1. Introduction
There are several factors involved in failure of rehabilitation using dental implants. The bacterial colonization is one of the major reasons of implant loss, and it is a necessary condition for the development of peri-implantitis. Peri-implantitis is characterized by inflammation in the tissues surrounding an osseointegrated implant, resulting in the progressive loss of supporting bone [
1,
2]. In this sense, the biological seal surrounding the implant is considered an important factor for the successful maintenance of peri-implant health [
3].
The literature suggests that the epithelial barrier formed by the peri-implant tissues is of lower quality compared to the junctional epithelium [
3]. This limitation can be explained by the fact that the attachment between the implant surface and the non-keratinized peri-implant epithelium by internal basal lamina and hemidesmosomes being limited to the most apical area of the implant-tissue interface. Additionally, the orientation of collagen fibers in the peri-implant connective tissue, mostly circular or oblique, also contributes to the formation of a poor epithelial barrier when compared to Sharpey’s fibers that are perpendicular to the tooth surface [
3].
Although Titanium alloys are the most used as implant material, Zirconia has revealed biological and mechanical properties similar to Titanium with superior aesthetic characteristics to the latter [
4,
5]. These characteristics of Zirconia could be an advantage when it comes to rehabilitation in the anterior jaw and in cases where patients are sensitive to metal [
5]. Additionally, the literature has reported that corrosion of Titanium implant can change the oral microbiome. On the other hand, several in vitro studies that have compared Zirconia and Titanium have demonstrated that Zirconia surfaces can lead to a significant decrease in the adhesion of periodontal pathogens [
5]. Despite all the beneficial properties previously described, Zirconia is a biologically inert material [
6]. In this sense, several surface treatments techniques and protocols have been developed with the aim of increasing its bioactivity, namely sandblasting, acid etching, lasers, and surface coatings [
4,
6]. In addition to the base material, implant surface treatments can significantly influence the success of osseointegration [
6].
With the aim of optimizing the biological response, inhibiting bacterial adhesion and biofilme formation on implant and abutment surfaces, several strategies involving mechanisms of repulsion or elimination of bacteria have been developed [
7]. The first is based on the prevention of biofilm formation, while the second consists of bacterial surfaces that lead to the disruption of bacterial cells [
7]. Surfaces functionalization with coatings that provide antibacterial properties is a potential technique for eliminating bacteria adhered to implants and surrounding areas [
7,
8]. The coating acts as reservoirs of bactericidal agents and allow their local release, contributing to a more efficient action [
7]. However, there is no defined optimal strategy to prepare antibacterial surfaces and for this reason, it is essential develop and investigate different approaches combinations and characterize these new surfaces [
4].
Mineral Trioxide Aggregate (MTA) chemically consists of tricalcium silicate, dicalcium silicate, tricalcium aluminum, tetra calcium aluminum ferrite, calcium sulfate and bismuth oxide [
9,
10,
11]. Due to its excellent chemical, physical and biological properties, in particular its biocompatibility and osteoconductive potential, it has been used in Dentistry for over 20 years [
9,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21], in particular in endodontic treatments [
22]. Its uses is also recommended in vital pulp therapy to preserve the vitality of dental pulp [
23]. Simultaneously, and although there are controversial studies, several demonstrate antibacterial and antifungal properties of this material [
17,
24,
25]. Despite the potential of MTA as an antibacterial agent, there are no studies of its use in implantology [
26].
In this study, we proposed a new surface treatment approach that combines two techniques: texturing using Nd:YAG laser and the use of MTA as a bioactive agent [
27]. The use of laser to create the texture is due to the fact that we can achieve a controlled texture without direct contact with the surface, as shown in previously studies [
28]. On the other hand, the surface texture is expected to mechanically retain the MTA coating. In this sense, this study investigates the biological response of peri implant cells in contact with MTA -coated Zirconia surfaces, as well as their microbiological response.
2. Materials and Methods
2.1. Samples Processing
Zirconia discs were produced using the cold-pressing technique from a partially-stabilize zirconia powder with uniform dispersion of 3 mol% yttria – TZ-3YB-E (Tosoh Corporation
©, Tokyo, Japan).
Table 1 presented the chemical composition of Zirconia powder. Discs with 3 mm of thickness and 8 mm of diameter were compacted in a stainless-steel mold using a uniaxial pressure of 25 MPa for 1 min.
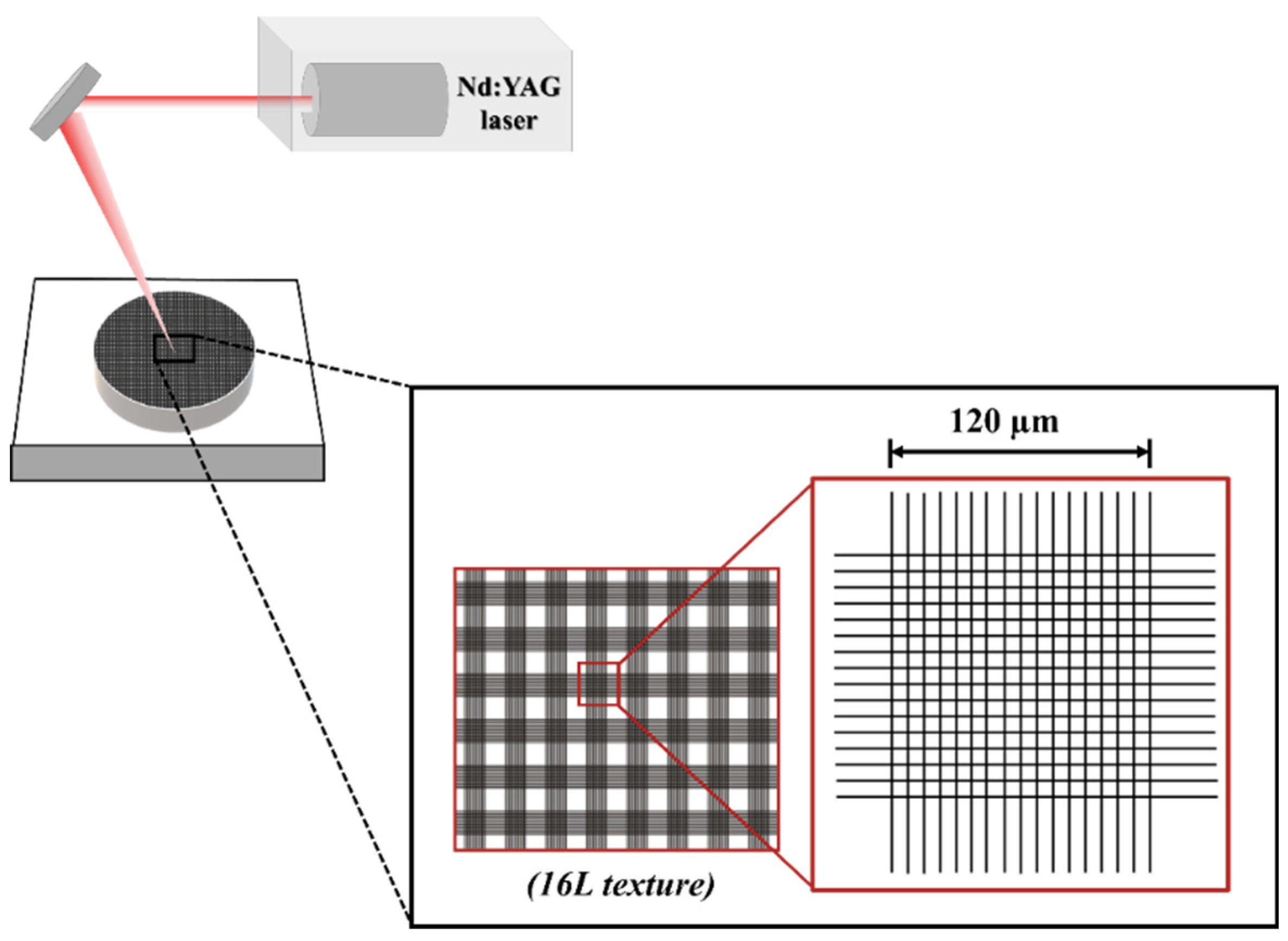
Green test samples were laser textured using Nd:YAG laser (OEM Plus, Sisma, Italy), with a focus distance of 328 mm, 3 μm of focal spot size, 6 W of output power, a pulse width around 35 ns and a wavelength of 1064 nm. Surface texture consisted in a square crosslinked pattern with 16 lines in each direction over the surface (
Figure 1). The texture was performed in normal air und atmospheric pressure using the following laser parameters: power of 40% (2.4 W), a scan speed of 128 mm/s and three laser passages, based on previous work [
29,
30,
31].
Samples were then sintered for 2 h in a sintering furnace – Zirkonofen 700 (Zirkonzahn®, Italy) at 1500 ºC and with heating and cooling rate of 8 ºC/min. At the end, surfaces were ultrasonically cleaned for 1 min.
Mineral Trioxide Aggregate powder (MTA Angelus
®, Angelus, Brazil) was used to produce the bioactive coating in laser textured surfaces. Chemical composition of MTA was described in
Table 2.
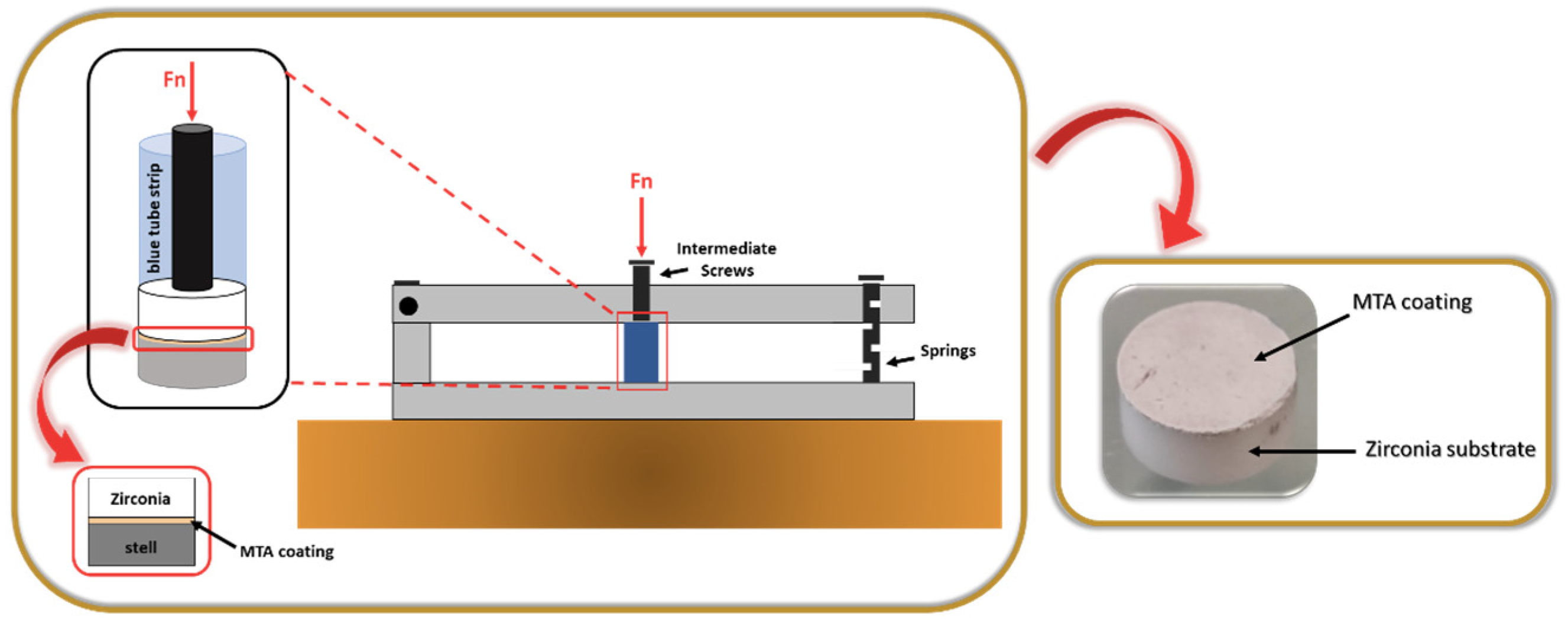
MTA powder was prepared according to the fabricator’s instructions and quantities. The MTA creamy mixture was applied to the textured surface using a spatula. To enhance the adherence of the coating to the surface, we created a home-made device composed of parallel bars attached to the metallic support by means of springs (
Figure 2).
The samples were placed inside the small blue tube strips, and a punch was positioned on the uncoated side of the zirconia substrate (textured surface). The strips were then placed under the intermediate screws of the developed device, and the pressure was applied for about 3-4 h, through the grip of the screws. After that time, the pressure was released, and the MTA -coated zirconia structured samples were kept inside the tubes for 24 h to ensure effective compaction of the coating. At the end, the samples were removed and subjected to a highly energetic and aggressive ultrasonic adhesion test for 1 min.
Titanium discs with 2 mm of thickness and 8 mm of diameter were produced from Ti grade V (Ti6Al4V) powder using hot-pressing technique, according to previously described methods [
8,
32]. The mold containing powder was heated up to 1200 ºC at 31 ºC/min. At 1100 ºC, the pressure on the samples was raised up to 20 MPa for 30 min. Titanium discs were wet ground on SiC papers down to 400 mesh, and then polished till a near-mirror finishing using aluminum oxide suspension (1 μm). Then samples were cleaned ultrasonically.
In this study, four sample groups were considered for comparison purposes of the experimental results, as shown in
Table 3.
2.2. Samples Characterization
2.2.1. Surface roughness
The Ra parameter – the arithmetic mean value between the peak and valley height values in the effective roughness profile – was measured by a mechanical 2D profilometer (Surftest SJ 201, Mitutoyo, Japan). Surface roughness was carried out according to ISO 4288:1996 standard. Measurements were performed in different areas always changing the scanning directions. The scanning speed used was 0.25 mm/s and 3 x 1.5 mm lines were scanned.
2.3. Cell Cultures
Human fetal osteoblasts – hFOB 1.19 (ATCC®, CRL- 11372TM; American Culture Collection, USA) were incubated in an controlled atmosphere of 5% of CO2, 98% of humidity and temperature of 37 ºC with a culture medium composed of (1:1 v/v) of Ham’s F12 Medium (Sigma- Aldrich®, USA) and Dulbecco’s Modified Eagle’s Medium – DMEM (BioWhittaker®, LonzaTM, USA) supplemented with 10% of fetal bovine serum – FBS (Biowest©, France) and 0.3 mg/mL of G418 (InvivoGen, France).
Immortalized Human Gingival Fibroblasts – HGF hTERT (T0026; Applied Biological Materials Inc., Richmond, BC, Canada) were cultured under the same atmospheric conditions as osteoblasts in a culture medium composed of DMEM (BioWhittaker®, LonzaTM, USA) supplemented with 10% of FBS (Biowest©, France), 100 U mL-1 penicillin, and 100 μg/mL streptomycin (LonzaTM, Switzerland).
When cells reach 80% of confluence, cells were detached using trypsin-EDTA (LonzaTM, Switzerland), centrifuged approximately 100 x g for 5 minutes and re-suspended in culture media. Cells were cultured on discs, distributed in 48’well culture plates (Corning®, USA) at a density of 1 x 104 cells/well for biological tests. The experiments were conducted using a fifth passage. Cells cultured directly on treated polystyrene surface of the well were used as a positive control.
2.3.1. Cell Viability
Cell viability was evaluated (n=15) using Cell-Titer Blue® reagent (Promega, USA) - viability assay based in resazurin conversion into a fluorescent product, according to the supplier’s instructions. The conversion rate after 1, 3, 7 and 14 days of culture was quantified as fluorescence intensity in arbitrary fluorescence units (AU). Fluorescence intensity was perceived at excitation wavelength of 530/30nm and emission wavelength of 595/10nm using a multimode microplates reader (VICTOR NivoTM HH3500, PerkinElmer®, UK).
2.3.2. Cell Morphology
Osteoblasts and fibroblasts were cultured on discs for 1 day. Culture wells were washed with Phosphate Buffered Saline – PBS (VWR®, USA) and then fixed with 2,5% glutaraldehyde (VWR®, USA) for 1h. A dehydration process took place by serial dilution of ethanol. Samples were metallized using a gold target in a JEOL JFC 1200 sputtering chamber. Samples were observed under JEOL JSM5200-LV and secondary images were carried out at an acceleration voltage of 15 kV and 25 kV and at different magnifications (100, 150, 180, 200x). Two calibrated researchers performed the image analysis considering cell morphology and adhesion to the materials and cell spreading.
2.3.3. Interleukin 8
Interleukin 8 (IL-8) is an important neutrophil chemotactic factor in response to inflammation. The quantification of IL-8 was achieved at 1 and 3 days of osteoblasts and fibroblasts cultures (n=4) by Human IL-8/ CXCL8 DuoSet ELISA (R&D Systems, Inc., USA), using a multimode microplate reader (VICTOR NivoTM HH3500, PerkinElmer®, UK). The results were obtained in absorbance units (AU) relative to the values of light intensity and were converted in pg/mL according to the calibration curve performed.
2.3.4. Osteocalcin
Osteocalcin is a non-collagenous protein in bone and is expressed in osteoblasts. Osteocalcin quantification was carried out in osteoblasts culture (n=4) after 1 and 3 days, using Human Osteocalcin DuoSet ELISA (R&D Systems, Inc., USA), by a technique of luminescence. The results in absorbance units (AU) were obtained using a multimode microplate reader (VICTOR NivoTM HH3500, PerkinElmer®, UK ) and converted in pg/mL according to the curve of calibration performed.
2.4. Bacterial Strain and Growth Conditions
The Streptococcus oralis CECT 907T strain was cultured on a plate with an enriched blood agar in an anaerobic atmosphere at 37 °C for 72 h, with a gas mixture of 10% CO2, 10% H2, and the remainder N2. Afterward, a single colony was transferred to 15 mL of Brain-Heart Infusion Modified Medium (BHI-2) in anaerobic conditions at 37 °C until it reached the exponential growth phase. The optical density (OD) of suspension was standardized to 0.4. and was measured at 550 nm using a Camspec M50 spectrophotometer to confirm the growth.
2.4.1. Colony Forming Unit (CFU)
In this experiment, discs from all groups were randomly selected and placed in 24 -well plate (Corning®, USA) with S. oralis in the exponential phase and incubated in anaerobic conditions at 37 ºC. The efficacy of MTA as a bactericidal or bacteriostatic agent was assessed by counting the Colony Forming Units (CFUs) after 24 h of S.oralis culture. To determine the number of viable bacterial cells that adhered to the discs over time, the discs were washed once with filtered PBS (VWR®, USA) and placed in a falcon with 3 mL of PBS (VWR®, USA). The falcon was then vortexed at 16 rpm x 100 for 1 minute, followed by ultrasonication for 4 minutes and another vertexing for 2 minutes at 16 rpm x100. Ten-fold serial dilutions were made up to 10-6, and 20 μL of each dilution were plated in triplicate on supplemented Brain-Heart Infusion Agar (BHIA), and were then incubated at 37 °C under anaerobic conditions.
2.5. Statistical Analysis
Statistical analysis was conducted using IBM® SPSS® 27.0 statistics software for Mac (SPSS, Chicago, USA). Kolmogorov-Smirnov test was used to test data for normality. Comparisons between groups for roughness values, cell viability, interleukin 8, osteocalcin levels and CFUs were performed using a factorial analysis of variance ANOVA or Kruskal-Wallis tests as appropriate. Significant differences between groups were identified with Tukey’s post-hoc test. Significance level was set as p<0.05. All data is presented as mean ± standard deviation (SD).
3. Results
3.1. Samples charactetization
3.1.1. Surface roughness
Before biological assays, surface roughness measurement was performed for all samples. Roughness values of Ra (μm) presented as mean and standard deviation (SD) are described in
Table 4. The results showed similar Ra values between Zr MTA, Zr and Ti samples, without no significant differences between them (p>0.05). However, Zr Textured Ra values were significantly higher comparing to the other groups (p<0.05).
3.2. Cell Culture
3.2.1. Cell Viability
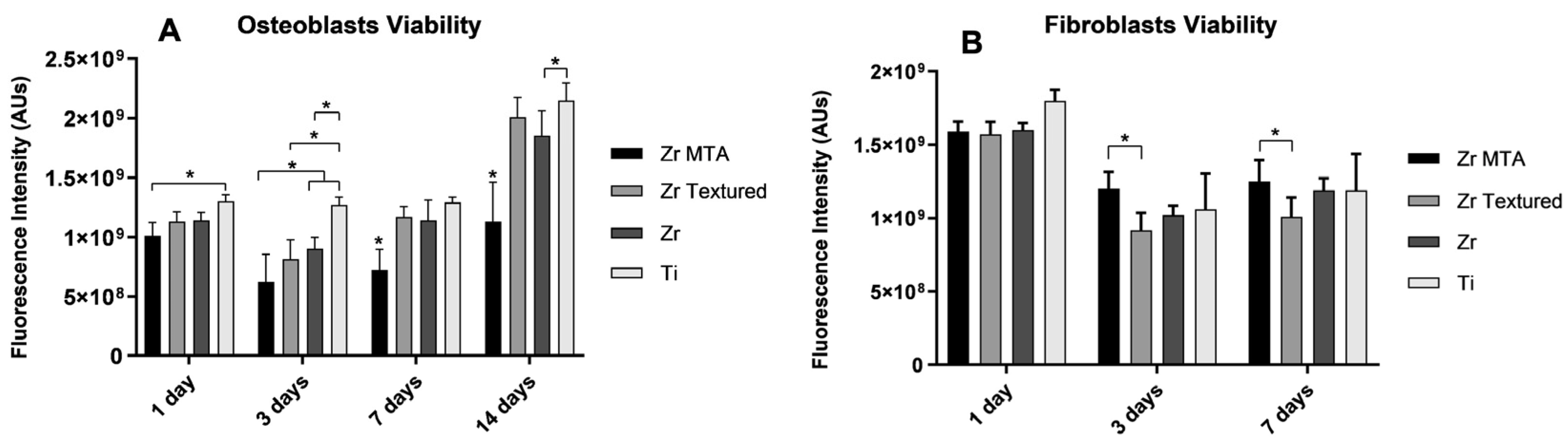
Cell viability results were obtained for 1, 3, 7 and 14 days for osteoblasts culture and 1, 3 and 7 days for fibroblasts culture (as shown in
Figure 3A and
3B, respectively). Cell viability decrease from 1 to 3 days of both cultures and then increased over time for all groups. On osteoblasts culture, Zr MTA samples showed significantly lower viability (p<0.05,
one-way ANOVA) unlike Ti group showed significantly higher viability values compared to Zr MTA (1day), to Zr MTA, Zr Textured and Zr (3days) and to Zr MTA and Zr (14days) (p<0.05,
one-way ANOVA). Unlike osteoblast culture, Zr MTA showed significantly higher values of fibroblasts viability comparing to Zr Textured at 3 and 7 days (p<0.05,
one-way ANOVA).
3.2.2. Cell Morphology
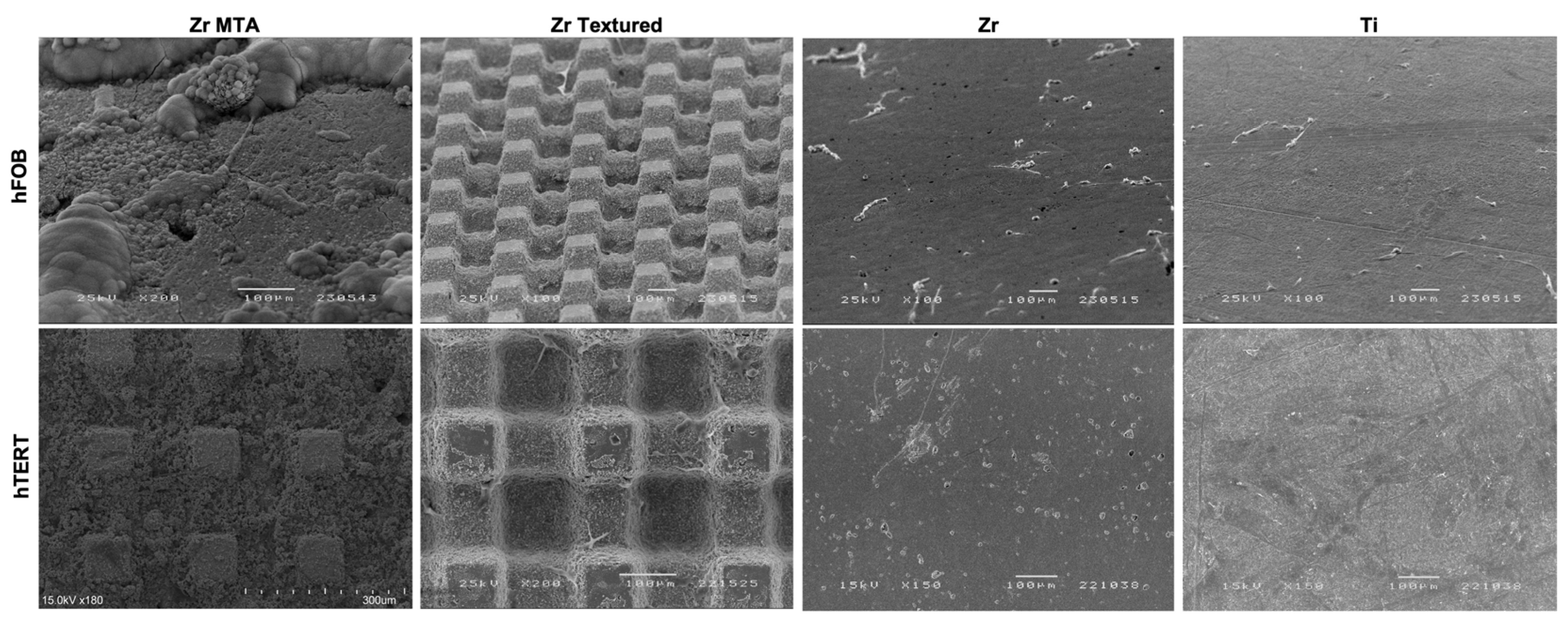
SEM images (
Figure 4) obtained on samples after 1 day of osteoblasts and fibroblasts cultures are presented with the respective magnification. Images showed adherent cells in all samples after 1 day of culture in both osteoblasts and fibroblasts cultures. However, Zr MTA samples appear to have fewer osteoblasts comparing to the other surfaces, unlike fibroblast cell bodies seems to be homogeneously adhered to all samples. Concerning cell morphology, the images showed that osteoblasts culture in Zr and Ti samples presented an elongated shape with some projecting processes, while in Zr MTA and Zr Textured osteoblasts have a more prismatic cell conformation. Fibroblasts presented a typical elongated veil-shape and filopodia formation, with an evidenced higher cell bodies in Zr MTA and Zr Textured samples.
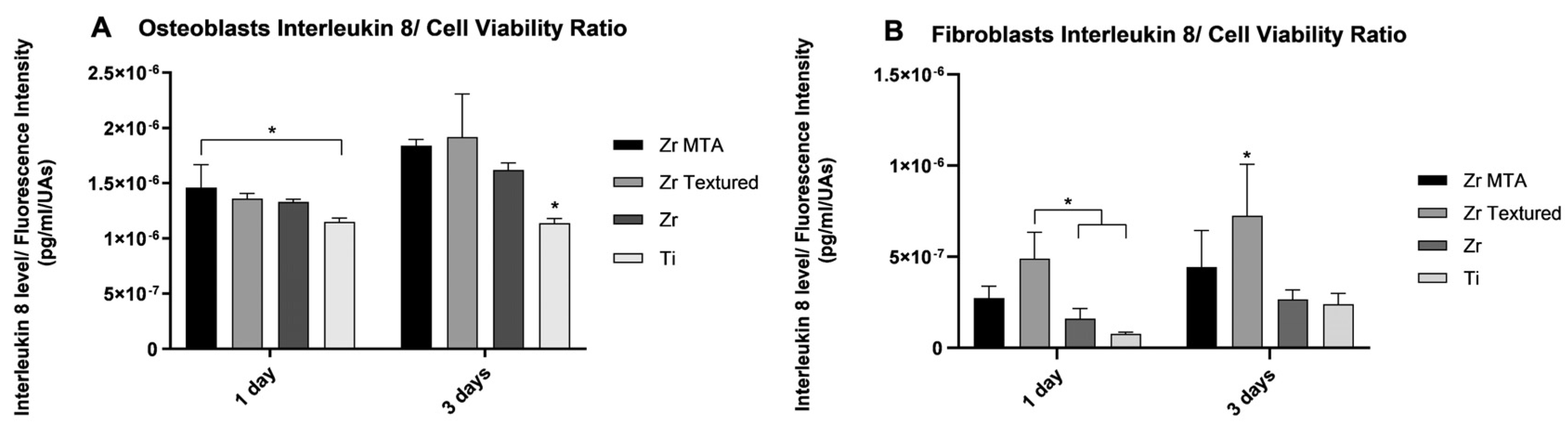
3.2.3. Interleukin 8
Interleukin 8 secretion by osteoblasts and fibroblasts at 1 and 3 days was obtained and is represented in
Figure 5 as an interleukin 8 concentration/ cell viability ratio. The results revealed that Zr MTA group showed significantly higher osteoblasts IL-8 secretion compared to Ti at 1 day, and at 3 days Ti group revealed significantly lower values comparing to Zr MTA, Zr Textured and Zr groups (p<0.05,
one-way ANOVA). Concerning fibroblasts IL-8 secretion, Zr Textured group showed significantly higher values comparing to Zr and Ti at 1 day (p<0.05,
one-way ANOVA) and comparing to all groups at 3 days of culture (p<0,05,
one-way ANOVA).
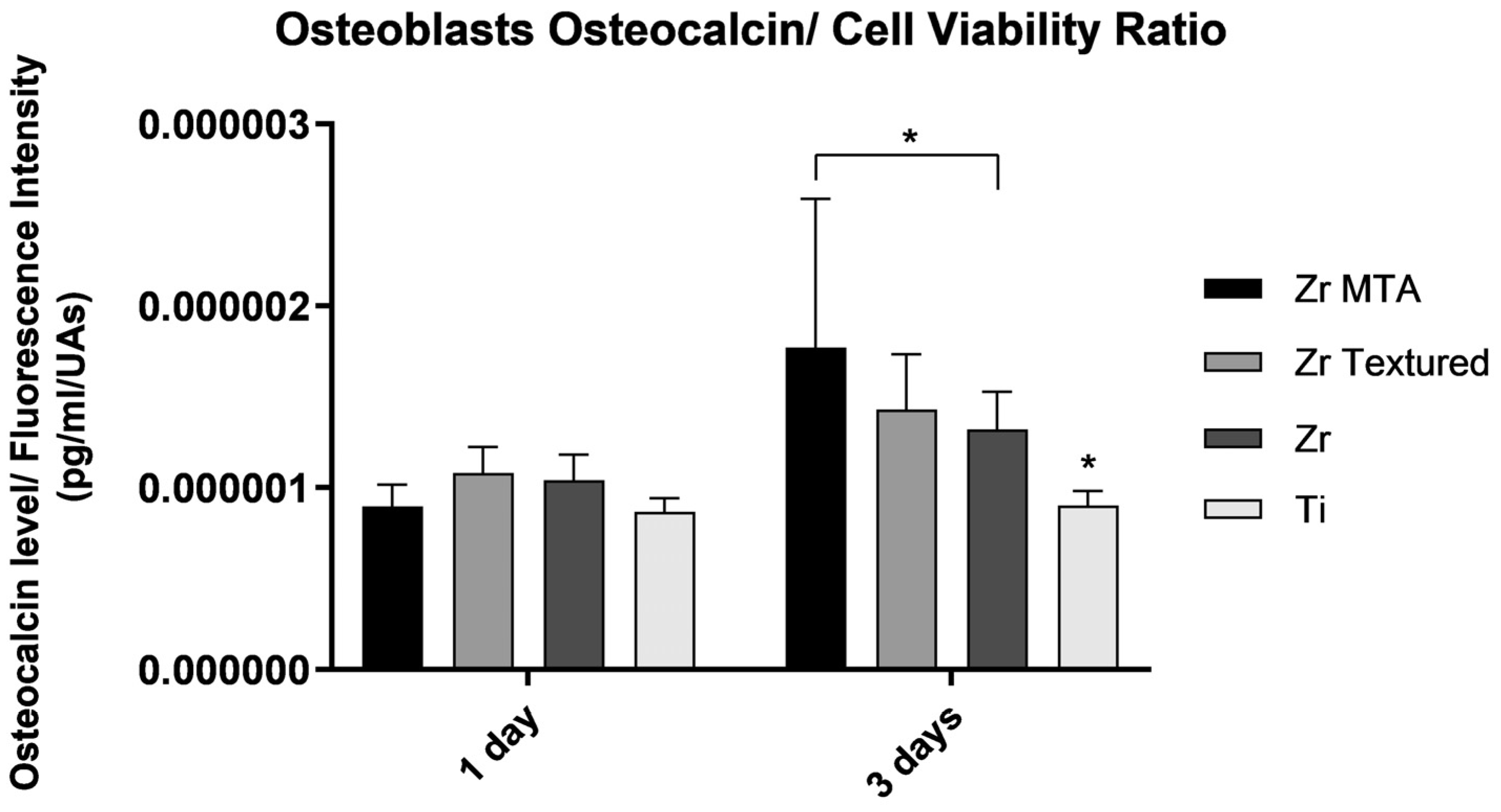
3.2.4. Osteocalcin
Osteocalcin results were also presented as osteocalcin concentration per cell viability (
Figure 6). The results showed an increased production of osteocalcin from day 1 to 3 in all groups. Although no significant differences between groups were found at 1 day of culture and higher levels were found in Zr MTA group compared to Zr and Ti groups at 3 days (p<0.05,
one-way ANOVA).
3.3. Bacterial Growth
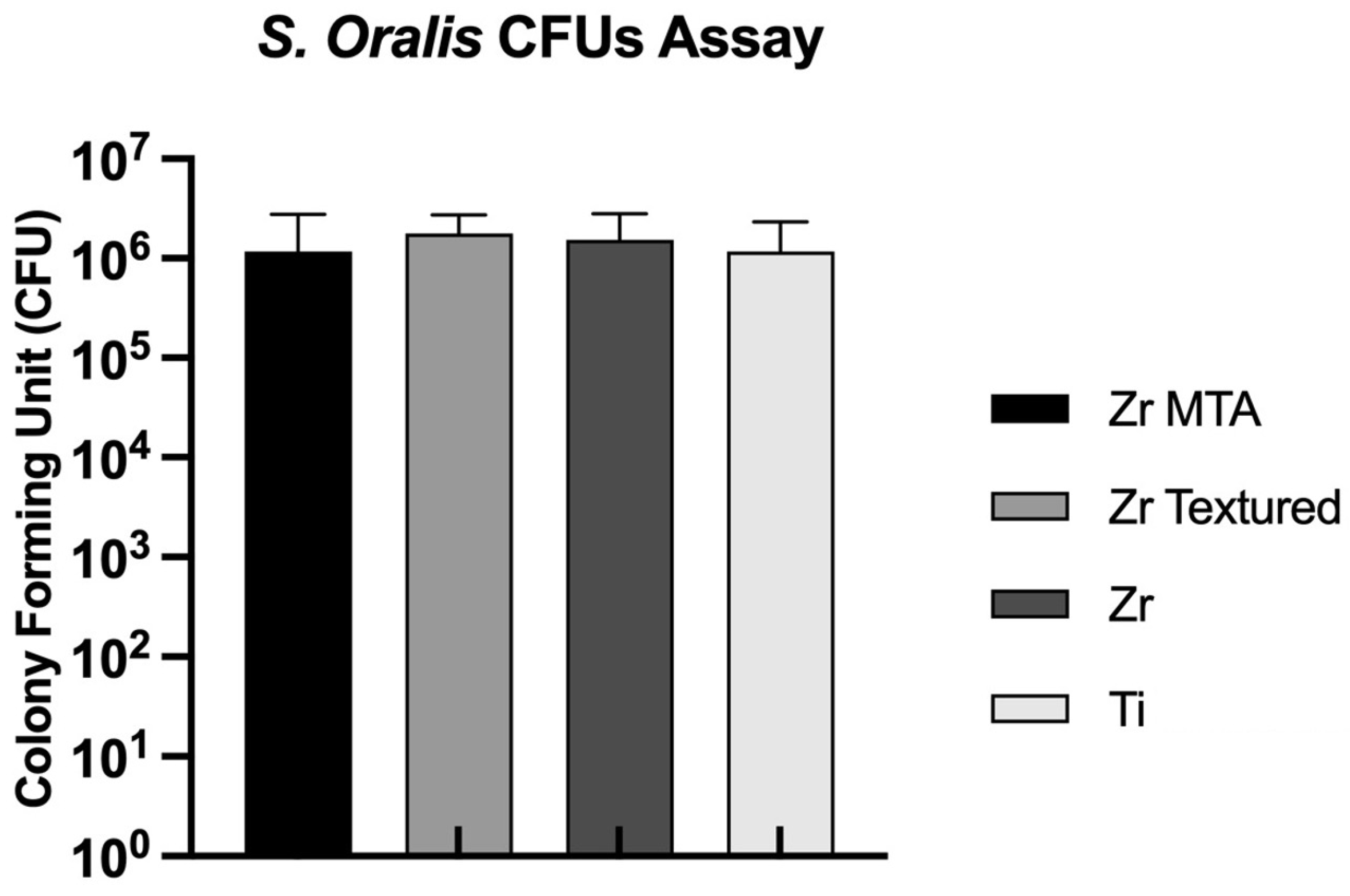
3.3.1. Colony Forming Unit (CFU)
The CFUs per milliliter of
Streptococcus oralis on the discs were evaluated at 24 hours of culture, as shown in
Figure 7. All samples showed CFU values around 10
6, and no statistically significant differences were observed between groups (p>0.05,
one-way ANOVA).
4. Discussion
Despite continuous research on implant biomaterials improvements, bacterial colonization continues to be one of the biggest causes of dental implant failure [
4]. Zirconia dental implant surfaces have demonstrated low bacterial colonization along with its biological, physical, and aesthetic properties [
4] However, Zirconia requires surface treatments to increase its bioactivity [
6]. Surface functionalization with coatings could be a possible approach for improve peri-implant cell response and, simultaneously, reduce bacterial adhesion and consequently biofilm formation [
7,
8].
Due to its biological and antibacterial properties and its extensive use in Dentistry for more than two decades [
7,
10,
11,
12,
13,
14,
15,
16,
17], we proposed the use of MTA as an antibacterial coating and functionalization approach for implant and abutment zirconia surfaces. In the present study, yttria-stabilized Zirconia samples laser textured and coated with MTA were used with the aim of better understanding the cellular behavior of peri-implant tissues in contact with these implant surfaces.
The MTA powder was prepared according to manufacturer’s protocol and the creamy mixture were applied to the Nd:YAG laser textured surface using a spatula. The discs were placed inside a tube in a home-device constructed to apply force for approximately 3-4h. Then, samples were left to dry for 24h (MTA drying time). The adhesion of the cement to the surface was tested using an energetic ultrasound for 1 minute.
In order to mimic the in vivo peri implant cell environment, immortalized human fetal osteoblasts (hFOB 1.19) and immortalized human gingival fibroblasts (HGF hTERT) were used in this study. Our results showed that osteoblasts viability was significantly lower in the Zr MTA group in all measured timepoints. These values are in agreement with SEM images, which revealed smaller quantity of osteoblasts adhered to the Zr MTA samples after 24 hours of culture compared to other groups, contrary to what would be expected [
13,
14,
18,
29]. However, most published studies evaluate the cellular response in contact with MTA cement without being on a textured Zirconia implant surface. Therefore, the comparison of our results with previous literature is limited. Interestingly, although osteoblasts viability was lower in Zr MTA group, fibroblasts viability was shown to be significantly higher in Zr MTA group compared to Zr Textured group at 3 and 7 days. The obtained fibroblasts viability result is in line with a study by Khedmat et al. which evaluated the cell viability of gingival fibroblasts in contact with MTA-Angelus [
33]. The difference in cell viability between osteoblasts and fibroblasts can be explained by the phagocytic activity of osteoblasts, which may inhibit cell proliferation because cells keep their metabolic energy for the phagocytosis and intracellular digestion [
34]. Another possible explanation for the reduced number of osteoblasts in the viability assay is the fact that the presence of MTA accelerates the process of differentiation of osteoblasts and consequently reduces their proliferation [
34,
35]. There is also a third hypothesis to explain this cell viability result, which is the fact that fibroblasts have a greater speed of adhesion growth and proliferations compared to osteoblasts [
28].
Although promising results have not been observed in Zr MTA group regarding the osteoblasts viability, osteocalcin release was significantly higher in this group compared to the other groups at 3 days and significantly higher values of IL-8 release by osteoblasts were also observed compared to the Ti group on the 1st day of culture. The existing literature [
36] demonstrates that osteoblasts in contact with MTA are stimulated to release greater amounts of cytokines (involved in bone turnover) as well as osteocalcin – plays a regulatory role in the bone mineralization [
37]. These results suggest that although there are fewer osteoblasts in Zr MTA group, these osteoblasts are more differentiated, supporting the previously described theory. Although fibroblasts viability values were significantly higher in Zr MTA group at 3 and 7 days, IL-8 results showed that Zr Textured group showed significantly higher values comparing to Zr and Ti groups at 1 day and comparing to all groups at 3 days of culture. These results suggest that MTA does not induce differentiation in fibroblasts, but the texture associated with the increased surface roughness may help in this process.
In order to evaluate the antibacterial effect of MTA coating, we culture S. oralis on the samples. The choice of this bacterial strain is because is one of the primary colonizers.
No differences in CFUs of
S. oralis culture were observed between groups, suggesting that this MTA coating technique does not seem to confer antibacterial properties to the samples. In vitro studies in literature describe that zirconia surfaces can lead a significantly decrease of periodontal microbiome adhesion when compared to titanium surfaces [
5]. In our study, we were not able to obtain these results –no differences were observed between zirconia and titanium surfaces (
Figure 7). However, the evaluation of bacterial growth was only carried out at a single time and the evaluation of biofilm mass could have revealed differences should be considered in future works.
This study evaluates for the first time the effect of MTA -coated textured zirconia surfaces in cell response of soft and hard human peri-implant tissues. The results of this study demonstrate that this technique of coating samples with MTA appears to induce differentiation in osteoblasts, but the same effect was not observed in fibroblasts, despite inducing their proliferation. Additionally, this MTA coating technique does not appear to confer antibacterial properties to the samples. However, the coating stability of this strategy was not optimal and that could impact the results. In this sense, further studies with new approaches to incorporating MTA into implant surfaces, improved mechanical and surface characterization methods and evaluation of cellular response should be carried out. Beyond this, it should be noted that it is an in vitro study and in vivo cell behavior integrated into complex biological systems should be evaluated to validate these findings.
5. Conclusions
The results obtained in this study demonstrated that despite the potential beneficial properties of MTA regarding biocompatibility and antibacterial capacity, the proposed strategy of coating MTA into textured zirconia implant surfaces does not seem to confer additional antibacterial properties to the samples. However, the addition of MTA to Zirconia laser textured samples increased differentiation in osteoblasts and fibroblasts proliferation.
Author Contributions
Conceptualization, M.B.D.C. and H.F.; methodology, Ó.C. and J.F.M.; software, B.F.F. and N.S.; formal analysis, B.F.F. and N.S.; investigation, B.F.F. and N.S.; resources, G.G.; Ó.C.; F.S.; data curation, G.G. and Ó.C.; writing—original draft preparation, B.F.; writing—review and editing, M.B.D.C. and J.F.M.; supervision, Ó.C., H.F. and J.F.M.; project administration, A.M., F.S. and J.F.M; funding acquisition, A.M. and F.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by FCT project POCI-01-0145-FEDER-030498 – Portugal, by FEDER funds through the COMPETE 2020 – Programa Operacional Competitividade e Internacionalização (POCI).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ferreira Ribeiro, C. , Cogo-Muller K., Franco G.C., Silva-Concilio L.R., Sampaio Campos M., de Mello Rode S., et al. Initial oral biofilm formation on titanium implants with different surface treatments: An in vivo study. Arch Oral Biol. 2016, 69, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Tarnow, D.P. Increasing Prevalence of Peri-implantitis: How Will We Manage? J Dent Res. 2016, 95, 7–8. [Google Scholar] [CrossRef]
- Chai, W.L. , Brook I.M., Palmquist A., van Noort R., Moharamzadeh K. The biological seal of the implant-soft tissue interface evaluated in a tissue-engineered oral mucosal model. J R Soc Interface. 2012, 9, 3528–3538. [Google Scholar] [CrossRef]
- Kunrath, M.F. , Gupta S., Lorusso F., Scarano A., Noumbissi S. Oral Tissue Interactions and Cellular Response to Zirconia Implant-Prosthetic Components: A Critical Review. Materials (Basel) 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, F. , Noumbissi S., Francesco I., Rapone B., Khater A.G.A., Scarano A. Scientific Trends in Clinical Research on Zirconia Dental Implants: A Bibliometric Review. Materials (Basel) 2020, 13. [Google Scholar] [CrossRef]
- Fernandes, B.F. , da Cruz M.B., Marques J.F., Madeira S., Carvalho O., Silva F.S., et al. Laser Nd:YAG patterning enhance human osteoblast behavior on zirconia implants. Lasers Med Sci. 2020, 35, 2039–2048. [Google Scholar] [CrossRef] [PubMed]
- Olmo J.A.-D.R.-R., L.; Pérez-Alvarez, L.; Sáez-Martínez, V.; Vilas-Vilela, J.L. Antibacterial Coatings for Improving the Performance of Biomaterials. Coatings. 2020, 10, 139. [Google Scholar] [CrossRef]
- da Cruz, M.B. , Marques J.F., Peñarrieta-Juanito G.M., Costa M., Souza J.C., Magini R.S., et al. Hard and Soft Tissue Cell Behavior on Polyetheretherketone, Zirconia, and Titanium Implant Materials. Int J Oral Maxillofac Implants. 2019, 34, 39–46. [Google Scholar] [CrossRef]
- Shi, W. , Mozumder M.S., Zhang H., Zhu J., Perinpanayagam H. MTA-enriched nanocomposite TiO(2)-polymeric powder coatings support human mesenchymal cell attachment and growth. Biomed Mater. 2012, 7, 055006. [Google Scholar] [CrossRef]
- Camilleri, J. , Montesin F.E., Brady K., Sweeney R., Curtis R.V., Ford T.R. The constitution of mineral trioxide aggregate. Dent Mater. 2005, 21, 297–303. [Google Scholar] [CrossRef]
- Monisha, R. , Manish R. MTA as A Revolution in Endodontics-A Review. IOSR Journal of Dental and Medical Sciences. 2013, 9, 18–21. [Google Scholar] [CrossRef]
- Ha, W.N. , Nicholson T., Kahler B., Walsh L.J. Mineral Trioxide Aggregate-A Review of Properties and Testing Methodologies. Materials (Basel) 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Tabari, M. , Seyed Majidi M., Hamzeh M., Ghoreishi S. Biocompatibility of Mineral Trioxide Aggregate Mixed with Different Accelerators: an Animal Study. J Dent (Shiraz). 2020, 21, 48–55. [Google Scholar] [PubMed]
- Saidon, J. , He J., Zhu Q., Safavi K., Spangberg L.S. Cell and tissue reactions to mineral trioxide aggregate and Portland cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003, 95, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Deller-Quinn, M. , Perinpanayagam H. Osteoblast expression of cytokines is altered on MTA surfaces. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009, 108, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Al-Rabeah, E. , Perinpanayagam H., MacFarland D. Human alveolar bone cells interact with ProRoot and tooth-colored MTA. J Endod. 2006, 32, 872–875. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M. , Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review--Part III: Clinical applications, drawbacks, and mechanism of action. J Endod. 2010, 36, 400–413. [Google Scholar] [CrossRef]
- Hinata, G. , Yoshiba K., Han L., Edanami N., Yoshiba N., Okiji T. Bioactivity and biomineralization ability of calcium silicate-based pulp-capping materials after subcutaneous implantation. Int Endod J 2017, 50 Suppl 2, e40-e51. [Google Scholar]
- Perinpanayagam, H. Cellular response to mineral trioxide aggregate root-end filling materials. J Can Dent Assoc. 2009, 75, 369–372. [Google Scholar]
- Koh, E.T. , McDonald F., Pitt Ford T.R., Torabinejad M. Cellular response to Mineral Trioxide Aggregate. J Endod. 1998, 24, 543–547. [Google Scholar] [CrossRef]
- Osorio, R.M. , Hefti A., Vertucci F.J., Shawley A.L. Cytotoxicity of endodontic materials. J Endod. 1998, 24, 91–96. [Google Scholar] [CrossRef]
- Main, C. , Mirzayan N., Shabahang S., Torabinejad M. Repair of root perforations using mineral trioxide aggregate: a long-term study. J Endod. 2004, 30, 80–83. [Google Scholar] [CrossRef]
- Karabucak, B. , Li D., Lim J., Iqbal M. Vital pulp therapy with mineral trioxide aggregate. Dent Traumatol. 2005, 21, 240–243. [Google Scholar] [CrossRef]
- Singh, G. , Gupta I., Elshamy F.M.M., Boreak N., Homeida H.E. In vitro comparison of antibacterial properties of bioceramic-based sealer, resin-based sealer and zinc oxide eugenol based sealer and two mineral trioxide aggregates. Eur J Dent. 2016, 10, 366–369. [Google Scholar] [CrossRef]
- Jonaidi-Jafari, N. , Izadi M., Javidi P. The effects of silver nanoparticles on antimicrobial activity of ProRoot mineral trioxide aggregate (MTA) and calcium enriched mixture (CEM). J Clin Exp Dent. 2016, 8, e22–26. [Google Scholar] [CrossRef]
- Naik, R.M. , Pudakalkatti P.S., Hattarki S.A. Can MTA be: Miracle trioxide aggregate? J Indian Soc Periodontol. 2014, 18, 5–8. [Google Scholar] [CrossRef]
- Torabinejad, M. , Parirokh M., Dummer P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview - part II: other clinical applications and complications. Int Endod J. 2018, 51, 284–317. [Google Scholar] [CrossRef]
- da Cruz, M.B. , Marques J.F., Fernandes B.F., Pinto P., Madeira S., Carvalho O., et al. Laser surface treatment on Yttria-stabilized zirconia dental implants: Influence on cell behavior. J Biomed Mater Res B Appl Biomater. 2022, 110, 249–258. [Google Scholar] [CrossRef]
- Faria, D. , Madeira S., Buciumeanu M., Silva F.S., Carvalho O. Novel laser textured surface designs for improved zirconia implants performance. Mater Sci Eng C Mater Biol Appl. 2020, 108, 110390. [Google Scholar] [CrossRef]
- Faria, D. , Henriques B., Souza A.C., Silva F.S., Carvalho O. Laser-assisted production of HAp-coated zirconia structured surfaces for biomedical applications. J Mech Behav Biomed Mater 2020, 112, 104049. [Google Scholar] [CrossRef]
- Faria, D.M.M. , de Castro Henriques B.A.P., De Souza A.C.B., da Silva F.S.C.P., Carvalho Ó.S.N. Laser-assisted manufacturing of 45S5 Bioglass-coated zirconia structured surfaces targeting medical implants: adhesive, wettability, mechanical, and bioactivity evaluation. The International Journal of Advanced Manufacturing Technology. 2022, 119, 1595–1612. [Google Scholar] [CrossRef]
- Peñarrieta-Juanito, G.M. , Costa M., Cruz M., Miranda G., Henriques B., Marques J., et al. Bioactivity of novel functionally structured titanium-ceramic composites in contact with human osteoblasts. J Biomed Mater Res A. 2018, 106, 1923–1931. [Google Scholar] [CrossRef]
- Khedmat, S. , Sarraf P., Seyedjafari E., Sanaei-Rad P., Noori F. Comparative evaluation of the effect of cold ceramic and MTA-Angelus on cell viability, attachment and differentiation of dental pulp stem cells and periodontal ligament fibroblasts: an in vitro study. BMC Oral Health. 2021, 21, 628. [Google Scholar] [CrossRef]
- Valverde, T.M. , Castro E.G., Cardoso M.H., Martins-Junior P.A., Souza L.M., Silva P.P., et al. A novel 3D bone-mimetic scaffold composed of collagen/MTA/MWCNT modulates cell migration and osteogenesis. Life Sci. 2016, 162, 115–124. [Google Scholar] [CrossRef]
- Deligianni, D.D. Multiwalled carbon nanotubes enhance human bone marrow mesenchymal stem cells' spreading but delay their proliferation in the direction of differentiation acceleration. Cell Adh Migr. 2014, 8, 558–562. [Google Scholar] [CrossRef]
- Koh, E.T. , Torabinejad M., Pitt Ford T.R., Brady K., McDonald F. Mineral trioxide aggregate stimulates a biological response in human osteoblasts. J Biomed Mater Res. 1997, 37, 432–439. [Google Scholar] [CrossRef]
- Rathinam, E. , Rajasekharan S., Chitturi R.T., Declercq H., Martens L., De Coster P. Gene Expression Profiling and Molecular Signaling of Various Cells in Response to Tricalcium Silicate Cements: A Systematic Review. J Endod. 2016, 42, 1713–1725. [Google Scholar] [CrossRef]
Figure 1.
Schematic representation of the laser surface texturing process and the correspondent design of texture.
Figure 1.
Schematic representation of the laser surface texturing process and the correspondent design of texture.
Figure 2.
Home-made device used to perform the pressing of the MTA coating.
Figure 2.
Home-made device used to perform the pressing of the MTA coating.
Figure 3.
Bar graphs showing osteoblasts (A) and fibroblasts (B) viability measured on Zr MTA, Zr Textured, Zr and Ti as mean exhibited in arbitrary units (AU) of fluorescence intensity. Standard deviation (SD) was represented by error bars. Statistical significance: *p<0.05, one-way ANOVA.
Figure 3.
Bar graphs showing osteoblasts (A) and fibroblasts (B) viability measured on Zr MTA, Zr Textured, Zr and Ti as mean exhibited in arbitrary units (AU) of fluorescence intensity. Standard deviation (SD) was represented by error bars. Statistical significance: *p<0.05, one-way ANOVA.
Figure 4.
SEM micrographs with osteoblasts (hFOB) and fibroblasts (hTERT) cultured on surfaces at 1 day (100x, 150x, 180x and 200x magnification).
Figure 4.
SEM micrographs with osteoblasts (hFOB) and fibroblasts (hTERT) cultured on surfaces at 1 day (100x, 150x, 180x and 200x magnification).
Figure 5.
Bar graphs showing osteoblasts (A) and fibroblasts (B) interleukin 8 secretion as a mean concentration expressed in pg/mL/UAs. Standard deviation (SD) was represented by error bars. Statistical significance: *p<0.05, one-way ANOVA.
Figure 5.
Bar graphs showing osteoblasts (A) and fibroblasts (B) interleukin 8 secretion as a mean concentration expressed in pg/mL/UAs. Standard deviation (SD) was represented by error bars. Statistical significance: *p<0.05, one-way ANOVA.
Figure 6.
Bar graphs showing osteocalcin levels on osteoblasts culture as mean concentration expressed in pg/mL/UAs. Standard deviation (SD) was represented by error bars. Statistical significance: *p<0.05, one-way ANOVA.
Figure 6.
Bar graphs showing osteocalcin levels on osteoblasts culture as mean concentration expressed in pg/mL/UAs. Standard deviation (SD) was represented by error bars. Statistical significance: *p<0.05, one-way ANOVA.
Figure 7.
Bar graphs showing Streptococcus oralis growth measured as mean and expressed in CFU. Standard deviation (SD) was represented by error bars.
Figure 7.
Bar graphs showing Streptococcus oralis growth measured as mean and expressed in CFU. Standard deviation (SD) was represented by error bars.
Table 1.
Chemical composition of TZ-3YB-E powder (according to manufacturer Tosoh Corporation©, Tokyo, Japan).
Table 1.
Chemical composition of TZ-3YB-E powder (according to manufacturer Tosoh Corporation©, Tokyo, Japan).
| Element |
Wt.% |
| ZrO2 + HfO2 + Y2O |
> 99.9 |
| Y2O3
|
5.15±0.20 |
| Al2O3
|
0.25±0.10 |
| SiO2
|
≦ 0.02 |
| Fe2O3
|
≦ 0.01 |
| Na2O |
≦ 0.04 |
Table 2.
Chemical composition of MTA powder (according to manufacturer MTA Angelus®, Angelus, Brazil).
Table 2.
Chemical composition of MTA powder (according to manufacturer MTA Angelus®, Angelus, Brazil).
| Element |
Wt.% |
| CaO |
49.20 |
| SiO2
|
18.58 |
| Bi2O3
|
8.26 |
| Al2O3
|
4.48 |
| MgO |
0.64 |
| SO3
|
0.19 |
| Na2O |
1.32 |
| Cl |
0.51 |
| H2O + CO2
|
16.82 |
Table 3.
Sample groups designation and description.
Table 3.
Sample groups designation and description.
| Samples designation |
Description |
| Zr MTA |
MTA -coated laser textured Zirconia samples |
| Zr Textured |
Laser textured Zirconia samples |
| Zr |
Zirconia samples |
| Ti |
Titanium samples |
Table 4.
Roughness values – Ra (μm) for Zr MTA, Zr Textured, Zr and Ti surfaces as mean and standard deviation.
Table 4.
Roughness values – Ra (μm) for Zr MTA, Zr Textured, Zr and Ti surfaces as mean and standard deviation.
| Sample |
Roughness – Ra (μm) |
Standard deviation (μm) |
| Zr MTA |
0.31 |
0.11 |
| Zr Textured |
27.73* |
3.22 |
| Zr |
0.19 |
0.14 |
| Ti |
0.49 |
0.14 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).