1. Introduction
Sacred and/or community forests have played a significant role in the socio-cultural live of local communities, assisting in the preservation of biodiversity [
1]–[
3] especially in areas where vegetation has significantly degraded or reduced. In Western cultures, the focus on forests has largely shifted towards recreation. However, traditional societies across the Global South, including Latin America, Asia, Africa, and Oceania, maintain strong cultural connections between communities and specific forests. Initially, these ecosystems serve as sanctuaries for deities, spirits, or ancestors of a community [
2], [
4].
In addition to the socio-cultural significance, these forests serve as a significant hotspot of local biodiversity, providing an insights into the floristic history and primary vegetation of the region. The conservation of biodiversity through sacred and community forests has become a matter of growing international concern in Africa and other parts of the world [
5]–[
7]. In the Savannah Region of Northern Togo, where forest cover, namely the protected areas are remarkably degraded [
8]–[
11], sacred and community forests serve as true sanctuaries for species that have either already disappeared or are in danger of extinction. These small woodlands, ranging from a few acres to several dozen hectares, are dispersed throughout a degraded landscape and remain natural habitats for specific plant and animal species that are threatened. Apart from protecting the biodiversity and cultural significance, community forests also contribute to conserving forest landscape and increasing community resilience against climate change [
7]. They also serve as a means economic revenues for residents [
12], [
13].
This study was carried out on the Nakpadjouak Community Forest (NCF) as part of the sustainable management of community forests in Togo. Its aims at: (i) analysing the dynamics of the forest’s ecosystem from the time of its installation (2015) until today, (ii) assessing the diversity of plant species, and (iii) characterizing the demographic structure of woody plants in NCF.
2. Materials and Methods
2.1. Study area
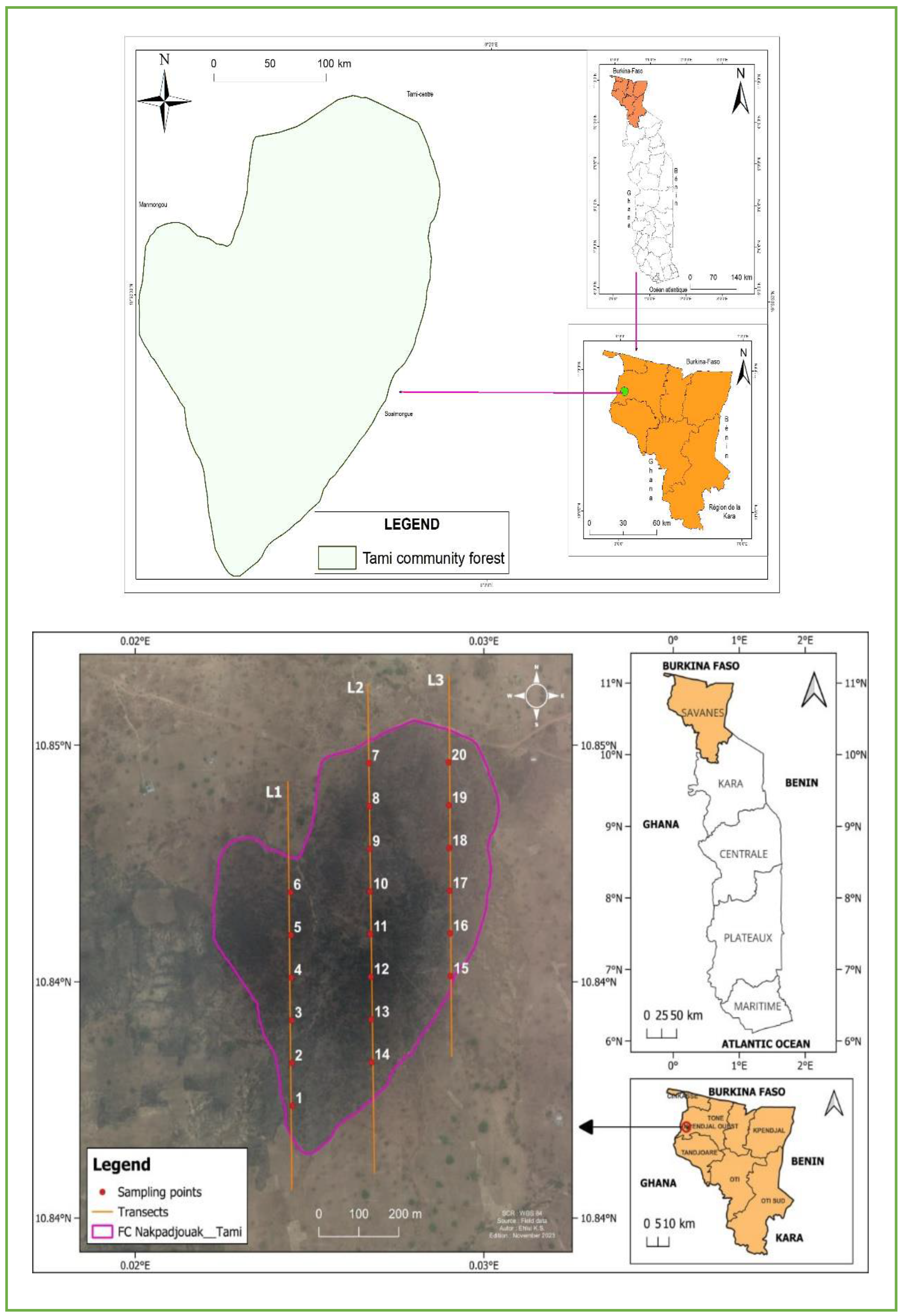
The studied forest, Nakpadjouak Community Forest (NCF) is located in the canton of Tami, prefecture of Tone in Savannahs’ region of Togo (
Figure 1.). The NCF is a part of the ecological zone I of Togo [
14] characterized by Soudanian Savannahs stands [
15]. Due to the soaring popularization, the native ecosystems were transformed to croplands and agroforestry parklands [
16].
2.2. Data collection
2.2.1. Sattelite image acquisition
The NCF map did not exist before this study. Therefore, the first activity was to demarcate the NCF. The mobile application LocustMap was used to record the routes and geographical coordinates. To digitise the land use units, the recorded route and GPS (Global Positioning System) coordinates were projected in Google Earth Pro software. Google Earth images provided by Astrium Service to Google Inc. in 2021 were used to map the land use units. The choice of these images was due to their high resolution (up to 1.5 m) and the small area of the NCF [
11], [
17].
2.2.2. Sampling design
Field data were collected along three transects 200 m apart. For the inventories, 100 m equidistant sampling points were established along each transect. This sampling method produced a total of 20 throughout the NCF. For phytosociological inventories of woody species, ecological inventories and forest inventories, the plot size was 50 m x 20 m [
18]. Phytosociological surveys of herbaceous flora were executed within plots of 10 m x 10 m [
19]. Three diagonally arranged 5 m x 5 m sub-plots, one (1) in the centre and the other two (2) in the opposite corners on either side of the central plot, were defined within each large 50 m x 20 m plot for the regeneration inventory[
19]–[
21]
2.2.3. Phytosociological and forest inventories
Phytosociology surveys were conducted using a standard phytosociology form with following information: plot code, survey date, locality, geographical coordinates of point and type of surface survey. All species (woody and herbaceous) present in the defined inventory areas were listed. They were assigned an abundance/dominance coefficient according to Braun-Blanquet [
22]. This scale is defined as follows: +: rare species, cover 0 to 1%; 1 = cover 1 to 5%; 2 = cover 5 to 25%; 3 = cover 25 to 50%; 4 = cover 50 to 75%; 5 = cover 75 to 100%.
Tree diameter, stem height and total height of woody plants with DBH ≥ 5 cm were measured during the forest inventory. Tree diameter was measured at 1.30m from the ground using a forestry compass. Visual scoring was used to assess the height of the trunk and total height. Crown diameter was assessed in north-south and east-west directions. Potential regeneration was defined as all woody species with a DBH < 5 cm [
23].
2.3. Data analysis
2.3.1. Land use unit mapping
The different land-use units were digitised using Google Earth Pro software while QGIS software was used to create the maps[
18], [
24]. The coordinates of the points of the different formations were recorded in the field using GPS. These were projected onto the Google Earth image to validate the maps previously produced. After digitisation, the layers generated were saved in KML format. They were then projected onto a background map of Togo, WGS 84_UTM Zone 31N, using QGIS 2.18.
2.3.2. Assessment of the floristic diversity
Microsoft Excel spreadsheets were used to record the data collected in the field. The analysis consisted of compiling a list of the species found. These were then grouped by family and genus. The classification used is that of APG IV[
5]. It can be consulted on the website
https://africanplantdatabase.ch/en. The biological and phytogeographical types of these species are identified in the following reference documents. [
25], [
26]. Phanerophytes (Ph), chamephytes (Ch), hemicryptophytes (He), geophytes (Ge), therophytes (Th) and epiphytes are the categories of biological types considered. Phanerophytes include: megaphanerophytes (MP) (trees over 30 m), mesophanerophytes (mP) (trees 10 to 30 m), microphanerophytes (mp, trees 2 to 10 m), nanophanerophytes (np, trees 0.4 to 2m), lianaceous forms (Lnp, Lmp, LmP, LMP) [
8].
2.3.3. Forest characteristics analysis
Dendrometry parameters such as trees density per hectare (D), mean Lorey height (HL in m), mean diameter (Dm in cm) and basal area (G in m
2/ha) were calculated[
27]. Tree density (N) is assessed as the number of trees per hectare according to the following formula N = n/s where “n” is the number of trees of DBH ≥ 5 cm on the plot and “s” is the area of the plot in hectares. Basal area (G, m²/ha) is the sum of the cross-sections at 1.30 m above the ground of all trees in a plot. It is converted into hectares. It is calculated according to the formula G = Σπd2/4s, where d is the diameter and s is the area of the group. Mean Lorey Height is calculated by averaging tree heights weighted by basal area [
28].
The trees are grouped into 10 cm diameter classes and 2 m height classes. Minimum diameter and height are 5 cm and 2 m respectivily. The 3 Weibull parameters (a = location parameter, b = scale or size parameter and c = shape parameter related to the diameter or height structure) were used to fit the demographic structures of trees to the theoretical distribution[
23].
3. Results
3.1. Land use units dynamic
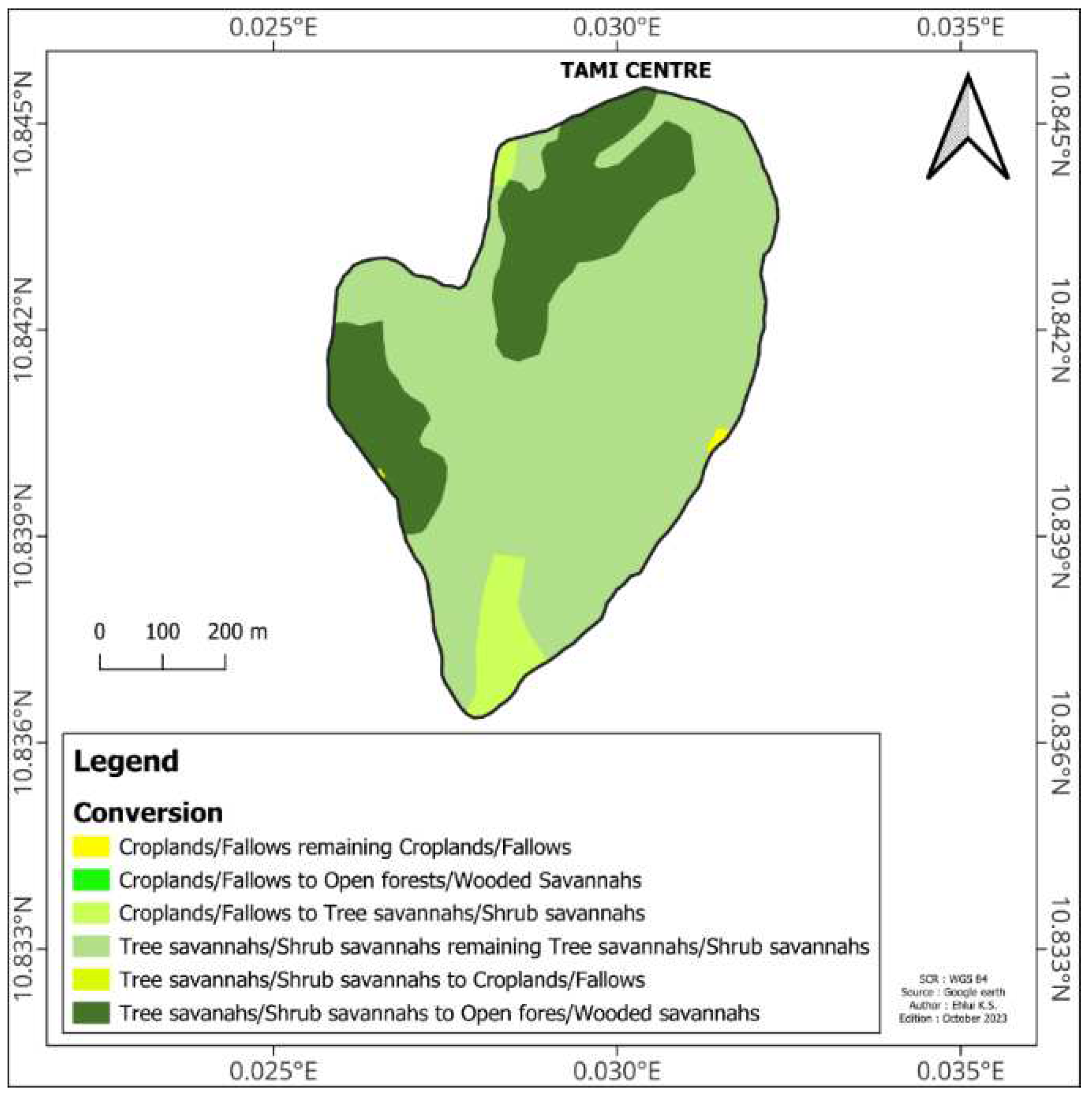
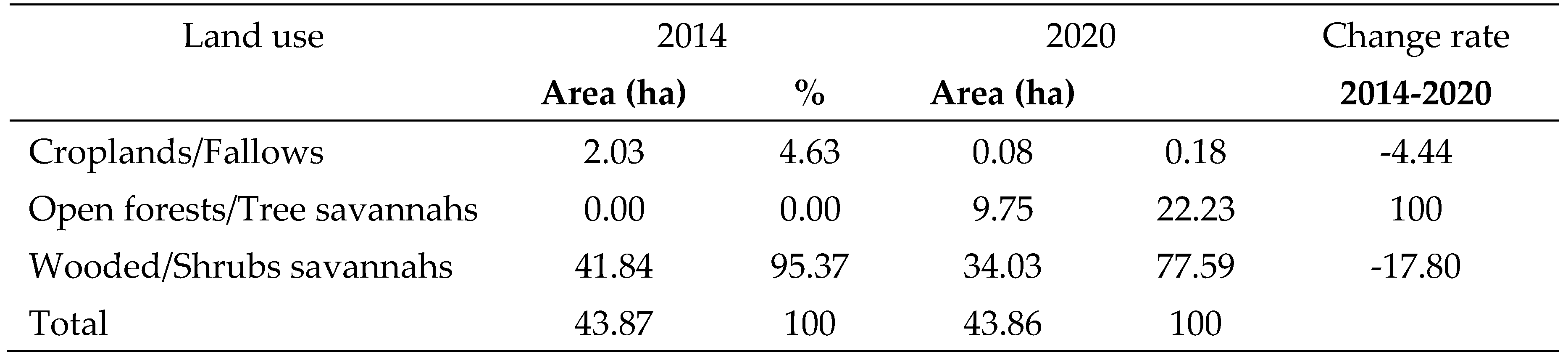
Nakpadjouak community forest consisted of two types of land use types in 2014 (
Figure 2). Wooded/shrub savannahs is the most common (95.37%). It is followed by croplands/fallows (4.63%). Three types of land use have been identified in 2020. These are wooded/shrub savannahs (77.59%), open forests/wooded savannahs (22.23%) and croplands/fallows (0.18%).
From 2014 to 2020, the
Nakpadjouak community forest was characterised by the emergence of open forests/wooded savannahs. The wooded/shrub savannah and the fields/fallows decreased by -17.80 % and -4.44 % respectively (
Table 1).
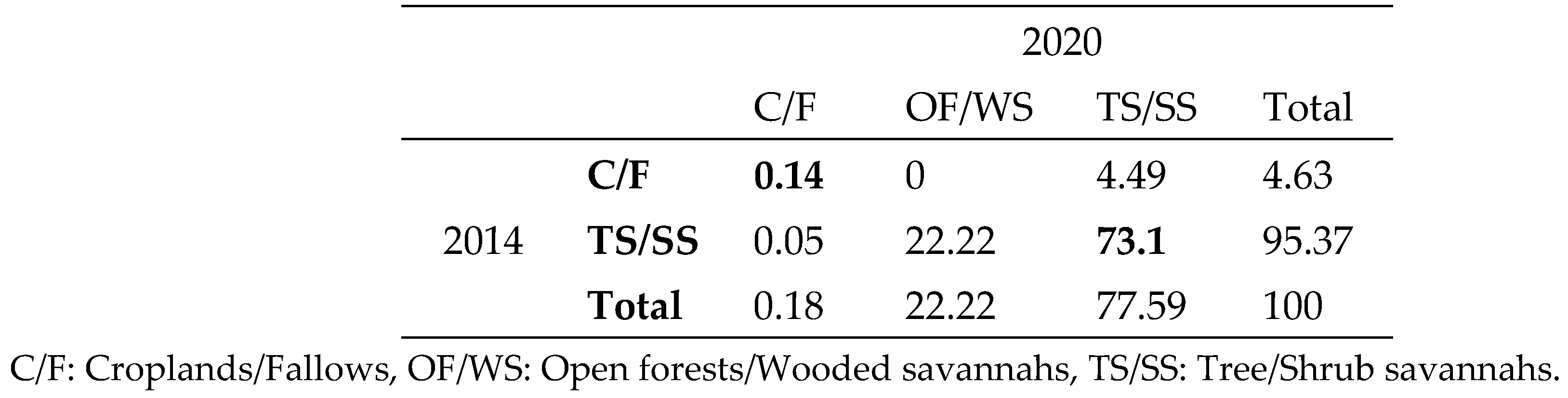
The main conversions that took place between 2014 and 2020 are identified by analysing the land use transition matrix for the NCF (
Figure 3). According to this analysis, 22.22% of wooded savannah/shrubland converted to open forest/wooded savannah and 4.49% of cropland to wooded savannah/shrubland (
Table 2).
C/F: Croplands/Fallows, OF/WS: Open forests/Wooded savannahs, TS/SS: Tree/Shrub savannahs.
3.2. Floristic diversity of NCF
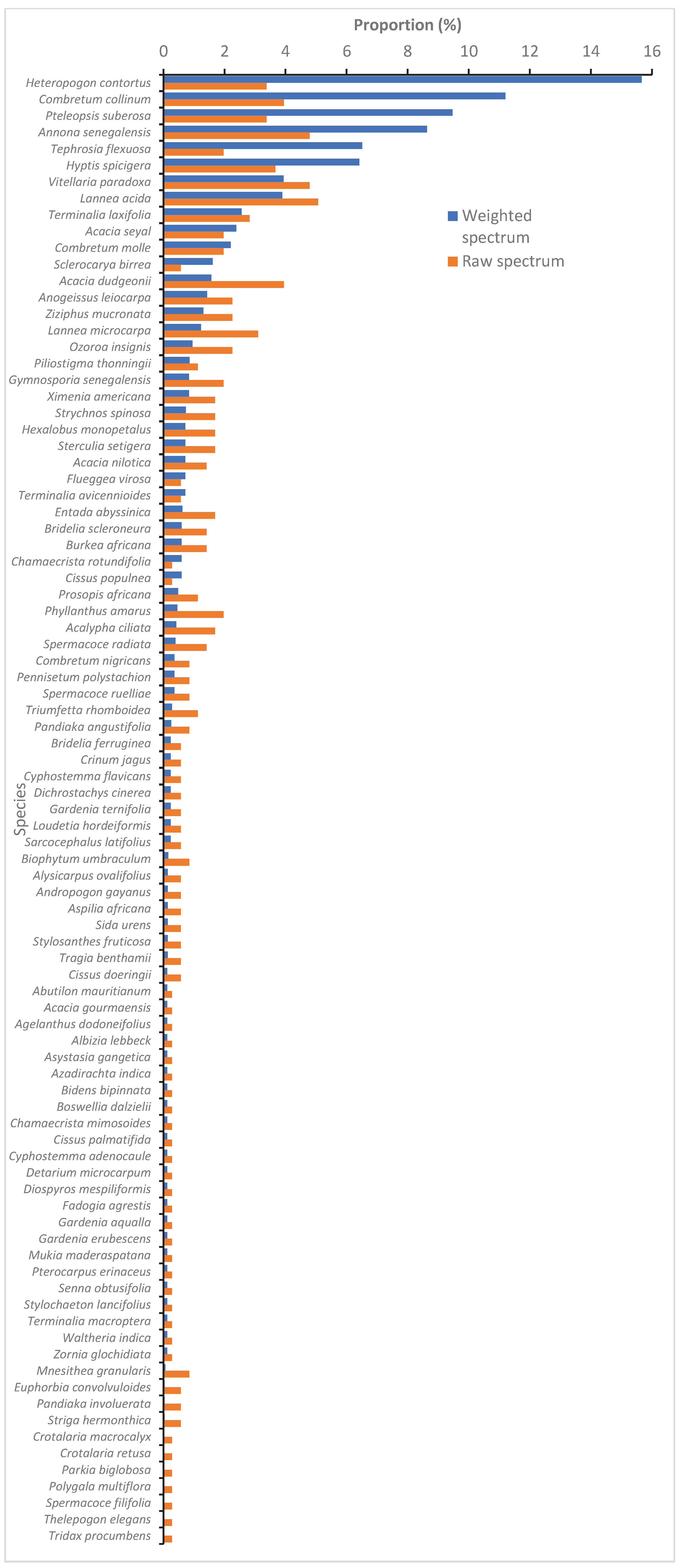
There are 89 species of plants that have been recorded in the Nakpadjouak community forest(Apendix A). These belong to 70 genera and 28 families. The dominant species are:
Hetopogon contortus (L.) P.Beauv. and
Combretum collinum Fresen., followed by
Pteleopsis suberosa Engl. & Diels,
Annona senegalensis Pers. (
Figure 4). The most frequent species are:
Lannea acida A.Rich. s.l.,
A. senegalensis,
Vitellaria paradoxa C.F.Gaertner subsp.
paradoxa,
C. collinum and
Acacia dudgeonii Craib ex Holland.
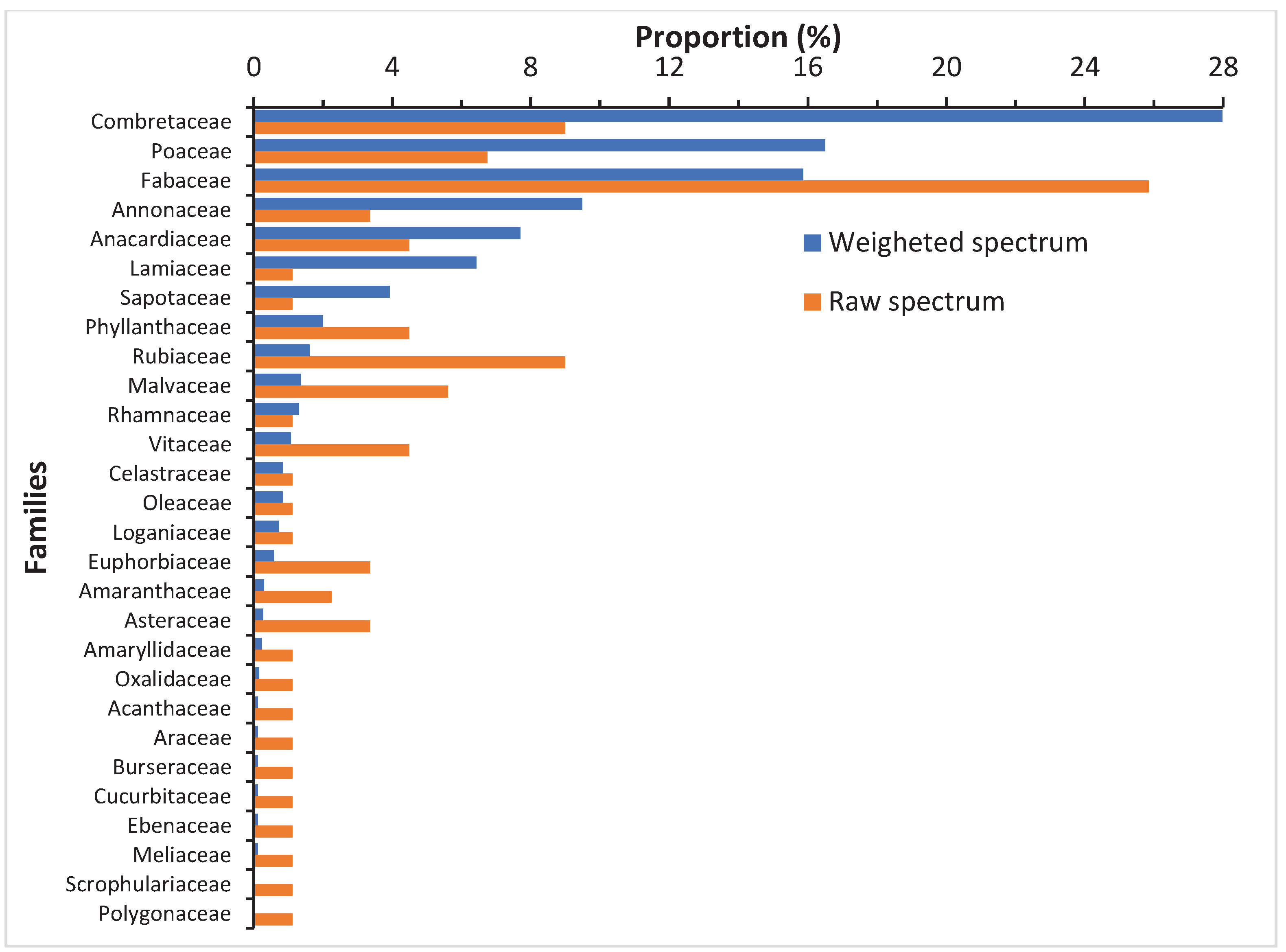
Fabaceae with 23 species is the most important family. This is followed by Combretaceae (8 species), Rubiaceae (8 species), Poaceae (6 species) and Malvaceae (5 species). Combretaceae, Poaceae and Fabaceae are the most dominant families based on the weighted spectrum (
Figure 5).
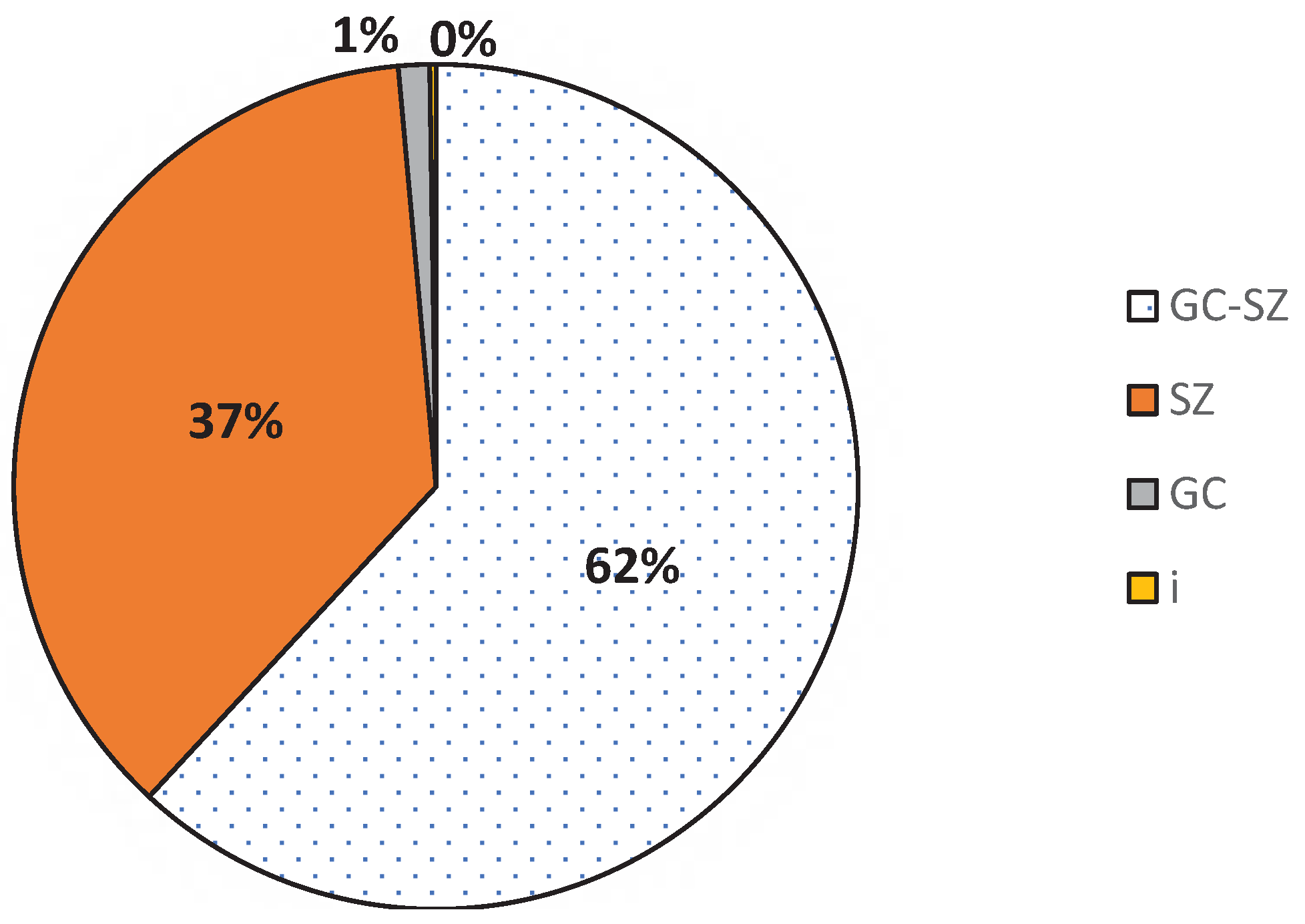
Within the NCF, four phytogeographical plant types are represented. Transitional species (GC-SZ, 61.89%) were the most common. They were followed by the Soudano-Zambezian species (SZ, 36.68%). Guineo-Congolese forest and introduced species were rarely present (
Figure 6). Looking at the raw spectrum based on the number of species per biological type, the trend is the same: 51.81%, 39.33%, 5.62 and 2.25% respectively.
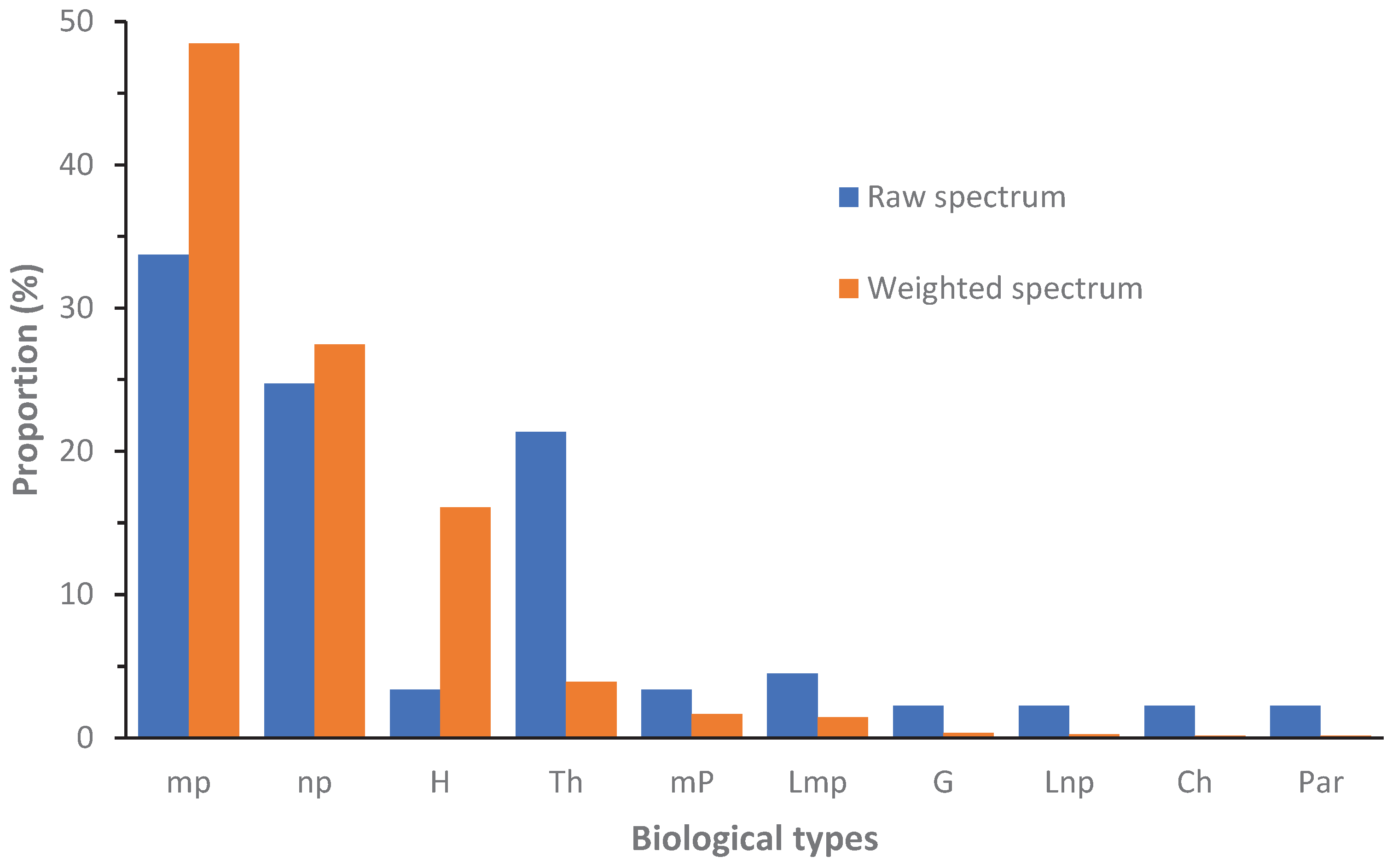
The weighted as raw spectrum showed that the most represented biological types in the NCF are the microphanerophytes and the nanophanerophytes (
Figure 7). The therophytes are more diversified than the hemicryptophytes. However, they are less abundant than the latter. The other biological types of plants are less abundant and less diversified in the NCF than the hemicryptophytes.
The NCF floras included one endangered species (Vitellaria paradoxa) and one threatened species (Pterocarpus erinaceus). The IUCN Red List criteria do not apply to almost half of the species (48.31%). A proportion of 43.82% of plants are of low concern.
3.3. Descriptrion of plant communities
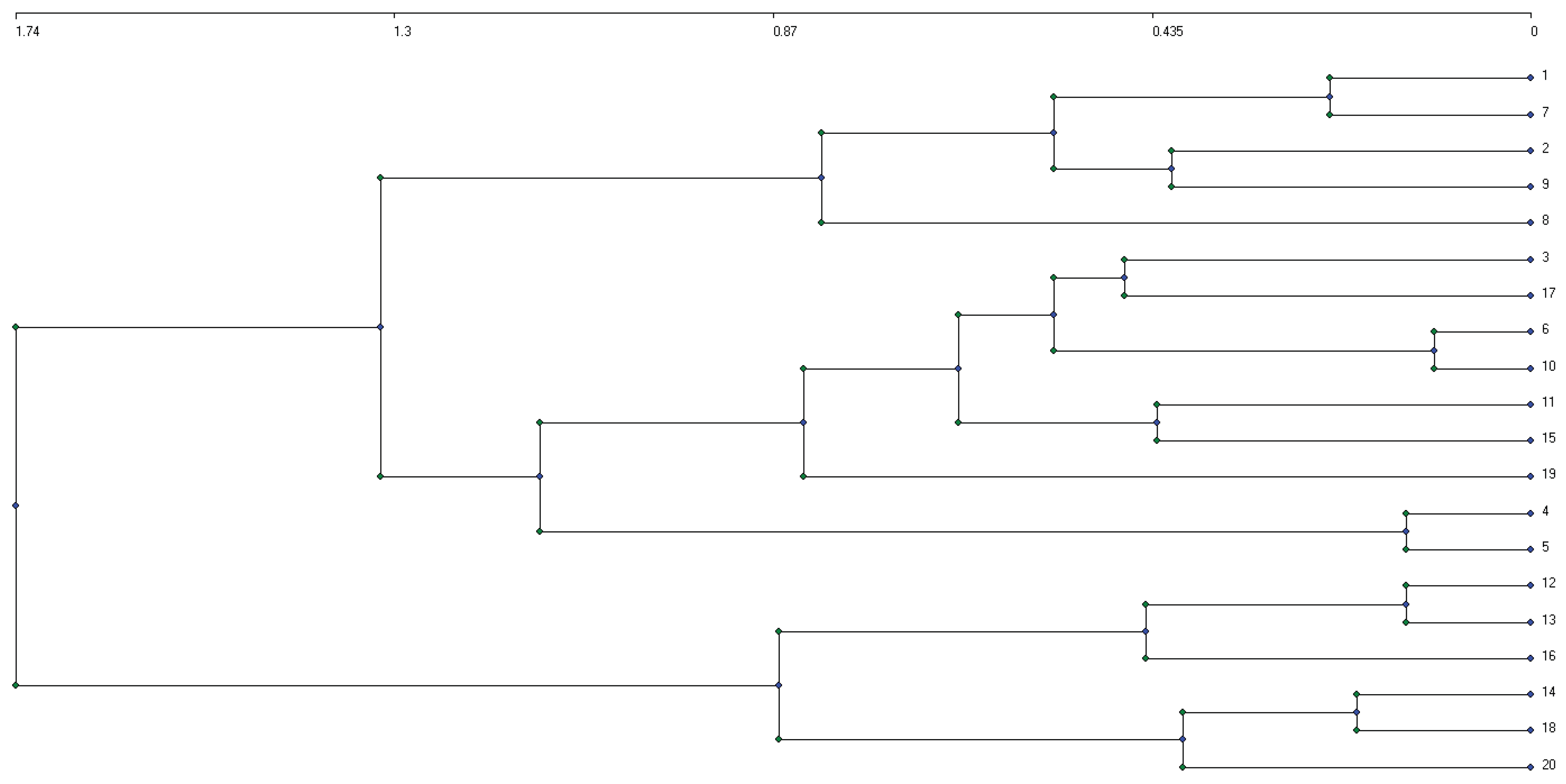
Three plants communities were discrimanated in NCF based on the hierarchical ascendant classification (HAC) at 1.3 of Euclidian distance (
Figure 8). These are: the plant community of
Lannea acida and
Combretum collinum (G1), the plant community of
Vitellaria paradoxa and
Acacia dodgoenii (G2), and the plant community of
Vitellaria paraxa and
Lannea acida (G3).
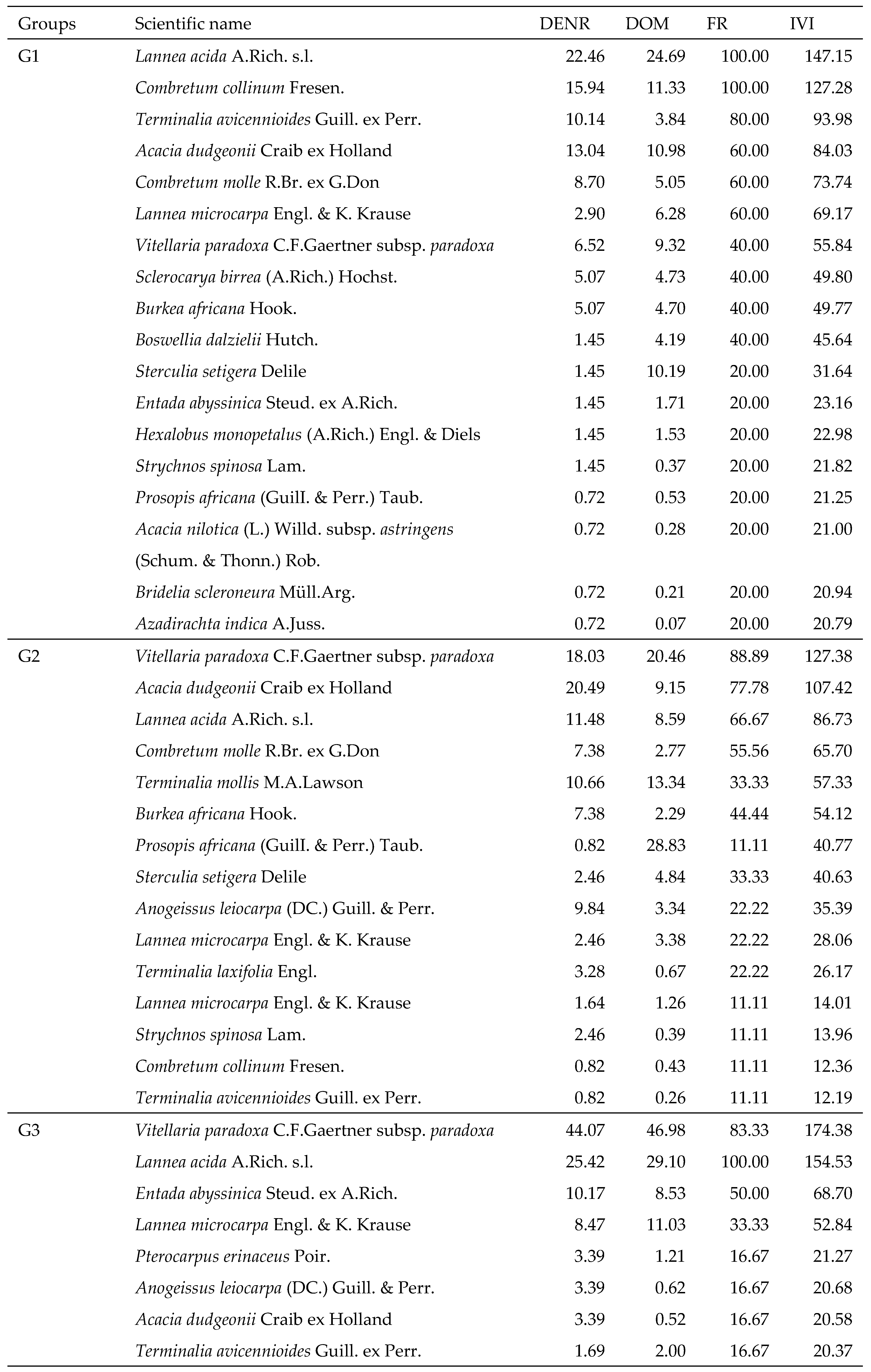
G1 of 5 wooded savannah plots is dominated by
Lannea acida and
Combretum collinum with IVI 147.15 and 127.28. These are followed by
Terminalia avicennioides,
Acacia dudgeonii and
Combretum molle. A total of 18 species of woody plants were recorded. They belong to 10 families. Anacardiaceae (FIV=82.80) and Combretaceae (71.67) are the most important families. The family with the greatest diversity is the Fabaceae, with 5 species (
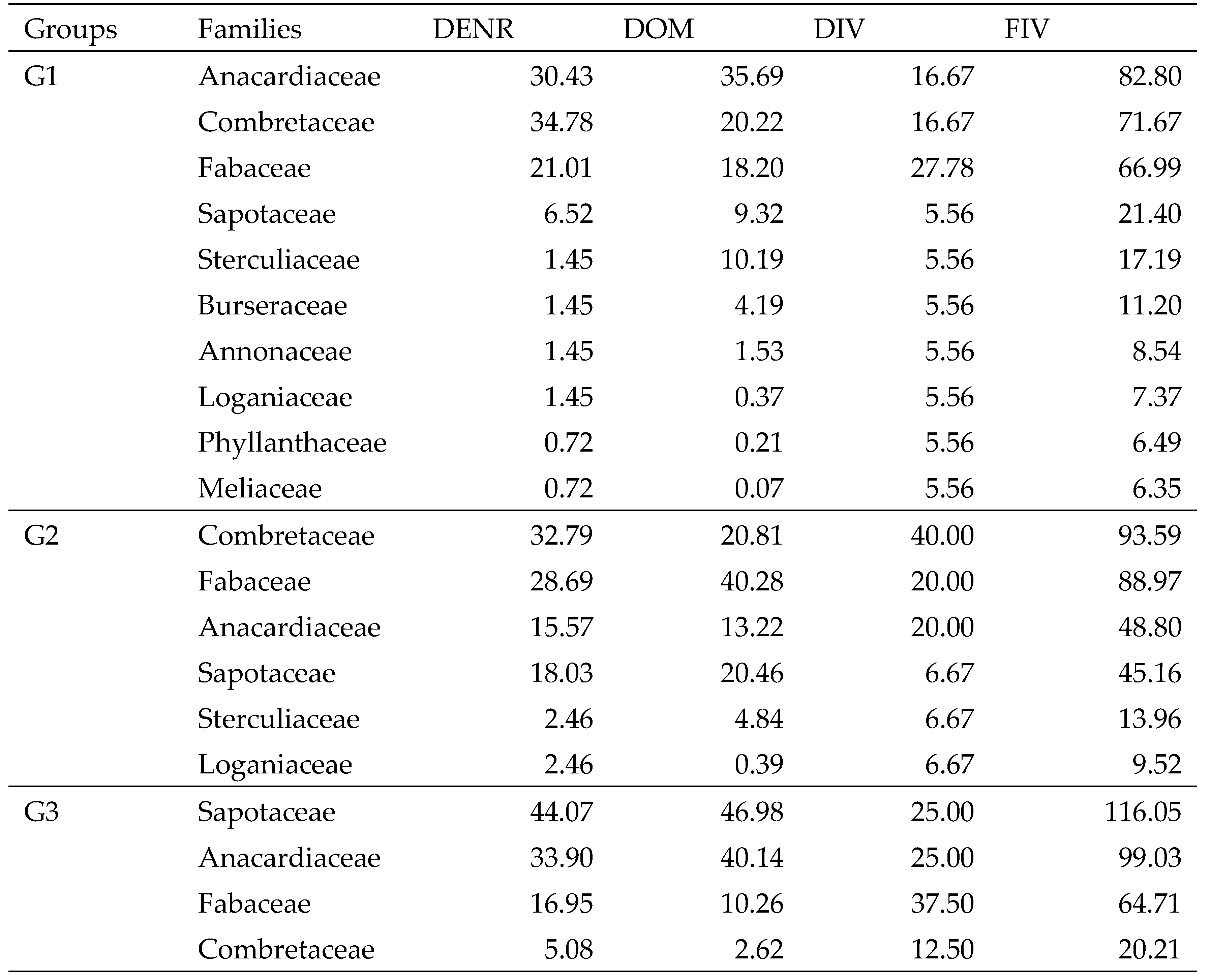
Table 3). The mean density was 276 stems/ha with a basal area of 3.61 m²/ha. The mean height (7.32 m) and mean diameter (12.91 cm) were the lowest.
G2 included 15 woody plant species recorded in 9 plots of shrub/wood savannahs. With IVI 127.38 and 107.42 respectively, Vitellaria paradoxa and Acacia dudgeonii dominated. Then came Lannea acida. The 15 species are distributed in 6 families. Combretaceae is the most diverse (6 species) and important (FIV, 93.59) family. It is followed by the Fabaceae (FIV, 88.97). The tree density corresponds to 136 stems/ha with 4.00 m²/ha. The average total height and diameter are 8.45 m and 19.39 cm respectively.
There are 6 tree savannah plots in G3. Eight species of woody plants that belong to four families in the census. Vitellaria paradoxa and Lannea acida are the most important. Their IVI is 174.38 and 154.53 respectively. Sapotaceae (116.01) and Anacardiaceae (99.03) are the most important families. The density is 98 stems/ha. The basal area is 4.08 m²/ha. This group has the highest mean total height (9.03 m) and mean diameter (22.99 cm).
3.4. Demographic structure
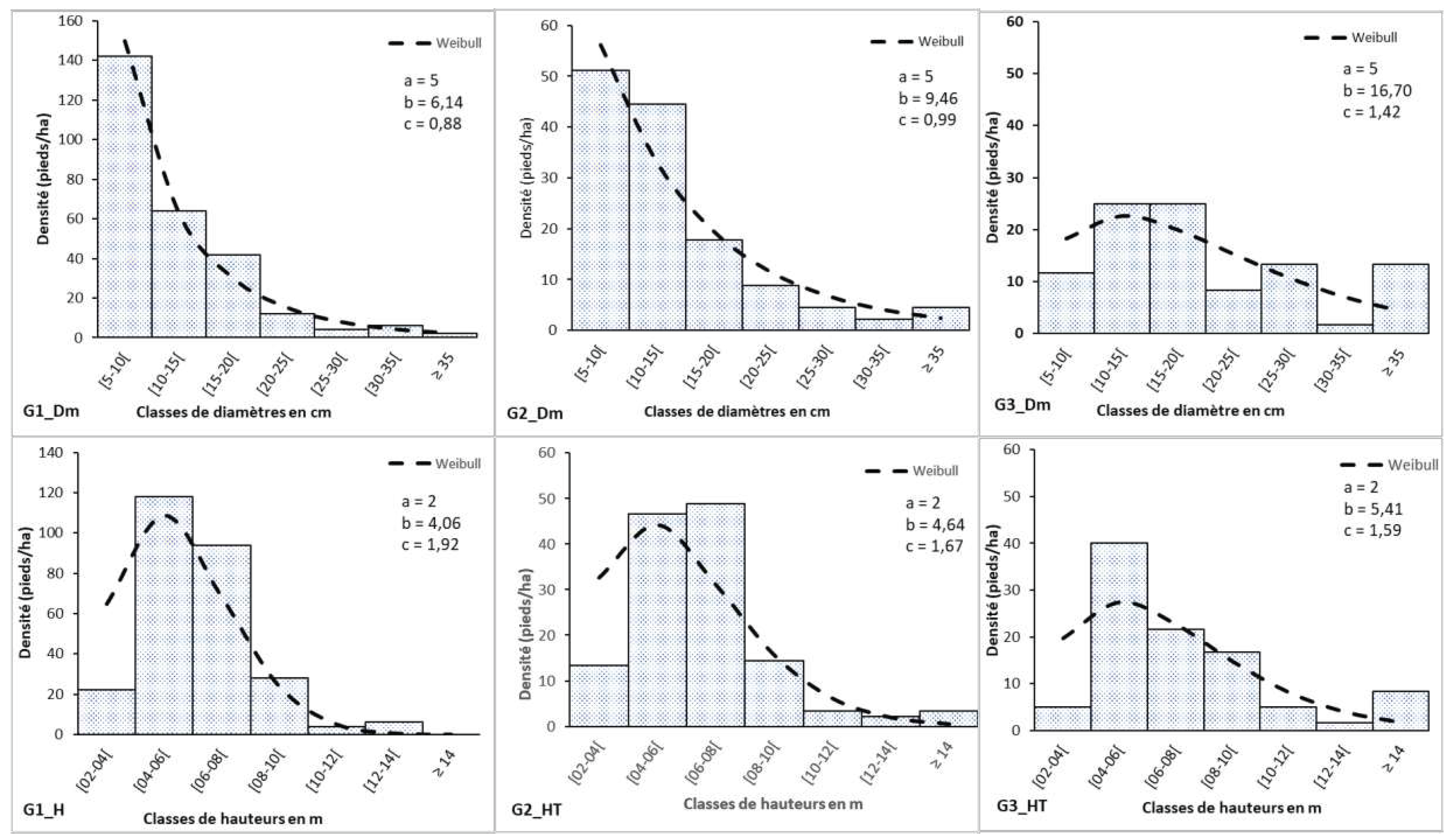
The distribution of trees by diameter class of G1 and G3 has a reversed J-shape (where c<1). The distribution is positively asymmetric for G3. Young trees are most dominant in these groups. The height distribution of individuals within clusters shows positive asymmetry. The theoretical shape coefficient of the Weibull distribution is between 1 and 3.6. Trees with low height are more represented in this structure.
4. Discussion
4.1. Land use change
Analysis of Google Earth images of the Nakpadjouak community forest shows a progressive vegetation dynamic between 2014 and 2020. A regression of tree savannah/shrub savannah and fields/ fallow land was observed. Tree savannas/shrub savannas have mostly regressed in favour of the open forest/wooded savannah class. Fields and fallows, on the other hand, regressed in favour of wooded savannah/shrub savannah. This result is similar to that of Egbelou et al. (2021) [
24]. He noted a progression of vegetation formations at the expense of fields in the Aboudjokopé forest over the period 2012-2018. The same observation was made by Kombate, B et al (2023) in the Alibi I community forest. [
7] The appearance of the open forest/forested savannah class in 2020 is due to the conservation measures implemented by the community. These measures have allowed certain parts of the savannah formation to grow and give rise to open forest/wooded savannah. These measures have also allowed certain parts of the fields to be converted to wooded savannah/shrub savannah[
18]. The result obtained in this study differs from that of Koumoi et al. (2013). They found a regression of vegetation formations in favour of anthropogenic formations. Polo-Akpisso et al. (2015) [
19]also found the same result as Koumoi et al. (2013) [
29] in the Oti-Keran-Mandouri National Park.
4.2. Floristic diversity
The survey of plant life conducted in Nakpadjouak community forest identified a variety of 89 species, classified into 70 genera and 29 families. Similarly, a comparable survey was carried out in a community forest located in the Dankpen prefecture, situated southwards in the same ecological region, yielding 84 species [
18]. The floricule of the NCF is comparatively lower than that of other community forests situated in Togo’s different ecological zones that receive higher amounts of rainfall. In these areas, the NCF’s floricule is only slightly more than double. The Afem-Boussou and Alibi 1 community forests in this ecological zone have a diversity of 163 and 229, respectively [
5], [
16]. In ecological zone 4, the floral diversity is lower than what was found by Egbelou et al. (2021) [
17]. Atakpama et al. (2018) recorded diversity of 109, 188, 217 and 264 in the community forests of Edouwossi-Copé, Agbedougbé, Aboudjokopé and Amavénou respectively.[
12], [
13], [
17]. In addition to the more advantageous conditions found in other ecological zones, the size of the forest and the lack of watercourses in the NCF contribute to limited floristic diversity.
The dominance of Combretaceae followed by Poaceae and Fabaceae characterizes the flora of Nakpadjouak community forest. This tendency points to generally dry climatic conditions in this area[
13], [
30], [
31]. The prevalence of these families is also documented in numerous studies of the region.[
3], [
31]. The prevalence of the Combretaceae family may be attributed to its natural regeneration ability through seedlings, suckering and/or stump sprouting [
32]. Additionally, its abundance highlights the susceptibility of this community forest’s ecosystems to wildfire[
31].
Four biological types were observed, with microphanerophytes being the most common. This prevalence of microphanerophytes has also been recognized by various authors in the same area[
8], [
33]. The dominance of microphanerophytes indicates the savannah nature of the vegetation.
Based on phytogeographic type, the most prominently featured species are the Guineo-Congolese/Sudano-Zambézian transition species (61.89%) and Sudano-Zambézian species (36.68%). This finding aligns with Atakpama et al.’s (2021c) results and with Dimobe
et al.’s (2012) [
3], [
8] observation of Sudano-Zambezian species’ dominance in the Oti-Mandouri wildlife reserve.
4.3. Demographic structure
The horizontal structure in groups G1 and G2 shows an "inverted J" diametric distribution. This result is comparable to that of Kombate et al. (2023) [
34]. This structure reflects the presence of strong anthropogenic pressure in both groups. It also suggests the presence of several future stems to ensure forest reconstitution[
35]. On the other hand, the diametric structure in group G3 shows a positive asymmetric distribution, with a predominance of individuals with diameters between 10 and 20m. This distribution is characteristic of stands with a predominance of small diameter individuals [
36].
The distribution is positively asymmetric, with a predominance of individuals between 4 and 6 m tall in groups G1 and G3, and a predominance of individuals between 6 and 8 m tall in group G2. This distribution reflects a strong representation of young, short individuals. This result is similar to that of Kombate, Bimare et al. (2023b) [
34].
5. Conclusion
Spatio-temporal dynamics indicate forest recovery in the Nakpadjouak community forest. A total of 89 plant species, belonging to 70 genera and 29 families, were identified (Apendix A). Hetopogon contortus (L.) P.Beauv. and Combretum collinum Fresen, followed by Pteleopsis suberosa Engl. & Diels, Annona senegalensis Pers are the dominant species. Dominant families are Combretaceae, Poaceae and Fabaceae. Nakpadjouak community forest is also home one endangered (Vitellaria paradoxa) and one threatened (Pterocarpus erinaceus) species. Forest demographic structures indicate a predominance of small-diamter and low-height trees. Protection against pasture use, wood removal and wildfire management are pivotal to forest land sustainability. The promotion of revenue-generating activities will help to the restoration of the forest to its optimal state.
Author Contributions
“Conceptualization, K.S.E.; methodology, K. S. E., W. A., A. B.; software, K.S.E., W. A.; validation, W.A., E.K., C.A-K.Y. and HvW.; formal analysis, K.S.E., W.A; investigation, K.S.E, W.A.; resources, K.S.E; data curation, K.S.E., W.A.; writing—original draft preparation, K.S.E.; writing—review and editing, K.S.E., HvW, W.A., C.A-K., E.K.; visualization, K.S.E.; supervision, C.A-K, K.E. and H.vW; project administration, K.S.E.; funding acquisition, K.S.E. All authors have read and agreed to the published version of the manuscript.”
Funding
This research was funded by the German Federal Ministry of Education and Research (BMBF), through the West Africa Science Service Center on Climate change and Adapted Land Use (WASCAL) program.
Data Availability Statement
WASCAL data is open access and will be made available when a formal request is received by the institution through the Data Administration Unit.
Acknowledgments
The authors are grateful to the German Federal Ministry of Education and Research (BMBF) for funding this study under the West African Science Service Centre on Climate Change and Adapted Land Use (WASCAL) program. We are grateful to all the stakeholders who invested their time and shared their experiences, and to the anonymous reviewers for their careful reading and comments on this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.”
Appendix A. List of plant species within Nakpadjouak Community Forest (NCF)
| Species |
Families |
Biological types |
Phytogeography |
|
Abutilon mauritianum (Jacq.) Medik. |
Malvaceae |
np |
GC-SZ |
|
Acacia dudgeonii Craib ex Holland |
Fabaceae |
mp |
SZ |
|
Acacia gourmaensis A.Chev. |
Fabaceae |
mp |
SZ |
|
Acacia nilotica (L.) Willd. subsp. astringens (Schum. & Thonn.) Rob. |
Fabaceae |
mp |
SZ |
|
Acacia seyal Delile var. seyal |
Fabaceae |
mp |
SZ |
|
Acalypha ciliata Forssk. |
Euphorbiaceae |
Th |
GC |
|
Agelanthus dodoneifolius (DC.) Polh. & Wiens |
Annonaceae |
Par |
SZ |
|
Albizia lebbeck (L.) Benth. |
Fabaceae |
mp |
i |
|
Alysicarpus ovalifolius (Schumach.) J.Léonard |
Fabaceae |
Th |
GC-SZ |
|
Andropogon gayanus Kunth var. bisquamulatus (Hochst.) Hack. |
Poaceae |
H |
GC-SZ |
|
Annona senegalensis Pers. |
Annonaceae |
np |
GC-SZ |
|
Anogeissus leiocarpa (DC.) Guill. & Perr. |
Combretaceae |
mP |
SZ |
|
Aspilia africana (Pers.) Adams |
Asteraceae |
np |
SZ |
|
Asystasia gangetica (L.) T.Anderson |
Acanthaceae |
np |
GC-SZ |
|
Azadirachta indica A.Juss. |
Meliaceae |
mp |
i |
|
Bidens bipinnata L. |
Asteraceae |
Th |
GC-SZ |
|
Biophytum umbraculum Welw. |
Oxalidaceae |
Th |
GC-SZ |
|
Boswellia dalzielii Hutch.i |
Burseraceae |
mP |
SZ |
|
Bridelia ferruginea Benth. |
Phyllanthaceae |
np |
GC-SZ |
|
Bridelia scleroneura Müll.Arg. |
Phyllanthaceae |
np |
SZ |
|
Burkea africana Hook. |
Fabaceae |
mp |
SZ |
|
Chamaecrista mimosoides (L.) Greene |
Fabaceae |
Th |
GC-SZ |
|
Chamaecrista rotundifolia (Pers.) Greene |
Fabaceae |
Th |
GC-SZ |
|
Cissus palmatifida (Baker) Planch. |
Vitaceae |
Lmp |
SZ |
|
Cissus populnea Guill. & Perr. |
Vitaceae |
Lmp |
GC-SZ |
|
Combretum collinum Fresen. |
Combretaceae |
mp |
GC-SZ |
|
Combretum molle R.Br. ex G.Don |
Combretaceae |
mp |
SZ |
|
Combretum nigricans Lepr. ex var. elliotii (Engl. & Diels) Aubrev. |
Combretaceae |
mp |
SZ |
|
Crinum jagus (J.Thomps.) Dandy |
Amaryllidaceae |
G |
GC-SZ |
|
Crotalaria macrocalyx Benth. |
Fabaceae |
np |
SZ |
|
Crotalaria retusa L. |
Fabaceae |
np |
GC-SZ |
|
Cyphostemma adenocaule (Steud.) Desc. |
Vitaceae |
Lmp |
GC-SZ |
|
Cyphostemma flavicans (Baker) Desc. |
Vitaceae |
H |
GC-SZ |
|
Detarium microcarpum Guill. & Perr. |
Fabaceae |
mp |
SZ |
|
Dichrostachys cinerea (L.) Wight & Am. |
Fabaceae |
mp |
GC-SZ |
|
Diospyros mespiliformis Hochst. ex A.DC. |
Ebenaceae |
mp |
GC-SZ |
|
Entada abyssinica Steud. ex A.Rich. |
Fabaceae |
Lmp |
GC-SZ |
|
Euphorbia convolvuloides Hochst. ex Benth. |
Euphorbiaceae |
Th |
GC |
|
Fadogia agrestis Schweinf. ex Hiern |
Rubiaceae |
np |
SZ |
|
Flueggea virosa (Roxb. ex Willd.) Voigt |
Phyllanthaceae |
np |
GC-SZ |
|
Gardenia aqualla Stapf & Hutch. |
Rubiaceae |
np |
GC-SZ |
|
Gardenia erubescens Stapf & Huteh. |
Rubiaceae |
np |
GC-SZ |
|
Gardenia ternifolia Schumaeh. & Thonn |
Rubiaceae |
np |
GC-SZ |
|
Gymnosporia senegalensis (Lam.) Loes. |
Celastraceae |
np |
SZ |
|
Heteropogon contortus (L.) P.Beauv. |
Poaceae |
H |
GC-SZ |
|
Hexalobus monopetalus (A.Rich.) Engl. & Diels |
Annonaceae |
mp |
SZ |
|
Hyptis spicigera Lam. |
Lamiaceae |
np |
GC-SZ |
|
Lannea acida A.Rich. s.l. |
Anacardiaceae |
mp |
GC-SZ |
|
Lannea microcarpa Engl. & K. Krause |
Anacardiaceae |
mp |
SZ |
|
Loudetia hordeiformis (Stapf) C.E.Hubbard |
Poaceae |
Th |
GC-SZ |
|
Mnesithea granularis (L.) Koning & Sosef |
Poaceae |
Th |
GC-SZ |
|
Mukia maderaspatana (L.) M.Roem. |
Cucurbitaceae |
Lnp |
GC-SZ |
|
Ozoroa insignis Delile |
Anacardiaceae |
np |
SZ |
|
Pandiaka angustifolia (Vahl) Hepper |
Amaranthaceae |
Th |
GC-SZ |
|
Pandiaka involuerata (Moq.) Hook.f. |
Amaranthaceae |
Th |
GC-SZ |
|
Parkia biglobosa (Jacq.) R.Br. ex Benth. |
Fabaceae |
mp |
GC-SZ |
|
Pennisetum polystachion (L.) Schult. |
Poaceae |
Th |
GC-SZ |
|
Phyllanthus amarus Schumach. & Thonn. |
Phyllanthaceae |
Th |
GC |
|
Piliostigma thonningii (Schumach.) Milne-Redh. |
Fabaceae |
np |
GC-SZ |
|
Polygala multiflora Poir. |
Polygonaceae |
Th |
GC-SZ |
|
Prosopis africana (GuilI. & Perr.) Taub. |
Fabaceae |
mp |
SZ |
|
Pteleopsis suberosa Engl. & Diels |
Combretaceae |
mp |
SZ |
|
Pterocarpus erinaceus Poir. |
Fabaceae |
mp |
SZ |
|
Sarcocephalus latifolius (Sm.) E.A.Bruce |
Rubiaceae |
mp |
GC-SZ |
|
Sclerocarya birrea (A.Rich.) Hochst. |
Anacardiaceae |
mp |
SZ |
|
Senna obtusifolia (L.) H.S.Irwin & Barneby |
Fabaceae |
np |
GC-SZ |
|
Sida urens L. |
Malvaceae |
np |
GC |
|
Spermacoce filifolia (Schumach. & Thonn.) J.-P.Lebrun & Stork |
Rubiaceae |
Th |
GC-SZ |
|
Spermacoce radiata (DC.) Hiem |
Rubiaceae |
Th |
GC-SZ |
|
Spermacoce ruelliae DC. |
Rubiaceae |
Th |
GC-SZ |
|
Sterculia setigera Delile |
Malvaceae |
mp |
SZ |
|
Striga hermonthica (DeliIe) Benth. |
Scrophulariaceae |
Par |
SZ |
|
Strychnos spinosa Lam. |
Loganiaceae |
mp |
SZ |
|
Stylochaeton lancifolius Kotsehy & Peyr. |
Araceae |
G |
GC-SZ |
|
Stylosanthes fruticosa (Retz.) Alston |
Fabaceae |
Ch |
GC-SZ |
|
Tephrosia flexuosa G.Don |
Fabaceae |
np |
GC-SZ |
|
Terminalia avicennioides Guill. ex Perr. |
Combretaceae |
mp |
SZ |
|
Terminalia laxifolia Engl. |
Combretaceae |
mp |
SZ |
|
Terminalia macroptera Guill. & Perr. |
Combretaceae |
mp |
SZ |
|
Thelepogon elegans Roth ex Roem. & Sehult. |
Poaceae |
Th |
SZ |
|
Tragia benthamii Baker |
Euphorbiaceae |
Lnp |
GC |
|
Tridax procumbens L. |
Asteraceae |
Ch |
GC-SZ |
|
Triumfetta rhomboidea Jacq. |
Malvaceae |
np |
GC-SZ |
|
Vitellaria paradoxa C.F.Gaertner subsp. paradoxa
|
Sapotaceae |
mp |
SZ |
|
Waltheria indica L. |
Malvaceae |
np |
GC-SZ |
|
Ximenia americana L. |
Oleaceae |
mp |
GC-SZ |
|
Ziziphus mucronata Willd. |
Rhamnaceae |
mp |
SZ |
|
Zornia glochidiata Rchb. ex DC. |
Fabaceae |
Th |
GC-SZ |
References
- K. Adjonou, M. K. Adjonou, M. Kpeli Poukpezi, K. N. Segla, and K. Kokou, “Impacts of traditional practices on biodiversity and structural characteristics of sacred groves in northern Togo, West Africa,” Acta Oecologica, vol. 110, no. 20, p. 103680, 2021. 20 October.
- W. Atakpama, B. W. Atakpama, B. Bilouktime, and Y. A. Woegan, “Ecologie des bosquets sacrés de la préfecture de Tone dans la Région des Savanes au Togo,” Rev. Espac. Geogr. Soc. Marocaine, vol. 56, no. January, p. 23, 2022.
- W. Atakpama, F. W. Atakpama, F. Fousseni, K. Mazama-esso, A. Firmin, and K. Gédéon, “Problématique de gestion durable de la biodiversité des bosquets sacrés de la Région des Savanes au Togo Challenge of sustainable management of biodiversity of sacred groves of the Region of Savannahs in Togo,” Rev. Sci. Technol., Synthèse, vol. 27, no. 22, pp. 22–32, 2021. 20 January.
- K. Kokou and N. Sokpon, “Les forêts sacrées du couloir du Dahomey,” Bois Forêts des Trop., vol. 288, no. 2, pp. 15–23, 2006.
- W. Atakpama, H. W. Atakpama, H. Egbelou, B. Kombate, S. Biaou, and K. Batawila, “Diversité et structure des formations végétales de la forêt communautaire d ’ Alibi -1 au Togo,” Rev. Sci. Technol., Synthèse, vol. 29, no. 1, pp. 06–20, 2023.
- W. Atakpama, H. W. Atakpama, H. Egbelou, M. Samarou, and F. Fousseni, “La foresterie communautaire au Togo: Où en sommes-nous?,” Rev. Marocaine des Sci. Agron. Vétérinaires, vol. 11, no. December, pp. 532–543, 2023.
- B. Kombate et al., “Dynamique de l’occupation de sol et modélisation du carbone de la Forêt Communautaire d’Alibi 1,” Ann. la Rech. For. en Algérie, vol. 12, no. 2, p. Sous presse, 2023.
- K. Dimobe, K. K. Dimobe, K. Wala, K. Batawila, M. Dourma, Y. A. Woegan, and K. Akpagana, “Analyse spatiale des différentes formes de pressions anthropiques dans la réserve de faune de l’Oti-Mandouri (Togo),” VertigO, no. Hors-série 14, 2012.
- F. Folega et al., “Assessment and impact of anthropogenic disturbances in protected areas of northern Togo,” For. Stud. China, vol. 14, no. 3, pp. 216–223, 2012.
- A. Polo-Akpisso et al., “Habitat biophysical and spatial patterns assessment within Oti-Keran-Mandouri protected area network in Togo,” Int. J. Biodivers. Conserv., vol. 10, no. 5, pp. 214–229, 2018.
- W. Atakpama, B. W. Atakpama, B. Badjare, E. Yawo, K. Aladji, and K. Batawila, “fosse de Doungh au Togo Alarming degradation of forest resources in the classified forest of Doungh pit in Togo,” African J. L. Policy Geospatial Sci., vol. 6, no. May, pp. 2657–2664, 2023.
- F. Folega et al., “Caractérisation écologique de la Foret Communautaire d’Edouwossi-Cope (région des Plateaux-Togo),” J. Rech. Sci. Univ. Lomé, vol. 19, no. 3, pp. 47–61, 2017.
- W. Atakpama, E. W. Atakpama, E. Asseki, E. Kpemissi Amana, C. Koudegnan, K. Batawila, and K. Akpagana, “mportance socio-économique de la forêt communautaire d’Edouwossi-copé dans la préfecture d’Amou au Togo.,” Rev. Marocaine des Sci. Agron. Vétérinaires, vol. 6, no. 1, pp. 55–63, 2018.
- H. Ern, “Die Vegetation Togos. Gliederung, Gefährdung, Erhaltung Author(s): Hartmut Ern Source:,” Willdenowi, vol. 9, pp. 295–312, 1979.
- J. F. Brunel, P. J. F. Brunel, P. Hiepko, and H. Scholz, “Flore analytique du Togo: Phanerogames,” Englera, no. 4, pp. 3–751, 1984.
- F. Folega et al., “Land Use Change and the Structural Diversity of Affem Boussou Community Forest in the Tchamba 1 Commune (Tchamba Prefecture, Togo),” Conservation, vol. 3, no. 3, pp. 346–362, 2023.
- H. Egbelou, W. H. Egbelou, W. Atakpama, M. Dourma, and K. Akpagana, “Dynamique spatio-temporelle et flore de la forêt d ’ Aboudjokopé au Togo,” Rev. Sci. Technol., Synth., vol. 27, no. December, pp. 37–50, 2021.
- W. Atakpama, H. W. Atakpama, H. Egbelou, F. Folega, C. Afo, K. Batawila, and K. Akpagana, “Diversité floristique des forêts communautaires de la préfecture de Dankpen au Togo,” Rev. Marocaine des Sci. Agron. Vétérinaires, vol. 10 (4), no. December, pp. 548–557, 2022.
- A. Polo-Akpisso et al., “Plant Species Characteristics and Woody Plant Community Types within the Historical Range of Savannah Elephant, Loxodonta africana Blumenbach 1797 in Northern Togo (West Africa),” Annu. Res. Rev. Biol., vol. 7, no. 5, pp. 283–299, 2015.
- A. Thiombiano, R. A. Thiombiano, R. Glèlè-Kakai, P. Bayen, J. I. Boussim, and A. Mahamane, “Méthodes et dispositifs d’inventaires forestiers en Afrique de l’Ouest : état des lieux et propositions pour une harmonisation,” Ann. des Sci. Agron., vol. 19, no. April, pp. 15–31, 2015.
- W. Atakpama, K. M. W. W. Atakpama, K. M. W. Agbetanu, L. L. Atara, S. Biaou, K. Batawila, and K. Akpagana, “Biodiversité et gestion des feux de végétation dans la réserve de faune d ’ Abdoulaye au Togo Biodiversity and management of burn fire within Abdoulaye Wildlife Forest in Togo,” Rev. Sci. Technol., Synthèse, vol. 27, no. December, pp. 51–64, 2021.
- J. Blanquet-Braun, Title: Plant Sociology: the Study of Plant Communities. 1933.
- M. Samarou et al., “Caractérisation écologique et structurale des parcs à tamarinier ( Tamarindus indica L., Fabaceae ) dans la zone soudanienne du Togo ( Afrique de l ’ Ouest ),” Rev Écosystèmes Paysages, vol. 02, no. July, pp. 109–125, 2022.
- H. Egbelou, W. H. Egbelou, W. Atakpama, M. Dourma, and K. Akpagana, “Dynamique spatio-temporelle et flore de la forêt d ’ Aboudjokopé au Togo,” Rev. Sci. Technol., Synth., vol. 27, no. December, pp. 37–50, 2021.
- F. White, La vegetation de l’Afrique. Paris: ORSTOM-UNESCO, 1986.
- L. Assi-Ake, “Reviews and Announcements,” Taxon, vol. 34, no. 4, pp. 739–753, 1985.
- D. M. Bawa, K. D. M. Bawa, K. Wala, F. Folega, and K. Akpagana, “Caractéristiques floristiques et structurales de la forêt communautaire d ’ Agbandi au centre du Togo ( Afrique de l ’ ouest ) Floristic and structural characteristics of Agbandi communi...,” Rev Écosystèmes Paysages, vol. 02, no. july, pp. 55–74, 2022.
- P. May. 2004.
- Z. Koumoi, A. Z. Koumoi, A. Alassane, M. Djangbedja, T. Boukpessi, and A.-E. Kouya, “Dynamique spatio-temporelle de l’occupation du sol dans le centre-Togo,” Rev. Géographie du LARDYMES, Univ. Lomé, vol. 7, no. 10, pp. 163–172, 2013.
- A. Aubreville, Flore forestière forestière soudano-guinéenne : A.O.F.-Cameroun-A.E.F., Societe d’. Paris: Office de la recherche scientifique d’Outre mer, 1950.
- W. Atakpama, K. B. W. Atakpama, K. B. Amegnaglo, B. Afelu, F. Folega, K. Batawila, and K. Akpagana, “Biodiversité et biomasse pyrophyte au Togo,” VertigO, vol. 19, no. 3, p. 21, 2019.
- R. Bellefontaine, “Pour de nombreux ligneux, la reproduction sexuée n’est pas la seule voie analyse de 875 cas,” Sécheresse, vol. 16, no. 4, pp. 315–317, 2005.
- H. Abdourhamane, B. H. Abdourhamane, B. Morou, H. Rabiou, and A. Amhamane, “Caractéristiques floristiques, diversité et structure de la végétation ligneuse dans le Centre-Sud du Niger : cas du complexe des forêts classées de Dan kada Dodo-Dan Gado,” Int. J. Biol. Chem. Sci., vol. 7, no. 3, p. 1048, 2013.
- et al. , “Structure et modélisation du carbone de la Forêt Classée de Missahohoé au Togo.,” African J. L. Policy Geospatial Sci. 6(1) 42-61., vol. 6, no. 1, pp. 42–61, 2023.
- R. Tsoumou, K. J. R. Tsoumou, K. J. Lumandé, J. P. Kampé, and J. D. Nzila, “Estimation de la quantité de carbone séquestré par la Forêt Modèle de Dimonika (Sud-ouest de la République du Congo),” Rev. Sci. Tech. Forêt &Environnement du Bassin du Congo, vol. 6, no. Avril, pp. 39–45, 2016.
- A. B. Kebenzikato et al., “Distribution et structure des parcs à Adansonia digitata L.(baobab) au Togo (Afrique de l’Ouest),” Afrique Sci., vol. 10, no. 2, pp. 434–449, 2014.
Figure 1.
Location and sampling disign of the Nakpadjouak Community Forest of in Togo, West Africa.
Figure 1.
Location and sampling disign of the Nakpadjouak Community Forest of in Togo, West Africa.
Figure 2.
Land use in Nakpadjouak community forest in 2014 and 2020.
Figure 2.
Land use in Nakpadjouak community forest in 2014 and 2020.
Figure 3.
Change of land use in NCF.
Figure 3.
Change of land use in NCF.
Figure 4.
Weighted and raw spectrums of the plant species in the NCF.
Figure 4.
Weighted and raw spectrums of the plant species in the NCF.
Figure 5.
Weighted and raw spectrums of families in the NCF.
Figure 5.
Weighted and raw spectrums of families in the NCF.
Figure 6.
Weighted phytogeographical spectrum of plant species in the NCF. GC = Guineo-Congolese, SZ = Soudano-Zambezian, GC-SZ = Guineo-Congolese/Soudano-Zambezian, I = Introduced.
Figure 6.
Weighted phytogeographical spectrum of plant species in the NCF. GC = Guineo-Congolese, SZ = Soudano-Zambezian, GC-SZ = Guineo-Congolese/Soudano-Zambezian, I = Introduced.
Figure 7.
Weighted and raw spectrums of plant biological types in the NCF. Ch = chamephyte, G = geophyte, H = hemicryptophyte, Lmp = liana mesophanerophyte, Lnp = liana microphanerophyte, np = nanophanerophyte, mp = microphanerophyte, mP = mesophanerophyte, Par = Parasit, Th = therophyte.
Figure 7.
Weighted and raw spectrums of plant biological types in the NCF. Ch = chamephyte, G = geophyte, H = hemicryptophyte, Lmp = liana mesophanerophyte, Lnp = liana microphanerophyte, np = nanophanerophyte, mp = microphanerophyte, mP = mesophanerophyte, Par = Parasit, Th = therophyte.
Figure 8.
Discrimination of plots according to Hierachical Ascendant Classification (HAC).
Figure 8.
Discrimination of plots according to Hierachical Ascendant Classification (HAC).
Figure 9.
Nakpadjouak Community Forest Plant Community Diameter and Height Structure.
Figure 9.
Nakpadjouak Community Forest Plant Community Diameter and Height Structure.
Table 1.
Change rate of land use of NCF from 2014 to 2020.
Table 1.
Change rate of land use of NCF from 2014 to 2020.
Table 2.
Land use transition matrix 2014-2020.
Table 2.
Land use transition matrix 2014-2020.
Table 3.
Importance Value Indices (IVI) of woody species within each plant community.
Table 3.
Importance Value Indices (IVI) of woody species within each plant community.
Table 4.
Table 4. Family Important Values (FIV) of woody plants within each plant community.
Table 4.
Table 4. Family Important Values (FIV) of woody plants within each plant community.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).