Submitted:
19 January 2024
Posted:
19 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods: Search Strategy & Data Sources
3. 3D Printing /Additive Manufacturing
3.1. 3D Printing Technologies
3.2. 3D Printing Materials
| FAMILY | TECHNIQUE | PRINCIPALS/ PROCESS | MATERIAL CONDITION | ACTIVATION SOURCE | TYPICALLY USED MATERIALS | ADVANTAGES | INCONVENIENCES |
|---|---|---|---|---|---|---|---|
| MOLTEN MATERIALS DEPOSITION |
FDM (Fused Deposition Modelling) |
Extrusion through a preheated nozzle, and deposition in thin layers that bind and fully solidify by cooling on the substrate | Filaments | Heat | -Ceramics -Edible materials, -Thermoplastics. |
-Good resistance, -Low cost, -Multi-material capability, -Production of complex 3D structures. |
-Clogging of the nozzle, -High roughness of the printed objects, -Layer by layer appearance, -Poor surface quality. |
|
FFF (Fused Filament Fabrication) | |||||||
| PHOTO-POLYMERIZATION |
SLA (Stéréo-lithography) |
Photosensitive liquid polymer exposed to laser (mainly UV) or free radicals solidifies through photopolymerization | Thermoset liquids | UV, LED | -Photocurable resin, -Photopolymers, - Thermoplastic polymers. |
- High printing resolution, - Precise geometries, -Reproducibility, - Smooth surface finish. |
-High cost, -Limitation of materials, -Relatively slow printing process, -Requires post-processing, -Release of toxic fumes during printing. |
|
DLP (Digital light processing) |
The polymer exposed to light projections (mainly UV) emitted by a digital projector solidifies by photopolymerization. | Soft materials | UV, LED | - Resins, - Waxes. |
-Cost effective, - High precision, - Reduced time compared to SLA, -Simultaneous printing of several compact objects with less detail. |
- Limited range of materials, -Need for adapted systems (ventilation ...), -SLA generally provides higher resolution and better surface finish than DLP technology, - Thickness limit, |
|
| MATERIAL JETTING |
3D InkJet |
The drops of photopolymer deposited on the working platform are exposed to UV light and solidified by light curing. |
Inks | UV |
- Gypsum, -Photo-polymers, - Polymers, - Waxes. |
- High level of precision and complexity, - Possibility of using several materials. |
- Expensive materials and printers, - Fixed resolution, - Long processing time, - Need for a material support. |
| Poly /Multijet | Printing layer by layer, projection of microdroplets and photopolymerization with UV light | Inks | UV | - Polymer resins. | - Advanced inkjet technology, - No post-processing, - Printing of objects combining several materials and colors, - Relatively low cost and printing time, - Smooth finish. |
- Highly sensitive to sun and temperature, - Slow process, - Weak finished producs. |
|
| POWDER BINDING |
SLS (Selective laser sintering) |
Using a highly powered CO2-laser beam to sinter the powder particles, another powder coating is then added and smoothed using a recoater. | Powder | Heat | -Ceramics, -Metals, - Polymers (especially polyamides and derivatives). |
- Ability to build articulated parts with various characteristics, - High level of complexity, - Good resistance, - Wide range of materials, - No need for support. |
- Accuracy limited to the fineness of the powder, - Rough and slightly granular finish, - Limited material range, - Powdery surface, - Requires post-processing, - High cost |
|
3DP (Agglomeration of powder bonding) |
Application of little colored glue droplets in various sizes to powdery layers until the desired effect is achieved. | Powder | Chemical | -All materials supplied in powder form are used. |
-Ambient processing environment, -Easy removal of carrier powder, -Low cost, -Multi-material capability, -Low installation cost. |
-Binder contamination, - Binder jet clogging, -Limited volume constructed, -Poor surface quality, -Poor porosity of the final product. |
|
| DMLS (Direct Metal Laser Sintering) |
A laser to deposit and fuse a metallic powder is used allowing for a layer-by-layer printing. | Powder | Heat | - Metals | - Complex geometries, - Dense components usage, - High construction speeds - Large objects production, - Remarkable objects strength, -Possibility of combining materials. |
-High cost, -Less complex and detailed objects, - Mandatory polishing step, - Use of X-rays. |
| Family | Materials | Properties | Applications/Industries |
|---|---|---|---|
| Plastics | ABS | Solid and resistant | Medical devices, Automotive, Aerospace. |
| Nylon | Good chemical resistance, high fatigue resistance and high impact resistance | The supply of high fatigue strength parts in the aerospace and automotive industries, such as antenna covers, custom production tools, friction inserts and pressure fits, appears to be of good quality and efficient in this material. | |
| PC | High tensile and flexural strength | Perfect for aerospace and automotive molding and blow molding, functional prototypes, tools and assembly. | |
| PET PETG |
Relatively hard and light, good impact resistance and firmer than ABS. | The manufacture of parts that must be both strong and flexible. | |
| PLA | Good tensile strength and surface quality | Suitable for mock-ups and prototypes for the home and office that involve visually pleasing and environmentally friendly elements. | |
| PP | Abrasion resistance and stress absorption. Good balance between stiffness and flexibility. | Mainly used in packaging activities, production of electrical items and equipment, automotive sector and household appliances manufacturing. | |
| PVA | Biodegradable and easily soluble, and allows quick cleaning of 3D printed structures. | Mainly used as a support material for printing PLA and/or ABS products. | |
| Resin | High resolution, smooth and delicate surface components with strong chemical bonding between layers and short build time. | Progressively developed for mass production. Resin 3D printing has a bright future, ranging from jewelry to construction projects to medical uses. | |
| Metals | Cobalt-chrome | Biocompatible, very high hardness, corrosion resistance, high strength and high ductility. | Cobalt chromium objects can be used in the fields of health and dental research, as well as in high-temperature areas such as jet engines. |
| Precious metals | Good ductility, inalterability and low mechanical resistance. | The additive manufacturing of precious metals is intended for the jewelry and dental sectors, as well as for various applications in industrial environments. | |
| Stainless steel | High wear resistance, corrosion resistance, high hardness and ductility. | Stainless steel components are used in the automotive sector, manufacturing industry, marine industry, medical technology and machine building. | |
| Titanium | Corrosion resistance, biocompatibility, low thermal expansion, high strength and low density. | Clinical technology, aviation, automotive, marine, jewelry and design are just some of the uses for titanium products. | |
| Ceramics |
UV curable monomers |
Thermal tolerance, toughness and mechanical performance are all excellent. Ceramic 3D printing allows the production of functional objects with high precision and technical ceramic qualities. |
Ceramic 3D printing has a wide range of applications, including construction, tableware, automotive, aerospace, telecommunications and electronics. |
| Composite materials | Possibility to create composites using computer models and then produce parts with optimized technical properties using 3D printing. | Less heavy, but also stronger and more rigid, and resistant to climate change and chemical exposure. With a longer life expectancy. There is also flexibility of shape: the material is much softer, making it easier to produce certain shapes. | Sensors, Fracture resistant composites and 3D piezoelectric polymers. |
| Smart materials | Shape memory polymers |
Delicate, adaptable and constantly changing. Capable of changing their physical characteristics (shape, color, elasticity...) or even having an effect on their environment when exposed to changes in temperature, pH, mechanical stress, light or electric field. |
Actuator, Sensor, Jewelery, Gripper. |
3.3. 3D Printing Materials and Technologies Commonly Employed Based on the Reviewed Literature
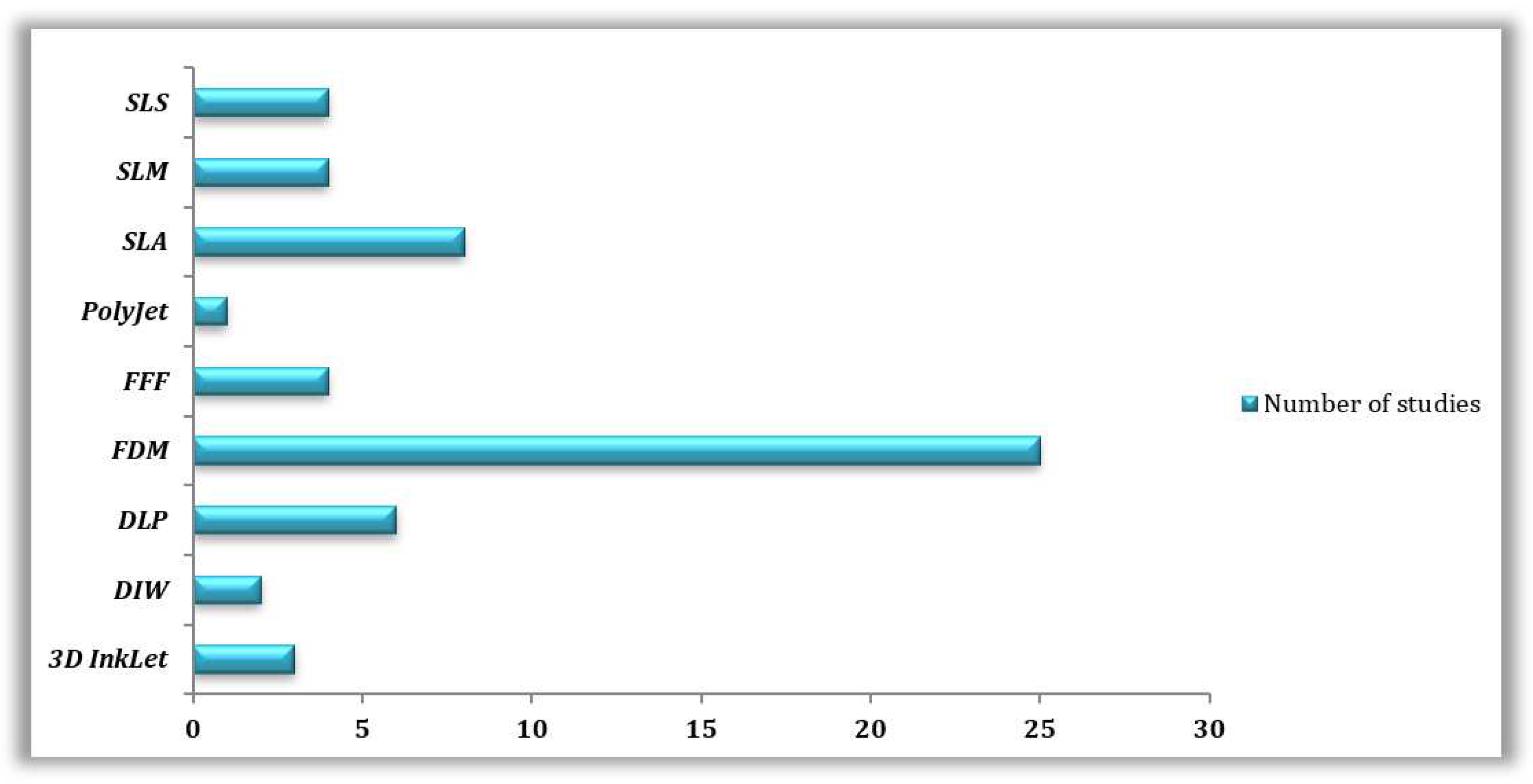
3.3.1. Reviewed 3D Printing Technologies
3.3.2. Reviewed 3D Printed Materials
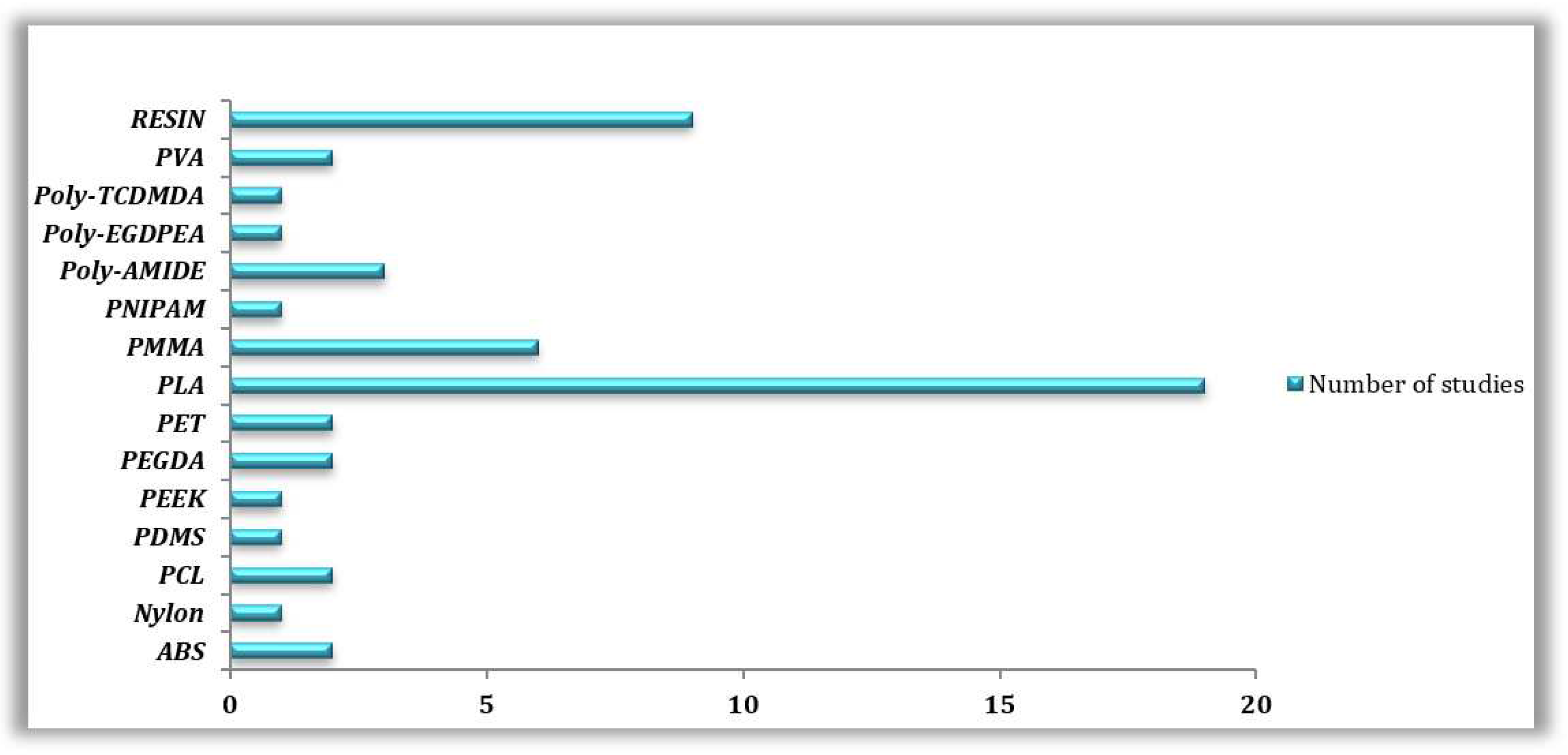
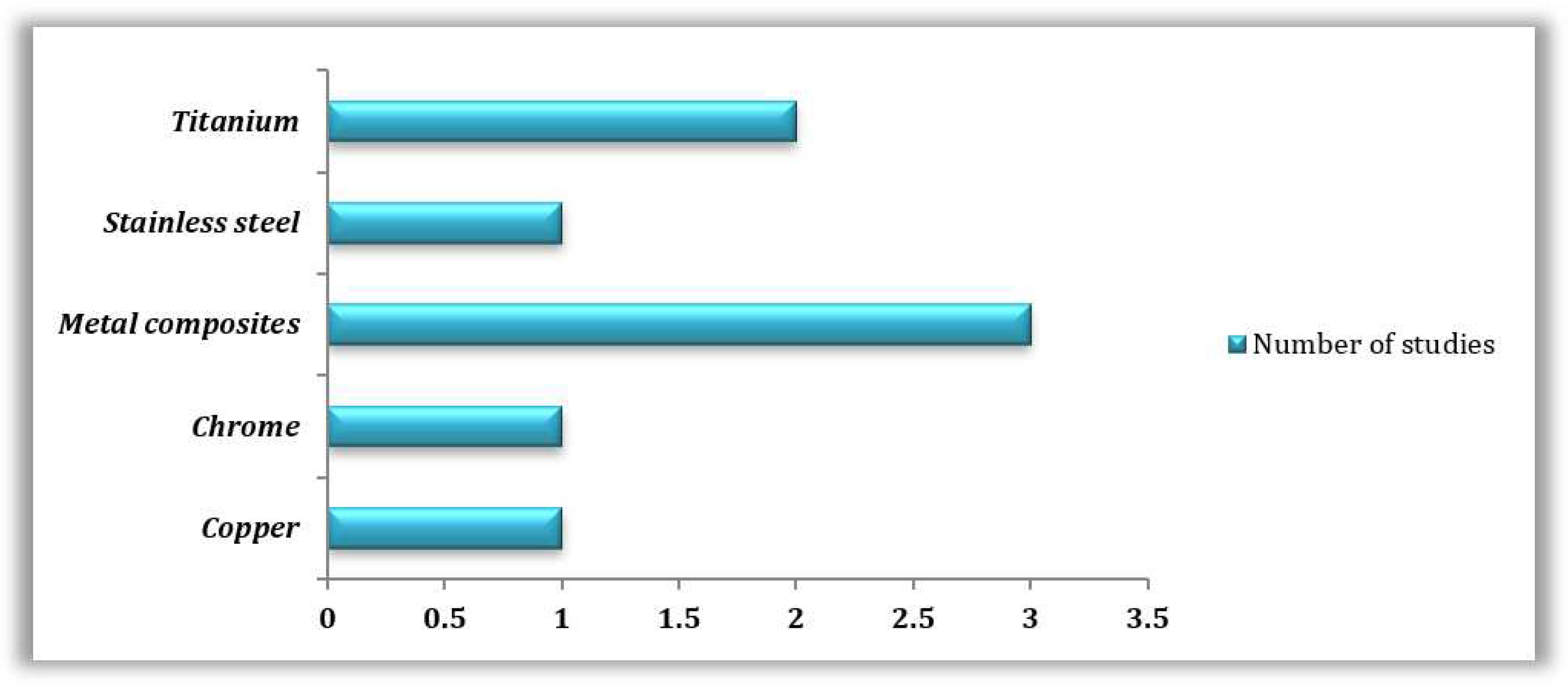
| 3D PRINTING TECHNOLOGY | 3D PRINTING MATERIAL | MICROORGANISM STUDIED | REFERENCES |
|---|---|---|---|
| FDM | PLA | Escherichia coli | [51] |
| N/A | [52] | ||
| Staphylococcusaureus | [53] | ||
| Staphylococcusepidermidis | |||
| Escherichia coli | [54] | ||
| Pseudomonas aeruginosa | |||
| Listeria monocytogenes | |||
| N/A | [55] | ||
| N/A | [56] | ||
| PLA 3D850 | N/A | [56] | |
| Staphylococcus aureus | [57] | ||
| PLA resin | Staphylococcus aureus | [58] | |
| Escherichia coli | |||
| DMHB resin | Escherichia coli | [59] | |
| Bacillus subtilis | |||
| PET | Escherichia coli | ||
| Bacillus subtilis | |||
| PCL | Marine Flora | [60] | |
| PVA | Mycobacterium abscessus | [61] | |
| Mycobacterium bovis | |||
| Mycobacterium smegmatis | |||
| PDMS | Marine Flora | [60] | |
| PEEK | Escherichia coli | [62] | |
| Staphylococcus aureus | |||
| SEBS | N/A | [52] | |
| Metal (Cu) | Escherichia coli | [58] | |
| Staphylococcus aureus | |||
| SLA | PLA Giahntarm |
Escherichia coli Staphylococcus aureus Pseudomonas aeruginosa |
[63] |
| Plactive™ |
Escherichia coli Staphylococcus aureus Pseudomonas aeruginosa |
||
| PCL | Marine Flora |
[60] |
|
| PDMS | Marine Flora | ||
| ABS (VisiJet®) | Marine Flora | ||
| VeroClear™ (Similair to PMMA) | Marine Flora | ||
| Elastic resin | N/A | [64] | |
| Acrylyc resin | Buccal Flora | [65] | |
| Bisacrylyc resin | |||
| Resin | Escherichia coli | [66] | |
| Bacillus cereus | |||
| PNIPAM Hydrogel | Escherichia coli | [67] | |
| PMMA | Candida albicans | [68] | |
| SLM | Titanium alloys | Staphylococcus aureus | [69] |
| Staphyloccocus epidermidis | |||
| Streptococcus mutans | |||
| Titanium Ti6Al4V | Staphylococcus aureus | [70] | |
| [71] | |||
| Staphylococcuspseudintermedius | [70] | ||
| Stainless steel | Staphylococcus aureus | ||
| Staphylococcuspseudintermedius | |||
| Cobalt-Chrome | Staphylococcuspseudintermedius | ||
| SLS | Acrylic resin | Buccal Flora | [72] |
| Bisacrylic resin | |||
| Stainless Steel Alloys |
Escherichia coli Bacillus cereus |
[66] | |
| PLA |
Escherichia coli Bacillus cereus |
||
| Polyamide |
Escherichia coli Bacillus cereus |
||
| Nylon |
Escherichia coli Bacillus cereus |
||
| Polyamide12 | Saccharomyces cerevisiae | [73] | |
| DLP | PEGDA 575 | Marine Bacteria | [74] |
| Resin (PMMA based) | Streptococcus mutans | [75] | |
| Flexible resin |
Pseudomonas aeruginosa Staphylococcus aureus |
[76] | |
| Hard ENG resin |
Pseudomonas aeruginosa Staphylococcus aureus |
||
| PEGDA | N/A | [77] | |
| InkJet | PEGDMA | N/A | |
| InkJet | Poly-TCDMDA |
Pseudomonas aeruginosa Staphylococcus aureus |
[78] |
| Poly-EGDPEA |
Pseudomonas aeruginosa Staphylococcus aureus |
||
| PET | Staphylococcus aureus | [79] | |
| Pseudomonas aeruginosa | |||
| PMMA | Staphylococcus aureus | [80] | |
| PolyJet | PCL | Marine Flora | [60] |
| PDMS | Marine Flora | ||
| ABS (VisiJet®) | Marine Flora | ||
| VeroClear™ (Similair to PMMA) | Marine Flora |
| 3D PRINTING TECHNOLOGY | 3D PRINTED MATERIAL | STUDIED MICROORGANISMS | REFERENCES |
|---|---|---|---|
|
FDM |
PLA (+Antimicrobials) | Escherichia coli | [11] |
| Staphylococcus aureus | |||
| Pseudomonas aeruginosa | |||
| PLA (+Graphene) | Pseudomonas aeruginosa | [81] | |
| PLA COS, (+ COS+ ZnHNTs + Ag) | Staphylococcusaureus | [53] | |
| Staphylococcusepidermidis | |||
| PLA (+AcAc) | Staphylococcus aureus | [54] | |
| Pseudomonas aeruginosa | |||
| PLA (+Ag) | Escherichia coli | [82] | |
| Staphylococcus aureus | |||
| Pseudomonas aeruginosa | |||
| PLA (+Ag NW) | Staphylococcus aureus | [83] | |
| Escherichia coli | |||
| PLA (+Col) PLA (+MH) PLA (+cHA) |
Staphylococcus aureus | [84] | |
| PLA (+NF) PLA (+HA) |
Staphylococcus aureus | [85] | |
| PCL (+ASA) | Staphylococcus aureus | [86] | |
| PMMA (+ATB) | Escherichia coli | [87] | |
| PLGA/HA (+HACC) | Staphylococcus aureus | [88] | |
| Metal (Cu + PLA resin) | Escherichia coli | [58] | |
| Staphylococcus aureus | |||
| Metal (Polished Bronze + PLA resin) | Escherichia coli | [58] | |
| Staphylococcus aureus | |||
| FFF | ABS ( +AgNPs) | Acinetobacter baumannii | [89] |
| Escherichia coli | |||
| Pseudomonas aeruginosa | |||
| Staphylococcus aureus | |||
| Candida albicans | |||
| PLA (+AcAc) PLA (+TEOS) |
Pseudomonas aeruginosa | [90] | |
| Staphylococcus aureus | |||
| Listeria monocytogenes | |||
| PLA (+Graphene) | N/A | [56] | |
| PLA (+Lingnin) | Staphylococcus aureus | [57] | |
| SLA | PMMA (+Nitrides) | Staphyloccocus epidermidis | [91] |
| Escherichia coli | |||
| Elastic resin (+Hydrochloride Lidocaine) | N/A | [64] | |
| PNIPAM (+CNF) | Escherichia coli | [67] | |
| Nanomodified Alumina | Listeria monocytogenes | [92] | |
| Staphylococcus aureus | |||
| Staphylococcus epidermidis | |||
| Escherichia coli | |||
| PLA (+NF) | Staphylococcus aureus | [14] | |
| SLM | Titanium (+HACC) | Staphylococcus aureus | [93] |
| SLS | Polyamide 12 (+ 1%B65003) | Staphylococcus aureus | [94] |
| Pseudomonas aeruginosa | |||
| Polyamide 12 (+UV stabilizer) | Saccharomyces cerevisiae | [73] | |
|
DLP |
GGMMA (+LNP ™ +AgNP) | Escherichia coli | [95] |
| Staphylococcus aureus | |||
| Resin (+ QAC) Resin (+SH-QAC) |
Escherichia coli | [96] | |
| Staphyloccocus epidermidis | |||
| DIW | Ceramic (+3Y-TZP) | Escherichia coli | [97] |
| Streptococcus salivarius | |||
| MG-PVA MG(+LEV)-PVA(+VAN) G(+RIF)MG(+LEV) PVA(+VAN) |
Escherichia coli | [98] | |
| Staphylococcus aureus | |||
|
Inkjet |
PMMA (+MPC) | Staphylococcus aureus | [99] |
| Streptococcus mutans | |||
| Klebsiella oxytoca | |||
| Klebsiella pneumonia | |||
| PMMA (+SB) | Staphylococcus aureus | ||
| Streptococcus mutans | |||
| Klebsiella oxytoca | |||
| Klebsiella pneumonia | |||
|
Plastic (+Gel +ATB) |
Escherichia coli | [100] |
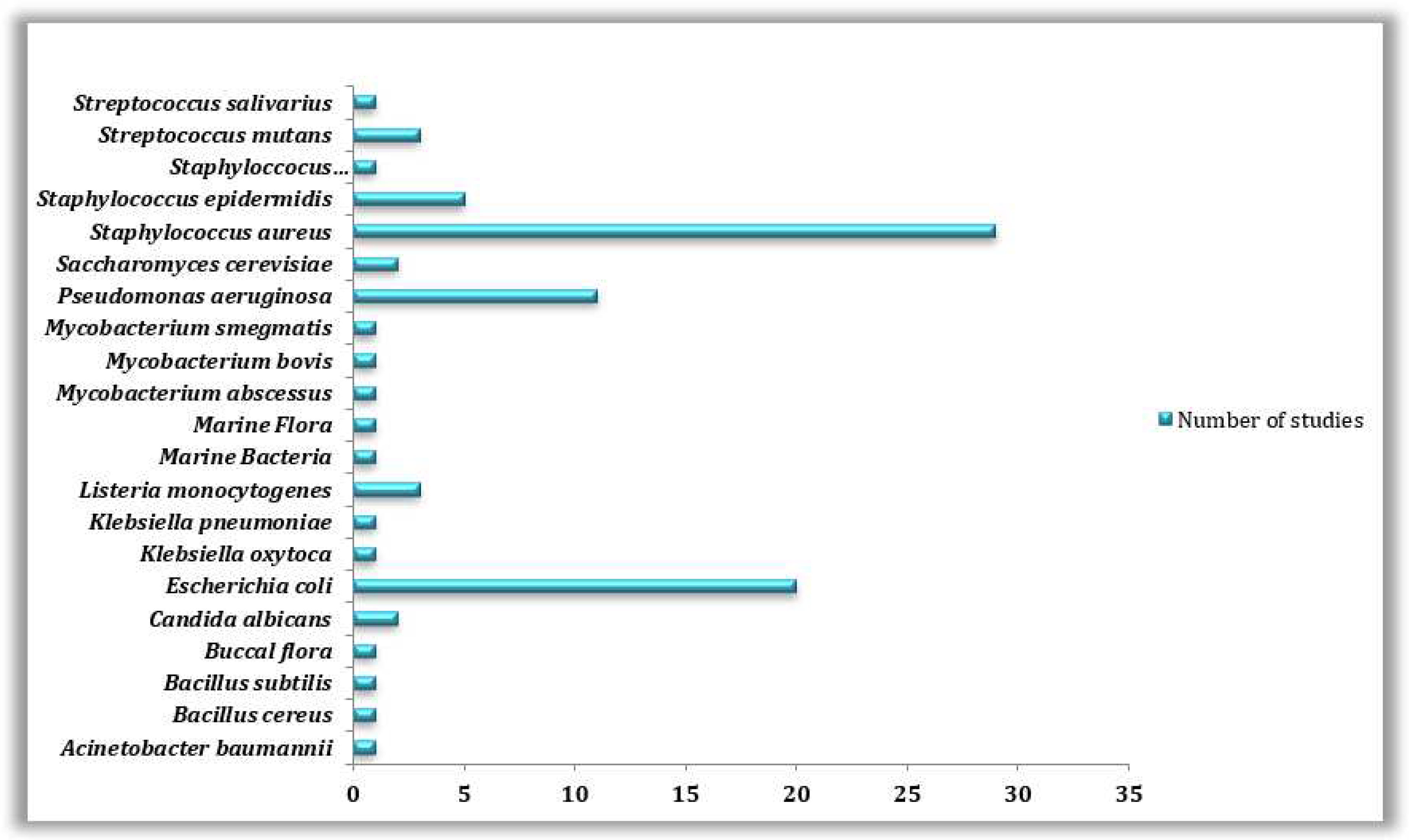
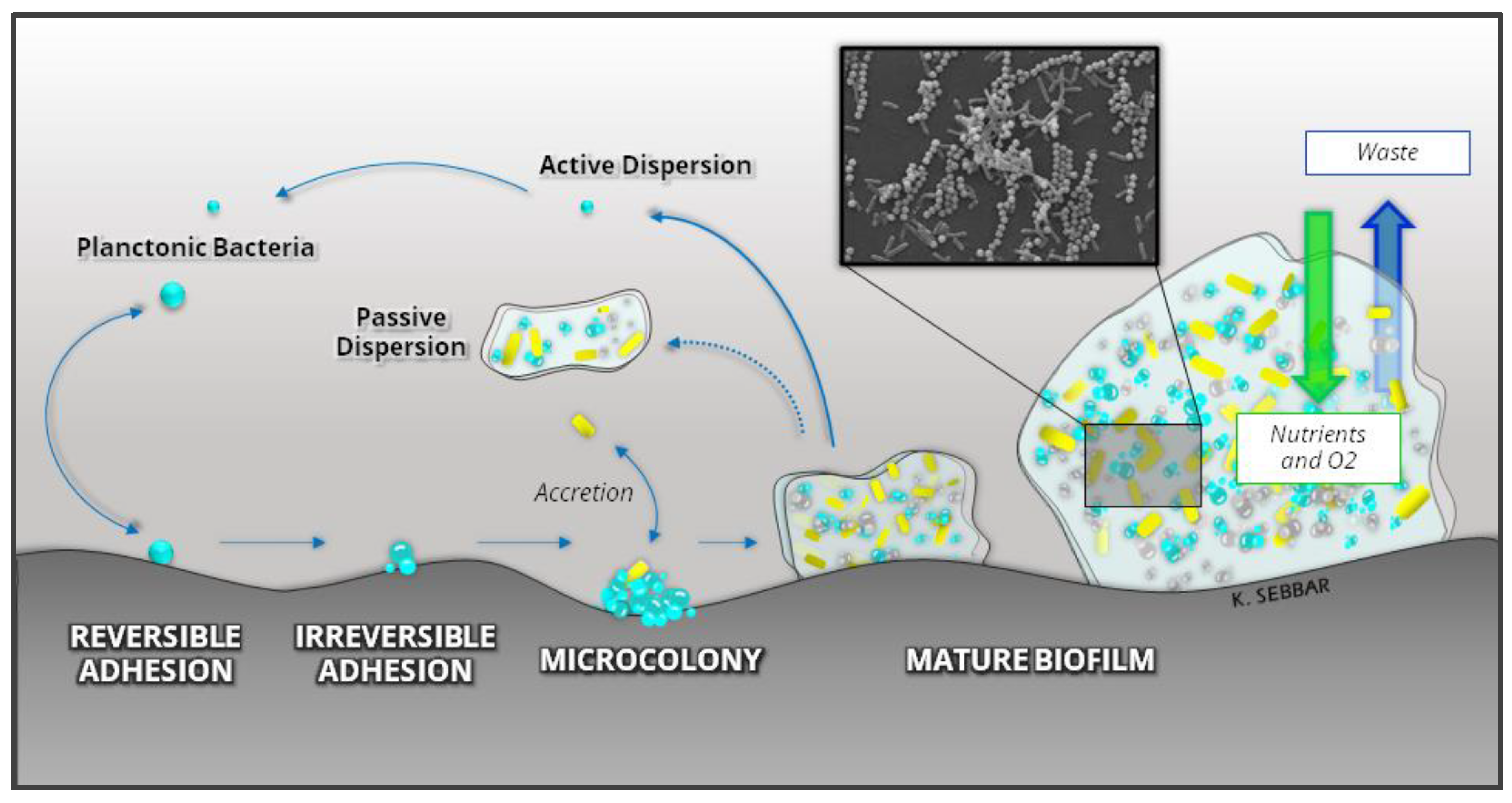
3.4. Microorganisms Involved in Biofilm Formation on 3D Printed Materials
| Studied microorganism | Field of study | Number of studies |
|---|---|---|
| Acinetobacter baumannii | Medical | 1 |
| Bacillus cereus | Others | 1 |
| Bacillus subtilis | Food industry | 1 |
| Buccal flora | Medical | 1 |
| Candida albicans | Others | 2 |
| Escherichia coli | Medical, Food industry, Environment, Biotechnology, Others | 20 |
| Klebsiella oxytoca | Medical | 1 |
| Klebsiella pneumoniae | Medical | 1 |
| Listeria monocytogenes | Food industry | 3 |
| Marine Bacteria | Environment | 1 |
| Marine Flora | Environment | 1 |
| Mycobacterium abscessus | Medical | 1 |
| Mycobacterium bovis | Medical | 1 |
| Mycobacterium smegmatis | Medical | 1 |
| Pseudomonas aeruginosa | Medical, Food industry, Environment, Others | 11 |
| Saccharomyces cerevisiae | Others | 2 |
| Staphylococcus aureus | Medical, Food industry, Environment, Others | 29 |
| Staphylococcus epidermidis | Medical, Food industry | 5 |
| Staphyloccocuspseudintermedius | Medical | 1 |
| Streptococcus mutans | Medical | 3 |
| Streptococcus salivarius | Medical | 1 |
4. Antimicrobial Approaches Against the Microbial Proliferation or the Formation of Biofilms Adopted by Recent Publications
| FIELD | ARTICLE |
TREATED MATERIAL |
TARGETED MICROORGANISMS | ANTIBIOFILM APPRAOCH | REFERENCES |
|---|---|---|---|---|---|
| MEDICAL |
|
PLA (+AcAc) | Pseudomonas aeruginosa& Staphylococcus aureus |
Acrylic acid (AcAc) coatings applied by plasma polymerization were deposited on 3D printed polylactic acid (PLA) Petri dishes. AcAc coatings with less number of plasma passes were more effective, and showed up to a 50% relative biofilm reduction compared to the untreated plates. |
[54] |
|
PLA (+Col) PLA (+Col+MH) PLA (+Col+MH+cHA) |
Staphylococcus aureus |
3D printed poly (lactic acid) (PLA) scaffolds with bioinspired surface coatings had the ability to reduce bacterial biofilm formation. PLA 3D printed scaffolds were further multifunctionalized with collagen (Col), minocycline (MH) and bioinspired citrate- hydroxyapatite nanoparticles (cHA). |
[84] | |
|
Ti (+HACC) | Staphylococcus aureus |
Mesh-like titanium (Ti) cages that anatomically fit into the discs were fabricated by 3D printing. Additionally, an antibacterial coating was applied with quaternized chitosan (Ti-HACC). All of the in vitro tests showed that Ti-HACC cages have antibacterial properties. Implanting Ti-HACC cages in vivo instead of normal Ti cages, the amount of bacteria in the removed cages decreased significantly. |
[93] | |
|
PEEK (+AgNPs) | Escherichia coli& Staphylococcus aureus |
In this study, they developed a novel Ag-decorated 3D printed PEEK via catecholamine chemistry, where silver nanoparticles (AgNPs) were evenly anchored on the surface. The Ag-decorated 3D PEEK scaffolds displayed significant antibacterial and antibiofilm effects towards Gram-negative and Gram-positive bacteria. |
[62] | |
|
PMMA (+GEN) PMMA (+TOB) PMMA (+NF) |
Escherichia coli &Staphylococcus aureus |
Gentamicin sulfate, tobramycin, and nitrofurantoin were doped into PMMA and antibiotic-doped 3D printed beads, disks, and fil- aments were successfully printed. Growth inhibition assays demonstrated the efficacy of antibiotic-loaded PMMA 3D printed constructs in inhibiting bacterial growth. |
[109] | |
|
PMMA (+Si) PMMA (+Zr) PMMA (+Hf) PMMA (+Al) |
Escherichia coli &Staphylococcusepidermidis. |
This study suggests that PMMA/nitride coatings can improve the antibacterial properties of PMMA implants. The application of nitride-PMMA composite coatings on 3D printed parts increased their resistance to bacteria colonization. Four different nitrides were tested: silicon, zirconium, hafnium and aluminum. |
[91] | |
|
PLGA (+HA+HACC) | Staphylococcus aureus |
In this study a HACC-grafted 3D-printed PLGA/HA porous scaffold endowed with a dual antibacterial and osteogenic functionality was manufactured. PLGA/HA/HACC composite scaffold exhibited enhanced anti-infection and bone repairing capability in two different infected bone defect models. |
[88] | |
|
Ceramic (+3Y-TZP) |
Escherichia coli & Streptococcus salivarius |
The novel porous zirconia scaffolds prepared using (PICN) and 3D-printing technologies by the deposition of (3Y-TZP) and Pluronic® hydrogel ceramic paste. The scaffolds exhibit antimicrobial properties similar to that of 3Y-TZP, as has been demonstrated by the adhesion and proliferation tests with E. coli and S. salivarius bacteria. |
[97] | |
|
Hydrogel (+HZJ phage) | Escherichia coli |
Phage-embedded hydrogel fibers were used to create porous wound dressing material using three-dimensional (3D) printing. This antibacterial dressing was capable of slowly releasing lytic phages and effectively suppressing bacterial growth for up to 24 h was produced in this study. This model represents an attractive means to reduce use of antibiotics and other additives in conventional dressings. |
[110] | |
|
Poly-TCDMDA Poly-EGDPEA |
Pseudomonas aeruginosa& Staphylococcus aureus | Bespoke devices were manufactured through ink-jetting using bacterial biofilm inhibiting formulations without the need for eluting antibiotics or coatings. The 3D printed poly-TCDMDA and poly-EGDPEA were selected on the basis of their in vitro bacterial biofilm inhibitory properties. P.aeruginosa biofilm formation on poly-TCDMDA was reduced by ~99% when compared with medical grade silicone. | [78] | |
|
MG-PVA MG(+LEV)-PVA(+VAN) G(+RIF)MG(+LEV) PVA(+VAN) |
Escherichia coli & Staphylococcus aureus |
Hierarchical 3D multidrug scaffolds based on nanocomposite bioceramic and polyvinyl alcohol (PVA) prepared by rapid prototyping with an external coating of gelatin-glutaraldehyde (Gel-Glu) have been fabricated. These 3D scaffolds contain three antimicrobial agents (rifampin, levofloxacin and vancomycin).This combined therapy is able to destroy Gram-positive and Gram-negative bacteria biofilms as well as inhibit the bacteria growth. |
[98] | |
|
PLA (+NF) | Staphylococcus aureus | In this study the incorporation of the antibiotic nitrofurantoin (NF) in the polymer carrier material and 3D printing of a model structure resulted in an inhibition of biofilm colonization. | [14] | |
|
CPS (+RIF+VAN) | Staphylococcus aureus |
Rifampin- and vancomycin-laden calcium phosphate scaffolds (CPS) were fabricated by (3D) printing to treat an implant-associated S. aureus bone infection. All vancomycin- and rifampin-laden CPS treatments significantly reduced the bacterial burden compared with vancomycin-laden PMMA. |
[80] | |
|
Resin (+QAC) Resin (+SH-QAC) |
Escherichia coli & Staphylococcus aureus |
In this contribution, a thiol–ene–acrylate ternary system was chosen as the antibacterial 3D printing matrix resin. Two quaternary ammonium salt-type antibacterial agents (QAC and SH-QAC) were designed and prepared to achieve contact antibacterial effect. Both antibacterial photo- sensitive resins have been successfully applied in DLP technology to fabricate tooth model with high precision. |
[96] | |
|
PMMA (+ MPC) PMMA (+ SB) |
Klebsiella oxytoca, Klebsiella pneumonia, Staphylococcus aureus & Streptococcus mutans |
This study indicates that the addition of MPC or SB into PMMA results in durable oral salivary Biofilm inhibition, with the maintenance of physical and mechanical properties. | [99] | |
|
Flexibale resin (+Cipro+FA) Hard resin (+Cipro+FA) |
Pseudomonas aeruginosa& Staphylococcus aureus |
Two polymer resins for 3DP, “ENG hard” and “Flexible” loaded with two antibiotics, ciprofloxacin and fluocinolone acetonide. All multi-drug-loaded devices exhibited a hydrophilic surface, excellent blood compatibility and anti-biofilm activity against P. aeruginosa and S. aureus. |
[76] | |
|
PCL (+10% ASA +1% RIF) | Staphylococcus aureus | PCL and ASA were used to prepare biodegradable antithrombotic vascular grafts using an extrusion-based 3D printing technique. Moreover, RIF was combined with ASA and PCL to obtain antimicrobial vascular grafts. These materials were capable of inhibiting the growth of S. aureus. | [86] | |
|
Titanium alloy Ti–6Al–4 V | Staphylococcus aureus |
In order to modify the surface topography of metallic implants for directed Staphylococcus aureus biofilm restriction, lowering the angle during SLM printing gave metallic surfaces lower roughness, lower hydrophobicity, higher surface energy, and fewer partially melted metal particles without altering the bulk surface chemistry, which directly correlated with significantly lower biofilm coverage and an associated reduction in microbial biomass. |
[71] | |
|
PLA (+LIG+TC) | Staphylococcus aureus |
3D printed meshes were prepared using PLA/LIG composite materials. These meshes can provide mechanical protection to the wound while providing antioxidant activity. Soluble patches containing drugs can be applied to the surface of the mesh. The drug can diffuse through the mesh pores to the wound. In the present work, they used TC an antibiotic compound which showed a significant reduction in bacterial adherence. |
[57] | |
|
PLA (+AgNPs) | Escherichia coli, Pseudomonas aeruginosa & Staphylococcus aureus |
A surgical retractor was created using a commercial polylactic acid (PLA) thermoplastic filament and a simple and scalable sonochemical deposition method to create a thin layer of silver (Ag) nanoparticles (NPs). S. aureus, P. aeruginosa, and E. coli, bacteria viability were all reduced when the PLA retractor was coated with Ag NPs (PLA@Ag). |
[82] | |
|
Titanium alloy Ti6Al4V Stainless steel 316L Cobalt chromium alloy CoCr |
Staphylococcus aureus & Streptococcus pyogenes |
This study suggests that metallic implants produced by laser powder bed fusion should be polished since the polishing of 3D printed titanium alloy, stainless steel, or cobalt chromium alloy disks has significantly reduced biofilm growth on these surfaces. |
[70] | |
|
LAB-made or commercially available PLA (+Antimicrobials) | Escherichia coli, Pseudomonas aeruginosa, & Staphylococcus aureus |
Biofilm formation depends on some of the polymer’s antibacterial activities. They compared their tested materials with commercially available antimicrobial PLA polymers. The greatest antimicrobial and antibiofilm activity were observed in the case of BRS PLA polymer. According to the manufacturer, this polymer contains about 40% metal. |
[11] | |
|
PLA (+NF ) PLA (+HA) |
Staphylococcus aureus |
Nitrofurantoin (NF) and hydroxyapatite (HA) were successfully mixed and extruded with up to 30% drug load with and without addition of 5% HA in polylactide strands, which were subsequently 3D-printed into model disc geometries. Disks with 30% drug loading were able to prevent surface-associated and planktonic growth of S. aureus over a period of 7 days. |
[85] | |
|
Resin GGMMA (+LNP +AgNPs) | Escherichia coli & Staphylococcus aureus |
A bio-based antimicrobial resin was developed for DLP printing engaging GGMMA as a photo-crosslinkable polymeric matrix and the nanocomposite lignin nanoparticles that are surface-embedded with silver nanoparticles (LNP@Ags) as a high-performance antimicrobial reagent. The GGMMA/LNP@Ag hydrogel also possesses high antimicrobial activity due to the bactericidal ability of Ag+ that was leached out of the hydrogel in a sustained manner. |
[95] | |
|
PMMA based resin (+ 0,1% ND) |
Streptococcus mutans |
The present study aimed to evaluate the role of nanodiamonds (NDs). Using a solution-based mixing technique, 0.1 wt% ND was incorporated into the PMMA, and specimens were 3D-printed for tribological and bacterial analysis. The addition of 0.1 wt% ND in the PMMA-based resin for 3D printing resulted in significant resistance to S. mutans. |
[75] | |
|
PLA (+ ZnHNTs-Ag-COS) | Staphylococcus aureus |
3D printed polylactic acid (PLA) constructs were alkali-treated to increase hydrophilicity and functionalized using a suspension of Zinc/HNTs-Ag-Chitosan Oligosaccharide Lactate (ZnHNTs-Ag-COS). Antibacterial evaluation confirmed the anti-biofouling potential of the PLA constructs (which was a function of the Ag content in the material). |
[53] | |
|
Ink A & Ink B |
Pseudomonas aeruginosa & Staphylococcus aureus |
This work has demonstrated the manufacture of MM-IJ3DP printed devices that are personalisable through generative design guided co-deposition of inks to create functional composites that are both resistant to bacterial biofilm formation and achieve a specific deformation profile. | [79] | |
|
ABS (+AgNPs) | Acinetobacter baumannii, Candida albicans, Escherichia coli, Pseudomonas aeruginosa & Staphylococcus aureus | This work describes a unique approach for attaching a layer of AgNPs to 3D-printed polymer acrylonitrile butadiene styrene (ABS) plastic using acetone. For all examined bacterial species, AgNP-coated ABS (AgNP-ABS) indicated considerable eradication of live bacteria after 4 hours, and for the tested fungal stain, it was within 19 hours. | [89] | |
| AGRI-FOOD-INDUSTRY |
|
PET (+CR or TML) DMHB resin(+CR or TML) |
Bacillus subtilis & Escherichia coli |
This study aimed to investigate the effect of thymol and carvacrol on the physicochemical characteristics of DMHB resin and PET using the contact angle method. Finally it was recommended to incorporate the studied major compounds into the composition of PET and resin materials in order to use them in the food industry. |
[59] |
|
PLA (+AcAc) PLA (+TEOS) |
Escherichia coli, Listeria monocytogenes. & Pseudomonas aeruginosa, |
Plasma-polymerized acrylic acid (AcAc) and tetraethyl orthosilicate (TEOS) coatings were used to minimize biofilm development on 3D printed PLA materials. The reduction in bacterial adhesion and biofilm development might be explained by chemical (hydration layer formation) and morphological (distance between peaks) changes induced by plasma-polymerized treatments. |
[90] | |
| ENVIRENO-MENTAL |
|
Resin (+ AgNPs) | Environmental microorganisms |
An acrylate resin containing silver nitrate (AgNO3) as a silver precursor is employed to generate silver nanoparticles (AgNPs). The fabricated silver-patterned devices exhibit different surface features that might be exploited in systems working in a marine environment to control Biofilm proliferation. |
[74] |
|
Plactive™ | Escherichia coli, Pseudomonas aeruginos &, Staphylococcus aureus |
In this study they analyzed a 3D printing material as a substitute for single-use face masks: Plactive™. Compared to unblended PLA (Giantarm™), Plactive™ PLA material has showed antimicrobial activities against Gram positive S. aureus but not for Gram negative P. aeruginosa and E. coli. |
[63] | |
| DIVERSE |
|
“Flexibile’’ , ‘‘ClearV2’’ & ‘‘TangoPlus’’ | Escherichia coli | Mass spectrometry was used to identify leached chemicals that inhibited bacterial growth. The FormLabs, ‘‘Flexibile’’ and ‘‘ClearV2,’’ and the Stratasys ‘‘TangoPlus’’ materials inhibited growth to varying degrees. | [111] |
|
PLA (+AgNW) | Escherichia coli & Staphylococcus aureus |
Antibacterial 3D printed nanocomposites : Silver nanowire (Ag NW) loaded polylactide (PLA) nanocomposites were investigated. The Ag NW loaded PLA nanocomposites show bactericidal activity against E. coli and S. aureus, which are the most common bacteria types living in public areas. |
[83] |
|
|
Nanoporous Alumina | Escherichia coli, Listeria monocytogenes, Staphylococcus aureus & Staphylococcus epidermidis |
Anodic nanoporous surfaces, the approach exploits anodisation to create alumina surfaces with cylindrical nanopores with diameters ranging from 15 to 100 nm. This method have effectively minimised bacterial attachment or biofilm formation by all the microorganisms tested. |
[92] |
|
|
Polyamide 12 (+ 1% B65003 silver phosphate glass) | Pseudomonas aeruginosa & Staphylococcus aureus |
A commercially available antimicrobial additive ( Biocote® B65003) was combined with a widely used Laser Sintering powder (polyamide 12, EOS PA2200) to create an antimicrobial material suitable for a range of potential uses. The composite material was able to reduce numbers of planktonic bacteria in its surroundings and numbers of biofilm bacteria attached to the surface. |
[94] | |
|
PMMA (with a modified printing orientation) | Candida albicans |
The goal was to see how printing orientation can affect 3D-printed denture base resin microbiological response. Denture base polymethyl methacrylate (PMMA) was used to print samples in three different printing orientations (0, 45, and 90 degrees). C. albicans response was assessed, With statistical significance, specimens printed at 90°<45°<0° orientation degrees included a greater percentage of C. albicans. |
[68] |
5. Conclusion
Declaration Of Competing Interest
Acknowledgements
List of Abbreviations
| 3DP | Three Dimensional Printing |
| 3Y-TZP | Yttrium-stabilized tetragonal zirconia polycrystal |
| AcAc | Acrylic acid |
| AgNP | Silver nanoparticle |
| AgNW | Silver nanowire |
| Al | Aluminum |
| AM | Additive Manufacturing |
| ASA | Acetylsalicylic acid |
| ATB | Antibiotic |
| Cipro | Ciprofloxacin |
| Col | Collagen |
| CPS | Calcium phosphate scaffolds |
| CUR | Curcumin |
| DLP | Digital Light Processing |
| EBM | Electron Beam Melting |
| EPS | Extracellular Polymeric Substances |
| FA | Fluocinolone Acetonide |
| FDM | Fused Deposition Modelling |
| Gel-Glu | Gelatin-glutaraldehyde |
| Gen | Gentamicin |
| GGMMA | Methacrylated O-acetyl-galactoglucomannan |
| HA | Hydroxyapatite |
| HACC | Quaternized chitosan |
| Hf | Hafnium |
| HZJ | Bacteriophage |
| LIG | Lignin |
| LNP | Lignin nanoparticle |
| MPC | 2-methacryloyloxyethyl phosphorylcholine |
| NDs (A-ND) | Amine-functionalized |
| NDs (ND) | Non-functionalized |
| NF ou NIT | Nitrofurantoin |
| PBF | Powder Bed Fusion |
| PEEK | Polyetheretherketone |
| PICN | Polymer-infiltrated ceramic network |
| PLGA | Polylactide-co-glycolide |
| Poly-EGDPEA | Polyethylene glycol dicyclopentenyl ether acrylate |
| Poly-TCDMDA | Pol-mers contained monomer D, tricylodecane-dimethanol diacrylate |
| RIF | Rifampin/ Rifampicin |
| SB | Sulfobetaine methacrylate |
| Si | Silicon |
| SLA | Stereolithography |
| SLM | Selective Laser Melting |
| SLS | Selective Laser Sintering |
| TEOS | Tetraethyl orthosilicate |
| Ti | Titanium |
| TOB | Tobramycin |
| VAN | Vancomycin |
| WOS | Web of science |
| Zr | Zirconium |
References
- Gibson and, D. Rosen, Additive Manufacturing Technologies : 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing. Springer New York: New York, NY, USA, 2015.
- Q. Yan et al., “A Review of 3D Printing Technology for Medical Applications,” Engineering, vol. 4, no. 8, pp. 729–742, 2018. [CrossRef]
- G. Shi, Y. Wang, S. Derakhshanfar, K. Xu, and W. Zhong, “Materials Science & Engineering C Biomimicry of oil infused layer on 3D printed poly ( dimethylsiloxane ): Non- fouling, antibacterial and promoting infected wound healing,” Mater. Sci. Eng. C, vol. 100, no. 3, pp. 915–927, 2019. [CrossRef]
- T. A. Campbell and O. S. Ivanova, “Additive Manufacturing as a Disruptive Technology : Implications of Three-Dimensional Printing,” Technol. Innov., vol. 15, no. 11, pp. 67–79, 2013. [CrossRef]
- D. G. Bekas, Y. Hou, Y. Liu, and A. Panesar, “3D printing to enable multifunctionality in polymer-based composites : A review,” Compos. Part B, vol. 179, no. 10, pp. 1–13, 2019. [CrossRef]
- J. M. Zuniga, “3D Printed Antibacterial Prostheses,” 2018. [CrossRef]
- J. W. Jung, J. Lee, and D. Cho, “3D printing system for printing of heterogeneous organ / tissue constructs,” Nat. Publ. Gr., no. 1, pp. 1–9, 2016. [CrossRef]
- Parietti, A. Maroni, A. Foppoli, A. Gazzaniga, and L. Zema, “Hot-melt extruded fi laments based on pharmaceutical grade polymers for 3D printing by fused deposition modeling,” Int. J. Pharm., vol. 509, no. 5, pp. 255–263, 2016. [CrossRef]
- S. A. M. Tofail, E. P. Koumoulos, A. Bandyopadhyay, S. Bose, L. O’Donoghue, and C. Charitidis, “Additive manufacturing: scientific and technological challenges, market uptake and opportunities,” Mater. Today, vol. 00, no. 7, pp. 1–16, 2017. [CrossRef]
- C. K. Chua, C. H. Wong, and W. Y. Yeong, Standards, Quality Control, and Measurement Sciences in 3D Printing and Additive Manufacturing, Brian Guer. Matthew Deans, 2017.
- D. C. Hall, P. Palmer, H. F. Ji, G. D. Ehrlich, and J. E. Król, “Bacterial Biofilm Growth on 3D-Printed Materials,” Front. Microbiol., vol. 12, no. 5, pp. 1–31, 2021. [CrossRef]
- S. Hawas, A. D. Verderosa, and M. Totsika, “Combination Therapies for Bio fi lm Inhibition and Eradication : A Comparative Review of Laboratory and Preclinical Studies,” Front. Cell. Infect. Microbiol., vol. 12, no. 2, pp. 1–19, 2022. [CrossRef]
- M. Jamal et al., “Bacterial biofilm and associated infections,” J. Chinese Med. Assoc., vol. 81, no. 7, pp. 7–11, 2018. [CrossRef]
- N. Sandler et al., “Towards fabrication of 3D printed medical devices to prevent biofilm formation,” Int. J. Pharm., vol. 459, no. 1–2, pp. 62–64, 2014. [CrossRef]
- M. Majumdar, T. K. Misra, and D. N. Roy, “In vitro anti-biofilm activity of 14-deoxy-11,12-didehydroandrographolide from Andrographis paniculata against Pseudomonas aeruginosa,” Brazilian J. Microbiol., vol. 51, no. 1, pp. 15–27, 2020. [CrossRef]
- B. Gottenbos, D. W. Grijpma, H. C. Van Der Mei, J. Feijen, and H. J. Busscher, Antimicrobial effects of positively charged surfaces on adhering Gram-positive and Gram-negative bacteria, vol. 48, no. 9. 2001.
- S. Kumar, D. N. Roy, and V. Dey, “A comprehensive review on techniques to create the anti-microbial surface of biomaterials to intervene in biofouling,” Colloids Interface Sci. Commun., vol. 43, no. 7, pp. 1–17, 2021. [CrossRef]
- S. Duan et al., “Progress in Materials Science Multifunctional antimicrobial materials : From rational design to biomedical applications,” Prog. Mater. Sci., vol. 125, no. 11, pp. 1–46, 2022. [CrossRef]
- S. T. Zivanovic, M. D. Popovic, N. M. Vorkapic, M. D. Pjevic, and N. R. Slavkovic, “An overview of rapid prototyping technologies using subtractive, additive and formative processes,” FME Trans., vol. 48, no. 11, pp. 246–253, 2020. [CrossRef]
- K. V Wong and A. Hernandez, “A Review of Additive Manufacturing,” Int. Sch. Res. Netw., no. 6, pp. 1–10, 2012. [CrossRef]
- J. Lee, J. An, and C. K. Chua, “Fundamentals and applications of 3D printing for novel materials,” Appl. Mater. Today, vol. 7, no. 2, pp. 120–133, 2017. [CrossRef]
- Kumnova, “Utilizing 3D Printing to Provide Customized Joysticks Designed to function universally across Volvo ’ s Bachelor thesis within the program Design and Product Development,” Chalmers University of Technology, Gothenburg, Sweden 2017, 2021.
- G. Wu and S. Hsu, “Review : Polymeric-Based 3D Printing for Tissue Engineering,” J. Med. Biol. Eng., vol. 35, no. 3, pp. 285–292, 2015. [CrossRef]
- G. Aspar, “L ’ impression 3D polymère appliquée au packaging en microélectronique tel-02148205,” Université Grenoble Alpes, 2019., 2019.
- C. Barnatt, 3D Printing: The Next Industrial Revolution. Amazon.com: Paperback or Kindle, 2013.
- P. Blyweert, V. Nicolas, V. Fierro, and A. Celzard, “3D printing of carbon-based materials: A review,” Carbon N. Y., vol. 183, no. 7, pp. 449–485, 2021. [CrossRef]
- Centre d’etude et de prospective industrielle, “Note Sur L’ Impression 3D,” 2018. [Online]. Available: www.tunisieindustrie.nat.tn.
- V. Chastand, “Etude du comportement mécanique et des mécanismes d’endommagement de pièces métalliques réalisées par fabrication additive,” 2016.
- C. De Leon, Q. Chen, N. B. Palaganas, J. O. Palaganas, J. Manapat, and R. C. Advincula, “High performance polymer nanocomposites for additive manufacturing applications,” React. Funct. Polym., vol. 103, no. 4, pp. 1–64, 2016. [CrossRef]
- J. R. C. Dizon, A. H. Espera, Q. Chen, and R. C. Advincula, “Mechanical characterization of 3D-printed polymers,” Addit. Manuf., vol. 20, no. 12, pp. 44–67, 2017. [CrossRef]
- Gebhardt and, M. Fateri, “3D Printing and Its Applications,” Lizenznehmer RTejournal, no. 1, pp. 1–12, 2013. [CrossRef]
- R. D. Goodridge et al., “Processing of a Polyamide-12/carbon nanofibre composite by laser sintering,” Polym. Test., vol. 30, no. 10, pp. 94–100, 2011. [CrossRef]
- M. Jacques-hulin, “Développement d’une méthode de conception de moules hybrides en fonderie,” UNIVERSITÉ DE REIMS CHAMPAGNE-ARDENNE ÉCOLE, 2019.
- G. D. Kim and Y. T. Oh, “A benchmark study on rapid prototyping processes and machines: Quantitative comparisons of mechanical properties, accuracy, roughness, speed, and material cost,” Proc. Inst. Mech. Eng. Part B J. Eng. Manuf., vol. 222, no. 9, pp. 201–215, 2008. [CrossRef]
- J. Z. Manapat, Q. Chen, P. Ye, and R. C. Advincula, “3D Printing of Polymer Nanocomposites via Stereolithography,” Macromol. Mater. Eng., vol. 302, no. 9, pp. 1–13, 2017. [CrossRef]
- Y. S. Zhang et al., “3D Bioprinting for Tissue and Organ Fabrication,” Ann Biomed Eng, vol. 45, no. 1, pp. 148–163, 2018. [CrossRef]
- K. Sood, R. K. Ohdar, and S. S. Mahapatra, “Parametric appraisal of mechanical property of fused deposition modelling processed parts,” Mater. Des., vol. 31, no. 6, pp. 287–295, 2010. [CrossRef]
- S. Vyavahare, S. Teraiya, D. Panghal, and S. Kumar, “Fused deposition modelling: a review,” Rapid Prototyp. J., vol. 26, no. 7, pp. 176–201, 2020. [CrossRef]
- X. Wang, M. Jiang, Z. Zhou, J. Gou, and D. Hui, “3D printing of polymer matrix composites: A review and prospective,” Compos. Part B Eng., vol. 110, no. 11, pp. 442–458, 2017. [CrossRef]
- S. C. Ligon, R. Liska, J. Stampfl, M. Gurr, and R. Mülhaupt, “Polymers for 3D Printing and Customized Additive Manufacturing,” Chem. Rev., vol. 117, no. 7, pp. 10212–10290, 2017. [CrossRef]
- T. D. Ngo, A. Kashani, G. Imbalzano, K. T. Q. Nguyen, and D. Hui, “Additive manufacturing (3D printing): A review of materials, methods, applications and challenges,” Compos. Part B Eng., vol. 143, no. 2, pp. 1–80, 2018. [CrossRef]
- G. R. Pereira, F. Gasi, and S. R. Lourenço, “Review, Analysis, and Classification of 3D Printing Literature : Types of Research and Technology Benefits,” Int. J. Adv. Eng. Res. Sci. (IJAERS, vol. 6, no. 6, pp. 167–187, 2019. [CrossRef]
- N. Shahrubudin, T. C. Lee, and R. Ramlan, “ScienceDirect ScienceDirect ScienceDirect An Overview on 3D Printing Technology : Technological, Materials, and Technology : Applications Technological, Materials, An Overview on 3D Printing and Applications,” Procedia Manuf., vol. 35, pp. 1286–1296, 2019. [CrossRef]
- M. Harris and E. C. Lee, “Improving Mechanical Performance of Injection Molded PLA by Controlling Crystallinity,” J. Appl. Polym. Sci., vol. 107, no. September, pp. 2246–2255, 2008. [CrossRef]
- Benwood, A. Anstey, J. Andrzejewski, M. Misra, and and A. K. Mohanty, “Improving the Impact Strength and Heat Resistance of 3D Printed Models: Structure, Property, and Processing Correlationships during Fused Deposition Modeling (FDM) of Poly(Lactic Acid),” ACS OMEGA, vol. 3, no. 5, pp. 4400–4411, 2018. [CrossRef]
- Amobonye, P. Bhagwat, S. Singh, and S. Pillai, “Plastic biodegradation: Frontline microbes and their enzymes,” Sci. Total Environ., vol. 759, no. 10, pp. 1–16, 2021. [CrossRef]
- R. Shah et al., “Exposure to polylactic acid induces oxidative stress and reduces the ceramide levels in larvae of greater wax moth (Galleria mellonella),” Environ. Res., vol. 220, no. 12, pp. 1–10, 2023. [CrossRef]
- Wittbrodt and J., M. Pearce, “The effects of PLA color on material properties of 3-D printed components,” 2015.
- T. Yang, “Effect of Extrusion Temperature on the Fiber-Reinforced Polylactic Acid Composite ( WFRPC ),” Polymers (Basel)., vol. 10, no. 976, pp. 1–11, 2018. [CrossRef]
- M. Savioli Lopes, A. L. Jardini, and R. Maciel Filho, “Poly (lactic acid) production for tissue engineering applications,” Procedia Eng., vol. 42, pp. 1402–1413, 2012. [CrossRef]
- J. Järvenpää, M. Perkkiö, R. Laitinen, and M. Lahtela-Kakkonen, “PE and PET oligomers’ interplay with membrane bilayers,” Sci. Rep., vol. 12, no. 2234, pp. 1–8, 2022. [CrossRef]
- R. Foresti et al., “3D Printed Masks for Powders and Viruses Safety Protection Using Food Grade Polymers : Empirical Tests,” Polymers (Basel)., vol. 13, no. 2, pp. 1–12, 2021. [CrossRef]
- Humayun, Y. Luo, A. Elumalai, D. K. Mills, and A. Humayun, “3D printed antimicrobial PLA constructs functionalised with zinc- coated halloysite nanotubes-Ag-chitosan oligosaccharide lactate ABSTRACT ARTICLE HISTORY,” Mater. Technol., no. 8, pp. 1–8, 2020. [CrossRef]
- Muro-Fraguas et al., “Antibiofilm coatings through atmospheric pressure plasma for 3D printed surgical instruments,” Surf. Coatings Technol., no. 7, pp. 1–49, 2020. [CrossRef]
- Beniak, P. Krizan, and M. Matus, “Mechanical properties of biodegradable pla plastic parts produced by 3d printing,” MM Sci. J., no. 3, pp. 2746–2750, 2019. [CrossRef]
- M. Á. Caminero, J. M. Chacón, E. García-Plaza, P. J. Núñez, J. M. Reverte, and and J. P. Becar, “Additive Manufacturing of PLA-Based Composites Using Fused Filament Fabrication : E ff ect of Graphene Nanoplatelet Reinforcement on Mechanical Properties, Dimensional Accuracy and Texture,” Polymers (Basel)., vol. 11, no. 5, pp. 2019; 22. [CrossRef]
- Dominguez-Robles et al., “Antioxidant PLA Composites Containing Lignin for 3D Printing Applications : A Potential Material for Healthcare Applications,” Pharmaceutics, vol. 11, no. 4, pp. 1–14, 2019. [CrossRef]
- T. Sato, T. Wakabayashi, and K. Saitoh, “Evaluation of Antibacterial and Mechanical Properties of 3D Shaped Metal-containing PLA resin,” in The 10th International Conference on Leading Edge Manufacturing in 21st Century (LEM21), 2021, p. 6, [Online]. Available: http://hdl.handle.net/10112/00025656.
- S. Er-rahmani, B. Errabiti, S. Er, E. Elharchli, A. Elaabedy, and S. Ibnsouda, “Reduction of biofilm formation on 3D printing materials treated with essential oils major compounds,” Ind. Crop. Prod., vol. 182, no. 3, pp. 1–8, 2022. [CrossRef]
- Ryley, M. Carve, R. Piola, A. J. Scardino, and J. Shimeta, “Comparison of biofouling on 3D-printing materials in the marine environment,” Int. Biodeterior. Biodegrad., vol. 164, no. 7, pp. 1–10, 2021. [CrossRef]
- Al-Taie et al., “3-D printed polyvinyl alcohol matrix for detection of airborne pathogens in respiratory bacterial infections,” Microbiol. Res., vol. 241, no. March, pp. 1–9, 2020. [CrossRef]
- Deng, Y. Deng, and K. Xie, “Colloids and Surfaces B : Biointerfaces AgNPs-decorated 3D printed PEEK implant for infection control and bone repair,” Colloids Surfaces B Biointerfaces, vol. 160, no. 9, pp. 483–492, 2017. [CrossRef]
- Kiel, B. P. Kaltschmidt, E. Asghari, A. Hütten, B. Kaltschmidt, and C. Kaltschmidt, “Bacterial Biofilm Formation on Nano-Copper Added PLA Suited for 3D Printed Face Masks,” Microorganisms, vol. 10, no. 2, pp. 1–14, Feb. 2022. [CrossRef]
- X. Xu et al., “Stereolithography (SLA) 3D printing of a bladder device for intravesical drug delivery,” Mater. Sci. Eng. C, vol. 120, no. 11, pp. 1–42, 2021. [CrossRef]
- M. Simoneti, T. Pereira-cenci, and M. B. F. dos Santos, “Comparison of material properties and bio fi lm formation in interim single crowns obtained by 3D printing and conventional methods,” J. Prosthet. Dent., pp. 1–5, 2020. [CrossRef]
- L. Wilson, K. Mohammad, T. Simmons-ehrhardt, and M. F. Bertino, “Customizable 3D printed di ff usion chambers for studies of bacterial pathogen phenotypes in complex environments,” J. Microbiol. Methods, vol. 162, no. 5, pp. 8–15, 2019. [CrossRef]
- X. Sun, P. Tyagi, S. Agate, M. G. Mccord, L. A. Lucia, and L. Pal, “Highly tunable bioadhesion and optics of 3D printable PNIPAm / cellulose nano fi brils hydrogels,” Carbohydr. Polym., vol. 234, no. January, pp. 1–8, 2020. [CrossRef]
- J. S. Shim, J. Kim, H. Jeong, J. Choi, and J. J. Ryu, “Printing accuracy, mechanical properties, surface characteristics, and microbial adhesion of 3D-printed resins with various printing orientations,” J. Prosthet. Dent., pp. 1–8, 2019. [CrossRef]
- J. Mazurek-popczyk, L. Palka, K. Arkusz, B. Dalewski, and K. Baldy-chudzik, “Personalized, 3D- printed fracture fixation plates versus commonly used orthopedic implant materials- biomaterials characteristics and bacterial biofilm formation,” Injury, no. 12, pp. 1–19, 2021. [CrossRef]
- Mcgaffey, Linden, N. Bachynski, M. Oblak, F. James, and J. S. Weese, “Manual polishing of 3D printed metals produced by laser powder bed fusion reduces biofilm formation,” PLoS One, no. 2, pp. 1–19, 2019. [CrossRef]
- Sarker, N. Tran, A. Rifai, M. Brandt, P. A. Tran, and M. Leary, “Rational design of additively manufactured Ti6Al4V implants to control Staphylococcus aureus biofilm formation,” Materialia, vol. 5, no. 2, pp. 1–13, 2019. [CrossRef]
- M. Simoneti, T. Pereira-Cenci, and M. B. F. dos Santos, “Comparison of material properties and biofilm formation in interim single crowns obtained by 3D printing and conventional methods,” J. Prosthet. Dent., vol. 127, no. 1, pp. 168–172, 2020. [CrossRef]
- T. H. Lücking, F. Sambale, B. Schnaars, D. Bulnes-Abundis, S. Beutel, and T. Scheper, “3D-printed individual labware in biosciences by rapid prototyping : In vitro biocompatibility and applications for eukaryotic cell cultures,” Eng. Life Sci., vol. 15, no. 7, pp. 57–64, 2015. [CrossRef]
- G. A. González Flores et al., “Single-Step 3D Printing of Silver-Patterned Polymeric Devices for Bacteria Proliferation Control,” Macromol. Mater. Eng., vol. 307, pp. 1–9, 2022. [CrossRef]
- U. Mangal et al., “Changes in tribological and antibacterial properties of poly(methyl methacrylate)-based 3D-printed intra-oral appliances by incorporating nanodiamonds,” J. Mech. Behav. Biomed. Mater., no. July, pp. 1–32, 2020. [CrossRef]
- M. Vivero-Lopez et al., “Anti-biofilm multi drug-loaded 3D printed hearing aids,” Mater. Sci. Eng. C, vol. 119, no. 10, pp. 1–38, 2020. [CrossRef]
- H. Kadry, S. Wadnap, C. Xu, and F. Ahsan, “Digital light processing ( DLP ) 3D-printing technology and photoreactive polymers in fabrication of modi fi ed-release tablets,” Eur. J. Pharm. Sci., vol. 135, no. 5, pp. 60–67, 2019. [CrossRef]
- Y. He et al., “Ink-jet 3D printing as a strategy for developing bespoke non-eluting biofilm resistant medical devices,” Biomaterials, vol. 281, no. 1, pp. 1–9, Feb. 2022. [CrossRef]
- Y. He et al., “Exploiting Generative Design for 3D Printing of Bacterial Biofilm Resistant Composite Devices,” Adv. Sci., vol. 8, no. 2100249, pp. 1–11, 2021. [CrossRef]
- J. A. Inzana, R. P. Trombetta, E. M. Schwarz, S. L. Kates, and H. A. Awad, “3D printed bioceramics for dual antibiotic delivery to treat implant-associated bone infection,” Eur. Cells Mater., vol. 30, pp. 232–247, 2015. [CrossRef]
- J. Slate et al., “Additive manufactured graphene-based electrodes exhibit beneficial performances in Pseudomonas aeruginosa microbial fuel cells,” J. Power Sources, vol. 499, no. 4, pp. 1–11, 2021. [CrossRef]
- L. Tzounis, P. I. Bangeas, A. Exadaktylos, and M. Petousis, “Three-Dimensional Printed Polylactic Acid ( PLA ) Surgical Retractors with Sonochemically Immobilized Silver Nanoparticles : The Next Generation of Low-Cost Antimicrobial Surgery Equipment,” nanomaterials, vol. 10, no. 5, pp. 1–14, 2020. [CrossRef]
- I. Bayraktar, Doganay, S. Coskun, C. Kaynak, G. Akca, and H. E. Unalan, “3D printed antibacterial silver nanowire/polylactide nanocomposites,” Compos. Part B, no. 5, pp. 1–31, 2019. [CrossRef]
- V. Martin et al., “Engineering a multifunctional 3D-printed PLA-collagen-minocycline-nanoHydroxyapatite scaffold with combined antimicrobial and osteogenic effects for bone regeneration,” Mater. Sci. Eng. C, no. March, pp. 1–38, 2019. [CrossRef]
- J. Water et al., “Three-dimensional printing of drug-eluting implants: Preparation of an antimicrobial polylactide feedstock material,” Pharm. Drug Deliv. Pharm. Technol. Three-Dimensional, no. 11, pp. 1–9, 2015. [CrossRef]
- Domínguez-Robles et al., “Use of 3D Printing for the Development of Biodegradable Antiplatelet Materials for Cardiovascular Applications,” Pharmaceuticals, vol. 14, no. 9, pp. 1–15, 2021. [CrossRef]
- K. Mills, U. Jammalamadaka, K. Tappa, and J. Weisman, “Studies on the cytocompatibility, mechanical and antimicrobial properties of 3D printed poly ( methyl methacrylate ) beads,” Bioact. Mater., vol. 3, no. 2, pp. 157–166, 2018. [CrossRef]
- Y. Yang et al., “Dual-functional 3D-printed composite scaffold for inhibiting bacterial infection and promoting bone regeneration in infected bone defect models,” Acta Biomater., no. 8, pp. 1–28, 2018. [CrossRef]
- Tse et al., “Antimicrobial Activity of 3D-Printed Acrylonitrile Butadiene Styrene (ABS) Polymer-Coated with Silver Nanoparticles,” Materials (Basel)., vol. 14, no. 12, pp. 1–13, 2021. [CrossRef]
- Muro-fraguas et al., “Atmospheric pressure cold plasma anti-biofilm coatings for 3D printed food tools,” J. Pre-proof, no. 5, pp. 1–35, 2020. [CrossRef]
- Marin, F. Boschetto, M. Zanocco, T. Honma, W. Zhu, and G. Pezzotti, “Explorative study on the antibacterial effects of 3D-printed PMMA/nitrides composites,” Mater. Des., vol. 206, no. 5, pp. 1–9, 2021. [CrossRef]
- Feng, Y. Cheng, S. Wang, D. A. Borca-tasciuc, R. W. Worobo, and C. I. Moraru, “Bacterial attachment and bio fi lm formation on surfaces are reduced by small-diameter nanoscale pores : how small is small enough ?,” Nat. Publ. Gr., vol. 1, no. 9, pp. 1–9, 2015. [CrossRef]
- Kodama, H. Chen, and T. Zhou, “Antibacterial efficacy of quaternized chitosan coating on 3D printed titanium cage in rat intervertebral disc space,” Spine J., vol. 21, no. 2, pp. 1217–1228, 2021. [CrossRef]
- R. D. Turner, J. R. Wingham, T. E. Paterson, J. Shepherd, and C. Majewski, “Use of silver-based additives for the development of antibacterial functionality in Laser Sintered polyamide 12 parts,” Sci. Rep., vol. 892, no. 10, pp. 1–11, 2020. [CrossRef]
- Wang et al., “Digital light processing (DLP) 3D-fabricated antimicrobial hydrogel with a sustainable resin of methacrylated woody polysaccharides and hybrid silver-lignin nanospheres †,” Green Chemestry, vol. 24, no. 2129, pp. 1–17, 2022. [CrossRef]
- Z. Li, C. Wang, W. Qiu, and R. Liu, “Antimicrobial Thiol–ene–acrylate Photosensitive Resins for DLP 3D Printing,” Photochem. Photobiol., vol. 95, no. 5, pp. 1219–1229, 2019. [CrossRef]
- Hodasova et al., “Polymer infiltrated ceramic networks with biocompatible adhesive and 3D-printed highly porous scaffolds,” Addit. Manuf., vol. 39, no. 1, pp. 1–12, 2021. [CrossRef]
- R. García-Alvarez, I. Izquierdo-Barba, and M. Vallet-Regí, “3D scaffold with effective multidrug sequential release against bacteria biofilm,” Acta Biomater., vol. 49, no. 11, pp. 113–126, 2017. [CrossRef]
- S. Kwon et al., “Durable oral biofilm resistance of 3d-printed dental base polymers containing zwitterionic materials,” Int. J. Mol. Sci., vol. 22, no. 12, pp. 1–13, 2021. [CrossRef]
- R. S. Han, G. Haghiashtiani, and M. C. Mcalpine, “3D Printing a Susceptibility Assay for Multidrug-Resistant Bacteria,” Chem Cell Press, vol. 1, no. 9, pp. 346–348, 2016. [CrossRef]
- E. Caselli, “Hygiene : microbial strategies to reduce pathogens and drug resistance in clinical settings,” Microb. Biotechnol., vol. 10, no. 6, pp. 1079–1083, 2017. [CrossRef]
- Bhattacharjee, M. Khan, M. Kleiman, and A. I. Hochbaum, “Effects of Growth Surface Topography on Bacterial Signaling in Coculture Biofilms Effects of Growth Surface Topography on Bacterial Signaling in Coculture Biofilms,” ACS Appl. Mater. Interfaces, no. 5, pp. 1–28, 2017, [Online]. Available: http://pubs.acs.org.
- R. Van Houdt and C. and Michiels, “Biofilm formation and the food industry, a focus on the bacterial outer surface,” J. Appl. Microbiol., vol. 109, no. 4, pp. 1117–1131, 2010. [CrossRef]
- Grenho, M. C. Manso, F. J. Monteiro, and M. P. Ferraz, “Adhesion of Staphylococcus aureus, Staphylococcus epidermidis, and Pseudomonas aeruginosa onto nanohydroxyapatite as a bone regeneration material,” J. Biomed. Mater. Res. - Part A, vol. 100 A, no. 7, pp. 1823–1830, 2012. [CrossRef]
- F. Reffuveille et al., “Staphylococcus aureus Biofilms and their Impact on the on the Medical Field,” INTECH, pp. 187–214, 2018. [CrossRef]
- Y. Wang, R. Blache, and X. Xu, “Selection of additive manufacturing processes,” Rapid Prototyp. J., vol. 23, no. 2, pp. 1–29, 2017. [CrossRef]
- S. Fujitani, H. Y. Sun, V. L. Yu, and J. A. Weingarten, “Pneumonia due to pseudomonas aeruginosa: Part I: Epidemiology, clinical diagnosis, and source,” Chest, vol. 139, no. 4, pp. 909–919, 2011. [CrossRef]
- J. A. Driscoll, S. L. Brody, and M. H. Kollef, “The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections,” Drugs, vol. 67, no. 3, pp. 351–368, 2007. [CrossRef]
- Mills, U. Jammalamadaka, K. Tappa, and J. Weisman, “Studies on the cytocompatibility, mechanical and antimicrobial properties of 3D printed poly(methyl methacrylate) beads,” Bioact. Mater., vol. 3, no. 1, pp. 157–166, 2018. [CrossRef]
- Shen, Z. Liu, J. Hong, M. Wu, S. Shiue, and H. Lin, “Controlled-release of free bacteriophage nanoparticles from 3D-plotted hydrogel fibrous structure as potential antibacterial wound dressing,” J. Control. Release, vol. 331, no. 1, pp. 154–163, 2021. [CrossRef]
- E. Walsh, A. Ostrinskaya, M. T. Sorensen, D. S. Kong, and P. A. Carr, “3D-printable materials for microbial liquid culture,” 3D Print. Addit. Manuf., vol. 3, no. 2, pp. 113–118, 2016. [CrossRef]
- Y. Wang, J. Shen, M. Yan, and X. Tian, “Poly ether ether ketone and its composite powder prepared by thermally induced phase separation for high temperature selective laser sintering,” Mater. Des., vol. 201, no. 1, pp. 1–9, 2021. [CrossRef]
- S. Yuan, J. Bai, C. K. Chua, J. Wei, and K. Zhou, “Material Evaluation and Process Optimization of CNT-Coated Polymer Powders for Selective,” Polymers (Basel)., vol. 8, no. 370, pp. 1–17, 2016. [CrossRef]
- J. Tiimob, G. Mwinyelle, W. Abdela, T. Samuel, S. Jeelani, and V. K. Rangari, “Nano-engineered eggshell-silver tailored co- polyester polymer blend film with antimicrobial properties,” Am. Chem. Soc., no. 2, pp. 1–37, 2017. [CrossRef]
- Y. He et al., “Exploiting Generative Design for 3D Printing of Bacterial Biofilm Resistant Composite Devices,” Adv. Sci., vol. 8, pp. 1–11, 2021. [CrossRef]
- 3Dnatives, (2022). All about 3d metal printing. https://www.3dnatives.com/impression-3d-metal/.
- Everett Hayley, (2021). 3D printing industry year in review: December 2021. Available online: https://3dprintingindustry.com/news/3dprinting-industry-year-inreview-december-2021-201491/(Consulted 04/30/2022).
- Formlabs, (2022). Guide to 3D printing materials: types, applications, and properties. https://formlabs.com/fr/blog/materiaux-impression-3d/(Accessed 06/09/2022).
- Gaget Lucie, 2017. Archive 2017 sculpteo blog on 3d printing. https://www.sculpteo.com/blog/fr/2017/(Accessed on: 4/20/22).
- Sculpteo, (2022). Resin 3D Printing: The Complete Guide. https://www.sculpteo.com/fr/centre-apprentissage/choisissez best-material-3d-printing/resin-3d-printing/ (Accessed 04/17/2022). https://www.sculpteo.com/fr/centre-apprentissage/choisissez best-material-3d-printing/resin-3d-printing/(Accessed on: 4/20/22).
- Sculpteo, (2022). The Ultimate Guide: What is 3D Printing? https://www.sculpteo.com/en/3d-learning-hub/basics-of 3dprinting/what-is-3d-printing/(Accessed 04/21/2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





