Submitted:
23 January 2024
Posted:
24 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. LA DDSs
3. Drug Release Kinetics from LA DDSs
4. Common Polymers in LA DDSs
4.1. Polyethylene Glycol (PEG)
4.2. Poloxamers
4.3. Ethylene-Vinyl Acetate (EVA)
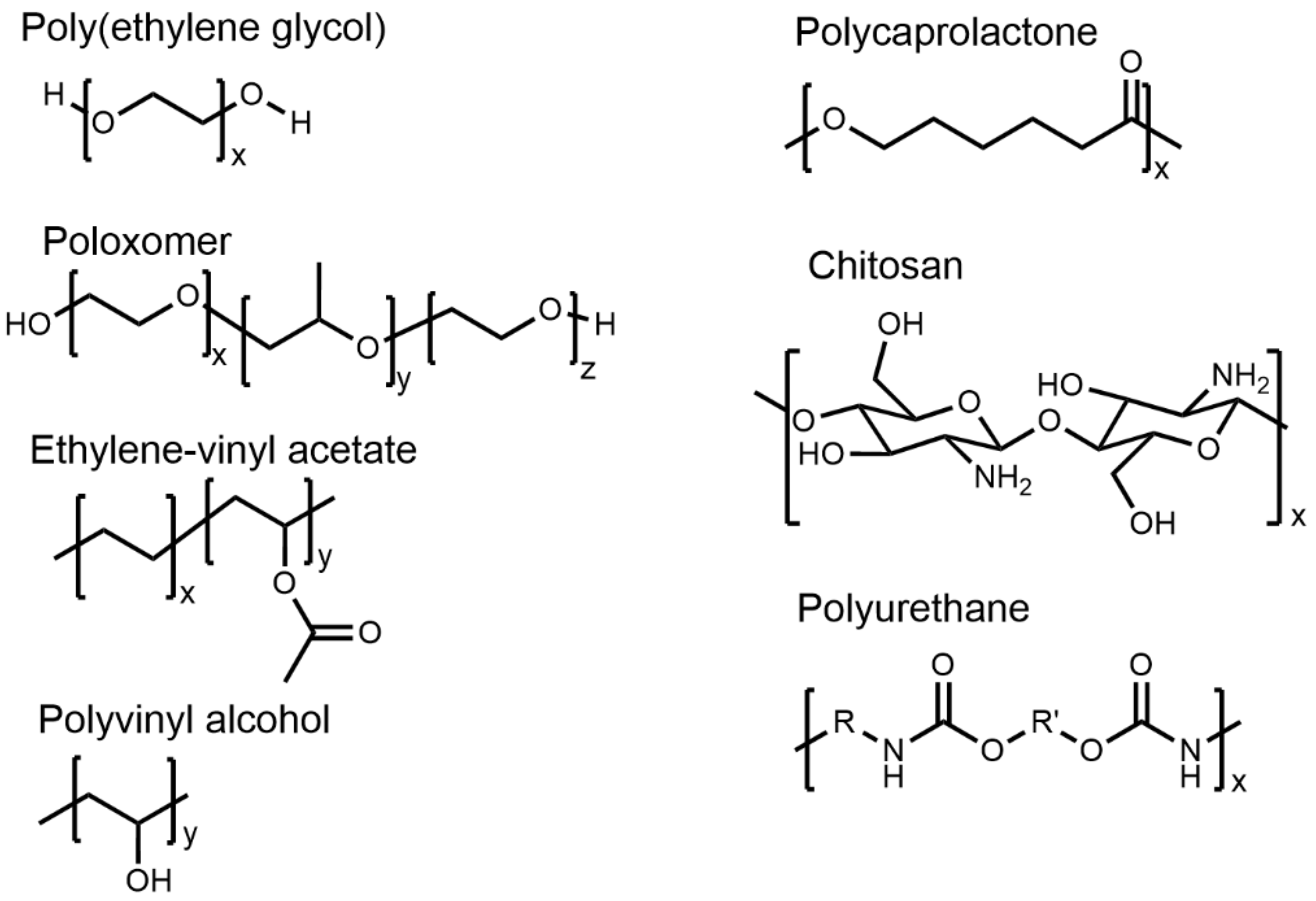
4.4. Polyvinyl Alcohol (PVOH)
4.5. Polyurethanes (PUs)
4.6. Polyesters
4.7. Chitosan and Hyaluronic Acid (HA)
4.8. Polymers and Polymer Blends
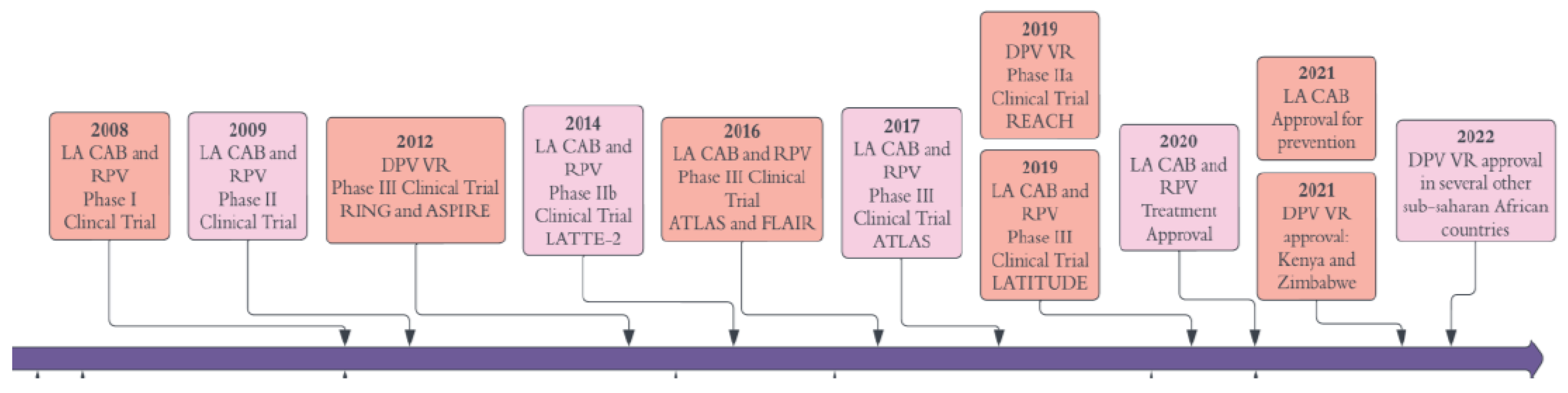
5. LA ARV Delivery
5.1. Implants
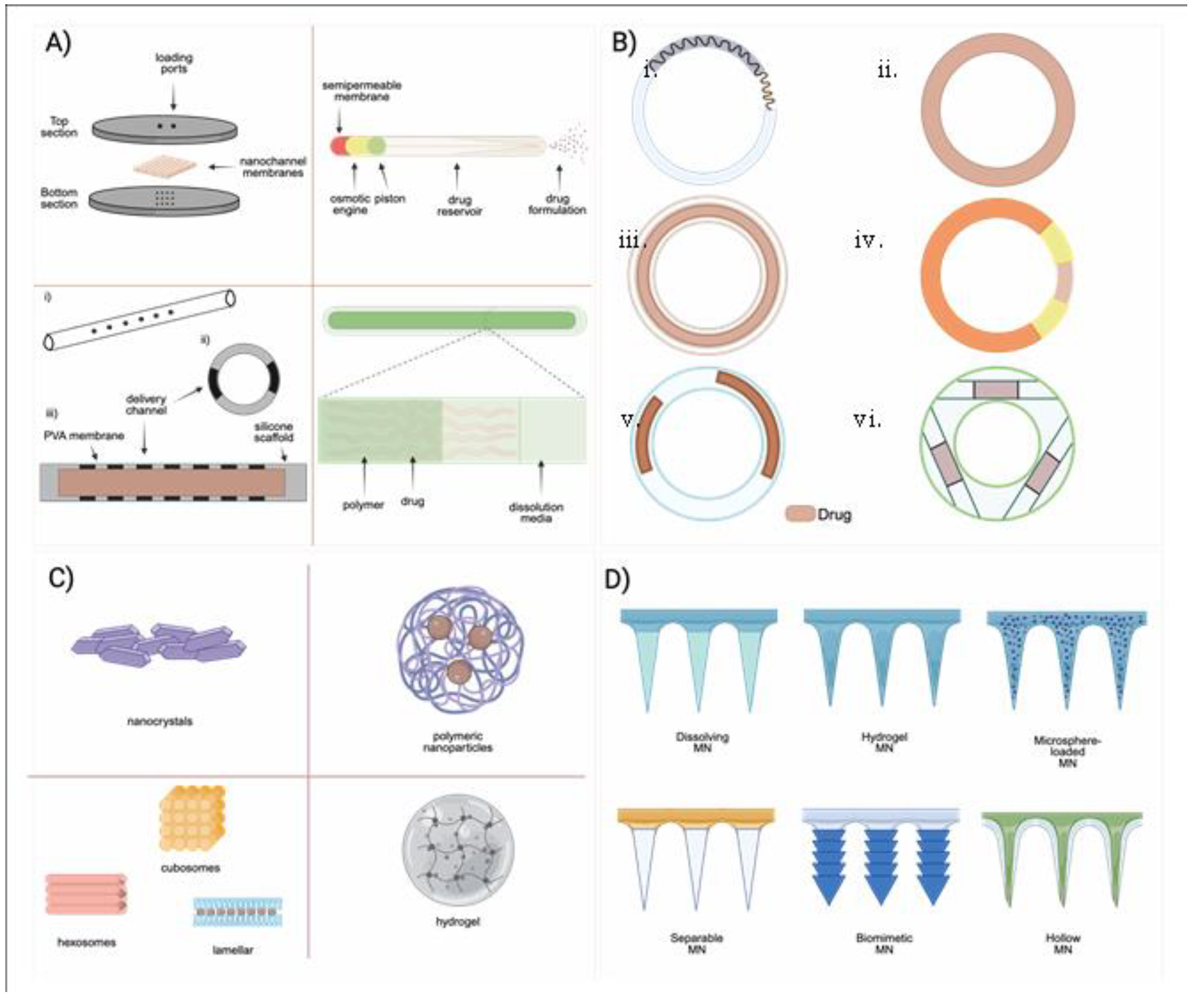
5.2. Vaginal Rings (VRs)
5.3. Microneedles (MNs)
5.4. Polymeric Micro- and Nanoparticles
5.4.1. PLGA Based Micro- and Nanoparticles
5.4.2. Nanocrystal Formulations
| Drugs | (CAB + RPV LA) | CAB LA |
| Brand name | Cabenuva | Apretude or Vocabria |
| Formulation characteristics | CAB nanocrystals are produced in an aqueous solution of polysorbate 20 (Tween 20), PEG3350, and mannitol with particle size of 200 nm.RPV nanocrystals are produced in an aqueous solution of Poloxamer 338 with an average particle size of 200 nm | Same as Cabenuva. |
| Indication | Treatment of HIV-1 infection | Prevention of HIV-1 infection |
| Population | Virologically suppressed PLWH | Adults and adolescents weighing at least 35 kg (77 lbs) who are at risk of sexually acquiring HIV |
| Dosage regimen | Two initial injection of CAB 600 mg/ 3 mL and RPV 900 mg/ 3 mL given 1 month apart for two consecutive months and then given every two months (CAB 600 mg/ 3 mL and 900 mg/ 3 mL) thereafter. Injection is given in separate gluteal muscles. | Two initial injections (600 mg; 3 mL) given one month apart for two consecutive months, followed by maintenance doses (600 mg; 3 mL) given every 2 months thereafter. An OLI of CAB tablets may, or may not, be given for one month prior to starting CAB to assess tolerability. |
| Approval | First approved by Health Canada in March 2020, followed by the EMA in October of 2020, and the US FDA in January of 2021. | First approved by the US FDA in December of 2021 and the EMA in October of 2022. |
| Efficacy in the clinical trials | Several phase IIb and phase III clinical trials (LATTE-2, FLAIR, ATLAS, ATLAS-2M) proved non-inferiority for maintaining viral suppression compared to standard daily oral therapy [11,14,16,267]. | Proved superior than approved oral PrEP agent TDF/FTC in 2 phase III clinical trials (HPTN-083 and 084) [268,269]. |
| Side effects | Mild ISRs were the most reported AEs during clinical trials, but none of them caused treatment withdrawal. | Same as Cabenuva. |
| PLWH – people living with HIV. EMA – European Medicines Agency. US FDA – US Food and Drug Administration. OLI – oral lead in. AEs – adverse events. ISRs – injection site reactions. | ||
6. Conclusion and Future Perspective of la Drug Delivery Systems
Author Contributions
Funding
References
- Benhabbour, S.R.; Kovarova, M.; Jones, C.; Copeland, D.J.; Shrivastava, R.; Swanson, M.D.; Sykes, C.; Ho, P.T.; Cottrell, M.L.; Sridharan, A. Ultra-long-acting tunable biodegradable and removable controlled release implants for drug delivery. Nature communications 2019, 10, 4324. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.M.; Krovi, S.A.; Li, L.; Girouard, N.; Demkovich, Z.R.; Myers, D.; Creelman, B.; van der Straten, A. Characterization of a Reservoir-Style Implant for Sustained Release of Tenofovir Alafenamide (TAF) for HIV Pre-Exposure Prophylaxis (PrEP). Pharmaceutics 2019, 11, 315. [Google Scholar] [CrossRef] [PubMed]
- Gunawardana, M.; Remedios-Chan, M.; Miller, C.S.; Fanter, R.; Yang, F.; Marzinke, M.A.; Hendrix, C.W.; Beliveau, M.; Moss, J.A.; Smith, T.J. Pharmacokinetics of long-acting tenofovir alafenamide (GS-7340) subdermal implant for HIV prophylaxis. Antimicrobial agents and chemotherapy 2015, 59, 3913–3919. [Google Scholar] [CrossRef] [PubMed]
- Tekko, I.A.; Vora, L.K.; Volpe-Zanutto, F.; Moffatt, K.; Jarrahian, C.; McCarthy, H.O.; Donnelly, R.F. Novel Bilayer Microarray Patch-Assisted Long-Acting Micro-Depot Cabotegravir Intradermal Delivery for HIV Pre-Exposure Prophylaxis. Advanced Functional Materials 2022, 32, 2106999. [Google Scholar] [CrossRef]
- Barrett, S.E.; Teller, R.S.; Forster, S.P.; Li, L.; Mackey, M.A.; Skomski, D.; Yang, Z.; Fillgrove, K.L.; Doto, G.J.; Wood, S.L.; et al. Extended-Duration MK-8591-Eluting Implant as a Candidate for HIV Treatment and Prevention. Antimicrobial Agents and Chemotherapy 2018, 62. [Google Scholar] [CrossRef]
- Deodhar, S.; Sillman, B.; Bade, A.N.; Avedissian, S.N.; Podany, A.T.; McMillan, J.M.; Gautam, N.; Hanson, B.; Dyavar Shetty, B.L.; Szlachetka, A. Transformation of dolutegravir into an ultra-long-acting parenteral prodrug formulation. Nature communications 2022, 13, 3226. [Google Scholar] [CrossRef]
- Malcolm, R.K.; Fetherston, S.M.; McCoy, C.F.; Boyd, P.; Major, I. Vaginal rings for delivery of HIV microbicides. Int J Womens Health 2012, 4, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Deanesly, R.; Parkes, A.S. Testosterone. British Medical Journal 1936, 1, 527–528. [Google Scholar] [CrossRef] [PubMed]
- Paolini, M.S.; Fenton, O.S.; Bhattacharya, C.; Andresen, J.L.; Langer, R. Polymers for extended-release administration. Biomedical Microdevices 2019, 21, 45. [Google Scholar] [CrossRef]
- Vora, L.K.; Moffatt, K.; Tekko, I.A.; Paredes, A.J.; Volpe-Zanutto, F.; Mishra, D.; Peng, K.; Raj Singh Thakur, R.; Donnelly, R.F. Microneedle array systems for long-acting drug delivery. European Journal of Pharmaceutics and Biopharmaceutics 2021, 159, 44–76. [Google Scholar] [CrossRef]
- Swindells, S.; Andrade-Villanueva, J.-F.; Richmond, G.J.; Rizzardini, G.; Baumgarten, A.; Masiá, M.; Latiff, G.; Pokrovsky, V.; Bredeek, F.; Smith, G. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. New England Journal of Medicine 2020, 382, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.H.R.; Henry, W.K.; Podzamczer, D.; Masiá, M.D.M.; Bettacchi, C.J.; Arasteh, K.; Jaeger, H.; Khuong-Josses, M.A.; Montes-Ramírez, M.L.; Stellbrink, H.J.; et al. Efficacy, Safety, and Durability of Long-Acting Cabotegravir and Rilpivirine in Adults With Human Immunodeficiency Virus Type 1 Infection: 5-Year Results From the LATTE-2 Study. Open Forum Infect Dis 2021, 8, ofab439. [Google Scholar] [CrossRef] [PubMed]
- Parikh, U.M.; Koss, C.A.; Mellors, J.W. Long-Acting Injectable Cabotegravir for HIV Prevention: What Do We Know and Need to Know about the Risks and Consequences of Cabotegravir Resistance? Curr HIV/AIDS Rep 2022, 19, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Margolis, D.A.; Gonzalez-Garcia, J.; Stellbrink, H.-J.; Eron, J.J.; Yazdanpanah, Y.; Podzamczer, D.; Lutz, T.; Angel, J.B.; Richmond, G.J.; Clotet, B. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. The Lancet 2017, 390, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Orkin, C.; Oka, S.; Philibert, P.; Brinson, C.; Bassa, A.; Gusev, D.; Degen, O.; García, J.G.; Morell, E.B.; Tan, D.H.S.; et al. Long-acting cabotegravir plus rilpivirine for treatment in adults with HIV-1 infection: 96-week results of the randomised, open-label, phase 3 FLAIR study. Lancet HIV 2021, 8, e185–e196. [Google Scholar] [CrossRef] [PubMed]
- Orkin, C.; Arasteh, K.; Górgolas Hernández-Mora, M.; Pokrovsky, V.; Overton, E.T.; Girard, P.-M.; Oka, S.; Walmsley, S.; Bettacchi, C.; Brinson, C. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. New England Journal of Medicine 2020, 382, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, T.A.; Bade, A.N.; Sillman, B.; Shetty, B.L.D.; Wojtkiewicz, M.S.; Gautam, N.; Hilaire, J.R.; Sravanam, S.; Szlachetka, A.; Lamberty, B.G. A year-long extended release nanoformulated cabotegravir prodrug. Nature materials 2020, 19, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Young, I.C.; Massud, I.; Cottrell, M.L.; Shrivastava, R.; Maturavongsadit, P.; Prasher, A.; Wong-Sam, A.; Dinh, C.; Edwards, T.; Mrotz, V.; et al. Ultra-long-acting in-situ forming implants with cabotegravir protect female macaques against rectal SHIV infection. Nature Communications 2023, 14, 708. [Google Scholar] [CrossRef] [PubMed]
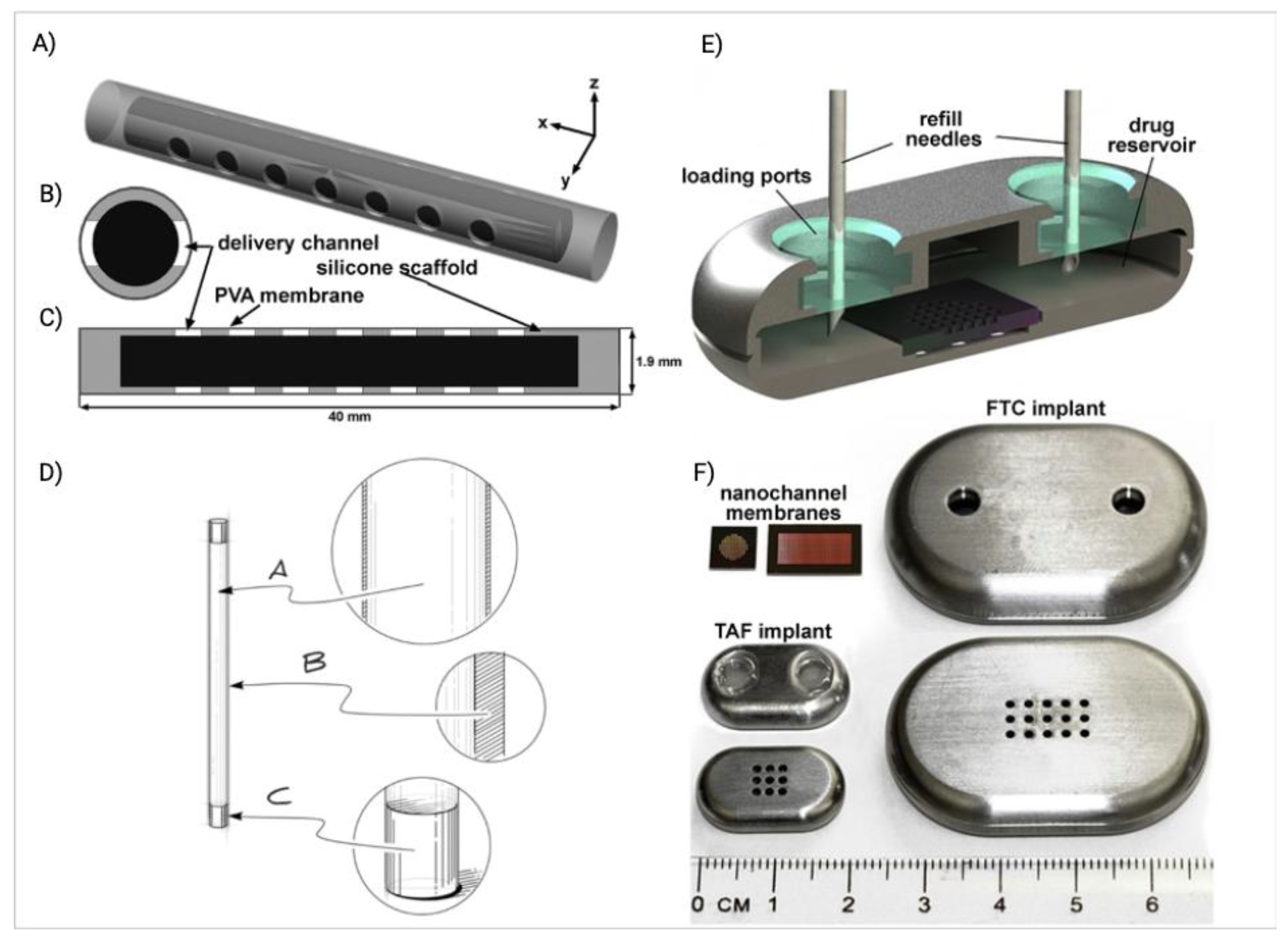
- Chua, C.Y.X.; Jain, P.; Ballerini, A.; Bruno, G.; Hood, R.L.; Gupte, M.; Gao, S.; Di Trani, N.; Susnjar, A.; Shelton, K.; et al. Transcutaneously refillable nanofluidic implant achieves sustained level of tenofovir diphosphate for HIV pre-exposure prophylaxis. J Control Release 2018, 286, 315–325. [Google Scholar] [CrossRef]
- Simpson, S.M.; Widanapathirana, L.; Su, J.T.; Sung, S.; Watrous, D.; Qiu, J.; Pearson, E.; Evanoff, A.; Karunakaran, D.; Chacon, J.E.; et al. Design of a Drug-Eluting Subcutaneous Implant of the Antiretroviral Tenofovir Alafenamide Fumarate. Pharmaceutical Research 2020, 37, 83. [Google Scholar] [CrossRef]
- Markowitz, M.; Grobler, J.A. Islatravir for the treatment and prevention of infection with the human immunodeficiency virus type 1. Current Opinion in HIV and AIDS 2020, 15, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Baeten, J.M.; Palanee-Phillips, T.; Mgodi, N.M.; Mayo, A.J.; Szydlo, D.W.; Ramjee, G.; Gati Mirembe, B.; Mhlanga, F.; Hunidzarira, P.; Mansoor, L.E.; et al. Safety, uptake, and use of a dapivirine vaginal ring for HIV-1 prevention in African women (HOPE): an open-label, extension study. Lancet HIV 2021, 8, e87–e95. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.J.; Wood, L.; Billingsley, J.M.; Ray, L.L.; Goymer, J.; Sinclair, S.; McGinn, A.P.; Marzinke, M.A.; Frank, B.; Srinivasan, S.; et al. Tenofovir disoproxil fumarate intravaginal ring for HIV pre-exposure prophylaxis in sexually active women: a phase 1, single-blind, randomised, controlled trial. Lancet HIV 2019, 6, e498–e508. [Google Scholar] [CrossRef] [PubMed]
- Boyd, P.; Variano, B.; Spence, P.; McCoy, C.F.; Murphy, D.J.; Dallal Bashi, Y.H.; Malcolm, R.K. In vitro release testing methods for drug-releasing vaginal rings. Journal of Controlled Release 2019, 313, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, E.T.; van der Straten, A.; Chitukuta, M.; Reddy, K.; Woeber, K.; Atujuna, M.; Bekker, L.G.; Etima, J.; Nakyanzi, T.; Mayo, A.J.; et al. Acceptability and use of a dapivirine vaginal ring in a phase III trial. Aids 2017, 31, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; van Niekerk, N.; Kapiga, S.; Bekker, L.-G.; Gama, C.; Gill, K.; Kamali, A.; Kotze, P.; Louw, C.; Mabude, Z.; et al. Safety and Efficacy of a Dapivirine Vaginal Ring for HIV Prevention in Women. New England Journal of Medicine 2016, 375, 2133–2143. [Google Scholar] [CrossRef] [PubMed]
- Mc Crudden, M.T.C.; Larrañeta, E.; Clark, A.; Jarrahian, C.; Rein-Weston, A.; Lachau-Durand, S.; Niemeijer, N.; Williams, P.; Haeck, C.; McCarthy, H.O.; et al. Design, formulation and evaluation of novel dissolving microarray patches containing a long-acting rilpivirine nanosuspension. Journal of Controlled Release 2018, 292, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Deanesly, R.; Parkes, A.S. Biological properties of some new derivatives of testosterone. Biochemical Journal 1937, 31, 1161. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.W. Novel drug delivery systems. Drugs and the pharmaceutical sciences 1992, 50. [Google Scholar]
- Dreyfuss, J.; Ross Jr, J.J.; Shaw, J.M.; Miller, I.; Schreiber, E.C. Release and elimination of 14C-fluphenazine enanthate and decanoate esters administered in sesame oil to dogs. Journal of pharmaceutical sciences 1976, 65, 502–507. [Google Scholar] [CrossRef]
- Wright, J.C.; Hoffman, A.S. Historical overview of long acting injections and implants. In Long acting injections and implants; Springer, 2011; pp. 11–24. [Google Scholar]
- Higuchi, T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. Journal of pharmaceutical sciences 1963, 52, 1145–1149. [Google Scholar] [CrossRef]
- Folkman, J.; Long Jr, D.M.; Rosenbaum, R. Silicone rubber: a new diffusion property useful for general anesthesia. Science 1966, 154, 148–149. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, K. Therapeutic systems: rate-controlled drug delivery: concept and development; 1984.
- Frazza, E.; Schmitt, E. A new absorbable suture. Journal of biomedical materials research 1971, 5, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Kent, J.S.; Lewis, D.H.; Sanders, L.M.; Tice, T.R. Microencapsulation of water soluble active polypeptides. 1987.
- Hoffman, A.S. The origins and evolution of “controlled” drug delivery systems. Journal of controlled release 2008, 132, 153–163. [Google Scholar] [CrossRef]
- Nowacek, A.S.; Miller, R.L.; McMillan, J.; Kanmogne, G.; Kanmogne, M.; Mosley, R.L.; Ma, Z.; Graham, S.; Chaubal, M.; Werling, J.; et al. NanoART synthesis, characterization, uptake, release and toxicology for human monocyte-macrophage drug delivery. Nanomedicine (Lond) 2009, 4, 903–917. [Google Scholar] [CrossRef] [PubMed]
- Baert, L.; van‘t Klooster, G.; Dries, W.; François, M.; Wouters, A.; Basstanie, E.; Iterbeke, K.; Stappers, F.; Stevens, P.; Schueller, L. Development of a long-acting injectable formulation with nanoparticles of rilpivirine (TMC278) for HIV treatment. European Journal of Pharmaceutics and Biopharmaceutics 2009, 72, 502–508. [Google Scholar] [CrossRef]
- Penrose, K.J.; Parikh, U.M.; Hamanishi, K.A.; Else, L.; Back, D.; Boffito, M.; Jackson, A.; Mellors, J.W. Selection of rilpivirine-resistant HIV-1 in a seroconverter from the SSAT 040 trial who received the 300-mg dose of long-acting rilpivirine (TMC278LA). The Journal of infectious diseases 2016, 213, 1013–1017. [Google Scholar] [CrossRef]
- Venkatesan, P. Long-acting injectable ART for HIV: a (cautious) step forward. Lancet Microbe 2022, 3, e94. [Google Scholar] [CrossRef]
- Langer, R. New methods of drug delivery. Science 1990, 249, 1527–1533. [Google Scholar] [CrossRef]
- Rahnfeld, L.; Luciani, P. Injectable lipid-based depot formulations: where do we stand? Pharmaceutics 2020, 12, 567. [Google Scholar] [CrossRef]
- U.S. Pharmacopeia, N.F.; Effective date, pp. 1–36.
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly (lactic-co-glycolic acid)-based drug delivery systems—A review. International journal of pharmaceutics 2011, 415, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Berchane, N.; Carson, K.; Rice-Ficht, A.; Andrews, M. Effect of mean diameter and polydispersity of PLG microspheres on drug release: Experiment and theory. International Journal of Pharmaceutics 2007, 337, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Kao, W.J. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert opinion on drug delivery 2010, 7, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annual review of chemical and biomolecular engineering 2010, 1, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Webber, W.L.; Lago, F.; Thanos, C.; Mathiowitz, E. Characterization of soluble, salt-loaded, degradable PLGA films and their release of tetracycline. Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and the Australian Society for Biomaterials 1998, 41, 18–29. [Google Scholar] [CrossRef]
- Marin, E.; Briceño, M.I.; Caballero-George, C. Critical evaluation of biodegradable polymers used in nanodrugs. International journal of nanomedicine 2013, 3071–3091. [Google Scholar]
- Uhrich, K.E.; Cannizzaro, S.M.; Langer, R.S.; Shakesheff, K.M. Polymeric systems for controlled drug release. Chemical reviews 1999, 99, 3181–3198. [Google Scholar] [CrossRef] [PubMed]
- Maroni, A.; Zema, L.; Cerea, M.; Foppoli, A.; Palugan, L.; Gazzaniga, A. Erodible drug delivery systems for time-controlled release into the gastrointestinal tract. Journal of Drug Delivery Science and Technology 2016, 32, 229–235. [Google Scholar] [CrossRef]
- Keraliya, R.A.; Patel, C.; Patel, P.; Keraliya, V.; Soni, T.G.; Patel, R.C.; Patel, M. Osmotic drug delivery system as a part of modified release dosage form. International Scholarly Research Notices 2012, 2012. [Google Scholar] [CrossRef]
- Huang, X.; Brazel, C.S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. Journal of controlled release 2001, 73, 121–136. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Saltzman, W.M. Drug delivery: engineering principles for drug therapy. Oxford University Press, 2001. [Google Scholar]
- Kim, J.M.; Seo, K.S.; Jeong, Y.K.; Lee, H.B.; Kim, Y.S.; Khang, G. Co-effect of aqueous solubility of drugs and glycolide monomer on in vitro release rates from poly (D, L-lactide-co-glycolide) discs and polymer degradation. Journal of Biomaterials Science, Polymer Edition 2005, 16, 991–1007. [Google Scholar] [CrossRef]
- Lu, L.; Peter, S.J.; Lyman, M.D.; Lai, H.-L.; Leite, S.M.; Tamada, J.A.; Uyama, S.; Vacanti, J.P.; Langer, R.; Mikos, A.G. In vitro and in vivo degradation of porous poly (DL-lactic-co-glycolic acid) foams. Biomaterials 2000, 21, 1837–1845. [Google Scholar] [CrossRef]
- Ehrenstein, G.W. Polymeric materials: structure, properties, applications. Carl Hanser Verlag GmbH Co KG, 2012. [Google Scholar]
- Odian, G. Principles of polymerization. John Wiley & Sons, 2004. [Google Scholar]
- Allcock, H.R.; Lampe, F.W.; Mark, J.E.; Allcock, H. Contemporary polymer chemistry. Prentice-Hall Englewood Cliffs: NJ, USA, 1981. [Google Scholar]
- Muller, W.E.; Wang, X.; Schroder, H. Biomedical Inorganic Polymers; Springer, 2016. [Google Scholar]
- Ginjupalli, K.; Shavi, G.V.; Averineni, R.K.; Bhat, M.; Udupa, N.; Upadhya, P.N. Poly (α-hydroxy acid) based polymers: A review on material and degradation aspects. Polymer Degradation and Stability 2017, 144, 520–535. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Progress in polymer science 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Dash, A.; Cudworth II, G. Therapeutic applications of implantable drug delivery systems. Journal of pharmacological and toxicological methods 1998, 40, 1–12. [Google Scholar] [CrossRef]
- Gan, M.; Zhou, Q.; Ge, J.; Zhao, J.; Wang, Y.; Yan, Q.; Wu, C.; Yu, H.; Xiao, Q.; Wang, W. Precise in-situ release of microRNA from an injectable hydrogel induces bone regeneration. Acta Biomaterialia 2021, 135, 289–303. [Google Scholar] [CrossRef]
- Rahme, K.; Dagher, N. Chemistry Routes for Copolymer Synthesis Containing PEG for Targeting, Imaging, and Drug Delivery Purposes. Pharmaceutics 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Cano, C.; Carril, M. Recent Developments in the Design of Non-Biofouling Coatings for Nanoparticles and Surfaces. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Dirisala, A.; Uchida, S.; Toh, K.; Li, J.; Osawa, S.; Tockary, T.A.; Liu, X.; Abbasi, S.; Hayashi, K.; Mochida, Y. Transient stealth coating of liver sinusoidal wall by anchoring two-armed PEG for retargeting nanomedicines. Science Advances 2020, 6, eabb8133. [Google Scholar] [CrossRef]
- Zhang, D.; Zheng, H.; Geng, K.; Shen, J.; Feng, X.; Xu, P.; Duan, Y.; Li, Y.; Wu, R.; Gou, Z. Large fuzzy biodegradable polyester microspheres with dopamine deposition enhance cell adhesion and bone regeneration in vivo. Biomaterials 2021, 272, 120783. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, K.; Hussain, I.; Oderinde, O.; Yao, F.; Zhang, J.; Fu, G. A Conductive Self-Healing Double Network Hydrogel with Toughness and Force Sensitivity. Chemistry–A European Journal 2018, 24, 6632–6638. [Google Scholar] [CrossRef] [PubMed]
- Apostolides, D.E.; Patrickios, C.S.; Sakai, T.; Guerre, M.; Lopez, G.r.; Améduri, B.; Ladmiral, V.; Simon, M.; Gradzielski, M.; Clemens, D. Near-model amphiphilic polymer conetworks based on four-arm stars of poly (vinylidene fluoride) and poly (ethylene glycol): Synthesis and characterization. Macromolecules 2018, 51, 2476–2488. [Google Scholar] [CrossRef]
- Xue, S.; Li, X.; Li, S.; Chen, N.; Zhan, Q.; Long, L.; Zhao, J.; Hou, X.; Yuan, X. Bone fracture microenvironment responsive hydrogel for timing sequential release of cargoes. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2021, 629, 127413. [Google Scholar] [CrossRef]
- Le Fer, G.; Dilla, R.A.; Wang, Z.; King, J.; Chuang, S.S.; Becker, M.L. Clustering and Hierarchical Organization of 3D Printed Poly (propylene fumarate)-block-PEG-block-poly (propylene fumarate) ABA Triblock Copolymer Hydrogels. Macromolecules 2021, 54, 3458–3468. [Google Scholar] [CrossRef]
- Roy, D.; Cambre, J.N.; Sumerlin, B.S. Future perspectives and recent advances in stimuli-responsive materials. Progress in Polymer Science 2010, 35, 278–301. [Google Scholar] [CrossRef]
- Alexandridis, P.; Zhou, D.; Khan, A. Lyotropic liquid crystallinity in amphiphilic block copolymers: temperature effects on phase behavior and structure for poly (ethylene oxide)-b-poly (propylene oxide)-b-poly (ethylene oxide) copolymers of different composition. Langmuir 1996, 12, 2690–2700. [Google Scholar] [CrossRef]
- Pozzo, D.C.; Hollabaugh, K.R.; Walker, L.M. Rheology and phase behavior of copolymer-templated nanocomposite materials. Journal of Rheology 2005, 49, 759–782. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of poloxamers for drug delivery. Journal of functional biomaterials 2018, 9, 11. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, X.; Lin, X.; Yao, C.; Shen, L.; Feng, Y. Poloxamer-based in situ hydrogels for controlled delivery of hydrophilic macromolecules after intramuscular injection in rats. Drug delivery 2015, 22, 375–382. [Google Scholar] [CrossRef]
- Yang, L.; Alexandridis, P. Controlled Drug Delivery. 2000.
- Van Hemelryck, S.; Dewulf, J.; Niekus, H.; van Heerden, M.; Ingelse, B.; Holm, R.; Mannaert, E.; Langguth, P. In vitro evaluation of poloxamer in situ forming gels for bedaquiline fumarate salt and pharmacokinetics following intramuscular injection in rats. International journal of pharmaceutics: X 2019, 1, 100016. [Google Scholar] [CrossRef] [PubMed]
- Demarteau, J.; Kermagoret, A.; Jérôme, C.; Detrembleur, C.; Debuigne, A. Controlled Synthesis of Ethylene-Vinyl Acetate Based Copolymers by Organometallic Mediated Radical Polymerization. In Controlled Radical Polymerization: Materials; ACS Symposium Series; American Chemical Society: 2015; Volume 1188, pp. 47–61.
- Schneider, C.; Langer, R.; Loveday, D.; Hair, D. Applications of ethylene vinyl acetate copolymers (EVA) in drug delivery systems. Journal of Controlled Release 2017, 262, 284–295. [Google Scholar] [CrossRef]
- Arsac, A.; Carrot, C.; Guillet, J. Determination of primary relaxation temperatures and melting points of ethylene vinyl acetate copolymers. Journal of Thermal Analysis and Calorimetry 2000, 61, 681–685. [Google Scholar] [CrossRef]
- Wang, X.; Venkatraman, S.S.; Boey, F.Y.; Loo, J.S.; Tan, L.P. Controlled release of sirolimus from a multilayered PLGA stent matrix. Biomaterials 2006, 27, 5588–5595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lin, W.; Yang, G.; Chen, Q. Studies on the phase structure of ethylene-vinyl acetate copolymers by solid-state 1H and 13C NMR. Journal of Polymer Science Part B: Polymer Physics 2002, 40, 2199–2207. [Google Scholar] [CrossRef]
- Wang, L.; Fang, P.; Ye, C.; Feng, J. Solid-state NMR characterizations on phase structures and molecular dynamics of poly(ethylene-co-vinyl acetate). Journal of Polymer Science Part B: Polymer Physics 2006, 44, 2864–2879. [Google Scholar] [CrossRef]
- Almeida, A.; Possemiers, S.; Boone, M.N.; De Beer, T.; Quinten, T.; Van Hoorebeke, L.; Remon, J.P.; Vervaet, C. Ethylene vinyl acetate as matrix for oral sustained release dosage forms produced via hot-melt extrusion. European Journal of Pharmaceutics and Biopharmaceutics 2011, 77, 297–305. [Google Scholar] [CrossRef]
- McKeen, L.W. The effect of sterilization on plastics and elastomers; William Andrew, 2018. [Google Scholar]
- Gaaz, T.S.; Sulong, A.B.; Akhtar, M.N.; Kadhum, A.A.; Mohamad, A.B.; Al-Amiery, A.A. Properties and Applications of Polyvinyl Alcohol, Halloysite Nanotubes and Their Nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef]
- Song, S.I.; Kim, B.C. Characteristic rheological features of PVA solutions in water-containing solvents with different hydration states. Polymer 2004, 45, 2381–2386. [Google Scholar] [CrossRef]
- Marin, E.; Rojas, J.; Ciro, Y. A review of polyvinyl alcohol derivatives: promising materials for pharmaceutical and biomedical applications. African J Pharm Pharmacol 2014, 8, 674–684. [Google Scholar]
- Rowe, R.C.; Sheskey, P.; Quinn, M. Handbook of pharmaceutical excipients; Libros Digitales-Pharmaceutical Press: 2009.
- Dediu, V.; Busila, M.; Tucureanu, V.; Bucur, F.I.; Iliescu, F.S.; Brincoveanu, O.; Iliescu, C. Synthesis of ZnO/Au nanocomposite for antibacterial applications. Nanomaterials 2022, 12, 3832. [Google Scholar] [CrossRef] [PubMed]
- Fatema, U.K.; Rahman, M.M.; Islam, M.R.; Mollah, M.Y.A.; Susan, M.A.B.H. Silver/poly (vinyl alcohol) nanocomposite film prepared using water in oil microemulsion for antibacterial applications. Journal of colloid and interface science 2018, 514, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Meira, J.; Madeira, C.; Falcão-Reis, F.; Figueira, L. Sustained control from recurring non-infectious uveitic macular edema with 0.19 mg fluocinolone acetonide intravitreal implant–a case report. Ophthalmology and Therapy 2019, 8, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Highley, C.B.; Yeh, Y.C.; Galarraga, J.H.; Uman, S.; Burdick, J.A. Three-dimensional extrusion bioprinting of single-and double-network hydrogels containing dynamic covalent crosslinks. Journal of Biomedical Materials Research Part A 2018, 106, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, J.; Liu, J.; Long, Y.; Fang, L.; Wang, Q.; Liu, T. Highly compressible and superior low temperature tolerant supercapacitors based on dual chemically crosslinked PVA hydrogel electrolytes. Journal of Materials Chemistry A 2020, 8, 6219–6228. [Google Scholar] [CrossRef]
- Chao, Y.; Chen, Q.; Liu, Z. Smart injectable hydrogels for cancer immunotherapy. Advanced Functional Materials 2020, 30, 1902785. [Google Scholar] [CrossRef]
- Lanman, T.; Martin, N.; Vinters, H. The pathology of encephalic arteriovenous malformations treated by prior embolotherapy. Neuroradiology 1988, 30, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hulman, G.; Kirkham, J. Ivalon Sponge Presenting as an Extrarectal Mass. In Proceedings of the Journal of Pathology; 1990; p. 171. [Google Scholar]
- Fassi, A.; Naidoo, N. Irritation associated with tear-replacement ophthalmic drops-a pharmaceutical and subjective investigation. South African Medical Journal 1989, 75, 233–235. [Google Scholar]
- Singh, B.; Varshney, L.; Francis, S. Designing tragacanth gum based sterile hydrogel by radiation method for use in drug delivery and wound dressing applications. International journal of biological macromolecules 2016, 88, 586–602. [Google Scholar] [CrossRef]
- Nasef, S.M.; Khozemy, E.E.; Kamoun, E.A.; El-Gendi, H. Gamma radiation-induced crosslinked composite membranes based on polyvinyl alcohol/chitosan/AgNO3/vitamin E for biomedical applications. International journal of biological macromolecules 2019, 137, 878–885. [Google Scholar] [CrossRef]
- Yuan, H.J.; Wei, Z.J.; Yu, X.Z. Study on Polyvinyl Alcohol Hydrogel Materials for Improving the Performance of Artificial Articular Cartilage. Advanced Materials Research 2013, 703, 29–32. [Google Scholar] [CrossRef]
- DeMerlis, C.; Schoneker, D. Review of the oral toxicity of polyvinyl alcohol (PVA). Food and chemical Toxicology 2003, 41, 319–326. [Google Scholar] [CrossRef]
- Rodwell, D.; Kelly, C.; DeMerlis, C.; Schoneker, D.; Borzelleca, J. Effects of polyvinyl alcohol administered in the diet to rats on fertility, early embryonic development, growth and development. Food and chemical toxicology 2003, 41, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.; DeMerlis, C.; Schoneker, D.; Borzelleca, J. Subchronic toxicity study in rats and genotoxicity tests with polyvinyl alcohol. Food and chemical toxicology 2003, 41, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Tian, J.; Chen, L.; He, S.; Wang, J. Improvement of biodegradability of PVA-containing wastewater by ionizing radiation pretreatment. Environmental Science and Pollution Research 2012, 19, 3178–3184. [Google Scholar] [CrossRef]
- Chou, W.-L.; Wang, C.-T.; Huang, K.-Y. Investigation of process parameters for the removal of polyvinyl alcohol from aqueous solution by iron electrocoagulation. Desalination 2010, 251, 12–19. [Google Scholar] [CrossRef]
- Jabbari, E.; Khakpour, M. Morphology of and release behavior from porous polyurethane microspheres. Biomaterials 2000, 21, 2073–2079. [Google Scholar] [CrossRef]
- Kääriä, K.; Hirvonen, A.; Norppa, H.; Piirilä, P.; Vainio, H.; Rosenberg, C. Exposure to 4, 4′-methylenediphenyl diisocyanate (MDI) during moulding of rigid polyurethane foam: determination of airborne MDI and urinary 4, 4′-methylenedianiline (MDA). Analyst 2001, 126, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Wei, K.; Zhao, N.; Zhang, S.; Chen, J. Drug distribution within poly (ɛ-caprolactone) microspheres and in vitro release. Journal of Materials Processing Technology 2009, 209, 348–354. [Google Scholar] [CrossRef]
- Thompson, D.G.; Osborn, J.C.; Kober, E.M.; Schoonover, J.R. Effects of hydrolysis-induced molecular weight changes on the phase separation of a polyester polyurethane. Polymer degradation and stability 2006, 91, 3360–3370. [Google Scholar] [CrossRef]
- Kaur, M.; Gupta, K.M.; Poursaid, A.E.; Karra, P.; Mahalingam, A.; Aliyar, H.A.; Kiser, P.F. Engineering a degradable polyurethane intravaginal ring for sustained delivery of dapivirine. Drug delivery and translational research 2011, 1, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Christenson, E.M.; Dadsetan, M.; Wiggins, M.; Anderson, J.M.; Hiltner, A. Poly (carbonate urethane) and poly (ether urethane) biodegradation: in vivo studies. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials 2004, 69, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Rychlý, J.; Lattuati-Derieux, A.; Lavédrine, B.; Matisová-Rychlá, L.; Malíková, M.; Csomorová, K.; Janigová, I. Assessing the progress of degradation in polyurethanes by chemiluminescence and thermal analysis. II. Flexible polyether-and polyester-type polyurethane foams. Polymer degradation and stability 2011, 96, 462–469. [Google Scholar] [CrossRef]
- Arifin, D.Y.; Lee, L.Y.; Wang, C.-H. Mathematical modeling and simulation of drug release from microspheres: Implications to drug delivery systems. Advanced drug delivery reviews 2006, 58, 1274–1325. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.J.; Gupta, K.M.; Fabian, J.; Albright, T.H.; Kiser, P.F. Segmented polyurethane intravaginal rings for the sustained combined delivery of antiretroviral agents dapivirine and tenofovir. European Journal of Pharmaceutical Sciences 2010, 39, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. Journal of controlled release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. International journal of pharmaceutics 2008, 364, 328–343. [Google Scholar] [CrossRef]
- Storey, R.F.; Hickey, T.P. Degradable polyurethane networks based on d, l-lactide, glycolide, ε-caprolactone, and trimethylene carbonate homopolyester and copolyester triols. Polymer 1994, 35, 830–838. [Google Scholar] [CrossRef]
- Guelcher, S.; Srinivasan, A.; Hafeman, A.; Gallagher, K.; Doctor, J.; Khetan, S.; McBride, S.; Hollinger, J. Synthesis, in vitro biocompatibility and biodegradation, and mechanical properties of two-component polyurethane scaffolds: effects of water and polyol composition. Tissue Eng 2007, 13, 2321–2333. [Google Scholar] [CrossRef]
- Labow, R.S.; Erfle, D.J.; Santerre, J.P. Elastase-induced hydrolysis of synthetic solid substrates: poly (ester-urea-urethane) and poly (ether-urea-urethane). Biomaterials 1996, 17, 2381–2388. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical Applications of Biodegradable Polymers. J Polym Sci B Polym Phys 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Feng, P.; Jia, J.; Liu, M.; Peng, S.; Zhao, Z.; Shuai, C. Degradation mechanisms and acceleration strategies of poly (lactic acid) scaffold for bone regeneration. Materials & Design 2021, 210, 110066. [Google Scholar] [CrossRef]
- Kuppermann, B.D.; Patel, S.S.; Boyer, D.S.; Augustin, A.J.; Freeman, W.R.; Kerr, K.J.; Guo, Q.; Schneider, S.; López, F.J. Phase 2 Stusy of the Safety and Efficacy of Brimonidine Drug Delivery System (BRIMO DDS) Generation 1 in Patients with Geographic Atrophy Secondary to Age-related Macular Degeneration. Retina 2021, 41, 144–155. [Google Scholar] [CrossRef]
- Pişkin, E. Biodegradable polymers as biomaterials. J Biomater Sci Polym Ed 1995, 6, 775–795. [Google Scholar] [CrossRef] [PubMed]
- Pisecky, L.; Luger, M.; Klasan, A.; Gotterbarm, T.; Klotz, M.C.; Hochgatterer, R. Bioabsorbable implants in forefoot surgery: a review of materials, possibilities and disadvantages. EFORT Open Rev 2021, 6, 1132–1139. [Google Scholar] [CrossRef]
- Wan, B.; Bao, Q.; Burgess, D. Long-acting PLGA microspheres: Advances in excipient and product analysis toward improved product understanding. Advanced Drug Delivery Reviews 2023, 198, 114857. [Google Scholar] [CrossRef] [PubMed]
- Blasi, P.; D'Souza, S.S.; Selmin, F.; DeLuca, P.P. Plasticizing effect of water on poly (lactide-co-glycolide). Journal of Controlled Release 2005, 108, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Rustgi, R. Biodegradable polymers. Progress in polymer science 1998, 23, 1273–1335. [Google Scholar] [CrossRef]
- Cayuela, J.; Bounor-Legaré, V.; Cassagnau, P.; Michel, A. Ring-opening polymerization of ε-caprolactone initiated with titanium n-propoxide or titanium phenoxide. Macromolecules 2006, 39, 1338–1346. [Google Scholar] [CrossRef]
- Natta, F.J.v.; Hill, J.W.; Carothers, W.H. Studies of polymerization and ring formation. XXIII. 1 ε-Caprolactone and its polymers. Journal of the American Chemical Society 1934, 56, 455–457. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Progress in polymer science 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Sinha, V.; Bansal, K.; Kaushik, R.; Kumria, R.; Trehan, A. Poly-ϵ-caprolactone microspheres and nanospheres: an overview. International journal of pharmaceutics 2004, 278, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Lu, X.; Dalai, S.; Zhang, J. Thermophysical properties of polycaprolactone/chitosan blend membranes. Thermochimica Acta 2009, 487, 33–38. [Google Scholar] [CrossRef]
- Perez, M.H.; Zinutti, C.; Lamprecht, A.; Ubrich, N.; Astier, A.; Hoffman, M.; Bodmeier, R.; Maincent, P. The preparation and evaluation of poly (ϵ-caprolactone) microparticles containing both a lipophilic and a hydrophilic drug. Journal of controlled release 2000, 65, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Gorna, K.; Gogolewski, S. In vitro degradation of novel medical biodegradable aliphatic polyurethanes based on ϵ-caprolactone and Pluronics® with various hydrophilicities. Polymer Degradation and Stability 2002, 75, 113–122. [Google Scholar] [CrossRef]
- Ren, D.; Yi, H.; Wang, W.; Ma, X. The enzymatic degradation and swelling properties of chitosan matrices with different degrees of N-acetylation. Carbohydrate Research 2005, 340, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. Reactive and Functional Polymers 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Fonte, P.; Araújo, F.; Silva, C.; Pereira, C.; Reis, S.; Santos, H.A.; Sarmento, B. Polymer-based nanoparticles for oral insulin delivery: Revisited approaches. Biotechnology advances 2015, 33, 1342–1354. [Google Scholar] [CrossRef]
- Li, P.; Fujimoto, K.; Bourguingnon, L.; Yukl, S.; Deeks, S.; Wong, J.K. Exogenous and endogenous hyaluronic acid reduces HIV infection of CD4(+) T cells. Immunol Cell Biol 2014, 92, 770–780. [Google Scholar] [CrossRef]
- Agrahari, V.; Meng, J.; Ezoulin, M.J.; Youm, I.; Dim, D.C.; Molteni, A.; Hung, W.T.; Christenson, L.K.; Youan, B.C. Stimuli-sensitive thiolated hyaluronic acid based nanofibers: synthesis, preclinical safety and in vitro anti-HIV activity. Nanomedicine (Lond) 2016, 11, 2935–2958. [Google Scholar] [CrossRef]
- Jouyban, A.; Fakhree, M.A.A.; Shayanfar, A. Review of pharmaceutical applications of N-methyl-2-pyrrolidone. Journal of Pharmacy & Pharmaceutical Sciences 2010, 13, 524–535. [Google Scholar]
- Guide, M. Highlights of Prescribing Information.
- Rivera-Briso, A.L.; Serrano-Aroca, Á. Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate): Enhancement Strategies for Advanced Applications. Polymers (Basel) 2018, 10. [Google Scholar] [CrossRef]
- Avella, M.; Martuscelli, E.; Raimo, M. Review Properties of blends and composites based on poly (3-hydroxy) butyrate (PHB) and poly (3-hydroxybutyrate-hydroxyvalerate)(PHBV) copolymers. Journal of materials science 2000, 35, 523–545. [Google Scholar] [CrossRef]
- Mofokeng, J.; Luyt, A. Morphology and thermal degradation studies of melt-mixed poly (hydroxybutyrate-co-valerate)(PHBV)/poly (ε-caprolactone)(PCL) biodegradable polymer blend nanocomposites with TiO 2 as filler. Journal of Materials Science 2015, 50, 3812–3824. [Google Scholar] [CrossRef]
- Hedayati, A.; Yazdi, S.G.; Dehghankelishadi, P.; Javan, N.B.; Akbari, H.; Dorkoosh, F.A. Preparation, Optimization and Physicochemical Characterization of Aripiprazole Loaded Nano-porous in situ Forming Implant. Pharm Nanotechnol 2017, 5, 138–147. [Google Scholar] [CrossRef]
- Acemoglu, M.; Nimmerfall, F.; Bantle, S.; Stoll, G.H. Poly(ethylene carbonate)s, part I: Syntheses and structural effects on biodegradation. Journal of Controlled Release 1997, 49, 263–276. [Google Scholar] [CrossRef]
- Sasanuma, Y.; Takahashi, Y. Structure–Property Relationships of Poly(ethylene carbonate) and Poly(propylene carbonate). ACS Omega 2017, 2, 4808–4819. [Google Scholar] [CrossRef] [PubMed]
- Stoll, G.H.; Nimmerfall, F.; Acemoglu, M.; Bodmer, D.; Bantle, S.; Müller, I.; Mahl, A.; Kolopp, M.; Tullberg, K. Poly(ethylene carbonate)s, part II: degradation mechanisms and parenteral delivery of bioactive agents. J Control Release 2001, 76, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kemmer, A.; Keim, K.; Curdy, C.; Petersen, H.; Kissel, T. Poly(ethylene carbonate) as a surface-eroding biomaterial for in situ forming parenteral drug delivery systems: A feasibility study. European Journal of Pharmaceutics and Biopharmaceutics 2010, 76, 222–229. [Google Scholar] [CrossRef]
- Panja, S.; Maji, S.; Maiti, T.K.; Chattopadhyay, S. A Smart Magnetically Active Nanovehicle for on-Demand Targeted Drug Delivery: Where van der Waals Force Balances the Magnetic Interaction. ACS Applied Materials & Interfaces 2015, 7, 24229–24241. [Google Scholar] [CrossRef]
- Panja, S.; Dey, G.; Bharti, R.; Mandal, P.; Mandal, M.; Chattopadhyay, S. Metal Ion Ornamented Ultrafast Light-Sensitive Nanogel for Potential in Vivo Cancer Therapy. Chemistry of Materials 2016, 28, 8598–8610. [Google Scholar] [CrossRef]
- Panja, S.; Dey, G.; Bharti, R.; Kumari, K.; Maiti, T.K.; Mandal, M.; Chattopadhyay, S. Tailor-Made Temperature-Sensitive Micelle for Targeted and On-Demand Release of Anticancer Drugs. ACS Applied Materials & Interfaces 2016, 8, 12063–12074. [Google Scholar] [CrossRef]
- Lan, T.; Guo, Q. Phenylboronic acid-decorated polymeric nanomaterials for advanced bio-application. Nanotechnology Reviews 2019, 8, 548–561. [Google Scholar] [CrossRef]
- Feng, R.; Zhu, L.; Teng, F.; Wang, M.; Chen, S.; Song, Z.; Li, H. Phenylboronic acid-modified polymaleic anhydride-F127 micelles for pH-activated targeting delivery of doxorubicin. Colloids and Surfaces B: Biointerfaces 2022, 216, 112559. [Google Scholar] [CrossRef]
- Mahalingam, A.; Jay, J.I.; Langheinrich, K.; Shukair, S.; McRaven, M.D.; Rohan, L.C.; Herold, B.C.; Hope, T.J.; Kiser, P.F. Inhibition of the transport of HIV in vitro using a pH-responsive synthetic mucin-like polymer system. Biomaterials 2011, 32, 8343–8355. [Google Scholar] [CrossRef]
- Jay, J.I.; Shukair, S.; Langheinrich, K.; Hanson, M.C.; Cianci, G.C.; Johnson, T.J.; Clark, M.R.; Hope, T.J.; Kiser, P.F. Modulation of Viscoelasticity and HIV Transport as a Function of pH in a Reversibly Crosslinked Hydrogel. Adv Funct Mater 2009, 19, 2969–2977. [Google Scholar] [CrossRef]
- Folkman, J.; Long, D.M. The use of silicone rubber as a carrier for prolonged drug therapy. Journal of Surgical Research 1964, 4, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, E.; Johengen, D.; Luecke, E.; Rothrock, G.; McGowan, I.; van der Straten, A.; Desai, T. A tunable, biodegradable, thin-film polymer device as a long-acting implant delivering tenofovir alafenamide fumarate for HIV pre-exposure prophylaxis. Pharmaceutical research 2016, 33, 1649–1656. [Google Scholar] [CrossRef]
- Li, L.; Johnson, L.M.; Krovi, S.A.; Demkovich, Z.R.; van der Straten, A. Performance and stability of tenofovir alafenamide formulations within subcutaneous biodegradable implants for HIV pre-exposure prophylaxis (PrEP). Pharmaceutics 2020, 12, 1057. [Google Scholar] [CrossRef]
- Su, J.T.; Simpson, S.M.; Sung, S.; Tfaily, E.B.; Veazey, R.; Marzinke, M.; Qiu, J.; Watrous, D.; Widanapathirana, L.; Pearson, E. A subcutaneous implant of tenofovir alafenamide fumarate causes local inflammation and tissue necrosis in rabbits and macaques. Antimicrobial agents and chemotherapy 2020, 64, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Joiner, J.B.; Prasher, A.; Young, I.C.; Kim, J.; Shrivastava, R.; Maturavongsadit, P.; Benhabbour, S.R. Effects of Drug Physicochemical Properties on In-Situ Forming Implant Polymer Degradation and Drug Release Kinetics. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Romano, J.W.; Baum, M.M.; Demkovich, Z.R.; Diana, F.; Dobard, C.; Feldman, P.L.; Garcia-Lerma, J.G.; Grattoni, A.; Gunawardana, M.; Ho, D.K.; et al. Tenofovir Alafenamide for HIV Prevention: Review of the Proceedings from the Gates Foundation Long-Acting TAF Product Development Meeting. AIDS Res Hum Retroviruses 2021, 37, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Ullah Nayan, M.; Sillman, B.; Hasan, M.; Deodhar, S.; Das, S.; Sultana, A.; Thai Hoang Le, N.; Soriano, V.; Edagwa, B.; Gendelman, H.E. Advances in long-acting slow effective release antiretroviral therapies for treatment and prevention of HIV infection. Advanced Drug Delivery Reviews 2023, 200, 115009. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, D.; Simpson, S.M.; Su, J.T.; Bryndza-Tfaily, E.; Hope, T.J.; Veazey, R.; Dobek, G.; Qiu, J.; Watrous, D.; Sung, S.; et al. Design and Testing of a Cabotegravir Implant for HIV Prevention. J Control Release 2021, 330, 658–668. [Google Scholar] [CrossRef]
- Nevoralová, M.; Koutný, M.; Ujčić, A.; Starý, Z.; Šerá, J.; Vlková, H.; Šlouf, M.; Fortelný, I.; Kruliš, Z. Structure Characterization and Biodegradation Rate of Poly(ε-caprolactone)/Starch Blends. Frontiers in Materials 2020, 7. [Google Scholar] [CrossRef]
- Markowitz, M.; Sarafianos, S.G. EFdA (4′-ethynyl-2-fluoro-2′-deoxyadenosine, MK-8591): a novel HIV-1 reverse transcriptase translocation inhibitor. Current opinion in HIV and AIDS 2018, 13, 294. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Burgess, D.J. Drug release from in situ forming implants and advances in release testing. Adv Drug Deliv Rev 2021, 178, 113912. [Google Scholar] [CrossRef]
- Phaechamud, T.; Senarat, S.; Puyathorn, N.; Praphanwittaya, P. Solvent exchange and drug release characteristics of doxycycline hyclate-loaded bleached shellac in situ-forming gel and-microparticle. International journal of biological macromolecules 2019, 135, 1261–1272. [Google Scholar] [CrossRef]
- Amini-Fazl, M.S. Biodegradation study of PLGA as an injectable in situ depot-forming implant for controlled release of paclitaxel. Polymer Bulletin 2021, 1–14. [Google Scholar] [CrossRef]
- Kempe, S.; Mäder, K. In situ forming implants—an attractive formulation principle for parenteral depot formulations. Journal of controlled release 2012, 161, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, N.; Sinha, V.R. In situ forming depot as sustained-release drug delivery systems. Critical Reviews™ in Therapeutic Drug Carrier Systems 2019, 36. [Google Scholar] [CrossRef]
- Yu, L.; Ding, J. Injectable hydrogels as unique biomedical materials. Chemical Society Reviews 2008, 37, 1473–1481. [Google Scholar] [CrossRef]
- Ibrahim, T.M.; Ayoub, M.M.; El-Bassossy, H.M.; El-Nahas, H.M.; Gomaa, E. Investigation of Alogliptin-Loaded In Situ Gel Implants by 23 Factorial Design with Glycemic Assessment in Rats. Pharmaceutics 2022, 14, 1867. [Google Scholar] [CrossRef]
- Wex, J.; Sidhu, M.; Odeyemi, I.; Abou-Setta, A.M.; Retsa, P.; Tombal, B. Leuprolide acetate 1-, 3-and 6-monthly depot formulations in androgen deprivation therapy for prostate cancer in nine European countries: evidence review and economic evaluation. ClinicoEconomics and Outcomes Research 2013, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Johnson, C.E.; Schmalstig, A.A.; Annis, A.; Wessel, S.E.; Van Horn, B.; Schauer, A.; Exner, A.A.; Stout, J.E.; Wahl, A. A long-acting formulation of rifabutin is effective for prevention and treatment of Mycobacterium tuberculosis. Nature communications 2022, 13, 4455. [Google Scholar] [CrossRef]
- Cox, M.C.; Scripture, C.D.; Figg, W.D. Leuprolide acetate given by a subcutaneous extended-release injection: less of a pain? Expert review of anticancer therapy 2005, 5, 605–611. [Google Scholar] [CrossRef]
- Agarwal, P.; Rupenthal, I.D. Injectable implants for the sustained release of protein and peptide drugs. Drug Discov Today 2013, 18, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Packhaeuser, C.; Schnieders, J.; Oster, C.; Kissel, T. In situ forming parenteral drug delivery systems: an overview. European Journal of Pharmaceutics and Biopharmaceutics 2004, 58, 445–455. [Google Scholar] [CrossRef]
- Griffin, J.B.; Ridgeway, K.; Montgomery, E.; Torjesen, K.; Clark, R.; Peterson, J.; Baggaley, R.; van der Straten, A. Vaginal ring acceptability and related preferences among women in low-and middle-income countries: a systematic review and narrative synthesis. PloS one 2019, 14, e0224898. [Google Scholar] [CrossRef]
- Macht, D.I. On the absorption of drugs and poisons through the vagina. Journal of Pharmacology and Experimental Therapeutics 1918, 10, 509–522. [Google Scholar]
- Malcolm, R.K.; Boyd, P.J.; McCoy, C.F.; Murphy, D.J. Microbicide vaginal rings: Technological challenges and clinical development. Advanced drug delivery reviews 2016, 103, 33–56. [Google Scholar] [CrossRef]
- McConville, C.; Andrews, G.P.; Laverty, T.P.; Woolfson, A.D.; Malcolm, R.K. Rheological evaluation of the isothermal cure characteristics of medical grade silicone elastomers. Journal of applied polymer science 2010, 116, 2320–2327. [Google Scholar] [CrossRef]
- Monteiro, I.; Guazzelli, C.F.; Bahamondes, L. Advances in contraceptive vaginal rings: What does the future hold? Expert Opinion on Pharmacotherapy 2018, 19, 1685–1691. [Google Scholar] [CrossRef]
- Speroff, L.; Group, U.S.V.I. Efficacy and tolerability of a novel estradiol vaginal ring for relief of menopausal symptoms. Obstetrics & Gynecology 2003, 102, 823–834. [Google Scholar]
- Woolfson, A.; Malcolm, R.; Gallagher, R. Design of a silicone reservoir intravaginal ring for the delivery of oxybutynin. Journal of controlled release 2003, 91, 465–476. [Google Scholar] [CrossRef]
- Leonhard, M.; Moser, D.; Reumueller, A.; Mancusi, G.; Bigenzahn, W.; Schneider-Stickler, B. Comparison of biofilm formation on new phonax and provox 2 voice prostheses—a pilot study. Head & neck 2010, 32, 886–895. [Google Scholar]
- MacCallum, N.; Howell, C.; Kim, P.; Sun, D.; Friedlander, R.; Ranisau, J.; Ahanotu, O.; Lin, J.J.; Vena, A.; Hatton, B. Liquid-infused silicone as a biofouling-free medical material. ACS Biomaterials Science & Engineering 2015, 1, 43–51. [Google Scholar]
- Fundeanu, I.; Klee, D.; Schouten, A.J.; Busscher, H.J.; Van der Mei, H.C. Solvent-free functionalization of silicone rubber and efficacy of PAAm brushes grafted from an amino-PPX layer against bacterial adhesion. Acta biomaterialia 2010, 6, 4271–4276. [Google Scholar] [CrossRef]
- Rodrigues, L.; Van der Mei, H.; Teixeira, J.A.; Oliveira, R. Biosurfactant from Lactococcus lactis 53 inhibits microbial adhesion on silicone rubber. Applied microbiology and biotechnology 2004, 66, 306–311. [Google Scholar] [CrossRef]
- Everaert, E.; Belt-Gritter, B.V.D.; Van der Mei, H.; Busscher, H.; Verkerke, G.; Dijk, F.; Mahieu, H.; Reitsma, A. In vitro and in vivo microbial adhesion and growth on argon plasma-treated silicone rubber voice prostheses. Journal of Materials Science: Materials in Medicine 1998, 9, 147–157. [Google Scholar]
- Everaert, E.P.; Mahieu, H.F.; van de Belt-Gritter, B.; Peeters, A.J.G.; Verkerke, G.J.; van der Mei, H.C.; Busscher, H.J. Biofilm formation in vivo on perfluoro-alkylsiloxane–modified voice prostheses. Archives of Otolaryngology–Head & Neck Surgery 1999, 125, 1329–1332. [Google Scholar]
- Traore, Y.L.; Chen, Y.; Bernier, A.-M.; Ho, E.A. Impact of hydroxychloroquine-loaded polyurethane intravaginal rings on lactobacilli. Antimicrobial Agents and Chemotherapy 2015, 59, 7680–7686. [Google Scholar] [CrossRef] [PubMed]
- Roddy, R.E.; Zekeng, L.; Ryan, K.A.; Tamoufe, U.; Weir, S.S.; Wong, E.L. A controlled trial of nonoxynol 9 film to reduce male-to-female transmission of sexually transmitted diseases. New England Journal of Medicine 1998, 339, 504–510. [Google Scholar] [CrossRef]
- Malcolm, R.K.; Woolfson, A.D.; Toner, C.F.; Morrow, R.J.; McCullagh, S.D. Long-term, controlled release of the HIV microbicide TMC120 from silicone elastomer vaginal rings. Journal of Antimicrobial Chemotherapy 2005, 56, 954–956. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Smythe, S.; Young, K.; Malcolm, K.; McCoy, C.; Rosenberg, Z.; Romano, J. Safety and Pharmacokinetics of Dapivirine Delivery From Matrix and Reservoir Intravaginal Rings to HIV-Negative Women. JAIDS Journal of Acquired Immune Deficiency Syndromes 2009, 51, 416–423. [Google Scholar] [CrossRef]
- Nel, A.; Bekker, L.-G.; Bukusi, E.; Hellstrӧm, E.; Kotze, P.; Louw, C.; Martinson, F.; Masenga, G.; Montgomery, E.; Ndaba, N. Safety, acceptability and adherence of dapivirine vaginal ring in a microbicide clinical trial conducted in multiple countries in Sub-Saharan Africa. PloS one 2016, 11, e0147743. [Google Scholar] [CrossRef] [PubMed]
- Baeten, J.M.; Palanee-Phillips, T.; Brown, E.R.; Schwartz, K.; Soto-Torres, L.E.; Govender, V.; Mgodi, N.M.; Matovu Kiweewa, F.; Nair, G.; Mhlanga, F. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. New England Journal of Medicine 2016, 375, 2121–2132. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.J.; Clark, M.R.; Albright, T.H.; Nebeker, J.S.; Tuitupou, A.L.; Clark, J.T.; Fabian, J.; McCabe, R.T.; Chandra, N.; Doncel, G.F.; et al. A 90-day tenofovir reservoir intravaginal ring for mucosal HIV prophylaxis. Antimicrob Agents Chemother 2012, 56, 6272–6283. [Google Scholar] [CrossRef]
- Mesquita, P.M.; Rastogi, R.; Segarra, T.J.; Teller, R.S.; Torres, N.M.; Huber, A.M.; Kiser, P.F.; Herold, B.C. Intravaginal ring delivery of tenofovir disoproxil fumarate for prevention of HIV and herpes simplex virus infection. Journal of antimicrobial chemotherapy 2012, 67, 1730–1738. [Google Scholar] [CrossRef]
- Smith, J.M.; Rastogi, R.; Teller, R.S.; Srinivasan, P.; Mesquita, P.M.; Nagaraja, U.; McNicholl, J.M.; Hendry, R.M.; Dinh, C.T.; Martin, A.; et al. Intravaginal ring eluting tenofovir disoproxil fumarate completely protects macaques from multiple vaginal simian-HIV challenges. Proc Natl Acad Sci U S A 2013, 110, 16145–16150. [Google Scholar] [CrossRef]
- Su, J.T.; Teller, R.S.; Srinivasan, P.; Zhang, J.; Martin, A.; Sung, S.; Smith, J.M.; Kiser, P.F. A Dose Ranging Pharmacokinetic Evaluation of IQP-0528 Released from Intravaginal Rings in Non-Human Primates. Pharm Res 2017, 34, 2163–2171. [Google Scholar] [CrossRef] [PubMed]
- Quinn, H.L.; Kearney, M.-C.; Courtenay, A.J.; McCrudden, M.T.; Donnelly, R.F. The role of microneedles for drug and vaccine delivery. Expert opinion on drug delivery 2014, 11, 1769–1780. [Google Scholar] [CrossRef]
- Permana, A.D.; McCrudden, M.T.; Donnelly, R.F. Enhanced intradermal delivery of nanosuspensions of antifilariasis drugs using dissolving microneedles: A proof of concept study. Pharmaceutics 2019, 11, 346. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.J.; Kaestner, S.A.; Sutter, D.E.; Harvey, N.G.; Mikszta, J.A.; Pettis, R.J. Microneedle-based intradermal delivery enables rapid lymphatic uptake and distribution of protein drugs. Pharmaceutical research 2011, 28, 107–116. [Google Scholar] [CrossRef]
- Moga, K.A.; Bickford, L.R.; Geil, R.D.; Dunn, S.S.; Pandya, A.A.; Wang, Y.; Fain, J.H.; Archuleta, C.F.; O'Neill, A.T.; DeSimone, J.M. Rapidly–dissolvable microneedle patches via a highly scalable and reproducible soft lithography approach. Advanced Materials 2013, 25, 5060–5066. [Google Scholar] [CrossRef]
- Römgens, A.; Bader, D.; Bouwstra, J.; Baaijens, F.; Oomens, C. Monitoring the penetration process of single microneedles with varying tip diameters. journal of the mechanical behavior of biomedical materials 2014, 40, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.; Prausnitz, M. Effect of microneedle design on pain in human subjects. clin. J. Pain 2008, 24, 585–594. [Google Scholar]
- Moffatt, K.; Quinn, C.; McCague, P.J.; Donnelly, R.F. Exploration into the opinions of patients with HIV, healthcare professionals and the lay public of the use of microneedles in clinical practice: highlighting the translational potential for their role in HIV infection. Drug Delivery and Translational Research 2021, 11, 1199–1217. [Google Scholar] [CrossRef]
- Chen, M.; Quan, G.; Sun, Y.; Yang, D.; Pan, X.; Wu, C. Nanoparticles-encapsulated polymeric microneedles for transdermal drug delivery. Journal of Controlled Release 2020, 325, 163–175. [Google Scholar] [CrossRef]
- Xie, L.; Zeng, H.; Sun, J.; Qian, W. Engineering microneedles for therapy and diagnosis: A survey. Micromachines 2020, 11, 271. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, J.-H.; Prausnitz, M.R. Dissolving microneedles for transdermal drug delivery. Biomaterials 2008, 29, 2113–2124. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Hirono, M.; Fukushima, K.; Sugioka, N.; Takada, K. Two-layered dissolving microneedles formulated with intermediate-acting insulin. International journal of pharmaceutics 2012, 436, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.Y.; Choi, S.-O.; Prausnitz, M.R. Fabrication of dissolving polymer microneedles for controlled drug encapsulation and delivery: bubble and pedestal microneedle designs. Journal of pharmaceutical sciences 2010, 99, 4228–4238. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, C.; Yan, L.; Huang, L.; Zhu, X.; Chen, B.; Sant, H.J.; Niu, X.; Zhu, G.; Yu, K. Improved polyvinylpyrrolidone microneedle arrays with non-stoichiometric cyclodextrin. Journal of Materials Chemistry B 2014, 2, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Vora, L.K.; Vavia, P.R.; Larrañeta, E.; Bell, S.E.; Donnelly, R.F. Novel nanosuspension-based dissolving microneedle arrays for transdermal delivery of a hydrophobic drug. Journal of interdisciplinary nanomedicine 2018, 3, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Allen, M.G.; Prausnitz, M.R. Polymer microneedles for controlled-release drug delivery. Pharmaceutical research 2006, 23, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, S.; Tekko, I.A.; Vora, L.; Larrañeta, E.; Permana, A.D.; Donnelly, R.F. Nanosuspension-based dissolving microneedle arrays for intradermal delivery of curcumin. Pharmaceutics 2019, 11, 308. [Google Scholar] [CrossRef]
- Li, W.; Terry, R.N.; Tang, J.; Feng, M.R.; Schwendeman, S.P.; Prausnitz, M.R. Rapidly separable microneedle patch for the sustained release of a contraceptive. Nature Biomedical Engineering 2019, 3, 220–229. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, S.; Yang, G.; Zhou, Z.; Gao, Y. Exploring Trehalose on the release of levonorgestrel from implantable PLGA microneedles. Polymers 2020, 12, 59. [Google Scholar] [CrossRef]
- He, M.; Yang, G.; Zhao, X.; Zhang, S.; Gao, Y. Intradermal implantable PLGA microneedles for etonogestrel sustained release. Journal of Pharmaceutical Sciences 2020, 109, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Than, A.; Liang, K.; Xu, S.; Sun, L.; Duan, H.; Xi, F.; Xu, C.; Chen, P. Transdermal delivery of anti-obesity compounds to subcutaneous adipose tissue with polymeric microneedle patches. Small Methods 2017, 1, 1700269. [Google Scholar] [CrossRef]
- Chen, M.-C.; Wang, K.-W.; Chen, D.-H.; Ling, M.-H.; Liu, C.-Y. Remotely triggered release of small molecules from LaB6@ SiO2-loaded polycaprolactone microneedles. Acta biomaterialia 2015, 13, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Mc Crudden, M.T.C.; Larrañeta, E.; Clark, A.; Jarrahian, C.; Rein-Weston, A.; Creelman, B.; Moyo, Y.; Lachau-Durand, S.; Niemeijer, N.; Williams, P.; et al. Design, Formulation, and Evaluation of Novel Dissolving Microarray Patches Containing Rilpivirine for Intravaginal Delivery. Advanced Healthcare Materials 2019, 8, 1801510. [Google Scholar] [CrossRef] [PubMed]
- Abbate, M.T.A.; Ramöller, I.K.; Sabri, A.H.; Paredes, A.J.; Hutton, A.J.; McKenna, P.E.; Peng, K.; Hollett, J.A.; McCarthy, H.O.; Donnelly, R.F. Formulation of antiretroviral nanocrystals and development into a microneedle delivery system for potential treatment of HIV-associated neurocognitive disorder (HAND). International Journal of Pharmaceutics 2023, 640, 123005. [Google Scholar] [CrossRef]
- Zhang, C.; Vora, L.K.; Tekko, I.A.; Volpe-Zanutto, F.; Peng, K.; Paredes, A.J.; McCarthy, H.O.; Donnelly, R.F. Development of dissolving microneedles for intradermal delivery of the long-acting antiretroviral drug bictegravir. Int J Pharm 2023, 642, 123108. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; McCrudden, M.T.; Zaid Alkilani, A.; Larrañeta, E.; McAlister, E.; Courtenay, A.J.; Kearney, M.-C.; Singh, T.R.R.; McCarthy, H.O.; Kett, V.L. Hydrogel-forming microneedles prepared from “super swelling” polymers combined with lyophilised wafers for transdermal drug delivery. PloS one 2014, 9, e111547. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, L.; Xu, C. Recent advances in the design of polymeric microneedles for transdermal drug delivery and biosensing. Lab on a Chip 2017, 17, 1373–1387. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Lin, C.-C.; Metters, A.T. Hydrogels in controlled release formulations: network design and mathematical modeling. Advanced drug delivery reviews 2006, 58, 1379–1408. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Singh, T.R.R.; Garland, M.J.; Migalska, K.; Majithiya, R.; McCrudden, C.M.; Kole, P.L.; Mahmood, T.M.T.; McCarthy, H.O.; Woolfson, A.D. Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery. Advanced functional materials 2012, 22, 4879–4890. [Google Scholar] [CrossRef] [PubMed]
- Blasi, P. Poly (lactic acid)/poly (lactic-co-glycolic acid)-based microparticles: An overview. Journal of Pharmaceutical Investigation 2019, 49, 337–346. [Google Scholar] [CrossRef]
- Abdelkader, H.; Fathalla, Z.; Seyfoddin, A.; Farahani, M.; Thrimawithana, T.; Allahham, A.; Alani, A.W.G.; Al-Kinani, A.A.; Alany, R.G. Polymeric long-acting drug delivery systems (LADDS) for treatment of chronic diseases: Inserts, patches, wafers, and implants. Advanced Drug Delivery Reviews 2021, 177, 113957. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K. Functional coatings and microencapsulation: a general perspective. Functional Coatings: by polymer microencapsulation, 2006; 1–28. [Google Scholar]
- Yeo, Y.; Baek, N.; Park, K. Microencapsulation methods for delivery of protein drugs. Biotechnology and Bioprocess Engineering 2001, 6, 213–230. [Google Scholar] [CrossRef]
- Tomaro-Duchesneau, C.; Saha, S.; Malhotra, M.; Kahouli, I.; Prakash, S. Microencapsulation for the therapeutic delivery of drugs, live mammalian and bacterial cells, and other biopharmaceutics: current status and future directions. Journal of pharmaceutics 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, N.V.N.; Prasanna, P.M.; Sakarkar, S.N.; Prabha, K.S.; Ramaiah, P.S.; Srawan, G. Microencapsulation techniques, factors influencing encapsulation efficiency. Journal of microencapsulation 2010, 27, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Bansode, S.; Banarjee, S.; Gaikwad, D.; Jadhav, S.; Thorat, R. Microencapsulation: a review. International journal of pharmaceutical sciences review and research 2010, 1, 38–43. [Google Scholar]
- Dubey, R. Microencapsulation technology and applications. Defence Science Journal 2009, 59, 82. [Google Scholar]
- Sharifi, F.; Otte, A.; Yoon, G.; Park, K. Continuous in-line homogenization process for scale-up production of naltrexone-loaded PLGA microparticles. Journal of Controlled Release 2020, 325, 347–358. [Google Scholar] [CrossRef]
- Chereddy, K.K.; Vandermeulen, G.; Préat, V. PLGA based drug delivery systems: Promising carriers for wound healing activity. Wound Repair and Regeneration 2016, 24, 223–236. [Google Scholar] [CrossRef]
- Ramazani, F.; Chen, W.; van Nostrum, C.F.; Storm, G.; Kiessling, F.; Lammers, T.; Hennink, W.E.; Kok, R.J. Strategies for encapsulation of small hydrophilic and amphiphilic drugs in PLGA microspheres: state-of-the-art and challenges. International journal of pharmaceutics 2016, 499, 358–367. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, H.J.; Ji, H.; Cho, S.H.; Cho, E.-H.; Han, H.D.; Shin, B.C. Marbofloxacin-encapsulated microparticles provide sustained drug release for treatment of veterinary diseases. Materials Science and Engineering: C 2016, 60, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Shahjin, F.; Patel, M.; Machhi, J.; Cohen, J.D.; Nayan, M.U.; Yeapuri, P.; Zhang, C.; Waight, E.; Hasan, M.; Abdelmoaty, M.M.; et al. Multipolymer microsphere delivery of SARS-CoV-2 antigens. Acta Biomaterialia 2023, 158, 493–509. [Google Scholar] [CrossRef]
- van de Weert, M.; Hennink, W.E.; Jiskoot, W. Protein instability in poly (lactic-co-glycolic acid) microparticles. Pharmaceutical research 2000, 17, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, L.I.; Gracia, I.; de Lucas, A.; Rodríguez, J.F. Novel model for the description of the controlled release of 5-fluorouracil from PLGA and PLA foamed scaffolds impregnated in supercritical CO2. Industrial & Engineering Chemistry Research 2014, 53, 15374–15382. [Google Scholar]
- Khuroo, T.; Dharani, S.; Mohamed, E.M.; Immadi, S.; Wu, Z.; Khan, M.A.; Lu, D.; Nehete, P.; Rahman, Z. Ultra-long acting prodrug of dolutegravir and delivery system – Physicochemical, pharmacokinetic and formulation characterizations. International Journal of Pharmaceutics 2021, 607, 120889. [Google Scholar] [CrossRef]
- Collett, B.M. Scanning electron microscopy: A review and report of research in wood science. Wood and Fiber Science 1970, 113–133. [Google Scholar]
- Doty, A.C.; Weinstein, D.G.; Hirota, K.; Olsen, K.F.; Ackermann, R.; Wang, Y.; Choi, S.; Schwendeman, S.P. Mechanisms of in vivo release of triamcinolone acetonide from PLGA microspheres. Journal of Controlled Release 2017, 256, 19–25. [Google Scholar] [CrossRef]
- Shah, R.B.; Schwendeman, S.P. A biomimetic approach to active self-microencapsulation of proteins in PLGA. Journal of Controlled Release 2014, 196, 60–70. [Google Scholar] [CrossRef]
- Srikar, G.; Rani, A.P. Study on influence of polymer and surfactant on in vitro performance of biodegradable aqueous-core nanocapsules of tenofovirdisoproxil fumarate by response surface methodology. Brazilian Journal of Pharmaceutical Sciences 2019, 55. [Google Scholar] [CrossRef]
- Kazazi-Hyseni, F.; Landin, M.; Lathuile, A.; Veldhuis, G.J.; Rahimian, S.; Hennink, W.E.; Kok, R.J.; van Nostrum, C.F. Computer modeling assisted design of monodisperse PLGA microspheres with controlled porosity affords zero order release of an encapsulated macromolecule for 3 months. Pharmaceutical research 2014, 31, 2844–2856. [Google Scholar] [CrossRef] [PubMed]
- Samadi, N.; Abbadessa, A.; Di Stefano, A.; Van Nostrum, C.; Vermonden, T.; Rahimian, S.; Teunissen, E.; Van Steenbergen, M.; Amidi, M.; Hennink, W. The effect of lauryl capping group on protein release and degradation of poly (d, l-lactic-co-glycolic acid) particles. Journal of Controlled Release 2013, 172, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Kang, G.; Prathipati, P.K.; Zhou, Y.; Fan, W.; Li, Q.; Destache, C.J. Nanoencapsulation introduces long-acting phenomenon to tenofovir alafenamide and emtricitabine drug combination: A comparative pre-exposure prophylaxis efficacy study against HIV-1 vaginal transmission. Journal of controlled release 2019, 294, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Prathipati, P.K.; Kang, G.; Zhao, Y.; Yuan, Z.; Fan, W.; Li, Q.; Destache, C.J. Tenofovir alafenamide and elvitegravir loaded nanoparticles for long-acting prevention of HIV-1 vaginal transmission. AIDS (London, England) 2017, 31, 469. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Prathipati, P.K.; Sunagawa, S.W.; Destache, C.J. A concept evaluation study of a new combination bictegravir plus tenofovir alafenamide nanoformulation with prolonged sustained-drug-release potency for HIV-1 preexposure prophylaxis. Antimicrobial Agents and Chemotherapy 2021, 65, 10–1128. [Google Scholar] [CrossRef]
- Mandal, S.; Kang, G.; Prathipati, P.K.; Fan, W.; Li, Q.; Destache, C.J. Long-acting parenteral combination antiretroviral loaded nano-drug delivery system to treat chronic HIV-1 infection: A humanized mouse model study. Antiviral research 2018, 156, 85–91. [Google Scholar] [CrossRef]
- Prathipati, P.K.; Mandal, S.; Pon, G.; Vivekanandan, R.; Destache, C.J. Pharmacokinetic and Tissue Distribution Profile of Long Acting Tenofovir Alafenamide and Elvitegravir Loaded Nanoparticles in Humanized Mice Model. Pharmaceutical Research 2017, 34, 2749–2755. [Google Scholar] [CrossRef] [PubMed]
- DeYoung, M.B.; MacConell, L.; Sarin, V.; Trautmann, M.; Herbert, P. Encapsulation of exenatide in poly-(D, L-lactide-co-glycolide) microspheres produced an investigational long-acting once-weekly formulation for type 2 diabetes. Diabetes technology & therapeutics 2011, 13, 1145–1154. [Google Scholar]
- Jindal, A.B.; Bhide, A.R.; Salave, S.; Rana, D.; Benival, D. Long-acting parenteral drug delivery systems for the treatment of chronic diseases. Advanced Drug Delivery Reviews 2023, 198, 114862. [Google Scholar] [CrossRef]
- Trezza, C.; Ford, S.L.; Spreen, W.; Pan, R.; Piscitelli, S. Formulation and pharmacology of long-acting cabotegravir. Current Opinion in HIV and AIDS 2015, 10, 239. [Google Scholar] [CrossRef]
- Overton, E.T.; Richmond, G.; Rizzardini, G.; Jaeger, H.; Orrell, C.; Nagimova, F.; Bredeek, F.; García Deltoro, M.; Swindells, S.; Andrade-Villanueva, J.F.; et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 48-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet 2021, 396, 1994–2005. [Google Scholar] [CrossRef] [PubMed]
- Delany-Moretlwe, S.; Hughes, J.P.; Bock, P.; Ouma, S.G.; Hunidzarira, P.; Kalonji, D.; Kayange, N.; Makhema, J.; Mandima, P.; Mathew, C. Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. The Lancet 2022, 399, 1779–1789. [Google Scholar] [CrossRef]
- Landovitz, R.J.; Donnell, D.; Clement, M.E.; Hanscom, B.; Cottle, L.; Coelho, L.; Cabello, R.; Chariyalertsak, S.; Dunne, E.F.; Frank, I. Cabotegravir for HIV prevention in cisgender men and transgender women. New England Journal of Medicine 2021, 385, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Sillman, B.; Bade, A.N.; Dash, P.K.; Bhargavan, B.; Kocher, T.; Mathews, S.; Su, H.; Kanmogne, G.D.; Poluektova, L.Y.; Gorantla, S. Creation of a long-acting nanoformulated dolutegravir. Nature communications 2018, 9, 443. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Su, H.; Dash, P.; Lin, Z.; Shetty, B.L.D.; Kocher, T.; Szlachetka, A.; Lamberty, B.; Fox, H.S.; Poluektova, L. Creation of a nanoformulated cabotegravir prodrug with improved antiretroviral profiles. Biomaterials 2018, 151, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Cobb, D.A.; Smith, N.; Deodhar, S.; Bade, A.N.; Gautam, N.; Shetty, B.L.D.; McMillan, J.; Alnouti, Y.; Cohen, S.M.; Gendelman, H.E. Transformation of tenofovir into stable ProTide nanocrystals with long-acting pharmacokinetic profiles. Nature communications 2021, 12, 5458. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Zhou, T.; Araínga, M.; Palandri, D.; Gautam, N.; Bronich, T.; Alnouti, Y.; McMillan, J.; Edagwa, B.; Gendelman, H.E. Creation of a long-acting nanoformulated 2′, 3′-dideoxy-3′-thiacytidine. Journal of Acquired Immune Deficiency Syndromes (1999) 2017, 74, e75. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.M.; Bade, A.N.; Lin, Z.; Soni, D.; Wojtkiewicz, M.; Dyavar Shetty, B.L.; Gautam, N.; McMillan, J.M.; Alnouti, Y.; Edagwa, B.J. Synthesis and characterization of a long-acting emtricitabine prodrug nanoformulation. International journal of nanomedicine 2019, 6231–6247. [Google Scholar] [CrossRef]
- Ogunnaike, M.; Das, S.; Raut, S.S.; Sultana, A.; Nayan, M.U.; Ganesan, M.; Edagwa, B.J.; Osna, N.A.; Poluektova, L.Y. Chronic Hepatitis B Infection: New Approaches towards Cure. Biomolecules 2023, 13, 1208. [Google Scholar] [CrossRef]
- Das, S.; Wang, W.; Ganesan, M.; Fonseca-Lanza, F.; Cobb, D.A.; Bybee, G.; Sun, Y.; Guo, L.; Hanson, B.; Cohen, S.M. An ultralong-acting tenofovir ProTide nanoformulation achieves monthslong HBV suppression. Science Advances 2022, 8, eade9582. [Google Scholar] [CrossRef]
- Spreen, W.; Ford, S.L.; Chen, S.; Wilfret, D.; Margolis, D.; Gould, E.; Piscitelli, S. GSK1265744 Pharmacokinetics in Plasma and Tissue After Single-Dose Long-Acting Injectable Administration in Healthy Subjects. JAIDS Journal of Acquired Immune Deficiency Syndromes 2014, 67, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Ford, S.; Chiu, J.; Lovern, M.; Spreen, W.; Kim, J. Population PK approach to predict cabotegravir (CAB, GSK1265744) long-acting injectable doses for phase 2b. In Proceedings of the Proceedings of the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, 2014; pp. 5–9.
- Soriano, V.; Barreiro, P.; Benitez, L.; Peña, J.M.; de Mendoza, C. New antivirals for the treatment of chronic hepatitis B. Expert opinion on investigational drugs 2017, 26, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Scarsi, K.K. Chasing the cabotegravir tail: implications for prevention. The Lancet HIV 2020, 7, e451–e453. [Google Scholar] [CrossRef] [PubMed]
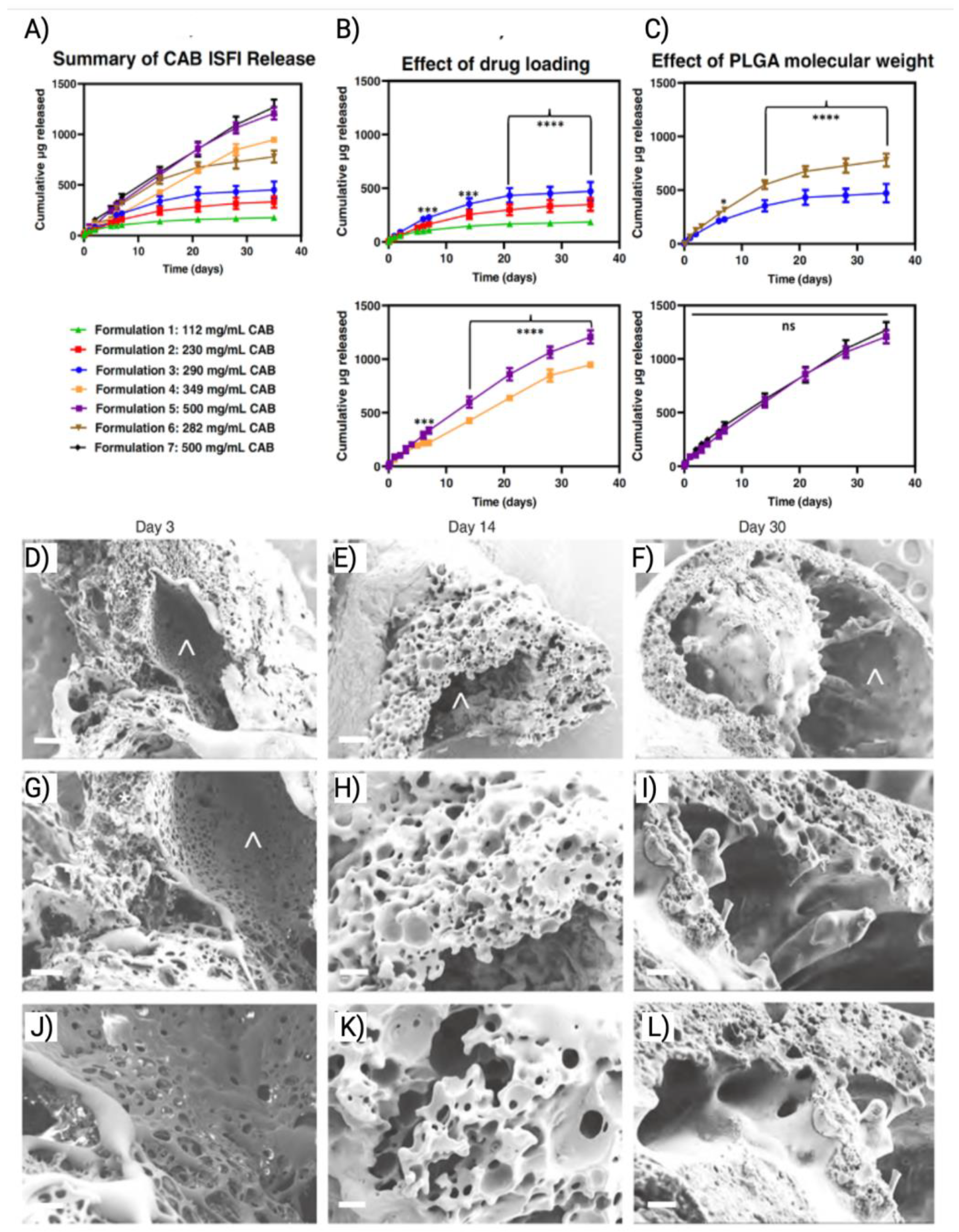
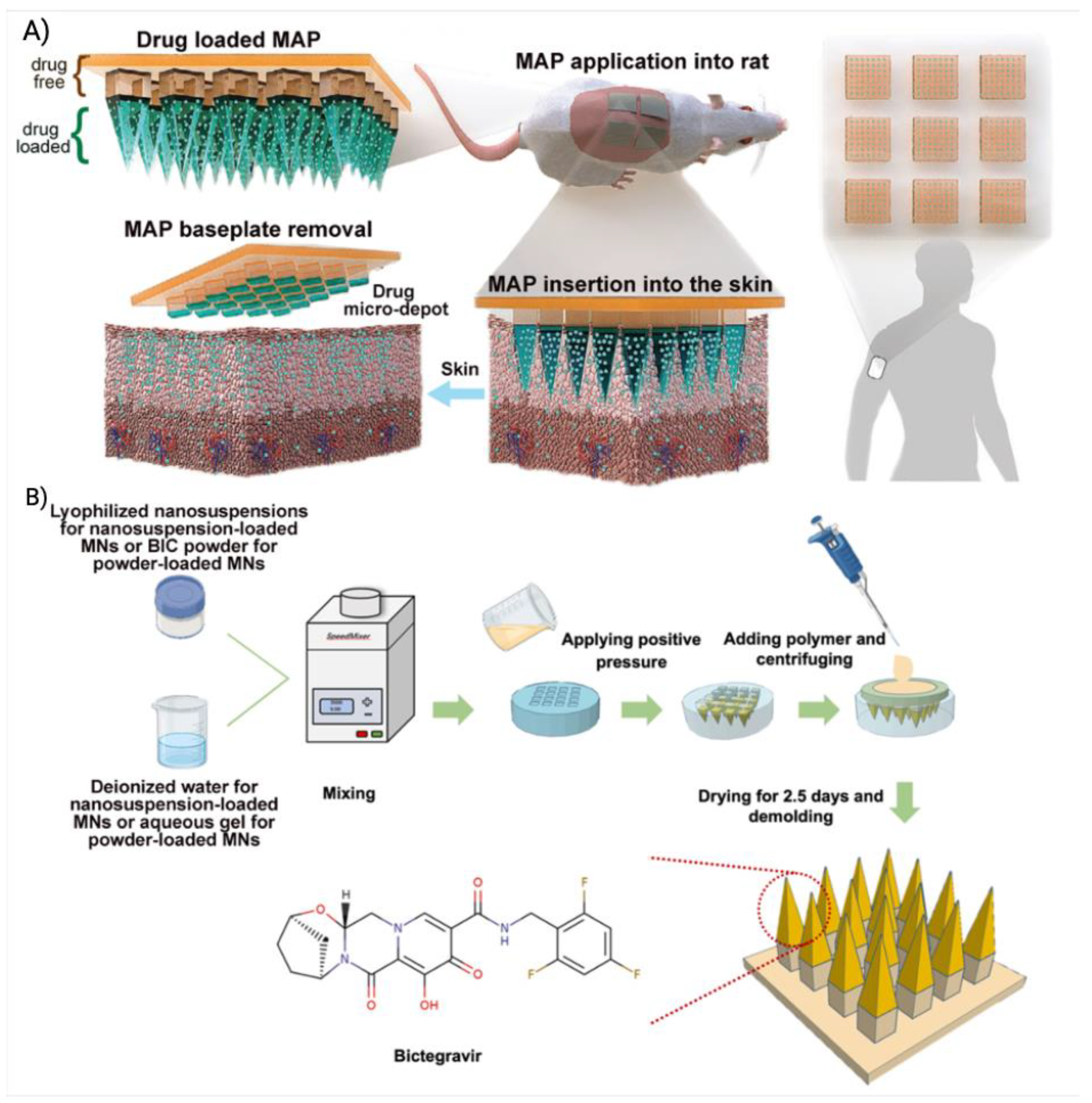
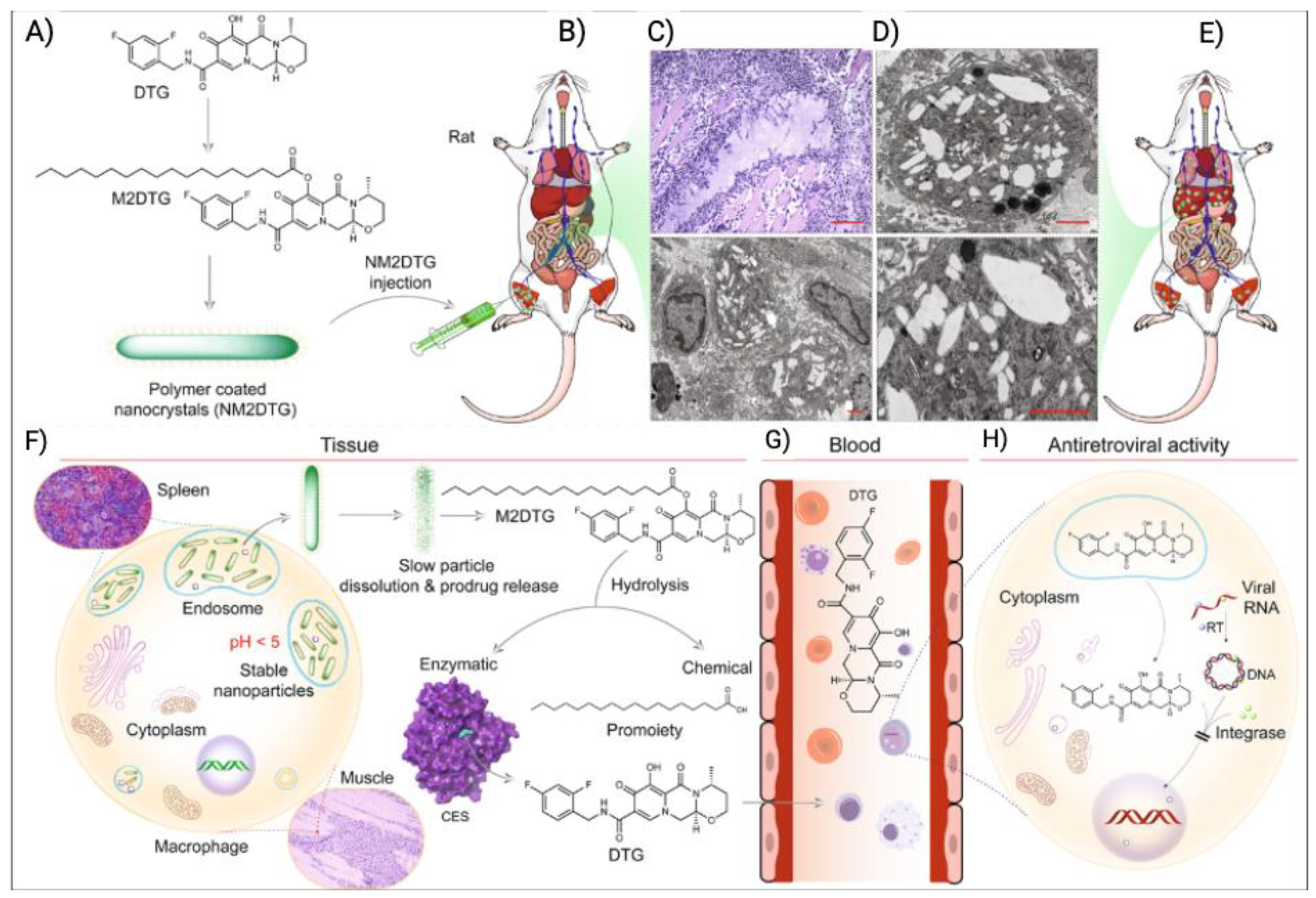
| Delivery system | ARV drug | Polymer | Formulation details | Predicted dosage regimen (month) | Clinical trial | Ref. |
|---|---|---|---|---|---|---|
| Implant | TAF | Silicone and PVA | TAF powder is encapsulated in a silicone tube, which is curated with several drug delivery channel coated with PVA. Drug release rate can be controlled by controlling the number of channel or thickness of PVA membrane | 6 | Phase I | [3] |
| TAF | Silicone | Transcutaneous refillable nanochannel delivery implant, where drug reservoir is made of titanium and drug is released through TaN coated slit nanochannel. | 6 | Preclinical | [19] | |
| TAF |
PUs |
TAF formulation pellet is encased in PU-based drug reservoir and the drug is released through diffusion through the PU membrane. | 3 | [166] | ||
| CAB | CAB formulation is converted into pellets and then encased in PUs membrane containing drug reservoir. PUs membrane act as RCM for controlling drug release through diffusion. | 3 | [170] | |||
| TAF | PCL | TAF formulation containing TAF and castor oil was loaded into PCL tubes and the drug was released through the diffusion and bulk erosion of PCL. Drug release rate can be controlled through controlling MW of PCL. | 3 | [164] | ||
| ISL | EVA | Crystalline ISL is uniformly dispersed in EVA and the implant is prepared through hot melt extrusion. | 12 | On hold | [5] | |
| ISFIs | CAB |
PLGA |
CAB and PLGA were solubilized in NMP:DMSO and injected subcutaneously. Upon injection, the solution went through phase inversion and formed solid implant. | 6 | Preclinical | [55] |
| DTG | Same as CAB PLGA ISFI | 6 | [1] | |||
| VRs | TDF | PUs | TDF formulation was loaded in hydrophilic PUs tubes and the PUs tube was end-sealed using induction welding. | 1 | [204] | |
| TDF | PUs | TDF and NaCl formulation was loaded onto PUs extruded tube and end sealed to prepare the VRs. | 1 | [23] | ||
| MNs | CAB |
PVP and PVA |
MNs tips were generated by using a hydrogel composed of 20:20:60 of PVA:PVP:CAB and the baseplate of MNs was made using PVP and PVA. The MNs tips dissolved quick to release the CAB and the CAB formed drug depot at the administration site. | 1 | Preclinical | [4] |
| BIC | MNs tips were made of PVP, PVA and BIC. The baseplate was made of PVP and glycerol. | 1 | [231] | |||
| RPV | MNs tips were made of PVP and RPV. The baseplate was made of PVA and glycerol. | 1 | [27] | |||
| Microparticle | DTG | PLGA | DTG was transformed into a hydrophobic prodrug and encapsulated into PLGA-based microparticles by organic and aqueous solvents emulsification-evaporation. | 3 | [252] | |
| Prodrug nanocrystal | TFV |
Poloxamer, Polysorbate and PEG |
TFV was converted into lipophilic ProTideS and then formulated as aqueous nanocrystals | ≥3 | [272] | |
| CAB | Lipohilic ester prodrugs of cabotegravir were synthesized and formulated as aqueous nanocrystals | 12 | [17] | |||
| DTG | Fatty acid ester prodrugs of DTG were syntheiszed and nanoformulated as aqueos nanocrystals | ≥6 | [6] | |||
| ISL – islatravir. TFV – tenofovir. TDF – tenofovir disoproxil fumarate. TAF – tenofovir alafenamide fumarate, CAB – cabotegravir, BIC – bictegravir, DTG – dolutegravir, RPV – rilpivirine. | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





