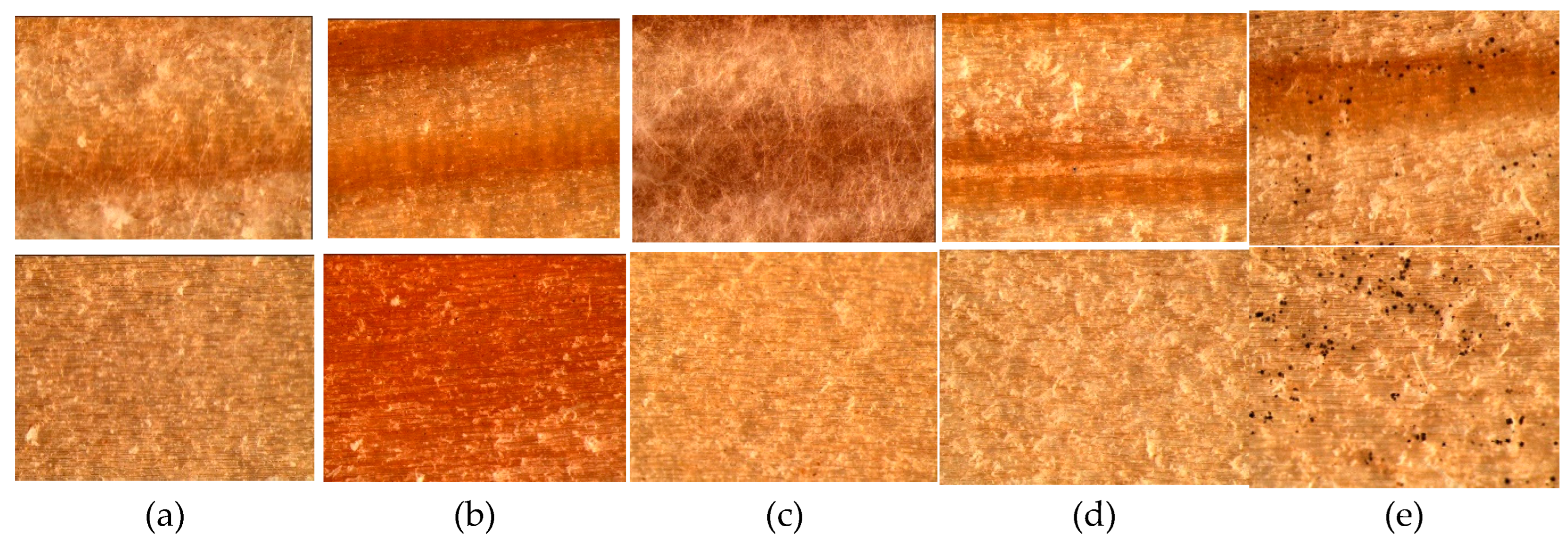
Table 2 and supplemented by microscopical surface view in
Figure 2.
As it is seen from the
Figure 2 the mold fungi growth on the wood controls is significantly different depending on fungi species and even wood surface. The growth of fungi A and C was observed only on the radial surface of wood specimens covering it up to 60% by the fluffy hyphae (
Figure 2a,c). The growth of fungi B was rated with 1 meaning that it covered the control specimens surface up to 20% by the small black points evenly on both tangential and radial surfaces (
Figure 2b). The growth of fungi D was not observed on both wood surfaces (
Figure 2d) similar as for control samples incubated without fungi inoculation. Only the growth of fungi E was detected on both wood surfaces covering it up to 60% by obvious black points (
Figure 2e).
3.2. Mould Fungal Colonization on Loose-Fill LCB
3.2.1. Fungal Colonization on Wheat Straw LCB Samples
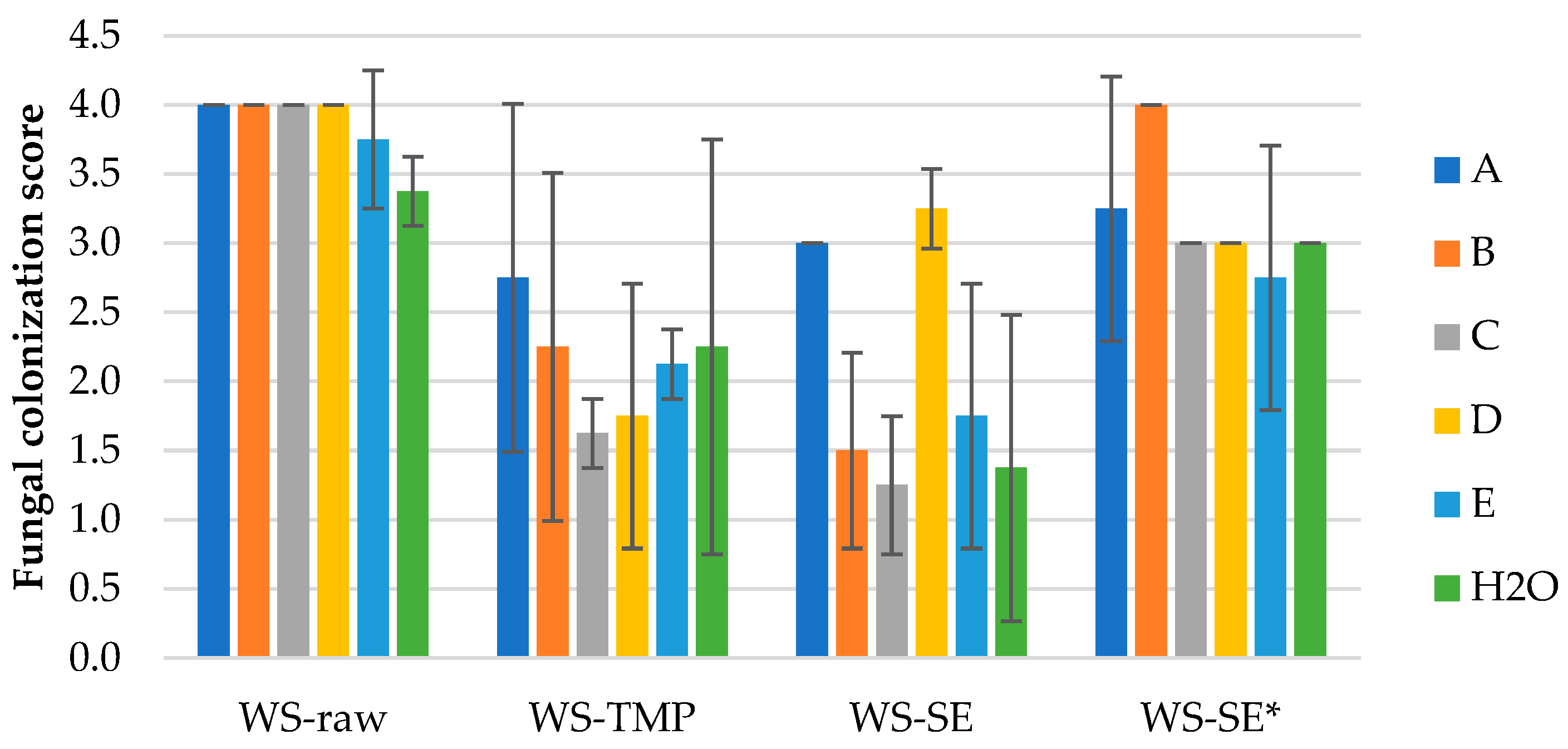
Results of mold fungal colonization on loose-fill wheat straw LCB samples depending on fungi species are summarized in
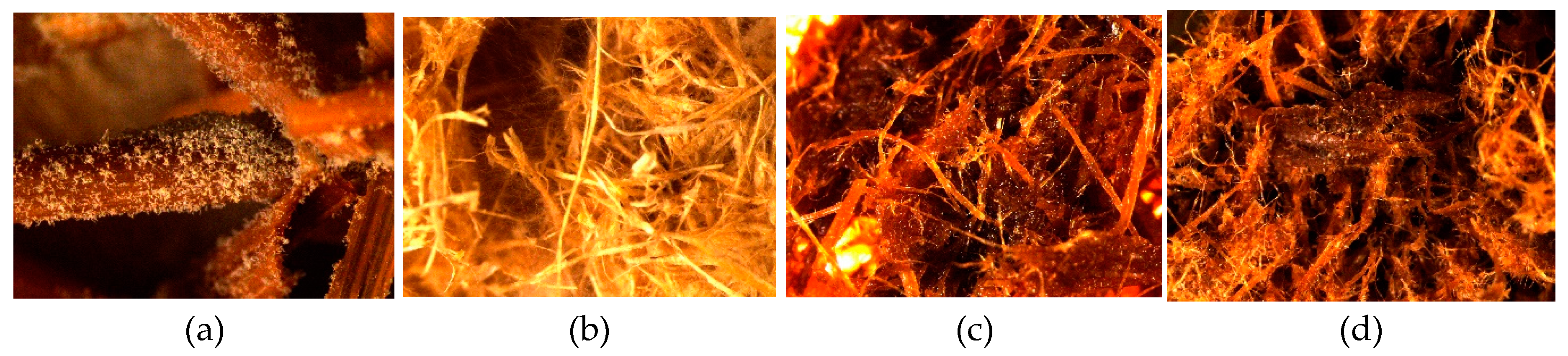
Figure 3 and supplemented by the surface views in
Figures S1.1–S1.4 of Supplementary Materials. As it is seen, all WS samples were affected by fungus growth rated in average from 1.3 ± 0.5 (WS-SE with fungi C) to 4.0 ± 0.0 (WS-raw with fungus A–D). There was not detected significant difference of fungal colonization between all WS-raw samples infected with individual fungus A–E (
Figure 4a and
Figure S1.1); except of control sample (H
2O) that demonstrated moderate colonization score of 3.4 ± 0.3 (
Figure 3).
The fungal colonization of WS-TMP samples varies in average range of 1.6–2.8 (small to moderate,
Figure 3 and
Figure S1.2); however, due to the high standard variation the difference between the samples was calculated as insignificant (P-value 0.698). The microscopical surface view of the sample affected by fungi B-
Chaetomium globulosum with developed sparse hypha is shown in
Figure 4b and
Figure S1.2b.
The fungal colonization of WS-SE samples (
Figure S1.3) varies in average range of 1.3–3.3 (small to moderate) with calculated significant difference between the samples (
Figure 3). There is no significant difference between fungal colonization scores of the samples infected with A and D fungus, and between fungus B, C, E and control, respectively. This observation means that the fungal colonization score of WS-SE samples depends on individual fungi in spite of fungicide presence. In turn, the fungal colonization score of WS-SE* samples varies in average range of 2.8–4.0 (moderate to heavy) indicating significant influence of fungicide absence. However, the only
T. viride demonstrates insignificant colonization difference between the SEP samples with and without fungicide addition, respectively (
Figures S1.3a and S1.4a). The last observation fits the previous tendency that the colonization score of WS-SE samples depends on individual fungi in spite of fungicide presence that could be seen in
Figure 4c,d,
Figures S1.3 and S1.4.
Summarizing the mold fungal colonization scores on WS samples, it could be said that these depend on individual fungi, the sample processing and fungicide application. As well, the used fungi are viable on all WS samples independent on used processing, including LCB control samples (H2O) without added fungi (
Figure 3).
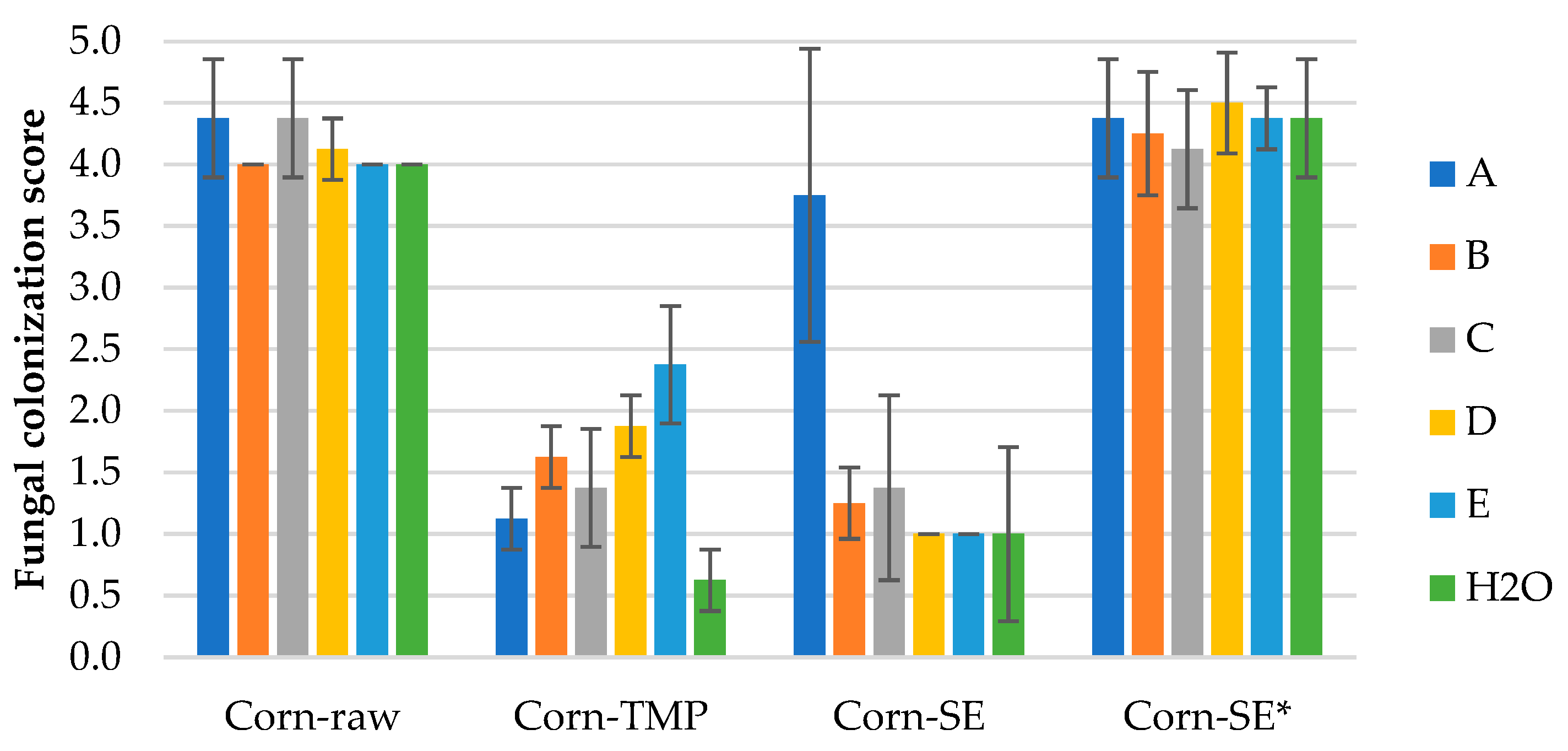
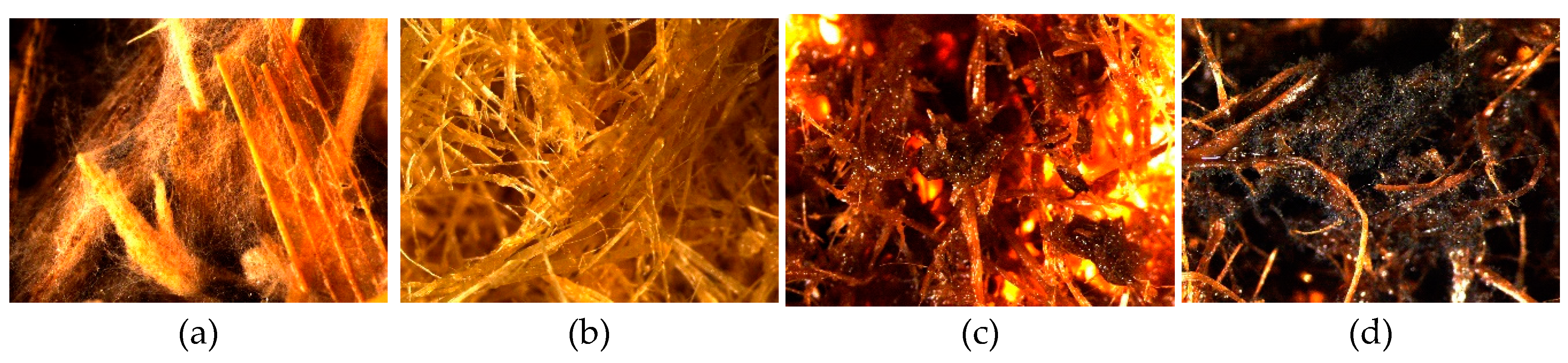
3.2.2. Mold Fungal Colonization on Corn Stalk LCB Samples
Results of mold fungal colonization on loose-fill corn stalk LCB samples depending on fungi species are summarized in
Figure 5 and supplemented by the surface views in
Figures S2.1–S2.4. As in the case of WS samples, all Corn samples were also affected by fungus growth rated in average from 0.6 ± 0.3 (Corn-TMP, H2O) to 4.5 ± 0.4 (Corn-SE* with fungus D). If compare with WS samples, the fungal colonization was higher for Corn-raw and Corn-SE* samples, but lower for Corn-TMP and Corn-SE samples (
Figure 3 and
Figure 5). This observation could be associated to the specific structural difference of LCB species and their susceptibility of mold fungi depending on processing.
The fungal colonization of Corn-raw samples depending on fungi species vary insignificantly in range of 4.0–4.4 that is characterized as heavy (
Figure 6a and
Figure S2.1). The fungal colonization of Corn-TMP samples depending on fungi species vary in range of 0.6–2.4, that is small to sparse (
Figure 6b and
Figure S2.2), and the variation is significant. That means the different resistance level of the material on individual mold fungi. The best mold fungi resistance demonstrates control Corn-TMP sample (0.6 ± 0.3), but the worse resistance was detected with inoculated fungi E-
Aspergillus niger (2.4 ± 0.5).
The fungal colonization of Corn-SE samples was rated in range of 1.0–3.8, that is small to heavy (
Figure 6c and
Figure S2.3), and the variation is significant. The small colonization was detected on the samples inoculated with fungi D-
Penicillium pinophilum (
Figure S2.3d) and E-
Aspergillus niger (
Figure S2.3e), and on the control sample (
Figure S2.3f). In turn, the heavy growth was detected on the sample inoculated by fungi A-
Trichoderma viride (
Figure S2.3a). The fungal colonization of Corn-SE* samples depending on fungi species vary insignificantly in range of 4.1–4.5 that is characterized as heavy to very heavy (
Figure 6d and
Figure S2.4). This means that the Corn-SE* material, similar to Corn-raw, is very susceptible to all mold fungi species including just the conditions of humid environment that was tested by the control sample.
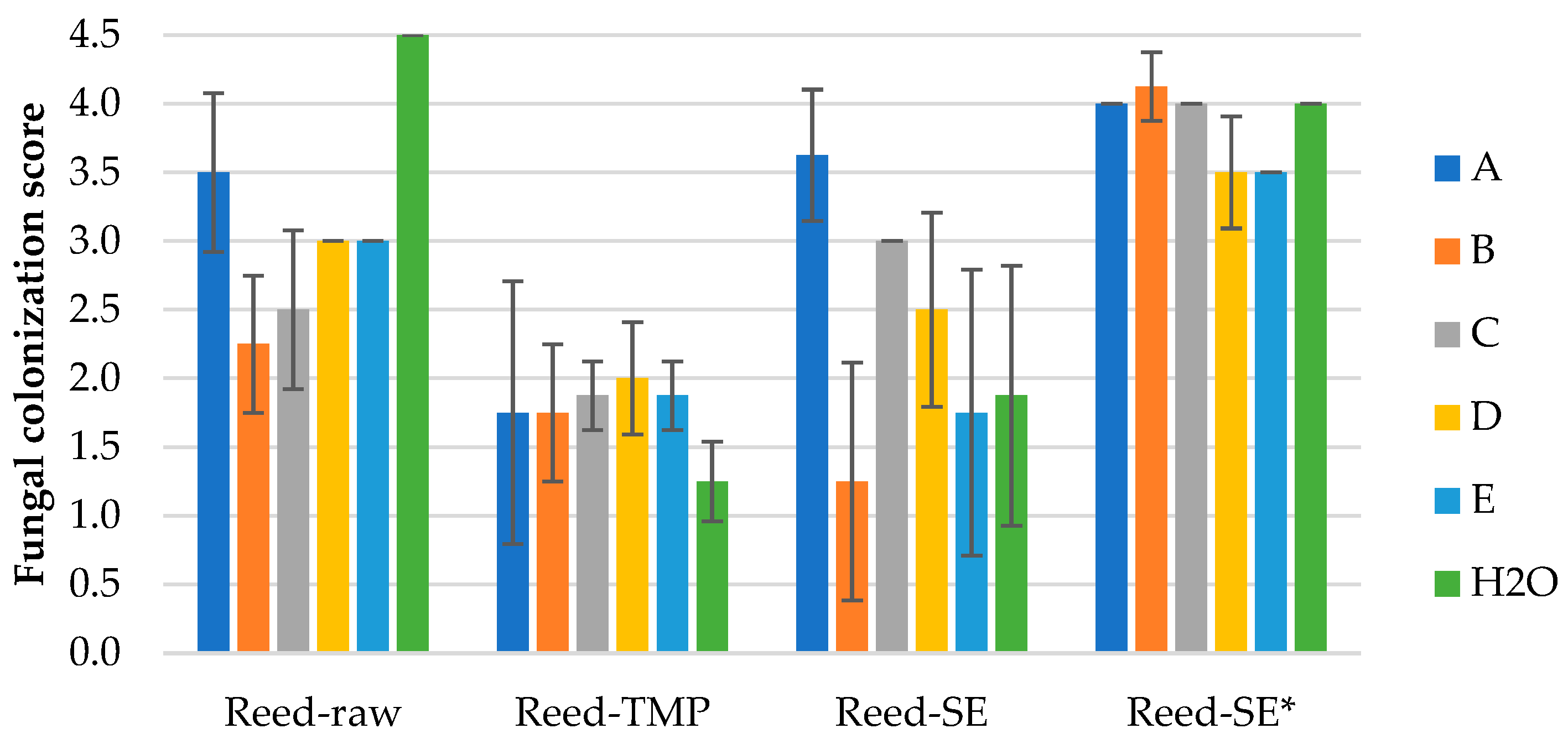
3.1.3. Mold Fungal Colonization on Water Reed LCB Samples
Results of mold fungal colonization on water reed LCB samples depending on fungi species are summarized in
Figure 7 and supplemented by the surface views in
Figures S3.1–S3.4. The fungal colonization of all Reed-raw samples varies in average range of 2.3–4.5 that is characterized as moderate to very heavy (
Figure 8a and
Figure S3.1), and the difference between the samples is significant. The moderate growth (2.3 ± 0.5) was observed by fungi B-
Chaetomium globulosum (
Figure S3.1b), but the very heavy (4.5 ± 0.0) – on the control sample without inoculated fungi (
Figure S3.1f).
The fungal colonization of all Reed-TMP samples varies in average range of 1.3–2.0 that is characterized as small to sparse (
Figure S3.2). The ANOVA approved insignificant differences between the samples spored with different fungi. The lowest fungal colonization score (1.3 ± 0.3) was observed on the control Reed-TMP sample (
Figure 8b and
Figure S3.2f), differently if compare with Reed-raw sample (
Figure 7).
The fungal colonization of all Reed-SE samples varies significantly in range of 1.3–3.6 that is characterized as small to heavy (
Figure 8c and
Figure S3.3). The lowest fungal colonization score (1.3 ± 0.9) was observed by fungi B-
Chaetomium globulosum (
Figure S3.3b), but the heavy growth (3.6 ± 0.5) – by fungi A-
Trichoderma viride (
Figure 3.3a). The fungal colonization of SEP samples without added fungicide (Reed-SE*) demonstrates a lower resistance varying in range of 3.5–4.1 characterizing heavy growth (
Figure 8d and
Figure S3.4) independing on fungi species. Despite of the small range of fungi growth, the ANOVA approved significantly lower results for Reed-SE* samples inoculated by fungus D-
Penicillium pinophilum (3.5 ± 0.4) and E-
Aspergillus niger (3.5 ± 0.0),
Figure 7.
4. Discussion
In general, wood control samples demonstrate a higher resistance to mold fungi when comparing to LCB samples (
Table 2,
Figure 3,
Figure 5 and
Figure 7). Particularly, taking into account that wood controls were not pretreated by fungicide. The different intensity of mold fungi growth regarding radial and tangential surface could be explained by a higher amount of soluble sugars found on sapwood surface as reported by [
27]. From another point of view, the loose-fill LCB structure is more accessible to mold fungi than the surface of rigid wood. Another difference between the samples could be considered is the moisture content that, possibly, increased faster in the LCB samples than in wood due to the high humidity of the test. Moreover, the achieved equilibrium moisture content even at the same conditions depends on LCB species. For e.g., equilibrium moisture content of wheat and corn grains at air conditions of 28 ℃ and RH 90% achieves 16.7% and 18.9%, respectively [
28] making the difference between LCB species for fungi development. It was shown that mold fungi growth rate of wheat straw-polypropylene composites was evaluated as 1 after one week exposure, and only after 4 weeks it was reached 4 covering > 60% of the composite surface [
29].
The detected mold fungi growth at small to very heavy levels of all control LCB samples (H
2O) could be explained by the presence of airborne mold fungi. As it was reported, airborne mold samples were consistent with bulk cellulosic insulation sample analyses relative to mold types present [
7]. Regarding the average values of mold fungal colonization of the control LCB samples, in general, they were at lower levels comparing to the fungi-inoculated LCB samples (
Figure 3,
Figure 5 and
Figure 7). Sufficient resistance to mold growth independent on raw LCB species showed TMP (1.4 ± 1.1) and SEP (1.4 ± 0.9) samples indicating to the most appropriate processing combined with fungicide addition.
The highest intensity of mold fungi growth independent on fungi species was observed for unprocessed samples in spite of the fungicide treatment. From them, the lowest mold fungi resistance shows corn samples, 4.1 ± 0.1 (
Figure 5), followed by wheat straw, 3.9 ± 0.3 (
Figure 3), and reed samples, 3.1 ± 0.8 (
Figure 7). The difference between the samples average values being significant. That phenomena could be explained by a large amount of soluble sugars providing nutrients for mold fungi [
25]. The most potential soluble sugars of raw LCB used in this study is the content of hemicelluloses that varied in range of 24–30% [
23]. The highest resistance to fungi growth of reed raw LCB could be associated to the natural plant growing conditions in water. However, as was reported by Malheiro et al. [
30] the mold growth intensity of neat giant reed was detected between 4 and 6 covering 50–80% of sample area that fit our results. In any case the used fungicide content in raw LCB samples is not enough to prevent the mold growth in acceptable levels (< 3).
The lowest fungal growth between the tested LCB samples was detected on all TMP samples (1.8 ± 0.8) that is acceptable for product use. The difference between the LCB species independent on fungi species was found to be significant (p-value 0.017). It is expected that the obtained results are influenced by the technological feature which includes the cooking with NaOH and then defibration in water medium. It was shown that bamboo timber treated with 1% of NaOH resisted
Trichoderma viride and
Penicillium citrinum efficiently, however, the fungi resistance against
Aspergillus niger was lower [
17]. The insulation panel of alkaline-treated and thermally compressed rape straw showed no visible mold fungi growth demonstrating perfect fungi resistance [
12].
The effect of applied fungicide on loose-fill LCB in the framework of this study is obvious, however, it is not enough in all cases. For e.g., comparing all the SEP samples independent on raw LCB and fungi species, the fungal colonization scores of the fungicide-sprayed samples is ~2× lower (2.0 ± 1.1) than for those without fungicide application (3.8 ± 0.7). The positive effect of steam explosion treatment on mold growth reduction on hemp fibers was also approved by Nykter et al. [
15]. If compare the fungal colonization of all raw (3.7 ± 0.7) and SEP (2.0 ± 1.1) samples the SE effect is significant that is associated with reduced amount of hemicelluloses in SEP samples resulting to a lower wettability [
31]. However, it doesn’t work without fungicide. For that reason, additional water rinsing especially combined with NaOH addition after the SE process may be suggested to release the residual hydrophilic hemicelluloses on the fiber surfaces. Possibly, this would result to decrease of fungi growth expecting that a fungicide would not be necessary, taking into account that insulation materials are anticipated to be located inside a construction under the conditions lighter than in the performed mold fungi test. For e.g., there was observed on chemically untreated wet-sprayed cellulose insulation that mold fungi growth significantly correlates with sample moisture and RH in terms when these units decrease the fungi growth decrease too down from 75% to 25% [
8].
It should be noted that the fungal colonization of used LCB samples was different depending on fungi species and used LCB processing. The different fungal activity at varying conditions depending on individual fungi was demonstrated by Li and Wadso [
32] concluding the effect of optimal fungi colonization conditions. The processing effect of LCB could be evaluated by the growth intensity of fungi A-
Trichoderma viride that was detected as moderate for Reed-raw sample (3.5 + 0.6),
Figure 7; the same intensity of the fungi was detected after SE with used fungicide (Reed-SEP, 3.6 + 0.5) and increased on the sample without fungicide (Reed-SEP* 4.0 + 0.0); however, the fungi A growth intensity decreased significantly after TMP process (Reed-TMP, 1.8 ± 1.0). If compare the detected average growth intensity of used fungi species independent on LCB and processing, the highest intensity shows fungi A-
Trichoderma viride (3.3 ± 1.2), but the lowest (2.7 ± 1.1) – fungi E-
Aspergillus niger.
The same results variation tendency depending on LCB processing is observed also on control (H
2O) samples which average mold growth intensity was calculated as 2.6 ± 1.5. The best fungal growth resistance between these samples was observed for Corn-TMP (1.5 ± 0.6) and Corn-SEP (1.6 ± 1.1) samples (
Figure 5) demonstrating the effective processing influence on the end products which are suggested for insulation applications. However, the fungal growth resistance of the sample Corn-SE* (without fungicide) in average was rated by 4.4 ± 0.5 with fungi-covered area of about 87% proving the influence and necessity of fungicide.
The performed study gives knowledge about mold fungal resistance of processed LCB for application in thermal insulation. Since the selected LCB species are widely available around the world the results of the study are of high importance. Taking into account that TMP and SEP processing resulted to the best results for all used species, the study will be continued investigating other important properties like settlement, water vapor diffusion, reaction to fire, and volatile organic compounds. We sincerely hope that achieved results will be useful for development and increase of LCB-based thermal insulation market share contributing to the mitigation of climate change.
5. Conclusions
In the framework of the study mold fungal resistance was evaluated for newly investigated loose-fill thermal insulation materials produced from wheat straw, corn stalk and water reed using different processing. In spite of fungicides addition (8% of boric acid and 7% of tetraborate), in average, heavy mold growth was detected on all raw materials covering around 75% of sample area. The same growth was detected also for steam-exploded pulp (SEP) without added fungicides. The SEP samples with fungicides addition demonstrated sparse mold fungi growth covering around 40% of sample area. The highest mold fungi resistance was demonstrated by thermal-mechanical pulp (TMP) samples in average covering around 35% of sample area. The TMP was performed by adding 4% of NaOH that significantly affected and decreased the mold growth. Therefore, the substrates of SEP and TMP of all used raw materials with fungicide admixture are the most suitable end products for thermal insulation application demonstrating the resistance with sparse mold growth. The highest resistance to internal and externally applied mold colonization among the tested source materials is demonstrated by Corn-TMP and Corn-SEP samples (~30%) followed by Reed-TMP (~35%), wheat straw SEP and TMP (~40–43%) and Reed-SEP (~47%).
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1.1: Microscopical surface view (2.5×) of WS-raw samples after 4 weeks incubation colonized with: (a) T. viride, (b) C. globulosum, (c) P. variotii, (d) P. pinophilum, (e) A. niger, (f) control (H2O). Figure S1.2: Microscopical surface view (2.5×) of WS-TMP samples after 4 weeks incubation colonized with: (a) T. viride, (b) C. globulosum, (c) P. variotii, (d) P. pinophilum, (e) A. niger, (f) control (H2O). Figure S1.3: Microscopical surface view (2.5×) of WS-SE samples after 4 weeks incubation colonized with: (a) T. viride, (b) C. globulosum, (c) P. variotii, (d) P. pinophilum, (e) A. niger, (f) control (H2O). Figure S1.4: Microscopical surface view (2.5×) of WS-SE* samples after 4 weeks incubation colonized with: (a) T. viride, (b) C. globulosum, (c) P. variotii, (d) P. pinophilum, (e) A. niger, (f) control (H2O). Figure S2.1: Microscopical surface view (2.5×) of Corn-raw samples after 4 weeks incubation colonized with: (a) T. viride, (b) C. globulosum, (c) P. variotii, (d) P. pinophilum, (e) A. niger, (f) control (H2O). Figure S2.2: Microscopical surface view (2.5×) of Corn-TMP samples after 4 weeks incubation colonized with: (a) T. viride, (b) C. globulosum, (c) P. variotii, (d) P. pinophilum, (e) A. niger, (f) control (H2O). Figure S2.3: Microscopical surface view (2.5×) of Corn-SE samples after 4 weeks incubation colonized with: (a) T. viride, (b) C. globulosum, (c) P. variotii, (d) P. pinophilum, (e) A. niger, (f) control (H2O). Figure S2.4: Microscopical surface view (2.5×) of Corn-SE* samples after 4 weeks incubation colonized with: (a) T. viride, (b) C. globulosum, (c) P. variotii, (d) P. pinophilum, (e) A. niger, (f) control (H2O). Figure S3.1: Microscopical surface view (2.5×) of Reed-raw samples after 4 weeks incubation colonized with: (a) T. viride, (b) C. globulosum, (c) P. variotii, (d) P. pinophilum, (e) A. niger, (f) control (H2O). Figure S3.2: Microscopical surface view (2.5×) of Reed-TMP samples after 4 weeks incubation colonized with: (a) T. viride, (b) C. globulosum, (c) P. variotii, (d) P. pinophilum, (e) A. niger, (f) control (H2O). Figure S3.3: Microscopical surface view (2.5×) of Reed-SE samples after 4 weeks incubation colonized with: (a) T. viride, (b) C. globulosum, (c) P. variotii, (d) P. pinophilum, (e) A. niger, (f) control (H2O). Figure S3.4: Microscopical surface view (2.5×) of Reed-SE* samples after 4 weeks incubation colonized with: (a) T. viride, (b) C. globulosum, (c) P. variotii, (d) P. pinophilum, (e) A. niger, (f) control (H2O).
Author Contributions
Conceptualization, R.T., M.A., J.L. and Z.O.; methodology, A.B., G.P., M.A., J.L. and Z.O.; validation and data curation, J.L. and Z.O.; formal analysis, K.T.B.; investigation, Z.O., K.T.B. and R.T.; resources, M.A., A.B., G.P. and J.L.; writing—original draft preparation, R.T.; writing—review and editing, R.T., A.B. and Z.O.; visualization, Z.O., K.T.B. and R.T; supervision and funding acquisition, R.T.; project administration, R.T. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research and APC of the article were funded by the LATVIAN COUNCIL OF SCIENCE, project “Investigation of eco-friendly thermal insulation materials from sustainable and renewable industrial crops residuals”, number lzp-2021/1-0599.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The raw data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
References
- Danish; Zhang, B.; Wang, Z.; Wang, B. Energy Production, Economic Growth and CO2 Emission: Evidence from Pakistan. Nat. Hazards 2018, 90, 27–50. [CrossRef]
- Benduski, M. Paris Agreement vs Kyoto Protocol [Comparison Chart] Available online: https://www.careaboutclimate.org/blog/paris-agreement-vs-kyoto-protocol-comparison-chart.
- 3. European Comission A European Green Deal Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en.
- Asdrubali, F.; D’Alessandro, F.; Schiavoni, S. A Review of Unconventional Sustainable Building Insulation Materials. Sustain. Mater. Technol. 2015, 4, 1–17. [CrossRef]
- Cintura, E.; Nunes, L.; Esteves, B.; Faria, P. Agro-Industrial Wastes as Building Insulation Materials: A Review and Challenges for Euro-Mediterranean Countries. Ind. Crops Prod. 2021, 171, 113833. [CrossRef]
- Schritt, H.; Pleissner, D. Recycling of Organic Residues to Produce Insulation Composites: A Review. Clean. Waste Syst. 2022, 3, 100023. [CrossRef]
- Godish, T.J.; Godish, D.R. Mold Infestation of Wet Spray-Applied Cellulose Insulation. J. Air Waste Manag. Assoc. 2006, 56, 90–95. [CrossRef]
- Herrera, J. Assessment of Fungal Growth on Sodium Polyborate-Treated Cellulose Insulation. J. Occup. Environ. Hyg. 2006, 2, 626–632. [CrossRef]
- Nguyen, D.M.; Grillet, A.-C.; Diep, T.M.H.; Thuc, C.N.H.; Woloszyn, M. Hygrothermal Properties of Bio-Insulation Building Materials Based on Bamboo Fibers and Bio-Glues. Constr. Build. Mater. 2017, 155, 852–866. [CrossRef]
- Hellová, K.E.; Unčík, S.; Cabanová, T. Sorption Properties of Thermal Insulation Composed of Flax or Hemp Fibers. Slovak J. Civ. Eng. 2020, 28, 47–52. [CrossRef]
- Nykter, M. MICROBIAL QUALITY OF HEMP (Cannabis Sativa L .) AND FLAX (Linum Usitatissimum L .) FROM PLANTS TO THERMAL INSULATION. Univ. Helsinki 2006, 97.
- Viel, M.; Collet, F.; Lecieux, Y.; François, M.L.M.; Colson, V.; Lanos, C.; Hussain, A.; Lawrence, M. Resistance to Mold Development Assessment of Bio-Based Building Materials. Compos. Part B Eng. 2019, 158, 406–418. [CrossRef]
- Klamer, M.; Morsing, E.; Husemoen, T. Fungal Growth on Different Insulation Materials Exposed to Different Moisture Regimes. Int. Biodeterior. Biodegrad. 2004, 54, 277–282. [CrossRef]
- Palumbo, M.; Lacasta, A.M.; Navarro, A.; Giraldo, M.P.; Lesar, B. Improvement of Fire Reaction and Mould Growth Resistance of a New Bio-Based Thermal Insulation Material. Constr. Build. Mater. 2017, 139, 531–539. [CrossRef]
- Nykter, M.; Kymalainen, H.-R.; Thomsen, A.B.; Lilholt, H.; Koponen, H.; Sjober, A.-M.; Thygesen, A. Effects of Thermal and Enzymatic Treatments and Harvesting Time on the Microbial Quality and Chemical Composition of Fibre Hemp (Cannabis Sativa L.). Biomass and Bioenergy 2008, 32, 392–399. [CrossRef]
- Ogar, A.; Tylko, G.; Turnau, K. Antifungal Properties of Silver Nanoparticles against Indoor Mould Growth. Sci. Total Environ. 2015, 521–522, 305–314. [CrossRef]
- Sun, F.; Zhou, Y.; Bao, B.; Chen, A.; Du, C. Influence of Solvent Treatment on Mould Resistance of Bamboo. BioResources 2011, 6, 2091–2100. [CrossRef]
- Garzón-Barrero, N.M.; Shirakawa, M.A.; Brazolin, S.; de Barros Pereira, R.G. de F.N.; de Lara, I.A.R.; Savastano, H. Evaluation of Mold Growth on Sugarcane Bagasse Particleboards in Natural Exposure and in Accelerated Test. Int. Biodeterior. Biodegrad. 2016, 115, 266–276. [CrossRef]
- Imken, A.A.P.; Brischke, C.; Kögel, S.; Krause, K.C.; Mai, C. Resistance of Different Wood-Based Materials against Mould Fungi: A Comparison of Methods. Eur. J. Wood Wood Prod. 2020, 78, 661–671. [CrossRef]
- Zheng, C.; Li, D.; Ottenhall, A.; Ek, M. Cellulose Fiber Based Fungal and Water Resistant Insulation Materials. Holzforschung 2017, 71, 633–639. [CrossRef]
- Berzins, A.; Tupciauskas, R.; Andzs, M.; Pavlovichs, G. Development of Thermal Insulation Materials from Plant Fibres by Steam Explosion Pre-Treatment. In Proceedings of the Proceedings of the 18th Annual Meeting of the Northern European Network for Wood Science and Engineering; Brischke, C., Buschalsky, A., Eds.; University of Göttingen: Göttingen, 2022; pp. 177–180.
- Berzins, A.; Tupciauskas, R.; Andzs, M.; Pavlovichs, G. Potential of Some Latvian Industrial Crops Residuals for Conversion to Bio-Based Thermal Insulation Material. Mater. Sci. Forum 2022, 1071, 139–146. [CrossRef]
- Tupciauskas, R.; Berzins, A.; Pavlovics, G.; Bikovens, O.; Filipova, I.; Andze, L.; Andzs, M. Optimization of Thermal Conductivity vs Bulk Density of Steam Exploded Loose-Fill Annual Lignocellulosics. Materials (Basel). 2023, 16, 3654. [CrossRef]
- EN 15101-1+A1 Thermal Insulation Products for Buildings - In-Situ Formed Loose Fill Cellulose (LFCI) Products - Part 1: Specification for the Products before Installation. Eur. Stand. 2019.
- Ahmed, S.A.; Yang, Q.; Sehlstedt-Persson, M.; Morén, T. Accelerated Mold Test on Dried Pine Sapwood Boards: Impact of Contact Heat Treatment. J. Wood Chem. Technol. 2013, 33, 174–187. [CrossRef]
- Montgomery, Douglas, C. Design and Analysis of Experiments; Ninth edit.; John Wiley & Sons: Hoboken, NJ, 2017; ISBN 9781119113478.
- Terziev, N.; Boutelje, J. Effect of Felling Time and Kiln-Drying on Color and Susceptibility of Wood to Mold and Fungal Stain during an above-Ground Field Test. Wood Fiber Sci. 1998, 30, 360–367.
- Woloshuk, C. and Martínez, E.M. Molds and Mycotoxins in Stored Products. In Stored Product Protection. Part I: Ecology of Storage Systems; Kansas State University, 2012; pp. 1–6.
- He, C.; Yao, X.; Xue, J.; Xiong, J.; Zhao, L. Influences of Mold Fungi Colonization on Wheat Straw-Polypropylene Composites. For. Prod. J. 2016, 66, 472–479. [CrossRef]
- Malheiro, R.; Ansolin, A.; Guarnier, C.; Fernandes, J.; Amorim, M.T.; Silva, S.M.; Mateus, R. The Potential of the Reed as a Regenerative Building Material—Characterisation of Its Durability, Physical, and Thermal Performances. Energies 2021, 14. [CrossRef]
- Han, G.; Cheng, W.; Deng, J.; Dai, C.; Zhang, S.; Wu, Q. Effect of Pressurized Steam Treatment on Selected Properties of Wheat Straws. Ind. Crops Prod. 2009, 30, 48–53. [CrossRef]
- Li, Y.; Wadsö, L. Fungal Activities of Indoor Moulds on Wood as a Function of Relative Humidity during Desorption and Adsorption Processes. Eng. Life Sci. 2013, 13, 528–535. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).