1. Introduction
Kumquat is a citrus fruit belonging to the genus Fortunella of the Rutaceae family. It is also known as golden orange in Türkiye. Up to date, Kumquat is classified into four main types: Fortunella japonica, Fortunella margarita, Fortunella crassifolia and Fortunella hindsii (Lou and Ho 2017). Kumquat fruits exhibit an orange-yellow hue, an elliptical shape and measuring approximately 2 cm in diameter, and it is regarded as one of the smallest citrus fruits (Chen et al., 2017). Kumquats have numerous advantages, such as cold resistance, an eatable peel, small size, and adaptability to various soil types (Love et al., 2007 ; Palma et al., 2018). These advantageous make them easy to care for and ideal for smaller spaces, offering a versatile citrus option suitable for diverse environments, ranging from greenhouses to terraces, and as a cold-tolerant citrus fruit (Palma et al., 2018). Compared to other citrus fruits, kumquat can be consumed whole with its peel, which provides an advantage in the intake of bioactive substances. While the pulp part of the fruit is sour, the peel part has a characteristic aroma due to flavonoids and terpenoids, which are important in the essential oil composition (Wang et al., 2012 ; Yıldız Turgut et al., 2015). Because of its acidic taste and soft peel, kumquat is used in products such as jams, pickles and sauces, in addition to its natural consumption (Choi et al., 2005; Pawełczyk et al., 2021). Besides, considering its use in the food industry, kumquat contains a variety of phytochemicals, including carotenoids, essential oils, ascorbic acid and flavonoids. These components are small molecules that are not essential for the survival of plants but represent pharmacological activity (Ogawa et al., 2001). Kumquat is becoming increasingly important in traditional medicine because it contains many beneficial phytochemicals with diverse biological effects (Wang et al., 2012; Lou et al., 2016). Phytochemicals are known to have beneficial biological effects. These include antibacterial, anti-oxidative, anti-inflammatory, anti-cancer, as well as cardiovascular protective effects (Li X. et al., 2022; Xinmiao Lv et al., 2015; Al-Saman et al., 2019). For example, carotenoids, an important component in citrus peel, have the ability to detoxify free radicals in cells. An important feature of carotenoids is that they are precursors of vitamin A. The body can convert certain carotenoids into active vitamin A (Grune et al., 2010). Carotenoids are used as nutraceuticals in various diseases such as eye diseases, cardiovascular diseases, neurodegenerative diseases and cancer (Saini et al., 2022). It is crucial to find and analyse bioactive compounds in citrus peels. Many methods such as Gas Chromatography-Mass Spectrometry, High-Performance Liquid Chromatography, Fourier Transform Infrared Spectroscopy are used for biocomponent analysis of citrus peels (Yu et al., 2022; Chen J. et al., 2022; Niluxsshun MCD et al., 2021).
These methods for component analysis have the advantages of high sensitivity and accuracy, but also disadvantages such as complexity and time-consuming for sample preparation (Yang et al., 2017).
In this study Raman spectroscopy, which is a non-destructive method, is employed to determined and analysis the compounds of kumquat peel. Raman spectroscopy is an analytical technique where inelastic scattered photons is used to measure the vibrational energy modes of a molecules. When photons interact with a substance, the frequency of most of the scattered light does not change, which is called Rayleigh scattering. However, inelastic light scattering processes can also occur due to molecular vibrations, so-called Raman scattering (Sarcan et al. ,2014 ; Sarcan et al., 2023 ; Serebrennikova et al., 2021). The spectrum of scattered photons in Raman spectroscopy is fingerprint of the investigated material therefore allows easy identification of the molecule of interest. Raman spectroscopy has many advantages such as ease of sample preparation, non-destructive and the ability to work with aqueous samples. Because of these advantages, Raman spectroscopy has become a powerful alternative tool to other commonly used techniques. Accordingly, Raman spectroscopy has been used as a promising analytical tool in recent years as it provides a chemical fingerprint for molecular identification (Dodo et al., 2022; Serebrennikova et al., 2021). Raman spectroscopy is becoming increasingly popular in research on food, environment, medicine and many other fields. In various application areas such as pesticide detection (Chen et al., 2016), pathogen detection in food (Zhu et al., 2023), water pollution (Almaviva et al., 2022) and neurodegenerative disease diagnosis (Devitt et al., 2018)
In 2017, Yang et al. used Raman spectroscopy for chemical mapping of functional compounds in citrus peels. The relative amount and distribution of essential oils, carotenoids and flavonoids in citrus peels at different locations (flavedo, albedo and longitudinal section) were studied (Yang et al., 2017). To the best our knowledge, there is no study that uses Raman spectroscopy for the determination of functional components on kumquat fruit.
In this study, carotenoids were determined in different locations (flavedo, albedo and cross-section) of kumquat fruit peel without any extraction process and a comparison was made between two kinds of citrus fruits (orange and kumquat). The fact that kumquat fruit can be consumed with its peel unlike other citrus fruits thanks to the terpenoids and flavonoids in the peel composition makes our research valuable. Kumquat is becoming increasingly important in food and pharmacology due to its nutritional and phytochemical content (Li X. et al., 2022). Therefore, a rapid and non-destructive determination of shell composition is very critical. The results obtained in our research are valuable in terms of advances in the use of Raman spectroscopy for the detection of biocomponents in citrus peels.
2. Materials and Methods
Sample Preparation
Kumquats and oranges from Verita (Verita, Istanbul, Türkiye) were soaked in saline for 10 min. The fruits were then washed three times with distilled water to remove the chemicals in the peel. Citrus peels were peeled. Pieces were cut for the Flavedo and Albedo parts. Cross-sectional pieces were also taken to understand the component distribution. Samples were adhered to the slide with double-sided tape and measured.
Raman Spectroscopy
Raman spectroscopy measurements was carried out using a free space custom modular micro-spectroscopy set-up equipped a thermoelectric cooled CCD (Newton BEX2-DD, Andor) with a 1800 grooves/mm of grating in a spectrometer (Shamrock 500i, Andor). To excite the samples, a 532 nm CW laser (Gem532, Novanta Photonics) was used, and the excitation laser beam is focused to a spot of 1.2 m in diameter and on the devices placed on a XYZ sample stage. Raman spectra from the samples were collected via a 50x objective (NA = 0.42).
3. Results and Discussion
Results
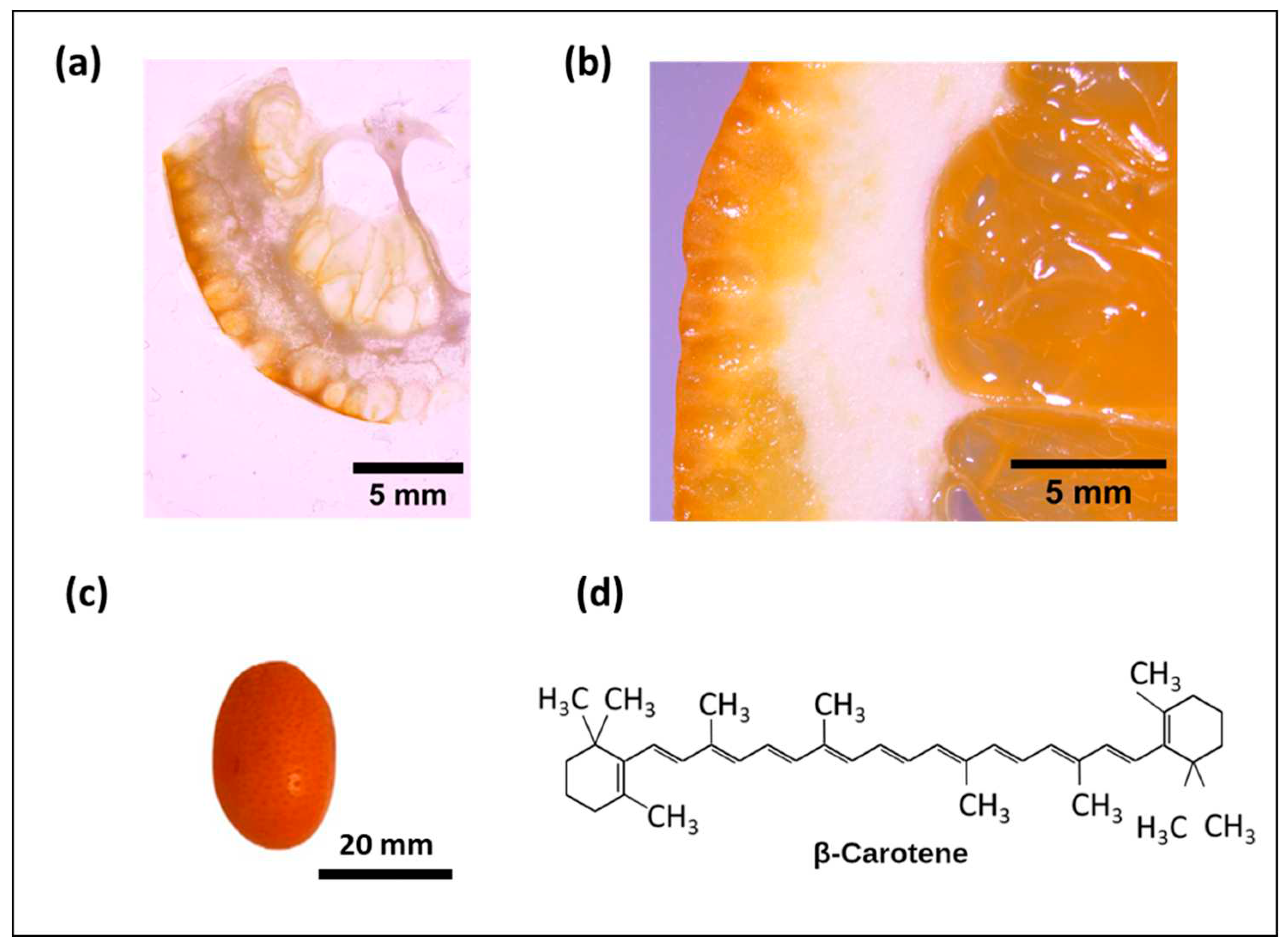
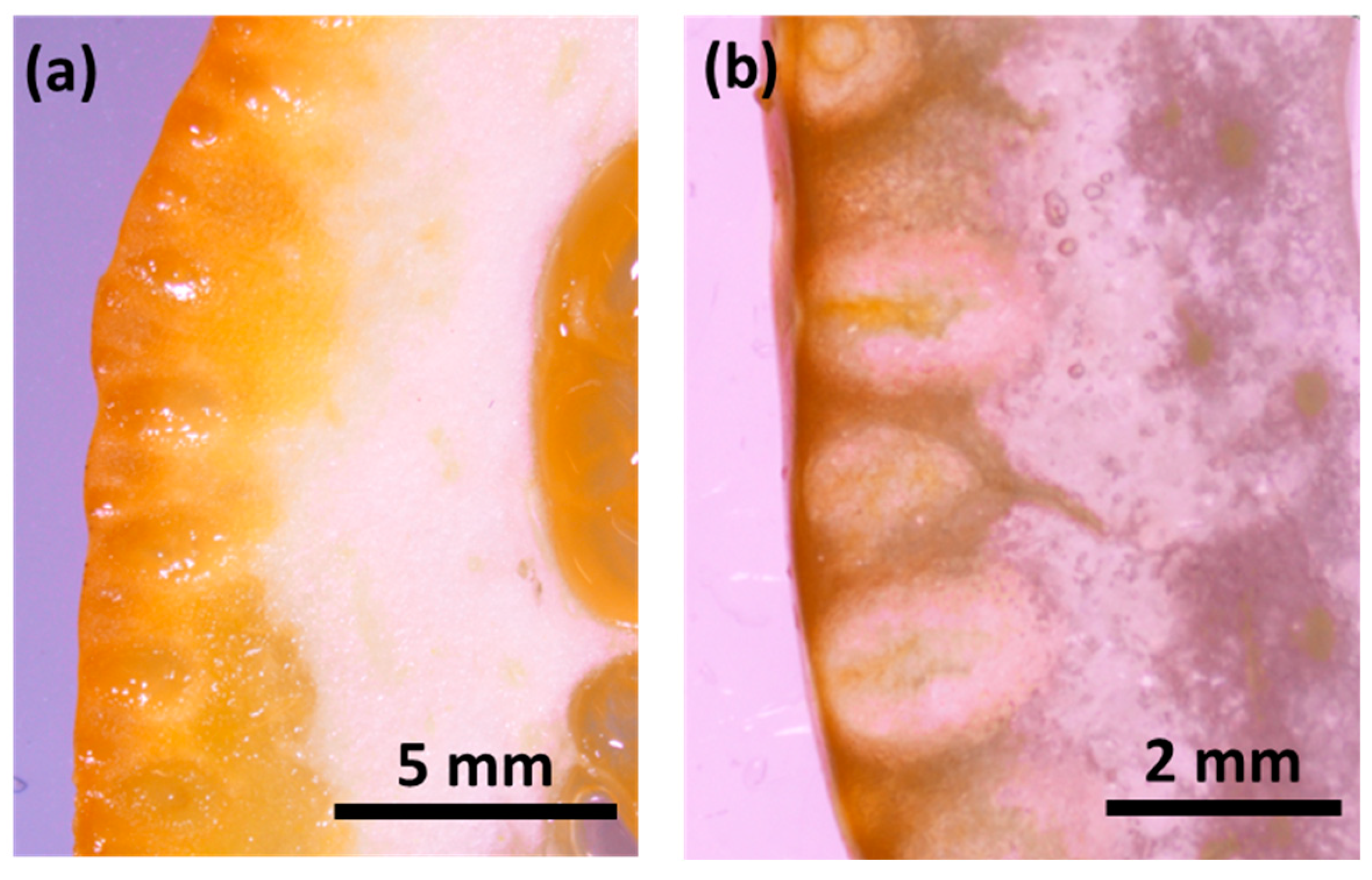
The flavedo and albedo structures of kumquat and orange fruits were observed by optical microscope (Figure 2). It was noted that the flavedo layer of the kumquat (N~1,5 mm) was 2.5 times thinner than that of the orange (N~4 mm).
The thinner flavedo part makes the kumquat fruit eatable with its peel. In addition to that the thinner flavedo layer of kumquat compared to orange is important for obtaining a higher concentration of beneficial components from a smaller part of the fruit.
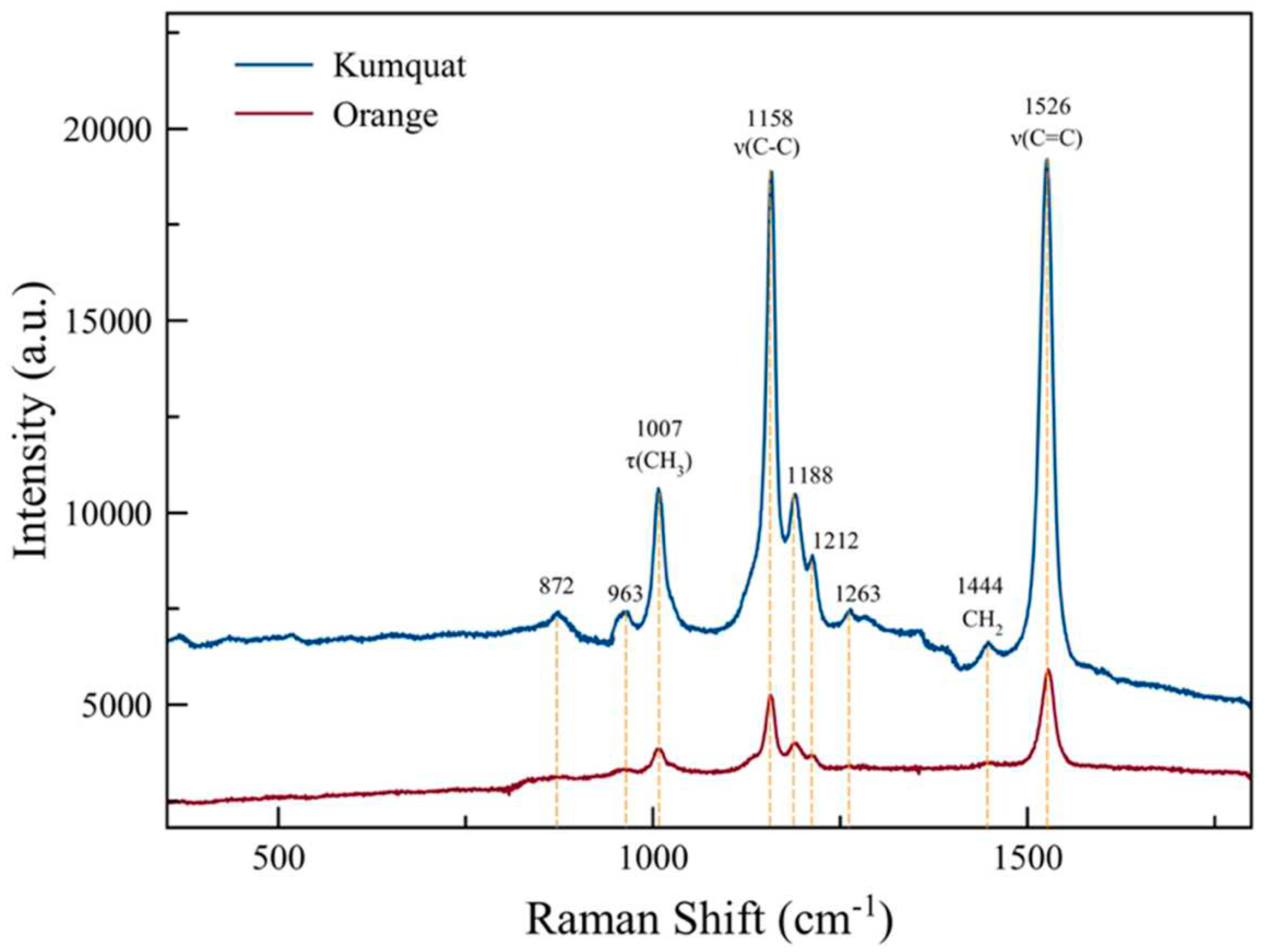
Figure 3 shows the Raman spectra of kumquat and orange peel. The strong Raman peaks were observed at 1007, 1158 and 1526 cm
-1 as well as the relatively weak peaks were observed at 872, 963, 1188, 1212 and 1263 cm
-1 (Figure 3). Peaks at 1007, 1158 and 1526 cm
-1 are assigned to carotenoids, a vitamin A precursor according to previous literature data (Yang
et al., 2017).
β-carotene containing 9 conjugated double bonds (Figure 1d) has been detected by Raman spectroscopy in foods such as tomatoes, carrots and pumpkin in previous studies. The bands attributed to carotenoids in the studies are shown in the
Table 1.
Figure 1.
Optical microscope image of a) kumquat and (b) orange. c) Image of kumquat and d) Chemical structures of β -carotene.
Figure 1.
Optical microscope image of a) kumquat and (b) orange. c) Image of kumquat and d) Chemical structures of β -carotene.
Figure 2.
Cross-sectional optical microscope images of a) orange and b) kumquat.
Figure 2.
Cross-sectional optical microscope images of a) orange and b) kumquat.
Figure 3.
Raman Spectra of Orange and Kumquat.
Figure 3.
Raman Spectra of Orange and Kumquat.
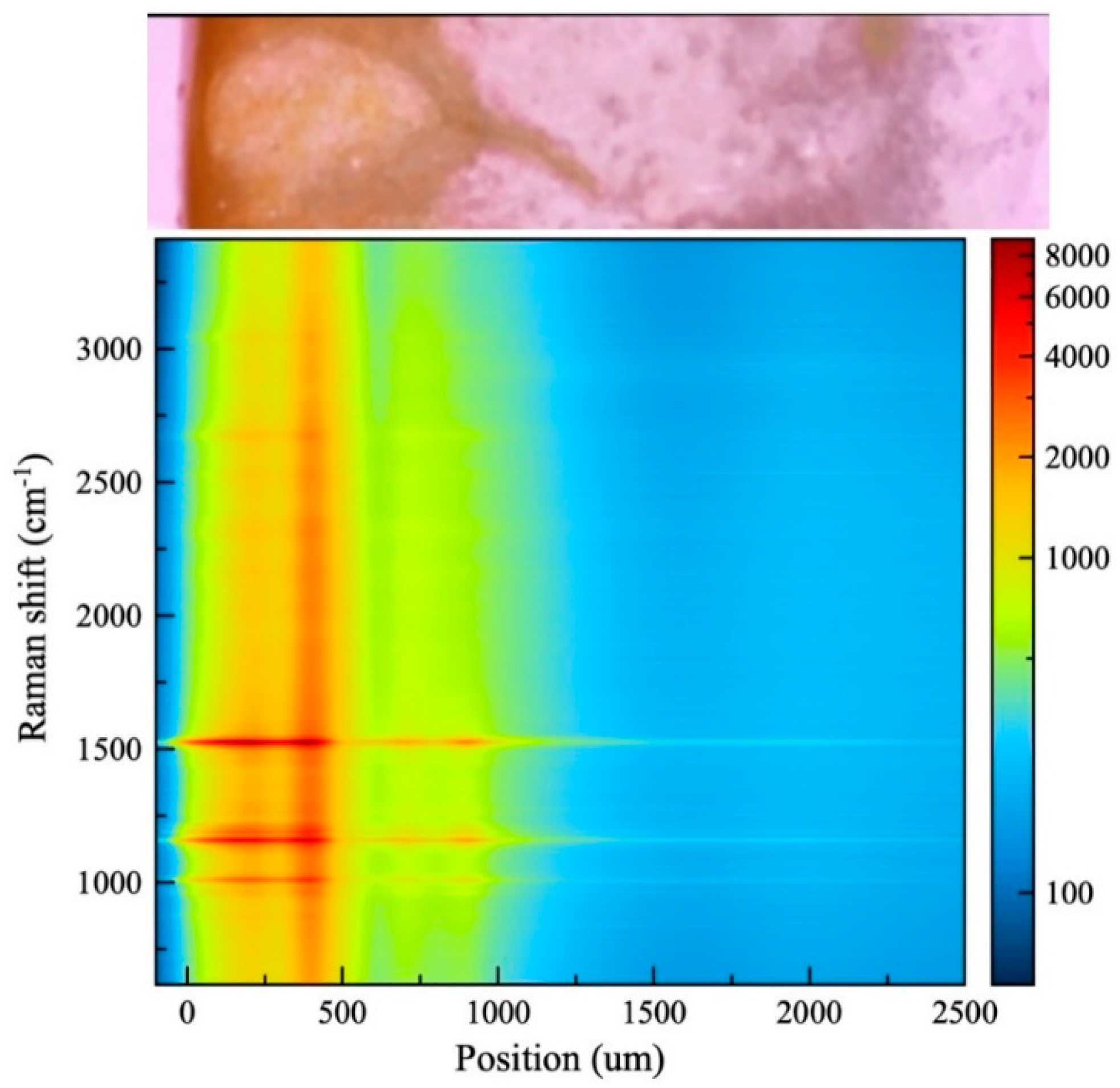
Figure 4.
Raman spectrum mapping of transverse section of kumquat peel.
Figure 4.
Raman spectrum mapping of transverse section of kumquat peel.
Raman spectrum of carrot showed strong bands at 1520, 1156 and 1007 cm-1 assigned to ν(C=C), ν(C-C) and τ(CH3) of β -carotene , respectively; in the Raman spectrum of tomato puree, β -carotene was observed with three intense bands at 1510 (ν1), 1156 (ν2) and 1005 cm-1 (ν3) (Schulz et al. 2005; Baranska et al. 2006).
According to the reported values in the literature, the results obtained confirmed the characteristic Raman bands at 1526 cm-1 and 1158 cm-1 assigned to the in-phase C=C and C-C stretching vibrations of the polyene chain and 1007 cm-1 assigned to the in-plane rocking modes of CH3 groups attached to the polyene chain joined by C-C bonds (De Gelder et al. 2007; Park et al. 2023; Yang et al. 2017). In addition, the peak at 1444 cm-1 is assigned to the CH3-CH2 bending modes indicates essential oil (Schulz et al. 2002; Vargas Jentzsch et al. 2014).
It is observed that the Raman peak intensities of both carotenoid and essential oils are 6 times stronger on the kumquat peel compared to that of orange. This makes kumquat a strong alternative for vitamin A intake beyond its advantageous properties such as cold resistance, compatibility with various soil types and edibility with its peel compared to other citrus fruits.
In the cross-sectional piece taken from kumquat fruit, the carotenoid content was investigated from the albedo layer to the flavedo layer. Looking at the distribution of carotenoids, it was found that the carotenoid density decreased when going from the albedo layer to the flavedo layer (Figure 4).
4. Conclusion
In conclusion, we have used Raman spectroscopy for a rapid and non-destructive detection of carotenoids in kumquat and compared that of orange peels. Raman spectrum analysis of kumquat peel have revealed distinct characteristic bands at 1007, 1158 and 1526 cm-1 associated with carotenoids, particularly dominated by β-carotene. This result indicates the potential of kumquat to be a valuable alternative among citrus fruits for obtaining vitamin A. It has been observed that the concentration of carotenoids is more pronounced in the flavedo part compared to the albedo part. The findings of this study address the differences in the biochemical profiles of citrus fruit peels and highlight the potential health benefits associated with kumquat peel consumption. The information obtained from our study will contribute to the quantitative analysis of the materials in the fruits. Furthermore, the successful application of Raman spectroscopy as a robust analytical tool for non-destructive compositional analysis in the field of food science and nutrition research.
Acknowledgments
This work was supported in part by the Scientific Research Projects Coordination Unit of Istanbul University (FBG-2022-38573, FBG-2021-37896).
Conflicts of Interest
The authors declare no competing interests.
References
- Almaviva, S.; Artuso, F.; Giardina, I.; Lai, A.; Pasquo, A. Fast Detection of Different Water Contaminants by Raman Spectroscopy and Surface-Enhanced Raman Spectroscopy. Sensors 2022, 22, 8338. [Google Scholar] [CrossRef]
- Al-Saman, M.A; .Abdella, A.; Mazrou, K.E.; et al. Antimicrobial and antioxidant activities of different extracts of the peel of kumquat (Citrus japonica Thunb). Food Meas. 2019, 13, 3221–3229. [Google Scholar] [CrossRef]
- Baranska, M.; Schütze, W.; Schulz, H. Determination of lycopene and beta-carotene content in tomato fruits and related products: Comparison of FT-Raman, ATR-IR, and NIR spectroscopy. Anal Chem. 2006, 78, 8456–8461. [Google Scholar] [CrossRef]
- Chen, J.; Shi, Y.; Zhong, Y.; Sun, Z.; Niu, J.; Wang, Y.; Chen, T.; Chen, J.; Luan, M. Transcriptome Analysis and HPLC Profiling of Flavonoid Biosynthesis in Citrus aurantium L. during Its Key Dev. Stages. Biol. 2022, 11, 1078. [Google Scholar] [CrossRef]
- Chen, M.H.; Yang, K.M.; Huang, T.C.; Wu, M.L. Traditional Small-Size Citrus from Taiwan: Essential Oils, Bioactive Compounds and Antioxidant Capacity. Medicines 2017, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Y.; Kannan, P.; Zhang, L.; Lin, Z.; Zhang, J.; Chen, T.; Guo, L. Flexible and Adhesive Surface Enhance Raman Scattering Active Tape for Rapid Detection of Pesticide Residues in Fruits and Vegetables. Anal. Chem. 2016, 88, 2149–2155. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S. Characteristic odor components of kumquat (Fortunella japonica Swingle) peel oil. J. Agric. Food Chem. 2005, 53, 1642–1647. [Google Scholar] [CrossRef]
- Devitt, G.; Howard, K.; Mudher, A.; Mahajan, S. Raman Spectroscopy: An Emerging Tool in Neurodegenerative Disease Research and Diagnosis. ACS Chem. Neurosci. 2018, 9, 404–420. [Google Scholar] [CrossRef]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Moens, L. Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. 2007, 38, 1133–1147. [Google Scholar] [CrossRef]
- Dodo, K.; Fujita, K.; Sodeoka, M. Raman Spectroscopy for Chemical Biology Research. J. Am. Chem. Soc. 2022, 144, 19651–19667. [Google Scholar] [CrossRef]
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.; Biesalski, H.K. Β-carotene is an important vitamin a source for humans. J. Nutr. 2010, 140, 2268S–2285S. [Google Scholar] [CrossRef]
- Li, X.; Meenu, M.; Xu, B. Recent development in bioactive compounds and health benefits of kumquat fruits. Food Rev. Int. 2022, 39, 4312–4332. [Google Scholar] [CrossRef]
- Love, K.; Bowen, R. ; Fleming, K Twelve fruits with potential value-added and culinary uses. University of Hawai‘i College of Tropical Agriculture and Human Resources, Hawai. 2007.
- Lou, S.N.; Ho, C.T. Phenolic compounds and biological activities of small-size citrus: Kumquat and calamondin. J. Food Drug Anal. 2017, 25, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.N.; Lai, Y.C.; Hsu, Y.S.; Ho, C.T. Phenolic content, antioxidant activity and effective compounds of kumquat extracted by different solvents. Food Chem. 2016, 197 Pt A, 1–6. [Google Scholar] [CrossRef]
- Lv, X.; Zhao, S.; Ning, Z.; Zeng, H.; Shu, Y.; Tao, O.; Xiao, C.; Lu, C.; Liu, Y. Citrus fruits as a treasure trove of active natural metabolites that potentially provide benefits for human health. Chem Cent J. 2015, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Niluxsshun, M.C.D.; Masilamani, K.; Mathiventhan, U. Green Synthesis of Silver Nanoparticles from the Extracts of Fruit Peel of Citrus tangerina, Citrus sinensis, and Citrus limon for Antibacterial Activities. Bioinorg Chem Appl. 2021, 2021, 6695734. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Kawasaki, A.; Omura, M.; Yoshida, T.; Ikoma, Y.; Yano, M. 3',5'-Di-C-beta-glucopyranosylphloretin, a flavonoid characteristic of the genus Fortunella. Phytochemistry 2001, 57, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, V.E.d.; Castro, H.V.; Edwards, H.G.M.; Oliveira, L.F.C.d. Carotenes and carotenoids in natural biological samples: A raman spectroscopic analysis. J. Raman Spectrosc. 2009, 41, 642–650. [Google Scholar] [CrossRef]
- Palma, A.; D’Aquino, S. Kumquat—Fortunella japonica. Exotic Fruits. [CrossRef]
- Park, M.; Somborn, A.; Schlehuber, D.; Keuter, V.; Deerberg, G. Raman spectroscopy in crop quality assessment: Focusing on sensing secondary metabolites: A review. Hortic. Res. 2023, 10, uhad074. [Google Scholar] [CrossRef]
- Pawełczyk, A.; Żwawiak, J.; Zaprutko, L. Kumquat fruits as an important source of food ingredients and utility compounds. Food Rev. Int. 2021, 39, 875–895. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.S.; Lee, J.H. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits-A Review of Recent Advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef]
- Sarcan, F.; Donmez, O.; Kara, K.; Erol, A.; Akalin, E.; Arıkan, M.K.; Fontaine, C. Bismuth-induced effects on optical, lattice vibrational, and structural properties of bulk gaasbi alloys. Nanoscale Res. Lett. 2014, 9, 119. [Google Scholar] [CrossRef]
- Sarcan, F.; Fairbairn, N.J.; Zotev, P.G.; Severs-Millard, T.; Gillard, D.J.; Wang, X.; Wang, Y. Understanding the impact of heavy ions and tailoring the optical properties of large-area monolayer ws2 using focused ion beam. NPJ 2d Mater. Appl. 2023, 7, 23. [Google Scholar] [CrossRef]
- Schulz, H.; Barańska, M.; Barański, R. Potential of nir-ft-raman spectroscopy in natural carotenoid analysis. Biopolymers 2005, 77, 212–221. [Google Scholar] [CrossRef]
- Schulz, H.; Schrader, B.D.; Quilitzsch, R.; Steuer, B. Quantitative Analysis of Various Citrus Oils by ATR/FT-IR and NIR-FT Raman Spectroscopy. Appl. Spectrosc. 2002, 56, 117–124. [Google Scholar] [CrossRef]
- Serebrennikova, K.V.; Berlina, A.N.; Sotnikov, D.V.; Zherdev, A.V.; Dzantiev, B.B. Raman Scattering-Based Biosensing: New Prospects and Opportunities. Biosensors 2021, 11, 512. [Google Scholar] [CrossRef]
- Vargas Jentzsch, P.; Ciobotă, V. Raman spectroscopy as an analytical tool for analysis of vegetable and essential oils. Flavour Fragr. J. 2014, 29, 287–295. [Google Scholar] [CrossRef]
- Wang, Y.W.; Zeng, W.C.; Xu, P.Y.; Lan, Y.J.; Zhu, R.X.; Zhong, K.; Huang, Y.N.; Gao, H. Chemical composition and antimicrobial activity of the essential oil of kumquat (Fortunella crassifolia Swingle) peel. Int. J. Mol. Sci. 2012, 13, 3382–3393. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, X.; Zhao, C.; Tian, G.; Zhang, H.; Xiao, H.; He, L.; Zheng, J. Chemical Mapping of Essential Oils, Flavonoids and Carotnoids in Citrus Peels by Raman Microscopy. J. Food Sci. 2017, 82, 2840–2846. [Google Scholar] [CrossRef] [PubMed]
- Turgut, D.Y.; Gölükcü, M.; Tokgöz, H. Kamkat (Fortunella margarita Swing.) meyvesi ve reçelinin bazı fiziksel ve kimyasal özellikleri. Derim 2015, 32, 71–80. [Google Scholar] [CrossRef]
- Yu, X.; Chen, X.; Li, Y.; Li, L. Effect of Drying Methods on Volatile Compounds of Citrus reticulata Ponkan and Chachi Peels as Characterized by GC-MS and GC-IMS. Foods 2022, 11, 2662. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Ali, S.; Jiao, T.; Wang, Z.; Ouyang, Q.; Chen, Q. Advances in surface-enhanced Raman spectroscopy technology for detection of foodborne pathogens. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1466–1494. [Google Scholar] [CrossRef] [PubMed]
Table 1.
Wavenumbers of ν1, ν2 and ν3 modes (cm−1) of the predominant carotenoids obtained from several products by Raman spectroscopy.
Table 1.
Wavenumbers of ν1, ν2 and ν3 modes (cm−1) of the predominant carotenoids obtained from several products by Raman spectroscopy.
| Sample |
ν1 (C=C) |
ν2 (C–C) |
ν3(C–CH3) |
|
| Citrus |
1528 |
1156 |
1010 |
Yang et al. 2017 |
| Tomato |
1510 |
1156 |
1005 |
Baranska et al. 2006 |
| Carrot |
1520 |
1156 |
1007 |
Schulz et al. 2005 |
| Pumpkin |
1527 |
1157 |
1008 |
Oliveira et al. 2009 |
| Kumquat |
1526 |
1158 |
1007 |
This work |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).